Abstract
Background
This double-blind (DB), randomized, parallel-group study was designed to evaluate efficacy and safety of paliperidone palmitate 6-month (PP6M) formulation relative to paliperidone palmitate 3-month (PP3M) formulation in patients with schizophrenia.
Methods
Following screening, patients entered an open-label (OL) maintenance phase and received 1 injection cycle of paliperidone palmitate 1-month (PP1M; 100 or 150 mg eq.) or PP3M (350 or 525 mg eq.). Clinically stable patients were randomized (2:1) to receive PP6M (700 or 1000 mg eq., gluteal injections) or PP3M (350 or 525 mg eq.) in a 12-month DB phase; 2 doses of PP6M (corresponding to doses of PP1M and PP3M) were chosen.
Results
Overall, 1036 patients were screened, 838 entered the OL phase, and 702 (mean age: 40.8 years) were randomized (PP6M: 478; PP3M: 224); 618 (88.0%) patients completed the DB phase (PP6M: 416 [87.0%]; PP3M: 202 [90.2%]). Relapse rates were PP6M, 7.5% (n = 36) and PP3M, 4.9% (n = 11). The Kaplan-Meier estimate of the difference (95% CI) between treatment groups (PP6M − PP3M) in the percentages of patients who remained relapse free was −2.9% (−6.8%, 1.1%), thus meeting noninferiority criteria (95% CI lower bound is larger than the pre-specified noninferiority margin of −10%). Secondary efficacy endpoints corroborated the primary analysis. Incidences of treatment-emergent adverse events were similar between PP6M (62.1%) and PP3M (58.5%). No new safety concerns emerged.
Conclusions
The efficacy of a twice-yearly dosing regimen of PP6M was noninferior to that of PP3M in preventing relapse in patients with schizophrenia adequately treated with PP1M or PP3M.
Trial Registration
Clinical Trials.gov identifier: NCT03345342
Keywords: Paliperidone palmitate 3-month, paliperidone palmitate 6-month, relapse-free, schizophrenia
Significance Statement.
Medications for schizophrenia in the form of injections that are long-lasting, known as long-acting injectable (LAI) antipsychotics, are more beneficial compared with oral pills in preventing the risk of relapse. Currently available LAIs can be received every 2 weeks up to once every 3 months, and there is a need for LAIs that are longer lasting, especially for patients with serious adherence issues and those living in isolated areas with healthcare access problems. Paliperidone palmitate 6-month (PP6M) is the first LAI that can be administered once every 6 months, enabling just 2 injections per year for patients who have been adequately treated on the 1-monthly or 3-monthly LAIs of paliperidone palmitate. In this phase 3 clinical study, the efficacy of PP6M was noninferior or comparable with the 3-monthly equivalent formulation (PP3M) for prevention of relapses, and there were no new safety concerns.
Introduction
Long-acting injectable (LAI) antipsychotics encourage better treatment outcomes in patients with schizophrenia by offering numerous advantages, including infrequent dosing, stable plasma medication levels, and adherence transparency and provide clinicians, patients, and caregivers the opportunity to intervene before further symptomatic worsening occurs (Brissos et al., 2014; Correll et al., 2016). LAI antipsychotics have shown superior efficacy to their oral equivalents in improving adherence, reducing hospitalizations and relapses in schizophrenia (Brissos et al., 2014; Marcus et al., 2015; Schreiner et al., 2015; Alphs et al., 2016; Correll et al., 2016; Greene et al., 2018).
There is extensive clinical trial evidence for the use of paliperidone palmitate 1-month (PP1M) and paliperidone palmitate 3-month (PP3M) formulations for maintaining treatment continuity and preventing relapses and risk of hospitalizations in patients with schizophrenia (Gopal et al., 2010, 2011; Hough et al., 2010; Berwaerts et al., 2015; Savitz et al., 2016; Di Lorenzo et al., 2018; Garcia-Portilla et al., 2020). The evidence is further strengthened by findings from real-world studies (Emsley et al., 2018; Emond et al., 2019; Patel et al., 2020). Both PP1M and PP3M have demonstrated efficacy in maintaining symptom and functional stability and acceptable safety and tolerability for long-term use in patients with schizophrenia (Attard et al., 2014; Bioque and Bernardo, 2018; Mathews et al., 2019). LAIs with longer dosing durations could be more patient-centric as they enable fewer injections per year with sustained medication delivery, particularly useful for patients living in isolated areas who may have transportation limitations or healthcare access problems (Mace et al., 2019; Pietrini et al., 2019; Blackwood et al., 2020; Moreno et al., 2020). To address this need, a paliperidone palmitate 6-month (PP6M) formulation was developed and is the first LAI with a substantially longer dosing interval of 6 months, enabling just 2 injections per year. The PP6M LAI is not intended for acutely symptomatic patients. Patients need to be stabilized on the shorter-acting equivalents (PP1M or PP3M) before transitioning to the longer-acting treatment with PP6M.
The present study was an active-controlled, non-inferiority study to evaluate the efficacy of PP6M relative to PP3M in preventing relapses and assess the safety and pharmacokinetics (PK) of PP6M in clinically stabilized patients with schizophrenia.
MATERIALS AND METHODS
Ethical Practices
The study protocol and amendments were approved by an independent ethics committee or institutional review board (See list of institutional review boards and independent ethics committees in the online supplement). The trial was conducted in compliance with the ethical principles of the Declaration of Helsinki, Good Clinical Practices, and applicable regulatory requirements. All patients provided written informed consent before study participation.
Patients
Eligible patients were between 18 and 70 years of age, had a Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5) diagnosis of schizophrenia for ≥6 months before screening, and had a Positive and Negative Syndrome Scale (PANSS) total score of <70 points at screening. Patients were receiving treatment with paliperidone palmitate (PP1M [100 or 150 mg eq.] or PP3M [350 or 525 mg eq.], with dose timing that fits the current study schedule), injectable risperidone microspheres, or any oral antipsychotic (except clozapine), which in the opinion of the investigator could have been discontinued during screening (except for PP1M/PP3M).
Patients who met any of the following criteria were excluded from the study: active primary DSM-5 diagnosis other than schizophrenia; receiving any form of involuntary treatment (e.g., involuntary psychiatric hospitalization); attempted suicide within 12 months before screening and was at imminent risk of suicide or violent behavior, as clinically assessed by the investigator; DSM-5 diagnosis of moderate or severe substance use disorder (except for nicotine or caffeine) within 6 months of screening; history of neuroleptic malignant syndrome, tardive dyskinesia, or clinically significant and unstable medical illness; history of treatment-resistant schizophrenia (i.e., failure to respond to 2 adequate trials of different antipsychotic medications with adequate doses); or intolerability or severe reactions to moderate or higher doses of antipsychotic medications. Please see supplementary Methods for details on entry criteria.
Patients were recruited from various sites, and the recruitment method was dependent on the regulations of the respective independent ethics committee or institutional review board and meeting eligibility criteria. Acutely ill patients were excluded, and the recruitment was at the discretion of the investigators.
Study Design
This study (NCT03345342) was a double-blind (DB), randomized, active-controlled, parallel-group multicenter study conducted between November 2017 and May 2020 at 121 research sites in 20 countries.
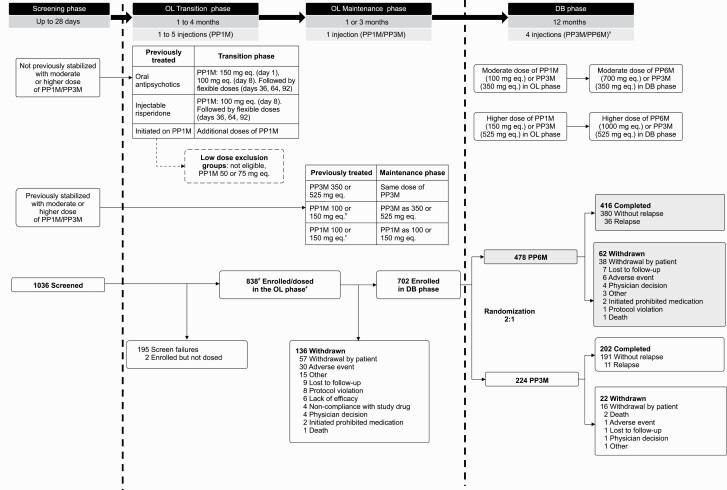
The study had 3 phases: (1) a screening phase (up to 28 days), (2) an open-label (OL) maintenance phase (duration of 1 or 3 months depending on treatment received [1 injection cycle of PP1M/PP3M]), and (3) DB phase (12 months) (Figure 1). Patients who entered the study on an oral antipsychotic, injectable risperidone microspheres, or PP1M previously initiated but not stabilized at study entry were eligible to participate in an OL transition phase just prior to the OL maintenance phase to initiate and/or continue treatment with PP1M for up to 4 months. Patients on PP1M or PP3M OL maintenance treatment could be randomized to DB treatment with equivalent doses of PP6M or PP3M.
Figure 1.
Study design and patient disposition. Abbreviations: DB, double-blind; OL, open label; PP1M, paliperidone palmitate once-monthly formulation; PP3M, paliperidone palmitate 3-month formulation; PP6M, paliperidone palmitate 6-month formulation. aPatients in PP6M group received 2 placebo injections. bBefore pre-randomization (approximately one-half of the total maintenance phase sample was treated with PP3M) criteria are met. cAfter pre-randomization criteria are met. dOne patient entered the OL transition phase, withdrew, then re-screened again and entered the DB phase. eAmong 838 patients who entered the OL phase, 71 withdrew during OL transition phase and 767 entered the OL maintenance phase.
