Abstract
Human papillomavirus (HPV) induced cervical cancer is becoming a major cause of mortality in women. The present research aimed to identify the natural inhibitors of HPV-18 E1 protein (1R9W) from Himalayan herbs with lesser toxicity and higher potency. In this study, one hundred nineteen phytoconstituents of twenty important traditional medicinal plants of Northwest Himalayas were selected for molecular docking with the target protein 1R9W of HPV-18 E1 Molecular docking was performed by AutoDock vina software. ADME/T screening of the bioactive phytoconstituents was done by SwissADME, admetSAR, and Protox II. A couple of best protein-ligand complexes were selected for 100 ns MD simulation. Molecular docking results revealed that among all the selected phytoconstituents only thirty-five phytoconstituents showed the binding affinity similar or more than the standard anti-cancer drugs viz. imiquimod (-6.1 kJ/mol) and podofilox (-6.9 kJ/mol). Among all the selected thirty-five phytoconstituents, eriodictyol-7-glucuronide, stigmasterol, clicoemodin and thalirugidine showed the best interactions with a docking score of -9.1, -8.7, -8.4, and -8.4 kJ/mol. Based on the ADME screening, only two phytoconstituents namely stigmasterol and clicoemodin selected as the best inhibitor of HPV protein. MD simulation study also revealed that stigmasterol and clicoemodin were stable inside the binding pocket of 1R9W, Stigmasterol and clicoemodin can be used as a potential investigational drug to cure HPV infections.
1. Introduction
Malignant tumors have cancerous cells that grow indefinitely and proliferate locally or to the nearby body parts. They can also metastasize to distant parts via the bloodstream or the lymphatic system and affects the liver, lungs, brain, bone, and other areas. Some of the viral infections including Epstein-Barr, Human immunodeficiency virus (HIV), Human papillomavirus (HPV), Human herpesvirus 8 (HHV-8), hepatitis B and C, etc. have been well documented in increasing the risks of various cancers. Cervical cancer is identified as the fourth most common malignancy among women worldwide and even ranked after breast cancer with 2·1 million cases, colorectal cancer with 0·8 million cases, and lung cancer with 0·7 million cases worldwide [1]. Each year 0.5 million women are diagnosed with cervical cancer [2].
Viral infections are responsible for enhancing the prevalence and as well as the risks associated with approximately 15–20% of the cancers in the total human population, whereby numerous viruses play a key role in the etiology of some malignant cancers at multiple stages. HPV infection is considered to be one of the common risk factors for the development of cervical cancer, wherein about 99% of the cases are associated with cervical cancer [3, 4]. HPV is a double-stranded DNA and a non-enveloped virus of the family Papovaviridae. It is 55 nm in diameter and has an icosahedral capsid comprising of 72 capsomeres, each one with at least two capsid proteins namely L1 and L2. HPV infection is widespread in humans and has been found in several animals as well, where it is unique to its host. It is reported that HPV is present in both symptomatic and asymptomatic populations and there are many more cancers that are cognate with HPV infections like anogenital, vulvar, vaginal, penile, head, and neck [5–8]. More than 120 HPV genotypes have been classified which infects human skin and among these only 13–15 types are associated with cancer involving high-risk and low-risk types depending on their oncogenic potential [9]. According to the earlier data, about 5% of the population in Europe is infected with HPV and 26% in the Sub-Saharan African countries [10], 21.4% in Eastern Europe, and 16.1% in Latin America [11]. In India, 6.3% of the population is infected with HPV [12], where 10.4% in Andhra Pradesh [13], 9.7% in Varanasi [6], 8.1% in Mumbai, 15.4% in New Delhi, 18.9% in Bangalore, 1.3% in Bhopal and 3.5% in Chennai [14] have been recorded. The high-risk group HPV genotypes are the primary cause for the development of pre-cancerous and cancerous cervix [15]. HPV-18 is one of the major cause of the development of cervical cancer in humans [11], whereas HPV-11 presents a low-risk for cervical cancer, usually causing benign hyperproliferative lesions as genital warts and oral papillomatosis. Transmission of the infection is caused by sexual contact which results in squamous intraepithelial lesions. Due to the immunological barriers, the induced lesions may disappear after 6 to 12 months, but in the case of chronic infections, it can lead to the development of cervical cancer [16].
The development of cervical cancer mainly depends upon the interference of the tumor suppressor proteins, p53 and pRB (retinoblastoma) with viral oncoproteins E6 and E7. A dysregulation in the cellular adhesion, cellular control, host cell immunomodulation, and genotoxicity is associated with the severity of the disease [17]. Infections can be cured by the use of chemotherapeutic agents which are non-specific to their targets or by the application of surgical and ablative techniques which are invasive and expensive alternatives [18]. Moreover, their availability is scarce to millions of patients, particularly in developing countries. Hence, one of the main alternatives to treat HPV-related diseases is the availability of highly effective natural therapeutics directed against the virus. Therefore, medicinal plants such as Allium sativum, Artemisia annua, Berberis aristata, Bergenia ligulata, Cannabis sativa, Cymbopogon citrates, Dactylorhiza hatagirea, Moringa oleifera, Myristica fragrans, Oxalis corniculata, Picrorhiza kurroa, Piper nigrum, Pleurospermum brunonis, Podophyllum hexandrum, Rheum emodi, Thalictrum foliolosum, Thymus serpyllum, Trillium grandiflorum, Viola odorata, and Zanthoxylum armatum which are traditionally used for the treatment of diabetes, gastrointestinal, hepatic, nervous, skin, and respiratory tract infections, and rheumatism [19–33], were investigated for their biochemical compositions. Earlier also, many natural and plant-based compounds have been identified as promising sources for developing cytotoxic agents for the treatment and prevention of cancer in recent years [34]. Stigmasterol, an active ingredient of B. aristata has also been shown to exert anti-angiogenic and anti-cancer effects. downregulation of TNF-alpha and VEGFR-2 [35]. Therefore, the present research is focused on to study the intraction of major phytocompounds of twenty important medicinal plants of North west Himalays with target protein of HPV-18 capsid protein L1 and to study the toxicity of active phytocomplounds docking.
2. Methodology
2.1 Bioinformatics tools
Computational facilities to perform in silico investigations of the important drug candidates included Open Babel GUI [36], UCSF Chimera 1.8.1 [37], PubChem (www.pubchem.com), RCSB PDB (http://www.rscb.org/pdb), Autodock 1.5.6/vina software [38] and discovery studio.
2.2 Ligand preparation
One hundred nineteen major phytoconstituents of twenty medicinal plants (Allium sativum, Artemisia annua, Berberis aristata, Bergenia ligulata, Cannabis sativa, Cymbopogon citrates, Dactylorhiza hatagirea, Moringa oleifera, Myristica fragrans, Oxalis corniculata, Picrorhiza kurroa, Piper nigrum, Pleurospermum brunonis, Podophyllum hexandrum, Rheum emodi, Thalictrum foliolosum, Thymus serpyllum, Trillium grandiflorum, Viola odorata and Zanthoxylum armatum) collected from the higher altitudes of Himachal Pradesh and Uttarakhand (India) were selected for the molecular docking studies. The 3-dimensional structures of the selected phytoconstituents and the two standard drugs of the HPV (podofilok and imiquimod) were downloaded from the PubChem database (www.pubchem.com) in.sdf formats. The.sdf files of the phytoconstituents were converted into PDB formats. All the selected ligands (phytoconstituents and drugs) were prepared using the open babel program from the command line on an Ubuntu terminal. The energy of all the selected phytocompounds was minimized by using chemdraw 3D 15.0. Phytoconstituents selected for the study, plant source, and their ethnomedicinal uses are enlisted in Table 1.
Table 1. Ethno-medicinal uses and plant sources of selected phytoconstituents.
