Abstract
A population pharmacokinetic (PK) analysis was conducted to determine if piperacillin and tazobactam exhibited linear or nonlinear PKs and if incremental changes in the daily dosage of piperacillin affected tazobactam PKs. Four dosage groups were evaluated after multiple dosing regimens. Concentrations of drug in plasma and amounts in urine were best fitted by using a linear two-compartment PK model. No significant difference between dosing groups was seen for any piperacillin or tazobactam PK parameters. Both drugs exhibited linear PKs when given at usual clinical doses. Tazobactam PKs did not appear to be affected by the different dosing regimens of piperacillin.
Piperacillin-tazobactam is currently recommended for the treatment of intra-abdominal, lower respiratory tract, skin and skin structure, and gynecologic infections. This β-lactamase inhibitor-antibiotic combination has been developed to overcome the ongoing problem of enzymatic degradation by β-lactamase enzymes (19, 27). There are some controversial reports in the literature concerning the pharmacokinetic (PK) behavior of these two drugs. During the past 2 decades, several authors have proposed that piperacillin exhibits nonlinear PKs, i.e., that its total clearance (CL) decreases or that its terminal elimination half-life (t1/2) increases with rising plasma drug concentrations (2, 3, 4, 15, 24), while others have proposed that the drug follows linear PKs (7, 8, 10, 13, 17, 18, 21, 22, 28–30). Results suggesting nonlinear elimination of piperacillin are, however, controversial. In essence, the basic principles that need to be met to conclude that the drug’s PKs is nonlinear were not completely fulfilled. Despite these existing contradictions, no attempts to reach a consensus on the PK behavior of piperacillin have been conducted.
Some authors have suggested that the concomitant administration of piperacillin influences the elimination of tazobactam (17, 21, 29). Although the PKs of tazobactam might be different when used alone, it is never used this way clinically. Because it is currently always administered concomitantly with piperacillin, it is relevant to determine if the PKs of tazobactam is different when administered with clinically used low or high dosages of piperacillin. The objectives of this study were therefore to determine if piperacillin and tazobactam exhibited linear or nonlinear PKs and if incremental changes in doses of piperacillin affected tazobactam PKs when we administer these two drugs at usual clinical dosages.
Data were obtained from previous PK studies (17) involving 27 healthy adult male volunteers (Wyeth Ayerst Inc.). Exclusion criteria included abnormalities in baseline chemistries, histories or clinical evidence of renal or hepatic diseases, and histories of hypersensitivity to β-lactam antibiotics or β-lactamase inhibitors. The subjects did not take any other medications for 7 days before and during the study period. All subjects were within 15% of their ideal weights for their ages and heights according to the standards established by the Metropolitan Life Insurance Company. We evaluated four dosage groups after 3 to 5 days of multiple dosing (8 to 18 g of piperacillin/day and 1 to 2.25 g of tazobactam/day). Piperacillin-tazobactam was administered intravenously at dosages of 2.25 g every 6 h to 5 subjects (group 1), 3.375 g every 6 h and 4.5 g every 8 h to 12 subjects (group 2), 4.5 g every 6 hours to 5 subjects (group 3), and 3.375 g every 4 hours to 5 subjects (group 4). Doses were given by 5 (groups 1 and 3)- or 30 (groups 2 and 4)-min infusion rates. Concentrations and amounts of piperacillin and tazobactam in plasma and urine, respectively, were determined by using previously validated high-performance liquid chromatography assays (unpublished data). Schedules for plasma and urine sampling were variable among the four dosage groups. The average number of plasma samples per subject was 30 (range, 24 to 39), while a mean of 11 (range, 9 to 12) urine collections were performed for each individual. All plasma and urine samples were stored at −70°C until analysis.
