Abstract
Purpose:
Exposure to narrow-band red light, which stimulates only the long-wavelength sensitive (LWS) cones, slows axial eye growth and produces hyperopia in tree shrews and monkeys. We asked whether exposure to amber light, which also stimulates only the LWS cones but with a greater effective illuminance than red light, has a similar hyperopia-inducing effect in tree shrews.
Methods:
Starting at 24±1 days of visual experience, 15 tree shrews (dichromatic mammals closely related to primates) received light treatment through amber filters (BPI 500/550 dyed acrylic) either atop the cage (Filter group, n=8, 300–400 human lux) or fitted into goggles in front of both eyes (Goggle group, n=7). Non-cycloplegic refractive error and axial ocular dimensions were measured daily. Treatment groups were compared with age-matched animals (Colony group, n=7) raised in standard colony fluorescent lighting (100–300 lux).
Results:
At the start of treatment, mean refractive errors were well-matched across the three groups (p=0.35). During treatment, the Filter group became progressively more hyperopic with age (p<0.001). By contrast, the Goggle and Colony groups showed continued normal emmetropization. When the treatment ended, the Filter group exhibited significantly greater hyperopia (mean (SE) = 3.5 (0.6) D) compared with the Goggle (0.2 (0.8) D, p=0.01) and Colony groups (1.0 (0.2) D, p=0.01). However, the refractive error in the Goggle group was not different from that in the Colony group (p=0.35). Changes in the vitreous chamber were consistent with the refractive error changes.
Conclusions:
Exposure to ambient amber light produced substantial hyperopia in the Filter group but had no effect on refractive error in the Goggle group. The lack of effect in the Goggle group could be due to the simultaneous activation of the short-wavelength sensitive (SWS) and LWS cones caused by the scattering of the broad-band light from the periphery of the goggles.
Keywords: Myopia, Animal Models, Refractive error, Wavelength, Longitudinal chromatic aberration, Induced hyperopia
Introduction
Most species, including humans, are born with a mismatch between the eye’s optical power (focal plane) and axial eye size, resulting in the formation of out-of-focus images of distant objects on the retina.1–7 During postnatal development, a feedback mechanism—termed emmetropization—regulates eye growth to reduce this mismatch to align, and then maintain the retina at the focal plane of the eye to attain a state of emmetropia, in which images of distant objects are in focus on the retina.8–11 To achieve this, the emmetropization mechanism actively derives sign information from defocus-related visual cues.12,13 For instance, during experimental manipulations of image focus (e.g., by imposing a positive or negative spherical lens), the emmetropization mechanism uses cues from the induced defocus to adjust the rate of axial elongation of the eye, moving the retina to the altered focal plane and compensating for the imposed defocus. This highly coordinated homeostatic mechanism of emmetropization is local to the eye,14 operating even in the absence of an intact central connection to the brain,15,16 or even when visual cues related to defocus are only available at local retinal regions.17,18 The emmetropization mechanism regulates axial eye growth in both infancy (where it serves to achieve emmetropia) and adolescence (where it serves to maintain emmetropia).19–21 At times the emmetropization mechanism fails to function normally, resulting in refractive errors such as myopia, which is rapidly rising in prevalence and severity worldwide, placing billions at risk of vision loss from blinding ocular pathology in later life and posing an urgent need for the identification of effective and novel interventions to slow its onset and progression.22–24
One potential strategy to treat myopia is to modify eye growth by tapping into the emmetropization pathway through deliberate manipulation of defocus-related visual cues involved in eye growth regulation. Indeed, the success of several optical treatments in slowing myopia progression suggests that the emmetropization mechanism continues to function in myopes, despite their deviation from emmetropia.25 Out-of-focus retinal images contain several potential cues that could provide defocus information, including longitudinal chromatic aberration (LCA). Across the range of the visible spectrum, vertebrate eyes have approximately 2 to 3 dioptres of LCA, with the longer wavelengths (red end of the spectrum) in focus further away from the cornea than the shorter wavelengths (blue end of the spectrum) in broadband lighting.26 Thus, the emmetropization mechanism could exploit the LCA inherent in the eye under broadband lighting to derive information about the state of focus. For instance, when longer wavelengths (e.g., red) are in better focus than the shorter wavelengths (e.g., blue), the emmetropization mechanism could infer that the eye is too long and signal to slow eye growth. Conversely, when shorter wavelengths are in better focus, the emmetropization mechanism could infer that the eye is too short and signal to accelerate eye growth.
As a dichromatic mammal, tree shrews contain two cone classes: short-wavelength sensitive (SWS, peak absorption at 428 ± 15 nm) cones and long-wavelength sensitive (LWS, peak absorption at 555 ± 6 nm, Figure 1).27 When tree shrews are exposed to a monochromatic light stimulus such as narrow-band red light, image contrast exists only for red, thereby activating only the LWS cones without the stimulation of SWS cones. In this scenario, the eyes slow their growth, producing hyperopia, suggesting that the emmetropization mechanism interprets red contrast in the absence of blue contrast as a sign that the eye is too long. This notion is consistent with a recently proposed opponent dual-detector spectral drive model of emmetropization. In this model, when red light in better focus than blue, it causes sharper image contrast on LWS cones and produces a negative “spectral drive” that signals the emmetropization mechanism to slow axial elongation.28
Figure 1.
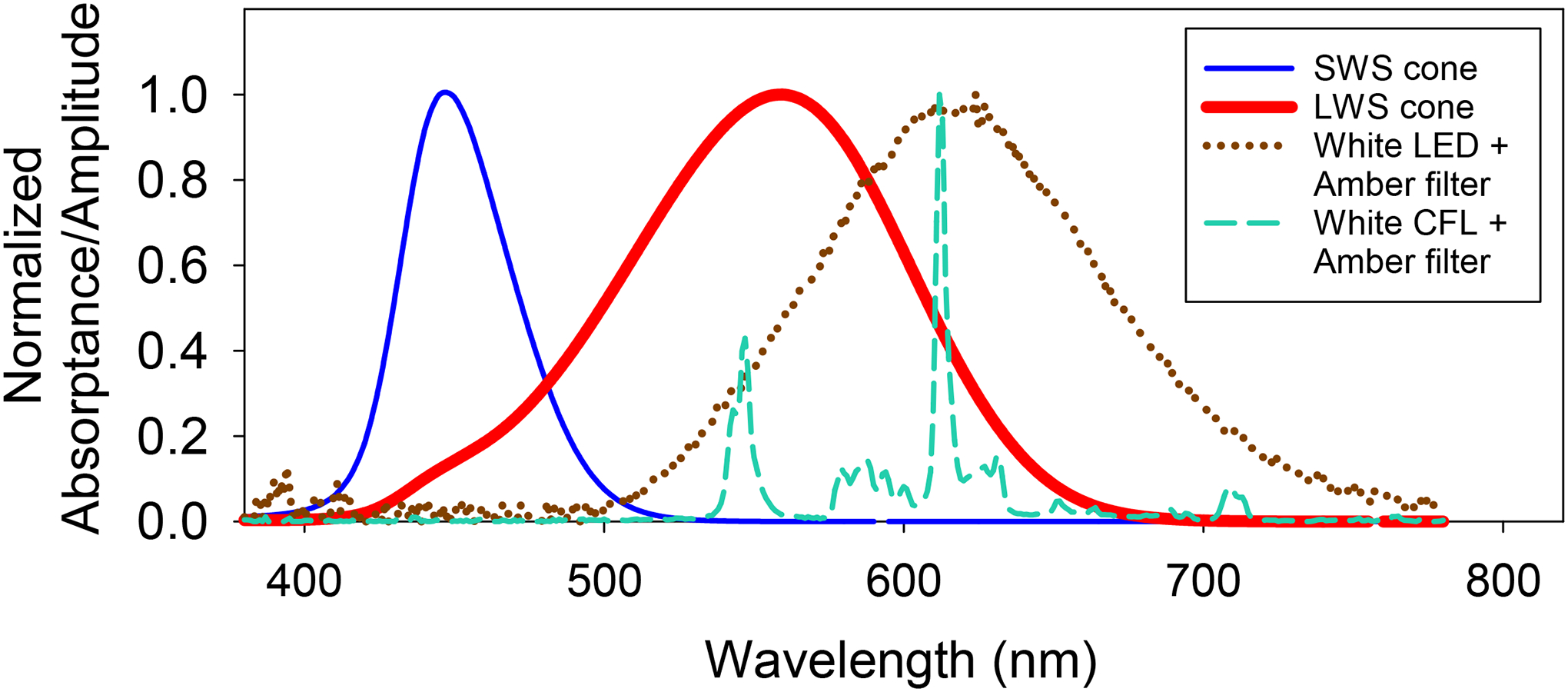
Dashed lines indicate the post-filter spectra of the light from commercial white LED bulbs (light-emitting diodes) and CFLs (compact fluorescent lights) taken at the cage floor. Both spectra have negligible light below 500 nm. Superimposed on the light spectra are the relative absorbances for the two classes of tree shrew cones (long-wavelength sensitive, LWS; short-wavelength sensitive, SWS), produced by modifying the toolbox in Lucas et al.73 using data from Petry and Hárosi.27
The ability of the emmetropization mechanism to regulate eye growth and its normal operating characteristics appears to rely on the presence of broadband light containing a wide range of wavelengths that allow comparisons of focus between longer and shorter wavelengths.29–32 Several studies have demonstrated that animals fail to emmetropize (i.e., develop refractive errors) in quasi-monochromatic light (visible spectrum restricted to a narrow-band light) that lacks LCA cues. These findings provide strong evidence that the feedback from LCA cues inherent in the broadband light is essential for the emmetropization mechanism to function properly.19,32–36 However, disparities exist in the way different species respond to varying wavelength conditions (see review37). While species more distant to humans like fish, chicken, guinea pigs and mice develop myopia in long-wavelength (red) light and hyperopia in short-wavelength (blue/violet) light,38–44 mammals more closely related to humans such as tree shrews and macaque monkeys show the opposite responses. Exposure to narrow-band red light (peak wavelength ~630 nm) slows axial elongation and produces substantial hyperopia in infantile tree shrews, contrary to the rapid decline in hyperopia typically observed when these animals are reared in broadband lighting.34 The hyperopia-inducing effect of narrow-band red light in tree shrews is highly nonlinear. Consistent with other stimuli that slow axial growth such as plus lenses and recovery from deprivation or minus-lens wear, even one hour per day of red light exposure is sufficient to produce significant amounts of hyperopia.45 Narrow-band red light promotes hyperopia development even in older animals in which the emmetropization mechanism no longer responds to a myopic defocus cue provided by an imposed positive spherical lens to restrain axial growth.19,46 Rhesus monkeys raised under ambient narrow-band red light or by wearing red filters in front of the eyes also show similar responses; developing hyperopia and even counteracting the myopiagenic effect of a negative spherical lens.32,35,36