| Major Phytoconstituents Selected for anti-carcinogenicity | Source of Phytoconstituents | Ethno-medicinal uses |
|---|---|---|
| Alizarin, Aloe-emodin, Anthraquinone, Anthrarufin, Anthrone, Chryophanol, Clicoemodin, Dantron, Emodin, Gallic acid, Juglone, Physicon, Piceatannol, Quinizarin, Rubiadin | Rheum emodi | Rheumatism [39], wounds healing and fractures [19], treatment of boils [40]. |
| 3-Octanone, 8-Prenylnaringenin, Apigenin, Apigenin-7-O-glucoside, Bornyl acetate, Camphene, Camphor, Carvacrol, Caryophyllene oxide, Catechin, Eriodictyol, Eriodictyol-7-glucuronide, Geranyl acetate, Linalool, Myrcene, Rutin, Spathulenol, Taxifolin, Thymol, Trans-Nerolidol, α-Pinene, β-Bisabolene, β-Caryophyllene, β-Pinene, δ-Cadinene. | Thymus serpyllum | Cold, flu, indigestion, hair loss treatment, diabetes, and respiratory diseases [20] |
| Citral alpha, Citronellal, Geraniol, lemonene, Nerol, Terpinolene | Cymbopogon citrates | Inflammation, fever, nervous disorders, pyrea, wound healing, hypertension, bone fracture [21] |
| Glucomoringin, Niazinicin A, Niazinin, Pterygospermin | Moringa oleifera | Skin infections, anxiety, asthma, wounds, fever, diarrhea, and sore throats [22], its seeds are used for purifying water [23] |
| 8-Oxyberberine, Berberine, Jatrorrhizine, Noroxyhydrastinine, Palmatine, Thalicarpine, Thalidasine, Thalirugidine, Thalirugine, Thalisopine, Thalrugosaminine, Thalrugosidine | Thalictrum foliolosum | Atonic dyspepsia, edema, skin diseases, and Jaundice [24]. |
| Berbamine, Columbamine, Isocorydine, Lambertine, Lupeol, Oleanolic acid, Oxyberberine, Oxycanthine, Stigmasterol | Berberis aristata | Skin diseases, menorrhagia, diarrhoea, Jaundice, and piles [25] |
| Guaiol, Piperazine, Piperine, Piperolein A, Piperolein B, Sabinene, β-caryophyllene | Piper nigrum | Skin diseases, Cough and cold, snake bite, and poisoning [26, 27]. |
| (E)-Ajoene, (Z)-Ajoene, Allicin, Alliin, Vinyllithium | Allium sativum | Skin diseases, Cough and cold [25] |
| 4-terpinol, 9-epoxylignan, Camphene, Elemicin, Isoelemicin, Methoxyeugenol, Meso-dihydroguaiaretic acid, Myristicin, Sabinene, Safrole, Trimyristin | Myristica fragrans | Hypertension, antispasmodic, diarrhea, abdominal pain, fever, and vomiting [28]. |
| Armatamide, Bergapten, Sesamin, Tambulin, Xanthyletin, β-Sitosterol-D-Glucoside | Zanthoxylum armatum | Dental problems, fever, dyspepsia, cold, cough, and abdominal pain [29]. |
| 3-O-b-galactopyranoside, Methyl a-L-arabinopyranoside, Sedanolide | Pleurospermum brunonis | Skin infection and fever [30]. |
| Cannabigerol, Cannabinoids, Cannabinol, Tetrahydrocannabinol | Cannabis sativa | Intoxicant, analgesic, narcotic, stomachic, antispasmodic, anodyne, and sedative [31, 32]. |
| Curcubitacine E, Pikuroside, Verbascoside | Picrorhiza kurroa | Improve appetite, and decoction of this with ajwain is used for the purification of blood and to cure skin infections [41]. |
| Isovitexin, Oleic acid, Palmitic acid | Oxalis corniculata | Dyspepsia [30]. |
| Artemisinin, Dihydroartemisinic acid | Artemisia annua | Fever, malaria cough, cold, and blood detoxifier [42]. |
| Bergenin, Pyrogallol | Bergenia ligulata | Excessive uterine hemorrhage, diseases of the urinary bladder, dysentery, menorrhagia, splenic enlargement, and heart diseases [33]. |
| Dactylorhin E | Dactylorhiza hatagirea | It is taken with milk in case of weakness [30]. |
| Podophyllin | Podophyllum hexandrum | It is given to cattle for bloating prevention [30]. |
| Octadecanoic acid | Trillium grandiflorum | Antiseptic, antispasmodic, diuretic poultice to the eye to reduce swelling, aching rheumatic joints [43, 44] & sore ear [45]. |
| Emetine | Viola odorata | Respiratory & gastrointestinal illness, cough, cold, and fever [46]. |
2.3 Protein preparation
Human papillomavirus type (HPV-18 E1) protein (PDB ID: 1R9W) [47] was selected for molecular docking with major bioactive phytoconstituents from the selected Himalayan medicinal plants as listed in Table 1 to identify its potential inhibitor. Podofilok and Imiquimod is used as drug target because of its small surface area and functional significance, the E1 dimerization interface is an obvious target for a small molecule disrupting DNA replication [47]. The 3-dimensional monomeric structure of HPV-18 E1 protein (1R9W) was downloaded from the protein databank (http://www.rscb.org/pdb). It consists of a pentamer structure, and its chain A was extracted for docking using Pymol software. Protein was prepared by using autoDock 1.5.6 program, during protein preparation all the water molecules were deleted, polar hydrogens were and kollaman charge (5.0) is added. The active site was predicted manually by grid box analysis (grid dimensions x = 40, y = 40, z = 40 Å, centered at x, y, z = 21.25, -0.773, 12.365 Å), TYR121, ASP124, VAL 126, SER129, TYR132, GLY133, GLY134, ASN135, PRO136, ASN259, ALA284, SER285, SER286, TYR288 are the key amino acids present in the active site of the protein.
2.4 Molecular docking using herbal phytoconstituents
The molecular docking of the selected ligands with the catalytic triad of the 1R9W protein was performed using AutoDock 1.5.6 program and responses were saved as a PDBQT file. Molecular docking was performed using vina Perl script [38] and the best conformation with the lowest docking energy was chosen. The.pdb complexes of the protein (1R9W) and ligands were analyzed on a Discovery Studio (https://discover.3ds.com/d) to study the list of interactions between the selected protein and ligands.
2.5 In silico pharmacokinetics and toxicity prediction of bioactive active phytoconstituents
ADME/T screening of the 35 selected bioactive phytoconstituents was done by using online web servers such as SwissADME (Molecular Modeling Group of the Swiss Institute of Bioinformatics, Lausanne, Switzerland), admetSAR (Laboratory of Molecular Modeling and Design, Shanghai, China), and ProTox (Charite University of Medicine, Institute for Physiology, Structural Bioinformatics Group, Berlin, Germany) [48–53].
2.6 MD simulation of protein-ligand complexes
To gain deeper insights into the binding interactions of 1R9W protein with clicoemodin and stigmasterol, MD simulations were performed that accelerated the graphics processing unit (GPU) in Amber18 [54]. Amber generalized force field (GAFF) was assigned to the ligands, whilst amber ff14SB to the protein [55, 56]. The atomic charges of the ligands were calculated using the restrained electrostatic potential (RESP) protocol at the HF/6-31G* level of theory 31, 32 using Gaussian 09 software [57]. The protonation states of the ionizable residues of protein structures were analyzed by pKa calculation at neutral pH using the H++ server. All the systems were prepared using tLeap program. Each system was neutralized with sodium ions and solvated using a cubic water box with TIP3P model. To eliminate the stearic clashes, each system was subjected to a total of four minimizations. First, all the sodium ions and water molecules were minimized by 2000 steps of steepest descent, followed by 3000 steps of the conjugate gradient algorithm. Consecutively, all the hydrogen and water molecules were relaxed using the same protocol. Finally, the whole system was energy-minimized for 5000 steps of steepest descent plus 5000 steps of conjugate gradients. The heat of the system was started from 0 to 300 K while running 200 ps of MD and, next, 300 ps of the density equilibration with position restraints on the protein atoms at a constant volume. Before performing the production step, all the protein-ligand systems were equilibrated with10 ns of MD without any positional restraints at a constant pressure. The temperature was maintained at 300 K by coupling to a Langevin thermostat using a collision frequency of 2 cm-1. A cutoff of 10 Å was employed for unbonded interactions, the Particle Mesh Ewald (PME) [56] method and the SHAKE [57] algorithm were used to restrict the bond lengths involving hydrogen atoms. Finally, the MD simulations (productions) were performed using 100 ns at a temperature of 300 K. The generated trajectories were used to analyze the behavior of each complex and to access the stability of the system. The deviations of the protein and protein-ligand complex system were analyzed by calculating the root mean square deviation (RMSD), root mean square fluctuation (RMSF), a radius of gyration (RG), and solvent accessible surface area (SASA) parameters [58, 59].
Furthermore, the total free energy of binding, the free energy of solvation (polar þ non-polar solvation energies), and the potential energy (electrostatic and van der Waal’s interactions) of each protein-ligand complex were calculated by molecular mechanics using Poisson–Boltzmann Surface Area (MM-PBSA) method [60]. The last 10 ns of the MD trajectory were taken for the calculation of MM-PBSA.
3. Results
3.1 Molecular docking of some medicinally important phytoconstituents with target protein of HPV-18 (1R9W)
One hundred nineteen major phytoconstituents of twenty important medicinal plants of Northwest Himalayas are selected based on their reported medicinal attributes as evidenced in the earlier literature. These are widely recommended in the Ayurvedic/Unani medicines for the treatment of diabetes, gastrointestinal, hepatic, nervous, skin, and respiratory tract infections, and rheumatism (as summarized in Table 1). 3D structures of these phytoconstituents and their target proteins were retrieved from PubChem and PDB databases. Molecular docking results showed that out of one hundred nineteen phytoconstituents, only thirty-five phytoconstituents of twelve medicinal plants showed docking scores more than the standard drugs imiquimod (-6.1 kJ/mol) and podofilok (-6.9 kJ/mol) interactions with HPV-18 (1R9W). Important phytoconstituents with their respective docking score, plant sources, and interactive amino acids are summarized in Table 2 and S1 Table. From this study, we found that six phytoconstituents of Thalictrum foliolosum and Thymus serpyllum, five of Berberis aristata, four of Rheum emodi, three each from Cannabis sativa and Picrorhiza kurroa, two each from Moringa oleifera and Zanthoxylum armatum, and one each from Myristica fragrans, Oxalis corniculata, Piper nigrum, and Pleurospermum brunonis showed docking score more than the standard anti-cancerous drugs.
Table 2. The table exhibits important bioactive phytoconstituents of herbal plants their docking with HPV-18 (1R9W) and interactive amino acids.
