Graphical abstract
Abbreviations: IDO, indoleamine (2,3) deoxygenase; IFN-γ, interferon gamma; GTP, guanosine triphosphate; GTP-CH, guanosine triphosphate cyclo-hydrolase-1; DHNTP, 7,8-dihydro-neopterin-triphosphate; PTPS, 6-pyruvoyl-tetrahydropterin synthase; ICAM, intracellular adhe- sion molecule-1; VCAM, vascular cell adhesion molecule-1; iNOS, nitric oxide synthase; CSF, cerebrospinal fluid; RP, adical prostatectomy; BCR, biochemical recurrence; IDO, Indoleamine 2,3-dioxygenase; MCP-1, monocyte chemoattractant protein; PTPS, pyruvoyl-tetrahydropterin synthase; LDL, Low-density lipoprotein
Keywords: SARS CoV-2, Neopterin, COVID-19, Prognosis
Abstract
Coronavirus Disease 2019 [COVID-19], caused by severe acute respiratory syndrome coronavirus 2 [SARS-CoV-2], has rapidly evolved into a global health emergency. Neopterin [NPT], produced by macrophages when stimulated with interferon [IFN-]gamma, is an essential cytokine in the antiviral immune response. NPT has been used as a marker for the early assessment of disease severity in different diseases. The leading cause of NPT production is the pro-inflammatory cytokine IFN-. Macrophage activation has also been revealed to be linked with disease severity in SARS-CoV-2 patients. We demonstrate the importance of NPT in the pathogenesis of SARS-CoV-2 and suggest that targeting NPT in SARS-CoV-2 infection may be critical in the early prediction of disease progression and provision of timely management of infected individuals.
1. Introduction
Severe acute respiratory syndrome coronavirus-2 [SARS-CoV-2] infection has spread rapidly worldwide since it's first appeared in China in late 2019. The data show that approximately percent 80 of COVID-19 patients have mild disease, percent 20 require hospitalization, and about percent 5 need intensive care admission [1]. COVID-19 has a poor prognosis in elderly, male patients and, in patients with comorbidities such as diabetes, cardiovascular disease, or chronic obstructive pulmonary disease [COPD] [2], [3], [4], [5]. In patients infected with SARS-CoV-2, hyper-inflammation and coagulopathy are associated with disease severity and death [6]. Elevated levels of inflammatory markers, including C-reactive protein, ferritin, D-dimer, inflammatory cytokines, and chemokines, and elevated neutrophil to lymphocyte ratios are associated with disease severity and mortality from COVID-19 [6]. High levels of circulating cytokines, profound lymphopenia, substantial mononuclear cell infiltration in the lungs and other organs have been reported in severe cases compared to mild COVID-19 cases [6]. Previous studies have shown that the proportion of mononuclear phagocytes increased in extreme cases, and the composition of macrophages changed in favor of monocyte-derived macrophages [6]. As a result, high levels of cytokines linked to macrophage activation, including interferon- [IFN-], have been reported in SARS-CoV-2 patients [7]. Neopterin (NPT) is produced by microstimulating-IFN-, a cytokine important in the antiviral immune response. Serum NPT levels reflect the activation phase of the cellular immune system, which is essential in the pathogenesis and progression of various diseases [8]. Previous studies have shown an association between serum NPT levels and prognosis in specific viral infections, such as influenza, human immunodeficiency viruses, hepatitis C virus, and dengue fever virus [9], [10], [11]. High levels of circulating cytokines have been reported in patients with severe COVID-19. Therefore, targeting NPT in SARS-CoV-2 infection may be necessary for the early prognosis of disease progression and timely treatment of infected patients. Serum NPT levels have been measured to assess the immune activation in several diseases, but only a few studies have been conducted on individuals infected with SARS-CoV-2. Therefore, this review is intended to elucidate the importance of NPT as a diagnostic and prognostic marker in COVID-19 patients.
2. Overview of Neopterin: biosynthesis, mechanisms of tryptophan and oxidative stress
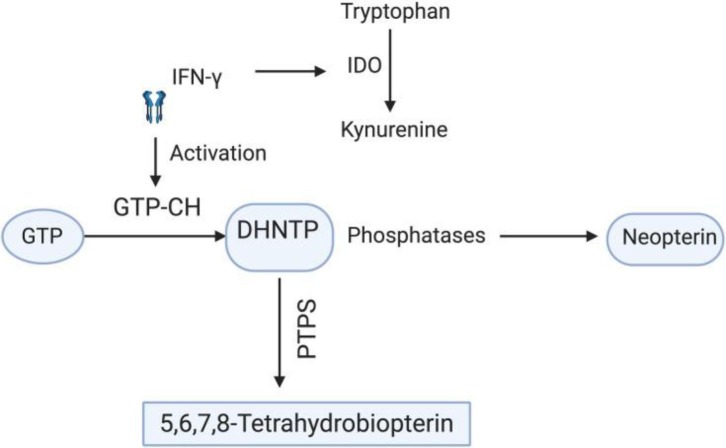
NPT [1′, 2′, 3′-D-erythro-trihydroxypropylpterin] belongs to a group of pteridine compounds containing 2-amino, 4-oxo, pyrimidine-pyrazine [pteridine ring], with a 3-carbon side-chain on carbon-6 [12] and is involved in several redox reactions in the body. NPT is biosynthetically derived in vivo from guanosine triphosphate [GTP], as shown in Graphical abstract. Inactivated monocytes, macrophages, dendritic cells, and endothelial cells, the reaction is released by macrophages in response to cytokines released by T lymphocytes and catalyzed by the enzyme GTP-cyclohydrolase-I [GTP-CH] mainly upon IFN- stimulation [13], [14]. The GTP-CH first cleaves GTP to synthesize 7, 8-dihydroneopterin triphosphate [DHNTP]. This intermediate is then converted by 6-pyruvoyl-tetrahydrobiopterin synthase [PTPS] to produce dihy- drobiopterin by biosynthesis 5,6,7,8-tetrahydrobiopterin. Because humans and primates are the only species lacking the PTPS enzyme, the DHNTP accumulates in the form of NPT [13], [14]. NPT is also produced by monocyte-derived dendritic cells, and its production is increased by IFN- stimulation [15] and lipopolysaccharide induction [15], [16]. After stimulation, dendritic cells degrade tryptophan in the tryptophan-kynurenine pathway, where N-formyl-kynurenine is the first intermediate formed by reaction with the enzyme indoleamine [2], [3]-dioxygenase enzyme [IDO] [17]. IDO is produced by vascular endothelial cells and activated via IFN- released by dendritic cells and T cells [18], [19]. IDO regulates the pathway of tryptophan kynurenine by tryptophan degradation. It has been shown that kynurenine may have a physiological role in suppressing the immune function of T cells and NK cells. NPT formation is associated with tryptophan catabolism, considering that both are stimulated by IFN-[20]. Thus, the NPT accumulation and tryptophan reduction could reflect IFN- induced macrophage activation. The accretion of NPT can be an indicator of systemic immune activation, particularly cell-mediated immunity [21]. On the other hand, increased serum NPT regulates reactive oxygen species [ROS]-mediated processes by regulating intracellular signaling cascades and activating ROS- sensitive transcription factor nuclear factor B [NF-B], which induces pro-inflammatory genes such as inducible nitric oxide synthase [iNOS] and further enhances inflammatory processes [22], [23]. NPT promotes the cytotoxic capacity of immune cells by stimulating iNOS gene expression at the mRNA level and subsequent nitric oxide [NO] production [24]. It has been shown that GTP-CH is inhibited during oxidative stress and was shown to inhibit NPT biosynthesis significantly. That is why NPT may also be used as an indicator of oxidative stress [25] (See Fig. 1 ).
Fig. 1.
Biosynthesis of neopterin: IFN-γ activates GTP cyclo-hydrolase that further cleaves GTP to 7,8-dihydro-neopterin triphosphate, and phosphatases, in turn, change this intermediate to neopterin. Since humans lack PTPS, an enzyme that converts DHNTP into 5,6,7,8-tetra hydrobiopetrin, the DHNTP is only biosynthesized to neopterin. On the other hand, IFN-γ initiates tryptophan degradation to kynurenine by activating the IDO enzyme. Thus, there is a direct correlation between increased neopterin and tryptophan degradation.
2.1. Neopterin in diseases
NPT, a sensitive marker of cell-mediated immune system activation, has been potentially studied as a disease marker and a nonspecific screening tool to facilitate conscious pathogen analysis [26]. As seen in Table 1 , NPT concentration increased in various diseases through different mechanisms. It has been defined that NPT, which is measured in many immune disorders, shows a significant increase in the amount of NPT in rheumatoid arthritis [RA] compared to healthy patients and is a marker that defines immune activation [27]. Although the relationship of NPT, a diagnostic or prognostic marker, with diabetes and hypertension remains unclear, Asci et al. In their research, the NPT level in hemodialysis patients caused by diabetes and hypertension was found to be higher than in healthy and control patients. It has been shown that NPT may be an early critical marker for the progression of nephropathy in the early stages in diabetic and hypertensive patients [28]. Since NPT is associated with tryptophan and kynurenine, a vital amino acid for growth, it is also related to many diseases in which this pathway is practical. The cytokine IFN- stimulates macrophages and monocytes to secrete NPT and induces Indoleamine 2,3-dioxygenase [IDO], which degrades tryptophan via kynurenine. Therefore, impaired tryptophan mechanism is directly related to depression and neuropsychiatric anomalies [29]. Increased NPT formation and increased tryptophan degradation have also been shown to affect the immune response in a group of patients with advanced Parkinson's disease [30]. In a study, when serum NPT concentrations of patients with dermatomyositis [DM] were examined, it was revealed that serum NPT levels increased significantly in DM patients compared to healthy controls and were closely related to disease severity [Table 1] [31]. Because NPT and fibrinogen play a role in inflammation-related diseases, the predictive effects of biomarkers in individuals with stable coronary artery disease [SCAD] have been investigated. They are associated with mortality [32]. When the risk of a diagnosis of prostate cancer [PCa] in a transrectal biopsy, the histopathological features of radical prostatectomy [RP] samples, and the effects of biomarkers on cancer-specific survival [CSS] after biochemical recurrence [BCR] were investigated, it was supported that NPT helps categorize it into prognostic groups [Table 1] [33]. In addition to many cancer studies, Yalcın et al. demonstrated that high NPT levels increase the risk of death in patients with lung cancer, whether it is possible to use these biomarkers in predicting tumor prognosis [Table 1] [34]. As can be seen, it has been proven by many studies that NPT is associated with many disease sources, regardless of disease, and provides information about the preliminary diagnosis and severity of infections.
Table 1.
The concentration of neopterin levels in some diseases.
| Disease | Neopterin concentration in the patient | Neopterin concentration in the healthy/mild | P-value | Reference |
|---|---|---|---|---|
| Behcet 'S Disease | 111.27 ± 37.49 nmol/L | 76.77 ± 38.27 nmol/L | P < 0.001 | [35] |
| Psoriasis Vulgaris | 2.26 ± 1.92 ng/ml | 1.19 ± 0.18 ng/ml | P = 0.001 | [36] |
| Dermatomyositis |
IQR 13·9–35·2 nmol/l | IQR 2·9–5·6 nmol/l | P < 0·001 | [31] |
| Prostat Cancer | 0.71 AUC | 0.75 AUC | P < 0.001 | [33] |
| Lung Cancer | 2.66–13.54 nmol/L | 3.36–51.70 nmol/L | P = 0.004 | [34] |
| Brucellosis | 79.07 ± 34.9 nmol/l | 39.71 ± 23.4 nmol/l | P = 0.002 | [37] |
| Polycystic Ovary Syndrome | 7.5–49.5 nmol/L | 6.5–12.9 nmol/L | p < 0.05 | [38] |
| Breast Cancer | 1.2–12.0 nmol/L | 0–23.6 nmol/L | P < 0.05 | [39] |
| Graves' Disease | 5.7 ± 2.4 | 4.1 ± 1.7 | p < 0.01 | [40] |
| 4.0 ± 1.5 | ||||
| Gastrointestinal Cancer | 4.84 ± 0.74 | 1.57 ± 0.13 | p < 0.001 | [41] |
| Thyroid Diseases | 7.14 ± 5.95 nmol/l | 4 0.10 ± 1.70 nmol/l | p < O.OOl | [42] |
| Renal Carcinoma | 7.09 ± 1.99 nmol/litre | 2.87 + 0.59 nmol/litre | P < 0.05 | [43] |
| Crohn’s Disease | 302 ± 15 nmol/mol | 163 ± 8 nmol/mol | P < 0.001 | [44] |
| Rheumatoid Arthritis | 11.46 ± 3.56 nmol/L | 4.74 ± 1.98 nmol/L | P < 0.0001 | [27] |
2.2. Neopterin in virus infection
NPT has been a marker of the disease by showing concentration changes in serum and urine in the early phase of many virus infections. Therefore, NPT levels are directly related to the disease and its activity. Thanks to this relationship, which is a sensitive indicator, it is balanced with the critical role played by NPT in virus infections [45], [46], [47]. The problems in immune response and regulation underlying the pathogenesis have been supported by many necessary pieces of evidence [46], [47], [48]. Cytokine-producing macrophages are the most critical targets in virus infection [27], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51]. During virus infection, cytokines are released by interacting with immunocompetent cells such as T lymphocytes. It is produced in increased amounts by NPT macrophages in the immune response triggered by a viral infection. Therefore, by determining the concentration of NPT in body fluid, the disease can be detected early in various conditions such as infections and autoimmune diseases, thanks to the activational changes in the immune response [51], [52], [53], [54]. NPT concentration in blood and urine samples is an early and sensitive marker of the presence of many viral infectious diseases, including human immunodeficiency virus type 1 [HIV-1] [55].
