Abstract
Epidural fat is commonly discarded during spine surgery to increase the operational field. However, mesenchymal progenitor cells (MPCs) have now been identified in human epidural fat and within the murine dura mater. This led us to believe that epidural fat may regulate homeostasis and regeneration in the vertebral microenvironment. Using two MPC lineage tracing reporter mice (Prx1 and Hic1), not only have we found that epidural fat MPCs become incorporated in the dura mater over the course of normal skeletal maturation, but have also identified these cells as an endogenous source of repair and regeneration post-dural injury. Moreover, our results reveal a partial overlap between Prx1+ and Hic1+ populations, indicating a potential hierarchical relationship between the two MPC populations. This study effectively challenges the notion of epidural fat as an expendable tissue and mandates further research into its biological function and relevance.
Keywords: regenerative medicine, epidural fat, stem cells, progenitor cells
Graphical Abstract
Graphical Abstract.
Significance Statement.
The epidural fat is commonly discarded during surgical procedures involving the spine. Our study demonstrates that at least two progenitor populations exist in the epidural fat and/or dura mater and these cells contribute to tissue homeostasis and injury repair. This study further challenges the notion of epidural fat as an incidental/biologically inert tissue.
Introduction
Since their discovery in bone marrow by A.J. Friedenstein in 1976,1 mesenchymal progenitor cells (MPCs) have been identified in almost all human tissues.2 Although tissue of origin confers site-specific differences in MPC characteristics—such as morphology, fate commitment biases, and immune-phenotype,3 overall, MPCs from all sources retain the ability to self-renew and differentiate into multiple cell types of mesodermal lineage. MPCs also possess bimodal immunomodulatory properties wherein they can enhance cells from both the innate and adaptive immune systems and inhibit the release of pro-inflammatory cytokines in damaged tissue.4,5 Using these properties and likely other mechanisms, MPCs home to and proliferate in injured/inflamed environments where they promote repair/regeneration, angiogenesis, and cellular recruitment.6 MPCs have been identified and/or derived from most mesodermally derived tissues within the body.7-9 Adipose tissue is an abundant source of MPCs, with less-invasive isolation methods and a higher MPC yield compared to bone marrow.10 As such, MPCs derived from adipose tissue have become a promising tool in regenerative medicine approaches for the treatment of numerous chronic and acute disorders.11-14 Adipose/fat is present throughout the body at varying quantities15 and is typically harvested/discarded during cosmetic procedures and/or surgical interventions.16 As fat is one of the largest organ systems in the body, and fat deposition typically increases,17 adipose tissue is regularly used as a viable source of MPCs.15,18 Furthermore, in vivo lineage tracing studies in mice have demonstrated that mesenchymal stem cells (MSCs) are present within white19 and brown fat throughout the body.20 While there is a substantial body of knowledge of adipose MPCs throughout the body, relatively little is known about the role of MPCs within epidural fat.
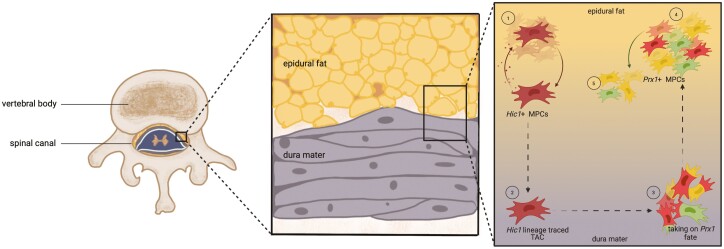
Epidural fat is found within the spinal canal21 and is unevenly distributed along the spinal canal, increasing craniocaudally and posteriorly.22 During development, epidural fat is found in greater abundance adjacent to the dural sac, whereas by adulthood, it becomes more discontinuous.23 Epidural fat has a lower density versus subcutaneous fat, and this is thought to allow it to conform better to the epidural space.21 While the localization of epidural fat is not associated with body size, the amount of epidural fat does vary in proportion with body size.24-27 Commonly, epidural fat is considered to act as a shock absorber,27 yet, little direct evidence supports this hypothesis. Clinically, however, it is commonly considered a space-filling tissue and is typically discarded during surgery to increase the operational field of view when present. This paradigm is being challenged by the recent discovery of MPC populations within human epidural fat.28,29 Furthermore, using a Prx1 lineage tracing mouse, it was also found that these epidural fat MPCs populated the dura mater.28 Adjacent to epidural fat is the dura mater, the outermost protective membrane surrounding the spinal cord, which contains the cerebrospinal fluid and plays a crucial role in anchoring and protecting the central nervous system.30
While there are many different MPC marker genes, controversy remains over which genes mark which types (or all) MPCs. One commonly used MPC lineage marker gene is paired related homeobox gene-1 (Prx1/Prrx1). Prx1 is a paired-type homeobox transcription factor, a class of transcription factors that control development and differentiation31 and are essentially master regulators of morphogenetic processes across species.32Prx1 is a transcription coactivator highly enriched in developing mesodermal tissues (eg, limb buds),33 is found in the ectomesenchyme of the face,34 and is also expressed in adult tissues like the heart,35 and regulates neural progenitors stemness.36 Targeted mutation of Prx1 has shown the essential role this gene plays in regulating limb skeletal development such that disruption led to perinatal death with limb/craniofacial deformations.37Prx1 expression has also been identified in MPCs capable of differentiating into bone,38 cartilage,39 and fat in vivo.40Prx1 has been previously shown as a robust adipose MPCs marker in white adipose tissue.40 Another lineage marker, hypermethylated in cancer 1 (Hic1), is a transcription factor gene ubiquitously expressed in normal tissues, however, in cancer cells, it is hypermethylated and under-expressed.41 Moreover, Hic1 is transcriptionally regulated by many cell cycle genes such as p53,42p21,43 and E2F1,44 highlighting its role in cell cycle regulation. Recently, Hic1 was identified in MPCs presented within skeletal muscle, yet was found to only be expressed in quiescent MPCs.45Hic1+ MPCs give rise to transit-amplifying cells (TACs) that support regeneration post-injury.45,46
In a previous study by Krawetz and Lyons,28 an adult MPC population was isolated from human epidural fat, and Prx1+ cells were found within mouse epidural fat and adjacent dura. Yet, the role of these cells (if any) remained elusive.
Therefore, in this study, we explored the possibility that cells originating from the epidural fat are responsible for tissue homeostasis (during growth and post-injury) in the dura mater. The previous literature on adipose-derived MPCs from other anatomical sources of fat would suggest MPCs within the epidural fat are a likely candidate to play a pivotal role in the health of the dura mater. Moreover, if these epidural fat MPCs are involved in the growth and/or maintenance of the dura, then these MPCs may also have the potential to respond to injury signals within the vertebral environment.
Materials and Methods
Experimental Outcome
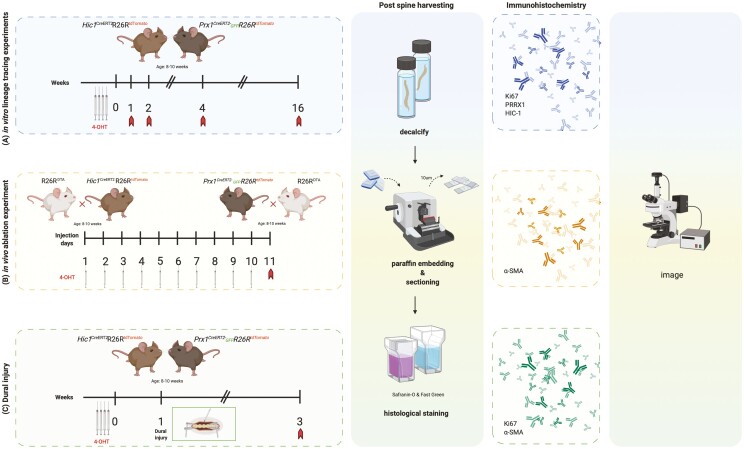
The experimental outline of the study is presented in Fig. 1. Briefly, Prx1 and Hic1 reporter mice were induced with 4 consecutive doses of tamoxifen to induce the expression of tdTomato, and mice were sacrificed at 1-, 2-, or 4-week post-induction to track the these MPC population (Fig. 1A). To ablate these MPC populations, Prx1 and Hic1 mice carrying a DTA (diphtheria toxin subunit A) transgene were induced with 10 consecutive doses of tamoxifen. Mice were sacrificed at 11 days after the first tamoxifen injection (Fig. 1B). To determine if these MPC populations respond to dural injury, Prx1 and Hic1 reporter mice were induced with 4 consecutive doses of tamoxifen to induce the expression of tdTomato. One week after the last tamoxifen injection, the mice underwent a dura injury. Mice were sacrificed at 2-week post-injury (Fig. 1C). Spines from all mice were processed for histological and immunohistochemical analysis.
