Abstract
Background
Disease-specific studies have reported impaired humoral responses after SARS-CoV-2 vaccination in patients with immune-mediated inflammatory disorders treated with specific immunosuppressants. Disease-overarching studies, and data on recall responses and third vaccinations are scarce. Our primary objective was to investigate the effects of immunosuppressive monotherapies on the humoral immune response after SARS-CoV-2 vaccination in patients with prevalent immune-mediated inflammatory disorders.
Methods
We did a cohort study in participants treated in outpatient clinics in seven university hospitals and one rheumatology treatment centre in the Netherlands as well as participants included in two national cohort studies on COVID-19-related disease severity. We included patients aged older than 18 years, diagnosed with any of the prespecified immune-mediated inflammatory disorders, who were able to understand and complete questionnaires in Dutch. Participants with immune-mediated inflammatory disorders who were not on systemic immunosuppressants and healthy participants were included as controls. Anti-receptor binding domain IgG responses and neutralisation capacity were monitored following standard vaccination regimens and a three-vaccination regimen in subgroups. Hybrid immune responses—ie, vaccination after previous SARS-CoV-2 infection—were studied as a proxy for recall responses.
Findings
Between Feb 2 and Aug 1, 2021, we included 3222 participants in our cohort. Sera from 2339 participants, 1869 without and 470 participants with previous SARS-CoV-2 infection were analysed (mean age 49·9 years [SD 13·7]; 1470 [62·8%] females and 869 [37·2%] males). Humoral responses did not differ between disorders. Anti-CD20 therapy, sphingosine 1-phosphate receptor (S1P) modulators, and mycophenolate mofetil combined with corticosteroids were associated with lower relative risks for reaching seroconversion following standard vaccination (0·32 [95% CI 0·19–0·49] for anti-CD20 therapy, 0·35 [0·21–0·55] for S1P modulators, and 0·61 [0·40–0·90] for mycophenolate mofetil combined with corticosteroids). A third vaccination increased seroconversion for mycophenolate mofetil combination treatments (from 52·6% after the second vaccination to 89·5% after the third) but not significantly for anti-CD20 therapies (from 36·8% to 45·6%) and S1P modulators (from 35·5% to 48·4%). Most other immunosuppressant groups showed moderately reduced antibody titres after standard vaccination that did not increase after a third vaccination, although seroconversion rates and neutralisation capacity were unaffected. In participants with previous SARS-CoV-2 infection, SARS-CoV-2 antibodies were boosted after vaccination, regardless of immunosuppressive treatment.
Interpretation
Humoral responses following vaccination are impaired by specific immunosuppressants. After standard vaccination regimens, patients with immune-mediated inflammatory disorders taking most immunosuppressants show similar seroconversion to controls, although antibody titres might be moderately reduced. As neutralisation capacity and recall responses are also preserved in these patients, this is not likely to translate to loss of (short-term) protection. In patients on immunosuppressants showing poor humoral responses after standard vaccination regimens, a third vaccination resulted in additional seroconversion in patients taking mycophenolate mofetil combination treatments, whereas the effect of a third vaccination in patients on anti-CD20 therapy and S1P modulators was limited.
Funding
ZonMw (The Netherlands Organization for Health Research and Development).
Research in context.
Evidence before this study
Before the COVID-19 pandemic, studies had shown impaired humoral immune responses after routine vaccinations in patients on immunosuppressants. Before the start of the Dutch SARS-CoV-2 vaccination campaign, we launched this study to investigate the immune response after SARS-CoV-2 vaccination in patients with immune-mediated inflammatory disorders and immunosuppressants. We searched PubMed and medRxiv for articles published in English between Dec 1, 2020, and Oct 29, 2021, focusing on humoral immune responses after vaccination against SARS-CoV-2 in patients with immunosuppressive treatment using the following terms: “SARS-CoV-2”, “vaccine”, “immunocompromised”, “immune-suppressed”, “immunosuppressive”, “auto-immune”, and “immune-mediated inflammatory disorder”, and identified 35 studies (three in preprint). Of those studies, 24 focused on specific immune-mediated inflammatory disorder groups (eg, rheumatological disorders, inflammatory bowel disorders, or multiple sclerosis), ten focused solely on other immunocompromised patients (eg, those who had undergone transplantation), and one focused both on immune-mediated inflammatory disorders and other immunocompromised categories. Studies varied markedly in humoral assays, timing of assessments, combination of monotherapy and combination therapies in treatment groups, composition of control groups, and controlling for potential previous SARS-CoV-2 infections. Anti-CD20 therapy, sphingosine 1-phosphate receptor (S1P) modulators, and mycophenolate mofetil were consistently associated with decreased seroconversion in studies investigating these treatments. For several other immunosuppressants, reduced antibody titres without reduced seroconversion rates have been reported.
Added value of this study
This large and disease-overarching study shows that specific immunosuppressants are associated with impaired humoral responses after SARS-CoV-2 vaccination. Moreover, this study shows that impaired responses can be rescued by an early third vaccination in patients on mycophenolate mofetil treatments, whereas the effect of the third vaccination in patients on anti-CD20 therapy and S1P modulators is limited. We show that most immunosuppressants had no effect on seroconversion rates, but some were associated with lower antibody titres. By studying hybrid immune responses—ie, vaccination responses after previous SARS-CoV-2 infections—and by studying third vaccinations in a subgroup, we show that recall responses are generally unaffected by immunosuppressants. Additionally, we report that antibody neutralisation capacity was not affected. The prospective, disease-overarching design with predefined immunotherapies allows for reliable estimates and comparison of risks between different treatments for the most prevalent immune-mediated inflammatory disorders, but also for rare disorders for which immunogenicity studies are unlikely to be done.
Implications of all the available evidence
In patients with immune-mediated inflammatory disorders, humoral responses are impaired by specific immunosuppressants. Results from this and other studies suggest that in a majority of patients with immune-mediated inflammatory disorders, humoral responses to vaccination are largely intact. Humoral responses can be improved through an early third vaccination, although effects for anti-CD20 therapy and S1P modulators are limited, with absent humoral responses in more than half of patients.
Introduction
Since the start of vaccination against SARS-CoV-2, concerns have been raised about the efficacy of vaccines in patients treated with immunosuppressants. Patients with immune-mediated inflammatory diseases, which include a wide range of disorders such as rheumatoid arthritis, inflammatory bowel disease, and multiple sclerosis, are frequently treated with either broad or targeted immunosuppressants. Several immunosuppressants used in immune-mediated inflammatory disorders have been associated with impaired seroconversion rates after SARS-CoV-2 vaccination, most notably anti-CD20 therapies, sphingosine 1-phosphate receptor (S1P) modulators, and mycophenolate mofetil.1, 2, 3, 4 For most other common immunosuppressants, such as methotrexate, reduced antibody titres without impaired seroconversion rates have been reported.2 On the basis of these observations, third vaccinations have been implemented for selected patients with immune-mediated inflammatory disorders to improve immune responses.