In infections caused by many human immunodeficiency viruses such as hepatitis C virus and dengue virus, a predictive relationship has been demonstrated by looking at the serum level of NPT. It has been reported that it is a beneficial biomarker for the early diagnosis of the severity of the disease in patients with severe acute respiratory syndrome, called SARS [10], [11], [56], [57]. Reibnegger et al., when they examined high NPT levels in blood or urine in patients with viral hepatitis, found that NPT levels were higher in infected patients[58]. In another study, NPT levels in dengue fever disease were examined, and healthy individuals, sick individuals, and controllers were compared in NPT concentrations. As a result, they stated that NPT levels were significantly higher in DF patients [59].
Similarly, in HIV-1 infection that causes AIDS, NPT levels in blood and serum were higher than in control and healthy individuals [60], [61]. The SARS COV-2 virus, which causes COVID-19, has also been associated with elevated cytokine levels, organ damage, increased phagocytes, and macrophage activation. NPT, which plays a role in viral infections and immune response and is produced by macrophages, can therefore be used in the early diagnosis of disease severity in cases of COVID-19 [62], [63], [64].
2.3. NF-B signaling and neopterin in COVID-19 infection
Results of some studies proved that During a COVID-19 infection, overexpression of NF-B leads to cytokine storm, abnormal production of reactive oxygen species [ROS] and adhesion molecules [e.g., intracellular adhesion molecule-1; ICAM, vascular cell adhesion molecule-1; VCAM, and E- selectin], resulting in organ damage [65], [66]. On the other hand, it has been shown that the NOD-, LRR-, and pyrin domain-containing protein 3 [NLRP3] inflammasome is activated by COVID-19 infection and contributes to tissue injury, for example, lung injury and ARDS [67]. Considering the previous results, it is found that NPT can attenuate inflammation by suppressing NF-B signaling and NLRP3 inflammasomes [68]. Furthermore, it should be noted that NPT plays a vital role in the modulation of monocyte chemoattractant protein [MCP-1], [ICAM-1], and [VCAM-1] [69]. Given the above, it seems that NPT probably relieves inflammation in patients with COVID-19 infection, and it is proposed that agonists of NPT may be hopeful in the treatment of COVID-19 [69]. Recent studies showed that inflammatory markers, e.g., C-reactive protein [CRP], procalcitonin, erythrocyte sedimentation rate [ESR], are positively associated with severity of COVID- 19 [70]. According to the evidence, increasing NPT level is regarded as macrophage activation in several diseases, such as; COVID-19 [71]. In Table 2 , detail of some study is summarized (See Table 3 ).
Table 2.
Some studies on neopterin.
| Year | References | Number of patients | Finding |
|---|---|---|---|
| 2021 | [71] | 34 [mild diseases, N = 15] [Sever diseases, N = 19] |
All severe cases had elevated neopterin concentrations [>9.1 nmol/L]. |
| 2020 | [72] | 115 | Elevated neopterin levels were significantly associated with disease severity. |
| 2021 | [73] | 6 | CSF neopterin [median 43.0 nmol/L] was increased in all patients. |
Table 3.
Some studies evaluate the effect of Zn supplementation on people in different condition.
| Year | References | Number of patients | Finding |
|---|---|---|---|
| 2004 | [85] | 214 | Taking Zn did not significantly affect the duration of symptoms versus the control group. |
| 2016 | [86] | 191 Zinc group 1 [n = 96] Without zinc group [n = 95] |
Zinc supplements did not improve the clinical efficacy of hydroxychloroquine. |
| 2008 | [87] | 91 | Zinc treatment did not attenuate the total symptom score. |
| 2011 | [88] | 153 | Zinc supplementation significantly reduces the duration of fever and very ill status in boys, but not in girls. |
| 2013 | [89] | 53 | Zinc treatment was able to increase the number of functional T. |
| 2019 | [90] | 50 | Zinc supplementation decreased both the production of inflammatory cytokines and oxidative stress markers: |
| 2018 | [91] | 108 | Zinc, selenium, and vitamin C treatment may alleviate symptoms in COPD. |
| 2006 | [92] | 301 | Zinc amino acid chelate had a better effect on the acute respiratory. |
| 2014 | [93] | 64 | Zinc supplementation reduced the number of days of ALRI in Thai children and their stay in hospital. |
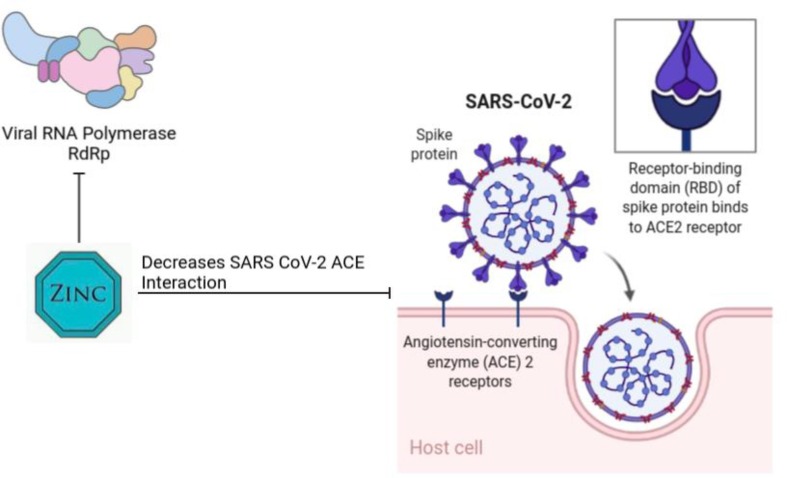
Zinc [Zn] is categorized as a trace element [74]. Numerous aspects of cellular metabolism such as development, growth, activation of enzymes [superoxide dismutase; SOD], and neurobehavioral are zinc-dependent [75], [76]. Current evidence suggests that zinc maintains a balanced immune system and oxidative stress status in the cell [77], [78]. It seems that there is a close relationship between Cu, Zn-superoxide dismutase, and NF-B. Indeed, overexpression of NF-B suppresses SOD-leading antioxidant defense [79]. Furthermore, Zinc deficiency probably affects phagocytosis of macrophages and inhibition of Natural Killer cells' activity [80]. It is shown that Zn acts as an antiviral mineral against several viruses, for example, COVID-19, via different mechanisms [Fig. 2 ].
Fig. 2.
Zn can inhibit ACE2 expression by reducing sirtuin1.
Also, Zn can suppress Replication of virus genomic and Translation of virus protein.
An experimental study suggests that Zn supplementation in Wistar rats affects the length of cilia and impresses several epithelial cells in the lung [81]. On the other hand, it is proved that Zn can inhibit replication by inactivation RNA-dependent RNA polymerase [RdRp] [82]. Some studies reported sirtuin1 is associated with ACE2 expression, and it is believed that sirtuin1 is reduced by zinc. Subsequently, ACE2 expression is inhibited [83], [84].
In the present pandemic, it is frequently observed that cytokine storm occurs in patients with COVID-19 infection. When respiratory epithelial tissue is infected by COVID-19 disease, inflammatory cytokines such as IL-1, IL-6, IL-8, IL-12, TNF- and other chemokines are locally realized. Subsequently, monocytes, macrophages, neutrophils, DCs, and NK cells are recruited by cytokines, resulting in the activation of CD4 + and CD8 + T cells to synthesize IFN- and TNF-, which induce lung injury. Furthermore, high IL-2, IFN-, GM-CSF, and TNF- leads to anemia by macrophage activation and erythro-phagocytosis [94], [95]. IFN- is considered a glycosylated protein of 25 kDa [96]. It is well established that IFNs are categorized into three categories: type I [IFN], type II [IFN], and type III [IFN] [97].
IFN is produced mainly by natural killer [NK] cells, natural killer T cells [NKT], activated lymphocytes such as CD4 T helper type 1 [Th1] cells, and CD8 cytotoxic T cells, B cells, and professional antigen-presenting cells [APCs] [98], [99], [100], [101], [102], [103]. It is now apparent that Janus activated kinases [JAKs] and binding IFN triggers STAT1 signal to IFNAR1 and IFNAR2 receptors. Attaching of IFN to IFNARs result in activation of tyrosine kinases JAK1 and JAK2 phosphorylating the transcription factor STAT1 to form dimer then dimers translocate to the nucleus and bind GAS to stimulate the transcription of these genes; for example, IFN stimulates the expression of immunoglobulin Fc receptors on phagocytes and improve the expression of MHC antigens facilitating antigen presentation to T lymphocytes [104], [105]. TNF is classified as a non-glycosylated protein with 157 residues [106] secreted by macrophages/monocytes. TNF gene is located on chromosome 6 [47]. TNF plays various roles in cells, for example, viral replication, cell growth modulation, tumorigenesis, and inflammation process [107], [108].
The expression of the TNF gene is controlled by nuclear factor kappa b [NFB] and nuclear factor activated T cells [NF-AT] [107], [108], [109], [110]. TNF signals through TNF receptor 1 [TNFR1] and TNF receptor 2 [TNFR2] [111]. Both pro-inflammatory and pro-apoptotic pathways are triggered by binding the soluble ligand TNF- and transmembrane to the TNF receptor [TNFR1] and TRAF2, respectively. TNFR1 stimulates NFB, MAPK, and Caspase-8, inducing inflammation, tissue degeneration, apoptosis. On the other hand, TRAF2 can activate MKLK leading to necroptosis [112], [113]. Interleukin: Interleukin [IL] refers to a class of cytokine prominently secreted by leukocytes [114]. ILs regulate numerous functions such as stimulation and differentiation of immune cells, proliferation, maturation. IL acts as a pro-inflammatory agent and has anti-inflammatory properties [114]. Mature IL-6 has 185 amino acids. The gene of IL-6 is located at chromosome 7p21. This pleiotropic cytokine exerts numerous functions such as inflammation, immune response, and hematopoiesis produced from T cells, macrophages, endothelial cells, fibroblasts, and monocytes [115]. The binding of IL-6 to its receptor initiates cascades of signaling through JAK/STAT3 stimulating the transcription of several factors such as other cytokines and adaptor proteins [116]. Taken together, Interleukins, TNF, and IFN play an inseparable role in a cytokine storm.
3. Association of neopterin with the severity of COVID-19
Statins, one of the best-selling prescription drug class HMG-CoA reductase enzyme inhibitors in the US, is known to have a favorable safety profile; They contain the world's bestselling prescription drug atorvastatin. When looking at their biochemical effects, the uncommon effects of statins extend far beyond the lipid profile and components such as LDL-C, HDL-C, and triglycerides ranging from nitric oxide and inflammatory markers to polyunsaturated fatty acids [117]. Since statins in the lipid-lowering drug class are inhibitors of 3-hydroxy-3-methylglutaryl coenzyme A reductase [118], many are at risk [119], including young/old, male/female individuals. It has well-documented benefits for moderate to high cardiovascular disease [117].
SARS-CoV-2 patients with a compromised cardiovascular system and comorbidities, including various cardiovascular diseases and hypertension, may suffer from acute respiratory distress syndrome and increased mortality. Statin can be given to the patient in the manufacturing field and office, where AR is “tiny and needs little in intensive care. In addition, in another study, the continued use of statins in patients with COPD had to be cautious about intubation. In addition to its benefits in existing techniques in the technology used in practice, it can also prove new roles that can benefit and can be achieved from technologies derived from anti-inflammatory, anti-thrombodultic, immuno-thrombodultic, and immuno-thrombodulatory and methods of sampling science. Still not explicitly applicable to the conversation, some hospital statins are included in education from COVID-19 [2]. In addition, an analysis of in-hospital deaths in 8910 COVID-19 patients from Asia, Europe, and North America demonstrated a favorable prognosis for statin use. At the same time, statins positively affect the endothelium under stress since viral use increases the endothelial damage, rendering thrombotic value [4]. If one person has something else across the U.S. due to COVID-19, more than 10,000, from admission to use, other prior use, comorbid conditions, hospital, and bear controlled more than 40% in the next and severely over 40. The result was associated with a greater than 25% risk of developing severe symptoms. The observations and teaching that statins were not considered ingredients still indicate preliminary information [120].
Some research results show that the statin effect is not significant for COVID-19 when the comparative data of users and non-users are examined. This is mainly because statin users with COVID-19 disease are shown to have a greater baseline risk driven by older age and a higher cardiovascular comorbidity burden. This could, in theory, hide the potential protective effect of statins in this subset of patients [121]. For this reason, multivariate analyzes can give more positive results than univariate analyzes. While it is known that there is a 30% reduction in fatal or severe COVID-19 infection according to multivariate meta-analyses, it does not confirm a significant lack of protection in the data reported in the univariate meta-analysis among statin users [122].
