Abstract
Sodium‐glucose cotransporter‐2 (SGLT‐2) inhibitors are antidiabetic drugs with associated safety concerns regarding the risk of genital and urinary tract infections. This study assessed the risk of genital and urinary tract infections associated with prescription of SGLT‐2 inhibitors as an add‐on therapy to metformin in patients with type 2 diabetes mellitus (T2DM) compared to dipeptidyl peptidase‐4 (DPP‐4) inhibitors, sulfonylurea (SU), and thiazolidinedione (TZD). We conducted a retrospective cohort study using the NHIS—National Health Insurance—Database in Korea from 2014 to 2017. Patients aged ≥19 years and those diagnosed with T2DM prior to drug prescription were enrolled. The outcomes were genital and urinary tract infections. Analysis was performed using Cox's proportional hazard model following 1:1 propensity score matching to calculate the hazard ratio (HR) with a 95% confidence interval (CI). Among the 107 131 patients included in the study, a total of 7738, 7145, and 2175 patients were assigned to the DPP‐4 inhibitors, SU, and TZD comparator groups, using the propensity score (PS) of each comparator based on 7741 people in the assessed drug SGLT‐2 inhibitor group. SGLT‐2 inhibitors were associated with a higher risk of genital infections than DPP‐4 inhibitors (HR: 2.39, 95% CI: 2.07–2.76), SU (HR: 3.23, 95% CI: 2.73–3.81), and TZD (HR: 3.23, 95% CI: 2.35–4.44), as an add‐on therapy to metformin. Similar results were observed for the risk of urinary tract infections. In conclusion, SGLT‐2 inhibitors are significantly associated with a higher risk of genital and urinary tract infections compared to DPP‐4 inhibitors, SU, and TZD.
Keywords: genital infection, SGLT‐2 inhibitors, type 2 diabetes mellitus, urinary tract infection
Our finding demonstrates that SGLT‐2 inhibitors are significantly associated with a higher risk of genital and urinary tract infections compared to DPP‐4 inhibitors, SU, and TZD.

Abbreviations
- CI
confidence interval
- DPP‐4
dipeptidyl peptidase‐4
- HR
hazard ratio
- PS
propensity score
- SGLT‐2
sodium‐glucose cotransporter‐2
- SU
sulfonylurea
- T2DM
type 2 diabetes mellitus
- TZD
thiazolidinedione
1. INTRODUCTION
The global prevalence of diabetes is continuously increasing with the rate among adults increasing from 4.7% in 1980 to 8.5% in 2014, according to the World Health Organization (WHO). 1 Moreover, considering that high blood glucose is associated with an increased risk of complications, including cardiovascular disease and kidney failure, glycemic control is imperative for diabetic patients. Hence, metformin is recommended as a first‐line therapy for type 2 diabetes mellitus (T2DM), 2 while combination therapy can be administered depending on the patient's comorbidities.
SGLT‐2 inhibitors lower blood glucose levels by inhibiting SGLT‐2 in the renal, reducing reabsorption of glucose, and promoting urinary excretion. Since the mechanism of action for these drugs is not related to insulin secretion, they have a lower risk of hypoglycemia compared to other glucose‐lowering agents. 3 , 4 Moreover, recent studies have shown that SGLT‐2 inhibitors offer cardiovascular benefits, 5 , 6 making them an appropriate option for patients with cardiovascular disease (CVD).
However, safety concerns have been raised regarding the increased risk of genital and urinary tract infections associated with the increased glucose concentration in the urinary tract induced by SGLT‐2 inhibitors. 7 , 8 In 2015, the U.S. Food and Drug Administration warned about the increased risk of urinary tract infections when taking SGLT‐2 inhibitors. 9 In 2018, it was further noted that rare cases of serious genital infections occurred following administration of SGLT‐2 inhibitors. 10 Although several studies have supported the association between SGLT‐2 inhibitors and an increased risk of genital infection, the results were inconsistent for urinary tract infections. 11 , 12 , 13
In Korea, specifically, 46 and 71 cases of genital and urinary infections were reported to the Korea Adverse Event Reporting System (KAERS) 15 between 2016 and 2020, with administration of SGLT‐2 inhibitors as the suspected cause. In contrast, 0 and 18 reports were made for other second‐line antidiabetic drugs during the same time period, respectively. Meanwhile, SGLT‐2 inhibitors have been on the market in Korea for a shorter time compared to other glucose‐lowering agents, and only few large‐scale studies have been conducted in Asian populations. Therefore, the current study sought to identify the risk associated with development of genital and urinary tract infections following administration of SGLT‐2 inhibitors as an add‐on therapy to metformin in patients with T2DM, using the national claims database in Korea.
2. MATERIALS AND METHODS
2.1. Data source
We conducted a retrospective cohort study using the NHIS‐customized data (NHIS‐2019‐4‐349) made available by National Health Insurance Service (NHIS). The NHIS database has covered almost 98% of the total population in Korea. It contains patient demographic information such as sex, date of birth, date of death, and medical treatment records, including details of disease and prescriptions. 16 The study was approved by the institutional review board of the Korean Institute of Drug Safety & Risk Management (KIDS; KIDS‐2019‐1).
2.2. Study population
This retrospective cohort study was comprised of patients aged ≥19 years (both inpatient outcome and outpatient visits), diagnosed with T2DM for the first time between January 1, 2014 and December 31, 2017, prescribed metformin as the first primary medication, and treated with at least one of the following classes as combination therapy: SGLT‐2 inhibitors, sulfonylurea (SU), meglitinide, thiazolidinedione (TZD), alpha‐glucosidase (AG) inhibitors, dipeptidyl peptidase‐4 (DPP‐4) inhibitors, or glucagon‐like peptide‐1 (GLP‐1) agonist. We identified an index date of each patient's first prescription with SGLT‐2 inhibitors, SU, meglitinide, TZD, AG inhibitors, DPP‐4 inhibitors, and GLP‐1 agonist. We excluded patients diagnosed with T2DM or those who were prescribed noninsulin antidiabetic drugs (ADs) in the 3 years prior to their index date. And patients diagnosed with T1DM, end‐stage renal disease, cancer, HIV infection, primary immunodeficiency, or aplastic anemia, as well as those treated with Foley/Nelaton catheterization or insulin within 1 year prior to the index date, were excluded. Also, we excluded patients if they did not receive metformin during the follow‐up period, were not diagnosed with T2DM before the index date, or were prescribed more than one class of second‐line medications (Figure 1).
