Abstract
Low-level alcohol consumption is commonly perceived as being inconsequential or even beneficial for overall health, with some reports suggesting that it may protect against dementia or cardiovascular risks. However, these potential benefits do not preclude the concurrent possibility of negative health outcomes related to alcohol consumption. To examine whether casual, non-heavy drinking is associated with premature brain aging, we utilized the Brain-Age Regression Analysis and Computational Utility Software package to predict brain age in a community sample of adults [n = 240, mean age 35.1 (±10.7) years, 4 8% male, 4 9% African American]. Accelerated brain aging was operationalized as the difference between predicted and chronological age (“brain age gap”). Multiple regression analysis revealed a significant association between previous 90-day alcohol consumption and brain age gap (β = 0.014, p = 0.023). We replicated these results in an independent cohort [n = 231 adults, mean age 34.3 (±11.1) years, 55% male, 28% African American: β = 0.014, p = 0.002]. Our results suggest that even low-level alcohol consumption is associated with premature brain aging. The clinical significance of these findings remains to be investigated.
Keywords: Brain age prediction, Alcohol, Community sample, accelerated aging
1. Introduction
Alcohol consumption is highly prevalent in the United States. According to the 2018 National Survey on Drug Use and Health, 87% of adults aged 21 years or older had consumed alcohol in their lifetime, with 71% of this age group reporting alcohol use in the past year (Substance Abuse and Mental Health Services Administration 2019). However, nationally representative surveys have estimated that only 12.6% of adults in the United States have engaged in “high-risk drinking” within the previous 12 months (defined as exceeding 3 standard drinks per day for women or 4 standard drinks per day for men at least once per week (Grant et al., 2017)). While it is well known that excessive alcohol consumption can lead to a variety of long-term health risks – including increased risk of breast cancer (Jung et al., 2016), liver disease (Bellentani et al., 1997), and hypertension (Fuchs et al., 2001) – less is known about risks associated with low or moderate levels of alcohol consumption. Given that the majority of adults in the United States fall into the latter category, it is important to investigate the effects of low-to-moderate alcohol consumption on the brain.
Current evidence suggests that, compared to non-drinkers and heavy drinkers, moderate alcohol consumption offers health benefits, including reduced risk of developing dementia (Orgogozo et al., 1997, Sabia et al., 2018) and cardiovascular disease (i.e., ischemic stroke and heart failure) (Bell et al., 2017). However, a recent meta-analysis has challenged these findings by considering the potential cardiovascular benefits of alcohol consumption in conjunction with the myriad negative health outcomes that are attributed to alcohol consumption, including tuberculosis, pancreatitis, cirrhosis, and cancers (GBD 2016 Alcohol Collaborators, 2018). Using data from 195 regions across the world collected from 1990–2016, the Global Burden of Disease (GBD) Alcohol Collaborators modelled the relative risk of several alcohol-related health consequences as a function of alcohol consumption levels (GBD 2016 Alcohol Collaborators, 2018). While there was evidence of a protective effect of low-level alcohol consumption on risk of ischemic heart disease, this protective effect was out-weighed by increased risk for other health conditions, particularly cancers including breast cancer and lip or oral cavity cancer (GBD 2016 Alcohol Collaborators, 2018). A weighted model of overall relative health risks associated with alcohol consumption indicated that the level of alcohol consumption that minimized overall risks to health was zero alcoholic drinks per day (GBD 2016 Alcohol Collaborators, 2018). Based on these results, the GBD Alcohol Collaborators emphasized that any level of alcohol use increases the risk of negative health consequences. In light of these striking results and the prevalence of low-to-moderate alcohol consumers in the United States, additional investigation of the effects of low-to-moderate alcohol use is warranted.
Many of the negative consequences of drinking are captured by the premature aging hypothesis, which posits that alcohol use accelerates or exaggerates natural aging processes (Guggenmos et al., 2017, Noonberg et al., 1985). Within the neurocognitive domain, the effects of chronic, heavy alcohol consumption on brain structure have been well documented in preclinical models, clinical studies (reviewed in (Zahr and Pfefferbaum, 2017)), and postmortem investigations (Harper and Blumbergs, 1982, Skullerud, 1985). Notably, magnetic resonance imaging (MRI) studies have reported significantly reduced grey matter volumes (GMV) in the frontal, temporal, parietal, cingulate, and insular cortices (Sullivan et al., 2018) and white matter integrity in frontal, temporal, parietal, and cerebellar tracts (Fortier et al., 2014) among heavy alcohol users when compared to non-alcoholic controls. However, whether the effects seen in heavy, chronic use of alcohol are also present (perhaps to a lesser degree) among low-level alcohol consumers is unknown.
In relation to the premature aging hypothesis, neuroimaging studies have investigated the interaction of alcohol use and aging on brain morphology by comparing age-related grey matter changes among a healthy community sample without major psychiatric disorders to those among heavy drinkers. While GMV decreased with age among both healthy controls without alcohol use problems and heavy alcohol users, individuals with a history of heavy alcohol consumption had greater GMV reductions in regionally specific areas including the cerebellum (Zhao et al., 2020), amygdala and hippocampus (Tomasi et al., 2021, Zahr et al., 2019), frontal cortex, precentral gyrus, and superior frontal gyrus (Sullivan et al., 2018). These results suggest that heavy alcohol drinkers may experience accelerated brain aging.
Further evidence for the premature aging hypothesis has been provided by a comprehensive study by Guggenmos et al. (Guggenmos et al., 2017). Using structural MRI data, the authors compared the grey matter changes seen in Alcohol Use Disorder (AUD) to the changes observed in normal aging among individuals without alcohol use problems. The widespread grey matter loss associated with aging (controlling for average yearly alcohol intake) was significantly, positively correlated with AUD-related grey matter loss (controlling for age), lending further evidence to the hypothesis that heavy alcohol use is associated with an acceleration of the typical brain aging process. To determine the extent of the observed acceleration of aging, Guggenmos et al. developed a “brain age” prediction algorithm. Brain age prediction models provide an individualized imaging-based biomarker for brain health (Franke et al., 2010, Wang and Pham, 2011). The brain age gap - the difference between the brain age prediction and chronological age - can be used to quantify accelerated or delayed brain aging at the individual level. Among heavy drinkers, the brain age was an average of 4 years greater than the chronological age. In a final step, Guggenmos et al. assessed the relationship between lifetime alcohol consumption and the brain age gap among heavy drinkers and found that the brain age gap was positively associated with lifetime alcohol consumption. Building on these findings, we aimed to investigate whether a similar association exists between the brain age gap and a continuous measure of alcohol consumption among casual drinkers.
The applications of brain age models are widespread. To date, accelerated brain aging has been reported among individuals with various neuropsychiatric disorders, including schizophrenia (Kaufmann et al., 2019, Koutsouleris et al., 2014, Nenadic et al., 2017, Schnack et al., 2016, Shahab et al., 2019), major depression (Koutsouleris et al., 2014), borderline personality disorder (Gaser et al., 2013, Koutsouleris et al., 2014), multiple sclerosis (Kaufmann et al., 2019), mild cognitive impairment (Gaser et al., 2013, Kaufmann et al., 2019, Koutsouleris et al., 2014, Liem et al., 2017), dementia (Kaufmann et al., 2019), Alzheimer’s disease (Gaser et al., 2013), and cigarette smoking (Ning et al., 2020). Associations between brain age predictions and various life factors have also been reported, including years of education (Steffener et al., 2016), physical activity (Steffener et al., 2016), menstrual cycle stages (Franke et al., 2015), postpartum stage (Luders et al., 2018), and negative fateful life events (Hatton et al., 2018). Among these studies, only Hatton et al. (Hatton et al., 2018) reported that alcohol was a significant covariate in their main analysis, however their cohort only included older men and thus is unlikely to generalize to the general population.
Smaller cohort studies can benefit from pre-existing, publicly available brain age prediction software. One such software is the Brain-Age Regression Analysis and Computation Utility Software (BARACUS) (Liem and Gorgolewski, 2017, Liem et al., 2017). This software utilizes measures of cortical thickness, cortical surface area, and subcortical volumes derived from structural MRI to predict brain age and has been shown to generalize to novel cohorts (Liem et al., 2017).
As such, we utilized BARACUS to investigate the relationship between the brain age gap and alcohol consumption patterns among a community sample to provide a better understanding of the neuroanatomical consequences of casual alcohol consumption. Our cohort consisted of 713 individuals from 2 independent samples which were a priori divided into a discovery sample (n = 313) and a replication sample (n = 400) prior to analyses. We hypothesized that there would be a direct association between alcohol consumption amount and the brain age gap.
