ABSTRACT
Neutrophils are capable of extruding neutrophil extracellular traps (NETs), a network of granule proteins and chromatin material, upon activation. NETs provide defense against extracellular microbes, but histones in NETs can also induce cytotoxicity and activate inflammatory responses. The relevance of NETs to bacterial pneumonias is beginning to be defined. In the present study, we found that the extracellular concentration of citrullinated histone H3, a component of NETs, was elevated in bronchoalveolar lavage fluid recovered from mice with diverse bacterial pneumonias and correlated with neutrophil infiltration and cell death in the lungs as well as levels of H4. Because the histone H4 component of NETs is sufficient to stimulate inflammation, we tested its effects in the air spaces of the lungs. Recombinant histone H4 in the noninflamed lung produced only modest effects, but in the setting of neutrophilic inflammation, H4 substantially increased pulmonary neutrophils, NETs, necrosis, and edema. However, blockade of histone H4 with a monoclonal antibody during pneumonia did not significantly alter measures of lung damage. Taken together, these results implicate NETs and extracellular histone H4 in exacerbating the lung injury resulting from bacterial pneumonia.
KEYWORDS: histone, H4, neutrophil extracellular traps, pneumonia, NETs
INTRODUCTION
Neutrophils, or polymorphonuclear leukocytes (PMNs), have long been known to be the dominant cellular infiltrate early in the alveolar spaces during bacterial pneumonia (1). By the end of the 20th century, neutrophils were recognized to perform a variety of effector immune functions and contain a variety of antimicrobial proteins in granules (2). More recently, it was discovered that as part of the response to infection, neutrophils were capable of extruding neutrophil extracellular traps (NETs), complexes of chromatin and granule proteins, in order to trap microbes and locally concentrate defense proteins such as myeloperoxidase (3). Although this discovery of NETs elucidated a role for these fiber-like structures in defense, NETs were soon found to play a role in pathological complications of infections, such as sepsis (4). NETs also are involved in the pathology of a variety of noninfectious diseases, such as vasculitis (5), rheumatoid arthritis (6), lupus (7), and thrombus formation (8). NETs correlate with lung injury from both systemic and local immune activation and are implicated across a variety of contexts in the lung, such as transfusion-related acute lung injury, trauma-associated lung injury, acute respiratory distress syndrome (ARDS), lung graft dysfunction, lung injury in response to viral infections including coronavirus disease 2019 (COVID-19), and ventilator-associated pneumonia (9–16).
Both the lysine-rich (H1, H2A, and H2B) and arginine-rich (H3 and H4) histones are components of NETs. Similar to NETs, the effects of histones were first noted to play a role in defense against microbes but have since been found to have deleterious effects on the host as well. A mixture of calf thymus histones can induce lung injury in vivo, and H4 can induce cytotoxicity of murine pneumocytes in vitro (17). In addition, H4 induces smooth muscle cell lysis that contributes to the inflammation in atherosclerosis, involving a positive feedback loop with neutrophil recruitment and extrusion of NETs (18). Finally, H4 is capable of directly activating neutrophils in vitro, producing membrane permeabilization, degranulation, and adhesion independent of Toll-like receptors (TLRs) (19). Thus, NETs and H4 specifically can stimulate multiple pathways of inflammatory injury.
Given their important antibacterial and possible deleterious effects, NETs may modify the balance between lung defense and inflammation-induced pathology for respiratory diseases (20). Investigations of NET-induced lung damage in response to infection have often compared outcomes by preventing the formation of NETs, such as through blockade or knockout of the protein arginine deiminase 4 (PAD4) enzyme necessary for histone citrullination (9, 21). While studies involving NETs have used multiple microbes, a design allowing for the direct comparison of the ability of different microbes to induce NET formation has not been performed, which could elucidate a correlation between varying degrees of NET formation and lung damage. Additionally, while studies have examined a role for histone H4 in chemical lung injury (22, 23), the effect of histone H4 alone on the lung in vivo has not previously been assessed. Given the current evidence on NETs in pneumonia and a cytotoxic effect of histone H4, we sought to investigate the following two key areas: (i) the presence of NETs in different bacterial pneumonias and their association with lung injury and (ii) the degree to which H4 is sufficient to elicit injury in the airspaces of the lungs. We establish that NETs are elevated in the bronchoalveolar lavage (BAL) specimens of bacterial pneumonias, that the level of NET induction differs across pathogens, and that NETs correlate with inflammatory injury. We also demonstrate that while H4 alone has minimal effects in an uninflamed lung, it is sufficient to significantly exaggerate neutrophil recruitment and pulmonary pathophysiology.
RESULTS
NETs in bacterial pneumonia.
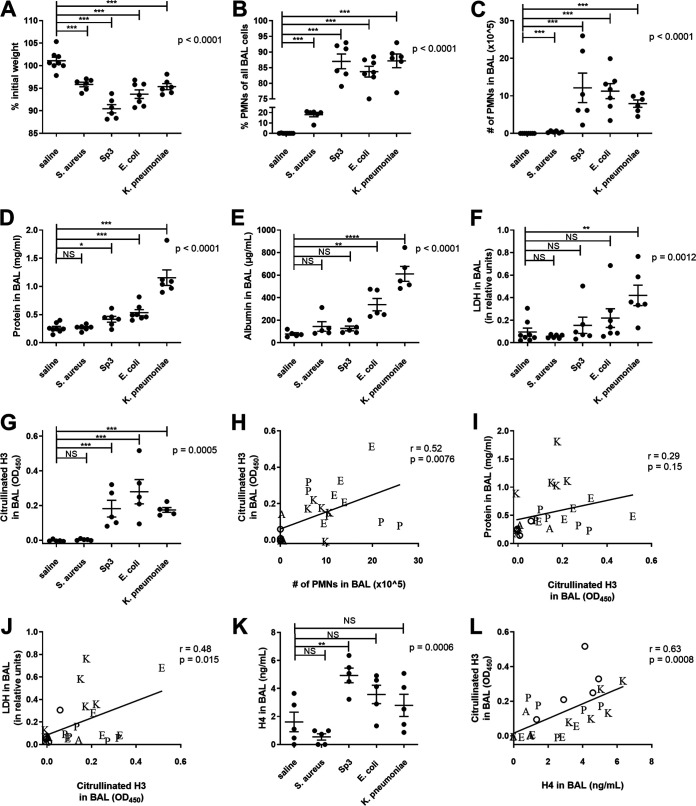
Murine models of bacterial pneumonias differ greatly in their inoculum dose, route of inoculation, and specified endpoints (24, 25). Thus, for the current study, we standardized our approach by using equal inocula, a uniform route of infection, and a consistent time point for analysis. Mice were examined 24 h after intratracheal instillations of 1 × 106 to 2 × 106 CFU of Staphylococcus aureus, Streptococcus pneumoniae, Escherichia coli, or Klebsiella pneumoniae. All pneumonic mice lost weight compared to controls who received saline instead of bacteria (Fig. 1A), demonstrating acute systemic morbidity due to respiratory infection. All bacterial species also produced a significant enrichment in the neutrophil fraction of the BAL (Fig. 1B) and increased absolute neutrophil counts (Fig. 1C). Neutrophil recruitment was modest during the S. aureus pneumonia but more pronounced and to similar levels following challenges with S. pneumoniae, E. coli, or K. pneumoniae. Thus, all 4 infections caused pulmonary inflammation and weight loss, with S. aureus being least severe. The concentration of protein in BAL fluid was significantly elevated for all bacterial pneumonias except for S. aureus (Fig. 1D), suggesting that S. pneumoniae, E. coli, and K. pneumoniae induced pulmonary edema. Albumin levels in the BAL were also measured (Fig. 1E), and differences between groups were similar to the differences in total protein, confirming total protein as a measure of pulmonary edema. In contrast, while analysis of variance (ANOVA) revealed that lactate dehydrogenase (LDH) concentrations in BAL fluids significantly differed among groups (P = 0.001), the only pneumonia with a significant elevation in LDH in post hoc analyses was K. pneumoniae (Fig. 1F) (P < 0.01). Since LDH is a cytoplasmic protein found extracellularly after necrosis, these data suggest that cell death was particularly prominent in the lungs infected by K. pneumoniae. Altogether, the results reveal that all of these bacteria cause acute pneumonia in mice. When delivered at comparable doses, K. pneumoniae induced the most injury, followed by significant and comparable amounts of injury from S. pneumoniae and E. coli, and only modest inflammation after S. aureus infection.
