Abstract
Objective:
To determine the pulpal blood flow (PBF) changes in anchorage teeth associated with the high forces of a rapid maxillary expansion (RME) appliance.
Materials and Methods:
The study was performed with 14 girls and 7 boys for a total of 21 patients between 10 and 15 years of age (mean, 13.1 ± 1.39 years). A modified acrylic bonded RME appliance was used as an expansion appliance. Laser Doppler flowmetry was used for the pulpal perfusion measurements. Records were taken from 42 upper central incisors, 28 canines, and 42 first molars at the following time intervals: just before expansion (T1); at the first week of expansion (T2); at the end of the expansion process (T3); and at the third (T4), seventh (T5), and 12th weeks of retention (T6). The data gained were statistically evaluated by parametric tests.
Results:
PBF values of the anchorage teeth were doubled at the first week of expansion; however, these values began to decrease because of separation of the median palatal suture. PBF values tended to reach their initial values during the retention period. Pulpal perfusion changes of all examined anchorage teeth were similar to each other from the beginning to the end of the evaluation.
Conclusion:
PBF changes that occur with RME are reversible.
Keywords: Rapid maxillary expansion, Laser Doppler flowmetry, Pulpal blood flow
INTRODUCTION
Rapid maxillary expansion (RME) is one of the most impressive orthopedic procedures. RME corrects maxillary arch constrictions, increases arch perimeter to reduce crowding, and corrects disharmonies in the transversal plane between maxillary and mandibular arches.1–6
The basis for the rapid expansion procedure is to produce immediate midpalatal suture separation by disruption of the sutural connective tissue with rapid maxillary expanders; this creates large forces at the sutural site over a short period of time.6,7 Forces produced by this appliance have been reported in the range of 16.6 to 34.8 pounds (7.54 to 15.8 kg).8 These heavy forces maximize skeletal separation of the midpalatal suture by overwhelming the suture before any dental movement or physiologic sutural adjustment can occur.6,9
Isaacson et al.7 and Zimring and Isaacson8 demonstrated that residual forces always develop during the active RME phase. Brosh et al.10 reported that strain level was preserved during the retention phase, after RME, as the result of relapse strains.
The relationship between orthopedic forces and the magnitude of pulpal impairment remains unclear. Dental pulp is a specialized tissue; it contains blood vessels and nerves that maintain tooth vitality and sensitivity. Pulpal responses after orthodontic forces were evaluated in the following ways: histologic observation,11,12 fluorescent microsphere injection,13,14 and pulp tissue respiration rate15,16 methods. However, these methods have technical limitations that allow observation after tooth extraction. Laser Doppler flowmetry (LDF) is a noninvasive method of determining pulp blood flow of the same tooth repeatedly without causing damage to the pulp.17 LDF recognizes heartbeat synchronous oscillations within teeth with a pulpal vasculature. It involves directing laser light through the enamel and dentin toward the pulp; some of the light may be reflected and Doppler-shifted by moving erythrocytes.18
Pulpal responses to orthodontic forces have previously been investigated19–24; however, few studies have evaluated the reaction of dental pulp to the orthopedic forces created by RME. Kayhan et al.25 and Taspinar et al.26 observed histologic changes after RME on extracted first premolar tooth pulp. They concluded that the orthopedic forces applied by RME appliances caused reversible vascular changes in pulpal tissue of anchor upper premolar teeth. However, these histologic studies give limited information on clinical changes in the pulp of the anchor tooth during orthopedic forces in human subjects. Another study by Cho et al.27 reported reversible changes after RME using an electric pulp tester and a cold test. The LDF method was reportedly more reliable than these two pulp vitality tests.28,29 Therefore the purpose of our study was to evaluate pulpal blood changes in human dental pulp with LDF from the beginning of RME to the end of retention.
