SUMMARY
The global pandemic of coronavirus disease 2019 (COVID-19) has disproportionately impacted global human health, economy, and security. Because of weaker health-care systems, existing comorbidities burden (HIV, malaria, tuberculosis, and non-communicable conditions), and poor socioeconomic determinants, initial predictive models had forecast a disastrous impact of COVID-19 in Africa in terms of transmission, severity, and deaths. Nonetheless, current epidemiological data seem not to have matched expectations, showing lower SARS-CoV-2 infection and fatality rates compared to Europe, the Americas and Asia. However, only few studies were conducted in low- and middle-income African settings where high poverty and limited access to health services worsen underlying health conditions, including endemic chronic infectious diseases such as HIV and tuberculosis. Furthermore, limited, and heterogeneous research was conducted to evaluate the indirect impact of the pandemic on general health services and on major diseases across African countries. International mitigation measures, such as resource reallocation, lockdowns, social restrictions, and fear from the population have had multi-sectoral impacts on various aspects of everyday life, that shaped the general health response. Despite the vast heterogeneity of data across African countries, available evidence suggests that the COVID-19 pandemic has severely impacted the control and prevention programs, the diagnosis capacity and the adherence to treatment of major infectious diseases (HIV, TB, and Malaria) - including neglected diseases - and non-communicable diseases. Future research and efforts are essential to deeply assess the medium- and long-term impact of the pandemic, and to implement tailored interventions to mitigate the standstill on decades of improvement on public health programs.
Keywords: COVID-19 impact, Africa, HIV/AIDS, tuberculosis, malaria, parasitic diseases, non-communicable diseases
INTRODUCTION
The global pandemic of coronavirus disease 2019 (COVID-19), caused by the severe acute respiratory syndrome coronavirus (SARS-CoV-2), has disproportionately impacted human health, economy, and security across the continents. Because of weaker health-care systems, existing comorbidities burden (HIV, tuberculosis, and noninfectious chronic conditions), and poor socioeconomic determinants, initial predictive models had forecast a disastrous impact of COVID-19 in Africa in terms of transmission, severity of disease and deaths [1–3]. Nonetheless, current epidemiological data seem not to have matched expectations, showing lower SARS-CoV-2 infection and fatality rates compared to Europe, the Americas and Asia [3].
Since the first African case of SARS-CoV-2 was notified by Egypt on 14 February 2020, the African continent has reported more than 10 million COVID-19 cases (3.2% of global cases) and 234,566 related deaths (4.2% of the worldwide deaths burden) as of 17 January 2022 despite more than 16% of the global population lives in Africa [4]. Deep heterogeneity in COVID-19 epidemic has been seen amongst different African countries, especially across sub-Saharan ones. South Africa has recorded the highest cumulative number of COVID-19 cases (3,559,230) followed by Morocco (1,048,653), Tunisia (788,012) and Ethiopia (457,322), together accounting for more than half of the whole confirmed COVID-19 cases in Africa [4]. Several hypotheses have been investigated to understand such varying COVID-19 epidemiology in Africa, including flawed capacity for large scale testing and reporting, weak surveillance and monitoring systems, younger age of population, cross-immunity from other human coronaviruses, climatic factors, low international air flows, as well as experience acquired from the public health response to other epidemics such as Ebola and Lassa fever [1, 3, 5]. Another crucial gap is that one year after the start of vaccination campaigns against COVID-19 around the world, a profound heterogeneity - and inequality - still characterizes the African continent. Alongside the 63% of Europeans who are fully vaccinated, only 10% of the African population is [6]. The vaccination coverage varies, ranging from more than 71% of the population fully vaccinated in Mauritius and 62.4% in Morocco to 0.17% in Democratic Republic of Congo. Despite reportedly being the country with the greatest burden of COVID-19 cases in the region, only 27.75% of the South Africa population is fully vaccinated. The only country on the continent not to have begun a vaccination program yet is Eritrea [7].
Since the beginning of the pandemic studies have identified older age, male sex, and underlying chronic non-communicable diseases as risk factors for COVID-19 severity and related death. However, only few studies were conducted in low- and middle-income African settings where high poverty and limited access to health services worsen underlying health conditions, including endemic chronic infectious diseases such as HIV and tuberculosis. Thus, the interactions between SARS-CoV-2, communicable and non-communicable diseases in shaping the prognosis of COVID-19 patients and the course of the pandemic on the African continent are still poorly understood [8]. Furthermore, limited, and heterogeneous research was conducted to evaluate the indirect impact of the pandemic on general health services and on other diseases. International mitigation measures, such as resource reallocation, lockdown, social restrictions, and fear from the population have had multi-sectoral impacts on various aspects of everyday life. After almost two years, the international community is trying to assess the indirect effects of the pandemic on major diseases across African countries. Here, we report the current major evidence on the main communicable and non-communicable diseases in the Continent.
Human Immunodeficiency Virus (HIV)
Current data on the association between HIV infection and adverse outcomes in COVID-19 have not been conclusive. A recent meta-analysis pointed to a potential greater risk of hospital admission for COVID-19 in people living with HIV (PLWH), with no clear evidence of increased risk of death or developing severe COVID-19 [9]. On the other hand, risk of COVID-19 mortality in PLWH may depend on the residence country [10]. Many studies from the U.S. and Europe have shown similar clinical outcomes with SARS-CoV-2 infection in PLWH than no-HIV infected people [11]. However, the great majority of the 38 million PLWH globally live in sub-Saharan Africa (26 million), may experience different comorbidities, hard socio-economic challenges, lower access to care, and antiretroviral therapy (ART) if compared to high-income settings [12].