These observations indicate that narrow-band red light is a pro-hyperopia/anti-myopia stimulus, raising the possibility of translating and clinically applying red light as a novel and easy-to-administer intervention to control myopia in children. However, filtering broad-band white light through long-wavelength (red) pass filters causes a substantial (about 95%) reduction in illuminance, requiring ambient lighting in the visual environment to be increased many fold to avoid light intolerance in children and to ensure sufficient image brightness for visual tasks. Such a lighting arrangement might be possible outdoors but would be extremely challenging, if not impractical, in a typical indoor environment.
By contrast, when broad-band white light is used in conjunction with “amber” filters that allow transmission of all wavelengths above 500 nm, this only reduces illumination by about 23%. This may potentially make amber filtered light more tolerable in children, and a viable intervention even in indoor lighting without the need to increase ambient illumination. Therefore, we asked whether exposure to amber light, which only contains wavelengths above 500 nm (Figure 1), promotes hyperopia development, and if so, whether it does as effectively as narrow-band red light. We hypothesized that the hyperopia-inducing effect of narrow-band red light is not due to the long-wavelength light directly but rather due to the removal of short wavelengths, resulting in the lack of activation of SWS cones, which causes the emmetropization mechanism to interpret the red image contrast as a signal to slow eye growth. Since amber light with a 500-nm lower cut-off only stimulates LWS cones, exposure to these wavelengths should also signal to the emmetropization mechanism that the eye is too long and thereby slow eye growth to produce hyperopia. We tested this hypothesis by raising juvenile tree shrews in ambient amber lighting (produced by filtering broad-band light through amber filters). Because wearing filters in goggles is much more practical as an intervention than attempting to illuminate the entire environment (particularly outdoors) with restricted wavelengths, we also raised a group of animals who wore amber goggles in broad-band light. A recent study in which dim broadband light was presented simultaneously with red light found that the presence of the broadband light greatly reduced the red-light hyperopia.47 Raising animals with amber goggles, which allowed some unfiltered white light to reach the retina from the periphery, would also enable us to study this possibility with amber light. For generalizability, we used two different broadband light sources, light-emitting diodes (LEDs) and compact fluorescent lights (CFLs).
Materials and Methods
The juvenile northern tree shrews48 (Tupaia belangeri) in this study were raised by their mothers in the University of Alabama at Birmingham (UAB) Tree Shrew Core. Tree shrews are born with their eyes closed and do not open the eyes until approximately three weeks postnatal. Therefore, we designated the first day of binocular eye-opening as the first day of visual experience (DVE). Amber light treatment started at the typical age of weaning after approximately three weeks of visual experience (six weeks of age). By this time, the initial emmetropization process is nearly complete, and refractive error plateaus at low hyperopia (approximately 1.4 D46). Starting treatment at this age provides a stable baseline for evaluating the effect of various wavelengths on emmetropization. All animal care adhered to the guidelines by the Association for Research in Vision and Ophthalmology for the use of animals in ophthalmic and visual research. The UAB Institutional Animal Care and Use Committee approved all experimental procedures in the study.
Experimental groups
Fifteen tree shrews (nine males/six females) were the subjects in this study. Animals were divided into the Filter group (n=8) or the Goggle group (n=7). All animals within a group came from different litters. They were exposed to binocular amber light through filters starting at 24 ± 1 DVE for 11 days. The amber filters were acrylic plates dyed with BPI (Brain Power Incorporated, callbpi.com) Diamond Dye 500/550 at 200°F for 60 minutes. The amber lenses were plano PMMA acrylic lenses with a base curve of 7.50 mm and a diameter of 12 mm (Conforma Laboratories, conforma.com) that were dyed similarly. All panels and lenses were tested with an Ocean Optics STS Microspectrometer (oceanoptics.com) (transmission spectrum shown in Figure S1), and those that did not cut off light below 500 nm were discarded. Approximately, one in five tested panels and lenses were discarded due to inconsistent filtering property achieved after dying the materials. Animals in the Filter group received amber light through amber filters atop the cage, whereas animals in the Goggle group received amber light through amber-dyed lenses fitted into a goggle frame that clipped onto a pedestal (see pedestal details below). Refractive error and ocular axial measurements (mean of right and left eyes) of the Filter and Goggle animals were compared with data from a group of animals raised in standard colony lighting (Colony group) in identical cages and environmental conditions.45 These animals received ambient illumination from fluorescent bulbs placed in the ceiling of the colony rooms.
Lighting Conditions
All animals were raised in a temperature-controlled colony maintained on a 14-hour light/10-hour dark cycle, with the light cycle starting at 08:30 hrs. Fluorescent bulbs (type F34CW RS WM ECO, General Electric Lighting,.gelighting.com) containing a wide range of wavelengths spanning the visible spectrum provided the colony lighting. The ambient illumination at the cage floor measured with a digital illuminance meter (LX1330B, Hisgadget, hisgadget.com) ranged from 100 to 300 lux during the daily 14-hour light cycle. These lighting conditions are similar to those used in previous studies from this laboratory.49–51
During amber light treatment, each animal was housed in its cage in a large dimly illuminated room, which contained two small bays separated from each other by black curtains to prevent light leakage. Each bay contained two side-by-side cages, having stainless-steel walls and measuring 60 cm × 60 cm × 60 cm. Each cage had an open mesh on the front and the top, and received ambient illumination from either the array of soft-white LED bulbs (Feit BPCEAG/500/4) or CFL bulbs (ecosmart EDXO-14) placed on top of the cage. With this ambient illumination, animals could see within the cage and up to approximately three meters outside the cage into the surrounding room. Ambient illumination for the Filter group was from either white LEDs (n=4) or white CFLs (n=4) and ranged from 300–400 lux at the centre of the cage floor. All animals in the Goggle group (n=7) received ambient illumination from the white CFLs.
Pedestal Installation
For accurate and consistent alignment of animals during refractive and axial component measurements and to hold the goggle frame containing the amber filters in the Goggle group, a dental acrylic pedestal was installed onto the skull of all experimental animals two to three days before treatment using procedures previously described.52 In brief, the animals were removed from their mother’s cage and anaesthetized with an intramuscular injection of 100 mg/kg ketamine and 7 mg/kg xylazine. After initial anaesthesia, but before the procedure began, they received administration of intraperitoneal atropine 0.27 mg/kg, intramuscular buprenorphine 0.02 mg/kg, and subcutaneous carprofen 5 mg/kg. Anaesthesia was supplemented with 0.5 to 2% isoflurane as needed. Following recovery from anaesthesia, the animals were weaned and housed singly, in broad-spectrum colony lighting. At the start of amber light treatment (24 ± 1 DVE), they were transferred into the treatment cages and housed individually.
