ABSTRACT
Despite the extensive efforts, there is still a lack of a licensed vaccine against Chlamydia trachomatis in humans. The mouse genital tract infection with Chlamydia muridarum has been used to both investigate chlamydial pathogenic mechanisms and evaluate vaccine candidates due to the C. muridarum’s ability to induce mouse hydrosalpinx. C. muridarum mutants lacking the entire plasmid or deficient in only the plasmid-encoded pGP3 are highly attenuated in inducing hydrosalpinx. We now report that intravaginal immunization with these mutants as live attenuated vaccines protected mice from hydrosalpinx induced by wild type C. muridarum. However, these mutants still productively infected the mouse genital tract. Further, the mutant-infected mice were only partially protected against the subsequent infection with wild type C. muridarum. Thus, these mutants as vaccines are neither safe nor effective when they are delivered via the genital tract. Interestingly, these mutants were highly deficient in colonizing the gastrointestinal tract. Particularly, the pGP3-deficient mutant failed to shed live organisms from mice following an oral inoculation, suggesting that the pGP3-deficient mutant may be developed into a safe oral vaccine. Indeed, oral inoculation with the pGP3-deficient mutant induced robust transmucosal immunity against both the infection and pathogenicity of wild type C. muridarum in the genital tract. Thus, we have demonstrated that the plasmid-encoded virulence factor pGP3 may be targeted for developing an attenuated live oral vaccine.
KEYWORDS: plasmid-free, pGP3 deficiency, Chlamydia muridarum, attenuated oral vaccine, transmucosal immunity, oral vaccine, plasmid, live attenuated, pGP3, transmucosal protection
INTRODUCTION
Chlamydia trachomatis is a leading cause of sexually transmitted bacterial infections (1), which may trigger sequelae in the upper genital tract such as tubal adhesion and hydrosalpinx, resulting in infertility (2). Chlamydia muridarum is known to cause hydrosalpinx and infertility in mice following intravaginal inoculation (3–5), closely mimicking the tubal adhesion/infertility observed in women (2, 6, 7). The C. muridarum murine model has been extensively used to investigate chlamydial pathogenesis and immunity (8–13). Interestingly, chlamydial organisms have also been frequently detected in the gastrointestinal (GI) tracts of both animals (14) and humans (15–18). However, the medical significance of the GI tract Chlamydia remains unclear.
The C. muridarum murine model-based studies have revealed that the order of exposure of mouse tissue sites to Chlamydia may determine the consequence of the GI tract Chlamydia. When a mouse is exposed to Chlamydia in the genital tract first, the genital Chlamydia may both ascend to the upper genital tract to infect tubal epithelial cells (to produce the 1st hit) and spread to the GI tract. The GI tract Chlamydia was found to promote chlamydial pathogenicity in the upper genital tract (19). It was hypothesized that the GI tract Chlamydia might induce antigen-specific lymphocytes such as CD8+ T cells for promoting pathological responses in the oviduct initially infected with genital Chlamydia. CD8+ T cells were indeed found both necessary and sufficient for the GI tract Chlamydia to promote genital chlamydial pathogenicity (20). The GI tract Chlamydia-induced lymphocytes may provide a 2nd hit for chlamydial induction of hydrosalpinx in the genital tract (21). Thus, the GI tract Chlamydia is pathogenic to the genital tract when the chlamydial organisms come from the genital tract. However, when a naive mouse is exposed to Chlamydia in the GI tract first, the GI tract Chlamydia may function as an oral vaccine.
We have recently reported that oral inoculation of Chlamydia can induce robust transmucosal immunity against subsequent chlamydial infections in both the genital tract (22) and airway (23). Because Chlamydia is nonpathogenic to the GI tract despite its long-lasting colonization, the above results suggest that it is feasible to develop an oral vaccine against Chlamydia in the genital tract. To improve the safety of the oral vaccine, an attenuated Chlamydia with mutations in chlamydial chromosomal genes was recently evaluated in mice (24). In the current study, we compared the protection efficacy of two attenuated Chlamydia mutants deficient in either the plasmid-encoded pGP3 (due to an engineered premature stop codon in the pgp3 gene, thus designated as CMpGP3S) or the entire plasmid (CMpf). Intravaginal inoculation with these attenuated mutants induced protection against pathology caused by subsequent wild type C. muridarum. However, these attenuated mutants still productively infected the mouse genital tract. Despite their infectivity in the genital tract, the intravaginal immunization only induced partial protection against subsequent infection with wild type C. muridarum. Interestingly, these attenuated mutants are significantly compromised in colonizing the gastrointestinal tract. The CMpGP3S mutant completely failed to shed live organisms from mice following an oral inoculation, suggesting that CMpGP3S may be developed into a safe oral vaccine. Oral inoculation with CMpGP3S indeed induced robust transmucosal immunity against both infection and pathogenicity of wild type C. muridarum in the genital tract. Thus, we propose that the plasmid-encoded virulence factor pGP3 may be targeted for developing a safe and effective live attenuated oral vaccine.
RESULTS
Chlamydia mutants deficient in plasmid-encoded pGP3 or the entire plasmid are attenuated in pathogenicity in mouse genital tract but can still induce protection against pathogenicity of wild type Chlamydia.
Intravaginal inoculation with wild type C. muridarum is known to induce hydrosalpinx (3, 5) and infertility (4) in mice. However, the C. muridarum mutants deficient in either the plasmid-encoded pGP3 (CMpGP3S) or free of the entire plasmid (CMpf) were found highly attenuated in inducing hydrosalpinx (Fig. 1). Although wild type C. muridarum (CMwt) induced 6 of the 7 C57BL/6j female mice to develop significant hydrosalpinx, CMpGP3S only induced hydrosalpinx in 1 out of 6 mice and CMpf failed to induce significant hydrosalpinx in any mice. These results have validated that both mutants are highly attenuated in mouse genital tract pathogenicity (25–28). When parallel groups of mice intravaginally inoculated with either CMpGP3S or CMpf were further challenged intravaginally with CMwt, the mutant-immunized mice were fully protected from upper genital tract pathology. None of the mice immunized with CMpGP3S (8 mice) or CMpf (7) developed significant hydrosalpinx when examined 56 days after the challenge infection with CMwt although 5 of the 6 control mice (mock-immunized with the buffer SPG or sucrose-phosphate-glucose) developed significant hydrosalpinx. Clearly, although both CMpGP3S and CMpf are highly attenuated in pathogenicity, they are still able to induce protective immunity against pathogenicity of the subsequent CMwt.