It is largely known that the most affected African country by COVID-19 pandemic is South Africa, where approximately 7 million people (19.1% of population aged 15–49 years) are infected with HIV [4, 12]. A multi-site observational study conducted in the Western Cape Province, South Africa, found a two-fold risk of COVID-19 death adjusted for age and sex in PLWH regardless of viremia or immunosuppression [13]. This result might be explained by the high numbers of PLWH having uncontrolled diabetes and active tuberculosis. In another large South African cohort of 219,265 individuals admitted to hospital with COVID-19, HIV infection, as well as tuberculosis, was found to be associated with a moderately increased risk of COVID-19 in-hospital mortality, similarly to chronic non-communicable diseases like diabetes, chronic renal disease, and cancer [14]. Even though Africa recorded lower COVID-19 incidence and mortality compared to the West, the pandemic’s indirect effects on HIV services are concerning. The introduction of strict COVID-19 lockdown measures as well as the reallocation of public health funds and health-care personnel from HIV/AIDS care to COVID-19 control have led to disruptions in HIV prevention, testing and retention-in-care services resulting in drop of HIV diagnosis and ART initiation, in certain low-income settings [15–18]. In South Africa, the average weekly HIV viral load testing and CD4+ cell testing fell by 22% and 33%, respectively compared to pre-lockdown periods [17]. In addition, a pre-exposure prophylaxis (PrEP) program for pregnant women in Cape Town, South Africa, reported more than 2-fold higher odds of missed antenatal care visits without changes in sexual activity since the start of lockdown measures [19]. Also fear of acquiring SARS-CoV-2 infection and increased levels of food insecurity may have contributed to reducing access to HIV clinics and ART adherence through mechanisms such as depression and competing interests [20, 21]. A prospective cohort study among PLWH in the slum of Kibera (Nairobi, Kenya) reported shortage of food in the household as the predominant factor (38%) that affected the uptake of medications after the onset of pandemic [18].
Models suggested that substantial increases in HIV related mortality by up to 40% are expected in next years in sub-Saharan Africa due to severe interruption of ART [15]. A mathematical model by researchers from Cameroon and Benin estimated that, in the worst-case scenario, a 6-month interruption of ART initiation and 50% reduction in HIV prevention/treatment use, would disproportionately impact key populations (especially female sex workers) with an increase of HIV incidence (+50%) and related deaths (+20%) over one year [22]. Moreover, several reports have predicted increases in mother-to-child HIV transmission rates and HIV drug resistance [15].
Anyway, real life findings from many sub-Saharan cohorts seem to be reassuring. A multi-site cohort study carried out in 1059 health facilities located in 11 sub-Saharan countries (Angola, Burundi, Cote d’Ivoire, Democratic Republic of Congo, Eswatini, Ethiopia, Kenya, Mozambique, South Sudan, and Zambia) suggested a transient negative effect on HIV testing and ART initiation followed by rapid recovery since more stringent measures were over. No impact was noted on viral load suppression [23]. Similar transient trend was documented in three African countries (Uganda, Kenya, Nigeria) registering a temporary decrease in HIV clinic visit adherence early in the pandemic, and an increase in viral suppression later in the pandemic [24]. In this latter case, ART adherence was unaffected by COVID-19. Likewise, an observational large cohort study conducted in Kampala, Uganda, did not document negative effects of COVID-19 pandemic on ART adherence and viral suppression despite the reduction in clinic attendance [16]. Given the priority of HIV testing as primary entry point to care, it is advisable guaranteeing access to HIV diagnosis even under restrictive measures. Among possible interventions, scaling up of a home-based HIV self-testing has been proposed as a successful option [25]. The pandemic has enhanced the use of telemedicine worldwide, as a useful strategy to continue HIV retention-in-care reducing exposure to COVID-19 [17]. However, the utilization of telemedicine in sub-Saharan Africa is greatly affected by insufficient technological infrastructure, limited network coverage and lack of internet connectivity, especially in rural communities [26]. Several gaps in the interplay between COVID-19 and HIV deserve to be addressed urgently. In contexts of scarce resources, understanding of whether HIV is associated with increased mortality becomes of huge importance for prioritization of COVID-19 care and vaccination programs and for optimal allocation of public health interventions [9, 14].
Tuberculosis
Globally, before the COVID-19 pandemic, an estimated 10.0 million people fell ill with tuberculosis (TB) in 2019 [27]. Geographically, most people who developed TB lived in the WHO regions of South-East Asia (44%), Africa (25%) and the Western Pacific (18%), with smaller percentages in the Eastern Mediterranean (8.2%), the Americas (2.9%) and Europe (2.5%). Just eight countries accounted for two thirds of the global incidence, including Nigeria (4.4%), and South Africa (3.6%). In 2019, about 85% of TB deaths (both HIV-positive and HIV-negative people) occurred in the WHO African and South-East Asia regions [27]. The COVID-19 pandemic posed dramatic challenges to the capacity of the global healthcare system, affecting tuberculosis control both directly and indirectly. Disruptions of TB services has led to a large global drop in diagnosis of TB, which decreased by 18% between 2019 and 2020, from 7.1 million to 5.8 million [28]. Despite being considered as a high TB burden area, the WHO African Region contributed to the global reduction in TB notifications just for 2.5%, while South-East Asia and the Western Pacific Regions accounted for a cumulative 84%. The pandemic has also reversed the positive trend in reducing global TB deaths, with the first year-on-year increase (of 5.6%) since 2005. The global trend of decline in TB deaths until 2019 followed by an increase in 2020 was evident in four of the six WHO regions; the WHO African and Western Pacific regions still have a flat trend [28]. The substantial variation in TB data between 2019 and 2020 reflects both supply- and demand-side disruptions to TB diagnostic and treatment services. Examples of such disruptions include reduced health system capacity to continue to provide services, less willingness and ability to seek care in the context of lockdowns and associated restrictions on movement, concerns about the risks of going to health care facilities during a pandemic, and stigma associated with similarities in the symptoms related to TB and COVID-19 [28].
