Abstract
Objective:
To test the hypothesis that sagittal mandibular development has no effects on the dimensions of the awake pharyngeal airway passage.
Materials and Methods:
Ninety-one subjects (age 15–25 years) with a normal vertical growth pattern of the mandible, normally positioned maxilla, and various sagittal mandibular developments were divided into three groups based on the sagittal mandibular development. Group I included 37 subjects who had a normally positioned mandible (76° ≤ angle between ‘S,’ ‘N,’ and ‘B’; it represents the antero-posterior position of the maxilla in relation to the anterior cranial base [SNB] ≤ 82°), Group II included 31 subjects in whom the mandible was retrognathic (SNB < 76°), and Group III included 23 subjects in whom the mandible was prognathic (SNB > 82°) in relation to the anterior cranial base. Lateral cephalograms were traced manually to evaluate the pharyngeal airway passage.
Results:
The length of the soft palate was significantly smaller in mandibular prognathism subjects than in subjects with mandibular retrognathism (P < .01). The thickness of the soft palate was significantly greater among subjects with mandibular prognathism than in subjects with normal (P < .01) and retrognathic (P < .001) mandibular development. The sagittal mandibular development had no effect on the dimensions of the nasopharyngeal and hypopharyngeal airway passage. The depth of the oropharynx was comparable among the subjects with normal and retrognathic mandibles but was greater (P < .001) among subjects with mandibular prognathism.
Conclusions:
The hypothesis is rejected. Sagittal mandibular development had significant effects on the dimensions of the awake pharyngeal airway passage.
Keywords: Sagittal mandibular development, Pharyngeal airway passage, Dimensions
INTRODUCTION
Narrowing of the pharyngeal airway passage (PAP) is a common feature in patients with breathing problems.1 There are significant relationships between the pharyngeal dimensions and craniofacial abnormalities.1 Craniofacial abnormalities such as maxillary retrusion, mandibular retrognathism, short mandibular body, and downward and backward rotation of the mandible in hyperdivergent patients may lead to narrowing of the PAP.2–4 Literature5 supports the notion that mandibular deficiency is frequently associated with a narrower PAP. It is believed6 that a retrognathic mandible and decreased space between the cranial column and the mandibular corpus might lead to a posterior postured tongue and soft palate, increasing the chances of impaired respiratory function and possibly causing nocturnal breathing problems.
There are plenty of studies in the literature assessing the PAP in subjects with craniofacial syndromes7 and obstructive sleep apnea,8 in subjects following orthognathic surgery,9,10 and in subjects with various jaw dysplasias.1,5,11,12 However, very few studies1,5,11,12 in the literature mention the PAP among subjects in various stages of sagittal mandibular development. In all of the previous studies, the vertical growth pattern of the mandible was not considered while evaluating the PAP among the subjects in various stages of sagittal mandibular development. Since the vertical growth pattern of the mandible has a significant effect on the PAP,2,4 it is necessary to include all of the subjects with similar vertical growth patterns of the mandible in order to eliminate any effect on PAP caused by changes in the vertical plane while evaluating the pharyngeal airway dimensions among subjects with various sagittal mandibular development. Thus, to overcome the lacuna present in the previous studies, the present study was designed to evaluate the PAP dimensions among subjects with normal, retrognathic, and prognathic mandibles who demonstrates a similar vertical growth pattern of the mandible.
MATERIALS AND METHODS
Pretreatment lateral cephalograms of 91 North Indian subjects aged 15–25 years with a normal vertical growth pattern of the mandible were selected for the study. Each subject met the following selection criteria:
They were 15–25 years of age;
They had a Frankfort mandibular plane angle (FMA) in the range of 20° to 25°; and
They had a normal position of the maxilla (angle between ‘S,’ ‘N,’ and ‘A’; it represents the antero-posterior position of the maxilla in relation to the anterior cranial base [SNA], 79°–83°) with various stages of sagittal mandibular development in relation to the anterior cranial base.
Subjects with a history of previous orthodontic treatment, functional jaw orthopedic treatment, any surgery involving the jaws, or surgery for adenoids; breathing disorders (such as snoring and obstructive sleep apnea); cleft lip and palate; and any systemic disease affecting normal growth were excluded from the study.
