Abstract
The extensive growth of energy and plastic demand has raised concerns over the depletion of fossil fuels. Moreover, the environmental conundrums worldwide integrated with global warming and improper plastic waste management have led to the development of sustainable and environmentally friendly biofuel (bioethanol) and biopolymer (lactic acid, LA) derived from biomass for fossil fuels replacement and biodegradable plastic production, respectively. However, the high production cost of bioethanol and LA had limited its industrial-scale production. This paper has comprehensively reviewed the potential and development of third-generation feedstock for bioethanol and LA production, including significant technological barriers to be overcome for potential commercialization purposes. Then, an insight into the state-of-the-art hydrolysis and fermentation technologies using macroalgae as feedstock is also deliberated in detail. Lastly, the sustainability aspect and perspective of macroalgae biomass are evaluated economically and environmentally using a developed cascading system associated with techno-economic analysis and life cycle assessment, which represent the highlights of this review paper. Furthermore, this review provides a conceivable picture of macroalgae-based bioethanol and lactic acid biorefinery and future research directions that can be served as an important guideline for scientists, policymakers, and industrial players.
Graphical abstract

Keyword: Bioethanol, High value-added bioproducts, Seaweed, Hydrolysis, Fermentation, Third generation
Introduction
In recent years, skyrocketing global energy demands and limited availability of fossil fuels due to urbanization and progressively growing of the world’s population have escalated renewable energy development. At the same time, due to the COVID-19 pandemic, increment of plastic waste generation is observed as a human propensity towards wearing personal protective equipment (PPE) such as face masks and hand gloves. Besides, the pandemic also slowly shifted human lives to depend on online platforms to get their meals, goods, and groceries delivered. Globally to date, nearly 140-fold of increment of plastic waste had been generated as compared to that produced in 2010 and reached approximately 8.3 billion tons of plastic waste in 2020 [1]. As a result, the interest in developing innovative biorefinery approaches for the production of bioenergies and biopolymers from renewable resources has intensified. The biorefinery concept offers a scheme to facilitate the circular bioeconomy that closes the loop of organic or fresh resources, minerals, carbon, and water. It can be defined as a green and sustainable bioprocess that utilizes the optimum energy potential of organic resources to produce bioenergy and bioproducts through the bioconversion process [2]. From the point of view of circular bioeconomy, the ideas focus on conserving the long-term usage of biomass resources, minimizing contamination on both environment and end-product, and guaranteeing food security while producing jobs for mankind [3]. To this extent, the macroalgae feedstocks tallying with these ideas strengthen the bioeconomy [4].
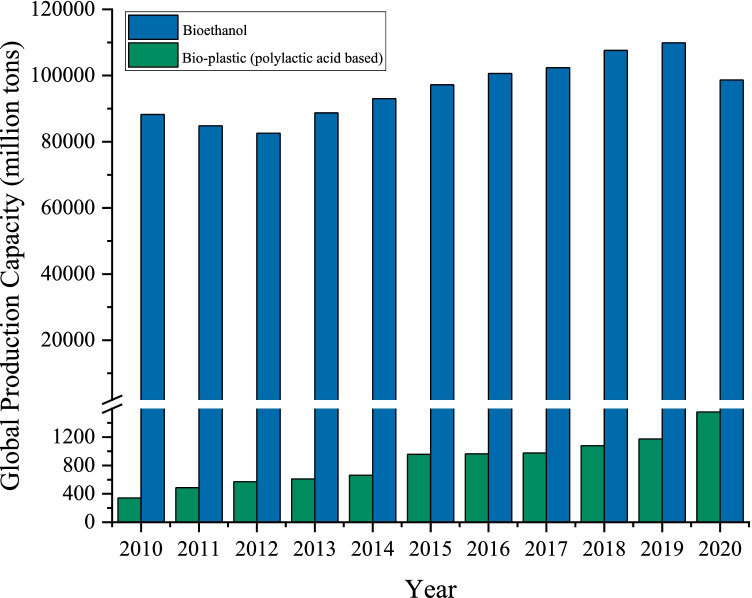
As a consequence, literature related to macroalgal biorefinery showed an increasing trend with expanding research outputs [5, 6]. Macroalgae can be processed towards a variety of rare sugars (glucose, galactose, mannose, and rhamnose) depending on the macroalgae strain, which can then be converted into a wide range of bioproducts by fermentation, including biofuels, biochemicals, biomaterials, and biofertilizer [4]. Among the renewable energies, bioethanol is considered a clean biofuel due to its renewability properties and biodegradability [7]. The global production of bioethanol continues to increase at an average of 5% annually from 2010 to 2019, but production fell worldwide in 2020 due to the pandemic and reached 98.65 billion tons (Fig. 1) [8]. Several nations such as the United States (US), China, India, Turkey, and Brazil have taken the initiative to develop bioethanol production as a commercial fuel [9–11]. Apart from being utilized as a fuel additive, bioethanol can also be converted into various derivatives such as acetic acid and ethylene, which can be further applied as raw material to produce a variety of green solvents and polymers [12].
Fig. 1.
Global production capacities of bioethanol and bioplastic 2010–2020. Adjusted from [8, 15]
In order to achieve sustainable bioeconomic growth, the production of high value-added bioproducts coupled with renewable energies generation in an integrated biorefinery should be prioritized. Apart from bioethanol, biochemical products, especially lactic acid (LA) or 2-hydroxypropionic acid, can also be generated from macroalgae biomass through biotechnological route by using lactic acid bacteria (LAB) to metabolize rare sugars. A large scale of the world’s commercial LA production is currently derived from food-grade sources [13]. On the other hand, large-scale synthesis of LA from edible bioresources may conflict with food and feed availability. Thus, non-edible macroalgae biomass is a better option for biochemical products synthesis due to their high compositional diversity. Moreover, LA is an essential building block for polylactic acid (PLA), a biodegradable and biocompatible aliphatic polyester with various applications. It can be found in the forms of D- and L-enantiomeric, where D( −)-LA and L( +)-LA are outlined as dextro-lactic acid and levo-lactic acid, respectively [14]. In fact, the applications of PLA in different fields have grown enormously in recent years, especially when produced from pure isomers (L( +)- or D( −)-lactic acid) and reached nearly 1.6 billion tons of global production capacity in 2020 [15]. Its applications range from packaging, fibers to foams and biomedical applications such as implants, sutures, bone fixation, scaffold in tissue engineering, and controlled drug delivery [9].
Moreover, the renewability and biodegradability properties of PLA have driven it to become one of the biopolymers that can be utilized as bioplastic. The main advantage of PLA as bioplastic is that the plastic can be degraded in a short time by the action of enzymes and microorganisms such as bacteria and fungi. The microbial degradation of bioplastic occurs with the changes in the chemical structure of the exposed material and normally requires a certain period, which ranges between 11 months and a few years. The degradation period of bioplastic mainly depends on the mechanical (crystallinity and melting temperature) and chemical (molecular weight distribution and chemical structure) properties of PLA and environment conditions (temperature, pH) [16]. Other than biodegradability, the production of plastic by using PLA can save approximately two-thirds of energy consumption compared to the production of petrochemical-based plastic. Furthermore, bioplastic derived from PLA will not increase the net emission of carbon dioxide into the atmosphere since PLA originates from cellulosic and macroalgae biomass. The macroalgae will absorb the carbon dioxide released during degradation [17].
Both bioproducts can be produced through two critical stages of macroalgae biorefinery, including hydrolysis of polysaccharides and fermentation of rare sugars extracted from macroalgae biomass. Thus, it is widely regarded as a superior approach for the sustainable valorization of biomass to meet the future multi-fold demand of commodities [18]. The fermentation process, which metabolize rare sugar to bioproducts, has taken place after the disruption of the cell wall which also can be defined as the hydrolysis process. Tan and Lee [19] reported that bioethanol fermentation could be done by selecting Saccharomyces cerevisiae to ferment the rare sugars from hydrolysates. The robust characteristics of S. cerevisiae that enable it to be used under a wide range of pH have promoted it to become the most commonly employed yeast in bioethanol production [20]. According to Alexandri et al. [21], Bacillus coagulans is favorable in the anaerobic conversion of rare sugars from hydrolysates to LA. Various configurations of hydrolysis and fermentation have been employed in bioethanol and biobased product generation. The configuration for both processes can be categorized into separate hydrolysis and fermentation, simultaneous saccharification and fermentation, and high cell density culture [22, 23].
This paper was systematically designed to critically review the prospects of biorefineries in transforming biomass into value-added products as a strategy for sustainability. Even though extensive reviews on biomass utilization had been published in the past few years, the current study focused on the latest trends and state-of-the-art technological development in this area. In addition, the advantages of different integration scenarios for bioethanol and LA production were also compared extensively. On the basis of the different integration scenarios, some recommendations were pointed out for future research directions on the seamless integration of third-generation bioethanol and LA production from macroalgae-based feedstocks. Therefore, this review provides essential technical information on the contemporary status and future trends of macroalgae biomass utilization to realize the pursuit of a green and sustainable economy.
Limitations and challenges of first and second generations of microbial bioethanol and lactic acid production
Bioethanol is one of the liquid alcohol–based biofuels, while LA is one of the acid- and alcohol-based biochemicals which can be produced by anaerobic conversion of carbohydrates extracted from various types of feedstocks such as food waste, woody biomass, agricultural residual, and edible crops using microorganisms and bacteria [24, 25]. In recent years, L-LA with high enantiomeric purity is displaying great potential for various applications in different industries (e.g. polymer, food, and pharmaceutical industries) as food packaging material, preservative, and flavoring agent [26]. In this section, several restrictions and drawbacks of existing bioethanol and LA production were discussed comprehensively, such as issues of using food carbohydrates as feedstocks for bioproducts synthesis, sensitivity to inhibitory compounds during pretreatment of lignocellulosic biomass, indirect utilization of polymeric sugars (cellulose in all macroalgae and xylan in green macroalgae), and impacts on bioproducts productivity due to the end-product inhibition (Table 1). Being the most demanded biofuel and biopolymer for resolving the energy and environmental issues, bioethanol and LA production have passed through several technological advancements to increase global productivity due to the technical and economic challenges with respect to first-generation feedstocks (edible crops, corn husk) and second-generation feedstocks (woody biomass, agricultural residual) in bioethanol and LA production [27].
Table 1.
Differences among bioethanol and lactic acid generations
| Generation | Feedstocks | Advantages | Limitations | Reference |
|---|---|---|---|---|
| First | Cereal crops (wheat, oats, grain sorghum) Edible oil seed (sunflower, cucumber, soybeans) Sugar crops (sugar beet, sugarcane, sweet sorghum) | Low production cost; Fairly simple conversion technology; Availability of industrial and commercial-scale equipment | Fluctuation of bioethanol selling price Food security Increasing global food price; Extensive demand on agricultural land and water consumption in cultivation phase; Bioethanol quality depends on environmental condition Massive usage of fertilizers and pesticides; Required laborious harvesting process | [28, 29] |
| Second | Energy crops (maize, sudan grass, millet) Lignocellulosic biomass (LCB) (corncobs, corn stover, wheat straw, grasses) Non-edible oil seed Waste stream | No food vs. energy competition; Abundancy of feedstocks at lower costs compared to edible crops; Availability of industrial and commercial-scale equipment; Lesser amount of fertilizers and pesticides compared to first-generation feedstocks | Extensive demand on agricultural land and water consumption in cultivation phase; Bioethanol quality depends on environmental condition Delignification is required for LCB; Complex and costly manufacturing, upgrading, and development process; Need for novel and emerging technologies to reduce the conversion costs | [30–32] |
| Third | Algae (microalgae, macroalgae, water hyacinth) | No food vs. energy competition; Fast growth rate; Producing algal biomass with high amounts of carbohydrates, proteins, and lipids Capable of yielding high amount of bioethanol per unit land area compared with terrestrial biomass; Contain lower amount of lignin compared to LCB; Capable of algal biomass to be cultivated in non-arable land and wastewater; Feasibility of algae-based wastewater treatment to eliminate the harmful components mainly phosphorus and nitrogen; Reduction of greenhouse gas emission level by fixation of carbon dioxide in the algae cultivation; Compatibility with co-production of multiple products by biorefinery process | Large-scale cultivation lead to change of nutrient content and water hydrology characteristics of marine ecosystem; Expensive algae harvesting process; Difficult scaling up of lab-scale production rate to industrial and commercial quantities; Lack of research and technological development for commercial and industrial-scale equipment | [4, 33] |
1G microbial bioethanol and lactic acid production
The feedstocks for first-generation (1G) bioethanol and LA are generally classified into food-based, starch-based (corn, barley, grain sorghum, wheat, and oats), and sugar-based crops (sugar beet, sugarcane juice, and sweet sorghum) [28, 34, 35]. The 1G bioethanol and LA can be produced from direct fermentation of hexose sugars or polysaccharides converted into rare sugars without pretreatment [36]. 1G bioethanol processing technologies in the US, Brazil, Turkey, and several countries in Europe have been commercialized for over two decades [29]. However, several studies reported that 1G bioethanol encounters economic issues such as fluctuating prices for commercial bioethanol production and inconsistent feed supply, which caused global food security as bioethanol is derived from food crops [34]. Renewable Fuels Association [37] had reported that maize was primarily used for 1G bioethanol and LA production in the US, which raised the conflict between bioethanol production and food consumption. The usage of edible food as feedstock poses a considerable ethical dilemma and strongly polarized debate, generally referred to as the “food vs. biofuel.” The supply of edible food as feedstock can also become a potential limiting factor due to the potential increased demand.
2G microbial bioethanol and lactic acid production
Second-generation (2G) biorefinery, also known as lignocellulosic biorefinery, is introduced to replace the 1G biorefinery approach for both bioethanol and LA production as its feedstocks are based on non-food raw materials that do not compete with the food supplies. One of the most common raw materials for 2G biorefinery is lignocellulosic biomass (LCB), which can be classified into woody biomass, agricultural residues (rice straw, grasses, and corncobs), forest residual, and energy crops [30–32]. Lignocellulosic waste contains three major chemical compositions: cellulose, hemicellulose, and lignin which can be processed into biofuels, biochemicals, and reinforcement agents for biopolymer, respectively [32]. The chemical compositions of different biomass can vary greatly from each other. However, several works of literature have reported that lignin is strongly bounded with cellulose-hemicellulose complex via hydrogen and covalent bonds, which render the structures to be highly stable and recalcitrant for depolymerization [38–40]. Thereby, delignification process is introduced with the usage of chemicals to remove the lignin complex and ensure the optimum yield of rare sugars can be attained [41]. Moreover, LCB requires a large scale of land for cultivations, which caused the issue of land-use competition [29]. Recently, European Parliament had raised a vote to phase out the usage of oil palm–based bioethanol as transport fuels from 2030 due to the European Union (EU) aimed to make the EU climate neutral by 2050. Owing to the cultivation of oil palm offers the highest indirect greenhouse gas (GHG) emissions, which is caused by the drainage of peatlands and deforestation [37]. Thus, lignocellulosic-derived bioethanol and LA are commercially limited due to the high production cost and environmentally unfavorable biorefinery processes [42].
Exploitation of macroalgae as a potential feedstock for 3G bioethanol and lactic acid production: a sustainable approach
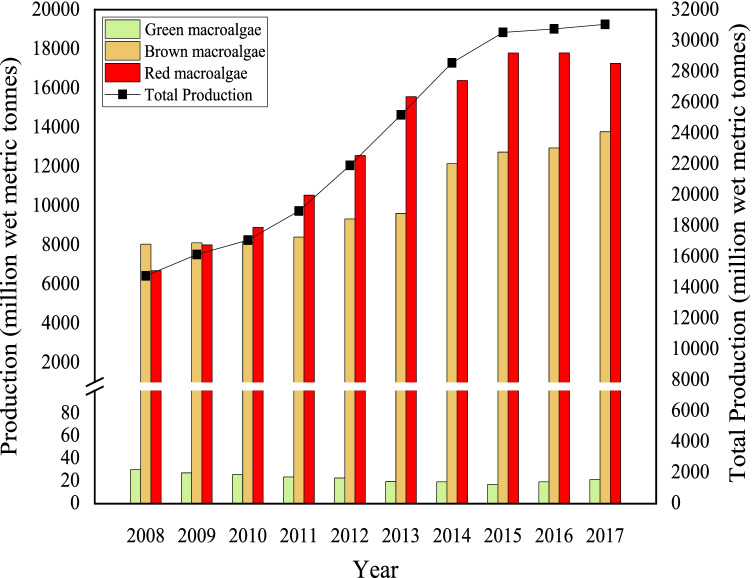
The development of a sustainable feedstock is needed to overcome the limitations encountered by 1G and 2G bioethanol and LA production. In view of this, algal biomass is a promising alternative feedstock as the third-generation (3G) energy and polymer resources. Saccharification of macroalgal polysaccharides to fermentable sugar and LA production is still yet to be studied. In this context, clarification on the algal biorefineries concept is paramount to attract the attention of researchers on the perspectives of algal-based bioethanol and LA production. Macroalgae have shown significant potential as feedstocks for bioethanol and LA production. Macroalgae, also known as seaweed, are photosynthetic and multicellular eukaryotic organisms present abundantly in oceans [4]. Red algae (Rhodophyta), green algae (Chlorophyta), and brown algae (Phaeophyta) are the main types of macroalgae that derive their colors based on chlorophyll and natural pigment synthesis. The carbohydrate-rich strain of macroalgae has driven it to become the most sustainable resource for the production of high rare sugar yield [43]. The world production of macroalgae had increased dramatically at an average increment rate of 10% annually over the past 10 years (2008–2017) and reached 31.05 million tons, which is worth over US$11.3 billion [44]. From Fig. 2, the cultivation of red and brown macroalgae has increased in the last 10 years. In recent years, the drastic growth in macroalgae production is mostly owing to increased demand for macroalgae applications in agricultural and biofuel production. Gajaria et al. [45] reported that green macroalgae were suitable to be applied as a sustainable source of bioactive compounds for biofertilizer production. Moreover, the red and brown species of macroalgae are mainly cultivated for the application of renewable energy production and wastewater treatment processes [19, 22, 46].
Fig. 2.
World production of farmed macroalgae from 2008 to 2017. Adjusted from Adjusted from [44]
Several laboratories work on the utilization of macroalgae for the generation of bioethanol and LA that had been reported in the literature, and the chemical compositions for selected macroalgae are shown in Table 2. These studies revealed that carbohydrates in the form of glucose polysaccharides such as cellulose could be found in macroalgae, laminarin can be found in brown algae, cellulose and starch can be found in both red and green algae, and other polysaccharides such as mannitol and alginate were contained in brown algae, agar, and carrageenan in red macroalgae and ulvan in green macroalgae [47–49]. Hence, macroalgae are generally considered sustainable sources for fermentable sugar. It also addresses the sustainability concerns related to food supplies and land cultivation suffered by the edible crops and LCBs [50, 51]. Unlike terrestrial plants, macroalgae possess many excellent properties such as abundance in supply, ability to grow in seawater (not competing with agricultural land for cultivation), and low lignin content [52]. Enormous quantities of macroalgae can be found in the oceans, so the rigidity conferred by lignin is pointless to the macroalgae. This highlights a major benefit of biorefinery processing because the delignification of the biomass is no longer required. This will further simplify the carbohydrates extraction and the saccharification process. Moreover, the detoxification or neutralization process, which is usually needed to remove the inhibitory compounds (5-HMF, furfural acid, and irreversible salts) produced during the delignification process of LCBs, can be eliminated leads to lower production cost [4]. Thus, macroalgae biomass is a cost-effective feedstock for 3G bioethanol and LA production [53].
Table 2.
Summary of polysaccharides in different macroalgae and major monosaccharides via hydrolysis
| Macroalgae group | Macroalgae | Polysaccharides | Major monosaccharides | Reference |
|---|---|---|---|---|
| Rhotophyta | Gracilaria sp. | Cellulose | Glucose | [22] |
| Agar | Galactose | |||
| Kappaphycus alvarezii | Cellulose | Glucose | [47] | |
| Carrageenan | D-galactose | |||
| Agar | Galactose | |||
| Gelidiopsis variabilis | Cellulose | Glucose | [57] | |
| Agar | Galactose | |||
| Chondrus crispus | Cellulose | Glucose | [58] | |
| Chlorophyta | Enteromorpha intestinalis | Cellulose | Glucose | [59] |
| Xylan | Xylose | |||
| Mannose | D-glucuronic acid | |||
| L-rhamnose | ||||
| Ulva lactuca | Ulvan | Glucose | [48] | |
| Xylose | ||||
| L-rhamnose | ||||
| Glucuronic acid | ||||
| Iduronic acid | ||||
| Cellulose | Glucose | |||
| Phaeophyta | Laminaria digitata | Alginate | Mannuronic acid | [49] |
| Guluronic acid | ||||
| Fucoidan | Frucose | |||
| D-xylose | ||||
| D-galactose | ||||
| D-mannose | ||||
| Glucuronic acid | ||||
| Cellulose | Glucose |
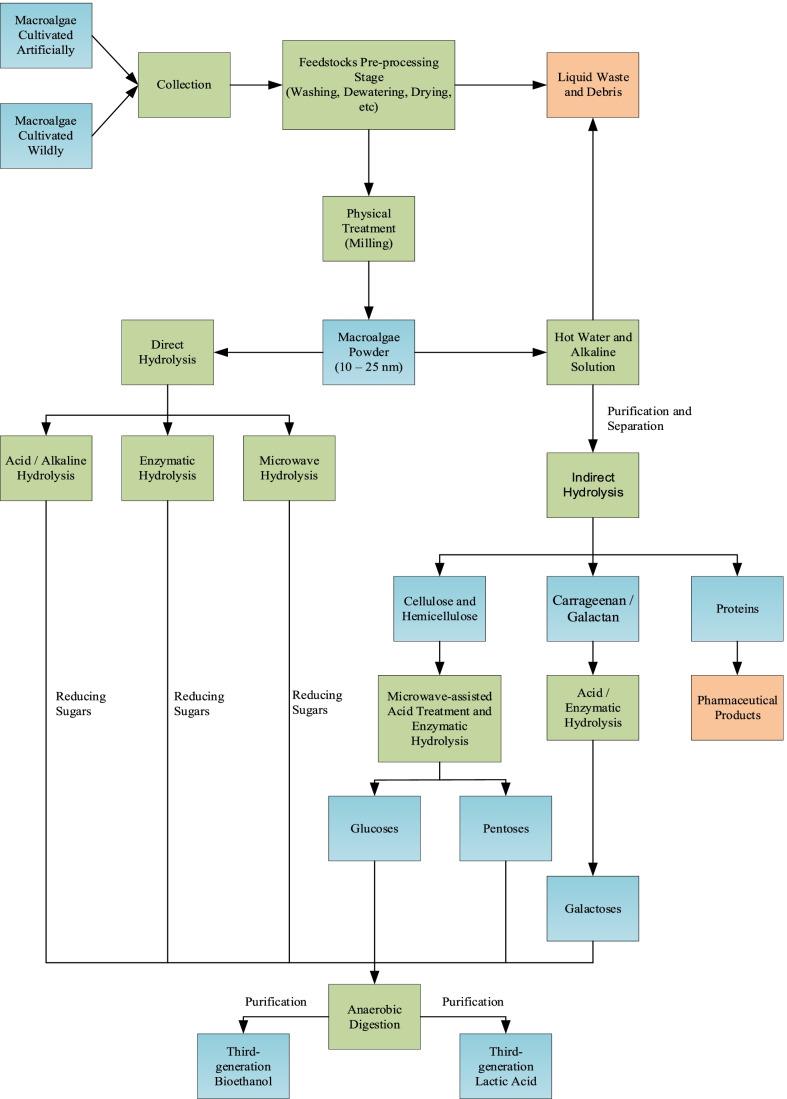
The macroalgae bioethanol and LA production processes include milling, pretreatment, hydrolysis (saccharification), fermentation, and distillation [54]. An overview of all stages for macroalgae-based bioethanol and LA generation is summarized in Fig. 3. Hydrolysis is essential to disintegrate and hydrolyze the cell wall of macroalgae to release the carbohydrates such as cellulose and other rare sugars for fermentative microorganisms [55]. The carbohydrates in the cell wall of macroalgae can be hydrolyzed easily to form monosaccharides via hydrolysis due to the low lignin content in macroalgae. However, in the work by Kostas et al. [49], it was highlighted that hydrolysis of pretreated brown macroalgae Laminaria digitata would yield a higher amount of rare sugars, which is approximately 93.80% as compared to hydrolysis of untreated biomass under the same loading. This was due to the pretreatment on macroalgae increasing the reaction surface area of carbohydrates in macroalgae and thus maximizing the fermentable sugar yield [56]. The fermentation process is followed after the disruption of the cell wall to produce fermentable sugar. The alcoholic fermentation is carried out using yeast under anaerobic conditions along with the hydrolysate [20]. Like 2G LA, 3G LA can be derived from the residual medium, which contains xylose and galactose hydrolyzed from macroalgae using LAB. In contrast, to glucose, which is a priority consumed by yeast strain for bioethanol production, conversion of xylose and galactose is slower due to the slower reaction kinetics [18, 20].