Several important questions remain. First, because disease-overarching studies are scarce, it is unknown whether results from one specific disease can be translated to other diseases, particularly rare immune-mediated inflammatory disorders in which large cohort studies are unlikely. Second, the clinical relevance of reduced antibody titres after vaccination is unclear because data on the minimal antibody threshold required for protection are absent.5 Third, it is unclear whether the neutralising capacity of antibodies is reduced in patients with reduced titres, as suggested in some studies of patients on methotrexate or mycophenolate mofetil.6, 7, 8, 9 Fourth, the effects of third vaccinations on humoral responses have not been studied in patients with immune-mediated inflammatory disorders. Finally, humoral recall responses in patients with immune-mediated inflammatory disorders have not yet been investigated extensively, although they are essential for the durability of SARS-CoV-2 immunity. In the absence of long-term follow-up studies investigating immunological changes in patients with immune-mediated inflammatory disorders on immunosuppressants, hybrid immune responses—ie, responses that develop after vaccination of individuals previously infected with SARS-CoV-2—can be used as a proxy model for recall responses since such responses rely greatly on memory B cells.10, 11
We aimed to investigate the effects of various prevalent immunosuppressive monotherapies on humoral SARS-CoV-2 vaccination response in a large, national, disease-overarching cohort of patients with prevalent immune-mediated inflammatory disorders. We also sought to investigate effects of immunosuppressants on repeated antigen exposure by assessing the effect of selected immunosuppressive therapies on humoral responses after third vaccinations in patients with immune-mediated inflammatory disorders without a previous SARS-CoV-2 infection, and to investigate hybrid immune responses in patients with immune-mediated inflammatory disorders who had SARS-CoV-2 infections before vaccination.
Methods
Study design and participants
We did a cohort study in participants treated in outpatient clinics in seven university hospitals and one rheumatology treatment centre in the Netherlands as well as participants included in two national cohort studies on COVID-19-related disease severity (appendix pp 3–4).12, 13
Eligible participants were aged older than 18 years, diagnosed with any of the prespecified immune-mediated inflammatory disorders (appendix pp 3–4), and were able to understand and complete questionnaires in Dutch. Participants with known pregnancy during study entry and those undergoing concomitant treatment with immunosuppressants (ie, chemotherapy) for cancer or organ transplantation (including stem-cell transplantation) were excluded.
Participants were actively recruited to fit into predefined monotherapy treatment groups as maintenance treatment for the primary analysis (appendix pp 3–4). These immunosuppressants and immunomodulatory treatments were prioritised based on relevance (ie, frequently prescribed and expected effect on vaccine efficacy) and feasibility. For brevity, immunosuppressive and immunomodulatory treatments are grouped as immunosuppressive treatments throughout the manuscript. For the secondary analysis, we recruited participants treated with less frequently prescribed, but clinically relevant, monotherapies and frequently prescribed combination therapies. To assess hybrid responses following vaccination, we actively recruited participants with documented previous SARS-CoV-2 infections before vaccination. A subset of participants without evidence of previous SARS-CoV-2 infection, previously vaccinated with two mRNA vaccines, and treated with either anti-CD20 therapy (monotherapy or in combination), S1P modulators, mycophenolate mofetil (monotherapy or in combination), methotrexate, tumour necrosis factor (TNF) inhibitors, or purine antagonists were enrolled for third vaccination analysis.
As controls, we recruited patients with immune-mediated inflammatory disorders not on systemic immunosuppressants and healthy controls (appendix pp 3–4). For healthy controls, two additional inclusion criteria applied: no active or previous autoimmune, oncological, or haematological disease; and no current or previous treatment with systemic immunosuppressive medication in the past year. Additional methods are described in the appendix (pp 3–4).
This study was approved by the medical ethical committee (NL74974.018.20 and EudraCT 2021-001102-30). All participants provided written informed consent.
Procedures
Clinical data were collected by the investigators using a standardised electronic case record form (eCRF) and by sending online questionnaires to participants. Questionnaires were used to register demographics and dates of any positive PCR for SARS-CoV-2. Investigators completed eCRFs using data from the electronic patient files. Patient files were used to register immune-mediated inflammatory disorder diagnosis, and start and stop dates for any immunosuppressant used since Jan 1, 2021, or Jan 1, 2020, for treatments with long-term effects (ie, anti-CD20 therapies or cyclophosphamide).
Serum samples were collected by venipuncture or by participants at home using a fingerprick set (custom set, DaklaPack Europe, Lelystad, Netherlands), which was sent at baseline (before the first vaccination), day 28 after first vaccination, and day 28 after the second vaccination (when applicable). Serum samples after vaccination were received at the central test laboratory at day 33 (median [IQR 30–39]). For the third vaccination, serum samples were collected on the day of vaccination, at day 7 after, and at day 28 after the third vaccination. Participants returned their serum tube (Minicollect 450548, Greiner Bio-One, Alphen aan den Rijn, Netherlands) to the central test laboratory (Sanquin, Amsterdam, Netherlands).
The presence of SARS-CoV-2 antibodies was investigated using three assays in the central laboratory; all assays were developed in house. The primary assay was a quantitative anti-receptor binding domain (RBD) IgG ELISA, as described previously.14, 15 Anti-RBD IgG titres were expressed as arbitrary units (AU) per mL and were compared with a serially diluted calibrator (arbitrarily assigned a value of 100 AU/mL) consisting of pooled convalescent plasma. Signals below the limits of detection were imputed as 0·1 AU/mL. Seroconversion after vaccination was defined as an antibody concentration of more than 4 AU/mL (99% specificity in pre-pandemic sera).14, 15
In addition, we used a semiquantitative total antibody bridging ELISA to detect antibodies against the RBD in baseline samples before vaccination to identify participants with previous SARS-CoV-2 infection. Sensitivity of the semiquantitative RBD bridging ELISA is higher than the anti-RBD IgG ELISA in very low antibody ranges (98·1% sensitivity and 99·5% specificity).14
To detect SARS-CoV-2 infection in samples after the first vaccination, we used a semiquantitative total antibody bridging ELISA to detect antibodies against nucleocapsid, as described previously, but using a truncated version of the nucleocapsid protein to enhance specificity (specificity >99%, sensitivity 95%).16
Antibody neutralisation capacity against SARS-CoV-2 WA1/2020 was investigated for monotherapy treatment groups in participants without previous SARS-CoV-2 infection using a competition assay that measures blocking antibodies by assessing inhibition of RBD-binding to angiotensin-converting enzyme 2 (ACE2). We have previously demonstrated good correlation between this assay and classic virus neutralisation assays.15
Definitions for immunosuppressants, active treatment, and grouping of combination therapies are described in the appendix (pp 3–4). In summary, immunosuppressants were defined as either immunosuppressive or immunomodulating treatment in the 3 months before first or third vaccination, or in the 12 months before vaccination in case of long-acting therapies. We predefined nine monotherapy groups: anti-CD20 therapy, calcineurin inhibitors, corticosteroids, dupilumab, intravenous or subcutaneous immunoglobulins, methotrexate, purine antagonists, TNF inhibitors, and ustekinumab. Combination therapy groups were grouped in the following order: any combination therapy involving anti-CD20 therapy, mycophenolate mofetil in combination with corticosteroids, purine antagonists or methotrexate in combination with TNF inhibitors, dual combination therapies of corticosteroids with any other immunosuppressant, and dual combination therapies with purine antagonists or methotrexate with any other immunosuppressant. We defined a completed SARS-CoV-2 vaccination as having had two vaccinations of the same type for ChAdOx1 nCoV-19 (Oxford–AstraZeneca), BNT162b2 (Pfizer–BioNtech), and CX-024414 (Moderna) vaccines, regardless of the interval, and one vaccination for Ad.26.COV2.S (Janssen). As a third dose, either CX-024414 or BNT162b2 was administered. A previous SARS-CoV-2 infection was defined as a self-reported positive PCR for SARS-CoV-2 with or without evidence of anti-RBD antibodies at baseline or anti-nucleocapsid antibodies at follow-up.