FIG 1.
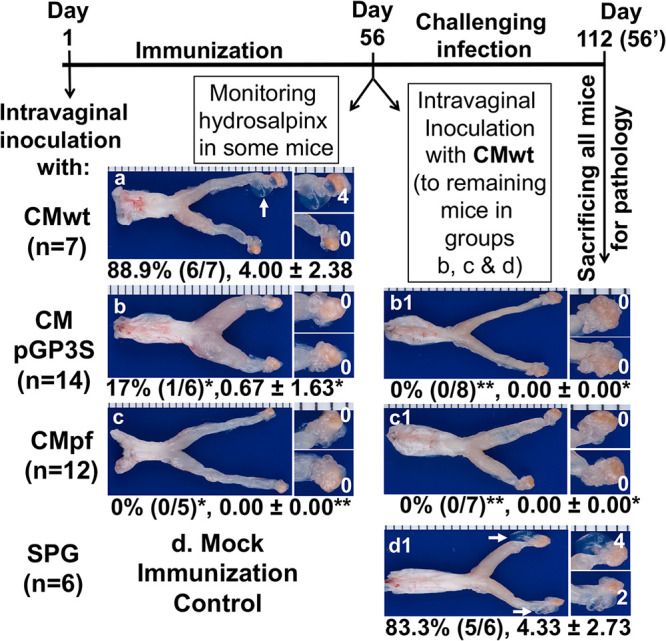
Effect of genital immunization with attenuated Chlamydia on the induction of hydrosalpinx by wild type Chlamydia. Groups of female C57BL/6J mice were intravaginally inoculated with wild type C. muridarum (CMwt, n = 7, designated as group a, panel a) or mutant C. muridarum deficient in the plasmid-encoded pGP3 (CMpGP3S, n = 14, b) or free of the entire plasmid (CMpf, n = 12, c) or the sucrose-phosphate-glucose buffer alone (SPG, n = 6), d as immunization. 56 days after the immunization, all mice from group “a” and some mice from groups “b” & “c” were sacrificed for observing hydrosalpinx (a-c). All remaining mice were intravaginally inoculated with CMwt as challenge infection and 56 days after the challenge infection, all mice were sacrificed for monitoring hydrosalpinx (b1-d1). Only one representative image of the entire genital tract was shown for each group. Oviducts positive for hydrosalpinx were marked with white arrows. The magnified images of oviduct/ovary regions (with hydrosalpinx scores indicated in white numbers) were shown on the right of the overall genital tract image. Both the hydrosalpinx incidence (along with group sample size) and severity score from each group were listed under the corresponding group images. *P < 0.05, **P < 0.01 (Fisher’s Exact for comparing incidences while Wilcoxon for scores). Data were from 2 or 3 independent experiments. Note that both mutants CMpGP3S and CMpf were highly attenuated in inducing hydrosalpinx but still able to induce complete protection against CMwt-induced pathogenicity.
The attenuated Chlamydia mutants can still productively infect the female mouse genital tract but induce only partial protection against challenge infection by wild type Chlamydia.
The infection time courses of the attenuated C. muridarum mutants CMpGP3S and CMpf in the female mouse genital tract were also monitored (Fig. 2). It was found that the two mutants developed similar time courses of live organism shedding. Comparing to CMwt, the mutants displayed more obvious drops in infectious titers starting on day 7. Nevertheless, the mutants’ shedding time courses were longer than that of CMwt. More importantly, after intravaginal challenge infection with CMwt, the mutant-immunized mice were significantly more resistant to the challenge infection than the SPG mock-immunized control mice. The infectious titers recovered at most time points were significantly lower in the mutant-immunized mice than the SPG control mice, indicating that the mutant-immunized mice developed protective immunity in the genital tract. Nevertheless, infectious organisms were still recovered from the genital tracts of the mutant-immunized mice on day 21 after the challenge infection, suggesting that the mutant-induced immunity is only partially effective against the challenge infection. Thus, we can conclude that although the pathogenicity-attenuated mutants are still competent in productively infecting female mouse genital tract, the mutant infection can only induce partial protection against CMwt. These observations suggest that the attenuated mutants may not be considered safe and effective vaccines when they are delivered via the genital tract.
FIG 2.
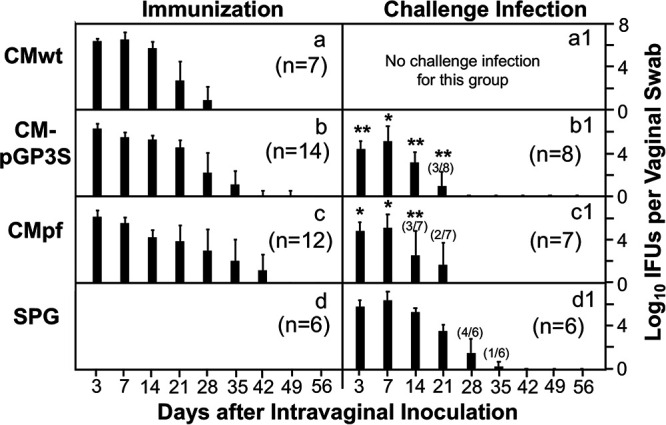
Effect of genital immunization with attenuated Chlamydia on the infection of wild type Chlamydia. The same 4 groups of C57BL/6J mice as described in Fig. 1 legend (listed on the left of the figure) were monitored for live Chlamydia burdens in the genital tract by taking vaginal swabs on days 3, 7 and weekly thereafter (X-axis) following the immunization (panels a-d) and challenge infection (a1-d1) respectively. The number of live organisms recovered from each vaginal swab was expressed as log10 inclusion forming unit (IFU). The numbers of mice detected positive for IFU were indicated in parenthesis if not all mice in the group were positive or negative for IFU. The group mean and standard deviation from each time point were displayed along the Y-axis. *P < 0.05, **P < 0.01 (Wilcoxon for comparing the mutant Chlamydia-immunized groups with the SPG control group). Data were from 2 or 3 independent experiments. Note that mice immunized with CMpGP3S or CMpf significantly reduced the live organism shedding in the genital tract following challenge infection with CMwt.
The pathogenicity-attenuated mutants are deficient in colonizing the gastrointestinal tract and oral immunization with these mutants induces protection against genital challenge infection by wild type Chlamydia.