Low-density lipoprotein (LDL); ApoB is a large particulate molecule with a molecular weight of 2,000 kDa, consisting of triacylglycerol, free cholesterol, cholesteryl ester, and phospholipid molecules. LDL, which contains 1600 cholesterol esters and 170 molecules of triglycerides in its core, consists of 700 phospholipid molecules and 600 cholesterol molecules in the surrounding layer. In its outer layer, there is an apo B100 molecule. Half of LDL fatty acids consist of polyunsaturated fatty acids (PUFA). PUFAs are protected from oxidation by antioxidants. Oxidized LDL (ox-LDL) is exposed to oxidative stress and inflammation in various diseases, resulting in oxidative stress and reactive oxygen derivatives. A high concentration of ox-LDL initiates cellular changes resulting in cell death, ROS formation, caspase, protein kinase activation, calcium homeostasis, and pro-apoptotic/antiapoptotic gene expression change [123]. NPT, a pyrazinopyrimidine compound, is a popular biomarker, especially in essential pathologies that activate cellular immune mechanisms [13]. Since high NPT production is associated with increased reactive oxygen species (ROS) production and low serum antioxidant concentrations such as alpha-tocopherol, NPT can also be considered a marker of ROS generated by the active cellular immune system. Therefore, NPT measurements can predict not only the extent of cellular immune activation but also the extent of oxidative stress [6], [124].
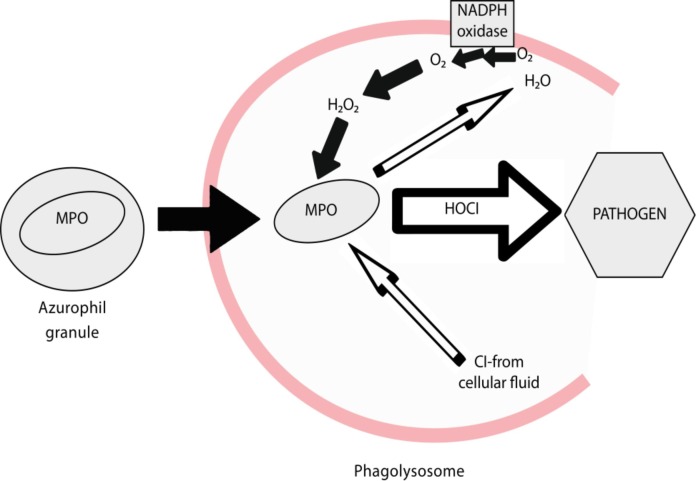
Hypochlorous acid (HOCl), an essential inorganic bactericidal compound of innate immunity, is effective against many microorganisms [125]. Stabilized at pH 3.5–5.5, HOCl is a weak acid that interacts with structural proteins or viral material to inactivate microorganisms [126]. HOCl is the most potent oxidant produced by neutrophils and is a powerful microbicidal agent within these cells. Because of its chemical nature, HOCl has never been used to treat an infection. A remarkable feature of the immune system is its ability to initiate an effective response against invading pathogens by deploying a group of highly reactive chemicals, including oxidized halogens, oxidizing radicals, and single oxygen (Fig. 3 ) [125]. HOCl is currently a disinfectant approved under different brands by the US Environmental Protection Agency for SARS-CoV-2. HOCl, which interacts with structural proteins such as the capsid or surface compounds of viruses, lipid envelope, and DNA/RNA materials, HOCl with concentrations as low as 20 ppm is effective in disinfecting surfaces, including porous rayon. In addition, it is not toxic to humans and is a disinfectant 80–200 times more effective than standard disinfection procedures [126].
Fig. 3.
HOCI mechanism acting on pathogens.
The current data show that HOCl oxidizes NH2 to form NPT. NH2 is described as an antioxidant and a potent radical scavenger. NPT remains stable at neutral pH, while NH2 is oxidized to 7,8-dihydroxyantopterine in an oxygen-saturated solution. Oxidizing agents in acidic solution, for example, MnO2 or I2, leave the NH2 side-chain unaffected and selectively oxidize the 7,8-dihydro structure of NH2 to form NPT.
The mechanism of oxidation remains unclear. The reaction mixture has a balance of hypochlorite and free acid and has oxidizing properties of dissolved chlorine (Cl2) resulting from the decomposition of HOCl. However, when HOCl content is quantified spectrophotometrically, the oxidative potential of Cl is not included. This may explain why NPT formed exceeds the amount of HOCl administered. Since the quantification of NPT by fluorescence detection is selective and sensitive in one study, the rate of formation of NPT was monitored. NH2 is a non-fluorescent compound. The UV/Vis signal overlaps NH2 and NPT, and apart from electrochemical detection, no suitable HPLC method is known to separate these two compounds. It was also concluded that alternative quantification of NH2 after iodine oxidation after HOCl oxidation is impossible due to additional products formed. NPT is thought to act instead as a pro-oxidant, depending on conditions such as the nature of the oxidant, pH, or absence/presence of iron ions. Evidence is provided for the first time that reactive species can independently act on the NPT/NH2 ratio, thereby altering these pteridines' redox modulatory properties. A dynamic balance has been noted in conformity with the idea that pteridines are redox modulators rather than a static model of stable NPT/NH2 excretion. HOCl has been shown to increase the oxidative potential in the local microenvironment by increasing the NPT-mediated prooxidative potency and decreasing the antioxidative capacity of NH2. A conversion of the NPT/NH2 ratio was also found, with NPT concentrations sometimes found higher than NH2 concentrations compared to NH2 alone [127].
COPD has been defined as an inflammatory disease with systemic consequences in recent years. COPD may also predispose individuals to the presence of other comorbidities, such as arterial hypertension, diabetes, and cardiovascular disease, which can potentially affect the outcome and severity of COPD. Therefore, the coexistence of associated conditions is common and may affect COPD disease progression and prognosis. According to the stated experience, higher NPT levels have been found in patients with cardiac and renal diseases, and these can be expressed as reflecting attacks of viral etiology [128]. Data support that mortality and NPT after pneumonia are risk factors for respiratory tract infection and cardiovascular events. The first line of these observations may have clinical implications when assessing COPD severity and exacerbation [129].
NPT can be potentially expressed as a promising inflammatory mediator. It has been reported to act as a mediator of cell immunity against intracellular pathogens such as viruses, parasites, and intracellular bacteria. It is widely accepted that COPD is associated with an increased systemic inflammatory response than controls. This inflammatory response is reported in patients with stable COPD at higher levels of NPT when compared to control groups [130]. NPT is released from monocytic cells after stimulation with interferon-gamma (IFN-γ) as a well-established biomarker of cellular activation. IFN-γ also promotes tryptophan degradation in the kynurenine pathway, producing several neuroactive metabolites, including quinolinic acid, which may contribute to neurological disorders [131]. Briefly, NPT is an oxidized form of dihydroneopterin during antioxidant reactions. High levels of NPT in serum and other biological fluids are associated with increased production of ROS and induction of oxidative stress (OS) during intense activation of cellular immunity [69]. According to studies, high concentrations of NPT have been reported to be detected in every neuro-COVID patient studied. NPT was elevated in cerebrospinal fluid samples of patients with COVID-19 and neurological abnormalities [131]. Also, the serum level of NPT can distinguish viral infection of the lower respiratory tract from a bacterial one; it can be noted a twofold higher increase in its viral state than a bacterial infection. In brain damage caused by COVID-19, NPT levels were elevated in patients' cerebrospinal fluid (CSF) [69].
It is accepted that uveitis patients with comorbidities such as diabetes mellitus, hypertension, and cardiovascular disease are at higher risk if they develop COVID-19. Asymptomatic retinal complications of SARS-CoV-2 infection have also been reported, but the prevalence is unknown. Research currently points to studies describing uveitis, retinitis, retinal vasculitis, and optic disc involvement in animals after coronavirus infection [132]. No reports of COVID-19-associated uveitis have been published to date, but thin retinal microvascular pathology and small lesions have been described in the ganglion cell and inner plexiform layers [133].
NPT plasma level has been measured in many autoimmune diseases. Due to the overstimulation of monocyte/macrophage cells by T lymphocytes in patients with RA, NPT may be an indicator of both cellular and innate immune activity in these patients. Higher NPT concentrations are associated with increased cardiovascular risk in the general population. Cardiovascular disorders are one of the most important causes of mortality in patients with RA. Studies have shown that NPT levels increase with age in both RA and control groups; In addition, it was found that RA patients increased with disease onset age and disease duration. The reason for higher NPT levels in male RA patients is not apparent. Still, it can be stated here that higher anti-CCP antibody contributes to increased inflammation and NPT levels in these patients [134], [135].
4. Association of neopterin with symptoms in COVID-19 patients
NPT is an independent prognostic factor for COVID-19 severity [53]. It appears in the blood before clinical symptoms arise in acute stages of viral infection and are linked to severe dyspnea, a more extended hospitalization period, and other complications [69]. While COVID-19 is generally identified as pulmonary infection, it brings disturbances to various organ systems in the body with their related symptoms. The association of non-pulmonary clinical signs and symptoms with NPT in patients with COVID-19 has not been thoroughly investigated. Some scarce studies reported the probable association of NPT in body fluids with gastrointestinal, neurologic, and renal signs and symptoms [69].
The pooled prevalence of gastrointestinal (GI) symptoms (including nausea, vomiting, diarrhea, abdominal pain, and anorexia) in patients with confirmed COVID-19 was 18%, with diarrhea being the most significant [136]. Some patients show gastrointestinal symptoms (e.g., nausea and diarrhea) as an initial manifestation of the disease [137], and patients with a severe form were more likely to experience GI symptoms.
Fecal NPT is assumed as a surrogate of cellular viral immune response and may be an indicator of intestinal inflammation in COVID-19 patients [10], [138]. SARS-CoV-2 can impose injuries to the gut mucosa by its ability to infect and replicate in the enterocytes. Intestinal epithelial cells simultaneously express two critical proteins for SARS-CoV-2 cell entry: ACE2 and transmembrane serine protease [139], making the oral-fecal route a potential route for infection [138].
In a study on 37 hospitalized COVID-19 patients (Non-ICU setting) with a median age of 62 years and a high level of C-reactive protein (evidence for systemic inflammation), fecal NPT values were elevated (more than 614.7 ng/g) in comparison with control healthy subjects. Seventeen patients with GI symptoms (diarrhea and nausea, and vomiting) demonstrated even higher NPT values in the stool. This subgroup of patients was also found to have elevated serum C-reactive protein concentration and body temperature on the day of stool sampling compared with the low NPT group, suggesting the presence of systemic inflammation. The fecal NPT did not significantly differ according to the GI sign or symptoms. The infected cells (including enterocytes) release selected cytokines and chemokines that induce intestinal inflammation and underlie GI symptoms [138].
Considering that the results of this study are based on a limited sample size, and SARS-COV-2 RNA was confirmed in only 35% of the patients, we should sound a note of caution about such findings. As SARS-CoV-2 infection is closely related to previous SARS in several aspects, it is assumed that SARS-CoV-2 RNA may have an ability to spread into the CNS via the membrane-bound ACE2, resulting in clinical neurological signs and symptoms [132].
NPT is an informative biomarker of central nervous system immune activation in various viral infectious settings, including HIV-1 infection and influenza [139], [140]. NPT level in the serum and cerebrospinal fluid (CSF) increased in 6 patients with moderate to severe COVID-19 illness who also presented neurological disorders. Neurologic symptoms were encephalopathy, extreme fatigue, memory loss, personality changes, mild neck stiffness, photophobia, drowsiness, dysgeusia, disorientation [132]. High CSF NPT may be inspired by a forceful systemic inflammatory response induced by SARS-CoV-2 infection [141]. This observation may outline the COVID-19-induced CSF inflammation and brain injury [69], [132]. There is still considerable ambiguity about the pathophysiological basis of profound elevated CSF NPT in COVID-19 infection and its use as a prognostic factor for neurological symptom development [141].
All we know about the role of NPT in COVID-19-induced acute kidney injury are in the light of studies evaluating NPT in severe COVID-19 setting. The severe form may be accompanied by acute kidney injury in about half of the cases [142]. Even though it is reported that elevated serum creatinine and blood urea concentrations are associated with high serum NPT, some other studies failed to provide a meaningful correlation in severe COVID-19 cases [71]. Many studies believed that high serum NPT concentrations related to the severity of the infection deteriorated renal function and higher temperature upon hospital admission [53]. Therefore, future studies on the current topic are required to elucidate the exact role of NPT in the clinical symptoms of patients with COVID-19 infection.
5. Measurement of neopterin in COVID-19
NPT is one of the measurable prognostic substances produced by humans' immune systems. Due to its cost-effective and easily detectable features, NPT has recently become a significant marker for usage in the clinic to predict disease progression. Because the high amount of NPT mirrors significantly activated cellular immunity, it has been used to diagnose several diseases and their treatment selections [10], [143]. Since the 19th-century [14], NPT levels were often detected and used as a disease progression prediction marker. Especially in infectious diseases like bacterial parasitic and viral, detecting NPT levels became highly useful in monitoring cellular immunity [144], [145]. Currently, we are facing the COVID-19 viral infection, and helpful information about the disease and its progression has become crucial. Several articles showed that measuring NPT levels can guide observing the infection degree of COVID-19 prognosis.
