Abstract
Background
Waning of vaccine protection against coronavirus disease 2019 (Covid-19) and the emergence of the omicron (or B.1.1.529) variant of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) have led to expedited efforts to scale up booster vaccination. Protection conferred by booster doses of the BNT162b2 (Pfizer–BioNTech) and mRNA-1273 (Moderna) vaccines in Qatar, as compared with protection conferred by the two-dose primary series, is unclear.
Methods
We conducted two matched retrospective cohort studies to assess the effectiveness of booster vaccination, as compared with that of a two-dose primary series alone, against symptomatic SARS-CoV-2 infection and Covid-19–related hospitalization and death during a large wave of omicron infections from December 19, 2021, through January 26, 2022. The association of booster status with infection was estimated with the use of Cox proportional-hazards regression models.
Results
In a population of 2,239,193 persons who had received at least two doses of BNT162b2 or mRNA-1273 vaccine, those who had also received a booster were matched with persons who had not received a booster. Among the BNT162b2-vaccinated persons, the cumulative incidence of symptomatic omicron infection was 2.4% (95% confidence interval [CI], 2.3 to 2.5) in the booster cohort and 4.5% (95% CI, 4.3 to 4.6) in the nonbooster cohort after 35 days of follow-up. Booster effectiveness against symptomatic omicron infection, as compared with that of the primary series, was 49.4% (95% CI, 47.1 to 51.6). Booster effectiveness against Covid-19–related hospitalization and death due to omicron infection, as compared with the primary series, was 76.5% (95% CI, 55.9 to 87.5). BNT162b2 booster effectiveness against symptomatic infection with the delta (or B.1.617.2) variant, as compared with the primary series, was 86.1% (95% CI, 67.3 to 94.1). Among the mRNA-1273–vaccinated persons, the cumulative incidence of symptomatic omicron infection was 1.0% (95% CI, 0.9 to 1.2) in the booster cohort and 1.9% (95% CI, 1.8 to 2.1) in the nonbooster cohort after 35 days; booster effectiveness against symptomatic omicron infection, as compared with the primary series, was 47.3% (95% CI, 40.7 to 53.3). Few severe Covid-19 cases were noted in the mRNA-1273–vaccinated cohorts.
Conclusions
The messenger RNA (mRNA) boosters were highly effective against symptomatic delta infection, but they were less effective against symptomatic omicron infection. However, with both variants, mRNA boosters led to strong protection against Covid-19–related hospitalization and death. (Funded by Weill Cornell Medicine–Qatar and others.)
Waning of vaccine protection against coronavirus disease 2019 (Covid-19)1-4 and the emergence of the immune-evasive omicron (or B.1.1.529) variant5 of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2),6,7 which is a variant of concern, have stimulated efforts to scale up Covid-19 booster vaccination. Qatar launched its third-dose (booster) vaccination program in mid-September 2021. Both the BNT162b2 (Pfizer–BioNTech) and mRNA-1273 (Moderna) messenger RNA (mRNA) vaccines8,9 were used. These are the same two vaccines that have been used since the launch of the Covid-19 immunization program in Qatar.10,11
Although eligibility for booster vaccination was initially restricted to older adults, immunocompromised persons, and persons with severe or multiple chronic conditions, it was later expanded, according to age group, to the rest of the population. Eligibility was first restricted to persons who had completed the primary series of two doses of vaccine at least 8 months previously, but subsequently this interval was shortened to 6 months. The same type of vaccine was used in both the primary series and booster vaccinations in the majority of the population, but the booster dose of mRNA-1273 was half the dose used in the primary series. As booster vaccination was being scaled up, the omicron variant was introduced into the population, leading to the largest epidemic wave of SARS-CoV-2 infections in Qatar.
We investigated the effectiveness of booster vaccination against symptomatic SARS-CoV-2 infection and Covid-19–related hospitalization and death, as compared with that of the two-dose primary series alone. Our study population consisted of the national cohort of vaccinated persons in Qatar during the wave of infections with the omicron variant.7
Methods
Data Sources
The study analyzed information from national, federated databases regarding Covid-19 vaccination, laboratory testing, hospitalization, and death. These data were retrieved from the integrated nationwide digital-health information platform. Databases included all SARS-CoV-2–related data and associated demographic information since the onset of the pandemic. These databases include, with no missing information, all polymerase-chain-reaction (PCR) tests and, more recently, rapid antigen tests conducted at health care facilities on or after January 5, 2022. They also include all vaccination records, Covid-19–related hospitalizations, infection severity and fatality classifications according to the World Health Organization (WHO) guidelines,12,13 and sex, age, and nationality information retrieved from the national registry. Further descriptions of these national databases, such as PCR testing and the process for assessing infection severity, have been reported previously.1,2,10,11,14-20 Details regarding laboratory methods for reverse-transcriptase–quantitative PCR testing are provided in Section S1 in the Supplementary Appendix, available with the full text of this article at NEJM.org.
Study Design
To estimate the effectiveness of booster vaccination with either the BNT162b2 or mRNA-1273 vaccines, as compared with that of the two-dose primary series, we used a matched retrospective cohort study design that emulated a target trial.21,22 The study compared the incidence of symptomatic breakthrough SARS-CoV-2 infection among persons who had received the booster dose more than 7 days previously (the booster cohort) with the incidence among persons who had not yet received a booster dose (the nonbooster cohort). The 7-day cutoff between the administration of the booster and the start of follow-up was informed by earlier studies22-24 to ensure sufficient time for the buildup of immune protection. A 14-day cutoff was also investigated in a sensitivity analysis.
All persons who had received at least two doses of the BNT162b2 vaccine between January 5, 2021 (the date of the first two-dose BNT162b2 vaccination series in Qatar), and January 26, 2022 (the end of the study), could be included in the eligible cohorts of the study, provided that they had no previous documented infection before the start of follow-up. The same inclusion criteria applied to persons who had received the mRNA-1273 vaccine, but the corresponding dates were January 24, 2021, and January 26, 2022, respectively.
