Abstract
Muscarinic acetylcholine receptors (mAChRs) have been shown to mediate alcohol consumption and seeking. Both M4 and M5 mAChRs have been highlighted as potential novel treatment targets for alcohol use disorders (AUD). Similarly, M1 mAChRs are expressed throughout reward circuitry, and their signaling has been implicated in cocaine consumption. However, whether the same effects are seen for alcohol consumption, or whether natural reward intake is inadvertently impacted is still unknown. To determine the role of M1 mAChRs in alcohol consumption, we tested operant self‐administration of alcohol under both fixed ratio (FR3) and progressive ratio (PR3‐4) schedules. Enhancing M1 mAChR signaling (via the M1 PAM‐Agonist PF‐06767832, 1 mg/kg, i.p.) reduced operant alcohol consumption on a fixed schedule but had no effect on motivation to acquire alcohol. To determine whether these actions were specific to alcohol, we examined the effects of M1 enhancement on natural reward (sucrose) self‐administration. Systemic administration of PF‐06767832 (1 mg/kg, i.p.) also reduced operant sucrose self‐administration, suggesting the actions of the M1 receptor may be non‐selective across drug and natural rewards. Finally, to understand whether this reduction extended to natural consummatory behaviors, we assessed home cage standard chow and water consumption. M1 enhancement via systemic PF‐06767832 administration reduced food and water consumption. Together our results suggest the M1 PAM‐agonist, PF‐06767832, non‐specifically reduces consummatory behaviors that are not associated with motivational strength for the reward. These data highlight the need to further characterize M1 agonists, PAMs, and PAM‐agonists, which may have varying degrees of utility in the treatment of neuropsychiatric disorders including AUD.
Keywords: addiction, alcohol use disorder, allosteric modulation, consummatory behavior, muscarinic receptor
The muscarinic M1 receptor PAM‐Agonist, PF‐06767832 was examined in rodent models of alcohol use disorder and general consummatory behaviors. We reveal that PF‐06767832 non‐specifically reduces consummatory behavior.

Abbreviations
- ACh
acetylcholine
- AD
Alzheimer's disease
- AUD
alcohol use disorders
- KO
knockout
- mAChRs
muscarinic acetylcholine receptors
- NHMRC
National Health and Medical Research Council
- PAM
positive allosteric modulator
- PD
Parkinson's disease
- PPI
prepulse inhibition
- RM
repeated measures
1. INTRODUCTION
Given that acetylcholine (ACh) mediates reward, 1 , 2 learning, memory, and other higher‐order cognitive processes, 3 the cholinergic system is an appealing pathway to target for the treatment of neuropsychiatric disorders, including addiction. Recently, we have implicated both the M4 and M5 muscarinic acetylcholine receptors (mAChRs) in alcohol consumption and seeking. 4 , 5 , 6 , 7 However, little research has focused on M1 mAChRs in drug and alcohol use disorders (AUD), with a primary focus surrounding cognition, including in Alzheimer's disease (AD), Parkinson's disease (PD), and schizophrenia. 8 , 9 , 10
M1 mAChRs are expressed in reward circuitry, including the amygdala, striatum, and hippocampus. 6 , 11 M1 mAChR knockout (KO) mice show decreased conditioned place preference to cocaine and morphine 12 ; however, this may be underpinned by corresponding deficits in a range of cognitive domains. 13 , 14 , 15 The M1/M4 mAChR preferring agonist xanomeline enhances cognitive functioning in rodent models 16 and in individuals with Alzheimer's Disease or schizophrenia. 17 , 18 , 19 Further, in mice, xanomeline administration reduced cocaine self‐administration, 20 , 21 and increased the choice for food over cocaine in rats. 22 Promising findings with xanomeline have driven the continued interest in the discovery and development of novel muscarinic activators, with a shift to subtype‐selective compounds as a safer and more effective treatment strategy. 23 , 24 , 25 These compounds can be classified into three types: (1) positive allosteric modulator (PAM) which bind a site topographically distinct from the orthosteric site (where endogenous ACh binds) and potentiate ACh signaling, but have no intrinsic activity per se; (2) agonists, which can bind to the orthosteric site, allosteric site, or bitopic (across both bindings sites) and cause direct activation of the M1 receptor; (3) PAM‐agonist, which act to potentiate ACh activity and can directly activate the M1 receptor in the absence of an orthosteric agonist. 24 , 26 The advantage of a PAM is the modification of M1 signaling in a more nuanced manner, preserving spatial and temporal receptor signaling, and enhancing receptor subtype selectivity. However, agonists (targeting the orthosteric or allosteric binding sites) are also being explored as they offer the benefit of action in the absence of endogenous acetylcholine, while PAMs require binding by the endogenous ligand before activity can be amplified. 24
A recent study using a bitopic M1 mAChR agonist, VU0364572, 27 showed acute systemic administration in rats reduced cocaine choice over food for a sustained period (up to 4 weeks). 28 However, whether this action is driven by cognitive or reward processes, generalizes across drug classes, or is mediated through positive allosteric modulation of ACh signaling is unknown. Another compound, PF‐06767832, was developed as a selective M1 compound, which shows both agonist and positive allosteric actions, classifying this compound as a PAM‐agonist. 29 Here, we assessed PF‐06767832 in alcohol consumption and motivation to consume alcohol using operant conditioning paradigms. To determine whether these actions were specific to alcohol, we also assessed the actions of PF‐06767832 in sucrose, food (chow), and water consumption.
