Abstract
Background
Ansofaxine (LY03005) extended-release tablet is a potential triple reuptake inhibitor of serotonin, norepinephrine, and dopamine. This study assessed the efficacy, safety, and appropriate dosage of ansofaxine for the treatment of major depressive disorder (MDD).
Methods
A multicenter, randomized, double-blind, placebo-controlled, dose-finding, Phase 2 clinical trial was conducted in China. Eligible patients with MDD (18–65 years) were randomly assigned to receive fixed-dose ansofaxine extended-release tablets (40, 80, 120, or 160 mg/d) or placebo for 6 weeks. The primary outcome measure was a change in the total score on the 17-item Hamilton Depression Rating Scale from baseline to week 6.
Results
A total of 260 patients were recruited from October 2015 to September 2017, and 255 patients received the study drug as follows: 40 mg (n = 52), 80 mg (n = 52), 120 mg (n = 51), and 160 mg (n = 51) ansofaxine and placebo (n = 49). Significant differences were found in mean changes in 17-item Hamilton Depression Rating Scale total scores at week 6 in the 4 ansofaxine groups vs placebo (−12.46; χ2 = −9.71, P = .0447). All doses of ansofaxine were generally well-tolerated. Treatment-related adverse events occurred in 141 patients (303 cases), yielding incidence rates of 51.92%, 65.38%, 56.86%, and 62.75% in the 40-, 80-, 120-, and 160-mg ansofaxine groups and 38.78% in the placebo group.
Conclusion
Active doses (40, 80, 120, and 160 mg/d) of ansofaxine in a controlled setting were safe, tolerated, and effective in improving depression symptoms in MDD patients.
Keywords: Ansofaxine, major depressive disorder, Phase 2 Clinical Trial
Significance Statement.
The global burden of depression is so heavy that the development of new antidepressant drugs with more powerful antidepressant effects and fewer side effects is still a top priority. We performed a Phase 2 clinical trial to explore the safety, efficacy, and tolerability of ansofaxine extended-release (ER) tablet for the treatment of MDD. This was a multicenter, randomized, double-blind, placebo-controlled, dose-finding trial in which patients received 6 weeks of treatment with ansofaxine ER tablets at doses of 40, 80, 120, or 160 mg/d or placebo. The results showed that 4 doses of ansofaxine ER tablet exerted antidepressant effects and were safe and well-tolerated. Triple reuptake inhibitors block the reuptake of serotonin, norepinephrine, and dopamine from the synapse, with a stronger effect on dopamine reuptake. The results of this trial support further evaluations of ansofaxine ER tablet as a new drug for the treatment of patients with MDD.
Introduction
Major depressive disorder (MDD) is a common illness that severely limits psychosocial functioning and diminishes quality of life. In 2008, the World Health Organization ranked MDD as the third leading cause of disease burden worldwide and projected that MDD will rank first by 2030. The 12-month prevalence of MDD varies considerably among countries but is approximately 6% overall (Kessler and Bromet, 2013). In China, depression is also one of the most common mental illnesses, with a 12-month prevalence of 3.6% and lifetime prevalence of 6.8% (Huang et al., 2019). For the treatment of depression, antidepressants that affect monoamine systems (dopamine [DA], serotonin [5-hydroxytryptamine (5-HT)], and norepinephrine [NE]) are primary or first-line medications, including tricyclic antidepressants, monoamine oxidase inhibitors, selective serotonin reuptake inhibitors (SSRIs), serotonin and NE reuptake inhibitors (SNRIs), and serotonin receptor partial agonist/reuptake inhibitors (Ashton et al., 2019). However, these drugs are characterized by a slow onset and may have some side effects. Triple reuptake inhibitors (TRIs) block the reuptake of 5-HT, NE, and DA from the synapse, with stronger efficacy on DA reuptake. They may have the additional effect of enhancing neurotransmission of all 3 monoamine systems and potential advantages of a rapid onset of action, amelioration of symptoms of anhedonia and sexual dysfunction, and improvements in cognitive function, reward-motivated function, and goal-oriented behavior (Tran et al., 2012).
Ansofaxine hydrochloride ([±]-4-[2-(dimethylamino)-1-(1-hydroxycyclohexyl) ethyl] phenyl 4-methylbenzoate hydrochloride dihydrate [LY03005, LPM570065]; see supplementary Figure 1; https://pubchem.ncbi.nlm.nih.gov/compound/56955395#section=Information-Sources) is a new chemical compound that is formulated as ansofaxine extended-release (ER) oral tablets for the treatment of adults with MDD. Ansofaxine has a high affinity for the DA transporter, NE transporter, and serotonin transporter and significantly inhibits the reuptake of DA, NE, and 5-HT, making it a potential TRI for the treatment of MDD (Zhang et al., 2014). Microdialysis studies showed that ansofaxine hydrochloride inhibited the reuptake of DA, NE, and 5-HT and increased extracellular 5-HT, DA, and NE levels in the rat striatum after acute and chronic administration (Zhang et al., 2014). The ability of ansofaxine to increase extracellular 5-HT and NE levels in the rat striatum is similar to desvenlafaxine, but its ability to increase extracellular DA levels is approximately 2 to 3 times stronger than desvenlafaxine. Although ansofaxine is a methyl benzoate of desvenlafaxine and can be quickly converted to desvenlafaxine in vivo, ansofaxine and desvenlafaxine can coexist in the blood and brain because of the liposolubility of ansofaxine, with similar concentrations of ansofaxine and desvenlafaxine in the brain when equivalent oral doses of the 2 drugs are given (Zhang et al., 2014; Guo et al., 2018; Ashton et al., 2019). Additionally, ansofaxine is the most balanced TRI that has been studied to date in clinical trials, in which the reuptake of DA, NE, and 5-HT was strongly inhibited. DA plays an important role in the pathogenesis of depression (Nestler and Carlezon, 2006; Grace, 2016). The mesocorticolimbic DA system has been linked to rewarding events and incentive-driven behaviors, and low dopaminergic activity may lead to loss of interest and anhedonia, a core symptom of depression (Nestler and Carlezon, 2006). Studies have shown that a rapid increase in DA levels in the synaptic cleft in the midbrain-cortical-marginal pathway can ameliorate the delay in the treatment of depression, whereas an increase in DA levels in the hypothalamus can enhance dopaminergic nerve stimulation of the nucleus accumbens, thereby improving pleasure, cognition, and sexual dysfunction and rewarding incentive-driven and goal-oriented behaviors.