FIGURE 1.
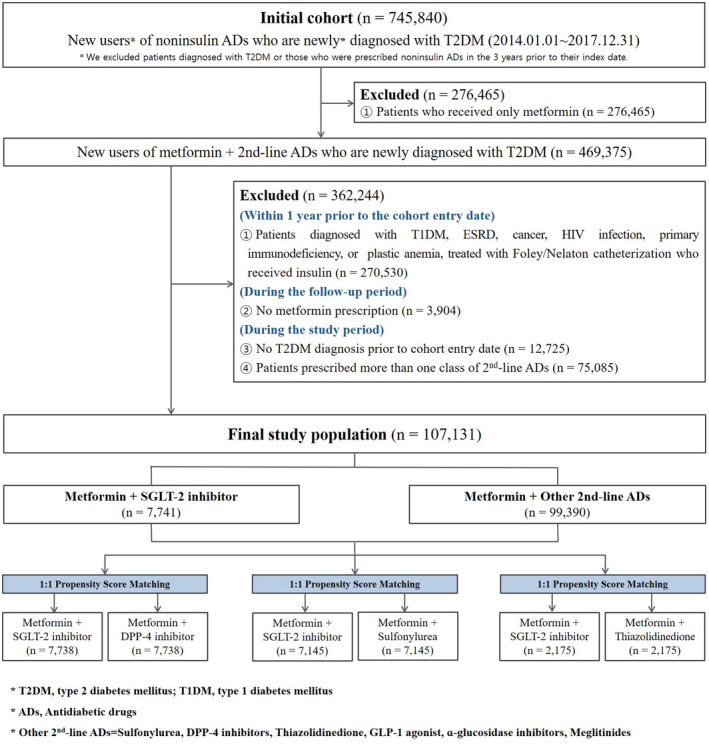
Flow chart
2.3. Outcomes and exposure
The primary outcomes were the occurrence of genital infections and urinary tract infections (UTIs). The definition varied by sex, since some diagnosis codes are sex specific. Outcomes for genital infection included KCD‐7 codes of Candida infections, vaginitis, vulvitis, gonococcal infections, and inflammatory disease of the uterus for female, whereas candidal balanitis, orchitis, epididymitis, and balanoposthitis were defined as the outcomes for male. UTIs were defined as pyelonephritis, cystitis, urethritis, and urethral syndrome for female and inflammatory diseases of the prostate in male (Table S1 in Appendix S1). The follow‐up was terminated when any of the following were first observed: (1) occurrence of a study outcome (genital infections, urinary tract infections); (2) death; and (3) end of the study period (December 31, 2017).
The exposure of main interest was the use of SGLT‐2 inhibitors, including dapagliflozin, empagliflozin, and ipragliflozin. We identified all of the SGLT‐2 inhibitors used in the year prior to the index date except for canagliflozin. The NHIS dataset included the Korean ingredient code of the drug, the date the prescription was written, the number of days of supply, and quantity. We used this data to identify prescriptions for SGLT‐2 inhibitors and any concomitantly used drugs.
2.4. Covariates
Based on previous studies 11 , 12 , 13 , 14 and advice from clinical experts, we described the demographic information (sex and age), drug use (e.g., broad‐spectrum antibiotics, NSAIDs, estrogen, antifungal drugs, antihypertensive drug, immunosuppressants, systemic steroid, anticonvulsants), medical treatment (e.g., Foley/Nelaton catheterization), medical history (e.g., diabetes, moderate or severe renal diseases, stroke, ischemic heart disease, hypertension, hyperlipidemia, congestive heart failure, cardiac arrhythmias, valvular disease, chronic pulmonary diseases, pulmonary circulation disorders, peripheral vascular disease, hemiplegia, neurodegenerative disorders, hypothyroidism, liver disease, peptic ulcer disease without bleeding, rheumatoid arthritis, collagen vascular diseases, coagulopathy, obesity, weight loss, fluid and electrolyte disorders, blood loss anemia, deficiency anemia, alcohol abuse, drug abuse, psychosis, depression, pregnancy), and Charlson Comorbidity Index (CCI) 17 within 1 year prior to the index date were individually collected.
2.5. Statistical analysis
The general characteristics of each drug exposure group (SGLT‐2 inhibitors, SU, meglitinide, TZD, AG inhibitors, DPP‐4 inhibitors, GLP‐1 agonist + metformin) were examined. With the exception of the small population groups (n < 1000) comprising meglitinide, AG inhibitors, and GLP‐1 against exposure groups, to mitigate the potential of confounding factors, 1:1 PS‐matched pairs for the SGLT‐2 inhibitor group versus DPP4‐inhibitor, SU, and TZD groups were modeled on cohort entry.
Baseline characteristics were summarized for patient groups in categorical variables as frequency and percentage and compared using the Chi‐square or Fisher's exact test or were summarized for patient groups as continuous variables using mean ± standard deviation (SD), and compared using the t‐test as appropriate. Then we used propensity score (PS) matching (caliper 1:1 matching) to reduce the potential selection bias in an observational study and balance the distribution between the two groups excluding the confounding variables. A logistic regression model was fitted to estimate the propensity score (i.e., probability of inclusion in the treatment group), 18 and standardized difference (STD) was the statistic used for the assessment of covariate balance after PS matching. An STD greater than 0.1 can be considered as a sign of a meaningful imbalance between the study groups.
We used the Cox proportional hazard regression and determined the hazard ratios (HR) with 95% confidence intervals (CI) to estimate the risk of genital infections and UTIs associated with SGLT‐2 inhibitors. We also conducted two subgroup analyses stratified by age and sex. Finally, we performed sensitivity in two ways described in previous studies, the first limiting the follow‐up period to 1 year. 11 According to a previous report (Dave et al.), urinary tract infections and genital infections occur mostly within 52 weeks of taking SGLT‐2 inhibitors. And, the second was an analysis of high‐risk patients over 60 years of age. 19 Conventionally, probabilities lower than 0.05 are considered significant or statistically significant, and HR cannot include unity (one) in 95% CI. We used the statistical software SAS Enterprise Guide 7.15 (SAS Institute) provided by the NHIS remote server. 20
3. RESULTS
During the 4‐year study period from January 1, 2014, to December 31, 2017, a total of 745 840 patients aged ≥19 years were diagnosed with T2DM and prescribed metformin as the primary and treated with type of ADs. After exclusion criteria were applied, a total of 107 131 patients were enrolled, of whom 78 808 (73.6%) were assigned to the DPP‐4 inhibitor group, 17 936 (16.7%) to the SU group, 7741 (7.2%) to the SGLT‐2 inhibitor group, 2264 (2.1%) to the TZD group, and 382 (0.4%) were allocated to the other noninsulin ADs (meglitinide, AG inhibitors, GLP‐1 agonist) group as add‐on therapies to metformin.