Because genital C. muridarum organisms readily spread to the gastrointestinal tract (29), mice intravaginally inoculated with the mutant C. muridarum organisms were also monitored for live organism burdens in the gastrointestinal tracts (Fig. 3). The spreading of CMpf to the gastrointestinal tract was significantly delayed and reduced while CMpGP3S completely failed to shed live organisms from the gastrointestinal tract following intravaginal inoculation. The mutants still failed to colonize the gastrointestinal tract even after oral inoculation as demonstrated previously (30–35). More importantly, mice orally inoculated with either CMpGP3S or CMpf became highly resistant against intravaginal challenge with CMwt (Fig. 4). Only minimal levels of infectious CMwt organisms were detected in the vaginal swabs of the orally immunized mice on days 3 or 7 following intravaginal challenge infection. However, the SPG mock-immunized mice developed a 28-day shedding course of live organisms following the same challenge infection. Finally, it is worth emphasizing that oral immunization with either mutant induced as robust protection as the oral immunization with the wild type C. muridarum.
FIG 3.
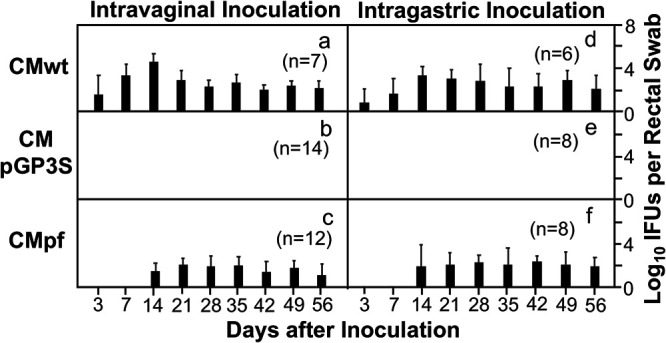
Colonization of CMpGP3S versus CMpf in the gastrointestinal tract. Groups of C57BL/6J mice as described in Fig. 1 legend were monitored for live Chlamydia burdens in the gastrointestinal tract by taking rectal swabs on days 3, 7 and weekly thereafter (X-axis) following the intravaginal inoculation for the purpose of immunization (panels a-c). Three parallel groups of mice were intragastrically inoculated with the same types of chlamydial organisms at the same amounts (panels d-f). Similarly, on days 3, 7 and weekly thereafter (X-axis) following the intragastric inoculation, rectal swabs were taken for monitoring live Chlamydia burdens in the gastrointestinal tract. The number of live organisms recovered from each rectal swab was expressed as log10IFU. The group mean and standard deviation from each time point were displayed along the Y-axis. Data were from two or three independent experiments. Note that regardless of the inoculation routes, mice inoculated with CMpGP3S failed to shed infectious organisms although CMpf did.
FIG 4.
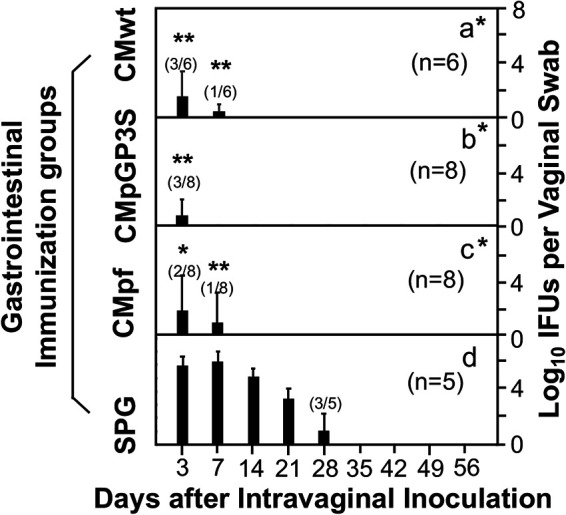
Effects of intragastric immunization with the attenuated Chlamydia on chlamydial infectivity in the genital tract. Groups of C57BL/6J mice intragastrically inoculated with CMwt (a, n = 6), CMpGP3S (b, n = 8) or CMpf (c, n = 8) as described in Fig. 3 legend plus a SPG buffer immunization control group (d, n = 5) were all challenged with CMwt via intravaginal inoculation on day 56 after the intragastric immunization. All mice were monitored for Chlamydia shedding from the genital tract via vaginal swabbing on days 3, 7 and weekly thereafter following the challenge infection. The results were expressed as Log10 IFUs per swab. The numbers of mice detected positive for IFU were indicated in parenthesis if not all mice in the group were either positive or negative for IFU. Note that mice orally immunized with either wild type or mutant Chlamydia organisms were protected from genital tract infection (*P < 0.05, **P < 0.01, Wilcoxon for comparing AUC or IFUs at different time points).
Oral immunization with the attenuated Chlamydia mutants induces transmucosal protection against chlamydial pathogenicity in the genital tract.
After observing the robust transmucosal protection against chlamydial challenge infection in the genital tract of the orally vaccinated mice, the genital pathologies in these mice were further carefully evaluated at both macroscopic (Fig. 5) and microscopic (Fig. 6) levels. The intravaginal infection with CMwt induced 4 of the 5 SPG buffer immunized control mice to develop significant hydrosalpinx. As a positive oral immunization control, oral immunization with CMwt prevented all 6 mice from developing any significant hydrosalpinx, which is consistent with our previous finding (22). Importantly, oral immunization with CMpGP3S prevented all 8 mice from developing any significant hydrosalpinx while oral immunization with CMpf protected 7 out of the 8 mice from developing hydrosalpinx. It is clear that following oral delivery, the genital pathogenicity-attenuated C. muridarum mutants are as competent as wild type C. muridarum in inducing transmucosal protection against chlamydial induction of hydrosalpinx. To validate these observations, the mouse genital tissues were further processed for histopathology evaluation under microscopy (Fig. 6). When the H&E-stained slides were observed under a 4x objective lens, clear oviduct dilation can be seen in mice with hydrosalpinx. Under a 40x lens, inflammatory infiltration can be identified in both the oviduct lumen and tissue. Following the semi-quantitation schemes described in the materials and method section, both oviduct dilation and inflammatory infiltration were scored. It was found that mice orally immunized with either the mutant or wild type C. muridarum were significantly protected from oviduct dilation and inflammatory infiltration induced by the genital challenge infection.
FIG 5.