Figure 1.
Schematic overview of the experimental design used in the current study. Prx1 and Hic1 reporter mice were induced with 4 consecutive doses of tamoxifen to induce the expression of tdTomato. Mice were sacrificed at 1-, 2-, or 4-week post-induction, and the spines were processed with histological and immunohistochemical analysis (A). Prx1 and Hic1 mice carrying a DTA transgene were induced with 10 consecutive doses of tamoxifen to specifically ablate the Prx1- and Hic1-positive cell populations. Mice were sacrificed at 11 days after the first tamoxifen injection and the spines were processed with histological and immunohistochemical analysis (B). Prx1 and Hic1 reporter mice were induced with 4 consecutive doses of tamoxifen to induce the expression of tdTomato. One week after the last tamoxifen injection, the mice underwent a dura injury. Mice were sacrificed at 2-week post-injury and the spines were processed with histological and immunohistochemical analysis (C). Abbreviations: DTA, diphtheria toxin subunit A; Hic1, hypermethylated in cancer 1; Prx1, paired related homeobox-1.
Ethics Statement
Animal studies were carried out in accordance with the recommendations in the Canadian Council on Animal Care Guidelines and approved by the University of Calgary Health Sciences Animal Care Committee (AC20-0042). An n = 3 mice were used per group per time point, based on the total number of mice used, it was not possible to have equal numbers of males and females. However, every group contained male and female mice.
Lineage Tracing
Prx1 CreERT2−GFP+/+R26RtdTomato+/+ (derived from stock no. 029211 and 007914 from The Jackson Laboratory; Supplementary Fig. 1) and Hic1CreERT2+/+R26RtdTomato+/+ (courtesy of Dr. T. Michael Underhill, University of British Columbia; Supplementary Fig. 2) reporter mice were used in this study. The active Z isomer of tamoxifen ((Z)-4-OHT, Sigma-Aldrich) was administered to both mice strains intraperitoneally (1 mg/injection) for 4 consecutive days to drive Cre-mediated recombination and permanently label the cells with tdTomato. Prx1 and Hic1 MPC lineage tracing was performed (mice aged 2 months) at 1-, 2-, and 4-week and at 4-month post-tamoxifen induction. Additionally, MPC lineage tracing was performed on aged mice (6 months) at 1-, 2-, and 4-week post-tamoxifen induction. Mice were sacrificed via CO2 asphyxiation, and intact spines were removed and fixed for 7 days in 10% neutral buffered formalin (NBF; Fisherbrand), then decalcified in 10% EDTA (pH = 7) for 14 days. After decalcification, samples underwent tissue processing and paraffin embedding. EverBrite Hardset Mounting Medium with 4ʹ,6-diamidino-2-phenylindole (DAPI) (emission wavelength 420-470 nm; Biotium) was applied to slides, and endogenous GFP and tdTomato fluorescence was assayed using an Axio Scan.Z1 Slide Scanner microscope (Carl Zeiss) outfitted with a Plan-Apochromat objective (10×/0.8 or 20×/0.8). The following filters were applied: DAPI (353 nm/465 nm), EGFP (493 nm/517 nm), DsRed (563 nm/581 nm).
Histology and Immunohistochemistry
Sections were deparaffinized with SlideBrite (Thermo Fisher Scientific) and then dehydrated with ethanol prior to being stained using safranin-O with fast green and hematoxylin counterstains to examine the general morphology of the spine as well as the presence of glycosaminoglycan. To prepare samples for immunostaining, serial sagittal paraffin sections (10 µm) were deparaffinized in CitriSolv (Thermo Fisher Scientific) and rehydrated through a series of graded ethanols to distilled water. Next, samples were subjected to antigen retrieval (10 mM sodium citrate, pH 6.0) and blocking (1:500 dilution; 100 µL goat serum: 50 mL Tris-buffered saline, 0.1% Tween 20 (TBST) for 1 hour) steps were performed prior to going through TBST wash and antibody application steps. Antibodies conjugated to fluorophores for cell proliferation (Ki67—AF647, Clone # SolA15, eBioscience), a dural marker (α-SMA—AF647, Clone # 1A4, Biolegend), Hic1 (Clone # H6, Santa Cruz), Prx1 (Novus Biologicals) were applied at 4°C overnight. Sections were then washed 3 times at 10 minutes/wash in TBST and mounted using EverBrite Mounting Medium with DAPI (Biotium) for nuclear counterstaining and coverslipped.
Cell Enumeration
Cells positive for specific markers were quantified within 2 regions of interest (area = 1.12 × 105 sq. µm): dura and epidural fat.47,48 Briefly, n = 3 tissue sections per animal were counted for each fluorescent filter (eg, EGFP, R-PE, APC) and in combination when applicable. Two independent observers counted all images and their values were averaged. All data were analyzed with GraphPad Prism 8. All datasets containing 2 experimental groups were analyzed using a two-tailed unpaired parametric t test with a 95% confidence interval (a = 0.05). All datasets containing more than 2 experimental groups were analyzed using a one-way analysis of variance (ANOVA) with a 95% confidence interval (a = 0.05).
MSC Ablation
Prx1 CreERT2−GFP+/+R26RtdTomato+/+ and Hic1CreERT2+/+R26RtdTomato+/+ mice were crossed with R26RDTA+/+ mice (DTA; stock no. 010527 from The Jackson Laboratory) mice to generate the Prx1CreERT2GFP+/−R26RDTA+/− and Hic1CreERT2+/−R26RDTA+/− strains. (Z)-4-hydroxytamoxifen (1 mg/injection) was administered to mice (aged 2 months) intraperitoneally for 10 days consecutively to drive Cre recombination, and subsequent release of DTA, to ablate the Prx1- and Hic1-expressing MPCs. Spines were harvested 1 day after the last injection of tamoxifen.
Dural Injuries
The injury model was performed on both induced Prx1 and Hic1 reporter mice. Mice were anesthetized (isoflurane 3.0 vol/vol% with 1 L/min O2), the skin of their back shaved and disinfected, and the dorsal aspect of the spinal column exposed at the L3 vertebrae. Paraspinal muscles were mobilized and retracted, with hemostasis secured by bipolar cautery. An L3 laminectomy was performed and the dura mater was focally punctured with a 30-gauge needle. Evident leakage of cerebrospinal fluid was used as indication of a successful puncture. The muscle was repaired with 6-0 vicryl and the skin closed with stainless clips. Mice were sacrificed 2-week post-injury for histology (Safranin-O and Fast Green) and immunohistochemistry.