| S.no | Phytoconstituents/Standard drug | Plant source | Docking score (-kJ/mol) | Interactive amino acids | |
|---|---|---|---|---|---|
| H- bonds | Hydrophobic interactions | ||||
| 1. | Chryophanol | Rheum emodi | -7 | - | ALA 213, MET 214, LYS 210, TRP 278, PHE 248, CYS 311, GLY 249 |
| 2. | Emodin | -7.1 | LYS 20 | GLY 249, PHE 248, TRP 278, CYS 311, ALA 213, MET 214 | |
| 3. | Rubiadin | -6.9 | - | GLY 249, CYS 311, TRP 278, LYS 210, PHE 248, ALA 213, MET 214 | |
| 4. | Clicoemodin | -8.4 | ASN 335, SER 337 | GLY 341, ILE 333, SER 334, GLY 332, TYR 329, GLYU 338, VAL 339, THR 343, GLN 348 | |
| 5. | Apigenin | Thymus serpyllum | -7.1 | TYR 268 | HIS 270, VAL 339, GLY 332, THR 343, GLN 348, TYR 329, ILE 347, TRP 328 |
| 6. | Catechin | -7.5 | TYR 268, TRP 328 | ILE 333, GLN 348, TYR 329, ILE 347, GLY 332, THR 343, HIS 270 | |
| 7. | Eriodictyol-7-glucuronide | -9.1 | VAL 339, LYS 259, THR 343 | ILE 333, TYR 329, HIS 270, TYR 268, GLY 332, SER 337, GLU 338 | |
| 8. | Eriodictyol | -7.5 | TYR 268 | TYR 329, ILE 347, TRP 328, GLY 332, THR 343, HIS 270, MET 340 | |
| 9. | Rutin | -8.3 | TYR 268, TRP 328, THR 343, GLY 341, SER 337, ASN 335 | HIS 270, ASP 342, GLY 332, MET 340, ILE 347, TYR 329, ILE 333, SER 334, GLU 338 | |
| 10. | Taxifolin | -7.2 | TYR 268, TRP 328 | GLN 348, THR 343, TYR 329, ILE 347, GLY 332, HIS 270, MET 340 | |
| 11. | Glucomoringin | Moringa oleifera | -7.4 | THR 343, GLY 332, TYR 268 | TYR 329, VAL 339, ILE 333, PRO 344, HIS 270, TRP 328, ILE 347, ARG 349, GLY 341, ASP 342, GLN 348, THR 351, ILE 353 |
| 12. | Pterygospermin | -7.6 | - | VAL 323, LEU 224, TYR 327, GLY 223, LEU 326, THR 331, ARG 330 | |
| 13. | 8-Oxyberberine | Thalictrum foliolosum | -7.4 | - | LYA 210, ALA 213, MET 214, TRP 278, PHE 248, GLY 249, VAL 307, CYS 311, THR 310 |
| 14. | Thalicarpine | -7.9 | - | TRP 328, ILE 333, VAL 339, ILE 347, TYR 329, THR 343, GLN 348, GLY 332, TYR 268, HIS 270, MET 340, GLY 341 | |
| 15. | Thalidasine | -7.8 | ARG 287 | LEU 319, LEU 350, TYR 346, ARG 349, ARG 320, CYS 240, THR 241, ASP 242, ARG 231, LEU 267 | |
| 16. | Thalirugidine | -8.4 | - | HIS 270, ILE 347, TYR 329, TYR 268, GLN 348, THR 343, GLY 341, ASP 342, ILE 333, VAL 339, GLY 332, SER 337, GLU 338 | |
| 17. | Thalirugine | -8 | ASP 125, TYR 288 | ASP 124, TYR 123, ALA 284, SER 129, VAL 126, GLU 127, PHE 257, HIS 290, VAL 289 | |
| 18. | Thalrugosaminine | -7.8 | - | VAL 280, CYS 273, PRO 252, GLU 256, ALA 255, VAL 280, LEU 274, ASP 275, ILE 336, ASN 335 | |
| 19. | Berbamine | Berberis aristata | -8.3 | THR 212 | GLY 180, LEU 210, GLY 265, PRO 183, MET 205, VAL 289, ASP 216, ARG 260, ALA 261, ILE 287, ASN 121, GLY 262, ASN 213 |
| 20. | Berberine | -6.9 | THR 241, ARG 287 | CYS 240, TRP 346, GLU 345, ARG 349, LEU 319, ASP 242, ARG 320, LEU 350 | |
| 21. | Lupeol | -8.2 | ASP 228 | TYR 222, THR 331, TYR 327, ARG 330, LEU 224, VAL 323 | |
| 22. | Oleanolic acid | -8 | VAL 323 | LEU 326, ARG 330, THR 331, GLN 272, LEU 274, ILE 336, EU 224, TYR 323, TYR 222 | |
| 23. | Stigmasterol | -8.7 | - | HIS 270, TYR 268, ILE 333, VAL 339, GLY 332, TYR 329, GLN 348 | |
| 24. | Piperolein B | Piper nigrum | -6.9 | - | SER 334, THR 331, GLY 223, TYR 222, ARG 330, LEU 326, TYR 327, VAL 323, LEU 224 |
| 25. | 9-epoxylignan | Myristica fragrans | -7 | GLY 341, THR 343 | MET 340, VAL 339, ILE 347, TYR 329, ILE 333, GLY 332, GLN 348 |
| 26. | Armatamide | Zanthoxylum armatum | -7.2 | - | LYS 210, MET 214, PRO 308, ALA 213, TRP 278, PHE 248, CYS 311, GLY 249, VAL 307, VAL 250, LEU 305, ASN 251, HIS 306 |
| 27. | β-Sitosterol-D-Glucoside | -7.7 | - | ASP 24, ARG 29, PHE 33, GLN 311, LYS 312, GLN 311, GLN 373, PHE 464, GLN 467, SER 468 | |
| 28. | 3-O-b-galactopyranoside | Pleurospermum brunonis | -7 | GLY 341, THR 343 | GLN 348, ILE 333, VAL 339, GLY 332, GLN 348, ILE 347, TYR 268, HIS 270, TRP 328, PRO 344 |
| 29. | Cannabigerol | Cannabis sativa | -7.2 | - | TYR 222, GLN 272, TYR 327, LEU 326, ARG 330, VAL 323, LEU 224, ILE 336, LEU 274, THR 331 |
| 30. | Cannabinol | -7.1 | GLY 223 | LEU 326, THR 331, ARG 330, TYR 222, LEU 274, GLN 272, VAL 323, TYR 327, LEU 224 | |
| 31. | Tetrahydrocannabinol | -7.3 | - | ASP 228, GLY 223, TYR 222, VAL 323, LEU 224, TYR 327, THR 331, GLN 272, LEU 274, LEU 326, ARG 330, ILE 336 | |
| 32. | Curcubitacine E | Picrorhiza kurroa | -7.7 | ASP 220, SER 225 | GLY 223, LYS 219, THR 227, ALA 216, LEU 215, PHE 226 |
| 33. | Pikuroside | -7.7 | THR 343, SER 334, ASN 335, SER 337, LYS 259, VAL 339 | ILE 347, TYR 268, HIS 270, GLY 332, TYR 329, MET 340, GLU 338, GLN 263, THR 260 | |
| 34. | Verbascoside | -7.8 | THR 241, TRP 346, GLU 345, ARG 287, ASP 342 | CYS 240, ASP 242, ARG 231, PRO 344, TYR 268, GLY 341 | |
| 35. | Isovitexin | Oxalis corniculata | -7.9 | VAL 339, ASN 335, ILE 333, GLY 332 | GLU 338, SER 337, SER 334, TYR 268, THR 343, GLN 348 |
| 36. | Podofilok | Standard drugs | -6.9 | - | GLY 341, MET 340, VAL 339, HIS 270, TRP 328, TYR 329, GLY 332, THR 343, ILE 333 |
| 37. | Imiquimod | -6.1 | - | GLN 348, TYR 329, ILE 347, TRP 328, HIS 270, TYR 268, THR 343, VAL 339, GLY 332 | |
Among all the active thirty-five phytoconstituents eriodictyol-7-glucuronide of Thymus serpyllum showed the best interactions with a docking score of -9.1 kJ/mol followed by stigmasterol (-8.7 kJ/mol) of Berberis aristata, clicoemodin (-8.4 kJ/mol) of Rheum emodi, and thalirugidine (-8.4 kJ/mol) of Thalictrum foliolosum. Eriodictyol-7-glucuronide makes hydrogen bonds with VAL 339, LYS 259, THR 343 and showed hydrophobic interactions with ILE 333, TYR 329, HIS 270, TYR 268, GLY 332, SER 337, GLU 338, while stigmasterol showed hydrophobic interactions with HIS 270, TYR 268, ILE 333, VAL 339, GLY 332, TYR 329, and GLN 348, clicoemodin makes hydrogen bonds with ASN 335, SER 337 and showed hydrophobic interactions with GLY 341, ILE 333, SER 334, GLY 332, TYR 329, GLYU 338, VAL 339, THR 343, GLN 348 while thalirugidine didn’t make any hydrogen bond, it only showed hydrophobic interactions with HIS 270, ILE 347, TYR 329, TYR 268, GLN 348, THR 343, GLY 341, ASP 342, ILE 333, VAL 339, GLY 332, SER 337, GLU 338 (Table 2). 2D and 3D structures of the best complexes are represented in Figs 1 and 2. The remaining phytoconstituents with higher docking scores such as bornyl acetate, cannabinoids, caryophyllene oxide, dactylorhin E1, dactylorhin E2, dactylorhin E3, dactylorhin E4, dihydroatemisinic acid, gallic acid, geranylacetate 2, geranylacetate, meso-dihydrogualaretic acid, methy a-L-arabinopyranoside, niazinicin A, oleanolic acid, oleic acid, palmitic acid, piperolin A, piperolin B and podophyllin do not show any interaction with HPV 18 (1R9W).
Fig 1.
2D structures of active phytoconstituents in complexation with 1R9W: where, (A) Thalirugonidine, (B) Clicoemodin, (C) Eriodictyol-7, and (D) Stigmasterol.
Fig 2.
3D structure of active phytoconstituents in complex with 1R9W: (A) Thalirugonidine, (B) Clicoemodin, (C) Eriodictyol-7, and (D) Stigmasterol.