Bellmann-Weiler and her colleagues used 115 patients' serum samples to measure NPT levels, and they found that the NPT levels were similar to the first study, which is above 40 nmol/L. Moreover, they concluded that the high amount of NPT [ 45 nmol/L] can be helpful for the early prediction of high-risk group COVID-19 patients [71]. NPT has been chiefly detected in serum and urine [146]. Also, it showed that it could be measured in the cerebrospinal fluid [147] and saliva [148] as well, and besides this, some studies detected NPT in the synovial fluid [149] and pancreatic secretion [150]. NPT is immensely simple in body samples, and it can be made with several techniques. Table 4 represents all studies measuring and pointing to the importance of NPT levels in the COVID-19. According to the table, ELISA has been the first choice for measuring the levels of NPT. ELISA is one of the labeled immunoassays. Furthermore, this technique uses the antibody-antigen interactions as an immunocomplex to detect the desired molecule in the sample.
Table 4.
Neopterin detection techniques and levels in COVID-19.
| Sample Size | Sample | Neopterin Level (Mean) | P-Value | Measurement Technique | Ref. |
|---|---|---|---|---|---|
| 103 patients | Serum | 46 nmol/L | p < 0.001 | ELISA | [52] |
| 34 patients | Serum | 42 nmol/L | p < 0.0001 | ELISA and HPLC | [72] |
| 115 patients | Serum | 56.6 nmol/L | p < 0.001 | ELISA | [71] |
| 37 patients | Fecal Sample (Stool) | >614.7 ng/g | – | ELISA | [137] |
| 45 patients | Serum | 44.90 nmol/L | p < 0.05 | ELISA | [159] |
Generally, a particular molecule binds to its antibody that contains unique binding sites for its specific antigen. Also, the antibodies can be detected with the ELISA tests [150]. For the detection and measurement of NPT, the ELISA test contains rabbit-anti-NPT, that is, antibody binding sites for both sample NPT and enzyme-attached antigen. These antigen–antibody complexes then bind to the specific surface of the test for detection. Due to its flexibility, the ELISA test can apply and design for various diseases. The test uses up to 96 well plates, allowing one to look at the multiple samples simultaneously [151]. Thus, many models can be observed, and the results can be obtained quickly. Also, its usage is straightforward then does not need exceptional learning. The other advantageous use of ELISA is its sensitivity and specificity [150]. With a small sample size, desired substances can be detected through specific antigen–antibody interactions.
Additionally, the test has some drawbacks. While applying fluid samples, it does not need pretreatment, but non– fluid samples like stool require pretreatment. One of the studies in Table 4 used the stool sample to measure NPT levels. They made some dilution processes before the applying test on the pieces. Then they used the supernatant of the models for test respectively [148]. Other potential methods measure and detect NPT. Lately, their usage did not present in COVID-19 infection, but they used it for measuring NPT levels in several diseases and conditions. The technique RIA is one of the labeled immunoassays[152], [153]. Unlike the ELISA, the RIA technique uses radioactive isotopes rather than enzymes as a label. Although the procedure is similar to ELISA, RIA has some differences and drawbacks [153]. The trained people need to prepare and do the experiment in the RIA test due to its labeled radioactive isotopes. Also, the storage and disposal of radioactive substances require special procedures that must do carefully.
Most importantly, if these isotopes are not disposed of correctly, they can cause radiation hazardous. Notwithstanding it has some difficulties, The RIA test successfully detects biomarkers. S'anchez-Regaña et al. concluded that the determination of NPT could be done with the RIA, and results showed that the RIA is highly accurate [151].
Another potentially used technique for measuring NPT is HPLC, which uses the liquid sample mixture, several pumps, and columns to specify biological substances. Additionally, the detector of the HPLC system is enabled to determine implications quantitatively [154]. Although high-pressure liquid chromatography [HPLC] for detecting fluorimetric signals has been widely used, many of the procedures described have practical limitations. This is mainly due to the difficulty of detecting contaminant peaks in blood samples. Carru et al. used the more extended column in their experiment. In these conditions, the NPT concentration achieved with phosphate buffer was resolved from impurities. The concentration achieved with water as eluents were also decreased by about 20% wing to several features of NPT, the measurement techniques should choose carefully. The specificity of ELISA enables it to detect NPT not only in serum samples but also in urine and other body materials.
Conversely to ELISA, the HPLC method should be used to detect NPT, mostly in urine samples, in NPT levels deficient in the serum rather than urine [155]. Also, the high fluorescent feature makes NPT easily detectable in the urine samples by HPLC. Furthermore, Werner et al. found that the NPT detection with RIA was uncertain in the urine samples [156]. Therefore, they conclude that the RIA should measure NPT in serum samples. Recently, biosensors have become promising devices for the measurement of NPT. Also, with the fastly growing human population, the demand for fast and accurate point of care biosensor devices increased. Biosensors are handy analytic devices with several good features such as portability, simplicity, and the best-desired quality its specificity [157]. As a chemosensor, Sharma et al. used the molecular imprinting method that generates an artificially synthesized receptor polymer for the NPT. They showed that their molecularly imprinted film was sensitive and gave a chance to differentiate NPT analogs from samples [158]. The specification of progression of COVID-19 patients is highly possible through the measurement of NPT levels. Therefore, the demand for improved measurement techniques for NPT has become essential for characterizing the disease severity and infectious degrees of patients with the SARS COV-2 virus.
6. Prognostic value of neopterin in COVID-19
There is incomplete evidence about predictive biomarkers that could ad- vantage physicians to categorize COVID-19 cases that are likely to improve a poor outcome. In COVID-19 patients, it is recognized that overexcited inflammation and coagulopathy are related to death in patients and disease severity. The results showed that compared with mild cases of COVID-19, high levels of circulating cytokines, profound lymphopenia, and significant infiltration of mononuclear cells into the lungs and other organs occur in very severe inflammatory conditions [6]er. High circulating biomarkers such as cytokines were described in severe COVID-19 patients. The serum rates of the immune motivation NPT have been revealed to be predictive value in patients with SARS-CoV-1 [55]. Early studies suggest that serum NPT may help classify SARS-CoV-2 patients [52], [72]. It has also been shown that high levels of cytokines associated with macrophage activation, including IFN-, are present in patients with SARS-CoV-2 [7]. Also proved based on different reports that raised kynurenine/ tryptophan ratio is usual in COVID-19 and associate narrowly with elevated NPT levels.
Like NPT, kynurenine/ tryptophan is also related to undesirable results [72]. The same observations have been made in other infections, such as HIV-1 [160]. Overall, the IFN-induced immune response to viral infections may lead to increased NPT concentrations, tryptophan degradation, and increased kynurenine to tryptophan ratio [13]. According to the different results of the studies, various scenarios have been presented about the role of neoprene and how it increases in people with COVID-19. Most are related to antioxidants in controlling ROS [160]. These include a variety of vitamins, especially vitamin B; a decrease in vitamin B levels is associated with an increase in homocysteine and NPT levels in people with the disease [161], [162]. It is also one of the possible mechanisms related to iron levels. Suppose the number of inflammatory factors in the blood increases. In that case, iron may be more likely to be stored [ferritin], which the results of some studies prove the same, and the amount of NPT is associated with an increase in ferritin [163], [164]. NPT is formed by macrophage cells on motivation with IFN-, which is a primary factor in the antiviral immune reaction. Therefore, it can forecast the severity of disease in COVID-19 cases [52]. In patients with infectious diseases, improved NPT levels were known in body fluid samples like saliva, urine, blood, and CSF, e.g., during cytokine therapies but also in conditions that are related to stimulation of the T-helper-1 immune cells such as autoimmune pathologies, mycobacterial infections and numerous types of tumor cells and with viral infections including SARS-CoV-1 and HIV-1 and recently also in COVID-19 with SARS-CoV-2 [21], [73], [165], [166]. The results of studies have shown that serum levels of NPT are closely related to the severity of COVID-19, with classes starting to rise from the 3–4 days of SARS-CoV-2 infection, being correlated with severe dyspnea, Take an extended stay hospital and other complications [68]. The different measurements of NPT levels can prepare valuable data in patients with current COVID-19 disease. Higher NPT levels emerge to describe an epidemic and widespread infection and thus to an improved infectious condition. In contrast, a standard or very low NPT reveals for quiet infection lacking fewer active infections [72]. Nevertheless, further studies are needed to confirm this conclusion, which is still in the early stages.
7. Challenges and future perspectives
COVID-19 is considered a cytokine storm and affects multi-organ inflammatory infection. The severity of inflammation and coagulation ascertains the mortality rate of this pulmonary and systemic injury. Due to this, prevention early diagnosis incorporation with effective therapeutic interventions is urgently needed for saving lives. NPT is an early critical marker for the progression and severity of immune disease or may be helpful together with the several inflammatory markers to suggest a diagnosis of SARS-CoV-2. It is also postulated as a sensitive marker of oxidative stress, which could decrease inflammation through suppressing NF-B signaling and NLRP3 inflammasomes. Other studies indicated that NPT contributes to high ROS and NF-B production, which leads to pro-inflammatory gene rise, including inducible nitric oxide synthase [iNOS], and triggers inflammatory processes.
Furthermore, we need to consider that the elevated level of NPT may be related to other pathological conditions or inflammation-related diseases not only confined to COVID-19, such as RA, nephropathy, neuropsychiatric anomalies, or cancers. Within this view, further studies are required to address the exact role of NPT in the clinical symptoms of patients with COVID-19 infection. Also, it is essential to highlight that among the knowledge gaps of COVID-19, there are diagnostic errors with laboratory testing and their interpretation in patient management. While NPT appears in the blood before the onset of clinical symptoms, it is considered as an independent prognostic factor for COVID-19 severity. However, what seems to issue from this evaluation is that NPT values have significantly enhanced in individuals with severe SARS-CoV-2 infection compared to those with milder forms of the disease. Therefore, it could be logical to assume immediate measurement of cellular immune activation marker, namely NPT in patients and subsequently longitudinal monitoring, to identify a subgroup of patients with progressive inflammatory situations.
