ABSTRACT
Amphibian populations have been declining around the world for more than five decades, and the losses continue. Although causes are complex, major contributors to these declines are two chytrid fungi, Batrachochytrium dendrobatidis and Batrachochytrium salamandrivorans, which both cause the disease termed chytridiomycosis. Previously, we showed that B. dendrobatidis impedes amphibian defenses by directly inhibiting lymphocytes in vitro and in vivo by release of soluble metabolites, including kynurenine (KYN), methylthioadenosine (MTA), and spermidine (SPD). Here, we show that B. salamandrivorans cells and cell-free supernatants also inhibit amphibian lymphocytes as well as a human T cell line. As we have shown for B. dendrobatidis, high-performance liquid chromatography (HPLC) and mass spectrometry revealed that KYN, MTA, and SPD are key metabolites found in the B. salamandrivorans supernatants. Production of inhibitory factors by B. salamandrivorans is limited to mature zoosporangia and can occur over a range of temperatures between 16°C and 26°C. Taken together, these results suggest that both pathogenic Batrachochytrium fungi have evolved similar mechanisms to inhibit lymphocytes in order to evade clearance by the amphibian immune system.
KEYWORDS: amphibian declines, Batrachochytrium dendrobatidis, Batrachochytrium salamandrivorans, chytrid, immunomodulation, kynurenine, lymphocyte, methylthioadenosine, polyamine, spermidine
INTRODUCTION
Chytridiomycosis is a skin disease caused by two pathogenic chytrid fungi, Batrachochytrium dendrobatidis and B. salamandrivorans. Batrachochytrium dendrobatidis was first described in 1998 (1–3) and is a globally distributed pathogen associated with declines of hundreds of amphibian species (4). Batrachochytrium salamandrivorans was first described in 2013 and is thought to be native to parts of Asia but was lethal when accidentally introduced into populations of fire salamanders (Salamandra salamandra) in northern Europe (5–7). Although published information about the number of amphibian species tested for susceptibility to B. salamandrivorans is limited, this pathogen appears to cause greater disease in urodeles (salamanders and newts) than in anurans (frogs and toads) (6, 8, 9). Previous studies showed that B. dendrobatidis releases soluble factors that can inhibit both amphibian and mammalian lymphocyte proliferation, induce apoptosis of lymphocytes and epithelial cells (10, 11), induce apoptosis in frog skin (12), and inhibit a delayed type of hypersensitivity (DTH) response in frog skin (13). The supernatants were shown to contain at least three small immunomodulatory metabolites, kynurenine (KYN), methylthioadenosine (MTA), and spermidine (SPD) (14, 15). Studies designed to examine an immune response to B. salamandrivorans in salamanders showed that prior exposure to this pathogen followed by heat-induced clearance did not appear to protect from subsequent exposures, suggesting that adaptive immunity is slow to develop or absent (7, 16). This suggests that B. salamandrivorans, like its most closely related sister species, B. dendrobatidis, has evolved mechanisms to evade immune recognition and clearance. Here, we investigated whether B. salamandrivorans also directly inhibits amphibian lymphocyte functions and produces a similar suite of inhibitory metabolites as those produced by B. dendrobatidis.
RESULTS
Effects of B. salamandrivorans cells and supernatants on proliferation of amphibian lymphocytes.
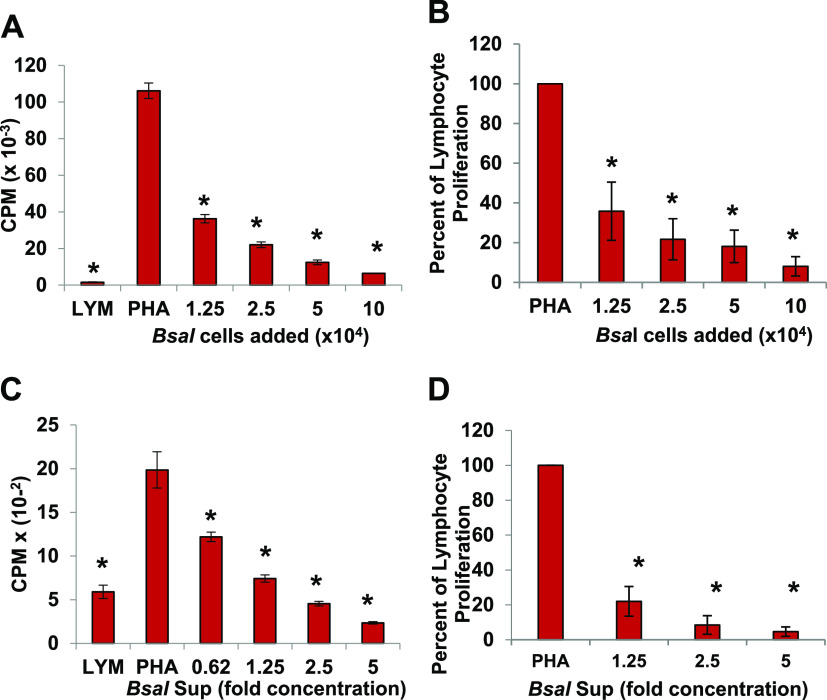
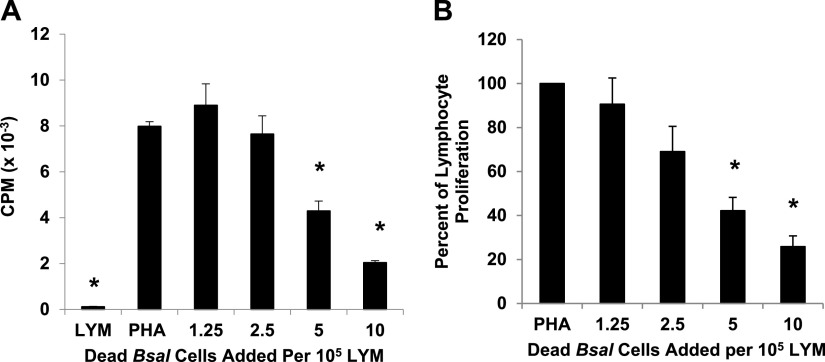
To examine the effects of B. salamandrivorans zoosporangia on activated amphibian lymphocytes, increasing numbers of zoosporangia were cocultured with the lymphocyte-enriched fraction of splenocytes (LYM) from Xenopus laevis that were induced to proliferate with the plant lectin, phytohemagglutinin (PHA). Frogs do not have lymph nodes, and thus, the greatest source of lymphocytes is the spleen. PHA specifically induces proliferation of the T lymphocyte subset (17). When 1.25 × 104 zoosporangia were added to 105 lymphocytes (a ratio of about 1 zoosporangium to 8 lymphocytes), proliferation of the lymphocytes was consistently and significantly reduced by approximately 75% (*, P ≤ 0.01 by one-way analysis of variance with Tukey post hoc test). As the number of added zoosporangia was increased, proliferation was reduced in a dose-dependent fashion and was almost completely inhibited when zoosporangia and lymphocytes were cultured in equal numbers (Fig. 1A and B; *, P ≤ 0.01 by one-way analysis of variance with Tukey post hoc test). To determine whether the inhibition of lymphocyte proliferation was due to direct cell-to-cell signaling, we prepared and tested cell-free supernatants from the B. salamandrivorans cultures (B. salamandrivorans Sups). The supernatant factors were also significantly inhibitory to proliferating lymphocytes. At a 5-fold concentration of the supernatants, proliferation of lymphocytes was almost completely prevented. Even at a 1.25-fold concentration of the supernatants, proliferation of the lymphocytes was reduced by greater than 75% (see Materials and Methods for supernatant preparation) (Fig. 1C and D; *, P ≤ 0.01 by one-way analysis of variance with Tukey post hoc test.). Although live fungal cells were very effective inhibitors of activated T lymphocytes, dead zoosporangia also inhibited proliferating lymphocytes. However, it required at least one fungal cell for each two lymphocytes (Fig. 2A and B; *, P ≤ 0.01 by one-way analysis of variance with Tukey post hoc test). This suggested that the heat-killed fungal cells are still capable of delivering an inhibitory signal.