2. MATERIALS AND METHODS
2.1. Animals
Inbred male Indiana alcohol‐preferring (iP) rats (~8 weeks at the start of the experiment, N = 67) were obtained from The Florey Institute of Neuroscience and Mental Health breeding colony. The parental stock was previously acquired from the late Professor T.K. Li (while at Indiana University). Rats were pair‐housed (except for experiment 5 for which they were individually housed) and kept on a 12‐h light‐dark cycle (lights on 7 am–7 pm) with food (Barastoc rat and mouse) and tap water freely available. All experimentation was undertaken between 10 am and 2 pm iP rats were used in this study as they voluntarily consume high levels of alcohol to intoxication and show high predictive, face, and construct validity for the study of AUD. 30 All efforts we made to minimise animal suffering and experiments were performed in accordance with the Prevention of Cruelty to Animals Act (2004), under the guidelines of the National Health and Medical Research Council (NHMRC) Australian Code of Practice for the Care and Use of animals for Experimental Purposes (2013) and approved by The Florey Institute of Neuroscience and Mental Health Animal Ethics Committee. Animal studies were reported in compliance with the ARRIVE 2.0 guidelines. 31
2.2. Compounds
The selective M1 PAM‐agonist PF‐06767832 (N‐[(3R,4S) −3‐Hydroxytetrahydro‐2H‐pyran‐4‐yl]‐5‐methyl‐4‐[4‐(1,3‐thiazol‐4‐yl)benzyl]pyridine‐2‐carboxamide) (Sigma‐Aldrich, NSW, AUS) dissolved in 10% Tween80 (Sigma‐Aldrich) in sterile saline was administered at 1 mg/kg i.p. (1 ml/kg) based on previous studies. 29
2.3. Locomotor activity apparatus
Locomotor activity (distance traveled in meters) was recorded using photobeam detectors in a 43.2 × 43.2 × 30.5 cm apparatus (Med Associates) as per our previous studies. 32 , 33
2.4. Home cage drinking procedure (intermittent access)
Rats received access to two bottles over three 24 h‐sessions per week, one containing 20% ethanol (v/v) and one containing tap water. 33 , 34 , 35 Solutions were prepared by diluting alcohol in tap water from 100% (v/v) pure ethanol. Daily access was given at 10.00 am. The alcohol bottle was exchanged 24 h later with a water bottle for the subsequent 24 h period (e.g., 24 h alcohol‐free). After this period, a water bottle was exchanged for a 20% alcohol bottle, and the position of the alcohol bottle was swapped from the previous session to prevent a side preference forming. The total alcohol consumption was calculated for each session (grams), using the difference in weight from the start to the end of the session multiplied by the density of 20% ethanol (0.97) and divided by the number of rats per cage.
2.5. Operant self‐administration apparatus
Operant self‐administration was conducted as per our previous reports. 7 , 35 , 36 Standard operant chambers (Med Associates) located in ventilated cubicles fitted with sound‐proofing were used. Individual chambers were equipped with two retractable levers, located on opposite sides of the chamber. Reward delivery on the active lever resulted in 0.1 ml ethanol (10% v/v, experiment 1 & 2) or 0.1 ml sucrose (1.25%–5% w/v, experiment 3) into the receptacle controlled by a 220v syringe pump driver connecting a 50 ml syringe to an 18‐gauge blunt needle via silastic tubing. Inactive lever pressing did not result in any delivery. Data were recorded by Med‐PC IV software (Med Associates) connected to the chambers via a computer.
2.6. Experimental design
2.6.1. Experiment 1: The role of M1 mAChRs in locomotor activity
To determine if PF‐06767832 had any effect on locomotor activity, alcohol naïve rats (n = 12) were habituated to daily i.p. injections (vehicle solution, 10% Tween80 in milliQ H2O). On the test day, rats were randomly assigned to receive a vehicle (1 ml/kg, i.p., n = 6) or PF‐06767832 (1 mg/kg, i.p., n = 6) injection, and were directly placed into the locomotor apparatus for 1 h to test spontaneous locomotor activity.
2.6.2. Experiment 2: The role of M1 mAChRs in fixed ratio alcohol self‐administration
Next, given the recent evidence that M1 receptor enhancement reduces cocaine choice in male rats, 28 we assessed whether enhancing M1 receptor activity reduced operant self‐administration for alcohol in a separate cohort of rats (n = 16). Rats first underwent 9–12 24‐h sessions of intermittent alcohol consumption in the home cage. Next, rats were trained to self‐administer alcohol on a FR3 schedule (3 lever presses were required for 1 alcohol delivery) for >30 sessions each lasting 20 min. Prior to testing, rats were habituated to daily vehicle injections for 4–5 days. On the test day, rats received either PF‐06767832 (1 mg/kg, i.p. n = 8) or vehicle (1 ml/kg, i.p. n = 8) and were placed back into their home cage. After 30 min, rats were placed into an operant chamber, and lever responding for 10% alcohol was assessed in a 20‐min test session. After 3 days of baseline FR3 self‐administration, rats undertook a second test, receiving the opposite treatment in a randomized and counterbalanced manner. Based on the sustained decrease in cocaine choice over food previously observed, 28 we also analyzed operant self‐administration data from the same rats when they underwent an operant self‐administration session 24 h after PF‐06767832 or vehicle administration.
2.6.3. Experiment 3: The role of M1 mAChRs in motivation to consume alcohol
In another cohort of rats, we next assessed whether PF‐06767832 reduced alcohol self‐administration via a reduction in motivation to acquire and consume alcohol using a progressive ratio (PR3‐4, n = 17). Similar to experiment 1, after home cage intermittent alcohol access (9–12 sessions), rats underwent operant self‐administration sessions (>30 session, each 20 min, FR3 schedule). Rats were habituated to daily vehicle injections for 4–5 days prior to testing. On test day, rats received either PF‐06767832 (1 mg/kg, i.p. n = 9) or vehicle (1 ml/kg, i.p. n = 8) and were placed back into their home cage. After 30 min, rats were placed into an operant chamber and lever responding for 10% alcohol assessed in a single 2‐h test session on a PR3‐4 schedule, in which 32 active lever responses are required for the 10th alcohol delivery. 37 We defined the breakpoint as the final ratio completed within the 2‐h session.
2.6.4. Experiment 4: The role of M1 mAChRs in sucrose self‐administration
To test whether PF‐06767832 reduced natural reward consumption, rats (n = 8) underwent self‐administration of sucrose (1.25–5% w/v) on a FR3 schedule. 5 After stabilization (10 sessions, 20 min), rats received either PF‐06767832 (1 mg/kg, i.p. n = 4) or vehicle (1 ml/kg, i.p. n = 4) and were placed back into their home cage. After 30 min, rats were placed into an operant chamber and lever responding for sucrose assessed in a 20 min test session. After 3 days of baseline FR3 sucrose self‐administration, rats undertook a second test, receiving the opposite treatment in a randomized and counterbalanced manner. Of note, these rats had been previously trained for alcohol self‐administration but had not undergone any pharmacological experimentation. Rats were given at least 2 weeks break between alcohol and sucrose self‐administration training.