MATERIALS AND METHODS
The study consisted of 21 patients—14 girls and 7 boys—between the ages of 10 and 15 years (mean, 13.1 ± 1.39 years), presenting bilateral posterior cross-bite of skeletal origin. The modified acrylic bonded RME appliance was used as an expansion appliance in all patients.30 This type of RME appliance provides control of vertical dimension changes that occur in growing patients during maxillary expansion. The RME appliance was cemented, in all subjects, with the use of glass ionomer cement (Ketac-Cem, Espe Dental AG, Seefeld, Germany). The appliance was activated one-quarter turn once a day during the expansion period until the desired suture opening was achieved. After removal, the appliance used in active treatment was cleaned and the screw was fixed with 0.014-inch ligature wire and reused as a removable retention appliance for 6 months. Table 1 shows the distribution of age and gender and average expansion periods and average retention periods of subjects. The purpose and the procedure were explained to the participants, and their informed consent for the experiment was obtained. This study was approved by the Ethical Committee at Cumhuriyet University Faculty of Dentistry.
Table 1.
Summary of Age, Gender Distribution, and Average Expansion and Retention Periods of Subjects
Only the teeth of patients who underwent a routine and successful RME procedure were chosen for the study. Records were taken from 42 central incisors, 28 canines, and 42 first molars at the following time intervals: just before expansion (T1); at the first week of expansion (T2) and at the end of expansion (T3); and at the third (T4), seventh (T5), and 12th weeks of retention (T6). Data were collected with a laser Doppler flowmeter (Periflex 4001 Master, Perimed AB, Stockholm, Sweden) and were simultaneously shown on a monitor (Figure 2). Evaluation took 10 seconds for each tooth. A special software package (Perisoft 5.1, Gastrosoft Inc, Jarfalla, Sweden) was used to calculate average perfusion unit (PU) values. The PU is the output value of the laser Doppler flowmeter that means the pulpal blood flow (PBF). A commercially available motility standard (Perimed) was used to calibrate the flowmeter, which was a bottle containing 2 to 5 cm3 of a colloidal suspension of latex particles. Light with a wavelength of 632.8 nm was produced by a 1-mW helium-neon (He-Ne) laser within the flowmeter and was sent along a flexible fiberoptic conductor inside the probe (Perimed) to the labial surface of the teeth. Before any measurement, the patients rested for 10 minutes. All measurements were done in the same unit. Probe holes were drilled 3 to 4 mm below the gingival margin of the teeth on the acrylic expansion appliances. These holes were made to insert and fix the probe, and to ensure accurate and reproducible positioning of the probe on the tooth during each session (Figure 1).
Figure 2.
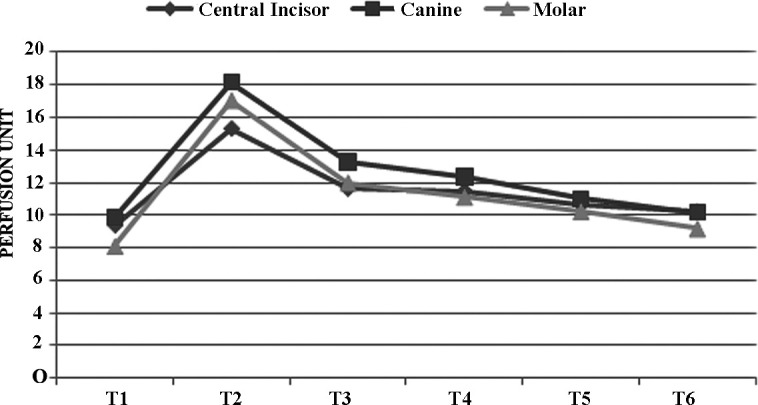
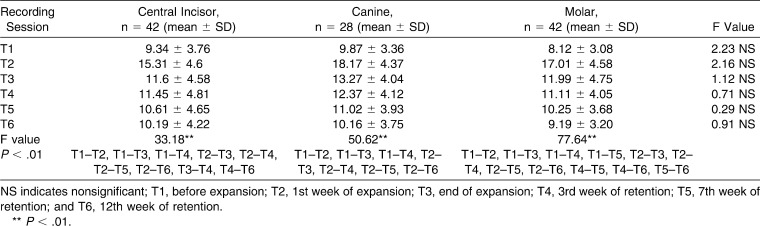
The change in mean PU is examined in three types of teeth. Y axis: perfusion unit; X axis: time points.