Treatment
Patients could have entered the study screening phase on PP1M (100 or 150 mg eq.), PP3M (350 or 525 mg eq.), injectable risperidone microspheres (50 mg), or oral antipsychotics (at any dosage with a reason to change, such as problems with efficacy, safety or tolerability, or a preference for a LAI) as prior medications. Patients without documented tolerability to any oral or injectable risperidone or paliperidone formulations received paliperidone extended-release/prolonged-release 6-mg tablets or risperidone 3 mg/d for 4–6 consecutive days during screening. Patients previously treated with oral antipsychotics, injectable risperidone microspheres, or a moderate or higher dose of PP1M but without previous stabilization (defined as ≥3 monthly injections, with the last 2 doses being the same dose strength) received additional doses of PP1M during the conditional OL transition phase (Figure 1).
In the OL maintenance phase, all patients received only 1 dose of PP1M (100 or 150 mg eq.) or PP3M (350 or 525 mg eq.), which was either matched (PP1M to PP1M, or PP3M to PP3M) or converted (PP1M to PP3M) to an equivalent of the dose last received in the OL transition phase or prior to enrollment (Figure 1). To ensure adequate numbers of PP1M- and PP3M-treated patients were switched to PP6M when randomized, some patients (after appropriate treatment with PP1M) were switched to PP3M on entry to the OL maintenance phase (until approximately one-half of the total maintenance phase sample was treated with PP3M) because low enrollment of patients previously treated with PP3M was anticipated. Injections of PP1M or PP3M during the OL phase were deltoid or gluteal in accordance with the prescribing information. In the DB phase, patients were randomized to PP6M or PP3M (2:1, respectively). Patients in the PP3M group who received OL PP1M doses (100 or 150 mg eq.) were assigned to DB PP3M doses (350 or 525 mg eq., respectively), and those on OL PP3M doses (350 or 525 mg eq.) continued at the same DB dose level (Figure 1). Patients in the PP6M group were transitioned to PP6M doses corresponding to the prescribed doses of PP1M (100 mg eq. or 150 mg eq.) and PP3M (350 mg eq. or 525 mg eq.) (supplementary Table 1). Patients who received “moderate” doses of OL PP1M (100 mg eq.) or PP3M (350 mg eq.) received the “moderate” dose of PP6M (700 mg eq.) in the DB phase, and those on the “high” doses of OL PP1M (150 mg eq.) or PP3M (525 mg eq.) received the “high” dose of PP6M (1000 mg eq.) in the DB phase. Injections (PP6M and PP3M) were administered dorsogluteally due to the larger volume of PP6M.
Randomization and Blinding
Randomization was based on a computer-generated randomization schedule, balanced using randomly permuted blocks, and stratified by study site and moderate or high dose in the maintenance phase. To maintain the blinding, patients treated with PP6M received injections of placebo at the 3-month time points between their 6-month doses. The placebo was 20% Intralipid® (200 mg/mL) injectable emulsion and matched the appearance of the active treatment. Therefore, the 12-month DB phase included a total of 4 injections at 3-month intervals, irrespective of treatment group. Due to differences in syringe sizes for PP6M vs PP3M, the study drug administrator was unblinded and not allowed to perform any other study-related procedures or communicate patient-related information with study-site personnel to ensure integrity of the blind.
PK Assessments
Efficacy Assessments
The primary endpoint was time to relapse during the DB phase. This noninferiority primary endpoint was based on the difference in the Kaplan-Meier 12-month estimate of survival (i.e., percentage of patients remaining relapse free) between PP6M and PP3M. The relapse criteria were identical to those used in previous clinical studies of PP3M and PP1M (Berwaerts et al., 2015; Savitz et al., 2016). Relapse was defined as ≥1 of the following: (1) psychiatric hospitalization due to exacerbation of schizophrenia symptoms (involuntary or voluntary admission); (2) 25% increase (for patients with PANSS scores >40 at randomization) or 10-point increase (for patients with PANSS scores ≤40 at randomization) in PANSS total score from randomization for 2 consecutive assessments between 3 to 7 days; (3) deliberate self-injury resulting in suicide or exhibited violent behavior resulting in clinically significant injury; (4) aggressive behavior, suicidal or homicidal ideation; (5) PANSS items scores ≥5 (if PANSS items was ≤3 at randomization); or ≥6 (PANSS items was 4 at randomization) after randomization for 2 consecutive assessments between 3 to 7 days on any of the following items: P1 (delusions), P2 (conceptual disorganization), P3 (hallucinatory behavior), P6 (suspiciousness/persecution), P7 (hostility), and G8 (uncooperativeness). The date of relapse was the date of the first assessment for symptoms of relapse (not the date of confirmation).
Secondary efficacy endpoints included changes from baseline during the 12 months of the DB phase in the following scales: PANSS total score and subscale scores (Kay et al., 1987), Clinical Global Impression-Severity (CGI-S) (Busner and Targum, 2007), and Personal and Social Performance (PSP) scale (Morosini et al., 2000). Additionally, the proportion of patients during the DB phase who met criteria for symptomatic remission (defined as having a score ≤3 on all of the following 8 PANSS items: P1, P2, P3, N1, N4, N6, G5, and G9 for the last 6 months of DB treatment, with 1 excursion allowed) was summarized (Andreasen et al., 2005).
Safety Assessments
Safety was assessed by treatment-emergent adverse events (TEAEs), 12-lead electrocardiograms, vital signs, clinical laboratory tests, and injection-site evaluations. Extrapyramidal symptoms (EPS) were assessed by the Simpson-Angus Scale, Barnes Akathisia Rating Scale, and Abnormal Involuntary Movement Scale. Suicidal ideation and behavior were assessed using Columbia Suicide Severity Rating Scale.
Statistical Analysis
Sample Size Determination
Sample size calculation for the DB phase was based on the primary endpoint, which was also tested for noninferiority. The study was designed to have a minimum of 80% power to demonstrate an 85% survival rate (percentage of patients remaining relapse free at 12 months) in the PP3M group based on 1-sided significance level of 2.5%. A total 549 patients were expected to be randomized (2:1, PP6M:PP3M) to demonstrate with 80% power that PP6M is no worse than PP3M by a noninferiority margin of −10% for the percentage of patients remaining relapse free at 12 months assuming efficacy similar as observed in previous studies of PP3M (rationale for the 10% noninferiority margin is described previously; Savitz et al., 2016). The study design assumed discontinuation rates of 20% and 40% during the transition and maintenance for patients who entered the study with or without previous PP1M or PP3M stability, respectively, and a dropout rate of 10% during the DB phase. Given these assumptions for discontinuation, the study targeted approximately 840 patients to enter the transition or maintenance phase.
Statistical Analyses
Plasma concentrations of paliperidone and paliperidone palmitate and the derived PK parameters were summarized descriptively for the PK data analysis set. Efficacy and safety during the OL phase were summarized for the OL intent-to-treat (ITT-OL) set (all patients who received ≥1 dose of OL study drug, including transition and maintenance phases). The primary efficacy analysis included all randomized patients who received ≥1 dose of treatment (PP3M or PP6M) during the DB phase (ITT-DB analysis set). Analyses involving changes from the DB baseline were provided for both observed case and last observation carried forward data. The ITT-DB set was used for all secondary efficacy endpoints. An additional analysis with the per-protocol analysis set (all patients who were randomized in the DB-phase received ≥1 dose of DB treatment and did not have any major protocol violations) was performed to evaluate consistency of results for the primary endpoint. Safety analysis during the DB phase was conducted using the DB safety analysis set (DB safety; same as the ITT-DB).
The null hypotheses to be tested using a 1-sided α = 0.025 level were as follows: H0: p6-p3≤−δ vs H1: p6-p3 >−δ; where p3 referred to the percentage of patients who remained relapse free at 12 months for the PP3M groups and p6 referred to the percentage of patients who remained relapse free at 12 months for the PP6M group. The Kaplan-Meier method was used to estimate the 12-month cumulative estimate of survival. Standard error (SE) estimates were based on Greenwood’s formula. Noninferiority of PP6M to PP3M was to be concluded if the lower limit of the 2-sided 95% confidence interval (CI) of the difference in the relapse-free rates between PP6M and PP3M exceeded −10%. Hazard ratios (HR) along with 95% CI were estimated using the Cox proportional hazards model with treatment as the only factor to compare treatment effects on the time to relapse of schizophrenia symptoms. The change from baseline (DB) at each visit in PANSS total and subscale scores and CGI-S and PSP scores during the DB phase was analyzed using an ANCOVA model with factors for treatment and country and baseline score as a covariate. Estimates of treatment effects as least square mean difference (PP6M − PP3M) and the accompanying 95% CI were presented. The proportion of patients achieving symptomatic remission in the DB phase was calculated using the Cochran-Mantel-Haenszel test controlling for country.
RESULTS
Patient Disposition
Of the 838 patients enrolled and dosed in the OL phases, 702 (83.8%) completed the OL phases and were randomized (PP6M, n = 478; PP3M, n = 224) in the DB phase (Figure 1). All 702 patients were included in the ITT-DB set. Withdrawal by patient (57/838 [6.8%]) or adverse events (30/838 [3.6%]) were the common reasons for discontinuations in the OL phases. A total of 618 (88.0%) patients completed the DB phase, with similar percentages in both treatment groups (PP3M, n = 202 [90.2%]; PP6M n = 416 [87.0%]). Withdrawal by patient (54/702 [7.7%]) was the most common reason for discontinuation during the DB phase.