Outcomes
For the primary objective to investigate effects of various prevalent immunosuppressive monotherapies on humoral SARS-CoV-2 vaccination response, the primary outcome was the relative risk with corresponding 95% CI for seroconversion after completed vaccination, for nine predefined monotherapy treatment groups. For the secondary objective to investigate effects of immunosuppressants on repeated antigen exposure, the outcomes were seropositivity rate and antibody titre after each vaccination.
Statistical analysis
For the primary objective, we a priori defined a difference in the proportion of patients not reaching seroconversion of at least 15% between a treatment and control group to be clinically meaningful, and we predefined nine monotherapy groups to be included in the primary analysis, yielding a sample size of 175 per monotherapy group (appendix pp 3–4).16 After the start of recruitment, we observed that recruitment lagged while other studies reported much larger effects than 15% for some immunosuppressants, such as anti-CD20 therapies.3, 17, 18 Therefore, we decided to include the predefined monotherapy groups in the primary analyses regardless of the number of observations, and adjusted the CI accordingly. Notably, calcineurin inhibitors were not included in the primary analysis because of a small number of observations in this group.
Only participants without a previous SARS-CoV-2 infection were included for the primary analysis. As a reference, we used a combined control group of patients with immune-mediated inflammatory disorders not taking immunosuppressants and healthy controls.
In the secondary analysis, we investigated the relative risk for seroconversion in other less frequent monotherapy treatment groups and combination therapy groups in participants without a previous SARS-CoV-2 infection. For both analyses, for participants with seroconversion after vaccination, we investigated the association, reported as fold change, between log-transformed anti-RBD IgG titres and treatment groups. Antibody neutralisation capacity was analysed using scatterplots showing log-transformed anti-RBD IgG titres and the percentage non-inhibited signal (appendix p 9). For the primary objective, all estimates derived from multivariate models from the primary and secondary analysis were corrected for age, sex, and vaccine type (categorised as vector or mRNA vaccines). 95% CIs for the primary analysis were adjusted using Bonferroni's correction; 95% CIs for secondary analyses were not adjusted.
For the secondary objective, we did the following analyses. For third vaccinations, we focused on the added value of a third vaccination by comparing the change in proportion of participants with seroconversion after second and third vaccination using McNemar's test. We calculated the proportion of participants who seroconverted from a third vaccination (participants without seroconversion after the second vaccination but with seroconversion after the third vaccination) and the proportion of participants with loss of seroconversion before the third vaccination (participants with seroconversion after the second vaccination but absent anti-RBD antibodies on the day of the third vaccination). Notably, for the loss of seroconversion calculation, some serum samples from the day of the third vaccination were missing, leading to altered group sizes for this calculation. We also investigated changes in antibody titres using Wilcoxon signed-rank test between the following timepoints: between day 28 after the second and third vaccination; and between the day of third vaccination and day 28 after the third vaccination. We investigated induction of hybrid immunity by comparing anti-RBD IgG titres at day 28 after the first and second vaccination between participants with and without a previous SARS-CoV-2 infection. For this analysis, immunosuppressants were grouped into poor responders based on low seroconversion percentage (ie, those on anti-CD20 therapy [monotherapy or combination therapy], S1P modulators, or mycophenolate mofetil [monotherapy or combination therapy]) and other immunosuppressants.
We did a sensitivity analysis to investigate potential effects of different immune-mediated inflammatory disorders on seroconversion when controlling for treatment groups (appendix p 11). We investigated the potential influences of our choices in selecting and grouping immunosuppressants for the primary and secondary analysis by using an alternative strategy in which we included all immunosuppressants separately in a logistic regression model (appendix p 12). Additionally, we investigated the effects of missing serological data by repeating primary analyses using a multivariate imputation model (appendix p 15). Data analysis was done with R (version 4.1.0). This study is registered at the Netherlands Trial Registry, NL8900.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
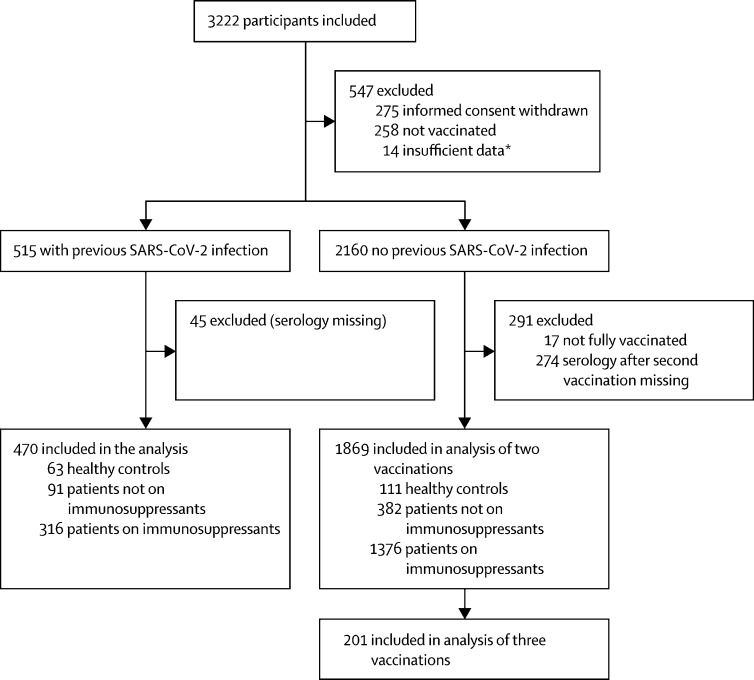
Between Feb 2 and Aug 1, 2021, we included 3222 participants in our cohort. 2339 participants (were included in the analyses, 1869 without previous SARS-CoV-2 infection and 470 with previous SARS-CoV-2 infection. The mean age of participants was 49·9 years [SD 13·7]; 1470 [62·8%] were women and 869 [37·2%] were men. Of the 1692 participants on immunosuppressants, 1273 were on monotherapy and 419 were on combination therapy. The control group consisted of 473 patients with immune-mediated inflammatory disorders not on immunosuppressants and 174 healthy controls. A subgroup of 201 participants without previous SARS-CoV-2 infection were analysed after a third vaccine dose (figure 1 ). Participant characteristics are shown in the table .