FIG 1.
B. salamandrivorans (Bsal) zoosporangia and supernatants inhibit proliferation of amphibian lymphocytes. (A) Lymphocytes (LYM) (105/well) from X. laevis were cultured alone or with phytohemagglutinin (PHA). PHA-stimulated lymphocytes were cultured alone or with increasing numbers of B. salamandrivorans zoosporangia. One representative experiment of six. *, significantly reduced [3H]-thymidine uptake detected as counts per minute (CPM) compared to control PHA-stimulated lymphocytes. (B) Summary of six experiments reported as percentage of proliferation of lymphocytes with added B. salamandrivorans zoosporangia in comparison with [3H]-thymidine uptake by PHA-stimulated control lymphocytes. (C) LYM (105/well) from X. laevis were cultured alone or with PHA. PHA-stimulated lymphocytes were cultured alone or with increasing concentrations of B. salamandrivorans supernatants (Bsal Sup). One representative experiment of four. *, significantly reduced [3H]-thymidine uptake detected as CPM compared to control PHA-stimulated lymphocytes. (D) Summary of four experiments reported as percent proliferation of lymphocytes with added Bsal Sup in comparison with [3H]-thymidine uptake by PHA-stimulated control lymphocytes. For all panels, comparisons were made to examine differences from PHA-stimulated lymphocytes. *, significant differences with P ≤ 0.01 by one-way analysis of variance with Tukey post hoc test. Lymphocytes and zoosporangia were cultured at 26°C.
FIG 2.
Dead B. salamandrivorans zoosporangia inhibit proliferation of amphibian lymphocytes. (A) Lymphocytes (LYM) (105/well) from X. laevis were cultured alone or with phytohemagglutinin (PHA). PHA-stimulated lymphocytes were cultured alone or with increasing numbers of heat-killed B. salamandrivorans zoosporangia (60°C for 10 min). One representative experiment of four. *, significantly reduced [3H]-thymidine uptake detected as counts per minute (CPM) compared to control PHA-stimulated lymphocytes. (B) Summary of four experiments reported as percentage of proliferation of LYM with added B. salamandrivorans zoosporangia in comparison with [3H]-thymidine uptake by PHA-stimulated control lymphocytes. For all comparisons to PHA-stimulated lymphocytes, significant differences are noted with an asterisk; *, P ≤ 0.01 by one-way analysis of variance with Tukey post hoc test. Lymphocytes and zoosporangia were cultured at 26°C.
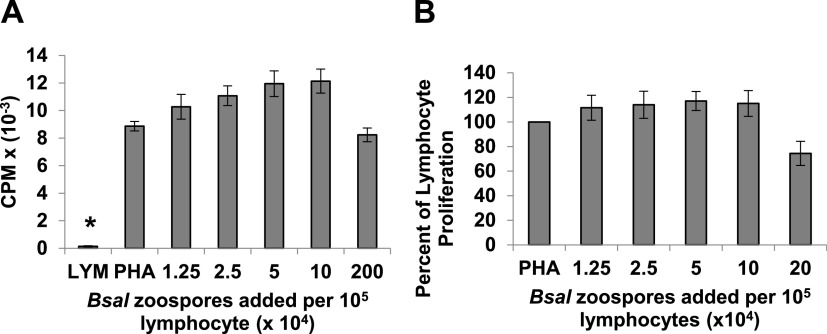
Batrachochytrium salamandrivorans is transmitted by a swimming flagellated zoospore that differentiates into the mature zoosporangium within the skin (5, reviewed in reference 18). The zoospores have been shown to express protease genes (16), and thus, it was important to test whether zoospores, as well as mature zoosporangia, could inhibit Xenopus lymphocyte proliferation. In contrast to the mature cell and cell-free supernatants of zoosporangia, purified zoospores did not inhibit lymphocyte proliferation in coculture assays (Fig. 3A and B).
FIG 3.
In coculture, purified B. salamandrivorans (Bsal) zoospores do not inhibit proliferation of PHA-stimulated amphibian lymphocytes. (A) Lymphocytes (LYM) (105/well) from X. laevis were cultured alone or with phytohemagglutinin (PHA). PHA-stimulated lymphocytes were cultured alone or with increasing numbers of purified B. salamandrivorans zoospores. One representative experiment of five. Only unstimulated control lymphocytes showed less proliferation than PHA stimulated lymphocytes; *, P ≤ 0.01 by one-way analysis of variance with Tukey post hoc test. (B) Summary of five experiments reported as percentage of lymphocyte proliferation with added B. salamandrivorans zoospores in comparison with [3H]-thymidine uptake by PHA-stimulated control lymphocytes. Lymphocytes and zoospores were cultured at 26°C.
Effects of temperature on capacity of B. salamandrivorans zoosporangia to inhibit lymphocyte proliferation.