Figure 1.
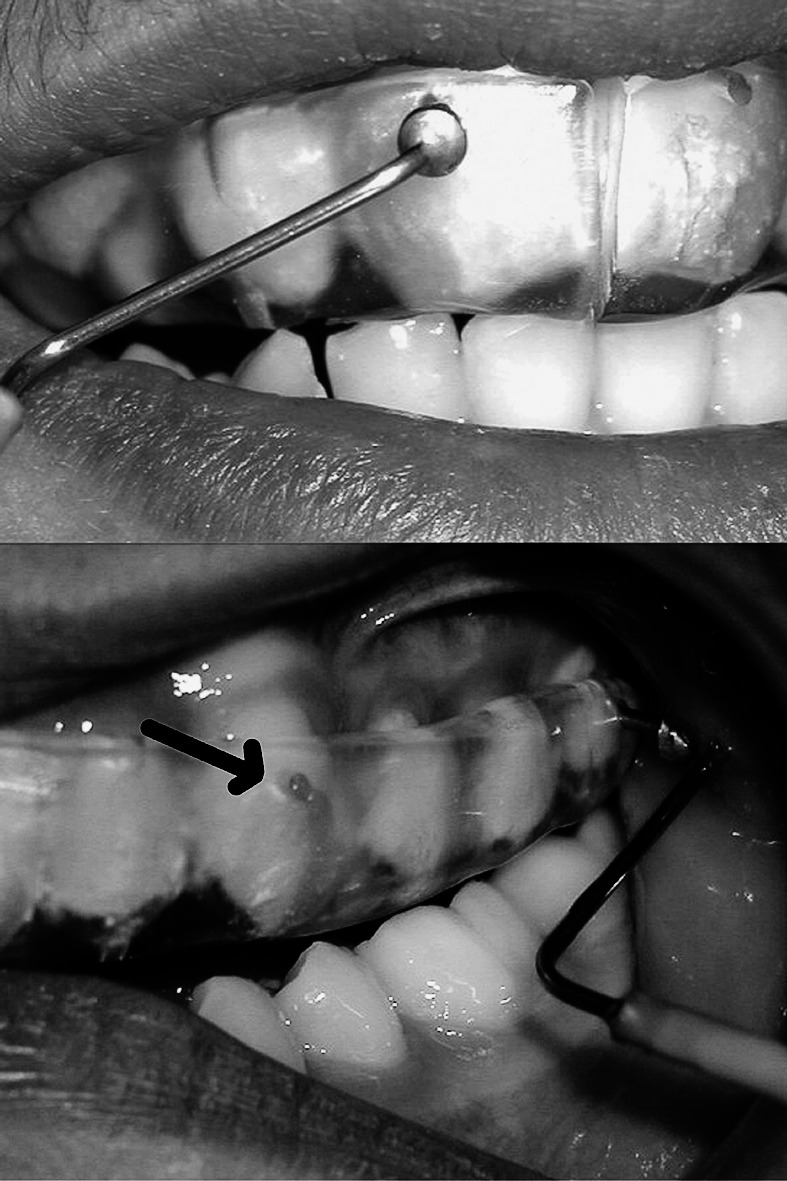
Holes are drilled 3 to 4 mm below the gingival margin of the modified acrylic bonded RME appliance to insert and fix the probe on the tooth.
Statistical Analysis
The Statistical Package for the Social Sciences (SPSS) package (version 10.0, SPSS Inc, Chicago, Ill) was used to analyze the results. A repeated measurement of variance analysis (ANOVA) was performed in the comparison of pulpal perfusion values of every tooth group according to time intervals. Variance analysis was performed in the comparison of intergroup differences in pulpal perfusion values of every tooth group according to time intervals. The Tukey test was performed to determine which time interval would show the difference.