Results
Prx1+ Cells Enrich the Dura Mater Over Time
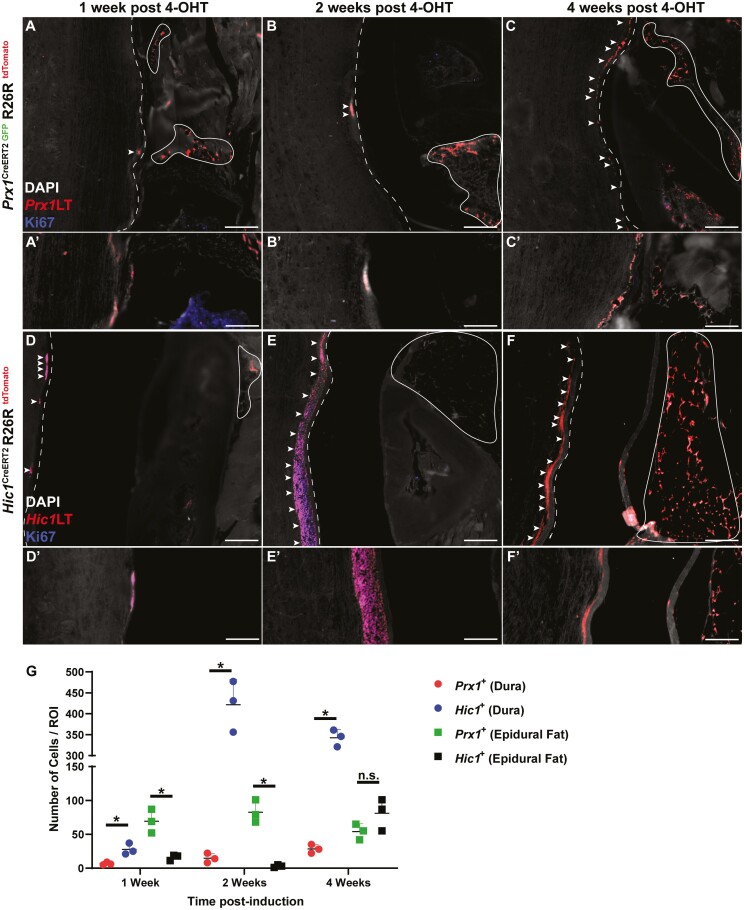
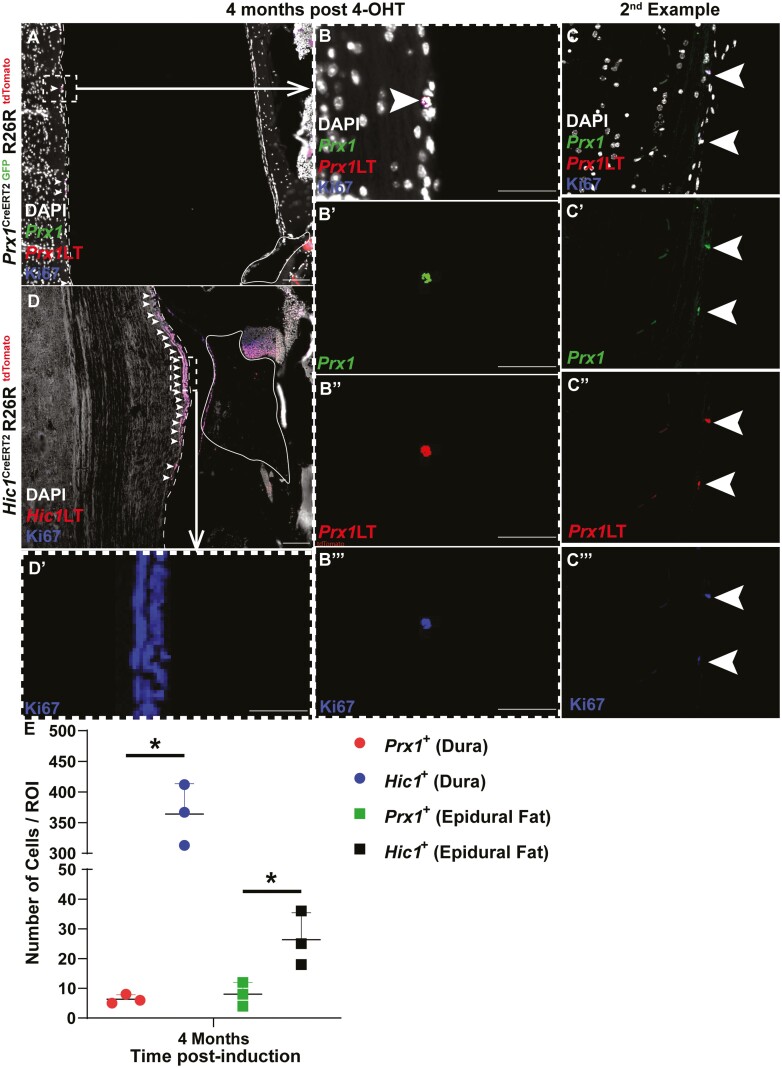
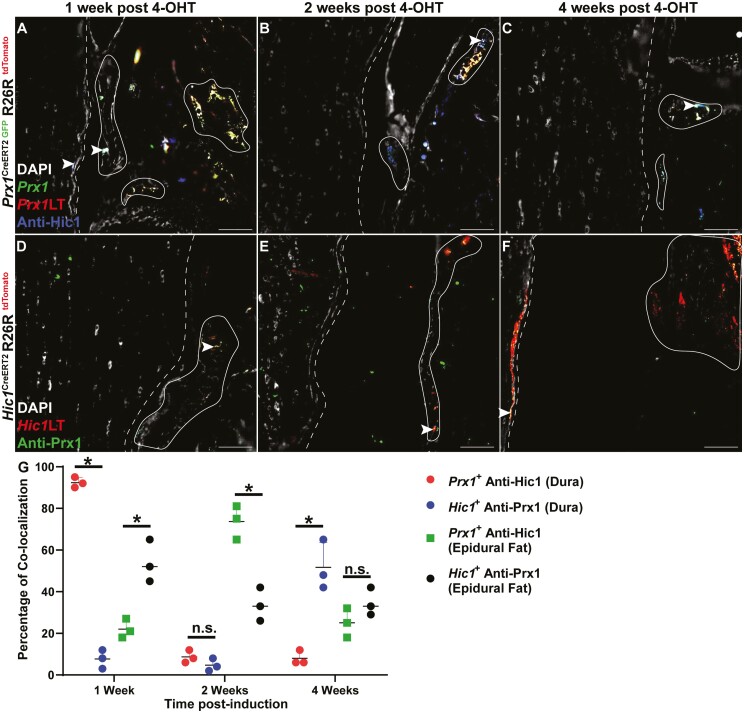
Post-tamoxifen induction in the Prx1CreERT2−GFP+/+R26RtdTomato+/+ model, if the cells continue to express Prx1, GFP, and tdTomato expression is co-localized (resulting in a yellow cell). Once Prx1+ cells commit to a fate decision, these cells lose Prx1 (and GFP) expression, yet retain tdTomato expression (resulting in a red cell). If a Prx1+ cell did not under recombination it would solely express GFP (resulting in a green cell). At 1- and 2-week post-tamoxifen induction, few Prx1+ cells were present within the dura mater (Fig. 2A, 2B), however, by 4-week post-induction, there was an enrichment of Prx1+ cells within the dura mater (Fig. 2C). While the abundance of Prx1+ cells was altered in the dura mater over time, the presence of Prx1+ cells in the epidural fat remained unchanged over the same time period (Fig. 2A-2C). It is also important to note that the Prx1+ MPCs within the dura mater remained Prx1+ as they retained the expression of GFP (Supplementary Fig. 3). These Prx1+ MPCs were non-proliferative within the dural tissue as Ki67 staining (blue) was absent over the time points examined (Fig. 2A-2C). Interestingly, in the skeletally mature mouse (6 months old; 4-month post-tamoxifen induction), single Prx1+ cells were observed interspaced every ~100 µm within the dura mater (Fig. 3). Similar to lineage traced Prx1+ cells in the younger mice, these cells also retained GFP expression suggesting they retained expression of Prx1 (Fig. 3). Yet, in contrast to the earlier time points, these cells expressed Ki67 (Fig. 3B, 3C), suggesting they were proliferative within the dural tissue (Fig. 3). To determine if the expansion of Prx1+ MPCs in the dura mater over time was an effect of growth/maturation or a normal cyclic phenomenon, 6-month-old mice were induced with tamoxifen (Supplementary Fig. 4). No expansion of Prx1+ MPCs was observed within the dura mater over time in the aged mouse, instead lineage tracing revealed that the sparse pattern of MPCs (GFP+) remained in the dura mater (Supplementary Fig. 4).
Figure 2.
Prx1 + (A-C) and Hic1+ (D-F) MPC lineage tracing 1-, 2-, and 4-week post-tamoxifen induction. Sections were also stained with Ki67 to identify proliferative cells (A-F). The dura mater is highlighted by the dashed line while the epidural fat is encircled by the solid line. Arrows indicate examples of Prx1+ and Hic1+ cells within the dura mater. Quantification of Prx1+ and Hic1+ MPCs within the dura mater and epidural fat (G). An n = 3 mice were used per group per time point. ∗P < .05. Scale bars = 100 µm. Abbreviations: Hic1, hypermethylated in cancer 1; MPCs, mesenchymal progenitor cells; Prx1, paired related homeobox-1.
Figure 3.