PK analyses were performed by using compartmental PK techniques (9). No evidence of a nonlinear PK elimination (i.e., elimination exhibiting the classic “hockey stick” effect, whereby concentrations fall very slowly at first [the handle of the hockey stick] and then very rapidly) (26) was seen by visual inspection of any individual subject’s concentration in plasma (logarithmic scale) versus time curves following piperacillin-tazobactam administrations. We therefore investigated linear PK models for the quality of fitting, which was assessed by visual inspection of graphs (concentrations versus time, weighted residuals versus observed concentrations) and computation of Akaike’s information criterion test (1). All concentrations and amounts of piperacillin or tazobactam in plasma and urine, respectively, were best fitted by using a linear two-compartment PK model. Individual PK parameter estimates (ADAPT-II) were used as priors for each dosing group, and population PK analyses were performed by using an iterative two-stage methodology (5, 6). All concentrations were fitted with a weighting factor of Wi = 1/Si2 where the variance Si2 was calculated for each observation by using the equation Si2 = (a × Yi) + (b)2. The slope (a) is related to the sum of all errors associated with each concentration, and the intercept (b) is related to the limit of detection of the analytical assay. The following series of differential equations describes the PK model:
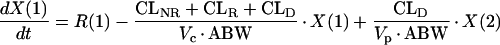 |
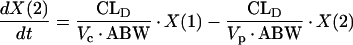 |
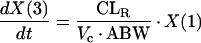 |
where R(1) is the zero-order infusion rate of either piperacillin or tazobactam (mg/h); X(1), X(2), and X(3) are the amounts of drug in the central, peripheral, and urinary compartments, respectively; Vc and Vp are the central and peripheral volumes of distribution (liters/kg), respectively; CLD, CLNR, and CLR are the distributional, nonrenal, and renal clearances of piperacillin or tazobactam, respectively; and ABW is the actual body weight. The observed concentrations [Y(1)] and amounts [Y(2)] of piperacillin or tazobactam in plasma and urine, respectively, were simultaneously fitted by the model by using the following output equations:
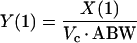 |
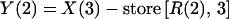 |
X(3) − store [R(2), 3] is the amount of tazobactam or piperacillin that was excreted unchanged in the urine during each specified collection interval to be fitted. Total volumes of distribution (VSS) were calculated as the sum of Vc and Vp. CL was calculated by adding CLNR and CLR. Maximum concentrations of drug in plasma (Cmax) were obtained directly from the observed concentrations versus time points. We calculated the estimated areas under the plasma drug concentration-time curve (AUC) of either compound during a dosing interval at steady state by using the linear trapezoidal rule.
Piperacillin and tazobactam PK parameters of the different dosage groups were compared by using a one-way analysis of variance (ANOVA) for unbalanced designs. The relationships between the AUC/dose, the CL, the VSS, and t1/2 versus the doses of piperacillin and tazobactam were determined by linear regression. We stipulated a priori that a P value of less than 0.05 would be associated with statistical significance.
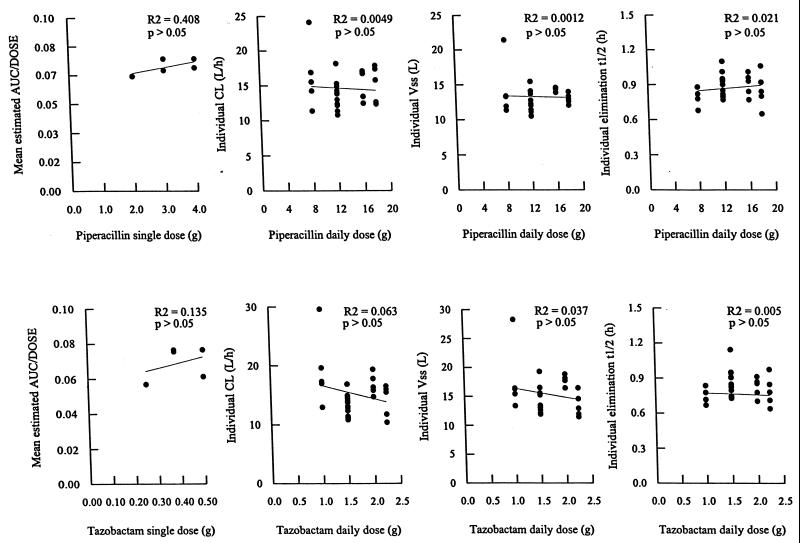
The proposed linear PK model predicted the concentrations of piperacillin and tazobactam in plasma and the amounts in urine very well, without any evidence of accumulation in each of the individual concentration-time data sets. The relationships between the dose and the estimated mean AUC/dose, the calculated individual CL, VSS, and elimination of t1/2 are illustrated for piperacillin and tazobactam in Fig. 1. The values of the estimated PK parameters for piperacillin and tazobactam were the same despite variations in the daily dosages of these compounds (ANOVA, P > 0.05), indicating that piperacillin and tazobactam exhibited linear PK behavior. The average percentages of dose-related variability observed in these PK parameters of piperacillin and tazobactam were 11 and 6%, respectively. Mean values for the different estimated PK parameters are presented for the four dosage groups for piperacillin and tazobactam in Table 1.