FIG 1.
Comparison of bacterial pneumonias. Mice were infected with 1 to 2 million CFU of Escherichia coli, Streptococcus pneumoniae serotype 3, Staphylococcus aureus, or Klebsiella pneumoniae via IT instillation and sacrificed 24 h later. Mouse weight was recorded prior to IT instillation and at sacrifice, and the percent of original weight was calculated (A). BAL was collected, and the cellular components analyzed for cell count and differential by cytospin to produce the fraction of PMNs (B) and the absolute number of PMNs (C) in the BAL. BAL fluid was then analyzed for total protein (D), albumin (E), LDH (F), citrullinated H3 as a marker of NETs (G), and histone H4 (K). Data shown as individual data points with the mean and standard error represented by solid lines. For all graphs, a one-way ANOVA was performed with P values reported, and Bonferroni’s posttests were performed. For graphs where a Bartlett’s test indicated unequal variances (B to F), the data was log transformed and then analyzed by one-way ANOVA with Bonferroni’s posttests performed. In panels H to J and L, a linear regression was performed, with correlation coefficient and P value reported. Data points are shown as the following letters corresponding to bacteria: A (Staphylococcus aureus), E (Escherichia coli), K (Klebsiella pneumoniae), and P (Streptococcus pneumoniae) or an open circle for saline mice. Data were from two independent experiments that totaled n = 6 to 8 mice per group. NS, not significant; *, P < 0.05; **, P < 0.01; ***, P < 0.001.
To assess NET formation in these infected lungs, we measured levels of citrullinated H3, a quantitative marker of NETs (26), in the BAL fluids. Citrullinated H3 was not detected in the mice who received saline or S. aureus (Fig. 1G) but was significantly increased in all other infections. These data indicate NET formation in lungs with bacterial pneumonia. We assessed the correlation between citrullinated H3 and parameters of inflammation and injury. Citrullinated H3 significantly associated with neutrophil numbers in the BAL (Fig. 1H), corroborating an interpretation of neutrophils as sources of NETs. Citrullinated H3 did not independently correlate with BAL protein (Fig. 1I), but it did positively associate with LDH in the BAL fluid (Fig. 1J). The statistically significant correlations were moderate (r ∼ 0.5) with substantial deviations from a linear relationship. The bacterial pneumonias causing detectable edema and necrosis exhibit NET formation in the infected air spaces, but the quantities of NETs reflect the degrees of inflammation or injury only weakly. We also assessed levels of H4 in the BAL fluids of the bacterial pneumonias, reflecting all cell lysis events, including NETosis plus other pathways of necrosis. Similar to the levels of citrullinated H3, total H4 trended higher in S. pneumoniae, E. coli, and K. pneumoniae pneumonias (Fig. 1K). Across the pneumonias, total H4 levels correlated with the levels of citrullinated H3 (r = 0.63) (Fig. 1L).
Histone H4 has modest effects in a healthy lung.
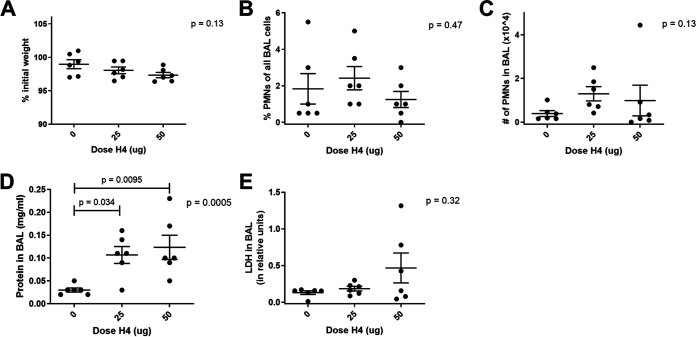
Since mixed histone extracts can induce pulmonary inflammation (17) and histone H4 is sufficient to trigger arterial inflammation (18), we investigated the effects of extracellular H4 in the air spaces of the normal lung. To assess dose response, we instilled 0, 25 μg, or 50 μg histone H4 intratracheally (IT) and measured inflammation and injury at 6 h (Fig. 2). There was no significant weight loss or enrichment of neutrophils at either dose after 6 h (Fig. 2A to C). There was a small but significant increase in BAL protein concentration (Fig. 2D), which could be consistent with detecting the instilled histone protein. Levels of LDH did not significantly differ (Fig. 2E). To assess time course, we used the higher 50-μg dose of H4 and measured effects after 6, 12, and 24 h. The histone H4 was sufficient to cause a significant reduction in body weight (Fig. 3A) and increase the neutrophil fraction (Fig. 3B), absolute neutrophil count (Fig. 3C), and BAL protein (Fig. 3D) (increasing over time and exceeding the quantity of instilled histone protein) but did not significantly affect LDH (Fig. 3E). Posttests demonstrated significance at individual time points for neutrophil recruitment but not for BAL protein, but the two-way ANOVA was significant for the effect of H4 on BAL protein (P = 0.0044). Albumin levels were also measured from the BAL at the 24-h time point and support the BAL protein data by indicating a significant increase in vascular leakage with H4 relative to saline (Fig. 3F). The amount of inflammation and injury induced by 50 μg H4 was modest but statistically significant, revealing that H4 in the lungs is sufficient to stimulate responses, including neutrophil recruitment and weight loss.
