Abstract
The tight packing of six fatty acids in the lipid A constituent of lipopolysaccharide (LPS) has been proposed to contribute to the unusually low permeability of the outer membrane of gram-negative enteric bacteria to hydrophobic antibiotics. Here it is shown that the Escherichia coli msbB mutant, which elaborates defective, penta-acylated lipid A, is practically as resistant to a representative set of hydrophobic solutes (rifampin, fusidic acid, erythromycin, clindamycin, and azithromycin) as the parent-type control strain. The susceptibility index, i.e., the approximate ratio between the MIC for the msbB mutant and that for the parent-type control, was maximally 2.7-fold. In comparison, the rfa mutant defective in the deep core oligosaccharide part of LPS displayed indices ranging from 20 to 64. The lpxA and lpxD lipid A mutants had indices higher than 512. Furthermore, the msbB mutant was resistant to glycopeptides (vancomycin, teicoplanin), whereas the rfa, lpxA, and lpxD mutants were susceptible. The msbB htrB double mutant, which elaborates even-more-defective, partially tetra-acylated lipid A, was still less susceptible than the rfa mutant. These findings indicate that hexa-acylated lipid A is not a prerequisite for the normal function of the outer membrane permeability barrier.
The outer membrane (OM) of gram-negative bacteria acts as an effective permeability barrier against external noxious agents, and lipopolysaccharide (LPS) is the key molecule for this function (9). In the OM of gram-negative enteric bacteria, the LPS molecules occupy the outer leaflet of the OM and leave no space for glycerophospholipids, which thus occupy the inner leaflet. The LPS monolayer is a highly ordered quasicrystalline structure with very low fluidity (7–9, 16). Such a structure may not allow rapid diffusion of hydrophobic solutes, and hydrophobic probe molecules have indeed been shown to partition very poorly into the hydrophobic interior portion of isolated LPS (8, 17).
Escherichia coli lpxA, lpxC, and lpxD mutants have profound defects in the biosynthesis of the lipid A part of the LPS and are extremely susceptible to hydrophobic antibiotics (10, 14, 18, 19). Since they synthesize greatly reduced amounts of LPS, the most probable explanation for their supersusceptibility is simply the lack of a continuous LPS layer in the outer leaflet and the resultant compensatory presence of glycerophospholipids in this leaflet (14, 16). This creates glycerophospholipid bilayers or patches in the OM that allow the diffusion of hydrophobic solutes. Such patches have been demonstrated in another group of antibiotic-supersusceptible mutants, the deep rough mutants that elaborate the very truncated LPS inner core (8, 9). The lpx mutants are also susceptible to large peptide antibiotics. Since these hydrophilic peptides cannot penetrate the OM of the lipid A mutants through the glycerophospholipid patches, they probably enter via transient ruptures (14, 16).
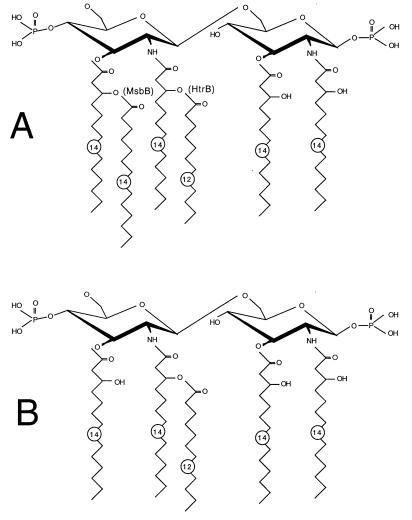
The highly ordered structure of the LPS layer is probably due to tight lateral interaction of the LPS molecules, mediated by divalent cations that bridge the anionic LPS molecules (8, 9). Contributing factors could also include the rigidifying effects of (i) the inner core oligosaccharide domain as well as (ii) the tightly packed set of six fatty acids in lipid A (four linked to the nonreducing glucosamine residue GlcN′ [8, 9]). Interestingly, lipid A mutants have recently been described that are defective in the transfer of the last two fatty acids (late acylations) to the nascent lipid A (1, 2, 4, 5, 11). In the present study we show that an msbB mutant that produces penta-acylated lipid A (only three fatty acids linked to GlcN′ [Fig. 1]) has an intact permeability barrier against both hydrophobic antibiotics and large glycopeptides. These findings indicate that hexa-acylation of lipid A, which results in very tight fatty acid packing of GlcN′, is not crucial to the function of the barrier. Furthermore, we show that the even-more-defective mutant (msbB htrB) which produces a partially tetra-acylated lipid A still possesses an OM permeability barrier that is less defective than those of the deep rough mutants and the lpx mutants.