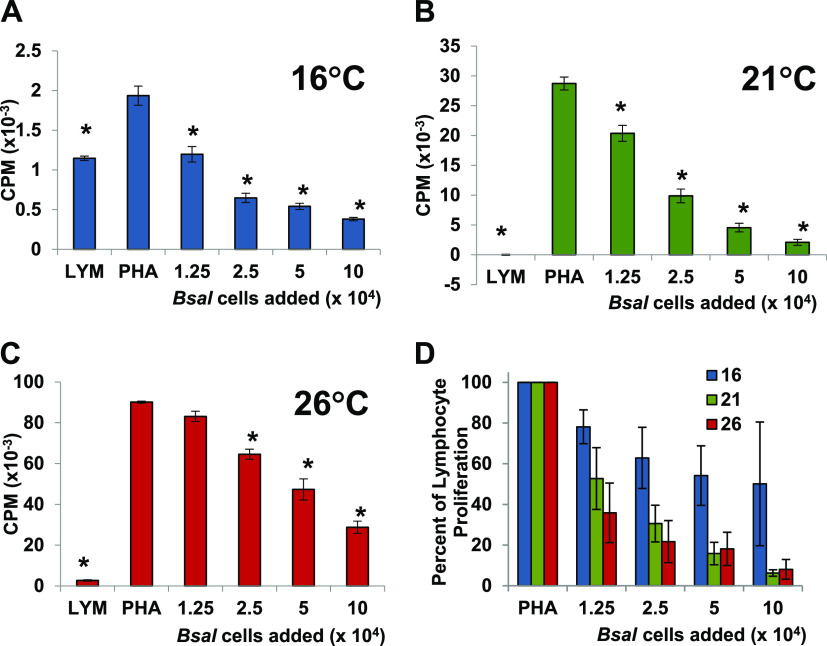
Xenopus laevis lymphocyte proliferation is optimal at about 26°C, and thus, the initial coculture assays with live B. salamandrivorans were conducted at this temperature (Fig. 1A and B). However, 26°C is likely to be a very stressful temperature for this cold-loving pathogen (5). Therefore, the capacity of the zoosporangia to inhibit lymphocyte proliferation was also tested at both 16°C and 21°C. In a series of coculture experiments, we tested the capacity of B. salamandrivorans zoosporangia to inhibit proliferation at all three temperatures, 16°C, 21°C, and 26°C. In a representative coculture experiment with lymphocytes from one large frog, significant inhibition was observed at all three temperatures in a similar dose-dependent fashion (*, P ≤ 0.01 by one-way analysis of variance with Tukey post hoc test). It should be noted that the lymphocyte proliferation is highly temperature dependent, and thus, the maximum proliferation was very different at each temperature (Fig. 4A to C). Although results were variable, multiple-replicate coculture experiments at the three different temperatures suggested that the warmer temperatures of 21°C and 26°C resulted in a greater level of inhibition (Fig. 4D).
FIG 4.
B. salamandrivorans (Bsal) zoosporangia cocultured with PHA-stimulated amphibian lymphocytes inhibit proliferation of lymphocytes at a wide range of temperatures. (A to C) Lymphocytes (LYM) (105/well) from one X. laevis were cultured alone or with phytohemagglutinin (PHA). PHA-stimulated lymphocytes were cultured alone or with increasing numbers of B. salamandrivorans zoosporangia at 16°C (A), 21°C (B), and 26°C (C). *, significantly reduced [3H]-thymidine uptake detected as counts per minute (CPM) compared to control PHA-stimulated lymphocytes; *, P ≤ 0.01 by one-way analysis of variance with Tukey post hoc test. (D) Summary of three experiments using individual frogs at 16°C, five experiments at 21°C, and six experiments at 26°C reported as percent proliferation of lymphocytes with added B. salamandrivorans zoosporangia in comparison with [3H]-thymidine uptake by PHA-stimulated control lymphocytes.
Effects of B. salamandrivorans supernatants on proliferation of a human T cell line.
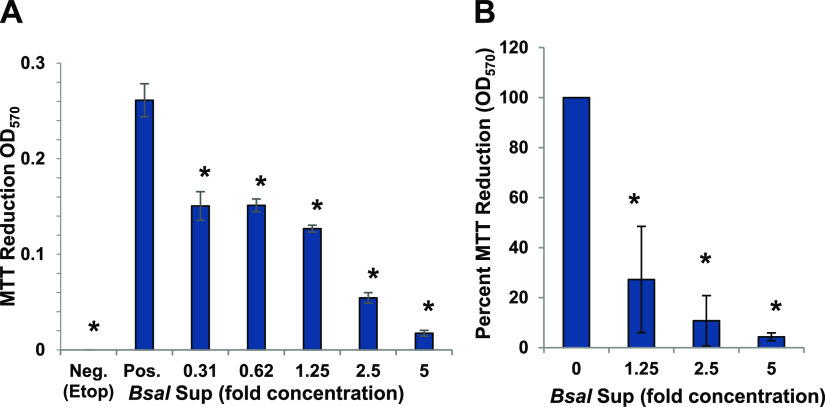
To determine whether the inhibition of lymphocytes was limited to amphibian cells, the capacity of B. salamandrivorans Sups to affect the survival and viability of a human T cell line, Jurkat T cells were also tested. Similar to the effects of the supernatants on amphibian lymphocytes, B. salamandrivorans Sups also significantly inhibited the survival of Jurkat T cells at concentrations greater than 1.25-fold (*, P ≤ 0.01 by one-way analysis of variance with Tukey post hoc test). There was greater than 95% reduction of survival at the highest concentration tested (5-fold) (Fig. 5A and B).
FIG 5.
B. salamandrivorans supernatants (Bsal Sup) inhibit proliferation of Jurkat T cells. (A) Jurkat T cells (2 × 105/mL) were cultured alone (Pos.) as a positive control for growth or with increasing concentrations of B. salamandrivorans Sup as shown or with 25 μg/mL etoposide (Etop) as a negative control for growth. Survival was measured as 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) reduction after 3 days. (A) One representative of two similar experiments (Sup generated by cells cultured at 107/mL). *, proliferation was significantly reduced determined by one-way analysis of variance, with Tukey post hoc test; P < 0.01. (B) Average of two similar experiments as percentage of positive growth in control wells (Sup generated by cells cultured at 107/mL or 108/mL). *, differences were significant by one-way analysis of variance with Tukey post hoc test; P < 0.05.
Comparison of B. salamandrivorans and B. dendrobatidis inhibition.
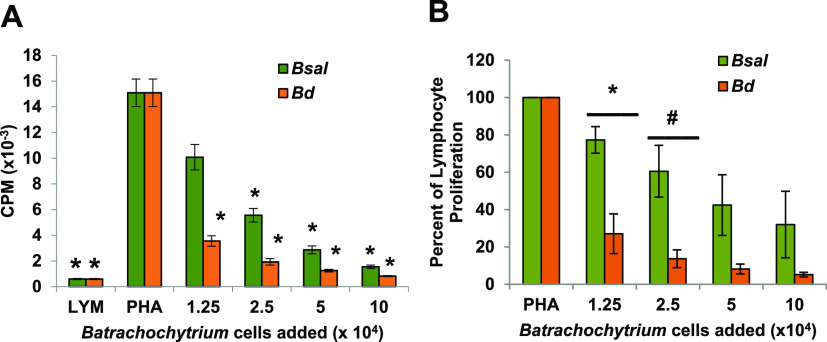
Because we hypothesized that both pathogens may use similar immune evasion strategies, the zoosporangia from each pathogen were tested in equal numbers for inhibition of the same sets of Xenopus lymphocytes incubated at 21°C, a temperature that is within the normal range of both pathogens (5, 19). In three experiments, inhibition by B. dendrobatidis zoosporangia was significantly greater than that of B. salamandrivorans on a per-cell basis for several concentrations of added cells (Fig. 6A; *, P ≤ 0.01 by one-way analysis of variance with Tukey post hoc test, and Fig. 6B, significantly different by multiple comparison of means with Tukey post hoc test; *, P < 0.05; #, P = 0.0668).