FIG 2.
Dose effects of IT histone H4. Mice received either saline, 25 μg of histone H4, or 50 μg of H4 via IT instillation and sacrificed 6 h later. Mouse weight was recorded prior to IT instillation and at sacrifice, and the percent of original weight was calculated (A). BAL was collected and the cellular components analyzed for cell count and differential by cytospin to produce the fraction of PMNs (B) and the absolute number of PMNs (C) in the BAL. BAL fluid was then analyzed for total protein (D) and LDH (E). Data shown as individual data points with the mean and standard error represented by solid lines. For all graphs, a one-way ANOVA was performed with P value reported, and Bonferroni’s posttests were performed with multiplicity-adjusted P values reported when significant. Data were from two independent experiments that totaled n = 6 mice per group.
FIG 3.
Time course of IT histone H4. Mice received either saline or 50 μg of histone H4 via IT instillation and sacrificed at 6, 12, or 24 h after IT. Mouse weight was recorded prior to IT instillation and at sacrifice, and the percent of original weight was calculated (A). BAL was collected and the cellular components analyzed for cell count and differential by cytospin to produce the fraction of PMNs (B) and the absolute number of PMNs (C) in the BAL. BAL fluid was then analyzed for total protein (D) and LDH (E). All individual data points are shown, with a line connecting the means. Black circles represent histone treatment, while gray circles represent saline. Albumin (F) was also measured from the 24-h BALs. For all graphs (except F), a two-way ANOVA was performed with the P value corresponding to the effect of histone treatment reported, and Bonferroni’s posttests were performed. In F, an unpaired t test was performed with P value reported. Data were from two independent experiments that totaled n = 6 mice per group. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Histone H4 significantly exacerbates lung injury in the setting of neutrophilic infiltration.
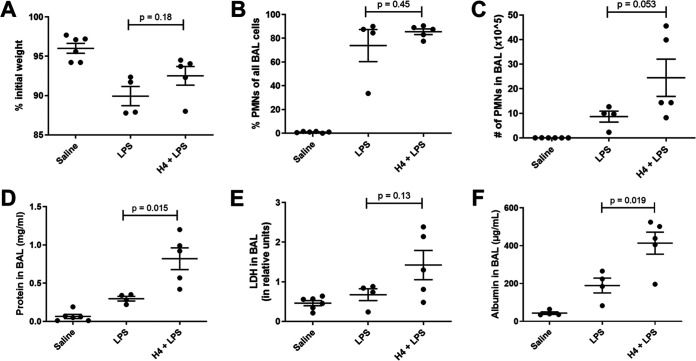
Because H4 can trigger proinflammatory activities of neutrophils (19), we considered that H4 might be more important when neutrophils were in the air spaces. We assessed the effect of H4 in the context of sterile lipopolysaccharide (LPS) as a means to induce neutrophilic infiltration. Compared to LPS alone (Fig. 4A to E), the combination of H4 with LPS yielded more neutrophils, more BAL protein, and more LDH relative to LPS alone, reaching significance at the P < 0.05 level for BAL protein. These data suggest that H4 is sufficient to exacerbate neutrophilic pulmonary inflammation. To confirm that the total BAL protein result was not influenced by the instilled histone, albumin was also measured in these samples (Fig. 4F) and demonstrated a significant increase in vascular leakage in the H4 with LPS group relative to LPS alone (P = 0.02).
FIG 4.
Effects of H4 combined with LPS at 24 h. Mice received either saline, 50 μg of LPS, or both 50 μg of H4 and LPS via IT instillation and sacrificed 24 h later. Mouse weight was recorded prior to IT instillation and at sacrifice, and the percent of original weight was calculated (A). BAL was collected and the cellular components analyzed for cell count and differential by cytospin to produce the fraction of PMNs (B) and the absolute number of PMNs (C) in the BAL. BAL fluid was then analyzed for total protein (D), LDH (E), and albumin (F). Data shown as individual data points with the mean and standard error represented by solid lines. For all graphs, an unpaired t test was performed comparing LPS and H4 + LPS with P value reported. Data were from two independent experiments that totaled n = 4 to 6 mice per group.
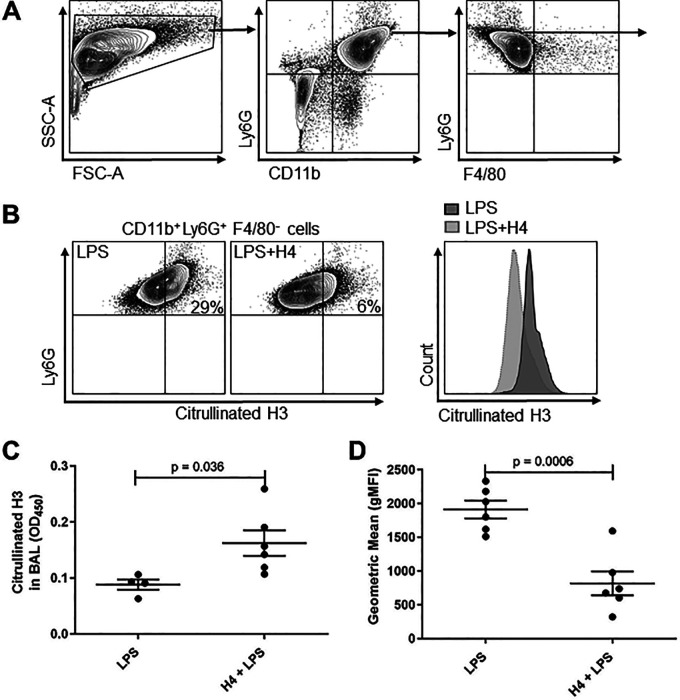
We analyzed differences in NETs between the LPS and H4-plus-LPS groups, both in the BAL fluid using enzyme-linked immunosorbent assay (ELISA) (as in Fig. 1) and colocalized with neutrophils using flow cytometry (Fig. 5A and B). Interestingly, H4 with LPS produced a significantly greater level of total NETs in the BAL fluid (Fig. 5C) but a significantly lower level of NETs that were colocalized with neutrophils (Fig. 5D). The elevated level of NETs as well as the increased lung damage seen in the LPS + H4 group support the bacterial pneumonia results (Fig. 1) that indicate that NETs correlate with lung damage. These data also indicate that one effect of H4 may be to further stimulate NET release from neutrophils, similar to what has previously been observed in vitro (18).
FIG 5.