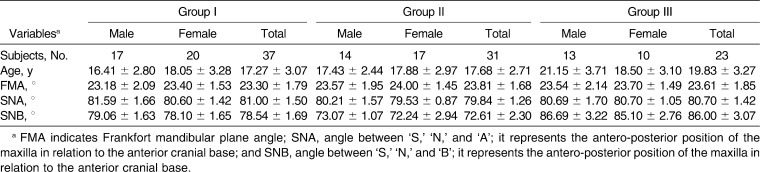
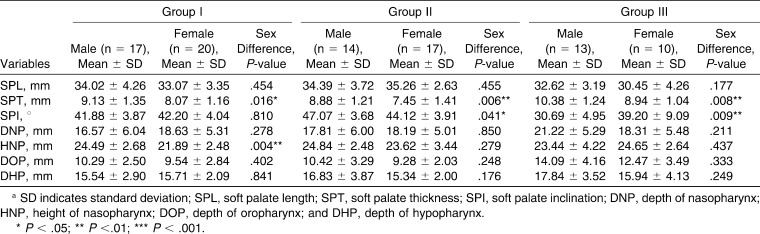
Based on the degree of sagittal mandibular development in relation to the anterior cranial base, all the subjects were divided into three groups. Group I included 37 (male = 17, female = 20) subjects who had a normally positioned maxilla and mandible (76° ≤ angle between ‘S,’ ‘N,’ and ‘B’; it represents the antero-posterior position of the maxilla in relation to the anterior cranial base [SNB] ≤ 82°) in relation to the anterior cranial base. Group II included 31 (male = 14, female = 17) subjects in whom the mandible was normally placed but in whom the mandible was retrognathic (SNB < 76°) in relation to the anterior cranial base. Group III included 23 (male = 13, female = 10) subjects in whom the maxilla was normally positioned but in whom the mandible was prognathic (SNB > 82°) in relation to the anterior cranial base. In addition, each group was also divided into subgroups according to sex. The characteristics of the subjects in each group are described in Table 1.