FIG 6.
B. salamandrivorans (Bsal) zoosporangia inhibit less well than B. dendrobatidis (Bd) zoosporangia at a common temperature. (A) Lymphocytes (LYM) (105/well) from X. laevis were cultured alone or with phytohemagglutinin (PHA). PHA-stimulated lymphocytes were cultured alone or with increasing numbers of B. salamandrivorans or B. dendrobatidis zoosporangia. One representative experiment of three. *, significantly reduced [3H]-thymidine uptake detected as counts per minute (CPM) compared to control PHA-stimulated lymphocytes; *, P ≤ 0.01 by one-way analysis of variance with Tukey post hoc test. Lymphocytes and zoosporangia were cultured at 21°C. (B) Summary of three independent experiments reported as percentage of proliferation of lymphocytes with added B. salamandrivorans or B. dendrobatidis zoosporangia in comparison with [3H]-thymidine uptake by PHA-stimulated control lymphocytes. Significantly different by multiple comparison of means with Tukey post hoc test; *, P < 0.05; #, P = 0.0668.
Detection of immunomodulatory metabolites in B. salamandrivorans cell-free supernatants.
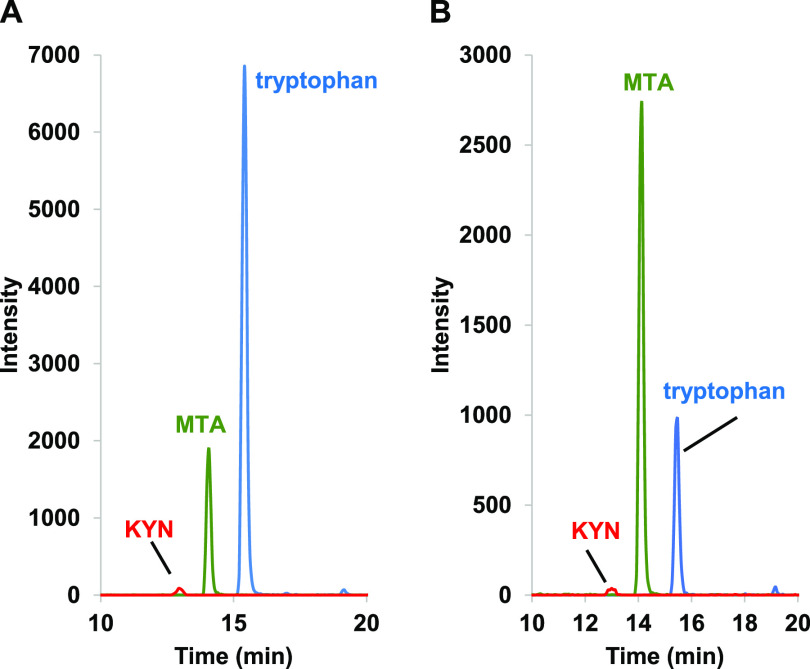
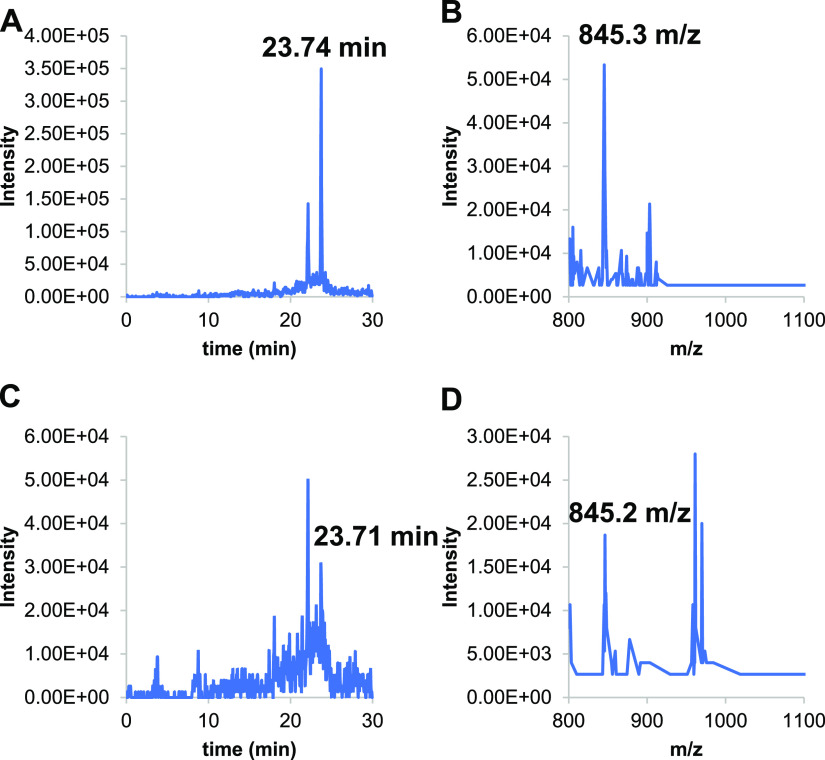
Since B. salamandrivorans Sups showed lymphocyte inhibition similar to that observed previously with B. dendrobatidis supernatants (B. dendrobatidis Sups), the concentrated B. salamandrivorans Sups were analyzed by high-performance liquid chromatography (HPLC), UV-visible spectroscopy (UV-Vis), and mass spectrometry (MS) using multiple-reaction monitoring (MRM) (see Materials and Methods). Three metabolites previously found in B. dendrobatidis Sups were also detected in B. salamandrivorans Sups generated at 16°C or 21°C. Cultures maintained at 26°C could not be maintained long enough to develop the high numbers of cells needed to produce B. salamandrivorans Sups at this temperature. The metabolites found in B. salamandrivorans Sups included high levels of the amino acid tryptophan, methylthioadenosine (MTA), and kynurenine (KYN) (Fig. 7). A third metabolite previously found in B. dendrobatidis Sups is the polyamine spermidine (SPD). SPD cannot be visualized with UV-Vis spectroscopy unless it is conjugated with a light-absorbing chromophore such as a dansyl group. Therefore, the B. salamandrivorans Sups were treated with dansyl chloride and then analyzed (see Materials and Methods). Both supernatants generated at 16°C and 21°C also contained spermidine (Fig. 8).