Prx1 + (A-C) and Hic1+ (D) MPC lineage tracking (LT) in skeletally mature mice (4-month post-tamoxifen induction). Individual GFP (Prx1), Tomato (Prx1LT), and Ki67 channel images (B/C’, B/C’’, B/C’’’) are presented to demonstrate that the Prx1+ LT cells remain undifferentiated. The dura mater is outlined by the dashed line while the epidural fat is encircled by the solid line. Arrows indicate examples of Prx1+ and Hic1+ cells in the dura mater. An individual Ki67 channel image (D) is presented to demonstrate that the Hic1+ LT cells remain proliferative. The dura mater is outlined by the dashed line while the epidural fat is encircled by the solid line (C). Quantification of Prx1+ and Hic1+ MPCs within the dura mater and epidural fat (E). An n = 3 mice were used per group per time point. ∗P < .05. Scale bars = 100 µm (A, C) and 50 µm (B-B’’). Abbreviations: GFP, green fluorescent protein; Hic1, hypermethylated in cancer 1; MPCs, mesenchymal progenitor cells; Prx1, paired related homeobox-1.
Hic1+ MPCs Cycle between the Dura Mater and Epidural Fat Over Time
In the Hic1CreERT2+/+R26RtdTomato+/+ model, Hic1-expressing MPCs and their differentiated progeny were permanently marked by tdTomato expression post-tamoxifen injection. Unlike Prx1+ MPCs, Hic1+ MPCs were present within the dura mater, with little to no tdTomato expression observed in the adjacent epidural fat at 1- and 2-week post-tamoxifen induction (Fig. 2D, 2E). However, by 4-week post-tamoxifen induction, the adjacent epidural fat was enriched with Hic1+ MPCs (Fig. 2F). Ki67 staining revealed that nearly all the Hic1+ MPCs within the dura mater were proliferative (Fig. 2D) with the exception of 4 weeks, where no Ki67 staining was present within Hic1+ MPCs (Fig. 2F). At 4-month post-tamoxifen induction, Hic1+ MPCs were once again observed within the dura mater, with minimal tdTomato expression observed within the epidural fat (Fig. 3D). Furthermore, these Hic1+ MPCs within the dura mater were proliferative as evidenced by Ki67 expression (Fig. 3D).
When comparing the number of Prx1+ or Hic1+ lineage traced MPCs in the dura and epidural fat over time, an inverse relationship between the 2 MPC populations was observed (Fig. 2G). Specifically, at all 3 time points examined (1-, 2-, and 4-week post-induction), there were increased Hic1+ versus Prx1+ lineage traced MPCs present in the dura, but there were increased Prx1+ versus Hic1+ lineage traced MPCs in the epidural fat; except for 4-week post-induction, where there was no difference (Fig. 2G). At the skeletally mature time point (4-month post-induction), increased numbers of Hic1+ versus Prx1+ lineage traced MPCs were observed in both the dura and epidural fat (Fig. 3E).
Prx1+ and Hic1+ MPC Populations Overlap
Prx1 CreERT2−GFP+/+R26RtdTomato+/+ spines were stained for Hic1 (Fig. 4A-4C, Supplementary Fig. 5) and Hic1CreERT2+/+R26RtdTomato+/+ spines with for Prx1 (Fig. 4D-4F, Supplementary Fig. 5). This was undertaken to determine if there was any overlap between the Prx1 and Hic1 MPC lineages during the time points examined (1-, 2-, and 4-week post-tamoxifen induction). In the dura of Prx1 lineage traced mice, ~90% of Prx1+ MPCs also expressed Hic1 at 1-week post-induction, however, this level decreased substantially at 2- and 4-week post-induction (~10%) (Fig. 4A-4C). This inverse pattern was observed in the dura of Hic1 lineage traced mice with Prx1 immunostaining (Fig. 4D-4F). Specially, minimal overlap was observed at 1- and 2-week post-induction (~10%), and this increased to ~50% by 4-week post-induction (Fig. 4D-4F).
Figure 4.
Hic1 protein expression in Prx1CreERT2−GFP+/+R26RtdTomato+/+ mice (A-C) and Prx1 protein expression in Hic1CreERT2+/+R26RtdTomato+/+ mice (D-F) 1-, 2-, and 4-week post-tamoxifen induction. The dura mater is outlined by the dashed line while the epidural fat is encircled by the solid line. Arrows indicate examples of colocalization between Prx1 and Hic1. Quantification of percentage of co-localized staining between Prx1+ with anti-Hic1 staining and Hic1+ with anti-Prx1 staining within the dura mater and epidural fat (G). An n = 3 mice were used per group per time point. ∗P < .05. Scale bars = 100 µm. Abbreviations: Hic1, hypermethylated in cancer 1; Prx1, paired related homeobox-1.
In the epidural fat of Prx1 lineage traced mice, ~20% of Prx1+ MPCs also expressed Hic1 at 1- and 4-week post-induction, however, this level temporality increased at 2-week post-induction (~75%) (Fig. 4A-4C). Once again, an inverse pattern was observed in the epidural fat of Hic1 lineage traced mice with Prx1 immunostaining (Fig. 4D-4F). Specially, ~50% overlap between Hic1 and Prx1 staining was observed at 1-week post-induction, and this decreased to ~30% by 2- and 4-week post-induction (Fig. 4D-4F).
Interestingly, we were able to identify instances of asymmetric cell division (~1 instance per 2 or 3 sections) in which a lineage traced cell (in this case Hic1) gave rise to one cell expressing Prx1 (or possibly the mother cell was Prx1-positive) while the other did not (Supplementary Fig. 6).
Ablation of Prx1+ or Hic1+ MPCs Results in a Loss of α-SMA Staining in the Dura
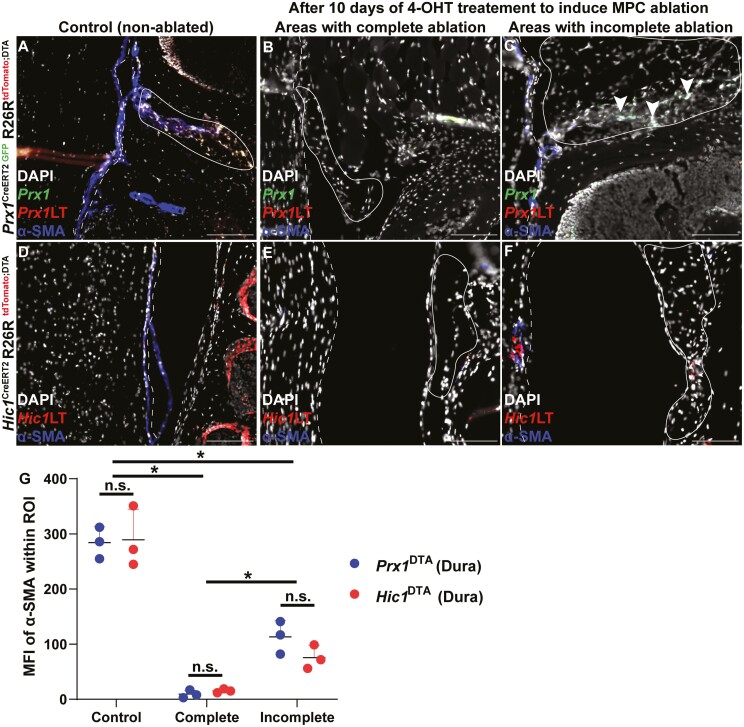
While Prx1+ and Hic1+ MPCs were observed in the epidural fat and adjacent dural tissue, it remained unknown if these cell populations play a functional role in this microenvironment. Therefore, Prx1CreERT2GFP+/−R26RDTA+/− and Hic1CreER2+/−R26RDTA+/− mice were used to ablate these populations and α-SMA immunostaining was undertaken to determine if the loss of these populations has a negative effect on the dura mater and/or epidural fat (Fig. 5). In the normal dura mater, α-SMA staining is fairly ubiquitous throughout (Fig. 5A, 5D), however, when Prx1+ or Hic1+ MPCs were ablated, nearly all α-SMA staining was lost in the dura mater (Fig. 5B, 5E, 5G). However, since genetic ablation is not 100% efficient, there were areas within the epidural fat and/or dura mater that retained Prx1+ or Hic1+ MPCs (Fig. 5C, 5F, 5G). In these cases, α-SMA expression was observed in the dura mater, however, the staining pattern was punctate and discontinuous (Fig. 5C, 5F). Furthermore, the moderate amount of α-SMA in the dura mater of these animals was only observed in close proximity to remaining Prx1+ or Hic1+ MPCs (Fig. 5C, 5F).