RESULTS
Mean baseline PBFs of the upper central incisors, canines, and first molars were similar (9.34 ± 4, 9.87 ± 3.36, and 8.12 ± 3 PU for central incisors, canines, and first molars, respectively). PBF increases nearly twofold at the end of the first week (T2) (15.31 ± 5.6, 18.17 ± 6.3, and 17.01 ± 5.58 PU for the central incisors, canines, and molars, respectively) and then decreases and tends to achieve baseline at the end of the third month of retention (10.19 ± 4.22, 10.16 ± 3.75, and 9.19 ± 3.2 PU for the central incisors, canines, and molars, respectively) (Table 2).
Table 2.
Mean Perfusion Units and SDs of Measurements Taken From Three Tooth Types at Each of Three Sessions
Differences in mean percentage values of PBF were compared for each set of teeth; the difference was found to be statistically insignificant at each time point (Table 2) (P > .01). Figure 2 shows the similarities between measurements of central incisors, canines, and first molars at each time point.
Differences from the beginning of expansion to the end of the third month of retention were assessed for each tooth (Figure 2, Table 2). PBF changes in central incisors, canines, and first molars were found to be statistically significant between the beginning of expansion (T1) and the first week of expansion (T2) (P < .01). The peak value at the end of the first week (T2) decreases significantly at the third week of expansion (T3) (P < .01). Mean PBF values decrease significantly during the retention period (T3–T6) (P < .01). Mean PBF values for each tooth tended to return to baseline level after 3 months of retention.
DISCUSSION
After orthodontic force application, pulpal response attracted the orthodontist's interest and was investigated in many aspects. Previous studies report that the type of movement may influence the observed tissue reaction.15,31 Orthodontic forces generally increase PBF as a result of the inflammatory process triggered by tooth movement, in which blood supply and inflammatory cells reach the area in an attempt to achieve tissue repair13 and generate new blood vessels.32 These results demonstrate the high capacity for adaptation of the pulp tissue to an aggression and also provide the biological limits for tolerance of the pulp.
Pulpal response after heavy force application was investigated both histologically and with pulp tests.25–27 Kayhan et al.25 evaluated the histologic and histomorphometric changes in pulpal tissue of anchor premolar teeth after the RME procedure. Although they noticed more pronounced fibrotic elements in the pulpal tissue, investigators observed no differences in pre-dentin width, no change in the number of vessels, and no calcification deposits or vacuoles after 3 months of retention. They concluded these results to be an adaptive vascular tissue response. Taspinar et al.26 investigated the effects of the heavy orthopedic forces produced by RME appliances on the pulpal tissue of upper premolar teeth using histopathologic techniques. They reported similar reversible vascular changes as Kayhan et al.25 in the pulpal tissue of upper premolar teeth. These histologic studies show inflammatory changes in the pulp tissue after orthopedic force application.25,26 Both described areas of the vessels that were increased because of the inflammatory process.
The other study that investigated pulp vitality after RME, using an electric pulp tester and a cold test, was performed by Cho et al.27 Investigators reported that all the teeth tested responded with signs of vitality after retention.
These previous studies reveal reversible vascular changes after RME. Our study shows that PBF increases because of this inflammation in the initial period, and this increase tends to return to baseline values with respect to a decrease in residual forces accumulated around the suture after therapy with RME is complete. Our results demonstrate reversible vascular changes as reported in previous studies.
Previous histologic studies report changes only at the first premolar site; however, changes in other teeth affected by heavy orthopedic forces remain unclear. All teeth examined here (central incisor, canine, and first molar) show similar pulpal perfusion changes at the checked time points (Figure 2). Our results suggest that these changes did not differ according to the type, location, or shape of the tooth. The teeth investigated in our report gave very convincing signals that were compatible with histologic findings.
