Abstract
OBJECTIVES
Acute kidney injury (AKI) following surgery involving the heart-lung-machine is associated with high mortality and morbidity. In addition to the known mechanisms, thrombotic microangiopathy (TMA) triggered by the dysregulation of complement activation was recently described as another pathophysiological pathway for AKI following aortic surgery. The aim of this retrospective study was to analyse incidence, predictors and outcome in these patients.
METHODS
Between January 2018 and September 2019, consecutive patients undergoing aortic surgery requiring hypothermic circulatory arrest were retrospectively reviewed. If suspected, diagnostic algorithm was initiated to identify a TMA and its risk factors, and postoperative outcome parameters were comparably investigated.
RESULTS
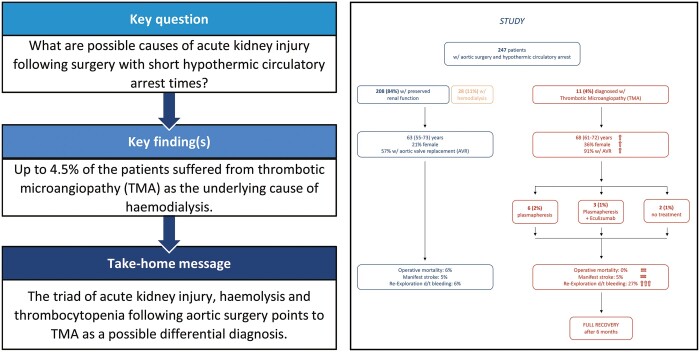
The incidence of TMA in the analysed cohort (n = 247) was 4.5%. Multivariable logistic regression indicated female gender {odds ratio (OR) 4.905 [95% confidence interval (CI) 1.234–19.495], P = 0.024} and aortic valve replacement [OR 8.886 (95% CI 1.030–76.660), P = 0.047] as independent predictors of TMA, while cardiopulmonary bypass, X-clamp and hypothermic circulatory arrest times showed no statistically significance. TMA resulted in postoperative AKI (82%), neurological disorders (73%) and thrombocytopaenia [31 (interquartile range 25–42) G/l], corresponding to the diagnostic criteria. Operative mortality and morbidity were equal to patients without postoperative TMA, despite a higher incidence of re-exploration for bleeding (27 vs 6%; P = 0.027). After 6 months, survival, laboratory parameters and need for dialysis were comparable between the groups.
CONCLUSIONS
TMA is a potential differential diagnosis for the cause of AKI following aortic surgery regardless of the hypothermic circulatory arrest time. Timely diagnosis and appropriate treatment resulted in a comparable outcome concerning mortality and renal function.
Keywords: Aortic surgery, Hypothermic circulatory arrest, Thrombotic microangiopathy, Haemolytic uraemic syndrome, Cardiopulmonary bypass, Acute kidney injury, Haemolytic anaemia, Thrombocytopaenia, Eculizumab
Acute kidney injury (AKI) not infrequently complicates the postoperative recovery in patients following surgical repair of the aorta.
INTRODUCTION
Acute kidney injury (AKI) not infrequently complicates the postoperative recovery in patients following surgical repair of the aorta. Especially if the thoracic aorta is involved and cardiopulmonary bypass (CPB) with or without hypothermic circulatory arrest (HCA) is required, the incidence of AKI ranges between 15% and 50% and renal replacement therapy (RRT) is required in 8–11% [1–4]. Postoperative AKI is associated with higher morbidity, mortality and a prolonged length of hospital stay [1]. Whereas the mechanism is evident in certain cases, for example, in aortic dissection with manifest malperfusion of the renal arteries, in the majority of cases the underlying mechanism for AKI is multifactorial and yet not fully understood. Various predictors for postoperative AKI have been reported in the literature. Apart from demographic characteristics and pre-existing comorbidities, the duration of CPB and hypothermic HCA have repeatedly been identified as independent risk factors [3, 5, 6]. Ischaemia–reperfusion injury causing tubular dysfunction and systemic inflammatory response syndrome are furthermore well-identified major contributing factors [7]. The activation of alternative complement pathways has also been discussed as a potential cause [8].
Recently, our group reviewed patients suffering from AKI following cardiac surgery and identified thrombotic microangiopathy (TMA) as another potential and so far underestimated mechanism [9].
TMA’s are systemic diseases, characterized by the triad of non-immune thrombocytopaenia, microangiopathic haemolytic anaemia and AKI, caused by endothelial damage and microvascular thrombosis. Extra-renal manifestations are mainly neurological, but cardiovascular, pulmonary and gastrointestinal symptoms have also been described [10].
TMA thereby refers to 3 different pathophysiological entities [10]:
Thrombotic thrombocytopenic purpura (TTP), caused by severe deficiency of the protease A Disintegrin and Metalloprotease with a Thrombospondin type 1 motif, member 13 (ADAMTS13);
Haemolytic uraemic syndrome (HUS), caused by Shiga-toxin producing Escherichia coli (STEC-HUS);
Atypical HUS (aHUS).
The rare clinical entity aHUS includes all phenotypical TMA cases after definitive exclusion of TTP and HUS and may be divided into a complement-mediated ‘primary’ form and secondary forms with defined triggering agents such as autoimmune diseases, solid-organ transplantation or certain drugs [11, 12].