8. Conclusion
In conclusion, NPT level has a significant correlation with the severity of COVID-19 and can be considered a macrophage activation and sensitive indicator to predict disease risk. Further studies should also be planned to clarify whether targeting NPT in SARS-CoV-2 infection may be critical in early assessing infected patients' disease progression and prognosis.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Zhao Q., Meng M., Kumar R., Wu Y., Huang J., Deng Y., Weng Z., Yang L., Lymphopenia is associated with severe coronavirus disease (COVID-19) infections: a systemic review and meta-analysis. Int. J. Infect. Dis. 2019;96(2020):131–135. doi: 10.1016/j.ijid.2020.04.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zheng Z., Peng F., Xu B., Zhao J., Liu H., Peng J., Li Q., Jiang C., Zhou Y., Liu S., Ye C., Zhang P., Xing Y., Guo H., Tang W. Risk factors of critical & mortal COVID-19 cases: a systematic literature review and meta-analysis. J. Infect. 2020;81(2):e16–e25. doi: 10.1016/j.jinf.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang Jing, Zheng Ya, Gou Xi, Pu Ke, Chen Zhaofeng, Guo Qinghong, Ji Rui, Wang Haojia, Wang Yuping, Zhou Yongning. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int. J. Infect. Dis. 2020;94:91–95. doi: 10.1016/j.ijid.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bentivegna E., Luciani M., Spuntarelli V., Speranza M.L., Guerritore L., Sentimentale A., Martelletti P. Extremely severe case of COVID-19 pneumonia recovered despite bad prognostic indicators: a didactic report. SN Compreh. Clin. Med. 2020;2(8):1204–1207. doi: 10.1007/s42399-020-00383-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.N. Kaur, I. Gupta, H. Singh, R. Karia, A. Ashraf, A. Habib, U.K. Patel, P. Malik, Epidemiological and Clinical Characteristics of 6635 COVID-19 Patients: a Pooled Analysis, SN comprehensive clinical medicine (2020) 1-5. [DOI] [PMC free article] [PubMed]
- 6.Merad M., Martin J.C. Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat. Rev. Immunol. 2020;20(6):355–362. doi: 10.1038/s41577-020-0331-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jungraithmayr Therese C, Reschke Marco, Grebe Scott O, Lange Harald, Radsak Klaus, Mueller Thomas F. Assessment of cytomegalovirus infections using neopterin and a new immunoblot. Clin. Chim. Acta. 2001;310(1):63–69. doi: 10.1016/s0009-8981(01)00528-9. [DOI] [PubMed] [Google Scholar]
- 8.Michalak Łukasz, Bulska Magdalena, Strząbała Karolina, Szcześniak Piotr. Neopterin as a marker of cellular immunological response. Postepy Hig Med Dosw (Online) 2017;71(1) doi: 10.5604/01.3001.0010.3851. [DOI] [PubMed] [Google Scholar]
- 9.Pizzini A., Kurz K., Santifaller J., Tschurtschenthaler C., Theurl I., Fuchs D., Weiss G., Bellmann-Weiler R. Assessment of neopterin and indoleamine 2,3-dioxygenase activity in patients with seasonal influenza: a pilot study, Influenza Other Respir. Viruses. 2019;13(6):603–609. doi: 10.1111/irv.12677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eisenhut M. Neopterin in diagnosis and monitoring of infectious diseases. J. Biomark. 2013;2013 doi: 10.1155/2013/196432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chan C.P.Y., Choi J.W.Y., Cao K.-Y., Wang M., Gao Y., Zhou D.-H., Di B., Xu H.-F., Leung M.-F., Bergmann A., Lehmann M., Nie Y.-M., Cautherley G.W.H., Fuchs D., Renneberg R., Zheng B.-J. Detection of serum neopterin for early assessment of dengue virus infection. J. Infect. 2006;53(3):152–158. doi: 10.1016/j.jinf.2005.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Müller Mathias M., Curtius Hans-Christoph, Herold Manfred, Huber Christoph H. Neopterin in clinical practice. Clin. Chim. Acta. 1991;201(1-2):1–16. doi: 10.1016/0009-8981(91)90019-9. [DOI] [PubMed] [Google Scholar]
- 13.Murr C., Widner B., Wirleitner B., Fuchs D. Neopterin as a marker for immune system activation. Curr. Drug Metab. 2002;3(2):175–187. doi: 10.2174/1389200024605082. [DOI] [PubMed] [Google Scholar]
- 14.Fuchs D., Weiss G., Wachter H. Neopterin, biochemistry and clinical use as a marker for cellular immune reactions. Int. Arch. Allergy Immunol. 1993;101(1):1–6. doi: 10.1159/000236491. [DOI] [PubMed] [Google Scholar]
- 15.Wirleitner B., Reider D., Ebner S., Böck G., Widner B., Jaeger M., Schennach H., Romani N., Fuchs D. Monocyte-derived dendritic cells release neopterin. J. Leukoc. Biol. 2003;72:1148–1153. [PubMed] [Google Scholar]
- 16.Sghiri R., Feinberg J., Thabet F., Dellagi K., Boukadida J., Ben Abdelaziz A., Casanova J.L., Barbouche M.R. Gamma interferon is dispensable for neopterin production in vivo. Clin. Diagn. Lab. Immunol. 2005;12(12):1437–1441. doi: 10.1128/CDLI.12.12.1437-1441.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Werner-Felmayer G., Werner E.R., Fuchs D., Hausen A., Reibnegger G., Wachter H. Tumour necrosis factor-alpha and lipopolysaccharide enhance interferon-induced tryptophan degradation and pteridine synthesis in human cells. Biol Chem Hoppe Seyler. 1989;370(9):1063–1069. doi: 10.1515/bchm3.1989.370.2.1063. [DOI] [PubMed] [Google Scholar]
- 18.Terness P., Bauer T.M., Röse L., Dufter C., Watzlik A., Simon H., Opelz G. Inhibition of allogeneic T cell proliferation by indoleamine 2,3-dioxygenase-expressing dendritic cells: mediation of suppression by tryptophan metabolites. J. Exp. Med. 2002;196(4):447–457. doi: 10.1084/jem.20020052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hol Jaap Willem, Stolker Robert J, Klimek Markus, Stronks Dirk L, Fekkes Durk. The tryptophan kynurenine pathway, neopterin and IL-6 during vulvectomy and abdominal hysterectomy. J. Biomed. Sci. 2014;21(1) doi: 10.1186/s12929-014-0102-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fuchs Dietmar, Möller Arnulf A., Reibnegger Gilbert, Werner Ernst R., Werner-Felmayer Gabriele, Dierich Manfred P., Wachter Helmut. Increased endogenous interferon-gamma and neopterin correlate with increased degradation of tryptophan in human immunodeficiency virus type 1 infection. Immunol. Lett. 1991;28(3):207–211. doi: 10.1016/0165-2478(91)90005-u. [DOI] [PubMed] [Google Scholar]
- 21.Hailemichael W., Kiros M., Akelew Y., Getu S., Andualem H. Neopterin: a promising candidate biomarker for severe COVID-19. J. Inflamm. .earch. 2021;14:245–251. doi: 10.2147/JIR.S290264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Toygar M., Aydin I., Agilli M., Aydin F.N., Oztosun M., Gul H., Macit E., Karslioglu Y., Topal T., Uysal B., Honca M. The relation between oxidative stress, inflammation, and neopterin in the paraquat-induced lung toxicity. Hum. Exp. Toxicol. 2015;34(2):198–204. doi: 10.1177/0960327114533808. [DOI] [PubMed] [Google Scholar]
- 23.Schobersberger W., Hoffmann G., Grote J., Wachter H., Fuchs D. Induction of inducible nitric oxide synthase expression by neopterin in vascular smooth muscle cells. FEBS Lett. 1995;377(3):461–464. doi: 10.1016/0014-5793(95)01393-8. [DOI] [PubMed] [Google Scholar]
- 24.Berdowska A., Zwirska-Korczala K. Neopterin measurement in clinical diagnosis. J. Clin. Pharm. Ther. 2001;26(5):319–329. doi: 10.1046/j.1365-2710.2001.00358.x. [DOI] [PubMed] [Google Scholar]
- 25.Svoboda Peter, Ko Seong-Hee, Cho BeLong, Yoo Sang-Ho, Choi Seong-Won, Ye Sang-Kyu, Kasai Hiroshi, Chung Myung-Hee. Neopterin, a marker of immune response, and 8-hydroxy-2'-deoxyguanosine, a marker of oxidative stress, correlate at high age as determined by automated simultaneous high-performance liquid chromatography analysis of human urine. Anal. Biochem. 2008;383(2):236–242. doi: 10.1016/j.ab.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 26.Wirleitner Barbara, Schroecksnadel Katharina, Winkler Christiana, Fuchs Dietmar. Neopterin in HIV-1 infection. Mol. Immunol. 2005;42(2):183–194. doi: 10.1016/j.molimm.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 27.El-Lebedy Dalia, Hussein Jihan, Ashmawy Ingy, Mohammed Asmaa M. Serum level of neopterin is not a marker of disease activity in treated rheumatoid arthritis patients. Clin. Rheumatol. 2017;36(9):1975–1979. doi: 10.1007/s10067-016-3433-4. [DOI] [PubMed] [Google Scholar]
- 28.Asci A., Baydar T., Cetinkaya R., Dolgun A., Sahin G. Evaluation of neopterin levels in patients undergoing hemodialysis. Hemodial Int. 2010;14(2):240–246. doi: 10.1111/j.1542-4758.2010.00439.x. [DOI] [PubMed] [Google Scholar]
- 29.Widner B., Laich A., Sperner-Unterweger B., Ledochowski M., Fuchs D. Neopterin production, tryptophan degradation, and mental depression—What is the link? Brain Behav. Immun. 2002;16:590–595. doi: 10.1016/s0889-1591(02)00006-5. [DOI] [PubMed] [Google Scholar]
- 30.Widner B., Leblhuber F., Fuchs D. Increased neopterin production and tryptophan degradation in advanced Parkinson's disease. J. Neural Transm. (Vienna) 2002;109(2):181–189. doi: 10.1007/s007020200014. [DOI] [PubMed] [Google Scholar]
- 31.Peng Q.L., Zhang Y.M., Liang L., Liu X., Ye L.F., Yang H.B., Zhang L., Shu X.M., Lu X., Wang G.C. A high level of serum neopterin is associated with rapidly progressive interstitial lung disease and reduced survival in dermatomyositis. Clin. Exp. Immunol. 2020;199(3):314–325. doi: 10.1111/cei.13404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mjelva Øistein, Svingen Gard, Pedersen Eva, Seifert Reinhard, Kvaløy Jan, Midttun Øivind, Ueland Per, Nordrehaug Jan, Nygård Ottar, Nilsen Dennis. Fibrinogen and neopterin is associated with future myocardial infarction and total mortality in patients with stable coronary artery disease. Thromb. Haemost. 2018;47(04):778–790. doi: 10.1055/s-0038-1629912. [DOI] [PubMed] [Google Scholar]
- 33.Pichler Renate, Fritz Josef, Heidegger Isabel, Steiner Eberhard, Culig Zoran, Klocker Helmut, Fuchs Dietmar. Predictive and prognostic role of serum neopterin and tryptophan breakdown in prostate cancer. Cancer Sci. 2017;108(4):663–670. doi: 10.1111/cas.13171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yalcin S., Demir M.E., Ozturk R., Kılınç A.Ş., Suer H., Karahan I. Prognostic effects of SuPAR and neopterin levels on patients with lung cancer. Pteridines. 2020;31(1):136–141. [Google Scholar]
- 35.Akyurek Fikret, Tuncez Akyurek Fatma. Investigation of pregnancy associated plasma protein-A and neopterin levels in Behçet's patients. Dermatol. Ther. 2020;33(4) doi: 10.1111/dth.v33.410.1111/dth.13443. [DOI] [PubMed] [Google Scholar]
- 36.Kemeriz F., Gönül M., Cengiz F.P., Emiroğlu N., Cemil B. Evaluation of neopterin level and disease severity in patients with psoriasis vulgaris treated with narrowband UVB. Ind. J. Dermatol. 2019;64(6):447–450. doi: 10.4103/ijd.IJD_53_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Akbulut H. Handan, Celik Ilhami, Akbulut Ayhan, Yuce Pinar, Kiliç S. Sirri. Serum neopterin levels in patients with brucellosis. J. Infect. 2005;51(4):281–286. doi: 10.1016/j.jinf.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 38.Barutcuoglu B., Bozdemir A.E., Dereli D., Parildar Z., Mutaf M.I., Ozmen D., Bayindir O. Increased serum neopterin levels in women with polycystic ovary syndrome. Ann. Clin. Lab. Sci. 2006;36(3):267–272. [PubMed] [Google Scholar]
- 39.Yildirim Yesim, Gunel Nazan, Coskun Ugur, Pasaoglu Hatice, Aslan Sabahattin, Cetin Abdullah. Serum neopterin levels in patients with breast cancer. Med. Oncol. 2008;25(4):403–407. doi: 10.1007/s12032-008-9054-2. [DOI] [PubMed] [Google Scholar]
- 40.Wagner R., Hayatghebi S., Rosenkranz M., Reinwein D. Increased serum neopterin levels in patients with Graves' disease. Exp Clin. Endocrinol. 1993;101(04):249–254. doi: 10.1055/s-0029-1211240. [DOI] [PubMed] [Google Scholar]
- 41.A. Hacışevki, Neopterin: a possible biomarker in gastrointestinal cancer, Ankara Üniversitesi Eczacılık Fakültesi Dergisi 42 (2018) 32-41.
- 42.Kondera-Anasz Zdzislawa, Mertas Anna. Level of serum neopterin and interleukin-6 in patients with thyroid diseases. Pteridines. 1999;10(4):197–201. [Google Scholar]
- 43.Höbarth K., Szabo N., Hallas A., Aulitzky W., Marberger M. Serum neopterin as a parameter for monitoring the course of renal cell carcinoma during interferon-gamma therapy. Clin. Immunol. Immunopathol. 1994;70(3):241–244. doi: 10.1006/clin.1994.1035. [DOI] [PubMed] [Google Scholar]
- 44.Nancey Stephane, Boschetti Gilles, Moussata Driffa, Cotte Eddy, Peyras Julie, Cuerq Charlotte, Haybrard Julie, Charlois Anne-Laure, Mialon Anne, Chauvenet Marion, Stroeymeyt Karine, Kaiserlian Dominique, Drai Jocelyne, Flourié Bernard. Neopterin is a novel reliable fecal marker as accurate as calprotectin for predicting endoscopic disease activity in patients with inflammatory bowel diseases. Inflamm. Bowel Dis. 2013;19(5):1043–1052. doi: 10.1097/MIB.0b013e3182807577. [DOI] [PubMed] [Google Scholar]
- 45.Bonagura V.R., Rosenthal D.W. Infections that cause secondary immune deficiency. Stiehm's Immune Deficiencies. 2020:1035–1058. [Google Scholar]
- 46.Lopes Mariana, Marques Patrícia, Silva Bruno, Cruz Gonçalo, Serra José Eduardo, Ferreira Eugenia, Alves Helena, da Cunha José Saraiva. Guillain-Barré syndrome as the first presentation of human immunodeficiency virus infection. BMC Neurol. 2021;21(1) doi: 10.1186/s12883-021-02350-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.R. Purrmann, Konstitution und Synthese des sogenannten Anhydroleukopterins. Über die Flügelpigmente der Schmetterlinge XII, Justus Liebigs Annalen der Chemie 548 (2006) 284-292.
- 48.H. Rembold, L. Buschmann, Struktur und Synthese des Neopterins, Chemische Berichte 96 (1963) 1406-1410.