2. Methods
2.1. Participants
Participants from a community sample (n = 313) were enrolled under several protocols approved by the Institutional Review Board of the National Institute on Drug Abuse Intramural Research Program (NIDA-IRP) and served as our discovery cohort (see section 2.6 below for details of the replication cohort). All participants provided written informed consent and were assessed with a comprehensive history and physical exam, general urine and blood laboratory panels, a Structured Clinical Interview for DSM-IV or DSM-5, and the Timeline Follow Back (TLFB) (Sobell and Sobell, 1992) to assess lifetime drug use. Left-handed subjects, those contraindicated for MRI scanning, individuals with current drug dependence other than nicotine, and individuals with cognitive impairment, learning disability or any major medical or psychiatric conditions were excluded as were those with missing data for years of education (n = 3), TLFB reported lifetime alcohol consumption exceeding 100,000 drinks (n = 3), and no alcohol consumption during the 90-day period preceding the MRI session (n = 67), resulting in a final sample of 240 adults (Supplementary Fig. S1).
We excluded left-handed subjects because these samples were convenience samples comprised of data from functional studies which routinely exclude left-handed participants. Handedness reflects hemispheric asymmetry in motor control (Amunts et al., 1996), which could be associated with structural asymmetries between right- and left-handed individuals. Indeed, subtle anatomical differences in cortical and subcortical structures have been reported in several (Foundas et al., 1998, Foundas et al., 1995, Powell et al., 2012, Szabo et al., 2001), but not all studies (Guadalupe et al., 2014, Herve et al., 2005). As such, including left-handed subjects could risk confounding the results.
We did not exclude nicotine smokers because the combined use of alcohol and nicotine is prevalent with approximately 22% of the general population using both substances (Falk et al., 2006) and over 40% of individuals with alcohol dependence reporting nicotine use (Falk et al., 2006, Hitschfeld et al., 2015). However, in consideration of the potential effects of cigarette use on the brain age estimates, we repeated the entire analysis after excluding smokers.
2.2. Drug use history
Although history of all drug use is captured in the TLFB, we focused only on participants’ history of alcohol use. We limited the scope of the alcohol use variables to the most recent 90-day period leading up to the date of the MRI scan as a proxy for lifetime use. Restricting the time frame to 90 days allowed us to evaluate the effects of recent drinking patterns and to limit the effects of recall bias on reports of past substance use (Perrine and Schroder, 2005, Searles et al., 2002). We used the TLFB responses to derive three alcohol use variables to characterize participants’ alcohol consumption during the most recent 90-days: total number of alcoholic drinks consumed, total number of drinking days, and average number of drinks consumed per drinking day.
2.3. MRI data acquisition
Structural MRI data were collected at the NIDA-IRP on either a Siemens Tim Trio 3T MRI scanner (n = 207; Erlangen, Germany) or a Siemens Prisma 3T MRI scanner (n = 33; Erlangen, Germany) equipped with phased array head coils [Trio: 32 channels (n = 179), or 12 channels (n = 28); Prisma: 32 channels (n = 20), or 20 channels (n = 13)]. High-resolution anatomical images were acquired using a three-dimensional (3D) magnetization prepared rapid gradient-echo (MPRAGE) T1-weighted sequence in 1mm3 isotropic voxels (Trio: TR=1,900 ms, TE=3.51 ms, flip angle=9°; Prisma: TR=1,900 ms, TE=3.42 ms, flip angle=9°).
2.4. Brain age predictions
The Brain-Age Regression Analysis and Computational Utility Software (BARACUS; https://github.com/BIDS-Apps/baracus (Liem and Gorgolewski, 2017)) was used to generate each participant’s brain age prediction. The software requires a T1-weighted structural MRI to compute the brain age prediction. In the first step, the software processes the structural MRI using FreeSurfer version 5.3.0. The FreeSurfer output is then used to generate the brain age prediction, with several model options. The prediction model was trained on the BARACUS database (default model: Liem2016__OCI_norm). We used the “stacked-anatomy” prediction, which has been shown to be the most accurate model from this software using only structural data as input (Liem et al., 2017). The input for the “stacked-anatomy” prediction model consists of 3 types of neuroimaging data: cortical thickness (5,124 features), cortical surface area (5,124 features), and subcortical volumes (66 features). For details on the brain age prediction model, readers should refer to Liem et al. (Liem et al., 2017), as a thorough discussion of the model is beyond the scope of this paper. The training cohort had a mean absolute error of 4.83 (± 4.01) years (Liem et al., 2017). While Liem et al do not comment on the relationship between chronological age and brain age prediction error, figure 3 in their paper illustrates a tendency for the brain age to be overestimated for younger participants and underestimated for older participants. Of note, previous literature has consistently demonstrated an age-related bias in brain age predictions (Liang et al., 2019, Cole et al., 2017) such that the predicted age is overestimated for younger individuals and underestimated for older individuals. To account for this bias, we followed the model used by Kaufmann et al and included age and age-squared as covariates in all regression models (Kaufmann et al., 2019). Scans were processed using the computational resources of the NIH High Performance Computing (HPC) Biowulf cluster (http://hpc.nih.gov).
2.5. Characterization measures
Participants’ education levels were assessed via self-report of total years of education, while IQ was assessed by the Wechsler Abbreviated Scale of Intelligence Full-Scale IQ-4 (WASI; (McCrimmon and Smith, 2013)). The WASI estimates intelligence among individuals aged 6 to 90 years old and is based on performance on four subtests that measure either verbal comprehension or perceptual reasoning. The WASI is normed to have an average score of 100.
Psychological measures of anxiety, depression, and childhood trauma were also collected. Anxiety symptoms were assessed using the Beck Anxiety Inventory (BAI; (Beck et al., 1988)). Scores on the BAI range from 0–63, with higher scores indicating more severe anxiety. Scores below 9 indicated normative levels of anxiety (Julian, 2011). The Beck Depression Inventory (BDI-II; (Beck et al., 1961)) was used to measure depression symptoms. Scores on the BDI-II range from 0–63, with higher scores indicating more severe depression. Scores less than 13 indicate minimal depression symptomology. The Childhood Trauma Questionnaire (CTQ; (Bernstein et al., 1994)) consists of 5 trauma subscales: emotional neglect, emotional abuse, physical neglect, physical abuse, and sexual abuse. The scores for each subscale range from 5–25 and lower scores indicate less exposure to the respective type of trauma.
The Fagerstrom Test for Nicotine Dependence (FTND; (Heatherton et al., 1991)) was used to characterize nicotine dependence in those participants who reported a history of nicotine use. Scores on the FTND have a possible range of 0–10, with a score of 0 indicating no nicotine dependence, scores less than 4 indicating low dependence, and scores above 7 indicating high dependence.
2.6. Replication cohort
An independent cohort (n = 400) collected at the Clinical NeuroImaging Research Core (CNIRC) at the National Institute on Alcohol Abuse and Alcoholism, Division of Intramural Clinical and Biological Research (NIAAA-DICBR) served as a replication sample. Similar to the discovery cohort, individuals in this sample completed an assessment of their alcohol consumption during the 90-day period prior to their MRI acquisition. Structural MRI data were collected on a Siemens 3T Skyra MRI scanner (Erlangen, Germany) at the NIH Clinical Center in Bethesda, MD. The structural scan was acquired using a T1-MPRAGE sequence (TR = 1,900 ms, TE=3.09 ms, flip angle=10°) using a 20-channel head coil. Psychological measures of anxiety, depression, and childhood trauma were not available for this cohort.
2.7. Statistical analyses
The brain age gap was calculated by subtracting the chronological age from the predicted age derived from BARACUS. Further analyses were conducted in R (version 3.6.2; (R Core Team 2019)). To ensure generalizability of BARACUS to our cohort, we conducted a Pearson correlation analysis comparing the predicted brain age to the chronological age. Previous groups have reported effects of race on the brain age predictions (Hatton et al., 2018). To assess for such an effect, we conducted a one-way ANOVA to compare the brain age gap between self-reported races. We also conducted a Welch two-tailed t-test to assess for sex differences in the brain age gap. Results were considered significant at p <0.05. Clinical characterization and alcohol use measures are shown as mean (±SD).
The relationship between the brain age gap and the total number of drinks consumed in 90 days was assessed using linear regression via the lm function in the stats package for R (R Core Team 2019). We included age, age squared, Euler number (a representation of the quality of the Freesurfer cortical reconstruction) and sex as covariates for the regression models. Additional covariates included years of education, race, and the scanner used for MRI acquisition. Finally, we investigated the unique effect of alcohol on the brain age gap by repeating the regression analysis without nicotine users.