TMAs are commonly mistaken for episodes of disseminated intravascular coagulation [13]. In contrast to disseminated intravascular coagulation, TMA, however, causes an isolated consumption of thrombocytes, leaving the plasmatic coagulation factors unaffected.
From the aforementioned analysis of AKI in cardiac surgery patients, a disproportionately high incidence of AKI was noted in aortic surgery patients due to TMA [9]. Thus, the aim of the present study was to evaluate incidence and potentially predictive factors of a TMA-associated AKI in these patients and to compare the results with contemporary patients undergoing the same operative procedure without consecutive TMA.
PATIENTS AND METHODS
Ethical statement
This study was designed as a retrospective single-centre analysis and approved by the local ethics committee of the Ludwig Maximilian University (Project No. 19-439 and 20-629). Informed consent was waived.
Study design
Between January 2018 and September 2019, consecutive patients undergoing ascending aortic surgery with extension into the arch and thus requiring HCA were reviewed and included into the study. Descending aortic replacements with or without distal arch procedures and thoraco-abdominal aortic repairs were excluded. Patients requiring chronic haemodialysis prior to surgery were also excluded from the study.
All patients, who were clinically suspected of postoperative TMA, underwent the below-mentioned diagnostic algorithm. Subsequently, the cohort was divided into 2 groups for further analysis: (i) patients who fulfilled the diagnostic criteria for TMA within the first 7 postoperative days (TMA group) and (ii) those who did not (non-TMA group) (see also Supplementary Material, Fig. S1).
All patients were routinely seen for follow-up 6 months after surgery. Evaluation of potential genetic mutations or the proof of complement consumption was not the aim of this retrospective study.
Diagnosis of postoperative thrombotic microangiopathy
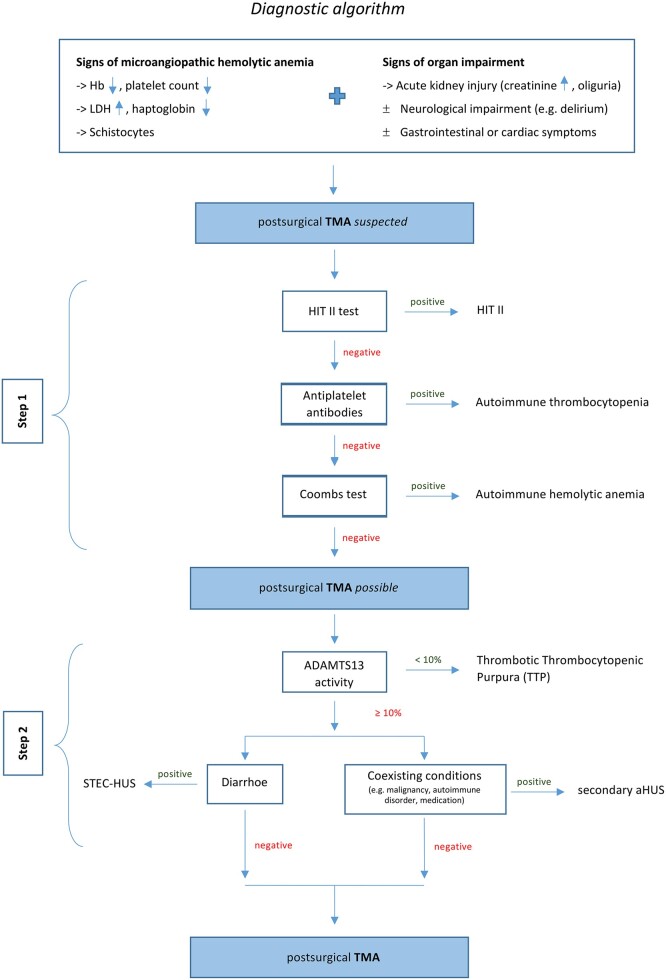
The diagnostic algorithm used here is depicted in Fig. 1 [14].
Figure 1:
Diagnostic algorithm for postsurgical TMA. aHUS: atypical haemolytic uraemic syndrome; Hb: hemoglobin; LDH: lactate dehydrogenase; TMA: thrombotic microangiopathy; TTP: thrombotic thrombocytopenic purpura.
Step 1: Exclusion of the more common differential diagnoses like heparin-induced thrombocytopenia type II, autoimmune causes for haemolysis (using Coombs Test) and autoantibody-induced thrombocytopenia.
Step 2: Exclusion of other forms of TMA. TTP was excluded by determining the enzymatic activity of von Willebrand Factor-cleaving protease, called ADAMTS13. STEC-HUS was excluded by microbiological testing. Coexisting medical conditions triggering secondary aHUS were excluded by the patient’s medical history and preoperative computed tomography.
Surgical technique
The perioperative management during arch surgery is described elsewhere [15]. Briefly, procedural conditions were moderate HCA at 25–26°C with selective antegrade cerebral perfusion at 22°C. At the proximal site, either aortic valve repair or aortic valve replacement with or without root replacement was performed. Distally, a proximal arch or a total arch replacement was performed. All the procedures included in the analysis were carried out with cardioplegic cardiac arrest using 3 applications of Bretschneider cardioplegia, which was not actively removed from the circulation.