- 49.Biron Christine A. Cytokines in the generation of immune responses to, and resolution of, virus infection. Curr. Opin. Immunol. 1994;6(4):530–538. doi: 10.1016/0952-7915(94)90137-6. [DOI] [PubMed] [Google Scholar]
- 50.Ding X., Li S., Zhu L. Potential effects of HMGB1 on viral replication and virus infection-induced inflammatory responses: a promising therapeutic target for virus infection-induced inflammatory diseases. Cytokine Growth Factor Rev. 2021;62:54–61. doi: 10.1016/j.cytogfr.2021.08.003. [DOI] [PubMed] [Google Scholar]
- 51.Sakurai A., Goto M. Neopterin: isolation from human urine. J. Biochem. 1967;61(1):142–145. doi: 10.1093/oxfordjournals.jbchem.a128513. [DOI] [PubMed] [Google Scholar]
- 52.Ozger Hasan Selcuk, Dizbay Murat, Corbacioglu Seref Kerem, Aysert Pinar, Demirbas Zehra, Tunccan Ozlem Guzel, Hizel Kenan, Bozdayi Gulendam, Caglar Kayhan. The prognostic role of neopterin in COVID-19 patients. J. Med. Virol. 2021;93(3):1520–1525. doi: 10.1002/jmv.26472. [DOI] [PubMed] [Google Scholar]
- 53.Nathan C F. Secretory products of macrophages. J. Clin. Investig. 1987;79(2):319–326. doi: 10.1172/JCI112815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hausen Arno, Fuchs Dietmar, Grünewald Kurt, Huber Heinz, König Klaus, Wechter Helmut. Urinary neopterine as marker for haematological neoplasias. Clin. Chim. Acta. 1981;117(3):297–305. doi: 10.1016/0009-8981(81)90117-0. [DOI] [PubMed] [Google Scholar]
- 55.Zheng Bojian, Cao Kai-Yuan, Chan Cangel P.Y., Choi Junet W.Y., Leung Wingman, Leung Manfai, Duan Zhao-Hui, Gao Yang, Wang Ming, Di Biao, Hollidt Jörg M., Bergmann Andreas, Lehmann Matthias, Renneberg Ilka, Tam John S.L., Chan Paul K.S., Cautherley George W.H., Fuchs Dietmar, Renneberg Reinhard. Serum neopterin for early assessment of severity of severe acute respiratory syndrome. Clin. Immunol. 2005;116(1):18–26. doi: 10.1016/j.clim.2005.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Petrova Velislava N., Russell Colin A. The evolution of seasonal influenza viruses. Nat. Rev. Microbiol. 2018;16(1):47–60. doi: 10.1038/nrmicro.2017.118. [DOI] [PubMed] [Google Scholar]
- 57.Reibnegger Gilbert, Auhuber Ingeborg, Fuchs Dietmar, Hausen Arno, Judmaier Gert, Prior Christian, Werner Ernst R., Wachter Helmut. Urinary neopterin levels in acute viral hepatitis. Hepatology. 1988;8(4):771–774. doi: 10.1002/hep.1840080412. [DOI] [PubMed] [Google Scholar]
- 58.Reibnegger G., Fuchs D., Hausen A., Werner E.R., Werner-Felmayer G., Wachter H. Neopterin and viral infections: diagnostic potential in virally induced liver disease. Biomed. Pharmacother. 1989;43(4):287–293. doi: 10.1016/0753-3322(89)90010-3. [DOI] [PubMed] [Google Scholar]
- 59.Nübling C.M., Chudy M., Volkers P., Löwer J. Neopterin levels during the early phase of human immunodeficiency virus, hepatitis C virus, or hepatitis B virus infection. Transfusion. 2006;46(11):1886–1891. doi: 10.1111/j.1537-2995.2006.00994.x. [DOI] [PubMed] [Google Scholar]
- 60.Mildvan D., Spritzler J., Grossberg S.E., Fahey J.L., Johnston D.M., Schock B.R., Kagan J. Serum neopterin, an immune activation marker, independently predicts disease progression in advanced HIV-1 infection. Clin. Infect. Dis. 2005;40(6):853–858. doi: 10.1086/427877. [DOI] [PubMed] [Google Scholar]
- 61.Hojyo S., Uchida M., Tanaka K., Hasebe R., Tanaka Y., Murakami M., Hirano T. How COVID-19 induces cytokine storm with high mortality. Inflamm Regen. 2020;40:37. doi: 10.1186/s41232-020-00146-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Robba C., Battaglini D., Pelosi P., Rocco P.R.M. Multiple organ dysfunction in SARS-CoV-2: MODS-CoV-2. Expert Rev Respir Med. 2020;14(9):865–868. doi: 10.1080/17476348.2020.1778470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.M. Abdelmoaty, P. Yeapuri, J. Machhi, K. Olson, F. Shahjin, Y. Zhou, L. Jingjing, K. Pandey, A. Acharya, S. Byrareddy, L. Mosley, H. Gendelman, Defining the Immune Responses for SARS-CoV-2-Human Macrophage Interactions, bioRxiv (2021). [DOI] [PMC free article] [PubMed]
- 64.Kandasamy M. NF-κB signalling as a pharmacological target in COVID-19: potential roles for IKKβ inhibitors. Naunyn Schmiedebergs Arch. Pharmacol. 2021;394(3):561–567. doi: 10.1007/s00210-020-02035-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hariharan Apurva, Hakeem Abdul Rahman, Radhakrishnan Subathra, Reddy Mettu Srinivas, Rela Mohamed. The Role and Therapeutic Potential of NF-kappa-B Pathway in Severe COVID-19 Patients. Inflammopharmacology. 2021;29(1):91–100. doi: 10.1007/s10787-020-00773-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Freeman T.L., Swartz T.H. Targeting the NLRP3 Inflammasome in Severe COVID-19. Front. Immunol. 2020;11:1518. doi: 10.3389/fimmu.2020.01518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.de Paula Martins Roberta, Ghisoni Karina, Lim Chai K., Aguiar Aderbal Silva, Guillemin Gilles J., Latini Alexandra. Neopterin preconditioning prevents inflammasome activation in mammalian astrocytes. Free Radic. Biol. Med. 2018;115:371–382. doi: 10.1016/j.freeradbiomed.2017.11.022. [DOI] [PubMed] [Google Scholar]
- 68.Al-Kuraishy H.M., Al-Gareeb A.I., Alzahrani K.J., Cruz-Martins N., Batiha G.E.-S. The potential role of neopterin in Covid-19: a new perspective. Mol. Cell. Biochem. 2021;476(11):4161–4166. doi: 10.1007/s11010-021-04232-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zeng Furong, Huang Yuzhao, Guo Ying, Yin Mingzhu, Chen Xiang, Xiao Liang, Deng Guangtong. Association of inflammatory markers with the severity of COVID-19: a meta-analysis. Int. J. Infect. Dis. 2020;96:467–474. doi: 10.1016/j.ijid.2020.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Palabiyik S.S., Girgin G., Tutkun E., Yilmaz O.H., Baydar T. Immunomodulation and oxidative stress in denim sandblasting workers: changes caused by silica exposure. Arh Hig Rada Toksikol. 2013;64(3):431–437. doi: 10.2478/10004-1254-64-2013-2312. [DOI] [PubMed] [Google Scholar]
- 71.Bellmann-Weiler R., Lanser L., Burkert F., Seiwald S., Fritsche G., Wildner S., Schroll A., Koppelstätter S., Kurz K., Griesmacher A., Weiss G. Neopterin predicts disease severity in hospitalized patients With COVID-19, open forum. Infect. Dis. 2021;8(1):ofaa521. doi: 10.1093/ofid/ofaa521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Robertson J., Gostner J.M., Nilsson S., Andersson L.M., Fuchs D., Gisslen M. Serum neopterin levels in relation to mild and severe COVID-19. BMC Infect. Dis. 2020;20(1):942. doi: 10.1186/s12879-020-05671-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Edén A., Kanberg N., Gostner J., Fuchs D., Hagberg L., Andersson L.M., Lindh M., Price R.W., Zetterberg H., Gisslén M. CSF Biomarkers in patients with COVID-19 and neurologic symptoms: a case series. Neurology. 2021;96(2):e294–e300. doi: 10.1212/WNL.0000000000010977. [DOI] [PubMed] [Google Scholar]
- 74.Samad N., Sodunke T.E., Abubakar A.R., Jahan I., Sharma P., Islam S., Dutta S., Haque M. The implications of zinc therapy in combating the COVID-19 global pandemic. J. Inflamm. Res. 2021;14:527–550. doi: 10.2147/JIR.S295377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Overbeck S., Rink L., Haase H. Modulating the immune response by oral zinc supplementation: a single approach for multiple diseases. Arch. Immunol. Ther. Exp. (Warsz) 2008;56(1):15–30. doi: 10.1007/s00005-008-0003-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Younus H. Therapeutic potentials of superoxide dismutase. Int. J. Health Sci. 2018;12:88–93. [PMC free article] [PubMed] [Google Scholar]
- 77.Allen J.I., Perri R.T., McClain C.J., Kay N.E. Alterations in human natural killer cell activity and monocyte cytotoxicity induced by zinc deficiency. J. Lab. Clin. Med. 1983;102(4):577–589. [PubMed] [Google Scholar]
- 78.Murgia C., Lang C., Truong-Tran A., Grosser D., Jayaram L., Ruffin R., Perozzi G., Zalewski P. Zinc and its specific transporters as potential targets in airway disease. Curr. Drug Targets. 2006;7:607–627. doi: 10.2174/138945006776818683. [DOI] [PubMed] [Google Scholar]
- 79.te Velthuis Aartjan J.W., van den Worm Sjoerd H.E., Sims Amy C., Baric Ralph S., Snijder Eric J., van Hemert Martijn J., Andino Raul. Zn2+ Inhibits coronavirus and arterivirus RNA polymerase activity in vitro and zinc ionophores block the replication of these viruses in cell culture. PLoS Pathog. 2010;6(11):e1001176. doi: 10.1371/journal.ppat.1001176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shang Jian, Wan Yushun, Luo Chuming, Ye Gang, Geng Qibin, Auerbach Ashley, Li Fang. Cell entry mechanisms of SARS-CoV-2. Proc. Natl. Acad. Sci. U.S.A. 2020;117(21):11727–11734. doi: 10.1073/pnas.2003138117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Patel Vaibhav B., Zhong Jiu-Chang, Grant Maria B., Oudit Gavin Y. Role of the ACE2/angiotensin 1–7 axis of the renin-angiotensin system in heart failure. Circ. Res. 2016;118(8):1313–1326. doi: 10.1161/CIRCRESAHA.116.307708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Thomas Suma, Patel Divyang, Bittel Barbara, Wolski Kathy, Wang Qiuqing, Kumar Anirudh, Il’Giovine Zachary J., Mehra Reena, McWilliams Carla, Nissen Steve E., Desai Milind Y. Effect of high-dose zinc and ascorbic acid supplementation vs usual care on symptom length and reduction among ambulatory patients with SARS-CoV-2 infection: the COVID A to Z randomized clinical trial. JAMA Netw Open. 2021;4(2):e210369. doi: 10.1001/jamanetworkopen.2021.0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Abd-Elsalam S., Soliman S., Esmail E.S., Khalaf M., Mostafa E.F., Medhat M.A., Ahmed O.A., El Ghafar M.S.A., Alboraie M., Hassany S.M. Do zinc supplements enhance the clinical efficacy of hydroxychloroquine?: a randomized multicenter trial. Biol Trace Elem. Res. 2021;199(10):3642–3646. doi: 10.1007/s12011-020-02512-1. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 84.Turner Ronald B. Ineffectiveness of intranasal zinc gluconate for prevention of experimental rhinovirus colds. Clin. Infect. Dis. 2001;33(11):1865–1870. doi: 10.1086/324347. [DOI] [PubMed] [Google Scholar]
- 85.Mahalanabis D., Lahiri M., Paul D., Gupta S., Gupta A., Wahed M.A., Khaled M.A. Randomized, double-blind, placebo-controlled clinical trial of the efficacy of treatment with zinc or vitamin A in infants and young children with severe acute lower respiratory infection. Am. J. Clin. Nutr. 2004;79(3):430–436. doi: 10.1093/ajcn/79.3.430. [DOI] [PubMed] [Google Scholar]
