SUMMARY
The impact of current SARS-CoV-2 pandemic on the healthcare services had serious consequences, especially for the most fragile populations such as HIV-positive subjects. In the period April to September 2020 we reported four cases of HIV-infected late presenters with an AIDS-defining life-threatening condition that, due to the difficult access to the hospital during the pandemic, were characterized by a delayed HIV recognition and institution of correct treatment. Even after two decades of highly active antiretroviral therapy late presenters HIV-infected patients still represent a serious clinical challenge.
Keywords: AIDS, opportunistic infections, COVID-19, late presenters
INTRODUCTION
The current SARS-CoV-2 pandemic had serious consequences on the healthcare service, especially for the most fragile populations such as HIV-infected subjects. These may have been unable to reach the hospital for the HIV testing and treatment supply, leading to a delayed diagnosis and a potential increased mortality [1].
Many HIV-outpatient services have been closed during the pandemic. Confinement measures and imposed limitations to the hospital access determined also a reduction of the outpatient activities [2, 3]. A survey over 98 Outpatient HIV-Services in 53 European countries reported a reduction of HIV testing up to 50% during the first months of pandemic in 2020 [4].
Eventually, as the SARS-CoV-2 continues to spread many clinicians are worried to rule out a COVID-19 diagnosis in every patient admitted with respiratory symptoms, with the risk of missing other possible diagnosis.
We reported here four cases of HIV-infected late presenters with an AIDS-defining life-threatening condition that were hospitalized from April to August 2020 after the beginning of COVID-19 pandemic.
CASE PRESENTATION 1
On April 29, 2020, a 53-year-old man presented to the hospital with breathlessness, low-grade fever and dry cough lasting for 7 days. He complained also ageusia and diarrhoea. On examination the patient had fever (38°C) and bilateral basal crackles were present at chest auscultation. His oxygen saturation (SpO2) was 89% on room air. Laboratory examinations showed leucocytosis (white blood count (WBC) 14,870/mmc; normal values 4.5–11,000 cells/mmc), and increased values of C-reactive protein (PCR) 24 mg/dL; n.v. <0.5 mg/dL) and procalcitonin (PCT) 1.08 mcg/L; n.v. <0.25 mcg/L). There were also high levels of lactate dehydrogenase (LDH; 493 U/L; n.v. <160 U/L) and D-dimer (4430 ng/mL; n.v. ≤300 mcg/L).
A chest computed-tomography (CT) revealed extensive bilateral ground glass opacities with air space consolidation. Two consecutive throat/nasal swabs (T/NS) were negative for SARS-COV-2 by reverse transcriptase PCR (RT-PCR). The patient was admitted in a general medicine ward, where an empiric antibiotic treatment for community-acquired pneumonia with ceftriaxone 2 g i.v. once daily, and azythromicin 500 mg i.v. once daily was started. Clinical respiratory conditions rapidly worsened in the following days so, when considering the high likelihood of COVID-19 pulmonary infection a bronchoscopy with broncho-alveolar lavage for SARS-COV-2 detection was planned. In the meantime, a markedly low T CD4 lymphocytes count was detected (54 cells/mmc; 4,2%) with a T CD4/T CD8 lymphocytes ratio of 0.07. A serology for HIV infection was therefore performed, and it resulted positive with a plasmatic HIV-RNA count of 14,407 copies/mL. With the suspicion of Pneumocystis jirovecii pneumonia (PJP) trimethoprim 20 mg/kg-sulfamethoxazole 75 mg/kg i.v. daily along with steroids treatment (starting with methylprednisolone 80 mg i.v. daily) was begun, and the patient’s clinical conditions quickly improved. Serum (1,3)-β-D-glucan was positive (9.28 pg/mL; reference value <7 pg/mL). Two weeks later highly active antiretroviral therapy with dolutegravir 50 mg + emtricitabine 200 mg/tenofovir alafenamide 25 mg once daily was started and a fast decrease of HIV viral load was obtained. At week 24 plasma HIV-RNA was undetectable, T CD4 lymphocytes count was 167 cells/mmc, with a T CD4/T CD8 lymphocytes ratio of 0.10.