Parameter and end points
Demographic characteristics, comorbidities, intraoperative data and postoperative outcome were comparatively analysed. Laboratory parameters (creatinine, lactate dehydrogenase and platelet counts) were determined and analysed at 3 different time pointes: preoperative, postoperative (daily) and 6 months after surgery. Follow-up data were assessed 6 months after surgery as part of our routine monitoring program.
AKI was defined by standardized three-staged AKIN criteria based on the serum creatinine level and the urine output (Supplementary Material, Table S1) and was assessed daily [16].
The primary end point was the incidence of TMA following aortic surgery with HCA. Secondary end points were to investigate (i) factors associated to TMA, such as demographic characteristics, comorbidities and surgical data and (ii) the postoperative outcome and follow-up after treatment compared to patients without TMA (non-TMA group).
Statistical analyses
Data were analysed using IBM SPSS® Statistics software (IBM Corporation, Armonk, NY, USA). Data are presented either as absolute frequency distribution and percentages for categorical variables or as median and interquartile range as measures of central tendency and dispersion of the continuous variables. The independent predictors for TMA (dependent variable) were tested by univariable and multivariable binary logistic regression models using a forward stepwise model and including variables with a P-value of ≤0.2 from univariable analysis. To investigated the postoperative results, the statistical difference between patients with and without postsurgical TMA was tested using the Pearson χ2 test or Fisher’s exact test for categorical variables, and the two-sample Wilcoxon rank-sum test for continuous variables. P-value ≤0.05 was considered statistically significant.
RESULTS
In total, 247 consecutive patients were included into the study. During the first 7 postoperative days, 39 patients (15.8%) developed AKI requiring RRT. Twenty-three of them (9.3%) showed refractory thrombocytopaenia and haemolytic anaemia and fulfilled the clinical criteria suspecting TMA (Supplementary Material, Fig. S2 and Supplementary Material, Table S2). Following the application of the diagnostic algorithm (Fig. 1), 9 patients (3.6%) were finally diagnosed with TMA. Two more patients (0.8%) retrospectively fulfilled the TMA criteria but showed predominantly extra-renal manifestation without the need of RRT. Thus, the overall incidence of TMA within the cohort was 4.5%.
Given the definitions of the subtypes, the TMA’s observed here should be classified as aHUSs. However, as surgery is not a commonly reported and accepted triggering event, such a subclassification is as yet not licit.
Association between thrombotic microangiopathy, and demographic characteristics, comorbidities and surgical data
Univariable and multivariable regression analyses identified female gender {P = 0.024; odds ratio (OR) 4.905 [95% confidence interval (CI) 1.234–19.495]} and aortic valve replacement [P = 0.047; OR 8.886 (95% CI 1.030–76.660)] as independent predictors of TMA (Table 1).
Table 1:
Demographic characteristics and underlying disease
| Study cohort (n = 247) | Incidence TMA (n = 11) | Univariable analysis |
||
|---|---|---|---|---|
| P-value | Odds ratio | |||
| Age (years) | 64 (56–73) | 68 (61–72) | 0.205 | 1.038 (0.980–1.099) |
| Female gender | 70 | 4 (6) | 0.015 | 4.806 (1.361–16.973) |
| BMI (kg/m2) | 26 (24–29) | 26 (21–30) | 0.524 | 0.956 (0.831–1.099) |
| Aortic pathology | ||||
| Aneurysm | 165 | 7 (4) | 0.820 | 0.864 (0.246–3.040) |
| Chronica/residualb dissection | 19 | 2 (11) | 0.200 | 2.863 (0.572–14.317) |
| Acute aortic syndromec | 53 | 1 (2) | 0.327 | 0.354 (0.044–2.828) |
| Endocarditis/prosthetic infection | 10 | 1 (1) | 0.401 | 2.522 (0.291–21.890) |
| Aortic valve pathology | ||||
| Stenosis | 50 | 2 (4) | 0.862 | 0.870 (0.182–4.161) |
| Insufficiency | 205 | 9 (4) | 0.915 | 0.918 (0.191–4.412) |
| Bicuspid morphology | 83 | 2 (2) | 0.400 | 0.504 (0.102–2.486) |
| Valve prosthesis in situ | 14 | 2 (14) | 0.089 | 4.148 (0.806–21.353) |
| Biological type | 7 | 1 (14) | 0.919 | 1.167 (0.059–22.937) |
Data are shown as median (0.25–0.75 percentile), n (%) or odds ratio (95% confidence interval), bold: statistically significant.
Occurrence ≥14 days ago.
Persistent/progredient disease after surgically corrected dissection.
Includes aortic dissection, intramural haematoma and symptomatic penetrating aortic ulcer.
BMI: body mass index; TMA: thrombotic microangiopathy.
There was no statistical significance among the underlying pathology leading to surgery; however, a trend was seen in residual aortic dissections (11%; P = 0.200), previous aortic valve replacement requiring CPB (14%; P = 0.089) and reoperation (10%; P = 0.132) (Table 1). Comorbidities, surgical procedures performed, CPB and HCA times did not show predictive validity. Further details are listed in Tables 2 and 3.