FIG. 1.
Structures of the lipid A part of the LPS of wild-type E. coli (A) and the msbB mutant of E. coli, which lacks the myristic acid (C14) residue (B). The msbB htrB double mutant also lacks part of the lauric acid residue (C12) (see text). Acylations catalyzed by MsbB and HtrB are shown in panel A.
MATERIALS AND METHODS
Strains.
E. coli strains defective in late acylations of lipid A were the knockout mutants MLK53 (htrB1::Tn10), MLK1067 (msbB::Ωcam), and MLK986 (htrB1::Tn10 msbB::Ωcam) (4), obtained from Chris Raetz (Durham, N.C.). MLK1067 lacks the msbB-encoded (Kdo)2-(lauroyl)-lipidIVA myristoyltransferase and produces penta-acylated lipid A that is devoid of the myristic acid residue (Fig. 1); only extremely small amounts of hexa-acylated lipid A can be detected (2). This lack has been verified by a report on another msbB mutant allele of E. coli (11). The msbB gene is not essential for bacterial growth, and MLK1067 grows well even at 42°C (4).
MLK53 and MLK986 are thermosensitive and grow well at 30°C but poorly at 37°C (4). They lack the htrB-encoded (Kdo)2-lipidIVA lauroyltransferase (1). At 30°C, MLK53 produces a mixture of tetra-, penta-, and hexa-acylated lipid A, apparently because the msbB-encoded acyltransferase is able to compensate partially for the loss of htrB (2). The double mutant MLK986 that lacks both htrB and msbB elaborates at 30°C lipid A with a significant fraction of the tetra-acylated form (2). Due to the compensatory activity of still other acyltransferases, part of the lipid A formed at 30°C by the double mutant is penta-acylated.
The other lipid A mutants were SM101 (lpxA2 [3]) and CDH23-213 (lpxD [the gene formerly known as omsA, firA, and ssc] [19]), both of which are thermosensitive. SM101 grows well at 28°C but not at 37°C; CDH23-213 grows well at 20 and 37°C but not at 42°C. Detailed phenotypic analyses of these strains as well as their isogenic control strains SM105 (lpxA+) and CDH23-210 (lpxD+) have been published previously (14, 18, 19).
The strain with a defective LPS inner core was E. coli D21f2 (rfa); it elaborates Re-type (heptoseless) LPS and has previously been well characterized (14). IH3080 (O18:K1:H7 [15]) was the smooth, encapsulated E. coli strain used. Micrococcus luteus ATCC 9341 was the representative antibiotic-susceptible gram-positive bacterium.
Susceptibility determinations.
Susceptibilities were measured by using E-test strips (AB Biodisk, Solna, Sweden) according to the methodology recommended by the manufacturer, with an incubation time of 18 h. The test was performed on Luria-Bertani agar plates (10 g of tryptone; Difco Laboratories, Detroit, Mich.) with 5 g of yeast extract (Oxoid, Columbia, Md.), 5 g of NaCl, and 15 g of agar (pH 7.2). The inoculum for the test consisted of Luria-Bertani agar-grown bacteria in their early stationary growth phase. The E test can be used to reliably determine MICs for supersusceptible enterobacterial mutants (15).
RESULTS AND DISCUSSION
The OM permeability barrier to hydrophobic antibiotics.
It has been reported previously, by using an insensitive agar streak method, that the htrB and msbB mutants show no increased susceptibility to rifampin (4). This interesting finding needs verification and further analysis. Since some mutants supersusceptible to hydrophobic antibiotics display no altered susceptibility to rifampin (12), a broader range of hydrophobic antibiotics was chosen for study of the permeability barrier. These included dibasic (azithromycin), monobasic (erythromycin and clindamycin), monoanionic (fusidic acid), and zwitterionic (rifampin) antibiotics.
