Abstract
Background:
Regular screening for colorectal cancer (CRC) reduces its mortality. We explored patterns of use of different CRC screening modalities and quantified the association between having a regular primary care provider and being up to date for CRC screening in a community-based population in Alberta, Canada.
Methods:
We conducted a cross-sectional study of adults between 50 and 74 years of age in Alberta, using Canadian Community Health Survey data (2015–2016). We defined being up to date for CRC screening as having completed a fecal occult blood test (FOBT) or fecal immunochemical test (FIT) within the previous 2 years, or having a colonoscopy or sigmoidoscopy in the previous 5 years before the survey. We analyzed data using multivariable logistic regression models.
Results:
Of 4600 surveyed adults, 62.6% were up to date for CRC screening, with 45.1% having completed a FIT or FOBT (45.1%), and 34.1% having undergone a colonoscopy or sigmoidoscopy. The adjusted odds ratio of being up to date for CRC screening was 0.25 (95% confidence interval 0.17–0.38) and the absolute probability of being up to date for CRC screening was 34.4% lower for adults who had no regular primary care provider, compared with those who had. This pattern was observed in both male and female subgroups.
Interpretation:
Our findings suggest a suboptimal uptake of CRC screening overall in Alberta, with high disparity between adults with and without a regular primary care provider. The use of customized, multicomponent intervention strategies that are shown to be effective in increasing participation in CRC screening may address this issue.
Colorectal cancer (CRC) is the third most commonly diagnosed cancer and the second most common cause of cancer death in Alberta, Canada.1 Screening for CRC reduces its incidence and mortality rate through early detection and treatment of precancerous polyps or CRC.2–6 However, high adherence to CRC screening is essential for achieving those benefits. The current Canadian Task Force on Preventive Health Care guideline recommends that CRC screening start from age 50 years and continue until age 74 years.7 The guideline recommends fecal occult blood testing (FOBT), with either guaiac FOBT or a fecal immunochemical test (FIT), every 2 years for the average-risk population and flexible sigmoidoscopy every 10 years for those at increased risk (such as family history of CRC).7
Implementation of the task force recommendations varies across Canadian provinces and territories.8,9 All provinces and territories, except Manitoba, currently use FIT as the primary screening modality for people at average risk for CRC.8,9 The Alberta CRC screening program (ACRCSP) implemented FIT in 2013. The current ACRCSP guideline recommends FIT every 1 to 2 years for people at average risk for CRC, and colonoscopy every 10 years for people with increased risk for CRC.10,11 The aim of ACRCSP is that at least 70% of the target population in Alberta participate in CRC screening.12 Similarly, the national target for FIT participation is at least 60%.13
According to an earlier study, based on the 2012 Canadian Community Health Survey, the prevalence of being up to date for CRC screening (defined as FOBT in the previous 2 years or colonoscopy or sigmoidoscopy in the previous 10 years) was 59.5% among adults aged 50–74 years in Alberta, with 38.1% receiving FOBT and 36.7% receiving colonoscopy or sigmoidoscopy.14 Overall, the available published data suggest that uptake of CRC screening and adherence to provincial CRC screening guidelines are suboptimal. 13–15 Continuous assessment of adherence to CRC screening is vital to monitor the progress and opportunities for improvement in provincial CRC screening programs.
In Alberta, although the current CRC screening guideline is population-based, the opportunity for CRC screening still depends largely on access to a primary care provider, primarily family physicians.10,11 Primary care providers determine patient eligibility for CRC screening and offer requisitions or referrals for FIT or colonoscopy during the clinic visit. After obtaining a FIT requisition, patients pick up the FIT kit from a laboratory site, collect the sample at home and then return the sample to the laboratory.
This practice is not optimal for the initiation of CRC screening among people who do not have a regular primary care provider, who constitute about 18% of Alberta’s total population.16 Although people who do not have their own regular provider can visit walk-in clinics for medical consultations, they most often visit these clinics for problems they believe require prompt medical attention rather than preventive practices such as CRC screening.17,18 Detailed quantification of patterns of up-to-date status for CRC screening of adults with no regular primary care provider and characterization of this population are lacking. Such quantification and subgroup characterization are an important step toward understanding the impact of this service disparity to develop tailored interventions for people with no regular primary care provider.
We explored patterns of modality use for CRC screening, and quantified the association between having a regular primary care provider and being up to date for CRC screening in a representative, community-based population.
Methods
Study design
We conducted a secondary analysis of data from the 2015–2016 Statistics Canada Canadian Community Health Survey (CCHS),19 focusing on the results of a CRC screening questionnaire module completed by older respondents in Alberta.
Data source
Briefly, the 2015–2016 CCHS is a nationally representative cross-sectional survey of the household population of people aged 12 years or older living in the 10 provinces and 3 territories of Canada (n = 109 700). We used the CCHS public meta file (PUMF), obtained from the Nesstar data portal of Statistics Canada (https://www.statcan.gc.ca/eng/microdata/dli/data).
The CRC screening questionnaire module was an optional component in the 2015–2016 CCHS that provinces could choose to include or not. It was included for respondents from Alberta aged 40 years or older during the survey period. Although the CRC screening component was also measured in Alberta during the 2017 and 2019 CCHS, the PUMFs of these individual survey years had not been released by Statistics Canada at the time of our study.
Participants
We included data from survey respondents between the ages of 50 and 74 years. The survey excluded people who lived in reserves and any other Indigenous settlements, fulltime members of the Canadian Armed Forces and people who lived in institutions; altogether, these groups accounted for around 3% of the total Canadian population aged 12 years or older.19
Measures
In the CCHS, the respondents were asked whether they had an FOBT (including FIT or guaiac FOBT), or a colonoscopy or sigmoidoscopy for the purpose of CRC screening at any time in their lifetime and, if yes, the last time they had each of those tests.