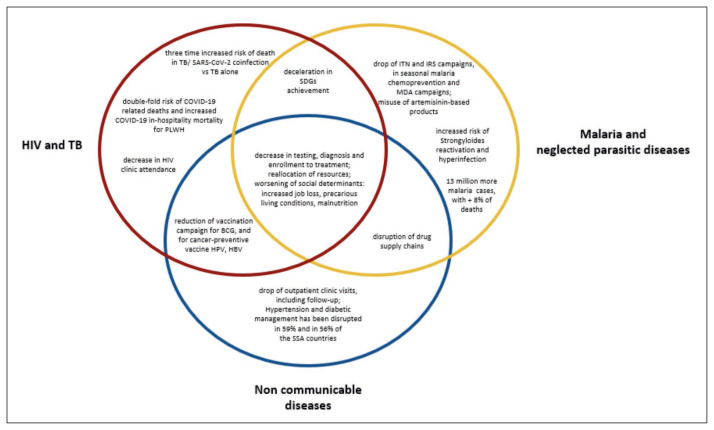
Figure 1.
impact of the COVID-19 of the COVID-19 pandemic on the major diseases in African countries.
Footnotes: HIV: human immunodeficiency virus; TB: tuberculosis; PLWH: people living with HIV; SDGs: sustainable development goals; BCG: Bacillus Calmette-Guérin; HPV: Human papilloma virus; HBV: viral hepatitis B; ITN: insecticide-treated net; IRS: indoor residual spraying; MDA: mass drug administration.
However, to really understand the impact that the pandemic has had on TB, you need to pull out a magnifying glass and observe how it has affected in heterogeneous settings for endemicity and health determinant factors. A review conducted in several countries (China, the Philippines, Nigeria, South Africa, Kenya, Malawi and Zimbabwe, Uganda, as well as Brazil and Vietnam), shows that testing for TB detection has decreased [29]. South Africa carries a disproportionate burden of TB and is listed as a priority country by the WHO. Since social restrictions have been implemented (16–27 March 2020) in response to the increase in COVID-19 cases, South Africa has seen a weekly reduction of 48% in TB Xpert testing and a decrease in TB positives by 33% [30]. The National Institute for Communicable Diseases affirmed that the decline in tests could not be explained by reduced testing capacity nor TB health service, as these were operational during the period. Rather, restrictions had limited patient mobility and thereby access to services, especially for people who did not have advanced TB [30]. Compared to the pre-pandemic period, during the COVID-19 period (March 2020-February 2021), Harare, Zimbabwe, experienced a decrease in people with presumptive pulmonary TB (40.6%), where the greater reduction was in children (71.3%), in bacteriologically confirmed diagnosis (30.1%), in enrollment for TB treatment (33.7%), in treatment success (11.6%) [31]. By adopting the same methodology, Mbithi I et al. found that in Nairobi, Kenya, COVID-19 pandemic resulted in a decrease in people with presumptive pulmonary TB (31.2%), active TB diagnoses (28.0%) and in those tested for HIV (50.5%). Interventions to overcome the setback were implemented in August 2020 and were associated with improvements in all parameters during the second half of 2020 [32]. In Lilongwe, Malawi, which did not have an official COVID-19 lockdown, COVID-19 was associated with a 45.6% decrease in people with presumptive pulmonary TB and 19.1% reduction in TB diagnosis. Blantyre, another city in Malawi, experienced a 35.9% reduction in TB notifications in April 2020 (where women and girls were the most affected), and further recovered to near pre-pandemic level by December 2020 [33]. The COVID-19 pandemic also negatively impacted TB care delivery in West Africa, where at the largest treatment center in Freetown, Sierra Leone, a 12.7% decrease in referrals for TB testing was registered. Unexpectedly, treatment success remarkably increased in 2020, for which anti-TB drugs self-administration were identified as independently predictors of success, a finding that may have policy implications for TB control. The high financial costs and the logistical and human resource implications of the Directly Observed Treatment (DOT) may not be an optimal response, especially during public health emergencies requiring social distancing measures and with restrictions on movements, such as the previous Ebola epidemic and the current COVID-19 pandemic [34]. Alongside, Nigeria experienced a progressive and significant decline in clinic attendance (63%), presumptive TB identification (64%), TB cases detection (73%) and treatment initiation (72%) during March–April 2020 [35]. COVID-19 also has a direct impact on TB patients, as data emerging from India and South Africa shows that people coinfected with TB and SARS-CoV-2 have three times higher mortality than people infected with TB alone, as reported by Stop TB Partnership. The pandemic has therefore posed unprecedented challenges in the global fight against TB, reducing progress and making people more vulnerable to the disease in the future. Disruptions of TB services could increase TB cases and deaths over the next 5 years, resulting in an additional 24,700 (16,100–44,700) TB cases and 12,500 deaths (8.8–17.8 thousand) in Kenya, as projected by a mathematical model [36]. A core factor to consider is that COVID-19 has put an unprecedented strain on immunization campaigns, including those for the TB-preventive Bacille-Calmette Guérin (BCG) vaccine. A decrease of more than 10% in routine vaccine doses provided in six African countries has been reported, due to pandemic-related gaps [37]. In particular, in rural Sierra Leone a devastating reduction of 53% in BCG vaccinations for children under five years of age, in a two-month period in 2020 compared to the same period in 2019 [38]. The pandemic has involved not only health services, but also the economic and social sphere. The well-known social and economic determinants of TB, such as undernutrition, could be triggered by loss of income and the shutdown of school food programs during lockdowns and school closures [39].