Table 1.
Characteristics of Subjects in the Various Groups
While recording the lateral cephalograms, patients were placed in the standing position with the FH plane parallel to the floor and the teeth in centric occlusion. The head of the patient was erect. The cephalogram was exposed at the end-expiration phase of the respiration. Subjects were instructed not to move their heads and tongues and not to swallow while during cephalogram exposure. All of the cephalograms were recorded with the same exposure parameters and in the same machine.
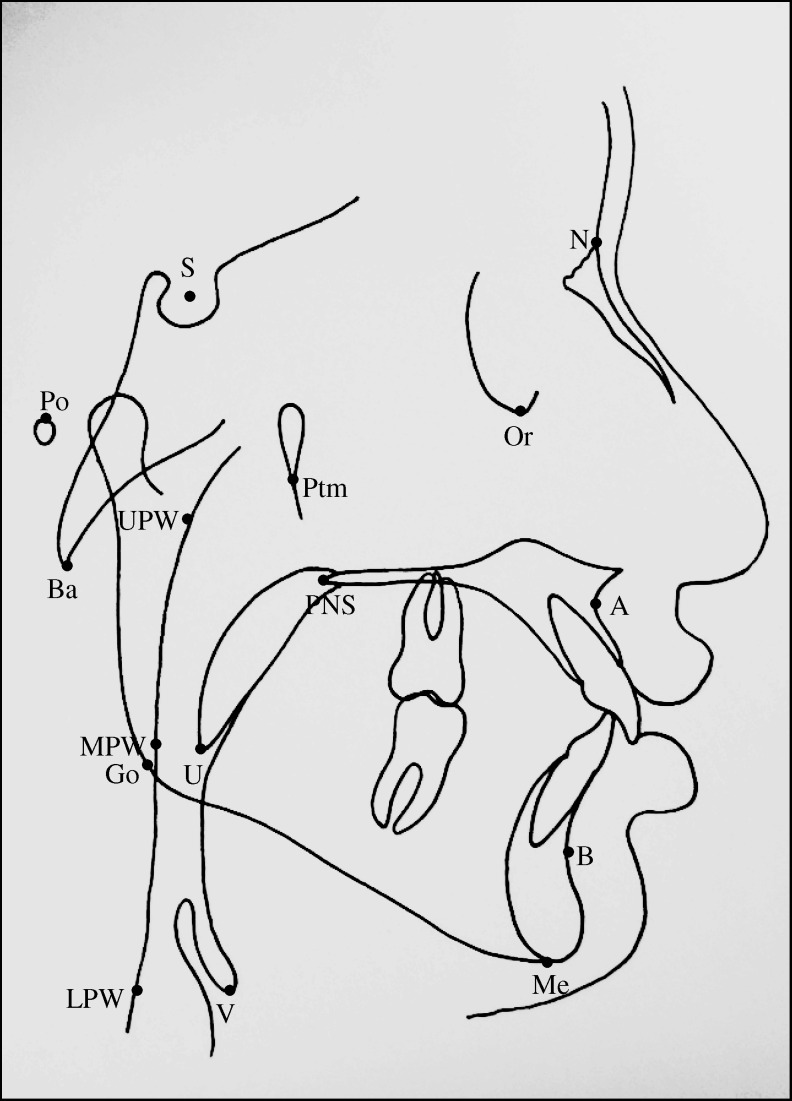
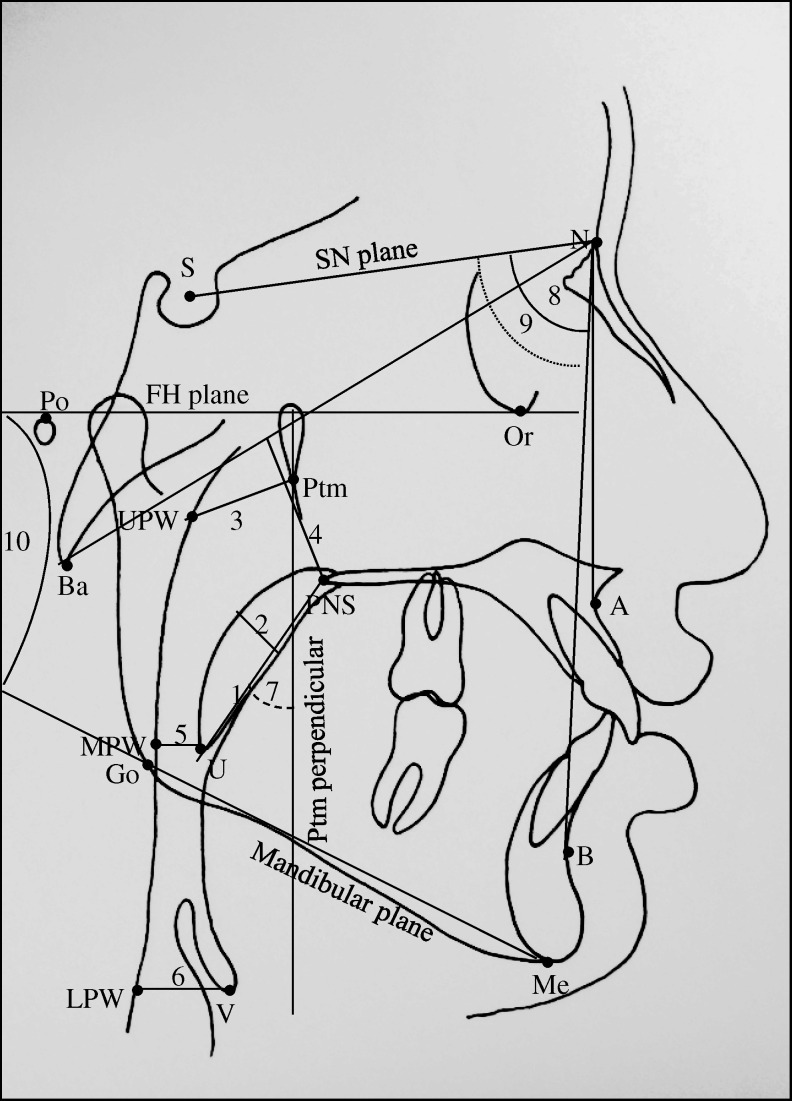
All lateral cephalograms were traced manually, and various landmarks were identified (Figure 1). Various reference planes, linear and angular parameters used for the evaluation of maxillary and mandibular position in relation to the anterior cranial base, vertical growth pattern of the mandible, and PAP dimensions are shown in Figure 2.
Figure 1.