CASE PRESENTATION 2
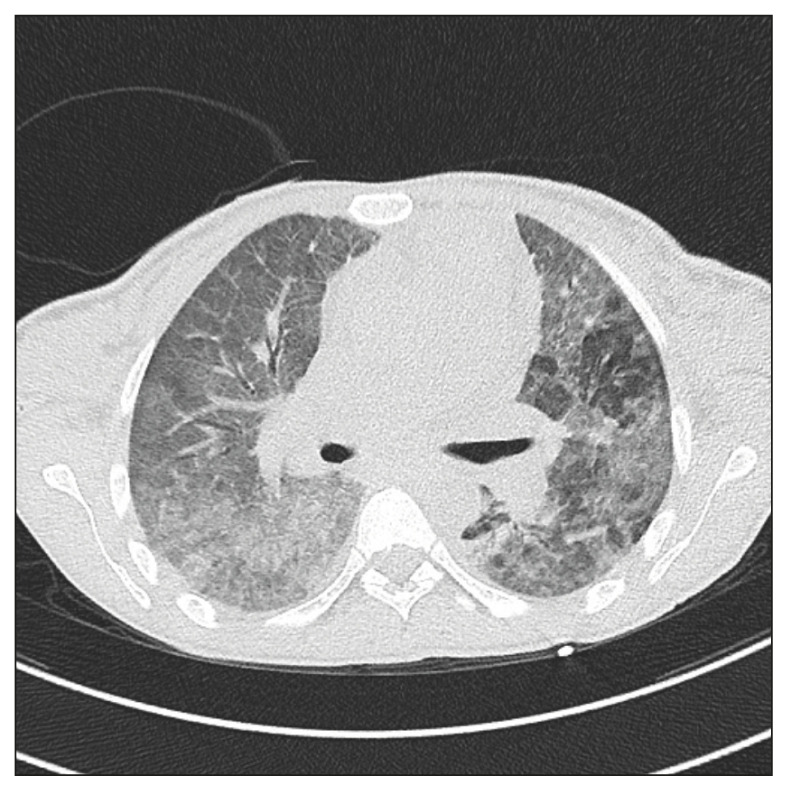
A 31-year-old female patient, recently immigrated from an eastern European country, presented to our hospital on 6 April 2020 with fevers, dry cough and dyspnoea. She was homeless and unemployed, and she had been complaining a weight loss for a month. Past history was also relevant for injecting drug use. The patient was admitted to the Medical Intensive Care Unit because a rapidly worsening respiratory failure, and she was intubated and placed on strict isolation precautions given concerns for SARS-CoV-2 infection. Her chest CT was notable for diffuse bilateral ground-glass opacities (Figure 1), but two repeated nasopharyngeal swabs were negative for SARS-CoV-2 RNA. Her HIV serology tested positive and T CD4 absolute cells count was 59/mmc (7%), with a TC4/T CD8 lymphocytes ratio of 0.10. Plasmatic HIV-RNA was 1,279,048 copies/mL. Serum (1,3)-β-D-glucan was positive (251 pg/mL; reference value <7 pg/mL). A presumptive diagnosis of PJP was made and treatment with trimethoprim 20 mg/Kg-sulphamethoxazole 75 mg/Kg i.v. daily was started. Clinical improvement was observed, on the tenth day of hospitalization the patient was extubated, and eight days later any oxygen supplement was discontinued. Trimethoprim-sulphamethoxazole was stopped after two weeks because of skin rash, severe anaemia and thrombocytopenia. This treatment was then substituted by pyrimethamine and dapsone. At week 24 plasma HIV-RNA was <40 copies/mL, T CD4 lymphocytes count was 358 cells/mmc (19.1%), with a T CD4/T CD8 lymphocytes ratio of 0.34.
Figure 1.
