Abstract
Objectives:
(1) To evaluate dental pulp sensitivity by electrical pulp testing and measure aspartate aminotransferase activity in the pulp after 14 days of orthodontic intrusion, and (2) to compare those measurements with measurements obtained in teeth after 7 days of intrusion and 7 days of rest.
Materials and Methods:
The study sample included 13 subjects (mean age = 16.5 ± 2.7 years). For every subject, before extraction, two contralateral premolars were included in a spring and loaded by a force. Two study groups were formed: Group A, teeth with 14 days of mechanical load, and Group B, teeth with 7 days of mechanical load plus 7 days of rest. Electrical pulp testing and aspartate aminotransferase activity measurements were performed after 14 days in all tested teeth. After extraction, aspartate aminotransferase activity in the pulp was determined spectrophotometrically at 20°C.
Results:
Mean aspartate aminotransferase activity values were 0.21 U/mg (SD = 0.15) in Group A and 0.27 U/mg (SD = 0.17) in Group B. Mean electrical pulp testing readings were 38.92 µA (SD = 24.61) in Group A and 36.77 µA (SD = 26.84) in Group B. Mean values of the intrusive force magnitude did not differ in both groups.
Conclusions:
Different durations of orthodontic intrusion, defined as 14 days of load and 7 days of load followed by 7 resting days, were not reflected by electrical pulp testing or by aspartate aminotransferase activity levels in the pulp of the affected teeth. However, the response threshold to electrical pulp stimulation was elevated in all tested teeth.
Keywords: Aspartate aminotransferase, Dental pulp, Pulp vitality, Orthodontic intrusion
INTRODUCTION
In medicine, clinical enzymology is used to help diagnose some localized inflammatory lesions before overt clinical symptoms develop. Aspartate aminotransferase (AST) is an intracellular enzyme that is released to the extracellular environment upon cell death.1 AST activity has been detected in healthy and inflamed dental pulp.2 Increased levels of AST activity in dental pulp or gingival crevicular fluids were observed in cases of orthodontic treatment.3–6 It was suggested that changes of AST activity reflect metabolic alterations that occur in the dental pulp during orthodontic treatment.
Intrusion is thought to have the greatest impact on the apical region7–9 during orthodontic treatment, and it may occlude the apical blood supply.10 In the literature, discussions about changes occurring in the dental pulp, in response to orthodontic intrusion, is mainly based on experiments with animals and is controversial.11–13
Electrical pulp testing (EPT) is used to acquire information about pulp vitality based on the patient's subjective sensations. There is no predictable relationship between tooth response and cellular changes within the pulp.14–17 In orthodontic patients where force application might have altered the physiological status of the pulpal elements, the pulp's response to electrical stimulation becomes inconsistent.15,18–20
Most of the studies with orthodontic patients were concentrated on short-term orthodontic force3,4,13,21–27 and its effects on pulp vitality. Relatively little information is available about possible changes in AST activity in orthodontically moved teeth with respect to different force duration and about variations in responses to EPT in affected teeth.
Recently, we demonstrated that after 7 days of orthodontic intrusion, AST activity and EPT parameters significantly increased in the affected teeth compared with the teeth without orthodontic load.4 The present study aimed (1) to investigate changes in the dental pulp after 14 days of orthodontic intrusion and compare them with AST activity levels measured in the pulp after 7 days of orthodontic intrusion and 7 days of rest and (2) to compare the pulp sensitivity in the same subjects on the basis of pulp response to electrical stimulus of the affected teeth.
MATERIAL AND METHODS
The study sample consisted of 13 healthy subjects who needed contralateral premolars extracted for orthodontic reasons. Subjects' ages ranged from 14 to 22 years (mean age = 16.5 ± 2.7 years). Criteria for subject inclusion were described previously.4
Informed consent was obtained from the patients and from the parents of those younger than 18 years before the study. The protocol was approved by the Ethical Committee of Kaunas University of Medicine.