We defined being up to date for CRC screening (i.e., the point prevalence of participation for CRC screening)20 as having completed FOBT within the previous 2 years, or either colonoscopy or sigmoidoscopy in the previous 5 years, before the survey.21 We used a conservative time cut-off because the CCHS questionnaire does not distinguish the type of endoscopy (colonoscopy or sigmoidoscopy) performed and whether it was performed in the previous 10 years before the survey.
The 2015–2016 CCHS collected data on whether the respondents have a regular health care provider and, if yes, the type of regular health care provider(s). If CCHS respondents indicated having a regular health care provider, we considered them to have a regular primary care provider because 99% of those who reported having a regular health care provider reported having a family physician. Other study variables included were sociodemographic characteristics (e.g., age, sex, race) and health behaviours (e.g., smoking, physical activity). We selected these sociodemographic and health behaviour variables as they are associated with disparities in CRC screening.14,15,22,23
Statistical analysis
We calculated the proportion of survey respondents who were up to date for CRC screening. We also calculated the proportion of respondents who had undergone a colonoscopy or sigmoidoscopy in the previous 5 years who had also had FOBT in the previous 2 years. We used bivariate analyses to assess up-to-date status by sociodemographic and health behaviour characteristics and by whether respondents had a regular primary care provider.
We developed a multivariable logistic regression model to assess the association between having a regular provider and being up to date for CRC screening, adjusting for variables such as age, marital status, education attainment and smoking.22 These variables were selected for adjustment in the model based on our previous knowledge that they are associated with both the outcome (participation in CRC screening) and exposure (having a primary care provider) but do not reside in the causal pathway of the relation between having a provider and participation in CRC screening. Being up to date for breast cancer screening was not included in the model so that we could compare between males and females in the same model.
We then stratified this model to assess further whether the association differs for male and female subgroups. Odds ratios (ORs) and 95% confidence intervals (CIs) were estimated from these models. In addition, we estimated the adjusted predicted probability of being up to date for CRC screening using these multivariable regression models. The distribution patterns of respondent characteristics, except race, were similar whether respondents received FOBT, or colonoscopy or sigmoidoscopy (Appendix 1, Supplementary Table 1, available at www.cmajopen.ca/content/10/1/E203/suppl/DC1); hence, the regression models were developed for overall participation in CRC screening (including FOBT, colonoscopy or sigmoidoscopy).
The CCHS used stratified, multistage sample selection techniques that included clustering and unequal selection probabilities. We weighted and bootstrapped all estimates to ensure the representativeness of the target population and to account for design effect, using survey weights and a set of 1000 replicate bootstrap sampling weights that were provided by Statistics Canada for use with the data file.
The proportion of missing data for each variable included in this study was less than 2%, except body mass index (< 4%) and race (< 4%). Missing data were deleted using a variablewise or pairwise deletion approach for univariate or bivariate analysis, and a listwise deletion approach for regression models. We used an α of less than 0.05 to determine statistical significance. All analyses were performed using STATA/IC 14.1. Reported numbers were rounded according to the reporting guidelines of Statistics Canada.
Ethics approval
Given that the PUMF of the CCHS is deidentified and publicly available, review and approval by our research ethics board, the Conjoint Health Research Ethics Board at the University of Calgary, was not required.
Results
Characteristics of survey respondents (n = 4600) are shown in Table 1. Overall, 11.0% of respondents did not have a regular primary care provider. Of the total respondents, 45.1% (95% CI 42.7%–47.4%) had FOBT in the previous 2 years, 34.1% (95% CI 32.0%–36.2%) had a colonoscopy or sigmoidoscopy in the previous 5 years, and 62.6% (95% CI 60.3%–64.9%) had either or both. Among those who had colonoscopy or sigmoidoscopy in the previous 5 years, 47.1% (i.e., 25% of those who were up to date on CRC screening) also had FOBT in the last 2 years, and 26.0% of these procedures were done as follow-up of FOBT (Table 2).