Various cephalometric landmarks used in the study: S indicates sella; N, nasion; Po, porion; Or, orbitale; Go, gonion; A, Point A; B, Point B; Me, menton; Ptm, pterygomaxillary fissure; Ba, basion; UPW, upper pharyngeal wall, the intersection of line Ptm–Ba and posterior pharyngeal wall; MPW, middle pharyngeal wall, the intersection of perpendicular line on Ptm perpendicular from ‘U’ with posterior pharyngeal wall; V, vallecula; and LPW, lower pharyngeal wall, the intersection of perpendicular line on Ptm perpendicular from ‘V’ with posterior pharyngeal wall.
Figure 2.
Various cephalometric reference planes and linear and angular parameters used in the study. Reference planes: SN plane indicates the line joining ‘S’ and ‘N’; FH plane, line joining ‘Po’ and ‘Or’; Ptm perpendicular (Ptm per), perpendicular plane on FH plane at ‘Ptm’; and Ba-N plane, line joining ‘Ba’ and ‘N.’ Linear parameters: 1. SPL (U–PNS) indicates linear distance between U and PNS; 2. SPT, the maximum thickness of the soft palate; 3. DNP (Ptm–UPW), linear distance between ‘Ptm’ and ‘UPW’; 4. HNP, the shortest linear distance from PNS to Ba-N plane; 5. DOP (U–MPW), linear distance between ‘U’ and ‘MPW’; and 6. DHP (V–LPW), linear distance from ‘V’ to ‘LPW.’ Angular parameters: 7. SPI (Ptm per × PNS-U), the angle between Ptm perpendicular and soft palate (PNS-U); 8. SNA, angle between ‘S,’ ‘N,’ and ‘A’; it represents the antero-posterior position of the maxilla in relation to the anterior cranial base; 9. SNB, angle between ‘S,’ ‘N,’ and ‘B’; it represents the antero-posterior position of the maxilla in relation to the anterior cranial base; and 10. FMA, angle between FH plane and mandibular plane (Go-Me).
Statistical Method
A master file was created, and the data were statistically analyzed on a computer with SPSS software (version 13). A data file was created under dBase and converted into a microstat file. The data were subjected to descriptive analysis for mean, standard deviation, range, and 95% confidence interval. One-way analysis of variance and post-hoc test (Bonferroni) for multiple comparisons were used. The differences between males and females were tested using the Student's t-test. The Pearson correlation coefficient test was used to detect any relationship between SNB angle and other variables. A probability (P value) of .05 was considered statistically significant.
RESULTS
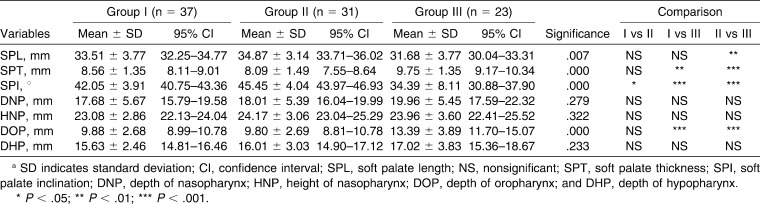
The results are shown in Tables 2–4. The soft palate length (SPL; posterior nasal spine [PNS]–tip of the soft palate [U]) was greatest among Group II subjects and was smallest among Group III subjects; the difference was statistically significant (P < .01). The difference in the soft palate thickness (SPT) was statistically significant between the subjects in Group I and Group III (P < .01) and between the subjects in Group II and Group III (P < .001). The SPT was significantly greater in males than in females in Group I (P < .05), Group II (P < .01), and Group III (P < .01). The soft palate inclination (SPI) was significantly different between subjects in Groups I and II (P < .05), Groups I and III (P < .001), and Groups II and III (P < .001). The SPI was comparable among males and females in Group I subjects. In Group II subjects, the SPI was significantly greater (P < .05) in males than females. However, in Group III subjects, the SPI was significantly greater (P < .01) among females than males. The depth of nasopharynx (DNP) and height of nasopharynx (HNP) were comparable among the subjects in all three groups. The DNP was comparable among males and females in all the groups. However, the HNP in male subjects was significantly greater (P < .01) than that of the female subjects in Group I. The depth of oropharynx (DOP) was comparable between the subjects of Groups I and II but was significantly different between the subjects of Groups I and III (P < .001) and Groups II and III (P < .001). The depth of hypopharynx (DHP) was comparable among the three groups of subjects.