FIG 7.
Mass spectrometry with multiple-reaction monitoring (MRM) of B. salamandrivorans supernatants to detect immunomodulatory compounds. Mass spectrometric detection of protonated kynurenine (red; 209→146 m/z), MTA (green; 298→136 m/z), and tryptophan (blue; 205→188 m/z) in B. salamandrivorans supernatants cultured at 16°C (A) and 21°C (B).
FIG 8.
Positive-ion electrospray ionization-mass spectrometry (ESI-MS) detection of tridansylated spermidine (expected retention time, 23.7 min; expected mass to charge [m/z], 845.3) in B. salamandrivorans supernatant samples from chytrids grown at 16°C (A and B) and at 21°C (C and D). (A) Extracted-ion chromatogram (XIC) of a 16°C sample showing detection of a compound eluting at 23.74 min. (B) Mass spectrogram of the compound eluting at 23.74 min in the 16°C sample with m/z of 845.3. (C) XIC of 21°C sample showing detection of a compound eluting at 23.71 min. (D) Mass spectrogram of the compound eluting at 23.71 min in the 21°C sample with m/z of 845.2.
DISCUSSION
Lymphocyte inhibition by B. salamandrivorans cells and cell-free metabolites.
Our results showed clearly that B. salamandrivorans, like its closest fungal relative, B. dendrobatidis, inhibited amphibian lymphocytes in coculture, and the factors produced by this fungus inhibited both amphibian and mammalian lymphocytes. The inhibitory molecules included kynurenine (KYN), methylthioadenosine (MTA), and spermidine (SPD). It should be noted that MTA is a by-product of spermidine synthesis, and the two can act together to enhance amphibian lymphocyte inhibition (15).
An effective immune response to a fungal pathogen in the skin would likely involve detection by resident antigen-presenting cells such as macrophages or Langerhans cells. Damaged skin cells would trigger activation of macrophages and neutrophils. The antigen-presenting cells would also recruit effector T lymphocytes. The T lymphocytes would proliferate and release cytokines to recruit additional phagocytes to clear the pathogen. Thus, it is thought that T cells are critical for effective antifungal responses (reviewed in reference 20). However, many fungal pathogens have evolved molecular defenses to evade immune responses (reviewed in reference 21). Here, we showed that B. salamandrivorans released at least three small metabolites that have previously been shown to inhibit T lymphocytes from amphibians and mammals (14, 15). These factors target fundamental processes that are shared by both amphibian and human lymphocytes. Previously, we and others showed that B. dendrobatidis induced apoptosis of frog lymphocytes and frog epithelial and skin cells (10–12). Thus, it is likely that the factors released by B. salamandrivorans may also induce apoptosis of lymphocytes and epithelial cells in the local environment of the skin. Cells undergoing apoptosis release a pool of small molecules, including spermidine that induces anti-inflammatory responses, including production of the immunosuppressive cytokine interleukin 10 (22). Thus, the metabolites released by B. salamandrivorans, as well as metabolites released by cells undergoing apoptosis, may create a local immunosuppressive environment that permits the fungus to survive.
Immune responses of other amphibians to B. salamandrivorans.
Because B. salamandrivorans was detected and described more recently than B. dendrobatidis, less is known about the immune responses of amphibians against this pathogen than those against its sister species; however, initial data are not encouraging in terms of a protective immune response. One small study, designed to demonstrate protection of fire salamanders (Salamandra salamandra) after multiple rounds of infection and clearance with heat, showed no significant difference in probability of infection or infection burden detected by quantitative PCR (qPCR) (7). Accordingly, fire salamanders surviving in the wild in areas where the pathogen is still present remain very susceptible (7). A further study of the transcriptome of the skin of another salamander species, the Wenxian knobby newt (Tylototriton wenxianensis), showed very little induction of immune response genes after B. salamandrivorans infection, whereas T. wenxianensis did respond by activation of immune genes against B. dendrobatidis (16). Other recent studies of the responses of eastern newts (Notophthalmus viridescens) to B. salamandrivorans demonstrated that this species is highly susceptible to lethal disease following experimental exposures (6, 23). Although N. viridescens appears to have some defensive factors and inhibitory bacteria in the skin secretions, these defenses did not sufficiently protect from lethal infections (6, 23). Studies of the skin transcriptome of N. viridescens infected with B. salamandrivorans showed that some immune genes were upregulated relative to control skin (e.g., genes involved in interferon signaling and antigen presentation), while other genes involved in T cell differentiation and signaling were downregulated (24). This demonstrated that the adaptive immune system was engaged, but the response was not effective. Thus, some studies suggested that there was little or no immune system activation following B. salamandrivorans infection in two salamander species, while the eastern newt study suggested immune activation that may have been dysregulated. Failure to respond and clear chytrid infections could be due to the stealthy infection mode by germ tube invasion demonstrated for B. dendrobatidis (25, 26) and presumed to occur in B. salamandrivorans (5). By injecting the contents of the encysted zoospore directly into the cytoplasm of the infected skin cells, the fungus may evade detection by patrolling antigen-presenting cells. Dying skin cells undergoing apoptosis (11, 12) may release some small metabolites, including spermidine that is immunosuppressive (22). However, our current data support the hypothesis that B. salamandrivorans appears to release inhibitory metabolites that have the potential to locally impair immunity in the skin. Thus, B. salamandrivorans, like B. dendrobatidis, has counterdefensive capabilities.
Possible effects of infection by both Batrachochytrium pathogens.
Because B. dendrobatidis is widespread globally, it is likely that accidental introduction of B. salamandrivorans into amphibian communities will result in a dual infection with both chytrid pathogens in the same hosts. Here, we compared the capacity of B. dendrobatidis and B. salamandrivorans to inhibit lymphocytes from the same frog in several replicate experiments. In our studies, the B. dendrobatidis zoosporangia were more suppressive at comparable ratios of chytrid cells to lymphocytes, suggesting that production or release of the inhibitory factors may differ in the two pathogens. Alternatively, because B. salamandrivorans prefers somewhat cooler temperatures (15°C is optimal) (5), 21°C may have been more favorable for production and release of inhibitory factors by B. dendrobatidis.