Effects of H4 combined with LPS at 24 h on NETs. (A) BAL cells were stained with surface markers CD11b-PE-Cy7, Ly6G-APC, F4/80-PE, and citrullinated H3-AF488, and gating strategy for selecting CD11b+ Ly6G+ F4/80− neutrophils is shown. (B) Representative flow cytometry analysis and overlay histograms for citrullinated H3 on neutrophils from LPS or LPS with H4 groups. (C) BAL from the mice in Fig. 4 were analyzed by ELISA for citrullinated H3 as a marker of NETs. (D) The experiment was repeated with LPS or H4 and LPS, and mice were sacrificed at 24 h, BAL collected and immediately fixed, and then analyzed by flow cytometry to report the mean fluorescence signal for citrullinated H3 on PMNs using the gating strategy shown in panels A and B. Data shown as individual data points with the mean and standard error represented by solid lines. Data were from two independent experiments that totaled n = 4 to 6 mice per group. FSC, forward scatter; SSC, side scatter.
The effects of H4 are dynamic (Fig. 3) as are NET responses to LPS (27). To determine whether the differences observed at 24 h were present at 12 h, we performed an independent set of experiments examining the lungs 12 h after LPS instillation. Similar to 24 h, at 12 h, there was no difference in weight loss (Fig. 6A), but increases in measures of pulmonary inflammation due to added H4 were detectable, including neutrophil recruitment, pulmonary edema, cellular necrosis, and vascular leakage (Fig. 6B–E, G). There was no difference in the quantities of NETs detected by ELISA at the 12-h time point (Fig. 6F). The combination of comparable levels of NETs in both groups with the increased neutrophils, edema, and cell death in the H4 and LPS combination group dissociates these sets of signals, suggesting that the more severe lung inflammation downstream of histone H4 is not exclusively through increased NET release. Additionally, the increases in LDH and the neutrophil fraction of BAL in the combined H4 with LPS group at 12 h were significant (P = 0.0059 and P = 0.0001), while the differences at 24 h were not (P = 0.20 and P = 0.45), which correlates with the results of the time course experiment, indicating a peak neutrophil influx from H4 alone at 12 h.
FIG 6.
Effects of H4 combined with LPS at 12 h. Mice received either 50 μg of LPS or both 50 μg of H4 and 50 μg of LPS via IT instillation and sacrificed 12 h later. Mouse weight was recorded prior to IT instillation and at sacrifice, and the percent of original weight was calculated (A). BAL was collected and the cellular components analyzed for cell count and differential by cytospin to produce the fraction of PMNs (B) and the absolute number of PMNs (C) in the BAL. BAL fluid was then analyzed for total protein (D), LDH (E), citrullinated H3 as a marker of NETs (F), and albumin (G). Data shown as individual data points with the mean and standard error represented by solid lines. For all graphs, an unpaired t test was performed with P value reported. Data were from two independent experiments that totaled n = 5 mice per group.
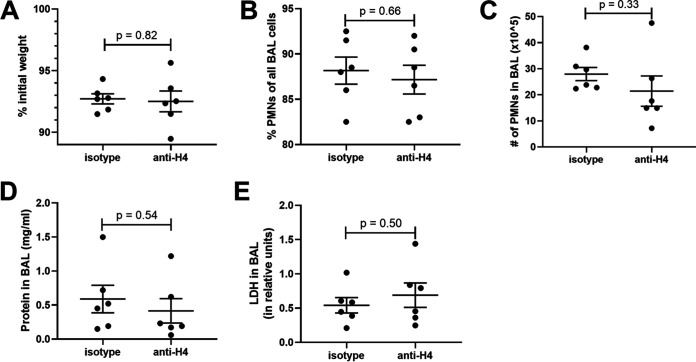
Blockade of histone H4 does not significantly improve severity of pneumonia.
To assess whether blocking H4 is protective in pneumonia, pneumonia was induced as before with K. pneumoniae with simultaneous IT administration of either anti-histone H4 or IgG control. There was no significant difference in weight loss, neutrophil recruitment, BAL protein, or LDH (Fig. 7A to E), indicating that blockade of H4 is insufficient to protect the lungs in pneumonia.
FIG 7.
Effects of H4 blockade during pneumonia. Mice were infected with 1 to 2 million CFU of Klebsiella pneumoniae with either 50 μg of anti-histone H4 antibody or IgG1, κ isotype via IT instillation and sacrificed 24 h later. Mouse weight was recorded prior to IT instillation and at sacrifice, and the percent of original weight was calculated (A). BAL was collected and the cellular components analyzed for cell count and differential by cytospin to produce the fraction of PMNs (B) and the absolute number of PMNs (C) in the BAL. BAL fluid was then analyzed for total protein (D) and LDH (E). Data shown as individual data points with the mean and standard error represented by solid lines. Data were from two independent experiments that totaled n = 6 mice per group.
DISCUSSION
In this study, we directly compared four different bacterial pneumonias caused by the same inoculum size and route of infection and examined at the same endpoint. As expected and based on prior studies in which higher doses of S. aureus in mouse lungs are needed to cause disease (24), S. aureus produced the least weight loss, neutrophil recruitment, BAL protein (a marker of edema), and LDH (a marker of cell death) and did not produce substantial NETs. In contrast, S. pneumoniae, E. coli, and K. pneumoniae all produced significant neutrophil recruitment, BAL protein, and NETs. Only K. pneumoniae significantly increased LDH, which is consistent with the ability of K. pneumoniae to induce abscess formation (24). Perhaps not surprisingly, the level of NETs correlated with the numbers of neutrophils in the BAL. The graphs of NETs versus BAL protein or LDH suggest that a direct relationship between NETs and lung damage may depend on other aspects unique to individual bacterial pneumonia models. Other toxic components from the large pool of recruited neutrophils likely contribute substantially to lung injury, and this also may explain the weaker correlations between NETs and markers of lung damage. The increase in total histone H4 from the pneumonic mice was only significant for S. pneumoniae, likely due to two saline mice with higher H4 levels. The process of collecting BAL involves some shear stress on cells being pulled through an angiocatheter, and damage to the cells can cause release of intracellular contents such as histones, likely contributing to the detection of H4 in uninfected mice. Further study is warranted to investigate other aspects across pneumonia models that may be influencing the interactions of NETs with the infected host.