Figure 5.
α-SMA expression in the dura mater of wild-type controls (A, D) and after Prx1 (B, C) or Hic1 (E, F) MPCs are ablated following 10 days of tamoxifen induction. The dura mater is outlined by the dashed line while the epidural fat is encircled by the solid line. Arrows indicate examples of Prx1+ and Hic1+ MPCs in the epidural fat. Quantification of mean fluorescent intensity (MFI) of α-SMA expression in Prx1+ and Hic1+ mice within the dura mater (G). An n = 3 mice were used per group per time point. ∗P < .05. Scale bars = 100 µm. Abbreviations: Hic1, hypermethylated in cancer 1; MPCs, mesenchymal progenitor cells; Prx1, paired related homeobox-1; α-SMA, alpha-smooth muscle actin.
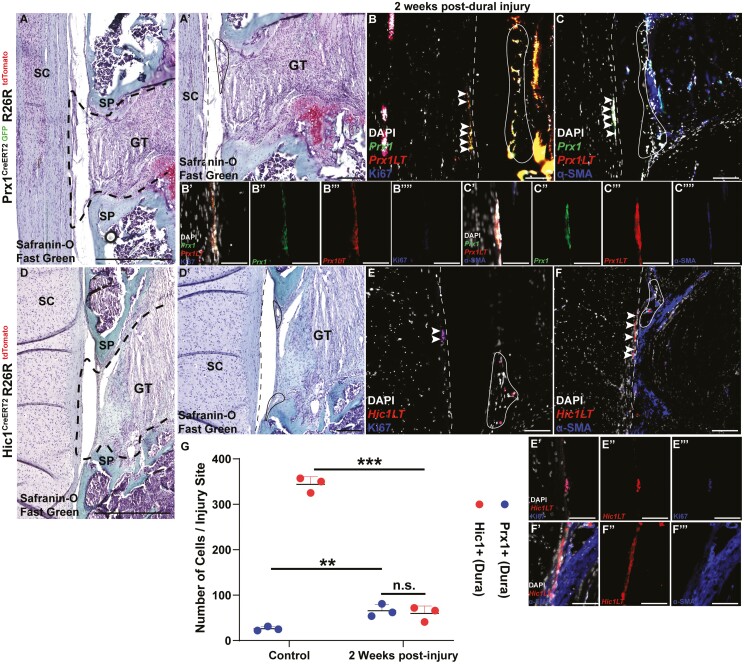
Prx1+ and Hic1+ MPCs Are Found at the Site of Dural Injury
The area of injury was determined by examining Safranin-O-stained slides in Prx1 and Hic1 reporter mice to identify granulation tissue that resulted due to the surgery (Fig. 6A, 6D). Two weeks post-injury, Prx1+ MPCs were found within the dural lesion. These MPCs retained the expression of Prx1 (GFP+) (Fig. 6B, 6C). These Prx1+ MPCs were also proliferative as they expressed Ki67 (Fig. 6B). However, while α-SMA staining was observed within the area of dural injury, it was not produced by these MPCs as α-SMA staining did not co-localize with GFP expression (Prx1+ MPCs) (Fig. 6C). Similarly, proliferating (Ki67+) Hic1+ MPCs were also found at the site of injury (Fig. 6E), yet these cells also did not express α-SMA staining as no co-localization was observed with tdTomato expression (Hic1+ MPCs) (Fig. 6F). There were no differences in the number of Prx1+ versus Hic1+ MPC within the injury site (Fig. 6G).
Figure 6.
Endogenous repair of injured dura mater in the Prx1CreERT2−GFP+/+R26RtdTomato+/+ (A-C) and Hic1CreERT2+/+R26RtdTomato+/+ mice (D-F). Histological (A, D) and corresponding Ki67 (B, E) and α-SMA (C, F) images are presented. The dura mater is outlined by the dashed line while the epidural fat is encircled by the solid line. Asterisks indicate granulation tissue adjacent to the dural injury (A, D). Arrows indicate examples of Prx1+ and Hic1+ MPCs within the dural injury site. Quantification of Prx1+ and Hic1+ MPCs within the dural injury site (G). An n = 3 mice were used per group. ∗∗P < .01, ∗∗∗P < .001. Scale bars = 100 µm. Abbreviations: Hic1, hypermethylated in cancer 1; MPCs, mesenchymal progenitor cells; Prx1, paired related homeobox-1; α-SMA, alpha-smooth muscle actin.
Discussion
Although we know that Prx1+ cells are present within epidural fat from lineage tracing experiments,28 the biological relevance of these cells in vivo has not been explored. In this study, we have expanded upon the contributions of 2 MPC populations within the epidural fat/dural microenvironment.
Prx1 (paired related homeobox-1) is a robust adipose MSC marker40 and it has previously been demonstrated that it marks presumptive MPCs within murine epidural fat.28 Recently, a novel potent MPC lineage marker, Hic1 (hypermethylated in cancer 1) was identified in skeletal/cardiac muscle-45,46 and skin-derived cells.49 Interestingly, while Prx1 is a transcription factor involved in early mesodermal fate commitment,33Hic1 is a transcriptional repressor50,51 found to be expressed only in quiescent MPCs.45,52 These Hic1+ MPCs give rise to TACs that support regeneration post-injury in the tissues examined to date.17,18,45 Since MPCs continue to demonstrate their vital importance in tissue homeostasis and regeneration, Prx1 and Hic1 reporter lineage tracing mice were used in this study, and their contribution to the dural environment was investigated. The current study has demonstrated epidural fat and/or dural MPCs contribute to the homeostasis of dural tissue over the course of normal growth and maturation, and that these MPCs are involved in tissue repair post-injury. We have demonstrated that there is an expansion of MPC populations during the growth/maturation period and when these animals reach skeletal maturity, there is a reduction of Prx1+ MPCs, while Hic1+ MPCs are maintained within the dura mater. When Prx1+ and Hic1+ MPCs are genetically ablated, there is a loss of dural tissue integrity (suggested by the loss of α-SMA expression). Moreover, when there is an injury in the dura mater, these MPCs are found within the injury site. These results strongly suggest that these cells are responsive to cues in the microenvironment and participate in growth, repair, and homeostasis. This behavior fits with previous studies of MPCs in other tissues39,45,49 and demonstrates that the epidural fat and dura mater have reservoirs of MPCs. We hypothesize that the Prx1+ MPCs are native to epidural fat (and not the dura) as Prx1 expression remained consistent in the epidural fat over the time points examined, whereas there was an expansion followed by reduction of this population over time in the dura mater. Furthermore, our data suggest that these Prx1+ MPCs are likely migrating from the epidural fat to the dura mater instead of proliferating within the dura. This is evidenced by the lack of Ki67 staining in the Prx1+ MPCs within the dura mater, with the exception of the late time point (4 months) in which we believe these cells are slow cycling, which is consistent with MPC in other adult tissues, such as synovium.53 However, to be sure, BrdU/EdU labeling could be used in future studies. This hypothesis is further supported by the appearance of the sparsely interspaced Prx1+ MPCs in the dura mater at skeletal maturity; which mimics MPC patterns in other adult tissues wherein the percentage of MPCs is negligible in comparison to the mature, differentiated cells of the tissue.54 For example, long bone growth occurs at the growth plate located between the epiphyseal and metaphyseal bones. Progenitor populations divide in the growth plate and differentiate into chondrocytes that synthesize large amounts of extracellular matrix proteins. Most of these cells eventually undergo apoptosis,55 which is also a possible outcome that explains the loss of Prx1+ MPCs in the dura by 6 months of age. Similarly, Prx1+ MPCs in the epidural fat could be maintaining the dura mater throughout growth and acting as a reservoir in the adult mouse similar to MPCs within other tissues such as periosteum.56 Additional studies will be required to investigate whether Prx1+ MPCs undergo apoptosis, terminally differentiate, and/or migrate away once the dural membrane reaches maturity.