FIG. 1.
Relationships between the mean estimated AUC/dose, the calculated individual CL, VSS, and elimination t1/2 versus the administered doses of piperacillin and tazobactam.
TABLE 1.
Piperacillin and tazobactam mean pharmacokinetic parameter estimates and their interindividual variability for the different dosage groups
| Piperacillin/tazobactam dose (g/day) | Parameter estimate for piperacillin [% CV]/estimate for tazobactam [% CV]a
|
|||
|---|---|---|---|---|
| CLNR (liters/h) | CLR (liters/h) | Vc (liters/kg) | Vp (liters/kg) | |
| 8/1 | 8.4 [30]/6.4 [28] | 8.0 [28]/12.9 [35] | 0.12 [37]/0.14 [49] | 0.07 [15]/0.095 [14] |
| 12/1.5 | 6.6 [14]/4.8 [9] | 6.9 [16]/8.6 [19] | 0.11 [13]/0.098 [21] | 0.053 [15]/0.084 [12] |
| 16/2 | 7.6 [17]/6.3 [13] | 7.0 [13]/10.6 [10] | 0.13 [12]/0.17 [13] | 0.070 [12]/0.087 [16] |
| 18/2.25 | 7.4 [15]/4.8 [10] | 7.8 [20]/9.3 [26] | 0.12 [5]/0.1 [17] | 0.048 [16]/0.077 [8] |
CV, interindividual variation.
Previous investigators have postulated that piperacillin exhibited a nonlinear PK behavior, inferring that its elimination does not follow first-order processes (2, 3, 4, 15, 24). Tjandramaga et al. (24) reported disproportionate AUCs, prolonged terminal elimination t1/2, and reduced CL and CLR with increasing doses of piperacillin when they administered 1 to 6 g as single-dose boluses to healthy volunteers. Batra et al. (3) observed similar trends after the administration of two multiple dosing regimens (4 g intravenously q8h and 6 g intravenously q6h) of piperacillin. The data obtained by Morrison and Batra (15) also suggested a dose-dependent effect on the PKs of piperacillin following bolus injections of piperacillin of 1 to 6 g. Bergan and Williams (4) reported similar findings when they evaluated the disposition of piperacillin when given at doses of 15, 30, and 60 mg/kg of body weight. Finally, Aronoff et al. (2) reported decreases in all clearances and prolonged elimination t1/2 following intravenous piperacillin administration of 15 and 60 mg/kg to seven adults with normal renal function.
Piperacillin is predominantly eliminated by active renal tubular secretion (2–4). Saturation of this process would result in a nonlinear elimination, a condition that Michaelis-Menten PK equations would best describe (12, 14, 25). In that case, the maximum velocity rate, Vmax, and the Km constant would govern the rate of elimination of piperacillin (12, 14). Nonlinear PKs will be observed in plasma piperacillin concentration-time curves only if the concentrations of the drug are at least equal to or greater than Km and if the tubular secretion process accounts for a minimum of 20% of its total clearance (14, 25). Since piperacillin is excreted by tubular secretion and glomerular filtration and via the bile, the possibility of saturable secretion associated with usual plasma drug concentrations is small (2, 3, 4, 14, 25).