Refractive Error and Ocular Axial Measurements
The refractive error of each eye was measured in awake and gently restrained animals using a Nidek ARK-700A infrared autorefractor (Marco Ophthalmic, marco.com). During autorefraction, the animal was aligned using the pedestal and 10 measurements were taken for each eye, with the five measures having the highest quality scores being averaged for the final result. As in previous studies from this laboratory, non-cycloplegic measures were recorded as they have been shown to provide a valid estimate of refractive error in tree shrews. When measured in the same animals, the difference in refractive error before and after cycloplegia (1% atropine) was small (approximately 0.8 D) and consistent irrespective of whether the eyes were myopic or not.53
Refractive error was measured in a dimly illuminated room between approximately 10:00 and 11:00 hrs daily, except in two goggle-wearing animals, in whom measurements were recorded every other day for technical reasons. Animals were kept in darkness while being transported from their treatment cage to the measurement room. During measurements, the internal incandescent target light of the autorefractor was disabled to avoid confounding effects of exposure to the same wavelength.
Immediately after the refractive measurements, axial component dimensions were measured in awake, gently restrained animals with the LenStar LS-900 optical biometer (Haag-Streit, haag-streit.com) using tree-shrew specific refractive indices.54 This system uses the principle of optical low-coherence optical interferometry to record axial dimensions and generally yields values consistent with A-scan ultrasonography, but with much better repeatability.55 Animals had daily axial component measurements recorded, except for two goggle-wearing animals, in whom axial component dimensions were measured every other day. These temporal measures of ocular component dimensions allowed us to evaluate whether any changes in refractive error were attributable to axial changes (e.g., vitreous chamber depth). Data (average of two eyes) obtained from the Filter and Goggle animals were compared with similar measurements recorded less frequently in the Colony animals raised in standard colony lighting.
Data Analysis
Refractive errors were expressed as spherical equivalent errors at the corneal plane, averaged, and corrected for the “small eye artefact” previously shown to be approximately +4 dioptres in tree shrews.56,57 Data (refractive error and axial component measurements) were imported and analysed in R (R project for Statistical Computing, r-project.org)58 and SigmaPlot Windows version 14.0 (Systat Software, systatsoftware.com).
As in previous studies,1,59 typically the right eye and left eye of an animal showed similar measurements (Supplementary Figure S2). For instance, the mean absolute difference in refractive error between the two eyes at the start of treatment was 0.6 D in the Filter animals (paired t7 = 1.10, p = 0.31) and 0.5 D in the Goggle animals (paired t6 = 0.16, p = 0.88). There was also a strong correlation between the refractive errors of the right and left eyes (r > 0.80). Therefore, outcome measures were the average of the right and left eyes. A two-way mixed analysis of variance (ANOVA) was performed with “DVE” as a within-subject factor and “Group” as a between-subject factor. If the interaction effect of these two factors on refractive error was significant, one-way ANOVA tests were used to compare the outcome measures across the experimental groups at the start (24 DVE) and end of treatment (35 DVE). If the group effect was significant, differences between any two groups were tested for statistical significance using post-hoc unpaired t-tests with Holm correction for multiple testing. A two-way, repeated measures (RM)-ANOVA with “DVE” and “Eye” as two within-subject factors was conducted to evaluate the temporal changes of outcome measures in a particular group. Results were verified with equivalent non-parametric tests. Data are presented as mean (SE) unless otherwise stated. P-values < 0.05 were considered statistically significant.
Results
Refractive error
Figure 2A and Table 1 shows the average refractive errors of the Filter and Goggle animals exposed to amber light during the 11-day treatment period. Data are compared with the refractive errors of the animals raised in standard colony lighting. A two-way mixed ANOVA showed a significant interaction effect of Group and DVE on refractive error (F2,82 = 11.6, p < 0.001). At the start of treatment, animals in both the Goggle and Filter groups had completed the initial emmetropization process and achieved an age-appropriate hyperopic refractive error, similar to the animals in the Colony group. Group refractive errors were 1.1 (0.4) D in the Filter group, 1.9 (0.5) D in the Goggle group and 1.7 (0.3) D in the Colony group; these starting refractive errors were not different amongst the groups (F2,19 = 0.99, p = 0.39). During treatment, refractive errors of the Filter group departed from the normal and became progressively more hyperopic with age (F11,66 = 12.22, p < 0.001). The temporal pattern of hyperopic development was not different between the two eyes in these animals (F1,6 = 0.01, p = 0.92, Supplementary Figure S2). By contrast with the Filter group, the Goggle and Colony groups exhibited a normal emmetropization response throughout the treatment period, showing a gradual decrease of refractive error toward emmetropia. The rapid shift towards hyperopia in the Filter group persisted throughout the treatment period, suggesting a continuing effect of the amber lighting on refractive errors.
Figure 2.
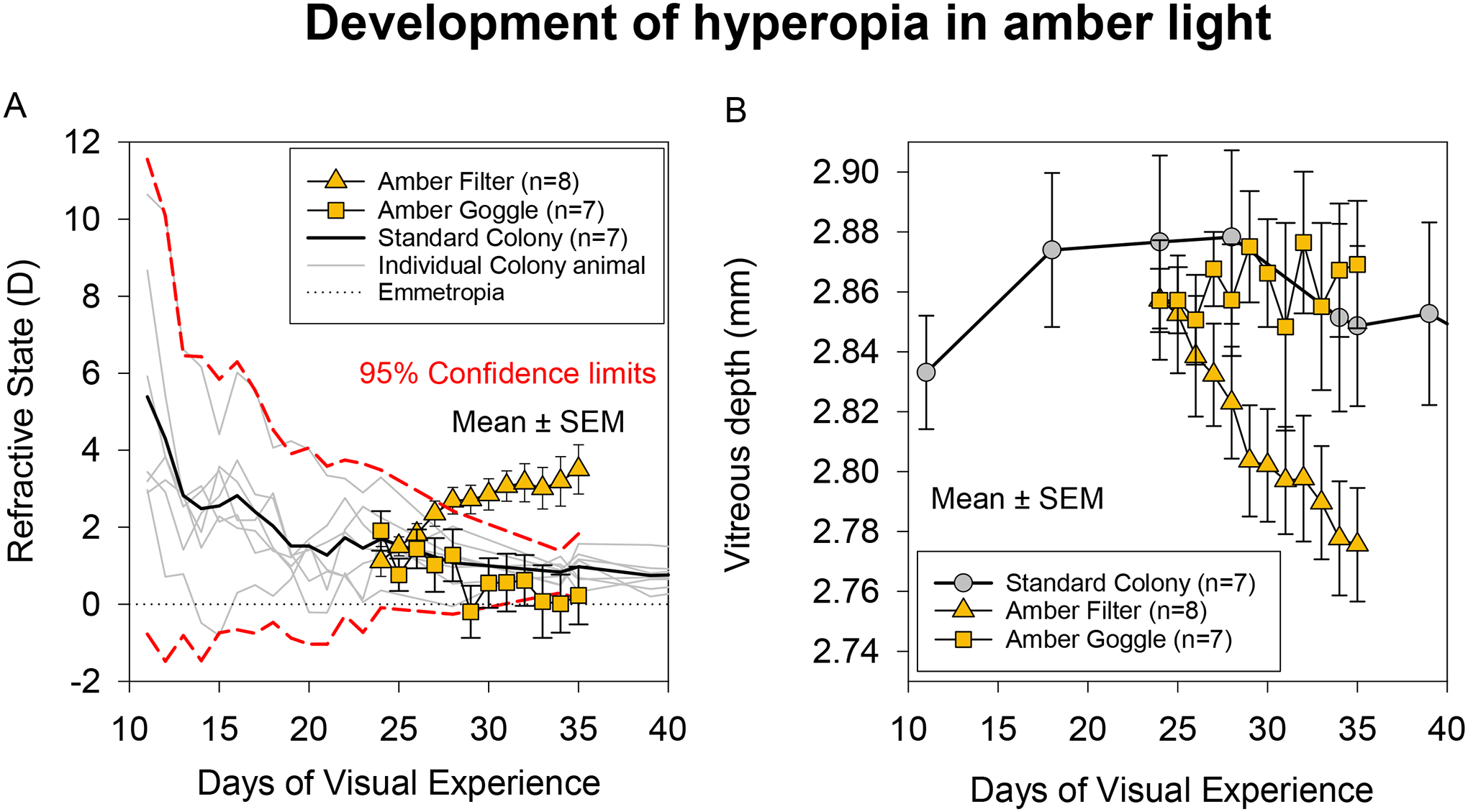
A) Refractive error and B) vitreous chamber depth of animals raised in amber light and standard colony light. Data are mean of the right and left eyes. The amber filled triangles are group means of animals reared under amber filtered ambient light, and the amber filled squares are group means of animals reared with goggles containing amber filters. The solid black line in A and grey circles in B are mean values of the animals raised in standard colony lighting. The grey lines are individual animals, and the red dashed lines are the 95% confidence intervals. The dotted line in A indicates emmetropia.
