ABSTRACT
Streptococcus pneumoniae is a serious human respiratory pathogen. It generates hydrogen peroxide (H2O2) as part of its normal metabolism, yet it lacks enzymes that remove this oxidant. Here we show that lactoperoxidase and myeloperoxidase, two host enzymes present in the respiratory tract, convert bacterial H2O2 into HOSCN that S. pneumoniae can resist. We found that incubation of S. pneumoniae with myeloperoxidase in chloride-rich buffer killed the bacteria due to formation of toxic hypochlorous acid (HOCl). However, the addition of physiological concentrations of thiocyanate protected the bacteria. Similarly, S. pneumoniae remained viable in the presence of lactoperoxidase and thiocyanate even though the majority of bacterial H2O2 was converted to hypothiocyanous acid (HOSCN). S. pneumoniae and Pseudomonas aeruginosa, another respiratory pathogen, were similarly sensitive to H2O2 and HOCl. In contrast, S. pneumoniae tolerated much higher doses of HOSCN than P. aeruginosa. When associated with neutrophil extracellular traps (NETs), S. pneumoniae continued to generate H2O2, which was converted to HOCl by myeloperoxidase (MPO) present on NETs. However, there was no loss in bacterial viability because HOCl was scavenged by the NET proteins. We conclude that at sites of infection, bacteria will be protected from HOCl by thiocyanate and extracellular proteins including those associated with NETs. Resistance to HOSCN may give S. pneumoniae a survival advantage over other pathogenic bacteria. Understanding the mechanisms by which S. pneumoniae protects itself from HOSCN may reveal novel strategies for limiting the colonization and pathogenicity of this deadly pathogen.
KEYWORDS: Streptococcus pneumoniae, hypothiocyanous acid, hypochlorous acid, hydrogen peroxide, thiocyanate, myeloperoxidase, lactoperoxidase, neutrophil extracellular traps, neutrophils
INTRODUCTION
Streptococcus pneumoniae is a commensal bacterium that colonizes the mucosal surfaces of the upper respiratory tract in humans. However, migration to other areas in the body can cause pneumonia, otitis media, sepsis, and meningitis (1). Despite the introduction of conjugate vaccines and antibiotic treatment, S. pneumoniae is still a serious threat to human health. It was identified as one of 12 priority pathogens for research and development of new treatment strategies by the World Health Organization in 2017 (2).
S. pneumoniae generates large amounts of the oxidant hydrogen peroxide (H2O2) as part of its normal metabolism (3), but lacks enzymes, such as catalase, that other bacteria commonly possess to detoxify H2O2 (4). As a consequence, H2O2 accumulates extracellularly. The quantity of H2O2 generated by S. pneumoniae during normal metabolism varies between bacterial strains (5, 6). H2O2 is primarily produced from oxygen and pyruvate by the enzyme pyruvate oxidase (SpxB) (5, 7). SpxB also helps protect S. pneumoniae from the deleterious effects of H2O2, potentially by providing ATP to repair H2O2-mediated DNA damage (6). The H2O2 produced by S. pneumoniae has been shown to kill or inhibit the growth of other bacterial species, including Haemophilus influenzae, Neisseria meningitidis, and Moraxella catarrhalis (5, 6), and is thought to provide S. pneumoniae with a survival advantage in the nasopharynx.
Lactoperoxidase (LPO), found in biological fluids such as saliva, tears, and airway lining fluid (8, 9), catalyzes the reaction between H2O2 and SCN− to form the bacteriostatic and bactericidal oxidant hypothiocyanous acid (HOSCN) (10). HOSCN has a pKa of 4.8 (11); therefore at physiological pH, the majority will be present as the weaker oxidant OSCN− (9). In human airway secretions, the concentration of LPO and SCN− is sufficient to generate an antibacterial effect against the respiratory tract pathogens Pseudomonas aeruginosa, Burkholderia cepacia, and H. influenzae when supplemented with H2O2 (8). As such, during S. pneumoniae colonization, bacterially produced H2O2 and SCN− in the extracellular milieu will likely be converted to HOSCN by LPO. H2O2 is also generated by the dual oxidases of epithelial cells lining the respiratory tract (12).
Neutrophils provide another source of oxidants during acute bacterial infection. Neutrophil activation leads to the assembly of an NADPH oxidase complex on the phagosomal or plasma membrane that converts oxygen into superoxide, which dismutates to H2O2 (13). This H2O2 is then used by the neutrophil enzyme myeloperoxidase (MPO) to generate hypochlorous acid (HOCl) or HOSCN (14). Chloride (Cl−) is considered the primary substrate of MPO due to its high concentration in plasma (100 to 110 mM) (15, 16) compared with thiocyanate (SCN−) (8 to 73 μM) (17–19); however, the substrate specificity of SCN− is approximately 700 times higher, meaning that MPO will convert up to half of the H2O2 to HOSCN in plasma (14). Higher concentrations of SCN− have been reported in lung airway fluid (30 to 650 μM) (8, 20), making HOSCN the major product. HOCl can also react rapidly with SCN− to produce HOSCN (21).
MPO is contained in pre-formed granules and comprises approximately 5% of total neutrophil protein (22). The contents of these granules are released intracellularly into the phagosome upon pathogen phagocytosis, or extracellularly through either degranulation or neutrophil extracellular trap (NET) release. NETs are comprised of neutrophil DNA and proteins and contain substantial quantities of enzymatically active MPO (23). NET formation can be induced by pathogens such as bacteria (24) and fungi (25). The severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), causing Coronavirus Disease 2019 (COVID-19), has been shown to activate the release of NETs from human neutrophils (26, 27), which can promote lung epithelial damage in vitro (26). NET formation has also been induced by the capsule of S. pneumoniae in a murine model of pneumonia (28). Therefore, in the airway it can be expected that S. pneumoniae will be exposed to NETs, and in turn, HOCl and HOSCN, even when they are not phagocytosed by neutrophils.
In this study, we have investigated how H2O2 production by S. pneumoniae affects the viability of these bacteria, especially when heme peroxidases and their substrates are present in the inflammatory complex milieu. We report that S. pneumoniae generate H2O2 while on NETs and it is converted to HOCl via NET-associated MPO. We found that the antibacterial activity of HOCl is limited by the presence of physiological concentrations of SCN− and NET proteins, and that S. pneumoniae is significantly more resistant to HOSCN than another respiratory tract pathogen P. aeruginosa.
RESULTS
S. pneumoniae produce high levels of H2O2 in buffer and on NETs.
S. pneumoniae are known to generate high levels of H2O2 as a part of their normal metabolism, with H2O2 generation varying between strains (5, 6). Here, we measured the rates of H2O2 production by log-phase S. pneumoniae strains SP264, D39, CIP 104340, and a lung clinical isolate (LU1), which ranged from 0.40 to 0.68 nmol/min/107 bacteria (Table 1). We then incubated S. pneumoniae SP264 with NETs to determine whether bacterially produced H2O2 rates differ when in the presence of NET-associated MPO (Table 2). Interestingly, the H2O2 generated by S. pneumoniae did not differ significantly when the bacteria were incubated in the presence or absence of NETs (P = 0.59).
TABLE 1.
| Strain | Serotype | H2O2 rate per minute (nmol/107 bacteria ± SEM) | Total H2O2 |
|---|---|---|---|
| SP264 | 23F | 0.60 ± 0.05 | 18.4 ± 1.3 |
| D39 | 2 | 0.40 ± 0.04 | 12.6 ± 1.3 |
| LU1 | Unknown | 0.68 ± 0.12 | 19.8 ± 3.4 |
| CIP 104340 | 19F | 0.58 ± 0.08 | 17.7 ± 2.3 |
Results are from three independent experiments.
No significant difference in H2O2 production between strains was identified (one-way ANOVA, P = 0.16).
TABLE 2.
| Oxidant | Condition | Total oxidant (nmol/107 bacteria ± SEM) |
|---|---|---|
| H2O2 | − NETs | 16.02 ± 4.43 |
| + NETs | 14.04 ± 3.47 | |
| HOCl | − NETs (no MPO) | 0.31 ± 0.13 |
| + NETs | 9.77 ± 1.60 |
Results are from four independent experiments.
Significance between conditions was tested with a two-tailed unpaired t test (H2O2 rate, P = 0.74; HOCl rate, P < 0.01).
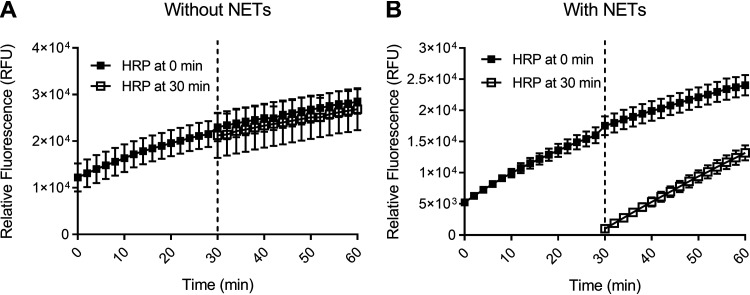
To quantify H2O2 production, an Amplex UltraRed assay was used. A function of this assay is to remove H2O2 from the system through a reaction with horseradish peroxidase (HRP), forming a colored product. However, the removal of H2O2 could potentially interfere with bacterial feedback inhibition mechanisms that would function in vivo. To check this and verify the applicability of our assay, the reaction mixture containing horseradish peroxidase was added to the bacteria in the absence of NETs after 30 min and compared with its addition at time 0, our usual assay condition. We found that the timing of the reaction mixture addition did not affect H2O2 measurements (Fig. 1A), indicating that there is no significant feedback inhibition mechanism acting in our assay and showing that H2O2 is not consumed by the bacteria. When tested with bacteria on NETs there was an absence of H2O2 in the buffer after the first 30-min period, consistent with H2O2 utilization by MPO (Fig. 1B).