In all previous studies claiming that piperacillin exhibits nonlinear PKs, noncompartmental PK analyses were used, which would give erroneous results if the behavior of piperacillin were not linear (2, 3, 4, 15, 24). The linear PK model with first-order elimination process that they used to describe the disposition of piperacillin implicitly assumes that, except for unextrapolated AUCs and Cmax, the PK parameters of piperacillin are constant after escalating or multiple-dose administration. These studies have also reported good fittings while using linear one- or two-compartment PK models. Another factor supporting piperacillin’s linear disposition is the absence of accumulation reported in multiple-dosing regimen studies (3, 17, 23). The slight differences observed in the elimination PK parameters with different doses of piperacillin reported by several studies could be the result of unaccounted noise and interindividual variability, as none was a population PK analysis. The use of microbiologic assays to determine piperacillin concentrations and suboptimal storage conditions for plasma and urine samples have also likely contributed to the variability present in these earlier studies (2, 3, 4, 15, 24). The limited sensitivity and specificity of a bioassay may preclude the detection of lower concentrations, resulting in wide intra- and intersubject fluctuations in piperacillin concentrations and therefore in calculated PK parameter values. Plasma and urine samples tested in most of these studies were stored at −20°C (2, 4, 24), whereas the optimal storage temperature recommended by the most recent stability studies is −70°C (11, 16). Our findings confirm that for the dosage range studied (8 to 18 g/day), piperacillin exhibits linear PKs that is unaltered in the presence of changing concentrations of tazobactam. The results of the present study agree with those obtained by several other investigators (7, 8, 10, 13, 17, 18, 21, 22, 28, 29, 30).
Analogous to piperacillin, controversial information suggesting a slower elimination for tazobactam with dose escalation (20, 21) or when coadministered with piperacillin (17, 21, 29) has been published. The mechanism involved in this potential dose-dependent elimination for tazobactam is not yet defined, but saturation of its tubular secretion process has been proposed (20, 21, 29). Sorgel and Kinzig (20) have evaluated the PKs of tazobactam alone over the dose range of 0.1 to 1 g in healthy volunteers. They reported considerable reductions in tazobactam CL and CLR with increasing doses while the terminal elimination t1/2 was prolonged. The differences in the PK parameter values were, however, essentially observed at the lower range of doses studied. Plasma tazobactam concentrations at 0.1- and 0.25-g doses were very close to the detection limit, which may have prevented the accurate calculations of the PK parameters for these dose levels compared with the higher doses administered. Zaghloul et al. (30) compared the PKs of tazobactam given alone (40 mg/kg) to the PKs of tazobactam when coadministered at the same dosage with piperacillin (320 mg/kg) in dogs. They concluded that piperacillin significantly reduced the elimination of tazobactam. The absence of a crossover design in the protocol and the small number of dogs in each arm (three per group) limit their comparison. Similarly, Wise et al. (29) reported decreases in tazobactam elimination when administered with piperacillin to healthy volunteers. As pointed out by different authors (13, 17), their PK parameter values were lower compared with the ones reported in similar healthy populations, making their interpretation difficult.
We designed the present study to assess if the PKs of tazobactam was linear between the dose range studied (1 to 2.25 g/day) and to see if using different doses of piperacillin modifies the PK profile of tazobactam. From this analysis, we conclude that the PKs of tazobactam is linear and unaffected by the coadministration of different doses of piperacillin. The results of the study conducted by Reed et al. (18) in a pediatric population, as well as those reported by Occhipinti et al. (17), support our conclusions.
Contrary to what has been proposed, neither piperacillin (8 to 18 g/day) nor tazobactam (1 to 2.25 g/day) exhibited nonlinear PKs with usual clinical dosing regimens. The different dosing regimens of piperacillin did not affect tazobactam PKs.