3. Results
3.1. Clinical characterization
The discovery community sample of 240 adults had a mean (±SD) age of 35.1 (±10.7) years (range 18 t-test 63 years, 48% male, 49% African American). In general, participants had relatively low scores on all psychological measures (see Table 1). The average rating of anxiety symptomology was 1.8 (±3.7), corresponding to normal levels of anxiety. Similarly, ratings of depression symptoms indicated minimal depression severity with an average of 3.0 (±4.3). The average scores on the CTQ indicated minimal to low levels of trauma on each subscale. Approximately 43% of study participants (n = 104) were nicotine users with a mean FTND (n = 87, FTND scores missing for n = 17 nicotine users) of 4.2 (±2.1), suggesting a low to moderate level of nicotine dependence severity.
Table 1.
Clinical characterization of the discovery cohort
| n | % | |
|---|---|---|
| Sex (M/F) | 116 / 124 | 48.3 / 51.7 |
| Race (African American/Caucasian/Other) | 118 / 95 / 27 | 49.2 / 39.6 / 11.3 |
| Ethnicity (Not Hispanic/Hispanic/Unknown) | 222 / 16 / 2 | 92.5 / 6.7 / 0.8 |
| Smoking Status (Non-smoker/Smoker) | 136 / 104 | 56.7 / 43.3 |
| Mean ± SD | Min - Max | |
| Brain-Age Gap | 6.1 ± 7.9 | −14.2 – 32.7 |
| Age | 35.1 ± 10.7 | 18 – 63 |
| Ys of Education | 14.6 ± 2.7 | 8 – 25 |
| WASI Full-4 IQ (n = 217) | 104.8 ± 13.0 | 76 – 139 |
| BDI-II Score (n = 230) | 3.0 ± 4.3 | 0 – 23 |
| BAI Score (n = 233) | 1.8 ± 3.7 | 0 – 40 |
| CTQ Emotional Abuse Score (n = 237) | 7.5 ± 4.2 | 5 – 25 |
| CTQ Emotional Neglect Score (n = 237) | 9.2 ± 4.8 | 5 – 25 |
| CTQ Physical Abuse Score (n = 237) | 7.0 ± 2.9 | 5 – 22 |
| CTQ Physical Neglect Score (n = 237) | 6.8 ± 3.1 | 5 – 25 |
| CTQ Sexual Abuse Score (n = 237) | 5.9 ± 2.7 | 5 – 22 |
| 90-Day Alcohol Use | ||
| Total Drinks | 44.7 ± 71.9 | 0.1 – 455 |
| Days of Use | 14.0 ± 18.4 | 1 – 90 |
| Average Drinks per Day of Use | 2.6 ± 2.2 | 0.1 – 24 |
| FTND Scorea (n = 87) | 4.2 ± 2.1 | 0 – 8 |
Participants were not excluded due to missing records of psychological measures. Counts are listed for each measure; n = 240 unless otherwise noted. Abbreviations: WASI, Wechsler Abbreviated Scale of Intelligence; BDI-II, Beck Depression Inventory; BAI, Beck Anxiety Inventory;FTND, Fagerstrom Test for Nicotine Dependence;CTQ, Childhood Trauma Questionnaire
FTND scores are shown for cigarette users only; FTND scores were missing for some cigarette users (n = 17)
3.2. Alcohol consumption
Participants were screened for either Alcohol Dependence (DSM-IV) or AUD (DSM-5); none met diagnostic criteria for a current alcohol use problem. Participants consumed an average of 44.7 (±71.9) alcoholic beverages in the 90-day TLFB period with a range between 0.1 and 455. The average number of drinking days was 14.0 (±18.4) days, corresponding to an alcohol consumption frequency of roughly 1 drinking day per week. Overall, participants consumed an average of 2.6(±2.2) alcoholic drinks per drinking day. More details on alcohol consumption can be found in (Table 1).
3.3. Brain age predictions
Participants’ chronological age and brain age predictions from BARACUS were strongly correlated (r = 0.70, R2=0.49, p < 0.0 0 01; Fig. 1), with a mean absolute error of 8.19 years in that model. The average brain age gap was 6.1 (±7.9) years. Females had a significantly greater brain age gap of 7.2 years compared to males (males= 4.9 years, t=−2.27, df=238, p=0.024). Additionally, we found that the brain age gap did not differ by self-reported race (F(2,237)=2.36, p=0.096).
Fig. 1. Correlation between chronological age and predicted brain age.
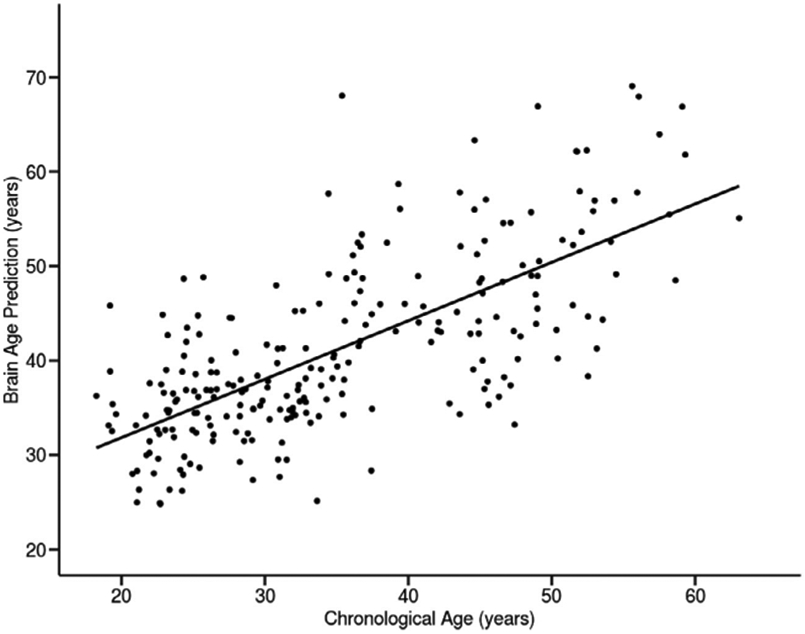
Fig. 1. Significant correlation between participants’ chronological ages and brain age predictions (n = 240, r = 0.70, R2 = 0.49, p < 0.0001).
3.4. Effects of alcohol consumption on the brain age gap
The total number of drinks consumed, as assessed by the TLFB, showed a significant, positive association with the brain age gap (β=0.014, p=0.023, partial R2=0.015; Fig. 2; Supplementary Fig. S3). The regression model indicated that the brain age gap increases by 0.014 years for each alcoholic drink consumed; this increase equates to an acceleration of the brain age gap by approximately 5 days per drink consumed in the previous 90-day period. Additionally, the regression model indicated a significant main effect of self-reported race (reference level: African American, Caucasian: β = −3.73, p<0.001; other race: β = −2.94, p=0.049). An exploratory analysis indicated that there was no significant race-by-drinks interaction (Caucasian interaction term: p=0.13; other race interaction term: p=0.8), and the main effect of drinks remained significant (β= 0.023, p=0.013). Further, the addition of the race*drinks interaction term did not improve the model (ANOVA p=0.31).
Fig. 2. Association between total number of drinks consumed over 90 days and the brain age gap in the discovery sample.
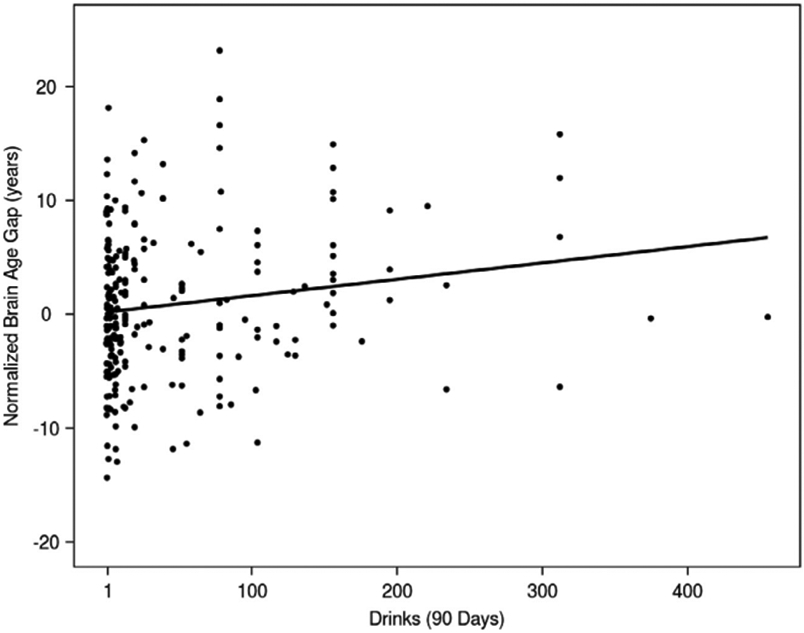
Fig. 2. Normalized brain age gap and its association with the number of drinks consumed in the previous 90-day assessment period in the discovery sample (n = 240). The normalized brain age gap was calculated by subtracting the mean brain age gap from each individual brain age gap. Therefore, a normalized brain age gap equal to zero indicates no difference from the mean brain age gap. The linear regression model predicting brain age gap showed a significant, positive association between the total number of drinks consumed in the 90-day period and the brain age gap (β = 0.014, p = 0.023, partial R2 = 0.015). The variability in the brain age gap indicates that alcohol consumption plays a small, but significant, role in accelerated brain age predictions.