Table 1:
Respondent characteristics
| Characteristic | Proportion of respondents, % (95% CI) n = 4600; weighted n = 1081168 |
|---|---|
| Age, yr | |
| 50–59 | 53.7 (51.7–55.6) |
| 60–74 | 46.3 (44.4–48.2) |
| Sex | |
| Female | 49.3 (48.8–49.9) |
| Male | 50.7 (50.1–51.2) |
| Marital status | |
| Widowed, divorced, separated or single | 24.6 (22.7–26.4) |
| Married or common law | 75.4 (73.6–77.3) |
| Highest education attainment | |
| Less than secondary graduation | 11.7 (10.3–13.2) |
| Secondary school education | 25.3 (23.3–27.4) |
| Postsecondary certificate or degree | 62.9 (60.7–65.1) |
| Total household income, $ | |
| No income or < 20 000 | 3.6 (3.0–4.1) |
| 20 000–39 999 | 10.6 (9.3–11.9) |
| 40 000–79 999 | 25.4 (23.5–27.2) |
| ≥ 80 000 | 60.4 (58.4–62.5) |
| Race | |
| White | 86.2 (84.1–88.2) |
| Indigenous or other visible minority | 13.8 (11.8–15.9) |
| Immigration status | |
| Born in Canada | 76.1 (73.9–78.3) |
| Permanent or nonpermanent resident | 23.9 (21.7–26.1) |
| Body mass index* | |
| Normal (18.5–24.9) | 27.1 (25.0–29.2) |
| Overweight (25.0–29.9) | 41.0 (38.6–43.4) |
| Obese (≥ 30.0) | 31.8 (29.6–34.0) |
| Moderate or vigorous physical activity,† min/wk | |
| < 150 (inadequate) | 44.9 (42.5–47.4) |
| ≥ 150 (adequate) | 55.0 (53.6–57.4) |
| Smoking status | |
| Never smoker | 46.0 (43.8–48.3) |
| Current smoker (daily or occasional) | 19.7 (17.9–21.6) |
| Past smoker (daily or occasional) | 34.2 (32.0–36.4) |
| Illicit drug used in previous 12 mo | 6.8 (5.6–7.9) |
| General health | |
| Poor or fair | 14.1 (12.6–15.5) |
| Good | 26.9 (25.0–28.8) |
| Very good | 36.8 (34.7–39.0) |
| Excellent | 22.2 (20.1–24.3) |
| Regular primary care provider | 89.0 (87.4–90.6) |
| Mammogram in previous 2 years (female) | 70.7 (67.7–73.8) |
Table 2:
Patterns of use of colorectal cancer screening modalities among adults aged 50–74 years in Alberta
| Colorectal cancer screening* | Weighted no. of respondents n = 1081168 |
Proportion of respondents, % (95% CI) n = 4600; weighted n = 1081168 |
|---|---|---|
| FOBT (guaiac FOBT or FIT), n = 1048500 | ||
| Had FOBT within previous 2 years | 472 500 | 45.1 (42.7–47.4) |
| Had FOBT earlier than the previous 2 years | 205 900 | 19.6 (17.8–21.5) |
| Colonoscopy or sigmoidoscopy, n = 1052500 | ||
| Had within previous 5 years | 359 000 | 34.1 (32.0–36.2) |
| Had earlier than the previous 5 years | 124 000 | 11.8 (10.2–13.4) |
| FOBT and/or colonoscopy, n = 1061000 | ||
| Had FOBT within last 2 years and/or colonoscopy or sigmoidoscopy within previous 5 years | 664 400 | 62.6 (60.3–64.9) |
| Had both FOBT within last 2 years and colonoscopy or sigmoidoscopy within previous 5 years | 167 100 | 15.8 (14.3–17.2) |
| Had FOBT within last 2 years, of those who had colonoscopy or sigmoidoscopy within previous 5 years, n = 354 700 | 167 100 | 47.1 (43.3–50.9) |
| Colonoscopy or sigmoidoscopy as follow-up of FOBT, among those who had colonoscopy or sigmoidoscopy within previous 5 years, n = 225 600 | 66 400 | 26.0 (22.3–29.7) |
Note: CI = confidence interval, FIT = fecal immunochemical test, FOBT = fecal occult blood test.
Provided numerators and denominators are weighted numbers, which were used to calculate the proportion, and numbers were rounded to the nearest 100 value according to the reporting guidelines of Statistic Canada; denominator varies owing to various levels of missing data across variables. Missing data were deleted on a variable basis.
Respondents who were aged 60–74 years, were married or common-law, were white, were nonsmokers or had a regular primary care provider were more up to date for CRC screening than their counterparts (Table 3). Similarly, respondent characteristics, including age, marital status, education status and smoking status, differed among those with and without a regular provider. Among respondents with a regular provider, 67.7% were up to date for CRC screening, whereas only 29.4% were up to date among those without a regular provider (Table 4).
Table 3:
Up-to-date status for colorectal cancer screening by respondent characteristics*
| Characteristic | Proportion of respondents, % (95% CI) | |
|---|---|---|
| Up to date | Not up to date | |
| Age, yr | ||
| 50–59 | 55.0 (51.6–58.3) | 45.0 (41.7–48.4) |
| 60–74 | 71.6 (68.7–74.5) | 28.4 (25.5–31.3) |
| Sex | ||
| Female | 61.6 (58.5–64.8) | 38.3 (35.2–41.5) |
| Male | 63.6 (60.2–66.9) | 36.4 (33.1–39.8) |
| Marital status | ||
| Widowed, divorced, separated or single | 55.1 (51.1–59.1) | 44.8 (40.9–48.9) |
| Married or common law | 65.1 (62.3–67.9) | 34.9 (32.1–37.7) |
| Highest education attainment | ||
| Less than secondary graduation | 56.0 (49.1–62.8) | 44.0 (37.2–50.8) |
| Secondary school education | 62.1 (57.1–67.1) | 37.9 (32.9–42.9) |
| Postsecondary certificate or degree | 64.1 (61.3–66.9) | 35.9 (33.1–38.7) |
| Total household income, $ | ||
| No income or < 20 000 | 55.5 (47.4–63.6) | 44.5 (36.4–52.6) |
| 20 000 to 39 999 | 60.5 (53.6–67.4) | 39.5 (32.5–46.4) |
| 40 000 to 79 999 | 63.7 (59.6–67.8) | 36.3 (32.2–40.4) |
| ≥ 80 000 | 62.9 (59.8–66.0) | 37.0 (33.9–40.1) |
| Race | ||
| White | 64.1 (61.8–66.4) | 35.9 (33.6–38.2) |
| Indigenous or other visible minority | 52.4 (43.5–61.4) | 47.6 (38.6–56.5) |
| Immigration status | ||
| Born in Canada | 64.4 (62.0–66.8) | 35.6 (33.2–37.9) |
| Permanent or nonpermanent resident | 57.3 (51.3–63.2) | 42.7 (36.7–48.6) |
| Body mass index† | ||
| Normal (18.5–24.9) | 60.6 (56.1–65.1) | 39.3 (34.9–43.8) |
| Overweight (25.0–29.9) | 62.0 (58.0–65.9) | 37.9 (34.0–41.9) |
| Obese (≥ 30.0) | 65.6 (61.8–69.3) | 34.4 (30.6–38.2) |
| Moderate or vigorous physical activity,‡ min/wk | ||
| < 150 (inadequate) | 58.3 (54.5–62.0) | 41.7 (37.9–45.5) |
| ≥ 150 (adequate) | 66.3 (63.5–69.2) | 33.7 (30.8–36.5) |
| Smoking status | ||
| Never smoker | 65.5 (62.0–68.9) | 34.5 (31.2–37.9) |
| Current smoker (daily or occasional) | 50.4 (45.2–55.6) | 49.6 (44.4–54.8) |
| Past smoker (daily or occasional) | 65.9 (61.9–69.8) | 34.1 (30.2–38.0) |
| Illicit drug used in previous 12 mo | 48.4 (39.8–57.1) | 51.6 (42.9–60.2) |
| General health | ||
| Poor or fair | 61.2 (55.4–66.9) | 38.8 (33.0–44.6) |
| Good | 60.9 (56.8–64.9) | 39.1 (35.1–43.2) |
| Very good | 65.3 (61.8–68.9) | 34.7 (31.1–38.2) |
| Excellent | 61.2 (55.6–66.8) | 38.8 (33.2–44.4) |
| Regular primary care provider | 68.5 (66.1–70.9) | 31.5 (29.1–33.9) |
| Mammogram in previous 2 years (female) | 71.7 (68.2–75.2) | 28.3 (24.8–31.7) |
Note: CI = confidence interval, FOBT = fecal occult blood test (either guaiac fecal occult blood test or fecal immunochemical test).