Fig. 3.
Flow chart of macroalgal bioethanol and lactic acid production process
Utilization of macroalgae biomass
Marine macroalgae are composed of different constituents (phycocolloids and celluloses), which could be fractionated into different constituents for refining separately or processed as whole biomass [4].
Extraction of phycocolloids from macroalgae biomass
Macroalgae is considered the natural resource of hydrocolloids which consist of various types of phycocolloid such as alginate, agar, carrageenan, fucoidan, and ulvan [60]. These phycocolloids are heterogeneous polysaccharides other than cellulose derived from macroalgae composed of sugars with unique chemical structures and commercially valued [61]. For instance, carrageenan extracted from red macroalgae consists of ester sulfate D-galactose and 3,6-anhydro-D-galactose (D-AHG). Herein, D-galactose is one of the abundantly used sugars in the carbohydrate-based biorefinery, while D-AHG has practical application for skin whitening and cell generation [62–64]. Meanwhile, sugars like 3,6-anhydro-L-galactose (L-AHG), L-rhamnose, L-fucose, and glucuronic acid can be found in agar from red macroalgae, ulvan from green macroalgae, fucoidan, and alginate from brown macroalgae, respectively [47–49]. Among the phycocolloids, agar, alginate, and carrageenan have been used widely as thickener and emulsifiers in food and textile industries to improve the viscosity of the aqueous solutions and the texture in foods [65]. Besides, the monomer sugars from phycocolloids could be used to generate bioethanol and LA through microbial fermentation [64].
Owing to the variety of macroalgae phycocolloids and their unique monomer sugars, which possess commercial significance for a wide range of applications, the extraction technology for macroalgae phycocolloids has been scarcely explored and upgraded over the years for enhancing the extraction yields. Various solvents, including distilled water, acidic or alkaline solution in stand-alone or in combinations employed for conventional and innovative phycocolloids extraction, are presented in Table 3. These studies revealed that specific approaches had been employed to extract the targeted phycocolloids from the cell wall of the specific macroalgae strain as the phycocolloid composition of macroalgae varies depending on the species. From the studies, the application of distilled water and alkaline in agar extraction is strain-dependent. For instance, an alkali extraction method is required for both Pyropia spp. and Gracilaria spp. to form L-AHG, which is responsible for producing a high-strength gel by eliminating the sulfate groups in agar, whereas this extraction method is not required for Gelidium spp. [66–68]. In contrast, both acidic and alkali extraction methods are required for alginate extraction. Acid such as hydrochloric acid (HCl) is mainly applied in alginate extraction for solubilizing the calcium alginate fraction in the brown macroalgae to alginic acid. To produce a more commercially valued phycocolloid, sodium carbonate (Na2CO3) is employed to transform the alginic acid to sodium alginate, a sodium salt that has a variety of applications, including hydrogels for cell immobilization and dental impression materials [69, 70]. In the case of carrageenan extraction, the alkali extraction method is not prioritized, but chelating agents such as calcium hydroxide (Ca(OH)2) and potassium hydroxide (KOH) can be utilized to improve the carrageenan-gel strength [71, 72].
Table 3.
Summary of pretreatment and extraction approach for macroalgal phycocolloids from macroalgae strain at laboratory and industrial level
| Targeted phycocolloid | Macroalgae strain | Pretreatment | Extraction procedure | Reference |
|---|---|---|---|---|
| Agar | Pyropia yezoensis | Washed with water | Ratio 1:30 algae/4% (v/v) NaOH Oil bath with 4% NaOH (80 °C, 2 h), followed by autoclave with 260 mL distilled water (130 °C, 1 h) | [66] |
| Agar | Gracilaria lemaneiformis | No pretreatment | Ratio 1:20 algae/reaction solution EAE in distilled water with 4 U/mL cellulases (50 °C, 1 h), followed by 3% NaOH (87 °C, 3 h) EAE in distilled water with 8 U/mL cellulases and 26.6 U/mL arylsulfatase (50 °C, 3 h) | [67] |
| Agar | Gelidium sesquipedale | Washed with water and milled into powder form | Ratio 1:10 algae/distilled water Maceration in distilled water (90 °C, 2 h), followed by screened through muslin cloth and freeze-dried (− 25 °C, 24 h) UAE (400 W, 24 kHz, 30 min) | [68] |
| Alginate | Sargassum muticum | Washed with water and oven-dried (65 °C) | Ratio 1: 10 algae/reaction solution Maceration in 0.2% CH2O (RT, 24 h), followed by 0.2 M HCl and washed with 3% Na2CO3 | [70] |
| Alginate | Sargassum binderi | Washed with water and macerated in 80% ethanol (RT, 24 h) | Ratio 1: 100 algae/distilled water Distilled water at pH 11.0, followed by UAE (150 W, 50 °C, 30 min) | [92] |
| Alginate | Sargassum angustifolium | 85% ethanol (1:4 g/mL, RT, 24 h). Rinsed with acetone and dried in fume hood (RT, 24 h) | Ratio 1:8 algae/reaction solution EAE in distilled water with 5% (w/w) alcalase (pH 8, 50 °C, 24 h) | [89] |
| Alginate | Sargassum muticum | Washed with water | Ratio 1:20 algae/distilled water UAE (150 W, 1.5 A, 40 Hz, RT,30 min) | [83] |
| Alginate | Nizamuddinia zanardinii | Washed with water and milled into powder form Pretreated with 0.2 M HCl using high voltage electrode discharge | Ratio 1:32 algae/reaction solution Maceration in 2% CH2O (RT, 200 rpm, 24 h), followed by 0.2 M HCl (60 °C, 150 rpm, 3 h) and 3% Na2CO3 (60 °C, 250 rpm, 2.5 h) | [93] |
| Carrageenan | Hypnea musciformis | Washed with water, oven-dried (60 °C) and milled into powder form | Ratio 1:50 algae/3% (v/v) KOH Macerated in 3% KOH (85 °C, 3.5 h) MAE (105 °C, 10 min, 2450 MHz) | [80] |
| Carrageenan | Kappaphycus alvarezii | Washed with water | Ratio 1:20 algae/0.5% (v/v) Ca(OH)2 Maceration in 0.5% Ca(OH)2 (RT, 2 h), followed by autoclave with 200 mL distilled water (107 °C, 1.5 h) | [71] |
| Carrageenan | Hypnea musciformis | Methanol-acetone mixture with a ratio of 1:1 (RT, 12 h) | Ratio 1:150 algae/3% (v/v) KOH Macerated in 3% KOH (80 °C, 4 h) UAE (500 W, RT, 20 min) | [72] |
| Carrageenan | Eucheuma denticulatum | Washed with water and macerated in 80% ethanol (RT, 24 h) | Ratio 1:100 algae/distilled water Distilled water at pH 7.0, followed by UAE (150 W, 50 °C, 30 min) | [92] |
| Carrageenan | Kappaphycus alvarezii | Washed with water and milled into powder form | Ratio 1:16 algae/1% (v/v) ionic liquid Ionic liquid assisted SWE (180 °C, 5 MPa, 200 rpm, 5 min) | [47] |
| Carrageenan | Eucheuma denticulatum | Washed with water and milled into powder form | Ratio 1:20 algae/distilled water Maceration in distilled water (90 °C, 1 h), followed by screened through 45 µm mesh and oven-dried (80 °C, 72 h) | [62] |
| Fucoidan | Splachnidium rugosum | Washed with water and oven-dried (45 °C, 72 h) | Ratio 1:100 algae/distilled water Maceration in distilled water (70 °C, 24 h), followed by screened through Whatman filter paper and freeze-dried (− 80 °C, 24 h) | [94] |
| Fucoidan | Nizamuddinia zanardinii | 85% ethanol (1:10 g/mL, 2000 rpm, RT, 24 h). Rinsed with acetone and dried in fume hood (RT, 24 h) | Ratio 1: 21 algae/distilled water SWE (150 °C, 7.5 bar, 1500 W, 29 min | [91] |
| Ulvan | Ulva pertusa | 80% ethanol (1:4 g/mL, 85 °C, 2 h), the precipitated was collected and oven-dried (50 °C) | Ratio 1:55.45 algae/distilled water MAE (600 W, 43.63 min, pH 6.57) | [81] |
| Ulvan | Ulva pertusa Kjellm | Milled into powder form and macerated in 80% ethanol (1:4 g/mL, 24 h) | Ratio 1:20 algae/distilled water Maceration in distilled water (90 °C, 3 h), followed by a precipitation of residue with 95% ethanol EAE in distilled water with 5% (w/w) 50,000 U/g cellulases (50 °C, 2.5 h) | [87] |
| Ulvan | Ulva fasciata | Dichloromethane (1:20 g/mL, 250 rpm, RT, 24 h) and ethanol (1:20 g/mL, 250 rpm, RT, 24 h). Rinsed with acetone and dried in a fume hood (RT, 24 h) | Ratio 1:20 algae/distilled water Maceration in distilled water (120 °C, 3 h), followed by screened through the non-woven fabric and dried in RT | [95] |
RT room temperature
Moreover, the extraction method is temperature-dependent based on the targeted phycocolloids. For agar extraction, operation temperatures above 80 °C are required for complete solubilization of agar from red macroalgae [66–68]. Besides, carrageenan is a group of water-soluble anionic sulfated polysaccharides soluble either in cold or hot water but depending on the genus of red macroalgae [73]. Das et al. [71] revealed that the carrageenan from Kappaphycus alvarezii can be solubilized in a 0.5% Ca(OH)2 solution at room temperature without being heated. On the other hand, ulvan from green macroalgae is only soluble in hot water with operation temperatures above 90 °C [74]. However, pH is the main solubilizing parameter for alginate extraction, and thus, the pH should be maintained above the pKa value of alginate (pKa > 3.65) [75]. From Table 3, hot water extraction (HWE) followed by filtration, centrifugation, and purification are the conventional phycocolloid extraction techniques employed by many researchers [62, 68, 71]. However, from the industrial point of view, the conventional extraction technique is constrained by requiring a high extraction temperature, longer extraction time that will cause severe depolymerization of phycocolloid chain, and effluents generated by this technique caused water pollution problems due to the usage of toxic chemicals [76, 77].
To improve the drawbacks of conventional extraction technology, innovative and eco-friendly extraction protocols are increasingly developed, including microwave-assisted extraction (MAE), ultrasound-assisted extraction (UAE), enzyme-assisted extraction (EAE), and subcritical water extraction (SWE) [78]. MAE technology is based on the application of electromagnetic radiation at frequencies and wavelength ranges between 0.3–300 GHz and 0.001–1 m, respectively, to transfer energy for rapid internal heating on the sample matrix and macroalgae cell wall disruption [79]. MAE has been applied successfully to extract carrageenan from Hypnea musciformis under 150 °C with an operation duration of 10 min [80]. MAE demonstrated to achieve higher carrageenan yields which are approximately 16.6% compared to the conventional alkali extraction method (85 °C, 3.5 h) that achieved approximately 3.74% yield per gram of biomass with a reduction of reaction time and volume of KOH used [80]. Ulvans from Ulva pertusa was also extracted by Le et al. [81] using MAE obtaining 41.91% yield at a microwave power of 600 W for 43.63 min.
On the other hand, UAE technology is based on the application of sound frequencies ranging between 0.2 and 10 MHz to treat the samples by applying agitation, pressure, shear force, compression-rarefaction, and radial formation on the sample matrix to enhance the cell wall disruption [82]. Martínez-Sanz et al. [68] concluded that UAE with non-alkali treatment (400 W, 24 kHz) and conventional HWE method achieved similar agar yields (10–12%) extracted from Gelidium sesquipedale; however, UAE successfully reduced the extraction time by fourfold. Alginates from Sargassum muticum were also extracted by Flórez-Fernández et al. [83] using UAE (150 W, 40 Hz, 30 min), obtaining 15% yield with a low mannuronic/guluronic ratio of 0.64 that resulted in a soft gel with high viscosity. Alginate gels with high guluronic acid content are essential in food and cosmetic industries, which are widely used as resistant gels in food and cosmetic products [84]. Besides achieving a higher yield of carrageenans from Hypnea musciformis, Rafiquzzaman et al. [72] also reported that the UAE method possesses specificity to extract pure kappa-carrageenan and eliminate the desulfation on the extracted carrageenan, which can enhance the properties of carrageenans. This is mainly due to carrageenans containing higher than 25% of sulfate groups being reported to have strong antiviral effects on both problematic enveloped and non-enveloped viruses such as hepatitis A, dengue virus, and human immunodeficiency virus [85].
As an emerging and innovative extraction technology, EAE was also explored to obtain phycocolloids from various macroalgae biomass. EAE technology is based on the application of enzymes secreted from microorganisms to disrupt the macroalgae cell wall for releasing the polysaccharides [86]. The use of EAE involving cellulase was explored by Chen et al. [87] to enhance the ulvan extraction from Ulva pertusa Kjellm. The yield of ulvans extracted through EAE was comparable to conventional HWE and UAE methods, in which the yields were 25.3%, 17.8%, and 20.6%, respectively [87]. Compared to the conventional extraction method that involved the use of calcium chelating agents to break the glycosidic bonding between the ulvan and cell wall matrix, EAE is considered as a simplified method that does not require the usage of chelating agents and dialysis process due to enzyme-assisted disruption of the macroalgae cell wall [88]. Borazjani et al. [89] extracted alginates from Sargassum angustifolium by EAE, using alcalase and cellulase. The use of both enzymes showed no significant differences in the alginates yield compared to the conventional HWE method, but the protein and polyphenol contents in the extracted alginates were significantly reduced coupled with enhanced purities. Furthermore, SWE is the advanced extraction method of HWE with the use of pressurized hot water for the isolation of phycocolloids from macroalgae [90]. Alboofetileh et al. [91] concluded that SWE (150 °C, 7.5 bar) successfully increased the fucoidan yields from Nizamuddinia zanardinii by approximately fivefold compared to the conventional HWE method, where the fucoidan yields were 25.98% and 5.2%, respectively. Besides, high temperatures observed in SWE facilitated reducing the extraction time by 12.4-fold compared to HWE [91]. It can be concluded that a considerable reduction in extraction times and increment in extraction yields can be achieved with minimal impact on the quality of phycocolloids extracted. Thus, the innovative extraction methods are considered the facile greener alternative to the conventional extraction methods for separating cellulose from macroalgal phycocolloids prior to being utilized for macroalgae-based bioethanol and LA production.
Synthetic pathway for rare sugars from macroalgae biomass
Besides being fractionated into different constituents and refined separately to high value-added bioproducts, macroalgae can be processed as whole biomass. The extraction of rare sugars such as glucose, galactose, and mannose from macroalgae has been explored extensively. Various hydrolysis techniques and rare sugar yields for bioethanol and LA production from macroalgae are described in Table 4. However, the extraction methods are technically similar to that for producing common sugars (glucose) from 1G- and 2G-based polysaccharides [96]. The main process is disrupting the cell wall and breaking the glycosidic bonds between polysaccharides to release rare sugars as the crystallinity of cellulose has provided greater stability and rigidity to the macroalgae cell wall. Hence, these structures have to be modified either by using chemo-catalytic, biocatalytic, thermal-catalytic, or innovative hydrolysis processes [97]. Before being processed using the chemical or biological hydrolysis method, macroalgae biomass is subjected to physical pretreatment to reduce the cellulose crystallinity in the cell wall matrix [98]. The mechanical comminution technique, which consists of the chipping and milling process, has been widely used to pretreat and reduce the biomass size to 10–25 nm. This will increase the reaction surface area of biomass to other hydrolysis reagents and reduced the crystallinity of cellulose [99].
Table 4.
Comprehensive review of various hydrolysis techniques for macroalgal biomass
| Macroalgae strain | Pretreatment | Hydrolysis technique | Hydrolysis procedure | Rare sugar yield (%) | Reference |
|---|---|---|---|---|---|
| Chemo-catalytic hydrolysis approach | |||||
| Gracilaria verrucosa | Washed with water, oven-dried (60 °C, 48 h), and crushing | Solid acid hydrolysis | S/L ratio of 1:7.5, 15% (w/w) Amberlyst-15 (140 °C, 2.5 h) | 51.90 | [100] |
| Eucheuma cottonii | Washed with water, oven-dried (40 °C), and crushing | Solid acid hydrolysis | 16% (w/v) biomass, 6% (w/v) Dowex™ Dr-G8 (120 °C, 1 h) | 43.20 | [19] |
| Eucheuma cottonii | Washed with water, oven-dried (40 °C), and crushing | Acid hydrolysis | 16% (w/v) biomass, 0.2 M H2SO4 (120 °C, 2.5 h) | 34.60 | [19] |
| Ulva fasciata | Washed with water, oven-dried (60 °C, 24 h), and crushing | Acid hydrolysis | S/L ratio of 0.1:5, 3% (w/w) H2SO4 (121 °C, 15 min) | 70.06 | [101] |
| Kappaphycus alvarezii | Milled into powder form | Hyper thermal acid hydrolysis | 10% (w/v) biomass, 360 mM H2SO4 (140 °C, 10 min) | 60.50 | [102] |
| Ulva rigida | Washed with water, oven-dried (60 °C, 24 h), and crushing | Thermal acid hydrolysis | 10% (w/v) biomass, 4% (v/v) H2SO4 (121 °C, 1 h, pH 7.0) | 60.20 | [103] |
| Ulva rigida | Washed with water, oven-dried (60 °C, 24 h), and crushing | Thermal acid hydrolysis | 15% (w/v) biomass, 5% (v/v) HCl (121 °C, 1 h, pH 7.0) | 30.00 | [103] |
| Gracilaria manilaensis | Washed with water, oven-dried (80 °C, 24 h), and crushing | Acid hydrolysis | S/L ratio of 1:20, 2.5% (w/v) H2SO4 (120 °C, 60 min) | 42.34 | [104] |
| Gelidium elegans | Washed with water, oven-dried (75 °C) | Acid hydrolysis | S/L ratio of 1:20, 2.5% (w/v) H2SO4 (120 °C, 40 min) | 39.42 | [105] |
| Biocatalytic hydrolysis approach | |||||
| Saccharina latissima | Washed with water, oven-dried (30 °C), and crushing | Enzymatic hydrolysis | 25% (w/v) biomass, 6.3 mg/g CellicCTec2 (37 °C, 3 h), 0.7 mg/g alginate lyase (50 °C, 17 h), 100 mM citric acid-sodium phosphate buffer (pH 6.3) | 48.65 | [106] |
| Enteromorpha sp. | Washed with water, air-dried, and crushing | Enzymatic hydrolysis | 3% (w/v) biomass, 10 FPU/g cellulase from Aspergillus niger (~ 0.8 U/g), 0.1 M sodium acetate buffer (50 °C, 96 h, pH 5.0) | 70.48 | [107] |
| Kappaphycus alvarezii | Washed with water, oven-dried (50 °C, 24 h), and crushing | Enzymatic hydrolysis | 1% (w/v) biomass, 60 Unit/g enzyme (Celluclast® + β-glucosidase), 0.1 M citrate buffer (50 °C, 8 h, pH 4.8) | 37.00 | [108] |
| Thermal-catalytic hydrolysis approach | |||||
| Sargassum muticum | Washed with water and crushing | Subcritical water hydrolysis | 14.3% (w/v) biomass, hydrolyzed (170 °C, 25 min) | 34.89 | [109] |
| Gelidium sesquipedale | Washed with water, oven-dried (40 °C), and crushing | Subcritical water hydrolysis | 4% (w/v) biomass, hydrolyzed (170 °C, 40 min) | 38.34 | [110] |
| Ulva intestinalis | Oven-dried (60 °C), and crushing | Steam explosion hydrolysis | 1 g biomass, steam exploded (121 °C, 1.72 bar, 15 min) | 51.70 | [111] |
| Advanced hydrolysis approach | |||||
| Ecklonia radiata | Washed with water, oven-dried (45 °C), and crushing | Microwave-assisted enzymatic hydrolysis | 1% (w/v) biomass, 100 µL enzyme (Ultraflo® L + Flavourzyme® 1000 L), 0.2 M phosphate buffer (50 °C, 3 h, pH 7.0) | 69.50 | [112] |
| Monostroma latissimum | Washed with water, lyophilized, and crushing | Microwave-assisted hydrothermal hydrolysis | 5% (w/v) biomass, microwave hydrolyzed (140 °C, 10 min) | 53.10 | [113] |
| Pyropia yezoensis | Washed with water, freeze-dried (− 20 °C), and crushing | Microwave-assisted enzymatic hydrolysis | S/E ratio of 10:1, amyloglucosidase, 0.1 M phosphate buffer (60 °C, 2 h, pH 4.5, 400 W) | 25.00 | [114] |
| Laminaria digitata | Washed with water, oven-dried (80 °C, 48 h), and crushing | Sequential acid and enzymatic hydrolysis | 25% (w/v) biomass, 1.5 M H2SO4 (121 °C, 24 min), enzymatic hydrolyzed (50 FPU/g CellicCTec2, 0.05 M sodium citrate buffer, 50 °C, 48 h, 120 rpm) | 93.80 | [49] |
| Eucheuma denticulatum | Washed with water, oven-dried (60 °C), and crushing | Microwave-assisted acid hydrolysis | 20% (w/v) biomass, 0.1 M H2SO4 (160 °C, 10 min) | 74.84 | [115] |
| Macrocystis pyrifera | Washed with water and crushing | Sequential acid and enzymatic hydrolysis | 33.3% (w/v) biomass, 2% (v/v) H2SO4 (120 °C, 60 min), enzymatic hydrolyzed (alginate lyases, oligoalginate lyases [25 °C, 12 h], CellicCTec2 [50 °C, 4 h]), 0.45 M McIlvaine buffer (pH 7.5) | 95.10 | [116] |
| Ulva lactuca | Washed with water, oven-dried (50 °C), and crushing | Sequential hydrothermal and enzymatic hydrolysis | 10% (w/v) biomass, hydrolyzed (135 °C, 20 min), enzymatic hydrolyzed (2.5% (w/w) cellulase (~ 2.32 U/g), 45 °C, 48 h) | 79.70 | [48] |
| Gracilaria verrucosa | Washed with water, freeze-dried, and crushing | Sequential acid and enzymatic hydrolysis | 7.5% (w/v) biomass, 0.1 M H2NSO3H (130 °C, 90 min), enzymatic hydrolyzed (CellicCTec2: Viscozyme: CellicHTec2 = 1:1:0.1 v/v/v ratio per dried biomass, 0.02% sodium azide, 50 °C, 72 h, 180 rpm) | 69.10 | [117] |
| Sargassum muticum | Washed with water and crushing | Sequential hydrothermal and enzymatic hydrolysis | 14.3% (w/v) biomass, hydrolyzed (170 °C, 25 min), enzymatic hydrolyzed (20 FPU/g CellicCTec2, 5 U/g Viscozyme, 0.05 M citric acid-sodium citrate buffer, pH 4.85, 48.5 °C, 28.6 h) | 94.40 | [109] |
| Gracilaria lemaneiformis | Washed with water, oven-dried (60 °C, 48 h), and crushing | Microwave-assisted acid hydrolysis | 5% (w/v) biomass, 0.2 M H2SO4 (180 °C, 20 min) | 73.30 | [118] |
| Saccharina latissima | Washed with water, crushing, and freeze-dried | Sequential microwave-assisted hydrothermal and enzymatic hydrolysis | 5% (w/v) biomass, microwave hydrolyzed (190 °C, 5 min), enzymatic hydrolyzed (50 °C, 20 h, 200 rpm, 0.7% (w/v) CellicCTec2) | 87.36 | [119] |
S/L solid-to-liquid ratio, S/E substrate-to-enzyme ratio
Chemo-catalytic hydrolysis approach
Recently, several studies have been conducted to develop chemo-catalytic hydrolysis approaches for the selective production of rare sugars from macroalgae. This process is principally based on the solvolysis in water to release rare sugars from their polymeric chains by using acid reagents as the catalyst, namely acid hydrolysis [120]. For acid hydrolysis, protic acid such as HCl and sulfuric acid (H2SO4) is mostly utilized because these catalysts are more effective in breaking the glycosidic bonding between polysaccharides with the intake of water molecules through nucleophilic substitution reaction [121]. Similar to other biomass, the hydrolytic efficiency of macroalgae through acidolysis is mainly dependent on the acid type used, the acid concentration used, biomass loading, hydrolysis duration, as well as reaction temperature (Table 4). El Harchi et al. [103] performed acidolysis of Ulva rigida under the condition of 121 °C for 1 h with a 1:10 of solid-to-liquid (S/L) ratio and enhanced the total rare sugar (rhamnose and glucose) yield in hydrolysate up to 60.20% when substituting the acid type from HCl to H2SO4 at the same concentration. Mild acid like dilute H2SO4 is preferable over HCl for acidolysis due to H2SO4 contains extra H+ ions, creating a more acidic environment that offers strength to hydrolyze and disrupt the acid-sensitive 1,3-glycosidic bonds, resulting in the generation of monosaccharides from polysaccharides with higher hydrolytic efficiency [115]. Hence, the H2SO4 concentration is a considerable parameter that requires to be optimized to enhance the rare sugar yield. Hessami et al. [105] conducted the acidolysis of Gelidium elegans using various concentrations of H2SO4 and verified that the total rare sugar (galactose and glucose) yield could be significantly enhanced from 5 to 39.42% by increasing the H2SO4 concentration from 0.5 to 2.5% (w/v). Similar research reported that the higher efficiency of acidolysis (70.95%) can be achieved from Gracilaria manilaensis by 2.5% diluted H2SO4 than that by 0.5% with a total rare sugar yield of 42.34% [104].