Next, we examined whether concurrent nicotine use could have confounded the results. We repeated the regression analysis including only non-smokers (n = 136). We found that the relationship between the number of alcoholic drinks consumed in the 90-day period and the brain age gap remained significant (β=0.039, p=0.020), suggesting that the observed association between alcohol consumption and the brain age gap cannot be fully attributed to the previously reported association between smoking and the brain age gap (Ning et al., 2020). We further explored the role of nicotine use in the relationship between alcohol consumption and the brain age gap by testing for an interaction between the 90-day alcohol consumption and nicotine use status. The interaction model revealed a significant effect of 90-day alcohol consumption (β=0.043, p=0.01), while the interaction between alcohol consumption and nicotine use status did not reach significance (β=−0.036, p=0.052).
Finally, to evaluate the potential effect of outliers in our original regression model (n = 240), we repeated the analysis using robust regressions (Imrob with MM-estimation from R package robustbase (Maechler et al., 2020)). The robust regression assessing the relationship between the brain age gap and the TLFB assessed number of drinks consumed yielded a similar regression coefficient to the previous model but failed to reach significance (β=0.013, p=0.08), suggesting that the original regression model was partially influenced by outliers, which may be due to the limited number of participants who consumed a high number of alcoholic drinks (greater than 150 drinks, n = 25). However, the replication cohort confirmed our initial findings without the effect of outliers (see section 3.6.4 below).
3.5. Replication sample
3.5.1. Clinical characterization
To best match this cohort to the discovery sample, participants from the replication cohort were excluded based on the following criteria: 90-day drink total greater than 460 drinks (n = 141), no alcohol consumption during 90-day period (n = 22), age greater than 65 years old (n = 1), or years of education less than 8 or greater than 30 years (n = 5). The resulting replication cohort consisted of a community sample of 231 adults (age range 21 – 65 years, mean age 34.3 (±11.1) years, 55% male, 28% African American; see Table 2). Among the 11% of this cohort who were smokers (n = 26), the average FTND score indicated mild nicotine dependence (3.1 ±2.2).
Table 2.
Clinical characterization of the replication cohort
| n | % | |
|---|---|---|
| Alcohol Use Disorder Lifetime Diagnosis (Y/N) | 83 / 148 | 35.9 / 64.1 |
| Sex (M/F) | 126 / 105 | 54.5 / 45.5 |
| Race (African American/Caucasian/Other) | 65 / 126 / 4540 | 28.1 / 54.5 / 17.3 |
| Ethnicity (Not Hispanic/Hispanic) | 204 / 27 | 88.3 / 11.7 |
| Smoking Status (Non-smoker/Smoker) | 205 / 26 | 88.7 / 11.3 |
| Mean ± SD | Min - Max | |
| Brain-Age Gap | 10.2 ± 9.0 | −10.9 – 42.7 |
| Age | 34.3 ± 11.1 | 21 – 65 |
| Years of Education | 15.8 ± 2.6 | 9 – 26 |
| WASI Full-4 IQ (n = 132) | 109.9 ± 15.3 | 78 – 141 |
| 90-Day Alcohol Use | ||
| Total Drinks | 112.3 ± 123.8 | 1 – 459 |
| Days of Use | 27.9 ± 21.4 | 1 – 90 |
| Average Drinks per Day of Use | 3.6 ± 3.5 | 1 – 32 |
| FTND Scorea (n = 26) | 3.1 ± 2.2 | 0 – 7.0 |
Participants (n = 253) were not excluded due to missing records of the WASI. Abbreviations: WASI, Wechsler Abbreviated Scale of Intelligence;FTND, Fagerstrom Test for Nicotine Dependence
FTND scores are shown for smokers only (n = 26)
3.5.2. Alcohol consumption
Participants in the replication cohort consumed an average of 112.3 (±123.8) alcoholic drinks in the assessed 90-day TLFB period. Participants reported consuming alcohol on an average of 27.9 (±21.4) days, or approximately 2 days per week. On average, participants consumed 3.6 (±3.5) alcoholic drinks per drinking day. Among this sample, 36% (n = 83) met criteria for a lifetime diagnosis of alcohol dependence or AUD (DSM-IV or DSM-5), and 29% (n = 68) met criteria for a current diagnosis of AUD. We opted not to exclude these participants from our analyses because their 90-day TLFB alcohol consumption amounts were similar to the discovery cohort and we suspect that the effects of alcohol on brain aging will be related to the amount of alcohol consumption rather than diagnostic status. Further details on alcohol consumption can be found in (Table 2).
3.5.3. Brain age predictions
There was a strong, positive correlation between the chronological brain age and the brain age prediction from BARACUS (r=0.68, R2=0.46, p<0.0001; Supplementary Fig. S2), with a mean absolute error of 11.1 years in that model. The average brain age gap was 10.2 (±9.0) years. Similar to the discovery cohort, there was no significant difference in the brain age gap by self-reported race (F(2,228)=1.48, p=0.23). Contrary to our findings in the discovery cohort, we found that the brain age gap did not differ by sex (males=11.1 years, females=9.1 years, t=1.64, df=227.86, p=0.10).
3.5.4. Effects of alcohol consumption on the brain age gap
The regression analysis revealed a significant, positive association between the number of alcoholic drinks consumed in the 90-day TLFB period and the brain age gap (β=0.014, p=0.002, partial R2=0.034; Fig. 3, Supplementary Fig. S4). These results suggest that the brain age gap increases by 0.014 years for each alcoholic drink consumed in the 90-day period, corresponding to approximately 5 days of brain aging for each drink. Similar to our findings in the discovery cohort, this association remained significant after removing smokers from the model (β=0.011, p=0.029). An exploratory analysis assessing the potential interaction of nicotine use and alcohol consumption further indicated a significant association between the amount of alcohol consumed in the 90-day period and the brain age gap (β=0.011, p=0.025) but no significant interaction between nicotine use status and alcohol consumption (β=0.019, p=0.17). Finally, to assess for an effect of outliers in the initial regression model, we conducted a robust regression, which produced results similar to the initial regression model (β=0.015, p=0.005, partial R2=0.032), suggesting that outliers are not driving model significance.
Fig. 3. Association between total number of drinks consumed over 90 days and the brain age gap in the replication cohort.
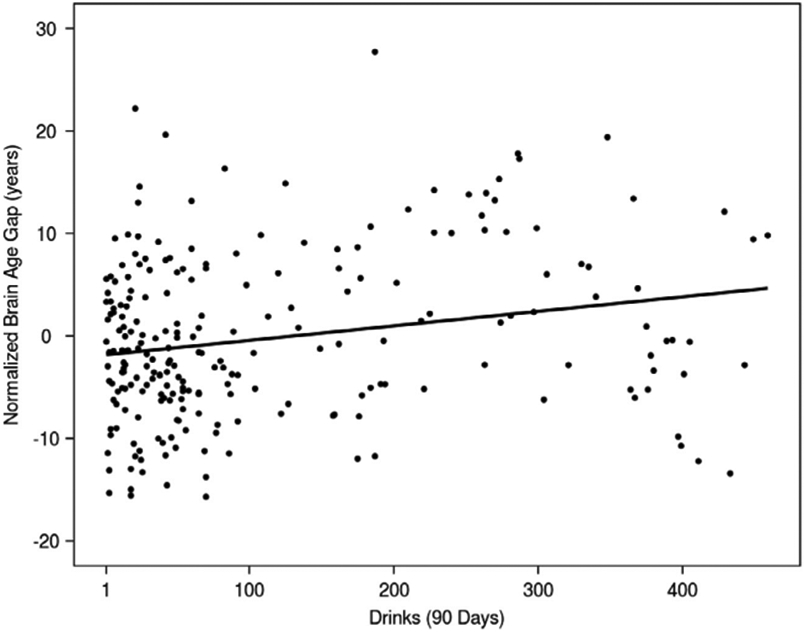
Fig. 3. Normalized brain age gap and its association with the number of drinks consumed in the previous 90-day assessment period in the replication cohort. The normalized brain age gap was calculated by subtracting the mean brain age gap from each individual brain age gap. Therefore, a normalized brain age gap equal to zero indicates no difference from the mean brain age gap. Similar to the discovery sample, the linear regression model predicting brain age gap showed a significant, positive association between the total number of drinks consumed in the previous 90-day period and the brain age gap (β = 0.014, p = 0.002, partial R2 = 0.034). The variability in the brain age gap indicates that alcohol consumption plays a small, but significant, role in accelerated brain age predictions.