FIG 1.
S. pneumoniae H2O2 production in the presence and absence of NETs. Amplex UltraRed oxidation of S. pneumoniae SP264 (1 × 107/mL) over 60 min in HBSS in the absence (A) or presence (B) of NETs (from 1 × 106 neutrophils/mL). Amplex UltraRed reaction mixture was added at 0 min (closed squares) or 30 min (open squares). Values are the mean ± SEM of three independent experiments.
S. pneumoniae are killed by HOCl generated by MPO from bacterially produced H2O2.
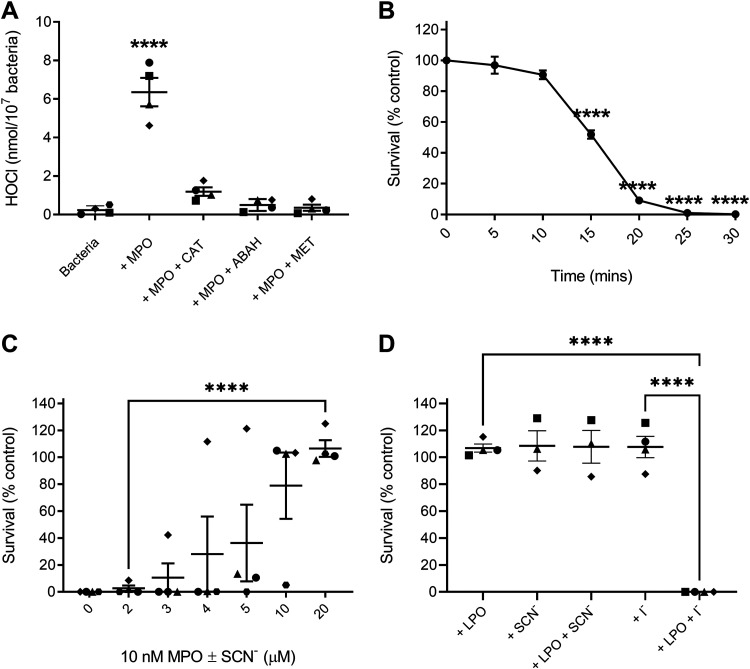
The addition of 10 nM MPO to bacteria in HBSS containing 145 mM Cl− resulted in the production of 6.4 ± 0.7 nmol of HOCl/107 bacteria, which is a conversion of approximately 20% of the H2O2 generated in that time (Fig. 2A). The addition of catalase, methionine, or 4-Aminobenzoic acid hydrazide (ABAH) reduced HOCl generation to control levels. In this system, there was nearly complete bacterial killing within 30 min (Fig. 2B). Surprisingly, the addition of only 20 μM SCN− was sufficient to completely protect S. pneumoniae from HOCl dependent killing (Fig. 2C). Therefore, despite being an effective antimicrobial agent on its own, HOCl will be rendered less effective by the presence of physiological levels of SCN−.
FIG 2.
Oxidant generation and S. pneumoniae survival after incubation with mammalian peroxidases and their substrates. Symbol shapes designate independent experiments. Bacterial survival is reported relative to untreated controls. (A) HOCl generation in HBSS with S. pneumoniae SP264 (5 × 107/mL) and 10 nM MPO with and without catalase (20 μg/mL), methionine (10 μM), and ABAH (10 μM) as quantified by taurine chloramine assay. (B) S. pneumoniae SP264 (5 × 107/mL) survival when incubated with 5 nM MPO compared with control for up to 30 min. (C) S. pneumoniae SP264 (5 × 107/mL) survival in HBSS containing 10 nM MPO and 2 to 20 μM SCN− for 30 min. (D) S. pneumoniae D39 (5 × 107/mL) survival with or without 10 nM LPO at pH 6.8 ± 800 μM SCN− or 100 μM I− after 30 min. A to D values are the mean ± SEM of 3 to 4 independent experiments, as designated by the symbols in A, C, and D. A, B, and D significant differences were identified using an unpaired one-way ANOVA with Dunnet’s (A and B) or Tukey’s (D) multiple comparisons tests. Significance between 2 and 20 μM SCN− in C was identified using an unpaired, two-way t test. Significance of **** indicates P < 0.0001.
As the MPO experiments suggested that S. pneumoniae can withstand at least 20 μM HOSCN without impacting bacterial survival, we examined bacterial survival with LPO, which cannot generate HOCl. In this experiment, S. pneumoniae strain D39 would have generated approximately 60 μM H2O2, based on H2O2 measurements (Table 1), which would be converted to approximately 60 μM HOSCN by LPO. Similar to what was seen in the MPO experiment (Fig. 2C), no bacterial killing was observed in the presence of the LPO-SCN−-H2O2 system (Fig. 2D). In contrast, bacterial killing was observed when the bacteria were incubated with LPO + I−, indicating that the bacteria were generating H2O2 and the enzyme was functional and produced bactericidal hypoiodous acid (HOI).
S. pneumoniae survival is not affected by high doses of HOSCN.
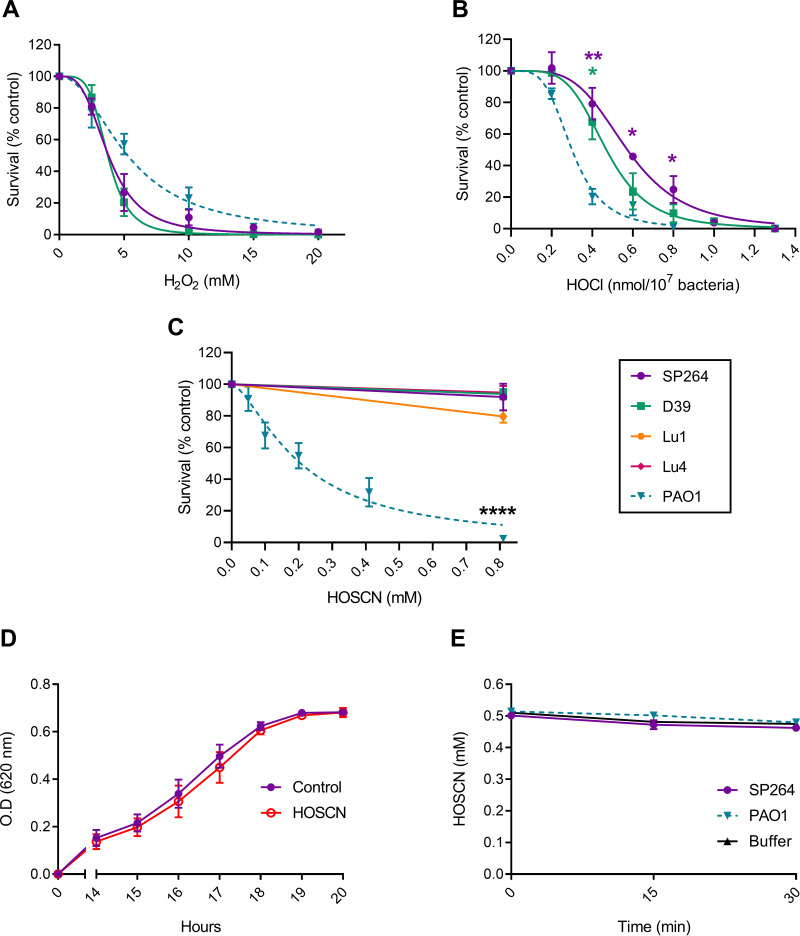
We measured S. pneumoniae survival on exposure to oxidants using strains SP264 and D39 to determine relative oxidant sensitivities. As the bacteria generate high concentrations of H2O2 (3) but lack the enzymes to detoxify it effectively (4), we first examined the sensitivity of S. pneumoniae to exogenous H2O2. We also tested the sensitivity of another respiratory bacterium, P. aeruginosa (strain PAO1) for comparison (Fig. 3A). Both S. pneumoniae strains and P. aeruginosa were similarly sensitive to a bolus of H2O2.
FIG 3.