Table 2:
Comorbidities
| Study cohort (n = 247) | Incidence TMA (n = 11) | Univariable analysis |
||
|---|---|---|---|---|
| P-value | Odds ratio | |||
| S/p stroke or CVA | 46 | 2 (4) | 0.969 | 0.970 (0.202–4.646) |
| Acute kidney injury | 17 | 1 (6) | 0.768 | 1.375 (0.165–11.425) |
| Chronic kidney disease | 29 | 1 (3) | 0.781 | 0.743 (0.092–6.025) |
| LVEF (%) | 60 | 60 (52–60) | 0.695 | 0.986 (0.918–1.059) |
| Coronary artery disease | 63 | 3 (5) | 0.891 | 1.100 (0.283–4.281) |
| Diabetes mellitus | 22 | 1 (5) | 0.983 | 1.024 (0.125–8.293) |
| w/insulin | 5 | 1 (20) | 0.131 | 5.800 (0.593–56.756) |
| Hypertension | 192 | 8 (4) | 0.684 | 0.754 (0.193–2.943) |
| COPD | 19 | 1 (5) | 0.859 | 1.211 (0.147–10.000) |
| Smoker | 85 | 2 (2) | 0.261 | 0.410 (0.086–1.940) |
| Obesity | 53 | 2 (4) | 0.787 | 0.806 (0.169–3.848) |
Data are shown as median (0.25–0.75 percentile), n (%) or odds ratio (95% confidence interval), bold: statistically significant.
COPD: chronic obstructive pulmonary disease; CVA: cerebrovascular accident; LVEF: left ventricular ejection fraction; TMA: thrombotic microangiopathy.
Table 3:
Surgical data
| Study cohort (n = 247) | Incidence TMA (n = 11) | Univariable analysis |
||
|---|---|---|---|---|
| P-value | Odds ratio | |||
| Extracorporeal circulation | ||||
| CPB time (min) | 203 (164–246) | 217 (164–267) | 0.418 | 1.004 (0.995–1.012) |
| Cross-clamp time (min) | 138 (111–165) | 147 (112–194) | 0.229 | 1.007 (0.995–1.020) |
| HCA time (min) | 25 (20–46) | 28 (21–36) | 0.820 | 0.996 (0.965–1.028) |
| SACP time (min) | 22 (16–41) | 26 (18–34) | 0.816 | 0.997 (0.970–1.024) |
| Lowest core temperature (°C) | 25 (23.7–25.5) | 24.4 (23.4–25.5) | 0.477 | 1.123 (0.816–1.545) |
| SACP temperature (°C) | 22.0 | 22.0 | 0.143 | 1.770 (0.824–3.802) |
| Cardioplegia (ml) | 2140 (1900–2440) | 2140 (1910–2440) | 0.935 | 1.000 (0.998–1.002) |
| Proximal site | ||||
| Aortic valve repair | 56 | 1 (2) | 0.294 | 0.329 (0.041–2.628) |
| David | 29 | 0 (–) | 0.998 | <0.001 (–) |
| Root-sparing (Frater) | 27 | 1 (4) | 0.842 | 0.808 (0.099–6.567) |
| Aortic valve replacement | 145 | 10 (7) | 0.057 | 7.481 (0.942–59.392) |
| Bentall | 98 | 7 (7) | 0.110 | 2.788 (0.794–9.792) |
| Root-sparing | 47 | 3 (6) | 0.480 | 1.636 (0.417–6.419) |
| Distal site | ||||
| Proximal arch replacement | 205 | 10 (5) | 0.484 | 2.103 (0.262–16.881) |
| Total arch replacement | 42 | 1 (2) | 0.484 | 0.476 (0.059–3.819) |
| w/FET | 34 | 1 (3) | 0.648 | 0.615 (0.076–4.965) |
| Concomitant procedures | 121 | 6 (5) | 0.707 | 1.263 (0.375–4.251) |
| Reoperation | 30 | 3 (10) | 0.132 | 2.903 (0.726–11.609) |
Data are shown as median (0.25–0.75 percentile), n (%) or odds ratio (95% confidence interval), bold: statistically significant.
CPB: cardiopulmonary bypass; FET: frozen elephant trunk; HCA: hypothermic circulatory arrest; SACP: selective antegrade cerebral perfusion; TMA: thrombotic microangiopathy.
However, Cox and Snell R2 and Nagelkerkes R2 were only 0.069 and 0.227, respectively. This seems to imply that there are other, presently unidentified factors, which are also important for the development of TMA in the surgical setting.
Laboratory parameters
The maximum values of creatinine, lactate dehydrogenase and thrombocyte count within the first 7 postoperative days, which represent the entry criteria for further TMA diagnostic testing, differ significantly between the groups, while preoperative values were comparable. Further details are listed in Table 4.
Table 4:
Laboratory parameters
| TMA (n = 11) | Non-TMA (n = 236) | P-value | |
|---|---|---|---|
| Preoperative values | |||
| Creatinine (mg/dl) | 0.9 (0.8–1.1) | 1 (0.9–1.2) | 0.172 |
| LDH (U/l) | 298 (258–301) | 280 (221.5–335.5) | 0.911 |
| Thrombocytes (G/l) | 193 (132–254) | 212 (173.5–255.5) | 0.307 |
| Postoperative values | |||
| Creatinine max (mg/dl) | 4.5 (3.7–6.7) | 1.3 (1.1–1.8) | <0.001 |
| LDH max (U/l) | 1436 (881–1562) | 452 (392–592) | <0.001 |
| Thrombocytes min (G/l) | 31 (25–42) | 97 (78–122) | <0.001 |
| Follow-up values | |||
| Creatinine max (mg/dl) | 0.85 (0.7–0.9) | 1 (0.9–1.1) | 0.118 |
| LDH max (U/l) | 223 (199–260) | 265 (221–334) | 0.315 |
| Thrombocytes (G/l) | 290 (210–419) | 233 (193–303) | 0.393 |
Data are shown as median (0.25–0.75 percentile), bold: statistically significant.