Acknowledgments
We are grateful to Wyeth Ayerst Canada Inc. for providing postdoctoral fellowship assistance to B.A.
REFERENCES
- 1.Akaike H. A Bayesian extension of the minimum AIC procedure of autoregressive model fitting. Biometrika. 1992;66:237–242. [Google Scholar]
- 2.Aronoff G R, Sloan R S, Brier M E, Luft F C. The effect of piperacillin dose on elimination kinetics in renal impairment. Eur J Clin Pharmacol. 1983;24:543–547. doi: 10.1007/BF00609901. [DOI] [PubMed] [Google Scholar]
- 3.Batra V K, Morrison J A, Lasseter K C, Joy V A. Piperacillin kinetics. Clin Pharmacol Ther. 1979;26:41–53. doi: 10.1002/cpt197926141. [DOI] [PubMed] [Google Scholar]
- 4.Bergan T, Williams J D. Dose dependence of piperacillin pharmacokinetics. Chemotherapy. 1982;28:153–159. doi: 10.1159/000238070. [DOI] [PubMed] [Google Scholar]
- 5.Collins D, Forrest A. IT2S user’s guide. Buffalo, N.Y: State University of New York at Buffalo; 1995. [Google Scholar]
- 6.D’Argenio D Z, Schumitzky A. ADAPT-II user’s guide. University of Southern CaliforniaLos Angeles, Calif.: Biomedical Simulation Resources; 1992. [Google Scholar]
- 7.Evans M A L, Leung T, Wilson P, Williams J D. Pharmacokinetics of intravenously administered antibiotics: a study of piperacillin, a new semi-synthetic penicillin. Drugs Exp Clin Res. 1979;5:111–116. [Google Scholar]
- 8.Evans M A L, Wilson P, Leung T, Williams J D. Pharmacokinetics of piperacillin following intravenous administration. J Antimicrob Chemother. 1978;4:255–261. doi: 10.1093/jac/4.3.255. [DOI] [PubMed] [Google Scholar]
- 9.Gibaldi M, Perrier D. Pharmacokinetics. 2nd ed. New York, N.Y: Marcel Dekker; 1982. [Google Scholar]
- 10.Johnson C A, Halstenson C E, Kelloway J S, Shapiro B E, Zimmerman S W, Tonelli A, Faulkner R, Dutta A, Haynes J, Greene D S, Kuye O. Single-dose pharmacokinetics of piperacillin and tazobactam in patients with renal disease. Clin Pharmacol Ther. 1992;51:32–41. doi: 10.1038/clpt.1992.5. [DOI] [PubMed] [Google Scholar]
- 11.Jung D, Mahajan N K. An improved micro-scale liquid-chromatographic assay for piperacillin in plasma and urine. Clin Chem. 1984;30:12–24. [PubMed] [Google Scholar]
- 12.Jusko W J. Pharmacokinetics of capacity-limited systems. J Clin Pharmacol. 1989;29:488–493. doi: 10.1002/j.1552-4604.1989.tb03369.x. [DOI] [PubMed] [Google Scholar]
- 13.Kinzig M, Sorgel F, Brismar B, Nord C E. Pharmacokinetics and tissue penetration of tazobactam and piperacillin in patients undergoing colorectal surgery. Antimicrob Agents Chemother. 1992;36:1997–2004. doi: 10.1128/aac.36.9.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ludden T M. Nonlinear pharmacokinetics: clinical implications. Clin Pharmacokinet. 1991;20:429–446. doi: 10.2165/00003088-199120060-00001. [DOI] [PubMed] [Google Scholar]
- 15.Morrison J A, Batra V K. Pharmacokinetics of piperacillin sodium in man. Drugs Exp Clin Res. 1979;5:105–110. [Google Scholar]
- 16.Ocampo A P, Hoyt K D, Wadgaonkar N, Carver A H, Puglisi C V. Determination of tazobactam and piperacillin in human plasma, serum, bile and urine by gradient elution reversed-phase high-performance liquid chromatography. J Chromatogr. 1989;496:167–179. doi: 10.1016/s0378-4347(00)82563-3. [DOI] [PubMed] [Google Scholar]