Table 2.
Values of Various Cephalometric Parameters among Subjects of the Three Groupsa
Table 3.
Values of Various Cephalometric Parameters for Male and Female Subjects of the Three Groupsa
Table 4.
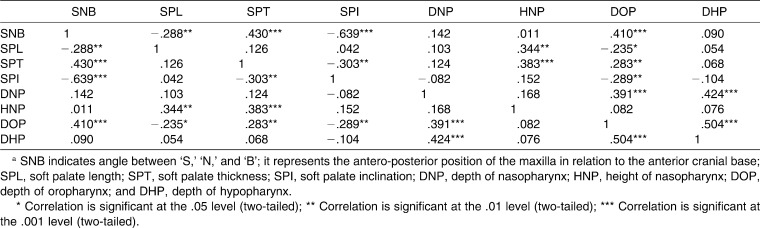
Correlation among All Variables in the Whole Sample (n = 91)a
The correlations between various parameters in the whole sample are described in Table 4. The SNB angle was significantly and negatively correlated with SPL (P < .01) and SPI (P < .001) but was significantly and positively correlated with SPT (P < .001) and DOP (P < .001). There was a negative correlation between SPL and DOP (P < .05). The SPT was positively correlated with DOP (P < .01). The SPI was negatively correlated with DOP (P < .01).
DISCUSSION
The pharynx is a tube-shaped structure that plays an important role in respiration and deglutition. The dimensions of the pharynx continue to grow rapidly until 13 years of age13 and then slow until adulthood.14 The depth of the upper pharyngeal airway increases with age, whereas the depth of the lower pharyngeal airway is established in early life.15 King16 reported no significant change in the DNP after 12 years of age. In the present study the age range of the subjects was 15–25 years to ensure that the pharyngeal structures had reached adult size.
In addition, the posture of the head has been suggested11 to influence the dimensions of the PAP. Thus, in order to eliminate the effects of head posture on the dimension of the PAP, patients were kept in a standing position with the head erect and with the FH plane parallel to the floor during cephalogram exposure.
We found an inverse correlation between the length of the soft palate and sagittal mandibular developments. The increased length of the soft palate among subjects with mandibular retrognathism could be the result of the backward position of the tongue, which compressed the soft palate and resulted in decreased thickness and increased length of the soft palate. Muto et al.5 also reported a similar observation. It was observed that a long soft palate was associated with smaller oropharyngeal depth17 and was more common among subjects who snored and had obstructive sleep apnea than among the normal subjects.18 In our study, the length of the soft palate was comparable among males and females. However, Allhaija and Al-Khateeb11 reported a longer soft palate in females than in males among subjects with mandibular retrognathism.
The soft palate was significantly thicker among subjects with a prognathic mandible. In subjects with mandibular prognathism the tongue was positioned forwardly away from the soft palate without touching it, whereas in subjects with mandibular retrognathism the tongue was positioned backwardly against the soft palate, which compressed the soft palate and decreased its thickness. Muto et al.5 also reported a similar observation. However, Allhaija and Al-Khateeb11 found a thinner soft palate among Class I subjects compared with Class II and Class III subjects. In our study, SNB angle was considered for the segregation of subjects into three groups, whereas Allhaija and Al-Khateeb11 considered ANB angle for the segregation of subjects, and this could be the reason for difference in our results.
The inclination of the soft palate was more horizontal among subjects with mandibular retrognathism, and it was more vertical in subjects with mandibular prognathism. The more backward position of the tongue among mandibular retrognathism subjects pushed the soft palate backward and increased its inclination. In mandibular prognathism subjects, the position of the tongue was more forward, away from the soft palate, which allowed the soft palate to hang freely and thus reduced the inclination. Muto et al.5 also observed maximum inclination of the soft palate among subjects with mandibular retrognathism, followed by those with a normal mandible and those with mandibular prognathism.