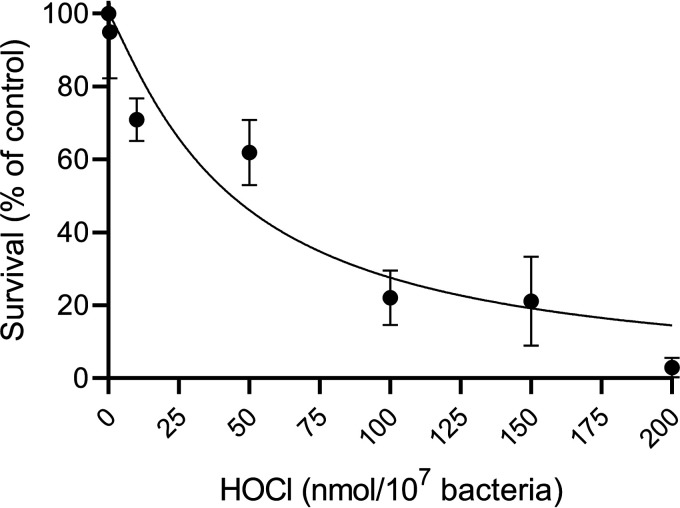
Sensitivity of S. pneumoniae and P. aeruginosa to H2O2, HOCl, and HOSCN. Strains are shown as S. pneumoniae SP264 (purple, circles), D39 (green, squares), Lu1 (orange, hexagons), Lu4 (pink, diamonds), and P. aeruginosa PAO1 (blue, inverted triangles, dotted line). Bacterial survival was assessed by plate counts, with treated samples compared to untreated controls. (A) S. pneumoniae (5 × 107/mL) and P. aeruginosa (5 × 107/mL) were incubated with increasing concentrations of H2O2 for 30 min. (B) S. pneumoniae (1 × 109/mL) and P. aeruginosa (1 × 108/mL) were incubated with increasing concentrations of HOCl for 15 min. (C) S. pneumoniae (2.5 × 105/mL) and P. aeruginosa (2.5 × 105/mL) where incubated with increasing concentrations of HOSCN for 30 min. (D) S. pneumoniae SP264 (2.5 × 105/mL) recovery following treatment with ± 0.8 mM HOSCN for 30 min. (E) The concentration of HOSCN present in the buffer of S. pneumoniae (SP264, 5 × 105/mL), P. aeruginosa (5 × 105/mL), and buffer alone (HBSS) were measured in 15-min intervals for 30 min. Values are the mean ± SEM of at least three independent experiments. A to C are fitted with [oxidant] versus normalized response – variable slope curves with top values constrained to 100 and bottom values constrained to 0. A to C significance was tested using a one-way ANOVA with Tukey’s multiple-comparison test at each dose, indicated by * P < 0.05, ** P < 0.01, **** P < 0.0001. D to E significance was tested using a two-way ANOVA with Šídák's (D) or Dunnett’s (E) multiple comparisons tests, with matched values stacked into a sub-column, indicated by * P < 0.05.
Next, we examined bacterial sensitivity to HOCl and HOSCN. Both S. pneumoniae strains and P. aeruginosa were killed at relatively low doses of HOCl, with 1 nmol of HOCl/107 bacteria killing > 95% of all strains tested (Fig. 3B). This is consistent with previous observations for Klebsiella pneumoniae, Escherichia coli, Staphylococcus aureus, P. aeruginosa, and B. cepacia (29–32). S. pneumoniae were more resistant to low doses of HOCl than P. aeruginosa with statistically significant differences in survival between S. pneumoniae strains and P. aeruginosa at several concentrations (0.4, 0.6, and 0.8 nmol/107 bacteria for SP264 versus PAO1 and 0.4 nmol for D39 versus PAO1) (Fig. 3B). HOCl is highly reactive and when used at low concentrations, significant proportions are lost in buffer alone. Therefore, we used higher concentrations and increased bacterial density accordingly. Rapid consumption means that HOCl levels are expressed as a dose.
In contrast, S. pneumoniae were significantly more resistant to HOSCN than P. aeruginosa (Fig. 3C). Four strains of S. pneumoniae were treated with HOSCN; SP264, D39, and two clinical lung isolates Lu1 and Lu4. While P. aeruginosa was killed in a dose-dependent manner, with an LC50 of 0.21 mM, the highest concentration tested 0.8 mM had no significant impact on the viability of any of the four S. pneumoniae strains after 30 min. The dose of HOSCN used was limited by the concentration of HOSCN able to be generated, as such, bacterial concentrations in this experiment were lowered to increase oxidant dose per bacterium. A control using decomposed HOSCN confirmed that killing of PAO1 was due to HOSCN, not a stable decomposition product (data not shown). To test whether there was a bacteriostatic effect under these conditions, bacteria were added to BHI media and bacterial growth was tracked for 20 h following HOSCN incubation (Fig. 3D). No difference in bacterial growth between treated and control bacteria was observed.
As S. pneumoniae were highly resistant to HOSCN-mediated killing and a bacteriostatic effect was not observed, we investigated whether S. pneumoniae were metabolizing HOSCN faster than P. aeruginosa. Under the conditions tested, HOSCN consumption by either species was not different compared with that in buffer alone (Fig. 3E). This lack of HOSCN consumption is similar to the inability of S. pneumoniae to consume H2O2 (Fig. 1), and is the reason why we report LC50 values for H2O2 and HOSCN (Table 3).
TABLE 3.
LC/LD50 for killing of S. pneumoniae and P. aeruginosa by H2O2, HOCl, and HOSCN
| Strain | Value (95% confidence interval) | ||
|---|---|---|---|
| H2O2 (mM) | HOCl (nmol/107 bacteria) | HOSCN (mM) | |
| S. pneumoniae SP264 | 3.80 (3.34, 4.34) | 0.57 (0.52, 0.63) | >0.8 |
| S. pneumoniae D39 | 3.74 (3.40, 4.12) | 0.47 (0.42, 0.52) | >0.8 |
| P. aeruginosa PAO1 | 5.28 (4.44, 6.20) | 0.30 (0.27, 0.33) | 0.21 (0.17, 0.26) |
S. pneumoniae produce H2O2 but remain viable in the presence of NETs.
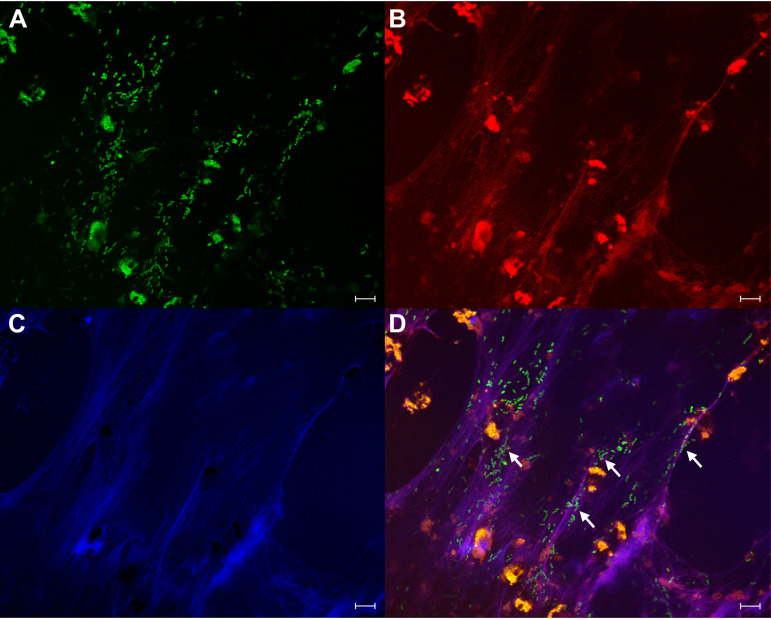
To monitor the interaction of S. pneumoniae with NETs, we fluorescein-5-isothiocyanate (FITC)-labeled S. pneumoniae SP264 and incubated them with pre-formed NETs. The sample was fixed and labeled with an antibody for MPO and DNA was stained with Hoechst. Bacteria were co-localized with strands of released DNA on which MPO was present (Fig. 4).
FIG 4.
S. pneumoniae SP264 co-localize with MPO on NETs. Neutrophils (1 × 106/mL) were stimulated on glass coverslips with 20 nM PMA to form NETs. After 4 h, FITC-labeled S. pneumoniae SP264 (2 × 107/mL) were added to NETs and co-incubated for 60 min. Cells were fixed, permeabilized, and then immunolabelled with an antibody to MPO and DNA-stained. (A) FITC-labeled S. pneumoniae SP264 (green), (B) Hoechst 33342-stained DNA (blue), (C) antibody-labeled MPO (red), and (D) overlay of images A to C. Arrows show co-localization of bacteria, NETs, and MPO. Scale bars are 20 μm. Images are representative of two independent experiments and 10 fields of view.
We examined the survival of S. pneumoniae in the presence of NETs by incubating SP264 for 60 min with neutrophils that had formed NETs. No loss of bacterial viability was observed in the presence of NETs compared with buffer alone (1.4 × 107 ± 1.9 × 106 vs 1.0 × 107 ± 1.4 × 106 CFU/mL, respectively, P = 0.12).
Endonuclease activity has been reported for several strains of S. pneumoniae, enabling them to escape from NETs and in turn survive NET-mediated killing (33, 34). Our early microscopy data indicated that S. pneumoniae SP264 does not degrade NETs. To examine this further, SP264 was incubated for 30 min with pre-formed NETs. The cell impermeable DNA dye Sytox green was used to compare NETs in the presence of bacteria to that of NETs alone. No significant difference in relative fluorescence units between NET samples with or without bacteria was observed (945.1 ± 221.3 vs 1047 ± 282.5 RFU, respectively, P = 0.8), indicating that strain SP264 does not possess the ability to degrade NETs. The addition of DNase in a single positive control experiment abolished the fluorescence signal (data not shown).
As the bacteria were localized on, and not degrading NETs, we assessed the ability of NET-associated MPO to generate HOCl from bacterially produced H2O2 using a taurine chloramine assay. In the presence of NETs, S. pneumoniae produced 14.04 ± 3.47 nmol of H2O2/107 bacteria on NETs and generated 9.77 ± 1.60 nmol of HOCl/107 bacteria (Table 2). The addition of catalase, methionine, or ABAH to bacteria associated with NETs significantly reduced HOCl generation to control levels (data not shown).
NETs protect S. pneumoniae from bactericidal levels of HOCl.