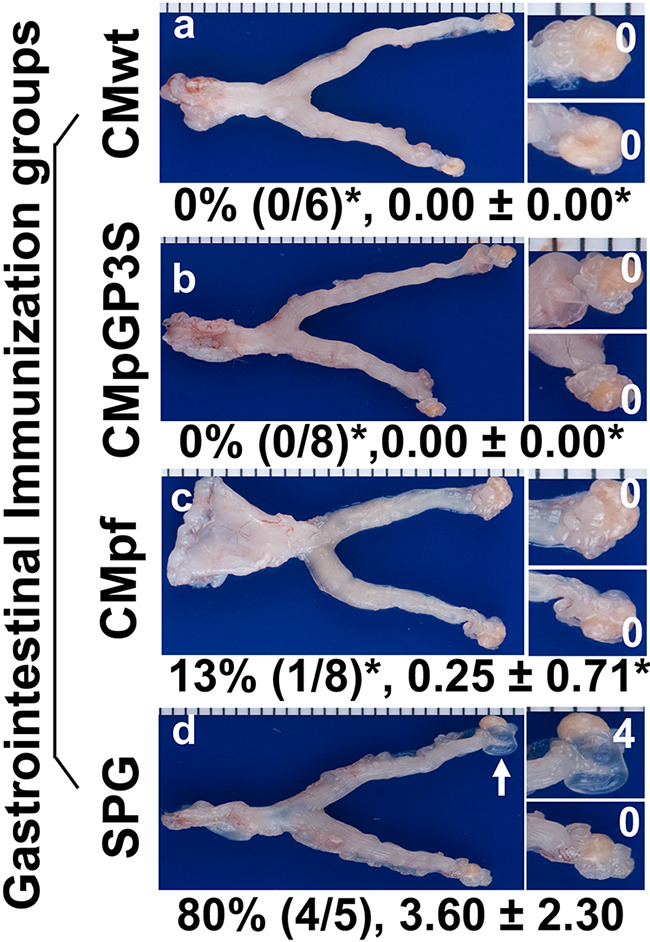
Effects of intragastric immunization with the attenuated Chlamydia on chlamydial induction of hydrosalpinx in the genital tract. The same groups of C57BL/6J mice intragastrically immunized and intravaginally challenged as described in Fig. 4 legend were sacrificed on day 56 after the intravaginal challenge infection for observing genital tract pathology. Hydrosalpinx was visually evaluated. Only one representative image of the entire genital tract was shown for each group. Oviducts positive for hydrosalpinx were marked with white arrows. The magnified images of oviduct/ovary regions (with hydrosalpinx scores indicated in white numbers) were shown on the right of the overall genital tract image. Both the hydrosalpinx incidence (along with group sample size) and severity score from each group were listed under the corresponding group images. *P < 0.05, (Fisher’s Exact for comparing incidences while Wilcoxon for scores). Data were from two or three independent experiments. Note that all orally immunized mice were protected from hydrosalpinx induction.
FIG 6.
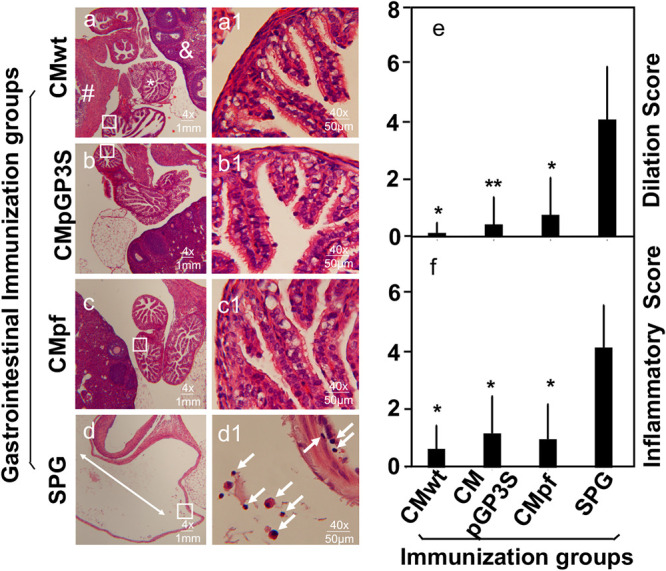
Effects of intragastric immunization with the attenuated Chlamydia on chlamydial induction of oviduct dilation and inflammatory infiltration in the upper genital tract. The same excised genital tract tissues described in Fig. 5 legend were processed for monitoring oviduct dilation and inflammatory infiltration under microscopy. After H&E staining, tissue sections of the genital tissues were first examined for the overall appearance of the oviduct tissues under a 4x objective lens (a-d). Representative normal oviduct tissue was labeled with a white star (*), ovary with “&” and uterine horn tissue with “#” while dilated oviducts were indicated with the white double arrowhead lines. Randomly views of oviduct tissues (as indicated with a black box) were further examined for inflammatory infiltration under a 40x subjective lens and representative images were displayed on the right side of the corresponding 4x images. Inflammatory infiltrates were indicated with white arrows (a1-d1). Both the oviduct dilation (e) and inflammatory infiltration (f) scores were semi-quantitatively measured as described the materials and method section. Note that all immunized groups showed significantly reduced oviduct dilation and inflammatory infiltration in the upper genital tract. *P < 0.05, **P < 0.01, Wilcoxon rank sum for comparing dilation and inflammatory scores between the control group and each experimental group. Data were acquired from two or three independent experiments.
DISCUSSION
There is still no licensed vaccine against Chlamydia trachomatis infection in humans despite serious health problems it causes. C. trachomatis is a leading cause of sexually transmitted bacterial infections (1), which may lead to pelvic inflammatory diseases, tubal adhesion and hydrosalpinx, resulting in infertility (2). Because C. muridarum can induce sequelae such as hydrosalpinx in the murine upper genital tract, the C. muridarum-mouse model has been used for investigating chlamydial pathogenic mechanisms and immunity. In the current study, we have used the C. muridarum-mouse model for evaluating the efficacy of the genital tract pathogenicity-attenuated Chlamydia mutants as oral vaccines in preventing chlamydial infection and pathogenicity in the genital tract.