Preclinical safety studies confirmed that ansofaxine hydrochloride has good safety and tolerability properties (Zhang et al., 2014; Li et al., 2018). Ansofaxine ER tablet has also completed 3 Phase 1 studies in health volunteers: a single ascending-dose study, a diet effect study, and a multiple ascending-dose study (registered at http://www.chinadrugtrials.org.cn/index.html; nos. CTR20130364, CTR20140333, and CTR20140418). The results of these Phase 1 studies showed that the pharmacokinetics of ansofaxine ER tablet have a dose-proportional relationship in the range of 20 to 200 mg/d and that diet had no significant effect on its pharmacokinetics. The results also indicated that ansofaxine ER tablet was safe and well-tolerated in the range of 20 to 200 mg/d in the single ascending-dose study or 40–160 mg/d in the multiple ascending-dose study. The main adverse reactions included nausea, vomiting, diarrhea, dizziness, and elevated total bilirubin or alanine aminotransferase. The full results, however, have not yet been published. In response to the Phase 1 study results, ansofaxine ER tablet was approved for Phase 2 clinical trials. Thus, to investigate the clinical effect of ansofaxine ER tablet, the present study (ClinicalTrial.gov; no. NCT03785652) was designed to explore the optimal dose range of ansofaxine ER tablet and preliminarily assess its safety, efficacy, and tolerability in patients with MDD.
METHODS
Study Design
This multicenter, randomized, double-blind, placebo-controlled, parallel-group, dose-finding, Phase 2 study was conducted at 10 hospitals in China (the hospitals are listed in “Additional Contributions” below) from October 2015 to September 2017. The protocol received independent ethics committee approval from the 10 hospitals before the trial began. The study was conducted in accordance with appropriate laws and regulations, including Good Clinical Practice and the Declaration of Helsinki. Written informed consent was obtained from all patients before screening. All researchers from the 10 hospitals underwent protocol consistency training, and all raters were given scale-rating consistency training to ensure consistency among hospitals in this multicenter trial.
Patients
Inclusion Criteria
—Individuals 18 to 65 years old with a diagnosis of MDD based on Diagnostic and Statistical Manual of Mental Disorders, 4th edition, text revision criteria who experienced a single episode or recurrent episodes without psychotic symptoms were included. At screening and baseline (day 0), total scores for eligibility were ≥20 on the 17-item Hamilton Depression Rating Scale (HAMD17), ≥2 on HAMD17 item 1 (depressive mood), and ≥4 on the Clinical Global Impressions-Severity (CGI-S) scale (moderately ill).
Exclusion Criteria
—Individuals who were resistant to venlafaxine and antidepressant treatment, had significant risk of suicide (HAMD17 item 3 score [suicide item] ≥3), significant placebo response (≥25% decrease in HAMD17 total score during the washout period from screening to baseline), another psychiatric diagnosis, unstable physical disease at screening, clinical abnormalities on physical examination, electrocardiogram (ECG), laboratory tests, or urine drug screen at screening, systematic psychotherapy (interpersonal relationship therapy, dynamic therapy, and cognitive behavioral therapy), or transcranial magnetic stimulation within 3 months prior to screening and light therapy within 2 weeks prior to screening (see supplementary Protocol) were excluded.
Randomization and Masking
Patients were randomly assigned to placebo or 1 of 4 fixed-dose ansofaxine ER tablets (40, 80, 120, or 160 mg/d) in a 1:1:1:1:1 manner using a web-based dynamic randomization system that generated unique container numbers instituted by an external independent third party. A randomized block schedule was used for the drug randomization lists. The sample size was designed to be 260 (52 per group). Patients, clinicians, and independent outcome raters were masked to treatment allocation until study completion (see supplementary Protocol).
Procedures
This trial comprised 3 periods: screening period of up to 1 week, placebo washout period of 1 week, and a double-blind treatment period of 6 weeks. In the screening period, the patients underwent physical and psychiatric examinations by researchers and laboratory and ECG examinations. After screening, MDD patients who met the inclusion criteria entered into the placebo washout period, during which they took 2 placebo tablets orally once daily in the morning for 7 consecutive days. Patients who still met the inclusion criteria after the placebo washout period were randomly assigned to placebo or 1 of 4 fixed-dose ansofaxine ER tablets. The last day of the placebo washout period was also the first day of the double-blind treatment period and was set as baseline (day 0). Participants returned for follow-up visits on study days 7, 14, 28, and 42 (see supplementary Protocol).
Outcomes
Primary Efficacy Measure
—The HAMD17 was applied at screening and baseline and during the double-blind treatment period on days 7, 14, 28, and 42 (or at the end of weeks 1, 2, 4, and 6). The primary efficacy endpoint was the change in HAMD17 total score from baseline to the end of week 6 or the final on-therapy evaluation.
Secondary Efficacy Measure
—Changes in Hamilton Anxiety Rating Scale (HAMA) total scores, Clinical Global Impressions-Improvement (CGI-I) scores, CGI-S scores, HAMD17 factor scores, and Visual Analog Scale of Pain Intensity (VAS-PI) scores were determined. Response rates, with a response defined as a ≥50% decrease in HAMD17 total scores from baseline to the final on-therapy evaluation, were also calculated. Finally, remission rates, defined as HAMD17 scores ≤7, were determined.
Safety and Tolerability Evaluations
—Adverse events (AEs), withdrawal caused by AEs, concomitant medications, weight, vital signs, physical examinations (at screening and on completion) at each visit, laboratory tests, and ECGs were determined (see supplementary Protocol).
Statistical Analysis
The data were analyzed using SAS 9.2 software (SAS Institute, Cary, NC, USA). The efficacy analysis was mainly based on the full-analysis set (FAS), which was defined as all randomized patients who received at least 1 dose of the study drug during the double-blind treatment period and had both baseline and at least 1 post-baseline measurement of primary efficacy. The FAS used last-observation carried forward imputation.