We considered respondents to be up to date for colorectal cancer screening if they had FOBT in the previous 2 years or colonoscopy or sigmoidoscopy in the previous 5 years.
Body mass index was calculated based on the international standard.24
Physical activity was defined based on Canadian physical activity guidelines.25
Table 4:
Characteristics of respondents with and without a regular primary care provider
| Characteristic | Proportion of respondents, % (95% CI)* | |
|---|---|---|
| Has primary care provider | No primary care provider | |
| Overall proportion, % | 89.0 | 11.0 |
| Age, yr | ||
| 50–59 | 50.7 (48.6–52.8) | 73.4 (67.5–79.2) |
| 60–74 | 49.3 (47.2–51.4) | 26.7 (20.8–32.5) |
| Sex | ||
| Male | 51.4 (50.3–53.6) | 33.5 (26.7–40.1) |
| Female | 48.6 (47.4–49.7) | 66.5 (59.9–73.1) |
| Marital status | ||
| Widowed, divorced, separated or single | 23.0 (21.1–24.8) | 39.9 (32.2–47.7) |
| Married or common law | 77.0 (75.2–78.9) | 60.1 (52.3–67.9) |
| Education | ||
| Less than secondary graduation | 10.9 (9.5–12.3) | 19.3 (13.1–25.5) |
| Secondary school education | 25.8 (23.7–27.9) | 20.7 (15.4–26.0) |
| Postsecondary certificate or degree | 63.3 (61.0–65.6) | 60.0 (52.7–67.2) |
| Total household income, $ | ||
| No income or < 20 000 | 3.5 (2.9–4.1) | 4.3 (2.6–6.1) |
| 20 000 to 39 999 | 10.2 (8.9–11.5) | 14.3 (8.8–19.7) |
| 40 000 to 79 999 | 25.5 (23.5–27.5) | 25.8 (19.6–32.0) |
| ≥ 80 000 | 60.8 (58.6–63.0) | 55.6 (47.9–63.3) |
| Race | ||
| White | 86.4 (83.4–88.4) | 86.9 (79.1–94.7) |
| Indigenous or other visible minority | 13.6 (11.6–15.6) | 13.0 (5.3–20.9) |
| Immigration status | ||
| Born in Canada | 76.5 (74.3.–78.7) | 74.4 (66.2–82.6) |
| Permanent or nonpermanent resident | 23.5 (21.9–25.7) | 25.6 (17.3–33.8) |
| Body mass index† | ||
| Normal (18.5–24.9) | 26.5 (24.5–28.6) | 33.7 (25.4–42.0) |
| Overweight (25.0–29.9) | 40.7 (38.2–43.1) | 40.7 (32.0–48.5) |
| Obese (≥ 30.0) | 32.8 (30.4–35.1) | 25.6 (19.3–31.9) |
| Moderate or vigorous physical activity,‡ min/wk | ||
| < 150 (inadequate) | 44.7 (42.2–47.2) | 45.7 (37.6–53.8) |
| ≥ 150 (adequate) | 55.3 (52.8–57.8) | 54.3 (46.2–62.4) |
| Smoking status | ||
| Never smoker | 46.6 (44.3–48.8) | 38.4 (30.4–46.4) |
| Current smoker (daily or occasional) | 18.5 (16.6–20.4) | 30.6 (23.8–37.4) |
| Past smoker (daily or occasional) | 34.9 (32.6–37.2) | 31.0 (24.1–37.9) |
| Illicit drug used in previous 12 mo | 6.0 (4.9–7.2) | 13.5 (8.5–18.6) |
| General health | ||
| Poor or fair | 14.0 (12.6–15.4) | 13.0 (6.5–19.4) |
| Good | 27.4 (25.4–29.4) | 23.4 (17.9–28.8) |
| Very good | 37.0 (34.7–39.3) | 38.5 (31.3–45.7) |
| Excellent | 21.6 (19.5–23.7) | 25.2 (17.2–33.2) |
| Mammogram in previous 2 years (female) | 74.3 (71.5–77.2) | 33.0 (23.1–43.0) |
| FOBT in the previous 2 years | 48.5 (46.1–51.0) | 18.0 (13.0–23.0) |
| Colonoscopy or sigmoidoscopy in previous 5 years | 36.4 (34.2–38.7) | 17.1 (10.8–23.5) |
| FOBT and/or colonoscopy or sigmoidoscopy | 67.7 (65.3–69.9) | 29.4 (22.6–36.2) |
Note: CI = confidence interval, FOBT = fecal occult blood test (either guaiac fecal occult blood test or fecal immunochemical test).