Given that HOCl was generated when S. pneumoniae were associated with NETs, yet no loss in viability was observed, we considered that NETs may protect S. pneumoniae against secondary oxidants. Due to its highly reactive nature, the generated HOCl will likely react with NETs and neutrophil debris before reaching the bacteria. To examine this further, the survival of SP264 associated with NETs was examined when exogenous HOCl was added. A much greater concentration of HOCl was needed to generate a killing response when associated with NETs, compared to when in PBS alone (Fig. 5). The LD50 for HOCl when SP264 were associated with NETs was 43.82 nmol/107 bacteria, 77-fold higher than the LD50 in PBS alone (0.57 nmol/107 bacteria). This result indicates that HOCl generated while S. pneumoniae are adherent to NETs will be an ineffective bactericidal agent because it is scavenged by extracellular proteins.
FIG 5.
S. pneumoniae SP264 survival on NETs when exposed to exogenous HOCl. S. pneumoniae SP264 (1 × 108/mL) were added to NETs (from 1 × 106 neutrophils/mL) then treated with increasing doses of HOCl. Bacterial viability was assessed after 5 min. Results are the mean ± SEM of three independent experiments fitted with a [HOCl] versus normalized response – variable slope curve.
DISCUSSION
Research into the oxidative stress response of S. pneumoniae has focused on its ability to generate and resist high concentrations of H2O2 (5–7). The role played by the secondary oxidants HOCl and HOSCN, that will be generated by host heme enzymes at S. pneumoniae infection sites, has been largely overlooked. In the present study, we investigated the ability of S. pneumoniae to survive exposure to these oxidants added in a bolus or generated by MPO and LPO systems. S. pneumoniae were killed readily upon incubation with free MPO in chloride-containing buffer. However, if physiological levels of SCN− were added to the system or the MPO was associated with NETs, bacterial survival was significantly improved.
The rate of H2O2 production of S. pneumoniae ranged from 0.40 to 0.68 nmol/min/107 bacteria among the four strains tested. When adjusted for size, this is approximately a third of the rate at which neutrophils produce H2O2 to kill bacteria. It has been proposed that the H2O2 generated by S. pneumoniae allows them to outcompete surrounding bacteria (5). Here, we found that S. pneumoniae and P. aeruginosa responded similarly to H2O2 exposure, but differed in their susceptibility to oxidants derived from H2O2. We therefore contend that, in addition to resisting H2O2 itself, defenses against these secondary oxidants may give S. pneumoniae a survival advantage.
Concentrations of SCN− in the lung airway fluid have been reported to reach up to 650 μM (8). As such, HOSCN will be the main oxidant which S. pneumoniae are exposed to in the lungs. Here, we found that S. pneumoniae are resistant to HOSCN. HOSCN-mediated killing has been previously examined in oral bacteria from the Streptococcus genus (35–41). In many of these strains, HOSCN protected the bacteria from H2O2- or HOCl-mediated killing (35, 36, 39, 41). The formation of HOSCN is also thought to have a protective effect on mammalian cells through the removal of harmful H2O2 and HOCl (42–44). Tenovuo et al. (35) examined the viability of Streptococcus mutans in the presence of LPO-SCN−-H2O2, MPO-SCN−-H2O2 and MPO-Cl−-H2O2 systems. While the MPO-Cl−-H2O2 system killed S. mutans at normal salivary concentrations of Cl−, the addition of SCN− at normal salivary concentrations protected the bacteria. Additionally, both the LPO-SCN−-H2O2 and MPO-SCN−-H2O2 systems did not affect bacterial viability. These findings are consistent with our data for both S. pneumoniae HOSCN resistance and the sensitivity to MPO-Cl−-H2O2 but not LPO-SCN−-H2O2 or MPO-SCN−-H2O2 systems. In contrast, Gingerich et al. (45) reported killing of S. pneumoniae by HOSCN when using a LPO-SCN−-H2O2 system with a long incubation period. It is possible that altering experimental conditions such as substrate availability or increasing oxidant exposure time, could result in bacterial killing.
The protection of S. pneumoniae by low concentrations of SCN− in our MPO-Cl−-H2O2 experiment was greater than we expected. Based on H2O2 measurements, up to 100 μM H2O2 could be generated over this time (not accounting for the loss of bacterial viability). If HOSCN was formed from all of the SCN− added, this accounts for 20 μM potential H2O2, leaving another 80 μM that could be converted to HOCl (16 nmol/107 bacteria). This far greater than the 1.3 nmol/107 bacteria required for complete bacterial killing. It therefore appears that another mechanism is providing a survival advantage to the bacteria. This could involve HOSCN inhibiting MPO by converting it to inactive compound II (46) or scavenging HOCl (47). In addition, when HOSCN reacts with thiols to form sulfenic acids, SCN− will be regenerated (48) so that it can act catalytically in the system rather than stoichiometrically.
The level of SCN− present in bodily fluids has been shown to vary significantly with dietary and smoking choices, with increased SCN− concentrations reported in smokers (17–19). Studies investigating the risk of respiratory tract and ear infections have reported an increased risk of infection in individuals who smoke actively or passively (49–53). Indeed, smoking is a leading risk factor for pneumococcal disease (49, 50). Increased carriage of S. pneumoniae in children with smoking mothers and the mothers themselves has also been reported (54). While cigarettes contain far more than just SCN−, the resistance of S. pneumoniae to HOSCN may contribute to the increased prevalence of infections reported.
During infection, neutrophils are recruited to infection sites where they employ both oxidative and non-oxidative mechanisms to kill invading organisms upon ingestion or degranulation (55, 56). An additional method employed by neutrophils is the formation of NETs, comprised of neutrophil DNA and proteins that can trap invasive organisms, and are thought to contribute to neutrophil antimicrobial activity (57). We found that S. pneumoniae viability and H2O2 production are unaffected by NETs, even though the NETs contain MPO which generated HOCl. The HOCl generated from bacterially produced H2O2 would be sufficient for bacterial killing in buffer alone, but in the presence of NETs, significantly higher concentrations were required. In fact, the LD50 for HOCl was 77-fold higher when bacteria were exposed on NETs than when bacteria were exposed in PBS.
While S. pneumoniae remained viable on NETs, bacterial killing on NETs has previously been reported with S. aureus (23). As S. aureus does not generate H2O2, the addition of H2O2 was required to generate a response. NET killing was reduced when ABAH or methionine was added, suggesting that the killing was through MPO generation of HOCl. However, much higher concentrations of HOCl would have been made in their system (≥1,000 nmol/107 bacteria, assuming that the H2O2 added was converted to HOCl at a 1:1 ratio by MPO) than what was generated in our experiment (≥57 nmol/107 bacteria). The LD50 of S. aureus in their experiment was more than 870-fold higher than what is required when S. aureus are exposed to HOCl in HBSS buffer alone (31, 58). In both systems, with either S. pneumoniae or S. aureus associated with NETs, a far greater concentration of HOCl was required to observe bacterial killing than in buffer alone. This suggests that the HOCl in the NET system is highly reactive and oxidants are being scavenged by NET material before reaching the trapped bacteria. As such, HOCl appears to be an ineffective bactericidal agent when bacteria are associated with NETs.
This study identifies the importance of the oxidant HOSCN in S. pneumoniae infections. While S. pneumoniae are sensitive to HOCl-mediated killing in buffer, they are resistant to HOSCN-mediated killing and can survive H2O2 and HOCl generation while associated with NETs. As SCN− is present in biological fluids along with the peroxidase enzymes LPO and MPO, HOSCN will be generated at infection sites from available H2O2. We conclude that the real competitive advantage of S. pneumoniae in this niche is its ability to resist the effects of HOSCN. An investigation into the protective strategies employed by S. pneumoniae against HOSCN may therefore reveal novel antimicrobial strategies for this deadly pathogen.
MATERIALS AND METHODS
Reagents.
Hanks’ balanced salt solution (HBSS) with sodium bicarbonate and without phenol red, phosphate-buffered saline (PBS), LPO from bovine milk (ϵ412 = 112,000 M−1 cm−1 [59]), horseradish peroxidase type IV, catalase from bovine liver, phorbol 12-myristate 13-acetate (PMA), paraformaldehyde, Tween 20, monosodium phosphate (NaH2PO4), disodium phosphate (Na2HPO4), sodium iodide, sodium acetate, sodium thiocyanate, diphenyleneiodonium chloride (DPI), potassium hydroxide (KOH), taurine, 4-(2-hydroxyethyl) piperazine-1-ethanesulfonic acid (HEPES), fetal bovine serum (FBS) (heat inactivated at 56°C) trypan blue, ethylenediaminetetraacetic acid (EDTA), l-methionine, and DNase I were purchased from Sigma-Aldrich (Merck, Darmstadt, Germany). MPO from human neutrophils (EC 1.11.2.2.) was obtained from Planta Natural Products (Vienna, Austria) (ϵ430 = 89,000 M−1 cm−1 per heme [60]). 4-Aminobenzoic acid hydrazide (ABAH) was from Fluka Chemicals (Buchs, Germany). Triton X-100 was purchased from Bio-Rad laboratories (Hercules, USA). Sytox Green and fluorescein-5-isothiocyanate (FITC) were from Molecular Probes (Eugene, USA). Amplex UltraRed and Hoechst 33342 were from Invitrogen (Waltham, MA, USA). Goat anti-rabbit Alexa Fluor DyLight 594 was from Abcam (Cambridge, UK) and polyclonal RAMPO (rabbit anti-human MPO) antibody was produced in-house (Christchurch, New Zealand), as described by Khalilova et al. (61). Hydrogen peroxide (30%) (H2O2, ε240 = 43.6 M−1 cm−1 [62]) and 3,3′,5,5′-tetramethylbenzidine (TMB) were from LabServ (Melbourne, Australia) and Fluka (Munich, Germany), respectively. Hypochlorous acid (HOCl, ε292 = 350 M−1 cm−1 for OCl− at pH 12 [63]) was purchased as a chlorine bleach solution from Household and Body Care (Auckland, New Zealand). Potassium iodide (I−) was from J.T. Baker Chemical Co. (Phillipsburg, USA). Roswell Park Memorial Institute medium (RPMI) without phenol red, and bovine serum albumin (BSA) were from Gibco (Waltham, USA). Heparin (5,000 U/mL) was from Pfizer Inc. (New York City, USA) and dextran (Mw 200,000 to 300,000 Da) was from MP Biomedicals Inc. (Santa Ana, USA). Ficoll and endotoxin-free water were from GE Healthcare (Chicago, USA) and Fluoromount-G was from eBioscience (San Diego, USA). TNB (2-nitro-5-thiobenzoate) was prepared from 5,5′-dithiobis-(2-nitrobenzoic acid) (DTNB, Sigma-Aldrich) through alkaline hydrolysis as described previously (11).