Malaria and neglected parasitic diseases
Malaria remains one of the major threats to public health in Africa. According to the WHO, 229 million cases of malaria occurred worldwide in 2019, of which 215 million (94.7%) in sub-Saharan Africa, mostly in Nigeria and the Democratic Republic of Congo. About 50% of global malaria deaths in 2019 were in Nigeria (23%), the Democratic Republic of the Congo (11%), the United Republic of Tanzania (5%), Mozambique (4%), Niger (4%) and Burkina Faso (4%). Substantial progress had been made since 2000 to reduce the global burden of malaria in low-income countries, with funded interventions aimed at reduction of transmission and early treatment, which determined a decrease of malaria-attributable deaths over the years. However, given that a large portion of malaria endemic countries depends on international economic aid, experts have suggested that funds for malaria control may be affected by resource reallocation to fight COVID-19, and this could have jeopardized resources for malaria efforts in the low-income regions [40]. Furthermore, with lockdowns going on in many countries, the pharmaceutical and medical supply chains were cut short, leaving African countries with declining supplies. Several countries in sub-Saharan Africa had deferred the insecticide-treated-mosquito net (ITNs) and indoor residual spraying (IRS) campaigns during the COVID-19 pandemic. In Nigeria, only 11% of the 22.7 million mosquito nets envisaged in the distribution plan had been delivered in the first 6 months of the pandemic [41]. Between 30–40% of malaria endemic countries reported some level of disruption to malaria diagnostic and treatment services (39% of 59 countries), ITNs distribution through mass campaigns (39% of 49 countries), IRS campaigns (33% of 43 countries) and seasonal malaria chemoprevention campaigns (30% of 10 countries) [42]. COVID-19 has also directly impacted malaria health services. A survey by The Global Fund across 22 African countries shows a 17% decrease in malaria diagnosis and a 15% decrease in malaria treatment, during April – September 2020. In addition, 21% of facilities in Africa were stocked out of the antimalarial medicine dosage for children under 5 years of age [43]. On the other hand, a study performed in Zimbabwe shows that during the first six months of 2020, an excess of over 30,000 malaria cases were reported compared to the same period in 2017, 2018 and 2019, as well as the number of malaria deaths recorded in this period exceeded the total annual for 2018 and 2019. It is likely that several factors could explain the observed increase in malaria morbidity and mortality, including COVID-19-related disruptions in the malaria prevention and control activities, changes of health-seeking behavior, and possible variation in case management. Furthermore, given the partial overlapping symptoms between COVID-19 and malaria, individuals with fever, headache, and body aches, who normally would not sought health care, may have promptly sought care due to increased awareness and vigilance [44].
Furthermore, many rapid test-manufacturing companies repurposed their production to supply COVID tests, thus causing a shortage in anti-malarial tests [40]. This tendency can lead to the infeasibility to diagnose malaria and therefore to empirically use anti-malarial drugs at each febrile appearance, contributing to the development of resistance. An indirect influence that the pandemic has had on malaria control is the false belief that artemisinin-based products could be used to minimize the effects of COVID-19. Indeed, it has been reported that Madagascar suggested the beneficial use of an Artemisinin (ACT)-plant based extract to fight SARS-CoV-2 [40]. The potential hazard of misusing one of the most effective drugs against malaria could lead to the decrease in the availability of ACT-based drugs, and the worrying risk of development of ACT resistant plasmodia. Alongside, also other anti-malarial drugs such as chloroquine and hydroxychloroquine have been widely used to treat COVID-19 patients, resulting in global shortage of anti-malarial drugs [45]. The WHO 2021 malaria report shows that in Africa in 2020 there were 13 million more cases than in 2019, with an increase of 8% in the number of deaths. These data show how 20 years of progress can rapidly reverse in only one year, and how costly it is in human lives to de-prioritize malaria in Africa. A study using a geostatistical model, estimates that under pessimistic scenarios, COVID-19-related disruption in malaria control could have double malaria mortality in 2020 in Africa, and potentially lead to even greater increases in further years [46].
Along with malaria, other parasitic infections have suffered from a setback due to the pandemic. Preserving the tradition of the name “neglected”, neglected tropical diseases (NTDs) services have been found to be among the most frequently affected by COVID-19 [42]. Disruption of community-based interventions, such as mass treatment-preventive chemotherapy is one of the mechanisms through which the COVID-19 has most affected NTD health services. Indeed, during the earlier phase of the pandemic, WHO advised that NTD surveys, active case detection activities and mass drug administration (MDA) campaigns had to be postponed [47]. A WHO global survey, as of early 2021, indicated that alterations of NTDs services occurred in 44% of the countries (48/109), of which 60% reported disruptions of large-scale preventive chemotherapy campaigns. Moreover, the proportion of countries reporting severe disruptions of NTD activities was the highest among all health services (19%) [42]. However, by December 2020, 11 West African countries (Benin, Burkina Faso, Cameroon, Cote d’Ivoire, Ghana, Guinea, Mali, Niger, Senegal, Sierra Leone, and Togo) had restarted the MDA and the community-based surveys to monitor NTD prevalence [48]. Among all NTDs, Strongyloides stercoralis infection deserves a special mention due to clinical implications in COVID-19 patients. The use of immunomodulatory drugs such as corticosteroids and biological drugs in case of COVID-19 pneumonia increases the risk of Strongyloides reactivation and hyperinfection, resulting in a potentially fatal outcome [49]. In this context, empiric treatment may be considered in endemic areas if steroids are used [50]. In Amhara, Ethiopia the referral leprosy and skin NTDs center was closed during the first months of the pandemic to serve as a dedicated COVID-19 treatment hospital. This was at the expense of thousands of patients who needed care. In addition, several leprosy rehabilitation services were closed [51]. Considering that Africa contributes to 39% of the 1.5 billion of the world population affected by NTDs, causing an estimated 200,000 deaths per year in the continent, as suggested by a model-based analysis of the WHO, the main public-health consequences of these disruptions will be an increased burden of NTDs, in terms of both mortality and morbidity [52].