For every subject, two randomly selected contralateral maxillary or mandibular premolars were included in a spring from the first molar and loaded with the intrusive force. One of those was subjected to mechanical stress for 14 days, and the other for 7 days. All selected teeth were not restored, were asymptomatic, and had no evidence of caries, periapical radiolucency, or root resorption. Orthodontic brackets (Roth 0.022 × 0.030 inch, Dentaurum, Inspringen, Germany) were bonded in the center of the buccal surfaces of the teeth. Crown orthodontic bands with buccal tubes were cemented on the first molars. To avoid side effects (tipping, extrusion), first molars were connected with a palatal or lingual arch into a rigid unit. A spring (0.016 × 0.022 inch stainless steel, Dentaurum) was fabricated for every patient to generate intrusion force toward their longitudinal axis. Tipping and torque moments were reduced to the minimum.
The magnitude of the intrusive tipping force for every experimental tooth was calculated using ANSYS 10.0 software (Finity element analysis system, ANSYS Inc, Canonsburg, PA). The spring was ligated to the bracket using wire ligature and bent in both sides to avoid mesiodistal displacement of teeth.4 The spring was removed after 7 days or after 14 days of intrusion (depending on the site).
Measurements of pulp sensitivity and AST activity were performed after 14 days. Thus, two study groups were formed: Group A, teeth with 14 days of mechanical load, and Group B, teeth with 7 days of mechanical load plus 7 days of rest.
Pulp sensitivity was investigated using electrical stimulation by Pulptester PT1 (UAB Lumen, Kaunas, Lithuania). Pulptester PT1 is characterized by a gradual linear increase of the impulses on the 1–200 unit scale (1 unit = 1 µA)28 and, subsequently, by providing threshold sensations with the least discomfort to the patient. The test application procedure was described previously.4 During testing the current flow was slowly increased from the initial zero current state by adjusting the variable voltage control. Readings were recorded as the perception threshold stimulating current in microamperes. Testing was repeated after a 3-minute interval to reduce subjective fatigue and to minimize the possibility of nerve accommodation. The kappa values for the repeat recordings of the pulp response in the tested teeth varied between 0.8 and 1.0.
After EPT, the teeth were extracted under local anesthesia, and the pulp samples were removed from all teeth. To evaluate AST activity, the extracted teeth were longitudinally grooved on the buccal and lingual surfaces under extensive water irrigation using a diamond bur, taking care not to penetrate the canal space, and then split in half. The pulp samples were washed twice with ice-cold, heparinized, sterile saline and then dried and frozen at −25°C.
Immediately before AST activity measurements, the residual liquid was removed from the pulp specimens with filter paper, the specimens were weighed using an A&D precision balance HA-202M (Tokyo, Japan) and homogenized and investigated for AST activity.4
Measurements of AST activity were carried out at room temperature (20°C) using a spectrophotometer (Heλios α, Thermo Electron Corporation, Waltham, Mass). Nicotinamide adenine dinucleotide (NADH) was added, and the oxidation of NADH was monitored as a decrease in absorbance at 340 nm.
Data analysis was performed using the Statistical Package for the Social Sciences, version 13.0 (SPSS Inc, Chicago, Ill). Each data set was tested for normality with the Shapiro-Wilk test. The paired t-test was used to assess the significance of the differences in AST activity and EPT response between the experimental teeth. The confidence intervals (CIs) at 95% of the difference between the mean values of the enzymatic activities of two groups were reported.
RESULTS
Mean values of magnitude of the intrusive tipping force for the teeth in Group A and Group B did not differ significantly: 82 g (SD = 73.6; range = 24–281 g) and 97 g (SD = 97.2; range = 21–324 g), respectively (P = .5).
Mean AST activity values measured in the pulp of the tested teeth were similar in both study groups: 0.27 U/mg (SD = 0.17) in the teeth with 7 days of mechanical load plus 7 days of rest (Group B), and 0.21 U/mg (SD = 0.15) in the teeth with 14 days of mechanical load (Group A). The 95% CI of the differences in mean values of AST activities ranged from −0.15 to 0.33 U/mg (P = .2).