Demographics and Baseline Characteristics
The demographics and baseline (OL) psychiatric characteristics of patients in the PP6M and PP3M groups in the DB phase were generally similar (Table 1). Overall demographics and baseline characteristics of dropouts/not randomized were similar to those randomized in the DB phase. Patients (n = 702) were a mean age of 40.8 (range, 18–69) years and were mostly men (68.4%), White (74.2%), non-Hispanic (84.6%), and ≤50 years of age (79.6%). The PP6M and PP3M treatment groups were matched in terms of baseline severity of illness as measured by the PANSS, CGI-S, and PSP scores and age at first diagnosis. Duration of psychiatric hospitalization was numerically longer in the PP6M group (mean [SD] duration of hospitalization: PP6M, 63.1 [70.25] days vs PP3M, 44.6 [53.09] days).
Table 1.
Demographic, Baseline Characteristics, and Psychiatric History (ITT-OL and ITT-DB Analysis Sets)
| DB phase (ITT-DB) | |||
|---|---|---|---|
| PP6M (n = 478) | PP3M (n = 224) | Total (n = 702) | |
| Age,a mean (SD), y | 41.2 (11.77) | 40.0 (10.98) | 40.8 (11.53) |
| Sex, n (%) | |||
| Men | 326 (68.2) | 154 (68.8) | 480 (68.4) |
| Race, n (%) | |||
| American Indian or Alaska Native | 0 | 0 | 0 |
| Asianb | 66 (13.8) | 30 (13.4) | 96 (13.7) |
| Black or African American | 49 (10.3) | 23 (10.3) | 72 (10.3) |
| Native Hawaiian or Other Pacific Islander | 3 (0.6) | 0 | 3 (0.4) |
| White | 353 (73.8) | 168 (75.0) | 521 (74.2) |
| Other | 0 | 0 | 0 |
| Multiple | 3 (0.6) | 2 (0.9) | 5 (0.7) |
| Not reported | 4 (0.8) | 1 (0.4) | 5 (0.7) |
| Ethnicity, n (%) | |||
| Hispanic or Latino | 75 (15.7) | 25 (11.2) | 100 (14.2) |
| Not Hispanic or Latino | 397 (83.1) | 197 (87.9) | 594 (84.6) |
| Not reported | 6 (1.3) | 2 (0.9) | 8 (1.1) |
| Weight-baseline (OL), mean (SD), kg | 81.9 (16.86) | 80.8 (17.01) | 81.5 (16.90) |
| BMI-baseline (OL), mean (SD), kg/m2 | 27.9 (4.96) | 27.5 (4.96) | 27.7 (4.96) |
| Age at schizophrenia diagnosis, mean (SD), y | 27.7 (9.01) | 27.5 (9.05) | 27.6 (9.02) |
| Prior hospitalization,c n (%) | |||
| N | 356 | 168 | 524 |
| None | 205 (57.6) | 98 (58.3) | 303 (57.8) |
| Once | 97 (27.2) | 47 (28.0) | 144 (27.5) |
| Twice | 37 (10.4) | 18 (10.7) | 55 (10.5) |
| Three times | 11 (3.1) | 4 (2.4) | 15 (2.9) |
| Four times or more | 6 (1.7) | 1 (0.6) | 7 (1.3) |
| PANSS total, n | |||
| Baseline (MA), mean (SD) | 53.1 (9.19) | 52.9 (9.62) | 53.0 (9.33) |
| Baseline (DB), mean (SD) | 51.9 (9.60) | 51.4 (9.77) | 51.7 (9.65) |
| PSP | |||
| Baseline (MA), mean (SD) | 65.6 (12.37) | 65.2 (11.78) | 65.5 (12.18) |
| Baseline (DB), mean (SD) | 66.3 (12.50) | 66.5 (11.82) | 66.4 (12.28) |
| CGI-S | |||
| Baseline (MA), mean (SD) | 3.1 (0.78) | 3.1 (0.76) | 3.1 (0.78) |
| Baseline (DB), mean (SD) | 3.0 (0.78) | 3.0 (0.77) | 3.0 (0.78) |
Abbreviations: BMI, body mass index; CGI-S, Clinical Global Impression-Severity; DB, double-blind; ITT, intent-to-treat; MA, maintenance phase; OL, open label; PANSS, Positive and Negative Symptom Scale; PP3M, paliperidone palmitate 3-month formulation; PP6M, paliperidone palmitate 6-month formulation PSP, Personal and Social Performance; SD, standard deviation.
a Age at screening.
b Asian subcategories include Chinese, Korean, Japanese, Filipino, Asian Indian, Thai, Malaysian, and Asian (other).
c Number of hospitalizations for psychosis within 24 months prior to study start.
Prior and Concomitant Therapies
At DB phase entry, the proportion of patients on prior psychotropic medication was similar between the PP3M and PP6M group (supplementary Table 2). Before study entry, 701/702 (99.9%) patients received psychotropic medications. Atypical antipsychotics (61.0%) were most commonly used, 18.4% of patients received benzodiazepines, and 14.2% were on anti-EPS medications.
During the DB phase, the concomitant use of benzodiazepines (23.6% vs 21.0%), anti-EPS medications such as anticholinergic medications (15.5% vs 12.9%), antihistamines (6.1% vs 8.5%), and beta-blockers (propranolol: 2.1% vs 1.8%; metoprolol: 1.3% vs 1.8%) were similar between PP6M and PP3M groups (supplementary Table 3). A total of 415/702 (59.1%) patients received concomitant medications other than benzodiazepines during the DB phase (PP6M: 60.5% vs PP3M: 56.3%), the common ones being acetaminophen (9.7%), metformin (8.3%), and biperiden (7.7%).
Drug Exposure
A total of 106/838 (12.6%) patients underwent oral tolerability testing, and none reported tolerability concerns. Of the 568 patients treated in the OL transition phase, 272 (47.9%) received 5 injections of PP1M and 217 (38.2%) received 4 injections; 458 (80.6%) patients received PP1M for ≥91 days. Of the 767 patients treated in the OL maintenance phase, 362 patients received PP1M (100 mg eq., n = 175; 150 mg eq., n = 187) and 405 received PP3M (350 mg eq. n = 193; 525 mg eq., n = 212). In the DB phase, the mean (SD) duration of exposure was 329.8 (86.97) days in the PP6M group and 336.4 (80.89) days in the PP3M group. The mean (SD) dose of PP6M was 855.6 (150.05) mg eq. and the mean (SD) dose of PP3M was 442.2 (87.57) mg eq. Overall, 86.4% of patients in the PP6M group received 2 and 84.8% in the PP3M group received 4 active injections.
Pharmacokinetics
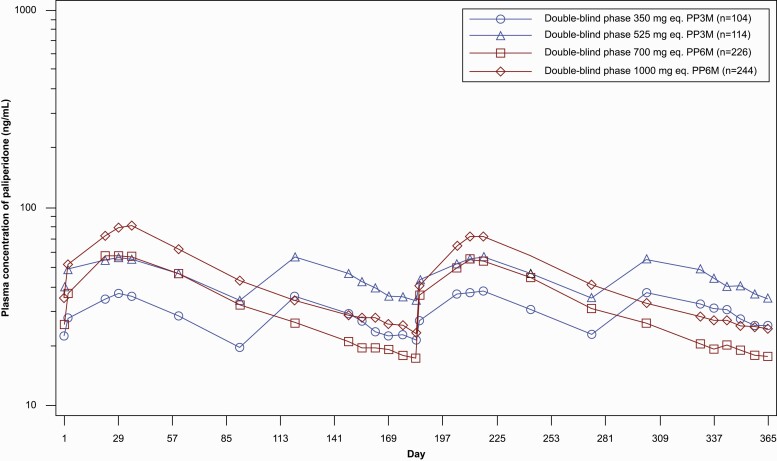
In the DB phase for all treatments and administered dosages, mean Cmax was achieved around 1 month after each dose (Figure 2). After achieving mean Cmax, concentrations gradually declined for the remainder of the dosing cycle. Patients who received PP6M had approximately 20%–25% lower trough concentrations (dose normalized Ctrough) compared with patients who received PP3M. In the DB phase, mean peak paliperidone concentrations (dose normalized Cmax) after PP6M dosing was slightly higher (1.4- to 1.5-fold) than after PP3M dosing, and mean total paliperidone exposure (dose normalized AUC6month) was comparable after PP3M and PP6M dosing (see supplementary Results for more details).
Figure 2.
Semi-logarithmic mean (SD) plasma concentration time profiles of paliperidone after administration of PP3M at 350 or 525 mg eq. and PP6M at 700 or 1000 mg eq. (PK data analysis set) in the DB phase. The x-axis displays the day relative to the day of dosing (day 1) in the DB phase. Abbreviations: DB, double-blind; PK, pharmacokinetics; PP3M, paliperidone palmitate 3-month formulation; PP6M, paliperidone palmitate 6-month formulation.