Among the 107 131 patients included in the study, a total of 7738, 7145, and 2175 patients were assigned to the DPP‐4 inhibitors, SU, and TZD comparator groups, using the PS of each comparator based on 7741 people in the assessed drug SGLT‐2 inhibitor group. We used 1:1 PS matching within a maximum caliper of 0.005 through a multiple logistic regression analysis. The DPP‐4 inhibitor group, SU group, and TZD group were comparable regarding the baseline covariates with no STD exceeding 10% (Table 1). An STD >10% can be considered as a sign of meaningful imbalance between study groups.
TABLE 1.
Baseline patient characteristics after 1:1 PS matching
| Category | SGLT‐2 inhibitors (7738) | DPP‐4 inhibitors (7738) | STD (%) | SGLT‐2 inhibitors (7145) | SU (7145) | STD (%) | SGLT‐2 inhibitors (2175) | TZD (2175) | STD (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | N | % | N | % | N | % | ||||
| Sex | |||||||||||||||
| Male | 5181 | 66.96 | 5184 | 66.99 | −0.1 | 4877 | 68.26 | 4862 | 68.05 | 0.5 | 1489 | 68.46 | 1509 | 69.38 | −2.0 |
| Female | 2557 | 33.04 | 2554 | 33.01 | 0.1 | 2268 | 31.74 | 2283 | 31.95 | −0.5 | 686 | 31.54 | 666 | 30.62 | 2.0 |
| Age | |||||||||||||||
| Mean (SD) | 47.6 (11.0) | 48.0 (0.9) | −5.1 | 48.3 (10.8) | 48.9 (10.9) | −5.5 | 52.5 (11.3) | 53.1 (11.3) | −5.3 | ||||||
| 19–29 | 401 | 5.18 | 386 | 4.99 | 0.9 | 277 | 3.88 | 277 | 3.88 | 0.0 | 63 | 2.90 | 61 | 2.80 | 0.6 |
| 30–39 | 1335 | 17.25 | 1363 | 17.61 | −1.0 | 1143 | 16.00 | 1140 | 15.96 | 0.1 | 177 | 8.14 | 172 | 7.91 | 0.8 |
| 40–49 | 2571 | 33.23 | 2587 | 33.43 | −0.4 | 2370 | 33.17 | 2353 | 32.93 | 0.5 | 546 | 25.10 | 541 | 24.87 | 0.5 |
| 50–59 | 2414 | 31.20 | 2398 | 30.99 | 0.4 | 2348 | 32.86 | 2337 | 32.71 | 0.3 | 783 | 36.00 | 796 | 36.60 | −1.2 |
| 60–69 | 853 | 11.02 | 841 | 10.87 | 0.5 | 844 | 11.81 | 866 | 12.12 | −0.9 | 467 | 21.47 | 482 | 22.16 | −1.7 |
| 70–79 | 143 | 1.85 | 144 | 1.86 | −0.1 | 142 | 1.99 | 147 | 2.06 | −0.5 | 121 | 5.56 | 107 | 4.92 | 2.9 |
| 80+ | 21 | 0.27 | 19 | 0.25 | 0.5 | 21 | 0.29 | 25 | 0.35 | −1.0 | 18 | 0.83 | 16 | 0.74 | 1.0 |
| Drug use | |||||||||||||||
| Broad spectrum antibiotic | 1742 | 22.51 | 1712 | 22.12 | 0.9 | 1526 | 21.36 | 1504 | 21.05 | 0.8 | 440 | 20.23 | 411 | 18.90 | 3.4 |
| NSAIDs | 2749 | 35.53 | 2750 | 35.54 | 0.0 | 2511 | 35.14 | 2539 | 35.54 | −0.8 | 771 | 35.45 | 737 | 33.89 | 3.3 |
| Estrogen | 36 | 0.47 | 36 | 0.47 | 0.0 | 31 | 0.43 | 31 | 0.43 | 0.0 | 5 | 0.23 | 8 | 0.37 | −2.5 |
| Antifungal drugs | 695 | 8.98 | 682 | 8.81 | 0.6 | 592 | 8.29 | 611 | 8.55 | −1.0 | 163 | 7.49 | 158 | 7.26 | 0.9 |
| Antihypertensive agent | 3215 | 41.55 | 3187 | 41.19 | 0.7 | 2903 | 40.63 | 2882 | 40.34 | 0.6 | 916 | 42.11 | 934 | 42.94 | −1.7 |
| Anticonvulsants | 5 | 0.06 | 4 | 0.05 | 0.5 | 4 | 0.06 | 8 | 0.11 | −1.9 | 1 | 0.05 | 1 | 0.05 | 0.0 |
| Immunosuppressive drug | — | — | — | — | 0.0 | — | — | — | — | 0.0 | — | — | — | — | 0.0 |
| Systemic steroid | 23 | 0.30 | 15 | 0.19 | 2.1 | 21 | 0.29 | 15 | 0.21 | 1.7 | 8 | 0.37 | 6 | 0.28 | 1.6 |
| CCI | |||||||||||||||
| Mean (SD) | 1.5 (1.0) | 1.5 (1.0) | 0.0 | 1.5 (1.2) | 1.5 (1.3) | 0.0 | 1.5 (1.1) | 1.5 (1.1) | 0.0 | ||||||
| 0 | 1699 | 21.96 | 1723 | 22.27 | −0.7 | 1684 | 23.57 | 1682 | 23.54 | 0.1 | 495 | 22.76 | 535 | 24.60 | −4.3 |
| 1 | 2329 | 30.10 | 2316 | 29.93 | 0.4 | 2197 | 30.75 | 2225 | 31.14 | −0.8 | 597 | 27.45 | 580 | 26.67 | 1.8 |
| 2 | 2192 | 28.33 | 2137 | 27.62 | 1.6 | 1989 | 27.84 | 1898 | 26.56 | 2.9 | 601 | 27.63 | 608 | 27.95 | −0.7 |
| 3+ | 1518 | 19.62 | 1562 | 20.19 | −1.4 | 1275 | 17.84 | 1340 | 18.75 | −2.4 | 482 | 22.16 | 452 | 20.78 | 3.4 |
| Medical treatment | |||||||||||||||
| Diabetes, complicated | 1270 | 16.41 | 1256 | 16.23 | 0.5 | 1058 | 14.81 | 1052 | 14.72 | 0.2 | 367 | 16.87 | 377 | 17.33 | −1.2 |
| Diabetes, uncomplicated | 4357 | 56.31 | 4369 | 56.46 | −0.3 | 3915 | 54.79 | 3985 | 55.77 | −2.0 | 1184 | 54.44 | 1180 | 54.25 | 0.4 |
| Renal diseases | 46 | 0.59 | 51 | 0.66 | −0.8 | 35 | 0.49 | 37 | 0.52 | −0.4 | 12 | 0.55 | 11 | 0.51 | 0.6 |