Changes in HAMD17 total scores from baseline were analyzed using ANCOVA, with treatment and study site as fixed factors and HAMD17 total score at baseline as the covariate. The least-squares mean, together with the 95% or 90% confidence interval (CI), was estimated for each treatment group and for differences between groups, respectively. The secondary endpoints included HAMA scores, CGI-S scores, HAMD17 factor scores, and VAS-PI scores, which were analyzed the same way as the primary endpoint but with replacement of the corresponding baseline with the respective baseline value. The other secondary endpoints included CGI-I scores, response rates, and remission rates, which were analyzed using the Cochran-Mantel-Haenszel χ² test with adjustment for the study site factor.
Safety analyses were based on the safety population (safety set), which included all randomized patients who received at least 1 dose of the study drug during the double-blind treatment period. Listings and summary tabulations of AEs were reported. AEs were classified according to Medical Dictionary for Regulatory Activities terminology.
This was a dose-finding study, and all statistical tests were conducted using 2-sided tests with 10% type 1 error rates unless otherwise stated. Values of P < .1 were considered statistically significant.
RESULTS
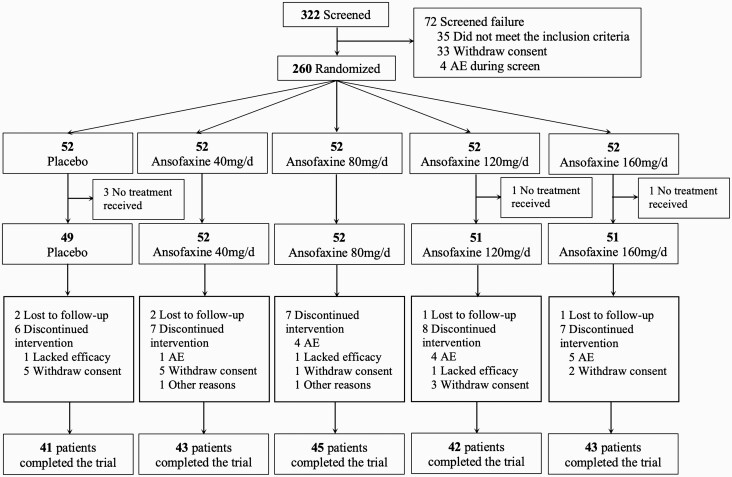
We screened 332 patients for this trial. Screening failure occurred in 72 patients, and 260 patients were randomized. Five patients did not receive the drug treatment, and 255 patients received the study drug (safety set) as follows: placebo (n = 49) and ansofaxine ER tablets at doses of 40 mg/d (n = 52), 80 mg/d (n = 52), 120 mg/d (n = 51), or 160 mg/d (n = 51). During the double-blind treatment period, 17.69% of the patients (n = 46, 46/260) withdrew from the study. The reasons for discontinuation are shown in Figure 1. Withdrawal rates did not significantly differ among the 5 groups (P = .9017). Nine of the randomized patients did not have at least 1 post-baseline measurement of primary efficacy; thus, the FAS included 246 patients: 49 patients in the placebo group, 49 patients in the 40-mg/d ansofaxine group, 50 patients in the 80-mg/d ansofaxine group, 50 patients in the 120-mg/d ansofaxine group, and 48 patients in the 160-mg/d ansofaxine group. Table 1 shows the demographic and baseline characteristics in the FAS set. There were no significant differences among the treatment groups (all P > .1).
Figure 1.
Study design and disposition of patients. AE, adverse event.
Table 1.
Demographic and Baseline Characteristics (FAS)
| Placebo (n = 49) |
Ansofaxine ER tablets | P | |||||
|---|---|---|---|---|---|---|---|
| 40 mg (n = 49) |
80 mg (n = 50) |
120 mg (n = 50) |
160 mg (n = 48) |
||||
| Age (y), mean (SD) | 34.93 (11.54) | 35.29 (11.44 | 34.38 (11.57) | 33.75 (11.63) | 35.14 (12.16) | .9650a | |
| Sex, no. (%) | Male | 18 (36.73) | 20 (40.82) | 19 (38.00) | 19 (38.00) | 14 (29.17) | .8082b |
| Female | 31 (63.27) | 29 (59.18) | 31 (62.00) | 31 (62.00) | 34 (70.83) | ||
| Han ethnicity, no. (%) | 47 (95.92) | 48 (97.96) | 4 8(96.00) | 50 (100.0) | 45 (93.75) | .4815a | |
| Weight (kg), mean (SD) | 61.73 (11.02) | 57.97 (8.59) | 59.94 (10.97) | 61.11 (12.27) | 56.91 (10.22) | .1315a | |
| BMI (kg/m2), mean (SD) | 22.56 (3.27) | 21.52 (2.62) | 22.33 (3.44) | 22.03 (3.07) | 21.11 (2.96) | .1322a | |
| Length of current MDE (mo), mean (SD) | 10.49 (13.82) | 8.33 (7.37) | 12.69 (25.96) | 9.02 (10.98) | 8.78 (13.86) | .5905a | |
| HAMD17 score, mean (SD) | 23.63 (3.40) | 22.69 (2.55) | 23.18 (2.54) | 23.68 (3.00) | 23.96 (3.05) | .2308a | |
| CGI-S score, mean (SD) | 4.63 (0.67) | 4.51 (0.58) | 4.46 (0.54) | 4.56 (0.64) | 4.60 (0.68) | .6508a |
Abbreviations: BMI, body mass index; FAS, full analysis set; ER, extended-release; HAMD17, 17-item Hamilton Depression Rating Scale; CGI-S, Clinical Global Impressions-Severity.
a ANOVA/eank sum test.
b χ 2 test.