3.2 In silico pharmacokinetics and toxicity prediction of the bioactive phytoconstituents
The pharmacokinetics and toxicity of pharmacologically important phytoconstituents were analyzed in silico using computational facilities of SWISS ADME, admetSAR, and Protox II servers. Thirty-five active phytoconstituents of twelve medicinal plants and two standard drugs (Podofilox and Imiquimod) were selected for the pharmacokinetics and toxicity studies. Among all the thirty-five phytoconstituents, only 29 phytoconstituents follow Lipinski’s rule of five, and phytoconstituents such as 3-O-b-galactopyranoside, eriodictyol-7-glucuronide, glucomoringin, pikuroside, rutin, and verbascoside do not follow Lipinski’s rule of five, therefore these cannot be used as investigational drug candidates. Whereas 30 phytocompounds follows the Veber’s rule. Phytocompounds such as 3-O-b-galactopyranoside, 8-Oxyberberine, Apigenin, Armatamide, Catechin, Clicoemodin, Clicoemodin, Eriodictyol-7-glucuronide, Eriodictyol, Glucomoringin, Isovitexin, Pikuroside, Piperolein B, Soluble, Rutin, Taxifolin, Verbascoside are water soluble. The GI-absorption of 8-Oxyberberine, 9-epoxylignan, Apigenin, Armatamide, Berbamine, Berberine, Cannabigerol, Cannabinol, Catechin, Chrysophanol, Clicoemodin, Emodin, Eriodictyol are found high. All the active phytocompounds were found non-carcinogenic in nature. Chrysophanol and Oleanolic acid are found hepetotoxic in nature. Eriodictyol, Berberine, Curcubitacine E, Armatamide, 9-epoxylignan, 8-Oxyberberine, 3-O-b-galactopyranoside Phytocompounds showed Carcinogenicity.Protox II server predict that phytocompounds such as 3-O-b-galactopyranoside, 8-Oxyberberine, 9-epoxylignan, Armatamide, Berbamine, Berberine, Cannabinol, Chrysophanol, Clicoemodin, Curcubitacine E, Eriodictyol-7-glucuronide¸ Glucomoringin, Lupeol, Lupeol, Pikuroside, Piperolein B, Rubiadin, Rutin, Stigmasterol, Tetrahydrocannabinol, Thalicarpine, Thalidasine, Thalirugidine, Thalirugine, Thalisopine, Thalrugosaminine, Thalrugosidine, Verbascoside and β-Sitosterol Glucoside are found immunotoxic in nature. 8-Oxyberberine, Armatamide, Berbamine, Berberine, Clicoemodin, Emodin, Isovitexin, Rubiadin, Taxifolin, Thalicarpine, Thalidasine, Thalirugidine, Thalirugine, Thalisopine, Thalrugosaminine, Thalrugosidine are found mutagenic in nature. 3-O-b-galactopyranoside, 8-Oxyberberine, Berberine are cytotoxic in nature. Results are summarized in Table 3 and S1–S3 Figs.
Table 3. Pharmacokinetics and toxicity prediction of the active phytoconstituents of Himalayan herbs.
| Phyto-compounds | SWISS ADME | Admet SAR | Protox-II | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | |
| 3-O-b-galactopyranoside | -2.09 | Soluble | Low | No | No | 182.4 | No | - | + | - | Non-carcinogen | -2.93 | 2.5194 (III) | 500 (class 5) | Inactive | Inactive | Active | Inactive | Active |
| 8-Oxyberberine | 3.05 | Soluble | High | Yes | Yes | 58.92 | No | + | + | + | Non-carcinogen | -2.79 | 2.40(III) | 400 (class 4) | Inactive | Active | Active | Active | Active |
| 9-epoxylignan | 2.99 | Moderately soluble | High | Yes | Yes | 77.38 | No | + | + | + | Non-carcinogen | -2.73 | 2.48 (III) | 2000 (class 4) | Inactive | Inactive | Active | Inactive | Inactive |
| Apigenin | 2.11 | Soluble | High | Yes | Yes | 90.9 | Yes | + | - | - | Non-carcinogen | -2.77 | 1.14(III) | 2500 (class 5) | Inactive | Inactive | Inactive | Inactive | Inactive |
| Armatamide | 3.26 | Soluble | High | Yes | Yes | 56.79 | No | + | + | + | Non-carcinogen | -3.30 | 1.74 (III) | 1000 (class4) | Inactive | Active | Active | Active | Inactive |
| Berbamine | 5.16 | Poorly soluble | High | Yes | Yes | 72.86 | No | + | + | + | Non-carcinogen | -2.63 | 2.82 (III) | 1700 (class 4) | Inactive | Inactive | Active | Active | Inactive |
| Berberine | 2.53 | Moderately soluble | High | Yes | Yes | 40.8 | No | + | + | + | Non-carcinogen | -2.97 | 1.54(III) | 200 (class3) | Inactive | Active | Active | Active | Active |
| Cannabigerol | 5.74 | Poorly soluble | High | Yes | Yes | 40.46 | No | + | + | + | Non-carcinogen | -3.42 | 2.52(III) | 500 (class4) | Inactive | Inactive | Inactive | Inactive | Inactive |
| Cannabinol | 5.21 | Moderately soluble | High | Yes | Yes | 29.46 | No | + | + | + | Non-carcinogen | -3.97 | 2.99 (III) | 13500 (class6) | Inactive | Inactive | Active | Inactive | Inactive |
| Catechin | 0.85 | Soluble | High | Yes | Yes | 110.3 | Yes | + | - | - | Non-carcinogen | -3.10 | 2.14 (IV) | 10000 (class6) | Inactive | Inactive | Inactive | Inactive | Inactive |
| Chrysophanol | 2.38 | Moderately soluble | High | Yes | Yes | 74.6 | No | - | - | + | Non-carcinogen | -3.12 | 2.91(II) | 1190 (class4) | Active | Inactive | Active | In active | In active |
| Clicoemodin | 0.19 | Soluble | High | Yes | No | 178.9 | No | + | - | - | Non-carcinogen | -2.36 | 2.82(III) | 3000 (class5) | Inactive | Inactive | Active | Active | Inactive |
| CurcubitacineE | 3.47 | Moderately soluble | Low | Yes | Yes | 138.2 | No | + | + | - | Non-carcinogen | -4.50 | 4.81 (I) | 340 (class4) | Inactive | Active | Active | Inactive | Inactive |
| Emodin | 1.87 | Soluble | High | Yes | Yes | 94.83 | Yes | + | - | + | Non-carcinogen | -3.01 | 2.56(III) | 5000 (class5) | Inactive | In active | In active | Active | Inactive |
| Eriodictyol-7-glucuronide | -0.37 | Soluble | Low | No | No | 203.4 | No | + | - | - | Non-carcinogen | -3.71 | 1.85 (II) | 2300 (class5) | Inactive | Inactive | Active | Inactive | Inactive |
| Eriodictyol | 1.45 | Soluble | High | Yes | Yes | 107.2 | Yes | + | - | - | Non-carcinogen | -3.44 | 2.41 (II) | 2000 (class4) | Inactive | Active | Inactive | Inactive | Inactive |
| Glucomoringin | -1.82 | Very soluble | Low | No | No | 278.9 | No | - | + | - | Non-carcinogen | -3.16 | 2.56(III) | 3750 (class5) | Inactive | Inactive | Active | Inactive | Inactive |
| Isovitexin | 0.05 | Soluble | Low | Yes | No | 181.0 | No | + | - | - | Non-carcinogen | -2.39 | 2.81(IV) | 159 (class3) | Inactive | Inactive | Inactive | Active | Inactive |
| Lupeol | 7.31 | Poorly soluble | Low | Yes | Yes | 20.23 | No | + | + | - | Non-carcinogen | -4.41 | 3.85 (III) | 2000 (class4) | Inactive | Inactive | Active | Inactive | Inactive |
| Oleanolic acid | 6.06 | Poorly soluble | Low | Yes | Yes | 57.53 | No | + | - | + | Non-carcinogen | -4.38 | 2.70(III) | 2000 (class4) | Active | Active | Active | Inactive | Inactive |
| Pikuroside | -1.53 | Very soluble | Low | No | No | 214.0 | No | + | - | - | Non-carcinogen | -1.98 | 2.87(III) | 2260 (class5) | Inactive | Inactive | Active | Inactive | Inactive |
| Piperolein B | 4.47 | Soluble | High | Yes | Yes | 38.77 | No | + | + | + | Non-carcinogen | -3.51 | 1.68(III) | 1000 (class4) | Inactive | Active | Active | Inactive | Inactive |
| Pterygospermin | 3.75 | Moderately soluble | High | Yes | Yes | 89.12 | No | + | + | - | Non-carcinogen | -4.32 | 2.41(III) | 898 (class4) | Inactive | Inactive | Inactive | Inactive | Inactive |
| Rubiadin | 2.23 | Soluble | High | Yes | Yes | 74.6 | Yes | + | - | + | Non-carcinogen | -3.11 | 2.09(III) | 7000 (class5) | Inactive | Inactive | Active | Active | Inactive |
| Rutin | -1.12 | Soluble | Low | No | No | 269.4 | No | + | - | - | Non-carcinogen | -2.77 | 2.59(III) | 5000 (class5) | Inactive | Inactive | Active | Inactive | Inactive |
| Stigmasterol | 6.96 | Poorly soluble | Low | Yes | Yes | 20.23 | No | + | + | + | Non-carcinogen | -4.70 | 3.28 (I) | 890 (class4) | Inactive | Inactive | Active | Inactive | Inactive |
| Taxifolin | 0.63 | Soluble | High | Yes | Yes | 127.4 | Yes | + | - | - | Non-carcinogen | -2.999 | 3.26(II) | 2000 (class4) | Inactive | Active | Inactive | Active | Inactive |
| Tetrahydrocannabinol | 5.28 | Poorly soluble | High | Yes | Yes | 29.46 | No | + | + | + | Non-carcinogen | -4.321 | 3.61(III) | 482 (class4) | Inactive | Inactive | Active | Inactive | Inactive |
| Thalicarpine | 5.68 | Poorly soluble | High | Yes | Yes | 80.32 | No | + | + | - | Non-carcinogen | -3.26 | 2.37(III) | 1500 (class4) | Inactive | Inactive | Active | Active | Inactive |
| Thalidasine | 5.34 | Poorly soluble | High | Yes | No | 71.09 | No | + | + | - | Non-carcinogen | -2.81 | 2.41(III) | 1700 (class4) | Inactive | Active | Active | Active | Inactive |
| Thalirugidine | 5.2 | Poorly soluble | High | Yes | Yes | 102.3 | No | + | + | - | Non-carcinogen | -2.83 | 2.38(III) | 1180 (class4) | Inactive | Inactive | Active | Active | Inactive |
| Thalirugine | 5.26 | Poorly soluble | High | Yes | No | 93.09 | No | + | + | - | Non-carcinogen | -2.83 | 2.25(III) | 1180 (class4) | Inactive | Inactive | Active | Active | Inactive |
| Thalisopine | 5.09 | Poorly soluble | High | Yes | Yes | 82.09 | No | + | + | + | Non-carcinogen | -2.50 | 3.09 (III) | 1700 (class4) | Inactive | Inactive | Active | Active | Inactive |
| Thalrugosaminine | 5.43 | Poorly soluble | High | Yes | Yes | 71.09 | No | + | + | + | Non-carcinogen | -2.86 | 2.53 (III) | 1700 (class4) | Inactive | Inactive | Active | Active | Inactive |
| Thalrugosidine | 5.05 | Poorly soluble | High | Yes | Yes | 82.09 | No | + | + | + | Non-carcinogen | -2.63 | 2.55(III) | 1700 (class4) | Inactive | Inactive | Active | Active | Inactive |
| Verbascoside | -0.43 | Soluble | Low | No | Yes | 245.29 | No | + | - | - | Non-carcinogen | -1.67 | 2.79(III) | 5000 (class5) | Inactive | Inactive | Active | Inactive | Inactive |
| β-Sitosterol Glucoside | 5.51 | Moderately soluble | Low | Yes | Yes | 99.8 | No | + | + | - | Non-carcinogen | -4.40 | 2.91 (III) | 1190 (class 4) | Active | Inactive | Active | In active | In active |
| Podofilox | 2.27 | Soluble | High | Yes | Yes | 92.98 | No | + | + | + | Non-carcinogen | -3.12 | 3.00(III) | 100 (class 3) | Inactive | Active | Active | Inactive | Inactive |
| Imiquimod | 2.34 | Soluble | High | Yes | Yes | 56.73 | No | + | + | + | Non-carcinogen | -4.03 | 2.09 (III) | 300 (class3) | Inactive | Active | Inactive | Active | Inactive |
where, 1-Consensus log P o/w, 2-water solubility, 3-GI-absorption, 4-Lipinski rule, 5- Veber’s rule, 6-TPSA (Å2), 7-Lead likeness, 8-Human intestinal absorption, 9-Blood brain barrier penetration, 10-Caco-2 cell permeability, 11-Carcinogens, 12-Aquatic & terrestrial toxicity (Log S value), 13-Rate acute toxicity (LD50), 14-LD50,(mg/kg), 15-Organ toxicity hepatotoxicity, 16-Carcinogenicity, 17-Immunotoxicity, 18-Mutagenicity, 19-Cytotoxicity, “+” Positive and “-” Negative.