Chest Computed Tomography: bilateral diffuse interstitial tickening with “ground-glass” and “crazy pavement” pattern.
CASE PRESENTATION 3
A 57-year-old female of Moldovan origin was admitted to our ward on August 22, 2020, with high fever and malaise. Two months before she presented to Haematology Service because of severe pancytopenia and idiopathic myelofibrosis was diagnosed. She then started a treatment with monoclonal antibody rulotuxinib, but it was stopped on July 2020 when the serology for HIV infection tested positive. Initial investigations at our ward revealed a low level of T CD4 cells count (10 cells/mmc; 3.4%) as well as the T CD4/T CD8 ratio (0.04) and a high plasmatic HIV-RNA (1,943,350 copies/mL). A chest CT scan showed bilateral interstitial infiltrates, with some aspect of tree in bud on both lungs and some enlarged lymph nodes in the mediastinum. Sputum smear sample was positive for acid-fast bacilli, while nucleic acid amplification for Mycobacterium tuberculosis complex was negative. Serum (1, 3) - β-D - glucan was positive, and plasmatic CMV-DNA was 14,504 copies/mL. Cultures from a bone marrow sample were negative for bacteria, mycobacteria and fungi. Instead cultures from sputum and bronchoalveolar lavage were positive for Mycobacterium avium complex. The patient was at first treated with trimethoprim-sulphamethoxazole 20/75 mg/kg i.v. daily and methylprednisolone 40 mg i.v. twice daily for a presumptive PJP. Ganciclovir 5 mg/kg twice daily i.v. for CMV disseminated infection, and ethambutol 1200 mg daily plus azithromycin 500 mg daily for the pulmonary mycobacterial infection were also commenced. The treatment was well tolerated but several blood transfusions were needed to correct anaemia. Three weeks later the antiretroviral treatment for HIV infection with co-formulated dolutegravir 50 mg/lamivudine 300 mg/abacavir 600 mg was begun achieving a fast decrease of plasmatic HIV viral load.
CASE PRESENTATION 4
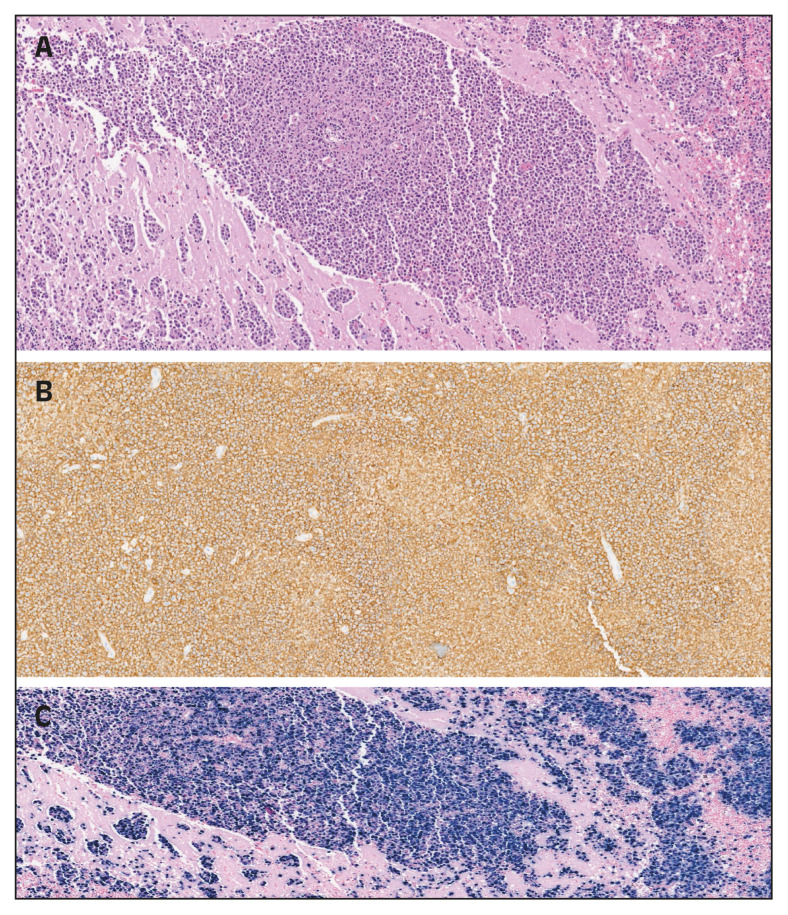
A 55-years-old man was carried to our hospital on April 24, 2020, after he was found lying on the ground of his bedroom, confused and unable to get up. Two months before he had been hospitalized for seven days because of a community-acquired pneumonia not related to SARS-CoV-2. Since the discharge he was complaining a growing difficulty to walk, and weight loss up to 10 kg. At the presentation the patient had dysarthria and gait ataxia. He had neither fever nor respiratory symptoms. Brain magnetic resonance imaging (MRI) showed a diffuse high signal intensity lesion, on T-2 weighted images (T2WI), at the left temporal cortex and left hippocampus, with surrounding cerebral oedema. Left cerebellar hemisphere was also involved. Remarkable haematology