EPT readings did not differ significantly between the two groups of teeth with different periods of mechanical load: 38.92 µA (SD = 24.61) in group A, and 36.77 µA (SD = 26.84) in Group B. The 95% CI of the differences in mean values of the EPT response ranged from −11.1 to 15.4 µA. (P = .7).
The distribution of AST activity values and EPT values in the teeth loaded for 7 days and for 14 days is presented in Figure 1. No significant correlation was observed between AST activity and EPT responses in the orthodontically intruded teeth of both groups. No relationship between the orthodontic force magnitude and the patients' age, pulp weight, and AST activity and EPT response was observed.
Figure 1.
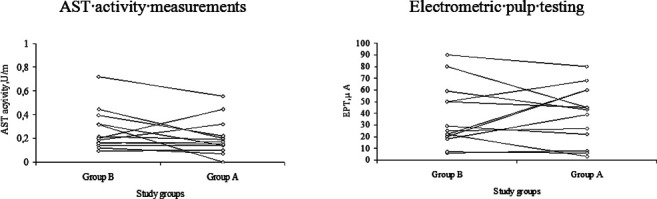
Distribution of AST activity and EPT values in teeth orthodontically loaded for 14 days (Group A) and for 7 days followed by 7 days of rest (Group B).
Results of this study were compared with the previously published findings in teeth with 7 days of mechanical load and in teeth not subjected to mechanical load 4 and are presented in Table 1.
Table 1.
Mean Values of AST and EPT in Teeth Subjected to Different Modes of Orthodontic Intrusion and in Teeth Without Intrusion
Thus, the mean AST activity in the pulp tissue was significantly higher after 7 days of load compared with AST activity in the pulp of teeth with 14 days and 7 days of load followed by 7 days of rest and in teeth not subjected to mechanical load. Mean AST values did not differ significantly between the groups of teeth without mechanical load, the teeth with 14 days of load, and the teeth with 7 days of load followed by 7 days of rest (Table 1).
Mean EPT values differed significantly between these groups: the teeth not subjected to mechanical load exhibited lower sensitivity to electrical stimulation than all other groups of teeth characterized with different modes of mechanical loading (Table 1).
DISCUSSION
The purpose of this study was to investigate how orthodontic intrusion by fixed appliances, applied for a period of 14 days, was reflected in the pulp response of the affected teeth in terms of sensitivity to electrical stimuli and AST activity in the pulp tissue after tooth extraction. Furthermore, these results were compared with findings obtained in teeth with 7 days of orthodontic intrusion followed by 7 days of rest in order to study possible differences of pulp behavior in response to different durations of mechanical loading.
We found that the mean values and distribution of AST and EPT were similar in both study groups. Moreover, compared with the results of our previous study of pulp response in orthodontically treated teeth,4 it appeared that measurements of AST activity in orthodontically treated teeth were significantly higher after 7 days of intrusion than after a 14-day period in both groups, those with intrusion or with partial rest. Measurements of AST activity obtained in the current study are close to those obtained in teeth not subjected to any mechanical load (Table 1). We realize that direct comparison of the results from the present study with those published previously does not allow firm conclusions to be drawn. However, it is impossible to obtain a sample of teeth to subject to several modes of treatment in the same patient (only contralateral premolars that are being extracted for orthodontic reasons can be used). Therefore, it was interesting to observe difference in the outcomes in relation to different modes of treatment used in two studies following a similar design. Although there was increased AST activity in the pulp of teeth subjected to 7 days of orthodontic intrusion, the presently estimated AST activity levels after 14 days of intrusion are similar to those measured previously in untreated teeth; therefore, we suggest a possibility of reversible metabolic changes in the pulp.