Efficacy
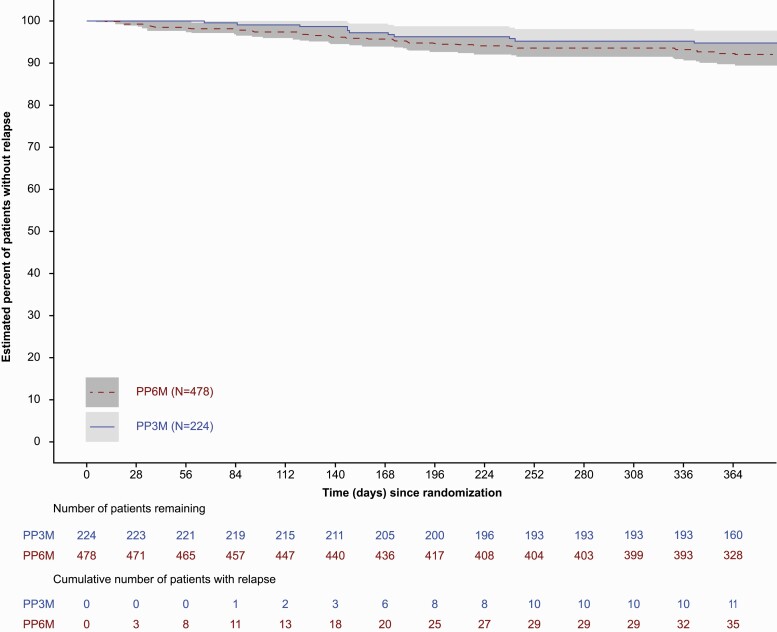
Thirty-six (7.5%) patients in the PP6M group and 11 (4.9%) in the PP3M group experienced a relapse event during the DB phase (ITT-DB). The Kaplan-Meier estimate of the difference (95% CI) between the treatment groups (PP6M − PP3M) in the percentages of patients who remained relapse free was −2.9% (−6.8%, 1.1%). Thus, PP6M was noninferior to PP3M based on the lower bound of the 95% CI being larger than the pre-specified noninferiority margin of −10% (Figure 3). Analysis using the per-protocol set corroborated the ITT-DB analysis, with the lower bound of 95% CI of Kaplan-Meier estimate of treatment difference being larger than the pre-specified noninferiority margin of −10% (supplementary Figure 1). Thus, PP6M was declared noninferior to PP3M for the primary efficacy endpoint. The median time to relapse (the time at which the cumulative survival function equals 0.5 [or 50%]) was not estimable for either the PP6M or PP3M groups due to the low number of relapses during the DB phase (Table 2; Figure 3). The most common reasons for relapses were an increase of ≥25% in PANSS total score (PP6M: 16 [3.3%]; PP3M: 5 [2.2%]), PANSS item (P1, P2, P3, P6, P7, G8) score of ≥5 after randomization (PP6M: 13 [2.7%]; PP3M: 5 [2.2%]), and psychiatric hospitalizations (PP6M: 11 [2.3%]; PP3M: 6 [2.7%]). The ratio (95% CI) of the instantaneous risk of relapse for a patient who received PP6M treatment vs the risk for a patient who received PP3M in the DB phase was 1.57 (0.80, 3.08).
Figure 3.
Kaplan-Meier plot and 95% pointwise confidence-based percentage of patients without relapse during the DB phase (ITT-DB analysis set). Abbreviations: DB, double-blind; ITT, intent-to-treat; PP3M, paliperidone palmitate 3-month formulation; PP6M, paliperidone palmitate 6-month formulation.
Table 2.
Time to Relapse During DB Phase and Number of Patients Who Remained Relapse Free at End of DB Phase (ITT-DB Analysis Set)
| PP6M (n = 478) | PP3M (n = 224) | Total (n = 702) | |
|---|---|---|---|
| No. assessed | 478 | 224 | 702 |
| No. censored (%)a | 442 (92.5) | 213 (95.1) | 655 (93.3) |
| No. of relapse (%) | 36 (7.5) | 11 (4.9) | 47 (6.7) |
| Time to relapse (days)b | |||
| 25% Quantile (95% CI) | NE | NE | NE |
| Median (95% CI) | NE | NE | NE |
| 75% Quantile (95% CI) | NE | NE | NE |
| Relapse freeb | |||
| End of 12 mo (d 365 [DB]) | |||
| Percentage relapse free | 91.9 | 94.8 | |
| Difference (PP6M − PP3M) | −2.9 | ||
| 95% CI | (−6.8; 1.1) |
Abbreviations: CI, confidence interval; DB, double-blind; ITT, intent-to-treat; NE, not estimable; PP3M, paliperidone palmitate 3-month formulation; PP6M, paliperidone palmitate 6-month formulation.
a Censored include patients who completed the DB phase without relapses and patients who withdrew early during the DB phase.
b Based on Kaplan-Meier product limit estimates.
Results for the secondary endpoints corroborated the primary efficacy analysis, indicating comparable improvements in PANSS total, subscale and Marder factor scores, and CGI-S and PSP scores between PP6M and PP3M groups from DB baseline to DB endpoint (Table 3; supplementary Figures 2 and 3). Changes in CGI-S and PSP scores indicated persistence of clinical stability over the 12-month DB period. The percentage of patients with ≥20% improvement from DB baseline to DB endpoint in PANSS total scores was numerically higher in the PP6M (38.9%) compared with PP3M (32.1%); similar percentage of patients in the PP6M and PP3M treatment groups showed an improvement of ≥30% and ≥40% in PANSS total score (Table 3). More than 60% of patients in both treatment groups (PP6M: 66.3%; PP3M: 70.1%) achieved symptomatic remission during the DB phase.
Table 3.
Summary of Change From DB Baseline in Secondary Efficacy Measures During the DB Phase (ITT-DB Analysis Set)
| PP6M (n = 478) | PP3M (n = 224) | Between-group difference LS means (SE), (95% CI) | |
|---|---|---|---|
| PANSS total scorea | |||
| Baseline, mean (SD) | 51.9 (9.60) | 51.4 (9.77) | −0.1 (0.67) |
| Change from baseline, mean (SD) | −1.8 (8.92) | −1.6 (7.40) | (−1.44; 1.19) |
| PANSS subscale scores,a mean (SD) | |||
| Positive subscale | |||
| Baseline | 11.0 (3.21) | 10.8 (2.98) | 0.0 (0.25) |
| Change from baseline | −0.1 (3.30) | −0.1 (2.82) | −0.46; 0.51 |
| Negative subscale | |||
| Baseline | 16.0 (4.20) | 15.9 (4.18) | −0.1 (0.21) |
| Change from baseline | −0.7 (2.70) | −0.6 (2.61) | −0.48; 0.35 |
| General psychopathology subscale | |||
| Baseline | 24.9 (4.78) | 24.7 (5.05) | −0.0 (0.36) |
| Change from baseline | −1.0 (4.86) | −0.9 (4.18) | −0.76; 0.66 |
| PANSS Marder standardized factor scores,a mean (SD) | |||
| Positive symptoms | |||
| Baseline | 13.9 (4.10) | 13.7 (3.73) | 0.0 (0.27) |
| Change from baseline | −0.4 (3.65) | −0.4 (3.19) | −0.50; 0.56 |
| Negative symptoms | |||
| Baseline | 14.9 (4.07) | 14.8 (4.12) | −0.0 (0.21) |
| Change from baseline | −0.8 (2.82) | −0.7 (2.61) | −0.45; 0.38 |
| Disorganized thoughts | |||
| Baseline | 12.6 (3.39) | 12.5 (3.47) | −0.2 (0.19) |
| Change from baseline | −0.4 (2.35) | −0.2 (2.56) | −0.59; 0.16 |
| Uncontrolled hostility/excitement | |||
| Baseline | 4.8 (1.38) | 4.7 (1.15) | 0.1 (0.12) |
| Change from baseline | 0.1 (1.71) | 0.0 (1.31) | −0.14; 0.32 |
| Anxiety/depression | |||
| Baseline | 5.7 (1.94) | 5.7 (2.05) | 0.0 (0.16) |
| Change from baseline | −0.3 (2.24) | −0.3 (2.17) | −0.28; 0.34 |
| CGI-S score,a n | 477 | 224 | |
| Baseline, mean (SD) | 3.0 (0.78) | 3.0 (0.77) | −0.0 (0.05) |
| Change from baseline, mean (SD) | 0.0 (0.70) | 0.0 (0.63) | −0.11; 0.09 |
| PSP score,a n | 478 | 224 | |
| Baseline, mean (SD) | 66.3 (12.50) | 66.5 (11.82) | −0.2 (0.57) |
| Change from baseline, mean (SD) | 1.0 (7.12) | 1.1 (8.11) | −1.27; 0.97 |
| Improvement in PANSS total, n (%) | Relative risk (95% CI)b | ||
| ≥20% | 183 (38.9) | 70 (32.1) | 1.12 (1.00; 1.25) |
| <20% | 287 (61.1) | 148 (67.9) | |
| ≥30% | 119 (25.3) | 52 (23.9) | 1.02 (0.93; 1.12) |
| <30% | 351 (74.7) | 166 (76.1) | |
| ≥40% | 77 (16.4) | 33 (15.1) | 1.01 (0.94; 1.08) |
| <40% | 393 (83.6) | 185 (84.9) | |
| DB 6-month remission status,c n (%) | 478 | 224 | |
| Achieved | 317 (66.3) | 157 (70.1) | 0.89 (0.71; 1.13) |
Abbreviations: CGI-S, Clinical Global Impression-Severity; CI, confidence interval; DB, double-blind; ITT, intent-to-treat; LS, least squares; PANSS, Positive and Negative Symptom Scale; PP3M, paliperidone palmitate 3-month formulation; PP6M, paliperidone palmitate 6-month formulation; PSP, Personal and Social Performance; SD, standard deviation; SE, standard error;
a Based on ANCOVA model with treatment (PP6M vs PP3M) and country as factors, and baseline value as a covariate.
b Point estimate (95% CI) of relative risk is based on Cochran-Mantel-Haenszel test controlling for country.
c Remission is defined as having a score of ≤3 on all of the following 8 PANSS items: P1, P2, P3, N1, N4, N6, G5, and G9 for the last 6 months of DB treatment, with 1 excursion allowed.