Citrullination of histone H3 is a component of NET formation, and extracellular citrullinated H3 was used in our study to differentiate levels of NETs between pneumonia models. Several bacterial species, including the gram positives S. aureus and S. pneumoniae, are known to degrade extracellular DNA to evade trapping by NETs (28, 29). It is possible that by measuring only citrullinated H3, we were only able to assess NET production and not whether the NETs were intact and functional at the time of analysis. Mice that received pneumococcus had less BAL protein and LDH compared to mice receiving K. pneumoniae, despite roughly equal levels of NETs, which could be explained by a degradation of NETs. Alternatively, the mechanism through which NETosis is induced may also explain this disparity. Neutrophils can undergo a suicidal lytic NETosis or vital NETosis (30), and the proportion to which these occur in pneumonia may depend on the bacterial species. Future direct in vitro comparisons between these bacteria in the presence of neutrophils could elucidate this. Any conclusions drawn between bacterial species seen in our study are also limited by the strains used and may not apply to other strains of the same species. For example, carbapenem-resistant K. pneumoniae has been shown to inhibit NETosis (31), while NETosis was prevalent with administration of the strain of K. pneumoniae in this study. Since the carbapenem-resistant K. pneumoniae produces significant persistence and mortality, it is possible that more virulent strains of bacteria have also evolved mechanisms to evade capture by host NETosis.
A direct role of histone H4 in lung injury in vivo in the absence of other stimuli has not previously been examined, so based on our bacterial results and prior in vitro reports of H4 cytotoxicity (18, 19), we were interested to see if H4 influenced pulmonary inflammation. Even at relatively large doses, H4 alone produced only modest increases in neutrophils and BAL protein, but in the setting of an LPS-induced neutrophilic inflammation, however, H4 substantially increased neutrophil recruitment, BAL protein, and LDH. These results were accompanied by a significantly increased level of NETs but a lower level of PMNs with colocalized NETs. H4 has been found in vitro to promote NET formation and direct membrane lysis, in addition to increasing proinflammatory activities such as peroxide generation and degranulation (19, 32). Because H4 amplifies multiple proinflammatory actions of neutrophils, a mechanism by which H4 aggravates lung injury could be through its effects on recruited neutrophils in vivo, producing more inflammatory stimuli that leads to greater recruitment of neutrophils. In addition, H4 may act to partially dissociate NETs from the dying cells, preventing their clearance and prolonging inflammatory activities. Since the observed results are a result of purified H4, histones once dissociated may act in a positive feedback loop to further dissociate NETs. Other leukocytes, such as macrophages, have also been recognized to undergo a similar process of extracellular trap formation (33). As alveolar macrophages are present in the airspace, it is possible that some of the traps measured as total citrullinated H3 are macrophage-derived, and this could be contributing somewhat to the dissociation between our ELISA and flow cytometry data. In the BAL specimens where citrullinated H3 was measured, >80% of cells were neutrophils, so the majority of extracellular traps are most likely derived from neutrophils. Because the recombinant H4 was of prokaryotic origin and not complexed with other proteins or DNA, the results demonstrate that H4 is sufficient to exert proinflammatory activities in neutrophilic lungs but do not elucidate possible further roles of posttranslational modifications of H4 and/or macromolecular aggregations of H4 with nuclear or granular contents of NETs. We propose the model that H4 from NETs produces a positive feedback loop with neutrophil recruitment that exacerbates inflammatory damage to the lungs during bacterial pneumonia. Future studies investigating the effects of modified histone H4 may help further elucidate the role of released H4 in pathophysiologic contexts.
As our results indicate that H4 aggravates lung injury, we examined whether blockade with an anti-H4 antibody was protective in pneumonia. During K. pneumoniae infection, the administration of anti-H4 antibody was insufficient to decrease weight loss, neutrophil recruitment, or measures of lung inflammation and injury. Other inflammatory factors may drive inflammation in this setting even when H4 is blocked, such as pathogen-associated molecular patterns (PAMPs) (including LPS but also lipoproteins, cyclic dinucleotides, unmethylated CpG DNA, n-formylated peptides, etc.) as well as damage-associated molecular patterns (DAMPs) (including H4 plus other NET components as well as other non-NET DAMPS like nuclear proteins, mitochondrial proteins, cleaved matrix proteins, etc.) plus generation of proinflammatory mediators like cytokines (tumor necrosis factor alpha [TNF-α], interleukin-1β [IL-1β], IL-6, etc.), eicosanoids (leukotrienes, prostaglandins, etc.), and more (complement peptides, neurotransmitters, vasoactive peptides, etc.). Thus, H4 is sufficient to exacerbate inflammation elicited by bacterial products in the lung, and it is present in the air spaces during multiple bacterial infections, but it is not essential for the pulmonary inflammation occurring during severe and life-threatening bacterial pneumonia. While our results do not address possible uses of H4 blockade as an adjunct to other antibiotic, anti-inflammatory, and/or supportive therapies, anti-H4 alone may be inadequate to mitigate inflammatory injury during severe bacterial pneumonia.
Our results support a deleterious effect of NETs in pneumonic lungs, aligning with a growing body of evidence from human studies that associate NETs with poor clinical outcomes. NETs in BAL correlate with bacterial burden and alveolar inflammation in ventilator-associated pneumonia (10). Levels of citrullinated H3 in tracheal aspirates of patients increase with disease severity and diminish with improvement in clinical markers of lung function (34). Higher levels of serum NETs are found in patients with pneumonia relative to healthy controls, and patients with the highest serum NETs at admission have worse outcomes, including a 4-fold increase in all-cause mortality at 30 days (35). NETs also appear to be necessary for biofilm formation for some bacteria, contributing to bacterial resilience (36). DNase treatment of pneumonic mice reduces lung damage, and the serum NET/DNase ratio correlates with more severe ARDS in humans (9), further supporting a deleterious role for NETs. However, the classical role of NETs in defense against bacteria applies also to pneumonia, as deficiency in NET formation contributes to higher bacterial burdens (9, 37). As a result, the increase in inflammation and NET release due to H4 in the inflamed lung may be important to NET-mediated defense in bacterial pneumonia.
In conclusion, the accumulation of NETs in the air spaces of lungs is induced by pulmonary infections of diverse etiologies relevant to bacterial pneumonia. NET induction correlates, albeit modestly, with markers of inflammation and injury in the pneumonic lung. Histone H4, a component of NETs that is capable of activating or killing cells, can amplify inflammatory injury when in the air spaces of the lungs. These data prompt next rounds of study, which should investigate contributions of neutrophil-released H4 in pathophysiological consequences of bacterial pneumonia.
MATERIALS AND METHODS
Mice.
C57BL/6J mice were obtained from Jackson Laboratories (Bar Harbor, ME). Experiments were initiated when mice were 8 weeks of age. Mice were housed in the Boston University animal facility and all animal protocols were approved by the Boston University Institutional Animal Care and Use Committee. Mice were weighed prior to intratracheal instillations and at sacrifice.
Bacterial pneumonias.