2.6.5. Experiment 5: The role of M1 mAChRs in home cage food and water consumption
Single housed rats received either PF‐06767832 (1 mg/kg, i.p. n = 7) or vehicle (n = 7) and were placed back into their home cage. After 30 min food (standard chow) and water were provided to rats and their consumption was measured 2 and 24 h later to examine any effect on general home cage consummatory behaviors as per our previous studies. 7 , 33 Rats were given 3 days rest before testing with the counterbalanced treatment. These rats had previously been trained for alcohol self‐administration and undergone experimentation (not related to the current study). Rats were given at least 2 weeks break after prior testing before examining food and water intake.
2.7. Statistical analysis
GraphPad Prism 9 statistical software was used for analysis. Locomotor timecourse data were analyzed by repeated measures (RM) two‐way ANOVA (time × treatment) and total distance by unpaired student's t‐test. For alcohol and sucrose self‐administration, operant self‐administration data were analyzed by RM two‐way ANOVA (lever × treatment) followed by Bonferroni post hoc analyses. Progressive ratio breakpoint and total distance in the locomotor test were analyzed by unpaired Student's t‐test. All operant timecourse data were analyzed by RM two‐way ANOVA (time × treatment) with Bonferroni post hoc analysis and latency to first lever press analyzed by paired or unpaired Student's t‐test. Previous studies examining similar behaviors were used to determine sample sizes. All data are expressed as mean ± SEM.
3. RESULTS
3.1. Experiment 1: PF‐06767832 does not alter locomotor activity
First, we established whether systemically administered PF‐06767832 (1 mg/kg i.p.) altered spontaneous locomotor activity (Figure 1A). Rats were tested in a 1‐h locomotor test; PF‐06767832 did not alter locomotor activity across time [two‐way ANOVA: main effect of time F (11,110) = 59.23, p < .0001, no effect of treatment F (1,10) = 0.6532, p = .438, no time × treatment interaction F (11,110) = 1.356, p = .203] (Figure 1B), or total locomotor activity [unpaired t‐test: t (10) = 0.808, p = .438] (Figure 1C).
FIGURE 1.
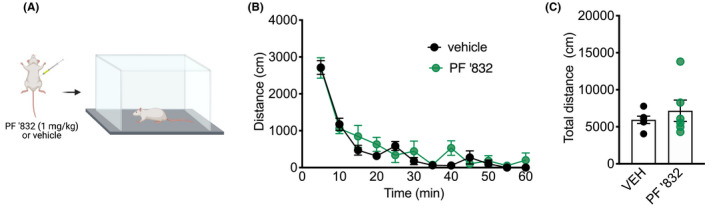
PF‐06767832 does not alter locomotor activity. (A) Schematic of experimental timeline. PF‐06767832 (PF ‘832; 1 mg/kg i.p) administered directly prior to a 1‐h locomotor test does not alter (B) distance traveled across time or (C) total distance traveled. Data expressed as mean ± SEM, n = 6/group. Created with BioRender.com
3.2. Experiment 2: PF‐06767832 decreases alcohol self‐administration
Given PF‐06767832 (1 mg/kg) did not alter locomotor activity, we next sought to examine the functional role of M1 mAChR in alcohol consumption by assessing whether systemic PF‐06767832 administration reduced operant self‐administration under a fixed ratio schedule (Figure 2A). During the last 10 days of training rats averaged 113 ± 6 active lever and 1.6 ± 0.2 inactive lever responses. Two‐way ANOVA revealed a main effect of lever (F (1,15) = 149.3, p < .0001), treatment (F (1,15) = 6.218, p < .05) and treatment × lever interaction (F (1,15) = 6.012, p < .05). Additional Bonferroni post hoc analysis revealed a difference in active lever (p = .0067), but not inactive lever (p > .999) responding following PF‐06767832 administration (Figure 2B). Additional analysis of lever pressing across time [two‐way ANOVA: main effect of time F (3,45) = 31.09, p < .0001, treatment F (1,15) = 10.65, p < .01 but no treatment × time interaction F (3,45) = 0.3778, p = .7694. Bonferroni post hoc analysis revealed a significant difference in lever responding at 10 min (p < .05), with rats administered PF‐06767832 showing reduced active lever responding (Figure 2C). Paired t‐test of latency to first active lever press showed no difference between treatment groups [t (15) = 1.271 p = .223] (Figure 2D). Based on previous findings, which showed a sustained decrease in cocaine choice over food following treatment with the selective M1 bitopic agonist VU0364572, 28 we examined subsequent operant alcohol responding the day after PF‐06767832 administration. No differences in operant alcohol self‐administration were observed 24 h following PF‐06767832 or vehicle administration [two‐way ANOVA revealed a main effect of lever (F (1,15) = 134.1, p < .0001), but no effect of treatment (F (1,15) = 0.0168, p = .898) or treatment × lever interaction (F (1,15) = 0.040, p = .8436) (Figure 2E).
FIGURE 2.