Non-communicable diseases
Non-communicable diseases (NCDs) in Africa have risen significantly in the last 20 years, influenced by broader demographic, socioeconomic, cultural, and behavioral changes, as an increased exposure to risk factors. In sub-Saharan Africa, cardiovascular diseases went from being the sixth cause of death in 1990 (8.2% of total deaths) to the second in 2019 (13.1% of total deaths) as reported by the Global Burden of Disease. The continent is experiencing its epidemiological transition, as estimates predict that NCDs will become Africa’s first cause of mortality by 2030 [53]. Around 22% of the monthly per capita household expenditure is dedicated to chronic non-communicable diseases in rural Malawi. Alongside, one third of the average monthly income is spent on diabetes and hypertension cure in Uganda [53].
In the context of sub-Saharan Africa, with limited resources and weak health-care systems, ensuring prevention, early diagnosis, and appropriate care for patients with NCD is extremely challenging. The pandemic has therefore exacerbated the already pre-existing vulnerabilities. Moreover, considering that evidence has clearly demonstrated that NCDs, especially cardiovascular diseases, obesity, and type 2 diabetes, are risk factors for severe COVID-19 and death, the risk of disruption of NCDs control systems is even more threatening. In a preliminary WHO survey of 41 countries in sub-Saharan Africa, 22% of countries reported that only emergency inpatient care for chronic conditions is available, while 37% of countries reported that outpatient care is limited. Hypertension management has been disrupted in 59% of the countries, while diabetic complications management has been disrupted in 56% of the countries [54]. A study in two primary care sites in the Cape Town Metro, South Africa, has highlighted the impact of COVID-19 on NCD services disruption showing a sharp decline in the number of HbA1c tests performed (up to −59%) in March–April 2020 compared with 2019, and the proportion of patients with uncontrolled diabetes was higher (up to +11%) [55].
As of June 2020, a significant reduction in immunization campaigns was reported in 89% of WHO African region countries, including cancer-preventive vaccination against Hepatitis B Virus and Human papillomavirus (HPV) [56]. Given that the supply of drugs and other sanitary materials in most sub-Saharan African countries depends almost exclusively on international production and import, delays in trade due to international lockdowns and the reallocation of resources, including pharmaceuticals, could make drugs’ availability even more difficult, especially in semi-urban and rural contexts. In Rwanda, with the emergence of COVID-19, the drug supply system was interrupted leaving both local pharmacies and the big medical stores running out of stock [57]. More than half (59.6%) of respondents reported having difficulties accessing medicinal drugs, as shown by a cross-sectional online survey in Zimbabwe [58]. Furthermore, the indictment of lockdowns, or even the fear of patients to contract SARS-CoV-2, disrupted the planning of periodic follow-up visits and distribution of medicines, or delayed first diagnoses, leading to the development of advanced-stage NCDs, with an increased risk of severe complications, including severe COVID19. The shutdown of public transport in addition to patients’ fear led to a relevant decrease in the number of consultations for breast cancer in Tunisia, from 2,701 in May 2019 to 945 in May 2020. Similarly, a 40% decrease in new outpatient cases was observed in an oncology center in Morocco [59]. During periods of constrained mobility and closure of services, worldwide evidence has shown the strategic role of technology in health, such as tele-health and tele monitoring, when used appropriately. However, it is even more challenging to apply this solution in contexts with scarce resources, where not all the population has easy access to telephony or the internet [53]. Lockdown measures could also have consequences themselves, by increasing job loss, food prices and precarious living conditions. These factors can affect the lifestyle, favoring unhealthy diets, sedentariness, and increased stress with a deterioration of pre-existing chronic conditions and onset of new NCDs and psychological or psychiatric disorders [58].
CONCLUSIONS
In spite of the vast heterogeneity of data across African countries, available evidence suggests that the COVID-19 pandemic has severely impacted the control and prevention programs (ITNs and IRS delivery, vaccination and MDA campaigns), the diagnosis capacity and the adherence to treatment of major infectious diseases - including neglected parasites - and non-communicable diseases.
It is largely known that COVID-19 hampers global inequity, even more in constrained economies and in vulnerable population groups. One of the major concerns regards the deep disparity in SARS-CoV-2 vaccination coverage in comparison to high-income economies and within African countries. Furthermore, the ongoing epidemiological transition in the Continent, has led to the increase of patients with chronic conditions, such as diabetes and cardiovascular diseases, which have already been impacted by the pandemic, but which are not even protected by the COVID-19 vaccine. This highlights the importance of strengthening the vaccination campaign, not just to prevent overwhelming a weak and burdened health care system, but to guarantee the universal right to health.