and biochemistry were severe lymphopenia (600 lymphocytes/μL; n.v. 770–4,500 μL), mild thrombocytopenia (115,000 μL; n.v. 150,000–350,000 μL) and hypergammaglobulinemia. Serology for HIV infection was positive, with plasma HIV-RNA of 343,052 copies/mL. T CD4 lymphocytes cells count was 365 μL (0.22%). Cerebrospinal fluid (CSF) examination was performed, and it revealed HIV-RNA (364,382 copies/mL). CSF sample was also positive for EBV-DNA. The patient was treated with pyrimethamine (200 mg on the first day, then 75 mg once daily), and sulphadiazine 1.5 g four times daily) for a presumptive cerebral toxoplasmosis, and steroid also were added for cerebral oedema. We observed a transient improvement, but a week later there was a worsening of neurological symptoms and cerebral MRI picture. The patient died six weeks after admission, and autoptic examination of the brain was performed, which showed an EBV-related diffuse large B-cells primary cerebral lymphoma (Figures 2A, 2B and 2C).
Figure 2.
Brain autoptic findings showing an infiltrate composed of large lymphoid cells; hematoxylin-eosin staining x20 (A); immunoreactive for CD-20 (B) and for Epstein Barr virus by in situ hybridization staining (C).
DISCUSSION
On June 2020 the Global Fund did a survey across 106 countries that showed a serious disruption on HIV, tuberculosis and malaria service delivery as a result of COVID-19 pandemic. Challenges to HIV testing and case findings, cancelled or delayed prevention activities, medical and laboratory staff fully engaged to the fight against COVID-19 are some more important reasons why 85% of HIV programs reported disruption to service delivery [5].
Early HIV diagnosis and treatment are of paramount importance in order to maximize individual and public health benefits of antiretroviral treatment. However, even in high-income countries such as in European region, late presentation (LP) of HIV (HIV diagnosis at a T lymphocytes CD4 below 350 cells/mmc or with an AIDS-defining event regardless of CD4 cell count in the six months following HIV diagnosis) accounts from 36.9 to 64.2% of all HIV diagnosis, according to data of two large European cohort studies in the period 2010–2016, and to ECDC data [6, 7]. In Europe, LP was associated on average with a 9-fold higher incidence of AIDS events/deaths within 1 year of HIV-diagnosis (adjusted incidence rate ratio 9.3 [95%CI 7.2–12.0]), compared to non-LP. In Italy late presenters represented 60% of people newly-diagnosed with HIV infection in 2020, and this figure was higher among heterosexual men. In addition the amount of Italian patients who were diagnosed with HIV infection in the last six months before AIDS presentation increased from 48.2% to 80.4% between 2000 and 2020 [8].