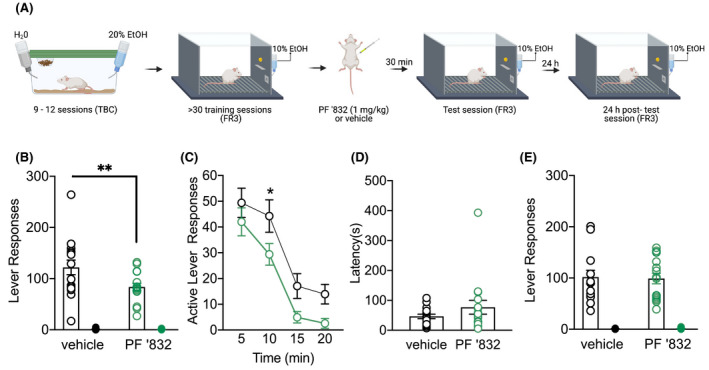
PF‐06767832 reduces alcohol self‐administration. (A) Schematic of experimental timeline. PF‐06767832 (PF ‘832; 1 mg/kg i.p) (B) decreases alcohol self‐administration on the active, but not an inactive lever. (C) Timecourse reveals a decrease at the 10 min time point. (D) No difference in latency to first lever press was observed in rats that received PF’832. (E) The reduction in alcohol self‐administration induced by PF ‘832 was not observed 24 h following administration. Data expressed as mean ± SEM, n = 16. TBC: two‐bottle choice; FR3: fixed ratio 3. Open circle = active lever, closed circle = inactive lever. Created with BioRender.com
3.3. Experiment 3: PF‐06767832 does not alter progressive ratio responding for alcohol
To determine whether the reduction in operant alcohol responding was driven by altering motivation to obtain alcohol, we next examined the effect of systemic PF‐06767832 administration on lever pressing under a progressive ratio schedule (PR3‐4, Figure 3A). During the last 10 days of training rats averaged 90 ± 4 active lever and 2.3 ± 0.3 inactive lever responses. M1 mAChR enhancement with PF‐06767832 did not alter breakpoint on the PR3‐4 schedule [unpaired t‐test: t (15) = 0.804, p = .4139] (Figure 3B). Further, no difference in lever responding was observed over the 2‐hour session [two‐way ANOVA: main effect of time F (3,45) = 36.90 p < .0001, no effect of treatment F (1,15) = 0.3168 p = .5819 or time × treatment interaction F (3,45) = 0.3070 p = .802] (Figure 3C). PF‐06767832 did, however, increase latency to first active lever response from an average of 17 ± 5 to 67 ± 12 s [unpaired t‐test: t (15) = 3.864, p < .01] (Figure 3D).
FIGURE 3.
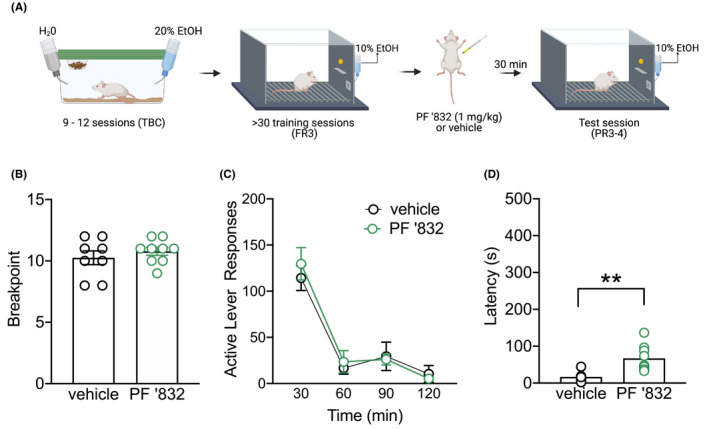
PF‐06767832 does not alter motivation for alcohol. (A) Schematic of experimental timeline. PF‐06767832 (PF ‘832; 1 mg/kg i.p) did not alter (B) breakpoint on the PR3‐4 schedule, nor (C) lever pressing across time. (D) PF’832 increased latency to first active lever press. Data expressed as mean ± SEM, n = 8–9/group. TBC: two‐bottle choice; FR3: fixed ratio 3. Open circle = active lever, closed circle = inactive lever. Created with BioRender.com
3.4. Experiment 4: PF‐06767832 decreases sucrose self‐administration
To determine whether PF‐06767832 reduction in alcohol consumption was specific to alcohol, or generalized to natural reward consumption, we next tested an effect on operant sucrose self‐administration under FR3 (Figure 4A). During training rats averaged 155 ± 28 active lever and 1.5 ± 0.1 inactive lever presses. Administration of PF‐06767832 (1 mg/kg, i.p.) reduced sucrose self‐administration [two‐way ANOVA: main effect of Lever F (1,7) = 109.1, p < .0001, treatment F (1,7) = 7.755, p = .027 and treatment × lever interaction F (1,7) = 8.697, p = .021] (Figure 4B). A significant main effect of time (F (3,21) = 14.94, p < .0001) and treatment were observed (F (1,7) = 8.28, p = .024), but no treatment × time interaction (F (3,21) = 0.2980, p = .826; Figure 4C), nor any difference in latency to first active lever press (paired t‐test: t (7) = 0.787, p = .4572) (Figure 4D).
FIGURE 4.
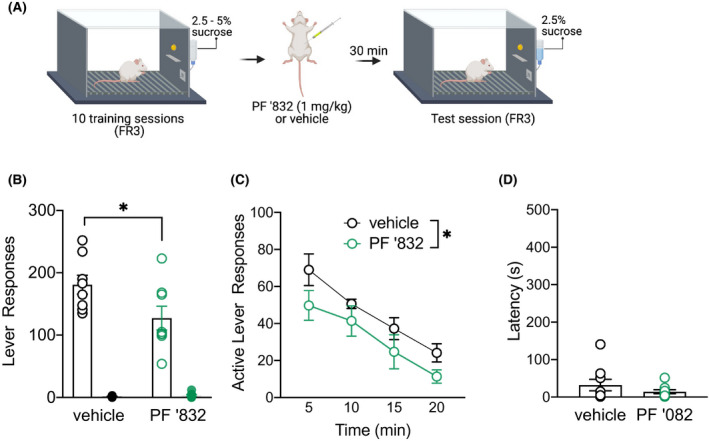
PF‐06767832 reduces sucrose self‐administration. (A) Schematic of experimental timeline. PF‐06767832 (PF ‘832; 1 mg/kg i.p) reduces (B) sucrose self‐administration, (C) with a difference in treatment groups observed across timecourse. (D) No difference in latency to first lever press was observed in rats which received the M1 PAM. Data expressed as mean ± SEM, n = 8. FR3: fixed ratio 3. Open circle = active lever, closed circle = inactive lever. Created with BioRender.com
3.5. Experiment 5: PF‐06767832 decreases home cage food and water consumption
Finally, based on our observations that PF‐06767832 reduced operant responding for both alcohol and sucrose, we next assessed whether it impacted natural consummatory behavior (standard chow and water) in the home cage. PF‐06767832 (1 mg/kg i.p.) decreased food consumption at 2 h [paired t‐test: t (13) = 4.485, p < .001] (Figure 5A), and 24 h [paired t‐test: t (13) = 3.611, p < .01] (Figure 5B). PF‐06767832 also reduced water consumption at 2 h [paired t‐test: t (13) = 4.036, p < .01] (Figure 5C), but this effect did not persist at the 24 h time point [paired t‐test: t (13) = 1.615, p = .130] (Figure 5D).