In animal studies, experimental coinfection of eastern newts with both B. dendrobatidis and B. salamandrivorans demonstrated greater mortality in the coinfected hosts than those infected by a single pathogen (27), raising the possibility that dual infection is more harmful. Related transcriptomic analysis of the skin and spleen showed differential gene expression patterns in the skin and spleen of coinfected newts in comparison with singly infected or control newts. Immune suppression was suggested in the coinfected newts (24). Another recent study of urodele species suggested that prior infection with nonvirulent isolates of global panzootic lineages of B. dendrobatidis (B. dendrobatidis GPL) had variable effects on susceptibility to B. salamandrivorans, depending on the species. That is, marbled newts (Triturus marmoratus) appeared to be protected, but fire salamanders were not (28). Much more research is needed to further understand the immune evasion capabilities of each Batrachochytrium pathogen. If coinfections occur, it is likely that environmental temperature will affect growth of each pathogen, immune defenses of the host, and counterdefenses of the chytrids.
MATERIALS AND METHODS
Culture of Batrachochytrium pathogens and enrichment of zoospores.
Batrachochytrium dendrobatidis isolate JEL 197 was cultured in 1% tryptone broth (T-broth) at 21°C and subcultured twice weekly as previously described (10, 29). Batrachochytrium salamandrivorans isolate AMFP13/1 was a generous gift of An Martel and Frank Pasmans, Ghent University, and was cultured and maintained in tryptone-gelatin hydrolysate-lactose (TGhL) broth (16 g tryptone, 4 g gelatin hydrolysate, and 2 g lactose in 1 L distilled water). The culture was incubated at 16°C and passaged once or twice weekly, depending on the observed rate of growth of cells. Zoospores were purified as previously described by washing the agar surface of 5- to 7-day-old cultures of B. dendrobatidis growing on 1% tryptone agar at 21°C or B. salamandrivorans growing on TGhL agar plates at 16°C three times using 3 to 5 mL of sterile broth (10, 29). The combined broth containing zoospores was passed over sterile nylon spectra/mesh filters (BioDesign Inc. of New York, Carmel, NY, USA) of 20-μm mesh opening size to remove mature zoosporangia.
Preparation of fungal supernatants.
Concentrated cell-free supernatants were prepared as previously described (10, 15). Briefly, a large volume of cells was cultured in TGhL broth at 16°C to ensure there would be sufficient material. These cells were grown for about 7 to 10 days, counted, and resuspended in sterile distilled water at cells/mL (unless indicated in figure legends), counting only mature zoosporangia. After a 24-h incubation at 16°C, the cells were centrifuged, and supernatants were passed through 0.2-μm filters to remove any remaining cells. Supernatants were frozen and lyophilized. Lyophilized supernatants were resuspended at 1/10 of the original volume and diluted in culture to achieve 0.625-fold to 5-fold concentration above the original concentration. For addition of supernatant factors to cultures of lymphocytes, the lyophilized supernatants were resuspended in complete Leibovitz-15 (L-15) medium, which had been adjusted to amphibian osmolarity and supplemented with 100 IU/mL penicillin, 100 μg/mL streptomycin, 12.5 mM sodium bicarbonate, 50 μM 2-mercaptoethanol, 2 mM l-glutamine, and 1% heat-inactivated fetal calf serum.
Frog lymphocyte culture.
Splenic lymphocytes from Xenopus laevis were obtained and enriched over a Ficoll gradient as previously described (10). Briefly, the lymphocytes were cultured in complete L-15 medium at a density of 5 × 105/mL (105 per well, replicates of five or six per parameter tested), and T lymphocytes were induced to proliferate by addition of phytohemagglutinin (PHA) at a final concentration of 2 μg/mL. Cells were incubated at temperatures ranging from 16°C to 26°C in the presence of 5% CO2-95% air for 3 days and were pulsed with 0.5 μCi [3H]-thymidine (5 μCi/mL; specific activity, 2 Ci/mmol) (Perkin Elmer, Waltham, MA, USA) during the last 24 h prior to harvesting. Proliferation was measured by uptake of [3H]-thymidine in the last 24 h and recorded as counts per minute (CPM). Use of animals was approved by the Institutional Animal Care and Use Committee (IACUC) of Vanderbilt University School of Medicine.
Coculture of lymphocytes with B. salamandrivorans or B. dendrobatidis zoosporangia or supernatant factors.
To determine the effects of coculture of cells from B. salamandrivorans or B. dendrobatidis or cell-free supernatants of B. salamandrivorans (B. salamandrivorans Sup) on frog lymphocyte proliferation, lymphocytes were cultured at a density of 5 × 105/mL (105 per well, five or six replicates) alone or stimulated with PHA (2 μg/mL). PHA-stimulated cells received the addition of various numbers of maturing zoosporangia or zoospores ranging from 1.25 × 104 to 1 × 105 per well or with B. salamandrivorans supernatant at a 0.31- to 5-fold concentration of the supernatant. For each set of cells or supernatants tested, there were five or six replicate cultures. Control wells contained lymphocytes only with no addition of PHA (negative) or lymphocytes with PHA but no added cells (positive). For experiments using dead B. salamandrivorans zoosporangia, the cells were killed by heat treatment at 60°C for 10 min (10). Proliferation was measured by uptake of [3H]-thymidine in the last 24 h of a 3-day culture and recorded as CPM.
Detection of the presence of inhibitory metabolites in cell-free supernatants of B. salamandrivorans.
Cell-free supernatants prepared in the Vanderbilt laboratory were lyophilized and sent to the Villanova laboratory for chemical identification of potential immunomodulatory components as previously described (14, 15). Briefly, a lyophilized cell-free supernatant (CFS) was reconstituted in 1.0 mL of HPLC-grade methanol and analyzed by liquid chromatography-mass spectrometry (LCMS) (Shimadzu LC-20 liquid chromatograph equipped with an ACE C18 column [3 μm, 150 by 4.6 mm], a Shimadzu SPD-M20A diode array detector, and an Applied Biosystems Sciex API 2000 triple quadrupole mass spectrometer). After elution (ramping from acidified H2O to acidified acetonitrile as per Umile et al. [30]), the chromatogram was compared to that of lyophilized water and commercial standards of KYN, MTA, and phenylalanine.
The mass spectrometric detection of MTA, kynurenine, and tryptophan was accomplished by building a multiple-reaction monitoring (MRM) method to isolate both the molecular ion [M+H]+ and a significant fragment ion. The following terms apply to the Sciex mass spectrometry source optimization and output. DP refers to declustering potential; FP refers to focusing potential; EP refers to entrance potential; CE refers to collision energy; CXP refers to collision cell exit potential. Tryptophan (205→188 m/z) shows the loss of an amine (DP = 80; FP = 400; EP = 10, CE = 25; CXP = 3). MTA (298→136 m/z) demonstrates a probable loss of the methythiopentose ring (DP = 80; FP = 400; EP = 10, CE = 25; CXP = 3). Kynurenine (209→146 m/z) has a loss of the amine followed by the loss of a carboxylic acid (DP = 60; FP = 400; EP = 10, CE = 35; CXP = 3).