Matching was used to identify a cohort of patients with similar baseline characteristics. Persons in the booster cohort and those in the nonbooster cohort were matched exactly in a 1:1 ratio according to sex, 10-year age group, and nationality to control for known differences in the risk of exposure to SARS-CoV-2 infection in Qatar.15,25-28 In a previous study that had a similar design, matching according to these factors was shown to provide adequate control of bias arising from differences in this risk. In that study, no meaningful difference between the matched mRNA-1273–vaccinated and BNT162b2-vaccinated cohorts was noted in the incidence of infection in the first 2 weeks after administration of the first dose,11 as had been expected, given the negligible vaccine protection in this 2-week period.8,9 Moreover, in previous studies using other designs but the same matching, no meaningful difference was observed between vaccinated persons and unvaccinated persons with respect to the incidence of infection in the first 2 weeks after administration of the first dose.1,2,20,29
In our study, persons were also matched exactly according to the calendar week of the second-dose vaccination in order to control for the time since vaccination and the waning of vaccine immunity over time.1,2,10,11 Matching was performed through an iterative process that ensured that each control person in the nonbooster cohort was alive, infection-free, and had not received the third dose of vaccine by the beginning of follow-up. For each matched pair, follow-up began on the eighth day after the person in the booster cohort received the booster dose, provided this day occurred during the wave of infections with the omicron variant (e.g., on or after December 19, 2021). The large exponential-growth phase of this wave of infections started on December 19, 2021, and reached its peak in mid-January 2022.7,30
Viral whole-genome sequencing of 315 random SARS-CoV-2–positive specimens collected between December 19, 2021, and January 22, 2022, was performed on a GridION sequencing device (Oxford Nanopore Technologies). Of these specimens, 300 (95.2%) were confirmed to be omicron infections and 15 (4.8%) were confirmed to be delta (or B.1.617.2)5 infections.7,30 No cases of infection with the delta variant were detected in the viral whole-genome sequencing after January 8, 2022.
Persons in the booster cohort who had received the booster dose at least 7 days before the onset of the wave of omicron infections were followed along with their matched controls in the nonbooster cohort beginning on December 19, 2021. Controls in the nonbooster cohort who received the booster dose at a future date were eligible for recruitment into the booster cohort, provided they were alive and infection-free at the start of follow-up. Accordingly, some persons contributed follow-up time both as persons who had received only a two-dose primary series and as persons who had received a booster, but at different times.
To ensure exchangeability, data on both members of each matched pair were censored once the control received the booster dose.22 Accordingly, follow-up continued until the first of one of these events: a documented SARS-CoV-2 infection (defined as the first positive PCR or rapid antigen test after the start of follow-up, regardless of the presence of symptoms or the reason for testing [this information was available only for PCR tests]), booster vaccination of the control (with matched pair censoring), death, or the end of study censoring (on January 26, 2022).
Study Outcomes
The primary outcome of the study was symptomatic infection, which was defined as a positive PCR test of a nasopharyngeal swab obtained because of clinical suspicion based on symptoms that were compatible with a respiratory tract infection. The secondary outcome was any severe,12 critical,12 or fatal13 case of Covid-19. Classification of severe Covid-19 cases (acute-care hospitalizations),12 critical Covid-19 cases (hospitalizations in an intensive care unit),12 and fatal Covid-19 cases (Covid-19–related deaths)13 followed WHO guidelines, and assessments were made by trained medical personnel, independent of the study investigators, through individual chart reviews, as part of a national protocol for every hospitalized patient with Covid-19. Details of the Covid-19 severity, criticality, and fatality classification are provided in Section S2.
Every hospitalized patient with Covid-19 underwent the infection severity assessment every 3 days until discharge or death. Persons who had progression to severe, critical, or fatal Covid-19 between the time of the positive PCR or rapid antigen test and the end of the study were classified according to the worst outcome, starting with death,13 followed by critical disease,12 and then severe disease.12
Study Oversight
This retrospective study was approved by the institutional review boards at Hamad Medical Corporation and Weill Cornell Medicine–Qatar, with waiver of informed consent. Reporting of the study followed Strengthening the Reporting of Observational Studies in Epidemiology guidelines (Table S1 in the Supplementary Appendix). The funders of the study had no role in the study design, data collection, data analysis, data interpretation, or writing of the manuscript. All the authors contributed to data collection and acquisition, database development, discussion and interpretation of the results, and to the writing of the manuscript. All the authors read and approved the final manuscript.
Statistical Analysis
The characteristics of the eligible and matched cohorts were described with the use of frequency distributions and measures of central tendency. Groups were compared with the use of standardized mean differences, with a standardized mean difference of less than 0.1 indicating adequate matching.31 The cumulative incidence of symptomatic infection was defined as the percentage of persons at risk in whom symptomatic infection (the primary end point) occurred during follow-up, and this incidence was estimated in each cohort with the use of the Kaplan–Meier estimator method.32 The incidence rate of symptomatic infection in each cohort, which was defined as the number of identified symptomatic infections divided by the number of person-weeks contributed by all persons in the cohort, was estimated, along with its 95% confidence interval, with the use of a Poisson log-likelihood regression model with the Stata software, version 17.0, stptime command.
The hazard ratio for the between-cohort comparison of the incidence of symptomatic infection and the corresponding 95% confidence interval were calculated with the use of Cox regression, with adjustment for the matching factors with the Stata software, version 17.0, stcox command. Schoenfeld residuals and log–log plots for the survival curves were used to test the proportional-hazards assumption and to investigate its adequacy. The 95% confidence intervals were not adjusted for multiplicity and thus should not be used to infer definitive differences between the cohorts. Interactions were not considered. The effectiveness of booster vaccination, as compared with that of the two-dose primary series, was estimated with the following equation: vaccine effectiveness=1−adjusted hazard ratio for the incidence of symptomatic infection.
An additional analysis was conducted to estimate vaccine effectiveness against symptomatic infection with the delta variant.5 In that analysis, the end of the study period was December 1, 2021 (i.e., right before the date of the first detection of the omicron variant in Qatar).7,30 During this specific follow-up period, Qatar had a low-incidence phase dominated by the delta variant.1,16,30,33,34 This analysis was possible only for the BNT162b2 vaccine because the time of follow-up was limited for the mRNA-1273 cohorts.
A second additional analysis was conducted to estimate vaccine effectiveness according to the time between the second dose and the booster dose. Moreover, because the full effectiveness of the booster dose may require more than 7 days to develop, the main analyses were repeated, but with the start of follow-up on the 15th day after booster vaccination instead of on the 8th day after booster vaccination. All the analyses were conducted with the use of Stata/SE software, version 17.0.