FIGURE 5.
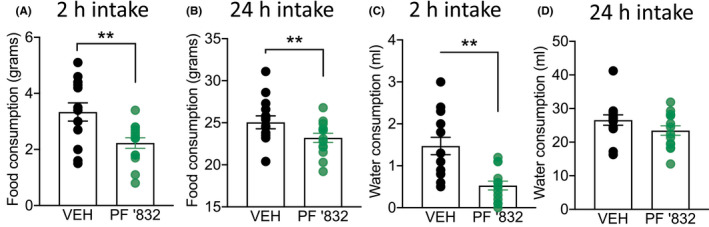
PF‐06767832 reduces home cage food and water consumption. PF‐06767832 (PF ‘832; 1 mg/kg i.p) decreases food consumption at (A) 2‐ and (B) 24‐h timepoints and decreases water consumption at (C) 2‐, but not (D) 24‐h. Data expressed as mean ± SEM, n = 14
3.6. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY, 38 and are permanently archived in the Concise Guide to PHARMACOLOGY 2019/20. 39
4. DISCUSSION
Here, we provide evidence that systemic administration of the M1 PAM‐agonist PF‐06767832 reduces consummatory behavior in male iP rats across both caloric and non‐caloric solutions. We observed that M1 enhancement, at a dose that did not alter locomotor activity, reduced operant alcohol self‐administration on a FR3 schedule. This is in line with previous studies showing involvement of the M1 receptor in other drug consumption. 12 , 28 However, our subsequent experiments also revealed that PF‐06767832 reduced self‐administration of natural reward (sucrose), suggesting the reduction in alcohol consumption is likely due to a general action of PF‐6767832 in reducing all consummatory behaviors. Given these data, and the known role of the M1 mAChR in cognitive domains 40 , 41 we next sought to determine whether our results related to interference with an instrumental process or generalized to task‐free consummatory behaviors (standard show and water within the home cage environment). PF‐06767832 treated rats showed reduced intake of both food and water 2 h after systemic administration, which persisted for 24 h in the case of food (but not water) consumption. Previous studies showed that fasting increased M1 mAChR expression in the frontal cortex and hippocampus, but not the amygdala of mice, which was decreased after food intake, 42 supporting a potential role for M1 enhancement to modulate food consumption. Interestingly, PF‐06767832 at higher doses (10–45 mg/kg) can increase food intake and weight gain in rats and dogs 29 and the bitopic M1 agonist VU0364572 increased food choice over cocaine. 28 M1 receptors are also expressed on salivary glands where they modulate the secretion of saliva by acinar cells. 43 It is, therefore, possible that differential effects of dose or different ligand classes on “food intake” may be indirect and related to differential effects on salivation. Overall, these results suggest a potential dose‐dependent action of M1 modulation in relation to food consumption.
Our observed reduction in operant alcohol self‐administration was transient, with no difference in alcohol consumption noted when animals were returned to the operant chamber 24 h after treatment. This result differs from recent findings using the M1 agonist, VU0364572, which induced a prolonged reduction in cocaine choice over food for up to 4 weeks, accompanied by reductions in dopamine and glutamate outflow after administration. 28 The transient actions of PF‐06767832 (PAM‐agonist), compared with VU0364572 (bitopic agonist) may be driven by differences that exist between studies (e.g., rodent strain, drug class, and experimental paradigm). Indeed, experimental procedures employed would elicit differential cognitive requirements on the animals. The choice paradigms involve a second‐order schedule, whereby an observer lever must be pressed to allow the animal to choose between the cocaine‐paired or food‐paired lever. 22 , 28 This task requires higher‐order cognitive processing, while experiments in our study required lesser (first‐order operant conditioning) or very little (home cage consumption) cognitive demand. Several selective orthosteric agonists have also shown that M1 stimulation enhances behavioral flexibility and pro‐cognitive effects. 40 , 44 , 45 Therefore, VU0364572 may reduce cocaine choice over food through altering cognitive processing that also relates to the lasting effect. Another distinct possibility is that VU0364572 may gradually reduce the reinforcing effect of cocaine, but not food, over time. We did not empirically test the possibility of such a profile developing with time in the case of PF‐06767832 because our acute data showed no differential between alcohol versus sucrose. Another factor to consider when comparing our alcohol data with prior cocaine data is that alcohol has a caloric value in addition to being rewarding and M1 signaling may also modulate energy intake. In agreement with this notion is the finding that the M1 antagonist, biperiden, increased breakpoint for a milkshake in mice, although on an FR5 schedule did not alter consumption. 46 Nevertheless, cholinergic signaling has been linked to modulating satiety at the level of the nucleus accumbens (for review see 47 ).