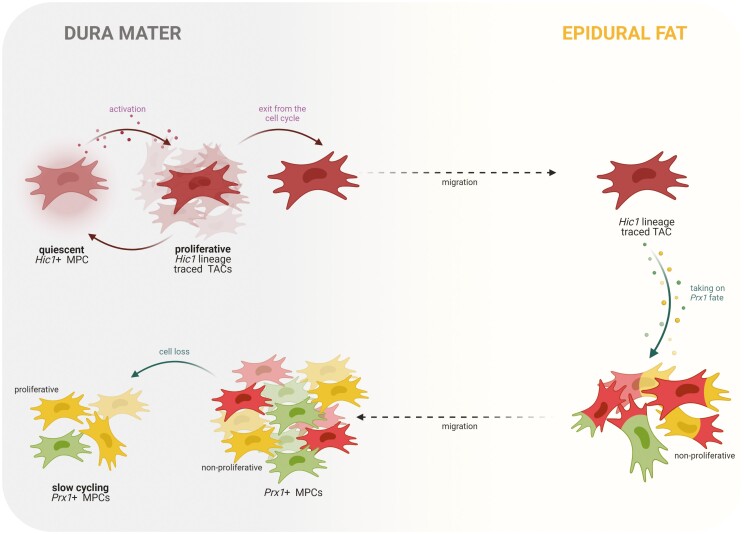
Interestingly, the localization pattern of Hic1+ MPCs was nearly inverse to that of Prx1+ MPCs such that increased Hic1 expression in the epidural fat was complemented by a decrease in expression in the dura mater over the 1- to 4-week time points examined. Based on these results, it is plausible that these 2 populations of MPCs are spatially distinct in the spinal canal and possess different fate trajectories. However, at skeletal maturity, and distinct from what was observed in Prx1 lineage traced animals, the dura mater was once again enriched with Hic1+ MPCs. This led us to hypothesize that Prx1 and Hic1 do not mark distinct progenitor populations in the dural environment and this was then supported by our finding of colocalization between Prx1/Hic1 lineage traced cells and Prx1/Hic1 protein expression. Our results clearly demonstrate that some (but not all) Prx1+/Hic1+ MPCs also express the other marker, and we have also seen evidence of asymmetrical cell division in these populations with mother/daughter cells expressing different combinations of Prx1/Hic1. However, this poses the question: is there a hierarchy between cells that express 1 of the 2 MPC markers and if so, which is the apex MPC marker in this case? Previous studies have shown that MPCs differentiate according to a discrete hierarchical model.57-59 In this case, does asymmetrical division in Hic1+ cells result in the loss or gain of Prx1 expression (and vice versa), and what significance does this hierarchical relationship hold to the anatomical region under study? Based on the pattern of Prx1 and Hic1 expression in the epidural fat and dura mater over time and that Hic1 marks only quiescent MPCs, while Prx1 marks committed progenitor populations, we propose a hypothetical model in which Hic1 identifies MPC with greater potency than Prx1 (Fig. 7). In this model, quiescent Hic1+ MPCs in the dura mater are activated in response to some biological cue (such as growth, injury, maintenance) and begin to proliferate. Once a sufficient TAC pool is obtained, these MPCs exit the cell cycle (Fig. 2F) after which these TACs migrate to the epidural fat where they commit to a mesodermal fate and gain Prx1 expression. With normal growth/maturation, these non-proliferative Prx1+ MPCs migrate back to the dura mater where they remain non-proliferative until the mice reach skeletal maturity at which point they take an apparent slow cycling phenotype, most likely to maintain this population within the dura mater. While our current data suggest migration, it does not prove that it occurs, therefore, it is also possible that these MPCs enter and leave quiescence in each respective tissue (dura vs epidural fat) which would result in the absence/presence of Hic1 expression while most retain Prx1 expression due to their mesodermal fate commitment. With our current transgenic models, this hypothesis cannot be confirmed as the migration of Prx1+ and Hic1+ cells between tissues remains an assumption. However, tracing of transplanted Prx1+/Hic1+ MPCs into the epidural fat of a wild-type mouse could provide insight into this assumption.
Figure 7.
Hypothetical model proposed for the Hic1 and Prx1 hierarchy. Hic1+ MPCs native to the dura mater proliferate in response to biological cues (Fig. 2D, 2E). These MPCs exit from the cell cycle while in the dura mater (Fig. 2F) and then these Hic1+ lineage traced TACs migrate to the adjacent epidural fat (Fig. 2F). In the epidural fat, these MPCs/TACs acquire a Prx1+ mesodermal fate, as seen by the colocalization of Hic1 and Prx1 expression in cells (Fig. 4; Supplementary Fig. 5). These Prx1+ MPCs migrate back to the dura mater and do not re-enter the cell cycle. The expansion of Prx1+ MPCs in the dura mater over time (Fig. 2A-2C) is a product of the migration of these cells from the epidural fat and not due to cell proliferation. At skeletal maturity, Prx1+ MPCs in the dura mater are lost aside from a few interspaced MPCs, which are now proliferative (Fig. 3) and most likely undergo slow cycling in this tissue to maintain homeostasis and respond to future activation cues/insults (Fig. 6). Abbreviations: Hic1, hypermethylated in cancer 1; MPCs, mesenchymal progenitor cells; Prx1, paired related homeobox-1; TACs, transit-amplifying cells.
The similar phenotype observed in the dura mater (loss of α-SMA staining) when both Prx1+ and Hic1+ MPCs were ablated also supports the ideas that Prx1+ and Hic1+ MPCs are not entirely unique populations and are at the least somewhat functionally similar as they contribute to maintaining the dura mater. Similarly, Prx1+ and Hic1+ MPCs both responded to the dural injury. However, the lack of α-SMA staining at the injury site suggests that these cells do not directly reconstitute the dural tissue (as neither population gives rise to α-SMA+ cells), but instead likely play an immunomodulatory and cell-signaling role to promote repair and regeneration, as is widely accepted to be a role of MPCs in vivo.5,45,57,60-62 Moreover, Hic1+ cells in the wound were proliferative, which is supported by previous research demonstrating that these cells give rise to TACs that support wound healing.45 In vitro experiments using purified progenitors focusing on proliferation and/or wound healing (eg, scratch assays) could begin to address differences in underlying mechanisms governing behavioral differences between these MPC types. Furthermore, additional transgenic mice carrying fibroadipose63,64 markers such as Sca1 and/or CD140a could be used to better understand the progenitor landscape in the epidural fat and dura mater.
MSCs/MPCs delivered exogenously in preclinical trials of wound healing have been found to home to sites of injury as a chemotactic response to local influences such as inflammation and hypoxia.65-68 However, systemically delivered MPCs face mechanical barriers in small diameter vessels where they can passively arrest,69 and even when cleared from the blood, MPCs are commonly found entrapped in the lung.67 Specific to our study, the need to repair dural defects has led to the search for a functional substitute that possesses the same physiological characteristics, such as biomechanical properties and fiber architecture, as the dura mater, while ensuring biocompatibility and functional integration.70 Our study demonstrates that an endogenous cell source exists that participates in dural maintenance and repair; and that if this cell population could be mobilized, it may have the ability to stimulate repair in patients suffering from dural injuries/pathologies.
Conclusion
Only recently, a progenitor population within epidural fat has been identified,28,29 yet the role of these MPCs remains unknown. In the current study, we have demonstrated that MPCs within the dura mater and adjacent epidural fat are essential for the maintenance of the dura mater throughout growth and post-injury. Moreover, we have demonstrated partial overlap (marker expression and function) between Prx1+ and Hic1+ MPC populations. This finding opens new avenues of research into the hierarchical relationship between these 2 progenitor populations in the spinal microenvironment in addition to other tissues (such as bone, periosteum). The findings of the current study further challenge the notion that epidural fat is an incidental/biologically inert tissue23,28 and suggest additional research should be directed toward unveiling the molecular and functional differences between epidural fat and other types of white adipose tissue in specific regards to homeostasis and in disease states.
Supplementary Material
Contributor Information
Sophia Shah, McCaig Institute for Bone and Joint Health, University of Calgary, Calgary, AB, Canada; Biomedical Engineering Graduate Program, University of Calgary, Calgary, AB, Canada.
Sathvika Mudigonda, McCaig Institute for Bone and Joint Health, University of Calgary, Calgary, AB, Canada.
Tully Michael Underhill, Department of Cellular and Physiological Sciences, University of British Columbia, Vancouver, BC, Canada.