Based on the molecular docking drug-likeness data and toxicity data, two best phytoconstituents (stigmasterol from Berberis aristata and clicoemodin from Rheum emodi) were selected for further MD simulation analysis.
3.3 MD simulation of protein-ligand complexes
The structural stability of the protein-ligand complex was determined using RMSD analysis during a specific time (100 ns). Molecular dynamic simulation of the selected protein-ligand complexes revealed that stigmasterol is stable inside the binding pocket of 1R9W. RMSD of stigmasterol was stable from the beginning of the simulation (0 ns) and remain stable up to 100ns period, which indicates fitness of the ligand inside the binding pocket of the target protein. RMSD has a little fluctuation between 0.5–1 Å, which is acceptable (Fig 3A). Similarly, the RMSD of clicoemodin, in complex with 1R9W is stable from the beginning (0 ns) and remains stable up to 100 ns periods, and only a little fluctuation was observed which is in the acceptable range (0.5–1 Å) (Fig 3B).
Fig 3.
RMSD of active phytoconstituents in complexation with 1R9W: A- Stigmasterol and B-Clicoemodin.
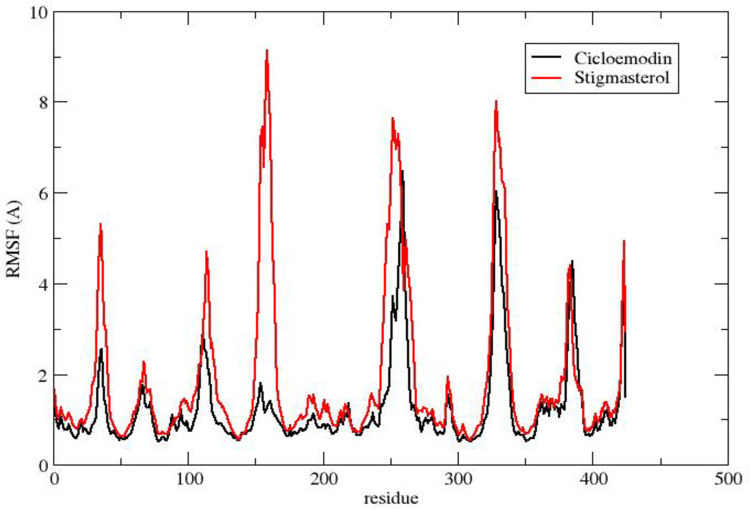
The RMSF value reflects the particle’s deviation from the initial position of macromolecules in a protein’s three-dimensional structure. The RMSF plots of stigmasterol and clicoemodin in complexation with 1R9W revealed that the interactive amino acids of stigmasterol and clicoemodin have lesser fluctuations in the alpha-helix and beta-strand of the target protein (Fig 4).
Fig 4. RMSF of active phytoconstituents in complexation with 1R9W.
The solvent-accessible range of stigmasterol is 8500–9500 Å, whereas in the case of clicoemodin, it lies between 8000–8500 Å (Fig 5). The radius of gyration plot established the compactness of stigmasterol and clicoemodin complexes with 1R9W, which confirms the stability of the protein-ligand complexes (Fig 6).
Fig 5. SASA of active phytoconstituents in complex with 1R9W: Black color is indicating clicoemodin and red color indicates stigmasterol.
Fig 6. Radius of gyration of active phytoconstituents in complex with 1R9W: Black color is indicating clicoemodin and red color indicates stigmasterol.
To understand the impact of inhibitors upon complexation in terms of their binding affinities, MM-PBSA binding free energy method was utilized to calculate the binding free energies and the energy components of their complexes are shown inTable 4.
Table 4. Binding free energies and their components for the two complexes: Clicoemodin-1R9W and stigmasterol-1R9W using the MM-PBSA method.
The energy components are in kcal/mol.
| Complex | Clicoemodin -2R5K | Stigmasterol -2R5K |
|---|---|---|
| ΔEvdW | − 45.61 | − 33.09 |
| ΔEel | − 39.38 | − 10.88 |
| ΔGPB | 63.38 | 29.51 |
| ΔGNP | -4.02 | -3.67 |
| ΔGbind | − 25.63 | − 18.13 |
As it is evident in Table 4, the total binding free energies (ΔGbind) of clicoemodin-1R9W and stigmasterol-1R9W were -25.63 kcal/mol and -18.13 kcal/mol, respectively. Accordingly, among the two studied complexes, Clicoemodin-1R9W depicted the most favorable of ΔGbind with its lowest values of -25.63 kcal/mol. At this point, it is interesting to address the key contributions of each binding component that can impose to the total binding free energies.
4. Discussion
HPV has been described as the principal cause for the development of cervical cancer, and this potential is linked with its ability to produce various oncoproteins. Women infected by the human immunodeficiency virus (HIV) also have a very high susceptibility to HPV infections with the presence of multiple HPV types being typically reported [61]. HPV infections cause several benign proliferations including warts, epithelial cysts, anogenital, oro-laryngeal and -pharyngeal papillomas, intraepithelial neoplasias, keratoacanthomas, and other forms of hyperkeratoses. Brown and co-workers detected a high-risk HPV viral DNA on condylomata acuminata lesions from HIV immunosuppressed and otherwise healthy patients, always in association with a low-risk HPV type, HPV 6 or 11 [62].
In the last decades, the pharmaceutical industry has been employing computer-aided drug design (CADD) techniques to accelerate drug development, intending to reduce time, costs, and failures, mainly in the final stage [63, 64]. CADD attempts to improve the efficacy of the hit discovery process through in silico methodologies by testing and screening a large number of compounds to identify a small group of candidates with desirable pharmacological properties. The virus particle binds to its specific cell surface receptors, which allows it to enter the host cell. Virions can first bind to the basement membrane before being transferred to the basal keratinocyte cell surface [65, 66]. The structure-based approaches, including docking and the application of molecular dynamics simulations, use the 3D structure of the target molecule to screen potential ligands. These methods evaluate ligand recognition by the target molecule and the prediction and characterization of binding sites as well as their binding affinities [63, 67].
Computational-based approaches hold a great promise for improving drug development and revolutionizing clinical research by providing appropriate interventions for women diagnosed with cervical cancer. In the current study, we found that among all the selected one hundred nineteen phytoconstituents of the twenty unexplored medicinal plants of northwestern Himalaya, stigmasterol from Berberis aristata, and clicoemodin from Rheum emodi can be used as a potent drug to cure the HPV. In our previous research, Kumar et al. [68] studied the interactions of traditionally used phytoconstituents (ferulic acid, ursolic acid, jaceosidin, curcumin, gingerol, indol-3-carbinol, silymarin, resveratrol, berberine, and artemisinin) against E6 oncoprotein of high-risk HPV 18 by using Autodock 4.2 tool. Sookmai et al. [69] reported the anti-papillomavirus activity of Clinacanthus nutans extract; they also suggested C. nutans as prevention of HPV. Earlier a study of Moghadamtousi and co-workers, also showed a high potential of curcumin to cure high-risk HPV 16 and 18 [70]. Similarly, various researchers revealed the anticancerous potential of stigmasterol in the treatment of skin carcinoma [71], ovarian cancer [72], and gastric cancer [73, 74]. Previous reports also revealed that crude extracts or phytoconstituents obtained from medicinal plants of the Northwest Himalayas have various therapeutic attributes and show synergism of the in silico and in vitro investigations [75–83] and even Rolta et al. [84] reported the antiviral activity of phytoconstituents of important medicinal plants of the Northwest Himalayas against SARS-CoV-2. According to Cha et al. [85], emodin a major phytocompound of Rheum emodi inhibits the cell growth of the bladder cancer cell line. Thymus serpyllum essential oil and its two phytoconstituents thymol and carvacrol have antitumor activity against P815 mastocytoma cell line [86], HeLa (cervical carcinoma), and HepG2 (hepatocellular carcinoma) cells as reported by Hussain and co-workers, 2013 [87].