Safety
A total of 341 of 838 patients (40.7%) had ≥1 TEAE in the OL phase; 23 (2.7%) patients had ≥1 serious TEAE, 31 (3.7%) had TEAEs leading to withdrawal of the study drug, and 1 (0.1%) death (due to completed suicide) was reported in the OL phase (Table 4).
Table 4.
Summary of Treatment-Emergent Adverse Events During the OL and DB Phases (DB Safety Analysis Set)
| ITT-OL | ITT-DB safety analysis sets | ||
|---|---|---|---|
| No. of patients (%) | |||
| PP1M/PP3M (n = 838) | PP6M (n = 478) | PP3M (n = 224) | |
| Patients with ≥1 TEAEs | 341 (40.7) | 297 (62.1) | 131 (58.5) |
| Patients with ≥1 serious TEAEs | 23 (2.7) | 24 (5.0) | 15 (6.7) |
| Most common (>5 patients) serious TEAE | |||
| Schizophrenia | 6 (0.7) | 8 (1.7) | 1 (0.4) |
| TEAEs leading to drug withdrawala | 31 (3.7) | 16 (3.3) | 6 (2.7) |
| Most common (>5 patients) TEAEs leading to drug withdrawala | |||
| Schizophrenia | 6 (0.7) | 8 (1.7%) | 1 (0.4) |
| TEAEs leading to death | 1 (0.1) | 1 (0.2) | 2 (0.9) |
| Most common (≥2% of patients) TEAEs | |||
| Weight increase | 8 (1.0) | 40 (8.4) | 17 (7.6) |
| Injection site pain | 72 (8.6) | 37 (7.7) | 9 (4.0) |
| Headache | 16 (1.9) | 32 (6.7) | 12 (5.4) |
| Upper respiratory tract infection | 19 (2.3) | 24 (5.0) | 9 (4.0) |
| Nasopharyngitis | 22 (2.6) | 22 (4.6) | 13 (5.8) |
| Akathisia | 21 (2.5) | 17 (3.6) | 8 (3.6) |
| Insomnia | 27 (3.2) | 15 (3.1) | 5 (2.2) |
| Anxiety | 25 (3.0) | 15 (3.1) | 1 (0.4) |
| Influenza | 11 (1.3) | 13 (2.7) | 4 (1.8) |
| Urinary tract infection | 4 (0.5) | 13 (2.7) | 2 (0.9) |
| Schizophrenia | 9 (1.1) | 11 (2.3) | 3 (1.3) |
| Weight decrease | 4 (0.5) | 8 (1.7) | 7 (3.1) |
| Suicidal ideation | 9 (1.1) | 4 (0.8) | 6 (2.7) |
| Patients with ≥1 EPS-related TEAEs | 53 (6.3) | 46 (9.6) | 19 (8.5) |
| Most common (>5 patients) EPS-related TEAEs | |||
| Parkinsonian rest tremor | 11 (1.3) | 9 (1.9) | 2 (0.9) |
| Muscle rigidity | 7 (0.8) | 2 (0.4) | 0 (0.0) |
| Parkinsonism | 7 (0.8) | 6 (1.3) | 2 (0.9) |
| Akathisia | 21 (2.5) | 17 (3.6) | 8 (3.6) |
| Dyskinesia | 11 (1.3) | 6 (1.3) | 2 (0.9) |
| Dystonia | 3 (0.4) | 1 (0.2) | 0 |
| Tremor | 1 (0.1) | 1 (0.2) | 0 (0.0) |
| Patients with ≥1 injection site-related TEAEs | 89 (10.6) | 59 (12.3) | 11 (4.9) |
| Injection site pain | 72 (8.6) | 37 (7.7) | 9 (4.0) |
| Injection site swelling | 8 (1.0) | 8 (1.7) | 1 (0.4) |
| Injection site induration | 8 (1.0) | 7 (1.5) | 2 (0.9) |
| Pain in extremity | 6 (0.7) | 7 (1.5) | 3 (1.3) |
| Injection site discomfort | 2 (0.2) | 3 (0.6) | 1 (0.4) |
| Injection site erythema | 2 (0.2) | 3 (0.6) | 1 (0.4) |
| Musculoskeletal pain | 2 (0.2) | 3 (0.6) | 0 (0.0) |
| Injection site haemorrhage | 0 | 1 (0.2) | 0 (0.0) |
| Injection site nodule | 0 | 1 (0.2) | 0 (0.0) |
| Injection site oedema | 3 (0.4) | 1 (0.2) | 0 (0.0) |
Abbreviations: DB, double-blind; EPS, extrapyramidal syndrome; ITT, intent-to-treat; OL, open label; PP1M, paliperidone palmitate 1-month formulation; PP3M, paliperidone palmitate 3-month formulation; PP6M, paliperidone palmitate 6-month formulation; TEAE, treatment-emergent adverse event.
a An adverse event that started in the open-label phase and resulted in study drug being discontinued in the double-blind phase is counted as treatment-emergent in the open-label phase.
In the DB phase, TEAEs were reported in a comparable percentage of patients in the PP6M (297/478 [62.1%]) and PP3M (131/224 [58.5%]) groups. The most common TEAEs (≥5% in either group) were increased weight, injection-site pain, headache, upper respiratory tract infections, and nasopharyngitis. Most TEAEs were mild or moderate in severity. In total, 24/478 (5.0%) patients in the PP6M group and 15/224 (6.7%) in the PP3M group experienced serious TEAEs that were mostly related to worsening of psychiatric symptoms; schizophrenia was the most frequent (PP6M: 1.7%; PP3M: 0.4%). Overall, 16/478 (3.3%) patients in the PP6M group and 6/224 (2.7%) in the PP3M group discontinued the DB phase due to TEAEs that were mostly psychiatric in nature, with schizophrenia (PP6M: 8 [1.7%]; PP3M: 1 [0.4%]) being the most common. Three deaths (PP6M: n = 1 [cause not specified]; PP3M: n = 2 [pulmonary embolism and sudden death, unknown cause, n = 1 each]) were reported in the DB phase; investigators considered these deaths as not related to study medication.
The occurrences of TEAEs of special interest related to EPS (46 [9.6%] vs 19 [8.5%]), suicidality (5 [1.0%] vs 6 [2.7%]), agitation and aggression (3 [0.6%] vs none), somnolence (9 [1.9%] vs 3 [1.3%]), tachycardia (7 [1.5%] vs 1 [0.4%]), orthostatic hypotension (2 [0.4%] vs 2 [0.9%]) and QT prolongation (2 [0.4%] vs 2 [0.9%]), and diabetes mellitus and hyperglycemia (15 [3.1%] vs 6 [2.7%]) were generally similar between the treatment groups (PP6M vs PP3M). There were no reported TEAEs for neuroleptic malignant syndrome or post-injection delirium/sedation syndrome during the study.
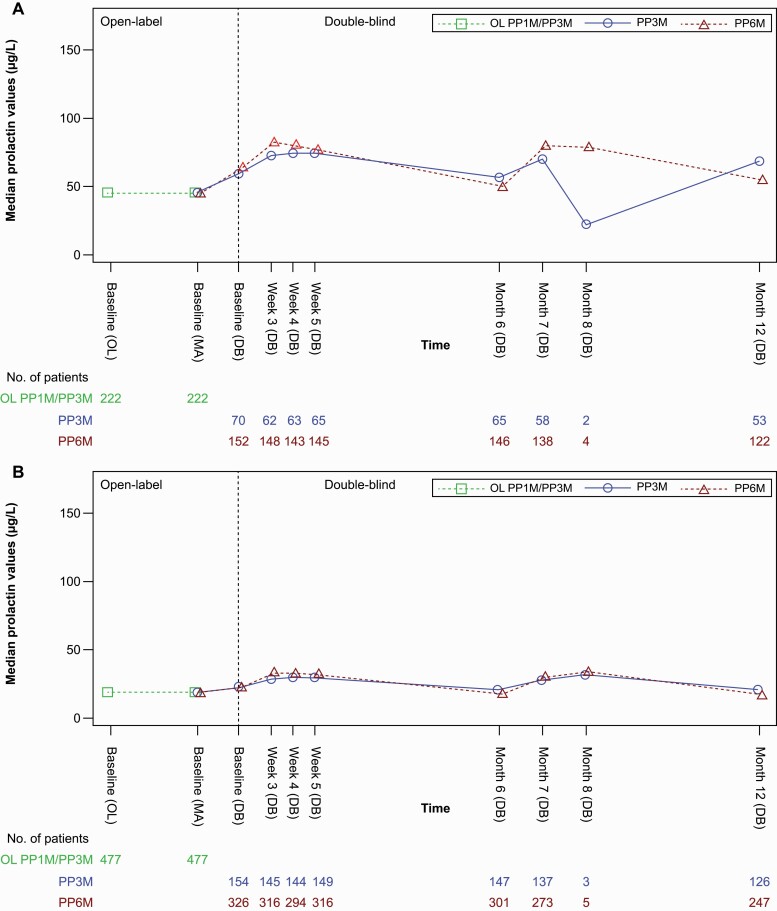
Prolactin-related TEAEs also occurred in a similar percentage of patients (PP6M: 18/478 [3.8%]; PP3M: 7/224 [3.1%]). The magnitude of change in mean (SD) levels of serum prolactin from OL or DB baseline to DB endpoint was greater in patients from the PP3M vs the PP6M group (Table 5). Median prolactin levels in men remained relatively stable throughout the OL and DB phases, whereas women showed an increase from OL baseline to DB baseline (continued to week 3 in the DB phase and remained relatively stable through month 6) (Figure 4). Incidences of TEAEs of weight gain in the DB phase were also similar between the groups: PP6M, 44/478 (9.2%) vs PP3M, 18/224 (8.0%). Injection site–related TEAEs were reported in 59/478 (12.3%) patients in the PP6M group and 11/224 (4.9%) in the PP3M group. Injection site pain was the most frequently reported TEAE in the PP6M (37 [7.7%]) and PP3M (9 [4.0%]) groups, and all other TEAEs including induration, redness, and swelling occurred in <2% of patients in both groups. Induration, redness, and swelling at the injection site, as noted by investigator evaluation, were observed in ≤6% of patients in both treatment groups and were mostly mild in severity. Reduction in mean (SD) value of injection site pain was observed in local tolerability assessments by patients using a visual analog scale from DB baseline to endpoint (Table 5).