Notably, unfavorable acid hydrolysis conditions could lead to the formation of undesirable by-products such as acetic acid, formic acid, 5-hydroxymethylfurfural (HMF), and levulinic acid [121]. The by-products can prevent the fermentation of rare sugars by damaging the DNA and hindering RNA and protein synthesis of fermentative microorganisms [122]. These inhibitors are formed from the carbonization or degradation of rare sugars caused by the high reaction temperature, long retention times, and high acid concentration [123]. Ra et al. [102] demonstrated that 34.85 g/L of rare sugar can be released during acidolysis of K. alvarezii using an extremely high temperature of 140 °C with 360 mM H2SO4 for 10 min, which resulting in a hydrolytic yield of 60.50%. However, Ra et al. [102] reported that increasing the temperature up to 200 °C would give rise to the loss of rare sugars from K. alvarezii to 7.20 g/L due to conversion of glucose and galactose to undesirable by-products. In addition, a long hydrolysis duration will increase the interaction between the acid and rare sugars, bringing about a low hydrolytic efficiency and total rare sugar concentration [101, 102, 105]. The degradation of rare sugars is the side reaction of acid hydrolysis, which is unable to suppress or avoid completely. Consequently, a neutralization or detoxification process is necessary to be carried out to minimize the detrimental impacts of by-products on the fermentation performance of the microorganisms [124]. Ra et al. [125] found that 6 g/L of 5-HMF was removed completely from acid-modified Gelidium amansii hydrolysates by using 3% (w/v) activated carbon in a shaking water bath at 100 rpm and 50 °C for 5 min, but activated carbon also removed approximately 5 g/L of total rare sugars present in the hydrolysate. Similar research reported that the higher HMF removal efficiency (41.6%) can be achieved from acid-modified Eucheuma spinosum hydrolysates by filtering through 2.5% (w/v) activated carbon powder in shaking water bath at 100 rpm and 50 °C for 2 min [126]. Alternatively, a bacterial strain, called Burkholderia cepacia H-2, has been found capable of degrading furfural and 5-HMF in acid-modified Chaetomorpha linum hydrolysates to furfuryl alcohol and 2,5-furan-dicarboxylic acid, respectively [127]. These organic acids were found to have no detrimental effect on rare sugars fermentation when accumulated in the fermentation medium [128].
Acid hydrolysis is preferred for rare sugar extraction in terms of high hydrolytic efficiency and mass transfer rate. Nevertheless, the sustainable use of liquid acid catalysts is constrained by the difficulty of catalyst recovery [129]. As an alternative for conventional liquid acid catalyst, solid acid catalyst (SAC) is preferred for dilute acid hydrolysis as it can be easily separated from reaction medium for recycling use, non-corrosive, and environmentally benign [130]. To ensure high hydrolytic efficiency, the SAC should have a high number of Brønsted acid sites, a high surface area, and good thermal stability [131]. Amberlyst™-15 and Dowex™ Dr-G8 resins were the most popular SAC in the organic synthesis process, mainly due to high thermal (up to 280 °C) and chemical stability [120]. Amberlyst™-15 resin is a strongly acidic catalyst that can selectively convert cellulose and other phycocolloids to rare sugars. About 51.90% of total rare sugar yield corresponding to 61 g/L of rare sugars was attained from milled G. verrucosa under acid hydrolysis reaction of 140 °C for 2.5 h with 15% (w/v) Amberlyst™-15 [100]. Besides possessing a microporous pore structure that allows the access of liquid or gaseous reactants to the H + ion sites, Dowex™ Dr-G8 resin also bearing with sulfonic acid sites could offer strength for simultaneous production of rare sugars from biomass and removal of by-products in the hydrolysates [132]. The use of Dowex™ Dr-G8 as SAC has been applied successfully to extract galactose from 16% (w/v) Eucheuma cottonii under the condition of 120 °C with 6% (w/v) catalyst loading for 1 h [19]. Dowex™ Dr-G8 achieved a higher galactose yield, which is approximately 43.20%, and no 5-HMF content in hydrolysate compared to the conventional dilute sulfuric acidolysis (120 °C, 2.5 h) that only achieved 34.60% of galactose yield with a reduction of reaction time [19].
Biocatalytic hydrolysis approach
Besides acid hydrolysis, the biocatalytic approach is an alternative method to hydrolyze macroalgae biomass. This process involves the utilization of enzymes or the direct addition of biological microorganisms (fungi or bacteria) to facilitate the cleavage of glycosidic bonds between the complex macroalgal polysaccharides into rare monomeric sugars generally known as enzymatic hydrolysis [133]. In addition, enzymatic hydrolysis is considered an effective disruption method due to its relatively low temperatures and the formation of minimum inhibitory compounds as compared to the chemo-catalytic hydrolysis method [2]. Similar to terrestrial plants, cellulose is the major component in the macroalgae biomass, but the macroalgae cell wall is composed of cellulose Iα which is different from cellulose Iβ in the plant cell wall. Cellulose Iα is the triclinic crystalline form of cellulose consisting of weaker hydrogen bonds with one cellobiose residue per unit cell, resulting in easy access to cellulolytic enzymes during enzyme hydrolysis [33]. The commonest enzyme utilized in the saccharification of macroalgae is cellulase [117, 134]. Cellulase is a mixture of different enzymes which consists of endocellulase, exocellulase, and β-glucosidase that function synergistically to convert cellulose into β-glucose without being consumed in the reaction [2]. Endocellulase is also known as endoglucanase, which is used to disrupt the cellulose chains and reduce the crystallinity of cellulose to improve hydrolysis efficiency. Exocellulase or cellobiohydrolase is used to break down the straight microfibrils cellulose ends for releasing the cellobiose molecules. Meanwhile, β-glucosidase or cellobiase is used to hydrolyze the glycosidic linkage of each soluble cellobiose molecule to release two molecules of β-glucose as final products [135].
The mechanism of cellulolytic enzymes on celluloses consists of three main stages: (1) adsorption of cellulase on the surface of the cellulose, (2) conversion of cellulose to β-glucose by hydrolysis, and (3) desorption of cellulase [33]. Cellulases are naturally secreted either by cellulolytic bacterial species of Cellulomonas, Clostridium, Bacillus, Erwinia, and Streptomyces or by fungal species of Aspergillus, Fusarium, Humicola, Trichoderma, and Penicillium [136, 137]. The use of cellulase derived from Aspergillus niger was explored by Jmel et al. [107] to enhance the glucose extraction from Enteromorpha sp. They revealed that enzymatic hydrolysis using cellulase from A. niger alone was sufficient to complete the saccharification of Enteromorpha sp. with glucose yields of 70.48%, primarily due to the only glucan was present in the macroalgae [107]. Moreover, Xue et al. [138] reported that the cellulase isolated from A. niger is composed of acidic and thermostable endoglucanase, which shows higher catalytic efficiency on cellulose hydrolysis compared to alkali-tolerant endoglucanase. This is mainly due to the acidic endoglucanase was able to enhance the cleavage of acid-sensitive 1,3-glycosidic bonds between the cellulosic polysaccharides and offers strength to hydrolyze polysaccharides across a wide range of pH conditions (pH 3–6) [139].
Unlike 1G and 2G feedstocks, polysaccharides of macroalgae are different in terms of macroalgae and sugar monomers species; a multiple-enzyme complex or also known as enzyme cocktail is thus needed to enhance the extraction of the rare sugars [140]. The use of enzyme cocktail (CellicCtec2 and alginate lyase) has been applied successfully for the complete hydrolysis of Saccharina latissimi [106]. The optimal total rare sugar (glucose and mannitol) yield of 48.65%, which corresponds to 74 g/L of sugars, was attained after inoculation with CellicCtec2 (37 °C, 3 h) and alginate lyase (50 °C, 17 h) to hydrolyze the cellulose and alginate, respectively. This study also revealed that the character of the enzyme was dependent on its species and could only perform well under their optimum conditions [106]. Besides using an enzyme cocktail for complete hydrolysis of various polysaccharides in the same biomass, an enzyme cocktail could be utilized for optimizing the extraction yield of the specific polysaccharide in the biomass. Rodrigues et al. [108] conducted the hydrolysis of K. alvarezii using cellulase alone and verified that the yield of rare sugars could be significantly enhanced from 31 to 37% by applying β-glucosidase as a supplement enzyme under the same hydrolysis duration and enzyme loading. This is mainly due to the addition of β-glucosidase could facilitate the cleavage of the glycosidic bonds between the cellobiose molecules and resolve the product inhibition setback caused by the single-enzyme process [141]. Although high rare sugar yield can be obtained, this process is constrained by the hydrolysis duration, which requires long residences times ranging between 1 and 4 days [106, 107]. Hence, the use of enzymatic hydrolysis usually implies with chemo-catalytic and thermo-catalytic hydrolysis approach to enhance the rare sugar productivity [133].
Thermo-catalytic hydrolysis approach
The thermo-catalytic hydrolysis approach, commonly known as hydrothermal hydrolysis, is principally based on the nucleophilic substitution in water or steam to release rare sugars from complex macroalgae polysaccharides at elevated levels of temperature and pressure in a closed system by changing their physiochemical properties [142]. Hydrothermal hydrolysis has been considered an environmentally friendly and cost-effective hydrolysis approach as this process possess several benefits on the macroalgal biorefinery route, including (1) the process does not require the addition of chemicals or catalysts as water is the only reagent, (2) limited corrosion problems on equipment, and (3) economical and simple operation [143]. Subcritical water (autohydrolysis) and steam explosion techniques can be considered hydrothermal hydrolysis, depending on the conditions of temperature and pressure employed [144]. In autohydrolysis processing, macroalgal biomass is exposed to water in the liquid state at high temperatures (150–380 °C) and pressure (5–28 MPa) to hydrolyze polysaccharides into a variety of rare monomeric sugars [145]. Autohydrolysis for rare sugar extraction was conducted by del Río et al. [109] with S. muticum in a pressurized batch reactor evaluating the effect of temperature and resistance time. A maximum rare sugar yield of 34.89% was achieved with a 1:7 S/L ratio at 180 °C and a residence time of 25 min. They revealed that temperature was the key factor for maximum rare sugar yield, followed by residence time [109]. Similar results were also found in the study of Gomes-Dias et al. [110] that the higher total rare sugar yield of 38.34% could be released from red macroalgae G. sesquipedale via autohydrolysis at the reaction temperature of 170 °C than that at 127.60 °C and 212.40 °C for 40 min. Moreover, Gomes-Dias et al. [110] concluded that increasing the reaction temperature up to 212.40 °C would give rise to the formation of 5-HMF from 1.04 to 3.23% in the G. sesquipedale hydrolysates. Wang et al. [146] reported that water at high temperatures will weaken the hydrogen bonds in the water molecules, resulting in the autoionization of water molecules into acidic hydronium ions (H3O+), which act as a catalyst to cleave the glycosidic bonds of macroalgal polysaccharides.
In contrast, the steam explosion hydrolysis technique has been widely employed as a lignocellulosic saccharification process. Nevertheless, it is still not highly explored as a thermal-catalytic hydrolysis approach for macroalgae as the macroalgae biomass is less recalcitrant due to the lack of lignin content [147]. The steam explosion technique utilizes high pressures of steam (1–50 bar) to treat the biomass followed by sudden depressurized so that the biomass will undergo explosive decompression. This quick pressure reduction comprises an initial temperature of 160 to 270 °C for a few seconds or minutes in saturated steam before exposure to atmospheric pressure [148]. Diffusion of the saturated steam into the macroalgal cell wall matrix leads to the dispersion of fibers and cleavage of the glycosidic bonds [149]. Compared to LCBs, the operating temperature and pressure for steam exploding of macroalgal biomass will be lower due to macroalgae possess high moisture content that facilitates a quick rise of pressure and temperature within the cells, allowing cell wall rupturing [144]. This aspect makes the steam explosion hydrolysis approach a simpler extraction method for macroalgal biomass. Rare sugar extraction from Ulva intestinalis by steam explosion obtaining yields of 51.70% under 121 °C and 1.75 bar for 15 min with no comparable values for control samples was reported [111].
Advanced hydrolysis approach
Despite the widespread usage of conventional hydrolysis protocols at the industrial level, there is a growing interest in incorporating innovative hydrolysis protocols to enhance rare sugar extraction. The aim of developing innovative hydrolysis processes is to improve the hydrolytic efficiency of the conventional hydrolysis protocols by increasing the sugar recovery from the biomass while decreasing the energy consumption and hydrolysis duration of macroalgal processing [78]. The most potential emerging hydrolysis protocols described in the literature involve the use of microwave irradiation, combined acids and enzymes, and combined hydrothermal process and enzymes [113, 114, 150]. The use of microwave irradiation is regarded to be a promising pretreatment process for macroalgae biomass as it utilized microwave-generated thermal and non-thermal effects in moisture and aqueous environment [151]. The thermal effect generated by microwave refers to the part of the process that generates heat for internal heating, which is dependent on the direct energy absorption by polar molecules or organic polymers [152]. On the other hand, the non-thermal effect refers to the effect caused by the dipole rotation of polar molecules and ionic conduction of dissolved ions [79]. The dipole rotation can be described as the realignment of polar molecules with the poles of the rapidly oscillating electromagnetic field of the microwave, resulting in the cleavage of the hydrogen bonds and glycosidic bonds between transmembrane domains of the cell [153]. Based on the abovementioned heating process, microwave heating offers several advantages over the conventional heating methods (autoclaving or water-bathing): (1) enhance the heat transfer between the biomass and solvent by applying volumetric and rapid internal heating; (2) the reaction temperature can be well controlled and stopped immediately; and (3) provide shorter reaction duration and can heat the biomass evenly in the whole reaction process, which enabled this method to be often utilized in combination with acids, enzymes, and thermal-catalytic hydrolysis approach to increase hydrolytic efficiency [150].
Acid hydrolysis was performed by Teh et al. [115] in an improved microwave oven (800 W) to evaluate the influence of temperature and acid concentration on the sugar recovery and by-product formation from Eucheuma denticulatum. The authors concluded that the red macroalgae E. denticulatum had been hydrolyzed effectively to achieve the sugar recovery rate of 74.84%, which corresponds to 51.47 g/L of sugars accompanied by a low by-product 5-HMF of 0.20 g/L with the involvement of microwave-assisted sulfuric acid (0.1 M) hydrolysis for 10 min [115]. Cao et al. [118] further applied higher microwave power (1900 W) to assist the acidolysis of red macroalgae Gracilaria lemaneiformis under the optimized condition of 180 °C with aided of 0.2 M H2SO4, and the maximum yield of rare sugars reached up to 73.30% using only 20 min of reaction time which is sixfold lesser than the conventional heating method. Boulho et al. [154] concluded that the superficial heat transfer environment offered by microwave heating to the biomass not only improved the sugar recovery rate from the biomass but also limited the formation of 5-HMF. Unlike microwave heating, conventional heating uses conduction and convection heat transfer, in which the heat energies are transferred from the surface to the center of biomass by conduction [155]. Thereby, the heating time of this process is longer than microwave heating for the solvent and biomass to achieve the targeted temperature [156]. As a result, it will lead to a reduction of the rare sugars and an increment of the 5-HMF due to the degradation of monosaccharides during the heating process [157].
The use of autohydrolysis involving microwave heating was studied by Tsubaki et al. [113] to enhance the extraction of rare sugars from Monostroma latissimum. They revealed that the microwave heating could increase the solubilization rate of M. latissimum probably due to the microwaves generate homogenous and uniform heating on the biomass, which allows penetration of subcritical water into the matrix polysaccharides to release the rare sugars, and the maximum total rare sugar yield of 53.10% was achieved under 140 °C for 10 min [113]. Furthermore, enzymatic hydrolysis could be enhanced by microwave irradiation, Charoensiddhi et al. [112] evaluated the production of rare sugars from brown macroalgae Ecklonia radiata by microwave-assisted enzymatic hydrolysis with carbohydrate hydrolytic enzymes: Viscozyme, Cellulast, Ultraflo, Alcalase, Neutrase, and Flavourzyme. The authors investigated different enzyme cocktail configurations in the same volume (100 µL) with microwave operating at 200 W. Enzyme cocktail of Ultraflo and Flavourzyme showed the highest extraction yield (69.50%) under working conditions of 50 °C. In addition, it was observed a synergic effect between microwave and enzyme cocktail, in which it shortens the time of hydrolyzing by eightfold and doubles the extraction yield when compared to conventional enzymatic hydrolysis [112]. A similar conclusion was found in a study by Lee et al. [114] that the rare sugar extraction yield from red macroalgae Pyropia yezoensis was improved from 5 to 25% with the involvement of microwave-assisted amyloglucosidase hydrolysis. This can be clarified by changing direction for the active sites on the enzyme due to the rotation and acceleration of the polysaccharide molecules done by microwave irradiation. Thus, the opportunity for the substrate bounded with the active sites on the enzyme per unit time to release rare sugar will increase, leading to the high productivity of rare sugars [158].
Besides using microwave irradiation as the heating source for the hydrolysis process, the hydrolytic efficiency and duration can be enhanced by employing an efficient pretreatment method. The establishment of the pretreatment method is to facilitate the hydrolytic efficiency to increase the sugar recovery rate and subsequently increase the productivity of bioethanol and LA [159]. Ravanal et al. [116] conducted additional enzymatic hydrolysis with enzyme cocktail (alginate lyase, oligoalginate lyase, and CellicCTec 2) for 17 h on the dilute H2SO4 pretreated green macroalgae Macrocystis pyrifera to increase rare sugars release content yield to 95.10%. Similar results were also achieved in the study of Park et al. [117] that the hydrolysis of red macroalgae G. verrucosa via the diluted sulfamic acid (H2NSO3H) and an enzyme cocktail composing of Viscozyme® L, Cellic® CTec2, and Cellic® HTec2 for 72 h led to a significantly increased production yield of rare sugars from 39.90 to 69.10%. Other than applied acidolysis as a pretreatment step prior to enzymatic hydrolysis, Poespowati et al. [48] added cellulase into the autohydrolyzed green macroalgae Ulva lactuca to achieve a maximal rare sugar yield of 79.70%. Del Río et al. [109] utilized ultrapure water and a mixed enzymatic system composing of Cellic® CTec2 and Viscozyme 1.5L to treat the brown macroalgae S. muticum, which resulted in the increment of total rare sugar yield from 34.89 to 94.40% by comparing with autohydrolysis only. The inorganic acids and subcritical water serve as a proton donor to break the intra- and inter-chain hydrogen bonds of the macroalgal cell wall matrix to release the hydrocolloids results in an increase of accessibility to enzymes for further degradation [160].
Biotechnological route for bioethanol and lactic acid
Fermentation of bioethanol and LA is followed after the pretreatment and hydrolysis of the macroalgae biomass. The overall process of fermentation can be described as the rare sugars that are produced as a result of disruption of the cell wall and depolymerization of phycocolloids and cellulose molecules before being subjected to fermentation by the relevant microorganisms or bacteria and converted into bioethanol and LA [31, 161].
Recommendations of microorganisms’ strain for 3G bioethanol and lactic acid conversion
To optimize the productivity of bioethanol and LA from macroalgal biomass through microbial fermentation, the strain of fermentative microorganisms implemented is considered as a crucial parameter for the fermentation process. This is due to different microbial strains possess different properties and metabolic pathways on the fermentable sugars extracted from the biomass. Furthermore, the derivatives of bioproduct generated by microbial fermentation are mainly dependent on the selected microbial strain [162, 163]. Thus, the selection of appropriate strains of microbial is crucial after deciding the target bioproduct for production. Several reports on the utilization of different fermentative microbial strains for the single production of bioethanol or LA are summarized in Table 5. Although there have been many bacterium and yeast strains utilized for the production of bioethanol from renewable resources, the results shown in Table 4 revealed that S. cerevisiae yeast is the dominant microbial that has been considered the most critical part was contributing to beneficial effects in bioethanol fermentation using reducing sugars as substrate. The eukaryotic microorganism S. cerevisiae is chosen over the other bacterium and yeast strains for bioethanol fermentation due to its offer strength to growth under a wide range of pH, less stringent nutritional requirements, and utmost resistance to contamination [163, 164]. Moreover, S. cerevisiae is also able to metabolize diverse fermentable sugars and possess the ability to produce a high titer of bioethanol as it can resist the contamination caused by high ethanol concentrations produced in the fermentation broth [164].
Table 5.