Results
Study Population, BNT162b2 Vaccine
Between January 5, 2021, and January 26, 2022, a total of 1,299,010 persons received at least two doses of BNT162b2 vaccine and 281,093 of those persons received a third (booster) dose of that vaccine. The median date of the first dose was May 3, 2021; the median date of the second dose was May 24, 2021; and the median date of the third dose was December 14, 2021 (Fig. S1). The median time between the first and second doses was 21 days (interquartile range, 21 to 22), and the median time between the second and booster doses was 249 days (interquartile range, 232 to 270).
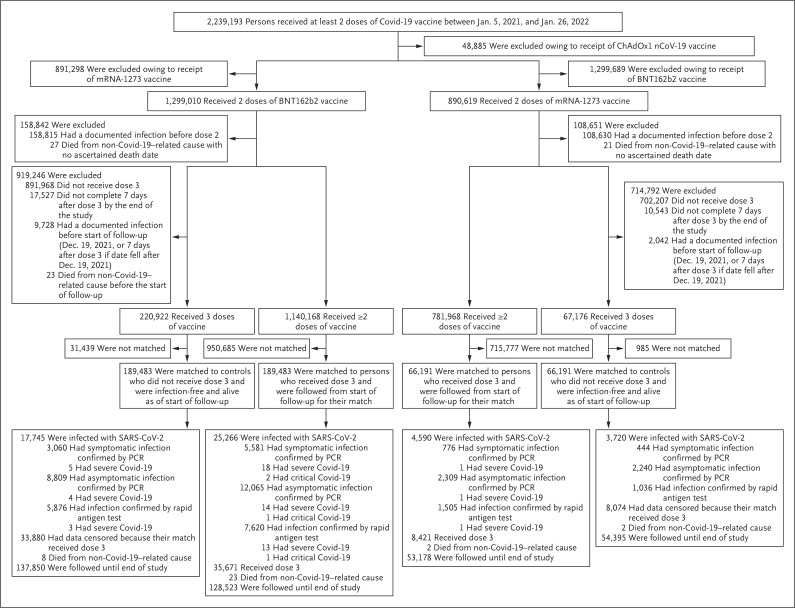
The process that was used to select the population for the BNT162b2 booster study is shown in Figure 1. Table 1 shows the baseline characteristics of the eligible and matched cohorts. The study was based on the total population of Qatar, and thus the study population is broadly representative of the diversity (according to national background) of that population, which is young and predominantly male (Table S2).
Figure 1. Cohort Selection for the Analysis of Effectiveness of BNT162b2 and mRNA-1273 Booster Vaccination.
Covid-19 denotes coronavirus disease 2019, PCR polymerase chain reaction, and SARS-CoV-2 severe acute respiratory syndrome coronavirus 2.
Table 1. Baseline Characteristics of the Eligible and Matched Booster and Nonbooster Cohorts.*.
| Characteristics | BNT162b2 Vaccine | mRNA-1273 Vaccine | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Eligible Cohorts | Matched Cohorts | Eligible Cohorts | Matched Cohorts | |||||||||
| Booster (N=220,922) |
No Booster (N=1,140,168) |
SMD† | Booster (N=189,483) |
No Booster (N=189,483) |
SMD† | Booster (N=67,176) |
No Booster (N=781,968) |
SMD† | Booster (N=66,191) |
No Booster (N=66,191) |
SMD† | |
| % | % | % | % | |||||||||
| Median age (IQR) — yr | 42 (35–52) | 36 (28–44) | 0.54 | 41 (34–50) | 41 (34–50) | 0.01 | 39 (33–46) | 35 (30–42) | 0.37 | 39 (33–46) | 39 (33–46) | 0.01 |
| Age group — no. (%) | ||||||||||||
| 0–19 yr | 4,968 (2.2) | 114,657 (10.1) | 0.56 | 4,706 (2.5) | 4,706 (2.5) | 0.00 | 657 (1.0) | 5,546 (0.7) | 0.37 | 570 (0.9) | 570 (0.9) | 0.00 |
| 20–29 yr | 20,392 (9.2) | 212,148 (18.6) | 19,504 (10.3) | 19,504 (10.3) | 8,390 (12.5) | 186,093 (23.8) | 8,292 (12.5) | 8,292 (12.5) | ||||
| 30–39 yr | 68,599 (31.1) | 391,199 (34.3) | 63,258 (33.4) | 63,258 (33.4) | 25,560 (38.0) | 334,256 (42.7) | 25,366 (38.3) | 25,366 (38.3) | ||||
| 40–49 yr | 58,137 (26.3) | 249,777 (21.9) | 52,384 (27.6) | 52,384 (27.6) | 21,647 (32.2) | 181,864 (23.3) | 21,357 (32.3) | 21,357 (32.3) | ||||
| 50–59 yr | 42,044 (19.0) | 115,261 (10.1) | 32,993 (17.4) | 32,993 (17.4) | 8,614 (12.8) | 60,574 (7.7) | 8,421 (12.7) | 8,421 (12.7) | ||||
| 60–69 yr | 20,363 (9.2) | 43,294 (3.8) | 13,018 (6.9) | 13,018 (6.9) | 1,995 (3.0) | 11,491 (1.5) | 1,915 (2.9) | 1,915 (2.9) | ||||
| ≥70 yr | 6,419 (2.9) | 13,832 (1.2) | 3,620 (1.9) | 3,620 (1.9) | 313 (0.5) | 2,144 (0.3) | 270 (0.4) | 270 (0.4) | ||||
| Sex — no. (%) | ||||||||||||
| Male | 139,405 (63.1) | 791,562 (69.4) | 0.13 | 122,435 (64.6) | 122,435 (64.6) | 0.00 | 45,867 (68.3) | 633,518 (81.0) | 0.30 | 45,443 (68.7) | 45,443 (68.7) | 0.00 |
| Female | 81,517 (36.9) | 348,606 (30.6) | 67,048 (35.4) | 67,048 (35.4) | 21,309 (31.7) | 148,450 (19.0) | 20,748 (31.3) | 20,748 (31.3) | ||||
| Nationality — no. (%)‡ | ||||||||||||
| Bangladeshi | 12,793 (5.8) | 126,930 (11.1) | 0.40 | 12,300 (6.5) | 12,300 (6.5) | 0.00 | 6,032 (9.0) | 152,344 (19.5) | 0.54 | 6,023 (9.1) | 6,023 (9.1) | 0.00 |
| Egyptian | 15,363 (7.0) | 63,783 (5.6) | 13,680 (7.2) | 13,680 (7.2) | 3,629 (5.4) | 30,614 (3.9) | 3,622 (5.5) | 3,622 (5.5) | ||||
| Filipino | 31,870 (14.4) | 103,726 (9.1) | 26,514 (14.0) | 26,514 (14.0) | 11,401 (17.0) | 69,085 (8.8) | 11,389 (17.2) | 11,389 (17.2) | ||||
| Indian | 62,744 (28.4) | 253,423 (22.2) | 54,682 (28.9) | 54,682 (28.9) | 26,097 (38.8) | 215,692 (27.6) | 26,068 (39.4) | 26,068 (39.4) | ||||
| Nepalese | 5,983 (2.7) | 96,176 (8.4) | 5,899 (3.1) | 5,899 (3.1) | 3,331 (5.0) | 109,048 (13.9) | 3,326 (5.0) | 3,326 (5.0) | ||||