| Stroke | 195 | 2.52 | 178 | 2.30 | 1.4 | 173 | 2.42 | 165 | 2.31 | 0.7 | 87 | 4.00 | 85 | 3.91 | 0.5 |
| Ischemic heart disease | 12 | 0.16 | 7 | 0.09 | 1.8 | 7 | 0.10 | 7 | 0.10 | 0.0 | 3 | 0.14 | — | — | 5.3 |
| Hypertension, uncomplicated | 295 | 3.81 | 283 | 3.66 | 0.8 | 217 | 3.04 | 227 | 3.18 | −0.8 | 65 | 2.99 | 60 | 2.76 | 1.4 |
| Hypertension, complicated | 3190 | 41.23 | 3165 | 40.90 | 0.7 | 2876 | 40.25 | 2855 | 39.96 | 0.6 | 889 | 40.87 | 901 | 41.43 | −1.1 |
| Hyperlipidemia | 4310 | 55.70 | 4333 | 56.00 | −0.6 | 3790 | 53.04 | 3839 | 53.73 | −1.4 | 1208 | 55.54 | 1206 | 55.45 | 0.2 |
| Congestive heart failure | 229 | 2.96 | 219 | 2.83 | 0.8 | 170 | 2.38 | 165 | 2.31 | 0.5 | 40 | 1.84 | 37 | 1.70 | 1.0 |
| Cardiac arrhythmias | 223 | 2.88 | 185 | 2.39 | 3.1 | 184 | 2.58 | 180 | 2.52 | 0.4 | 58 | 2.67 | 49 | 2.25 | 2.7 |
| Valvular disease | 28 | 0.36 | 21 | 0.27 | 1.6 | 15 | 0.21 | 18 | 0.25 | −0.9 | 10 | 0.46 | 9 | 0.41 | 0.7 |
| Chronic pulmonary diseases | 1008 | 13.03 | 991 | 12.81 | 0.7 | 862 | 12.06 | 907 | 12.69 | −1.9 | 297 | 13.66 | 294 | 13.52 | 0.4 |
| Pulmonary circulation disorders | 5 | 0.06 | 4 | 0.05 | 0.5 | 3 | 0.04 | 6 | 0.08 | −1.7 | 2 | 0.09 | — | — | 4.3 |
| Asthma | 8 | 0.10 | 2 | 0.03 | 3.1 | 4 | 0.06 | 5 | 0.07 | −0.6 | 3 | 0.14 | 1 | 0.05 | 3.0 |
| Peripheral vascular disease | 432 | 5.58 | 476 | 6.15 | −2.4 | 410 | 5.74 | 398 | 5.57 | 0.7 | 193 | 8.87 | 179 | 8.23 | 2.3 |
| Hemiplegia | 11 | 0.14 | 8 | 0.10 | 1.1 | 10 | 0.14 | 8 | 0.11 | 0.8 | 6 | 0.28 | 8 | 0.37 | −1.6 |
| Neurodegenerative disorders | 55 | 0.71 | 48 | 0.62 | 1.1 | 52 | 0.73 | 45 | 0.63 | 1.2 | 25 | 1.15 | 13 | 0.60 | 5.9 |
| Hypothyroidism | 316 | 4.08 | 316 | 4.08 | 0.0 | 264 | 3.69 | 266 | 3.72 | −0.1 | 84 | 3.86 | 85 | 3.91 | −0.2 |
| Liver disease | 2677 | 34.60 | 2655 | 34.31 | 0.6 | 2386 | 33.39 | 2464 | 34.49 | −2.3 | 727 | 33.43 | 699 | 32.14 | 2.7 |
| Peptic ulcer disease, no bleeding | 664 | 8.58 | 667 | 8.62 | −0.1 | 606 | 8.48 | 612 | 8.57 | −0.3 | 204 | 9.38 | 194 | 8.92 | 1.6 |
| Rheumatoid arthritis/collagen vascular diseases | 90 | 1.16 | 122 | 1.58 | −3.6 | 83 | 1.16 | 85 | 1.19 | −0.3 | 37 | 1.70 | 35 | 1.61 | 0.7 |
| Coagulopathy | 18 | 0.23 | 18 | 0.23 | 0.0 | 14 | 0.20 | 18 | 0.25 | −1.2 | 4 | 0.18 | 5 | 0.23 | −1.0 |
| Obesity | 39 | 0.50 | 33 | 0.43 | 1.1 | 15 | 0.21 | 13 | 0.18 | 0.6 | 4 | 0.18 | 5 | 0.23 | −1.0 |
| Weight loss | 22 | 0.28 | 13 | 0.17 | 2.4 | 22 | 0.31 | 21 | 0.29 | 0.3 | 8 | 0.37 | 5 | 0.23 | 2.5 |
| Fluid and electrolyte disorders | 225 | 2.91 | 191 | 2.47 | 2.7 | 186 | 2.60 | 166 | 2.32 | 1.8 | 50 | 2.30 | 46 | 2.11 | 1.3 |
| Blood loss anemia | 13 | 0.17 | 18 | 0.23 | −1.4 | 10 | 0.14 | 10 | 0.14 | 0.0 | 2 | 0.09 | 4 | 0.18 | −2.5 |
| Deficiency anemia | 281 | 3.63 | 245 | 3.17 | 2.6 | 258 | 3.61 | 256 | 3.58 | 0.2 | 82 | 3.77 | 86 | 3.95 | −1.0 |
| Alcohol abuse | 200 | 2.58 | 226 | 2.92 | −2.1 | 184 | 2.58 | 202 | 2.83 | −1.6 | 61 | 2.80 | 67 | 3.08 | −1.6 |
| Drug abuse | 1 | 0.01 | 3 | 0.04 | −1.6 | — | — | 2 | 0.03 | −2.4 | — | — | — | — | 0.0 |
| Psychosis | 64 | 0.83 | 73 | 0.94 | −1.2 | 63 | 0.88 | 61 | 0.85 | 0.3 | 23 | 1.06 | 21 | 0.97 | 0.9 |
| Depression | 231 | 2.99 | 208 | 2.69 | 1.8 | 198 | 2.77 | 212 | 2.97 | −1.2 | 83 | 3.82 | 81 | 3.72 | 0.5 |
| Pregnancy | 12 | 0.16 | 11 | 0.14 | 0.3 | 5 | 0.07 | 3 | 0.04 | 1.2 | 1 | 0.05 | 2 | 0.09 | −1.8 |
To estimate the risk of genital infections and UTIs associated with SGLT‐2 inhibitors, we used the Cox proportional hazard models and calculated the HR after PS matching. First, when patients with T2DM were prescribed metformin, the risk of genital infections with SGLT‐2 inhibitors was associated with a higher risk than that in DPP‐4 inhibitors (HR: 2.39, 95% CI: 2.07–2.76), SU (HR: 3.23, 95% CI: 2.73–3.81), and TZD (HR: 3.23, 95% CI: 2.35–4.44). Second, the use of SGLT‐2 inhibitors was associated with a significantly increased risk of UTIs compared to DPP‐4 inhibitors (HR: 1.57, 95% CI: 1.39–1.77), SU (HR: 1.66, 95% CI: 1.47–1.89), and TZD (HR: 1.69, 95% CI: 1.33–2.13; Table 2).