In silico docking examination by Muthukala et al. [88], has reported the inhibitory potential of quercetin and quercetin-like components towards human cervical cancer cell line proteins. Scheffner and coworkers also investigated that HPV oncoproteins i.e. E7 and E6 are activated in cervical cells during the progression of cervical cancer and also have been recognized to interact with tumor suppressor proteins viz. p53 and pRB respectively [89]. Through an in-silico approach, Kotadiya and Georrge [90] detected some putative drugs derived from the natural products against HPV, while Samant et al. [91] investigated the molecular interactions of HIV and anti-viral drugs targeting HPV-18 E6 protein. Inhibitory potential of natural products including colchicine, daphnoretin, curcumin, ellipticine, epigallocatechin-3-gallate, etc. towards HPV-16 E6 protein was also recognized by molecular docking [92]. Phytocompounds of Ficus carica is also reported as anticacinogenic drug [93]. Drug delivery systems can be developed to circumvent possible high toxicity and low availability of the compounds and, for instance, to combine this approach of L1 inhibitors with gene therapy to induce cancer cell apoptosis. Considering the costs of screening and treatment of cervical cancer and knowing that the highest incidence occurs in developing countries, a more cost-effective treatment is needed.
5. Conclusion
Major phytoconstituents of twenty traditionally important plants were screened against the HPV by molecular docking. Among the selected phytoconstituents stigmasterol from Berberis aristata, and clicoemodin from Rheum emodi were found to be the best inhibitors of HPV-18 (1R9W protein). ADME/T validated the proposal of stigmasterol and clicoemodin are safer drugs for the treatment of cervical cancer. MD simulations data showed that stigmasterol and clicoemodin are stable inside the binding pocket of the target protein. Since this study has only contemplated computational analysis, it is still necessary to validate the bioactivities of these important phytoconstituents in vitro and in vivo models before proposing their antiviral potential. We, also suggest that further research should take these results into account in both the discovery as well as the lead optimization stages to achieve the successful development of anti-HPV drugs.
Supporting information
(DOCX)
1) 3-O-b-galactopyranoside, 2) 8-Oxyberberine, 3) 9-epoxylignan, 4) Apigenin, 5) Armatamide, 6) Berbamine, 7) Berberine, 8) Cannabigerol, 9) Cannabinol, 10) Catechin, 11) Chrysophanol, 12) Clicoemodin.
(TIF)
1) CurcubitacineE, 2) Emodin, 3) Eriodictyol-7-glucuronide, 4) Eriodictyol, 5) Glucomoringin, 6) Isovitexin, 7) Lupeol, 8) Oleanolic acid, 9) Pikuroside, 10) Piperolein B, 11) Pterygospermin, 12) Rubiadin.
(TIF)
1) Rutin, 2) Stigmasterol, 3) Taxifolin, 4) Tetrahydrocannabinol, 5) Thalicarpine, 6) Thalidasine, 7) Thalirugidine, 8) Thalirugine, 9) Thalisopine, 10) Thalrugosaminine B, 11) Podofilox, 12) Imiquimod.
(PNG)
Acknowledgments
The authors acknowledge Shoolini University, Solan (India) and Kwangwoon University, Seoul (Korea) to support this research.
Data Availability
All relevant data are within the paper.
Funding Statement
This research was funded by the National Research Foundation (NRF) of Korea, funded by the Korean government (2021R1A6A1A03038785, 2021R1F1A1055694, 2021R1C1C1013875), and by Kwangwoon University in 2022.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer J Clinic. 2021; 71: 209–249. [DOI] [PubMed] [Google Scholar]
- 2.Venturas C, Umeh K. Health professional feedback on HPV vaccination roll-out in a developing country. Vaccine. 2017; 35(15); 1886–1891. doi: 10.1016/j.vaccine.2017.02.052 [DOI] [PubMed] [Google Scholar]
- 3.zur Hausen H. Papillomaviruses causing cancer: evasion from host-cell control in early events in carcinogenesis. J Natl Cancer Inst. 2000; 92(9): 690–698. doi: 10.1093/jnci/92.9.690 [DOI] [PubMed] [Google Scholar]
- 4.Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999; 189: 2–9. doi: [DOI] [PubMed] [Google Scholar]
- 5.Xue H, Lin X, Li T, Yan X, Guo K, Zhang Y. Prevalence and genotype distribution of human papillomavirus infection in asymptomatic women in Liaoning province, China J Med Virol. 2015; 87: 1248–1253. doi: 10.1002/jmv.24029 [DOI] [PubMed] [Google Scholar]
- 6.Srivastava S, Gupta S, Roy JK. High prevalence of oncogenic HPV-16 in cervical smears of asymptomatic women of eastern Uttar Pradesh, India: a population-based study. J Biosci. 2012; 37: 63–72. doi: 10.1007/s12038-012-9181-y [DOI] [PubMed] [Google Scholar]
- 7.Kerkar SC, Latta S, Salvi V, Pramanik JM. Human papillomavirus infection in asymptomatic population. Sex Reprod Health. 2011;2: 7–11. doi: 10.1016/j.srhc.2010.11.001 [DOI] [PubMed] [Google Scholar]
- 8.Petrelli A, Di Napoli A, Rossi PG, Rossi A, Luccini D, Di Marco I, et al. Prevalence of primary HPV in Djibouti: feasibility of screening for early diagnosis of squamous intraepithelial lesions. J low genit tract dis. 2016; 20: 321–326. doi: 10.1097/LGT.0000000000000240 [DOI] [PubMed] [Google Scholar]
- 9.Arbyn M, Castellsague X, DeSanjose S. Worldwide burden of cervical cancer. Ann Oncol. 2011; 22: 2675–2682. doi: 10.1093/annonc/mdr015 [DOI] [PubMed] [Google Scholar]
- 10.Clifford GM, Gallus S, Herrero R, Muñoz N, Snijders PJ, Vaccarella S, et al. Worldwide distribution of human papillomavirus types in cytologically normal women in the International Agency for Research on Cancer HPV prevalence surveys: a pooled analysis. Lancet. 2005; 366: 991–998. doi: 10.1016/S0140-6736(05)67069-9 [DOI] [PubMed] [Google Scholar]
- 11.Bruni L, Diaz M, Castellsagué M, Ferrer E, Bosch FX, de Sanjosé S. Cervical human papillomavirus prevalence in 5 continents: meta-analysis of 1 million women with normal cytological findings. J Infect Dis. 2010; 202: 1789–1799. doi: 10.1086/657321 [DOI] [PubMed] [Google Scholar]
- 12.Sankaranarayanan R, Chatterji R, Shastri SS, Wesley RS, Basu P, Mahe C, et al. Accuracy of human papillomavirus testing in primary screening of cervical neoplasia: results from a multicenter study in India. Int J Cancer. 2004;112:341–7. doi: 10.1002/ijc.20396 [DOI] [PubMed] [Google Scholar]
- 13.Sowjanya AP, Jain M, Poli UR, Padma S, Das M, Shah KV, et al. Prevalence and distribution of high-risk human papilloma virus (HPV) types in invasive squamous cell carcinoma of the cervix and in normal women in Andhra Pradesh, India. BMC Infect Dis. 2005:5:116. doi: 10.1186/1471-2334-5-116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sreedevi A, Javed R, Dinesh A. Epidemiology of cervical cancer with special focus on India. Int J Womens Health. 2015;7:405–14. doi: 10.2147/IJWH.S50001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wardak S. Human papillomavirus and cervical cancer. Med Dis Microbiol. 2016;73–84. [PubMed] [Google Scholar]
- 16.Zur Hausen H. Papillomaviruses and cancer: from basic studies to clinical application. Nat Rev Cancer. 2002; 2: 342–350. doi: 10.1038/nrc798 [DOI] [PubMed] [Google Scholar]
- 17.Galani E, Christodoulou C. Human papilloma viruses and cancer in the post-vaccine era. Clinical microbiology and infection. 2009; 15: 977–981. doi: 10.1111/j.1469-0691.2009.03032.x [DOI] [PubMed] [Google Scholar]
- 18.Hampson L, Martin-Hirsch P, Hampson IN. An overview of early investigational drugs for the treatment of human papilloma virus infection and associated dysplasia. Expert Opin Investig Drugs. 2015; 24: 1529–1537. doi: 10.1517/13543784.2015.1099628 [DOI] [PubMed] [Google Scholar]
- 19.Garbyal SS, Aggarwal KK, Babu CR. Traditionally used medicinal plants in Dharchula Himalayas of Pithoragarh district, Uttaranchal. Indian J Tradit Knowl. 2005; 4: 199–207. [Google Scholar]
- 20.Rowshan V, Bahmanzadegan A, Saharkhiz MJ. Influence of storage conditions on the essential oil composition of Thymus daenensis Celak Ind Crops Product. 2013; 49:97–101. [Google Scholar]
- 21.Tajidin NE, Ahmad SH, Rosenani AB, Azimah H, Munirah M. Chemical composition and citral content in lemongrass (Cymbopogon citratus) essential oil at three maturity stages. Afr J Biotechnol. 2012;11: 2685–2693. [Google Scholar]
- 22.Rani ANZ, Husain K, Kumolosasi E. Moringa genus: a review of phytochemistry and pharmacology. Front Pharmacol. 2018; 9: 108. doi: 10.3389/fphar.2018.00108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fahey JW. Moringa oleifera: a review of the medical evidence for its nutritional, therapeutic, and prophylactic properties. Part 1. Trees for life Journal. 2005; 1: 1–5. [Google Scholar]
- 24.Chopra RN, Nayar SL, Chopra IC. Glossary of Indian medicinal plants. New Delhi: Council of Scientific & Industrial Research. 1956; 138–139. [Google Scholar]