Table 1.
Refractive errors of the Amber Filter and Amber Goggle animals compared with standard colony lighting animals (Ward et al., 2018).
| DVE | Colony (C) (n=7) | Filter (F) (n=8) | Goggle (G) (n=7) | One-way ANOVA (p-value) | Unpaired t-test (adjusted p-value) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SEM | Mean | SEM | Mean | SEM | C v F | C v G | F v G | ||
| 24 | 1.7 | 0.3 | 1.1 | 0.4 | 1.9 | 0.5 | 0.39 | |||
| 25 | 1.5 | 0.2 | 0.8 | 0.4 | ||||||
| 26 | 1.8 | 0.3 | 1.4 | 0.5 | ||||||
| 27 | 2.4 | 0.3 | 1.0 | 0.7 | ||||||
| 28 | 1.1 | 0.3 | 2.7 | 0.3 | 1.3 | 0.7 | ||||
| 29 | 2.7 | 0.4 | −0.2 | 0.7 | ||||||
| 30 | 2.9 | 0.4 | 0.6 | 0.6 | ||||||
| 31 | 3.1 | 0.4 | 0.6 | 0.8 | ||||||
| 32 | 3.2 | 0.5 | 0.6 | 0.7 | ||||||
| 33 | 3.0 | 0.5 | 0.1 | 0.9 | ||||||
| 34 | 0.8 | 0.1 | 3.2 | 0.6 | 0.0 | 0.8 | ||||
| 35 | 1.0 | 0.2 | 3.5 | 0.6 | 0.2 | 0.8 | 0.002 | 0.01 | 0.35 | 0.01 |
Mixed ANOVA: DVE*Group: F2,82 = 11.6, p < 0.001
All values are diopters (D); DVE: Days of Visual Experience
When the treatment ended, animals in the Filter group had developed significant hyperopia (3.5 (0.6) D), whereas refractive errors in the Goggle animals (0.2 (0.8) D) and the Colony animals (1.0 (0.2) D) were near emmetropia. The final refractive error in the Filter group was, on average, 2.5 D more hyperopic than the Colony group and 3.3 D more hyperopic than the Goggle group (F2,19 = 8.93, p = 0.002). Post-hoc comparisons showed a significant difference in refractive error between the Filter and Goggle animals (t13 = 3.35, p = 0.01) and between the Filter and Colony animals (t13 = 3.60, p = 0.01) at the end of treatment. However, the final refractive error in the Goggle group was not different from that in the Colony group (t12 = −0.98, p = 0.35). No obvious differences were apparent in the pattern of hyperopic development between the two subgroups of Filter animals that received illumination from either CFLs or LEDs. After the 11-day amber light treatment, the mean change in refractive error was 2.4 D in the Filter CFL subgroup and 2.3 D in the Filter LED subgroup.
Axial component dimensions
Figure 2B and Table 2 shows the average vitreous chamber depths of the Filter and Goggle animals exposed to amber light treatment compared with the vitreous chamber depth of the animals raised in standard colony lighting. A two-way mixed ANOVA showed a significant interaction effect of Group and DVE on vitreous depth (F2,81 = 3.43, p = 0.04). At the start of treatment, vitreous depths were 2.86 (0.02) mm in the Filter group and 2.86 (0.01) mm in the Goggle group. These measures in the treated groups were well-matched with the starting vitreous depth in the Colony group (2.88 (0.03) mm, F2,19 = 0.28, p = 0.76). During amber light treatment, the vitreous chamber depth of the Filter animals decreased with a similar time course to the increase in hyperopia. In contrast, the depth of the vitreous chamber in the Goggle group remained relatively stable and followed a similar trend to the Colony animals. When the treatment ended at 35 DVE, there was a significant difference in vitreous chamber depth across the groups (F2,19 = 5.05, p = 0.02); vitreous chamber depth in the Filter group was on average 0.09 mm smaller than the Goggle group and 0.07 mm smaller than the Colony group. Post-hoc comparisons showed that the differences in end-of-treatment vitreous chamber depth were statistically significant between the Filter and Goggle groups (t13 = 3.29, p = 0.02) and between the Filter and Colony groups (t13 = 2.27, unadjusted p = 0.04, adjusted p = 0.08) but not between the Colony and Goggle groups (t12 = −0.6, p = 0.56).
Table 2.
Vitreous chamber depth of the Amber Filter and Amber Goggle animals compared with the standard colony lighting animals (Ward et al., 2018).
| DVE | Colony (C) (n=7) | Filter (F) (n=8) | Goggle (G) (n=7) | One-way ANOVA (p-value) | Unpaired t-test (adjusted p-value) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SEM | Mean | SEM | Mean | SEM | C v F | C v G | F v G | ||
| 24 | 2.88 | 0.03 | 2.86 | 0.02 | 2.86 | 0.01 | 0.76 | |||
| 25 | 2.88 | 0.03 | 2.85 | 0.02 | 2.86 | 0.01 | ||||
| 26 | 2.84 | 0.02 | 2.85 | 0.01 | ||||||
| 27 | 2.83 | 0.02 | 2.87 | 0.01 | ||||||
| 28 | 2.82 | 0.02 | 2.86 | 0.02 | ||||||
| 29 | 2.80 | 0.02 | 2.88 | 0.02 | ||||||
| 30 | 2.80 | 0.02 | 2.87 | 0.02 | ||||||
| 31 | 2.80 | 0.02 | 2.85 | 0.03 | ||||||
| 32 | 2.80 | 0.02 | 2.88 | 0.02 | ||||||
| 33 | 2.79 | 0.02 | 2.86 | 0.03 | ||||||
| 34 | 2.85 | 0.03 | 2.78 | 0.02 | 2.87 | 0.02 | ||||
| 35 | 2.85 | 0.03 | 2.78 | 0.02 | 2.87 | 0.02 | 0.02 | 0.08† | 0.56 | 0.02 |
Mixed ANOVA: DVE*Group: F2,81 = 3.43, p = 0.04
All values are millimeters (mm); DVE: Days of Visual Experience
Unadjusted p = 0.04
For other ocular component dimensions, choroidal thickness was the only parameter that differed significantly across the groups when the 11-day amber light treatment ended at 35 DVE (F2,19 = 5.01, p = 0.02, Figure 3). By the end of treatment, the Filter group had a significantly thicker choroid (0.09 (0.005) mm) than the Goggle group (0.06 (0.006) mm, t13 = 3.54, p = 0.01), but the difference between the Filter and Colony group was not significant (t13 = 1.71, p = 0.22).
Figure 3.