Figure 1.
Study flow chart
*Insufficient data to categorise SARS-CoV-2 infection status.
Table.
Demographic and clinical characteristics of the study participants
|
No previous SARS-CoV-2 infection |
Previous SARS-CoV-2 infection |
|||||||
|---|---|---|---|---|---|---|---|---|
| Patients on immunosuppressants (n=1376) | Patients not on immunosuppressants (n=382) | Healthy controls (n=111) | Patients on immunosuppressants (n=316) | Patients not on immunosuppressants (n=91) | Healthy controls (n=63) | |||
| Age, years | 50·4 (14·3) | 52·0 (12·9) | 49·4 (10·1) | 46·7 (13·3) | 48·2 (11·7) | 46·6 (11·6) | ||
| Sex | ||||||||
| Female | 855 (62·1%) | 256 (67·0%) | 77 (69·4%) | 187 (59·2%) | 54 (59·3%) | 41 (65·1%) | ||
| Male | 521 (37·9%) | 126 (33·0%) | 34 (30·6%) | 129 (40·8%) | 37 (40·7%) | 22 (34·9%) | ||
| Vaccine received | ||||||||
| BNT162b2 (Pfizer–BioNTech) | 812 (59·0%) | 186 (48·7%) | 52 (46·8%) | 198 (62·7%) | 62 (68·1%) | 14 (22·2%) | ||
| CX-024414 (Moderna) | 368 (26·7%) | 137 (35·9%) | 51 (45·9%) | 89 (28·2%) | 20 (22·0%) | 47 (74·6%) | ||
| ChAdOx1 nCoV-19 (Oxford–AstraZeneca) | 173 (12·6%) | 48 (12·6%) | 2 (1·8%) | 23 (7·3%) | 8 (8·8%) | 0 (0·0%) | ||
| Ad.26.COV2.S (Janssen) | 23 (1·7%) | 11 (2·9%) | 6 (5·4%) | 6 (1·9%) | 1 (1·1%) | 2 (3·2%) | ||
| Number of immunosuppressants per participant | ||||||||
| 1 | 1034 (75·1%) | .. | .. | 239 (75·6%) | .. | .. | ||
| 2 | 312 (22·7%) | .. | .. | 69 (21·8%) | .. | .. | ||
| ≥3 | 30 (2·2%) | .. | .. | 8 (2·5%) | .. | .. | ||
| Immune-mediated inflammatory disorders | ||||||||
| Rheumatic disorders | ||||||||
| Rheumatoid arthritis | 200 (14·5%) | 24 (6·3%) | .. | 41 (13·0%) | 3 (3·3%) | .. | ||
| Spondylarthritis | 79 (5·7%) | 18 (4·7%) | .. | 21 (6·6%) | 3 (3·3%) | .. | ||
| Systemic lupus erythematosus | 133 (9·7%) | 12 (3·1%) | .. | 29 (9·2%) | 0 (0·0%) | .. | ||
| Vasculitis* | 61 (4·4%) | 8 (2·1%) | .. | 5 (1·6%) | 0 (0·0%) | .. | ||
| Other rheumatic disease† | 29 (2·1%) | 7 (1·8%) | .. | 2 (0·6%) | 1 (1·1%) | .. | ||
| Inflammatory bowel disease | ||||||||
| Crohn's disease | 216 (15·7%) | 32 (8·4%) | .. | 53 (16·8%) | 11 (12·1%) | .. | ||
| Ulcerating colitis | 92 (6·7%) | 51 (13·4%) | .. | 23 (7·3%) | 10 (11·0%) | .. | ||
| Other inflammatory bowel diseases‡ | 37 (2·7%) | 4 (1·0%) | .. | 5 (1·6%) | 2 (2·2%) | .. | ||
| Neurological disorders | ||||||||
| Multiple sclerosis and neuromyelitis optica spectrum disorder§ | 179 (13·0%) | 86 (22·5%) | .. | 45 (14·2%) | 26 (28·6%) | .. | ||
| Inflammatory neuropathies and myopathies¶ | 123 (8·9%) | 11 (2·9%) | .. | 30 (9·5%) | 4 (4·4%) | .. | ||
| Myasthenia gravis | 69 (5·0%) | 40 (10·5%) | .. | 15 (4·7%) | 3 (3·3%) | .. | ||
| Dermatological disorders | ||||||||
| Atopic dermatitis | 82 (6·0%) | 2 (0·5%) | .. | 28 (8·9%) | 1 (1·1%) | .. | ||
| Other dermatological‖ | 76 (5·5%) | 87 (22·8%) | .. | 19 (6·0%) | 27 (29·7%) | .. | ||
Data are mean (SD) or n (%).
Including small-vessel, medium-vessel, and large-vessel vasculitis and other forms of vasculitis except giant cell arteritis.
Including Sjögren's syndrome, giant-cell arteritis, polymyalgia rheumatica, and others.
Including autoimmune hepatitis and autoimmune sclerosing cholangitis.
Including six patients with neuromyelitis optica spectrum disorder.
Including chronic inflammatory demyelinating polyneuropathy, multifocal motor neuropathy, and inflammatory myositis.
Including vitiligo, pemphigus, psoriasis, and others.