Bacterial culture and processing.
S. pneumoniae strain SP264, serotype 23F, (ATCC 700669) was obtained from the New Zealand reference culture collection. Strain D39, serotype 2, (NCTC 7466) was a kind gift from James Paton (University of Adelaide), who obtained it from the National Collection of Type Cultures (UK). Clinical isolate strains from patient’s lungs (Lu1 and Lu4) were obtained from Canterbury Health Laboratories (New Zealand). Strain CIP 104340 (ATCC 49619) and P. aeruginosa strain PAO1 (ATCC 47085) were obtained from the American Type Culture Collection (USA).
Bacteria were routinely cultured on Columbian sheep blood agar plates (Fort Richard Laboratories, Auckland, New Zealand). For experiments, S. pneumoniae were grown overnight (16 h to18 h) at 37°C with 5% CO2 without shaking in Brain Heart Infusion (BHI) media (Oxoid, Thermo Fisher Scientific, Waltham, USA), then diluted in fresh media and grown to log-phase (A620 of 0.4 to 0.7). P. aeruginosa were grown to stationary phase overnight at 37°C with shaking (200 rpm) in lysogeny broth (Millers, Thermo Fisher Scientific, Waltham, USA). Bacteria were washed twice in PBS (12,000 × g, 5 min) and suspended in HBSS. P. aeruginosa were subjected to an additional slow spin (100 × g, 5 min) to remove biofilm and bacterial aggregates from the suspension. The bacterial concentration was determined by absorbance measurements (A620 for S. pneumoniae, A550 for P. aeruginosa) using previously determined values from a standard curve.
S. pneumoniae H2O2 production.
H2O2 production for each S. pneumoniae strain was determined using an Amplex UltraRed fluorometric assay based on the method described by Mishin et al. (64), where in the presence of horseradish peroxidase, H2O2 oxidizes Amplex UltraRed to the fluorescent compound resorufin. Fluorescence detection by a plate reader and subsequent analysis against standards allows for H2O2 production over time to be calculated. Bacteria were diluted to approximately 1 × 107/mL and added to a clear 96-well plate alongside an H2O2 standard curve with a concentration range from 0 to 40 μM H2O2. Amplex UltraRed (100 μM) and horseradish peroxidase (200 mU/mL) were added to phosphate buffer (50 mM phosphate, pH 7.4) and added at a 1:1 ratio to bacteria/H2O2 standard wells. A PolarStar Galaxy (BMG Labtech, Ortenberg, Germany) plate reader or a BioTek Synergy™ Neo2 Hybrid Multi-Mode Microplate Reader (Winooski, VT, USA) were used to measure fluorescence at 37°C with fluorescence readings taken every 2 min (Ex/Em 544/590 nm or Ex/Em 530/590, respectively) for 30 min. Bacteria from the same stock sample were serially diluted, plated, and counted the following day to determine the bacterial concentration in each assay.
Neutrophil isolation.
Neutrophil isolation was carried out under sterile conditions using endotoxin-free solutions using an adapted method by Nauseef et al. (65). Fresh venous blood was collected from healthy volunteers with informed consent, approved by the Southern Health and Disability Ethics Committee NZ (URA/06/12/083) and mixed with heparin (10 U/mL). Our studies abide by the Declaration of Helsinki principles. Briefly, blood was diluted by one third with PBS, then dextran (1%) sedimentation was used to isolate white blood cells which were separated by density gradient centrifugation in Ficoll. The remaining red blood cells were removed by water lysis and the resulting neutrophil pellet was suspended in RPMI containing 2% FBS and 10 mM HEPES. Neutrophil purity (≥95%) was assessed by flow cytometry using a Cytomics FC 500 (Beckman Coulter Inc., USA). The concentration and viability of the neutrophils were calculated by hemocytometer counts and trypan blue-exclusion, respectively.
Formation of NETs with PMA.
To prepare NETs, neutrophils (1 × 106/mL) were stimulated with PMA (20 nM) then immediately added to a 12-, 24-, or 96-well plate and incubated at 37°C with 5% CO2 for 4 h. After incubation, the plates were centrifuged (200 × g, 10 min) before the media was carefully removed and replaced with bacterial samples in HBSS, then centrifuged (800 × g, 5 min) and incubated.
Assessing NET formation.
The cell-impermeable DNA-binding dye Sytox Green was used to assess NET formation. PMA-stimulated neutrophils, an unstimulated control, and a medium only blank were added to a black 96-well plate and incubated for 4 h at 37°C with 5% CO2. Following incubation, Sytox Green (3 μM) was added to all wells and incubated for 5 min. Fluorescence was read in a PolarStar Galaxy plate reader (Ex/Em 485/520 nm). Media fluorescence values were subtracted from all sample wells.
H2O2 production by S. pneumoniae when associated with NETs.
S. pneumoniae (1 × 107/mL) were added to pre-formed NETs in a clear 96-well plate and H2O2 production was measured by Amplex UltraRed as described above. In some wells, the Amplex UltraRed reaction mix was added after 30 min, not straight away. To prevent neutrophil generated H2O2 from contributing to the observed fluorescence, 10 μM DPI was added.
Immunofluorescence microscopy of NETs- and FITC-labeled S. pneumoniae.
Neutrophils were stimulated to form NETs with PMA (20 nM) on glass coverslips in a 24-well plate. S. pneumoniae were washed and suspended in 0.1 M sodium carbonate buffer. FITC (11 μM) was added to the bacteria and incubated in the dark for 30 min with end-over-end rotation. Labeled bacteria were then washed twice, diluted to 2 × 107/mL in HBSS and added to NETs. The plate was then incubated for 1 h at 37°C with 5% CO2. After incubation, the plate was centrifuged (500 × g, 5 min).
Cells were fixed in paraformaldehyde (4%) at 20 to 22°C for 20 min, coverslips were washed in PBS, and the cells were permeabilized by incubating in 0.5% Triton X-100 for 5 min. After washing, samples were blocked overnight with 10% BSA in PBS with 0.2% Tween 20 (PBST), then incubated with primary antibody (polyclonal rabbit anti-MPO, RAMPO), diluted 1 in 10 with 1% BSA in PBST for 1 h at 37°C or overnight at 4°C. Secondary antibody only controls were incubated in diluent alone without RAMPO. Samples were washed with PBST, then incubated with secondary antibody (goat anti-rabbit Alexa Fluor DyLight 594, diluted 1 in 5,000 with 1% BSA in PBST) for 1 h at 37°C or overnight at 4°C. After washes in PBST, samples were stained with Hoechst (10 μg/mL) for 5 min, washed with MilliQ water and mounted in Fluoromount-G. Samples were viewed on a Zeiss Axio Imager wide-field fluorescence microscope (Jena, Germany). Images were taken in DAPI, FITC, and Cy3 channels. Zen Blue software (Zeiss) was used to capture and process images. To better observe NETs, the gamma factor was changed from 1 to 0.45, which is recommended for dim structures to allow clear visualization without oversaturation of bright features (66).
Survival of S. pneumoniae in the presence of NETs.
S. pneumoniae (1 × 107/mL) were added to pre-formed NETs in a 12-well plate and incubated for 1 h at 37°C with 5% CO2. DNase I (final concentration 100 U) was added to each well for 10 min at 37°C to degrade the NETs and release the bacteria. The reaction was stopped with EDTA (5 mM). Surviving bacteria were harvested by gentle scraping, then centrifuged (150 × g, 5 min) to pellet neutrophils. All samples were serially diluted in PBS, plated, and counted the following day.
S. pneumoniae NET-degradation activity.
The NET-degradation activity of S. pneumoniae was measured by a Sytox green plate assay. NETs were pre-formed and incubated in the presence or absence of 1 × 107/mL S. pneumoniae for 30 min. Sytox Green (3 μM) was added and fluorescence was measured. NET degradation was determined by a decrease in relative fluorescence units.
HOCl generation in the presence of S. pneumoniae and MPO.