Future research and efforts are essential to deeply assess the medium- and long-term impact of the pandemic, and to implement tailored interventions to mitigate the standstill on decades of improvement on public health programs.
Footnotes
Conflict of interest
The Authors declare that they have no conflicts of interest with regard to the topics discussed in this manuscript.
The authors are responsible for the choice and presentation of views contained in this article and for opinions expressed therein, which are not necessarily those of UNESCO and do not commit the Organizations.
Funding statement
The manuscript was prepared without any external funding support.
REFERENCES
- 1.Bouba Y, Tsinda EK, Fonkou MDM, Mmbando GS, Bragazzi NL, Kong JD. The determinants of the low COVID-19 transmission and mortality rates in Africa: a cross-country analysis. Front Public Health. 2021;9:751197. doi: 10.3389/fpubh.2021.751197.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tessema SK, Nkengasong JN. Understanding COVID-19 in Africa. Nat Rev Immunol. 2021;21:469–70. doi: 10.1038/s41577-021-00579-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Okonji EF, Okonji OC, Mukumbang FC, et al. Understanding varying COVID-19 mortality rates reported in Africa compared to Europe, Americas and Asia. Trop Med Int Heal. 2021;26(7):716–9. doi: 10.1111/tmi.13575.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.African Center for Disease Control: Coronavirus Disease (COVID-19) AfricaCDC_COVIDBrief_18Jan22_ EN.pdf. [[accessed 30 January 2022]]. Available at: https://africacdc.org/download/outbreak-brief-105-coronavirus-disease-2019-covid-19-pandemic/
- 5.Snyman J, Sanders EJ, Ndung’u T. COVID-19 in Africa: preexisting immunity and HIV. AIDS. 2021;35(14):2391–3. doi: 10.1097/QAD.0000000000003079.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ritchie H, Mathieu E, Rodés-Guirao L, et al. Coronavirus Pandemic (COVID-19) [Last access January 28, 2022]. Published online at Our-WorldInData.org. Retrieved from: ‘ https://ourworld-indata.org/coronavirus’ [Online Resource]
- 7.Africa Center for Disease Control. COVID-19 Vaccination Latest updates from Africa CDC on progress made in COVID-19 vaccinations on the continent. [Last access January 28, 2022]. Available at https://africacdc.org/covid-19-vaccination/
- 8.Anjorin AA, Abioye AI, Asowata OE, et al. Comorbidities and the COVID-19 pandemic dynamics in Africa. Trop Med Int Health. 2021;26(1):2–13. doi: 10.1111/tmi.13504.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Danwang C, Noubiap JJ, Robert A, et al. Outcomes of patients with HIV and COVID-19 co-infection: a systematic review and meta-analysis. AIDS Res Ther. 2022;19(1):3. doi: 10.1186/s12981-021-00427-y. Published 2022 Jan 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dong Y, Li Z, Ding S, et al. HIV infection and risk of COVID-19 mortality: a meta-analysis. Medicine. 2021;100(26):e26573. doi: 10.1097/MD.0000000000026573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown LB, Spinelli MA, Gandhi M. The interplay between HIV and COVID-19: summary of the data and responses to date. Curr Opin HIV AIDS. 2021;16(1):63–73. doi: 10.1097/COH.0000000000000659.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Joint United Nations Programme on HIV/AIDS (UN-AIDS) UNAIDS DATA 2020. [[accessed 30 January 2022]]. Available at: https://www.unaids.org/sites/default/files/media_asset/2020_aids-data-book_en.pdf.
- 13.Western Cape Department of Health in collaboration with the National Institute for Communicable Diseases, South Africa. Risk Factors for Coronavirus Disease 2019 (COVID-19) Death in a Population Cohort Study from the Western Cape Province, South Africa. Clin Infect Dis. 2021;73(7):e2005–e2015. doi: 10.1093/cid/ciaa1198.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jassat W, Cohen C, Tempia S, et al. Risk factors for COVID-19-related in-hospital mortality in a high HIV and tuberculosis prevalence setting in South Africa: a cohort study. Lancet HIV. 2021;8(9):e554–e567. doi: 10.1016/S2352-3018(21)00151-X.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nachega JB, Kapata N, Sam-Agudu NA, et al. Minimizing the impact of the triple burden of COVID-19, tuberculosis, and HIV on health services in sub-Saharan Africa. Int J Infect Dis. 2021;113(Suppl 1):S16–S21. doi: 10.1016/j.ijid.2021.03.038.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aborode AT, Alexiou A, Ahmad S, et al. HIV/AIDS Epidemic and COVID-19 Pandemic in Africa. Front Genet. 2021;12:670511. doi: 10.3389/fgene.2021.670511.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abdool Karim Q, Baxter C. COVID-19: Impact on the HIV and Tuberculosis Response, Service Delivery, and Research in South Africa. Curr HIV/AIDS Rep. 2022:1–8. doi: 10.1007/s11904-021-00588-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muhula S, Opanga Y, Oramisi V, et al. Impact of the First Wave of the COVID-19 Pandemic on HIV/AIDS Programming in Kenya: Evidence from Kibera Informal Settlement and COVID-19 Hotspot Counties. Int J Environ Res Public Health. 2021;18(11):6009. doi: 10.3390/ijerph18116009.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davey DLJ, Bekker LG, Mashele N, et al. PrEP retention and prescriptions for pregnant women during COVID-19 lockdown in South Africa. Lancet HIV. 2020;7(11):e735. doi: 10.1016/S2352-3018(20)30226-5.