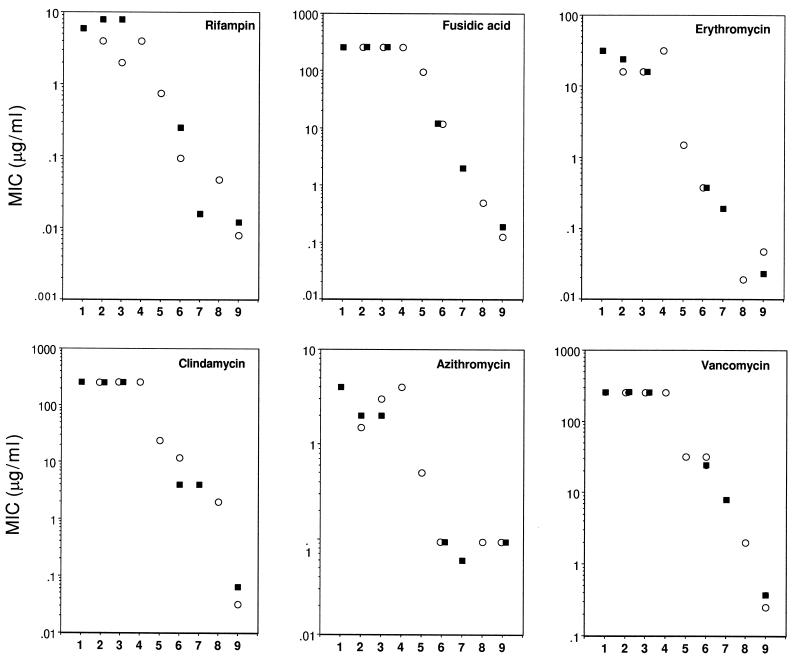
The MICs of all those antibiotics for the msbB mutant MLK1067 were almost as high as those for the reference E. coli K-12 strain SM105 as well as for strain IH3080 (Fig. 2). The susceptibility index, i.e., the approximate ratio between the MIC for strain SM105 and that for the mutant MLK1067, ranged between 2.7 and 0.7. Temperature had little effect on the MICs; MLK1067 remained resistant even at 42°C (data not shown).
FIG. 2.
MICs of rifampin (A), fusidic acid (B), erythromycin (C), clindamycin (D), azithromycin (E), and vancomycin (F) for E. coli mutants defective in the synthesis of LPS core oligosaccharide or lipid A as well as for their parent-type controls. Susceptibility determinations were performed at 28°C (○) and 37°C (■). Lanes: 1, strain IH3080 (smooth, encapsulated control); 2, strain SM105 (K-12 wild-type control); 3, strain MLK1067 (msbB); 4, strain MLK53 (htrB); 5, strain MLK986 (msbB htrB); 6, strain D21f2 (rfa, LPS chemotype Re); 7, strain CDH23-213 (lpxD); and 8, strain SM101 (lpxA). Susceptibility of a gram-positive bacterium, M. luteus ATCC 9341 (lanes 9), is shown for comparison.
The lack of supersusceptibility in MLK1067 indicates that hexa-acylated lipid A is not a prerequisite for the normal function of the OM permeability barrier. This function is severely impaired in the deep rough strain D21f2, which displayed susceptibility indices ranging from 20 to 64 (Fig. 2); yet its lipid A is not defective, in contrast to that of the msbB mutant (Fig. 1). The MICs for strain D21f2 were comparable to those previously determined for the Re-type strains of Salmonella typhimurium and Salmonella minnesota but still higher than those for the lipid A mutants SM101 (lpxA) and CDH-213 (lpxD), which displayed susceptibility indices higher than 512 (Fig. 2).
The msbB htrB double mutant (MLK986) was consistently more susceptible than the wild-type control and MLK1067 but less susceptible than the deep rough strain with the Re-type LPS (D21f2). The htrB single mutant MLK53 with the msbB-mediated compensatory mechanism working at 30°C (see above) displayed no increase in susceptibility.