A taurine chloramine assay was used to quantify HOCl generation based on the methods described by Kettle and Winterbourn (67) and Dypbukt et al. (68). TMB was used as the assay developer, which changes from colorless to blue upon oxidation. Bacteria were diluted to 5 × 107/mL and incubated in HBSS containing 5 mM taurine with or without 10 nM MPO for 30 min with end-over-end rotation at 37°C. Control treatments contained bacteria with either catalase (20 μg/mL), methionine (10 μM), or ABAH (10 μM). After incubation, samples were centrifuged (12,000 × g, 5 min) and supernatants (200 μL) transferred to a clear 96-well plate alongside taurine chloramine standards. TMB developer (1 mM TMB, 100 μM sodium iodide in 200 mM sodium acetate buffer, pH 5.4) was made and immediately added to each well to give a final concentration of 20 nM TMB. After 10 min at 20 to 22°C, the absorbance was read on either a VarioSkan Flash (Thermo Fisher Scientific) or MultiSkan Go (Thermo Fisher Scientific) plate reader at A650. The concentration of taurine chloramine in the samples was determined from the standard curve.
To measure HOCl generation when S. pneumoniae were incubated with NETs, S. pneumoniae (5 × 107/mL) were added to pre-formed NETs in 24-well plates in HBSS containing 5 mM taurine and incubated for 30 min at 37°C with 5% CO2. After incubation, the protocol was performed as without NETs.
MPO-mediated killing of S. pneumoniae.
Bacteria were diluted to 5 × 107/mL in HBSS. MPO (5 nM) was added and incubated at 37°C with 5% CO2 for up to 30 min. A sample was taken at time zero and after every 5 min. Each sample was immediately serially diluted in PBS containing methionine (1 mM), plated, and counted the following day. A sample without MPO was used as a control. In additional experiments, SCN− was added at varying concentrations (2 to 20 μM) with 10 nM MPO. A sample with 2 μM SCN− without MPO was used as an SCN− control.
LPO-mediated killing of S. pneumoniae.
Bacteria were diluted to 5 × 107/mL in HBSS, pH 6.8 before adding LPO to a final concentration of 10 nM, in the presence or absence of 800 μM SCN− or 100 μM I−. Samples with SCN− or I− alone were used as controls. After 30 min incubation at 37°C + 5% CO2, each sample was serially diluted in PBS, plated, and counted the following day.
S. pneumoniae and P. aeruginosa H2O2 sensitivity.
Bacteria were diluted to 5 × 107/mL in HBSS before adding H2O2 (2.5 to 25 mM). A catalase (20 μg/mL) control with 25 mM H2O2 was also included. Each sample was incubated for 30 min with end-over-end rotation at 37°C. After incubation, catalase (20 μg/mL) was added to remove any remaining H2O2. Each sample was serially diluted, plated, and counted the following day.
S. pneumoniae and P. aeruginosa HOCl sensitivity.
Bacteria were diluted to 1 × 109/mL (SP264 and D39) or 1 × 108/mL (PAO1) in PBS then warmed at 37°C for 10 min. A fresh 10 mM HOCl stock in PBS was used to make HOCl dilutions, which were added immediately to the bacteria at final concentrations of from 0.2 to 1.3 nmol/107 bacteria while vortexing. Bacteria were incubated for 15 min with end-over-end rotation at 37°C. After incubation, methionine (1 mM) was added to quench any remaining HOCl. Each sample was serially diluted, plated, and counted the following day.
To measure the sensitivity of S. pneumoniae to HOCl when associated with NETs, S. pneumoniae (1 × 108/mL) in PBS were added to pre-formed NETs in a 12-well plate. Reagent HOCl was diluted immediately before adding to each well. The plate was incubated for 5 min at 37°C with 5% CO2. After incubation, methionine (1 mM) was added to each sample to quench excess HOCl. The bacteria were harvested as in the NET survival experiment and were serially diluted, plated, and counted the following day.
S. pneumoniae and P. aeruginosa HOSCN sensitivity.
Bacteria were diluted to 2.5 × 105/mL in HBSS, pH 6.8. HOSCN was generated in the absence of bacteria from the reaction of LPO with SCN− and quantified by absorbance at A412, as described previously with modifications (11, 69). Briefly, H2O2 (0.75 mM H2O2) was added every minute in the dark for 4 min to phosphate buffer (10 mM, pH 6.6), LPO (100 μg/mL) and NaSCN (7.5 mM) (inverting gently after each addition), with HOSCN accumulating. LPO was removed by centrifugation in 10,000 MW cut-off filters (14,000 × g, 4°C). The concentration of HOSCN was determined using TNB and measuring the change in absorbance at A412, with a yellow to colorless change occurring upon TNB oxidation (ϵ412 = 14,100 M−1 cm−1). HOSCN was immediately diluted, added at final concentrations of 0.05 to 0.8 mM to bacteria, and incubated for 30 min with end-over-end rotation at 37°C. Each sample was then serially diluted, plated, and counted the following day. A control using a HOSCN stock solution that had been left to decompose overnight at 20 to 22°C was also included to test whether the observed effect was due to HOSCN or its decomposed products. HOSCN in the decomposed stock was measured before use and was found to be negligible. To determine whether HOSCN had a bacteriostatic effect on S. pneumoniae (SP264), additional experiments were carried out as above, but instead of plating the bacteria following HOSCN treatment, they were added to BHI media and bacterial growth was tracked over 20 h at 37°C with 5% CO2 using a BioTek Synergy Neo2 Hybrid Multi-Mode Microplate Reader (Winooski, VT, USA) at A620.
S. pneumoniae and P. aeruginosa HOSCN consumption.
Bacteria (SP264 and PAO1) were diluted to 5 × 105 CFU/mL in pH 6.8 HBSS and incubated with 0.5 mM HOSCN. HOSCN was also added to buffer alone. Samples were incubated for up to 30 min with end-over-end rotation at 37°C. At 15-min intervals, a sample of each treatment was taken and centrifuged to pellet bacteria (12,000 × g, 5 min). The HOSCN concentration in each sample was quantified using TNB. A sample of each bacteria was serially diluted, plated, and counted the following day to confirm bacterial numbers were similar across species and experiments.
Statistical analysis.
Graphs were plotted and statistical analysis tests were performed using GraphPad Prism 8.2.0. or 9.1.2. (San Diego, CA, USA). Tests carried out to determine significant differences between conditions are stated in figure/table legends where appropriate.
ACKNOWLEDGMENTS
We thank Nina Dickerhof for her critical reading of the manuscript. This research was supported by the Health Research Council of New Zealand (15/479), a University of Otago Doctoral Scholarship to H.L.S. and a University of Otago Postgraduate Publishing Bursary (Doctoral [H.L.S.] and Master’s [C.D.K.]).
We declare no conflicts of interest.
Contributor Information
Mark B. Hampton, Email: mark.hampton@otago.ac.nz.
Victor J. Torres, New York University School of Medicine
REFERENCES
- 1.Weiser JN, Ferreira DM, Paton JC. 2018. Streptococcus pneumoniae: transmission, colonization and invasion. Nat Rev Microbiol 16:355–367. 10.1038/s41579-018-0001-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tacconelli E, Carrara E, Savoldi A, Harbarth S, Mendelson M, Monnet DL, Pulcini C, Kahlmeter G, Kluytmans J, Carmeli Y, Ouellette M, Outterson K, Patel J, Cavaleri M, Cox EM, Houchens CR, Grayson ML, Hansen P, Singh N, Theuretzbacher U, Magrini N, WHO Pathogens Priority List Working Group . 2018. Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis 18:318–327. 10.1016/S1473-3099(17)30753-3. [DOI] [PubMed] [Google Scholar]
- 3.Yesilkaya H, Andisi VF, Andrew PW, Bijlsma JJ. 2013. Streptococcus pneumoniae and reactive oxygen species: an unusual approach to living with radicals. Trends Microbiol 21:187–195. 10.1016/j.tim.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 4.Tettelin H, Nelson KE, Paulsen IT, Eisen JA, Read TD, Peterson S, Heidelberg J, DeBoy RT, Haft DH, Dodson RJ, Durkin AS, Gwinn M, Kolonay JF, Nelson WC, Peterson JD, Umayam LA, White O, Salzberg SL, Lewis MR, Radune D, Holtzapple E, Khouri H, Wolf AM, Utterback TR, Hansen CL, McDonald LA, Feldblyum TV, Angiuoli S, Dickinson T, Hickey EK, Holt IE, Loftus BJ, Yang F, Smith HO, Venter JC, Dougherty BA, Morrison DA, Hollingshead SK, Fraser CM. 2001. Complete genome sequence of a virulent isolate of Streptococcus pneumoniae. Science 293:498–506. 10.1126/science.1061217. [DOI] [PubMed] [Google Scholar]