| Pakistani | 7,330 (3.3) | 48,869 (4.3) | 6,682 (3.5) | 6,682 (3.5) | 2,491 (3.7) | 42,114 (5.4) | 2,484 (3.8) | 2,484 (3.8) | ||||
| Qatari | 23,562 (10.7) | 158,879 (13.9) | 21,188 (11.2) | 21,188 (11.2) | 932 (1.4) | 14,351 (1.8) | 930 (1.4) | 930 (1.4) | ||||
| Sri Lankan | 5,271 (2.4) | 34,797 (3.1) | 5,038 (2.7) | 5,038 (2.7) | 2,580 (3.8) | 32,156 (4.1) | 2,572 (3.9) | 2,572 (3.9) | ||||
| Sudanese | 4,114 (1.9) | 25,218 (2.2) | 3,483 (1.8) | 3,483 (1.8) | 713 (1.1) | 13,285 (1.7) | 705 (1.1) | 705 (1.1) | ||||
| Other§ | 51,892 (23.5) | 228,367 (20.0) | 40,017 (21.1) | 40,017 (21.1) | 9,970 (14.8) | 103,279 (13.2) | 9,072 (13.7) | 9,072 (13.7) | ||||
| Dose 2 in 2021 — no. (%)¶ | ||||||||||||
| January | 9,901 (4.5) | 14,175 (1.2) | 1.08 | 3,882 (2.0) | 3,799 (2.0) | 0.01 | 1 (<0.1) | 3 (<0.1) | 1.30 | — | — | 0.01 |
| February | 28,260 (12.8) | 50,199 (4.4) | 17,977 (9.5) | 17,908 (9.5) | 5 (<0.1) | 16 (<0.1) | 2 (<0.1) | 2 (<0.1) | ||||
| March | 89,843 (40.7) | 213,326 (18.7) | 76,004 (40.1) | 76,156 (40.2) | 321 (0.5) | 1,578 (0.2) | 264 (0.4) | 275 (0.4) | ||||
| April | 40,632 (18.4) | 141,212 (12.4) | 39,871 (21.0) | 39,617 (20.9) | 25,650 (38.2) | 100,961 (12.9) | 25,205 (38.1) | 25,208 (38.1) | ||||
| May | 37,346 (16.9) | 242,738 (21.3) | 36,983 (19.5) | 37,178 (19.6) | 31,724 (47.2) | 197,069 (25.2) | 31,378 (47.4) | 31,358 (47.4) | ||||
| June | 13,496 (6.1) | 163,245 (14.3) | 13,354 (7.0) | 13,413 (7.1) | 7,061 (10.5) | 114,242 (14.6) | 6,966 (10.5) | 6,823 (10.3) | ||||
| July | 1,394 (0.6) | 113,127 (9.9) | 1,366 (0.7) | 1,364 (0.7) | 2,350 (3.5) | 95,632 (12.2) | 2,312 (3.5) | 2,461 (3.7) | ||||
| August | 22 (<0.1) | 62,357 (5.5) | 20 (<0.1) | 23 (<0.1) | 59 (0.1) | 213,908 (27.4) | 59 (0.1) | 59 (0.1) | ||||
| September | 16 (<0.1) | 72,448 (6.4) | 15 (<0.1) | 13 (<0.1) | 3 (<0.1) | 49,076 (6.3) | 3 (<0.1) | 3 (<0.1) | ||||
| October | 5 (<0.1) | 28,475 (2.5) | 5 (<0.1) | 6 (<0.1) | 1 (<0.1) | 3,357 (0.4) | 1 (<0.1) | 1 (<0.1) | ||||
| November | 2 (<0.1) | 20,584 (1.8) | 2 (<0.1) | 3 (<0.1) | 1 (<0.1) | 3,344 (0.4) | 1 (<0.1) | 1 (<0.1) | ||||
| December | 5 (<0.1) | 12,275 (1.1) | 4 (<0.1) | 3 (<0.1) | 0 | 551 (0.1) | — | — | ||||
| Dose 2 in January 2022 — no. (%)¶ | 0 | 6,007 (0.5) | — | — | 1 (<0.1) | 2,231 (0.3) | — | — | ||||
Cohorts were matched one to one according to sex, 10-year age group, nationality, and calendar week of the second-dose vaccination. Percentages may not total 100 because of rounding. IQR denotes interquartile range.
The standardized mean difference (SMD) was defined as the difference between the mean value of a covariate in one group and the corresponding mean value of a covariate in the other group, divided by the pooled standard deviation. An SMD of less than 0.1 indicates adequate matching.
Nationalities were chosen to represent the most populous groups in Qatar.
The category of other nationalities includes 162 other nationalities in Qatar in persons who received the third dose and 188 other nationalities in persons who did not receive the third dose in the unmatched cohorts of those who received the BNT162b2 vaccine, and 131 other nationalities in persons who received the third dose and 131 other nationalities in persons who did not receive the third dose in the matched cohorts of those who received the BNT162b2 vaccine. This category also includes 136 other nationalities in persons who received the third dose and 172 other nationalities in persons who did not receive the third dose in the unmatched cohorts of those who received the mRNA-1273 vaccine, and 106 other nationalities in persons who received the third dose and 106 other nationalities in persons who did not receive the third dose in the matched cohorts of those who received the mRNA-1273 vaccine.
Cohorts were matched exactly according to the calendar week of the second-dose vaccination, but we opted to report the distribution according to calendar month for brevity. Accordingly, members of the cohorts who were vaccinated in the same week may appear in different calendar months.