- 86.Barnett J., Dao M., Hamer D., Kandel R., Brandeis G., Wu D., Dallal G., Jacques P., Schreiber R., Kong E., Meydani S. Effect of zinc supplementation on serum zinc concentration and T cell proliferation in nursing home elderly: a randomized, double-blind, placebo-controlled trial1. Am. J. Clin. Nutr. 2016;103 doi: 10.3945/ajcn.115.115188. [DOI] [PubMed] [Google Scholar]
- 87.Prasad Ananda S., Beck Frances W.J., Bao Bin, Snell Diane, Fitzgerald James T. Duration and severity of symptoms and levels of plasma interleukin-1 receptor antagonist, soluble tumor necrosis factor receptor, and adhesion molecules in patients with common cold treated with zinc acetate. J. Infect. Dis. 2008;197(6):795–802. doi: 10.1086/528803. [DOI] [PubMed] [Google Scholar]
- 88.Isbaniah F., Wiyono W.H., Yunus F., Setiawati A., Totzke U., Verbruggen M.A. Echinacea purpurea along with zinc, selenium and vitamin C to alleviate exacerbations of chronic obstructive pulmonary disease: results from a randomized controlled trial. J. Clin. Pharm. Ther. 2011;36(5):568–576. doi: 10.1111/j.1365-2710.2010.01212.x. [DOI] [PubMed] [Google Scholar]
- 89.Sánchez Juliana, Villada Oscar Alonso, Rojas Maylen Liseth, Montoya Liliana, Díaz Alejandro, Vargas Cristian, Chica Javier, Herrera Ana Milena. Efecto del zinc aminoquelado y el sulfato de zinc en la incidencia de la infección respiratoria y la diarrea en niños preescolares de centros infantiles. Biomédica. 2013;34(1):79. doi: 10.1590/S0120-41572014000100011. [DOI] [PubMed] [Google Scholar]
- 90.Rerksuppaphol S., Rerksuppaphol L. A randomized controlled trial of zinc supplementation in the treatment of acute respiratory tract infection in Thai children. Pediatr Rep. 2019;11(2):7954. doi: 10.4081/pr.2019.7954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Al-Samkari Hanny, Berliner Nancy. Hemophagocytic Lymphohistiocytosis. Annu. Rev. Pathol. 2018;13(1):27–49. doi: 10.1146/annurev-pathol-020117-043625. [DOI] [PubMed] [Google Scholar]
- 92.Suntharalingam Ganesh, Perry Meghan R., Ward Stephen, Brett Stephen J., Castello-Cortes Andrew, Brunner Michael D., Panoskaltsis Nicki. Cytokine storm in a phase 1 trial of the anti-CD28 monoclonal antibody TGN1412. N. Engl. J. Med. 2006;355(10):1018–1028. doi: 10.1056/NEJMoa063842. [DOI] [PubMed] [Google Scholar]
- 93.Fam Christine M., Eisenberg Stephen P., Carlson Sharon J., Chlipala Elizabeth A., Cox George N., Rosendahl Mary S. PEGylation improves the pharmacokinetic properties and ability of interferon gamma to inhibit growth of a human tumor xenograft in athymic mice. J. Interferon Cytokine Res. 2014;34(10):759–768. doi: 10.1089/jir.2013.0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rabaan A.A., Al-Ahmed S.H., Muhammad J., Khan A., Sule A.A., Tirupathi R., Mutair A.A., Alhumaid S., Al-Omari A., Dhawan M., Tiwari R., Sharun K., Mohapatra R.K., Mitra S., Bilal M., Alyami S.A., Emran T.B., Moni M.A., Dhama K. Role of inflammatory cytokines in COVID-19 patients: a review on molecular mechanisms, immune functions, immunopathology and immunomodulatory drugs to counter cytokine storm. Vaccines (Basel) 2021;9(5) doi: 10.3390/vaccines9050436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kasahara T., Hooks J.J., Dougherty S.F., Oppenheim J.J. Interleukin 2-mediated immune interferon (IFN-gamma) production by human T cells and T cell subsets. J. Immunol. 1983;130(4):1784–1789. [PubMed] [Google Scholar]
- 96.Matsushita Hirokazu, Hosoi Akihiro, Ueha Satoshi, Abe Jun, Fujieda Nao, Tomura Michio, Maekawa Ryuji, Matsushima Kouji, Ohara Osamu, Kakimi Kazuhiro. Cytotoxic T lymphocytes block tumor growth both by lytic activity and IFNγ-dependent cell-cycle arrest. Cancer Immunol. Res. 2015;3(1):26–36. doi: 10.1158/2326-6066.CIR-14-0098. [DOI] [PubMed] [Google Scholar]
- 97.Girardi M., Glusac E., Filler R.B., Roberts S.J., Propperova I., Lewis J., Tigelaar R.E., Hayday A.C. The distinct contributions of murine T cell receptor (TCR)gammadelta+ and TCRalphabeta+ T cells to different stages of chemically induced skin cancer. J. Exp. Med. 2003;198(5):747–755. doi: 10.1084/jem.20021282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ribot J.C., deBarros A., Pang D.J., Neves J.F., Peperzak V., Roberts S.J., Girardi M., Borst J., Hayday A.C., Pennington D.J., Silva-Santos B. CD27 is a thymic determinant of the balance between interferon-gamma- and interleukin 17-producing gammadelta T cell subsets. Nat. Immunol. 2009;10(4):427–436. doi: 10.1038/ni.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yu J., Wei M., Becknell B., Trotta R., Liu S., Boyd Z., Jaung M.S., Blaser B.W., Sun J., Benson D.M., Jr., Mao H., Yokohama A., Bhatt D., Shen L., Davuluri R., Weinstein M., Marcucci G., Caligiuri M.A. Pro- and anti-inflammatory cytokine signaling: reciprocal antagonism regulates interferon-gamma production by human natural killer cells. Immunity. 2006;24(5):575–590. doi: 10.1016/j.immuni.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 100.Bao Yan, Liu Xingguang, Han Chaofeng, Xu Sheng, Xie Bin, Zhang Qian, Gu Yan, Hou Jin, Qian Li, Qian Cheng, Han Huanxing, Cao Xuetao. Identification of IFN-γ-producing innate B cells. Cell Res. 2014;24(2):161–176. doi: 10.1038/cr.2013.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.D. Griffin, Cytokines and Chemokines, 2008, pp. 620–624.
- 102.Lau J.F., Horvath C.M. Mechanisms of Type I interferon cell signaling and STAT-mediated transcriptional responses. Mt Sinai J. Med. 2002;69(3):156–168. [PubMed] [Google Scholar]
- 103.Platanias Leonidas C. Mechanisms of type-I- and type-II-interferon-mediated signalling. Nat. Rev. Immunol. 2005;5(5):375–386. doi: 10.1038/nri1604. [DOI] [PubMed] [Google Scholar]
- 104.Itai T., Tanaka M., Nagata S. Processing of tumor necrosis factor by the membrane-bound TNF-alpha-converting enzyme, but not its truncated soluble form. Eur. J. Biochem. 2001;268(7):2074–2082. doi: 10.1046/j.1432-1327.2001.02085.x. [DOI] [PubMed] [Google Scholar]
- 105.Spriggs D.R., Deutsch S., Kufe D.W. Genomic structure, induction, and production of TNF-alpha. Immunol. Ser. 1992;56:3–34. [PubMed] [Google Scholar]
- 106.Aggarwal B.B., Gupta S.C., Kim J.H. Historical perspectives on tumor necrosis factor and its superfamily: 25 years later, a golden journey. Blood. 2012;119(3):651–665. doi: 10.1182/blood-2011-04-325225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Vilcek J, Lee T H. Tumor necrosis factor. New insights into the molecular mechanisms of its multiple actions. J. Biol. Chem. 1991;266(12):7313–7316. [PubMed] [Google Scholar]
- 108.Banner D.W., D'Arcy A., Janes W., Gentz R., Schoenfeld H.J., Broger C., Loetscher H., Lesslauer W. Crystal structure of the soluble human 55 kd TNF receptor-human TNF beta complex: implications for TNF receptor activation. Cell. 1993;73(3):431–445. doi: 10.1016/0092-8674(93)90132-a. [DOI] [PubMed] [Google Scholar]
- 109.Locksley R.M., Killeen N., Lenardo M.J. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell. 2001;104(4):487–501. doi: 10.1016/s0092-8674(01)00237-9. [DOI] [PubMed] [Google Scholar]
- 110.Faustman D.L., Davis M. TNF Receptor 2 and Disease: Autoimmunity and Regenerative Medicine. Front. Immunol. 2013;4:478. doi: 10.3389/fimmu.2013.00478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kalliolias George D., Ivashkiv Lionel B. TNF biology, pathogenic mechanisms and emerging therapeutic strategies. Nat. Rev. Rheumatol. 2016;12(1):49–62. doi: 10.1038/nrrheum.2015.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.A.Q. Angel A. Justiz Vaillant, Interleukin, 2021.
- 113.Narazaki Masashi, Kishimoto Tadamitsu. The two-faced cytokine IL-6 in host defense and diseases. Int. J. Mol. Sci. 2018;19(11):3528. doi: 10.3390/ijms19113528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Velazquez-Salinas L., Verdugo-Rodriguez A., Rodriguez L.L., Borca M.V. The role of interleukin 6 during viral infections. Front. Microbiol. 2019;10 doi: 10.3389/fmicb.2019.01057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Golomb B.A., Evans M.A. Statin adverse effects : a review of the literature and evidence for a mitochondrial mechanism. Am. J. Cardiovasc Drugs. 2008;8(6):373–418. doi: 10.2165/0129784-200808060-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Fan Yongzhen, Guo Tao, Yan Feifei, Gong Ming, Zhang Xin A., Li Chenze, He Tao, Luo Huimin, Zhang Lin, Chen Ming, Wu Xiaoyan, Wang Hairong, Deng Ke-Qiong, Bai Jiao, Cai Lin, Lu Zhibing. Association of statin use with the in-hospital outcomes of 2019-coronavirus disease patients: a retrospective study. Frontiers in Medicine. 2020;7 doi: 10.3389/fmed.2020.584870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chow Ronald, Im James, Chiu Nicholas, Chiu Leonard, Aggarwal Rahul, Lee Jihui, Choi Young-Geun, Prsic Elizabeth Horn, Shin Hyun Joon, Ghavami Saeid. The protective association between statins use and adverse outcomes among COVID-19 patients: a systematic review and meta-analysis. PLoS ONE. 2021;16(6):e0253576. doi: 10.1371/journal.pone.0253576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Vuorio A., Kovanen P.T. Statins as adjuvant therapy for COVID-19 to calm the stormy immunothrombosis and beyond. Front. Pharmacol. 2021;11 doi: 10.3389/fphar.2020.579548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Daniels Lori B., Ren Junting, Kumar Kris, Bui Quan M., Zhang Jing, Zhang Xinlian, Sawan Mariem A., Eisen Howard, Longhurst Christopher A., Messer Karen, Zivkovic Aleksandar R. Relation of prior statin and anti-hypertensive use to severity of disease among patients hospitalized with COVID-19: findings from the american heart association's COVID-19 cardiovascular disease registry. PLoS ONE. 2021;16(7):e0254635. doi: 10.1371/journal.pone.0254635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Aparisi Á., Amat-Santos I.J., López Otero D., Marcos-Mangas M., González-Juanatey J.R., J.A San Román, [Impact of statins in patients with COVID-19] Rev. Esp. Cardiol. 2021;74(7):637–640. doi: 10.1016/j.rec.2021.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Scheen A.J. Statins and clinical outcomes with COVID-19: Meta-analyses of observational studies. Diabetes Metab. 2021;47(6) doi: 10.1016/j.diabet.2020.101220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ö. Kurnaz GÖMleksİZ, H. Yilmaz AydoĞAn, Okside LDL Reseptörü-1 (LOX-1) ve Kardiyovasküler Hastalıklarla İlişkisi, Tıp Araştırmaları Dergisi 12(3) (2014) 145-152.
- 123.S.P. Terken BAYDAR, Gönül ŞAHİN, Neopterin: Günümüzün Popüler Biyogöstergesi mi?, Türkiye Klinikleri Tıp Bilimleri Dergisi 29(5) (2009) 1280-1291.