Paul T Salo, McCaig Institute for Bone and Joint Health, University of Calgary, Calgary, AB, Canada; Department of Surgery, Cumming School of Medicine, University of Calgary, Calgary, AB, Canada.
Alim P Mitha, Biomedical Engineering Graduate Program, University of Calgary, Calgary, AB, Canada; Department of Clinical Neurosciences, Cumming School of Medicine, University of Calgary, Calgary, AB, Canada; Hotchkiss Brain Institute, University of Calgary, Calgary, AB, Canada.
Roman J Krawetz, McCaig Institute for Bone and Joint Health, University of Calgary, Calgary, AB, Canada; Biomedical Engineering Graduate Program, University of Calgary, Calgary, AB, Canada; Department of Surgery, Cumming School of Medicine, University of Calgary, Calgary, AB, Canada; Department of Cell Biology and Anatomy, Cumming School of Medicine, University of Calgary, Calgary, AB, Canada.
Funding
S.S. is supported by an NSERC CGSM Studentship. S.M. is supported by an O’Brien Undergraduate Studentship. R.J.K. is supported by a Tier 2 Canada Research Chair. This work from supported by grants from the Alberta Spine Foundation, The McCaig Institute for Bone and Joint Health, and the University of Calgary Section of Orthopedics.
Conflict of Interest
A.P.M. declared patent holder and stock ownership with Fluid Biotech Inc., Advisory role with Cerus Endovascular, and Research funding from Stryker Neurovascular. All of the other authors declared no potential conflicts of interest.
Author Contributions
S.S., P.T.S., R.J.K.: conception and design. S.S., S.M., R.J.K.: analysis and interpretation of the data. S.S.: drafting of the article. S.S., S.M., T.M.U., A.P.M., P.T.S., R.J.K.: critical revision of the article for important intellectual content. S.S., S.M., T.M.U., A.P.M., P.T.S., R.J.K.: final approval of the article. T.M.U., R.J.K.: provision of study materials. P.T.S., R.J.K.: obtaining of funding. S.S., S.M., P.T.S., R.J.K.: collection and assembly of data.
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1. Mafi R. Sources of adult mesenchymal stem cells applicable for musculoskeletal applications—a systematic review of the literature. Open Orthop J. 2011;5:242-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Berebichez-Fridman R, Montero-Olvera PR. Sources and clinical applications of mesenchymal stem cells state-of-the-art review. Sultan Qaboos Univ Med J. 2018;18:e264-e277. 10.18295/squmj.2018.18.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Klimczak A, Kozlowska U. Mesenchymal stromal cells and tissue-specific progenitor cells: their role in tissue homeostasis. Stem Cells Int. 2016;4285215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Patel SA, Sherman L, Munoz J, et al. Immunological properties of mesenchymal stem cells and clinical implications. Arch Immunol Ther Exp (Warsz). 2008;56:1-8. [DOI] [PubMed] [Google Scholar]
- 5. Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8:726-736. [DOI] [PubMed] [Google Scholar]
- 6. DiMarino AM, Caplan AI, Bonfield TL. Mesenchymal stem cells in tissue repair. Front Immunol. 2013;4:201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen Q, Shou P, Zheng C, et al. Fate decision of mesenchymal stem cells: adipocytes or osteoblasts? Cell Death Differ. 2016;23:1128-1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zuk PA, Zhu M, Ashjian P, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;12:4279-4295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. da Silva Meirelles L, Chagastelles PC, Nardi NB. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci. 2006;119:2204-2213. [DOI] [PubMed] [Google Scholar]
- 10. Alonso-Goulart V, Ferreira LB, Duarte CA, et al. Mesenchymal stem cells from human adipose tissue and bone repair: a literature review. Biotechnol Res Innov. 2018;2:74-80. [Google Scholar]
- 11. Musiał-Wysocka A, Kot M, Majka M. The pros and cons of mesenchymal stem cell-based therapies. Cell Transplant. 2019;28:801-812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Moreno-Manzano V, Mellado-López M, Morera-Esteve MJ, et al. Human adipose-derived mesenchymal stem cells accelerate decellularized neobladder regeneration. Regen Biomater. 2020;7:161-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Moreno-Manzano V, Zaytseva-Zotova D, López-Mocholí E, et al. Injectable gel form of a decellularized bladder induces adipose-derived stem cell differentiation into smooth muscle cells in vitro. Int J Mol Sci. 2020;21:1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Serrano-Aroca A, Vera-Donoso CD, Moreno-Manzano V. Bioengineering approaches for bladder regeneration. Int J Mol Sci. 2018;19:1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Baer PC. Adipose-derived stem cells and their potential to differentiate into the epithelial lineage. Stem Cells Dev. 2011;20:1805-1816. [DOI] [PubMed] [Google Scholar]
- 16. Schneider S, Unger M, Van Griensven M, et al. Adipose-derived mesenchymal stem cells from liposuction and resected fat are feasible sources for regenerative medicine. Eur J Med Res. 2017;1:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mancuso P, Bouchard B. The impact of aging on adipose function and adipokine synthesis. Front Endocrinol (Lausanne). 2019;10:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Secunda R, Vennila R, Mohanashankar AM, et al. Isolation, expansion and characterisation of mesenchymal stem cells from human bone marrow, adipose tissue, umbilical cord blood and matrix: a comparative study. Cytotechnology. 2015;67:793-807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Baglioni S, Francalanci M, Squecco R, et al. Characterization of human adult stem-cell populations isolated from visceral and subcutaneous adipose tissue. FASEB J. 2009;10:3494-3505. [DOI] [PubMed] [Google Scholar]
- 20. Silva FJ, Holt DJ, Vargas V, et al. Metabolically active human brown adipose tissue derived stem cells. Stem Cells. 2014;32:572-581. [DOI] [PubMed] [Google Scholar]
- 21. Reina MA, Franco CD, López A, et al. Clinical implications of epidural fat in the spinal canal. A scanning electron microscopic study. Acta Anaesthesiol Belg. 2009;60:7-17. [PubMed] [Google Scholar]
- 22. Gareau R. Atlas of Functional Anatomy for Regional Anesthesia and Pain Medicine. Vol. 63. Springer; 2016. [Google Scholar]
- 23. Beaujeux R, Wolfram-Gabel R, Kehrli P, et al. Posterior lumbar epidural fat as a functional structure? Histologic specificities. Spine (Phila Pa 1976). 1997;22:1264-1268; discussion 1269. [DOI] [PubMed] [Google Scholar]
- 24. Gala FB, Aswani Y. Imaging in spinal posterior epidural space lesions: a pictorial essay. Indian J Radiol Imaging. 2016;26:299-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dixon AK. Who has most epidural fat? Information from computed tomography. Br J Radiol. 1986;59:475-480. [DOI] [PubMed] [Google Scholar]
- 26. Wu HTH, Schweitzer ME, Parker L. Is epidural fat associated with body habitus? J Comput Assist Tomogr. 2005;29:99-102. [DOI] [PubMed] [Google Scholar]
- 27. Reina MA, Pulido P, Castedo J, et al. Características y distribución de la grasa epidural humana normal. Rev Esp Anestesiol Reanim. 2006;53:363-372. [PubMed] [Google Scholar]
- 28. Al-Jezani N, Cho R, Masson AO, et al. Isolation and characterization of an adult stem cell population from human epidural fat. Stem Cells Int. 2019;2175273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lee GW, Seo MS, Kang KK, et al. Epidural fat-derived mesenchymal stem cell: first report of epidural fat-derived mesenchymal stem cell. Asian Spine J. 2019;13:361-367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schütze G. Epiduroscopic Diagnostics. Epiduroscopy—Spinal Endoscopy. Springer; 2009:9-36. [Google Scholar]
- 31. Gehring WJ. Homeo boxes in the study of development. Science. 1987;236:1245-1252. [DOI] [PubMed] [Google Scholar]
- 32. Gehring WJ, Affolter M, Bürglin T. Homeodomain proteins. Annu Rev Biochem. 1994;63:487-526. [DOI] [PubMed] [Google Scholar]