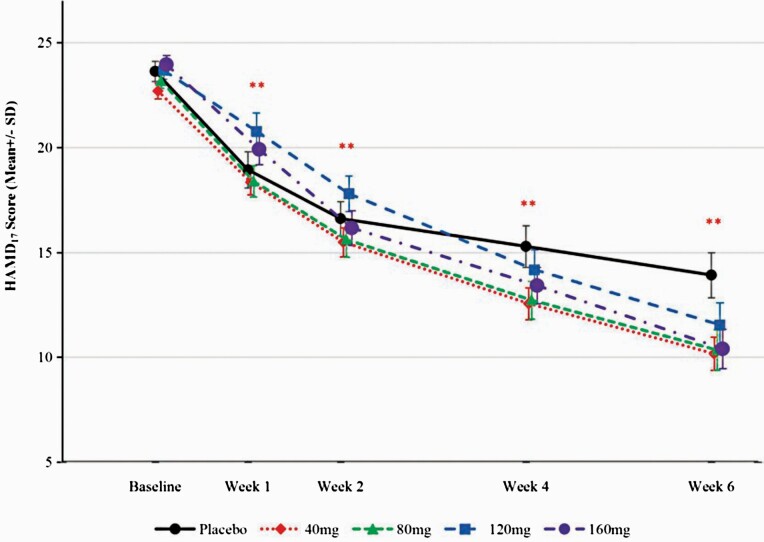
Mean changes from baseline in HAMD17 total scores at week 6 were −9.71, −12.53, −12.84, −12.14, and −13.56 in the placebo and 40-, 80-, 120-, and 160-mg/d ansofaxine groups, respectively. The differences among the 5 treatment groups were statistically significant (ANCOVA, χ2 = −9.71, P = .0447). Adjusted mean differences in HAMD17 total scores (95% CI) that were obtained by comparing placebo with the 4 ansofaxine dose groups were statistically significant (−2.92 [−6.09 to 0.24] for the 40-mg group, −3.08 [−6.22 to 0.05] for the 80-mg group, −2.43 [−5.56 to 0.70] for the 120-mg group, and −3.69 [−6.85 to −0.52] for the 160-mg group; Table 2). The 4 groups presented a greater reduction of HAMD17 total scores over time compared with the placebo group, and the difference was statistically significant at the end of week 1 (Figure 2).
Table 2.
Changes of HAMD17 Total Scores, HAMA Total Scores, HAMA Somatic Anxiety Factor, and HAMD17 Anxiety/Somatization Factor at the End of Week 6 (FAS, LOCF)
| Placebo (n = 49) | Ansofaxine ER tablets | P (a) a | ||||
|---|---|---|---|---|---|---|
| 40 mg (n = 49) | 80 mg (n = 50) | 120 mg (n = 50) | 160 mg (n = 48) | |||
| HAMD17 | ||||||
| LSMEAN | −9.54 | −12.46 | −12.62 | −11.97 | −13.23 | .0447 |
| Average difference from placebo groupb (90% CI) | −2.92 (−5.05, −0.80) |
−3.08 (−5.19, −0.98) |
−2.43 (−4.53, −0.33) |
−3.69 (−5.82, −1.56) |
||
| Average difference from placebo groupb (95% CI) | −2.92 (−6.09, 0.24) |
−3.08 (−6.22, 0.05) |
−2.43 (−5.56, 0.70) |
−3.69 (−6.85, −0.52) |
||
| HAMA total scores | ||||||
| LSMEAN | −6.99 | −9.43 | −10.24 | −8.80 | −10.31 | .0329 |
| Average difference from placebo group (90% CI) | −2.44 (−4.39, −0.49) |
−3.24 (−5.17, −1.31) |
−1.81 (−3.74, 0.12) |
−1.81 (−3.74, 0.12) |
||
| HAMA somatic anxiety factor | ||||||
| LSMEAN | −2.20 | −3.44 | −3.80 | −2.88 | −3.74 | .0295 |
| Average difference from placebo group (90% CI) | −1.23 (−2.18, −0.29) |
−1.59 (−2.54, −0.65) |
−0.68 (−1.62, 0.27) |
−1.53 (−2.49, −0.58) |
||
| HAMD17 anxiety/somatization factor | ||||||
| LSMEAN | −2.50 | −3.35 | −3.56 | −3.22 | −3.68 | .0724 |
| Average difference from placebo group (90% CI) | −0.85 (−1.59, −0.12) |
−1.06 (−1.79, −0.33) |
−0.72 (−1.45, 0.01) |
−1.18 (−1.92, −0.45) |
Abbreviations: CI, confidence interval; ER, extended-release; FAS, full analysis set; HAMD17, 17-item Hamilton Depression Rating Scale; LOCF, last observation carried forward; LSMEAN, least square mean.
a P value (a) by regression of ANCOVA for comparisons across study groups.
b Average differences and 90% CIs were obtained from the regression model (least-square approach) after adjusting other model factors.
Figure 2.
HAMD17 total scores during the double-blind treatment phase (FAS). **P < .01, compared with placebo. FAS, full analysis set; HAMD17, 17-item Hamilton Depression Rating Scale.
At the end of week 6, mean changes in HAMA total scores, the HAMA somatic anxiety factor, and the HAMD17 anxiety/somatization factor were significantly higher for 3 of the ansofaxine groups (40, 80, and 160 mg/d) but not for the 120-mg ansofaxine group (P < .1; Table 2) compared with placebo.
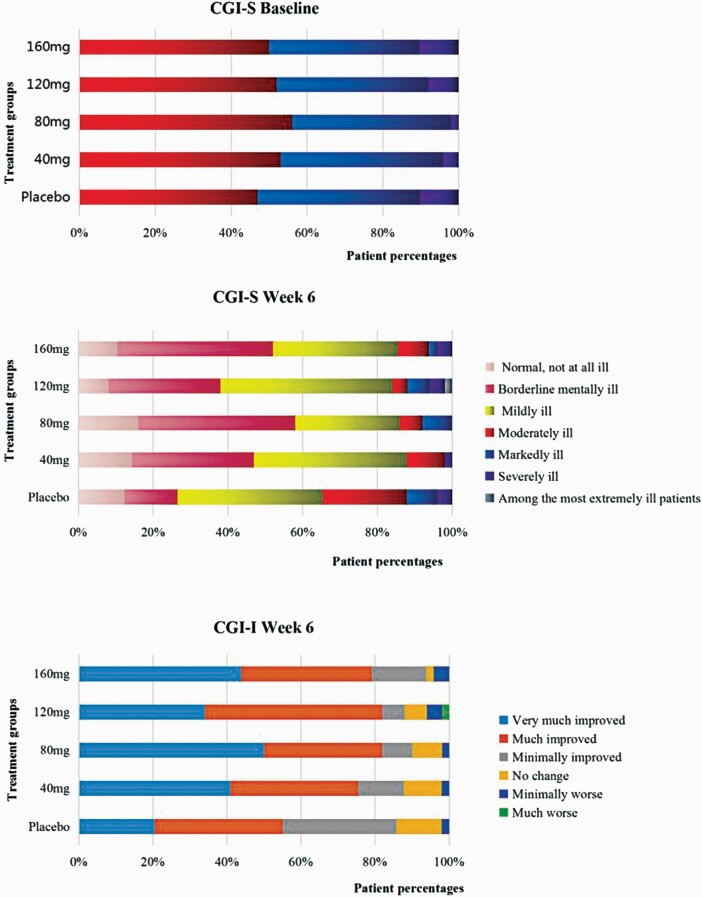
CGI-I scores were significantly higher in the 4 ansofaxine groups than in the placebo group (all P < .1). The total number and proportion of “normal, borderline mentally ill, and mildly ill” participants, assessed by the CGI-S at the end of week 6, were significantly higher compared with the placebo group (Figure 3).