The levels of AST activity reported by Perinetti et al3 after 7 days of orthodontic treatment were comparable with AST levels in teeth with reversible pulpitis.2 However, there were no marks of enzymatic activity in dental pulp after 2 weeks of orthodontic treatment. Rohaya et al5 reported that AST activity in the gingival crevicular fluid was highest in the first week and gradually reduced during the following 3 weeks. These results support the hypothesis that orthodontic treatment can cause temporary metabolic changes in pulp tissue during orthodontic treatment that are reversible. According to Grünheid et al,13 a number of pathological signs of rat pulp tissue peaked 24 and 72 hours after force application and returned to the initial values after 168 hours. The authors concluded that controlled mechanical forces during orthodontic treatment, if not excessive, might cause only transient pulpal changes as tissue regeneration was initiated almost immediately after the onset of tooth movement.13
Orthodontic load application can induce circulatory and respiratory disturbances21,25–26,29,30 in altered tissue22,31 that consequently lead to reduced oxygen levels in the pulp.3,7
Numerous studies of changes in pulp tissue vascularity during orthodontic tooth movement suggest that blood flow to the dental pulp decreases initially after orthodontic force application. However, it increases thereafter until it reaches a peak 7 days after the application of force.30–33 These processes, depending on the degree of their disturbances, may cause changes in metabolic cell activity, cell damage, or defense reactions. Orthodontic forces affect the dental pulp inducing vascular changes that are inflammatory in nature.7,29 As demonstrated in the rat model, the inflammatory vascular reactions subside within 3 weeks in all tissues.30,33
We found that after 14 days of orthodontic tooth intrusion there was an increased response threshold to EPT. Furthermore, the pulp sensitivity to electrical stimulation remained decreased in teeth that were subjected to orthodontic force for 7 days only and to a 7-day rest period. Although EPT is based on a subjective judgment provided by the patient, the observed differences between the measurements in healthy teeth4 and teeth subjected to orthodontic intrusion suggest a possibility of certain alterations in the pulp. Orthodontic tooth movement has been shown to produce changes in tissue respiration with a resulting reduction in blood flow and possible anoxia of A nerves.15,21 Gopikrishna and coworkers15 and Cave and coworkers34 reported that orthodontic force increased the response threshold to EPT. The effect was almost instantaneous and could persist for up to 9 months after treatment.
CONCLUSIONS
Different duration of orthodontic tooth intrusion, defined as 14 days of permanent load and 7 days of permanent load followed by 7 days of rest, were not reflected by the levels of AST activity in the pulp of the affected teeth.
The remaining increased response threshold to electrical pulp stimulation after 14 days of mechanical load and after 7 days of rest indicates alterations of the nerve.
Acknowledgments
We gratefully acknowledge Research Foundation of Kaunas University of Medicine, Lithuania, for supporting this study.
REFERENCES
- 1.Schmidt F, Schmidt W. Aminotransferases in human pathology and clinical chemistry. In: Christian P, Metzler D. E, editors. Transaminases. New York: John Wiley & Sons; 1985. pp. 586–590. [Google Scholar]
- 2.Spoto G. S, Fioroni M, Rubini C, Tripodi D, Perinetti G, Piattelli A. Aspartate aminotransferase activity in human healthy and inflamed dental pulps. J Endod. 2001;27:394–395. doi: 10.1097/00004770-200106000-00005. [DOI] [PubMed] [Google Scholar]