Summary of fermentative microbial strain utilized in the single production of bioethanol or LA
| Fermentative bacterium | Biomass | Fermentable sugar | Product | Reference |
|---|---|---|---|---|
| Saccharomyces cerevisiae Baker’s yeast | Chaetomorpha linum | Glucose | Bioethanol | [174] |
| Ambrosiozyma angophorae | Laminaria digitata | Glucose Laminarin | Bioethanol | [175] |
| Ethanologenic Escherichia coli | Arundo donax | Arabinose Glucose Xylose | Bioethanol | [176] |
| Saccharomyces cerevisiae KCTC 1126 | Gracilaria verrucosa | Galactose Glucose | Bioethanol | [177] |
| Candida glabrata | Gracilaria fisheri | Galactose Glucose | Bioethanol | [178] |
| Escherichia coli SL100 | Olive tree pruning biomass | Galactose Glucose Xylose | Bioethanol | [179] |
| Saccharomyces cerevisiae YRH400 | Populus deltoides | Glucose Xylose | Bioethanol | [180] |
| Saccharomyces cerevisiae Ethanol Red® | Sargassum muticum | Galactose Glucose Mannose | Bioethanol | [109] |
| Saccharomyces cerevisiae PE-2 | Sargassum spp. | Glucose | Bioethanol | [181] |
| Bacillus coagulans NBRC 12,714 | Corn stover | Glucose Xylose | L-lactic acid | [182] |
| Lactobacillus plantarum | Gracilaria vermiculophylla | Galactose Glucose | L-lactic acid | [183] |
| Bacillus coagulans DSM No. 2314 | Beechwood | Glucose Xylose | L-lactic acid | [184] |
| Bacillus coagulans LA-15–2 | Rice straw | Glucose Xylose | L-lactic acid | [185] |
| Bacillus coagulans DSM ID 14–300 | Sugarcane bagasse hemicellulosic material | Arabinose Glucose Xylose | L-lactic acid | [186] |
| Lactobacillus delbrueckii CECT 286 | Orange peel waste | Fructose Galactose Glucose | D-lactic acid | [187] |
| Bacillus coagulans ATCC 7050 | Eucheuma denticulatum cellulosic residue | Glucose | L-lactic acid | [62] |
| Lactobacillus rhamnosus ATCC 7469 | Brewer’s spent grain | Arabinose Galactose Glucose Mannose Xylose | L-lactic acid | [188] |
| Pediococcus acdilactici ZP26 | Picea abies | Glucose Mannose | D-lactic acid | [189] |
The large-scale production of LA is mostly done by employing the use of LAB as the bacteria for fermentation and the selected bacterium strain can be shown in Table 5. Among thousand types of identified LAB strains, B. coagulans has become one of the most popular bacteria employed in either laboratory- or industrial-scale LA production due to its characteristics and mild operating conditions. A typical superiority of B. coagulans strain for LA fermentation is offered better acid tolerance compared to other LAB strains, resistance to heat up to 50 °C, and less stringent nutritional requirements [21, 165]. Moreover, B. coagulans strain could improve the biorefinery performance and increase fermentable sugar digestibility as it is capable to metabolize both C6 and C5 sugars by secreting several types of thermostable enzymes, including glucokinase, α-galactosidase, and xylanase [166]. As a matter of fact, B. coagulans strains will metabolize C6 and C5 sugars through the homofermentative pathway and pentoses phosphate pathway, respectively, to produce LA as the major end metabolic product of carbohydrate fermentation [167].
By using the microbial fermentation route for LA production, the main concern of this production route is the enantiomer of LA produced is mainly dependent on the lactate dehydrogenase (LDH) specificity of the fermentative strain employed [168]. In this case, B. coagulans strain is considered an excellent producer of L-lactic acid (L-LA) as it contains L-lactate dehydrogenase (L-LDH) enzyme, which promotes the formation of L-LA [169]. As reported in the literature, high crystalline PLA can be prepared either from an optically pure L-LA isomer or D-lactic acid (D-LA) isomer via ring-opening polymerization [170, 171]. However, L-LA isomer was chosen over D-LA isomer as the monomer of PLA due to poly-L-lactic acid (PLLA) possess higher melting temperature (170–200 ) and tensile strength (15.5–150 MPa) as compared to poly-D-lactic acid (PDLA) [172]. Furthermore, PLLA is the material of choice for biomedical applications as D-LA is considered a harmful enantiomer of LA on human health which can cause neurotoxicity on the human body [173]. Thus, given the multiple traits described above, B. coagulans strain is a promising candidate for the production of LA at the industrial level to meet the high demands of PLA as bioplastics.
Synthetic pathway for 3G bioethanol and lactic acid
Bioethanol and LA fermentation can be classified into two methods, which include solid-state fermentation and submerged fermentation. The solid-state fermentation method is the bioconversion of the carbohydrates from macroalgal biomass in its natural state in which the biomass is introduced to the surface of a thin layer of water [190]. Moreover, water is also known as an essential solvent for the submerged fermentation method, where it is used for creating fermentation mash, which is mixed with the hydrolyzed biomass [191]. The solid-state fermentation method is preferred over submerged fermentation methods as the solid-state fermentation method is more energy-efficient due to smaller fermenter volume and requires no excess water in the fermenter, leading to less amount of water needed to be heated [192]. Currently, there are numerous solid-state fermentation approaches employed to convert rare sugars extracted from macroalgae into bioproducts (bioethanol and LA). The processes are denoted as follows: (1) separate hydrolysis and fermentation (SHF); (2) simultaneous saccharification and fermentation (SSF); and (3) high cell density culture (HCDC) [193–195].
Separate hydrolysis and fermentation (SHF)
SHF process is one of the most common combinations of hydrolysis and fermentation methods employed for the bioethanol and LA production process [196]. In the SHF process, the hydrolysis and the fermentation processes are operated separately, in which the carbohydrates of macroalgae biomass are first decomposed into monosaccharides via the hydrolysis process, and the fermentation of rare sugars are carried out later in separate units with different operating conditions [197]. The production of bioethanol and LA by using the SHF method on various types of macroalgae are summarized in Table 6. These studies revealed that the production of bioethanol and LA from macroalgae biomass using the SHF method was operated under batch mode. Batch mode is chosen over the continuous and fed-batch modes for the bioproducts fermentation process due to it offers the highest conversion rate as complete biomass can be utilized [198]. Hessami et al. [104] demonstrated that 18.16 g/L (67.90%) of bioethanol can be achieved during the fermentation of acid-modified G. manilaensis hydrolysates using 5% (v/v) S. cerevisiae Ethanol Red® directly under batch mode at 30 °C for 96 h. Under the same yeast cell volume, the fermentation process for the acid-modified G. elegans hydrolysates was optimized to achieve a bioethanol yield of 63.30%, corresponding to 13.27 g/L of bioethanol [105]. Saravanan et al. [22] also utilized S. cerevisiae yeast cell for fermentation of other red macroalgae Gracilaria sp. hydrolysates, and the maximal bioethanol yield obtained after 96 h fermentation at 30 °C was 28.70 g/L, which corresponded to a 50.98% of the theoretical yield.
Table 6.
Bioethanol and lactic acid production from SHF method on various macroalgae
| Macroalgae strain | Hydrolysis technique | Fermentation conditions | Bioethanol yield | Lactic acid yield | Reference |
|---|---|---|---|---|---|
| Whole macroalgae biomass | |||||
| Gracilaria manilaensis | Acid hydrolysis | 5% (v/v) Saccharomyces cerevisiae Ethanol Red®, 6 g/L yeast extract (30 °C, 150 rpm, pH 5.0, 96 h) | 67.90% | - | [104] |
| Ulva lactuca | Sequential acid and enzymatic hydrolysis | 106 CFU/g Lactobacillus plantarum BCRC 10,069 (37 °C, pH 5.5, 24 h) | - | 0.58 g/g RS | [200] |
| Gracilaria fisheri | Acid hydrolysis | 1% (v/v) Candida glabrata, 3 g/L yeast extract, 5 g/L peptone (37 °C, 120 rpm, pH 6.5, 96 h) | 0.03 g/g RS | - | [178] |
| Gracilaria sp. | Sequential acid and enzymatic hydrolysis | 2% (v/v) Saccharomyces cerevisiae MTCC174, 0.5 g/L yeast extract, 0.5 g/L (NH4)2SO4, 87.5 mg/L KH2PO4, 12.5 mg/L K2HPO4, 10 mg/L NaCl, 50 mg/L MgSO4·7H2O, 10 mg/L CaCl2·2H2O, 10 mg/L CuSO4·5H2O (30 °C, 125 rpm, pH 5.0, 96 h) | 50.98% | - | [22] |
| Ulva rigida | Thermal acid hydrolysis | 5% (v/v) Pachysolen tannophilus, 10 g/L yeast extract, 10 g/L peptone, 20 g/L glucose (30 °C, 120 rpm, 96 h) | 0.37 g/g RS | - | [103] |
| Gelidium elegans | Acid hydrolysis | 5% (v/v) Saccharomyces cerevisiae NBRC 10,217, 10 g/L yeast extract, 20 g/L peptone, 20 g/L dextrose (30 °C, 150 rpm, pH 6.0, 48 h) | 63.30% | - | [105] |
| Gracilaria sp. | Sequential acid and enzymatic hydrolysis | 6% (v/v) Lactobacillus acidophilus BCRC 10,695 and Lactobacillus plantarum BCRC 12,327 (30 °C, pH 5.6, 72 h) | - | 64.72% | [195] |
| Macroalgae residual biomass | |||||
| Mixed brown macroalgae extracted sodium alginate | Subcritical water hydrolysis | 2% (w/w) CaO (200 °C, 600 rpm, 1 h) | - | 12.66% | [203] |
| Gracilaria corticata residues | Acid hydrolysis | 10% (v/v) Saccharomyces cerevisiae Baker’s yeast, 10 g/L yeast extract, 20 g/L peptone, 20 g/L dextrose, 1 g/L (NH4)2SO4, 0.5 g/L Na2HPO4, 2.5 g/L KH2PO4, 1 mg/L FeSO4, 1 g/L MgSO4, 1 g/L Urea (34 °C, 50 rpm, pH 5.3, 120 h) | - | 0.02 g/g RS | [61] |
| Industrial spent Eucheuma spinosum residues | Acid hydrolysis | 0.5 g of Saccharomyces cerevisiae Baker’s yeast (30 °C, pH 4.5, 24 h) | 11.60 g/g algae | - | [204] |
| Eucheuma cottonii residues | Enzymatic hydrolysis | 12% (v/v) Saccharomyces cerevisiae ATCC 200,062, 10 g/L yeast extract, 20 g/L peptone, 20 g/L dextrose, 20 g/L agar (32 °C, pH 5.2, 72 h) | 0.40 g/g RS | - | [6] |
RS rare sugars
Notably, the bioethanol fermentation from macroalgae hydrolysates is limited by the inability of common ethanologenic yeast strains such as S. cerevisiae to metabolize a wide range of rare sugars extracted from macroalgae hydrolysis. This is mainly due to glucose extracted from the cellulose of macroalgae that will cause catabolic repression in the uptake of other rare sugars such as galactose, mannose, and rhamnose from carrageenan, fucoidan, and ulvan, respectively, which led to these sugars that were not fermented by the ethanologenic yeast and resulting in poor bioethanol productivity from macroalgae biomass [199]. In this regard, evolutionary and genetic engineering approaches for wild-type strains with the capability of fermenting a wide range of rare sugars have been developed to increase the rare sugars consumption [178]. El Harchi et al. [103] revealed that both rare sugars (glucose and rhamnose) in the acid-modified U. rigida hydrolysates can be fermented simultaneously to achieve a bioethanol yield of 11.92 g/L, which corresponded to 0.37 g/g rare sugars by using Pachysolen tannophilus. Similarly, the Candida glabrata strain isolated from the surface of Gracilaria fisheri has been developed with bioconversion yield up to 0.03 g/g rare sugars from acid-modified G. fisheri hydrolysates containing galactose and glucose for bioethanol production [178]. Apart from being applied successfully for bioethanol production, SHF is also being employed for LA production from macroalgal biomass. Wu et al. [200] conducted the fermentation of U. lactuca (green macroalgae) hydrolysates at 37 °C for 24 h by using LAB cells of Lactobacillus plantarum BCRC 10,069 and enhanced the LA titer in the fermentation broth up to 7.02 g/L, corresponding to 0.58 g/g rare sugars. In addition, Lin et al. [195] used combined LAB cells of L. acidophilus BCRC 10,695 and L. plantarum BCRC 12,327 for LA fermentation from red macroalgae Gracilaria sp. hydrolysates. The LA yield obtained after 72 h fermentation at 30 °C from 29.85 g/L of rare sugars was 64.72% or corresponding to a conversion yield of 0.19 g/g rare sugars [195].
As per current industrial applications, macroalgae biomass is widely utilized as a feedstock of value-added products (natural minerals, thickeners, and pigments) [201]. Additionally, the industrial fractionation of macroalgae for value-added products generates organic waste that mainly consists of cellulose and some amount of phycocolloids. These organic wastes could be a potential feedstock for bioethanol and LA production and considered as a green pathway for the macroalgae biorefinery [202]. Jeon et al. [203] explored the usage of mixed brown macroalgae extracted alginate and was fermented using calcium oxide (CaO). An optimum LA conversion yield of 12.66% was attained after 1 h of fermentation at 200 °C [203]. Besides, a study on bioconversion of cellulose from cellulosic residues of Gracilaria corticata indicated that acid hydrolysis followed by fermentation using S. cerevisiae Baker’s yeast under optimum conditions (34 °C, 120 h) can produce up to 0.02 g/g rare sugars of bioethanol [61]. Jambo et al. [6] reported that bioethanol production from enzymatic hydrolyzed E.cottonii residues resulted in 0.40 g of bioethanol from 1 g of rare sugars extracted, which corresponds to 9.77 g/L bioethanol. Alfonsín et al. [204] further adopted another acid hydrolyzed cellulosic residue of Eucheuma spinosum (red macroalgae) to ferment with S. cerevisiae Baker’s yeast, and the optimal conditions were set to 30 °C and 24 h to attain 11.60 g/g substrate of bioethanol. Thus, the industrial waste of macroalgae biomass can be utilized as an eco-friendly and cost-effective resource for bioethanol and LA production to encounter future energy and biopolymer requirements.
Simultaneous saccharification and fermentation (SSF)
SSF is also known as one of the configurations that are widely employed for biomass biorefinery processes to achieve value-added bioproducts. In the SSF method, the hydrolysis and fermentation processes are operated within the same unit, where the rare sugars released via saccharification of carbohydrates molecules by the enzymes can be metabolized directly by the yeasts or microorganisms into bioethanol and LA [176]. This combination posed several advantages over the SHF method, such as high production yield, reduced risk of contamination, reduced enzyme loading for depolymerization, and required less energy consumption. Thereby, SSF method is usually preferred over the SHF method [205]. The rapid metabolism of reducing sugars to bioethanol and LA can neutralize the inhibition effect of hydrolytic products on the cellulase activities and reduce the usage of enzymes for the depolymerization process of the carbohydrates [206]. Table 7 shows the comparative studies of different yeast and microorganism strains, fermentation conditions, and bioproducts (bioethanol and LA) yield using the SSF and SHF method on various types of macroalgae. From Table 7, the SSF method is identified to be more efficient than the SHF method in terms of the resulting bioethanol and LA concentration.
Table 7.
A comparison study of SSF and SHF methods on bioethanol and lactic acid production using macroalgae
| Macroalgae strain | Bacterium strain | Hydrolysis and fermentation mode | Nutrient source | Fermentation conditions | Bioethanol yield | Lactic acid yield | Reference |
|---|---|---|---|---|---|---|---|
| Whole macroalgae biomass | |||||||
| Gelidium amansii | Saccharomyces cerevisiae KCTC 7906 | Sequential hydrothermal and enzymatic hydrolysis, SHF | 10 g/L yeast extract, 20 g/L peptone | 1% (v/v) S.cerevisiae KCTC 7906 (30 °C, pH 4.8, 6 h) | 74.70% 3.33 g/L | - | [5] |
| Gelidium amansii | Saccharomyces cerevisiae KCTC 7906 | Subcritical water hydrolysis, SSF | 10 g/L yeast extract, 20 g/L peptone | 1% (v/v) S.cerevisiae KCTC 7906, 8 g/L cellulase, 4 g/L β-glucosidase (37 °C, pH 4.8, 12 h) | 84.90% 3.78 g/L | - | [5] |
| Saccharina latissima | Rhizopus oryzae F-814 | Sequential acid and enzymatic hydrolysis, SHF | 50 g/L glucose, 2.36 /L (NH4)2SO4, 0.2 g/L MgSO4·7H2O, 0.07 g/L ZnSO4·7H2O, 1 g/L K2HPO4 | 15 g/L R.oryzae F-814 (28 °C, pH 5.0, 40 h) | - | 0.11 g/g algae 11.3 g/L | [23] |
| Saccharina latissima | Rhizopus oryzae F-814 | Acid hydrolysis, SSF | 50 g/L glucose, 2.36 g/L (NH4)2SO4, 0.2 g/L MgSO4·7H2O, 0.07 g/L ZnSO4·7H2O, 1 g/L K2HPO4 | 15 g/L R.oryzae F-814, 10 mg/g Celluclast 1.5L, 10 mg/g Viscozyme L, 10 mg/g Laminarinase, 1 mg/g alginate lyase (33 °C, pH 5.0, 40 h) | - | 0.13 g/g algae 13.1 g/L | [23] |
| Gracilaria tenuispititata | Rhizopus oryzae F-814 | Acid hydrolysis, SSF | 50 g/L glucose, 2.36 /L (NH4)2SO4, 0.2 g/L MgSO4·7H2O, 0.07 g/L ZnSO4·7H2O | 15 g/L R.oryzae F-814, 10 mg/g Celluclast 1.5L, 10 mg/g Viscozyme L, 450 U/g agarase (33 °C, pH 5.0, 40 h) | - | 0.10 g/g algae 9.6 g/L 0.24 g/Lh | [23] |
| Sargassum muticum | Saccharomyces cerevisiae PE2 | Subcritical water hydrolysis, SSF | 10 g/L yeast extract, 20 g/L peptone, 20 g/L glucose | 1.8 g/L S.cerevisiae PE2, 20 FPU/g CellicCTec2, 5 U/g Viscozyme (35 °C, 150 rpm, pH 5.0, 30 h) | 81.00% 14.1 g/L | - | [109] |
| Rhizoclonium sp. | Saccharomyces cerevisiae I | Microwave heating, SSF | 0.5% yeast extract, 0.5% peptone | 12.5 g/L enzyme cocktail (cellulase: amylase: xylanase: pectinase = 5:3:1:1 v/v/v/v), 10% (v/v) S.cerevisiae I (32 °C, pH 6.0, 72 h) | 0.19 g/g RS 20.51 g/L | - | [207] |
| Macroalgae residual biomass | |||||||
| Eucheuma cottonii residues | Saccharomyces cerevisiae YSC2, type II | Solid acid hydrolysis, SSF | 10 g/L yeast extract, 20 g/L peptone, 20 g/L galactose, 17.5 mg/L K2HPO4 | 17.5 g/L S.cerevisiae YSC2, 45 FPU/g cellulase, 52 CBU/g β-glucosidase (43 °C, 130 rpm, pH 4.8, 3.5 h) | 92.70% 11.7 g/L | - | [19] |
| Mixed red macroalgae processing solid waste | Saccharomyces cerevisiae | Subcritical water hydrolysis, SSF | Potato dextrose broth | 10% (v/v) S.cerevisiae, 10% (v/v) cellulase from Trichoderma reesei (35 °C, 150 rpm, pH 4.8, 72 h) | 1.07 g/g RS | - | [208] |
| Eucheuma denticulatum residues | Bacillus coagulans ATCC 7050 | Microwave-assited hydrothermal hydrolysis, PSSF | MRS broth, 10 g/L yeast extract | 20 FPU/g cellulolytic enzyme blend SAE0020 (50 °C, 100 rpm, pH 4.8, 6 h), 10% (v/v) B.coagulans ATCC 7050 (37 °C, 100 rpm, pH 4.8, 15 h) | - | 98.60% 14.02 g/L | [62] |
RS rare sugars
A comparative study on SHF and SSF for the bioethanol production from red macroalgae G. amansii in the batch fermentation process has been reported. The yield of bioethanol was enhanced by 13.65% with SSF as compared to the SHF approach. Moreover, the biorefinery process duration was decreased dramatically as the entire bioconversion duration for using SSF was 13 h (1 h autohydrolysis, 12 h SSF), while for the entire SHF process, it was 31 h (1 h autohydrolysis, 24 h enzymatic hydrolysis, 6 h fermentation) [5]. Another study reported the production of LA from acid pretreated brown macroalgae S. latissima via SHF and SSF. The fraction of phycocolloids and cellulosic in the pretreated S. latissima was hydrolyzed by using the enzyme cocktail. The highest LA conversion yield of 0.13 g/g substrate and concentration of 13.10 g/L has been achieved via SSF with Rhizopus oryzae. This study concluded that LA productivity and titer can be improved with the SSF approach as compared to the SHF approach [23]. Hence, these results revealed that not only is SSF more efficient than SHF but it also serves as a time-effective process. In addition, Maslova et al. [23] investigated the production of LA through SSF from acid-treated red macroalgae Gracilaria tenuispititata using R. oryzae F-814 and enzyme cocktail (Celluclast 1.5L, Viscozyme L, agarase). They revealed that the highest LA yield (0.10 g/g substrate), productivity (0.24 g/L h), and titer (9.60 g/L) were successfully obtained.
Furthermore, del Río et al. [109] demonstrated that 14.10 g/L of bioethanol, which corresponds to 81% conversion yield, can be attained during SSF of hydrothermally treated S. muticum (brown macroalgae) using the cocktail enzyme (CellicCTec2 and Viscozyme) for saccharification of polysaccharides in S. muticum and fermented by S. cerevisiae PE2 yeast, and Sharma et al. [207] also applied SSF successfully on another microwave-treated green macroalga strain Rhizoclonium sp. to achieve maximum bioethanol yield of 0.19 g/g rare sugars (20.51 g/L). More recently, the feasibility of bioethanol production from E. cottonii residues by S. cerevisiae via the SSF process has been explored. The highest titer of bioethanol 11.70 g/L and yield of 92.70% has been achieved at the optimum conditions (43 °C, 130 rpm, pH 4.8, 3.5 h) [19]. The use of enzymatic-assisted SSF was also studied by Hakim et al. [208] to enhance the bioethanol production from hydrothermally treated mixed red macroalgae processing solid waste. The highest bioethanol conversion yield of 1.07 g/g rare sugars was achieved using S. cerevisiae yeast strain and cellulase from Trichoderma reesei [208]. Additionally, the prehydrolysis and simultaneous saccharification and fermentation (PSSF) approach in batch fermentation has been reported for LA production from pretreated biomass of E. denticulatum residues with microwave-assisted hydrothermal hydrolysis. A maximum of 98.60% (14.02 g/L) LA was attained under prehydrolysis condition of 50 °C with 0.05 M sodium citrate buffer and 20 FPU/g biomass cellulolytic enzyme blend for 6 h followed by SSF approach at 37 °C for 15 h [62].
High cell density culture (HCDC)
The volumetric productivities of bioethanol and LA are mainly relying on the chemical composition of the biomass, product inhibition, microbial strain, operating temperature, and pH value [209]. However, the more efficient way to improve the production efficiency of bioethanol and LA is to increase the biocatalyst loading. The initial amount of yeast or LAB is the main factor in determining the overall conversion efficiency and outcomes during the bioethanol and LA production [210]. A rapid and complete fermentation process of reducing sugars is required for maximizing the productivity and profitability of the process. Therefore, HCDC is currently employed accompanied by either SHF or SSF approach to enhance the productivity and conversion efficiency of value-added bioproducts from macroalgal biomass. HCDC can offer higher volumetric productivity of fermentation processes by providing a shorter metabolization rate than at low cell density culture in the same reactor [168]. Moreover, HCDC can be used to reduce the cost of cell propagation, as most of the cells are reused, recycled, or retained in the reactor. Thus, the unproductive lag phase of yeast or LAB cells during the cell growth phases can be eliminated since they are being reused during the fermentation process [211]. As a result, a smaller fermenter volume can be used for the anaerobic conversion of rare sugars to value-added bioproducts [212].