We have demonstrated that the plasmid-encoded pGP3 may be targeted for developing live attenuated oral vaccines because oral delivery of the CMpGP3S mutant (that was installed with a premature stop codon in the pgp3 gene) induced a robust transmucosal protection against both chlamydial infection and pathogenicity in the genital tract. First, CMpGP3S was highly attenuated in pathogenicity when it was directly incubated into the mouse genital tract. It was as attenuated as the plasmid-free C. muridarum (CMpf). Second, although intravaginal immunization with live CMpGP3S induced full protection against hydrosalpinx induced by wild type C. muridarum (CMwt), the immunity only provided partial protection against the infection of CMwt in the genital tract. More importantly, both the pathogenicity-attenuated mutants CMpGP3S and CMpf still productively infected the mouse genital tract. Thus, it is neither effective nor safe to use these mutants as vaccines when they are delivered via intravaginal inoculation. Third, the genital pathogenicity-attenuated mutants were also found highly deficient in colonizing the gastrointestinal (GI) tract. Especially, the CMpGP3S mutant completely failed to produce infectious particles in the rectal swabs following oral inoculation. Thus, orally inoculated CMpGP3S won’t be able to spread between the orally immunized hosts, suggesting that it can be a safe oral vaccine. Fourth, the orally inoculated CMpGP3S induced robust transmucosal protection against infection of CMwt in the genital tract. Finally, oral immunization with CMpGP3S fully protected mice from developing pathology induced by CMwt in the upper genital tract. The protection against pathology was confirmed both macroscopically and microscopically.
It is worth noting that despite the lack of live organism recovery from the mouse rectal swabs following oral inoculation with CMpGP3S, the orally inoculated mice were fully protected against both infection and pathogenicity of CMwt in the genital tract. To determine the GI tissue sites where CMpGP3S induced the transmucosal immunity, the live CMpGP3S organisms were monitored in different segments of the GI tract over time following oral inoculation. It was found that CMpGP3S colonized the small intestine but without reaching the large intestine (32). The lack of the orally incubated CMpGP3S organisms in the large intestine was probably caused by a blockade of spreading of CMpGP3S from the small intestine to the large intestine because CMpGP3S successfully colonized the large intestine following an intracolonic inoculation (35). The blockade of the orally inoculated CMGP3S from reaching the colon was dependent on a CD4+ T cell-mediated immunity (34). Thus, the orally inoculated CMpGP3S is able to activate lymphocyte responses in the GI tract despite its blockade from reaching the colon. Efforts are ongoing to further determine whether the same CD4+ T cell-mediated immunity responsible for blocking CMpGP3 spreading in the GI tract also contributes to the transmucosal immunity in the genital tract.
The roles of plasmid and/or the plasmid-encoded pGP3 in chlamydial pathogenesis has been evaluated extensively (21). It is now known that pGP3 is a major virulence factor encoded by the plasmid (25, 36) although pGP5 was also found to play a role in chlamydial pathogenicity (37). In the current study, it was found that CMpGP3S continued to shed live organisms from the genital tract but failed to shed any from the GI tract, suggesting that Chlamydia may have acquired pGP3 for improving its survival in the GI tract but not in the genital tract. Investigating how pGP3 improves chlamydial survival in the GI tract may provide important information for advancing our understanding of chlamydial pathogenic mechanisms. Regardless of the precise mechanisms by which pGP3 promotes chlamydial pathogenicity, the findings from the current study suggest that depletion of the virulence factor pGP3 may represent a promising approach for developing a live attenuated oral vaccine.
It is worth pointing out that the current manuscript has not only provided additional tools for developing live attenuated vaccines but also demonstrated a principle that the chlamydial plasmid or plasmid-encoded genes can be targeted for developing attenuated Chlamydia oral vaccines. Targeting the plasmid or plasmid-encoded genes for developing vaccines may offer advantages over the chromosomal gene mutation-based approach (24). First, it is more feasible to produce the desired loss of function mutations in the chlamydial plasmid genes than in the chromosomal genes. At this moment, there is still no reproducible approach to introduce the desired mutations to the chlamydial chromosomal genes while approaches for genetic manipulation of chlamydial plasmid genes have been very successful. Because all live attenuated chlamydial vaccines evaluated in the mouse model are based on the mouse-adapted C. muridarum species, the identified mutations will have to be introduced to the human C. trachomatis species for developing human vaccines. Thus, it is more feasible to use the mutation information provided in the current study for developing live attenuated Chlamydia for humans. Secondly, the chromosomal gene-based attenuated vaccine contains multiple mutations in multiple chromosomal genes (24). It will be difficult to reproduce these many different mutations in C. trachomatis even if a reliable mutagenesis method is developed for C. trachomatis chromosomal genes in the future. In addition, introducing many mutations into C. trachomatis may significantly reduce the immunogenicity of the attenuated vaccine because some mutations may inevitably disrupt some dominant protective epitopes. In contrast, our pGP3-deficient vaccine only lacks a single plasmid-encoded protein. Introducing a loss of function mutation to the C. trachomatis pgp3 gene is convenient and may minimally affect the immunogenicity of the attenuated C. trachomatis vaccine. Finally, our current study has shown that plasmid-free C. muridarum is sufficient for inducing transmucosal protection against chlamydial pathogenicity in the genital tract. Interestingly, plasmid-free C. trachomatis organisms have been isolated from humans. Thus, it will be worth investigating whether subjects who carry plasmid-free C. trachomatis still develop pathology in the genital tract and whether the same individuals can develop protective immunity against subsequent infection by wild type C. trachomatis. These retrospective human studies may serve as plasmid-free C. trachomatis vaccine trials or provide relevant information for designing human vaccine trials.
We are also aware of the limitation of the current study. Because all findings were made using the C. muridarum-mouse model, caveats should be taken when applying the knowledge to C. trachomatis infection in humans. The evolutionary relationship between C. muridarum and mice may differ from that between C. trachomatis and humans. Clearly, C. muridarum may be more efficiently transmitted between mice via an oral-fecal route than sexual contact. However, C. trachomatis may be forced to transmit between humans via sexual contacts because the oral-fecal route is cut off in modern humans. Nevertheless, pGP3 is highly conserved and C. trachomatis deficient in pGP3 is also attenuated in pathogenicity in the mouse genital tract (36). It will be worth testing whether the pGP3-deficinet C. trachomatis is also attenuated in the genital tract of nonhuman primates. Furthermore, it will be interesting to test whether pGP3-deficent C. muridarum or -C. trachomatis can induce transmucosal protection in the genital tract of nonhuman primates or humans.
MATERIALS AND METHODS
Chlamydial organism growth.