The most frequent AIDS-defining disease in Italian HIV-infected patients have been PJP for up 10 years, accounting in 2019 for 25% of AIDS diagnosis. PJP shares some clinical and radiological features with COVID-19 that could make difficult a fast diagnosis and institution of appropriate treatment. Fever, dry cough, dyspnoea, and diffuse bilateral ground-glass opacities at the CT-scan are present in both entities [9]. Lymphopenia and increased serum LDH are also common in both PJP and COVID-19. In the case 1 the correct diagnosis was delayed because of a very low suspicion of PJP in a male patient with interstitial pneumonia without clear risk factors for HIV infection. This patient presented to our hospital during the rage of the first pandemic wave, so all the physician’s worry was to rule out COVID-19, even when two consecutive throat swabs were negative for SARS-CoV-2 RNA detection. A delayed PJP diagnosis clearly explains the severe course of pneumonia in both the Case 1 and Case 2. In the second case we described a very severe PJP with respiratory failure that required mechanical ventilation for ten days. The PJP treatment in this case was also complicated by cutaneous and haematological side effects of pneumocystosis treatment. In the third case we presented a woman that was previously follow-up at a Haematology service for a probable idiopathic myelofibrosis that was secondary to HIV infection. Anaemia is one of the most common laboratory abnormalities in HIV-infected subjects, it is independently associated with HIV infection, with a low quality of life and high death rate [10, 11]. In this patient there was a delayed HIV diagnosis and the retention in care in an HIV Service was possible only after the patient was hospitalized with an advanced disease and multiple AIDS-defining overlapping conditions, such as non-tuberculous mycobacterial pulmonary infection and CMV disseminated infection. A difficult and delayed recognition of an AIDS-defining disease also occurred in the case 4. His symptoms worsened during the first wave of pandemic, when many hospital outpatient services were closed because of the lock-down restrictions. The empirical treatment for cerebral toxoplasmosis did not achieve a clinical improvement and the diagnosis of cerebral lymphoma was obtained only at autoptic examination. Primary central nervous system lymphoma (PCNSL) is an uncommon extranodal non-Hodgkin lymphoma, which accounts approximately for 4% of all intracranial malignancies, and 5% of all extranodal lymphoma. PNCSL is more common in immunocompromised subjects such as HIV-positive patients [12]. The advent of highly active antiretroviral therapy (HAART) in the first decade of 21th century improved dramatically the outcome of HIV-infected patients with PCNSL [13]. In an US epidemiological survey on 4,158 cases of PCNSL from 1992 to 2011, 1512 (36%) were diagnosed in HIV-positive patients, but the fraction occurring in HIV-infected people declined from 64.1% in the period 1992–1996 to 12.7% in 2007–2011, and it was determined by the availability of the HAART [14]. Survival at 5 years after diagnosis was poorer in HIV-infected subjects (9.0%) than in HIV-negative patients (26.2%). Clinical and radiological diagnosis are still challenging, since clinical presentation vary greatly and initial symptoms are often non-specific [15]. The barriers to reach hospital service during the lock-down months could have delayed a prompt recognition of PCNSL neurological signs in our patient.
CONCLUSIONS
COVID-19 pandemic dramatically disrupted public health structures priorities and organization, with the risk to compromise the advances in the fight against HIV infection that we obtained in the last decades. The cases we reported highlights the risk of misdiagnosing some pulmonary opportunistic conditions for SARS-CoV-2 infection. The difficult management of some uncommon and complex AIDS-defining diseases in a time of restriction to hospital access represent a serious challenge for the treatment of late presenters HIV-infected patients. Priority should be done to preparedness to avoid reducing routine HIV care, as well as to the availability of dedicated HIV wards for patients requiring hospitalization.
Footnotes
Conflict of interest The Authors state that they do not have any conflict of interest to declare.
Ethical approval This study was conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from the patient.
Funding
None.