- 5.Pericone CD, Overweg K, Hermans PW, Weiser JN. 2000. Inhibitory and bactericidal effects of hydrogen peroxide production by Streptococcus pneumoniae on other inhabitants of the upper respiratory tract. Infect Immun 68:3990–3997. 10.1128/IAI.68.7.3990-3997.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pericone CD, Park S, Imlay JA, Weiser JN. 2003. Factors contributing to hydrogen peroxide resistance in Streptococcus pneumoniae include pyruvate oxidase (SpxB) and avoidance of the toxic effects of the Fenton reaction. J Bacteriol 185:6815–6825. 10.1128/JB.185.23.6815-6825.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spellerberg B, Cundell DR, Sandros J, Pearce BJ, Idänpään‐Heikkilä I, Rosenow C, Masure HR. 1996. Pyruvate oxidase, as a determinant of virulence in Streptococcus pneumoniae. Mol Microbiol 19:803–813. 10.1046/j.1365-2958.1996.425954.x. [DOI] [PubMed] [Google Scholar]
- 8.Wijkstrom-Frei C, El-Chemaly S, Ali-Rachedi R, Gerson C, Cobas MA, Forteza R, Salathe M, Conner GE. 2003. Lactoperoxidase and human airway host defense. Am J Respir Cell Mol Biol 29:206–212. 10.1165/rcmb.2002-0152OC. [DOI] [PubMed] [Google Scholar]
- 9.Chandler JD, Day BJ. 2012. Thiocyanate: a potentially useful therapeutic agent with host defense and antioxidant properties. Biochem Pharmacol 84:1381–1387. 10.1016/j.bcp.2012.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aune TM, Thomas EL. 1977. Accumulation of hypothiocyanite ion during peroxidase‐catalyzed oxidation of thiocyanate ion. Eur J Biochem 80:209–214. 10.1111/j.1432-1033.1977.tb11873.x. [DOI] [PubMed] [Google Scholar]
- 11.Nagy P, Jameson GN, Winterbourn CC. 2009. Kinetics and mechanisms of the reaction of hypothiocyanous acid with 5-thio-2-nitrobenzoic acid and reduced glutathione. Chem Res Toxicol 22:1833–1840. 10.1021/tx900249d. [DOI] [PubMed] [Google Scholar]
- 12.van der Vliet A. 2008. NADPH oxidases in lung biology and pathology: host defense enzymes, and more. Free Radic Biol Med 44:938–955. 10.1016/j.freeradbiomed.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Winterbourn CC, Kettle AJ, Hampton MB. 2016. Reactive oxygen species and neutrophil function. Annu Rev Biochem 85:765–792. 10.1146/annurev-biochem-060815-014442. [DOI] [PubMed] [Google Scholar]
- 14.van Dalen JC, Whitehouse WM, Winterbourn CC, Kettle JA. 1997. Thiocyanate and chloride as competing substrates for myeloperoxidase. Biochem J 327:487–492. 10.1042/bj3270487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hull J, Skinner W, Robertson C, Phelan P. 1998. Elemental content of airway surface liquid from infants with cystic fibrosis. Am J Respir Crit Care Med 157:10–14. 10.1164/ajrccm.157.1.9703045. [DOI] [PubMed] [Google Scholar]
- 16.Joris L, Dab I, Quinton PM. 1993. Elemental composition of human airway surface fluid in healthy and diseased airways. Am Rev Respir Dis 148:1633–1637. 10.1164/ajrccm/148.6_Pt_1.1633. [DOI] [PubMed] [Google Scholar]
- 17.Morgan PE, Pattison DI, Talib J, Summers FA, Harmer JA, Celermajer DS, Hawkins CL, Davies MJ. 2011. High plasma thiocyanate levels in smokers are a key determinant of thiol oxidation induced by myeloperoxidase. Free Radic Biol Med 51:1815–1822. 10.1016/j.freeradbiomed.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 18.Nedoboy P, Morgan P, Mocatta T, Richards A, Winterbourn C, Davies MJ. 2014. High plasma thiocyanate levels are associated with enhanced myeloperoxidase-induced thiol oxidation and long-term survival in subjects following a first myocardial infarction. Free Radic Res 48:1256–1266. 10.3109/10715762.2014.947286. [DOI] [PubMed] [Google Scholar]
- 19.Tsuge K, Kataoka M, Seto Y. 2000. Cyanide and thiocyanate levels in blood and saliva of healthy adult volunteers. J Health Science 46:343–350. 10.1248/jhs.46.343. [DOI] [Google Scholar]
- 20.Thomson E, Brennan S, Senthilmohan R, Gangell CL, Chapman AL, Sly PD, Kettle AJ, Balding E, Berry LJ, Carlin JB, Carzino R, de Klerk N, Douglas T, Foo C, Garratt LW, Hall GL, Harrison J, Kicic A, Laing IA, Logie KM, Massie J, Mott LS, Murray C, Parsons F, Pillarisetti N, Poreddy SR, Ranganathan SC, Robertson CF, Robins-Browne R, Robinson PJ, Skoric B, Stick SM, Sutanto EN, Williamson E, Fibrosis ARESTfC . 2010. Identifying peroxidases and their oxidants in the early pathology of cystic fibrosis. Free Radic Biol Med 49:1354–1360. 10.1016/j.freeradbiomed.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 21.Ashby MT, Carlson AC, Scott MJ. 2004. Redox buffering of hypochlorous acid by thiocyanate in physiologic fluids. J Am Chem Soc 126:15976–15977. 10.1021/ja0438361. [DOI] [PubMed] [Google Scholar]
- 22.Schultz J, Kaminker K. 1962. Myeloperoxidase of the leucocyte of normal human blood. I. Content and localization. Arch Biochem Biophys 96:465–467. 10.1016/0003-9861(62)90321-1. [DOI] [PubMed] [Google Scholar]
- 23.Parker H, Albrett AM, Kettle AJ, Winterbourn CC. 2012. Myeloperoxidase associated with neutrophil extracellular traps is active and mediates bacterial killing in the presence of hydrogen peroxide. J Leukoc Biol 91:369–376. 10.1189/jlb.0711387. [DOI] [PubMed] [Google Scholar]
- 24.Ramos-Kichik V, Mondragón-Flores R, Mondragón-Castelán M, Gonzalez-Pozos S, Muñiz-Hernandez S, Rojas-Espinosa O, Chacón-Salinas R, Estrada-Parra S, Estrada-García I. 2009. Neutrophil extracellular traps are induced by Mycobacterium tuberculosis. Tuberculosis (Edinb) 89:29–37. 10.1016/j.tube.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 25.Branzk N, Lubojemska A, Hardison SE, Wang Q, Gutierrez MG, Brown GD, Papayannopoulos V. 2014. Neutrophils sense microbe size and selectively release neutrophil extracellular traps in response to large pathogens. Nat Immunol 15:1017–1025. 10.1038/ni.2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Veras FP, Pontelli MC, Silva CM, Toller-Kawahisa JE, de Lima M, Nascimento DC, Schneider AH, Caetité D, Tavares LA, Paiva IM, Rosales R, Colón D, Martins R, Castro IA, Almeida GM, Lopes MIF, Benatti MN, Bonjorno LP, Giannini MC, Luppino-Assad R, Almeida SL, Vilar F, Santana R, Bollela VR, Auxiliadora-Martins M, Borges M, Miranda CH, Pazin-Filho A, da Silva LLP, Cunha LD, Zamboni DS, Dal-Pizzol F, Leiria LO, Siyuan L, Batah S, Fabro A, Mauad T, Dolhnikoff M, Duarte-Neto A, Saldiva P, Cunha TM, Alves-Filho JC, Arruda E, Louzada-Junior P, Oliveira RD, Cunha FQ. 2020. SARS-CoV-2-triggered neutrophil extracellular traps mediate COVID-19 pathology. J Exp Med 217:e20201129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arcanjo A, Logullo J, Menezes CCB, de Souza Carvalho Giangiarulo TC, dos Reis MC, de Castro GMM, da Silva Fontes Y, Todeschini AR, Freire-de-Lima L, Decoté-Ricardo D, Ferreira-Pereira A, Freire-de-Lima CG, Barroso SPC, Takiya C, Conceição-Silva F, Savino W, Morrot A. 2020. The emerging role of neutrophil extracellular traps in severe acute respiratory syndrome coronavirus 2 (COVID-19). Sci Rep 10:1–11. 10.1038/s41598-020-76781-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moorthy AN, Rai P, Jiao H, Wang S, Tan KB, Qin L, Watanabe H, Zhang Y, Teluguakula N, Chow VTK. 2016. Capsules of virulent pneumococcal serotypes enhance formation of neutrophil extracellular traps during in vivo pathogenesis of pneumonia. Oncotarget 7:19327–19340. 10.18632/oncotarget.8451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ashby LV, Springer R, Hampton MB, Kettle AJ, Winterbourn CC. 2020. Evaluating the bactericidal action of hypochlorous acid in culture media. Free Radic Biol Med 159:119–124. 10.1016/j.freeradbiomed.2020.07.033. [DOI] [PubMed] [Google Scholar]
- 30.Dickerhof N, Paton L, Kettle AJ. 2020. Oxidation of bacillithiol by myeloperoxidase-derived oxidants. Free Radic Biol Med 158:74–83. 10.1016/j.freeradbiomed.2020.06.009. [DOI] [PubMed] [Google Scholar]
- 31.Chapman AL, Hampton MB, Senthilmohan R, Winterbourn CC, Kettle AJ. 2002. Chlorination of bacterial and neutrophil proteins during phagocytosis and killing of Staphylococcus aureus. J Biol Chem 277:9757–9762. 10.1074/jbc.M106134200. [DOI] [PubMed] [Google Scholar]
- 32.Bonvillain RW, Painter RG, Ledet EM, Wang G. 2011. Comparisons of resistance of CF and non-CF pathogens to hydrogen peroxide and hypochlorous acid oxidants in vitro. BMC Microbiol 11:112. 10.1186/1471-2180-11-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beiter K, Wartha F, Albiger B, Normark S, Zychlinsky A, Henriques-Normark B. 2006. An endonuclease allows Streptococcus pneumoniae to escape from neutrophil extracellular traps. Curr Biol 16:401–407. 10.1016/j.cub.2006.01.056. [DOI] [PubMed] [Google Scholar]