All Chlamydia muridarum (CM) clones used in the current study were derived from strain Nigg3 (GenBank accession# CP009760.1), including a plasmid-free clone CMUT3G5 (GenBank accession# CP006974.1) designated as CMpf. CMpf was used for transformation with pGFP:CM to create CMpGFP or pGFP:CM with a premature stop codon in pgp3 gene to create CMpGP3S as described previously (25, 38). CMpGFP was used as the wild type C. muridarum designated as CMwt. All chlamydial organisms were propagated in HeLa cells and purified as elementary bodies (EBs) as reported previously (29, 39). Aliquots of the purified EBs were stored at −80°C until use.
Mouse immunization and challenge infection.
Mouse experiments were carried out in accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Committee on the Ethics of Laboratory Animal Experiments of the University of Texas Health Science Center at San Antonio.
Purified C. muridarum EBs were used to inoculate 6-to-7-week-old female C57BL/6j mice (000664, Jackson Laboratories, Inc., Bar Harbor, Maine) intragastrically (as the oral immunization route) or intravaginally (as both immunization and challenge infection routes) as described previously (5, 29, 30, 40). The inoculation dose (for all experiments, all routes and all Chlamydia strains) was 2 × 105 inclusion forming units (IFUs). Following each inoculation, vaginal and/or rectal swabs were taken periodically for monitoring viable C. muridarum colonization as described previously (29, 40, 41).
Titrating live chlamydial organisms recovered from swabs.
To quantitate live chlamydial organisms in vaginal or rectal swabs, each swab was soaked in 0.5 mL of SPG, vortexed with glass beads, and the chlamydial organisms released into the supernatants were titrated on HeLa cell monolayers in duplicate. The infected cultures were processed for immunofluorescence assay as described previously (42) and below. Inclusions were counted in five random fields per coverslip under a fluorescence microscope. For coverslips with less than one IFU per field, entire coverslips were counted. Coverslips showing obvious cytotoxicity of HeLa cells were excluded. The total number of IFUs per swab was calculated based on the mean IFUs per view, the ratio of the view area to that of the well, dilution factor, and inoculation volumes. Where possible, a mean IFU/swab was derived from the serially diluted and duplicate samples for any given swab. The total number of IFUs/swab was converted into log10, which was used to calculate the mean and standard deviation across mice of the same group at each time point.
Immunofluorescence assay.
For immunofluorescence labeling of C. muridarum in HeLa cells, a rabbit antibody (designated as R1604, raised with purified C. muridarum EBs) was used as a primary antibody to label C. muridarum, which was visualized with a goat anti-rabbit IgG conjugated with Cy2 (green, cat#111-225-144, Jackson ImmunoResearch Laboratories, INC., West Grove PA). The DNA dye Hoechst 3328 (blue, Sigma-Aldrich, St. Louis, MO) was used to visualize nuclei. The doubly labeled samples were used for counting for C. muridarum under a fluorescence microscope (IX80, Olympus) equipped with a CCD camera (Hamamatsu).
Evaluating genital tract gross pathology hydrosalpinx macroscopically.
On day 56 after intravaginal infection, mice were euthanized for evaluating genital tract pathology. The focus was on the upper genital tract hydrosalpinx. Before the tissues were removed, an in situ gross examination was performed for evidence of oviduct hydrosalpinx or any other related abnormalities of oviducts. The severity of oviduct hydrosalpinx was scored based on the following criteria: no hydrosalpinx (0), hydrosalpinx detectable only under microscopic examination (1), hydrosalpinx clearly visible with naked eyes but the size was smaller than the ovary on the same side (2), equal to the ovary on the same side (3) or larger than the ovary on the same side (4). Bilateral hydrosalpinx severity was calculated for each mouse as the summed scores of the left and right oviducts. Hydrosalpinx incidence was calculated as the number of mice with a bilateral score of 1 or higher divided by the total number of mice in the group.
Evaluating oviduct dilation and inflammatory infiltration microscopically.
After macroscopic evaluation of hydrosalpinx and photographing for documenting hydrosalpinx, the same mouse genital tissues were fixed in 10% neutral formalin, embedded in paraffin and serially sectioned longitudinally (with 5 μm/each section). Efforts were made to include the cervix, both uterine horns, and the oviducts, as well as lumenal structures of each tissue in the same section. The sections were stained with hematoxylin and eosin (H&E). Three representative sections separated by 15 μM or more from each other were used for evaluating both oviduct dilation and inflammatory infiltration. The oviduct dilation was assessed under a 4x subjective lens. The severity of oviduct dilation was semi-quantitatively scored using the following criteria: 0, no significant oviduct lumenal dilatation; 1, mild dilation of a single cross section; 2, one to three dilated cross sections; 3, more than three dilated cross sections; and 4, confluent pronounced dilation. The median of the three scores served as a single value for each oviduct unilateral dilation score. Both unilateral scores for each mouse were combined to form a bilateral dilation score. Oviduct dilation incidence was calculated as the number of mice with a bilateral score of 1 or higher divided by the total number of mice in the group. The inflammatory infiltrates were semi-quantitatively scored under a 40X objective lens using the following criteria: 0 represents no cellular foci (of mononuclear cells), 1 indicates a single focus, 2 indicates two to four loci, 3 indicates more than four foci, and 4 indicates confluent infiltration in the tissue or one or more clusters of >3 mononuclear cells in the oviduct lumen. The median of the three scores served as a single value for each oviduct unilateral inflammation score, and both unilateral scores for each mouse were combined to form a bilateral score.
Statistics analyses.
The time courses of live organism shedding (IFUs) were compared using “area-under-the-curve or AUC” between two groups using Wilcoxon rank sum test. The individual data point IFUs and pathology scores were also analyzed with Wilcoxon rank sum test. The category data including the % of mice positive for live organism shedding or pathology were analyzed using Fisher's Exact test. Prior to any two designated group comparisons, an ANOVA was used to determine whether there was an overall significant difference among all groups in the same experiment (https://goodcalculators.com/one-way-anova-calculator/).
ACKNOWLEDGMENTS
This study is supported in part by US NIH grants (R01AI047997 & R01AI121989 to G.Z.) and a grant from the Natural Science Foundation of China (31800774 to L.W.).
Contributor Information
Luying Wang, Email: luyingwang2020@outlook.com.
Guangming Zhong, Email: Zhongg@UTHSCSA.EDU.