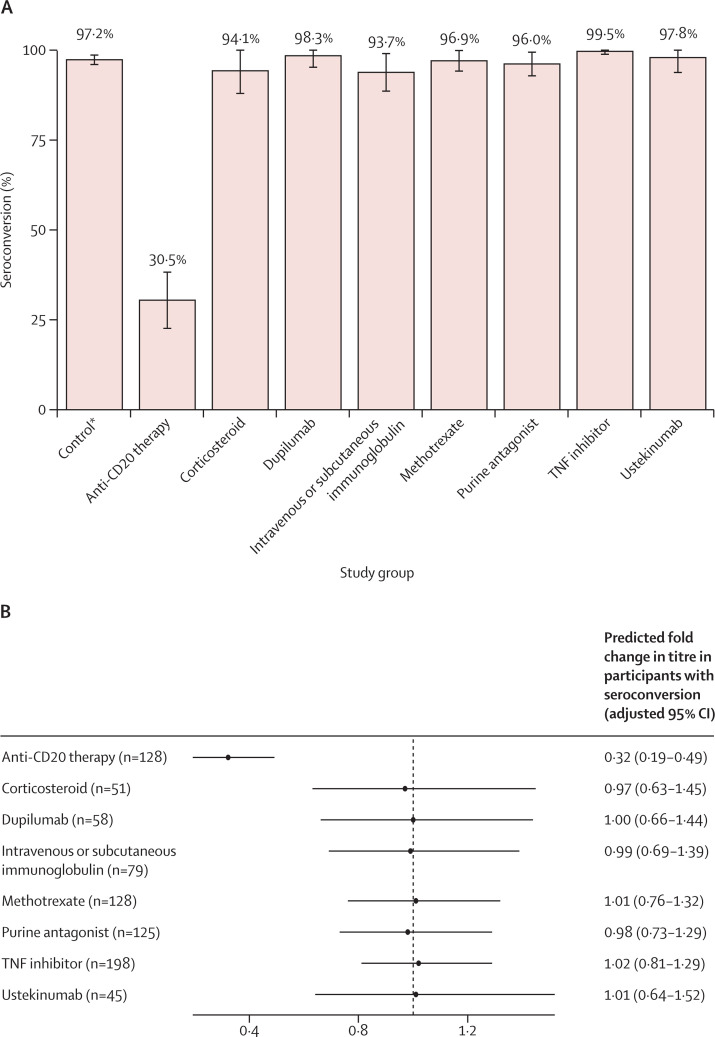
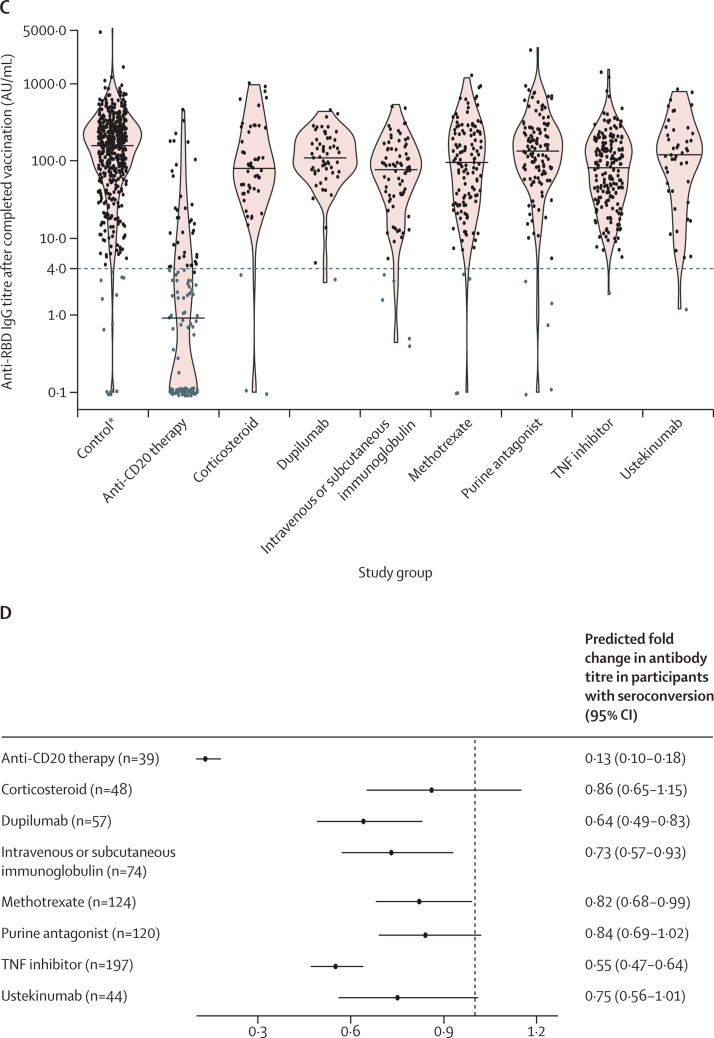
In the primary analysis, relative risk for seroconversion for anti-CD20 therapy was 0·32 (95% CI 0·19–0·49) after completed vaccination, and the relative risks for other immunosuppressants were not significantly reduced compared with controls (39 [30·5%] of 128 on anti-CD20 therapy seroconverted vs 479 [97·2%] of 493 controls; figure 2A, 2B ; appendix p 9). In participants who seroconverted, anti-CD20 therapy was associated with substantial reductions in anti-RBD IgG titres (figure 2C, 2D). TNF inhibitors, dupilumab, intravenous and subcutaneous immunoglobulin, and methotrexate were associated with moderate reductions in anti-RBD IgG titres (figure 2C, 2D). For purine antagonists, ustekinumab, and corticosteroids, fold change estimates were similar to other groups but were not significant (figure 2C, 2D).
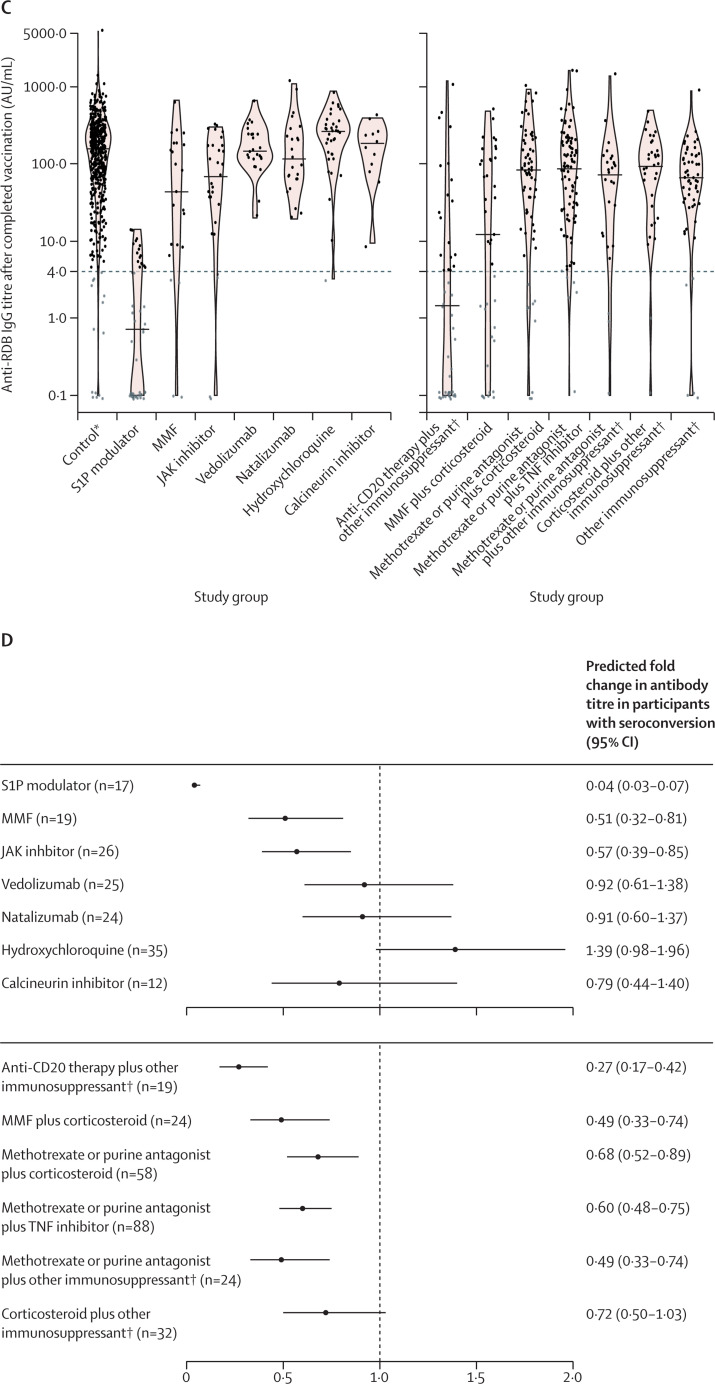
Figure 2.