In our study, the dimensions of the soft palate were different among males and females. Although the length of the soft palate was comparable among males and females in each group, the thickness and inclination were significantly different. Martina et al.19 also reported different nasopharyngeal soft tissue patterns in men and women with ideal occlusions. However, Allhaija and Al-Khateeb11 did not find any sex differences in the dimensions of the soft palate. Allhaija and Al-Khateeb11 found a thicker soft palate in Class III females, but we found the thickest soft palate in Class III males and the thinnest palate in Class II females.
Although the dimensions of the nasopharynx were slightly larger among the subjects with mandibular retrognathism, these dimensions were comparable among the three groups of subjects. Thus, sagittal mandibular development had no effect on the dimensions of the nasopharynx. This could be the case because the dimensions of the bony nasopharynx are a relative independent variable in relation to other dimensions of the facial complex.20 Many previous studies1,21 also reported no differences in the nasopharyngeal dimension among subjects with different morphologic configurations of the dentofacial structures and maxillomandibular relations. However, in contrast to our findings, Kerr22 found greater nasopharyngeal airway dimensions in Class II malocclusion subjects than in normal occlusion subjects.
The DOP in the present study was almost equal among subjects with normal and retrognathic mandibles. However, the DOP among subjects with a prognathic mandible was significantly greater than that for subjects with normal and retrognathic mandibles. The sagittal position of mandible had a positive correlation with the DOP. In contrast to our result, Muto et al.5 found significantly smaller oropharyngeal depths among subjects with retrognathic mandibles when compared with those subjects with normal and prognathic mandibles. In the previous study,5 the mandibular growth pattern among subjects with mandibular retrognathism was hyperdivergent, whereas among subjects with mandibular prognathism it was hypodivergent, and this could be the reason for the smaller oropharyngeal depths among subjects with retrognathic mandibles then among those subjects with normal and prognathic mandibles. When the mandible was both retruded and rotated in downward and backward directions, it caused the tongue base to be positioned more posteriorly and inferiorly and, thus, further reduced the oropharyngeal depth.2,4
It is known that in subjects with mandibular retrognathism, the tongue position is more backward, with contact to the soft palate resulting in the posterior displacement of the soft palate and narrowing of the oropharyngeal airway.5 However, in our study, the DOP was almost identical among subjects with normal and retrognathic mandibles. The relatively decreased thickness in the soft tissue posterior pharyngeal wall (as a compensatory mechanism) could be the reason for adequate oropharyngeal depth among subjects with mandibular retrognathism. As the mandible was very large and anteriorly positioned in subjects with mandibular prognathism, the tongue was positioned more anteriorly away from the soft palate and resulted in greater oropharyngeal airway depth. Our result agrees with those of many previous studies1,5,17 that also reported a significant correlation between maxillo-mandibular relations and oropharyngeal depth. However, many previous studies also reported no relation between pharyngeal structures and maxillo-mandibular relationship,1,21,23 and the antero-posterior dimension of the upper airway was usually maintained by the adaptation of both tongue and hyoid bone.24 We found comparable oropharyngeal depths among males and females. This finding was in agreement with those of many previous studies.11,25 However, a few studies1,26 also reported a significant sex difference in terms of oropharyngeal depth. The depth of the hypopharynx was comparable among the three groups of subjects, showing that the lower pharyngeal airway space was independent of sagittal mandibular development.
It is believed that snoring and obstructive sleep apnea are common among subjects with retrognathic mandible,7,27 although there are many reports in the literature that mention that a short and backwardly placed mandible is a common feature among subjects who exhibit snoring and obstructive sleep apnea. But in the present study, none of the subjects with a retruded mandible had a history of breathing problems. Thus, mandibular retrognathism in all subjects cannot be considered an etiology of snoring and obstructive sleep apnea. When the mandible is severely retruded, as in the case of subjects with Pierre Robin syndrome, mandibular dysostosis, etc., it holds the tongue back (glossoptosis) and thus restricts the PAP, resulting in snoring and obstructive sleep apnea.7,27,28 However, Opdebeeck et al.4 found that mandibular hypoplasia with or without glossoptosis may compromise the airway. Although the role of fat deposition in the pharyngeal wall and the resulting narrowing of the pharyngeal airway space are not clear in the literature, they might have an important role in airway obstruction.29
CONCLUSIONS
Sagittal mandibular development had significant effects on the dimensions of the awake pharyngeal airway passage.
The length of the soft palate was smaller among subjects with mandibular prognathism than among subjects with normal and retrognathic mandibles.