Craig R. Roy, Yale University School of Medicine
REFERENCES
- 1.CDC. 2017. Sexually Transmitted Disease Surveillance, 2016. Atlanta: Department of Health and Human Services. https://www.cdc.gov/std/stats16/default.htm. [Google Scholar]
- 2.Budrys NM, Gong S, Rodgers AK, Wang J, Louden C, Shain R, Schenken RS, Zhong G. 2012. Chlamydia trachomatis antigens recognized in women with tubal factor infertility, normal fertility, and acute infection. Obstet Gynecol 119:1009–1016. 10.1097/AOG.0b013e3182519326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shah AA, Schripsema JH, Imtiaz MT, Sigar IM, Kasimos J, Matos PG, Inouye S, Ramsey KH. 2005. Histopathologic changes related to fibrotic oviduct occlusion after genital tract infection of mice with Chlamydia muridarum. Sex Transm Dis 32:49–56. 10.1097/01.olq.0000148299.14513.11. [DOI] [PubMed] [Google Scholar]
- 4.de la Maza LM, Pal S, Khamesipour A, Peterson EM. 1994. Intravaginal inoculation of mice with the Chlamydia trachomatis mouse pneumonitis biovar results in infertility. Infect Immun 62:2094–2097. 10.1128/iai.62.5.2094-2097.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen J, Zhang H, Zhou Z, Yang Z, Ding Y, Zhou Z, Zhong E, Arulanandam B, Baseman J, Zhong G. 2014. Chlamydial induction of hydrosalpinx in 11 strains of mice reveals multiple host mechanisms for preventing upper genital tract pathology. PLoS One 9:e95076. 10.1371/journal.pone.0095076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rodgers AK, Budrys NM, Gong S, Wang J, Holden A, Schenken RS, Zhong G. 2011. Genome-wide identification of Chlamydia trachomatis antigens associated with tubal factor infertility. Fertil Steril 96:715–721. 10.1016/j.fertnstert.2011.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rodgers AK, Wang J, Zhang Y, Holden A, Berryhill B, Budrys NM, Schenken RS, Zhong G. 2010. Association of tubal factor infertility with elevated antibodies to Chlamydia trachomatis caseinolytic protease. P Am J Obstet Gynecol 203:494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vlcek KR, Li W, Manam S, Zanotti B, Nicholson BJ, Ramsey KH, Murthy AK. 2016. The contribution of Chlamydia-specific CD8(+) T cells to upper genital tract pathology. Immunol Cell Biol 94:208–212. 10.1038/icb.2015.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de la Maza LM, Peterson EM. 2002. Vaccines for Chlamydia trachomatis infections. Curr Opin Invest Drugs 3:980–986. [PubMed] [Google Scholar]
- 10.Morrison RP, Caldwell HD. 2002. Immunity to murine chlamydial genital infection. Infect Immun 70:2741–2751. 10.1128/IAI.70.6.2741-2751.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu C, Peng B, Li Z, Lei L, Li Z, Chen L, He Q, Zhong G, Wu Y. 2013. Induction of protective immunity against Chlamydia muridarum intravaginal infection with the chlamydial immunodominant antigen macrophage infectivity potentiator. Microbes Infect 15:329–338. 10.1016/j.micinf.2013.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson RM, Kerr MS, Slaven JE. 2014. An atypical CD8 T-cell response to Chlamydia muridarum genital tract infections includes T cells that produce interleukin-13. Immunology 142:248–257. 10.1111/imm.12248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rockey DD, Wang J, Lei L, Zhong G. 2009. Chlamydia vaccine candidates and tools for chlamydial antigen discovery. Expert Rev Vaccines 8:1365–1377. 10.1586/erv.09.98. [DOI] [PubMed] [Google Scholar]
- 14.Campos-Hernandez E, Vazquez-Chagoyan JC, Salem AZ, Saltijeral-Oaxaca JA, Escalante-Ochoa C, Lopez-Heydeck SM, de Oca-Jimenez RM. 2014. Prevalence and molecular identification of Chlamydia abortus in commercial dairy goat farms in a hot region in Mexico. Trop Anim Health Prod 46:919–924. 10.1007/s11250-014-0585-6. [DOI] [PubMed] [Google Scholar]
- 15.Peters RP, Dubbink JH, van der Eem L, Verweij SP, Bos ML, Ouburg S, Lewis DA, Struthers H, McIntyre JA, Morre SA. 2014. Cross-sectional study of genital, rectal, and pharyngeal Chlamydia and gonorrhea in women in rural South Africa. Sex Transm Dis 41:564–569. 10.1097/OLQ.0000000000000175. [DOI] [PubMed] [Google Scholar]
- 16.Gratrix J, Singh AE, Bergman J, Egan C, McGinnis J, Drews SJ, Read R. 2014. Prevalence and characteristics of rectal chlamydia and gonorrhea cases among men who have sex with men after the introduction of nucleic acid amplification test screening at 2 Canadian sexually transmitted infection clinics. Sex Transm Dis 41:589–591. 10.1097/OLQ.0000000000000176. [DOI] [PubMed] [Google Scholar]
- 17.Gratrix J, Singh AE, Bergman J, Egan C, Plitt SS, McGinnis J, Bell CA, Drews SJ, Read R. 2015. Evidence for increased Chlamydia case finding after the introduction of rectal screening among women attending 2 Canadian sexually transmitted infection clinics. Clin Infect Dis 60:398–404. 10.1093/cid/ciu831. [DOI] [PubMed] [Google Scholar]
- 18.Musil K, Currie M, Sherley M, Martin S. 2016. Rectal chlamydia infection in women at high risk of chlamydia attending Canberra Sexual Health Centre. Int J STD AIDS 27:526–530. 10.1177/0956462415586317. [DOI] [PubMed] [Google Scholar]
- 19.Tian Q, Zhou Z, Wang L, Abu-Khdeir AH, Huo Z, Sun X, Zhang N, Schenken R, Wang Y, Xue M, Zhong G. 2020. Gastrointestinal coinfection promotes chlamydial pathogenicity in the genital tract. Infect Immun 88:e00905-19. 10.1128/IAI.00905-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tian Q, Zhou Z, Wang L, Sun X, Arulanandam B, Xu D, Xue M, Zhong G. 2021. Gastrointestinal Chlamydia-induced CD8+ T cells promote chlamydial pathogenicity in the female upper genital tract. Infect Immun 89. 10.1128/IAI.00205-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhong G. 2018. Chlamydia spreading from the genital tract to the gastrointestinal tract - A two-hit hypothesis. Trends Microbiol 26:611–623. 10.1016/j.tim.2017.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang L, Zhu C, Zhang T, Tian Q, Zhang N, Morrison S, Morrison R, Xue M, Zhong G. 2018. Nonpathogenic colonization with chlamydia in the gastrointestinal tract as oral vaccination for inducing transmucosal protection. Infect Immun 86:e00630-17. 10.1128/IAI.00630-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu C, Lin H, Tang L, Chen J, Wu Y, Zhong G. 2018. Oral Chlamydia vaccination induces transmucosal protection in the airway. Vaccine 36:2061–2068. 10.1016/j.vaccine.2018.03.015. [DOI] [PubMed] [Google Scholar]