Jambo et al. [6] performed the fermentation of enzymatic hydrolyzed red macroalgae E. cottonii residues at pH 5.2 and 32 °C for 72 h with a 2% (w/v) E. cottonii residue hydrolysates and improved the bioethanol concentration in the fermentation broth up to 9.77 g/L when increasing the inoculum concentration of S. cerevisiae ATCC 200,062 from 10 to 12% (v/v). In another study, Sayed et al. [213] reported that the usage of 118 mg/L of S. cerevisiae CLIB 95 was able to fully assimilate both glucose and galactose from synthetic Ulva sp. hydrolysates for bioethanol production within 144 h of fermentation time. The results showed that the ethanol ratio (ethanol observed over ethanol theoretically produced) with theoretical bioethanol yield of 68% per dry cell biomass could be significantly increased from 92.50 to 97.70% by raising the inoculum concentration from 58.70 to 118 mg/L [213]. Lin et al. [195] demonstrated that after fermentation by combined 6% (v/v) L. acidophilus BCRC 10,695 and L. plantarum BCRC 12,327 at 30 °C for 72 h, the LA conversion yield of sequential acid and enzyme hydrolysates from Gracilaria sp. reached 15.02 g/L, which was markedly higher than the LA concentration of 14.57 g/L using 1% (v/v) of combined LAB. In addition, Hakim et al. [208] further analyzed SSF efficiency of the hydrothermally treated mixed red macroalgae processing solid waste using various inoculum concentrations of S. cerevisiae at 35 °C for 72 h and reported that the bioethanol conversion yield was enhanced from 0.60 to 1.07 g/g rare sugars with an increment of 78.33% when 10% (v/v) S. cerevisiae was employed compared to only 5% (v/v). These findings revealed that HCDC could enhance the productivity of bioethanol and LA from macroalgae biomass, indicating a significant opportunity for large-scale macroalgae-based bioethanol and LA production.
A perspective on novel cascading macroalgae bioethanol and lactic acid biorefinery system
The co-production of multiple products in a biorefinery process is considered a viable approach to address the dilemma of macroalgae bioproducts and improve the economics of high value-added products [214]. Process optimization, along with the selection of an effective macroalgae strain and corresponding biorefinery pathway, is necessary for the continuous production of grid quality bioethanol and biochemicals such as LA, succinic acid, and citric acid [215]. This is because the strain and the composition of macroalgae should be fundamental and essential in defining the targeted products and will affect the corresponding preprocessing and pretreatment techniques such as extraction, cell wall disruption, and anaerobic digestion (AD) [7]. Figure 4 summarizes the proposed decision-making algorithm for selecting the most effective biorefinery pathway from a perspective of macroalgae strains. In order to make an effective approach for choosing the preferable bioproducts, each possible product is ranked according to their equivalent selling prices (ESP) per kilogram is proposed. For instance, bioethanol, LA, and succinic acid equivalent selling prices are usually ranged between US$0.47–1.59/kg, US$3.00–4.00/kg, and US$0.92–0.99/kg, respectively [216–218]. Therefore, each alternative could then be rated in terms of profitability on the basis of the cost of production of the bioproducts. Other than the direct application of whole macroalgae as fertilizer and animal feed, all other bioproducts, including biofuels such as bioethanol and biobutanol, require additional processing and purification of the extracted fractions of macroalgae during the biorefinery stage so that the bioproducts can be obtained [219]. The cell wall disruption stage is an essential and costly biorefinery stage to facilitate the release of all the compounds in the cell wall matrix (carbohydrates, proteins, lipids, and ash) for further processing, estimated to be US$0.93–1.54/kg dry macroalgae biomass for biofuels production and US$0.30–1.98/kg dry macroalgae biomass for high value-added products production [195, 220]. The cost is greatly affected by the level of purity.
Fig. 4.
Decision-making algorithm for macroalgae application in a biorefinery approach from the perspective of macroalgae composition
Several potential circular energy systems for biorefineries have been proposed for three types of macroalgae (Rhodophyta, Chlorophyta, and Phaeophyta). For Rhotophyta macroalgae–based bioethanol production, K. alvarezii, Gracilaria sp., Chondrus crispus, G. sesquipedale, and Porphyra sp. are the most common feedstocks [4]. The bioethanol production using red macroalgae as feedstock can use either whole algae biomass or algae solid waste. By using algae solid waste for bioethanol production, the macroalgae should be subjected to carrageenan and agar extraction to separate the phycocolloids and solid waste. Carrageenan can be used for synthetic pigment production, which is commercially valued as thickener and food colorant in food industries and colorants for cosmetics and pharmaceutical applications [65]. Similar to LCBs, a feedstock preprocessing technique is required for macroalgae-based bioethanol production to increase the reaction surface area of the biomass, as discussed in Section 4. Except for bioethanol, several other bioproducts can be produced along with bioethanol, such as LA, succinic acid, fertilizer, antioxidants, and polyunsaturated fatty acids (PUFAs). During the extraction method, compounds such as proteins, lipids, ashes, and carbohydrates will also be liberated. Further purification of lipids and ashes can produce chemically and pharmaceutically valuable pigments for antioxidants and fertilizer, respectively [78]. Hexose sugars produced during hydrolysis of cellulose such as glucose and glycerol are mainly utilized for bioethanol production. At the same time, the galactose from agar and carrageenan will be metabolized using LAB for LA production. As the carbohydrate content in Phaeophyta macroalgae is as much as in Rhotophyta macroalgae [4], therefore, Phaeophyta macroalgae–based bioethanol and biochemical production steps are similar to red macroalgae.
Chlorophyta macroalgae contain lesser carbohydrates (25–50%) as compared to Rhodophyta and Phaeophyta macroalgae (30–60%) [4]. Thus, it is mainly utilized for biofertilizer, biomethane, and bioactive compounds production [45, 221]. Green macroalgae can be utilized directly for biomethane and biofertilizer production without the need for energy-intensive and costly cell disruption techniques [221]. Conversely, the extraction of valuable compounds from green macroalgae, including carbohydrates and ulvans in the biorefinery phase, is an attractive alternative. Carbohydrates can be utilized for bioethanol and LA production, while ulvans are rich in L-rhamnose that has several market applications as a synthetic spice, food additive, and biochemical reagent [222]. Furthermore, macroalgae-based bioproducts can be derived and produced directly from the wet macroalgae using hydrothermal treatments and AD, which can reduce the production cost [223]. During hydrothermal liquefaction, macromolecules in macroalgae such as lipids, proteins, and carbohydrates will break down at high pressure, which ranged between 5 and 20 MPa, intermediate-temperature range between 250 and 350 °C, and in the presence of a catalyst to partially oxygenate hydrocarbons as well as gaseous (biogas), aqueous (biooil), and solid by-products. The gaseous by-product can be further processed to become biomethane, while the aqueous solution is rich in sugars that can utilize to produce bioethanol and LA via fermentation. Its solid by-product can also be used to produce biofertilizer and biochemical for wastewater treatment [224].
Techno-economic evaluation of integrated 3G bioethanol and lactic acid production
In general, the market value of bioethanol and LA is directly proportional to the type of feedstocks implemented for biorefinery which embody several aspects including the cost of feedstock, cultivation and harvesting techniques, the origin of feedstock, transportation cost, equipment cost, and technologies cost [33]. To establish a sustainable and circular bioeconomy for a biotechnological industry, a techno-economic assessment (TEA) must be performed to assess the economic performance of an industrial process for cost-effective plant development [225]. The TEA is a crucial practice for assessing the biorefinery process and quality of production by identifying and managing prospective investment and finance processes for the future industry [226]. To date, TEA of bioethanol and LA production from renewable resources has been extensively investigated and reported by many researchers to evaluate economic feasibility for an industrial scale and design using different strategies by varying the biomass strain, solid biomass loading, and software programs [14, 29]. However, studies on 3G bioethanol and LA production cost from macroalgae are limited in the literature.
Barbot et al. [227] revealed that the economic aspects of macroalgae biomass to bioethanol and LA could be classified into two scenarios to evaluate the design of a biorefinery plant: (1) harvesting the biomass, which includes reconditioning and transportation to the processing site and (2) pretreatment, bioconversion, refinement of end-product, biomass storage, and waste treatment. To improve the economic viability, macroalgae biomass is mainly used to extract high value-added bioproducts such as LA along with renewable energies such as biofuels in an integrated biorefinery. Principal, macroalgae cultivation has been growing globally as it can grow 20–30 times faster than food crops and produce up to 30 times more fuel than an equivalent amount of other bioethanol resources, making a high yield for ensuring year-round availability [228]. Around 31 million tons of macroalgae were produced globally in 2017; the principal macroalgae strains are Eucheuma, Gracilaria, and Gelidium sp. [44]. Sadhukhan et al. [144] highlighted the capability of macroalgae to produce up to 60% of their biomass in the form of transportation fuels such as bioethanol. Assuming the bioethanol potential of algae biomasses is similar for all cultivated, harvested, and processed macroalgae species, 31 million tons of macroalgae could generate up to 18.6 million tons of bioethanol per year which satisfy the policy made by the government of the US where the bioethanol produced were sufficient to meet at least 5% of demand for transportation fuels [144, 229].
A comparative study with diverse feedstock was conducted to better analyze and discuss the differences between the macroalgae biorefinery and lignocellulosic biorefinery (Table 8). The reported minimum product selling price (MPSP) of lignocellulosic-based bioethanol and LA was US$1.70–2.13/kg [230, 231] and US$2.66–3.21/kg [232, 233], respectively, which is economically unfeasible as compared to macroalgae biorefinery. The pretreatment, delignification process in lignocellulosic biorefinery with the involvement of chemicals and equipment indicated higher production costs as both raw material cost and energy consumption increased [233]. Thereby, with the exception of pretreatment in macroalgae biorefinery, macroalgae is considered as a feasible feedstock for bioethanol and LA production. Chong et al. [234] developed a techno-economic study of red macroalgae E. cottonii as a cellulosic residue into bioethanol production by simulation using Aspen Plus V10 software. The sensitivity analysis revealed the design is potentially viable. The simulation showed that 66 million liters of anhydrous bioethanol is obtained by 132 thousand tons of E. cottonii residue per year with the minimum ethanol selling price (MESP) of US$0.54/kg. Brigljević et al. [235] reported an industrial biorefinery bioethanol plant (40,000 dry metric ton brown macroalgae input per year) that modeled using Aspen Plus V10 software associated with the fast pyrolysis of S. japonica in a fixed bed reactor and combined with a Rankine power cycle using the biochar by-product to produce bioelectricity. As a result, 23.65 million tons of bioethanol can be produced in this scenario with MESP of US$0.59/kg, which indicated that bioethanol production from brown macroalgae S. japonica is feasible. Another comparative analysis of techno-economic studied by Nazemi et al. [236] uses brown macroalgae Nizimuddinia zanardini under two different scenarios: (1) only-fuel approach in which only bioethanol and bioelectricity will be produced and (2) biorefinery approach in which co-producing high value-added products along with bioethanol and bioelectricity. Results expand the system boundary (total capital investment, sum of inside battery limits investment, outside battery limits investment, working capital, and contingency charges) to determine a complete macroalgae biorefinery. In this way, the results suggest that the biorefinery approach was economically superior over the only-fuel approach with the maximum dry seaweed price of US$374/ton and US$-64/ton [236]. This study indicated that any macroalgae biomass purchasing price below or equal to US$374/ton will result in a profitable process, while in the only-fuel scenario, the plant could not be economically feasible even by using cost-free macroalgae biomass.
Table 8.
Comparison of macroalgae biorefinery with lignocellulosic biorefinery on techno-economic aspect
| Feedstock | Software | Unit price of product (US$/kg) | Feedstock price (US$/kg) | Energy usage (MWh/year) | Reference | |
|---|---|---|---|---|---|---|
| Bioethanol | Lactic acid | |||||
| Eucheuma cottonii cellulosic residue | Aspen Plus V10 | 0.54 | - | 0.073 | 2.61 | [234] |
| Saccharina japonica | Aspen Plus V10 | 0.59 | - | 0.068 | - | [235] |
| Nizimuddinia zanardini | Aspen Plus | 0.62 | - | 0.100 | 1.28 | [236] |
| Eucheuma cottonii cellulosic residue | Aspen Plus V10 | 0.80 | 2.49 | 0.056 | 2.25 | [237] |
| Sugarcane bagasse | Aspen Plus V9 | - | 3.21 | 0.054 | - | [232] |
| Rice straw | Aspen Plus | 2.13 | - | 0.014 | 9.70 | [230] |
| Sugarcane | Aspen Plus | - | 2.66 | - | 50.75 | [233] |
| Corn stover | Aspen Plus V7.4 | 1.70 | - | 0.047 | 2.26 | [231] |
Wong et al. [237] conducted a TEA of red macroalgae cellulosic residue using 3G biorefinery; the study found that obtaining 15,883.3 kg/h of E. cottonii residue was required to produce 3856.8 kg/h of bioethanol, 4479.48 kg/h of fertilizer, and 6488.04 kg/h of LA with a MPSP of US$0.80/kg, US$0.24/kg, and US$2.49/kg, respectively. This TEA study reveals that it has commercial potential and economic feasibility for industrial-scale development: for instance, the developed 3G biorefinery attempts to convert to the real economy by involving on-site seed train for on-site cultivation of cellulase enzyme, yeast, and LAB for hydrolysis and fermentation to reduce the raw material cost. These recent researches contribute to standardizing and optimizing the 3G bioethanol and LA process to blend as a potential alternative to gasoline and petrochemical polymers. Today, it can be argued that current commercial macroalgae-based production is inefficient, unreliable, and mainly small-scale [195]. Thus, research and development activities will be required for technological advancement to maximize the bioethanol and LA productivity from macroalgae and improve the harvesting techniques, which would reduce the cost of the algal biomass production to a more competitive level. Moreover, González-Gloria et al. [238] suggest that standardization of the equipment design model is required to scale up to pilot or industrial scale to validate reliable data and prices for the socio-economic development of cost-effective and scalable technologies.
Environmental impact of the integrated 3G bioethanol and lactic acid production
Apart from the techno-economic concerns, it is also important to provide an analysis of the environmental impacts of the combined processing of 3G bioethanol and LA. The production of bioethanol using macroalgae biomass has been reported to contribute significantly to the reduction of GHGs, which pose problems for climatic stability due to its high tolerance to high carbon dioxide (CO2) concentration and can capture the CO2 from industrial flue gases [4]. Seghetta et al. [239] revealed that the negative environmental impact of 1G and 2G bioethanol and LA production was higher as compared to that of 3G, such as land-use transition, water utilization during cultivation, and delignification process of 2G feedstocks. Unlike edible crops and LCBs, macroalgae are present abundantly in oceans and can be cultivated either off-shore or artificial, which can overcome the limitations of 1G bioethanol and LA in terms of land occupational and competition with food. Moreover, the cultivation of macroalgae can improve the water quality in their habitat. By incorporating macroalgae together with fish farms, macroalgae can oxygenate water using the ammonia excreted by the fish [219]. In terms of climate change, Seghetta et al. [239] reported that macroalgae cultivation and processing exerted less impact on climate than that of the system without macroalgae cultivation. Besides, the cultivation of macroalgae as 3G feedstocks for bioethanol production can be used to substitute gasoline production and utilization, which can resolve approximately 70% of all negative impacts contributed by GHG emissions from the combustion of fuel gases [240]. Furthermore, the cultivation of macroalgae for biobased products generation, such as LA, proteins, and pigments in the biorefinery phase also contributes about 25% positive impacts as all the residue wastes from bioethanol production can be fully utilized to produce value-added products [239]. However, a substantial expansion in macroalgae cultivation to attain high global demands for fuels may subject the marine and coastal environments to some risks, such as changes in natural habitats, nutrient content, and water hydrology characteristics of marine ecosystems [219]. In order to minimize the negative impact of macroalgae cultivations on the marine environment, the cultivation can be done via transplantation. By using the transplantation approach, the macroalgae are grown indoors, then culture in greenhouse tanks, resulting in lower environmental risks compared to off-shore cultivation [241].
From the point of view of biorefinery, considering the bioethanol production from brown macroalgae Ecklonia maxima, Zhang et al. [242] evaluated a cradle-to-grave life cycle assessment (LCA) of three different hydrolysis methods: (1) microwave heating; (2) HWE; (3) SWE. The process included E. maxima cultivation, raw material preparation, sugar mill, industrial activities related to auxiliary biochemicals, and processing of E. maxima for bioethanol. HWE and SWE demonstrated higher environmental burdens compared to microwave heating by producing global warming potential (GWP) of 13.53 kg CO2eq and 25.665 kg CO2eq per kg of dry E. maxima, respectively, mainly due to the requirement of a large amount of electricity, natural gas, and catalysts to reach the targeted reaction temperature. In conclusion, microwave heating proved to be the most environmentally friendly hydrolysis approach [242]. In an evaluation of the environmental impacts of a biorefinery producing bioethanol and bioelectricity from brown macroalgae S. japonica using attributional and consequential LCA approach, the authors found that the best case was the integration of the production chains compared to stand-alone production which results in an 86.56% reduction to the net system emission by achieving 0.043 kg CO2eq/kg biomass of GWP compared to petrochemical processing [235].
Moreover, bioethanol and LA production using 3G feedstocks exhibited a lower environmental impact than 2G feedstocks due to the lower amount of acid or alkaline required for the delignification process of LCBs [243]. The utilization of other sugars (galactose, mannose, and rhamnose) in the bioethanol and LA production instead of biodigesting it to produce biomethane may also minimize the environmental effect of the 3G integrated process and improve the techno-economic feasibility [33]. Mhatre et al. [244] revealed that 3G integration involving co-fermentation for all the reducing sugars and the inclusion of residues for bioethanol and LA production has the least environmental impact compared to other fermentation methods such as SHF and SSF. However, the economic analysis suggested that the combined processing of 3G bioethanol and LA process with the least environmental impact was the most expensive processing method [244]. Therefore, further studies should concentrate on the trade-off between the technical, economic, and environmental feasibility on the production process of 3G bioethanol and LA.
Challenges and future prospectives
Research on bioethanol and biochemical processing from macroalgae has been described as one of the sustainable and clean processes as a result of the high growth rate and yield of macroalgae. However, several challenges still exist to restrict 3G bioethanol and biochemical commercialization, such as biorefinery approaches and existing technology for biomass conversion [245]. In addition, most of the macroalgal bioethanol production is constrained to only laboratory scale; thereby, process feasibility at a continuous system is not reliable for large-scale commercial operation in the industrial setting [246]. Hence, the hydrolysis and fermentation steps have to be more optimized and refined for successful scaling up at larger quantities. Furthermore, implementation of engineered enzymes or enzyme cocktail, which is a mixture of various enzymes in the hydrolysis process, will be an alternate route for increasing the fermentable sugar content as it can optimize the hydrolysis of biomass [247]. Moreover, macroalgae competitiveness can be further increased by maximizing the extraction of all available high-value components through cascading biorefinery (proteins, lipids, pigments, ashes as fertilizer) [4]. Furthermore, macroalgae can also be considered the feedstock for fourth-generation (4G) bioethanol and LA, as 4G bioproducts are mainly generated by genetically modified macroalgae and yeast [248]. From an economic perspective, it can be deduced that the production cost of 3G bioethanol is still higher compared to fossil fuels [249]. The absence of an efficient and reliable established technology is known as the main challenge in commercializing macroalgae-based energy and fuels. Moreover, the current incoherent technologies have strongly reduced the investor’s interest in commercializing bioethanol due to the huge revenue uncertainty [250]. Nevertheless, researchers are still focused on the improvement of algal bioethanol technologies along with the increasing investments throughout the world [213]. Most of the research in bioethanol and the LA industry focused on the optimization of different factors (feedstocks, process parameters, biomass loading, and enzyme loading) to obtain better reproducible results [6, 51, 251].
In terms of biorefinery, the flexibility of process design should be maintained since the feedstock efficiency for 3G bioethanol and LA could change depending on the location and market. The process design of algal bioethanol and LA has to take into account biomass variation in geological distribution, cultivation techniques, growing and harvesting seasons, and cultivation parameters (temperature, pH, nutrients, etc.) on the account that it is a challenging task to copy the same scenario elsewhere. Consequently, the implementation of genetic engineering for the production of transgenic macroalgal strains is considered one of the best approaches to address the viability of 3G bioethanol and LA [244]. Furthermore, the production of macroalgae-based bioethanol and biochemical is also constrained by the shortage of water resources for algal cultivation. This is mainly due to bioethanol, and LA production using algae biomass may use large amounts of freshwater, which ranged between 40 and 1600 L per liter of products depending on the macroalgae biomass loading. For commercial-scale production, the consumption may reach billions of gallons of water, which is enormous [59]. Therefore, an integrated design of the water supply system is a promising option that can be done to avoid the shortage of water resources during the cultivation process. Cuevas-Castillo et al. [252] have reported that the recycle stream and evaporation control have to be equipped in the water system design to reduce the utility cost and the water will recirculate within the system to avoid the shortage of water resources.
Conclusion
Carbohydrate-rich macroalgae biomass has demonstrated tremendous potential for the production of bioethanol and LA in more sustainable, environmentally, and economically friendly manners. The application of biorefinery systems and integration processes such as bioethanol, LA, and biofertilizer lead to a cost-effective process. In the near future, the outlook of the bioethanol and LA market is continued growth to cater the energy and plastic demand coupled with the urge to curb the GHG footprint in both sectors. Currently, the investments in the macroalgae biorefinery are focused on using novel substrates and technologies with genetic engineering tools to enhance the microorganism performance and achieve a better conversion yield of bioproducts. It promises to be the most potential and attractive biorefinery model with more innovation in the near future. This review presents the basic parameters and state-of-art biorefinery processes that should be considered throughout the 3G bioethanol and LA production system, the perspective on novel cascading macroalgae biorefinery systems along with techno-economic evaluation, environmental impact, and challenges and future prospectives, as well as the most recent achievements of macroalgae biorefinery.
Acknowledgements
The authors would like to acknowledge Curtin University Malaysia for supporting this research through the Curtin Malaysia Postgraduate Research Scheme (CMPRS).
Funding
Financial supports were given by the Fundamental Research Grant Scheme (FRGS/1/2019/TK02/CURTIN/03/2 and FRGS/1/2018/TK10/CURTIN/03/2) from the Ministry of Higher Education (MOHE), Malaysia.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Highlights
• 3G bioethanol and lactic acid were prepared by different techniques.
• High carbohydrate content offers a potential pathway for bioproduct generation.
• Combined acid and enzymatic hydrolysis offer a high yield of reducing sugars.