Kayhan et al.25 and Taspinar et al.26 suggested that repeated measurements of PBF at different stages of force application for evaluation of pulp hemodynamics were better than a single observation during force application. LDF can repeatedly measure PBF of the same tooth without causing damage to the pulp. In dentistry, the principal diagnostic role of LDF to date has been in assessment of the pulp condition of traumatized teeth that are not responsive to conventional tests.28,29 PBF measurement using LDF has been described as a more sensitive technique for evaluating tooth pulp vitality compared with conventional methods such as electric and thermal pulp testing.28,29,33,34
The modified acrylic bonded RME appliance, which decreases tipping forces and also controls vertical dimensions, was used as an expansion appliance because the heavy forces created by the screw were distributed among several anchorage teeth. Furthermore, the holes, made to insert and fix the probe on the tooth during each session, were standardized at each tooth, including central incisors. It is important to insert and fix the probe at the same point on the teeth during each session to ensure the reliability of the measurements.35 The acrylic portion of the expansion appliance was extended buccally to reduce noise from surrounding tissues during recording of the PBF. Our baseline measurements for the examined teeth are similar to the baseline measurements of Norer et al.,36 who measured the perfusion units of all teeth during three sessions with a time interval of 7 days between sessions using the same laser Doppler flowmeter.
CONCLUSIONS
PBF increased nearly twofold during the first week of expansion when heavy forces applied with the expander accumulated on the anchor teeth before sutural separation. After sutural opening, PBF decreased and tended to reach its initial values during the retention period.
PBF changes in central incisors, canines, and first molars due to RME were similar to each other.
REFERENCES
- 1.Angell E. H. Treatment of irregularities of the permanent or adult teeth. Dent Cosmos. 1860;1:540–544. [Google Scholar]
- 2.Haas A. J. Rapid expansion of the maxillary dental arch and nasal cavity by opening the midpalatal suture. Angle Orthod. 1961;31:73–90. [Google Scholar]
- 3.Bishara S. E, Staley R. N. Maxillary expansion: clinical implications. Am J Orthod Dentofacial Orthop. 1987;91:3–14. doi: 10.1016/0889-5406(87)90202-2. [DOI] [PubMed] [Google Scholar]
- 4.Haas A. J. The treatment of maxillary deficiency by opening the midpalatal suture. Angle Orthod. 1965;35:200–217. doi: 10.1043/0003-3219(1965)035<0200:TTOMDB>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 5.Haas A. J. Longterm post treatment evaluation of rapid palatal expansion. Angle Orthod. 1980;50:189–217. doi: 10.1043/0003-3219(1980)050<0189:LPEORP>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 6.Wertz R. A. Skeletal and dental changes accompanying rapid midpalatal suture opening. Am J Orthod. 1970;58:41–66. doi: 10.1016/0002-9416(70)90127-2. [DOI] [PubMed] [Google Scholar]
- 7.Isaacson R. J, Wood J. L, Ingram A. H. Forces produced by rapid maxillary expansion. I and II. Angle Orthod. 1964;34:256–270. doi: 10.1043/0003-3219(1965)035<0178:FPBRME>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 8.Zimring J. F, Issacson R. J. Forces produced by rapid maxillary expansion. III: forces present during retention. Angle Orthod. 1965;35:178–186. doi: 10.1043/0003-3219(1965)035<0178:FPBRME>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 9.Haas A. J. Palatal expansion: just the beginning of dentofacial orthopedics. Am J Orthod. 1970;57:219–255. doi: 10.1016/0002-9416(70)90241-1. [DOI] [PubMed] [Google Scholar]