3.5.5. Potential effect of different scanners/coils for data acquisition on the results
Our study utilized data from several previously completed experiments, which used different scanners and a variety of head coils for MRI acquisition. To verify that the coil used for image acquisition did not impact our results, we conducted several follow up analyses. Given that our reported analysis included the MRI scanner as a covariate in the regression model, we repeated the analysis without any variables representing the equipment used for imaging. We then compared this model to our reported regression model using ANOVA; the ANOVA indicated that the inclusion of the scanner variable does not improve the regression model (p=0.58). Next, we added a factor to represent head coils to the regression model (without the scanner variable); this model also included interaction terms for coil*(amount of alcohol consumed). The results of this analysis (β = 0.015, p = 0.048) were similar to the reported regression model and indicated no main effect of head coil on the brain age gap. Finally, we compared models with and without the coil factors and found no statistical preference for the more complex (i.e. with coil) model; F(6,231)=0.95, p=0.46.
4. Discussion
In this study, we provide initial evidence of a significant association between light alcohol consumption – roughly 2 drinks per week on average – and accelerated brain aging as measured by the brain age gap. Specifically, our results identified that the brain age gap in an adult community sample increases by approximately 5 days for each alcoholic drink consumed in the most recent 90-day period. Notably, we replicated these findings in an independent cohort of individuals also reporting relatively low levels (but higher than the discovery cohort) of regular alcohol consumption, with an average alcohol consumption of approximately 7 drinks per week. These intriguing results build upon the observations made by Guggenmos et al. (Guggenmos et al., 2017) by revealing, for the first time to our knowledge, that the dose-dependent acceleration of brain aging originally noted for high alcohol use extends to low levels of alcohol consumption.
Additionally, given the prevalence of concurrent alcohol and nicotine use (Falk et al., 2006, Hitschfeld et al., 2015), we repeated our analyses while excluding smokers and found that the alcohol-brain age relationship was maintained, suggesting that the previously reported smoking-related acceleration of brain aging (Ning et al., 2020) does not fully account for the observed alcohol-related brain aging acceleration. These findings do not suggest that smoking had no effect on brain aging. Preclinical models show that chronic exposure to cigarette smoke induces synaptic changes and other neuropathological alterations reminiscent of changes that take place during aging (Ho et al., 2012). Along the same lines, Ning et al. (Ning et al., 2020) found that frequent cigarette use was significantly associated with accelerated brain aging. Among the regular smokers in that cohort, Ning et al. also found a positive association between accelerated brain aging and alcohol consumption amount. Of note, their analysis could not fully separate the effects of smoking and alcohol consumption on accelerated brain aging as all individuals in their sample were frequent cigarette users. In contrast, the current cohort included both smokers and nonsmokers, allowing us to isolate the unique effects of alcohol consumption on the brain age gap independent of nicotine use.
While these findings indicate that the effect of alcohol can be distinguished from that of cigarettes, the interactive effect of concomitant nicotine smoking and alcohol consumption on brain aging process is not necessarily additive. In the discovery cohort, an interaction model assessing the effects of concurrent nicotine and alcohol use revealed a larger effect of alcohol consumption on the brain age gap among nonsmokers compared to smokers; the interaction itself trended toward significance (p=0.052). Similarly, preclinical models have demonstrated that concurrent exposure to alcohol and nicotine significantly reduced the total number of cerebellar Purkinje cells in rats (Sarna and Hawkes, 2003). However, this combined exposure to alcohol and nicotine did not lead to an additive effect in depleting Purkinje cells compared with the severity of cell loss after either alcohol or nicotine exposure alone. The underlying mechanisms behind this unexpected interaction is not entirely clear (Chen and Harle, 2005).
Our results, together with those from Guggenmos et al. (Guggenmos et al., 2017) and Ning et al. (Ning et al., 2020), suggest a dose-dependent association between alcohol consumption (at least as reflected by the most recent 90-day period) and an acceleration of brain aging among four independent cohorts. Furthermore, our results specifically suggest that even low levels of alcohol consumption may have a negative impact on the brain and are consistent with the known negative impact of heavy alcohol consumption on the brain (reviewed in (Abrahao et al., 2017)). This striking observation coincides with a recent large-scale epidemiological study (GBD 2016 Alcohol Collaborators, 2018) showing that the level of alcohol consumption that minimizes overall health risks was zero alcoholic drinks per day.
It should be noted that the clinical significance of the observed acceleration of brain aging associated with alcohol use were not tested and our findings should not be over-interpreted. Specifically, our study did not include measures of cognitive ability and cannot speak to whether the observed acceleration of brain aging is associated with any measurable functional consequences. While previous studies have investigated the relationship between brain aging and cognitive impairment (Liem et al., 2017), we did not explore potential mediating effects of alcohol use on the relationship between brain aging and cognitive outcomes and this topic should be addressed in the future. Further, the persistence of the observed alcohol-related acceleration of brain aging is unclear. Previous research has found that the grey matter volume changes seen in chronic alcohol abuse begin to partially recover within the first 2 weeks of abstinence (van Eijk et al., 2013), and brain atrophy in alcohol dependence may recover in direct relation to the length of sobriety (Sawyer et al., 2017). Thus, it is possible that the observed effect of alcohol consumption on the brain age gap is transient and reversible if the individual ceases alcohol use. The cross-sectional nature of our study does not provide an answer to this relevant question and further longitudinal studies are needed.
It should be reiterated that we only assessed relatively recent alcohol consumption over a previous 90-day period, as accuracy of self-reports in this time frame may be more reliable than reports of lifetime usage. Based on the age of our cohorts and general drinking patterns, we infer that their drinking was relatively stable beyond this time window, although that too was explicitly not tested herein. It is possible that the use of a relatively brief time period resulted in an inflated observation of the relationship between alcohol consumption and the brain age gap. While our analyses indicated that the brain age gap increased by about 5 days per alcoholic drink consumed in the 90-day period, the aforementioned study by Guggenmos et al. (Guggenmos et al., 2017) reported that the brain age increased by half a day for every 71 standard alcoholic drinks consumed in the lifetime. Thus, our assessment of alcohol consumption throughout a 90-day period may indeed reflect the cumulative effects of a general drinking pattern rather than the amount of alcohol consumed during the specifically assessed time frame. This observation suggests that the role of alcohol use recency as it relates to the effect of alcohol consumption on the brain age gap requires further exploration. Given that the brain age gap reflects the cumulative lifetime acceleration of brain aging, a pattern of consuming one alcoholic drink every 90 days may correspond to a total lifetime brain age acceleration of 5 days, rather than each drink in the lifetime corresponding to an additional 5 days of brain aging. Therefore, the observed aging acceleration of 5 days per drink could be an overestimation of the effect of alcohol on the brain age gap.
Moreover, in the replication cohort, we included all individuals whose 90-day alcohol consumption was within the range of alcohol consumption found in the discovery sample, regardless of AUD diagnosis. Given that the DSM-5 criteria for AUD do not consider the amount of alcohol consumed, it is possible for someone with relatively low/moderate levels of alcohol consumption to be diagnosed with AUD based on behavioral symptoms. In fact, some participants in the discovery cohort who do not have an AUD diagnosis actually reported greater alcohol consumption than some participants in the replication cohort who had a positive AUD diagnosis. In the replication cohort, individuals with AUD reported a minimum of 4 drinks in the assessed 90-day period.