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wagner Z, Mukasa B, Nakakande J, et al. Impact of the COVID-19 Pandemic on use of HIV care, anti-retroviral therapy adherence, and viral suppression: an observational cohort study from Uganda. J Acquir Immune Defic Syndr. 2021;88(5):448–56. doi: 10.1097/QAI.0000000000002811.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Campbell LS, Masquillier C, Knight L, et al. Stayat-Home: the impact of the COVID-19 lockdown on household functioning and ART adherence for people living with HIV in three sub-districts of Cape Town, South Africa. AIDS Behav. 2022:1–18. doi: 10.1007/s10461-021-03541-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Silhol R, Geidelberg L, Mitchell KM, et al. Assessing the Potential Impact of Disruptions Due to COVID-19 on HIV Among Key and Lower-Risk Populations in the Largest Cities of Cameroon and Benin. J Acquir Immune Defic Syndr. 2021;87(3):899–911. doi: 10.1097/QAI.0000000000002663.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harris TG, Jaszi E, Lamb MR, et al. Effects of the COVID-19 pandemic on HIV services: findings from 11 Sub-Saharan African Countries. Clin Infect Dis. 2021:ciab951. doi: 10.1093/cid/ciab951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dear N, Duff E, Esber A, et al. Transient reductions in Human Immunodeficiency Virus (HIV) clinic attendance and food security during the Coronavirus Disease 2019 (COVID-19) pandemic for people living with HIV in 4 African countries. Clin Infect Dis. 2021;73(10):1901–5. doi: 10.1093/cid/ciab379.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mhango M, Chitungo I, Dzinamarira T. COVID-19 Lockdowns: impact on facility-based HIV testing and the case for the scaling up of home-based testing services in Sub-Saharan Africa. AIDS Behav. 2020;24(11):3014–6. doi: 10.1007/s10461-020-02939-6.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chitungo I, Mhango M, Mbunge E, et al. Utility of telemedicine in sub-Saharan Africa during the COVID-19 pandemic. A rapid review. Hum Behav Emerg Technol. 2021 Nov 2; doi: 10.1002/hbe2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Global tuberculosis report 2020. Geneva: World Health Organization; 2020. [Last access January 28, 2022]. Licence: CC BY-NC-SA 3.0 IGO. Available at https://www.who.int/publications/i/item/9789240013131. [Google Scholar]
- 28.Global tuberculosis report 2021. Geneva: World Health Organization; 2021. [Last access January 28, 2022]. Licence: CC BY-NC-SA 3.0 IGO. Available at https://www.who.int/publications/i/item/9789240037021. [Google Scholar]
- 29.McQuaid CF, Vassall A, Cohen T, et al. The impact of COVID-19 on TB: a review of the data. Int J Tuberc Lung Dis. 2021;25(6):436–46. doi: 10.5588/ijtld.21.0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ismail N, Moultrie H. Impact of COVID-19 intervention on TB testing in South Africa. Johannesburg, South Africa: National Institute for Communicable Diseases; [Last access January 28, 2022]. Available at https://www.nicd.ac.za/wp-content/uploads/2020/05/Impact-of-Covid-19-interventions-on-TB-testing-in-South-Africa-10-May-2020.pdf. [Google Scholar]
- 31.Thekkur P, Takarinda KC, Timire C, et al. Operational research to assess the real-time impact of COVID- 19 on TB and HIV services: the experience and response from health facilities in Harare, Zimbabwe. Trop Med Infect Dis. 2021;6(2):94. doi: 10.3390/tropicalmed6020094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mbithi I, Thekkur P, Chakaya JM, et al. Assessing the real-time impact of COVID-19 on TB and HIV services: the experience and response from Selected Health Facilities in Nairobi, Kenya. Trop Med Infect Dis. 2021;6(2):74. doi: 10.3390/tropicalmed6020074.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Soko RN, Burke RM, Feasey HRA, et al. Effects of Coronavirus Disease Pandemic on Tuberculosis Notifications, Malawi. Emerg Infect Dis. 2021;27(7):1831–9. doi: 10.3201/eid2707.210557.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lakoh S, Jiba DF, Baldeh M, et al. Impact of COVID-19 on tuberculosis case detection and treatment outcomes in Sierra Leone. Trop Med Infect Dis. 2021;6(3):154. doi: 10.3390/tropicalmed6030154.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Odume B, Falokun V, Chukwuogo O, et al. Impact of COVID-19 on TB active case finding in Nigeria. Public Health Action. 2020;10(4):157–62. doi: 10.5588/pha.20.0037.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cilloni L, Fu H, Vesga JF, Arinaminpathy N, et al. The potential impact of the COVID-19 pandemic on the tuberculosis epidemic a modelling analysis. E Clinical Medicine. 2020;28:100603. doi: 10.1016/j.eclinm.2020.100603.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Masresha BG, Luce R, Jr, Shibeshi ME, et al. The performance of routine immunization in selected African countries during the first six months of the COVID-19 pandemic. Pan Afr Med J. 2020;37(Suppl 1):12. doi: 10.11604/pamj.supp.2020.37.12.26107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Buonsenso D, Cinicola B, Kallon MN, et al. Child healthcare and immunizations in Sub-Saharan Africa during the COVID-19 pandemic. Front Pediatr. 2020;8:517. doi: 10.3389/fped.2020.00517.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Coker M, Folayan MO, Michelow IC, et al. Things must not fall apart: the ripple effects of the COVID-19 pandemic on children in sub-Saharan Africa. Pediatr Res. 2021;89(5):1078–86. doi: 10.1038/s41390-020-01174-y.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aborode AT, David KB, Uwishema O, et al. Fighting COVID-19 at the expense of malaria in Africa: the consequences and policy options. Am J Trop Med Hyg. 2021;104(1):26–29. doi: 10.4269/ajtmh.20-1181.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guerra CA, Tresor Donfack O, Motobe Vaz L, et al. Malaria vector control in sub-Saharan Africa in the time of COVID-19: no room for complacency. BMJ Glob Health. 2020;5(9):e003880. doi: 10.1136/bmjgh-2020-003880.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.World Health Organization. Second round of the national pulse survey on continuity of essential health services during the COVID-19 pandemic: January-March 2021: Interim report, 22 April 2021. Geneva: World Health Organization; 2021. [[accessed 31 January 2022]]. Available at: https://apps.who.int/iris/handle/10665/340937. [Google Scholar]