- 124.Wang L., Bassiri M., Najafi R., Najafi K., Yang J., Khosrovi B., Hwong W., Barati E., Belisle B., Celeri C., Robson M.C. Hypochlorous acid as a potential wound care agent: part I. Stabilized hypochlorous acid: a component of the inorganic armamentarium of innate immunity. J. Burns Wounds. 2007;6 [PMC free article] [PubMed] [Google Scholar]
- 125.Nguyen K., Bui D., Hashemi M., Hocking D.M., Mendis P., Strugnell R.A., Dharmage S.C. The potential use of hypochlorous acid and a smart prefabricated sanitising chamber to reduce occupation-related COVID-19 exposure, risk manag healthc. Policy. 2021;14:247–252. doi: 10.2147/RMHP.S284897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Widner Bernhard, Mayr Christiane, Wirleitner Barbara, Fuchs Dietmar. Oxidation of 7,8-dihydroneopterin by hypochlorous acid yields neopterin. Biochem. Biophys. Res. Commun. 2000;275(2):307–311. doi: 10.1006/bbrc.2000.3323. [DOI] [PubMed] [Google Scholar]
- 127.Lacoma A., Prat C., Andreo F., Lores L., Ruiz-Manzano J., Ausina V., Domínguez J. Value of procalcitonin, C-reactive protein, and neopterin in exacerbations of chronic obstructive pulmonary disease. Int. J. Chron Obstruct Pulmon Dis. 2011;6:157–169. doi: 10.2147/COPD.S16070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Garrod R., Marshall J., Barley E., Fredericks S., Hagan G. The relationship between inflammatory markers and disability in chronic obstructive pulmonary disease (COPD) Prim Care Respir J. 2007;16(4):236–240. doi: 10.3132/pcrj.2007.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Rovina N., Koutsoukou A., Koulouris N.G. Inflammation and immune response in COPD: where do we stand? Mediators Inflamm. 2013;2013:413735. doi: 10.1155/2013/413735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lacoma A., Prat C., Andreo F., Dominguez J. Biomarkers in the management of COPD. Eur. Respirat. Rev. 2009;18(112):96–104. doi: 10.1183/09059180.00000609. [DOI] [PubMed] [Google Scholar]
- 131.Achar A., Ghosh C. COVID-19-associated neurological disorders: the potential route of CNS invasion and blood-brain relevance. Cells. 2020;9(11) doi: 10.3390/cells9112360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zierhut Manfred, De Smet Marc D., Gupta Vishali, Pavesio Carlos, Nguyen Quan Dong, Chee Soon-Phaik, Cunningham Emmett T., Agrawal Rupesh. Evolving consensus experience of the IUSG-IOIS-FOIS with uveitis in the time of COVID-19 infection. Ocul. Immunol. Inflamm. 2020;28(5):709–713. doi: 10.1080/09273948.2020.1780273. [DOI] [PubMed] [Google Scholar]
- 133.Smith Justine R., Lai Timothy Y.Y. Managing uveitis during the COVID-19 pandemic. Ophthalmology. 2020;127(9):e65–e67. doi: 10.1016/j.ophtha.2020.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Arshadi D., Nikbin B., Shakiba Y., Kiani A., Jamshidi A.R., Boroushaki M.T. Plasma level of neopterin as a marker of disease activity in treated rheumatoid arthritis patients: association with gender, disease activity and anti-CCP antibody. Int. Immunopharmacol. 2013;17(3):763–767. doi: 10.1016/j.intimp.2013.08.022. [DOI] [PubMed] [Google Scholar]
- 135.Cheung K.S., Hung I.F.N., Chan P.P.Y., Lung K.C., Tso E., Liu R., Ng Y.Y., Chu M.Y., Chung T.W.H., Tam A.R., Yip C.C.Y., Leung K.H., Fung A.Y., Zhang R.R., Lin Y., Cheng H.M., Zhang A.J.X., To K.K.W., Chan K.H., Yuen K.Y., Leung W.K. Gastrointestinal manifestations of SARS-CoV-2 infection and virus load in fecal samples from a hong kong cohort: systematic review and meta-analysis. Gastroenterology. 2020;159(1):81–95. doi: 10.1053/j.gastro.2020.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zhang Jilei, Garrett Shari, Sun Jun. Gastrointestinal symptoms, pathophysiology, and treatment in COVID-19. Genes Dis. 2021;8(4):385–400. doi: 10.1016/j.gendis.2020.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Grabherr Felix, Effenberger Maria, Pedrini Alisa, Mayr Lisa, Schwärzler Julian, Reider Simon, Enrich Barbara, Fritsche Gernot, Wildner Sophie, Bellmann-Weiler Rosa, Weiss Günter, Scholl-Bürgi Sabine, Müller Thomas, Moschen Alexander, Adolph Timon E., Tilg Herbert. Increased fecal neopterin parallels gastrointestinal symptoms in COVID-19. Clin. Transl. Gastroenterol. 2021;12(1):e00293. doi: 10.14309/ctg.0000000000000293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Macdonald-Laurs Emma, Koirala Archana, Britton Philip N., Rawlinson William, Hiew Chee Chung, Mcrae Jocelynne, Dale Russell C., Jones Cheryl, Macartney Kristine, McMullan Brendan, Pillai Sekhar. CSF neopterin, a useful biomarker in children presenting with influenza associated encephalopathy? Eur. J. Paediatr. Neurol. 2019;23(1):204–213. doi: 10.1016/j.ejpn.2018.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hirsch Jamie S., Ng Jia H., Ross Daniel W., Sharma Purva, Shah Hitesh H., Barnett Richard L., Hazzan Azzour D., Fishbane Steven, Jhaveri Kenar D., Abate Mersema, Andrade Hugo Paz, Barnett Richard L., Bellucci Alessandro, Bhaskaran Madhu C., Corona Antonio G., Chang Bessy Flores, Finger Mark, Fishbane Steven, Gitman Michael, Halinski Candice, Hasan Shamir, Hazzan Azzour D., Hirsch Jamie S., Hong Susana, Jhaveri Kenar D., Khanin Yuriy, Kuan Aireen, Madireddy Varun, Malieckal Deepa, Muzib Abdulrahman, Nair Gayatri, Nair Vinay V., Ng Jia H., Parikh Rushang, Ross Daniel W., Sakhiya Vipulbhai, Sachdeva Mala, Schwarz Richard, Shah Hitesh H., Sharma Purva, Singhal Pravin C., Uppal Nupur N., Wanchoo Rimda, Bessy Suyin Flores Chang, Ng Jia Hwei. C.-R.C. Northwell Nephrology, Acute kidney injury in patients hospitalized with COVID-19. Kid. Int. 2020;98(1):209–218. doi: 10.1016/j.kint.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Werner E.R., Bichler A., Daxenbichler G., Fuchs D., Fuith L.C., Hausen A., Hetzel H., Reibnegger G., Wachter H. Determination of neopterin in serum and urine. Clin. Chem. 1987;33(1):62–66. [PubMed] [Google Scholar]
- 141.Wachter H., Fuchs D., Hausen A., Reibnegger G., Werner E.R. Neopterin as marker for activation of cellular immunity: immunologic basis and clinical application. Adv. Clin. Chem. 1989;27:81–141. doi: 10.1016/s0065-2423(08)60182-1. [DOI] [PubMed] [Google Scholar]
- 142.Denz H., Fuchs D., Hausen A., Huber H., Nachbaur D., Reibnegger G., Thaler J., Werner E.R., Wachter H. Value of urinary neopterin in the differential diagnosis of bacterial and viral infections. Klin Wochenschr. 1990;68(4):218–222. doi: 10.1007/BF01662720. [DOI] [PubMed] [Google Scholar]
- 143.Cesur S., Aslan T., Hoca N.T., Çimen F., Tarhan G., Çifçi A., Ceyhan İ., Şipit T. Clinical importance of serum neopterin level in patients with pulmonary tuberculosis. Int. J. Mycobacteriol. 2014;3(1):5–8. doi: 10.1016/j.ijmyco.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 144.Hagberg L., Cinque P., Gisslen M., Brew B.J., Spudich S., Bestetti A., Price R.W., Fuchs D. Cerebrospinal fluid neopterin: an informative biomarker of central nervous system immune activation in HIV-1 infection. AIDS Res. Ther. 2010;7(1):15. doi: 10.1186/1742-6405-7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Mahendra L., Mahendra J., Borra S.K., Nagarajan A. Estimation of salivary neopterin in chronic periodontitis. Indian J. Dent. Res. 2014;25(6):794–796. doi: 10.4103/0970-9290.152207. [DOI] [PubMed] [Google Scholar]
- 146.Zhou Shi-Jun, Sun Zhi-Xia, Liu Jun. Neopterin concentrations in synovial fluid may reflect disease severity in patients with osteoarthritis. Scand. J. Clin. Lab. Invest. 2013;73(4):344–348. doi: 10.3109/00365513.2013.783228. [DOI] [PubMed] [Google Scholar]
- 147.Tilg H., Konigsrainer A., Krausler R., Aulitzky W.E., Margreiter R., Reibnegger G., Wachter H., Huber C. Urinary and pancreatic juice neopterin excretion after combined pancreas-kidney transplantation. Transplantation. 1992;53(4):804–807. doi: 10.1097/00007890-199204000-00020. [DOI] [PubMed] [Google Scholar]
- 148.Ma L.-N., Zhang J., Chen H.-T., Zhou J.-H., Ding Y.-Z., Liu Y.-S. An overview on ELISA techniques for FMD. Virol. J. 2011;8(1):419. doi: 10.1186/1743-422X-8-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Centi Sonia, Tombelli Sara, Puntoni Mariarita, Domenici Claudio, Franek Milan, Palchetti Ilaria. Detection of biomarkers for inflammatory diseases by an electrochemical immunoassay: the case of neopterin. Talanta. 2015;134:48–53. doi: 10.1016/j.talanta.2014.10.053. [DOI] [PubMed] [Google Scholar]
- 150.M.F. Clark, R.M. Lister, M. Bar-Joseph, ELISA techniques, Methods in Enzymology, Academic Press1986, pp. 742-766.
- 151.Sánchez-Regaña M., Catasús M., Creus L., Umbert P. Serum neopterin as an objective marker of psoriatic disease activity. Acta Derm. Venereol. 2000;80(3):185–187. doi: 10.1080/000155500750042934. [DOI] [PubMed] [Google Scholar]
- 152.Yalow R.S. The limitations of radioimmunoassay RIA. Trends Anal. Chem. 1982;1:128–131. [Google Scholar]
- 153.Miles L.E.M., Hales C.N. Labelled antibodies and immunological assay systems. Nature. 1968;219(5150):186–189. doi: 10.1038/219186a0. [DOI] [PubMed] [Google Scholar]
- 154.Dutov A.A., Nikitin D.A., Rinchinov Z. Ts., Tereshkov P.P., Tsydendambaev P.P., Fedotova A.A. HPLC determination of neopterin in biological liquids for clinical purposes. Russ. J. Phys. Chem. A. 2007;81(3):421–423. [Google Scholar]
- 155.Carru Ciriaco, Zinellu Angelo, Sotgia Salvatore, Serra Roberta, Usai Maria Franca, Pintus Gian Franco, Pes Giovanni Mario, Deiana Luca. A new HPLC method for serum neopterin measurement and relationships with plasma thiols levels in healthy subjects. Biomed. Chromatogr. 2004;18(6):360–366. doi: 10.1002/bmc.325. [DOI] [PubMed] [Google Scholar]
- 156.Werner-Felmayer Gabriele, Werner Ernst Robert, Fuchs Dietmar, Hausen Arno, Reibnegger Gilbert, Wachter Helmut. Characteristics of interferon induced tryptophan metabolism in human cells in vitro. Biochim. Biophys. Acta, Mol. Cell. Res. 1989;1012(2):140–147. doi: 10.1016/0167-4889(89)90087-6. [DOI] [PubMed] [Google Scholar]
- 157.Turner A.P.F. Biosensors: sense and sensibility. Chem. Soc. Rev. 2013;42(8):3184–3196. doi: 10.1039/c3cs35528d. [DOI] [PubMed] [Google Scholar]
- 158.Sharma Piyush Sindhu, Wojnarowicz Agnieszka, Sosnowska Marta, Benincori Tiziana, Noworyta Krzysztof, D’Souza Francis, Kutner Wlodzimierz. Potentiometric chemosensor for neopterin, a cancer biomarker, using an electrochemically synthesized molecularly imprinted polymer as the recognition unit. Biosens. Bioelectron. 2016;77:565–572. doi: 10.1016/j.bios.2015.10.013. [DOI] [PubMed] [Google Scholar]
- 159.Ogutmen Koc D., Sipahi H., Sürmeli C., Çalık M., Bireroğlu N., Öksüz S., Baydar T., Şahin G. Serum neopterin levels and the clinical presentation of COVID-19. Pteridines. 2020;31:185–192. [Google Scholar]
- 160.D. Fuchs, M. Gisslén, Laboratory diagnostic value of neopterin measurements in patients with COVID-19 infection, Pteridines 32 (2021) 1–4.
- 161.Gostner Johanna M., Becker Kathrin, Ueberall Florian, Fuchs Dietmar. The good and bad of antioxidant foods: an immunological perspective. Food Chem. Toxicol. 2015;80:72–79. doi: 10.1016/j.fct.2015.02.012. [DOI] [PubMed] [Google Scholar]
- 162.Ponti G., Roli L., Oliva G., Manfredini M., Trenti T., Kaleci S., Iannella R., Balzano B., Coppola A., Fiorentino G., Ozben T., Paoli V.D., Debbia D., De Santis E., Pecoraro V., Melegari A., Sansone M.R., Lugara M., Tomasi A. Homocysteine (Hcy) assessment to predict outcomes of hospitalized Covid-19 patients: a multicenter study on 313 Covid-19 patients. Clin. Chem. Lab. Med. 2021;59(9):e354–e357. doi: 10.1515/cclm-2021-0168. [DOI] [PubMed] [Google Scholar]
- 163.M. Vargas-Vargas, C. Cortés-Rojo, Ferritin levels and COVID-19, Revista Panamericana de Salud Pública 44 (2020) 1. [DOI] [PMC free article] [PubMed]
- 164.Lin Zhi, Long Fei, Yang Yong, Chen Xiangyu, Xu Linyong, Yang Minghua. Serum ferritin as an independent risk factor for severity in COVID-19 patients. J. Infect. 2020;81(4):647–679. doi: 10.1016/j.jinf.2020.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Reibnegger G., Fuchs D., Fuith L.C., Hausen A., Werner E.R., Werner-Felmayer G., Wachter H. Neopterin as a marker for activated cell-mediated immunity: application in malignant disease. Cancer Detect. Prev. 1991;15(6):483–490. [PubMed] [Google Scholar]
- 166.Rainer Timothy H., Chan Cangel P.Y., Leung Man Fai, Leung Wingman, Ip Margaret, Lee Nelson, Cautherley George W.H., Graham Colin A., Fuchs Dietmar, Renneberg Reinhard. Diagnostic utility of CRP to neopterin ratio in patients with acute respiratory tract infections. J. Infect. 2009;58(2):123–130. doi: 10.1016/j.jinf.2008.11.007. [DOI] [PubMed] [Google Scholar]