• Proving fast production rate of 3G bioproducts for high cell density culture
• Cascading biorefinery resolves the production, economic, and environmental issues.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Benson NU, Bassey DE, Palanisami T. COVID pollution: impact of COVID-19 pandemic on global plastic waste footprint. Heliyon. 2021;7:e06343. doi: 10.1016/J.HELIYON.2021.E06343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tan IS, Lam MK, Foo HCY, et al. Advances of macroalgae biomass for the third generation of bioethanol production. Chinese J Chem Eng. 2020;28:502–517. doi: 10.1016/J.CJCHE.2019.05.012. [DOI] [Google Scholar]
- 3.Balina K, Romagnoli F, Blumberga D. Seaweed biorefinery concept for sustainable use of marine resources. Energy Procedia. 2017;128:504–511. doi: 10.1016/J.EGYPRO.2017.09.067. [DOI] [Google Scholar]
- 4.Cesário MT, da Fonseca MMR, Marques MM, de Almeida MCMD. Marine algal carbohydrates as carbon sources for the production of biochemicals and biomaterials. Biotechnol Adv. 2018;36:798–817. doi: 10.1016/J.BIOTECHADV.2018.02.006. [DOI] [PubMed] [Google Scholar]
- 5.Kim HM, Wi SG, Jung S, et al. Efficient approach for bioethanol production from red seaweed Gelidium amansii. Bioresour Technol. 2015;175:128–134. doi: 10.1016/J.BIORTECH.2014.10.050. [DOI] [PubMed] [Google Scholar]
- 6.Jambo SA, Abdulla R, Marbawi H, Gansau JA. Response surface optimization of bioethanol production from third generation feedstock - Eucheuma cottonii. Renew Energy. 2019;132:1–10. doi: 10.1016/J.RENENE.2018.07.133. [DOI] [Google Scholar]
- 7.Sirajunnisa AR, Surendhiran D. Algae – A quintessential and positive resource of bioethanol production: A comprehensive review. Renew Sustain Energy Rev. 2016;66:248–267. doi: 10.1016/J.RSER.2016.07.024. [DOI] [Google Scholar]
- 8.US Department of Energy (2021) Alternative Fuels Data Center: Maps and Data - Clean Cities Alternative Fuel Vehicle Inventory. https://afdc.energy.gov/data/10581. Accessed 3 Feb 2022
- 9.Kim SN, Choi BH, Kim HK, Bin CY. Poly(lactic-co-glycolic acid) microparticles in fibrin glue for local, sustained delivery of bupivacaine. J Ind Eng Chem. 2019;75:86–92. doi: 10.1016/J.JIEC.2019.02.028. [DOI] [Google Scholar]
- 10.Lee OK, Lee EY. Sustainable production of bioethanol from renewable brown algae biomass. Biomass Bioenerg. 2016;92:70–75. doi: 10.1016/J.BIOMBIOE.2016.03.038. [DOI] [Google Scholar]
- 11.Melikoglu M, Turkmen B. Food waste to energy: Forecasting Turkey’s bioethanol generation potential from wasted crops and cereals till 2030. Sustain Energy Technol Assessments. 2019;36:100553. doi: 10.1016/J.SETA.2019.100553. [DOI] [Google Scholar]
- 12.Choi S, Song CW, Shin JH, Lee SY. Biorefineries for the production of top building block chemicals and their derivatives. Metab Eng. 2015;28:223–239. doi: 10.1016/J.YMBEN.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 13.Darwin C-R, Charles W. Ethanol and lactic acid production from sugar and starch wastes by anaerobic acidification. Eng Life Sci. 2018;18:635–642. doi: 10.1002/ELSC.201700178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alves de Oliveira R, Komesu A, Vaz Rossell CE, Maciel Filho R. Challenges and opportunities in lactic acid bioprocess design—From economic to production aspects. Biochem Eng J. 2018;133:219–239. doi: 10.1016/J.BEJ.2018.03.003. [DOI] [Google Scholar]
- 15.European Bioplastics (2020) Global production capacities of bioplastics 2019–2025
- 16.Bátori V, Åkesson D, Zamani A, et al. Anaerobic degradation of bioplastics: A review. Waste Manag. 2018;80:406–413. doi: 10.1016/J.WASMAN.2018.09.040. [DOI] [PubMed] [Google Scholar]
- 17.Qi X, Ren Y, Wang X. New advances in the biodegradation of Poly(lactic) acid. Int Biodeterior Biodegradation. 2017;117:215–223. doi: 10.1016/J.IBIOD.2017.01.010. [DOI] [Google Scholar]
- 18.Cubas-Cano E, López-Gómez JP, González-Fernández C, et al. Towards sequential bioethanol and l-lactic acid co-generation: Improving xylose conversion to l-lactic acid in presence of lignocellulosic ethanol with an evolved Bacillus coagulans. Renew Energy. 2020;153:759–765. doi: 10.1016/J.RENENE.2020.02.066. [DOI] [Google Scholar]
- 19.Tan IS, Lee KT. Comparison of different process strategies for bioethanol production from Eucheuma cottonii: An economic study. Bioresour Technol. 2016;199:336–346. doi: 10.1016/J.BIORTECH.2015.08.008. [DOI] [PubMed] [Google Scholar]
- 20.Mohd Azhar SH, Abdulla R, Jambo SA, et al. Yeasts in sustainable bioethanol production: A review. Biochem Biophys Reports. 2017;10:52–61. doi: 10.1016/J.BBREP.2017.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alexandri M, Neu A-K, Schneider R, et al. Evaluation of various Bacillus coagulans isolates for the production of high purity L-lactic acid using defatted rice bran hydrolysates. Int J Food Sci Technol. 2019;54:1321–1329. doi: 10.1111/IJFS.14086. [DOI] [Google Scholar]
- 22.Saravanan K, Duraisamy S, Ramasamy G, et al. Evaluation of the saccharification and fermentation process of two different seaweeds for an ecofriendly bioethanol production. Biocatal Agric Biotechnol. 2018;14:444–449. doi: 10.1016/J.BCAB.2018.03.017. [DOI] [Google Scholar]
- 23.Maslova O, Stepanov N, Senko O, Efremenko E. Production of various organic acids from different renewable sources by immobilized cells in the regimes of separate hydrolysis and fermentation (SHF) and simultaneous saccharification and fermentation (SFF) Bioresour Technol. 2019;272:1–9. doi: 10.1016/J.BIORTECH.2018.09.143. [DOI] [PubMed] [Google Scholar]
- 24.da Silva ARG, Ortega CET, Rong BG. Effects of Bioethanol Pretreatments on the Broth Concentration and its Impacts in the Optimal Design of Product Separation and Purification Processes. Comput Aided Chem Eng. 2016;38:583–588. doi: 10.1016/B978-0-444-63428-3.50102-8. [DOI] [Google Scholar]
- 25.Daful AG, Haigh K, Vaskan P, Görgens JF. Environmental impact assessment of lignocellulosic lactic acid production: Integrated with existing sugar mills. Food Bioprod Process. 2016;99:58–70. doi: 10.1016/J.FBP.2016.04.005. [DOI] [Google Scholar]
- 26.Abudi ZN, Hu Z, Sun N, et al. Batch anaerobic co-digestion of OFMSW (organic fraction of municipal solid waste), TWAS (thickened waste activated sludge) and RS (rice straw): Influence of TWAS and RS pretreatment and mixing ratio. Energy. 2016;107:131–140. doi: 10.1016/J.ENERGY.2016.03.141. [DOI] [Google Scholar]
- 27.Gavahian M, Munekata PES, Eş I, et al. Emerging techniques in bioethanol production: from distillation to waste valorization. Green Chem. 2019;21:1171–1185. doi: 10.1039/C8GC02698J. [DOI] [Google Scholar]
- 28.Nazli RI. Evaluation of different sweet sorghum cultivars for bioethanol yield potential and bagasse combustion characteristics in a semiarid Mediterranean environment. Biomass Bioenerg. 2020;139:105624. doi: 10.1016/J.BIOMBIOE.2020.105624. [DOI] [Google Scholar]
- 29.Ayodele BV, Alsaffar MA, Mustapa SI. An overview of integration opportunities for sustainable bioethanol production from first- and second-generation sugar-based feedstocks. J Clean Prod. 2020;245:118857. doi: 10.1016/J.JCLEPRO.2019.118857. [DOI] [Google Scholar]
- 30.Hernández D, Riaño B, Coca M, García-González MC. Saccharification of carbohydrates in microalgal biomass by physical, chemical and enzymatic pre-treatments as a previous step for bioethanol production. Chem Eng J. 2015;262:939–945. doi: 10.1016/J.CEJ.2014.10.049. [DOI] [Google Scholar]
- 31.Zabed H, Sahu JN, Boyce AN, Faruq G. Fuel ethanol production from lignocellulosic biomass: An overview on feedstocks and technological approaches. Renew Sustain Energy Rev. 2016;66:751–774. doi: 10.1016/J.RSER.2016.08.038. [DOI] [Google Scholar]
- 32.Ma J, Shi S, Jia X, et al. Advances in catalytic conversion of lignocellulose to chemicals and liquid fuels. J Energy Chem. 2019;36:74–86. doi: 10.1016/J.JECHEM.2019.04.026. [DOI] [Google Scholar]
- 33.Ramachandra TV, Hebbale D. Bioethanol from macroalgae: Prospects and challenges. Renew Sustain Energy Rev. 2020;117:109479. doi: 10.1016/J.RSER.2019.109479. [DOI] [Google Scholar]
- 34.Chen S, Xu Z, Li X, et al. Integrated bioethanol production from mixtures of corn and corn stover. Bioresour Technol. 2018;258:18–25. doi: 10.1016/J.BIORTECH.2018.02.125. [DOI] [PubMed] [Google Scholar]
- 35.Mączyńska J, Krzywonos M, Kupczyk A, et al. Production and use of biofuels for transport in Poland and Brazil – The case of bioethanol. Fuel. 2019;241:989–996. doi: 10.1016/J.FUEL.2018.12.116. [DOI] [Google Scholar]
- 36.Zhang C, Su H, Baeyens J, Tan T. Reviewing the anaerobic digestion of food waste for biogas production. Renew Sustain Energy Rev. 2014;38:383–392. doi: 10.1016/J.RSER.2014.05.038. [DOI] [Google Scholar]
- 37.Renewable Fuels Association (2020) Focus Forward 2020 Ethanol Industry Outlook. https://ethanolrfa.org/wp-content/uploads/2020/02/2020-Outlook-Final-for-Website.pdf. Accessed 1 Sep 2021
- 38.Gómez-Monedero B, Pilar Ruiz M, Bimbela F, Faria J. Selective depolymerization of industrial lignin-containing stillage obtained from cellulosic bioethanol processing. Fuel Process Technol. 2018;173:165–172. doi: 10.1016/J.FUPROC.2018.01.021. [DOI] [Google Scholar]
- 39.Chen X, Che Q, Li S, et al. Recent developments in lignocellulosic biomass catalytic fast pyrolysis: Strategies for the optimization of bio-oil quality and yield. Fuel Process Technol. 2019;196:106180. doi: 10.1016/J.FUPROC.2019.106180. [DOI] [Google Scholar]
- 40.Kumar B, Bhardwaj N, Agrawal K, et al. Current perspective on pretreatment technologies using lignocellulosic biomass: An emerging biorefinery concept. Fuel Process Technol. 2020;199:106244. doi: 10.1016/J.FUPROC.2019.106244. [DOI] [Google Scholar]
- 41.Rajendran K, Drielak E, Sudarshan Varma V, et al. Updates on the pretreatment of lignocellulosic feedstocks for bioenergy production–a review. Biomass Convers Biorefinery. 2017;8:471–483. doi: 10.1007/S13399-017-0269-3. [DOI] [Google Scholar]
- 42.Komesu A, Maciel MRW, Filho RM (2017) Separation and purification technologies for lactic acid - A brief review. BioResources 12:6885–6901. 10.15376/BIORES.12.3.6885-6901
- 43.Hebbale D, Chandran MDS, Joshi NV, Ramachandra TV. Energy and Food Security from Macroalgae. J Biodivers. 2017;8:1–11. doi: 10.1080/09766901.2017.1351511. [DOI] [Google Scholar]
- 44.Fisheries and Aquaculture (FAO) (2019) Fisheries and Aquaculture Department - Yearbook of Fishery and Aquaculture Statistics - Aquaculture production. http://www.fao.org/fishery/static/Yearbook/YB2017_USBcard/navigation/index_content_aquaculture_e.htm. Accessed 1 Sep 2021
- 45.Gajaria TK, Suthar P, Baghel RS, et al. Integration of protein extraction with a stream of byproducts from marine macroalgae: A model forms the basis for marine bioeconomy. Bioresour Technol. 2017;243:867–873. doi: 10.1016/J.BIORTECH.2017.06.149. [DOI] [PubMed] [Google Scholar]
- 46.Mazur LP, Cechinel MAP, de Souza SMAGU, et al. Brown marine macroalgae as natural cation exchangers for toxic metal removal from industrial wastewaters: A review. J Environ Manage. 2018;223:215–253. doi: 10.1016/J.JENVMAN.2018.05.086. [DOI] [PubMed] [Google Scholar]
- 47.Gereniu CRN, Saravana PS, Chun BS. Recovery of carrageenan from Solomon Islands red seaweed using ionic liquid-assisted subcritical water extraction. Sep Purif Technol. 2018;196:309–317. doi: 10.1016/J.SEPPUR.2017.06.055. [DOI] [Google Scholar]
- 48.T Poespowati A Riyanto Hazlan, et al 2018 Enzymatic hydrolysis of liquid hot water pre-treated macro-alga (Ulva lactuca) for fermentable sugar production MATEC Web Conf 156 10.1051/MATECCONF/201815601015
- 49.Kostas ET, White DA, Cook DJ. Development of a bio-refinery process for the production of speciality chemical, biofuel and bioactive compounds from Laminaria digitata. Algal Res. 2017;28:211–219. doi: 10.1016/J.ALGAL.2017.10.022. [DOI] [Google Scholar]
- 50.Rajkumar R, Yaakob Z, Takriff MS (2014) Potential of the micro and macro algae for biofuel production: A brief review. BioResources 9:. 10.15376/BIORES.9.1.1606-1633
- 51.Shukla R, Kumar M, Chakraborty S, et al. Process development for the production of bioethanol from waste algal biomass of Gracilaria verrucosa. Bioresour Technol. 2016;220:584–589. doi: 10.1016/J.BIORTECH.2016.08.096. [DOI] [PubMed] [Google Scholar]
- 52.Tong KTX, Tan IS, Foo HCY, et al. Third-generation L-Lactic acid production by the microwave-assisted hydrolysis of red macroalgae Eucheuma denticulatum extract. Bioresour Technol. 2021;342:125880. doi: 10.1016/J.BIORTECH.2021.125880. [DOI] [PubMed] [Google Scholar]
- 53.Konda NVSNM, Singh S, Simmons BA, Klein-Marcuschamer D. An Investigation on the Economic Feasibility of Macroalgae as a Potential Feedstock for Biorefineries. BioEnergy Res. 2015;8:1046–1056. doi: 10.1007/S12155-015-9594-1. [DOI] [Google Scholar]
- 54.Dave N, Selvaraj R, Varadavenkatesan T, Vinayagam R. A critical review on production of bioethanol from macroalgal biomass. Algal Res. 2019;42:101606. doi: 10.1016/J.ALGAL.2019.101606. [DOI] [Google Scholar]
- 55.Aditiya HB, Mahlia TMI, Chong WT, et al. Second generation bioethanol production: A critical review. Renew Sustain Energy Rev. 2016;66:631–653. doi: 10.1016/J.RSER.2016.07.015. [DOI] [Google Scholar]
- 56.Kwon O-M, Kim S-K, Jeong G-T. Potential of phosphoric acid-catalyzed pretreatment and subsequent enzymatic hydrolysis for biosugar production from Gracilaria verrucosa. Bioprocess Biosyst Eng. 2016;39:1173–1180. doi: 10.1007/S00449-016-1593-X. [DOI] [PubMed] [Google Scholar]
- 57.Baghel RS, Reddy CRK, Jha B. Characterization of agarophytic seaweeds from the biorefinery context. Bioresour Technol. 2014;159:280–285. doi: 10.1016/J.BIORTECH.2014.02.083. [DOI] [PubMed] [Google Scholar]
- 58.Kulshreshtha G, Burlot A-S, Marty C, et al. Enzyme-Assisted Extraction of Bioactive Material from Chondrus crispus and Codium fragile and Its Effect on Herpes simplex Virus (HSV-1) Mar Drugs. 2015;13:558. doi: 10.3390/MD13010558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim DH, Lee SB, Jeong GT. Production of reducing sugar from Enteromorpha intestinalis by hydrothermal and enzymatic hydrolysis. Bioresour Technol. 2014;161:348–353. doi: 10.1016/J.BIORTECH.2014.03.078. [DOI] [PubMed] [Google Scholar]
- 60.A Bayu T Handayani 2018 High-value chemicals from marine macroalgae: Opportunities and challenges for marine-based bioenergy development IOP Conf Ser Earth Environ Sci 209 10.1088/1755-1315/209/1/012046
- 61.Sudhakar MP, Merlyn R, Arunkumar K, Perumal K. Characterization, pretreatment and saccharification of spent seaweed biomass for bioethanol production using baker’s yeast. Biomass Bioenerg. 2016;90:148–154. doi: 10.1016/J.BIOMBIOE.2016.03.031. [DOI] [Google Scholar]
- 62.Chai CY, Tan IS, Foo HCY, et al. Sustainable and green pretreatment strategy of Eucheuma denticulatum residues for third-generation l-lactic acid production. Bioresour Technol. 2021;330:124930. doi: 10.1016/J.BIORTECH.2021.124930. [DOI] [PubMed] [Google Scholar]
- 63.Lee SB, Kim JA, Lim HS. Metabolic pathway of 3,6-anhydro-D-galactose in carrageenan-degrading microorganisms. Appl Microbiol Biotechnol. 2016;100:4109–4121. doi: 10.1007/S00253-016-7346-6. [DOI] [PubMed] [Google Scholar]
- 64.Mohd Azhar SH, Abdulla R. Bioethanol production from galactose by immobilized wild-type Saccharomyces cerevisiae. Biocatal Agric Biotechnol. 2018;14:457–465. doi: 10.1016/J.BCAB.2018.04.013. [DOI] [Google Scholar]
- 65.McKim JM. Food additive carrageenan: Part I: A critical review of carrageenan in vitro studies, potential pitfalls, and implications for human health and safety. Crit Rev Toxicol. 2014;44:211–243. doi: 10.3109/10408444.2013.861797. [DOI] [PubMed] [Google Scholar]
- 66.Sasuga K, Yamanashi T, Nakayama S, et al. Optimization of yield and quality of agar polysaccharide isolated from the marine red macroalga Pyropia yezoensis. Algal Res. 2017;26:123–130. doi: 10.1016/J.ALGAL.2017.07.010. [DOI] [Google Scholar]
- 67.Xiao Q, Weng H, Ni H, et al. Physicochemical and gel properties of agar extracted by enzyme and enzyme-assisted methods. Food Hydrocoll. 2019;87:530–540. doi: 10.1016/J.FOODHYD.2018.08.041. [DOI] [Google Scholar]
- 68.Martínez-Sanz M, Gómez-Mascaraque LG, Ballester AR, et al. Production of unpurified agar-based extracts from red seaweed Gelidium sesquipedale by means of simplified extraction protocols. Algal Res. 2019;38:101420. doi: 10.1016/J.ALGAL.2019.101420. [DOI] [Google Scholar]
- 69.Catanzano O, D’Esposito V, Acierno S, et al. Alginate–hyaluronan composite hydrogels accelerate wound healing process. Carbohydr Polym. 2015;131:407–414. doi: 10.1016/J.CARBPOL.2015.05.081. [DOI] [PubMed] [Google Scholar]
- 70.Mazumder A, Holdt SL, De Francisci D, et al. Extraction of alginate from Sargassum muticum: process optimization and study of its functional activities. J Appl Phycol. 2016;28:3625–3634. doi: 10.1007/S10811-016-0872-X. [DOI] [Google Scholar]
- 71.Das AK, Sharma M, Mondal D, Prasad K. Deep eutectic solvents as efficient solvent system for the extraction of κ-carrageenan from Kappaphycus alvarezii. Carbohydr Polym. 2016;136:930–935. doi: 10.1016/J.CARBPOL.2015.09.114. [DOI] [PubMed] [Google Scholar]
- 72.Rafiquzzaman SM, Ahmed R, Lee JM, et al. Improved methods for isolation of carrageenan from Hypnea musciformis and its antioxidant activity. J Appl Phycol. 2016;28:1265–1274. doi: 10.1007/S10811-015-0605-6. [DOI] [Google Scholar]
- 73.Yang D, Yang H. The temperature dependent extraction of polysaccharides from eucheuma and the rheological synergistic effect in their mixtures with kappa carrageenan. LWT. 2020;129:109515. doi: 10.1016/J.LWT.2020.109515. [DOI] [Google Scholar]
- 74.Tran T, Truong H, Tran N, et al. Structure, conformation in aqueous solution and antimicrobial activity of ulvan extracted from green seaweed Ulva reticulata. Nat Prod Res. 2018;32:2291–2296. doi: 10.1080/14786419.2017.1408098. [DOI] [PubMed] [Google Scholar]
- 75.JJ Chuang YY Huang SH Lo et al 2017 Effects of pH on the Shape of Alginate Particles and Its Release Behavior Int J Polym Sci 10.1155/2017/3902704
- 76.M Garcia-Vaquero G Rajauria B Tiwari 2020 Conventional extraction techniques: Solvent extraction Sustain Seaweed Technol 171–189 10.1016/B978-0-12-817943-7.00006-8
- 77.M Garcia-Vaquero V Ummat B Tiwari G Rajauria 2020 Exploring Ultrasound, Microwave and Ultrasound-Microwave Assisted Extraction Technologies to Increase the Extraction of Bioactive Compounds and Antioxidants from Brown Macroalgae Mar Drugs 18 10.3390/MD18030172 [DOI] [PMC free article] [PubMed]
- 78.Gomez LP, Alvarez C, Zhao M, et al. Innovative processing strategies and technologies to obtain hydrocolloids from macroalgae for food applications. Carbohydr Polym. 2020;248:116784. doi: 10.1016/J.CARBPOL.2020.116784. [DOI] [PubMed] [Google Scholar]
- 79.Yuan Y, Macquarrie D. Microwave assisted extraction of sulfated polysaccharides (fucoidan) from Ascophyllum nodosum and its antioxidant activity. Carbohydr Polym. 2015;129:101–107. doi: 10.1016/J.CARBPOL.2015.04.057. [DOI] [PubMed] [Google Scholar]
- 80.Vázquez-Delfín E, Robledo D, Freile-Pelegrín Y. Microwave-assisted extraction of the Carrageenan from Hypnea musciformis (Cystocloniaceae, Rhodophyta) J Appl Phycol. 2014;26:901–907. doi: 10.1007/S10811-013-0090-8. [DOI] [Google Scholar]
- 81.B Le KS Golokhvast SH Yang S Sun 2019 Optimization of Microwave-Assisted Extraction of Polysaccharides from Ulva pertusa and Evaluation of Their Antioxidant Activity Antioxidants 8 10.3390/ANTIOX8050129 [DOI] [PMC free article] [PubMed]
- 82.M Garcia-Vaquero G Rajauria B Tiwari et al 2018 Extraction and Yield Optimisation of Fucose, Glucans and Associated Antioxidant Activities from Laminaria digitata by Applying Response Surface Methodology to High Intensity Ultrasound-Assisted Extraction Mar Drugs 16 10.3390/MD16080257 [DOI] [PMC free article] [PubMed]
- 83.Flórez-Fernández N, Domínguez H, Torres MD. A green approach for alginate extraction from Sargassum muticum brown seaweed using ultrasound-assisted technique. Int J Biol Macromol. 2019;124:451–459. doi: 10.1016/J.IJBIOMAC.2018.11.232. [DOI] [PubMed] [Google Scholar]
- 84.W Jiao W Chen Y Mei et al 2019 Effects of Molecular Weight and Guluronic Acid/Mannuronic Acid Ratio on the Rheological Behavior and Stabilizing Property of Sodium Alginate Molecules 24 10.3390/MOLECULES24234374 [DOI] [PMC free article] [PubMed]