- 10.Brosh T, Vardimon A. D, Ergatudes C, Spiegler A, Lieberman M. Rapid palatal expansion. Part 3: strains developed during active and retention phases. Am J Orthod Dentofacial Orthop. 1998;114:123–133. doi: 10.1053/od.1998.v114.a85568. [DOI] [PubMed] [Google Scholar]
- 11.Wong V. S, Freer T. J, Joseph B. K, Daley T. J. Tooth movement and vascularity of the dental pulp: a pilot study. Aust Orthod J. 1999;15:246–250. [PubMed] [Google Scholar]
- 12.Santamaria M, Jr, Milagres D, Stuani A. S, Stuani M. B, Ruellas A. C. Initial changes in pulpal microvasculature during orthodontic tooth movement: a stereological study. Eur J Orthod. 2006;28:217–220. doi: 10.1093/ejo/cji117. [DOI] [PubMed] [Google Scholar]
- 13.Vandevska-Radunovic V, Kristiansen A. B, Heyeraas K. J, Kvinnsland S. Changes in blood circulation in teeth and supporting tissues incident to experimental tooth movement. Eur J Orthod. 1994;16:361–369. doi: 10.1093/ejo/16.5.361. [DOI] [PubMed] [Google Scholar]
- 14.Kvinnsland S, Heyeraas K, Ofjord E. S. Effect of experimental tooth movement on periodontal and pulpal blood flow. Eur J Orthod. 1989;11:200–205. doi: 10.1093/oxfordjournals.ejo.a035986. [DOI] [PubMed] [Google Scholar]
- 15.Unsterseher R. E, Nieberg L. G, Weimer A. D, Dyer J. K. The response of human pulpal tissue after orthodontic force application. Am J Orthod Dentofacial Orthop. 1987;92:220–224. doi: 10.1016/0889-5406(87)90415-x. [DOI] [PubMed] [Google Scholar]
- 16.Hamersky P. A, Weimer A. D, Taintor J. F. The effect of orthodontic force application on the pulpal tissue respiration rate in the human premolar. Am J Orthod. 1980;77:368–378. doi: 10.1016/0002-9416(80)90103-7. [DOI] [PubMed] [Google Scholar]
- 17.Gazelius B, Olgart L, Edwall B, Edwall L. Non-invasive recording of blood flow in human dental pulp. Endod Dent Traumatol. 1986;2:219–221. doi: 10.1111/j.1600-9657.1986.tb00148.x. [DOI] [PubMed] [Google Scholar]
- 18.Gazelius B, Lindh-Strömberg U, Pettersson H, Oberg P. A. Laser Doppler technique—a future diagnostic tool for tooth pulp vitality. Int Endod J. 1993;26:8–9. doi: 10.1111/j.1365-2591.1993.tb00530.x. [DOI] [PubMed] [Google Scholar]
- 19.McDonald F, Pitt Ford T. R. Blood flow changes in permanent maxillary canines during retraction. Eur J Orthod. 1994;16:1–9. doi: 10.1093/ejo/16.1.1. [DOI] [PubMed] [Google Scholar]
- 20.Barwick P. J, Ramsay D. S. Effect of brief intrusive force on human pulpal blood flow. Am J Orthod Dentofacial Orthop. 1996;110:273–279. doi: 10.1016/s0889-5406(96)80011-4. [DOI] [PubMed] [Google Scholar]
- 21.Brodin P, Linge L, Aars H. Instant assessment of pulpal blood flow after orthodontic force application. J Orofac Orthop. 1996;57:306–309. doi: 10.1007/BF02197551. [DOI] [PubMed] [Google Scholar]
- 22.Ikawa M, Fujiwara M, Horiuchi H, Shimauchi H. The effect of short-term tooth intrusion on human pulpal blood flow measured by laser Doppler flowmetry. Arch Oral Biol. 2001;46:781–787. doi: 10.1016/s0003-9969(01)00049-8. [DOI] [PubMed] [Google Scholar]
- 23.Sano Y, Ikawa M, Sugawara J, Horiuchi H, Mitani H. The effect of continuous intrusive force on human pulpal blood flow. Eur J Orthod. 2002;24:159–166. doi: 10.1093/ejo/24.2.159. [DOI] [PubMed] [Google Scholar]