LDH: lactate dehydrogenase; TMA: thrombotic microangiopathy.
Treatment of postsurgical thrombotic microangiopathy
The treatment of postsurgical TMA is generally based on the clinical practice guidelines for the management of TMA. However, these guidelines were established for non-surgical patients and, thus, treatment of the patients reported here required individual management and interdisciplinary discussion [17].
Nine patients (82% out of the TMA group) were treated by plasmapheresis. The number of treatment cycles was dependent on the clinical evolution and averaged at 4 cycles (range 3–5). Because of insufficient remission after 3 cycles of plasmapheresis, 3 of the 9 patients (27%) received Eculizumab.
As mentioned above, 2 patients were diagnosed retrospectively after reviewing the patient’s medical record and thus received no specific treatment.
In-hospital outcome and follow-up
Corresponding to the diagnostic criteria, the TMA group showed a significant higher incidence of neurological symptoms (73% vs 30%; P = 0.005), predominantly delirium (46% vs 18%; P = 0.041).
Patients with TMA experienced more frequently re-exploration due to bleeding and/or tamponade (27% vs 6%; P = 0.027). Operative mortality and morbidities did not differ between the groups. The parameters analysed are shown in Table 5.
Table 5:
In-hospital outcome
| TMA (n = 11) | Non-TMA (n = 236) | P-value | |
|---|---|---|---|
| Operative mortalitya | 0 (–) | 13 (6) | 1.000 |
| Neurological outcome | |||
| Neurological disorders | 8 (73) | 70 (30) | 0.005 |
| Focal neurological deficits | 1 (9) | 12 (5) | 0.455 |
| Delirium | 5 (46) | 43 (18) | 0.041 |
| Delayed awakening | 2 (18) | 18 (8) | 0.220 |
| Renal outcome | |||
| Postoperative dialysis | 9 (82) | 30 (13) | <0.001 |
| Haemodialysis @ discharge | 0 (–) | 11 (5) | 1.000 |
| Morbidities | |||
| Re-exploration d/t bleeding | 3 (27) | 13 (6) | 0.027 |
| Postoperative v-a ECMO | 1 (9) | 11 (5) | 0.429 |
| Multiple organ failure | 3 (27) | 22 (9) | 0.088 |
| Catecholamines | |||
| Adrenalin (mg/h) | 0.2 (0–0.4) | 0.35 (0–0.6) | 0.086 |
| Noradrenalin (mg/h) | 0.7 (0.2–1) | 1.1 (0.5–2.1) | 0.105 |
| Vasopressin (IE/h) | 0 (0–0.5) | 0.5 (0–1.5) | 0.079 |
Data are shown as n (%) or median (0.25–0.75 percentile), bold: statistically significant.
Classified as in-hospital mortality plus 30-day mortality of discharged patients.
TMA: thrombotic microangiopathy; v-a ECMO: veno-arterial extracorporeal membrane oxygenation.
No patient in the TMA group required RRT at the time of discharge.
After 6 months, survival (100% vs 92%; P = not significant) and the need for RRT (0 vs 1%; P = not significant) were comparable between the groups. However, the 2 retrospectively identified and non-treated patients still suffered from relevant impairment of the renal function without, however, the need of RRT. Follow-up was completed in 100% of the TMA and 78% of the non-TMA group.
DISCUSSION
This study identified TMA with an incidence of 4.5% in patients having undergone aortic surgery with HCA. TMA patients predominantly suffered from the typical triad—AKI with or without the need of RRT, haemolytic anaemia and thrombocytopaenia—complemented by neurological impairment as a symptom of extra-renal manifestation [18, 19]. The patient’s status improved after treatment and all extra-renal manifestations proved only temporary. None of the affected patients showed a worse long-term outcome than the unaffected patients. Female gender and aortic valve replacement were statistically identified as potential predictors; however, the power of the model does not allow a more precise conclusion. The incidence per se may be overestimated as the diagnostic algorithm for classical, non-surgical forms of TMA was applied. However, TMA is clearly an unrecognized relevant cause of AKI in aortic surgery patients complicating the postoperative course especially if unrecognized and untreated.
All affected cases may theoretically be subclassified as aHUS, a rare form of TMA [10]. Historically, aHUS is the diagnosis assigned to all presentations of TMA, once TTP and STEC-HUS have been excluded. Primary aHUS is caused by an altered, dysfunctional activation of the alternative complement pathway and leads to endothelial damage, microvascular thrombosis and end-organ failure. For the clinical manifestation of secondary aHUS, specific triggering conditions have been defined [11, 20]. In ∼60–70%, genetic variants have been described as possible predisposing factors and in 5–10% autoantibodies against complement factor H are present [12]. However, surgery is not yet reported as potential triggering event. At present, the phenomenon described here has thus to be considered as a non-subspecified form of TMA.