Another major difference in our study compared to previous findings is the class of M1 compounds employed. Our study used a selective PAM‐agonist, PF‐06767832, which shows good oral bioavailability and robust in vivo activity. 29 In contrast, bitopic M1 agonists, which span both orthosteric and allosteric binding sites, may lack some receptor selectivity and are thought to cause a greater incidence of adverse incidences. For example, the bitopic M1 agonist GSK1034702 has shown pro‐cognitive effects in rodents 40 ; however, adverse gastrointestinal effects of this molecule were seen in clinical trials. 48 Previous studies have suggested drug candidates that have high M1 receptor selectivity, and low levels of intrinsic agonist activity (e.g., selective PAMs) provide the most potential as compounds for AD and other diseases. 49 In contrast, allosteric modulation only occurs in the presence of the endogenous ligand, maintaining the natural spatiotemporal scale and allowing nuanced manipulation of ACh signaling. 24 However, one possibility is M1 PAM‐agonists, which have both agonist and allosteric actions may produce similar adverse effects as traditional orthosteric agonists through over‐activation of the M1 receptor. 50 , 51 Indeed, M1 PAMs lacking agonist activity are thought to provide a more optimal profile for enhancing higher‐order processing. 25 , 50
4.1. Limitations
While our current data suggest the M1 PAM‐agonist PF‐06767832 reduces alcohol and natural consummatory behaviors, several limitations exist that should be addressed in future work. A major limitation of our study is the solitary examination of PF‐06767832; a direct comparison of M1 agonist, PAM‐agonist, and PAM compounds should be conducted in the future to understand differential contributions to cognitive effects, drug consumption/seeking, and side effects. Such a study should also incorporate a longitudinal testing component to examine persistence and/or the emergence of effects. Another limitation of our study is the lack of a dose‐response curve. PF‐06767832 has been administered up to 30 mg/kg in rats and 45 mg/kg in dogs. In dogs, doses ranging from 3 to 15 mg/kg showed gastrointestinal side effects, while 45 mg/kg also resulted in ataxia and convulsions, however, no adverse gastrointestinal side effects were observed at 1 mg/kg. 29 In rats, 1 mg/kg was sufficient to reduce amphetamine‐induced locomotor activity and amphetamine‐disrupted prepulse inhibition (PPI), an effect which was not observed at a lower dose (0.32 mg/kg). 29 Further, the reductions observed across fluids/food observed within our study were of similar magnitudes, with 2 h water showing the greatest magnitude in reduction (64%) following PF‐06767832 administration, followed by food (33%), alcohol (30%), and sucrose (30%). Together this suggests lower doses of PF‐06767832 would likely act similarly across all consummatory behaviors we tested. Additionally, in the current study, we only assessed male rats. This is a limitation given the rising prevalence of AUD in women 52 and the potential roles of sex hormones in drug and alcohol consumption. 53 , 54 , 55 PF‐06767832 showed similar dose‐dependent side effects in both male and female subjects, suggesting no overt differences in the pharmacology between sexes, 29 however, this should be empirically addressed in the future studies. Finally, little is known about the muscarinic system in Indiana alcohol‐preferring (iP) rats. We have previously shown that allosteric modulation of the M4 and M5 receptors specifically reduces alcohol consumption in male iP rats. 4 , 5 , 7 Further, we show similar striatal M4 mAChR dysregulation across human and rodent species following chronic alcohol consumption. 5 Whether the same alcohol‐induced dysregulation is observed in outbred strains has not been thoroughly examined, however, iP rats are a strain that voluntarily consumes enough alcohol to achieve intoxication and dependence. 30 , 56
4.2. Conclusions
Collectively, our data show a non‐specific effect for PF‐06767832 on consummatory behavior, without altering motivation or locomotor activity. Our data highlight potential idiosyncrasies with M1 muscarinic compounds, whereby agonist versus PAM versus PAM‐agonist may have different actions on drug and alcohol consumption, and/or cognitive demand in different behavioral processes that may influence the ability of M1 compounds to modulate drug/alcohol consumption. Understanding the selectivity and pharmacokinetics/dynamics of specific M1 agonists, PAM‐agonists and PAMs is required to further understand their utility for clinical development to treat alcohol and drug use disorders.
DISCLOSURE
Authors report no biomedical financial interests or potential conflicts of interest.
AUTHOR CONTRIBUTIONS
The study was conceived, planned, and supervised by LCW, CJL, and AJL. LCW, EJC, KLH, and NAC performed experiments and analyzed data. The manuscript was written by LCW and AJL (original draft) with input from CJL. All authors approved the manuscript prior to submission.
ACKNOWLEDGMENTS
This research was supported by a National Health and Medical Research Council project grant (Grant No. 1120576 [to AJL and CJL]), of which AJL is a principal research fellow (Grant No. 1116930).
Walker LC, Campbell EJ, Huckstep KL, Chen NA, Langmead CJ, Lawrence AJ. M1 muscarinic receptor activation decreases alcohol consumption via a reduction in consummatory behavior. Pharmacol Res Perspect.2022;10:e00907. doi: 10.1002/prp2.907
Contributor Information
Leigh C. Walker, Email: leigh.walker@florey.edu.au.
Andrew J. Lawrence, Email: andrew.lawrence@florey.edu.au.
DATA AVAILABILITY STATEMENT
The data from this study are available from the corresponding authors upon reasonable request.