Bacteria were grown on blood agar plates at 37°C with 5% CO2 and suspended in sterile saline prior to infecting mice. Mice were anesthetized with an intraperitoneal (i.p.) injection of ketamine (50 mg/kg) and xylazine (5 mg/kg). Experimental infections were induced by intratracheal (IT) instillation of 50 μL saline containing approximately 1 × 106 to 2 × 106 CFU of Escherichia coli serotype O6:K2:H1 (ATCC 19138), Streptococcus pneumoniae serotype 3 (Sp3; ATCC 6303), Staphylococcus aureus (ATCC 25923), or Klebsiella pneumoniae serotype 2 (ATCC 43816). The concentration of viable bacteria was estimated by optical density and subsequently verified by enumerating CFU from serial dilutions grown on blood agar plates. IT instillation was accomplished by inserting a 24-gauge angiocatheter through a tracheostomy and into the left bronchus for instillation of 50 μL of either saline or bacterial solution. Mice used in the bacterial pneumonia models were sacrificed 24 h after IT instillation.
Histone and/or LPS IT instillation.
In the dose-response experiment, 50 μL of saline alone or saline containing 25 or 50 μg recombinant histone H4 (New England BioLabs) was instilled IT using the same method as above, and specimens were collected after 6 h. The histone H4 was expressed in E. coli, and preparations contained <0.015 endotoxin units (EU)/mL endotoxin (19). In the time course experiment, either 50 μL of saline or saline containing 50 μg H4 was instilled IT and mice sacrificed at 6, 12, and 24 h after IT administration. In the histone-LPS experiment, 50 μL of saline, saline containing 50 μg LPS from Escherichia coli (serotype O111:B4; Sigma-Aldrich), saline containing 50 μg H4, or saline containing both 50 μg LPS and 50 μg H4 were instilled IT and mice sacrificed 24 h after IT administration. The 50 μg LPS and 50 μg LPS plus 50 μg H4 groups were also compared in a separate set of experiments at the 12-h time point; because these time points were not run together in the same set of experiments, the two time points should not be directly compared.
Histone H4 blockade.
Experimental infections were induced as before by IT instillation of 50 μL saline containing approximately 1 × 106 to 2 × 106 CFU of Klebsiella pneumoniae serotype 2 (ATCC 43816). The 50 μL of bacterial solution also contained 50 μg of either mouse anti-mouse histone H4 antibody (clone BWA3) or Ultra-LEAF purified mouse IgG1, κ isotype (BioLegend). Mice were sacrificed 24 h after IT instillation.
Bronchoalveolar lavage.
At the specified time point (6, 12, or 24 h after IT instillation), mice were euthanized by isoflurane overdose. The trachea was cannulated with an 18-gauge angiocatheter, and the lungs were lavaged 5 times with 1 mL of ice-cold phosphate-buffered saline (PBS). The BAL fluid was centrifuged at 300 g for 5 min to isolate cells. The supernatant from the first wash was kept isolated for protein analysis. The pellets of all washes were combined for cell analysis. Cell counts were obtained using LUNA-FL dual fluorescence cell counter (Logos Biosystems) and differentiated using cytocentrifuge preparations stained with Diff-Quik (VWR).
BAL fluid analysis.
Total protein in the BAL fluid was quantified using the Pierce bicinchoninic acid (BCA) assay according to manufacturer protocol (Thermo Fischer Scientific). Relative concentrations of LDH were analyzed using the CytoTox 96 cytotoxicity assay according to manufacturer protocol (Promega).
Flow cytometry.
Mice were administered 50 μg LPS with or without 50 μg H4 IT and sacrificed at 12 or 24 h postinstillation. BAL was collected using 3 washes of 1 mL cold PBS (Thermo Fisher Scientific, Waltham, MA). A total of 4% paraformaldehyde (Santa Cruz Biotechnology, Dallas, TX) was added and incubated for 10 min for fixation followed by centrifugation at 400 × g for 5 min. After blocking in 2% bovine serum albumin (BSA) (Sigma-Aldrich) and CD16/32 blocking antibody (1:40, TrueStain FcX; BioLegend, San Diego, CA) for 10 min, the following antibodies were added and incubated for 20 min using the following dilutions: allophycocyanin (APC)-conjugated Ly6G 1:400 (clone 1A8; BioLegend), PE-conjugated F4/80 1:100 (clone BM8; BioLegend), PE-Cy7-conjugated CD11b 1:200 (clone M1/70; BioLegend), BV421-conjugated Siglec-F 1:400 (clone S17007L; BioLegend), and unlabeled rabbit anti-histone H3 (citrulline R2 + R8 + R17) antibody 1:200 (clone ab5103; Abcam, Cambridge, United Kingdom). This was followed by incubation with AF488-conjugated goat anti-rabbit IgG 1:200 (clone ab150077; Abcam) for 20 min. After washing, stained cells were analyzed by flow cytometry using a BD LSRII flow cytometer (Becton, Dickinson, Franklin Lakes, NJ). Raw data was analyzed using FlowJo v10. Polymorphonuclear leukocytes (PMNs) were identified as Ly6G+CD11b+F4/80−Siglec F−, and the geometric mean (gMFI) AF488 fluorescence was recorded to compare the level of neutrophil extracellular traps (NETs) colocalized on PMNs.
Citrullinated H3 ELISA.
The level of NETs in BAL fluid was analyzed by quantifying the level of citrullinated H3. We modified the use of a cell death detection kit ELISA (11774425001; Roche) (38, 39) by using an alternative plate, generated by coating an ELISA plate with 100 μL per well of 4 μg/mL anti-histone H3 (citrulline R2 + R8 + R17) as capture antibody (clone ab5103; Abcam) in carbonate-bicarbonate buffer. Volumes of 20 μL of undiluted BAL fluid were added to this anti-H3 coated plate. The immunoreagent was prepared using the supplied detection anti-DNA antibody without including the capture antibody supplied in the kit, after which we proceeded per the standard manufacturer protocol. The optical density was read at 450 nm.
Histone H4 ELISA.
The level of Histone H4 in BAL was analyzed using a commercially available ELISA (ab156909; abcam) according to manufacturer protocol.
Albumin ELISA.
The level of albumin in BAL was analyzed using a commercially available ELISA (ab108792; Abcam) according to manufacturer protocol.
Statistical analysis.
Statistical analyses were performed using GraphPad Prism version 9.1. Comparisons were performed with a one-way ANOVA or two-way ANOVA with Bonferroni post hoc analysis, except in instances of only two conditions in which an unpaired t test was performed. When data did not pass Bartlett’s test for homogeneity of variance, they were log-transformed to improve comparisons by ANOVA. Differences were considered statistically significant when P was <0.05.
ACKNOWLEDGMENTS
We thank Konstantinos Kontodimas for technical assistance in performing the ELISA assays.
Studies were funded by the U.S. NIH, including R35-HL135756, R01-AI115053, and R33-HL137081 to J.P.M. and R01HL141513, R01HL139641, and R01AI153613 to M.B.