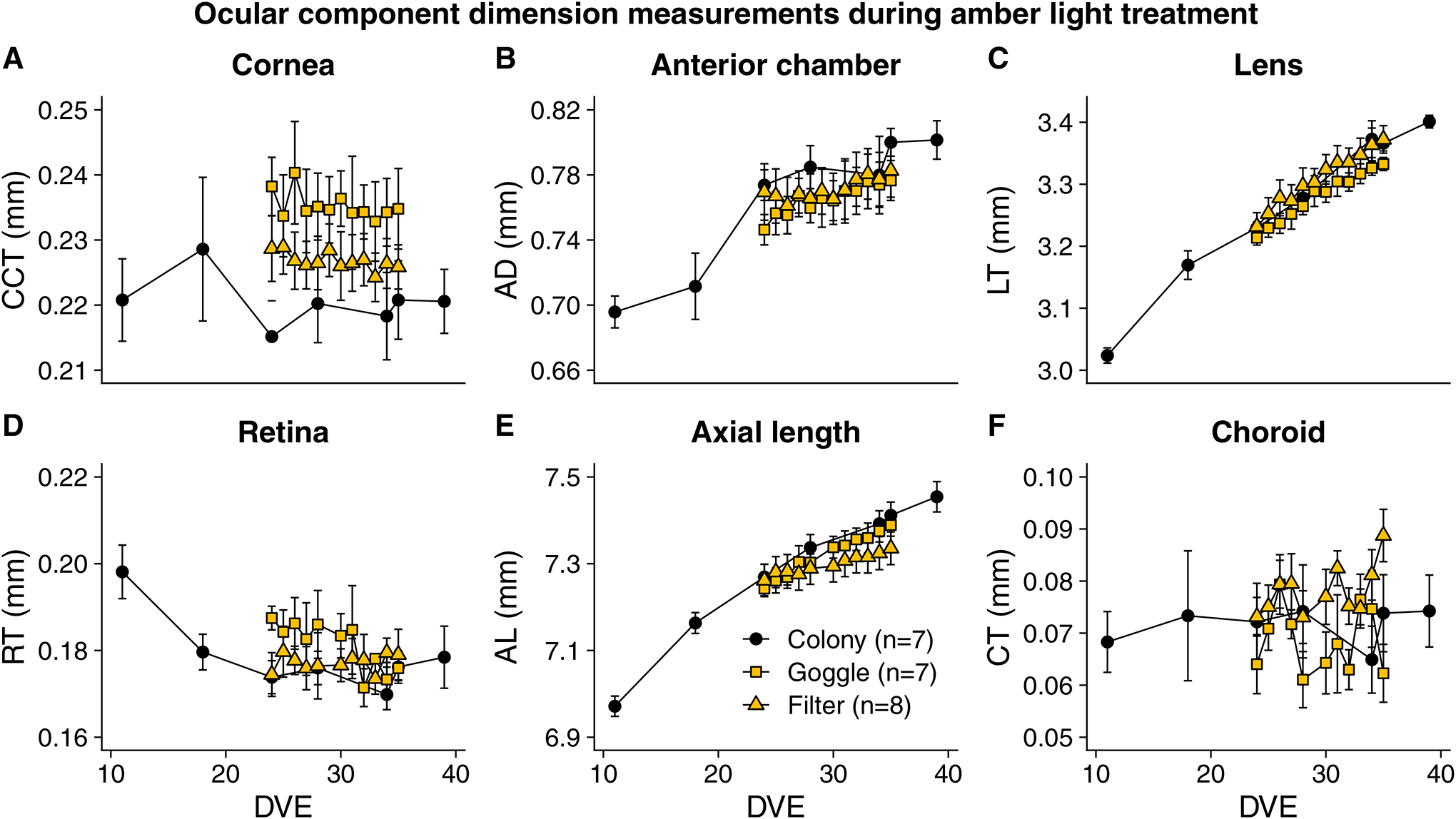
Ocular component dimension measurements during amber light treatment in the Filter and Goggle groups, excluding vitreous chamber depth (shown in Fig. 2B). A) Central Corneal Thickness (CCT), B) Anterior Chamber Depth (ACD) C) Lens Thickness (LT), D) Retinal Thickness (RT), E) Axial Length (AL) and F) Choroidal Thickness (CT). Among these components, choroidal thickness was the only one that was significantly different across groups at the end of treatment. After 11-day amber light treatment, the Filter group had significantly thicker choroid (0.09 ± 0.005 mm) than the Goggle group (0.06 ± 0.006 mm, p = 0.01).
Discussion
This study examined the effect of amber light on emmetropization in juvenile tree shrews. We exposed animals initially raised in broadband lighting to amber light treatment for 11 days after they had completed the initial emmetropization process and achieved a refractive state of near emmetropia. We found that: 1) amber filtered ambient light produced a significant hyperopic shift in the tree shrew’s refractive error and 2) the hyperopia-inducing effect of amber lighting failed to occur when animals wore goggles fitted with amber filters. The results from this study provide evidence that exposure to amber-filtered lighting can promote hyperopia development with a considerably less reduction in illumination and substantially greater light transmittance than red filters, thus avoiding the need to increase light intensity to maintain ambient illumination.
The two modes of amber light treatment showed contrasting effects in terms of refractive development. Unlike the animals exposed to amber filtered ambient light that developed hyperopia, animals wearing amber filters goggles did not develop hyperopia. Rather, they maintained a relatively constant vitreous chamber depth with no axial growth above that of the colony animals, suggesting that the emmetropization mechanism functioned normally in the goggle animals. Why the same amber filters used in two different modes would produce contrasting results remains uncertain. One possibility is that the eyes of the Filter and Goggle animals experienced different light levels to the Colony animals. Although it was not possible to measure the light level reaching the eye with the goggle in place, when unclipped from the head, the transmission characteristics (including the amount of attenuation) of the light measured through the goggle was identical to that of the light passing through the filters positioned atop the cage. Furthermore, while emmetropia is not maintained in very dim (~ 50 lux) colony lighting,60 and much higher lighting (15,000 lux) biases tree shrew eyes toward hyperopia,61 the lighting levels used here as well as previous studies without goggles are within a range that does not seem to affect emmetropization.62 The most likely reason for the difference in the response of the Filter and Goggle groups is that some unfiltered white light reached the eyes, as the coverage was incomplete with a rim of unrestricted broadband light in the far periphery. In the presence of both amber light and broadband light, one would expect that the emmetropization mechanism would use the LCA-feedback loop to guide the growth of the eye to maintain emmetropia. This interpretation is supported by the findings from a recent study where adding even dim broadband light with red light dramatically reduced the red-light hyperopia.47
In the present study, we exposed the tree shrews to a relatively long duration of constant amber light (14 hours light/10 hours of darkness), which would be impractical for use as a therapy for human children. However, a previous study in tree shrews using brief exposures to red light without any broadband light “contamination” showed that even one hour a day of red light, with 13 hours a day exposure to white light, could still have a significant hyperopic effect.45 This is consistent with other studies which found stimuli that produce retinal “stop” signals (brief removal of negative lens, form deprivation or the imposition of positive lenses) show “temporal nonlinearity” – i.e., brief periods produce powerful effects (see review63). Further, multiple briefer periods spaced across the day are even more powerful.63–65 Granted that we have not specifically demonstrated this nonlinearity with amber light, nevertheless it seems likely that amber light would exhibit the same property. In human children undergoing progressive myopia, the eyes seem to ignore myopic defocus cues that one might expect would return them to emmetropia. The amber light—like the red light—might stimulate the emmetropization mechanism to produce hyperopia that could counteract the myopic progression, suggesting that it could have a potential therapeutic effect in humans.
In broadband light, a hyperopic refractive error provides a strong defocus cue for the emmetropization mechanism to increase the growth rate of the eye (consequently reducing the refractive error) to achieve emmetropia. However, in amber filtered ambient light, the effect of wavelength appears to interfere with utilising the cues related to hyperopic defocus. When the amber light treatment started, animals showed an increase in hyperopia within 1–2 days. However, despite the increase in hyperopic refractive error, the emmetropization mechanism did not increase axial elongation to restore emmetropia. Rather, it decreased the rate of vitreous chamber elongation, producing the resultant hyperopia. This development of hyperopia suggests that the growth-inhibiting (“STOP”) signals generated by the amber light prevented the emmetropization mechanism from utilising hyperopic defocus to increase axial elongation and reduce the hyperopia. A similar effect was found in red light where the emmetropization mechanism slowed the axial elongation of the eye despite a rapid and sustained development of hyperopia.19,35 The absence of short wavelengths in the visual environment presumably leads to the genesis of STOP signals, which interfere with the intrinsic ability of the emmetropization mechanism to utilize the defocus-related cue and to signal the eye to modify its growth appropriately.
The hyperopic effect of amber filtered ambient light observed in the present study may have been less potent than that of the narrow-band red light reported previously.19 As shown in Figure 4, the juvenile animals reared in red light were slightly older (exposure starting at 35 vs. 24 DVE) and had somewhat higher starting refractive error (1.5 vs. 1.1 D) than the amber filter animals at the start of treatment. These differences and the comparison with a previously-studied group make a direct comparison between animals raised in amber and red light difficult and warrant some caution. Nevertheless, the two groups on average showed a similar hyperopic shift over the treatment duration. After 11 days of treatment, the change in refractive error was 2.3 D in animals raised in filtered red light and 2.4 D in animals raised in filtered amber light. Despite the minimal difference in the hyperopic shift (from the baseline) between the red light-treated and amber light-treated groups (~0.1 D), the pattern of hyperopic development was slightly different between the groups: hyperopia in the red-light group rose rapidly over the first few days and then plateaued, whereas hyperopia in the amber Filter group rose more slowly. However, the absolute amount of hyperopia after 11 days of treatment was similar; 3.5 (0.6) D in amber light and 3.8 (1.0) D in red light.
Figure 4.