Unless indicated otherwise.
Body mass index was calculated based on the international standard.24
Physical activity was defined based on Canadian physical activity guidelines.25
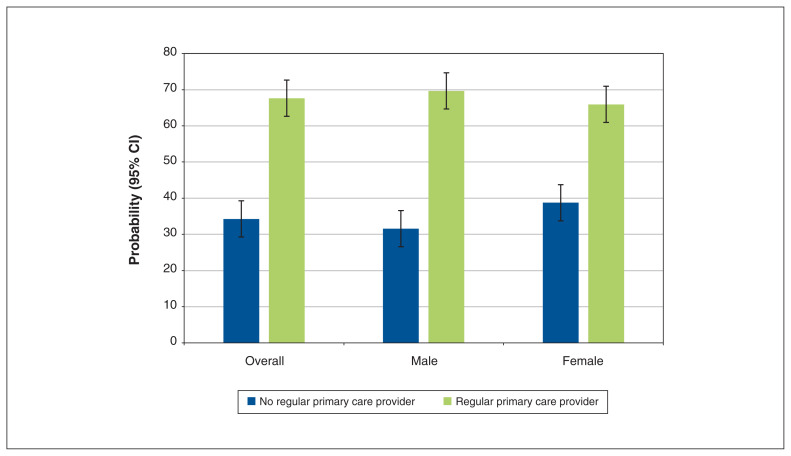
Having a regular primary care provider was significantly associated with being up to date for CRC screening. The odds of being up to date for CRC screening for respondents without a regular provider were significantly lower than for those with a regular provider (adjusted OR 0.25, 95% CI 0.17–0.38). This association remained significant for both male and female subgroups, although it was stronger among males than females (Table 5). Overall, the adjusted absolute probability of being up to date for CRC screening was significantly lower for respondents without a regular primary care provider (33.4%, 95% CI 25.4%–41.5%) than for those with a regular provider (67.8%, 95% CI 65.4%–70.3%). This pattern was observed in both male and female subgroups (Figure 1).
Table 5:
Association between having a regular primary care provider and being up to date for colorectal cancer screening*†
| Variable | Overall | Stratified | ||
|---|---|---|---|---|
|
|
|
|||
| Unadjusted OR (95% CI) | Adjusted OR (95% CI) | Male Adjusted OR (95% CI) | Female Adjusted OR (95% CI) | |
| Regular primary care provider | ||||
|
| ||||
| Yes (Ref.) | 1.00 | 1.00 | 1.00 | 1.00 |
|
| ||||
| No | 0.23 (0.16–0.33) | 0.25 (0.17–0.38) | 0.20 (0.11–0.35) | 0.34 (0.19–0.59) |
|
| ||||
| Age, yr | ||||
|
| ||||
| 50–59 (Ref.) | 1.00 | 1.00 | 1.00 | 1.00 |
|
| ||||
| 60–74 | 2.08 (1.71–2.54) | 1.86 (1.48–2.35) | 1.53 (1.08–2.18) | 2.22 (1.63–3.00) |
|
| ||||
| Marital status | ||||
|
| ||||
| Widowed, divorced, separated or single (Ref.) | 1.00 | 1.00 | 1.00 | 1.00 |
|
| ||||
| Married or common law | 1.52 (1.24–1.88) | 1.44 (1.14–1.83) | 1.57 (1.06–2.32) | 1.29 (0.95–1.74) |
|
| ||||
| Education attainment | ||||
|
| ||||
| Less than secondary graduation (Ref.) | 1.00 | 1.00 | 1.00 | 1.00 |
|
| ||||
| Secondary school education | 1.29 (0.91–1.83) | 1.09(0.70–1.72) | 0.76 (0.38–1.49) | 1.66 (0.94–2.91) |
|
| ||||
| Postsecondary certificate or degree | 1.45 (1.06–1.97) | 1.13 (0.74,1.74) | 0.82 (0.44–1.52) | 1.62 (0.96–2.74) |
|
| ||||
| Race | ||||
|
| ||||
| White (Ref.) | 1.00 | 1.00 | 1.00 | 1.00 |
|
| ||||
| Indigenous or other visible minority | 0.64 (0.43–0.93) | 0.69 (0.41–1.15) | 0.85 (0.36–1.99) | 0.57 (0.31–1.04) |
|
| ||||
| Immigration status | ||||
|
| ||||
| Born in Canada (Ref.) | 1.00 | 1.00 | 1.00 | 1.00 |
|
| ||||
| Permanent or nonpermanent resident | 0.76 (0.58–0.99) | 0.93 (0.66–1.37) | 0.73 (0.40–1.30) | 1.24 (0.78–1.99) |
|
| ||||
| Smoking status | ||||
|
| ||||
| Never smoker (Ref.) | 1.00 | 1.00 | 1.00 | 1.00 |
|
| ||||
| Current smoker (daily or occasional) | 0.52 (0.40–0.67) | 0.61 (0.43–0.87) | 0.68 (0.39–1.17) | 0.54 (0.35–0.83) |
|
| ||||
| Past smoker (daily or occasional) | 1.00 (0.79–1.27) | 0.94 (0.71–1.22) | 1.08 (0.72–1.62) | 0.81 (0.56–1.18) |
|
| ||||
| Illicit drug use in previous 12 mo | ||||
|
| ||||
| No (Ref.) | 1.00 | 1.00 | 1.00 | 1.00 |
|
| ||||
| Yes | 0.54 (0.37–0.77) | 0.69 (0.43–1.09) | 0.67 (0.39–1.16) | 0.61 (0.22–1.67) |
|
| ||||
| Body mass index‡ | ||||
|
| ||||
| Normal (18.5–24.9) (Ref.) | 1.00 | 1.00 | 1.00 | 1.00 |
|
| ||||
| Overweight (25.0–29.9) | 1.06 (0.82–1.37) | 0.98 (0.60–1.13) | 0.95 (0.60–1.50) | 0.97 (0.67–1.38) |
|
| ||||
| Obese (≥ 30.0) | 1.27 (0.98–1.63) | 1.09 (0.82–1.45) | 1.00 (0.64–1.56) | 1.09 (0.75–1.59) |
|
| ||||
| Moderate or vigorous physical activity,§ min/wk | ||||
|
| ||||
| < 150 (inadequate) (Ref.) | 1.00 | 1.00 | 1.00 | 1.00 |
|
| ||||
| ≥ 150 (adequate) | 1.38 (1.12–1.69) | 1.39 (1.09–1.76) | 1.33 (0.91–1.95) | 1.47 (1.08–2.02) |
|
| ||||
| General health | ||||
|
| ||||
| Poor or fair (Ref.) | 1.00 | 1.00 | 1.00 | 1.00 |
|
| ||||
| Good | 1.39 (0.92–2.11) | 0.76 (0.54–1.09) | 0.85 (0.48–1.52) | 0.68 (0.44–1.07) |
|
| ||||
| Very good | 1.54 (1.03–2.29) | 0.89 (0.61–1.24) | 1.03 (0.58–1.84) | 0.76 (0.49–1.19) |
|
| ||||
| Excellent | 1.41 (0.94–2.12) | 0.75 (0.50–1.12) | 0.71 (0.36–1.39) | 0.81 (0.50–1.31) |
Note: CI = confidence interval, FOBT = fecal occult blood test (either guaiac FOBT or fecal immunochemical test), OR = odds ratio, Ref. = reference.
We considered respondents to be up to date for colorectal cancer screening if they had FOBT in the previous 2 years or colonoscopy or sigmoidoscopy in the previous 5 years.