Humoral response following SARS-CoV-2 vaccination (primary analysis)
(A) Percentage (with error bars indicating 95% CIs) of participants with seroconversion (ie, anti-RBD IgG titre >4 AU/mL) for each group. (B) The predicted relative risk for seroconversion compared with controls, adjusted for confounders and multiple comparisons. (C) Anti-RBD IgG titres for each group. Grey dots indicate titres below the threshold for seroconversion (indicated by the dashed grey line), and black dots are above this threshold. Solid horizontal lines indicate the median per group. (D) The predicted fold change in anti-RBD titres for participants with seroconversion compared with controls. AU=arbitrary units. RBD=receptor binding domain. TNF=tumour necrosis factor. *The control group is composed of healthy controls and patients with immune-mediated inflammatory disorders not on immunosuppressants. Seroconversion and anti-RBD IgG titres at day 28 after completed SARS-CoV-2 vaccination for participants without previous SARS-CoV-2 infection treated with immunosuppressants.
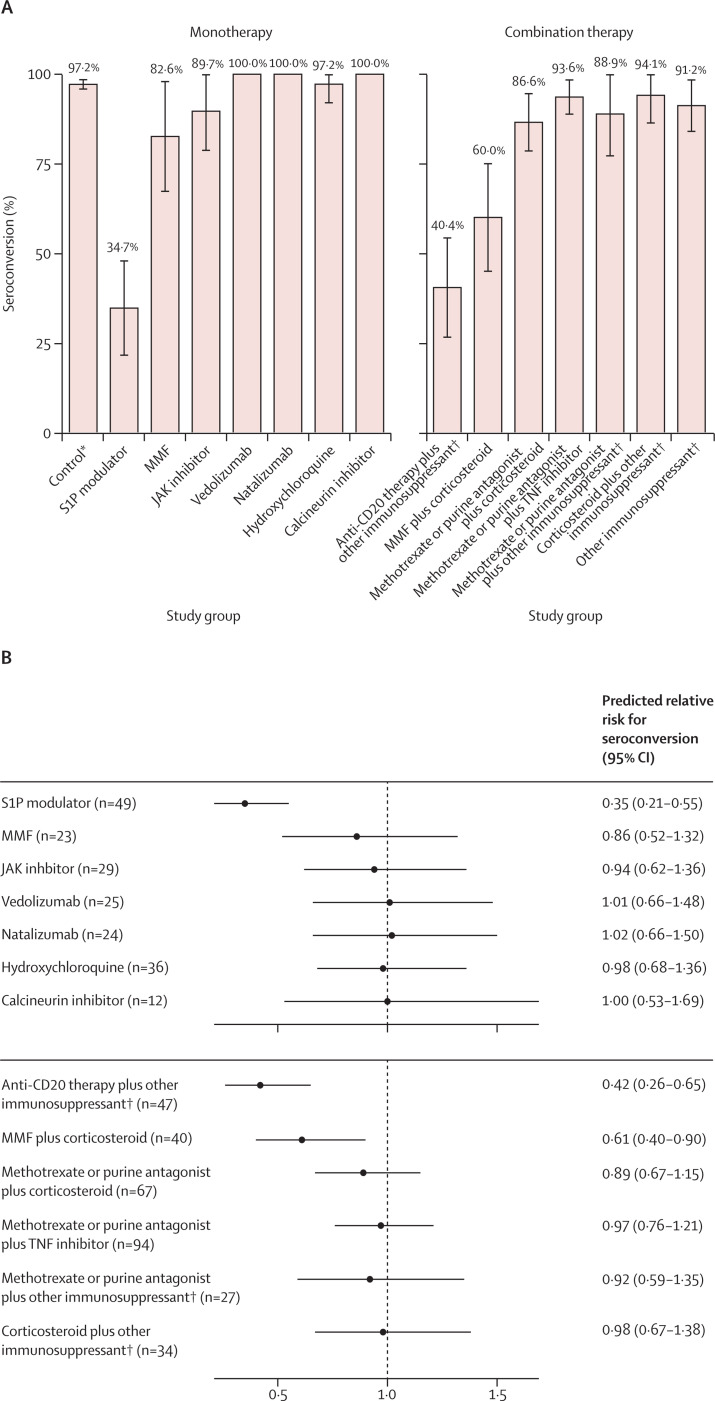
In the secondary analysis, relative risks for seroconversion were decreased for S1P modulators, anti-CD20 combination therapies, and mycophenolate mofetil in combination with corticosteroids (figure 3A, 3B ; appendix p 9). Mycophenolate mofetil and JAK inhibitors, and most combination treatment groups, were associated with moderate reductions in anti-RBD IgG titres (figure 3C, 3D).
Figure 3.
Humoral response following SARS-CoV-2 vaccination (secondary analysis)
Seroconversion and anti-RBD IgG titres at day 28 after completed SARS-CoV-2 vaccination for participants without previous SARS-CoV-2 infections treated with immunosuppressants included in the secondary analysis. (A) The percentage (with error bars indicating 95% CIs) of participants with seroconversion (ie, anti-RBD IgG titre >4 AU/mL) for each group. (B) The predicted relative risk for seroconversion compared with controls. (C) Anti-RBD IgG titres for each group. Grey dots indicate titres below the threshold for seroconversion (indicated by the dashed grey line); black dots are above this threshold. Solid horizontal lines indicate the median per group. (D) The predicted fold change in anti-RBD titres for participants with seroconversion compared with controls. AU=arbitrary unit. JAK=Janus kinase. MMF=mycophenolate mofetil. RBD=receptor binding domain. S1P=sphingosine 1-phosphate receptor. TNF=tumour necrosis factor. *The control group is composed of healthy controls and patients with immune-mediated inflammatory disorders not on immunosuppressants. †Details on other immunosuppressants are shown in the appendix (pp 5–6).