- 85.A Pagarete AS Ramos P Puntervoll et al 2021 Antiviral Potential of Algal Metabolites—A Comprehensive Review Mar Drugs 19 10.3390/MD19020094 [DOI] [PMC free article] [PubMed]
- 86.Vásquez V, Martínez R, Bernal C. Enzyme-assisted extraction of proteins from the seaweeds Macrocystis pyrifera and Chondracanthus chamissoi: characterization of the extracts and their bioactive potential. J Appl Phycol. 2019;31:1999–2010. doi: 10.1007/S10811-018-1712-Y. [DOI] [Google Scholar]
- 87.Chen J, Zeng W, Gan J, et al. Physicochemical properties and anti-oxidation activities of ulvan from Ulva pertusa Kjellm. Algal Res. 2021;55:102269. doi: 10.1016/J.ALGAL.2021.102269. [DOI] [Google Scholar]
- 88.Hardouin K, Bedoux G, Burlot AS, et al. Enzyme-assisted extraction (EAE) for the production of antiviral and antioxidant extracts from the green seaweed Ulva armoricana (Ulvales, Ulvophyceae) Algal Res. 2016;16:233–239. doi: 10.1016/J.ALGAL.2016.03.013. [DOI] [Google Scholar]
- 89.Borazjani NJ, Tabarsa M, You SG, Rezaei M. Effects of extraction methods on molecular characteristics, antioxidant properties and immunomodulation of alginates from Sargassum angustifolium. Int J Biol Macromol. 2017;101:703–711. doi: 10.1016/J.IJBIOMAC.2017.03.128. [DOI] [PubMed] [Google Scholar]
- 90.Luo X, Duan Y, Yang W, et al. Structural elucidation and immunostimulatory activity of polysaccharide isolated by subcritical water extraction from Cordyceps militaris. Carbohydr Polym. 2017;157:794–802. doi: 10.1016/J.CARBPOL.2016.10.066. [DOI] [PubMed] [Google Scholar]
- 91.Alboofetileh M, Rezaei M, Tabarsa M, et al. Subcritical water extraction as an efficient technique to isolate biologically-active fucoidans from Nizamuddinia zanardinii. Int J Biol Macromol. 2019;128:244–253. doi: 10.1016/J.IJBIOMAC.2019.01.119. [DOI] [PubMed] [Google Scholar]
- 92.Youssouf L, Lallemand L, Giraud P, et al. Ultrasound-assisted extraction and structural characterization by NMR of alginates and carrageenans from seaweeds. Carbohydr Polym. 2017;166:55–63. doi: 10.1016/J.CARBPOL.2017.01.041. [DOI] [PubMed] [Google Scholar]
- 93.Abka Khajouei R, Keramat J, Hamdami N, et al. Effect of high voltage electrode discharge on the physicochemical characteristics of alginate extracted from an Iranian brown seaweed (Nizimuddinia zanardini) Algal Res. 2021;56:102326. doi: 10.1016/J.ALGAL.2021.102326. [DOI] [Google Scholar]
- 94.January GG, Naidoo RK, Kirby-McCullough B, Bauer R. Assessing methodologies for fucoidan extraction from South African brown algae. Algal Res. 2019;40:101517. doi: 10.1016/J.ALGAL.2019.101517. [DOI] [Google Scholar]
- 95.Madany MA, Abdel-Kareem MS, Al-Oufy AK, et al. The biopolymer ulvan from Ulva fasciata: Extraction towards nanofibers fabrication. Int J Biol Macromol. 2021;177:401–412. doi: 10.1016/J.IJBIOMAC.2021.02.047. [DOI] [PubMed] [Google Scholar]
- 96.Bayu A, Warsito MF, Putra MY, et al. Macroalgae-derived rare sugars: Applications and catalytic synthesis. Carbon Resour Convers. 2021;4:150–163. doi: 10.1016/J.CRCON.2021.04.002. [DOI] [Google Scholar]
- 97.Maurya DP, Singla A, Negi S (2015) An overview of key pretreatment processes for biological conversion of lignocellulosic biomass to bioethanol. 3 Biotech 5:597–609. 10.1007/S13205-015-0279-4 [DOI] [PMC free article] [PubMed]
- 98.Elgharbawy AA, Alam MZ, Moniruzzaman M, Goto M. Ionic liquid pretreatment as emerging approaches for enhanced enzymatic hydrolysis of lignocellulosic biomass. Biochem Eng J. 2016;109:252–267. doi: 10.1016/J.BEJ.2016.01.021. [DOI] [Google Scholar]
- 99.Venkateswar Rao L, Goli JK, Gentela J, Koti S. Bioconversion of lignocellulosic biomass to xylitol: An overview. Bioresour Technol. 2016;213:299–310. doi: 10.1016/J.BIORTECH.2016.04.092. [DOI] [PubMed] [Google Scholar]
- 100.Jeong GT, Kim SK, Park DH. Application of solid-acid catalyst and marine macro-algae Gracilaria verrucosa to production of fermentable sugars. Bioresour Technol. 2015;181:1–6. doi: 10.1016/J.BIORTECH.2015.01.038. [DOI] [PubMed] [Google Scholar]
- 101.Hamouda RA, Sherif SA, Dawoud GTM, Ghareeb MM. Enhancement of bioethanol production from Ulva fasciata by biological and chemical saccharification. Rend Lincei. 2016;27:665–672. doi: 10.1007/S12210-016-0546-2. [DOI] [Google Scholar]
- 102.Ra CH, Nguyen TH, Jeong GT, Kim SK. Evaluation of hyper thermal acid hydrolysis of Kappaphycus alvarezii for enhanced bioethanol production. Bioresour Technol. 2016;209:66–72. doi: 10.1016/J.BIORTECH.2016.02.106. [DOI] [PubMed] [Google Scholar]
- 103.El Harchi M, Fakihi Kachkach FZ, El Mtili N. Optimization of thermal acid hydrolysis for bioethanol production from Ulva rigida with yeast Pachysolen tannophilus. South African J Bot. 2018;115:161–169. doi: 10.1016/J.SAJB.2018.01.021. [DOI] [Google Scholar]
- 104.Hessami MJ, Phang S-M, Salleh A, Rabiei R. Evaluation of tropical seaweeds as feedstock for bioethanol production. Int J Environ Sci Technol. 2018;15:977–992. doi: 10.1007/S13762-017-1455-3. [DOI] [Google Scholar]
- 105.Hessami MJ, Cheng SF, Ambati RR, et al (2019) Bioethanol production from agarophyte red seaweed, Gelidium elegans, using a novel sample preparation method for analysing bioethanol content by gas chromatography. 3 Biotech 9:1–8. 10.1007/S13205-018-1549-8 [DOI] [PMC free article] [PubMed]
- 106.Sharma S, Horn SJ. Enzymatic saccharification of brown seaweed for production of fermentable sugars. Bioresour Technol. 2016;213:155–161. doi: 10.1016/J.BIORTECH.2016.02.090. [DOI] [PubMed] [Google Scholar]
- 107.Jmel MA, Ben Messaoud G, Marzouki MN, et al. Physico-chemical characterization and enzymatic functionalization of Enteromorpha sp. cellulose. Carbohydr Polym. 2016;135:274–279. doi: 10.1016/J.CARBPOL.2015.08.048. [DOI] [PubMed] [Google Scholar]
- 108.EL Rodrigues BC Fonseca VC Gelli et al 2019 Enzymatically and/or thermally treated Macroalgae biomass as feedstock for fermentative H2 production Matéria (Rio Janeiro) 24 10.1590/S1517-707620190002.0678
- 109.del Río PG, Domínguez E, Domínguez VD, et al. Third generation bioethanol from invasive macroalgae Sargassum muticum using autohydrolysis pretreatment as first step of a biorefinery. Renew Energy. 2019;141:728–735. doi: 10.1016/J.RENENE.2019.03.083. [DOI] [Google Scholar]
- 110.Gomes-Dias JS, Romaní A, Teixeira JA, Rocha CMR. Valorization of Seaweed Carbohydrates: Autohydrolysis as a Selective and Sustainable Pretreatment. ACS Sustain Chem Eng. 2020;8:17143–17153. doi: 10.1021/ACSSUSCHEMENG.0C05396. [DOI] [Google Scholar]
- 111.Ruangrit K, Chaipoot S, Phongphisutthinant R, et al. Environmental-friendly pretreatment and process optimization of macroalgal biomass for effective ethanol production as an alternative fuel using Saccharomyces cerevisiae. Biocatal Agric Biotechnol. 2021;31:101919. doi: 10.1016/J.BCAB.2021.101919. [DOI] [Google Scholar]
- 112.Charoensiddhi S, Franco C, Su P, Zhang W. Improved antioxidant activities of brown seaweed Ecklonia radiata extracts prepared by microwave-assisted enzymatic extraction. J Appl Phycol. 2015;27:2049–2058. doi: 10.1007/S10811-014-0476-2. [DOI] [Google Scholar]
- 113.Tsubaki S, Oono K, Hiraoka M, et al. Microwave-assisted hydrothermal extraction of sulfated polysaccharides from Ulva spp. and Monostroma latissimum. Food Chem. 2016;210:311–316. doi: 10.1016/J.FOODCHEM.2016.04.121. [DOI] [PubMed] [Google Scholar]
- 114.Lee JH, Kim HH, Ko JY, et al. Rapid preparation of functional polysaccharides from Pyropia yezoensis by microwave-assistant rapid enzyme digest system. Carbohydr Polym. 2016;153:512–517. doi: 10.1016/J.CARBPOL.2016.07.122. [DOI] [PubMed] [Google Scholar]
- 115.Teh YY, Lee KT, Chen WH, et al. Dilute sulfuric acid hydrolysis of red macroalgae Eucheuma denticulatum with microwave-assisted heating for biochar production and sugar recovery. Bioresour Technol. 2017;246:20–27. doi: 10.1016/J.BIORTECH.2017.07.101. [DOI] [PubMed] [Google Scholar]
- 116.Ravanal MC, Sharma S, Gimpel J, et al. The role of alginate lyases in the enzymatic saccharification of brown macroalgae, Macrocystis pyrifera and Saccharina latissima. Algal Res. 2017;26:287–293. doi: 10.1016/J.ALGAL.2017.08.012. [DOI] [Google Scholar]
- 117.Park M-R, Kim S-K. Jeong G-T (2018) Biosugar Production from Gracilaria verrucosa with Sulfamic Acid Pretreatment and Subsequent Enzymatic Hydrolysis. Biotechnol Bioprocess Eng. 2018;233(23):302–310. doi: 10.1007/S12257-018-0090-2. [DOI] [PubMed] [Google Scholar]
- 118.Cao L, Yu IKM, Cho DW, et al. Microwave-assisted low-temperature hydrothermal treatment of red seaweed (Gracilaria lemaneiformis) for production of levulinic acid and algae hydrochar. Bioresour Technol. 2019;273:251–258. doi: 10.1016/J.BIORTECH.2018.11.013. [DOI] [PubMed] [Google Scholar]
- 119.Abeln F, Fan J, Budarin VL, et al. Lipid production through the single-step microwave hydrolysis of macroalgae using the oleaginous yeast Metschnikowia pulcherrima. Algal Res. 2019;38:101411. doi: 10.1016/J.ALGAL.2019.101411. [DOI] [Google Scholar]
- 120.Onda A, Onda S, Koike M, et al. Catalytic Hydrolysis of Polysaccharides Derived from Fast-Growing Green Macroalgae. ChemCatChem. 2017;9:2638–2641. doi: 10.1002/CCTC.201700100. [DOI] [Google Scholar]
- 121.Meinita MDN, Marhaeni B, Winanto T, et al. Catalytic efficiency of sulfuric and hydrochloric acids for the hydrolysis of Gelidium latifolium (Gelidiales, Rhodophyta) in bioethanol production. J Ind Eng Chem. 2015;27:108–114. doi: 10.1016/J.JIEC.2014.12.024. [DOI] [Google Scholar]
- 122.Feldman D, Kowbel DJ, Glass NL, et al. (2015) Detoxification of 5-hydroxymethylfurfural by the Pleurotus ostreatus lignolytic enzymes aryl alcohol oxidase and dehydrogenase. Biotechnol Biofuels. 2015;81(8):1–11. doi: 10.1186/S13068-015-0244-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Shobana S, Kumar G, Bakonyi P, et al. A review on the biomass pretreatment and inhibitor removal methods as key-steps towards efficient macroalgae-based biohydrogen production. Bioresour Technol. 2017;244:1341–1348. doi: 10.1016/J.BIORTECH.2017.05.172. [DOI] [PubMed] [Google Scholar]
- 124.Ran H, Zhang J, Gao Q, et al. (2014) Analysis of biodegradation performance of furfural and 5-hydroxymethylfurfural by Amorphotheca resinae ZN1. Biotechnol Biofuels. 2014;71(7):1–12. doi: 10.1186/1754-6834-7-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ra CH, Jeong G-T, Kim S-K. Hyper-thermal acid hydrolysis and adsorption treatment of red seaweed, Gelidium amansii for butyric acid production with pH control. Bioprocess Biosyst Eng. 2017;40:403–411. doi: 10.1007/S00449-016-1708-4. [DOI] [PubMed] [Google Scholar]
- 126.Chae HR, Jang HJ, In YS, et al. Detoxification of Eucheuma spinosum Hydrolysates with Activated Carbon for Ethanol Production by the Salt-Tolerant Yeast Candida tropicalis. J Microbiol Biotechnol. 2015;25:856–862. doi: 10.4014/JMB.1409.09038. [DOI] [PubMed] [Google Scholar]
- 127.Yang CF, Huang CR. Biotransformation of 5-hydroxy-methylfurfural into 2,5-furan-dicarboxylic acid by bacterial isolate using thermal acid algal hydrolysate. Bioresour Technol. 2016;214:311–318. doi: 10.1016/J.BIORTECH.2016.04.122. [DOI] [PubMed] [Google Scholar]
- 128.Yang CF, Huang CR. Isolation of 5-hydroxymethylfurfural biotransforming bacteria to produce 2,5-furan dicarboxylic acid in algal acid hydrolysate. J Biosci Bioeng. 2018;125:407–412. doi: 10.1016/J.JBIOSC.2017.11.005. [DOI] [PubMed] [Google Scholar]
- 129.Hong Y, Wu YR. Acidolysis as a biorefinery approach to producing advanced bioenergy from macroalgal biomass: A state-of-the-art review. Bioresour Technol. 2020;318:124080. doi: 10.1016/J.BIORTECH.2020.124080. [DOI] [PubMed] [Google Scholar]
- 130.Jiménez Toro MJ, Dou X, Ajewole I, et al. (2017) Preparation and Optimization of Macroalgae-Derived Solid Acid Catalysts. Waste Biomass Valorization. 2017;104(10):805–816. doi: 10.1007/S12649-017-0101-0. [DOI] [Google Scholar]
- 131.Lei Y, Zhang M, Li Q, et al. A Porous Polymer-Based Solid Acid Catalyst with Excellent Amphiphilicity: An Active and Environmentally Friendly Catalyst for the Hydration of Alkynes. Polymers (Basel) 2019;11:2091. doi: 10.3390/POLYM11122091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ansanay Y, Kolar P, Sharma-Shivappa R, et al. Pre-treatment of biomasses using magnetised sulfonic acid catalysts. J Agric Eng. 2017;48:117–122. doi: 10.4081/JAE.2017.594. [DOI] [Google Scholar]
- 133.Thompson TM, Young BR, Baroutian S. Advances in the pretreatment of brown macroalgae for biogas production. Fuel Process Technol. 2019;195:106151. doi: 10.1016/J.FUPROC.2019.106151. [DOI] [Google Scholar]
- 134.Meinita MDN, Marhaeni B, Jeong G-T, Hong Y-K. Sequential acid and enzymatic hydrolysis of carrageenan solid waste for bioethanol production: a biorefinery approach. J Appl Phycol. 2019;31:2507–2515. doi: 10.1007/S10811-019-1755-8. [DOI] [Google Scholar]
- 135.Trivedi N, Reddy CRK, Lali AM. Marine Microbes as a Potential Source of Cellulolytic Enzymes. Adv Food Nutr Res. 2016;79:27–41. doi: 10.1016/BS.AFNR.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 136.Singh A, Rodríguez Jasso RM, Gonzalez-Gloria KD, et al. The enzyme biorefinery platform for advanced biofuels production. Bioresour Technol Reports. 2019;7:100257. doi: 10.1016/J.BITEB.2019.100257. [DOI] [Google Scholar]
- 137.S Jayasekara R Ratnayake 2019 Microbial Cellulases: An Overview and Applications Cellulose 10.5772/INTECHOPEN.84531
- 138.D Xue sheng, Zeng X, Lin D, Yao S, 2018 Ethanol tolerant endoglucanase from Aspergillus niger isolated from wine fermentation cellar Biocatal Agric Biotechnol 15 19 24 10.1016/J.BCAB.2018.04.016
- 139.Mandeep LH, Shukla P. Synthetic Biology and Biocomputational Approaches for Improving Microbial Endoglucanases toward Their Innovative Applications. ACS Omega. 2021;6:6055–6063. doi: 10.1021/ACSOMEGA.0C05744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Pathiraja D, Lee S, Choi I-G. Model-Based Complete Enzymatic Production of 3,6-Anhydro-l-galactose from Red Algal Biomass. J Agric Food Chem. 2018;66:6814–6821. doi: 10.1021/ACS.JAFC.8B01792. [DOI] [PubMed] [Google Scholar]
- 141.Zabed H, Sahu JN, Suely A, et al. Bioethanol production from renewable sources: Current perspectives and technological progress. Renew Sustain Energy Rev. 2017;71:475–501. doi: 10.1016/J.RSER.2016.12.076. [DOI] [Google Scholar]
- 142.DE Cervantes-Cisneros D Arguello-Esparza A Cabello-Galindo et al 2017 Hydrothermal Processes for Extraction of Macroalgae High Value-Added Compounds Hydrothermal Process Biorefineries Prod Bioethanol High Added-Value Compd Second Third Gener Biomass 461–481 10.1007/978-3-319-56457-9_20
- 143.Michalak I, Chojnacka K. Algae as production systems of bioactive compounds. Eng Life Sci. 2015;15:160–176. doi: 10.1002/ELSC.201400191. [DOI] [Google Scholar]
- 144.Sadhukhan J, Gadkari S, Martinez-Hernandez E, et al. Novel macroalgae (seaweed) biorefinery systems for integrated chemical, protein, salt, nutrient and mineral extractions and environmental protection by green synthesis and life cycle sustainability assessments. Green Chem. 2019;21:2635–2655. doi: 10.1039/C9GC00607A. [DOI] [Google Scholar]
- 145.del Río PG, Gomes-Dias JS, Rocha CMR, et al. Recent trends on seaweed fractionation for liquid biofuels production. Bioresour Technol. 2020;299:122613. doi: 10.1016/J.BIORTECH.2019.122613. [DOI] [PubMed] [Google Scholar]
- 146.Wang T, Zhai Y, Zhu Y, et al. A review of the hydrothermal carbonization of biomass waste for hydrochar formation: Process conditions, fundamentals, and physicochemical properties. Renew Sustain Energy Rev. 2018;90:223–247. doi: 10.1016/J.RSER.2018.03.071. [DOI] [Google Scholar]
- 147.S Maneein JJ Milledge BV Nielsen PJ Harvey (2018) A Review of Seaweed Pre-Treatment Methods for Enhanced Biofuel Production by Anaerobic Digestion or Fermentation. Ferment, 2018 Vol 4 Page 100 4 100 10.3390/FERMENTATION4040100
- 148.J Baruah BK Nath R Sharma et al 2018 Recent Trends in the Pretreatment of Lignocellulosic Biomass for Value-Added Products Front Energy Res 141 10.3389/FENRG.2018.00141
- 149.D Özçimen B Inan 2015 An Overview of Bioethanol Production From Algae Biofuels - Status Perspect 10.5772/59305
- 150.Kostas ET, Beneroso D, Robinson JP. The application of microwave heating in bioenergy: A review on the microwave pre-treatment and upgrading technologies for biomass. Renew Sustain Energy Rev. 2017;77:12–27. doi: 10.1016/J.RSER.2017.03.135. [DOI] [Google Scholar]
- 151.Yuan Y, Xu X, Jing C, et al. Microwave assisted hydrothermal extraction of polysaccharides from Ulva prolifera: Functional properties and bioactivities. Carbohydr Polym. 2018;181:902–910. doi: 10.1016/J.CARBPOL.2017.11.061. [DOI] [PubMed] [Google Scholar]
- 152.Ninomiya K, Yamauchi T, Ogino C, et al. Microwave pretreatment of lignocellulosic material in cholinium ionic liquid for efficient enzymatic saccharification. Biochem Eng J. 2014;90:90–95. doi: 10.1016/J.BEJ.2014.05.013. [DOI] [Google Scholar]
- 153.Li H, Qu Y, Yang Y, et al. Microwave irradiation – A green and efficient way to pretreat biomass. Bioresour Technol. 2016;199:34–41. doi: 10.1016/J.BIORTECH.2015.08.099. [DOI] [PubMed] [Google Scholar]
- 154.Boulho R, Marty C, Freile-Pelegrín Y, et al. Antiherpetic (HSV-1) activity of carrageenans from the red seaweed Solieria chordalis (Rhodophyta, Gigartinales) extracted by microwave-assisted extraction (MAE) J Appl Phycol. 2017;29:2219–2228. doi: 10.1007/S10811-017-1192-5. [DOI] [Google Scholar]
- 155.Kundu C, Lee JW. Optimization conditions for oxalic acid pretreatment of deacetylated yellow poplar for ethanol production. J Ind Eng Chem. 2015;32:298–304. doi: 10.1016/J.JIEC.2015.09.001. [DOI] [Google Scholar]
- 156.A Amini K Ohno ichiro, Maeda T, Kunitomo K, 2019 A kinetic comparison between microwave heating and conventional heating of FeS-CaO mixture during hydrogen-reduction Chem Eng J 374 648 657 10.1016/J.CEJ.2019.05.226
- 157.Dussán KJ, Silva DDV, Moraes EJC, et al. Dilute-acid Hydrolysis of Cellulose to Glucose from Sugarcane Bagasse. Chem Eng Trans. 2014;38:433–438. doi: 10.3303/CET1438073. [DOI] [Google Scholar]
- 158.Conesa C, Seguí L, Laguarda-Miró N, Fito P. Microwaves as a pretreatment for enhancing enzymatic hydrolysis of pineapple industrial waste for bioethanol production. Food Bioprod Process. 2016;100:203–213. doi: 10.1016/J.FBP.2016.07.001. [DOI] [Google Scholar]
- 159.Kim SW, Hong CH, Jeon SW, Shin HJ. High-yield production of biosugars from Gracilaria verrucosa by acid and enzymatic hydrolysis processes. Bioresour Technol. 2015;196:634–641. doi: 10.1016/J.BIORTECH.2015.08.016. [DOI] [PubMed] [Google Scholar]
- 160.Azizi N, Najafpour G, Younesi H. Acid pretreatment and enzymatic saccharification of brown seaweed for polyhydroxybutyrate (PHB) production using Cupriavidus necator. Int J Biol Macromol. 2017;101:1029–1040. doi: 10.1016/J.IJBIOMAC.2017.03.184. [DOI] [PubMed] [Google Scholar]
- 161.Kumar D, Juneja A, Singh V. Fermentation technology to improve productivity in dry grind corn process for bioethanol production. Fuel Process Technol. 2018;173:66–74. doi: 10.1016/J.FUPROC.2018.01.014. [DOI] [Google Scholar]
- 162.Chen Z, Ni D, Zhang W, et al. Lactic acid bacteria-derived α-glucans: From enzymatic synthesis to miscellaneous applications. Biotechnol Adv. 2021;47:107708. doi: 10.1016/J.BIOTECHADV.2021.107708. [DOI] [PubMed] [Google Scholar]
- 163.Selim KA, El-Ghwas DE, Easa SM, Hassan MIA. Bioethanol a Microbial Biofuel Metabolite; New Insights of Yeasts Metabolic Engineering. Fermentation. 2018;4:16. doi: 10.3390/FERMENTATION4010016. [DOI] [Google Scholar]
- 164.Perez-Samper G, Cerulus B, Jariani A, et al. The crabtree effect shapes the saccharomyces cerevisiae lag phase during the switch between different carbon sources. MBio. 2018;9:1–18. doi: 10.1128/MBIO.01331-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Majeed M, Nagabhushanam K, Natarajan S, et al. Evaluation of genetic and phenotypic consistency of Bacillus coagulans MTCC 5856: a commercial probiotic strain. World J Microbiol Biotechnol. 2016;32:60. doi: 10.1007/S11274-016-2027-2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.R Zhao R Zhao Y Tu et al 2018 A novel α-galactosidase from the thermophilic probiotic Bacillus coagulans with remarkable protease-resistance and high hydrolytic activity PLoS ONE 13 10.1371/JOURNAL.PONE.0197067 [DOI] [PMC free article] [PubMed]