- 25.Chauhan NS. Medicinal and aromatic plants of Himachal Pradesh. Indus publishing: Delhi, India, 1999. [Google Scholar]
- 26.Sharma R, Manhas RK. Ethnoveterinary plants for the treatment of camels in Shiwalik regions of Kathua district of Jammu & Kashmir, India. J ethnopharmacol. 2015; 169: 170–175. doi: 10.1016/j.jep.2015.04.018 [DOI] [PubMed] [Google Scholar]
- 27.Takooree H, Aumeeruddy MZ, Rengasamy KR, Venugopala KN, Jeewon R, Zengin G, et al. A systematic review on black pepper (Piper nigrum L.): From folk uses to pharmacological applications. Crit rev food sci nutr. 2019; 59: S210–43. doi: 10.1080/10408398.2019.1565489 [DOI] [PubMed] [Google Scholar]
- 28.Ross IA. Myristica fragrans. In Medicinal Plants of the World 2001. (pp. 333–352). Humana Press, Totowa, NJ. [Google Scholar]
- 29.Paul A, Kumar A, Singh G. Choudhary A. Medicinal, pharmaceutical and pharmacological properties of Zanthoxylum armatum: A Review. J Pharmacogn Phytochem. 2018; 7: 892–900. [Google Scholar]
- 30.Rana D, Bhatt A, Lal B. Ethnobotanical knowledge among the semi-pastoral Gujjar tribe in the high altitude (Adhwari’s) of Churah subdivision, district Chamba, Western Himalaya. J. Ethnobiol Ethnomedic. 2019; 15: 1–21. doi: 10.1186/s13002-019-0286-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Russo E, Guy GW. A tale of two cannabinoids: the therapeutic rationale for combining tetrahydrocannabinol and cannabidiol. Medical hypotheses. 2006; 66: 234–246. doi: 10.1016/j.mehy.2005.08.026 [DOI] [PubMed] [Google Scholar]
- 32.Kuddus M, Ginawi IA, Al-Hazimi A. Cannabis sativa: An ancient wild edible plant of India. Emir J Food Agric. 2013; 736–745. [Google Scholar]
- 33.Kirtikar KR, Basu BD. Text book of Indian medicinal plants. Dehradun, India: International Book Distributors. 2005; 2: 993–994. doi: 10.1080/10286020310001608949 [DOI] [PubMed] [Google Scholar]
- 34.Gordaliza M. Natural products as leads to anticancer drugs. Clinical and Translational Oncology. 2007; 9:767–76. doi: 10.1007/s12094-007-0138-9 [DOI] [PubMed] [Google Scholar]
- 35.Kangsamaksin T, Chaithongyot S, Wootthichairangsan C, Hanchaina R. Tangshewinsirikul C, Svasti J. Lupeol and stigmasterol suppress tumor angiogenesis and inhibit cholangiocarcinoma growth in mice via downregulation of tumor necrosis factor-α. PLoS One. 2017:12; e0189628. doi: 10.1371/journal.pone.0189628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O’Boyle NM, Banck M, James CA, Morley C, Vandermeersch T, Hutchison GR. Open Babel: An open chemical toolbox. J cheminformatics. 2011; 3:1–4. doi: 10.1186/1758-2946-3-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, et al. UCSF Chimera-a visualization system for exploratory research and analysis. J computat chem. 2004; 25: 1605–1612. doi: 10.1002/jcc.20084 [DOI] [PubMed] [Google Scholar]
- 38.Trott O, Olson AJ. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem. 2010;31: 455–461. doi: 10.1002/jcc.21334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bisht VK, Negi JS, Bh AK, Sundriyal RC. Traditional use of medicinal plants in district Chamoli, Uttarakhand, India. J Med Plant Res. 2013;7:918–929. [Google Scholar]
- 40.Lal B, Singh KN. Indigenous herbal remedies used to cure skin disorders by the natives of Lahaul-Spiti in Himachal Pradesh. 2008; 7:237–241. [Google Scholar]
- 41.Guleria V, Vasishth A. Ethnobotanical uses of wild medicinal plants by Guddi and Gujjar tribes of Himachal Pradesh. Ethnobot leafl. 2009; 2009: 8. [Google Scholar]
- 42.Van Geldre E, De Pauw I, Inzé D, Van Montagu M, Van den Eeckhout E. Cloning and molecular analysis of two new sesquiterpene cyclases from Artemisia annua L. Plant Science. 2000; 158: 163–171. doi: 10.1016/s0168-9452(00)00322-8 [DOI] [PubMed] [Google Scholar]
- 43.Coffey T. The History and Folklore of North American Wild Flowers. Facts on File. 1993; ISBN 0-8160-2624-6.
- 44.Weiner MA. Earth Medicine, Earth Food. Ballantine Books 1980; ISBN 0-449-90589-6.
- 45.Moerman D. Native American Ethnobotany Timber Press. Oregon. 1998; ISBN 0-88192-453-9.
- 46.Mahboubi M, Kashani LMT. A Narrative study about the role of Viola odorata as traditional medicinal plant in management of respiratory problems. Adv Integr Med. 2018; 5: 112–118. [Google Scholar]
- 47.Auster AS, Joshua-Tor L. The DNA-binding domain of human papillomavirus type 18 E1: crystal structure, dimerization, and DNA binding. J Biolog Chem. 2004;279:3733–42. [DOI] [PubMed] [Google Scholar]
- 48.Banerjee P, Eckert AO, Schrey AK, Preissner R. ProTox-II: a webserver for the prediction of toxicity of chemicals. Nucl Acids Res. 2018; 46(W1): W257–W263. doi: 10.1093/nar/gky318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang H, Lou C, Sun L, Li J, Cai Y, Wang Z, et al. AdmetSAR 2.0: web-service for prediction and optimization of chemical ADMET properties. Bioinfo. 2019; 35: 1067–1069. doi: 10.1093/bioinformatics/bty707 [DOI] [PubMed] [Google Scholar]
- 50.Salaria D, Rolta R, Sharma N, Dev K, Sourirajan A, Kumar V. In silico and In vitro evaluation of the anti-inflammatory and antioxidant potential of Cymbopogon citratus from North-western Himalayas. J Biomol Struct Dyn. 2021. doi: 10.1080/07391102.2021.2001371 [DOI] [PubMed] [Google Scholar]
- 51.Eswaramoorthy R, Hailekiros H, Kedir F, Endale M. In silico Molecular Docking, DFT Analysis and ADMET Studies of Carbazole Alkaloid and Coumarins from Roots of Clausena anisata: A Potent Inhibitor for Quorum Sensing. Advances and Applications in Bioinformatics and Chemistry: AABC. 2021;14:13. doi: 10.2147/AABC.S290912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Anza M, Endale M, Cardona L, Cortes D, Eswaramoorthy R, Zueco J, et al. Antimicrobial Activity, in silico Molecular Docking, ADMET and DFT Analysis of Secondary Metabolites from Roots of Three Ethiopian Medicinal Plants. Advances and Applications in Bioinformatics and Chemistry: AABC. 2021;14:117. doi: 10.2147/AABC.S323657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lipinski CA. Lead-and drug-like compounds: the rule-of-five revolution. Drug discovery today: Technologies. 2004. Dec 1;1(4):337–41. doi: 10.1016/j.ddtec.2004.11.007 [DOI] [PubMed] [Google Scholar]
- 54.Lee TS, Cerutti DS, Mermelstein D, Lin C, LeGrand S, Giese TJ, et al. GPU-accelerated molecular dynamics and free energy methods in Amber18: performance enhancements and new features. J Chem Inf Model. 2018; 58: 2043–2050. doi: 10.1021/acs.jcim.8b00462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang J, Wolf RM, Caldwell JW, Kollman PA, Case DA. Development and testing of a general amber force field. J comput chem. 2004; 25: 1157–1174. doi: 10.1002/jcc.20035 [DOI] [PubMed] [Google Scholar]
- 56.Song LF, Lee TS, Zhu C, York DM, Merz KM Jr. Using AMBER18 for relative free energy calculations. J Chem Inf Model. 2019;59;3128–3135. doi: 10.1021/acs.jcim.9b00105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, et al. Gaussian 16. Gaussian, Inc., Wallingford CT, GaussView 5.0. Wallingford, E.U.A. 2016. [Google Scholar]
- 58.Frisch RE, Snow RC, Johnson LA, Gerard B, Barbieri R, Rosen B. Magnetic resonance imaging of overall and regional body fat, estrogen metabolism, and ovulation of athletes compared to controls. J Clin Endocrinol Metab. 1993; 77: 471–7. doi: 10.1210/jcem.77.2.8345054 [DOI] [PubMed] [Google Scholar]
- 59.Ryckaert JP, Ciccotti G, Berendsen HJ. Numerical integration of the cartesian equations of motion of a system with constraints: molecular dynamics of n-alkanes. Journal of computational physics. 1977; 23:327–41. [Google Scholar]
- 60.Sun H, Duan L, Chen F, Liu H, Wang Z, Pan P, et al. Assessing the performance of MM/PBSA and MM/GBSA methods. 7. Entropy effects on the performance of end-point binding free energy calculation approaches. Phys Chem Chem Phys. 2018; 20: 14450–14460. doi: 10.1039/c7cp07623a [DOI] [PubMed] [Google Scholar]
- 61.Levi JE, Fernandes S, Tateno AF, Motta E, Lima LP, Eluf-Neto J. Presence of multiple human papillomavirus types in cervical samples from HIV-infected women. Gynecol Oncol. 2004; 92: 226–232. doi: 10.1016/j.ygyno.2003.10.004 [DOI] [PubMed] [Google Scholar]
- 62.Brown DR, Schrieder JM, Bryan JT, Stoler MH, Fife KH. Detection of multiple human papillomavirus types in Condylomata acuminata lesions from otherwise healthy and immunosupressed patents. J Clin Microbiol. 1999;37:3316–3322. doi: 10.1128/JCM.37.10.3316-3322.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Macalino SJ, Gosu V, Hong S, Choi S. Role of computer-aided drug design in modern drug discovery. Arch. Pharm Res. 2015; 38: 1686–1701. doi: 10.1007/s12272-015-0640-5 [DOI] [PubMed] [Google Scholar]