The finding of the supersusceptibility of MLK986 is novel but compatible with the report that the amount of LPS is decreased in the OM of the htrB mutant at 42°C (6), the temperature at which the msbB-mediated compensatory mechanism is abolished (2). This decrease apparently results in the observed (6) increase in glycerophospholipids that creates gates for hydrophobic diffusion, i.e., phospholipid bilayer patches (see above).
The OM permeability barrier to glycopeptides.
Vancomycin and teicoplanin are hydrophilic glycopeptides and are effectively excluded by the intact enterobacterial OM. As the lpxA and lpxD mutants are very susceptible to vancomycin, their OM is probably fragile and transiently ruptured (14). Another indication of such ruptures is that both mutants leak remarkable amounts of their periplasmic proteins into the growth medium.
As shown in Fig. 2, MLK1067 was resistant to vancomycin. Accordingly, it can be concluded that its OM is not fragile. MLK1067 remained resistant to vancomycin even at 42°C (data not shown). In contrast, the MIC of vancomycin for the double mutant MLK986 was rather low, indicating a tendency for the formation of ruptures in the OM. Teicoplanin appeared to be a less-sensitive probe than vancomycin (data not shown).
General conclusions.
This report shows that hexa-acylation of lipid A, which results in very tight fatty acid packing of GlcN′, is not a prerequisite for normal function of the OM permeability barrier. Surprisingly, penta-acylated lipid A, which lacks the myristic acid residue of the nonreducing glucosamine, can completely replace the hexa-acyl form in this function. The very tight fatty acid packing of lipid A, and especially of its GlcN′, could contribute to the rigidity of the hydrophobic interior of LPS monolayer and thus to the relatively poor diffusion of hydrophobic solutes through this layer (8, 9). Tight packing of fatty acids, such as that in the glycolipids of the extreme thermophilic eubacterium Thermus, is expected to strengthen the hydrophobic and van der Waal’s interactions between neighboring molecules (8). However, despite the loss of one fatty acid residue, the remaining penta-acylated lipid A remains extremely richly acylated, and therefore the above-mentioned finding cannot be seen as a serious challenge to the validity of the theory. The study of the tetra-acylated lipid A would be interesting, but the pleiotropic changes in the structure of the OM of the htrB msbB mutant (see above) as well as the compensatory effects of additional fatty acid transferases would make interpretation of the results impossible. Therefore, hydrophobic permeability measurement techniques that employ artificial LPS mono- or bilayers and synthetic or purified lipid A and LPS derivatives are needed.
msbB mutants such as MLK1067 are becoming increasingly valuable in research on gram-negative bacterial infections (20). They grow at the same rate as their wild-type parents and are less virulent and much less active in eliciting macrophage responses. Because these strains can provide new insights into endotoxin research and novel opportunities for vaccine development, they should be characterized in detail. As shown in the present study, OM permeability barrier function, one of the main functions of LPS, is unimpaired in the msbB mutant.
Acknowledgments
The excellent technical assistance of Raija Lahdenperä and Sirkka-Liisa Skogberg is gratefully acknowledged.