SPD present in B. salamandrivorans cell-free supernatant was derivatized as previously reported (15); in a scintillation vial, 100 μL of supernatant was mixed with 200 μL saturated sodium carbonate and 400 μL of a dansyl chloride solution (7.5 mg/mL; 28 mM) in acetone. Reactions were stirred at 40°C in the dark for 1 to 2 h for a complete reaction. After the reaction was completed, 400 μL of 28 mM aqueous proline was added to quench any unreacted dansyl chloride. Dansylated products were extracted with dichloromethane, the dichloromethane was evaporated under a gentle air stream, and the residue was reconstituted in 1 mL of methanol for LCMS analysis as above.
Statistical analysis.
Differences in lymphocyte proliferation were assessed by one-way analysis of variance (ANOVA) with Tukey post hoc tests using the VassarStats online program (http://vassarstats.net/anova1u.html). For most experiments, control lymphocytes were cultured in sets of five or six replicate wells, and each experimental parameter (B. salamandrivorans cells or B. salamandrivorans supernatant concentrations added) was tested in sets of five or six replicate wells. A P value of ≤0.05 was considered to be statistically significant.
Data availability.
All data needed to understand these results are summarized in the figures and figure legends. Further details about the data are available upon request.
ACKNOWLEDGMENTS
Work from the Rollins-Smith lab was supported by National Science Foundation grants IOS-1557634, DEB-1814520 (M. Gray and D. Miller, Co-PIs., subcontract to Vanderbilt), and IOS-2011291. Work from the Minbiole and Umile laboratories was supported by National Science Foundation grant IOS-1557592.
We thank Frank Pasmans and An Martel, Ghent University, for the gift of the isolate of Batrachochytrium salamandrivorans used in these studies.
Contributor Information
Louise A. Rollins-Smith, Email: louise.rollins-smith@vanderbilt.edu, louise.rollins-smith@vumc.org.
Mairi C. Noverr, Tulane School of Medicine
REFERENCES
- 1.Berger L, Speare R, Daszak P, Green DE, Cunningham AA, Goggin CL, Slocombe R, Ragan MA, Hyatt AD, McDonald KR, Hines HB, Lips KR, Marantelli G, Parkes H. 1998. Chytridiomycosis causes amphibian mortality associated with population declines in the rain forests of Australia and Central America. Proc Natl Acad Sci USA 95:9031–9036. 10.1073/pnas.95.15.9031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Longcore JE, Pessier AP, Nichols DK. 1999. Batrachochytrium dendrobatidis gen. et sp. nov., a chytrid pathogenic to amphibians. Mycologia 91:219–227. 10.2307/3761366. [DOI] [Google Scholar]
- 3.Pessier AP, Nichols DK, Longcore JE, Fuller MS. 1999. Cutaneous chytridiomycosis in poison dart frogs (Dendrobates spp.) and White's tree frogs (Litoria caerulea). J Vet Diagn Invest 11:194–199. 10.1177/104063879901100219. [DOI] [PubMed] [Google Scholar]
- 4.Scheele BC, Pasmans F, Skerratt LF, Berger L, Martel A, Beukema W, Acevedo AA, Burrowes PA, Carvalho T, Catenazzi A, De la Riva I, Fisher MC, Flechas SV, Foster CN, Frías-Álvarez P, Garner TWJ, Gratwicke B, Guayasamin JM, Hirschfeld M, Kolby JE, Kosch TA, La Marca E, Lindenmayer DB, Lips KR, Longo AV, Maneyro R, McDonald CA, Mendelson J, III, Palacios-Rodriguez P, Parra-Olea G, Richards-Zawacki CL, Rödel M-O, Rovito SM, Soto-Azat C, Toledo LF, Voyles J, Weldon C, Whitfield SM, Wilkinson M, Zamudio KR, Canessa S. 2019. Amphibian fungal panzootic causes catastrophic and ongoing loss of biodiversity. Science 363:1459–1463. 10.1126/science.aav0379. [DOI] [PubMed] [Google Scholar]
- 5.Martel A, Spitzen-van der Sluijs A, Blooi M, Bert W, Ducatelle R, Fisher MC, Woeltjes A, Bosman W, Chiers K, Bossuyt F, Pasmans F. 2013. Batrachochytrium salamandrivorans sp. nov. causes lethal chytridiomycosis in amphibians. Proc Natl Acad Sci USA 110:15325–15329. 10.1073/pnas.1307356110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martel A, Blooi M, Adriaensen C, Van Rooij P, Beukema W, Fisher MC, Farrer RA, Schmidt BR, Tobler U, Goka K, Lips KR, Muletz C, Zamudio KR, Bosch J, Lötters S, Wombwell E, Garner TWJ, Cunningham AA, Spitzen-van der Sluijs A, Salvidio S, Ducatelle R, Nishikawa K, Nguyen TT, Kolby JE, Van Bocxlaer I, Bossuyt F, Pasmans F. 2014. Recent introduction of a chytrid fungus endangers Western Palearctic salamanders. Science 346:630–631. 10.1126/science.1258268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stegen G, Pasmans F, Schmidt BR, Rouffaer LO, Van Praet S, Schaub M, Canessa S, Laudelout A, Kinet T, Adriaensen C, Haesebrouck F, Bert W, Bossuyt F, Martel A. 2017. Drivers of salamander extirpation mediated by Batrachochytrium salamandrivorans. Nature 544:353–356. 10.1038/nature22059. [DOI] [PubMed] [Google Scholar]
- 8.Carter ED, Miller DL, Peterson AC, Suggon WB, Cusaac JPW, Spatz JA, Rollins-Smith L, Reinert L, Bohanon B, Williams LA, Upchurch A, Gray MJ. 2020. Conservation risk of Batrachochytrium salamandrivorans to endemic lungless salamanders. Conserv Lett 13:e12675. 10.1111/conl.12675. [DOI] [Google Scholar]
- 9.Towe AE, Gray MJ, Carter ED, Wilber MQ, Ossiboff RJ, Ash K, Bohanon M, Bajo BA, Miller DL. 2021. Batrachochytrium salamandrivorans can devour more than salamanders. J Wildl Dis 57:942–948. 10.7589/JWD-D-20-00214. [DOI] [PubMed] [Google Scholar]