About 9% of the total sample (n = 4600, weighted n = 1 081 161) were excluded from the models owing to missing data across variables. The models included 4300 respondents (weighted n = 997 495). Adjusted models are simultaneously adjusted for every other variable included in the model. We did not include the mammogram variable to enable comparisons across males and females.
Body mass index was calculated based on the international standard.24
Physical activity was defined based on Canadian physical activity guidelines. 25
Figure 1:
Absolute probability of being up to date for colorectal cancer screening among respondents who had a regular primary care provider and those who did not. Adjusted for age, marital status, education status, race, immigration status, smoking status, illicit drug use, physical activity, body mass index and general health. Error bars are 95% confidence intervals (CIs).
Interpretation
Overall, 62.6% of study respondents were up to date for CRC screening by FOBT (45.1%), or by colonoscopy or sigmoidoscopy (34.1%). People without a regular primary care provider were significantly less likely to be up to date on CRC screening than those with a regular primary care provider. This was persistent in both male and female subgroups.
Our study findings show that the prevalence of being up to date for CRC screening in Alberta during the study period was suboptimal, although it was slightly higher than the prevalence in 2012 (59.5%).14 The only other province that responded to the 2015–2016 CCHS module on CRC screening was Prince Edward Island, where, using these data, the overall prevalence of being up to date on CRC screening was 60.9% (FOBT 47.2%, colonoscopy or sigmoidoscopy 35.8%). In PEI, comprehensive recruitment methods are used to promote uptake of CRC screening (i.e., mailed invitation letter, physician referral and self-referral).8
The observed uptake of FOBT, the primary screening modality for the average-risk population in Alberta, was disproportionally low. In contrast, the use of colonoscopy or sigmoidoscopy may be disproportionately high. Colonoscopies or sigmoidoscopies are recommended as first-line CRC screening modalities only for the population at high risk for CRC,10,11 which is estimated to be less than 15% of the total population eligible for CRC screening in Alberta.15 Furthermore, a large proportion of people had used both screening modalities. These findings indicate that screening resources may have been used suboptimally at the time of survey and that there may be an opportunity for better allocation of resources. However, temporal or longitudinal data on the use of CRC screening modalities and detailed data on indications for colonoscopy or sigmoidoscopy will be required to understand the use of screening resources.
The likelihood of up-to-date status for CRC screening was lower in the group with no regular primary care provider than among those with a regular provider. These findings reflect the fact that CRC screening in Alberta is largely opportunistic, with access provided only through primary care providers. Although studies comparing CRC screening rates between people with and without a regular primary care provider are scarce, our findings are in line with strong evidence that indicates a clinician’s recommendation is the most important independent predictor of up-to-date CRC screening.22 Disparities in CRC screening rates across sociodemographic and health behaviour characteristics are well documented.14,15,22,23 Our study shows that having a regular primary care provider is a strong predictor of up-to-date CRC screening, independent of these characteristics.
These findings provide guidance for the improvement of the population-based CRC screening program in Alberta. Our study used CCHS data collected from a population-based, representative sample in Alberta; thus, the findings are generalizable to the target population. In general, multicomponent interventions that target different levels (i.e., the patient, provider or health care system), involving improved provider screening routines, patient education and follow-up, and FIT kit access, are valuable to address overall low rates of CRC screening participation.26,27 Such strategies should be tailored to people without a regular primary care provider to improve their access to CRC screening. This will involve shifting from the current opportunistic screening approach to a universal or organized approach, whereby every eligible person receives a screening invitation and has timely access to FIT kits, regardless of access to primary care providers.28 Particular supports and strategies will be needed for vulnerable populations through enhanced outreach strategies.