TABLE 2.
Risk of genital and urinary tract infections associated with SGLT‐2 inhibitors compared to DPP‐4 inhibitors, Sulfonylurea, and Thiazolidinedione
| Outcome | SGLT‐2 inhibitors | Compare group | HR (95% CI) | ||
|---|---|---|---|---|---|
| No. of events | Incidence rate (Per 1000 PY) | No. of events | Incidence rate (Per 1000 PY) | ||
| SGLT‐2 inhibitors (n = 7738) versus DPP‐4 inhibitors (n = 7738) | |||||
| Genital infections | 473 | 67.2 | 356 | 32.2 | 2.39 (2.07–2.76) |
| Urinary tract infections | 543 | 77.0 | 613 | 55.4 | 1.57 (1.39–1.77) |
| SGLT‐2 inhibitors (n = 7145) versus Sulfonylurea (n = 7145) | |||||
| Genital infections | 413 | 63.1 | 275 | 15.0 | 3.23 (2.73–3.81) |
| Urinary tract infections | 492 | 75.1 | 608 | 45.5 | 1.66 (1.47–1.89) |
| SGLT‐2 inhibitors (n = 2175) versus Thiazolidinedione (n = 2175) | |||||
| Genital infections | 114 | 56.7 | 74 | 21.2 | 3.23 (2.35–4.44) |
| Urinary tract infections | 146 | 72.7 | 180 | 51.6 | 1.69 (1.33–2.13) |
We carried out further subgroup analyses by sex and age to evaluate associations between the risk of genital infections and UTIs with SGLT‐2 inhibitors compared to DPP‐4 inhibitors, SU, and TZD. In the subgroup analyses, according to sex, using SGLT‐2 inhibitors compared to DPP‐4 inhibitors was associated with a risk of genital tract infections in female (HR: 2.60, 95% CI: 2.24–3.02) and in male (HR: 2.43, 95% CI: 1.31–4.51). In addition, the risk of UTIs with SGLT‐2 inhibitors was associated with a higher risk than that with DPP‐4 inhibitors among female (HR: 1.70, 95% CI: 1.43–2.01) and male (HR: 1.53, 95% CI: 1.29–1.81). Other comparators had similar results in both outcomes. As for the age groups, the association between the risk of genital infections and UTIs with SGLT‐2 inhibitor use remained statistically significant for each age group; however, the most pronounced increases were observed in individuals >60 years of age (Table 3). Subgroup analysis revealed an increased risk of infection in patients aged over 60 years taking SGLT‐2 inhibitors.
TABLE 3.
Risk of genital and urinary tract infections associated with SGLT‐2 inhibitors in subgroup analysis
| Comparator | DPP‐4 inhibitors | Sulfonylurea | Thiazolidinedione | |||
|---|---|---|---|---|---|---|
| Outcome | Genital infections | Urinary tract infections | Genital infections | Urinary tract infections | Genital infections | Urinary tract infections |
| Total | 2.39 (2.07–2.76) | 1.57 (1.39–1.77) | 3.23 (2.73–3.81) | 1.66 (1.47–1.89) | 3.23 (2.35–4.44) | 1.69 (1.33–2.13) |
| Sex | ||||||
| Male | 2.43 (1.31–4.51) | 1.53 (1.29–1.81) | 2.34 (1.26–4.32) | 1.54 (1.29–1.83) | 2.57 (0.68–9.74) | 1.79 (1.30–2.46) |
| Female | 2.60 (2.24–3.02) | 1.70 (1.43–2.01) | 3.67 (3.08–4.37) | 1.92 (1.60–2.31) | 3.59 (2.58–5.01) | 1.66 (1.17–2.35) |
| Age group | ||||||
| 19–29 | 1.45 (0.81–2.60) | 0.82 (0.44–1.52) | 1.65 (0.37–7.41) | 1.82 (0.30–11.17) | 2.09 (0.99–4.45) | 1.80 (0.81–3.98) |
| 30–39 | 1.87 (1.37–2.55) | 1.52 (1.15–2.01) | 1.31 (0.56–3.07) | 1.58 (0.68–3.66) | 2.16 (1.49–3.15) | 1.46 (1.06–2.02) |
| 40–49 | 1.64 (1.29–2.07) | 1.43 (1.16–1.77) | 1.94 (0.97–3.89) | 1.35 (0.80–2.26) | 1.92 (1.47–2.51) | 1.42 (1.14–1.77) |
| 50–59 | 2.26 (1.70–3.00) | 1.27 (1.03–1.57) | 2.73 (1.60–4.65) | 1.57 (1.05–2.34) | 3.05 (2.22–4.18) | 1.34 (1.08–1.66) |
| 60–69 | 3.21 (1.97–5.24) | 1.62 (1.13–2.32) | 3.20 (1.80–6.11) | 1.36 (0.87–2.11) | 3.38 (2.09–5.46) | 1.55 (1.10–2.19) |
| 70–79 | 3.23 (0.83–12.62) | 1.30 (0.60–2.84) | 2.08 (0.49–8.81) | 1.30 (0.55–3.10) | 3.18 (0.82–12.38) | 1.32 (0.62–2.83) |
| ≥80 | — | — | — | — | — | — |
Data are shown as HR (95% CI).