Figure 3.
CGI-S and CGI-I scores at baseline and the end of week 6 (FAS). FAS, full analysis set; CGI-I, Clinical Global Impressions-Improvement; CGI-S, Clinical Global Impressions-Severity.
At the end of week 6, there was no significant difference in VAS-PI scores (total pain, headache, back pain, joint pain, abdominal pain, or others) among the 5 groups (P > .1). Response rates, based on the HAMD17, were higher in the 40-, 80-, 120-, and 160-mg/d ansofaxine groups than in the placebo group (FAS, P < .05; supplementary Table 1; supplementary Figure 2).
A total of 174 patients (among a total of 457 cases) experienced treatment-emergent AEs (TEAEs) based on the investigators’ judgment: 30 (61.22%) in the placebo group, 34 (65.38%) in the 40-mg ansofaxine group, 39 (75.00%) in the 80-mg ansofaxine group, 36 (70.59%) in the 120-mg ansofaxine group, and 35 (68.63%) in the 160-mg ansofaxine group. No significant differences were found in the incidence of TEAEs among groups (P = .6423). In terms of severity, 119 patients (46.67%) had mild TEAEs, 43 (16.86%) had moderate TEAEs, and 12 (4.71%) had severe TEAEs.
A total of 141 participants (303 cases) experienced AEs that might be associated with the study drug (TRAEs) based on the investigators’ judgment. Incidence rates of TRAEs were 51.92% (27 patients, 65 cases), 65.38% (34 patients, 68 cases), 56.86% (29 patients, 70 cases), and 62.75% (32 patients, 70 cases) in the 40-, 80-, 120-, and 160-mg/d ansofaxine groups, respectively, and 38.78% (19 patients, 30 cases) in the placebo group. The severity of TRAEs was mild in 103 patients (40.39%), moderate in 30 patients (11.76%), and severe in 8 patients (3.14%).
Fourteen patients withdrew from the double-blind treatment period because of TEAEs, including 1 patient (1.92%) in the 40-mg ansofaxine group, 4 patients (7.69%) in the 80-mg ansofaxine group, 4 patients (7.84%) in the 120-mg ansofaxine group, and 5 patients (9.80%) in the 160-mg ansofaxine group. No patients withdrew in the placebo group. The difference among groups was statistically significant (P = .0948). TEAEs that resulted in withdrawal were mainly nausea, headache, and dizziness and judged to be definitely, probably, or possibly related to ansofaxine.
TRAEs with a ≥5% incidence in any dose group and with an incidence at least twice as high as in the placebo group included decreased appetite, chest discomfort, fatigue, lethargy, constipation, nausea, dry mouth, palpitations, and blurred vision. The incidences of nausea and chest discomfort significantly differed among groups (P = .0665 and P = .0913, respectively). The 3 most common TRAEs were nausea, lethargy, and decreased appetite (Table 3). Notably, throughout the whole trial, only 1 case reported sexual dysfunction in the 80-mg ansofaxine group.
Table 3.
Adverse Events With an Incidence ≥5% and at Least Twice as High as in the Placebo Group During the Double-Blind Period (SS)
| Adverse Event, no. (%) | Placebo (n = 49) |
Ansofaxine ER tablets | Fisher Pa | |||
|---|---|---|---|---|---|---|
| 40 mg (n = 52) |
80 mg (n = 52) |
120 mg (n = 51) |
160 mg (n = 51) |
|||
| Nausea | 3 (6.12) | 10 (19.23) | 10 (19.23) | 11 (21.57) | 14 (27·45) | .0665 |
| Decreased appetite | 1 (2.04) | 3 (5.77) | 1 (1.92) | 4 (7.84) | 0 (0) | .1811 |
| Chest discomfort | 0 (0) | 0 (0) | 1 (1.92) | 0 (0) | 3 (5·88) | .0913 |
| Fatigue | 1 (2.04) | 0 (0) | 2 (3.85) | 2 (3.92) | 3 (5·88) | .5342 |
| Lethargy | 0 (0) | 1 (1.92) | 2 (3.85) | 5 (9.8) | 3 (5.88) | .1278 |
| Constipation | 0 (0) | 2 (3.85) | 2 (3.85) | 1 (1.96) | 3 (5·88) | .6251 |
| Dry mouth | 1 (2.04) | 1 (1.92) | 3 (5.77) | 2 (3.92) | 2 (3·92) | .9040 |
| Palpitations | 1 (2.04) | 0 (0) | 2 (3.85) | 2 (3.92) | 4 (7·84) | .2743 |
| Blurred vision | 0 (0) | 3 (5.77) | 2 (3.85) | 0 (0) | 3 (5·88) | .1577 |
Abbreviations: ER, extended-release; no., number; SS, safety set. Treatment drug-related adverse events.
a Fishers exact test was used for comparisons between groups.
During the study, 2 serious AEs (SAEs) occurred, both in the 40-mg/d ansofaxine group, including hypomania and the exacerbation of depression. Both events were judged to be related to the study drug and had alleviated by the end of the study. No deaths occurred.
Concomitant medications are shown in supplementary Table 2. Ansofaxine ER tablets might increase supine diastolic pressure and orthostatic diastolic pressure (supplementary Tables 3 and 4). Ansofaxine ER tablets did not appear to influence supine or orthostatic pulse, weight, or physical examination. Two patients had abnormal ECGs with clinical significance, as determined by the researcher (1 case in the placebo group and 1 case in the 40-mg/d ansofaxine group). Ansofaxine ER tablets slightly influenced the laboratory tests (supplementary Information).