- 167.Aulitto M, Fusco S, Bartolucci S, et al. Bacillus coagulans MA-13: a promising thermophilic and cellulolytic strain for the production of lactic acid from lignocellulosic hydrolysate. Biotechnol Biofuels. 2017;10:1–15. doi: 10.1186/S13068-017-0896-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Genzel Y, Vogel T, Buck J, et al. High cell density cultivations by alternating tangential flow (ATF) perfusion for influenza A virus production using suspension cells. Vaccine. 2014;32:2770–2781. doi: 10.1016/J.VACCINE.2014.02.016. [DOI] [PubMed] [Google Scholar]
- 169.Cao J, Yu Z, Liu W, et al. Probiotic characteristics of Bacillus coagulans and associated implications for human health and diseases. J Funct Foods. 2020;64:103643. doi: 10.1016/J.JFF.2019.103643. [DOI] [Google Scholar]
- 170.Laaziz SA, Raji M, Hilali E, et al. Bio-composites based on polylactic acid and argan nut shell: Production and properties. Int J Biol Macromol. 2017;104:30–42. doi: 10.1016/J.IJBIOMAC.2017.05.184. [DOI] [PubMed] [Google Scholar]
- 171.Rahmayetty WY, Sukirno, , et al. Use of Candida rugosa lipase as a biocatalyst for L-lactide ring-opening polymerization and polylactic acid production. Biocatal Agric Biotechnol. 2018;16:683–691. doi: 10.1016/J.BCAB.2018.09.015. [DOI] [Google Scholar]
- 172.Yu B, Cao Y, Sun H, Han J. The Structure and Properties of Biodegradable PLLA/PDLA for Melt-Blown Nonwovens. J Polym Environ. 2016;25:510–517. doi: 10.1007/S10924-016-0827-Y. [DOI] [Google Scholar]
- 173.M Pohanka 2020 D-Lactic Acid as a Metabolite: Toxicology, Diagnosis, and Detection Biomed Res Int 2020 10.1155/2020/3419034 [DOI] [PMC free article] [PubMed]
- 174.Ben Yahmed N, Jmel MA, Ben Alaya M, et al. A biorefinery concept using the green macroalgae Chaetomorpha linum for the coproduction of bioethanol and biogas. Energy Convers Manag. 2016;119:257–265. doi: 10.1016/J.ENCONMAN.2016.04.046. [DOI] [Google Scholar]
- 175.Adams JMM, Bleathman G, Thomas D, Gallagher JA. The effect of mechanical pre-processing and different drying methodologies on bioethanol production using the brown macroalga Laminaria digitata (Hudson) JV Lamouroux. J Appl Phycol. 2017;29:2463–2469. doi: 10.1007/S10811-016-1039-5. [DOI] [Google Scholar]
- 176.Loaces I, Schein S, Noya F. Ethanol production by Escherichia coli from Arundo donax biomass under SSF, SHF or CBP process configurations and in situ production of a multifunctional glucanase and xylanase. Bioresour Technol. 2017;224:307–313. doi: 10.1016/J.BIORTECH.2016.10.075. [DOI] [PubMed] [Google Scholar]
- 177.Nguyen TH, Ra CH, Sunwoo I, et al. Bioethanol production from Gracilaria verrucosa using Saccharomyces cerevisiae adapted to NaCl or galactose. Bioprocess Biosyst Eng. 2017;40:529–536. doi: 10.1007/S00449-016-1718-2. [DOI] [PubMed] [Google Scholar]
- 178.Rattanasaensri S, Nunraksa N, Muangmai N, et al. Ethanol production from Gracilaria fisheri using three marine epiphytic yeast species. J Appl Phycol. 2018;30:3311–3317. doi: 10.1007/S10811-018-1527-X. [DOI] [Google Scholar]
- 179.Fernandes-Klajn F, Romero-García JM, Díaz MJ, Castro E. Comparison of fermentation strategies for ethanol production from olive tree pruning biomass. Ind Crops Prod. 2018;122:98–106. doi: 10.1016/J.INDCROP.2018.05.063. [DOI] [Google Scholar]
- 180.Zhu J, Chen L, Gleisner R, Zhu JY. Co-production of bioethanol and furfural from poplar wood via low temperature (≤90 °C) acid hydrotropic fractionation (AHF) Fuel. 2019;254:115572. doi: 10.1016/J.FUEL.2019.05.155. [DOI] [Google Scholar]
- 181.Aparicio E, Rodríguez-Jasso RM, Pinales-Márquez CD, et al. High-pressure technology for Sargassum spp biomass pretreatment and fractionation in the third generation of bioethanol production. Bioresour Technol. 2021;329:124935. doi: 10.1016/J.BIORTECH.2021.124935. [DOI] [PubMed] [Google Scholar]
- 182.Ma K, Hu G, Pan L, et al. Highly efficient production of optically pure l-lactic acid from corn stover hydrolysate by thermophilic Bacillus coagulans. Bioresour Technol. 2016;219:114–122. doi: 10.1016/J.BIORTECH.2016.07.100. [DOI] [PubMed] [Google Scholar]
- 183.Cabrita ARJ, Maia MRG, Sousa-Pinto I, Fonseca AJM. Ensilage of seaweeds from an integrated multi-trophic aquaculture system. Algal Res. 2017;24:290–298. doi: 10.1016/J.ALGAL.2017.04.024. [DOI] [Google Scholar]
- 184.Glaser R, Venus J. Co-fermentation of the main sugar types from a beechwood organosolv hydrolysate by several strains of Bacillus coagulans results in effective lactic acid production. Biotechnol Reports. 2018;18:e00245. doi: 10.1016/J.BTRE.2018.E00245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Chen H, Huo W, Wang B, et al. L-lactic acid production by simultaneous saccharification and fermentation of dilute ethylediamine pre-treated rice straw. Ind Crops Prod. 2019;141:111749. doi: 10.1016/J.INDCROP.2019.111749. [DOI] [Google Scholar]
- 186.Alves de Oliveira R, Schneider R, Vaz Rossell CE, et al. Polymer grade l-lactic acid production from sugarcane bagasse hemicellulosic hydrolysate using Bacillus coagulans. Bioresour Technol Reports. 2019;6:26–31. doi: 10.1016/J.BITEB.2019.02.003. [DOI] [Google Scholar]
- 187.de la Torre I, Acedos MG, Ladero M, Santos VE. On the use of resting L. delbrueckii spp. delbrueckii cells for D-lactic acid production from orange peel wastes hydrolysates. Biochem Eng J. 2019;145:162–169. doi: 10.1016/J.BEJ.2019.02.012. [DOI] [Google Scholar]
- 188.Radosavljević M, Lević S, Belović M, et al. Encapsulation of Lactobacillus rhamnosus in Polyvinyl Alcohol for the production of L-(+)-Lactic Acid. Process Biochem. 2021;100:149–160. doi: 10.1016/J.PROCBIO.2020.10.006. [DOI] [Google Scholar]
- 189.Campos J, Bao J, Lidén G. Optically pure lactic acid production from softwood-derived mannose by Pediococcus acidilactici. J Biotechnol. 2021;335:1–8. doi: 10.1016/J.JBIOTEC.2021.06.007. [DOI] [PubMed] [Google Scholar]
- 190.Trivedi N, Baghel RS, Bothwell J, et al. An integrated process for the extraction of fuel and chemicals from marine macroalgal biomass. Sci Rep. 2016;6:1–8. doi: 10.1038/srep30728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Lin TH, Guo GL, Hwang WS, Huang SL. The addition of hydrolyzed rice straw in xylose fermentation by Pichia stipitis to increase bioethanol production at the pilot-scale. Biomass Bioenerg. 2016;91:204–209. doi: 10.1016/J.BIOMBIOE.2016.05.012. [DOI] [Google Scholar]
- 192.Baeyens J, Kang Q, Appels L, et al. Challenges and opportunities in improving the production of bio-ethanol. Prog Energy Combust Sci. 2015;47:60–88. doi: 10.1016/J.PECS.2014.10.003. [DOI] [Google Scholar]
- 193.Westman JO, Franzén CJ. Current progress in high cell density yeast bioprocesses for bioethanol production. Biotechnol J. 2015;10:1185–1195. doi: 10.1002/BIOT.201400581. [DOI] [PubMed] [Google Scholar]
- 194.MC Ravanal C Camus AH Buschmann et al 2019 Production of Bioethanol From Brown Algae Adv Feed Convers Technol Altern Fuels Bioprod New Technol Challenges Oppor 69–88 10.1016/B978-0-12-817937-6.00004-7
- 195.H-TV Lin M-Y Huang T-Y Kao (2020) Production of Lactic Acid from Seaweed Hydrolysates via Lactic Acid Bacteria Fermentation. Ferment, et al 2020 Vol 6 Page 37 6 37 10.3390/FERMENTATION6010037
- 196.Dahnum D, Tasum SO, Triwahyuni E, et al. Comparison of SHF and SSF Processes Using Enzyme and Dry Yeast for Optimization of Bioethanol Production from Empty Fruit Bunch. Energy Procedia. 2015;68:107–116. doi: 10.1016/J.EGYPRO.2015.03.238. [DOI] [Google Scholar]
- 197.Rastogi M, Shrivastava S (2018) Current Methodologies and Advances in Bio-ethanol Production. J Biotechnol Bioresearch 1:. 10.31031/JBB.2018.01.000505
- 198.Ahmad A, Banat F, Taher H. A review on the lactic acid fermentation from low-cost renewable materials: Recent developments and challenges. Environ Technol Innov. 2020;20:101138. doi: 10.1016/J.ETI.2020.101138. [DOI] [Google Scholar]
- 199.Yun EJ, Kim HT, Cho KM, et al. Pretreatment and saccharification of red macroalgae to produce fermentable sugars. Bioresour Technol. 2016;199:311–318. doi: 10.1016/J.BIORTECH.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 200.Wu Z-Z, Li D-Y, Cheng Y-S. Application of ensilage as a green approach for simultaneous preservation and pretreatment of macroalgae Ulva lactuca for fermentable sugar production. Clean Technol Environ Policy. 2018;20:2057–2065. doi: 10.1007/S10098-018-1574-7. [DOI] [Google Scholar]
- 201.Bleakley S, Hayes M. Algal Proteins: Extraction, Application, and Challenges Concerning Production. Foods. 2017;6:1–34. doi: 10.3390/FOODS6050033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Chisti Y. Biorefinery: Integrated Sustainable Processes for Biomass Conversion to Biomaterials, Biofuels, and Fertilizers. Biotechnol Adv. 2019;37:107464. doi: 10.1016/J.BIOTECHADV.2019.107464. [DOI] [Google Scholar]
- 203.Jeon W, Ban C, Park G, et al. Hydrothermal conversion of macroalgae-derived alginate to lactic acid catalyzed by metal oxides. Catal Sci Technol. 2016;6:1146–1156. doi: 10.1039/C5CY00966A. [DOI] [Google Scholar]
- 204.Alfonsín V, Maceiras R, Gutiérrez C. Bioethanol production from industrial algae waste. Waste Manag. 2019;87:791–797. doi: 10.1016/J.WASMAN.2019.03.019. [DOI] [PubMed] [Google Scholar]
- 205.Szambelan K, Nowak J, Szwengiel A, et al. Separate hydrolysis and fermentation and simultaneous saccharification and fermentation methods in bioethanol production and formation of volatile by-products from selected corn cultivars. Ind Crops Prod. 2018;118:355–361. doi: 10.1016/J.INDCROP.2018.03.059. [DOI] [Google Scholar]
- 206.Mithra MG, Jeeva ML, Sajeev MS, Padmaja G. Comparison of ethanol yield from pretreated lignocellulo-starch biomass under fed-batch SHF or SSF modes. Heliyon. 2018;4:e00885. doi: 10.1016/J.HELIYON.2018.E00885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Sharma P, Sharma N, Sharma N (2019) Exploration of Rhizoclonium sp. algae potential under different ethanol production strategies with SEM analysis of biomass and detoxification of hydrolysate. Life Sci J 16:. 10.7537/marslsj160619.12
- 208.Hakim A, Chasanah E, Uju U, Santoso J (2017) Bioethanol Production from Seaweed Processing Waste by Simultaneous Saccharification and Fermentation (SSF). Squalen Bull Mar Fish Postharvest Biotechnol 12:41. 10.15578/SQUALEN.V12I2.281
- 209.Lippi L, Bähr L, Wüstenberg A, et al. Exploring the potential of high-density cultivation of cyanobacteria for the production of cyanophycin. Algal Res. 2018;31:363–366. doi: 10.1016/J.ALGAL.2018.02.028. [DOI] [Google Scholar]
- 210.Tapia F, Vázquez-Ramírez D, Genzel Y, Reichl U. Bioreactors for high cell density and continuous multi-stage cultivations: options for process intensification in cell culture-based viral vaccine production. Appl Microbiol Biotechnol. 2016;100:2121–2132. doi: 10.1007/S00253-015-7267-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Bähr L, Wüstenberg A, Ehwald R. Two-tier vessel for photoautotrophic high-density cultures. J Appl Phycol. 2016;28:783–793. doi: 10.1007/S10811-015-0614-5. [DOI] [Google Scholar]
- 212.A Guljamow M Kreische K Ishida et al 2017 High-Density Cultivation of Terrestrial Nostoc Strains Leads to Reprogramming of Secondary Metabolome Appl Environ Microbiol 83 10.1128/AEM.01510-17 [DOI] [PMC free article] [PubMed]
- 213.Sayed W, Cabrol A, Abdallah R, et al. Enhancement of ethanol production from synthetic medium model of hydrolysate of macroalgae. Renew Energy. 2018;124:3–10. doi: 10.1016/J.RENENE.2017.10.094. [DOI] [Google Scholar]
- 214.Chemodanov A, Robin A, Golberg A. Design of marine macroalgae photobioreactor integrated into building to support seagriculture for biorefinery and bioeconomy. Bioresour Technol. 2017;241:1084–1093. doi: 10.1016/J.BIORTECH.2017.06.061. [DOI] [PubMed] [Google Scholar]
- 215.Osman AI, Abdelkader A, Farrell C, et al. Reusing, recycling and up-cycling of biomass: A review of practical and kinetic modelling approaches. Fuel Process Technol. 2019;192:179–202. doi: 10.1016/J.FUPROC.2019.04.026. [DOI] [Google Scholar]
- 216.GlobalPetrolPrices (2021) Ethanol prices around the world, 15-Aug-2021 | GlobalPetrolPrices.com. https://www.globalpetrolprices.com/ethanol_prices/. Accessed 17 Aug 2021
- 217.Pharmacompass (2021) Lactic Acid | API Reference Price . https://www.pharmacompass.com/active-pharmaceutical-ingredients/lactic-acid/api-price-information/api-reference-price. Accessed 17 Aug 2021
- 218.Ghayur A, Verheyen TV, Meuleman E. Techno-economic analysis of a succinic acid biorefinery coproducing acetic acid and dimethyl ether. J Clean Prod. 2019;230:1165–1175. doi: 10.1016/J.JCLEPRO.2019.05.180. [DOI] [Google Scholar]
- 219.Ghadiryanfar M, Rosentrater KA, Keyhani A, Omid M. A review of macroalgae production, with potential applications in biofuels and bioenergy. Renew Sustain Energy Rev. 2016;54:473–481. doi: 10.1016/J.RSER.2015.10.022. [DOI] [Google Scholar]
- 220.M Soleymani KA Rosentrater 2017 Techno-Economic Analysis of Biofuel Production from Macroalgae (Seaweed) Bioengineering 4 10.3390/BIOENGINEERING4040092 [DOI] [PMC free article] [PubMed]
- 221.Akila V, Manikandan A, Sahaya Sukeetha D, et al. Biogas and biofertilizer production of marine macroalgae: An effective anaerobic digestion of Ulva sp. Biocatal Agric Biotechnol. 2019;18:101035. doi: 10.1016/J.BCAB.2019.101035. [DOI] [Google Scholar]
- 222.Cardoso S, Carvalho L, Silva P, et al. Bioproducts from Seaweeds: A Review with Special Focus on the Iberian Peninsula. Curr Org Chem. 2014;18:896–917. doi: 10.2174/138527281807140515154116. [DOI] [Google Scholar]
- 223.Milledge JJ, Smith B, Dyer PW, Harvey P (2014) Macroalgae-Derived Biofuel: A Review of Methods of Energy Extraction from Seaweed Biomass. Energies 2014, Vol 7, Pages 7194–7222 7:7194–7222. 10.3390/EN7117194
- 224.Velazquez-Lucio J, Rodríguez-Jasso RM, Colla LM, et al (2018) Microalgal biomass pretreatment for bioethanol production: a review. Biofuel Res J 17:780–791. 10.18331/BRJ2018.5.1.5
- 225.Manhongo TT, Chimphango A, Thornley P, Röder M. An economic viability and environmental impact assessment of mango processing waste-based biorefineries for co-producing bioenergy and bioactive compounds. Renew Sustain Energy Rev. 2021;148:111216. doi: 10.1016/J.RSER.2021.111216. [DOI] [Google Scholar]
- 226.Tunå P, Hulteberg C. Woody biomass-based transportation fuels – A comparative techno-economic study. Fuel. 2014;117:1020–1026. doi: 10.1016/J.FUEL.2013.10.019. [DOI] [Google Scholar]
- 227.YN Barbot H Al-Ghaili R Benz 2016 A Review on the Valorization of Macroalgal Wastes for Biomethane Production Mar Drugs 14 10.3390/MD14060120 [DOI] [PMC free article] [PubMed]
- 228.Ullah K, Ahmad M, Sofia, , et al. Algal biomass as a global source of transport fuels: Overview and development perspectives. Prog Nat Sci Mater Int. 2014;24:329–339. doi: 10.1016/J.PNSC.2014.06.008. [DOI] [Google Scholar]
- 229.EIA USEIA (2021) Use of energy for transportation - U.S. Energy Information Administration (EIA). https://www.eia.gov/energyexplained/use-of-energy/transportation.php. Accessed 4 Jan 2022
- 230.Peng J, Xu H, Wang W, et al. Techno-economic analysis of bioethanol preparation process via deep eutectic solvent pretreatment. Ind Crops Prod. 2021;172:114036. doi: 10.1016/J.INDCROP.2021.114036. [DOI] [Google Scholar]
- 231.Hossain MS, Theodoropoulos C, Yousuf A. Techno-economic evaluation of heat integrated second generation bioethanol and furfural coproduction. Biochem Eng J. 2019;144:89–103. doi: 10.1016/J.BEJ.2019.01.017. [DOI] [Google Scholar]
- 232.Munagala M, Shastri Y, Nalawade K, et al. Life cycle and economic assessment of sugarcane bagasse valorization to lactic acid. Waste Manag. 2021;126:52–64. doi: 10.1016/J.WASMAN.2021.02.052. [DOI] [PubMed] [Google Scholar]
- 233.Marchesan AN, Leal Silva JF, Maciel Filho R, Wolf Maciel MR. Techno-Economic Analysis of Alternative Designs for Low-pH Lactic Acid Production. ACS Sustain Chem Eng. 2021;9:12120–12131. doi: 10.1021/ACSSUSCHEMENG.1C03447/SUPPL_FILE/SC1C03447_SI_001.PDF. [DOI] [Google Scholar]
- 234.Chong TY, Cheah SA, Ong CT, et al. Techno-economic evaluation of third-generation bioethanol production utilizing the macroalgae waste: A case study in Malaysia. Energy. 2020;210:118491. doi: 10.1016/J.ENERGY.2020.118491. [DOI] [Google Scholar]
- 235.Brigljević B, Liu JJ, Lim H (2019) Comprehensive feasibility assessment of a poly-generation process integrating fast pyrolysis of S. japonica and the Rankine cycle. Appl Energy 254:113704. 10.1016/J.APENERGY.2019.113704
- 236.Nazemi F, Karimi K, Denayer JFM, Shafiei M. Techno-economic aspects of different process approaches based on brown macroalgae feedstock: A step toward commercialization of seaweed-based biorefineries. Algal Res. 2021;58:102366. doi: 10.1016/J.ALGAL.2021.102366. [DOI] [Google Scholar]
- 237.Wong KH, Tan IS, Foo HCY, et al (2022) Third-generation bioethanol and L-lactic acid production from red macroalgae cellulosic residue: Prospects of Industry 5.0 algae. Energy Convers Manag 253:115155. 10.1016/J.ENCONMAN.2021.115155
- 238.KD González-Gloria RM Rodríguez-Jasso Shiva, et al 2021 Macroalgal biomass in terms of third-generation biorefinery concept: Current status and techno-economic analysis – A review Bioresour Technol Reports 16 100863 10.1016/J.BITEB.2021.100863
- 239.Seghetta M, Hou X, Bastianoni S, et al. Life cycle assessment of macroalgal biorefinery for the production of ethanol, proteins and fertilizers – A step towards a regenerative bioeconomy. J Clean Prod. 2016;137:1158–1169. doi: 10.1016/J.JCLEPRO.2016.07.195. [DOI] [Google Scholar]
- 240.Ghadge A, van der Werf S, Er Kara M, et al. Modelling the impact of climate change risk on bioethanol supply chains. Technol Forecast Soc Change. 2020;160:120227. doi: 10.1016/J.TECHFORE.2020.120227. [DOI] [Google Scholar]
- 241.Peteiro C, Sánchez N, Dueñas-Liaño C, Martínez B. Open-sea cultivation by transplanting young fronds of the kelp Saccharina latissima. J Appl Phycol. 2014;26:519–528. doi: 10.1007/S10811-013-0096-2. [DOI] [Google Scholar]
- 242.Zhang X, Border A, Goosen N, Thomsen M. Environmental life cycle assessment of cascade valorisation strategies of South African macroalga Ecklonia maxima using green extraction technologies. Algal Res. 2021;58:102348. doi: 10.1016/J.ALGAL.2021.102348. [DOI] [Google Scholar]
- 243.Rabemanolontsoa H, Saka S. Various pretreatments of lignocellulosics. Bioresour Technol. 2016;199:83–91. doi: 10.1016/J.BIORTECH.2015.08.029. [DOI] [PubMed] [Google Scholar]
- 244.Mhatre A, Gore S, Mhatre A, et al. Effect of multiple product extractions on bio-methane potential of marine macrophytic green alga Ulva lactuca. Renew Energy. 2019;132:742–751. doi: 10.1016/J.RENENE.2018.08.012. [DOI] [Google Scholar]
- 245.Zollmann M, Robin A, Prabhu M, et al. Green technology in green macroalgal biorefineries. Phycologia. 2019;58:516–534. doi: 10.1080/00318884.2019.1640516. [DOI] [Google Scholar]
- 246.Ocreto JB, Chen W-H, Ubando AT, et al. A critical review on second- and third-generation bioethanol production using microwaved-assisted heating (MAH) pretreatment. Renew Sustain Energy Rev. 2021;152:111679. doi: 10.1016/J.RSER.2021.111679. [DOI] [Google Scholar]
- 247.Rajak RC, Jacob S, Kim BS. A holistic zero waste biorefinery approach for macroalgal biomass utilization: A review. Sci Total Environ. 2020;716:137067. doi: 10.1016/J.SCITOTENV.2020.137067. [DOI] [PubMed] [Google Scholar]
- 248.B Abdullah SAF Syed Muhammad ad, Shokravi Z, et al 2019 Fourth generation biofuel: A review on risks and mitigation strategies Renew Sustain Energy Rev 107 37 50 10.1016/J.RSER.2019.02.018
- 249.Jambo SA, Abdulla R, Mohd Azhar SH, et al. A review on third generation bioethanol feedstock. Renew Sustain Energy Rev. 2016;65:756–769. doi: 10.1016/J.RSER.2016.07.064. [DOI] [Google Scholar]
- 250.Abinandan S, Shanthakumar S. Challenges and opportunities in application of microalgae (Chlorophyta) for wastewater treatment: A review. Renew Sustain Energy Rev. 2015;52:123–132. doi: 10.1016/J.RSER.2015.07.086. [DOI] [Google Scholar]
- 251.Das B, Roy AP, Bhattacharjee S, et al. Lactose hydrolysis by β-galactosidase enzyme: optimization using response surface methodology. Ecotoxicol Environ Saf. 2015;121:244–252. doi: 10.1016/J.ECOENV.2015.03.024. [DOI] [PubMed] [Google Scholar]
- 252.Cuevas-Castillo GA, Navarro-Pineda FS, Baz Rodríguez SA, Sacramento Rivero JC. Advances on the processing of microalgal biomass for energy-driven biorefineries. Renew Sustain Energy Rev. 2020;125:109606. doi: 10.1016/J.RSER.2019.109606. [DOI] [Google Scholar]