Contributor Information
Joseph P. Mizgerd, Email: jmizgerd@bu.edu.
Igor E. Brodsky, University of Pennsylvania
REFERENCES
- 1.Loosli CG. 1942. The pathogenesis and pathology of experimental type I pneumococcic pneumonia in the monkey. J Exp Med 76:79–92. doi: 10.1084/jem.76.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borregaard N, Lollike K, Kjeldsen L, Sengeløv H, Bastholm L, Nielsen MH, Bainton DF. 1993. Human neutrophil granules and secretory vesicles. Eur J Haematol 51:187–198. doi: 10.1111/j.1600-0609.1993.tb00629.x. [DOI] [PubMed] [Google Scholar]
- 3.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A. 2004. Neutrophil extracellular traps kill bacteria. Science 303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 4.Camicia G, Pozner R, de Larrañaga G. 2014. Neutrophil extracellular traps in sepsis. Shock 42:286–294. doi: 10.1097/SHK.0000000000000221. [DOI] [PubMed] [Google Scholar]
- 5.Kessenbrock K, Krumbholz M, Schönermarck U, Back W, Gross WL, Werb Z, Gröne HJ, Brinkmann V, Jenne DE. 2009. Netting neutrophils in autoimmune small-vessel vasculitis. Nat Med 15:623–625. doi: 10.1038/nm.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rohrbach AS, Hemmers S, Arandjelovic S, Corr M, Mowen KA. 2012. PAD4 is not essential for disease in the K/BxN murine autoantibody-mediated model of arthritis. Arthritis Res Ther 14:R104. doi: 10.1186/ar3829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garcia-Romo GS, Caielli S, Vega B, Connolly J, Allantaz F, Xu Z, Punaro M, Baisch J, Guiducci C, Coffman RL, Barrat FJ, Banchereau J, Pascual V. 2011. Netting neutrophils are major inducers of type I IFN production in pediatric systemic lupus erythematosus. Sci Transl Med 3:73ra20. doi: 10.1126/scitranslmed.3001201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fuchs TA, Brill A, Duerschmied D, Schatzberg D, Monestier M, Myers DD, Jr, Wrobleski SK, Wakefield TW, Hartwig JH, Wagner DD. 2010. Extracellular DNA traps promote thrombosis. Proc Natl Acad Sci USA 107:15880–15885. doi: 10.1073/pnas.1005743107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lefrançais E, Mallavia B, Zhuo H, Calfee CS, Looney MR. 2018. Maladaptive role of neutrophil extracellular traps in pathogen-induced lung injury. JCI Insight 3:e98178. doi: 10.1172/jci.insight.98178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mikacenic C, Moore R, Dmyterko V, West TE, Altemeier WA, Liles WC, Lood C. 2018. Neutrophil extracellular traps (NETs) are increased in the alveolar spaces of patients with ventilator-associated pneumonia. Crit Care 22:358. doi: 10.1186/s13054-018-2290-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caudrillier A, Kessenbrock K, Gilliss BM, Nguyen JX, Marques MB, Monestier M, Toy P, Werb Z, Looney MR. 2012. Platelets induce neutrophil extracellular traps in transfusion-related acute lung injury. J Clin Invest 122:2661–2671. doi: 10.1172/JCI61303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sayah DM, Mallavia B, Liu F, Ortiz-Muñoz G, Caudrillier A, DerHovanessian A, Ross DJ, Lynch JP, III, Saggar R, Ardehali A, Ware LB, Christie JD, Belperio JA, Looney MR, Lung Transplant Outcomes Group Investigators . 2015. Neutrophil extracellular traps are pathogenic in primary graft dysfunction after lung transplantation. Am J Respir Crit Care Med 191:455–463. doi: 10.1164/rccm.201406-1086OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cortjens B, de Boer OJ, de Jong R, Antonis AF, Sabogal Piñeros YS, Lutter R, van Woensel JB, Bem RA. 2016. Neutrophil extracellular traps cause airway obstruction during respiratory syncytial virus disease. J Pathol 238:401–411. doi: 10.1002/path.4660. [DOI] [PubMed] [Google Scholar]
- 14.Narasaraju T, Yang E, Samy RP, Ng HH, Poh WP, Liew AA, Phoon MC, van Rooijen N, Chow VT. 2011. Excessive neutrophils and neutrophil extracellular traps contribute to acute lung injury of influenza pneumonitis. Am J Pathol 179:199–210. doi: 10.1016/j.ajpath.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zuo Y, Yalavarthi S, Shi H, Gockman K, Zuo M, Madison JA, Blair C, Weber A, Barnes BJ, Egeblad M, Woods RJ, Kanthi Y, Knight JS. 2020. Neutrophil extracellular traps in COVID-19. JCI Insight 5:e138999. doi: 10.1172/jci.insight.138999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abrams ST, Zhang N, Manson J, Liu T, Dart C, Baluwa F, Wang SS, Brohi K, Kipar A, Yu W, Wang G, Toh CH. 2013. Circulating histones are mediators of trauma-associated lung injury. Am J Respir Crit Care Med 187:160–169. doi: 10.1164/rccm.201206-1037OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bosmann M, Grailer JJ, Ruemmler R, Russkamp NF, Zetoune FS, Sarma JV, Standiford TJ, Ward PA. 2013. Extracellular histones are essential effectors of C5aR- and C5L2-mediated tissue damage and inflammation in acute lung injury. FASEB J 27:5010–5021. doi: 10.1096/fj.13-236380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Silvestre-Roig C, Braster Q, Wichapong K, Lee EY, Teulon JM, Berrebeh N, Winter J, Adrover JM, Santos GS, Froese A, Lemnitzer P, Ortega-Gómez A, Chevre R, Marschner J, Schumski A, Winter C, Perez-Olivares L, Pan C, Paulin N, Schoufour T, Hartwig H, González-Ramos S, Kamp F, Megens RTA, Mowen KA, Gunzer M, Maegdefessel L, Hackeng T, Lutgens E, Daemen M, von Blume J, Anders HJ, Nikolaev VO, Pellequer JL, Weber C, Hidalgo A, Nicolaes GAF, Wong GCL, Soehnlein O. 2019. Externalized histone H4 orchestrates chronic inflammation by inducing lytic cell death. Nature 569:236–240. doi: 10.1038/s41586-019-1167-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hsieh IN, Deluna X, White MR, Hartshorn KL. 2021. Histone H4 directly stimulates neutrophil activation through membrane permeabilization. J Leukoc Biol 109:763–775. doi: 10.1002/JLB.3A0620-342R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Twaddell SH, Baines KJ, Grainge C, Gibson PG. 2019. The emerging role of neutrophil extracellular traps in respiratory disease. Chest 156:774–782. doi: 10.1016/j.chest.2019.06.012. [DOI] [PubMed] [Google Scholar]