REFERENCES
- 1.Clementz T, Bednarski J J, Raetz C R. Function of the htrB high temperature requirement gene of Escherichia coli in the acylation of lipid A: HtrB catalyzed incorporation of laurate. J Biol Chem. 1996;271:12095–12102. doi: 10.1074/jbc.271.20.12095. [DOI] [PubMed] [Google Scholar]
- 2.Clementz T, Zhou Z, Raetz C R H. Function of the Escherichia coli msbB gene, a multicopy suppressor of htrB knockouts, in the acylation of lipid A. J Biol Chem. 1996;272:10353–10360. doi: 10.1074/jbc.272.16.10353. [DOI] [PubMed] [Google Scholar]
- 3.Galloway S, Raetz C R H. A mutant of Escherichia coli defective in the first step of endotoxin biosynthesis. J Biol Chem. 1990;265:6394–6402. [PubMed] [Google Scholar]
- 4.Karow M, Georgopoulos C. Isolation and characterization of the Escherichia coli msbB gene, a multicopy suppressor of null mutations in the high-temperature requirement gene htrB. J Bacteriol. 1992;174:702–710. doi: 10.1128/jb.174.3.702-710.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karow M, Fayet O, Cegielska A, Ziegelhoffer T, Georgopoulos C. Isolation and characterization of the Escherichia coli htrB gene, whose product is essential for bacterial viability above 33°C in rich media. J Bacteriol. 1991;173:741–750. doi: 10.1128/jb.173.2.741-750.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karow M, Fayet O, Georgopoulos C. The lethal phenotype caused by null mutations in the Escherichia coli htrB gene is suppressed by mutations in the accBC operon, encoding two subunits of acetyl coenzyme A carboxylase. J Bacteriol. 1992;174:7407–7418. doi: 10.1128/jb.174.22.7407-7418.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Labischinski H, Barnickel G, Bradaczek H, Naumann D, Rietschel E T, Giesbrecht P. High state of order of isolated bacterial lipopolysaccharide and its possible contribution to the permeability barrier properties of the outer membrane. J Bacteriol. 1985;162:9–20. doi: 10.1128/jb.162.1.9-20.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nikaido H. Permeability of the lipid domains of bacterial membranes. Adv Membr Fluid. 1990;4:165–190. [Google Scholar]
- 9.Nikaido H. Outer membrane. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B Jr, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C: American Society for Microbiology; 1996. pp. 29–47. [Google Scholar]
- 10.Raetz C R H. Lipopolysaccharide biosynthesis. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B Jr, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C: American Society for Microbiology; 1996. pp. 1035–1063. [Google Scholar]
- 11.Somerville J E, Jr, Cassiano L, Bainbridge B, Cunningham M D, Darveau R P. A novel Escherichia coli lipid A mutant that produces an antiinflammatory liposaccharide. J Clin Investig. 1996;97:359–365. doi: 10.1172/JCI118423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vaara M. Antimicrobial susceptibility of Salmonella typhimurium carrying the outer membrane permeability mutation SS-B. Antimicrob Agents Chemother. 1990;34:853–857. doi: 10.1128/aac.34.5.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vaara M. Agents that increase the permeability of the outer membrane. Microbiol Rev. 1992;56:395–411. doi: 10.1128/mr.56.3.395-411.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vaara M. Antibiotic-supersusceptible mutants of Escherichia coli and Salmonella typhimurium. Antimicrob Agents Chemother. 1993;37:2255–2260. doi: 10.1128/aac.37.11.2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vaara M. Quantitative antimicrobial susceptibility testing of outer membrane-altered mutant bacteria by the E test. J Antimicrob Chemother. 1993;31:171–173. doi: 10.1093/jac/31.1.171-a. [DOI] [PubMed] [Google Scholar]
- 16.Vaara, M. LPS and the permeability of the outer membrane. In H. Brade, D. Morrison, S. Opal, and S. N. Vogel (ed.), Endotoxin in health and disease. Marcel Dekker Inc., in press.
- 17.Vaara M, Plachy W Z, Nikaido H. Partitioning of hydrophobic probes into lipopolysaccharide bilayers. Biochim Biophys Acta. 1990;1024:152–158. doi: 10.1016/0005-2736(90)90218-d. [DOI] [PubMed] [Google Scholar]
- 18.Vuorio R, Vaara M. The lipid A biosynthesis mutation lpxA2 of Escherichia coli results in drastic antibiotic supersusceptibility. Antimicrob Agents Chemother. 1992;36:826–829. doi: 10.1128/aac.36.4.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vuorio R, Vaara M. Mutants carrying conditionally lethal mutations in outer membrane genes omsA and firA (ssc) are phenotypically similar, and omsA is allelic to firA. J Bacteriol. 1992;174:7090–7097. doi: 10.1128/jb.174.22.7090-7097.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wyckoff T, Raetz C R H, Jackman J E. Antibacterial and anti-inflammatory agents that target endotoxin. Trends Microbiol. 1998;6:154–159. doi: 10.1016/s0966-842x(98)01230-x. [DOI] [PubMed] [Google Scholar]