The thickness of the soft palate was greater among mandibular prognathic subjects than among subjects with normal and retrognathic mandibles.
Sagittal mandibular development had a significant influence on the inclination of the soft palate.
The dimensions of the nasopharynx and hypopharynx were independent of sagittal mandibular development.
The depth of the oropharynx was greater among subjects with mandibular prognathism than among subjects with normal and retrognathic mandibles.
REFERENCES
- 1.Ceylan I, Oktay H. A study on the pharyngeal size in different skeletal patterns. Am J Orthod Dentofacial Orthop. 1995;108:69–75. doi: 10.1016/s0889-5406(95)70068-4. [DOI] [PubMed] [Google Scholar]
- 2.Joseph A. A, Elbaum J, Cisneros G. J, Eisig S. B. A cephalometric comparative study of the soft tissue airway dimensions in persons with hyperdivergent and normodivergent facial pattern. J Oral Maxillofac Surg. 1998;56:135–139. doi: 10.1016/s0278-2391(98)90850-3. [DOI] [PubMed] [Google Scholar]
- 3.Liano Y, Huang C, Chuang M. The utility of cephalometry with the Muller maneuver in evaluating the upper airway and its surrounding structures in Chinese patients with sleep disordered breathing. Laryngoscope. 2003;113:614–619. doi: 10.1097/00005537-200304000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Opdebeeck H, Bell W. H, Eisenfeld J, Mishelevich D. Comparative study between the SFS and LFS rotation as a possible morphologic mechanism. Am J Orthod. 1978;74:509–521. doi: 10.1016/0002-9416(78)90026-x. [DOI] [PubMed] [Google Scholar]
- 5.Muto T, Yamazaki A, Takeda S. A cephalometric evaluation of the pharyngeal airway space in patients with mandibular retrognathia and prognathia, and normal subjects. Int J Oral Maxillofac Surg. 2008;37:228–231. doi: 10.1016/j.ijom.2007.06.020. [DOI] [PubMed] [Google Scholar]
- 6.Ozbek M. M, Miyamoto K, Lowe A. A, Fleetham J. A. Natural head posture, upper airway anatomy and obstructive sleep apnea severity in adults. Eur J Orthod. 1998;20:133–143. doi: 10.1093/ejo/20.2.133. [DOI] [PubMed] [Google Scholar]
- 7.Figueroa A. A, Glupker T. J, Fitz M. G, BeGole E. A. Mandible, tongue and airway in Pierre Robin Sequence: a longitudinal cephalometric study. Cleft Palate Craniofac J. 1991;28:425–434. doi: 10.1597/1545-1569_1991_028_0425_mtaaip_2.3.co_2. [DOI] [PubMed] [Google Scholar]
- 8.Tangugsorn V, Skatvedt O, Krogstad O, Lyberg T. Obstructive sleep apnea: a cephalometric study. Part-II. Uvulo-glossopharyngeal morphology. Eur J Orthod. 1995;17:57–67. doi: 10.1093/ejo/17.1.57. [DOI] [PubMed] [Google Scholar]
- 9.Muto T, Yamazaki A, Takeda S, Sato Y. Effect of bilateral sagittal split ramus osteotomy setback on the soft palate and pharyngeal airway space. Int J Oral Maxillofac Surg. 2008;37:419–423. doi: 10.1016/j.ijom.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 10.Degerliyurt K, Ueki K, Hashiba Y, et al. The effect of mandibular setback or two-jaws surgery on pharyngeal airway among different genders. Int J Oral Maxillofac Surg. 2009;38:647–652. doi: 10.1016/j.ijom.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 11.Allhaija E. S. A, Al-Khateeb S. N. Uvulo-glossopharyngeal dimensions in different anteroposterior skeletal patterns. Angle Orthod. 2005;75:1012–1018. doi: 10.1043/0003-3219(2005)75[1012:UDIDAS]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 12.Trenouth M. J, Timms D. J. Relationship of the functional oropharynx to craniofacial morphology. Angle Orthod. 1999;69:419–423. doi: 10.1043/0003-3219(1999)069<0419:ROTFOT>2.3.CO;2. [DOI] [PubMed] [Google Scholar]