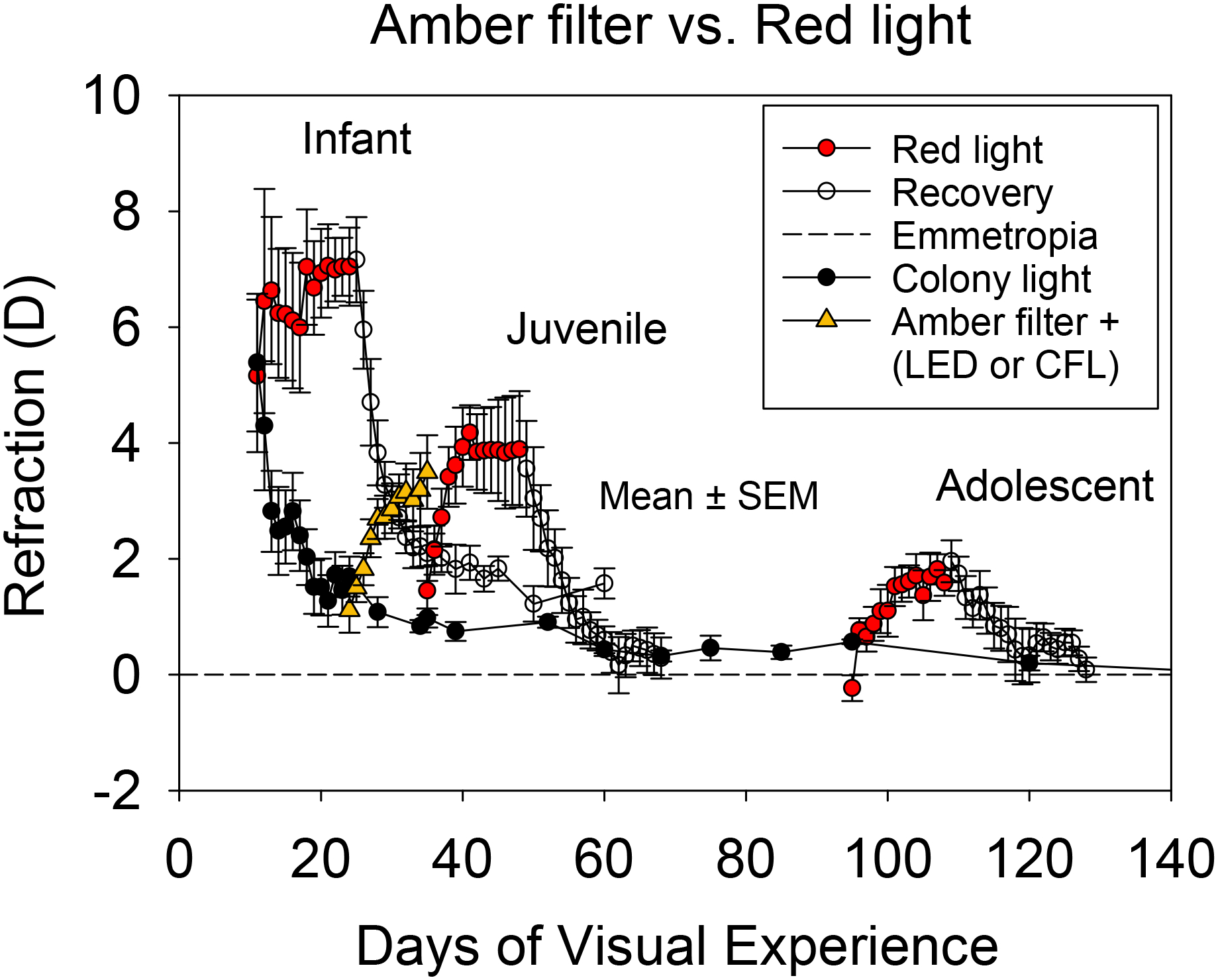
Hyperopia development in amber filter animals compared with animals exposed to red light. Data for the red light-treated animals are redrawn from Gawne et al.19. The rate of hyperopia development in animals raised in filtered ambient amber light was approximately similar to that of juvenile animals raised in filtered ambient red light.
The apparent similarity in the potency of amber and red light to produce hyperopia despite age differences between the two treatment groups is slightly surprising, as long-wavelength treatment typically produces faster and more robust responses in younger animals.19 However, the slower rate of hyperopia development in amber light compared with red light suggests that amber light may have produced a weaker than expected response because the lower cut-off of the amber light was right at the edge of the SWS absorbance profile, causing some SWS activation (Figure 1). Regardless of the response potency, the ability of both amber and red light to produce hyperopia indicates that avoiding significant activation of the SWS cones could be the key to slowing axial elongation and achieving hyperopia with long-wavelength light. In both wavelength treatment conditions, the spectral profile of the light overlaps the absorbance spectrum of the LWS cones, with little to no overlap with the absorbance spectrum of the SWS cones (Figure 1). This hypothesis is consistent with a recently proposed model in which an imbalance between LWS and SWS image statistics perturbs normal emmetropization and dysregulates eye growth.28
In tree shrews and other animal models of myopia studied to date, the normal emmetropization response appears to depend on the presence of a continuous band of wavelengths across the spectrum in the visual environment. When animals are exposed to a limited band of wavelengths (i.e., quasi-monochromatic light), they do not emmetropize, but develop substantial refractive errors. Tree shrews (mammals closely related to primates) raised in broadband light failed to maintain emmetropia when transferred and exposed to narrow-band light; they developed substantial hyperopia in long-wavelength (red) light (peak 632 nm)19,34 but showed variable refractive errors and ultimately developed myopia in short-wavelength (blue) light;33 developing hyperopia in amber light limited to wavelengths above 500 nm as observed in the present study. Macaque monkeys (non-human primates) showed similar responses, developing hyperopia under narrow-band long-wavelengths35 and variable refractive errors under short-wavelength lighting.66 Other species such as fish,42 chicks,38,41 mice40 and guinea pigs39,44 also failed to emmetropize normally when exposed to narrow-band quasi-monochromatic lighting. However, in contrast to tree shrews and monkeys, the emmetropization responses of species more distant to the primate line were opposite, with exposure to long-wavelength light promoting myopia development, while exposure to short-wavelength light (blue/violet) promoted hyperopic development.38–42 Taken together, these results suggest that the emmetropization responses to narrow-band lighting appear to depend not only on the wavelength composition but also on the species being tested.
The exact reasons for the variability in responses to narrow-band light across species remain unclear but could be related to inter-species differences in habitual environment, ocular transmission characteristics, cone types and their absorption peaks, the interaction of wavelength with cone-pigment absorptions, sex hormones, temporal sensitivity and circadian rhythms.32,37 Other methodological factors, such as age, luminous intensity, bandwidth, specific wavelength distributions and duration of exposure could also be involved.32,37 It is also likely that the operational characteristics of the emmetropization mechanism are different across species; the mechanism could be using wavelength information as a target in some species, whereby the eye grows to match the focal plane of the narrow-band light, or as a size cue in others, whereby the presence of narrow-band light signals whether the eye is too short or long. Nonetheless, a commonality among all species is that the emmetropization mechanism is unable to produce or maintain emmetropia when the visual environment contains only a narrow range of wavelengths. To that end, the results from this study indicate a possibility of exploiting the dependence of the emmetropization mechanism on a full spectrum of wavelengths to direct the mechanism to guide eyes toward hyperopia. However, the processing of wavelength cues by the emmetropization mechanism may be more complex and could involve a combination of strategies, including sensitivity to SWS cones,67 temporal frequency, colour contrast68,69 and spatial and temporal luminance contrast.70 It is also possible that the emmetropization mechanism uses additional cues besides LCA to achieve or maintain emmetropia.
In conclusion, exposure to amber light produces hyperopic effects in tree shrews, with a similar potency to red light but considerably less reduction in ambient illuminance, thus avoiding the need to enhance light intensity substantially to mitigate reduced ambient illumination as would be necessary with deep red filters. These findings provide a basis for investigating if periods of amber light treatment might be developed as a novel and easy-to-administer anti-myopia intervention. There is already some evidence in healthy human adults that short-term exposure to ambient lighting influences choroidal thickness, which is linked to longer-term changes in eye growth.71,72 The findings from this study also suggest that if wearing amber filters were to be used as an anti-myopia therapy, they would possibly not be effective in open-framed spectacle lenses but would have to be in goggles with a good peripheral light seal, such as those used for motorcycles or paintball. Further knowledge of the exact parameters governing the hyperopia-inducing effect of amber light may provide a better understanding of how to maximize the efficacy and utility of amber light treatment in promoting hyperopia or reducing myopia.
Supplementary Material
Figure S2. Refractive errors of the right and left eyes of individual animals reared under amber filtered ambient light (Filter#) and with goggles containing amber filters (Goggle#). The red dashed lines indicate 95% confidence limits for the standard colony-raised animals. CFL: Compact fluorescent light; LED: Light-emitting diode.
Figure S1. Transmission spectra of the amber filter.
Key Points.