It is also important to note that alcohol consumption is not the only factor that impacts the brain age gap. In our cohorts, the variation in the brain age gap that can be explained by the amount of alcohol consumed in 90 days is small (discovery cohort partial R2=0.015, replication cohort partial R2=0.034). Similar results were reported in the aforementioned study by Ning et al. (R2= 0.015 (Ning et al., 2020)). Therefore, it is clear that alcohol use alone cannot explain the error in the brain age predictions. It is known that brain age can be affected by several additional factors such as traumatic brain injury (Green et al., 2014, Cole et al., 2015), which is prevalent among individuals with AUD (Chen et al., 2012) and may compromise our ability to determine the effects of alcohol on brain aging. However, in this study we excluded participants with head injury associated with loss of consciousness or followed by post-concussion sequelae. Similarly, negative life events could impact the brain’s aging process (Hatton et al., 2018). It is difficult to account for such a variable. However, the childhood trauma questionnaire indicated that participants in the discovery cohort had minimal to low levels of trauma on average.
Additionally, in the discovery cohort only, we observed sex difference in brain age prediction where females had significantly greater brain age gap compared to males (7.2 vs. 4.9 years, p=0.024). It is known that hormonal changes associated with menstruation and postpartum stages may impact brain age predictions (Franke et al., 2015, Luders et al., 2018). We did not explore the sex*drinking interaction. However, few studies have found sex differences in heavy drinking samples suggesting that the female brain is more sensitive to alcohol-related deterioration, although findings in this regard have been mixed (Pfefferbaum et al., 2009, Monnig et al., 2015, Hommer et al., 2001, Hommer et al., 1996) and the concept of female-specific vulnerability to medical complications of alcohol drinking (Hernandez-Avila et al., 2004) has been recently challenged (Keyes et al., 2010). One study reported that female brain aging process is less accelerated compared to males due to more efficient glucose uptake as observed by FDG PET (Beheshti et al., 2021). On the other hand, more advanced brain aging in females with family history of Alzheimer’s disease has been recently reported (Subramaniapillai et al., 2021). Similarly, males, but not females, with higher BMI (Chin Fatt et al., 2021) and less physical activity (Bittner et al., 2021), seem to have greater brain aging. These conflicting reports support the need for more research on the effect of social drinking on brain aging processes in both males and females.
Further limitations may be associated with the brain age prediction model itself. As previously mentioned, extant literature involving brain age predictions has shown a consistent, age-related bias in brain age predictions (Liang et al., 2019, Cole et al., 2017). We accounted for this bias by including age and age-squared as covariates in our regression models (Kaufmann et al., 2019). Additionally, the brain age predictions may be improved by a multimodal approach, as indicated by the model comparisons in Liem et al. (Liem et al., 2017). However, this study was limited in that the BARACUS software accepts only structural MRI as input. Along the same lines, the BARACUS software accepts input only from an older version of the FreeSurfer software (5.3.0), and it is possible that newer versions may provide more accurate measurements for brain age estimation. Further investigation of the relationship between the brain age and low-level alcohol consumption should utilize a multimodal approach when possible. Finally, a mechanistic limitation of this study is the inability to identify specific brain regions, circuits, or networks implicated in the association of alcohol consumption and the brain age gap. The applied multivariate model does not indicate whether atrophy/dysregulation to a specific brain region or regions drives the association between the brain age gap and alcohol consumption. As such, this study serves as a starting point for additional investigations of the mechanisms by which low-level alcohol consumption impacts the brain.
In conclusion, we provide evidence of a direct association between 90-day alcohol consumption and an acceleration of brain aging within a community sample. While further research is necessary to elucidate the underlying mechanisms responsible for, and implications of, the observed aging acceleration, our findings call attention to the low levels of alcohol consumption endorsed by a community sample of adults in the United States. The dose-dependent effect of alcohol on the brain age gap warrants further investigation as low-level alcohol use has previously been assumed to be negligible. It will be important to investigate the differences between non-heavy drinkers and non-drinkers in other brain imaging studies. Understanding the effects of low-level alcohol consumption on the brain will be necessary to determine whether or when it is appropriate to include low-level drinkers with those who do not use alcohol within healthy control comparison groups when comparing to target populations such as individuals with AUD. Furthermore, it will be important for future research to reveal the mechanisms underlying this effect and to determine the cognitive and/or affective consequences of low-level alcohol consumption, which can provide valuable public health insight into the safety and long-term implications of low levels of social alcohol use.
Supplementary Material
Acknowledgements
This research was supported by the Intramural Research Programs of the National Institute on Drug Abuse and the National Institute of Alcohol Abuse and Alcoholism, NIH.
This work utilized the computational resources of the NIH HPC Biowulf cluster. (http://hpc.nih.gov)
We thank Erika Almira of the NIAAA Clinical NeuroImaging Research Core for contributing to this project.
Footnotes
Disclosure statement
The authors have no conflict of interest to declare.
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.neurobiolaging.2021.11.008.
Data availability
Data files are available upon request
References
- Abrahao KP, Salinas AG, Lovinger DM, 2017. Alcohol and the Brain: Neuronal Molecular Targets, Synapses, and Circuits. Neuron 96 (6), 1223–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amunts K, Schlaug G, Schleicher A, Steinmetz H, Dabringhaus A, Roland PE, Zilles K, 1996. Asymmetry in the human motor cortex and handedness. Neuroimage 4 (3 Pt 1), 216–222. [DOI] [PubMed] [Google Scholar]
- Beck AT, Epstein N, Brown G, Steer RA, 1988. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol 56 (6), 893–897. [DOI] [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J, 1961. An inventory for measuring depression. Arch Gen Psychiatry 4, 561–571. [DOI] [PubMed] [Google Scholar]
- Beheshti I, Nugent S, Potvin O, Duchesne S, 2021. Disappearing metabolic youth-fulness in the cognitively impaired female brain. Neurobiol Aging 101, 224–229. [DOI] [PubMed] [Google Scholar]
- Bell S, Daskalopoulou M, Rapsomaniki E, George J, Britton A, Bobak M, Casas JP, Dale CE, Denaxas S, Shah AD, Hemingway H, 2017. Association between clinically recorded alcohol consumption and initial presentation of 12 cardiovascular diseases: population based cohort study using linked health records. BMJ 356, j909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellentani S, Saccoccio G, Costa G, Tiribelli C, Manenti F, Sodde M, Saveria Croce L, Sasso F, Pozzato G, Cristianini G, Brandi G, 1997. Drinking habits as cofactors of risk for alcohol induced liver damage. The Dionysos Study Group. Gut 41 (6), 845–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein DP, Fink L, Handelsman L, Foote J, Lovejoy M, Wenzel K, Sapareto E, Ruggiero J, 1994. Initial reliability and validity of a new retrospective measure of child abuse and neglect. Am J Psychiatry 151 (8), 1132–1136. [DOI] [PubMed] [Google Scholar]