Effectiveness of BNT162b2 Booster against Omicron Variant
The median follow-up time was 22 days (interquartile range, 12 to 38 days) in the booster cohort and 21 days (interquartile range, 11 to 38 days) in the nonbooster BNT162b2 cohort (Fig. S1). A total of 17,745 infections were recorded in the booster cohort 8 days or more after receipt of the booster (Figure 1 and Table S3). Twelve of these infections progressed to severe Covid-19, but none progressed to critical or fatal disease. A total of 25,266 infections were recorded in the nonbooster cohort. Of these infections, 45 progressed to severe Covid-19, 4 progressed to critical Covid-19, and none progressed to fatal Covid-19.
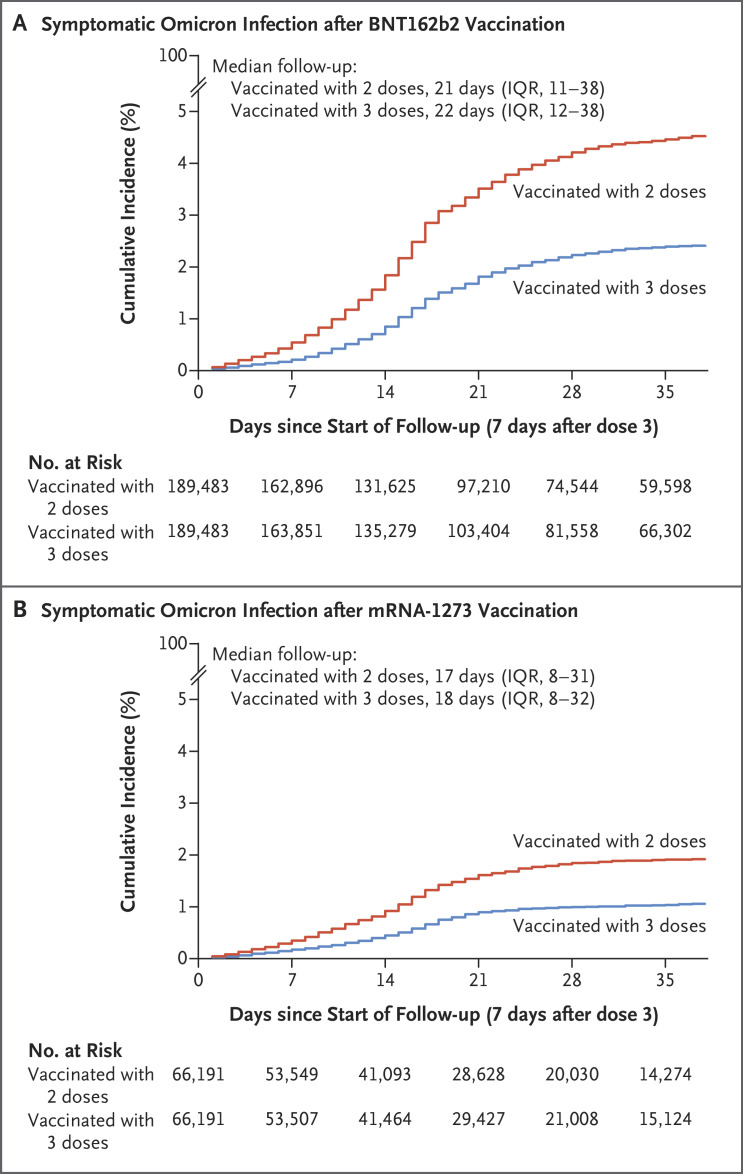
At 35 days after the start of follow-up, the estimated cumulative incidence of symptomatic infection was 2.4% (95% confidence interval [CI], 2.3 to 2.5) in the booster cohort and 4.5% (95% CI, 4.3 to 4.6) in the nonbooster cohort (Figure 2A). The estimated hazard ratio for symptomatic infection, adjusted for sex, 10-year age group, nationality group (of which there were 10), and calendar week of the second-dose vaccination, was 0.51 (95% CI, 0.48 to 0.53) (Table 2). The estimated effectiveness of the BNT162b2 booster against symptomatic omicron infection, as compared with that of the two-dose primary series, was 49.4% (95% CI, 47.1 to 51.6).
Figure 2. Estimated Cumulative Incidence of Symptomatic Omicron Infection in the Matched Booster and Nonbooster Cohorts.
Shown are Kaplan–Meier estimates of the cumulative incidence of symptomatic infection with the omicron variant of SARS-CoV-2, according to the type and number of doses of vaccine received. IQR denotes interquartile range.
Table 2. Effectiveness of BNT162b2 and mRNA-1273 Booster Vaccination against Infection with the Omicron Variant.
| Epidemiologic Measure | BNT162b2 Vaccine | mRNA-1273 Vaccine | ||
|---|---|---|---|---|
| Estimate | Effectiveness of Booster vs. Two-Dose Series (95% CI) |
Estimate | Effectiveness of Booster vs. Two-Dose Series (95% CI) |
|
| percent | percent | |||
| Total follow-up time — person-wk | ||||
| Booster cohort | 625,033 | — | 185,850 | — |
| Nonbooster cohort | 600,539 | — | 182,986 | — |
| Incidence rate of symptomatic infection per 10,000 person-wk (95% CI) | ||||
| Booster cohort | 49.0 (47.3–50.7) | — | 23.9 (21.8–26.2) | — |
| Nonbooster cohort | 92.9 (90.5–95.4) | — | 42.4 (39.5–45.5) | — |
| Adjusted hazard ratio for symptomatic infection (95% CI)* | 0.51 (0.48–0.53) | 49.4 (47.1–51.6) | 0.53 (0.47–0.59) | 47.3 (40.7–53.3) |
| Adjusted hazard ratio for Covid-19–related hospitalization and death (95% CI)† | 0.23 (0.12–0.44) | 76.5 (55.9–87.5) | — | — |
Cox proportional-hazards regression models were used to adjust for sex, 10-year age group, 10 nationality groups, and calendar week of the second-dose vaccination.
No estimate could be derived for the mRNA-1273 vaccine because there were no events in the booster cohort and few events in the nonbooster cohort.
The estimated adjusted hazard ratio for any severe, critical, or fatal Covid-19 (leading to hospitalization or death due to omicron infection) was 0.23 (95% CI, 0.12 to 0.44) (Table 2). The estimated effectiveness of the BNT162b2 booster against any severe, critical, or fatal Covid-19 (leading to hospitalization or death due to omicron infection), as compared with that of the two-dose primary series, was 76.5% (95% CI, 55.9 to 87.5).