- 13.Jeans W. D, Fernando D. C. J, Maw A. R, Leighton B. C. A longitudinal study of the growth of the nasopharynx and its contents in normal children. Br J Radiol. 1981;54:117–121. doi: 10.1259/0007-1285-54-638-117. [DOI] [PubMed] [Google Scholar]
- 14.Tourne L. P. M. Growth of the pharynx and its physiologic implications. Am J Orthod Dentofacial Orthop. 1991;99:129–139. doi: 10.1016/0889-5406(91)70115-D. [DOI] [PubMed] [Google Scholar]
- 15.Tsai H. H. Developmental changes of pharyngeal airway structures from young to adult persons. Clin Pediatr Dent. 2007;31:219–221. doi: 10.17796/jcpd.31.3.023h753711p24273. [DOI] [PubMed] [Google Scholar]
- 16.King E. W. A roentgenographic study of pharyngeal growth. Angle Orthod. 1952;22:32–37. [Google Scholar]
- 17.Muto T, Yamazaki A, Takeda S, et al. Relationship between the pharyngeal airway space and craniofacial morphology, taking into account head posture. Int J Oral Maxillofac Surg. 2006;35:132–136. doi: 10.1016/j.ijom.2005.04.022. [DOI] [PubMed] [Google Scholar]
- 18.Maltais F, Carrier G, Cormier Y, Series F. Cephalometric measurements in snorers, non-snorers and patients with sleep apnea. Thorax. 1991;46:419–423. doi: 10.1136/thx.46.6.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martina O, Muelas L, Vinas M. J. Nasopharyngeal cephalometric study of ideal occlusions. Am J Orthod Dentofacial Orthop. 2006;130:436.e1–436.e9. doi: 10.1016/j.ajodo.2006.03.022. [DOI] [PubMed] [Google Scholar]
- 20.Linder-Aronson S, Woodside D. G. The growth in the sagittal depth of the bony nasopharynx in relation to some other facial variables. Trans Eur Orthod Soc. 1977:69–83. [Google Scholar]
- 21.Mergen D. C, Jacobs R. M. The size of nasopharynx associated with normal occlusion and Class II malocclusion. Angle Orthod. 1970;40:342–346. doi: 10.1043/0003-3219(1970)040<0342:TSONAW>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 22.Kerr W. J. S. The nasopharynx, face height and overbite. Angle Orthod. 1985;55:31–36. doi: 10.1043/0003-3219(1985)055<0031:TNFHAO>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 23.Sosa F. A, Graber T. M, Muller T. P. Postpharyngeal lymphoid tissue in Angle Class I and Class II malocclusions. Am J Orthod. 1982;81:299–309. doi: 10.1016/0002-9416(82)90216-0. [DOI] [PubMed] [Google Scholar]
- 24.Graber L. W. Hyoid changes following orthopedic treatment of mandibular prognathism. Angle Orthod. 1978;48:33–38. doi: 10.1043/0003-3219(1978)048<0033:HCFOTO>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 25.Solow B, Siersbaek-Nielsen S, Greve E. Airway adequacy, head posture, and craniofacial morphology. Am J Orthod. 1984;86:214–223. doi: 10.1016/0002-9416(84)90373-7. [DOI] [PubMed] [Google Scholar]
- 26.Sheng C. M, Lin L. H, Su Y, Tsai H. H. Developmental changes in pharyngeal airway depth and hyoid bone position from childhood to young adulthood. Angle Orthod. 2009;79:484–490. doi: 10.2319/062308-328.1. [DOI] [PubMed] [Google Scholar]
- 27.Shprintzen R. J, Croft C, Berkman M. D, Rakoff S. J. Pharyngeal hypoplasia in Trecher Collins Syndrome. Arch Otolaryngol. 1979;105:127–131. doi: 10.1001/archotol.1979.00790150017005. [DOI] [PubMed] [Google Scholar]
- 28.Schafer M. E. Upper airway obstruction and sleep disorders in children with craniofacial anomalies. Clin Plast Surg. 1982;9:555–567. [PubMed] [Google Scholar]
- 29.Chen F, Terada K, Hanada K, Saito I. Predicting the pharyngeal airway space after mandibular setback surgery. J Oral Maxillofac Surg. 2005;63:1509–1514. doi: 10.1016/j.joms.2005.06.007. [DOI] [PubMed] [Google Scholar]