- 24.Konno Y, Daimaruya T, Iikubo M, Kanzaki R, Takahashi I, Sugawara J, Sasano T. Morphologic and hemodynamic analysis of dental pulp in dogs after molar intrusion with the skeletal anchorage system. Am J Orthod Dentofacial Orthop. 2007;132:199–207. doi: 10.1016/j.ajodo.2005.07.029. [DOI] [PubMed] [Google Scholar]
- 25.Kayhan F, Küçükkeles N, Demirel D. A histological and histomorphometric evaluation of pulpal reactions following rapid palatal expansion. Am J Orthod Dentofacial Orthop. 2000;117:465–473. doi: 10.1016/s0889-5406(00)70167-3. [DOI] [PubMed] [Google Scholar]
- 26.Taspinar F, Akgül N, Simşek G, Ozdabak N, Gündoğdu C. The histopathological investigation of pulpal tissue following heavy orthopaedic forces produced by rapid maxillary expansion. J Int Med Res. 2003;31:197–201. doi: 10.1177/147323000303100305. [DOI] [PubMed] [Google Scholar]
- 27.Cho J. J, Efstratiadis S, Hasselgren G. Pulp vitality after rapid palatal expansion. Am J Orthod Dentofacial Orthop. 2010;137:254–258. doi: 10.1016/j.ajodo.2008.04.023. [DOI] [PubMed] [Google Scholar]
- 28.Olgart L, Gazelius B, Lindh-Stromberg U. Laser Doppler flowmetry in assessing vitality in luxated permanent teeth. International Endodontic Journal. 1988;21:300–306. doi: 10.1111/j.1365-2591.1988.tb01139.x. [DOI] [PubMed] [Google Scholar]
- 29.Mesaros S. V, Trope M. Revascularization of traumatized teeth assessed by laser Doppler flowmetry: case report. Endod Dent Traumatol. 1997;13:24–30. doi: 10.1111/j.1600-9657.1997.tb00005.x. [DOI] [PubMed] [Google Scholar]
- 30.Basciftci F. A, Karaman A. I. Effects of a modified acrylic bonded rapid maxillary expansion appliance and vertical chin cap on dentofacial structures. Angle Orthod. 2002;72:61–71. doi: 10.1043/0003-3219(2002)072<0061:EOAMAB>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 31.Nixon C. E, Saviano J. A, King G. J, Keeling S. D. Histomorphometric study of dental pulp during orthodontic tooth movement. J Endod. 1993;19:13–16. doi: 10.1016/S0099-2399(06)81034-4. [DOI] [PubMed] [Google Scholar]
- 32.Derringer K. A, Jaggers D. C, Linden R. W. Angiogenesis in human dental pulp following orthodontic tooth movement. J Dent Res. 1996;75:1761–1766. doi: 10.1177/00220345960750100901. [DOI] [PubMed] [Google Scholar]
- 33.Evans D, Reid J, Strang R, Stirrups D. A comparison of laser Doppler flowmetry with other methods of assessing the vitality of traumatized anterior teeth. Endod Dent Traumatol. 1999;15:284–290. doi: 10.1111/j.1600-9657.1999.tb00789.x. [DOI] [PubMed] [Google Scholar]
- 34.Roeykens H, Van Maele G, Martens L, De Moor R. A two-probe laser Doppler flowmetry assessment as an exclusive diagnostic device in a long-term follow-up of traumatized teeth: a case report. Dent Traumatol. 2002;18:86–91. doi: 10.1034/j.1600-9657.2002.180208.x. [DOI] [PubMed] [Google Scholar]
- 35.Oztürk M, Doruk C, Ozeç I, Polat S, Babacan H, Biçakci A. A. Pulpal blood flow: effects of corticotomy and midline osteotomy in surgically assisted rapid palatal expansion. J Craniomaxillofac Surg. 2003;31:97–100. doi: 10.1016/s1010-5182(02)00188-9. [DOI] [PubMed] [Google Scholar]
- 36.Norer B, Kranewitter R, Emshoff R. Pulpal blood-flow characteristics of maxillary tooth morphotypes as assessed with laser Doppler flowmetry. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;87:88–92. doi: 10.1016/s1079-2104(99)70301-x. [DOI] [PubMed] [Google Scholar]