- 24.Morrison SG, Giebel AM, Toh E, Banerjee A, Nelson DE, Morrison RP. 2020. A genital infection-attenuated Chlamydia muridarum mutant infects the gastrointestinal tract and protects against genital tract challenge. mBio 11. 10.1128/mBio.02770-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu Y, Huang Y, Yang Z, Sun Y, Gong S, Hou S, Chen C, Li Z, Liu Q, Wu Y, Baseman J, Zhong G. 2014. Plasmid-encoded Pgp3 is a major virulence factor for Chlamydia muridarum to induce hydrosalpinx in mice. Infect Immun 82:5327–5335. 10.1128/IAI.02576-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lei L, Chen J, Hou S, Ding Y, Yang Z, Zeng H, Baseman J, Zhong G. 2014. Reduced live organism recovery and lack of hydrosalpinx in mice infected with plasmid-free Chlamydia muridarum. Infect Immun 82:983–992. 10.1128/IAI.01543-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O’Connell CM, Ingalls RR, Andrews CW, Scurlock AM, Darville T. 2007. Plasmid-deficient Chlamydia muridarum fail to induce immune pathology and protect against oviduct disease. J Immunol 179:4027–4034. 10.4049/jimmunol.179.6.4027. [DOI] [PubMed] [Google Scholar]
- 28.Zhong G. 2017. Chlamydial Plasmid-Dependent Pathogenicity. Trends Microbiol 25:141–152. 10.1016/j.tim.2016.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang Q, Huang Y, Gong S, Yang Z, Sun X, Schenken R, Zhong G. 2015. In vivo and ex vivo imaging reveals a long-lasting Chlamydial infection in the mouse gastrointestinal tract following genital tract inoculation. Infect Immun 83:3568–3577. 10.1128/IAI.00673-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shao L, Melero J, Zhang N, Arulanandam B, Baseman J, Liu Q, Zhong G. 2017. The cryptic plasmid is more important for Chlamydia muridarum to colonize the mouse gastrointestinal tract than to infect the genital tract. PLoS One 12:e0177691. 10.1371/journal.pone.0177691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shao L, Zhang T, Melero J, Huang Y, Liu Y, Liu Q, He C, Nelson DE, Zhong G. 2018. The genital tract virulence factor pGP3 is essential for Chlamydia muridarum colonization in the gastrointestinal Tract. Infect Immun 86:e00429-17. 10.1128/IAI.00429-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koprivsek JJ, Zhang T, Tian Q, He Y, Xu H, Xu Z, Zhong G. 2019. Distinct roles of chromosome- versus plasmid-encoded genital tract virulence factors in promoting Chlamydia muridarum colonization in the gastrointestinal tract. Infect Immun 87:e00265-19. 10.1128/IAI.00265-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ma J, He C, Huo Z, Xu Y, Arulanandam B, Liu Q, Zhong G. 2020. The cryptic plasmid improves Chlamydia fitness in different regions of the gastrointestinal tract. Infect Immun 88:e00860-19. 10.1128/IAI.00860-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.He C, Xu Y, Huo Z, Wang J, Jia T, Li X, Zhong G. 2021. Regulation of Chlamydia spreading from small intestine to large intestine via an immunological barrier. Immunology and Cell Biology 99:611–621. 10.1111/imcb.12446. [DOI] [PubMed] [Google Scholar]
- 35.Huo Z, He C, Xu Y, Jia T, Wang J, Zhong G. 2020. Chlamydia deficient in plasmid-encoded pGP3 is prevented from spreading to large intestine. Infect Immun 88:e00120-20. 10.1128/IAI.00120-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ramsey KH, Schripsema JH, Smith BJ, Wang Y, Jham BC, O'Hagan KP, Thomson NR, Murthy AK, Skilton RJ, Chu P, Clarke IN. 2014. Plasmid CDS5 influences infectivity and virulence in a mouse model of Chlamydia trachomatis urogenital infection. Infect Immun 82:3341–3349. 10.1128/IAI.01795-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang Y, Zhang Q, Yang Z, Conrad T, Liu Y, Zhong G. 2015. Plasmid-encoded Pgp5 is a significant contributor to Chlamydia muridarum induction of hydrosalpinx. PLoS One 10:e0124840. 10.1371/journal.pone.0124840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu Y, Chen C, Gong S, Hou S, Qi M, Liu Q, Baseman J, Zhong G. 2014. Transformation of Chlamydia muridarum reveals a role for Pgp5 in suppression of plasmid-dependent gene expression. J Bacteriol 196:989–998. 10.1128/JB.01161-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fan T, Lu H, Hu H, Shi L, McClarty GA, Nance DM, Greenberg AH, Zhong G. 1998. Inhibition of apoptosis in Chlamydia-infected cells: blockade of mitochondrial cytochrome c release and caspase activation. J Exp Med 187:487–496. 10.1084/jem.187.4.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang L, Zhang Q, Zhang T, Zhang Y, Zhu C, Sun X, Zhang N, Xue M, Zhong G. 2016. The Chlamydia muridarum organisms fail to auto-inoculate the mouse genital tract after colonization in the gastrointestinal tract for 70 days. PLoS One 11:e0155880. 10.1371/journal.pone.0155880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dai J, Zhang T, Wang L, Shao L, Zhu C, Zhang Y, Failor C, Schenken R, Baseman J, He C, Zhong G. 2016. Intravenous inoculation with Chlamydia muridarum leads to a long-lasting infection restricted to the gastrointestinal tract. Infect Immun 84:2382–2388. 10.1128/IAI.00432-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tang L, Zhang H, Lei L, Gong S, Zhou Z, Baseman J, Zhong G. 2013. Oviduct infection and hydrosalpinx in DBA1/j mice is induced by intracervical but not intravaginal inoculation with Chlamydia muridarum. PLoS One 8:e71649. 10.1371/journal.pone.0071649. [DOI] [PMC free article] [PubMed] [Google Scholar]