Discussion
This trial was a dose-finding study that preliminarily explored the effective dose, efficacy, and safety of ansofaxine ER tablets. Significant differences were observed in mean changes at week 6 in HAMD17 total scores in the 4 ansofaxine groups (40, 80, 120, and 160 mg/d) vs placebo in patients with MDD. We also found that all doses of ansofaxine were generally well-tolerated and safe.
The active ingredient of ansofaxine ER tablets is a p-hydroxybenzoic acid, O-desmethylvenlafaxine (ODV). With oral administration, ODV has a Tmax of approximately 6 hours and t1/2 of approximately 11 hours. It is expected that a steady-state plasma concentration would be reached after 3 doses. ODV is an active metabolite of venlafaxine after first-pass metabolism and has a longer half-life than venlafaxine (Klamerus et al., 1992). Venlafaxine is a 5-HT and NE reuptake inhibitor, with a stronger inhibitory action on 5-HT reuptake and weak affinity for α-adrenergic receptors, DA D2 receptors, 5-HT1A and 5-HT2 receptors, opioid receptors, and benzodiazepine binding sites (Schweizer et al., 1997). Compared with venlafaxine, ODV is metabolized by CYP2D6 and is a weak inhibitor of CYP3A4, which is less affected by liver function (Lessard et al., 1999; Perry and Cassagnol, 2009; Pae, 2011). Unlike ODV, ansofaxine is a potential TRI that has high affinity for the recombinant human DA transporter, recombinant human NE transporter, and recombinant human 5-HT transporter. Dopamine transporter reuptake inhibition may ameliorate MDD patients’ anhedonia and the decline in positive emotion and improve cognition reward motivation and goal-oriented behavior (Zhang et al., 2014; Carvalho et al., 2016; Guo et al., 2018; Meder et al., 2019). It may be less likely to cause sexual dysfunction (Lane, 2015) and have fewer residual symptoms after patients achieve clinical recovery. Additionally, it may be effective against treatment-resistant depression (Clayton et al., 2014).
In this trial, the results showed ansofaxine ER tablets were superior to placebo in all 4 dose groups, which might provide strong evidence for the mechanism explorations in the future. At the end of week 6, changes in HAMD17 scores from baseline (controlled by placebo) in the 80- and 160-mg/d groups were better than −3 (Dunlop et al., 2012; Boulenger et al., 2014), which is considered clinically significant according to National Institute for Health and Care Excellence guidelines (https://www.nice.org.uk/guidance/CG23). The results were consistent with previous studies (Brownstone et al., 2012; Boulenger et al., 2014). The treatment response rates were superior to the placebo group and better than vortioxetine, vilazodone, and levomilnacipran in previous studies, which used Montgomery and Asberg Depression Rating Scale scores as the evaluation criterion (Kennedy et al., 2009; Montgomery et al., 2015; Thase et al., 2016; Kornstein et al., 2018). Our results suggest that ansofaxine ER tablets had a significant and rapid antidepressant effect, which became statistically significant at the end of week 1 compared with placebo. It also had an apparent effect in patients with anxiety symptoms. The mechanism of action of ansofaxine may be related to an increase in the inhibition of DA reuptake, which quickly improves depressive symptoms.
The incidences of TRAEs in the 40-, 80-, 120-, and 160-mg/d ansofaxine groups were 51.92%, 65.38%, 56.86%, and 62.75%, respectively. As previously reported, the incidences of AEs were 75.5% for levomilnacipran (Montgomery et al., 2013), 84% for vilazodone, 55% for vortioxetine (Deardorff and Grossberg, 2014), and 77%–80% for desmethylvenlafaxine succinate sustained-release tablets (Boyer et al., 2008; Liebowitz et al., 2008). A meta-analysis that compared AEs among escitalopram and other SSRIs (i.e., citalopram, fluoxetine, paroxetine, and sertraline) and SNRIs (i.e., venlafaxine and duloxetine) found incidences of 73.3%–73.6%, 78.2%, and 77.4%, respectively. The percentages of withdrawal that was attributable to AEs were 1.92%, 7.69%, 7.84%, and 9.80% in the 40-, 80-, 120-, and 160-mg/d ansofaxine groups in the present study. The percentages of withdrawal attributable to AEs were 6.8% for vortioxetine (Boulenger et al., 2014), 9.4% for levomilnacipran (Montgomery et al., 2013), 5.3%–5.4% for escitalopram, 6.3% for other SSRIs (i.e., citalopram, fluoxetine, paroxetine, and sertraline), and 12.9% for SNRIs (i.e., venlafaxine and duloxetine) (Kennedy et al., 2009). These findings showed that the incidences of AEs and rate of discontinuation attributable to AEs of ansofaxine ER tablets were similar to SSRIs and lower than SNRIs, suggesting that the safety and tolerability of ansofaxine ER tablets were similar to these SSRIs and better than other SNRIs. Most AEs that occurred in the present study were mild to moderate, with no unexpected adverse reactions. The most common AEs that were related to the study drug (>5% incidence and twofold higher incidence vs placebo) were nausea, lethargy, decreased appetite, and dry mouth, which were similar to SSRIs and SNRIs (Kennedy et al., 2009; Montgomery et al., 2013). Two SAEs occurred in the 40-mg/d ansofaxine dose group, namely hypomania (mild) and the exacerbation of depression (severe), which were determined to be related to the study drug and stable during follow-up. Similar SAEs have been reported for desmethylvenlafaxine succinate sustained-release tablets Desvenlafaxine Medical Review(s), 2008. All of the above comparisons suggest that ansofaxine ER tablets are safe for MDD patients.
Sexual dysfunction is common during antidepressant treatment. The prevalence of sexual dysfunction in depressive patients who are treated with antidepressants has been found to be 2 times higher than healthy controls (50% vs 24%) (Angst, 1998). The mechanisms that underlie sexual dysfunction are complex (Montejo et al., 2018). Two studies indicated that 27%–65% of female patients and 26%–57% of male depressed patients experienced either a worsening of preexisting difficulties or the emergence of new sexual difficulties in the early weeks of SSRI or SNRI treatment (Williams et al., 2006, 2010). In the present study, the incidence of sexual dysfunction was quite low relative to previous studies, indicating that ansofaxine may not have unwanted sexual side effects. The reason why ansofaxine is associated with a lower incidence of sexual dysfunction is unclear. A meta-analysis of 58 randomized controlled trials and 5 observational studies found some advantages of bupropion, with significantly lower sexual dysfunction compared with SSRIs, such as escitalopram, fluoxetine, paroxetine, and sertraline, which may be related to the mechanisms of action on NE and DA (Reichenpfader et al., 2014). Ansofaxine is a potential TRI with high affinity for the recombinant human DA transporter, recombinant human NE transporter, and recombinant human 5-HT transporter, which may be a mechanism that underlies the lower occurrence of sexual function–related AEs. Further studies should directly investigate the effects of ansofaxine on sexual dysfunction.