- 34.Jhelum H, Sori H, Sehgal D. 2018. A novel extracellular vesicle-associated endodeoxyribonuclease helps Streptococcus pneumoniae evade neutrophil extracellular traps and is required for full virulence. Sci Rep 8:7985. 10.1038/s41598-018-25865-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tenovuo J, Anttilla O, Lumikari M, Sievers G. 1988. Antibacterial effect of myeloperoxidase against Streptococcus mutans. Oral Microbiol Immunol 3:68–71. 10.1111/j.1399-302x.1988.tb00084.x. [DOI] [PubMed] [Google Scholar]
- 36.Carlsson J, Iwami Y, Yamada T. 1983. Hydrogen peroxide excretion by oral streptococci and effect of lactoperoxidase-thiocyanate-hydrogen peroxide. Infect Immun 40:70–80. 10.1128/iai.40.1.70-80.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oram J, Reiter B. 1966. The inhibition of streptococci by lactoperoxidase, thiocyanate and hydrogen peroxide. The effect of the inhibitory system on susceptible and resistant strains of group N streptococci. Biochem J 100:373–381. 10.1042/bj1000373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lumikari M, Soukka T, Nurmio S, Tenovuo J. 1991. Inhibition of the growth of Streptococcus mutans, Streptococcus sobrinus and Lactobacillus casei by oral peroxidase systems in human saliva. Arch Oral Biol 36:155–160. 10.1016/0003-9969(91)90078-9. [DOI] [PubMed] [Google Scholar]
- 39.Thomas EL, Milligan TW, Joyner RE, Jefferson MM. 1994. Antibacterial activity of hydrogen peroxide and the lactoperoxidase-hydrogen peroxide-thiocyanate system against oral streptococci. Infect Immun 62:529–535. 10.1128/iai.62.2.529-535.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thomas E, Pera K, Smith K, Chwang A. 1983. Inhibition of Streptococcus mutans by the lactoperoxidase antimicrobial system. Infect Immun 39:767–778. 10.1128/iai.39.2.767-778.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Adamson M, Carlsson J. 1982. Lactoperoxidase and thiocyanate protect bacteria from hydrogen peroxide. Infect Immun 35:20–24. 10.1128/iai.35.1.20-24.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chandler JD, Nichols DP, Nick JA, Hondal RJ, Day BJ. 2013. Selective metabolism of hypothiocyanous acid by mammalian thioredoxin reductase promotes lung innate immunity and antioxidant defense. J Biol Chem 288:18421–18428. 10.1074/jbc.M113.468090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu Y, Szép S, Lu Z. 2009. The antioxidant role of thiocyanate in the pathogenesis of cystic fibrosis and other inflammation-related diseases. Proc Natl Acad Sci USA 106:20515–20519. 10.1073/pnas.0911412106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wagner BA, Reszka KJ, McCormick ML, Britigan BE, Evig CB, Patrick Burns C. 2004. Role of thiocyanate, bromide and hypobromous acid in hydrogen peroxide-induced apoptosis. Free Radic Res 38:167–175. 10.1080/10715760310001643302. [DOI] [PubMed] [Google Scholar]
- 45.Gingerich AD, Doja F, Thomason R, Tóth E, Bradshaw JL, Douglass MV, McDaniel LS, Rada B. 2020. Oxidative killing of encapsulated and nonencapsulated Streptococcus pneumoniae by lactoperoxidase-generated hypothiocyanite. PLoS One 15:e0236389. 10.1371/journal.pone.0236389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van Dalen CJ, Kettle AJ. 2001. Substrates and products of eosinophil peroxidase. Biochem J 358:233–239. 10.1042/0264-6021:3580233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ashby MT. 2012. Hypothiocyanite. Adv Inorg Chem 64:263–303. [Google Scholar]
- 48.Thomas EL, Aune TM. 1978. Lactoperoxidase, peroxide, thiocyanate antimicrobial system: correlation of sulfhydryl oxidation with antimicrobial action. Infect Immun 20:456–463. 10.1128/iai.20.2.456-463.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nuorti JP, Butler JC, Farley MM, Harrison LH, McGeer A, Kolczak MS, Breiman RF, Team ABCS. 2000. Cigarette smoking and invasive pneumococcal disease. N Engl J Med 342:681–689. 10.1056/NEJM200003093421002. [DOI] [PubMed] [Google Scholar]
- 50.Jover F, Cuadrado J-M, Andreu L, Martínez S, Cañizares R, de la Tabla VO, Martin C, Roig P, Merino J. 2008. A comparative study of bacteremic and non-bacteremic pneumococcal pneumonia. Eur J Intern Med 19:15–21. 10.1016/j.ejim.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 51.Jones LL, Hassanien A, Cook DG, Britton J, Leonardi-Bee J. 2012. Parental smoking and the risk of middle ear disease in children: a systematic review and meta-analysis. Arch Pediatr Adolesc Med 166:18–27. 10.1001/archpediatrics.2011.158. [DOI] [PubMed] [Google Scholar]
- 52.Bakoula CG, Kafrista Y, Kavadias GD, Lazopoulou DD, Theodoridou MC, Matsaniotis N, Maravelias K. 1995. Objective passive-smoking indicators and respiratory morbidity in young children. Lancet 346:280–281. 10.1016/S0140-6736(95)92167-2. [DOI] [PubMed] [Google Scholar]
- 53.Almirall J, González CA, Balanzó X, Bolíbar I. 1999. Proportion of community-acquired pneumonia cases attributable to tobacco smoking. Chest 116:375–379. 10.1378/chest.116.2.375. [DOI] [PubMed] [Google Scholar]
- 54.Greenberg D, Givon-Lavi N, Broides A, Blancovich I, Peled N, Dagan R. 2006. The contribution of smoking and exposure to tobacco smoke to Streptococcus pneumoniae and Haemophilus influenzae carriage in children and their mothers. Clin Infect Dis 42:897–903. 10.1086/500935. [DOI] [PubMed] [Google Scholar]
- 55.Hampton MB, Kettle AJ, Winterbourn CC. 1998. Inside the neutrophil phagosome: oxidants, myeloperoxidase, and bacterial killing. Blood 92:3007–3017. [PubMed] [Google Scholar]
- 56.Nauseef WM. 2007. How human neutrophils kill and degrade microbes: an integrated view. Immunol Rev 219:88–102. 10.1111/j.1600-065X.2007.00550.x. [DOI] [PubMed] [Google Scholar]
- 57.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A. 2004. Neutrophil extracellular traps kill bacteria. Science 303:1532–1535. 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 58.Coker MS, Forbes LV, Plowman-Holmes M, Murdoch DR, Winterbourn CC, Kettle AJ. 2018. Interactions of staphyloxanthin and enterobactin with myeloperoxidase and reactive chlorine species. Arch Biochem Biophys 646:80–89. 10.1016/j.abb.2018.03.039. [DOI] [PubMed] [Google Scholar]
- 59.Paul KG, Ohlsson PI. 1985. The chemical structure of lactoperoxidase. p 15–29. In Pruitt K.M., Tenovuo J.O. (ed), The lactoperoxidase system, chemistry and biological significance. Marcel Dekker, New York. [Google Scholar]
- 60.Odajima T, Yamazaki I. 1970. Myeloperoxidase of the leukocyte of normal blood. I. Reaction of myeloperoxidase with hydrogen peroxide. Biochimica et Biophysica Acta (BBA)-Enzymology 206:71–77. 10.1016/0005-2744(70)90083-5. [DOI] [PubMed] [Google Scholar]
- 61.Khalilova IS, Dickerhof N, Mocatta TJ, Bhagra CJ, McClean DR, Obinger C, Kettle AJ. 2018. A myeloperoxidase precursor, pro-myeloperoxidase, is present in human plasma and elevated in cardiovascular disease patients. PLoS One 13:e0192952. 10.1371/journal.pone.0192952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Beers RF, Sizer IW. 1952. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem 195:133–140. [PubMed] [Google Scholar]
- 63.Morris JC. 1966. The acid ionization constant of HOCl from 5 to 35. J Phys Chem 70:3798–3805. 10.1021/j100884a007. [DOI] [Google Scholar]
- 64.Mishin V, Gray JP, Heck DE, Laskin DL, Laskin JD. 2010. Application of the Amplex red/horseradish peroxidase assay to measure hydrogen peroxide generation by recombinant microsomal enzymes. Free Radic Biol Med 48:1485–1491. 10.1016/j.freeradbiomed.2010.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nauseef WM. 2014. Isolation of human neutrophils from venous blood, p 13–18, In Quinn MT, DeLeo FR (ed), Neutrophil methods and protocols. Springer, New York. [DOI] [PubMed] [Google Scholar]
- 66.Brown CM. 2007. Fluorescence microscopy-avoiding the pitfalls. J Cell Sci 120:1703–1705. 10.1242/jcs.03433. [DOI] [PubMed] [Google Scholar]
- 67.Kettle AJ, Winterbourn CC. 1994. Assays for the chlorination activity of myeloperoxidase. Methods Enzymol 233:502–512. [DOI] [PubMed] [Google Scholar]
- 68.Dypbukt JM, Bishop C, Brooks WM, Thong B, Eriksson H, Kettle AJ. 2005. A sensitive and selective assay for chloramine production by myeloperoxidase. Free Radic Biol Med 39:1468–1477. 10.1016/j.freeradbiomed.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 69.Bozonet SM, Scott-Thomas AP, Nagy P, Vissers MC. 2010. Hypothiocyanous acid is a potent inhibitor of apoptosis and caspase 3 activation in endothelial cells. Free Radic Biol Med 49:1054–1063. 10.1016/j.freeradbiomed.2010.06.028. [DOI] [PubMed] [Google Scholar]