TMA entities mainly affected adults (94%) with a mean age of 53.3 years. Prevailing female gender is a common epidemiological observation of unknown cause. Schönermarck et al. [20] analysed 232 patients admitted with suspected TMA, revealing that, among other things, 54% of the adult patients were female. The most common organ manifestation was AKI. Surprisingly, gastrointestinal symptoms were the most common extra-renal manifestation not only in patients suffering from STEC-HUS but also in the TMA group (51%). In our cohort, none of the patients developed gastrointestinal symptoms during their TMA episode. However, these may have been masked by the typical symptoms that follow HCA, such as gastroparesis and obstipation during the early postoperative days. Neurological disorders, the most common extra-renal manifestation in our study, were present in 58% of TTP patients but only in 39% of aHUS patients in Schönermarck’s work.
No patient suffered from TMA here showed dermal or other manifestation such as purpura.
Plasmapheresis aiming to stabilize uncontrolled complement activation is accepted as first-line therapy of primary, complement-mediated aHUS, if TTP remains a potential differential diagnosis. Remission is described in 55–80% of the patients, depending on the genetic variants in the complement system [21]. However, no randomized controlled trial has been reported. Once TTP has been definitively excluded, Eculizumab, a humanized monoclonal IgG antibody against the complement protein C5, becomes the primary choice of treatment. It improves outcome by preventing the ongoing formation of microthrombi [20]. Several studies have shown the efficacy and the beneficial effect on renal function and avoidance of RRT [21, 22]. However, its use in secondary aHUS and the necessary duration of therapy remains controversial [20, 22, 23].
In the present cohort, plasmapheresis and Eculizumab were both used with good results. However, a differential analysis of the treatment strategies cannot be performed due to the small number of patients, the lack of a control group and a standardized treatment protocol.
CPB is a known trigger for complement activation due to the extensive and prolonged contact of the patients’ blood with artificial surfaces but has not been recognized as a trigger for the development of TMA so far [9]. Only few reports of TMA following cardiac surgery exist in the literature [24–26].
Ikushima et al. [24] presented a case of an acute aortic dissection type A, who developed thrombocytopaenia and AKI requiring RRT shortly after surgery. TMA diagnosis was established by confirming schistocytes in the blood smear.
Although the results of our present study did not suggest any association of TMA with acute aortic dissection, residual/chronic aortic dissection seems to be more frequent in TMA patients. Whether this is due to the largely increased surface area of the aorta, which is only partially endothelialized, or a mere reflection of the fact, that these patients have had aortic surgery in the past, remains unclear.
Markakis et al. [25] described one case of aHUS after solitary biological aortic valve replacement due to combined aortic valve disease in a 78-year-old woman presenting the typical clinical triad. No other triggers of aHUS were present, suggesting surgical aortic valve replacement as the only potential cause of the aHUS. This agrees with our present data that hypothesized prosthetic valves as 1 potential predictor. It remains hypothetical, however, whether the foreign surface itself (activation of innate immune system) or the preservation and sterilization process serves as trigger. Other conceivable pathogenetic mechanisms are a local activation of the complement system by the valve stenosis per se or release of the anaphylatoxins C3a and C5a during the decalcification process of the annulus [27].
Oda et al. [28] described a correlation between temperature during deep HCA (22–23°C) and the extent of complement activation. The complement activation led to a decrease in key proteins in complement regulation suppressing the activity of this part of the innate immune system. However, this down-regulation of the proteins only lasts until rewarming. Analysis of plasma samples obtained after rewarming showed an up-regulated complement activation, potentially caused by ischaemia–reperfusion injury. Bisschops et al. [29] demonstrated a decrease in activity of the innate immune system including the complement system following therapeutic hypothermia in patients following cardiac arrest.
It remains undetermined, however, if such an imbalance of complement regulation may trigger aHUS in perhaps genetically predisposed patients. The presented data do not delineate the effect of temperature, as only patients with HCA were included.
Presently, it appears that cardiovascular surgery-induced TMA may be the result of an excessive overactivation of the complement system by the surgery, presumably in patients with a predisposition. A correlation with HCA seems unlikely, based on our findings. Further studies are necessary in order to confirm this theory and to possibly identify the responsible genetic mutations. From a clinical standpoint, the identification of preoperative markers would be of great value.
Limitations
This is a retrospective single-centre study with the inherent limitation of such an analysis. Second, the results are further limited by the small number of TMA patients and, thus, univariable and multivariable regression models are of limited power. Third, a prevalence incidence bias (Neyman Bias) cannot be excluded. Fourth, the temporal sequence of TMA and each postoperative parameter is unknown and, thus, the competing postoperative outcome (Table 5) has to be interpreted with caution. However, suggesting surgery as TMA trigger, the comparability should be given, but the lack of a non-treated TMA cohort weakens the conclusions. Prospective studies are necessary to confirm and expand the findings of this study.
CONCLUSION
TMA is a potential cause of AKI following aortic surgery with HCA and appears to be independent of its duration. The presence of the triad—AKI, severe haemolysis and refractory thrombocytopaenia—should heighten the awareness of the possible diagnosis (Fig. 1). If diagnosed and adequately treated in time, the outcome seems as good as in non-affected patients.