To verify the consistency of the results, we performed sensitivity analysis. First, we limited the follow‐up period to less than 1 year after cohort entry. The risk of genital infections with SGLT‐2 inhibitors was associated with a higher risk than that in DPP‐4 inhibitors (HR: 3.77, 95% CI: 3.03–4.70). And use of SGLT‐2 inhibitors was associated with a significantly increased risk of UTIs compared to DPP‐4 inhibitors (HR: 2.64, 95% CI: 2.21–3.14). Also, other comparators (SU and TZD) had similar patterns. Second, high‐risk age groups were defined as “age >60 years,” SGLT‐2 inhibitors were associated with a significantly increased risk of genital infections compared to DPP‐4 inhibitors (HR: 4.20, 95% CI: 2.63–6.71), SU (HR: 4.27, 95% CI: 2.70–6.75), and TZD (HR: 4.11, 95% CI: 2.25–7.50). Also, the risk of UTIs was higher than DPP‐4 inhibitors (HR: 1.82, 95% CI: 1.32–2.52), SU (HR: 1.72, 95% CI: 1.27–2.34), and TZD (HR: 1.50, 95% CI: 1.03–2.18) (Table 4).
TABLE 4.
Risk of genital and urinary tract infections associated with SGLT‐2 inhibitors in sensitivity analysis
| Comparator | DPP‐4 inhibitors | Sulfonylurea | Thiazolidinedione | |||
|---|---|---|---|---|---|---|
| Outcome | Genital infections | Urinary tract infections | Genital infections | Urinary tract infections | Genital infections | Urinary tract infections |
| Limiting follow‐up duration | ||||||
| ≤1 year | 3.77 (3.03–4.70) | 2.64 (2.21–3.14) | 4.74 (3.67–6.14) | 2.33 (1.96–2.78) | 6.26 (3.63–10.80) | 2.45 (1.77–3.39) |
| Restricting age | ||||||
| ≥60 years old | 4.20 (2.63–6.71) | 1.82 (1.32–2.52) | 4.27 (2.70–6.75) | 1.72 (1.27–2.34) | 4.11 (2.25–7.50) | 1.50 (1.03–2.18) |
Data are shown as HR (95% CI).
4. DISCUSSION
In this study, we found that SGLT‐2 inhibitors, compared with DPP‐4 inhibitors, SU, and TZD, in combination with metformin, were significantly associated with an increased risk of genital infections and UTIs in patients with T2DM.
Similarly, a previous retrospective longitudinal cohort study in Australia, 13 a systematic review in China, 12 and a retrospective cohort study in the United States 11 have reported an increased risk of genital infections associated with SGLT‐2 inhibitors. Moreover, Gadzhanova et al. reported the risk of genital infections with SGLT‐2 inhibitors is increased compared with DPP‐4 inhibitors (HR: 3.50, 95% CI: 1.95–5.89). 13 Liu et al. and Dave et al. have also reported that the risk of genital infections is increased compared with placebo (relative risk: 2.87, 95% CI: 2.27–3.62) 12 and DPP‐4 inhibitors (HR: 2.81, 95% CI: 2.64–2.99), 11 respectively.
However, these study results did not exhibit significant differences in the risk of UTIs between SGLT‐2 inhibitors. Gadzhanova et al. reported the risk of UTIs with SGLT‐2 inhibitors compared with DPP‐4 inhibitors (HR: 0.90, 95% CI: 0.66–1.22), 6 Liu et al. reported the risk compared with active drugs (relative risk: 1.10, 95% CI: 0.96–1.26), 12 and Dave et al. reported the risk compared with DPP‐4 inhibitors (HR: 0.98, 95% CI: 0.68–1.41); according to their research, SGLT‐2 inhibitors did not increase the risk of UTIs. 21 The reason for the difference from the results of this study was that the statistical approach was different. For instance, Gadzhanova et al. 13 lacked recorded data and did not implement PS matching, Liu et al. 12 conducted a systematic review, and Dave et al. 21 showed that the diagnostic code for UTIs is limited to severe cases. UTIs are among the most common infections, with 40%–50% of female suffering from infection at least once. 19 Hence, making direct correlations between UTIs and specific drugs can be challenging. In other words, specific individual characteristics of a patient can lead to their being selected for specific treatments, which may introduce selection bias and impact the statistical results of studies. To address this issue, we controlled for covariate imbalance using PS matching.
In this study, the sensitivity analysis of the association between the risk of genital infections and UTIs with SGLT‐2 inhibitor use was conducted by limiting the follow‐up period to 365 days and age to over 60 years, the result of which was similar to that of prior studies. 11 , 12 , 13 A retrospective cohort study in the United States showed that the risk of genital infections within 365 days of initiating SGLT‐2 inhibitor use was significantly higher than that with DPP‐4 inhibitors. 21 An observational study using the General Practice Research Database in the British population reported the incidence of UTIs over 60 years of age. 19 Comprehensively, SGLT‐2 inhibitors were found to be significantly associated with a higher risk of genital infections and UTIs in patients over 60 years of age in our study. Therefore, the administration of SGLT‐2 inhibitors to elderly patients should be closely monitored.