- 10.Fites JS, Ramsey JP, Holden WM, Collier SP, Sutherland DM, Reinert LK, Gayek AS, Dermody TS, Aune TM, Oswald-Richter K, Rollins-Smith LA. 2013. The invasive chytrid fungus of amphibians paralyzes lymphocyte responses. Science 342:366–369. 10.1126/science.1243316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Verbrugghe E, Van Rooij P, Favoreel H, Martel A, Pasmans F. 2019. In vitro modeling of Batrachochytrium dendrobatidis infection of amphibian skin. PLoS One 14:e0225224. 10.1371/journal.pone.0224224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brannelly LA, Roberts AA, Skerratt LF, Berger L. 2017. Epidermal cell death in frogs with chytridiomycosis. PeerJ 5:e2925. 10.7717/peerj.2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fites JS, Reinert LK, Chappell TM, Rollins-Smith LA. 2014. Inhibition of local immune responses by the frog-killing fungus, Batrachochytrium dendrobatidis. Infect Immun 82:4698–4706. 10.1128/IAI.02231-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rollins-Smith L, Fites JS, Reinert LK, Shiakolas AR, Umile TP, Minbiole KPC. 2015. Immunomodulatory metabolites released by the frog-killing fungus, Batrachochytrium dendrobatidis. Infect Immun 83:4565–4570. 10.1128/IAI.00877-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rollins-Smith LA, Ruzzini AC, Fites JS, Reinert LK, Hall EM, Joosse BA, Ravikumar VI, Huebner MI, Aka A, Kehs MH, Gillard BM, Doe E, Tasca JA, Umile TP, Clardy J, Minbiole KPC. 2019. Metabolites involved in immune evasion by Batrachochytrium dendrobatidis include the polyamine spermidine. Infect Immun 87:e00035-19. 10.1128/IAI.00035-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Farrer RA, Martel A, Verbrugghe E, Abouelleil A, Ducatelle R, Longcore JE, James TY, Pasmans F, Fisher MC, Cuomo CA. 2017. Genomic innovations linked to infection strategies across emerging pathogenic chytrid fungi. Nat Commun 8:14742. 10.1038/ncomms14742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Green N, Cohen N. 1979. Phylogeny of immunocompetent cells: III. Mitogen response characteristics of lymphocyte subpopulations from normal and thymectomized frogs (Xenopus laevis). Cell Immunol 48:59–70. 10.1016/0008-8749(79)90099-6. [DOI] [PubMed] [Google Scholar]
- 18.Van Rooij P, Martel A, Haesebrouck F, Pasmans F. 2015. Amphibian chytridiomycosis: a review with focus on fungus-host interactions. Vet Res 46:137. 10.1186/s13567-015-0266-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Piotrowski JS, Annis SL, Longcore JE. 2004. Physiology of Batrachochytrium dendrobatidis, a chytrid pathogen of amphibians. Mycologia 96:9–15. 10.2307/3761981. [DOI] [PubMed] [Google Scholar]
- 20.d'Enfert C, Kaune A-K, Alaban L-R, Chakraborty S, Cole N, Delavy M, Kosmala D, Marsaux B, Fróis-Martins R, Morelli M, Rosati D, Valentine M, Xie Z, Emritloll Y, Warn PA, Bequet F, Bougnoux M-E, Bornes S, Gresnigt MS, Hube B, Jacobsen ID, Legrand M, Leibundgut-Landmann S, Manichanh C, Munro CA, Netea MG, Queiroz K, Roget K, Thomas V, Thoral C, Van den Abbeele P, Walker AW, Brown AJP. 2021. The impact of the fungus-host-microbiota interplay upon Candida albicans infections: current knowledge and new perspectives. FEMS Microbiol Rev 45:fuaa060. 10.1093/femsre/fuaa060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.König A, Müller R, Mogavero S, Hube B. 2021. Fungal factors involved in host immune evasion, modulation and exploitation during infection. Cell Microbiol 23:e13272. 10.1111/cmi.13272. [DOI] [PubMed] [Google Scholar]
- 22.Medina CB, Mehrotra P, Arandjelovic S, Perry JSA, Guo Y, Morioka S, Barron B, Walk SF, Ghesquière B, Krupnick AS, Lorenz U, Ravichandran KS. 2020. Metabolites released from apoptotic cells act as tissue messengers. Nature 580:130–135. 10.1038/s41586-020-2121-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carter ED, Bletz MC, Le Sage M, LaBumbard B, Rollins-Smith LA, Woodhams DC, Miller DL, Gray MJ. 2021. Winter is coming – temperature affects immune defenses and susceptibility to Batrachochytrium salamandrivorans. PLoS Pathog 17:e1009234. 10.1371/journal.ppat.1009234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McDonald CA, Longo AV, Lips KR, Zamudio KR. 2020. Incapacitating effects of fungal coinfection in a novel pathogen system. Mol Ecol 29:3173–3186. 10.1111/mec.15452. [DOI] [PubMed] [Google Scholar]
- 25.Van Rooij P, Martel A, D'Herde K, Brutyn M, Croubels S, Ducatelle R, Haesebrouck F, Pasmans F. 2012. Germ tube mediated invasion of Batrachochytrium dendrobatidis in amphibian skin is host dependent. PLoS One 7:e41481. 10.1371/journal.pone.0041481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Greenspan SE, Longcore JE, Calhoun AJK. 2012. Host invasion by Batrachochytrium dendrobatidis: fungal and epidermal ultrastructure in model anurans. Dis Aquat Organ 100:201–210. 10.3354/dao02483. [DOI] [PubMed] [Google Scholar]
- 27.Longo AV, Fleischer RC, Lips KR. 2019. Double trouble: co-infections of chytrid fungi will severely impact widely distributed newts. Biol Invasions 21:2233–2245. 10.1007/s10530-019-01973-3. [DOI] [Google Scholar]
- 28.Greener MS, Verbrugghe E, Kelly M, Blooi M, Beukema W, Canessa S, Carranza S, Croubels S, De Troyer N, Fernandez-Giberteau D, Goethals P, Lens L, Li Z, Stegen G, Strubbe D, van Leeuwenberg R, Van Praet S, Vila-Escale M, Vervaeke M, Pasmans F, Martel A. 2020. Presence of low virulence chytrid fungi could protect European amphibians from more deadly strains. Nat Commun 11:5393. 10.1038/s41467-020-19241-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rollins-Smith LA, Carey C, Longcore J, Doersam JK, Boutte A, Bruzgal JE, Conlon JM. 2002. Activity of antimicrobial skin peptides from Ranid frogs against Batrachochytrium dendrobatidis, the chytrid fungus associated with global amphibian declines. Dev Comp Immunol 26:471–479. 10.1016/s0145-305x(01)00088-x. [DOI] [PubMed] [Google Scholar]
- 30.Umile TP, McLaughlin PJ, Johnson KR, Honarvar S, Blackman AL, Burzynski EA, Davis RW, Teotonio TL, Hearn GW, Hughey CA, Lagalante AF, Minbiole KPC. 2014. Nonlethal amphibian skin swabbing of cutaneous natural products for HPLC fingerprinting. Anal Methods 6:3277–3284. 10.1039/C4AY00566J. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data needed to understand these results are summarized in the figures and figure legends. Further details about the data are available upon request.