REFERENCES
- 1. Cragg SJ, Exley R, Clements MA. Striatal acetylcholine control of reward‐related dopamine signalling. The Basal Ganglia VIII. Springer; 2005:99‐108. [Google Scholar]
- 2. Williams MJ, Adinoff B. The role of acetylcholine in cocaine addiction. Neuropsychopharmacology. 2008;33(8):1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pepeu G, Giovannini MG. Changes in acetylcholine extracellular levels during cognitive processes. Learning & Memory. 2004;11(1):21‐27. [DOI] [PubMed] [Google Scholar]
- 4. Berizzi AE, Perry CJ, Shackleford DM, et al. Muscarinic M5 receptors modulate ethanol seeking in rats. Neuropsychopharmacology. 2018;43(7):1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Walker LC, Berizzi AE, Chen NA, et al. Acetylcholine muscarinic M4 receptors as a therapeutic target for alcohol use disorder: converging evidence from humans and rodents. Biol Psychiat. 2020;88(12):898‐909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Walker LC, Lawrence AJ. Allosteric modulation of muscarinic receptors in alcohol and substance use disorders. Advances in Pharmacology. Elsevier; 2020:233‐275. [DOI] [PubMed] [Google Scholar]
- 7. Walker LC, Huckstep KL, Chen NA, et al. Muscarinic M4 and M5 receptor subtypes in the ventral subiculum differentially modulate alcohol seeking vs consumption in male alcohol preferring rats. Br J Pharmacol. 2021. [DOI] [PubMed] [Google Scholar]
- 8. Bosboom J, Stoffers D, Wolters EC. The role of acetylcholine and dopamine in dementia and psychosis in Parkinson’s disease. In: Giuseppe DG, Matteo VD, Ennio E, eds. Advances in Research on Neurodegeneration. Springer; 2003:185‐195. [DOI] [PubMed] [Google Scholar]
- 9. Mesulam M, Guillozet A, Shaw P, Quinn B. Widely spread butyrylcholinesterase can hydrolyze acetylcholine in the normal and Alzheimer brain. Neurobiol Dis. 2002;9(1):88‐93. [DOI] [PubMed] [Google Scholar]
- 10. Raedler TJ, Knable MB, Jones DW, et al. In vivo determination of muscarinic acetylcholine receptor availability in schizophrenia. Am J Psychiatry. 2003;160(1):118‐127. [DOI] [PubMed] [Google Scholar]
- 11. Lebois EP, Thorn C, Edgerton JR, Popiolek M, Xi S. Muscarinic receptor subtype distribution in the central nervous system and relevance to aging and Alzheimer's disease. Neuropharmacology. 2018;136:362‐373. [DOI] [PubMed] [Google Scholar]
- 12. Carrigan KA, Dykstra LA. Behavioral effects of morphine and cocaine in M1 muscarinic acetylcholine receptor‐deficient mice. Psychopharmacology. 2007;191(4):985‐993. [DOI] [PubMed] [Google Scholar]
- 13. Anagnostaras SG, Murphy GG, Hamilton SE, et al. Selective cognitive dysfunction in acetylcholine M1 muscarinic receptor mutant mice. Nat Neurosci. 2003;6(1):51‐58. [DOI] [PubMed] [Google Scholar]
- 14. Bartko SJ, Romberg C, White B, Wess J, Bussey TJ, Saksida LM. Intact attentional processing but abnormal responding in M1 muscarinic receptor‐deficient mice using an automated touchscreen method. Neuropharmacology. 2011;61(8):1366‐1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shinoe T, Matsui M, Taketo MM, Manabe T. Modulation of synaptic plasticity by physiological activation of M1 muscarinic acetylcholine receptors in the mouse hippocampus. J Neurosci. 2005;25(48):11194‐11200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Galloway CR, Lebois EP, Shagarabi SL, Hernandez NA, Manns JR. Effects of selective activation of M1 and M4 muscarinic receptors on object recognition memory performance in rats. Pharmacology. 2014;93(1‐2):57‐64. [DOI] [PubMed] [Google Scholar]
- 17. Ding JB, Guzman JN, Peterson JD, Goldberg JA, Surmeier DJ. Thalamic gating of corticostriatal signaling by cholinergic interneurons. Neuron. 2010;67(2):294‐307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Goldberg JA, Ding JB, Surmeier DJ. Muscarinic modulation of striatal function and circuitry. In: Fryer AD, Christopoulos A, Nathanson NM, eds. Muscarinic Receptors. Springer; 2012:223‐241. [DOI] [PubMed] [Google Scholar]
- 19. Shen W, Tian X, Day M, et al. Cholinergic modulation of Kir2 channels selectively elevates dendritic excitability in striatopallidal neurons. Nat Neurosci. 2007;10(11):1458‐1466. [DOI] [PubMed] [Google Scholar]
- 20. Thomsen M, Conn PJ, Lindsley C, et al. Attenuation of cocaine's reinforcing and discriminative stimulus effects via muscarinic M1 acetylcholine receptor stimulation. J Pharmacol Exp Ther. 2010;332(3):959‐969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Thomsen M, Lindsley CW, Conn PJ, et al. Contribution of both M1 and M4 receptors to muscarinic agonist‐mediated attenuation of the cocaine discriminative stimulus in mice. Psychopharmacology. 2012;220(4):673‐685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Thomsen M, Fulton BS, Caine SB. Acute and chronic effects of the M1/M4‐preferring muscarinic agonist xanomeline on cocaine vs. food choice in rats. Psychopharmacology. 2014;231(3):469‐479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Brady AE, Jones CK, Bridges TM, et al. Centrally active allosteric potentiators of the M4 muscarinic acetylcholine receptor reverse amphetamine‐induced hyperlocomotor activity in rats. J Pharmacol Exp Ther. 2008;327(3):941‐953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kruse AC, Ring AM, Manglik A, et al. Activation and allosteric modulation of a muscarinic acetylcholine receptor. Nature. 2013;504(7478):101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Moran SP, Maksymetz J, Conn PJ. Targeting muscarinic acetylcholine receptors for the treatment of psychiatric and neurological disorders. Trends Pharmacol Sci. 2019;40(12):1006‐1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Langmead CJ, Christopoulos A. Functional and structural perspectives on allosteric modulation of GPCRs. Curr Opin Cell Biol. 2014;27:94‐101. [DOI] [PubMed] [Google Scholar]
- 27. Digby GJ, Utley TJ, Lamsal A, et al. Chemical modification of the M1 agonist VU0364572 reveals molecular switches in pharmacology and a bitopic binding mode. ACS Chem Neurosci. 2012;3(12):1025‐1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Weikop P, Jensen KL, Thomsen M. Effects of muscarinic M1 receptor stimulation on reinforcing and neurochemical effects of cocaine in rats. Neuropsychopharmacology. 2020;45(12):1994‐2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Davoren JE, Lee CW, Garnsey M, et al. Discovery of the potent and selective M1 PAM‐agonist N‐[(3 R, 4 S)‐3‐hydroxytetrahydro‐2 H‐pyran‐4‐yl]‐5‐methyl‐4‐[4‐(1, 3‐thiazol‐4‐yl) benzyl] pyridine‐2‐carboxamide (PF‐06767832): evaluation of efficacy and cholinergic side effects. J Med Chem. 2016;59(13):6313‐6328. [DOI] [PubMed] [Google Scholar]