According to the recommendation of the Canadian Task Force on Preventive Health Care guideline,7 CRC screening should be started at age 50 years, the choice of screening modalities should depend on a person’s risk level and longitudinal adherence is required to achieve the full benefits of CRC screening. Given data limitations, we could not examine risk level and longitudinal adherence, but future studies may improve our understanding of patterns of patient adherence to CRC screening and of physician adherence to the provincial CRC screening guideline. An analysis of existing surveillance or administrative data, including colonoscopy or sigmoidoscopy databases and laboratory data for FIT, would be valuable for the evaluation of these issues. An electronic health system, Connect Care, has been implemented across Alberta, which will enable better monitoring of CRC screening practices and outcomes.29
Limitations
The CCHS data were self-reported and, thus, likely to include recall bias. The provincial CRC screening guideline considers adults who have received a sigmoidoscopy in the previous 10 years as up to date for CRC screening,10,11 whereas we defined adults as up to date if they had undergone a colonoscopy or sigmoidoscopy in the previous 5 years because of limitations of our data source. Although the reduced time frame may have reduced recall bias, this can underestimate the use of CRC screening. However, this discrepancy is unlikely to have affected our estimates substantially as few of our respondents reported having had colonoscopy or sigmoidoscopy more than 5 years before the survey, and a large proportion of respondents who underwent a colonoscopy in the previous 5 years also participated in FOBT in the previous 2 years and thus may have been included in our FOBT calculation (combined modality). Similarly, we could not exclude patients with a CRC diagnosis, who need colonoscopies for disease management.
The CCHS did not ask about participation in computed tomography (CT) colonography; people with negative results on CT colonography would not need FOBT or colonoscopy. However, this procedure is not a publicly funded screening modality, and is only performed in a few community clinics in Alberta. Based on personal communication with the ACRCSP (Dr. Huiming Yang, Alberta Health Services, Edmonton, Alta.: personal communication, 2021), the number of people who have received CT colonography is very small.
Although the 2017 and 2019 CCHS collected data on CRC screening in Alberta, we were unable to analyze these more recent data because PUMFs for those years were not available at the time of our study. Furthermore, we could not access those data at the University of Calgary Statistics Canada Research Data Centre because of facility access restrictions as a result of the COVID-19 pandemic. We believe that the use of data from the 2015–2016 CCHS still serves our main purpose, which was to explore the relation between having access to a regular primary care provider and being up to date with CRC screening, as the mechanism of access to CRC screening in Alberta has not changed since 2016.
Conclusion
Overall, we found that the proportion of adults who were up to date for CRC screening was suboptimal in the general population of Alberta. People who did not have a regular primary care provider were particularly unlikely to be up to date on CRC screening. Intervention strategies to improve the practice of CRC screening need to be multifaceted to reduce the structural barriers in access to CRC screening, to enhance providers’ service delivery practices of CRC screening and encourage individual demand for CRC screening. Tailored strategies to improve access to CRC screening for adults who do not have a regular primary care provider are needed to address the large disparity in CRC screening participation for this group. Future longitudinal assessments of CRC screening status across modalities and by risk group can offer an improved understanding of CRC screening status and guidance on future improvement and interventions.
Supplementary Material
Footnotes
Competing interests: None declared.
This article has been peer reviewed.
Contributors: All authors contributed to the conception and design of the study. Kamala Adhikari conducted the analysis, and all authors were involved in interpreting the data. Kamala Adhikari drafted the manuscript, and all authors revised it critically for important intellectual content, gave final approval of the version to be published and agreed to be accountable for all aspects of the work.
Funding: This research was funded by Alberta Health through the Alberta Cancer Prevention Legacy Fund (ACPLF). Provision of funding by Alberta Health does not signify that this project represents the policies or views of Alberta Health. The funders had no role in the design of the study; in the collection, analyses or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Data sharing: The public meta file of the 2015–2016 Canadian Community Health Survey is freely available from the Nesstar data portal of Statistics Canada. Additional information such as a statistical data file, statistical codes, text files, tables, additional charts and graphs necessary to understand the research can be provided upon request.
Supplemental information: For reviewer comments and the original submission of this manuscript, please see www.cmajopen.ca/content/10/1/E203/suppl/DC1.