- 3.Perinetti G, Varvara G, Festa F, Esposito P. Aspartate aminotransferase activity in pulp of orthodontically treated teeth. Am J Orthod Dentofacial Orthop. 2004;125:88–92. doi: 10.1016/j.ajodo.2003.02.006. [DOI] [PubMed] [Google Scholar]
- 4.Veberiene R, Smailiene D, Danielyte J, Dagys A, Toleikis A, Machiulskiene V. Effects of the intrusive force on selected determinants of pulp vitality. Angle Orthod. 2009;79:1114–1118. doi: 10.2319/110408-563R.1. [DOI] [PubMed] [Google Scholar]
- 5.Rohaya M. A. W, Shahrul Hisham Z. A, Khazlina K. Preliminary study of aspartate aminotransferase activity in gingival crevicular fluids during orthodontic tooth movement. J Appl Sci. 2009;9:1393–1396. [Google Scholar]
- 6.Perinetti G, Paolantonio M, D'Archivio D, Dolci M, Femminella B. Aspartate aminotransferase activity in the gingival crevicular fluid during orthodontic treatment. A controlled short-term longitudinal study. J Periodontol. 2003;74:145–152. doi: 10.1902/jop.2003.74.2.145. [DOI] [PubMed] [Google Scholar]
- 7.Stenvik A, Mjor I. A. Pulp and dentine reactions to experimental tooth intrusion—a histological study of the initial changes. Am J Orthod. 1970;57:370–385. doi: 10.1016/s0002-9416(70)90219-8. [DOI] [PubMed] [Google Scholar]
- 8.Dellinger E. L. A histologic and cephalometric investigation of premolar intrusion in the Macaca speciosa monkey. Am J Orthod. 1969;53:325–355. doi: 10.1016/0002-9416(67)90100-5. [DOI] [PubMed] [Google Scholar]
- 9.Dermout L. R, De Munk A. Apical root resorption of upper incisors caused by intrusive tooth movement: a radiographic study. Am J Orthod. 1986;90:321–326. doi: 10.1016/0889-5406(86)90088-0. [DOI] [PubMed] [Google Scholar]
- 10.Butcher E. O, Taylor A. C. The vascularity of the monkey and its alteration by tooth retraction. J Dent Res. 1952;31:239–247. doi: 10.1177/00220345520310020901. [DOI] [PubMed] [Google Scholar]
- 11.Konno Y, Daimaruya T, Iikubo M, Kanzaki R, Takahashi I, Sugawara J, Sasano T. Morphologic and hemodynamic analysis of dental pulp in dogs after molar intrusion with the skeletal anchorage system. Am J Orthod Dentofacial Orthop. 2007;132:199–207. doi: 10.1016/j.ajodo.2005.07.029. [DOI] [PubMed] [Google Scholar]
- 12.Brodin P, Linge L, Aars H. Instant assessment of pulpal blood flow after orthodontic force application. J Orofac Orthop. 1996;57:306–309. doi: 10.1007/BF02197551. [DOI] [PubMed] [Google Scholar]
- 13.Grünheid T, Morbach B. A, Zenter A. Pulpal cellular reactions to experimental tooth movement in rats. Oral Surg Med Oral Pathol Oral Radiol Endod. 2007;104:434–441. doi: 10.1016/j.tripleo.2007.03.022. [DOI] [PubMed] [Google Scholar]
- 14.Chambers I. G. The role and methods of pulp testing in oral diagnosis: a review. Int Endod J. 1982;15:1–15. doi: 10.1111/j.1365-2591.1982.tb01331.x. [DOI] [PubMed] [Google Scholar]
- 15.Gopikrishna V, Pradeep G, Venkateshbabu N. Assessment of pulp vitality: a review. Int J Paediatr Dent. 2009;19:3–15. doi: 10.1111/j.1365-263X.2008.00955.x. [DOI] [PubMed] [Google Scholar]
- 16.Abd-Elmeguid Ashraft, Yu Donald C. Dental pulp neurophysiology: part2. Current diagnostic tests to Assess pulp vitality. Journal of the Canadian Dental Association. 2009;75(2):139–143. [PubMed] [Google Scholar]
- 17.Dummer P. M. H, Hicks R, Huws D. Clinical signs and symptoms in pulp disease. Int Endod J. 1980;13:27–35. doi: 10.1111/j.1365-2591.1980.tb00834.x. [DOI] [PubMed] [Google Scholar]
- 18.Bender I. B, Landau M. A, Fonsecca S, Trowbridge H. O. The optimum placement-site of electrode in electrical pulp testing of the 12 anterior teeth. J Am Dent Assoc. 1989;118:305–310. doi: 10.14219/jada.archive.1989.0096. [DOI] [PubMed] [Google Scholar]