Table 5.
Summary of Changes From Baseline in Serum Prolactin Levels, ECG, EPS Scales, and Injection Site Evaluations During the DB Phase (DB Safety Analysis Set)
| PP6M (n = 478) | PP3M (n = 224) | |
|---|---|---|
| Prolactin, µg/L | n = 477 | n = 221 |
| Mean (SD) change from baseline | −3.03 (22.37) | 3.89 (27.93) |
| QTcF, n (%), msec | n = 474 | n = 220 |
| ≤30 | 422 (89.0) | 194 (88.2) |
| >30-60 | 50 (10.5) | 26 (11.8) |
| >60 | 2 (0.4) | 0 |
| AIMS total score, median (range) | n = 477 | n = 221 |
| Changes from baseline | 0.0 (−7, 14) | 0.0 (−3, 2) |
| BARS global clinical rating of akathisia, DB baseline, n (%) | n = 478 | n = 224 |
| Absent | 453 (94.8) | 218 (97.3) |
| Questionable | 16 (3.3) | 6 (2.7) |
| Mild akathisia | 9 (1.9) | 0 (0.0) |
| Moderate akathisia | 0 (0.0) | 0 (0.0) |
| Marked akathisia | 0 (0.0) | 0 (0.0) |
| Severe akathisia | 0 (0.0) | 0 (0.0) |
| BARS global clinical rating of akathisia, DB end point, n (%) | n = 477 | n = 221 |
| Absent | 451 (94.5) | 212 (95.9) |
| Questionable | 19 (4.0) | 7 (3.2) |
| Mild akathisia | 6 (1.3) | 2 (0.9) |
| Moderate akathisia | 1 (0.2) | 0 (0.0) |
| Marked akathisia | 0 (0.0) | 0 (0.0) |
| Severe akathisia | 0 (0.0) | 0 (0.0) |
| SAS global score, median (range) | n = 477 | n = 220 |
| Change from baseline | 0.0 (−1;2) | 0.0 (−1;2) |
| Injection site evaluationa | ||
| Redness, DB baseline | n = 478 | n = 224 |
| Absent | 473 (99.0) | 222 (99.1) |
| Mild | 5 (1.0) | 2 (0.9) |
| DB endpoint | n = 477 | n = 223 |
| Absent | 476 (99.8) | 222 (99.6) |
| Mild | 1 (0.2) | 1 (0.4) |
| Induration/swelling, DB baseline | n = 478 | n = 224 |
| Absent | 469 (98.1) | 220 (98.2) |
| Mild | 9 (1.9) | 4 (1.8) |
| DB endpoint | n = 477 | n = 223 |
| Absent | 475 (99.6) | 222 (99.6) |
| Mild | 2 (0.4) | 1 (0.4) |
| Tenderness, DB baseline | n = 478 | n = 224 |
| Absent | 425 (88.9) | 207 (92.4) |
| Mild | 48 (10.0) | 16 (7.1) |
| Moderate | 5 (1.0) | 1 (0.4) |
| DB endpoint | n = 477 | n = 223 |
| Absent | 474 (99.4) | 221 (99.1) |
| Mild | 3 (0.6) | 2 (0.9) |
| Injection site pain (mm)b | ||
| DB baseline, n | 478 | 224 |
| Mean (SD) | 17.2 (20.86) | 15.0 (18.98) |
| DB endpoint, n | 477 | 223 |
| Mean (SD) | 5.4 (10.78) | 4.54 (8.93) |
Abbreviations: AIMS, Abnormal Involuntary Movement Scale; BARS, Barnes Akathisia Rating Scale; DB, double-blind; ECG, electrocardiogram; EPS, extrapyramidal symptoms; ITT, intent-to-treat; PP3M, paliperidone palmitate 3-month formulation; PP6M, paliperidone palmitate 6-month formulation; QTcF, QT interval, corrected according to Fridericia’s formula; SAS, Simpson-Angus Scale.
a Investigator assessment.
b Assessment of local tolerability by the patient using a visual analog scale (VAS) (within 30 mins of injection) presented as a 100-mm horizontal line on which the patient’s pain intensity is represented by a point between the “no pain at all” (0) and “unbearably painful” (100).
Figure 4.
Median prolactin level over time (safety analysis set). (A) Women (B) Men. Abbreviations: DB, double-blind; OL, open label; PP1M, paliperidone palmitate once-monthly formulation; PP3M, paliperidone palmitate 3-monthly formulation; PP6M, paliperidone palmitate 6-monthly formulation.
Discussion
This multicenter, randomized, DB relapse prevention study demonstrated the noninferiority of PP6M at 700 and 1000 mg eq. doses in patients with schizophrenia, suggesting comparable efficacy with its 3-monthly equivalent formulation (PP3M) for patients who remained relapse free at the end of the 12-month DB phase. There were no appreciable differences in efficacy or safety when transitioning directly from PP1M or PP3M to PP6M. The safety profiles of PP6M and PP3M were largely similar aside from higher injection site pain with PP6M, which could be attributable to the larger injection volume.
The rate of relapse during the DB phase was low in both PP6M (7.5%) and PP3M (4.9%) groups, and a high proportion of patients remained relapse free and completed the 12-month DB phase (PP6M: 79.5%; PP3M: 85.3%). Results for the secondary efficacy analysis were supportive of the primary analysis. Overall, the type and incidence of TEAEs were comparable between PP6M and PP3M groups in the DB phase and consistent with the known profile of paliperidone palmitate (Hough et al., 2010; Kramer et al., 2010; Gopal et al., 2011; Berwaerts et al., 2015; Savitz et al., 2016). None of the TEAEs related to injection site, potentially prolactin-related, weight gain or diabetes mellitus and hyperglycemia—were reported as serious or resulted in treatment discontinuation during the OL or DB phase. Investigator evaluations of swelling, redness, and induration were similar across treatment groups, and patient assessment of injection site pain showed reduction from DB baseline to endpoint for both treatments, suggesting good tolerability and acceptance of the dorsogluteal injections.
Two doses of PP6M (moderate: 700 mg eq.; high: 1000 mg eq.) that resulted in a range of paliperidone plasma exposures similar to simulated exposures obtained with the most commonly prescribed monthly doses of PP1M (100 and 150 mg eq.), 3-monthly doses of PP3M (350 and 525 mg eq.), and corresponding doses of once-daily paliperidone ER (8 [dose used for comparing moderate-dose level] and 12 mg) were selected for assessment (data on file). Dose-normalized total exposure over a 6-month period (2 PP3M administrations vs 1 PP6M administration) were comparable between dose levels for both products. Overall, administration of PP6M once every 6 months, at doses of 700 and 1000 mg. eq., resulted in a range of paliperidone exposures that overlapped with the range of exposures obtained with corresponding doses of PP3M. Relapses were observed throughout dosing cycle and did not appear to be clustered near the end of dosing cycle for both treatments, implying that the comparably lower PP6M Ctrough is likely not the key determinant of relapse.
To date, the PP6M LAI has the longest available dosing interval of 6 months and is intended for use in stable patients with schizophrenia who have been adequately treated with PP1M and PP3M. Its widespread use will require a paradigm shift in how clinicians and patients place oral antipsychotics and LAIs in the management of schizophrenia. Longer-term treatment planning will be required. Evidence supporting patient-centric care also indicates that long-term treatment continuation in schizophrenia improves when treatment injections are less frequent or have longer dosing intervals (Citrome, 2017; Pietrini et al., 2019). Fewer injections are associated with less social stigma, which is a barrier in patients with severe mental illness (da Silva et al., 2020). The twice-yearly dosing regimen of PP6M represents a significant advancement over existing treatments for the management of a chronic illness such as schizophrenia. The use of PP6M would allow patients with limited access to healthcare (due to geographic or economic constraints) to have more consistent medication coverage. Real-world data from specific groups of clinical interest such as homeless patients who often have infrequent contact with outreach workers, patients with recent-onset schizophrenia who have high relapse rates within 5 years after initial recovery, or patients with a history of incarceration or self-harm could provide useful insights on the potential benefits of treatment with PP6M in these patients (Robinson et al., 1999; Morken et al., 2008; Sajatovic et al., 2013). Studies investigating LAIs in adults with first-episode schizophrenia have shown favorable neuropathological changes along with early symptom improvement and reduced risk of relapse (Taylor and Ng, 2013; Stevens et al., 2016). A recent guideline also recommended LAI use in stabilized adult patients after first episode and in early-phase patients to maintain treatment continuity (floridabhcenter.org, 2020). In accordance with a recent clinical guideline issued by Serious Mental Illness (SMI) Adviser (an initiative from the American Psychiatric Association and the Substance Abuse and Mental Health Services Administration), the use of LAIs such as PP6M can be of great value to ensure medication continuation while minimizing interpersonal contact and hospital visits (Moreno et al., 2020; SMI-Adviser, 2020). However, the 6-month dosing interval of PP6M does not dictate the frequency of clinical visits, which can be decided mutually by the patient and clinician.