- 21.Muraro SP, De Souza GF, Gallo SW, Da Silva BK, De Oliveira SD, Vinolo MAR, Saraiva EM, Porto BN. 2018. Respiratory syncytial virus induces the classical ROS-dependent NETosis through PAD-4 and necroptosis pathways activation. Sci Rep 8:14166. doi: 10.1038/s41598-018-32576-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Y, Wen Z, Guan L, Jiang P, Gu T, Zhao J, Lv X, Wen T. 2015. Extracellular histones play an inflammatory role in acid aspiration-induced acute respiratory distress syndrome. Anesthesiology 122:127–139. doi: 10.1097/ALN.0000000000000429. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Y, Zhao J, Guan L, Mao L, Li S, Zhao J. 2020. Histone H4 aggravates inflammatory injury through TLR4 in chlorine gas-induced acute respiratory distress syndrome. J Occup Med Toxicol 15:31. doi: 10.1186/s12995-020-00282-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dietert K, Gutbier B, Wienhold SM, Reppe K, Jiang X, Yao L, Chaput C, Naujoks J, Brack M, Kupke A, Peteranderl C, Becker S, von Lachner C, Baal N, Slevogt H, Hocke AC, Witzenrath M, Opitz B, Herold S, Hackstein H, Sander LE, Suttorp N, Gruber AD. 2017. Spectrum of pathogen- and model-specific histopathologies in mouse models of acute pneumonia. PLoS One 12:e0188251. doi: 10.1371/journal.pone.0188251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mizgerd JP, Skerrett SJ. 2008. Animal models of human pneumonia. Am J Physiol Lung Cell Mol Physiol 294:L387–L398. doi: 10.1152/ajplung.00330.2007. [DOI] [PubMed] [Google Scholar]
- 26.Masuda S, Nakazawa D, Shida H, Miyoshi A, Kusunoki Y, Tomaru U, Ishizu A. 2016. NETosis markers: quest for specific, objective, and quantitative markers. Clin Chim Acta 459:89–93. doi: 10.1016/j.cca.2016.05.029. [DOI] [PubMed] [Google Scholar]
- 27.Petretto A, Bruschi M, Pratesi F, Croia C, Candiano G, Ghiggeri G, Migliorini P. 2019. Neutrophil extracellular traps (NET) induced by different stimuli: a comparative proteomic analysis. PLoS One 14:e0218946. doi: 10.1371/journal.pone.0218946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beiter K, Wartha F, Albiger B, Normark S, Zychlinsky A, Henriques-Normark B. 2006. An endonuclease allows Streptococcus pneumoniae to escape from neutrophil extracellular traps. Curr Biol 16:401–407. doi: 10.1016/j.cub.2006.01.056. [DOI] [PubMed] [Google Scholar]
- 29.Thammavongsa V, Missiakas DM, Schneewind O. 2013. Staphylococcus aureus degrades neutrophil extracellular traps to promote immune cell death. Science 342:863–866. doi: 10.1126/science.1242255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yipp BG, Kubes P. 2013. NETosis: how vital is it? Blood 122:2784–2794. doi: 10.1182/blood-2013-04-457671. [DOI] [PubMed] [Google Scholar]
- 31.Birnberg-Weiss F, Castillo LA, Pittaluga JR, Martire-Greco D, Gómez SA, Landoni VI, Fernández GC. 2021. Modulation of neutrophil extracellular traps release by Klebsiella pneumoniae. J Leukoc Biol 109:245–256. doi: 10.1002/JLB.4MA0620-099R. [DOI] [PubMed] [Google Scholar]
- 32.Shi L, Aymonnier K, Wagner DD. 2021. Neutrophil stimulation with citrullinated histone H4 slows down calcium influx and reduces NET formation compared with native histone H4. PLoS One 16:e0251726. doi: 10.1371/journal.pone.0251726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.King PT, Sharma R, O'Sullivan K, Selemidis S, Lim S, Radhakrishna N, Lo C, Prasad J, Callaghan J, McLaughlin P, Farmer M, Steinfort D, Jennings B, Ngui J, Broughton BR, Thomas B, Essilfie AT, Hickey M, Holmes PW, Hansbro P, Bardin PG, Holdsworth SR. 2015. Nontypeable Haemophilus influenzae induces sustained lung oxidative stress and protease expression. PLoS One 10:e0120371. doi: 10.1371/journal.pone.0120371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ojima M, Yamamoto N, Hirose T, Hamaguchi S, Tasaki O, Kojima T, Tomono K, Ogura H, Shimazu T. 2020. Serial change of neutrophil extracellular traps in tracheal aspirate of patients with acute respiratory distress syndrome: report of three cases. J Intensive Care 8:25. doi: 10.1186/s40560-020-00444-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ebrahimi F, Giaglis S, Hahn S, Blum CA, Baumgartner C, Kutz A, van Breda SV, Mueller B, Schuetz P, Christ-Crain M, Hasler P. 2018. Markers of neutrophil extracellular traps predict adverse outcome in community-acquired pneumonia: secondary analysis of a randomised controlled trial. Eur Respir J 51:1701389. doi: 10.1183/13993003.01389-2017. [DOI] [PubMed] [Google Scholar]
- 36.Pylaeva E, Bordbari S, Spyra I, Decker AS, Häussler S, Vybornov V, Lang S, Jablonska J. 2019. Detrimental effect of type I IFNs during acute lung infection with Pseudomonas aeruginosa is mediated through the stimulation of neutrophil NETosis. Front Immunol 10:2190. doi: 10.3389/fimmu.2019.02190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cai S, Paudel S, Jin L, Ghimire L, Taylor CM, Wakamatsu N, Bhattarai D, Jeyaseelan S. 2021. NLRP6 modulates neutrophil homeostasis in bacterial pneumonia-derived sepsis. Mucosal Immunol 14:574–584. doi: 10.1038/s41385-020-00357-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chandrasekar B, Boylston WH, Venkatachalam K, Webster NJ, Prabhu SD, Valente AJ. 2008. Adiponectin blocks interleukin-18-mediated endothelial cell death via APPL1-dependent AMP-activated protein kinase (AMPK) activation and IKK/NF-kappaB/PTEN suppression. J Biol Chem 283:24889–24898. doi: 10.1074/jbc.M804236200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mei S, Gu H, Yang X, Guo H, Liu Z, Cao W. 2012. Prolonged exposure to insulin induces mitochondrion-derived oxidative stress through increasing mitochondrial cholesterol content in hepatocytes. Endocrinology 153:2120–2129. doi: 10.1210/en.2011-2119. [DOI] [PMC free article] [PubMed] [Google Scholar]