SUPPLEMENTARY MATERIAL
Supplementary material is available at ICVTS online.
Conflict of interest: Ulf Schönermarck received study fees, travel support and consultancy fees from Alexion. All other authors declared no conflict of interest.
Author contributions
Christine E. Kamla: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Visualization; Writing—original draft. Melissa Grigorescu-Vlass: Data curation; Visualization; Writing—original draft. Dietmar Wassilowsky: Data curation; Investigation. Michael Fischereder: Resources; Software; Supervision; Validation; Writing—review & editing. Christian Hagl: Resources; Software; Supervision; Validation; Writing—review & editing. Ulf Schönermarck: Methodology; Resources; Validation; Writing—review & editing. Maximilian A. Pichlmaier: Formal analysis; Investigation; Validation; Writing—review & editing; Language Editing. Sven Peterss: Conceptualization; Data curation; Formal analysis; Methodology; Visualization; Writing—original draft. Dominik Jóskowiak: Conceptualization; Methodology; Writing—original draft.
Reviewer information
Interactive CardioVascular and Thoracic Surgery thanks Thomas Schachner and the other, anonymous reviewer(s) for their contribution to the peer review process of this article.
Supplementary Material
ABBREVIATIONS
- aHUS
Atypical haemolytic uraemic syndrome
- AKI
Acute kidney injury
- CI
Confidence interval
- CPB
Cardiopulmonary bypass
- HCA
Hypothermic circulatory arrest
- HUS
Haemolytic uraemic syndrome
- OR
Odds ratio
- RRT
Renal replacement therapy
- STEC
Shiga-toxin producing Escherichia coli
- TMA
Thrombotic microangiopathy
- TTP
Thrombotic thrombocytopenic purpura
Contributor Information
Christine E Kamla, Department of Cardiac Surgery, LMU University Hospital, Munich, Germany.
Melissa Grigorescu-Vlass, Division Nephrology, Department of Internal Medicine IV, LMU University Hospital, Munich, Germany.
Dietmar Wassilowsky, Department of Anaesthesiology, LMU University Hospital, Munich, Germany.
Michael Fischereder, Division Nephrology, Department of Internal Medicine IV, LMU University Hospital, Munich, Germany.
Christian Hagl, Department of Cardiac Surgery, LMU University Hospital, Munich, Germany.
Ulf Schönermarck, Division Nephrology, Department of Internal Medicine IV, LMU University Hospital, Munich, Germany.
Maximilian A Pichlmaier, Department of Cardiac Surgery, LMU University Hospital, Munich, Germany.
Sven Peterss, Department of Cardiac Surgery, LMU University Hospital, Munich, Germany.
Dominik Jóskowiak, Department of Cardiac Surgery, LMU University Hospital, Munich, Germany.
REFERENCES
- 1. Arnaoutakis GJ, Bihorac A, Martin TD, Hess PJ Jr, Klodell CT, Ejaz AA et al. RIFLE criteria for acute kidney injury in aortic arch surgery. J Thorac Cardiovasc Surg 2007;134:1554–60. [DOI] [PubMed] [Google Scholar]
- 2. Englberger L, Suri RM, Greason KL, Burkhart HM, Sundt TM, Daly RC et al. Deep hypothermic circulatory arrest is not a risk factor for acute kidney injury in thoracic aortic surgery. J Thorac Cardiovasc Surg 2011;141:552–8. [DOI] [PubMed] [Google Scholar]
- 3. Kim WH, Lee SM, Choi JW, Kim EH, Lee JH, Jung JW et al. Simplified clinical risk score to predict acute kidney injury after aortic surgery. J Cardiothorac Vasc Anesth 2013;27:1158–66. [DOI] [PubMed] [Google Scholar]
- 4. Pacini D, Pantaleo A, Di Marco L, Leone A, Barberio G, Parolari A et al. Risk factors for acute kidney injury after surgery of the thoracic aorta using antegrade selective cerebral perfusion and moderate hypothermia. J Thorac Cardiovasc Surg 2015;150:127–33.e1. [DOI] [PubMed] [Google Scholar]
- 5. Ghincea C, Reece TB, Eldeiry M, Roda GF, Bronsert MR, Jarrett MJ et al. Predictors of acute kidney injury following aortic arch surgery. J Surg Res 2019;242:40–6. [DOI] [PubMed] [Google Scholar]
- 6. Xu S, Liu J, Li L, Wu Z, Li J, Liu Y et al. Cardiopulmonary bypass time is an independent risk factor for acute kidney injury in emergent thoracic aortic surgery: a retrospective cohort study. J Cardiothorac Surg 2019;14:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kamiya H, Hagl C, Kropivnitskaya I, Bothig D, Kallenbach K, Khaladj N et al. The safety of moderate hypothermic lower body circulatory arrest with selective cerebral perfusion: a propensity score analysis. J Thorac Cardiovasc Surg 2007;133:501–9. [DOI] [PubMed] [Google Scholar]
- 8. Bruins P, Te Velthuis H, Yazdanbakhsh AP, Jansen PG, van Hardevelt FW, de Beaumont EM et al. Activation of the complement system during and after cardiopulmonary bypass surgery: postsurgery activation involves C-reactive protein and is associated with postoperative arrhythmia. Circulation 1997;96:3542–8. [DOI] [PubMed] [Google Scholar]