- Bittner N, Jockwitz C, Franke K, Gaser C, Moebus S, Bayen UJ, Amunts K, Caspers S, 2021. When your brain looks older than expected: combined lifestyle risk and BrainAGE. Brain Struct Funct 226 (3), 621–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CM, Yi HY, Yoon YH, Dong C, 2012. Alcohol use at time of injury and survival following traumatic brain injury: results from the National Trauma Data Bank. J Stud Alcohol Drugs 73 (4), 531–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WJ, Harle LK, 2005. Interactive effect of alcohol and nicotine on developing cerebellum: an investigation of the temporal pattern of alcohol and nicotine administration. Alcohol Clin Exp Res 29 (3), 437–442. [DOI] [PubMed] [Google Scholar]
- Chin Fatt CR, Jha MK, Minhajuddin A, Mayes T, Trivedi MH, 2021. Sex-specific differences in the association between body mass index and brain aging in young adults: Findings from the human connectome project. Psychoneuroendocrinology 124, 105059. [DOI] [PubMed] [Google Scholar]
- Cole JH, Leech R, Sharp DJ, Alzheimer’s Disease Neuroimaging I, 2015. Prediction of brain age suggests accelerated atrophy after traumatic brain injury. Ann Neurol 77 (4), 571–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole JH, Poudel RP, Tsagkrasoulis D, Caan MW, Steves C, Spector TD, Montana GJN, 2017. Predicting brain age with deep learning from raw imaging data results in a reliable and heritable biomarker 163, 115–124. [DOI] [PubMed] [Google Scholar]
- Collaborators, G.B.D.A., 2018. Alcohol use and burden for 195 countries and territories, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 392 (10152), 1015–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk DE, Yi HY, Hiller-Sturmhofel S, 2006. An epidemiologic analysis of co-occurring alcohol and tobacco use and disorders: findings from the National Epidemiologic Survey on Alcohol and Related Conditions. Alcohol Res Health 29 (3), 162–171. [PMC free article] [PubMed] [Google Scholar]
- Fortier CB, Leritz EC, Salat DH, Lindemer E, Maksimovskiy AL, Shepel J, Williams V, Venne JR, Milberg WP, McGlinchey RE, 2014. Widespread effects of alcohol on white matter microstructure. Alcohol Clin Exp Res 38 (12), 2925–2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foundas AL, Hong K, Leonard CM, Heilman KM, 1998. Hand preference and magnetic resonance imaging asymmetries of the central sulcus. Neuropsychiatry Neuropsychol Behav Neurol 11 (2), 65–71. [PubMed] [Google Scholar]
- Foundas AL, Leonard CM, Heilman KM, 1995. Morphologic cerebral asymmetries and handedness. The pars triangularis and planum temporale. Arch Neurol 52 (5), 501–508. [DOI] [PubMed] [Google Scholar]
- Franke K, Hagemann G, Schleussner E, Gaser C, 2015. Changes of individual BrainAGE during the course of the menstrual cycle. Neuroimage 115, 1–6. [DOI] [PubMed] [Google Scholar]
- Franke K, Ziegler G, Kloppel S, Gaser C, Alzheimer’s Disease Neuroimaging, I., 2010. Estimating the age of healthy subjects from T1-weighted MRI scans using kernel methods: exploring the influence of various parameters. Neuroimage 50(3), 883–892. [DOI] [PubMed] [Google Scholar]
- Fuchs FD, Chambless LE, Whelton PK, Nieto FJ, Heiss G, 2001. Alcohol consumption and the incidence of hypertension: The Atherosclerosis Risk in Communities Study. Hypertension 37 (5), 1242–1250. [DOI] [PubMed] [Google Scholar]
- Gaser C, Franke K, Kloppel S, Koutsouleris N, Sauer H, Alzheimer’s Disease Neuroimaging, I., 2013. BrainAGE in Mild Cognitive Impaired Patients: Predicting the Conversion to Alzheimer’s Disease. PLoS One 8 (6), e67346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Chou SP, Saha TD, Pickering RP, Kerridge BT, Ruan WJ, Huang B, Jung J, Zhang H, Fan A, Hasin DS, 2017. Prevalence of 12-Month Alcohol Use, High-Risk Drinking, and DSM-IV Alcohol Use Disorder in the United States, 2001-2002 to 2012-2013: Results From the National Epidemiologic Survey on Alcohol and Related Conditions. JAMA Psychiatry 74 (9), 911–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green RE, Colella B, Maller JJ, Bayley M, Glazer J, Mikulis DJ, 2014. Scale and pattern of atrophy in the chronic stages of moderate-severe TBI. Front Hum Neurosci 8, 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guadalupe T, Willems RM, Zwiers MP, Arias Vasquez A, Hoogman M, Hagoort P, Fernandez G, Buitelaar J, Franke B, Fisher SE, Francks C, 2014. Differences in cerebral cortical anatomy of left- and right-handers. Front Psychol 5, 261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guggenmos M, Schmack K, Sekutowicz M, Garbusow M, Sebold M, Sommer C, Smolka MN, Wittchen HU, Zimmermann US, Heinz A, Sterzer P, 2017. Quantitative neurobiological evidence for accelerated brain aging in alcohol dependence. Transl Psychiatry 7 (12), 1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper CG, Blumbergs PC, 1982. Brain weights in alcoholics. J Neurol Neurosurg Psychiatry 45 (9), 838–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatton SN, Franz CE, Elman JA, Panizzon MS, Hagler DJ Jr., Fennema-Notestine C, Eyler LT, McEvoy LK, Lyons MJ, Dale AM, Kremen WS, 2018. Negative fateful life events in midlife and advanced predicted brain aging. Neurobiol Aging 67, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO, 1991. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict 86 (9), 1119–1127. [DOI] [PubMed] [Google Scholar]
- Hernandez-Avila CA, Rounsaville BJ, Kranzler HR, 2004. Opioid-, cannabis- and alcohol-dependent women show more rapid progression to substance abuse treatment. Drug Alcohol Depend 74 (3), 265–272. [DOI] [PubMed] [Google Scholar]
- Herve PY, Mazoyer B, Crivello F, Perchey G, Tzourio-Mazoyer N, 2005. Finger tapping, handedness and grey matter amount in the Rolando’s genu area. Neuroimage 25 (4), 1133–1145. [DOI] [PubMed] [Google Scholar]
- Hitschfeld MJ, Schneekloth TD, Ebbert JO, Hall-Flavin DK, Karpyak VM, Abulseoud OA, Patten CA, Geske JR, Frye MA, 2015. Female smokers have the highest alcohol craving in a residential alcoholism treatment cohort. Drug Alcohol Depend 150, 179–182. [DOI] [PubMed] [Google Scholar]
- Ho YS, Yang X, Yeung SC, Chiu K, Lau CF, Tsang AW, Mak JC, Chang RC, 2012. Cigarette smoking accelerated brain aging and induced pre-Alzheimer-like neuropathology in rats. PLoS One 7 (5), e36752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hommer D, Momenan R, Kaiser E, Rawlings R, 2001. Evidence for a gender-related effect of alcoholism on brain volumes. Am J Psychiatry 158 (2), 198–204. [DOI] [PubMed] [Google Scholar]
- Hommer D, Momenan R, Rawlings R, Ragan P, Williams W, Rio D, Eckardt M, 1996. Decreased corpus callosum size among alcoholic women. Arch Neurol 53 (4), 359–363. [DOI] [PubMed] [Google Scholar]
- Julian LJ, 2011. Measures of anxiety: State-Trait Anxiety Inventory (STAI), Beck Anxiety Inventory (BAI), and Hospital Anxiety and Depression Scale-Anxiety (HAD-S-A). Arthritis Care Res (Hoboken) 63 (Suppl 11), S467–S472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung S, Wang M, Anderson K, Baglietto L, Bergkvist L, Bernstein L, van den Brandt PA, Brinton L, Buring JE, Eliassen AH, Falk R, Gapstur SM, Giles GG, Goodman G, Hoffman-Bolton J, Horn-Ross PL, Inoue M, Kolonel LN, Krogh V, Lof M, Maas P, Miller AB, Neuhouser ML, Park Y, Robien K, Rohan TE, Scarmo S, Schouten LJ, Sieri S, Stevens VL, Tsugane S, Visvanathan K, Wilkens LR, Wolk A, Weiderpass E, Willett WC, Zeleniuch-Jacquotte A, Zhang SM, Zhang X, Ziegler RG, Smith-Warner SA, 2016. Alcohol consumption and breast cancer risk by estrogen receptor status: in a pooled analysis of 20 studies. Int J Epidemiol 45 (3), 916–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann T, van der Meer D, Doan NT, Schwarz E, Lund MJ, Agartz I, Alnaes D, Barch DM, Baur-Streubel R, Bertolino A, Bettella F, Beyer MK, Boen E, Borgwardt S, Brandt CL, Buitelaar J, Celius EG, Cervenka S, Conzelmann A, Cordova-Palomera A, Dale AM, de Quervain DJF, Di Carlo P, Djurovic S, Dorum ES, Eisenacher S, Elvsashagen T, Espeseth T, Fatouros-Bergman H, Flyckt L, Franke B, Frei O, Haatveit B, Haberg AK, Harbo HF, Hartman CA, Heslenfeld D, Hoekstra PJ, Hogestol EA, Jernigan TL, Jonassen R, Jonsson EG, Karolinska Schizophrenia P, Kirsch P, Kloszewska I, Kolskar KK, Landro NI, Le Hellard S, Lesch KP, Lovestone S, Lundervold A, Lundervold AJ, Maglanoc LA, Malt UF, Mecocci P, Melle I, Meyer-Lindenberg A, Moberget T, Norbom LB, Nordvik JE, Nyberg L, Oosterlaan J, Papalino M, Papassotiropoulos A, Pauli P, Pergola G, Persson K, Richard G, Rokicki J, Sanders AM, Selbaek G, Shadrin AA, Smeland OB, Soininen H, Sowa P, Steen VM, Tsolaki M, Ulrichsen KM, Vellas B, Wang L, Westman E, Ziegler GC, Zink M, Andreassen OA, Westlye LT, 2019. Common brain disorders are associated with heritable patterns of apparent aging of the brain. Nat Neurosci 22 (10), 1617–1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyes KM, Martins SS, Blanco C, Hasin DS, 2010. Telescoping and gender differences in alcohol dependence: new evidence from two national surveys. Am J Psychiatry 167 (8), 969–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutsouleris N, Davatzikos C, Borgwardt S, Gaser C, Bottlender R, Frodl T, Falkai P, Riecher-Rossler A, Moller HJ, Reiser M, Pantelis C, Meisenzahl E, 2014. Accelerated brain aging in schizophrenia and beyond: a neuroanatomical marker of psychiatric disorders. Schizophr Bull 40 (5), 1140–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang H, Zhang F, Niu X, 2019. Investigating systematic bias in brain age estimation with application to post-traumatic stress disorders 40 (11), 3143–3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liem F and Gorgolewski C (2017). BIDS-Apps/baracus: v1.1.2 (Version v1.1.2). Zendo. [Google Scholar]