- 43.The Global Fund to fight AIDS, Tuberculosis and Malaria. [[accessed 31 January 2022]];The impact of COVID-19 on HIV, TB and malaria services and systems for health: a snapshot from 502 facilities across Africa and Asia. 2021 April; Available at: The impact of COVID-19 on HIV, TB and malaria services and systems for health - Updates - The Global Fund to Fight AIDS, Tuberculosis and Malaria. [Google Scholar]
- 44.Gavi S, Tapera O, Mberikunashe J, Kanyangarara M. Malaria incidence and mortality in Zimbabwe during the COVID-19 pandemic: analysis of routine surveillance data. Malar J. 2021;20(1):233. doi: 10.1186/s12936-021-03770-7.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Diptyanusa A, Zablon KN. Addressing budget reduction and reallocation on health-related resources during COVID-19 pandemic in malaria-endemic countries. Malar J. 2020;19(1):411. doi: 10.1186/s12936-020-03488-y.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weiss DJ, Bertozzi-Villa A, Rumisha SF, et al. Indirect effects of the COVID-19 pandemic on malaria intervention coverage, morbidity, and mortality in Africa: a geospatial modeling analysis. Lancet Infect Dis. 2021;21(1):59–69. doi: 10.1016/S1473-3099(20)30700-3.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.World Health Organization. COVID-19: WHO issues interim guidance for implementation of NTD programmes. 2020. [[Last access: 30 January 2022].]. Available at: https://www.who.int/neglected_diseases/news/COVID19-WHO-interim-guidance-implementation-NTD-programmes/en/
- 48.Kabore A, Palmer SL, Mensah E, et al. Restarting neglected tropical diseases programs in West Africa during the COVID-19 pandemic: lessons learned and best practices. Am J Trop Med Hyg. 2021;105(6):1476–82. doi: 10.4269/ajtmh.21-0408.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Marchese V, Crosato V, Gulletta M, et al. Strongyloides infection manifested during immunosuppressive therapy for SARS-CoV-2 pneumonia. Infection. 2021;49(3):539–542. doi: 10.1007/s15010-020-01522-4.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.World Health Organization. [Last accessed January 30, 2022];WHO Therapeutics and COVID-19: living guideline. Seventh version, published 7 December 2021. Available at: https://www.who.int/publications/i/item/WHO-2019-nCoV-therapeutics-2022.1. [Google Scholar]
- 51.Abdela SG, van Griensven J, Seife F, et al. Neglecting the effect of COVID-19 on neglected tropical diseases: the Ethiopian perspective. Trans R Soc Trop Med Hyg. 2020;114(10):730–2. doi: 10.1093/trstmh/traa072.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.World Health Organization. Impact of the COVID-19 pandemic on seven neglected tropical diseases: a model based analysis. Geneva: World Health Organization; 2021. [accessed 30 January 2022]. Available at: https://apps.who.int/iris/handle/10665/343993. [Google Scholar]
- 53.Owopetu O, Fasehun LK, Abakporo U. COVID-19: implications for NCDs and the continuity of care in Sub-Saharan Africa. Glob Health Promot. 2021;28(2):83–6. doi: 10.1177/1757975921992693.. [DOI] [PubMed] [Google Scholar]
- 54.World Health Organization. The impact of the COVID-19 pandemic on noncommunicable disease resources and services: results of a rapid assessment. Geneva: World Health Organization; 2020. [[accessed 30 January 2022].]. Available at: https://apps.who.int/iris/rest/bitstreams/1299882/retrieve. [Google Scholar]
- 55.Delobelle PA, Abbas M, Datay I, et al. Non-communicable disease care and management in two sites of the Cape Town Metro during the first wave of COVID-19: A rapid appraisal PHCFM 141 10.4102/phcfm.v14i1.3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.World Health Organization (WHO) Special feature: immunization and COVID-19. 2020. [[accessed30 January 2022]]. [21/09/20]. Available at: https://www.who.int/immunization/monitoring_surveillance/immunization-and-covid-19/en/
- 57.Uwizeyimana T, Hashim HT, Kabakambira JD, et al. Drug supply situation in Rwanda during COVID-19: issues, efforts and challenges. J Pharm Policy Pract. 2021;14(1):12. doi: 10.1186/s40545-021-00301-2.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Matsungo TM, Chopera P. Effect of the COVID-19-induced lockdown on nutrition, health and lifestyle patterns among adults in Zimbabwe. BMJ Nutr Prev Health. 2020;3(2):205–12. doi: 10.1136/bm-jnph-2020-000124.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nnaji CA, Moodley J. Impact of the COVID-19 pandemic on cancer diagnosis, treatment and research in African health systems: a review of current evidence and contextual perspectives. E cancer medical science. 2021;15:1170. doi: 10.3332/ecancer.2021.1170.. [DOI] [PMC free article] [PubMed] [Google Scholar]