- 33. Mohamed FF, Franceschi RT. Skeletal stem cells: origins, functions, and uncertainties. Curr Mol Biol Rep. 2017;3:236-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kuratani S, Martin JF, Wawersik S, et al. The expression pattern of the chick homeobox gene gMHox suggests a role in patterning of the limbs and face and in compartmentalization of somites. Dev Biol. 1994;161:357-369. [DOI] [PubMed] [Google Scholar]
- 35. Leussink B, Brouwer A, El Khattabi M, et al. Expression patterns of the paired-related homeobox genes MHox Prx1 and S8 Prx2 suggest roles in development of the heart and the forebrain. Mech Dev. 1995;52:51-64. [DOI] [PubMed] [Google Scholar]
- 36. Shimozaki K, Clemenson GD, Gage FH. Paired related homeobox protein 1 is a regulator of stemness in adult neural stem/progenitor cells. J Neurosci. 2013;33:4066-4075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Martin JF, Bradley A, Olson EN. The paired-like homeo box gene MHox is required for early events of skeletogenesis in multiple lineages. Genes Dev. 1995;9:1237-1249. [DOI] [PubMed] [Google Scholar]
- 38. Duchamp de Lageneste O, Julien A, Abou-Khalil R, et al. Periosteum contains skeletal stem cells with high bone regenerative potential controlled by periostin. Nat Commun. 2018;9:773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Murao H, Yamamoto K, Matsuda S, et al. Periosteal cells are a major source of soft callus in bone fracture. J Bone Miner Metab. 2013;31:390-398. [DOI] [PubMed] [Google Scholar]
- 40. Sanchez-Gurmaches J, Hsiao WY, Guertin DA. Highly selective in vivo labeling of subcutaneous white adipocyte precursors with Prx1-Cre. Stem Cell Rep. 2015;4:541-550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wales MM, Biel MA, El Deiry W, et al. p53 activates expression of HIC-1, a new candidate tumour suppressor gene on 17p13.3. Nat Med. 1995;1:570-577. [DOI] [PubMed] [Google Scholar]
- 42. Britschgi C, Rizzi M, Grob TJ, et al. Identification of the p53 family-responsive element in the promoter region of the tumor suppressor gene hypermethylated in cancer 1. Oncogene. 2006;25:2030-2039. [DOI] [PubMed] [Google Scholar]
- 43. Dehennaut V, Loison I, Boulay G, et al. Identification of p21 (CIP1/WAF1) as a direct target gene of HIC1 (Hypermethylated In Cancer 1). Biochem Biophys Res Commun. 2013;430:49-53. [DOI] [PubMed] [Google Scholar]
- 44. Jenal M, Trinh E, Britschgi C, et al. The tumor suppressor gene hypermethylated in cancer 1 is transcriptionally regulated by E2F1. Mol Cancer Res. 2009;7:916-922. [DOI] [PubMed] [Google Scholar]
- 45. Scott RW, Arostegui M, Schweitzer R, et al. Hic1 defines quiescent mesenchymal progenitor subpopulations with distinct functions and fates in skeletal muscle regeneration. Cell Stem Cell. 2019;25:797-813.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Soliman H, Paylor B, Scott RW, et al. Pathogenic potential of Hic1-expressing cardiac stromal progenitors. Cell Stem Cell. 2020;26:205-220.e8. [DOI] [PubMed] [Google Scholar]
- 47. O’Brien K, Tailor P, Leonard C, et al. Enumeration and localization of mesenchymal progenitor cells and macrophages in synovium from normal individuals and patients with pre-osteoarthritis or clinically diagnosed osteoarthritis. Int J Mol Sci. 2017;18:774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Jablonski CL, Leonard C, Salo P, et al. CCL2 but not CCR2 is required for spontaneous articular cartilage regeneration post-injury. J Orthop Res. 2019;37:2561-2574. [DOI] [PubMed] [Google Scholar]
- 49. Abbasi S, Sinha S, Labit E, et al. Distinct regulatory programs control the latent regenerative potential of dermal fibroblasts during wound healing. Cell Stem Cell. 2020;27:396-412.e6. [DOI] [PubMed] [Google Scholar]
- 50. Pinte S, Stankovic-Valentin N, Beltour S, et al. The tumor suppressor gene HIC1 (hypermethylated in cancer 1) is a sequence-specific transcriptional repressor: definition of its consensus binding sequence and analysis of its DNA binding and repressive properties. J Biol Chem. 2004;279:38313-38324. [DOI] [PubMed] [Google Scholar]
- 51. Zhang B, Chambers KJ, Leprince D, et al. Requirement for chromatin-remodeling complex in novel tumor suppressor HIC1-mediated transcriptional repression and growth control. Oncogene. 2009;28:651-661. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 52. Coller HA, Sang L, Roberts JM. A new description of cellular quiescence. PLoS Biol. 2006;4:e830329-e830349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kurth TB, Dell’accio F, Crouch V, et al. Functional mesenchymal stem cell niches in adult mouse knee joint synovium in vivo. Arthritis Rheum. 2011;63:1289-1300. [DOI] [PubMed] [Google Scholar]
- 54. Fossett E, Khan WS. Optimising human mesenchymal stem cell numbers for clinical application: a literature review. Stem Cells Int. 2012:465259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Rauch F. Bone growth in length and width: the Yin and Yang of bone stability. J Musculoskelet Neuronal Interact. 2005;5:194-201. [PubMed] [Google Scholar]
- 56. Knight MN, Hankenson KD. Mesenchymal stem cells in bone regeneration. Adv Wound Care. 2013;2:306-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Nombela-Arrieta C, Ritz J, Silberstein LE. The elusive nature and function of mesenchymal stem cells. Nat Rev Mol Cell Biol. 2011;12:126-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Muraglia A, Cancedda R, Quarto R. Clonal mesenchymal progenitors from human bone marrow differentiate in vitro according to a hierarchical model. J Cell Sci. 2000;113(Pt 7):1161-1166. [DOI] [PubMed] [Google Scholar]
- 59. Sarugaser R, Hanoun L, Keating A, et al. Human mesenchymal stem cells self-renew and differentiate according to a deterministic hierarchy. PLoS One. 2009;4:e6498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Balaji S, Keswani SG, Crombleholme TM. The role of mesenchymal stem cells in the regenerative wound healing phenotype. Adv Wound Care. 2012;1:159-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Stappenbeck TS, Miyoshi H. The role of stromal stem cells in tissue regeneration and wound repair. Science. 2009;324:1666-1669. [DOI] [PubMed] [Google Scholar]
- 62. Singer NG, Caplan AI. Mesenchymal stem cells: mechanisms of inflammation. Annu Rev Pathol Mech Dis. 2011;6:457-478. [DOI] [PubMed] [Google Scholar]
- 63. Lemos DR, Paylor B, Chang C, et al. Functionally convergent white adipogenic progenitors of different lineages participate in a diffused system supporting tissue regeneration. Stem Cells. 2012;30:1152-1162. [DOI] [PubMed] [Google Scholar]
- 64. Paylor B, Fernandes J, McManus B, et al. Tissue-resident Sca1+ PDGFRα+ mesenchymal progenitors are the cellular source of fibrofatty infiltration in arrhythmogenic cardiomyopathy. F1000Res. 2013;2:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kidd S, Spaeth E, Dembinski JL, et al. Direct evidence of mesenchymal stem cell tropism for tumor and wounding microenvironments using in vivo bioluminescent imaging. Stem Cells. 2009;27:2614-2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. McFarlin K, Gao X, Liu YB, et al. Bone marrow-derived mesenchymal stromal cells accelerate wound healing in the rat. Wound Repair Regen. 2006;14:471-478. [DOI] [PubMed] [Google Scholar]
- 67. Rustad KC, Gurtner GC. Mesenchymal stem cells home to sites of injury and inflammation. Adv Wound Care. 2012;1:147-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Fu X, Fang L, Li X, et al. Enhanced wound-healing quality with bone marrow mesenchymal stem cells autografting after skin injury. Wound Repair Regen. 2006;14:325-335. [DOI] [PubMed] [Google Scholar]
- 69. Krueger TEG, Thorek DLJ, Denmeade SR, et al. Concise review: mesenchymal stem cell-based drug delivery: the good, the bad, the ugly, and the promise. Stem Cells Transl Med. 2018;7:651-663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Protasoni M, Sangiorgi S, Cividini A, et al. The collagenic architecture of human dura mater: laboratory investigation. J Neurosurg. 2011;114:1723-1730. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.