- 9. Grigorescu M, Kamla CE, Wassilowsky D, Joskowiak D, Peterss S, Kemner S et al. Severe acute kidney injury in cardiovascular surgery: thrombotic microangiopathy as a differential diagnosis to ischemia reperfusion injury. A retrospective study. J Clin Med 2020;9:2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. George JN, Nester CM. Syndromes of thrombotic microangiopathy. N Engl J Med 2014;371:654–66. [DOI] [PubMed] [Google Scholar]
- 11. Kavanagh D, Goodship TH, Richards A. Atypical hemolytic uremic syndrome. Semin Nephrol 2013;33:508–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Raina R, Krishnappa V, Blaha T, Kann T, Hein W, Burke L et al. Atypical hemolytic-uremic syndrome: an update on pathophysiology, diagnosis and treatment. Ther Apher Dial 2019;23:4–21. [DOI] [PubMed] [Google Scholar]
- 13. Wada H, Matsumoto T, Suzuki K, Imai H, Katayama N, Iba T et al. Differences and similarities between disseminated intravascular coagulation and thrombotic microangiopathy. Thromb J 2018;16:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nguyen MH, Mathew JJ, Denunzio TM, Carmichael MG. Diagnosis of atypical hemolytic uremic syndrome and response to eculizumab therapy. Hawaii J Med Public Health 2014;73(9 Suppl 1):22–4. [PMC free article] [PubMed] [Google Scholar]
- 15. Peterss S, Pichlmaier M, Curtis A, Luehr M, Born F, Hagl C. Patient management in aortic arch surgery. Eur J Cardiothorac Surg 2017;51:i4–14. [DOI] [PubMed] [Google Scholar]
- 16. Lopes JA, Jorge S. The RIFLE and AKIN classifications for acute kidney injury: a critical and comprehensive review. Clin Kidney J 2013;6:8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Scully M, Hunt BJ, Benjamin S, Liesner R, Rose P, Peyvandi F et al. ; British Committee for Standards in Haematology. Guidelines on the diagnosis and management of thrombotic thrombocytopenic purpura and other thrombotic microangiopathies. Br J Haematol 2012;158:323–35. [DOI] [PubMed] [Google Scholar]
- 18. Fidan K, Goknar N, Gulhan B, Melek E, Yildirim ZY, Baskin E et al. Extra-renal manifestations of atypical hemolytic uremic syndrome in children. Pediatr Nephrol 2018;33:1395–403. [DOI] [PubMed] [Google Scholar]
- 19. Formeck C, Swiatecka-Urban A. Extra-renal manifestations of atypical hemolytic uremic syndrome. Pediatr Nephrol 2019;34:1337–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schönermarck U, Ries W, Schröppel B, Pape L, Dunaj-Kazmierowska M, Burst V et al. Relative incidence of thrombotic thrombocytopenic purpura and haemolytic uraemic syndrome in clinically suspected cases of thrombotic microangiopathy. Clin Kidney J 2020;13:208–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Noris M, Caprioli J, Bresin E, Mossali C, Pianetti G, Gamba S et al. Relative role of genetic complement abnormalities in sporadic and familial aHUS and their impact on clinical phenotype. Clin J Am Soc Nephrol 2010;5:1844–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Legendre CM, Licht C, Muus P, Greenbaum LA, Babu S, Bedrosian C et al. Terminal complement inhibitor eculizumab in atypical hemolytic-uremic syndrome. N Engl J Med 2013;368:2169–81. [DOI] [PubMed] [Google Scholar]
- 23. Fakhouri F, Fila M, Provot F, Delmas Y, Barbet C, Chatelet V et al. Pathogenic variants in complement genes and risk of atypical hemolytic uremic syndrome relapse after eculizumab discontinuation. Clin J Am Soc Nephrol 2017;12:50–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ikushima E, Hisahara M, Nishijima T, Uchiyama H, Onzuka T, Ochiai Y et al. Atypical hemolytic uremic syndrome following acute type A aortic dissection. Case Rep Hematol 2020;2020:2467953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Markakis K, Westhoff TH, Pagonas N. Aortic valve replacement as a trigger of atypical hemolytic uremic syndrome. Ann Thorac Surg 2017;104:e255–6. [DOI] [PubMed] [Google Scholar]
- 26. Matsukuma E, Imamura A, Iwata Y, Takeuchi T, Yoshida Y, Fujimura Y et al. Postoperative atypical hemolytic uremic syndrome associated with complement c3 mutation. Case Rep Nephrol 2014;2014:784943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Helske S, Oksjoki R, Lindstedt KA, Lommi J, Turto H, Werkkala K et al. Complement system is activated in stenotic aortic valves. Atherosclerosis 2008;196:190–200. [DOI] [PubMed] [Google Scholar]
- 28. Oda T, Yamaguchi A, Yokoyama M, Shimizu K, Toyota K, Nikai T et al. Plasma proteomic changes during hypothermic and normothermic cardiopulmonary bypass in aortic surgeries. Int J Mol Med 2014;34:947–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bisschops LL, Hoedemaekers CW, Mollnes TE, van der Hoeven JG. Rewarming after hypothermia after cardiac arrest shifts the inflammatory balance. Crit Care Med 2012;40:1136–42. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.