REFERENCES
- 1.Jewell BL, Mudimu E, Stover J, et al. Potential effects of disruption to HIV programmes in Sub-Saharan Africa caused by COVID-19: results from multiple mathematical models. Lancet HIV. 2020;7:e629–e640. doi: 10.1016/S2352-3018(20)30211-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pinto RM, Park S. COVID-19 Pandemic disrupts HIV continuum of care and prevention: implications for research and practice concerning community-based organizations and frontline providers. AIDS Behav. 2020;24:2486–9. doi: 10.1007/s10461-020-02893-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mantica G, Riccardi N, Terrone C, et al. Non-COVID-19 visits to emergency departments during the pandemic: the impact of fear. Public Health. 2020;183:40–1. doi: 10.1016/j.puhe.2020.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simoes D, Stengaard AR, Combs L, et al. The EuroTEST COVID-19 impact assessment consortium of partners. Impact of COVID-19 pandemic on testing services for HIV, viral hepatitis and sexually transmitted infections in the WHO European Region, March to August 2020. Euro Surveill. 2020;25(47) doi: 10.2807/1560-7917.ES.2020.25.47.2001943. pii=2001943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.von Lingen A, Hows J, Hodgson I, et al. HIV Care in the COVID-19 Era: A Community Perspective In collaboration with the European AIDS Treatment Group (EATG) HIV Glasgow - Virtual, 5–8 October 2020. J Intern AIDS Soc. 2020;23:e25616. doi: 10.1002/jia2.25616. Available from: https/ [DOI] [Google Scholar]
- 6.Estimating the burden of HIV late presentation and its attributable morbidity and mortality across Europe 2010–2016. BMC Infect Dis. 2020;20:728. doi: 10.1186/s12879-020-05261-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.European Centre for Disease Prevention and Control/WHO Regional Office for Europe. HIV/AIDS surveillance in Europe 2020–2019 data. Copenhagen: WHO Regional Office for Europe; 2020. [Google Scholar]
- 8.Regine V, Pugliese L, Boros S, et al. Aggiornamento delle nuove diagnosi di infezione da HIV e dei casi di AIDS in Italia al 31 dicembre 2020. Notiziario Ist Sup San. 2021;34:3–59. [Google Scholar]
- 9.Coleman JJ, Manavi K, Marson EJ, et al. COVID-19: to be or not to be; that is the diagnostic question. Post-grad Med J. 2020;96:392–8. doi: 10.1136/postgradmedj-2020-137979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marchionatti A, Migliorini Parisi M. Anemia and thrombocytopenia in people living with HIV/AIDS: a narrative literature review. Internat Health. 2020;0:1–12. doi: 10.1093/inthealth/ihaa036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Akdag D, Knudsen AD, Thudium RF, et al. Increased risk of anemia, neutropenia, and thrombocytopenia in people with human immunodeficiency virus and well-controlled viral replication. J Infect Dis. 2019;220:1834–42. doi: 10.1093/infdis/jiz394. [DOI] [PubMed] [Google Scholar]
- 12.Grommes C, DeAngelis LM. Primary CNS lymphoma. J Clin Oncol. 2017;35:2410–8. doi: 10.1200/JCO.2017.72.7602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wolf T, Brodt HR, Fichtlscherer S. Changing incidence and prognostic factors of survival in AIDS related non-Hodgkin’s lymphoma in the era of highly active antiretroviral therapy (HAART) Leuk Lymphoma. 2005;46:207–15. doi: 10.1080/10428190400015733. [DOI] [PubMed] [Google Scholar]
- 14.Shiels MS, Pfeiffer RM, Besson C, et al. Trends in Primary Central Nervous System Lymphoma Incidence and Survival in the U.S. Br J Haematol. 2016;174:417–24. doi: 10.1111/bjh.14073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grommes C, Rubenstein JL, De Angelis LM, et al. Comprehensive approach to diagnosis and treatment of newly diagnosed primary CNS lymphoma. Neuro-Oncology. 2018;21:296–305. doi: 10.1093/neuonc/noy192. [DOI] [PMC free article] [PubMed] [Google Scholar]