Effectiveness of BNT162b2 Booster against Delta Variant
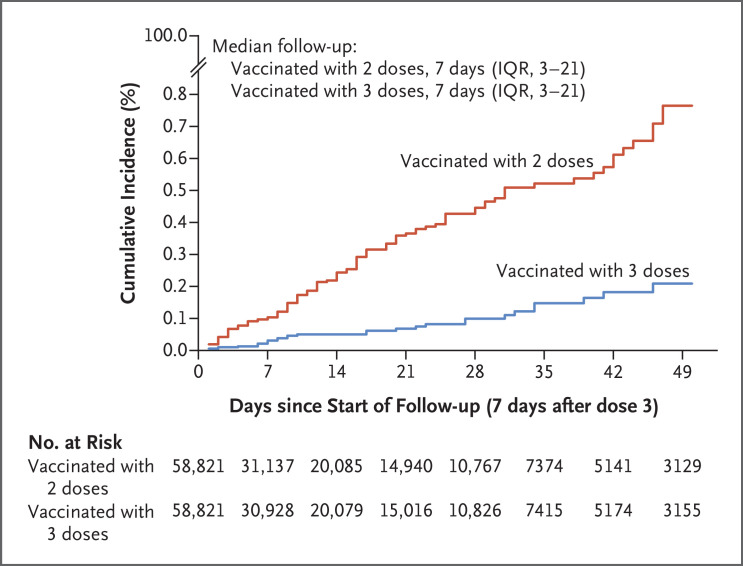
Figure 3 and Table 3 show the results of the delta-variant analysis. The estimated effectiveness of the BNT162b2 booster, as compared with that of the two-dose primary series, was 86.1% (95% CI, 67.3 to 94.1).
Figure 3. Estimated Cumulative Incidence of Symptomatic Delta Variant Infection in the Matched Booster (BNT162b2 Vaccine) and Nonbooster Cohorts.
Table 3. Effectiveness of BNT162b2 Booster Vaccination against Infection with the Delta Variant.
| Epidemiologic Measure | Estimate | Effectiveness of Booster vs. Two- Dose Series (95% CI) |
|---|---|---|
| percent | ||
| Total follow-up time — person-wk | ||
| Booster cohort | 120,914 | — |
| Nonbooster cohort | 120,599 | — |
| Incidence rate of symptomatic infection per 10,000 person-wk (95% CI) | ||
| Booster cohort | 0.6 (0.3–1.2) | — |
| Nonbooster cohort | 3.6 (2.6–4.8) | — |
| Adjusted hazard ratio for symptomatic infection (95% CI)* | 0.14 (0.06–0.33) | 86.1 (67.3–94.1) |
Cox proportional-hazards regression models were used to adjust for sex, 10-year age group, 10 nationality groups, and calendar week of the second-dose vaccination.
Study Population, mRNA-1273 Vaccine
Between January 24, 2021, and January 26, 2022, a total of 890,619 persons received at least two doses of mRNA-1273 vaccine and 92,829 received a third (booster) dose of that vaccine. The median date of the first dose was May 28, 2021; the median date of the second dose was June 27, 2021; and the median date of the third dose was January 1, 2022 (Fig. S1). The median time between the first and second doses was 28 days (interquartile range, 28 to 30 days), and the median time between the second and booster doses was 232 days (interquartile range, 211 to 250 days).
Figure 1 shows the population-selection process for the mRNA-1273 booster study. Table 1 shows the baseline characteristics of the eligible and matched cohorts. The study was based on the total population of Qatar, and thus the study population is broadly representative of this population (Table S2).
Effectiveness of mRNA-1273 Booster against Omicron Variant
The median follow-up time was 18 days (interquartile range, 8 to 32 days) in the booster cohort and 17 days (interquartile range, 8 to 31 days) in the nonbooster mRNA-1273 vaccine cohort (Fig. S1). A total of 3720 infections were recorded in the booster cohort 8 days or more after receipt of the booster (Figure 1 and Table S3). None of these infections progressed to severe, critical, or fatal Covid-19. A total of 4590 infections were recorded in the nonbooster cohort. Three of these infections progressed to severe Covid-19, but none progressed to critical or fatal Covid-19.
The estimated cumulative incidence of symptomatic infection was 1.0% (95% CI, 0.9 to 1.2) in the booster cohort and 1.9% (95% CI, 1.8 to 2.1) in the nonbooster cohort 35 days after the start of follow-up (Figure 2B). The estimated adjusted hazard ratio for symptomatic infection was 0.53 (95% CI, 0.47 to 0.59) (Table 2). The estimated effectiveness of the mRNA-1273 booster, as compared with that of the two-dose primary series, was 47.3% (95% CI, 40.7 to 53.3). The effectiveness of mRNA-1273 vaccine against any severe, critical, or fatal Covid-19 could not be calculated because no cases were recorded in the booster cohort and only three cases were seen in the nonbooster cohort (Figure 1).
Additional Analyses
For the BNT162b2 vaccine analysis, with the start of follow-up on the 15th day after the booster vaccination, the estimated effectiveness of the booster against symptomatic omicron infection, as compared with that of the two-dose primary series, was 49.9% (95% CI, 47.6 to 52.2) (Fig. S2 and Table S4). The corresponding estimated effectiveness of the mRNA-1273 vaccine was 52.0% (95% CI, 45.1 to 57.9). Both effectiveness estimates were similar to those in the main analysis.
The estimated effectiveness of the BNT162b2 vaccine booster against symptomatic omicron infection, as compared with that of the two-dose primary series, was 38.0% (95% CI, 28.8 to 46.0) in persons who received the booster 8 months or less after the second dose and 50.5% (95% CI, 48.2 to 52.8) in those who received it more than 8 months after the second dose. The corresponding estimates of the effectiveness of mRNA-1273 vaccine were 41.5% (95% CI, 32.3 to 49.5) and 56.8% (95% CI, 47.0 to 64.8).
Discussion
BNT162b2 booster vaccination was associated with an 86.1% reduction in the incidence of symptomatic delta variant infection and a 49.4% reduction in the incidence of symptomatic omicron infection. With the mRNA-1273 booster, the reduction in the incidence of symptomatic infection with the omicron variant was similar at 47.3%. Fewer cases of severe Covid-19 were observed in the booster cohorts than in the nonbooster cohorts, which is consistent with the strong protection against hospitalization and death associated with the booster. Moreover, cases of severe Covid-19 were rare in both the booster and nonbooster cohorts despite the large number of infections. These findings affirm the durability of vaccine protection against hospitalization and death several months after receipt of the second dose.1,2
In the context of a global expansion of the omicron variant and the dwindling incidence of the delta variant, these findings may suggest that a longer-term strategy for a global response is the development and administration of a new generation of broadly effective vaccines rather than continuing with a strategy of repeated booster vaccination with existing vaccines. Pan-coronavirus vaccines35 would target a broad range of existing and future SARS-CoV-2 variants.