The present study was adequately powered to assess efficacy using a noninferiority approach. The noninferiority margin of −10% (different from the PP3M study: −15%) was selected based on the efficacy results of a phase 3 non-inferiority study comparing PP3M to PP1M (Berwaerts et al., 2015; Savitz et al., 2016) and on advice from experts and health authorities and endorsed by the Committee for Medicinal Products for Human Use. The eligibility criteria for the present study were similar to the PP3M studies and designed to give a representative patient population likely to be treated with PP6M in clinical practice (Berwaerts et al., 2015; Savitz et al., 2016). Notably, at study entry (OL baseline), the criterion for PANSS total score (<70 indicating clinical stability) was different from the previous PP3M studies (between 70 and 120, not indicating clinical stability, although a PANSS <70 score was the criteria for DB entry).
Several study limitations should be noted. The noninferiority design was based on the principle of enrichment, that is, criteria of clinical stability were applied prior to entry into the DB phase; thus, the results may not reflect true efficacy for prevention of relapses in the overall population. However, the criterion of clinical stability was fundamental to the study design, and PP6M is not intended for use in acutely ill patients with schizophrenia. Furthermore, an OL long-term study (NCT04072575) with more real-world features is currently ongoing and will provide additional insight on the relapse prevention efficacy of PP6M. Also, the absence of a placebo group further limits interpretation of findings, and it is unknown how these findings compare with oral antipsychotics. The fixed doses evaluated during the DB phase were not directly informative of any changes in the dose of PP6M that could occur during long-term treatment in clinical practice or directly inform the dose response of PP6M for use as maintenance therapy, as patients were not randomly assigned to distinct dose levels of PP6M. Notably, patients in the PP6M group received injections every 3 months to maintain the blinding (2 active, 2 placebo injections), thus limiting the interpretation on injection site ratings, patient preference, caregiver burden, etc., that are directly related to different dosing intervals between the 2 formulations. The use of placebo injections could also introduce a potential placebo effect in the PP6M group, which is an inherent limitation of most randomized controlled trials.
Conclusions
The primary efficacy analysis of this clinical trial confirmed that the efficacy (measured by percentage of patients who remained relapse free) of PP6M (700 or 1000 mg eq.) was noninferior to PP3M (350 or 525 mg eq.) in clinically stable patients with schizophrenia adequately treated with PP1M for ≥4 months or PP3M for ≥1 injection cycle. Safety findings for PP6M were consistent with the known profile of paliperidone palmitate, and no new signals specific for PP6M emerged. There were no appreciable differences in efficacy or safety when transitioning directly from PP1M or PP3M to PP6M. Thus, PP6M offers the longest LAI dosing interval available to date and, along with PP1M and PP3M, provides flexible dosing regimens for a patient-centric approach in the management of schizophrenia. An OL extension study is currently underway to assess the long-term safety and tolerability of PP6M.
Supplementary Material
Acknowledgments
We acknowledge Priya Ganpathy, MPharm CMPP (SIRO Clinpharm Pvt. Ltd., India), for medical writing assistance and Ellen Baum, PhD (Janssen Global Services, LLC) for additional editorial support. We also thank the study patients and investigators for their support.
The following principal investigators participated in the study: Argentina: Hernán Alessandria, Ricardo Marcelo Corral, Héctor Fabian Lamaison, Christian Maria Rosa Lupo, Carlos Morra, Hernan David Ruggeri. Australia: Peter Farnbach, Dennis Liu. Brazil: Claudiane Daltio, Clarissa Severino Gama, Hamilton Grabowski, Acioly Lacerda, Moacyr Rosa, Sandra Ruschel, Wagner Gattaz. Bulgaria: Dora Atanasova, Svetlozar Georgiev, Veselin Palazov, Penka Grozeva, Ana Popova, Tony Donchev. Czech Republic: Jiri Masopust, Martin Anders, Marta Holanova, Lubos Janu, Simona Papezova, Alexander Nawka. France: Marie-OdileKrebs, David Misdrahi, Mocrane Abbar, Jérôme Attal, Jean-Luc Bartoli. Hong Kong: Yu-Hai, Eric Chen, Shiu-YinCatherine Chong. Hungary: Ágnes Fuchs, Eva Mathé, Judit Radics, Edina Edit Cserep. India: Prasad Rao Gundugurti, Ramasubramanian Chellamuthu, Vaishal Vora, Venu Gopal Jhanwar, Sathianathan Ramanathan, Sanjay Phadke, Ravish Thunga, P.S.V.N Sharma. Italy: Bernardo Carpiniello, Serafino De Giorgi, Silvana Galderisi, Maurizio Pompili. Republic of Korea: Young- Chul Chung, Sung-Wan Kim, Jun Soo Kwon, Sang- Hyuk Lee. Malaysia: Ahmad Hatim Sulaiman, Kok Yoon Chee, Arunakiri Muthukrishnan, Selvasingam Ratnasingam. Mexico: Erasmo Saucedo, Ontiveros Sánchez José Alfonso, Gabriel De Jesus Alejo Galarza, Carlos Arnaud, Miguel Angel Herrera Estrella. Poland: Hanna Badzio-jagiello, Barbara Janczewska, Ewa Kordyjak-Starczewska, Dariusz Malicki, Tomasz Markowski, Mariusz Perucki, Agata Szulc, Joanna Lazarczyk, Piotr Zalitacz, Jacek Turczynski. Russian Federation: Alexander Reznik, Oxana Olevskaya, Mikhail Popov, Alexey Agarkov, Alexander Golubev, Mikhail Ivanov, Alexander Parashchenko, Ekaterina Smetannkova, Vladislava Savitskaya, Alena Sidenkova, Anatoly Smulevich, Yuri Suchkov. Spain: Jose Maria Pelayo, Julio Bobes García, Sonia Bustamante Madariaga, Carlos Cañete Nicolas, Manuel Franco Martín, Josep Antoni Ramos Quiroga, Salvador Ros Montalbán. Taiwan, Province of China: Yen-Kuang Yang, Chia-Yih Liu, Ya-Mei Bai, Chen-Ju Lin. Turkey: Kursat Altinbas, Hasan Kaya, Atila Erol, Huseyin Gulec, Ersin Karslioglu. Ukraine: Serhiy Mykhnyak, Liudmyla Samsonova, Yuliya Blazhevych, Iryna Kosenkova, Nataliya Maruta, Oleksandr Mykhaylyukovych, Pavlo Palamarchuk, Oksana Serebrennikova, Gennadii Zilberblat; United States: Eric Achtyes, Michael Downing, Otto Dueno, Corinna Gamez, John Kane, Shlomo Pascal, Robert Billingsley, Paul Murphy, Sonia Rente, Robert Riesenberg, John Sonnenberg, Subhdeep Virk, David Walling, Charmaine Semeniuk, Alejandro Alva, Gustavo Alva, Fredrick W Reimherr, Oliver Freudenreich, Christian Kohler, Constantin Abuzatoaie, Jose M Rubio-Lorente, James Barker, Jason Bermak, Daniel Gruener, Manuel Melendez, Kelley Yokum.
This work was supported by Janssen Research & Development, LLC.
Interest Statement
D.N. is an employee of Janssen Scientific affairs, LLC and may hold company stocks or stock options. P.S., S.W., P.L., A.S., M.J.R., K.C., R.M., R.V., H.T., and S.G. are employees of Janssen Research & Development (a Johnson & Johnson company) and may hold company stocks or stock options. Alain Schotte was an employee of Janssen Research & Development, Beerse, Belgium, during the study and has now retired. Dr Walling reports grants from AbbVie, Acadia, Alkermes, Allergan, Avanir, Boehringer Ingelheim, CoMentis, Intra-Cellular Therapies, Janssen, Johnson & Johnson PRD, Lundbeck, Lupin, Novartis, Noven, Omeros, Otsuka, Pfizer, Roche, Sunovion, Takeda, and Zogenix and personal fees from Janssen and Otsuka. Prof. Galderisi has consulted with or served on advisory boards for Innova Pharma-Recordati Group, Janssen, Gedeon Richter-Recordati, and Angelini and also reports fees as speaker in Congresses and Webinars for Angelini, Janssen, and Recordati.