References
- 1.Canadian Cancer Statistics Advisory Committee. Canadian Cancer Statistics 2019. Toronto: Canadian Cancer Society; 2019. [accessed on 2020 Nov 17]. Available: cancer.ca/Canadian-Cancer-Statistics-2019-EN. [Google Scholar]
- 2.Heitman SJ, Hilsden RJ, Au F, et al. Colorectal cancer screening for average-risk North Americans: an economic evaluation. PLoS Med. 2010;7:e1000370. doi: 10.1371/journal.pmed.1000370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Colorectal cancer screening: IARC Handbook of Cancer Prevention. Vol. 17. Lyon (France): International Agency for Research on Cancer (IARC); 2019. [accessed 2020 Dec. 21]. pp. 1–300. Available: http://publications.iarc.fr/573. [Google Scholar]
- 4.Shaukat A, Lehenbauer KP. Screening for colorectal neoplasia. N Engl J Med. 2017;376:1599. doi: 10.1056/NEJMc1702535. [DOI] [PubMed] [Google Scholar]
- 5.Fitzpatrick-Lewis D, Ali MU, Warren R, et al. Screening for colorectal cancer: a systematic review and meta-analysis. Clin Colorectal Cancer. 2016;15:298–313. doi: 10.1016/j.clcc.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 6.Patient completion of screening tests. Health Quality Council of Alberta; [accessed 2020 Dec. 21]. Available: https://focus.hqca.ca/primaryhealthcare/screening/ [Google Scholar]
- 7.Canadian Task Force on Preventive Health Care. Recommendations on screening for colorectal cancer in primary care. CMAJ. 2016;188:340–8. doi: 10.1503/cmaj.151125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Colorectal cancer screening in Canada: environmental scan. Toronto: Canadian Partnership Against Cancer; 2018. revised 2019. [Google Scholar]
- 9.Colorectal cancer screening in Canada: environmental scan – Version 1.1, 2019–2020. Toronto: Canadian Partnership Against Cancer; updated 2021 Jan. 13. [Google Scholar]
- 10.Standards and guidelines for screening colonoscopy services: Alberta Colorectal Cancer Screening Program. Version 2.0. Edmonton: Alberta Health Services; 2014. [accessed 2020 Dec. 21]. Available: https://screeningforlife.ca/wp-content/uploads/2019/12/ACRCSP-Standards-and-Guidelines-for-Screening-Colonoscopy-Services-Feb-2014.pdf. [Google Scholar]
- 11.Colorectal cancer screening: clinical practice guideline. Edmonton: Toward Optimized Practice, Alberta Medical Association; 2013. [accessed 2020 Dec. 21]. revised 2020. Available: http://www.topalbertadoctors.org/download/1009/colorectal_guideline.pdf. [Google Scholar]
- 12.Alberta Colorectal Cancer Screening Program: standards and guidelines. Version 4.0. Edmonton: Alberta Health Services; 2014. [accessed 2020 Dec. 21]. Available: https://screeningforlife.ca/wp-content/uploads/2019/12/ACRCSP-Program-and-Practice-Standards-and-Guidelines-Jan-2014.pdf. [Google Scholar]
- 13.2014 Cancer system performance report. Toronto: Canadian Partnership Against Cancer; 2014. [Google Scholar]
- 14.Singh H, Bernstein CN, Samadder JN, et al. Screening rates for colorectal cancer in Canada: a cross-sectional study. CMAJ Open. 2015;3:E149–57. doi: 10.9778/cmajo.20140073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Solbak NM, Xu J-Y, Vena JE, et al. Patterns and predictors of adherence to colorectal cancer screening recommendations in Alberta’s Tomorrow Project participants stratified by risk. BMC Public Health. 2018;18:177. doi: 10.1186/s12889-018-5095-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Primary health care providers, 2016. Ottawa: Statistic Canada; 2017. [accessed 2021 Feb. 14]. Available: https://www150.statcan.gc.ca/n1/pub/82-625-x/2017001/article/54863-eng.htm. [Google Scholar]
- 17.Broekhuis SM, van Dijk WD, Giesen P, et al. Walk-in clinics in Quebec, Canada: patients and doctors do not agree on appropriateness of visits. Fam Pract. 2014;31:92–101. doi: 10.1093/fampra/cmt069. [DOI] [PubMed] [Google Scholar]
- 18.Barnsley J, Williams AP, Kaczorowski J, et al. Who provides walk-in services? Survey of primary care practice in Ontario. Can Fam Physician. 2002;48:519–26. [PMC free article] [PubMed] [Google Scholar]
- 19.Canadian Community Health Survey: annual component (CCHS) Ottawa: Statistics Canada; [accessed 2021 Jan. 28]. modified 2016 June 24. Available: https://www23.statcan.gc.ca/imdb/p2SV.pl?Function=getSurvey&Id=238854. [Google Scholar]
- 20.Chubak J, Hubbard R. Defining and measuring adherence to cancer screening. J Med Screen. 2016;23:179–85. doi: 10.1177/0969141316630766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blair A, Gauvin L, Ouédraogo S, et al. Area-level income disparities in colorectal screening in Canada: evidence to inform future surveillance. Curr Oncol. 2019;26:e128–37. doi: 10.3747/co.26.4279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gimeno García AZ. Factors influencing colorectal cancer screening participation. Gastroenterol Res Pract. 2012;2012:483417. doi: 10.1155/2012/483417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crouse A, Sadrzadeh SM, de Koning L, et al. Sociodemographic correlates of fecal immunotesting for colorectal cancer screening. Clin Biochem. 2015;48:105–9. doi: 10.1016/j.clinbiochem.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 24.World Health Organization. Obesity: preventing and managing the global epidemic. Report of a WHO consultation on obesity. Geneva: World Health Organization; 1998. [PubMed] [Google Scholar]
- 25.Tremblay MS, Warburton DER, Janssen I, et al. New Canadian physical activity guidelines. Appl Physiol Nutr Metab. 2011;36:36–46. doi: 10.1139/H11-009. [DOI] [PubMed] [Google Scholar]
- 26.Sabatino SA, Lawrence B, Elder R, et al. Community Preventive Services Task Force. Effectiveness of interventions to increase screening for breast, cervical, and colorectal cancers: nine updated systematic reviews for the guide to community preventive services. Am J Prev Med. 2012;43:97–118. doi: 10.1016/j.amepre.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 27.Increasing colorectal cancer screening: multicomponent interventions — Community Preventive Task Force finding and rationale statement, ratified August 2016. Atlanta: The Community Guide; 2016. [accessed 2021 Jan. 1]. Available: https://www.thecommunityguide.org/findings/cancer-screening-multicomponent-interventions-colorectal-cancer. [Google Scholar]
- 28.Melson JE, Imperiale TF, Itzkowitz SH, et al. AGA White Paper: roadmap for the future colorectal cancer screening in the United States. Clin Gastroenterol Hepatol. 2020;18:2667–78e2. doi: 10.1016/j.cgh.2020.06.053. [DOI] [PubMed] [Google Scholar]
- 29.Connect Care. Edmonton: Alberta Health Services; [accessed 2021 Jan. 1]. Available: https://insite.albertahealthservices.ca/cis/Page12170.aspx. Login required to access content. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.