The effectiveness of the BNT162b2 booster, as compared with that of the two-dose primary series, was slightly higher than that of the mRNA-1273 booster. This may be explained by the higher baseline mRNA-1273 vaccine effectiveness and slower waning of effectiveness after the second dose.2,10,11 Possibly because of less waning, the effectiveness of both mRNA vaccines, as compared with that of the two-dose primary series, was lower in persons who had received the booster 8 months or less after the second dose than in those who had received it more than 8 months after the second dose.
The estimates of effectiveness of the BNT162b2 and mRNA-1273 vaccines against the delta and omicron variants are broadly consistent with the growing evidence of the effectiveness of mRNA vaccines against these variants in other countries.22-24,36-39 However, in our study, the effectiveness of boosters was compared with that of the two-dose primary series; we did not compare the outcomes among persons who received boosters with those among unvaccinated persons. Nevertheless, with the waning of vaccine protection against infection over time after the second dose1,2 — and more so against infection with the omicron variant36 — the effectiveness of boosters as compared with that of the primary series should be only slightly lower than the protection afforded by boosters as compared with no vaccination.
This study has limitations. With the durable effectiveness conferred by a two-dose primary series of BNT162b2 and mRNA-1273 vaccines against Covid-19–related hospitalization and death,1,2 the lower severity of omicron as compared with that of previous variants,40 the young population of Qatar,15 and the time lag between infection and severe forms of Covid-19, we had insufficient data to estimate the effectiveness of the mRNA-1273 vaccine booster against Covid-19–related hospitalization and death. In this observational study, the vaccinated cohorts were aware of which vaccines they received and the participants did not undergo randomization, so potentially unmeasured or uncontrolled confounding cannot be ruled out. Although the cohorts were matched for sex, 10-year age group, nationality, and calendar week of the second-dose vaccination, matching was not possible for other factors such as coexisting conditions, occupation, or geographic location because such data were not available to the investigators. With the large wave of omicron variant infections, the use of rapid antigen testing was expanded to supplement PCR testing beginning on January 5, 2022, but information on symptoms and the reasons for testing was not available to be included in the analysis.
Matching was performed to control for factors that are known to affect exposure to SARS-CoV-2 infection in Qatar.15 Matching according to 10-year age group may have partially reduced potential bias due to coexisting conditions. The number of persons with severe chronic conditions is also small in the young population of Qatar.15,19 In the first phase of the vaccine rollout, the national list of vaccine prioritization included only 19,800 persons (of all age groups) with serious coexisting conditions.1 Matching according to nationality may have partially controlled for the differences in occupational risk, given the statistical association between the nationality of workers and occupation type in Qatar.15,25-28 Qatar is essentially a city-state, and the incidence of SARS-CoV-2 infection was broadly distributed across the country’s neighborhoods and areas; thus, geographic location is not likely to have been a confounding factor. The use of rapid antigen testing may not have differentially affected the investigated cohorts because it was broadly implemented. A strength of this study is the exclusion of persons with a documented previous infection, because the presence of a previous infection may affect booster protection.10
In this study, mRNA boosters were highly effective against infection with the delta variant but were less effective against infection with the omicron variant. However, these boosters led to strong protection against Covid-19–related hospitalization and death due to both variants. Given future waves of SARS-CoV-2 infections that may be driven by new variants, these findings may suggest the need for the development of a new generation of vaccines that target a broad range of variants to confront virus evolution, rather than a continued strategy of repeated boosters with existing vaccines.
Acknowledgments
We thank the many dedicated persons at Hamad Medical Corporation, the Ministry of Public Health, the Primary Health Care Corporation, Qatar Biobank, Sidra Medicine, and Weill Cornell Medicine–Qatar for the diligent efforts and contributions that made this study possible.
Supplementary Appendix
Disclosure Forms
This article was published on March 9, 2022, at NEJM.org.
Footnotes
Supported by the Biomedical Research Program and the Biostatistics, Epidemiology, and Biomathematics Research Core at Weill Cornell Medicine–Qatar; the Ministry of Public Health; Hamad Medical Corporation; and Sidra Medicine. The Qatar Genome Program and the Qatar University Biomedical Research Center provided the reagents needed for the viral genome sequencing.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Chemaitelly H, Tang P, Hasan MR, et al. Waning of BNT162b2 vaccine protection against SARS-CoV-2 infection in Qatar. N Engl J Med 2021;385(24):e83-e83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abu-Raddad LJ, Chemaitelly H, Bertollini R. Waning mRNA-1273 vaccine effectiveness against SARS-CoV-2 infection in Qatar. N Engl J Med. DOI: 10.1056/NEJMc2119432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andrews N, Tessier E, Stowe J, et al. Duration of protection against mild and severe disease by Covid-19 vaccines. N Engl J Med 2022;386:340-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nordström P, Ballin M, Nordström A. Risk of infection, hospitalisation, and death up to 9 months after a second dose of COVID-19 vaccine: a retrospective, total population cohort study in Sweden. Lancet 2022;399:814-823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization. Tracking SARS-CoV-2 variants (https://www.who.int/en/activities/tracking-SARS-CoV-2-variants/).
- 6.Planas D, Saunders N, Maes P, et al. Considerable escape of SARS-CoV-2 omicron to antibody neutralization. Nature 2022;602:671-675. [DOI] [PubMed] [Google Scholar]
- 7.Altarawneh HN, Chemaitelly H, Hasan MR, et al. Protection against the omicron variant from previous SARS-CoV-2 infection. N Engl J Med. DOI: 10.1056/NEJMc2200133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med 2020;383:2603-2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med 2021;384:403-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abu-Raddad LJ, Chemaitelly H, Ayoub HH, et al. Association of prior SARS-CoV-2 infection with risk of breakthrough infection following mRNA vaccination in Qatar. JAMA 2021;326:1930-1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abu-Raddad LJ, Chemaitelly H, Bertollini R. Effectiveness of mRNA-1273 and BNT162b2 vaccines in Qatar. N Engl J Med 2022;386:799-800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization. Living guidance for clinical management of COVID-19. November 23, 2021. (https://www.who.int/publications/i/item/WHO-2019-nCoV-clinical-2021-2).