- Liem F, Varoquaux G, Kynast J, Beyer F, Kharabian Masouleh S, Huntenburg JM, Lampe L, Rahim M, Abraham A, Craddock RC, Riedel-Heller S, Luck T, Loeffler M, Schroeter ML, Witte AV, Villringer A, Margulies DS, 2017. Predicting brain-age from multimodal imaging data captures cognitive impairment. Neuroimage 148, 179–188. [DOI] [PubMed] [Google Scholar]
- Luders E, Gingnell M, Poromaa IS, Engman J, Kurth F, Gaser C, 2018. Potential Brain Age Reversal after Pregnancy: Younger Brains at 4-6 Weeks Postpartum. Neuroscience 386, 309–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maechler Martin, Rousseeuw Peter, Croux Christophe, Todorov Valentin, Ruckstuhl Andreas, Salibian-Barrera Matias, Verbeke Tobias, Koller Manuel, Conceicao Eduardo L.T., di Palma Maria Anna, 2020. robustbase: Basic Robust Statistics. [Google Scholar]
- McCrimmon AW, Smith AD, 2013. Review of the Wechsler Abbreviated Scale of Intelligence. Second Edition (WASI-II) 31 (3), 337–341. [Google Scholar]
- Monnig MA, Yeo RA, Tonigan JS, McCrady BS, Thoma RJ, Sabbineni A, Hutchison KE, 2015. Associations of White Matter Microstructure with Clinical and Demographic Characteristics in Heavy Drinkers. PLoS One 10 (11), e0142042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nenadic I, Dietzek M, Langbein K, Sauer H, Gaser C, 2017. BrainAGE score indicates accelerated brain aging in schizophrenia, but not bipolar disorder. Psychiatry Res Neuroimaging 266, 86–89. [DOI] [PubMed] [Google Scholar]
- Ning K, Zhao L, Matloff W, Sun F, Toga AW, 2020. Association of relative brain age with tobacco smoking, alcohol consumption, and genetic variants. Sci Rep 10 (1), 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noonberg A, Goldstein G, Page HA, 1985. Premature aging in male alcoholics: "accelerated aging" or "increased vulnerability"?". Alcohol Clin Exp Res 9 (4), 334–338. [DOI] [PubMed] [Google Scholar]
- Orgogozo JM, Dartigues JF, Lafont S, Letenneur L, Commenges D, Salamon R, Renaud S, Breteler MB, 1997. Wine consumption and dementia in the elderly: a prospective community study in the Bordeaux area. Rev Neurol (Paris) 153 (3), 185–192. [PubMed] [Google Scholar]
- Perrine MW, Schroder KE, 2005. How many drinks did you have on September 11, 2001? J Stud Alcohol 66 (4), 536–544. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Rosenbloom M, Rohlfing T, Sullivan EV, 2009. Degradation of association and projection white matter systems in alcoholism detected with quantitative fiber tracking. Biol Psychiatry 65 (8), 680–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell JL, Kemp GJ, Roberts N, Garcia-Finana M, 2012. Sulcal morphology and volume of Broca’s area linked to handedness and sex. Brain Lang 121 (3), 206–218. [DOI] [PubMed] [Google Scholar]
- Core Team, R, 2019. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria, https://www.R-project.org/, 11/17/2021. [Google Scholar]
- Sabia S, Fayosse A, Dumurgier J, Dugravot A, Akbaraly T, Britton A, Kivimaki M, Singh-Manoux A, 2018. Alcohol consumption and risk of dementia: 23 year follow-up of Whitehall II cohort study. BMJ 362, k2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarna JR, Hawkes R, 2003. Patterned Purkinje cell death in the cerebellum. Prog Neurobiol 70 (6), 473–507. [DOI] [PubMed] [Google Scholar]
- Sawyer KS, Oscar-Berman M, Barthelemy OJ, Papadimitriou GM, Harris GJ, Makris N, 2017. Gender dimorphism of brain reward system volumes in alcoholism. Psychiatry Res Neuroimaging 263, 15–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnack HG, van Haren NE, Nieuwenhuis M, Hulshoff Pol HE, Cahn W, Kahn RS, 2016. Accelerated Brain Aging in Schizophrenia: A Longitudinal Pattern Recognition Study. Am J Psychiatry 173 (6), 607–616. [DOI] [PubMed] [Google Scholar]
- Searles JS, Helzer JE, Rose GL, Badger GJ, 2002. Concurrent and retrospective reports of alcohol consumption across 30, 90 and 366 days: interactive voice response compared with the timeline follow back. J Stud Alcohol 63 (3), 352–362. [DOI] [PubMed] [Google Scholar]
- Shahab S, Mulsant BH, Levesque ML, Calarco N, Nazeri A, Wheeler AL, Foussias G, Rajji TK, Voineskos AN, 2019. Brain structure, cognition, and brain age in schizophrenia, bipolar disorder, and healthy controls. Neuropsychopharmacology 44 (5), 898–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skullerud K, 1985. Variations in the size of the human brain. Influence of age, sex, body length, body mass index, alcoholism, Alzheimer changes, and cerebral atherosclerosis. Acta Neurol Scand Suppl 102, 1–94. [PubMed] [Google Scholar]
- Sobell LC, Sobell MB, 1992, Timeline Follow-Back. In: Litten RZ, Allen JP (Eds.). Humana Press, Totowa, NJ, pp. 41–72. [Google Scholar]
- Steffener J, Habeck C, O’Shea D, Razlighi Q, Bherer L, Stern Y, 2016. Differences between chronological and brain age are related to education and self-reported physical activity. Neurobiol Aging 40, 138–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramaniapillai S, Rajagopal S, Snytte J, Otto AR, Group, P.-A.R., Einstein G, Rajah MN, 2021. Sex differences in brain aging among adults with family history of Alzheimer’s disease and APOE4 genetic risk. Neuroimage Clin 30, 102620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration, 2019. Results from the 2018 National Survey on Drug Use and Health: Detailed tables. Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration, Rockville, MD: Retrieved from https://www.samhsa.gov/data/. [Google Scholar]
- Sullivan EV, Zahr NM, Sassoon SA, Thompson WK, Kwon D, Pohl KM, Pfefferbaum A, 2018. The Role of Aging, Drug Dependence, and Hepatitis C Comorbidity in Alcoholism Cortical Compromise. JAMA Psychiatry 75 (5), 474–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo CA, Xiong J, Lancaster JL, Rainey L, Fox P, 2001. Amygdalar and hippocampal volumetry in control participants: differences regarding handedness. AJNR Am J Neuroradiol 22 (7), 1342–1345. [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Wiers CE, Manza P, Shokri-Kojori E, Michele-Vera Y, Zhang R, Kroll D, Feldman D, McPherson K, Biesecker C, Schwandt M, Diazgranados N, Koob GF, Wang GJ, Volkow ND, 2021. Accelerated Aging of the Amygdala in Alcohol Use Disorders: Relevance to the Dark Side of Addiction. Cereb Cortex 31 (7), 3254–3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Eijk J, Demirakca T, Frischknecht U, Hermann D, Mann K, Ende G, 2013. Rapid partial regeneration of brain volume during the first 14 days of abstinence from alcohol. Alcohol Clin Exp Res 37 (1), 67–74. [DOI] [PubMed] [Google Scholar]
- Wang B, Pham TD, 2011. MRI-based age prediction using hidden Markov models. J Neurosci Methods 199 (1), 140–145. [DOI] [PubMed] [Google Scholar]
- Zahr NM, Pfefferbaum A, 2017. Alcohol’s Effects on the Brain: Neuroimaging Results in Humans and Animal Models. Alcohol Res 38 (2), 183–206. [PMC free article] [PubMed] [Google Scholar]
- Zahr NM, Pohl KM, Saranathan M, Sullivan EV, Pfefferbaum A, 2019. Hippocampal subfield CA2+3 exhibits accelerated aging in Alcohol Use Disorder: A preliminary study. Neuroimage Clin 22, 101764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Q, Pfefferbaum A, Podhajsky S, Pohl KM, Sullivan EV, 2020. Accelerated aging and motor control deficits are related to regional deformation of central cerebellar white matter in alcohol use disorder. Addict Biol 25 (3), e12746. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data files are available upon request