In ambient amber light produced by passing broad-band light through filters that transmit wavelengths above 500 nm, eyes slowed axial growth and developed hyperopic refractive errors.
Eyes wearing amber filters in goggles that allowed some white light to reach the retina from the periphery grew normally and maintained good focus.
Exposure to amber light uncontaminated by broad-band white light might be developed as a treatment to slow eye growth and counteract myopia in humans.
Acknowledgements:
The authors acknowledge the technical assistance of Russell Veale, Eric Worthington, Johanna Henry, Randle Tibbs, and the University of Alabama at Birmingham Animal Resources Program veterinarians and staff. The study was supported by National Institutes of Health National Eye Institute grants: NEI P30 EY003039 (core), NEI R21 EY025254, and NEI RO1 EY028578.
Footnotes
Disclosure: The authors have no conflict of interest to declare concerning this work.
References
- 1.Norton TT, McBrien NA. Normal development of refractive state and ocular component dimensions in the tree shrew (Tupaia belangeri). Vision Research. 1992; 32: 833–842. [DOI] [PubMed] [Google Scholar]
- 2.Graham B, Judge SJ. Normal development of refractive state and ocular component dimensions in the marmoset (Callithrix jacchus). Vision Res 1999; 39: 177–187. [DOI] [PubMed] [Google Scholar]
- 3.Bradley DV, Fernandes A, Lynn M, Tigges M, Boothe RG. Emmetropization in the rhesus monkey (Macaca mulatta): birth to young adulthood. Invest Ophthalmol Vis Sci 1999; 40: 214–229. [PubMed] [Google Scholar]
- 4.Cook RC, Glasscock RE. Refractive and ocular findings in the newborn. Am J Ophthalmol 1951; 34: 1407–1413. [DOI] [PubMed] [Google Scholar]
- 5.Wallman J, Adams JI, Trachtman J. The eyes of young chickens grow toward emmetropia. Invest Ophthalmol Vis Sci 1981; 20: 557–561. [PubMed] [Google Scholar]
- 6.Ofri R, Millodot S, Shimoni R, Horowitz IH, Ashash E, Millodot M. Development of the refractive state in eyes of ostrich chicks (Struthio camelus). Am J Vet Res 2001; 62: 812–815. [DOI] [PubMed] [Google Scholar]
- 7.Zhou X, Qu J, Xie R, Wang R, Jiang L, Zhao H et al. Normal development of refractive state and ocular dimensions in guinea pigs. Vision Res 2006; 46: 2815–2823. [DOI] [PubMed] [Google Scholar]
- 8.Norton TT. Animal Models of Myopia: Learning How Vision Controls the Size of the Eye. ILAR J 1999; 40: 59–77. [DOI] [PubMed] [Google Scholar]
- 9.Wallman J, Winawer J. Homeostasis of eye growth and the question of myopia. Neuron 2004; 43: 447–468. [DOI] [PubMed] [Google Scholar]
- 10.Schaeffel F, Feldkaemper M. Animal models in myopia research. Clin Exp Optom 2015; 98: 507–517. [DOI] [PubMed] [Google Scholar]
- 11.Troilo D, Smith EL 3rd, Nickla DL, Ashby R, Tkatchenko AV, Ostrin LA et al. IMI - report on experimental models of emmetropization and myopia. Invest Ophthalmol Vis Sci 2019; 60: M31–M88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith EL, Hung LF, Arumugam B. Visual regulation of refractive development: Insights from animal studies. Eye 2014; 28: 180–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wildsoet CF. Active emmetropization — evidence for its existence and ramifications for clinical practice. Ophthalmic Physiol Opt 1997; 17: 279–290. [PubMed] [Google Scholar]
- 14.Wallman J, Gottlieb MD, Rajaram V, Fugate-Wentzek LA. Local retinal regions control local eye growth and myopia. Science 1987; 237: 73–77. [DOI] [PubMed] [Google Scholar]
- 15.McBrien NA, Moghaddam HO, Cottriall CL, Leech EM, Cornell LM. The effects of blockade of retinal cell action potentials on ocular growth, emmetropization and form deprivation myopia in young chicks. Vision Res 1995; 35: 1141–1152. [DOI] [PubMed] [Google Scholar]
- 16.Wildsoet CF. Neural pathways subserving negative lens-induced emmetropization in chicks – Insights from selective lesions of the optic nerve and ciliary nerve. Curr Eye Res 2003; 27: 371–385. [DOI] [PubMed] [Google Scholar]
- 17.Smith EL, Huang J, Hung LF, Blasdel TL, Humbird TL, Bockhorst KH. Hemiretinal Form Deprivation: Evidence for Local Control of Eye Growth and Refractive Development in Infant Monkeys. Investigative Opthalmology & Visual Science 2009; 50: 5057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Norton TT, Siegwart JT. Local myopia produced by partial visual-field deprivation in tree shrew. Soc Neurosci Abstr 1991; 17. [Google Scholar]
- 19.Gawne TJ, Ward AH, Norton TT. Long-wavelength (red) light produces hyperopia in juvenile and adolescent tree shrews. Vision Res 2017; 140: 55–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Norton TT, Amedo AO, Siegwart JT Jr. The effect of age on compensation for a negative lens and recovery from lens-induced myopia in tree shrews (Tupaia glis belangeri). Vision Res 2010; 50: 564–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mutti DO, Hayes JR, Mitchell GL, Jones LA, Moeschberger ML, Cotter SA et al. Refractive error, axial length, and relative peripheral refractive error before and after the onset of myopia. Invest Ophthalmol Vis Sci 2007; 48: 2510–2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holden BA, Fricke TR, Wilson DA, Jong M, Naidoo KS, Sankaridurg P et al. Global Prevalence of Myopia and High Myopia and Temporal Trends from 2000 through 2050. Ophthalmology. 2016; 123: 1036–1042. [DOI] [PubMed] [Google Scholar]
- 23.Haarman AEG, Enthoven CA, Tideman JWL, Tedja MS, Verhoeven VJM, Klaver CCW. The Complications of Myopia: A Review and Meta-Analysis. Invest Ophthalmol Vis Sci 2020; 61: 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Flitcroft DI. The complex interactions of retinal, optical and environmental factors in myopia aetiology. Prog Retin Eye Res 2012; 31: 622–660. [DOI] [PubMed] [Google Scholar]
- 25.Brennan NA, Toubouti YM, Cheng X, Bullimore MA. Efficacy in myopia control. Prog Retin Eye Res 2020; : 100923. [DOI] [PubMed] [Google Scholar]
- 26.Mandelman T, Sivak JG. Longitudinal chromatic aberration of the vertebrate eye. Vision Res 1983; 23: 1555–1559. [DOI] [PubMed] [Google Scholar]
- 27.Petry HM, Hárosi FI. Visual pigments of the tree shrew (Tupaia belangeri) and greater galago (Galago crassicaudatus): a microspectrophotometric investigation. Vision Res 1990; 30: 839–851. [DOI] [PubMed] [Google Scholar]
- 28.Gawne TJ, Norton TT. An opponent dual-detector spectral drive model of emmetropization. Vision Res 2020; 173: 7–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gisbert S, Feldkaemper M, Wahl S, Schaeffel F. Interactions of cone abundancies, opsin expression, and environmental lighting with emmetropization in chickens. Exp Eye Res 2020; 200: 108205. [DOI] [PubMed] [Google Scholar]
- 30.Rucker FJ, Eskew RT Jr, Taylor C. Signals for defocus arise from longitudinal chromatic aberration in chick. Exp Eye Res 2020; 198: 108126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qian Y-F, Liu R, Dai J-H, Chen M-J, Zhou X-T, Chu R-Y. Transfer from blue light or green light to white light partially reverses changes in ocular refraction and anatomy of developing guinea pigs. J Vis 2013; 13. doi: 10.1167/13.11.16. [DOI] [PubMed] [Google Scholar]
- 32.Smith EL 3rd, Hung L-F, Arumugam B, Holden BA, Neitz M, Neitz J. Effects of long-wavelength lighting on refractive development in infant rhesus monkeys. Invest Ophthalmol Vis Sci 2015; 56: 6490–6500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gawne TJ, Ward AH, Norton TT. Juvenile Tree Shrews Do Not Maintain Emmetropia in Narrow-band Blue Light. Optometry and Vision Science. 2018; 95: 911–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gawne TJ, Siegwart JT Jr, Ward AH, Norton TT. The wavelength composition and temporal modulation of ambient lighting strongly affect refractive development in young tree shrews. Exp Eye Res 2017; 155: 75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hung L-F, Arumugam B, She Z, Ostrin L, Smith EL III. Narrow-band, long-wavelength lighting promotes hyperopia and retards vision-induced myopia in infant rhesus monkeys. Exp Eye Res 2018; 176: 147–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu R, Hu M, He JC, Zhou X-T, Dai J-H, Qu X-M et al. The effects of monochromatic illumination on early eye development in rhesus monkeys. Invest Ophthalmol Vis Sci 2014; 55: 1901–1909. [DOI] [PubMed] [Google Scholar]