- 13.World Health Organization. International guidelines for certification and classification (coding) of COVID-19 as cause of death. April 20, 2020. (https://www.who.int/classifications/icd/Guidelines_Cause_of_Death_COVID-19-20200420-EN.pdf?ua=1).
- 14.Abu-Raddad LJ, Chemaitelly H, Butt AA. Effectiveness of the BNT162b2 Covid-19 vaccine against the B.1.1.7 and B.1.351 variants. N Engl J Med 2021;385:187-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abu-Raddad LJ, Chemaitelly H, Ayoub HH, et al. Characterizing the Qatar advanced-phase SARS-CoV-2 epidemic. Sci Rep 2021;11:6233-6233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tang P, Hasan MR, Chemaitelly H, et al. BNT162b2 and mRNA-1273 COVID-19 vaccine effectiveness against the SARS-CoV-2 delta variant in Qatar. Nat Med 2021;27:2136-2143. [DOI] [PubMed] [Google Scholar]
- 17.Abu-Raddad LJ, Chemaitelly H, Ayoub HH, et al. Relative infectiousness of SARS-CoV-2 vaccine breakthrough infections, reinfections, and primary infections. Nat Commun 2022;13:532-532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abu-Raddad LJ, Chemaitelly H, Bertollini R. Severity of SARS-CoV-2 reinfections as compared with primary infections. N Engl J Med 2021;385:2487-2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seedat S, Chemaitelly H, Ayoub HH, et al. SARS-CoV-2 infection hospitalization, severity, criticality, and fatality rates in Qatar. Sci Rep 2021;11:18182-18182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chemaitelly H, Yassine HM, Benslimane FM, et al. mRNA-1273 COVID-19 vaccine effectiveness against the B.1.1.7 and B.1.351 variants and severe COVID-19 disease in Qatar. Nat Med 2021;27:1614-1621. [DOI] [PubMed] [Google Scholar]
- 21.Hernán MA, Robins JM. Using big data to emulate a target trial when a randomized trial is not available. Am J Epidemiol 2016;183:758-764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barda N, Dagan N, Cohen C, et al. Effectiveness of a third dose of the BNT162b2 mRNA COVID-19 vaccine for preventing severe outcomes in Israel: an observational study. Lancet 2021;398:2093-2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arbel R, Hammerman A, Sergienko R, et al. BNT162b2 vaccine booster and mortality due to Covid-19. N Engl J Med 2021;385:2413-2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Andrews N, Stowe J, Kirsebom F, et al. Effectiveness of COVID-19 booster vaccines against Covid-19 related symptoms, hospitalisation and death in England. Nat Med 2022. January 14 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ayoub HH, Chemaitelly H, Seedat S, et al. Mathematical modeling of the SARS-CoV-2 epidemic in Qatar and its impact on the national response to COVID-19. J Glob Health 2021;11:05005-05005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coyle PV, Chemaitelly H, Ben Hadj Kacem MA, et al. SARS-CoV-2 seroprevalence in the urban population of Qatar: an analysis of antibody testing on a sample of 112,941 individuals. iScience 2021;24:102646-102646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Al-Thani MH, Farag E, Bertollini R, et al. SARS-CoV-2 infection is at herd immunity in the majority segment of the population of Qatar. Open Forum Infect Dis 2021;8(8):ofab221-ofab221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jeremijenko A, Chemaitelly H, Ayoub HH, et al. Herd immunity against severe acute respiratory syndrome coronavirus 2 infection in 10 communities, Qatar. Emerg Infect Dis 2021;27:1343-1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abu-Raddad LJ, Chemaitelly H, Yassine HM, et al. Pfizer-BioNTech mRNA BNT162b2 Covid-19 vaccine protection against variants of concern after one versus two doses. J Travel Med 2021;28(7):taab083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Global Initiative on Sharing Avian Influenza Data. Qatar viral genome sequencing data: data on randomly collected samples (https://www.gisaid.org/phylodynamics/global/nextstrain/).
- 31.Austin PC. Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Commun Stat Simul Comput 2009;38:1228-1234. [Google Scholar]
- 32.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958;53:457-481. [Google Scholar]
- 33.Hasan MR, Kalikiri MKR, Mirza F, et al. Real-time SARS-CoV-2 genotyping by high-throughput multiplex PCR reveals the epidemiology of the variants of concern in Qatar. Int J Infect Dis 2021;112:52-54. [DOI] [PubMed] [Google Scholar]
- 34.Benslimane FM, Al Khatib HA, Al-Jamal O, et al. One year of SARS-CoV-2: genomic characterization of COVID-19 outbreak in Qatar. Front Cell Infect Microbiol 2021;11:768883-768883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morens DM, Taubenberger JK, Fauci AS. Universal coronavirus vaccines — an urgent need. N Engl J Med 2022;386:297-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Andrews N, Stowe J, Kirsebom F, et al. Covid-19 vaccine effectiveness against the omicron (B.1.1.529) variant. N Engl J Med. DOI: 10.1056/NEJMoa2119451. [DOI] [PMC free article] [PubMed]
- 37.Buchan SA, Chung H, Brown KA, et al. Effectiveness of COVID-19 vaccines against omicron or delta infection. January 1, 2022. (https://www.medrxiv.org/content/10.1101/2021.12.30.21268565v1). preprint. [DOI] [PMC free article] [PubMed]
- 38.Hansen CH, Schelde AB, Moustsen-Helm IR, et al. Vaccine effectiveness against SARS-CoV-2 infection with the omicron or delta variants following a two-dose or booster BNT162b2 or mRNA-1273 vaccination series: a Danish cohort study. December 21, 2021. (https://www.medrxiv.org/content/10.1101/2021.12.20.21267966v1). preprint.
- 39.Tartof SY, Slezak JM, Puzniak L, et al. Effectiveness of a third dose of BNT162b2 mRNA COVID-19 vaccine in a large US health system: a retrospective cohort study. December 21, 2021. (https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3989856). preprint. [DOI] [PMC free article] [PubMed]
- 40.Wolter N, Jassat W, Walaza S, et al. Early assessment of the clinical severity of the SARS-CoV-2 omicron variant in South Africa: a data linkage study. Lancet 2022;399:437-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.