- 19.Burnside R. R, Srenson F. M, Buck D. L. Electric vitality testing in orthodontic patients. Angle Orthod. 1974;44:213–217. doi: 10.1043/0003-3219(1974)044<0213:EVTIOP>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 20.Hall C. J, Freer T. J. The effects of early orthodontic force application on pulp test responses. Austr Dent J. 1998;43:359–361. doi: 10.1111/j.1834-7819.1998.tb00189.x. [DOI] [PubMed] [Google Scholar]
- 21.McDonald F, Pitt Ford T. R. Blood flow changes in permanent maxillary canines during retraction. Euro J Orthod. 1994;16:1–93. doi: 10.1093/ejo/16.1.1. [DOI] [PubMed] [Google Scholar]
- 22.Unterscher R. E, Nieberg L. G, Weimer A. D, Dyer J. K. The response of human pulpal tissue after orthodontic force application. Am J Orthod Dentofacial Orthop. 1987;92:220–224. doi: 10.1016/0889-5406(87)90415-x. [DOI] [PubMed] [Google Scholar]
- 23.Santamaria M, Milagres D, Sasso Stuani A, Sasso Stuani M. B, Oliveira Ruellas A. C. Initial changes in pulpal microvasculature during orthodontic tooth movement: a stereological study. Europ J Orthod. 2006;28:217–220. doi: 10.1093/ejo/cji117. [DOI] [PubMed] [Google Scholar]
- 24.Perinetti G, Varvara G, Salini L, Tete S. Alkaline phosphatase activity in dental pulp of orthodontically treated teeth. Am J Orthod Dentofacial Orthop. 2005;128:492–496. doi: 10.1016/j.ajodo.2004.07.042. [DOI] [PubMed] [Google Scholar]
- 25.Barwick P. J, Ramsay D. S. Effect of brief intrusive force on human pulpal blood flow. Am J Orthod Dentofac Orthop. 1996;110:273–279. doi: 10.1016/s0889-5406(96)80011-4. [DOI] [PubMed] [Google Scholar]
- 26.Sano Y, Ikawa M, Sugawara J, Horiuchi H, Mitani H. The effect of continuous intrusive force on human pulpal blood flow. Eur J Orthod. 2002;24:159–166. doi: 10.1093/ejo/24.2.159. [DOI] [PubMed] [Google Scholar]
- 27.Ikawa M, Fujiwara M, Horiuchi H, Shimauchi H. The effect of short-term tooth intrusion on human pulpal blood flow measured by laser Doppler flowmetry. Arch Oral Biol. 2001;46:781–787. doi: 10.1016/s0003-9969(01)00049-8. [DOI] [PubMed] [Google Scholar]
- 28.Martinaitis J, Masiulis R. Dasledavanne pulpy zuba elektraadontometram “Pulptester PT1.”. Stomatologicheski Zurnaly. 2002;2(7):48–49. [Google Scholar]
- 29.Guevara M, McClugage S. G. Effects of intrusive forces upon the microvasculature of dental pulp. Angle Orthod. 1980;50:129–134. doi: 10.1043/0003-3219(1980)050<0129:EOIFUT>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 30.Vandevska- Radunovic V, Kristiansen A. B, Heyeraas K. J, Kvinnsland S. Changes in blood circulation in teeth supporting tissues incident to experimental tooth movement. Eur J Orthod. 1994;16:361–369. doi: 10.1093/ejo/16.5.361. [DOI] [PubMed] [Google Scholar]
- 31.Hamersky P. A, Weimer A. D, Taintor J. F. The effect of orthodontic force application on the pulpal tissue respiration rate in human premolar. Am J Orthod. 1980;77:368–378. doi: 10.1016/0002-9416(80)90103-7. [DOI] [PubMed] [Google Scholar]
- 32.Nixon C. E, Saviano J. A, King G. J, Keeling S. D. Histomorphometric study of dental pulp during orthodontic tooth movement. J Endod. 1992;19(1):13–16. doi: 10.1016/S0099-2399(06)81034-4. [DOI] [PubMed] [Google Scholar]
- 33.Vandevska-Radunovic V, Kvinnsland S, Hals Kvinnsland I. Effect of experimental tooth movement on nerve fibers immunoreactive to calcitonin gene-related peptide, protein gene product 9.5, and blood vessel density and distribution in rats. Eur J Orthod. 1997b;19:517–529. doi: 10.1093/ejo/19.5.517. [DOI] [PubMed] [Google Scholar]
- 34.Cave S. G, Freer T. J, Podlich H. M. Pulp-test responses in orthodontic patients. Aust Orthod J. 2002;18:27–34. [PubMed] [Google Scholar]