There are several potential mechanisms by which genital infections and UTIs risk in those with SGLT‐2 inhibitors. The mechanism of action of SGLT‐2 inhibitors is to prevent reabsorption of glucose by inhibiting SGLT‐2 protein present in proximal convoluted tubules of the kidney and facilitate its excretion in urine. 22 , 23 Due to their mechanism of action, SGLT2 inhibitors were expected to increase glucosuria, a well‐recognized risk factor for genital infections. 24 And, this may stem from the increase in urinary glucose levels—and consequent predisposition to growth of commensal microorganisms—that is a consequence of hyperglycemia. A logical consequence of this would be a further increase in risk in association with SGLT2 inhibitor administration and a series of cases in which people with diabetes experienced progression of a UTI to urosepsis or pyelonephritis leading the FDA to issue a warning about the risk of serious UTIs in SGLT2 inhibitor‐treated patients. 25 In addition, SGLT2 inhibitors are associated with increased benign urinary symptoms (e.g., increased urinary output) due to osmotic diuresis. This may have potentially increased the diagnosis of infection in patients treated with SGLT2 inhibitors. 26
The key strength of this study is its representation of a large population using the Korean national claims data. The NHIS data using the national health insurance claim data included approximately 98% of the Korean medical service and prescribed medicines, making it readily applicable to the general Korean population. In particular, the results of this study are meaningful in that there has been no previous study in the Korean population to analyze the risk of genital infections and UTIs associated with SGLT‐2 inhibitors. We also sought to minimize the effect of confounding factors, such as underlying diseases and medication use, which are known to be related to genital infection and UTI risk, by applying statistical methods using PS matching. Furthermore, we generalized results considering one or more comparative drug groups. According to the 2016 prescription for diabetes treatment, the most commonly administered metformin‐based combined therapies include DPP‐4 inhibitor, SU, SGLT‐2 inhibitors, and TZD in this order. Although various prior studies have analyzed only DPP‐4 inhibitors as a comparative control group for SGLT‐2 inhibitors, this study also included the use of SU and TZD.
Nevertheless, our study has some limitations. First, diabetes, known as an underlying risk factor for the incidence of genital infections and UTIs, may have affected as a confounding factor affecting the results. Although the prevalence and moderate degree of diabetes may affect the results, there were limited data sources that utilize clinical information such as severity and detailed symptoms of each patient's disease. 27 Second, the period of follow‐up for the study drugs differed. Specifically, SGLT‐2 inhibitors were first marketed in 2014 in Korea and had a shorter follow‐up period than the other study drugs. Therefore, we conducted a sensitivity analysis with a constant follow‐up period applied to adjust for the observation period. However, a long‐term follow‐up further studies are required. Third, restricted information for each clinical site also imposes limitations to the study. For instance, it is difficult to clearly define the diagnostic criteria applied by each clinic for genital and urinary tract infections. Hence, although the risk of UTIs that require treatment is considered to be significantly associated with SGLT‐2 inhibitors, mild cases not requiring treatment may have also been included in the outcome variables for this study. In addition, potential confounders such as history of hospitalization were not considered. Lastly, potential confounders such as history of hospitalization were not considered. However, we tried to reduce confounding related to infection by adjusting infection‐related diseases and drug history.
In conclusion, as SGLT‐2 inhibitors are relatively new and effective agents, further studies are needed to clarify their adverse event and potential complication. And, mild to moderate genital infections and UTIs can be treated according to local guidelines. 28 , 29 , 30 Also, SGLT‐2 inhibitors have been shown to reduce cardiovascular disease, so we should consider the risks and benefits of SGLT‐2 inhibitors. 31 However, patients with a very high risk of genital infections and UTIs, such as perineal gangrene, recurrent and neurological bladder patients, it is probably recommended not to administer SGLT2 inhibitors. Therefore, the results of this study must be interpreted carefully when applying them to clinical settings.
In this national‐based, retrospective cohort study, the use of SGLT‐2 inhibitors in T2DM patients taking metformin as the primary drug was found to be associated with genital infections and UTIs compared to DPP‐4 inhibitors, SU, and TZD. In particular, patients over 60 years of age tended to have a higher risk of genital infections, indicating that careful monitoring of these patients is imperative.
ACKNOWLEDGMENTS
We appreciate the National Health Insurance Service for their cooperation in providing access to the database.
DISCLOSURE
The authors declare no competing interests.
ETHICS STATEMENT
This study was performed in accordingly with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines and was approved by the institutional review board of the Korean Institute of Drug Safety & Risk Management (KIDS; KIDS‐2019‐1).
AUTHOR CONTRIBUTIONS
Hyeri Yang and Eunmi Choi designed the study and prepared the first draft. Eunjun Park and Eonji Na and helped conduct the literature review and prepare the Materials and Methods and the Discussion sections of the text, Soo Youn Chung helped supervise the field activities and designed the study’s analytic strategy, Soon Young Han and Bonggi Kim designed the study and directed its implementation.
Supporting information
Table S1–S3
Yang H, Choi E, Park E, et al. Risk of genital and urinary tract infections associated with SGLT‐2 inhibitors as an add‐on therapy to metformin in patients with type 2 diabetes mellitus: A retrospective cohort study in Korea. Pharmacol Res Perspect. 2022;10:e00910. doi: 10.1002/prp2.910
Hyeri Yang and Eunmi Choi contributed equally to this work as first authors.
*These authors are co‐corresponding authors of this work
Contributor Information
Bonggi Kim, Email: bgkim@drugsafe.or.kr.
Soon Young Han, Email: soonyoungh@drugsafe.or.kr.
DATA AVAILABILITY STATEMENT
The data are available from the Korean National Health Insurance Sharing Service at https://nhiss.nhis.or.kr/bd/ay/bdaya001iv.do. Upon an individual researcher's request, NHIS provides customized data to the researcher through supervision and approval.
REFERENCES
- 1. World Health Organization . Global report on diabetes. 2020.
- 2. American Diabetes Association . Standards of medical care in diabetes. 2019.