Analysis of binding of RBD to ACE2, the receptor on SARS-CoV-2 target cells, was used to evaluate neutralisation capacity of antibodies in the serum samples. We found that between different monotherapy groups, antibody blocking capacity for the RBD protein was similar to that of the control group (appendix p 10). No effects of the different immune-mediated inflammatory disorders on seroconversion were observed when adjusted for immunosuppressants (appendix p 11). Moreover, a different grouping strategy of immunosuppressants and missing serological assessments did not affect the results (appendix pp 12–15). Serology results according to sex and vaccine type in the control group are shown in the appendix (pp 16–17).
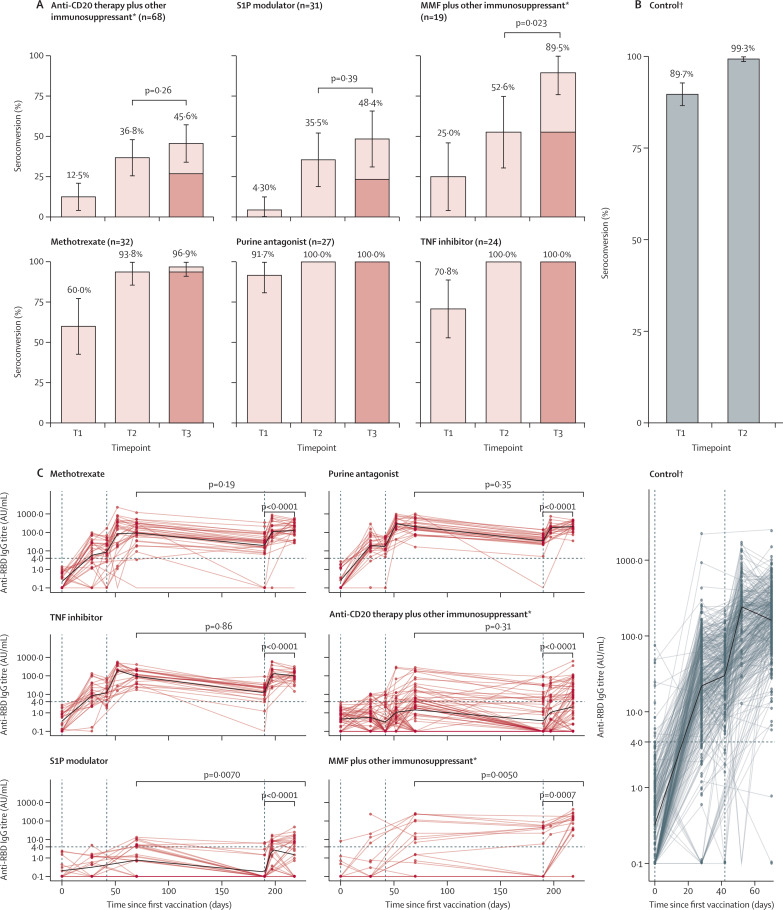
Figure 4 shows humoral responses after a third vaccination; clinical characteristics are shown in the appendix (pp 18–19). The median time between second and third vaccination was 111 days (IQR 100–119; appendix pp 18–19). After the third vaccination, 31 (45·6%) of 68 patients in the anti-CD20 group, 15 (48·4%) of 31 in the S1P modulator group, and 17 (89·5%) of 19 in the mycophenolate mofetil group seroconverted (figure 4A; appendix p 20). Compared with the second vaccination, 13 (19·1%) of 68 patients in the anti-CD20 therapy group, eight (25·8%) of 31 in the S1P modulator group, and seven (36·8%) of 19 in the mycophenolate mofetil group gained seroconversion after the third vaccination. Between the second vaccine dose and the day of the third dose, seroreversion—ie, loss of seroconversion—was seen in seven (10·6%) of 66 patients in the anti-CD20 therapy group, four (13·3%) of 30 in S1P modulator group, and none in the mycophenolate mofetil group (figure 4A; appendix p 20).
Figure 4.
Humoral response following a third SARS-CoV-2 vaccination
Seroconversion and anti-RBD IgG titres up to the third SARS-CoV-2 vaccination. The percentage (with error bars indicating 95% CIs) of participants with seroconversion (ie, anti-RBD IgG titre >4 AU/mL) 28 days after each vaccination for different immunosuppressant groups (A) and the control group (B) are shown. The dark coloured parts of the bars represent the proportion of participants who remained seropositive after their second vaccination. The anti-RBD IgG titres for different immunosuppressant groups (C) and the control group (D), from before first vaccination to after third vaccination, are shown. Vertical dashed lines represent first, second, and third vaccine doses. Dots represent serum samples before first vaccination, 28 days after first vaccination, at second vaccination (day 42; absent in MMF plus other immunosuppressant group and S1P modulator group), 10 days after second vaccination (day 52), 28 days after second vaccination (day 70), at third vaccination (day 190), 7 days after third vaccination (day 197; absent in MMF plus other immunosuppressant group), and 28 days after third vaccination (day 218). AU=arbitrary unit. MMF=mycophenolate mofetil. RBD=receptor binding domain. S1P=sphingosine 1-phosphate receptor. T1=28 days after first vaccination. T2=28 days after second vaccination. T3=28 days after third vaccination. TNF=tumour necrosis factor. *Details on other immunosuppressants are shown in the appendix (pp 5–6). †The control group is composed of healthy controls and patients with immune-mediated inflammatory disorders not on immunosuppressants.
In the S1P modulator group and the mycophenolate mofetil group, antibody titres at 28 days after the third vaccination were higher than antibody titres after second vaccination (figure 4C). In the other immunosuppressant groups, antibody titres did not increase further (figure 4C). Of note, all groups showed an increase in antibody titres between the day of third vaccination and day 28 after third vaccination (figure 4C). In most patients, this could be already be observed at day 7 after the third vaccination, indicating a rapid recall response (figure 4C).