- 30. Ciccocioppo R. Genetically selected alcohol preferring rats to model human alcoholism. In Sommer WH, Spanagel R, eds. Behavioral Neurobiology of Alcohol Addiction. Springer; 2012:251‐269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Percie du Sert N, Hurst V, Ahluwalia A, et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biology. 2020;18(7):e3000410. 10.1371/journal.pbio.3000410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Campbell EJ, Flanagan JPM, Walker LC, Hill MKRI, Marchant NJ, Lawrence AJ. Anterior insular cortex is critical for the propensity to relapse following punishment‐imposed abstinence of alcohol seeking. J Neurosci. 2019;39(6):1077‐1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Walker LC, Hand LJ, Letherby B, Huckstep KL, Campbell EJ, Lawrence AJ. Cocaine and amphetamine regulated transcript (CART) signalling in the central nucleus of the amygdala modulates stress‐induced alcohol seeking. Neuropsychopharmacology. 2021;46(2):325‐333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Walker LC, Kastman HE, Krstew EV, Gundlach AL, Lawrence AJ. Central amygdala relaxin‐3/relaxin family peptide receptor 3 signalling modulates alcohol seeking in rats. Br J Pharmacol. 2017;174(19):3359‐3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Walker LC, Kastman HE, Lawrence AJ. Pattern of neural activation following yohimbine‐induced reinstatement of alcohol seeking in rats. Eur J Neurosci. 2020;51(3):706‐720. [DOI] [PubMed] [Google Scholar]
- 36. Walker LC, Kastman HE, Koeleman JA, et al. Nucleus incertus corticotrophin‐releasing factor 1 receptor signalling regulates alcohol seeking in rats. Addict Biol. 2017;22(6):1641‐1654. [DOI] [PubMed] [Google Scholar]
- 37. Farid WO, Lawrence AJ, Krstew EV, et al. Maternally administered sustained‐release naltrexone in rats affects offspring neurochemistry and behaviour in adulthood. PLoS One. 2012;7(12):e52812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Harding SD, Sharman JL, Faccenda E, et al. The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucleic Acids Res. 2018;46(D1):D1091‐D1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Alexander SP, Christopoulos A, Davenport AP, et al. The Concise Guide to PHARMACOLOGY 2019/20: G protein‐coupled receptors. Br J Pharmacol. 2019;176:S21‐S141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Budzik B, Garzya V, Shi D, et al. Novel N‐substituted benzimidazolones as potent, selective, CNS‐penetrant, and orally active M1 mAChR agonists. ACS Med Chem Lett. 2010;1(6):244‐248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Davie BJ, Christopoulos A, Scammells PJ. Development of M1 mAChR allosteric and bitopic ligands: prospective therapeutics for the treatment of cognitive deficits. ACS Chem Neurosci. 2013;4(7):1026‐1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Saygı Bacanak M, Aydın B, Cabadak H, Nurten A, Gören MZ, Enginar N. Contribution of M1 and M2 muscarinic receptor subtypes to convulsions in fasted mice treated with scopolamine and given food. Behav Brain Res. 2019;364:423‐430. [DOI] [PubMed] [Google Scholar]
- 43. Proctor GB, Carpenter GH. Regulation of salivary gland function by autonomic nerves. Auton Neurosci. 2007;133(1):3‐18. [DOI] [PubMed] [Google Scholar]
- 44. Brandeis R, Sapir M, Hafif N, et al. AF150 (S): a new functionally selective M1 agonist improves cognitive performance in rats. Pharmacol Biochem Behav. 1995;51(4):667‐674. [DOI] [PubMed] [Google Scholar]
- 45. Ragozzino ME, Artis S, Singh A, Twose TM, Beck JE, Messer WS. The selective M1 muscarinic cholinergic agonist CDD‐0102A enhances working memory and cognitive flexibility. J Pharmacol Exp Ther. 2012;340(3):588‐594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hailwood JM, Heath CJ, Phillips BU, Robbins TW, Saksida LM, Bussey TJ. Blockade of muscarinic acetylcholine receptors facilitates motivated behaviour and rescues a model of antipsychotic‐induced amotivation. Neuropsychopharmacology. 2019;44(6):1068‐1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wiss DA, Avena N, Rada P. Sugar addiction: from evolution to revolution. Front Psychiatry. 2018;9:545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Nathan PJ, Watson J, Lund J, et al. The potent M1 receptor allosteric agonist GSK1034702 improves episodic memory in humans in the nicotine abstinence model of cognitive dysfunction. Int J Neuropsychopharmacol. 2013;16(4):721‐731. [DOI] [PubMed] [Google Scholar]
- 49. Bradley SJ, Molloy C, Bundgaard C, et al. Bitopic binding mode of an M1 muscarinic acetylcholine receptor agonist associated with adverse clinical trial outcomes. Mol Pharmacol. 2018;93(6):645‐656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Moran SP, Dickerson JW, Cho HP, et al. M 1‐positive allosteric modulators lacking agonist activity provide the optimal profile for enhancing cognition. Neuropsychopharmacology. 2018;43(8):1763‐1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Rook JM, Bertron JL, Cho HP, et al. A novel M1 PAM VU0486846 exerts efficacy in cognition models without displaying agonist activity or cholinergic toxicity. ACS Chem Neurosci. 2018;9(9):2274‐2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Grant BF, Chou SP, Saha TD, et al. Prevalence of 12‐month alcohol use, high‐risk drinking, and DSM‐IV alcohol use disorder in the United States, 2001–2002 to 2012–2013: results from the National Epidemiologic Survey on Alcohol and Related Conditions. JAMA Psychiatry. 2017;74(9):911‐923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Agabio R, Pisanu C, Gessa GL, Franconi F. Sex differences in alcohol use disorder. Curr Med Chem. 2017;24(24):2661‐2670. [DOI] [PubMed] [Google Scholar]
- 54. Arunogiri S, Crossin R, Rizzo D, Walker L, Ridley K, Gurvich C. A systematic review of the effect of ovarian sex hormones on stimulant use in females. Addict Biol. 2021;26(6):e13079. [DOI] [PubMed] [Google Scholar]
- 55. Erol A, Ho A‐C, Winham SJ, Karpyak VM. Sex hormones in alcohol consumption: a systematic review of evidence. Addict Biol. 2019;24(2):157‐169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kampov‐Polevoy AB, Matthews DB, Gause L, Morrow AL, Overstreet DH. P rats develop physical dependence on alcohol via voluntary drinking: changes in seizure thresholds, anxiety, and patterns of alcohol drinking. Alcohol Clin Exp Res. 2000;24(3):278‐284. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data from this study are available from the corresponding authors upon reasonable request.