- 3. Miller EM. Overview of the efficacy and safety of SGLT‐2 inhibitors in Type 2 diabetes mellitus. J Fam Pract. 2017;66:S5‐S12. [PubMed] [Google Scholar]
- 4. Tahrani AA, Barnett AH, Bailey CJ. SGLT inhibitors in management of diabetes. Lancet Diabetes Endocrinol. 2013;1(2):140‐151. [DOI] [PubMed] [Google Scholar]
- 5. d'Emden M, Amerena J, Deed G, Pollock C, Cooper ME. SGLT2 inhibitors with cardiovascular benefits: transforming clinical care in type 2 diabetes mellitus. Diabetes Res Clin Pract. 2018;136:23‐31. [DOI] [PubMed] [Google Scholar]
- 6. Rabizadeh S, Nakhjavani M, Esteghamati A. Cardiovascular and renal benefits of SGLT2 inhibitors: a narrative review. Int J Endocrinol Metab. 2019;17:e84353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hasan FM, Alsahli M, Gerich JE. SGLT2 inhibitors in the treatment of type 2 diabetes. Diabetes Res Clin Pract. 2014;104(3):297‐322. [DOI] [PubMed] [Google Scholar]
- 8. Nauck A. Update on developments with SGLT2 inhibitors in the management of type 2 diabetes. Drug Des Devel Ther. 2014;8:1335‐1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. FDA . FDA revises labels of SGLT2 inhibitors for diabetes to include warnings about serious urinary tract infections. 2015. https://www.fda.gov/files/drugs/published/FDA‐revises‐labels‐of‐SGLT2‐inhibitors‐for‐diabetes‐to‐include‐warnings‐about‐too‐much‐acid‐in‐the‐blood‐and‐serious‐urinary‐tract‐infections.pdf
- 10. FDA . FDA warns about rare occurrences of a serious infection of the genital area with SGLT2 inhibitors for diabetes. 2018. https://www.fda.gov/drugs/drug‐safety‐and‐availability/fda‐warns‐about‐rare‐occurrences‐serious‐infection‐genital‐area‐sglt2‐inhibitors‐diabetes
- 11. Dave CV, Schneeweiss S, Patorno E. Comparative risk of genital infections associated with sodium‐glucose co‐transporter‐2 inhibitors. Diabetes Obes Metab. 2019;21:434‐438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liu J, Li L, Li S, et al. Effects of SGLT2 inhibitors on UTIs and genital infections in type 2 diabetes mellitus: a systematic review and meta‐analysis. Sci Rep. 2017;7:2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gadzhanova S, Pratt N, Roughead E. Use of SGLT2 inhibitors for diabetes and risk of infection: analysis using general practice records from the NPS MedicineWise Medicine Insight program. Diabetes Res Clin Pract. 2017;130:180‐185. [DOI] [PubMed] [Google Scholar]
- 14. Li D, Wang T, Shen S, Fang Z, Dong Y, Tang H. Urinary tract and genital infections in patients with type 2 diabetes treated with sodium‐glucose co‐transporter 2 inhibitors: a meta‐analysis of randomized controlled trials. Diabetes Obes Metab. 2017;19:348‐355. [DOI] [PubMed] [Google Scholar]
- 15. Korea Institute of Drug Safety & Risk Management . Introduction of Korea Adverse Event Reporting System, KAERS. https://www.drugsafe.or.kr/iwt/ds/en/report/WhatIsKAERS.do
- 16. Lee J, Lee JS, Park S‐H, Shin SA, Kim K. Cohort profile: the National Health Insurance Service–National Sample Cohort (NHIS‐NSC), South Korea. Int J Epidemiol. 2016;dyv319. doi: 10.1093/ije/dyv319 [DOI] [PubMed] [Google Scholar]
- 17. Sundararajan V, Henderson T, Perry C, Muggivan A, Quan H, Ghali W. New ICD‐10 version of the Charlson comorbidity index predicted in‐hospital mortality. J Clin Epidemiol. 2004;57(12):1288‐1294. [DOI] [PubMed] [Google Scholar]
- 18. Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46:399‐424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hirji I, Guo Z, Andersson SW, Hammar N, Gomez‐Caminero A. Incidence of urinary tract infection among patients with type 2 diabetes in the UK General Practice Research Database (GPRD). J Diabetes Complications. 2012;26:513‐516. [DOI] [PubMed] [Google Scholar]
- 20. National Health Insurance Service . Introduction of National Health Insurance Sharing Service, NHISS. https://nhiss.nhis.or.kr/bd/ab/bdaba000eng.do
- 21. Dave CV, Schneeweiss S, Kim D, Fralick M, Tong A, Patorno E. Sodium‐glucose cotransporter 2 inhibitors and the risk of severe urinary tract infections. Ann Intern Med. 2019;171:248‐256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Komoroski B, Vachharajani N, Feng Y, Li L, Kornhauser D, Pfister M. Dapagliflozin, a novel, selective SGLT2 inhibitor, improved glycemic control over 2 weeks in patients with type 2 diabetes mellitus. Clin Pharmacol Ther. 2009;85(5):513‐519. [DOI] [PubMed] [Google Scholar]
- 23. Unnikrishnan AG, Kalra S, Purandare V, et al. Genital infections with sodium glucose cotransporter‐2 inhibitors occurrence and management in patients with type 2 diabetes mellitus. Indian J Endocrinol Metab. 2018;22(6):837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Geerlings S, Fonseca V, Castro‐Diaz D, List J, Parikh S. Genital and urinary tract infections in diabetes: impact of pharmacologically‐induced glucosuria. Diabetes Res Clin Pract. 2014;103(3):373‐381. [DOI] [PubMed] [Google Scholar]
- 25. Benfield T, Jensen JS, Nordestgaard BG. Influence of diabetes and hyperglycaemia on infectious disease hospitalisation and outcome. Diabetologia. 2007;50(3):549‐554. doi: 10.1007/s00125-006-0570-3 [DOI] [PubMed] [Google Scholar]
- 26. Nelinson DS, Sosa JM, Chilton RJ. SGLT2 inhibitors: a narrative review of efficacy and safety. J Osteopath Med. 2021;121(2):229‐239. [DOI] [PubMed] [Google Scholar]
- 27. Yun NR. Fungal infection in patients with diabetes mellitus. J Korean Diabetes. 2017;18(1):20. doi: 10.4093/jkd.2017.18.1.20 [DOI] [Google Scholar]
- 28. Johnsson KM, Ptaszynska A, Schmitz B, Sugg J, Parikh SJ, List JF. Vulvovaginitis and balanitis in patients with diabetes treated with dapagliflozin. J Diabetes Complications. 2013;27:479‐484. [DOI] [PubMed] [Google Scholar]
- 29. Kushner P. Benefits/risks of sodium‐glucose co‐transporter 2 inhibitor canagliflozin in women for the treatment of type 2 diabetes. Women’s Health. 2016;12:379‐388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lupsa BC, Inzucchi SE. Use of SGLT2 inhibitors in type 2 diabetes: weighing the risks and benefits. Diabetologia. 2018;61:2118‐2125. [DOI] [PubMed] [Google Scholar]
- 31. Lopaschuk GD, Verma S. Mechanisms of cardiovascular benefits of sodium glucose co‐transporter 2 (SGLT2) inhibitors. JACC. 2020;5(6):632‐644. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1–S3
Data Availability Statement
The data are available from the Korean National Health Insurance Sharing Service at https://nhiss.nhis.or.kr/bd/ay/bdaya001iv.do. Upon an individual researcher's request, NHIS provides customized data to the researcher through supervision and approval.