- 17.Occhipinti D J, Pendland S, Schoonover L L, Rypins E B, Danzinger L H, Rodvold K A. Pharmacokinetics and pharmacodynamics of two multiple-dose piperacillin-tazobactam regimens. Antimicrob Agents Chemother. 1997;41:2511–2517. doi: 10.1128/aac.41.11.2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reed M D, Goldfarb J, Yamashita T S, Lemon E, Blumer J L. Single-dose pharmacokinetics of piperacillin and tazobactam in infants and children. Antimicrob Agents Chemother. 1994;38:2817–2826. doi: 10.1128/aac.38.12.2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schoonover L L, Occhipinti D J, Rodvold K A, Danziger L H. Piperacillin/tazobactam: a new β-lactam/β-lactamase inhibitor combination. Ann Pharmacother. 1995;29:501–514. doi: 10.1177/106002809502900510. [DOI] [PubMed] [Google Scholar]
- 20.Sorgel F, Kinzig M. The chemistry, pharmacokinetics and tissue distribution of piperacillin/tazobactam. J Antimicrob Chemother. 1993;31(Suppl. A):39–60. doi: 10.1093/jac/31.suppl_a.39. [DOI] [PubMed] [Google Scholar]
- 21.Sorgel F, Kinzig M. Pharmacokinetics and tissue penetration of piperacillin/tazobactam with particular reference to its potential in abdominal and soft tissue infections. Eur J Surg. 1994;573(Suppl.):39–44. [PubMed] [Google Scholar]
- 22.Sullivan M C, Nightingale C H, Quintiliani R, Sweeney K. Comparison of the pharmacokinetic and pharmacodynamic activity of piperacillin and mezlocillin. Pharmacotherapy. 1993;13:607–612. [PubMed] [Google Scholar]
- 23.Tartaglione T A, Nye L, Vishniavsky N, Poynor W, Polk R. Multiple-dose pharmacokinetics of piperacillin and azlocillin in 12 healthy volunteers. Clin Pharm. 1986;5:911–916. [PubMed] [Google Scholar]
- 24.Tjandramaga, T. B., A. Mullie, R. Verbesselt, P. J. De Schepper, and L. Verbist. 1978. Piperacillin: human pharmacokinetics after intravenous and intramuscular administration. 14:829–837. [DOI] [PMC free article] [PubMed]
- 25.Van Ginneken C A M, Russel F G M. Saturable pharmacokinetics in the renal excretion of drugs. Clin Pharmacokinet. 1989;16:38–54. doi: 10.2165/00003088-198916010-00003. [DOI] [PubMed] [Google Scholar]
- 26.Wagner J G. Pharmacokinetics for the pharmaceutical scientist. Lancaster, Pa: Technomic Publishing Co.; 1993. [Google Scholar]
- 27.Walmsley S the Committee on Antimicrobial Agents. Piperacillin/tazobactam. Can J Infect Dis. 1997;8:79–84. [Google Scholar]
- 28.Welling P G, Craig W A, Bundtzen R W, Kwok F W, Gerber A U, Madsen P. Pharmacokinetics of piperacillin in subjects with various degrees of renal function. Antimicrob Agents Chemother. 1983;23:881–887. doi: 10.1128/aac.23.6.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wise R, Logan M, Cooper M, Andrews J M. Pharmacokinetics and tissue penetration of tazobactam administered alone and with piperacillin. Antimicrob Agents Chemother. 1991;35:1081–1084. doi: 10.1128/aac.35.6.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zaghloul I, Kuck N, Yacobi A. The effect of tazobactam on the pharmacokinetics and the antibacterial activity of piperacillin in dogs. Int J Pharm. 1997;153:115–121. [Google Scholar]