Pharmacological studies of desmethylvenlafaxine suggest that it has pain-relieving effects. Eight weeks of treatment with 100 mg/d desmethylvenlafaxine decreased VAS-PI scores. Although changes in VAS-PI scores that were caused by ansofaxine treatment were higher than in the placebo group, the differences were not statistically significant (Bonci and Hopf, 2005; Guiard et al., 2009). This may be related to the relatively small sample size, the patients’ low VAS-PI scores at baseline, and possibly insufficient drug dosages to elicit an antinociceptive effect in this trial.
One possible limitation of the present study was that the number of MDD patients was small. Further studies with a large sample size are required. The main objective of this trial was to determine the optimal dose of ansofaxine ER tablet and preliminarily assess its safety, efficacy, and tolerability. Another limitation of this study was that we did not explore the TRI effect of the different doses of ansofaxine ER tablet, which should be considered in future studies.
In conclusion, this Phase 2, randomized, placebo-controlled clinical trial of ansofaxine ER tablets (40–160 mg/d) demonstrated its safety, efficacy, and tolerability in patients with MDD. Our findings warrant further studies in Phase 3 clinical trials, including larger, multinational, randomized controlled trials.
Supplementary Material
Acknowledgments
This study was supported by grants from the National Science and Technology Major Project for Investigational New Drugs (nos. 2009ZX09103-100, 2013ZX09402201-002, 2017GSF218106, and 2018ZX09303015). Support was also received from the Beijing Municipal Commission of Science and Technology Clinical Application Special Fund (no. Z181100001718051). Shandong Luye Pharmaceutical Co., Ltd, provided Ansofaxine ER tablets and its placebo for the clinical trial. The sponsors had no role in the design or conduct of the study; data collection, management, analysis, or interpretation; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Statement of Interest
None.
References
- Angst J (1998) Sexual problems in healthy and depressed persons. Int Clin Psychopharmacol 13 Suppl 6:S1–S4. [DOI] [PubMed] [Google Scholar]
- Ashton MM, Berk M, Ng CH, Hopwood M, Dodd S, Turner A, Brown E, Jacka FN, Cotton SM, Khoo JP, Chatterton ML, Kavanagh BE, Nadjidai SE, Lo Monaco SL, Harvey BH, Sarris J, Malhi GS, Dowling NL, Dean OM (2019) Efficacy of adjunctive Garcinia mangostana Linn (mangosteen) pericarp for bipolar depression: study protocol for a proof-of-concept trial. Braz J Psychiatry 41:245–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonci A, Hopf FW (2005) The dopamine D2 receptor: new surprises from an old friend. Neuron 47:335–338. [DOI] [PubMed] [Google Scholar]
- Boulenger JP, Loft H, Olsen CK (2014) Efficacy and safety of vortioxetine (Lu AA21004), 15 and 20 mg/day: a randomized, double-blind, placebo-controlled, duloxetine-referenced study in the acute treatment of adult patients with major depressive disorder. Int Clin Psychopharmacol 29:138–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer P, Montgomery S, Lepola U, Germain JM, Brisard C, Ganguly R, Padmanabhan SK, Tourian KA (2008) Efficacy, safety, and tolerability of fixed-dose desvenlafaxine 50 and 100 mg/day for major depressive disorder in a placebo-controlled trial. Int Clin Psychopharmacol 23:243–253. [DOI] [PubMed] [Google Scholar]
- Brownstone L, Anderson K, Beenhakker J, Lock J, Le Grange D (2012) Recruitment and retention in an adolescent anorexia nervosa treatment trial. Int J Eat Disord 45:812–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho AF, Sharma MS, Brunoni AR, Vieta E, Fava GA (2016) The safety, tolerability and risks associated with the use of newer generation antidepressant drugs: a critical review of the literature. Psychother Psychosom 85:270–288. [DOI] [PubMed] [Google Scholar]
- Clayton AH, Croft HA, Handiwala L (2014) Antidepressants and sexual dysfunction: mechanisms and clinical implications. Postgrad Med 126:91–99. [DOI] [PubMed] [Google Scholar]
- Deardorff WJ, Grossberg GT (2014) A review of the clinical efficacy, safety and tolerability of the antidepressants vilazodone, levomilnacipran and vortioxetine. Expert Opin Pharmacother 15:2525–2542. [DOI] [PubMed] [Google Scholar]
- Desvenlafaxine Medical Review(s) (2008) https://www.accessdata.fda.gov/drugsatfda_docs/nda/2008/021992s000_MedR_P2.pdf. Accessed Janary 5, 2021.