- 64.Yella JK, Yaddanapudi S, Wang Y, Jegga AG. Changing Trends in Computational Drug Repositioning. Pharmaceuticals 2018; 11: 57. doi: 10.3390/ph11020057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Roberts JN, Buck CB, Thompson CD, Kines R, Bernardo M, Choyke PL, et al. Genital transmission of HPV in a mouse model is potentiated by nonoxynol-9 and inhibited by carrageenan. Nat Med. 2007; 13: 857–861. doi: 10.1038/nm1598 [DOI] [PubMed] [Google Scholar]
- 66.Horvath CA, Boulet GA, Renoux VM, Delvenne PO, Bogers JP. Mechanisms of cell entry by human papillomaviruses: an overview. Virol J. 2010; 7: 1–7. doi: 10.1186/1743-422X-7-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Prieto-Martínez FD, López-López E, Eurídice Juárez-Mercado K, Medina-Franco JL. Computational Drug Design Methods—Current and Future Perspectives. In In Silico Drug Design; Elsevier: Amsterdam, The Netherlands. 2019; 19–44. doi: 10.1007/978-3-030-14632-0_1 [DOI] [Google Scholar]
- 68.Kumar S, Jena L, Sahoo M, Kakde M, Daf S, Varma AK. In silico docking to explicate interface between plant-originated inhibitors and E6 oncogenic protein of highly threatening human papillomavirus 18. Genomics Inform. 2015;13: 60. doi: 10.5808/GI.2015.13.2.60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sookmai W, Ekalaksananan T, Pientong C, Sakdarat S, Kongyingyoes B. The anti-papillomavirus infectivity of Clinacanthus nutans compounds. Srinagarind Med J. 2011;26: 240–243. [Google Scholar]
- 70.Moghadamtousi ZS, Abdul Kadir H, Hassandarvish P, Tajik H, Abubakar S, Zandi K. A review on antibacterial, antiviral, and antifungal activity of curcumin. BioMed Res Int. 2014; 2014: 186864. doi: 10.1155/2014/186864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ali H, Dixit S, Ali D, Alqahtani SM, Alkahtani S, Alarifi S. Isolation and evaluation of anticancer efficacy of stigmasterol in a mouse model of DMBA-induced skin carcinoma. Drug Des Devel Ther. 2015; 9:2793–2800. doi: 10.2147/DDDT.S83514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bae H, Song G, Lim W. Stigmasterol causes ovarian cancer cell apoptosis by inducing endoplasmic reticulum and mitochondrial dysfunction. Pharmaceutics. 2020; 12(6): 488. doi: 10.3390/pharmaceutics12060488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li K, Yuan D, Yan R, Meng L, Zhang Y, Zhu K. Stigmasterol exhibits potent antitumor effects in human gastric cancer cells mediated via inhibition of cell migration, cell cycle arrest, mitochondrial mediated apoptosis and inhibition of JAK/STAT signalling pathway. J Buon. 2018; 23:1420–1425. [PubMed] [Google Scholar]
- 74.Zhao H, Zhang X, Wang M, Lin Y, Zhou S. Stigmasterol Simultaneously Induces Apoptosis and Protective Autophagy by Inhibiting Akt/mTOR Pathway in Gastric Cancer Cells. Front Oncol. 2021;11: 43. doi: 10.3389/fonc.2021.629008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mehta J, Dev K. Extraction, isolation and characterization of lariciresinol from Zingiber officinale (ginger) rhizome extract for their efflux pump inhibitory activity on Salmonella enterica serovar typhimurium strains. Studies in Indian Place Names. 2010; 40: 218–231. [Google Scholar]
- 76.Mehta J, Jandaik S, Urmila, Mehta BB. Antibacterial activity and phytochemical screening of selected medicinal plants against Salmonella enterica serovar Typhimurium strains. IJBPAS. 2016. a; 5: 2119–2130. [Google Scholar]
- 77.Mehta J, Jandaik S, Urmila. Evaluation of phytochemicals and synergistic interaction between plant extracts and antibiotics for efflux pump inhibitory activity against Salmonella enterica serovar typhimurium strains. Int J Pharm and Pharm Sci. 2016. b; 8: 217–223. [Google Scholar]
- 78.Urmila, Jandaik S, Mehta J, Mohan M. Synergistic and efflux pump inhibitory activity of plant extracts and antibiotics on staphylococcus aureus strains. Asian J Pharm Clin Res. 2016: 9;277–282. [Google Scholar]
- 79.Mehta J, Rolta R, Dev K. Role of medicinal plants from North Western Himalayas as an efflux pump inhibitor against MDR AcrAB-TolC Salmonella enterica serovar typhimurium: In vitro and In silico studies. J Ethnopharmacol. 2021;114589. doi: 10.1016/j.jep.2021.114589 [DOI] [PubMed] [Google Scholar]
- 80.Mehta J, Rolta R, Salaria D, Awofisayo O, Fadare OA, Sharma PP, et al. Phytocompounds from Himalayan Medicinal Plants as Potential Drugs to Treat Multidrug-Resistant Salmonella typhimurium: An In Silico Approach. Biomed. 2021; 9:1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rolta R, Salaria D, Sharma P, Sharma B, Kumar V, Rathi B, et al. Phytocompounds of Rheum emodi, Thymus serpyllum, and Artemisia annua Inhibit Spike Protein of SARS-CoV-2 Binding to ACE2 Receptor: In Silico Approach. Current Pharmacol. Rep. 2021; 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Salaria D, Rolta R, Patel CN, Dev K, Sourirajan A, Kumar V. In vitro and in silico analysis of Thymus serpyllum essential oil as bioactivity enhancer of antibacterial and antifungal agents. J Biomolecul Struct. Dynam. 2021;1–20. doi: 10.1080/07391102.2021.1943530 [DOI] [PubMed] [Google Scholar]
- 83.Rolta R, Salaria D, Kumar V, Patel CN, Sourirajan A, Baumler DJ, et al. Molecular docking studies of phytocompounds of Rheum emodi Wall with proteins responsible for antibiotic resistance in bacterial and fungal pathogens: in silico approach to enhance the bio-availability of antibiotics. J. Biomol. Struct. Dynam. 2020; 1–15. doi: 10.1080/07391102.2020.1850364 [DOI] [PubMed] [Google Scholar]
- 84.Rolta R, Yadav R, Salaria D, Trivedi S, Imran M, Sourirajan A, et al. In silico screening of hundred Phytoconstituents of ten medicinal plants as potential inhibitors of nucleocapsid phosphoprotein of COVID-19: an approach to prevent virus assembly. J biomol Struct Dyn. 2020; 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cha TL, Chuang MJ, Tang SH, Wu ST, Sun KH, Chen TT, et al. Emodin modulates epigenetic modifications and suppresses bladder carcinoma cell growth. Mol Carcinog. 2015; 54: 167–177. doi: 10.1002/mc.22084 [DOI] [PubMed] [Google Scholar]
- 86.Jaafari A, Mouse HA, Rakib EM, M’barek L.A, Tilaoui M, Benbakhta C, et al. Chemical composition and antitumor activity of different wild varieties of Moroccan thyme. Rev. bras. Pharmacogn. 2007; 17: 477–491. [Google Scholar]
- 87.Hussain AI, Anwar F, Chatha SAS, Latif S, Sherazi STH, Ahmad A, et al. Chemical composition and bioactivity studies of the essential oils from two Thymus species from the Pakistani flora. LWT-Food Science and Technology. 2013; 50:185–192. [Google Scholar]
- 88.Muthukala B, Sivakumari K, Ashok K. In silico docking of Quercetin compound against the Hela cell line proteins. Int J Curr Pharm Res. 2015; 7: 13–16. [Google Scholar]
- 89.Scheffner M, Münger K, Byrne JC, Howley PM. The state of the p53 and retinoblastoma genes in human cervical carcinoma cell lines. Proc Natl Acad Sci. 1991; 88: 5523–5527. doi: 10.1073/pnas.88.13.5523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kotadiya R, Georrge JJ. In silico approach to identify putative drugs from natural products for human papillomavirus (HPV) which cause cervical cancer. Life Sci Leafl. 2015; 63: 1–13. [Google Scholar]
- 91.Samant LR, Sangar VC, Chaudhary S, Chowdhary A. In silico molecular interactions of HIV antiviral drugs against HPV type 18 E6 protein. World J Pharm Pharm Sci. 2015; 4: 1056–1066. [Google Scholar]
- 92.Mamgain S, Sharma P, Pathak RK, Baunthiyal M. Computer aided screening of natural compounds targeting the E6 protein of HPV using molecular docking. Bioinformation. 2015: 11;236–242. doi: 10.6026/97320630011236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gurung AB, Ali MA, Lee J, Farah MA, Al-Anazi KM. Molecular docking and dynamics simulation study of bioactive compounds from Ficus carica L. with important anticancer drug targets. Plos one. 2021;16:e0254035. doi: 10.1371/journal.pone.0254035 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
1) 3-O-b-galactopyranoside, 2) 8-Oxyberberine, 3) 9-epoxylignan, 4) Apigenin, 5) Armatamide, 6) Berbamine, 7) Berberine, 8) Cannabigerol, 9) Cannabinol, 10) Catechin, 11) Chrysophanol, 12) Clicoemodin.
(TIF)
1) CurcubitacineE, 2) Emodin, 3) Eriodictyol-7-glucuronide, 4) Eriodictyol, 5) Glucomoringin, 6) Isovitexin, 7) Lupeol, 8) Oleanolic acid, 9) Pikuroside, 10) Piperolein B, 11) Pterygospermin, 12) Rubiadin.
(TIF)
1) Rutin, 2) Stigmasterol, 3) Taxifolin, 4) Tetrahydrocannabinol, 5) Thalicarpine, 6) Thalidasine, 7) Thalirugidine, 8) Thalirugine, 9) Thalisopine, 10) Thalrugosaminine B, 11) Podofilox, 12) Imiquimod.
(PNG)
Data Availability Statement
All relevant data are within the paper.