References
- Alphs L, Mao L, Lynn Starr H, Benson C (2016) A pragmatic analysis comparing once-monthly paliperidone palmitate versus daily oral antipsychotic treatment in patients with schizophrenia. Schizophr Res 170:259–264. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, Carpenter WT Jr, Kane JM, Lasser RA, Marder SR, Weinberger DR (2005) Remission in schizophrenia: proposed criteria and rationale for consensus. Am J Psychiatry 162:441–449. [DOI] [PubMed] [Google Scholar]
- Attard A, Olofinjana O, Cornelius V, Curtis V, Taylor D (2014) Paliperidone palmitate long-acting injection–prospective year-long follow-up of use in clinical practice. Acta Psychiatr Scand 130:46–51. [DOI] [PubMed] [Google Scholar]
- Berwaerts J, Liu Y, Gopal S, Nuamah I, Xu H, Savitz A, Coppola D, Schotte A, Remmerie B, Maruta N, Hough DW (2015) Efficacy and safety of the 3-month formulation of paliperidone palmitate vs placebo for relapse prevention of schizophrenia: a randomized clinical trial. JAMA Psychiatry 72:830–839. [DOI] [PubMed] [Google Scholar]
- Bioque M, Bernardo M (2018) The current data on the 3-month paliperidone palmitate formulation for the treatment of schizophrenia. Expert Opin Pharmacother 19:1623–1629. [DOI] [PubMed] [Google Scholar]
- Blackwood C, Sanga P, Nuamah I, Keenan A, Singh A, Mathews M, Gopal S (2020) Patients’ preference for long-acting injectable versus oral antipsychotics in schizophrenia: results from the Patient-Reported Medication Preference Questionnaire. Patient Prefer Adherence 14:1093–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brissos S, Veguilla MR, Taylor D, Balanzá-Martinez V (2014) The role of long-acting injectable antipsychotics in schizophrenia: a critical appraisal. Ther Adv Psychopharmacol 4:198–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busner J, Targum SD (2007) The clinical global impressions scale: applying a research tool in clinical practice. Psychiatry 4:28–37. [PMC free article] [PubMed] [Google Scholar]
- Citrome L (2017) Long-acting injectable antipsychotics update: lengthening the dosing interval and expanding the diagnostic indications. Expert Rev Neurother 17:1029–1043. [DOI] [PubMed] [Google Scholar]
- Correll CU, Citrome L, Haddad PM, Lauriello J, Olfson M, Calloway SM, Kane JM (2016) The use of long-acting injectable antipsychotics in schizophrenia: evaluating the evidence. J Clin Psychiatry 77:1–24. [DOI] [PubMed] [Google Scholar]
- da Silva AG, Baldaçara L, Cavalcante DA, Fasanella NA, Palha AP (2020) The impact of mental illness stigma on psychiatric emergencies. Front Psychiatry 11:573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lorenzo R, Cameli M, Piemonte C, Bolondi M, Landi G, Pollutri G, Spattini L, Moretti V, Ferri P (2018) Clinical improvement, relapse and treatment adherence with paliperidone palmitate 1-month formulation: 1-year treatment in a naturalistic outpatient setting. Nord J Psychiatry 72:214–220. [DOI] [PubMed] [Google Scholar]
- Emond B, Joshi K, Khoury ACE, Lafeuille MH, Pilon D, Tandon N, Romdhani H, Lefebvre P (2019) Adherence, healthcare resource utilization, and costs in medicaid beneficiaries with schizophrenia transitioning from once-monthly to once-every-3-months paliperidone palmitate. Pharmacoecon Open 3:177–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emsley R, Parellada E, Bioque M, Herrera B, Hernando T, García-Dorado M (2018) Real-world data on paliperidone palmitate for the treatment of schizophrenia and other psychotic disorders: a systematic review of randomized and nonrandomized studies. Int Clin Psychopharmacol 33:15–33. [DOI] [PubMed] [Google Scholar]
- floridabhcenter.org (2020) 2019–2020 Florida Best Practice Psychotherapeutic Medication Guidelines for Adults. In: The University of South Florida, Florida Medicaid Drug Therapy Management Program sponsored by the Florida Agency for Health Care Administration. Accessed June 7, 2021. https://floridabhcenter.org/adult-guidelines/2019-2020-treatment-of-adult-schizophrenia/ [Google Scholar]
- Garcia-Portilla MP, Llorca PM, Maina G, Bozikas VP, Devrimci-Ozguven H, Kim SW, Bergmans P, Usankova I, Pungor K (2020) Symptomatic and functional outcomes after treatment with paliperidone palmitate 3-month formulation for 52 weeks in patients with clinically stable schizophrenia. Ther Adv Psychopharmacol 10:2045125320926347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopal S, Hough DW, Xu H, Lull JM, Gassmann-Mayer C, Remmerie BM, Eerdekens MH, Brown DW (2010) Efficacy and safety of paliperidone palmitate in adult patients with acutely symptomatic schizophrenia: a randomized, double-blind, placebo-controlled, dose-response study. Int Clin Psychopharmacol 25:247–256. [DOI] [PubMed] [Google Scholar]
- Gopal S, Vijapurkar U, Lim P, Morozova M, Eerdekens M, Hough D (2011) A 52-week open-label study of the safety and tolerability of paliperidone palmitate in patients with schizophrenia. J Psychopharmacol 25:685–697. [DOI] [PubMed] [Google Scholar]
- Greene M, Yan T, Chang E, Hartry A, Touya M, Broder MS (2018) Medication adherence and discontinuation of long-acting injectable versus oral antipsychotics in patients with schizophrenia or bipolar disorder. J Med Econ 21:127–134. [DOI] [PubMed] [Google Scholar]
- Hough D, Gopal S, Vijapurkar U, Lim P, Morozova M, Eerdekens M (2010) Paliperidone palmitate maintenance treatment in delaying the time-to-relapse in patients with schizophrenia: a randomized, double-blind, placebo-controlled study. Schizophr Res 116:107–117. [DOI] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opler LA (1987) The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull 13:261–276. [DOI] [PubMed] [Google Scholar]
- Kramer M, Litman R, Hough D, Lane R, Lim P, Liu Y, Eerdekens M (2010) Paliperidone palmitate, a potential long-acting treatment for patients with schizophrenia. Results of a randomized, double-blind, placebo-controlled efficacy and safety study. Int J Neuropsychopharmacol 13:635–647. [DOI] [PubMed] [Google Scholar]
- Mace S, Chak O, Punny S, Sedough-Abbasian D, Vegad C, Taylor DM (2019) Positive views on antipsychotic long-acting injections: results of a survey of community patients prescribed antipsychotics. Ther Adv Psychopharmacol 9:2045125319860977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus SC, Zummo J, Pettit AR, Stoddard J, Doshi JA (2015) Antipsychotic adherence and rehospitalization in schizophrenia patients receiving oral versus long-acting injectable antipsychotics following hospital discharge. J Manag Care Spec Pharm 21:754–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews M, Gopal S, Nuamah I, Hargarter L, Savitz AJ, Kim E, Tan W, Soares B, Correll CU (2019) Clinical relevance of paliperidone palmitate 3-monthly in treating schizophrenia. Neuropsychiatr Dis Treat 15:1365–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno C, et al. (2020) How mental health care should change as a consequence of the COVID-19 pandemic. Lancet Psychiatry 7:813–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morken G, Widen JH, Grawe RW (2008) Non-adherence to antipsychotic medication, relapse and rehospitalisation in recent-onset schizophrenia. BMC Psychiatry 8:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morosini PL, Magliano L, Brambilla L, Ugolini S, Pioli R (2000) Development, reliability and acceptability of a new version of the DSM-IV Social and Occupational Functioning Assessment Scale (SOFAS) to assess routine social functioning. Acta Psychiatr Scand 101:323–329. [PubMed] [Google Scholar]
- Patel C, Emond B, Lafeuille MH, Côté-Sergent A, Lefebvre P, Tandon N, El Khoury AC (2020) Real-world analysis of switching patients with schizophrenia from oral risperidone or oral paliperidone to once-monthly paliperidone palmitate. Drugs Real World Outcomes 7:19–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrini F, Albert U, Ballerini A, Calò P, Maina G, Pinna F, Vaggi M, Boggian I, Fontana M, Moro C, Carpiniello B (2019) The modern perspective for long-acting injectables antipsychotics in the patient-centered care of schizophrenia. Neuropsychiatr Dis Treat 15:1045–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson D, Woerner MG, Alvir JM, Bilder R, Goldman R, Geisler S, Koreen A, Sheitman B, Chakos M, Mayerhoff D, Lieberman JA (1999) Predictors of relapse following response from a first episode of schizophrenia or schizoaffective disorder. Arch Gen Psychiatry 56:241–247. [DOI] [PubMed] [Google Scholar]
- Sajatovic M, Levin J, Ramirez LF, Hahn DY, Tatsuoka C, Bialko CS, Cassidy KA, Fuentes-Casiano E, Williams TD (2013) Prospective trial of customized adherence enhancement plus long-acting injectable antipsychotic medication in homeless or recently homeless individuals with schizophrenia or schizoaffective disorder. J Clin Psychiatry 74:1249–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitz AJ, Xu H, Gopal S, Nuamah I, Ravenstijn P, Janik A, Schotte A, Hough D, Fleischhacker WW (2016) Efficacy and safety of paliperidone palmitate 3-month formulation for patients with schizophrenia: a randomized, multicenter, double-blind, noninferiority study. Int J Neuropsychopharmacol 19:pyw018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiner A, Aadamsoo K, Altamura AC, Franco M, Gorwood P, Neznanov NG, Schronen J, Ucok A, Zink M, Janik A, Cherubin P, Lahaye M, Hargarter L (2015) Paliperidone palmitate versus oral antipsychotics in recently diagnosed schizophrenia. Schizophr Res 169:393–399. [DOI] [PubMed] [Google Scholar]
- SMI-Adviser (2020) Serious Mental Illness Adviser. What are clinical considerations for giving LAIs during the COVID-19 public health emergency? American Psychiatric Association.https://smiadviser.org/knowledge_post/what-are-clinical-considerations-for-giving-lais-during-the-covid-19-public-health-emergency [Google Scholar]
- Stevens GL, Dawson G, Zummo J (2016) Clinical benefits and impact of early use of long-acting injectable antipsychotics for schizophrenia. Early Interv Psychiatry 10:365–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor M, Ng KY (2013) Should long-acting (depot) antipsychotics be used in early schizophrenia? A systematic review. Aust N Z J Psychiatry 47:624–630. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.