- 37.Rucker FJ. Monochromatic and white light and the regulation of eye growth. Exp Eye Res 2019; 184: 172–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Foulds WS, Barathi VA, Luu CD. Progressive myopia or hyperopia can be induced in chicks and reversed by manipulation of the chromaticity of ambient light. Invest Ophthalmol Vis Sci 2013; 54: 8004–8012. [DOI] [PubMed] [Google Scholar]
- 39.Liu R, Qian Y-F, He JC, Hu M, Zhou X-T, Dai J-H et al. Effects of different monochromatic lights on refractive development and eye growth in guinea pigs. Exp Eye Res 2011; 92: 447–453. [DOI] [PubMed] [Google Scholar]
- 40.Strickland R, Landis EG, Pardue MT. Short-wavelength (Violet) light protects mice from myopia through cone signaling. Invest Ophthalmol Vis Sci 2020; 61: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Seidemann A, Schaeffel F. Effects of longitudinal chromatic aberration on accommodation and emmetropization. Vision Res 2002; 42: 2409–2417. [DOI] [PubMed] [Google Scholar]
- 42.Kröger RHH, Wagner H-J. The eye of the blue acara (Aequidens pulcher, Cichlidae) grows to compensate for defocus due to chromatic aberration. Journal of Comparative Physiology A 1996; 179: 837–842. [DOI] [PubMed] [Google Scholar]
- 43.Rucker FJ, Wallman J. Cone signals for spectacle-lens compensation: Differential responses to short and long wavelengths. Vision Res 2008; 48: 1980–1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Long Q, Chen D, Chu R. Illumination with monochromatic long-wavelength light promotes myopic shift and ocular elongation in newborn pigmented guinea pigs. Cutan Ocul Toxicol 2009; 00: 090825080943088–090825080943085. [DOI] [PubMed] [Google Scholar]
- 45.Ward AH, Norton TT, Huisingh CE, Gawne TJ. The hyperopic effect of narrow-band long-wavelength light in tree shrews increases non-linearly with duration. Vision Res 2018; 146–147: 9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Siegwart JT Jr, Norton TT. Binocular lens treatment in tree shrews: Effect of age and comparison of plus lens wear with recovery from minus lens-induced myopia. Exp Eye Res 2010; 91: 660–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Samal AV, Norton TT, Gawne TJ. The Effect of Contamination by White Light on Red-Light Induced Hyperopia in Juvenile Tree Shrews. American Academy of Optometry Annual Meeting, 2020. [Google Scholar]
- 48.Olson LE, Sargis EJ, Martin RD. Phylogenetic relationships among treeshrews (scandentia): A review and critique of the morphological evidence. J Mamm Evol 2004; 11: 49–71. [Google Scholar]
- 49.Guo L, Frost MR, He L, Siegwart JT Jr, Norton TT. Gene expression signatures in tree shrew sclera in response to three myopiagenic conditions. Invest Ophthalmol Vis Sci 2013; 54: 6806–6819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.He L, Frost MR, Siegwart JT Jr, Norton TT. Gene expression signatures in tree shrew choroid during lens-induced myopia and recovery. Exp Eye Res 2014; 123: 56–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McBrien NA, Norton TT. The development of experimental myopia and ocular component dimensions in monocularly lid-sutured tree shrews (Tupaia belangeri). Vision Res 1992; 32: 843–852. [DOI] [PubMed] [Google Scholar]
- 52.Siegwart JT Jr, Norton TT. Goggles for controlling the visual environment of small animals. Lab Anim Sci 1994; 44: 292–294. [PubMed] [Google Scholar]
- 53.Norton TT, Siegwart JT, German AJ, Robertson J, Wu WW. Comparison of cycloplegic streak retinoscopy with autorefractor measures in tree shrew eyes with, and without, induced myopia. In: Investigative Ophthalmology & Visual Science. ASSOC RESEARCH VISION OPHTHALMOLOGY INC 9650 ROCKVILLE PIKE, BETHESDA, MD: …, 2000, pp S563–S563. [Google Scholar]
- 54.El Hamdaoui M, Gann DW, Norton TT, Grytz R. Matching the LenStar optical biometer to A-Scan ultrasonography for use in small animal eyes with application to tree shrews. Exp Eye Res 2019; 180: 250–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Penha AM, Burkhardt E, Schaeffel F, Feldkaemper MP. Ultrasonography and optical low-coherence interferometry compared in the chicken eye. Optom Vis Sci 2012; 89: 916–921. [DOI] [PubMed] [Google Scholar]
- 56.Norton TT, Wu WW, Siegwart JT Jr. Refractive state of tree shrew eyes measured with cortical visual evoked potentials. Optom Vis Sci 2003; 80: 623–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sajdak BS, Salmon AE, Cava JA, Allen KP, Freling S, Ramamirtham R et al. Noninvasive imaging of the tree shrew eye: Wavefront analysis and retinal imaging with correlative histology. Exp Eye Res 2019; 185: 107683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.R Core Team. R: A Language and Environment for Statistical Computing. 2020. https://www.R-project.org/.
- 59.Norton TT, Amedo AO, Siegwart JT. Darkness Causes Myopia in Visually Experienced Tree Shrews. Investigative Opthalmology & Visual Science. 2006; 47: 4700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cohen Y, Belkin M, Yehezkel O, Solomon AS, Polat U. Dependency between light intensity and refractive development under light-dark cycles. Exp Eye Res 2011; 92: 40–46. [DOI] [PubMed] [Google Scholar]
- 61.Siegwart JT Jr, Ward AH, Norton TT. Moderately elevated fluorescent light levels slow form deprivation and minus lens-induced myopia development in tree shrews. Invest Ophthalmol Vis Sci 2012; 53: 3457–3457. [Google Scholar]
- 62.Norton TT, Siegwart JT Jr. Light levels, refractive development, and myopia--a speculative review. Exp Eye Res 2013; 114: 48–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhu X Temporal integration of visual signals in lens compensation (a review). Exp Eye Res 2013; 114: 69–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kee C-SS, Hung LF, Qiao-Grider Y, Ramamirtham R, Winawer J, Wallman J et al. Temporal constraints on experimental emmetropization in infant monkeys. Invest Ophthalmol Vis Sci 2007; 48: 957–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lan W, Feldkaemper M, Schaeffel F. Intermittent episodes of bright light suppress myopia in the chicken more than continuous bright light. PLoS One 2014; 9: e110906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hung L-F, Beach K, She Z, Arumugam B, Ostrin L, Smith EL. Effect of narrowband, short-wavelength ambient lighting on refractive development in infant rhesus monkeys. Invest Ophthalmol Vis Sci 2020; 61: 560–560. [Google Scholar]
- 67.Taylor CP, Shepard TG, Rucker FJ, Eskew RT Jr. Sensitivity to S-cone stimuli and the development of myopia. Invest Ophthalmol Vis Sci 2018; 59: 4622–4630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Watts NS, Taylor C, Rucker FJ. Temporal color contrast guides emmetropization in chick. Exp Eye Res 2021; 202: 108331. [DOI] [PubMed] [Google Scholar]
- 69.Lin G, Taylor C, Rucker F. Effect of duration, and temporal modulation, of monochromatic light on emmetropization in chicks. Vision Res 2020; 166: 12–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rucker FJ. The role of luminance and chromatic cues in emmetropisation. Ophthalmic Physiol Opt 2013; 33: 196–214. [DOI] [PubMed] [Google Scholar]
- 71.Read SA, Pieterse EC, Alonso-Caneiro D, Bormann R, Hong S, Lo CH et al. Daily morning light therapy is associated with an increase in choroidal thickness in healthy young adults. Sci Rep 2018; 8: 8200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lou L, Ostrin LA. Effects of narrowband light on choroidal thickness and the pupil. Invest Ophthalmol Vis Sci 2020; 61: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lucas RJ, Peirson SN, Berson DM, Brown TM, Cooper HM, Czeisler CA et al. Measuring and using light in the melanopsin age. Trends Neurosci 2014; 37: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S2. Refractive errors of the right and left eyes of individual animals reared under amber filtered ambient light (Filter#) and with goggles containing amber filters (Goggle#). The red dashed lines indicate 95% confidence limits for the standard colony-raised animals. CFL: Compact fluorescent light; LED: Light-emitting diode.
Figure S1. Transmission spectra of the amber filter.