- Dunlop BW, Thase ME, Wun CC, Fayyad R, Guico-Pabia CJ, Musgnung J, Ninan PT (2012) A meta-analysis of factors impacting detection of antidepressant efficacy in clinical trials: the importance of academic sites. Neuropsychopharmacology 37:2830–2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace AA (2016) Dysregulation of the dopamine system in the pathophysiology of schizophrenia and depression. Nat Rev Neurosci 17:524–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guiard BP, El Mansari M, Blier P (2009) Prospect of a dopamine contribution in the next generation of antidepressant drugs: the triple reuptake inhibitors. Curr Drug Targets 10:1069–1084. [DOI] [PubMed] [Google Scholar]
- Guo W, Gao Y, Jiang W, Li C, Lin F, Zhu H, Wang H, Ye L, Qi JG, Cen X, Tian J (2018) Toxicity effects of a novel potent triple reuptake inhibitor, LPM570065, on the fertility and early embryonic development in Sprague-Dawley rats. Regul Toxicol Pharmacol 100:45–51. [DOI] [PubMed] [Google Scholar]
- Huang Y, et al. (2019) Prevalence of mental disorders in China: a cross-sectional epidemiological study. Lancet Psychiatry 6:211–224. [DOI] [PubMed] [Google Scholar]
- Kennedy SH, Andersen HF, Thase ME (2009) Escitalopram in the treatment of major depressive disorder: a meta-analysis. Curr Med Res Opin 25:161–175. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Bromet EJ (2013) The epidemiology of depression across cultures. Annu Rev Public Health 34:119–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klamerus KJ, Maloney K, Rudolph RL, Sisenwine SF, Jusko WJ, Chiang ST (1992) Introduction of a composite parameter to the pharmacokinetics of venlafaxine and its active O-desmethyl metabolite. J Clin Pharmacol 32:716–724. [DOI] [PubMed] [Google Scholar]
- Kornstein S, Chang CT, Gommoll CP, Edwards J (2018) Vilazodone efficacy in subgroups of patients with major depressive disorder: a post-hoc analysis of four randomized, double-blind, placebo-controlled trials. Int Clin Psychopharmacol 33:217–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane RM (2015) Antidepressant drug development: focus on triple monoamine reuptake inhibition. J Psychopharmacol 29:526–544. [DOI] [PubMed] [Google Scholar]
- Lessard E, Yessine MA, Hamelin BA, O’Hara G, LeBlanc J, Turgeon J (1999) Influence of CYP2D6 activity on the disposition and cardiovascular toxicity of the antidepressant agent venlafaxine in humans. Pharmacogenetics 9:435–443. [PubMed] [Google Scholar]
- Li C, Jiang W, Gao Y, Lin F, Zhu H, Wang H, Ye L, Qi JG, Tian J (2018) Acute, subchronic oral toxicity, and genotoxicity evaluations of LPM570065, a new potent triple reuptake inhibitor. Regul Toxicol Pharmacol 98:129–139. [DOI] [PubMed] [Google Scholar]
- Liebowitz MR, Manley AL, Padmanabhan SK, Ganguly R, Tummala R, Tourian KA (2008) Efficacy, safety, and tolerability of desvenlafaxine 50 mg/day and 100 mg/day in outpatients with major depressive disorder. Curr Med Res Opin 24:1877–1890. [DOI] [PubMed] [Google Scholar]
- Meder D, Herz DM, Rowe JB, Lehéricy S, Siebner HR (2019) The role of dopamine in the brain - lessons learned from Parkinson’s disease. Neuroimage 190:79–93. [DOI] [PubMed] [Google Scholar]
- Montejo AL, Montejo L, Baldwin DS (2018) The impact of severe mental disorders and psychotropic medications on sexual health and its implications for clinical management. World Psychiatry 17:3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery SA, Mansuy L, Ruth A, Bose A, Li H, Li D (2013) Efficacy and safety of levomilnacipran sustained release in moderate to severe major depressive disorder: a randomized, double-blind, placebo-controlled, proof-of-concept study. J Clin Psychiatry 74:363–369. [DOI] [PubMed] [Google Scholar]
- Montgomery SA, Gommoll CP, Chen C, Greenberg WM (2015) Efficacy of levomilnacipran extended-release in major depressive disorder: pooled analysis of 5 double-blind, placebo-controlled trials. CNS Spectr 20:148–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ, Carlezon WA Jr (2006) The mesolimbic dopamine reward circuit in depression. Biol Psychiatry 59:1151–1159. [DOI] [PubMed] [Google Scholar]
- Pae CU (2011) Desvenlafaxine in the treatment of major depressive disorder. Expert Opin Pharmacother 12:2923–2928. [DOI] [PubMed] [Google Scholar]
- Perry R, Cassagnol M (2009) Desvenlafaxine: a new serotonin-norepinephrine reuptake inhibitor for the treatment of adults with major depressive disorder. Clin Ther 31 Pt 1:1374–1404. [DOI] [PubMed] [Google Scholar]
- Reichenpfader U, Gartlehner G, Morgan LC, Greenblatt A, Nussbaumer B, Hansen RA, Van Noord M, Lux L, Gaynes BN (2014) Sexual dysfunction associated with second-generation antidepressants in patients with major depressive disorder: results from a systematic review with network meta-analysis. Drug Saf 37:19–31. [DOI] [PubMed] [Google Scholar]
- Schweizer E, Thielen RJ, Frazer A (1997) Venlafaxine:a novel antidepressant compound. Expert Opin Investig Drugs 6:65–78. [DOI] [PubMed] [Google Scholar]
- Thase ME, Mahableshwarkar AR, Dragheim M, Loft H, Vieta E (2016) A meta-analysis of randomized, placebo-controlled trials of vortioxetine for the treatment of major depressive disorder in adults. Eur Neuropsychopharmacol 26:979–993. [DOI] [PubMed] [Google Scholar]
- The Global Burden of Disease: 2004 Update. https://www.mendeley.com/catalogue/50c6202e-0cbf-34f7-b0fe-032e42784817/. Accessed Feb 20, 2021.
- Tran P, Skolnick P, Czobor P, Huang NY, Bradshaw M, McKinney A, Fava M (2012) Efficacy and tolerability of the novel triple reuptake inhibitor amitifadine in the treatment of patients with major depressive disorder: a randomized, double-blind, placebo-controlled trial. J Psychiatr Res 46:64–71. [DOI] [PubMed] [Google Scholar]
- Williams VS, Baldwin DS, Hogue SL, Fehnel SE, Hollis KA, Edin HM (2006) Estimating the prevalence and impact of antidepressant-induced sexual dysfunction in 2 European countries: a cross-sectional patient survey. J Clin Psychiatry 67:204–210. [DOI] [PubMed] [Google Scholar]
- Williams VS, Edin HM, Hogue SL, Fehnel SE, Baldwin DS (2010) Prevalence and impact of antidepressant-associated sexual dysfunction in three European countries: replication in a cross-sectional patient survey. J Psychopharmacol 24:489–496. [DOI] [PubMed] [Google Scholar]
- Zhang R, Li X, Shi Y, Shao Y, Sun K, Wang A, Sun F, Liu W, Wang D, Jin J, Li Y (2014) The effects of LPM570065, a novel triple reuptake inhibitor, on extracellular serotonin, dopamine and norepinephrine levels in rats. PLoS One 9:e91775. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.