Previous SARS-CoV-2 infections were identified using both a self-reported positive PCR and positive anti-RBD or anti-nucleocapsid antibodies in 178 (37·9%) of 470 participants, positive anti-RBD or anti-nucleocapsid antibodies in 244 (51·9%) participants, or a self-reported positive PCR in 48 (10·2%) participants. The median time between a positive PCR and first vaccination was 166 days (IQR 86–220). The appendix (pp 22–23) shows results for the hybrid humoral responses among patients in poor-responder groups (ie, anti-CD20, S1P modulators, and mycophenolate mofetil combination treatments), other immunosuppressants, and controls. After first vaccination, seroconversion rates and anti-RBD IgG titres were higher in all groups of participants with previous SARS-CoV-2 infection than in groups of participants without previous SARS-CoV-2 infections, indicative of a boost response. In participants with previous SARS-CoV-2 infections, anti-RBD IgG titres after the first vaccination were lower in participants treated with other immunosuppressants or poor responder immunosuppressants than in controls. Higher anti-RBD IgG titres after a second vaccination were seen in participants with previous SARS-CoV-2 infection treated with immunosuppressants.
Discussion
In this large disease-overarching cohort of the most prevalent immune-mediated inflammatory disorders, we show that the type of immunosuppressive monotherapy or combination therapy is of major relevance for humoral responses following SARS-CoV-2 vaccination. After standard vaccination regimens, patients on most immunosuppressants showed seroconversion rates, neutralisation capacity, and recall responses similar to controls. Patients on methotrexate, TNF inhibitors, or purine antagonist showed moderate reductions in antibody titres that were not increased after a third vaccination. Patients on anti-CD20 monotherapy or combination therapies, S1P modulators, and mycophenolate mofetil with corticosteroids or other combinations showed impaired responses after standard vaccination. A third vaccination resulted in additional seroconversion in those on mycophenolate mofetil combination treatments, whereas effects for anti-CD20 therapy and S1P modulators were limited.
Our results confirm and expand findings from disease-specific studies in patients with immune-mediated inflammatory disorders and other patient groups on anti-CD20 therapies, mycophenolate mofetil with corticosteroids, and S1P modulators.1, 2, 4, 19 In addition, we now present data showing that patients with immune-mediated inflammatory disorders treated with mycophenolate mofetil could benefit from a third vaccination, as was previously demonstrated for other populations.20 In patients with immune-mediated inflammatory disorders on anti-CD20 therapies and S1P modulators, we observed marginal recall responses in participants with a previous SARS-CoV-2 infection or after a third vaccination, but overall humoral responses remained blunted. This finding is largely in line with what might be expected on the basis of the modes of action of these immunosuppressants. Previously, the formation of antigen-specific T cells after two vaccinations has been shown to be relatively unaffected by anti-CD20 therapy, whereas the sparse data available indicate severely reduced circulating antigen-specific T cells in those on S1P modulators.21 Intriguingly, increased susceptibility and increased severity of COVID-19 has not been demonstrated for patients with immune-mediated inflammatory disorders treated with S1P modulators, whereas for patients taking anti-CD20 therapies increased risks are observed. However, a considerable proportion of patients on anti-CD20 treatment might have asymptomatic or pauci-symptomatic SARS-CoV-2 infections.22, 23 Taken together, these findings indicate that solely relying on humoral responses to determine future booster strategies for these patient groups is probably inappropriate. We need more clinical data on COVID-19 susceptibility and disease severity after vaccination, as well as informative and reliable cellular biomarkers to predict the risk of severe COVID-19 and vaccine effects in these particular groups. Moreover, high clinical vigilance for breakthrough infections is needed and use of early treatments, such as recombinant anti-SARS-CoV-2 monoclonal antibodies or novel, effective antiviral treatments might be indicated in case of infection.24, 25
For patients taking most other monotherapy and combination therapies, we observed only moderately reduced antibody titres following standard vaccination, similar to what has been observed in other studies done in patients in specific immune-mediated inflammatory disorder groups.1, 2, 7 Currently, the clinical relevance of this observation is uncertain. A formal minimal antibody threshold for protection after vaccination has not been established for healthy individuals. Based on a predictive model, one study estimated that neutralising antibody levels of approximately 20% of the mean level in convalescents provide 50% protection against symptomatic infection, and levels of 3% of the mean level in convalescents provide 50% protection against severe disease.26 Our competition assay results using WA1/2020 suggest that the neutralisation capacity of the humoral response in patients taking most immunosuppressants is similar to healthy individuals and confirm studies in other populations showing that anti-RBD IgG correlates well with neutralisation capacity.7, 8, 27 However, cross-variant neutralisation was not assessed, and we cannot confirm that our findings also apply to other SARS-CoV-2 variants. In a previous study, using the same assay, median titre among convalescent individuals was 24 AU/mL.15 Moreover, recall responses, as observed in hybrid immune responses and after a third vaccination, were largely similar to those in controls, implying that formation of memory B cells is not affected to a relevant degree by most immunosuppressants.10, 11 Collectively, these results suggests that reductions in antibody titres for patients taking most immunosuppressants might not translate to a clinically significant loss of protection, at least not in the short term. Further studies on the clinical relevance of changes in antibody titres over time are needed to understand potential long-term effects in immunosuppressed patients and the need for subsequent booster vaccinations. Of note, a second vaccination further increased antibody titres in patients with previous SARS-CoV-2 infections treated with immunosuppressants. This finding supports completing a two-vaccination regimen in these patients and not reverting to a one-vaccine strategy, as was shown to be sufficient for healthy individuals after a previous SARS-CoV-2 infection.28
The strengths of this study include the prospective and standardised collection of serology and clinical data in a large, disease-overarching cohort that has been specifically recruited to investigate effects of clinically relevant monotherapy and combination therapies with immunosuppressants. By investigating standard vaccination regimens, third vaccinations, and hybrid immune responses, this study creates a comprehensive overview of SARS-CoV-2 vaccination responses in patients with immune-mediated inflammatory disorders taking immunosuppressants, compared with patients with these diseases but not taking immunosuppressants and healthy controls. Moreover, we used both PCR and two different SARS-CoV-2 antibody responses to detect participants with previous SARS-CoV-2 infection. The main limitation is that we did not reach the predefined sample size for most immunosuppressants to test the hypothesis of at least 15% difference in seroconversion. For these groups, we cannot exclude that seroconversion was lower than for controls, although we consider a 15% difference unlikely given the observed point estimates and findings of other studies. Additionally, we did not investigate potential dose effects on associations between immunosuppressants and humoral responses. Factors potentially influencing the hybrid immune response, such as the severity of SARS-CoV-2 infection,29 were also not addressed in this study.
In patients with immune-mediated inflammatory disorders, humoral responses following SARS-CoV-2 vaccination are impaired by specific immunosuppressants. After standard vaccination regimens, patients on most immunosuppressants show equal seroconversion to controls, although antibody titres might be moderately reduced. As neutralisation capacity and recall responses are preserved in patients showing optimal seroconversion rates after vaccination, the lower titres are not likely to translate in loss of (short-term) protection. Patients on mycophenolate mofetil combination treatments, anti-CD20 therapy, and S1P modulators showed poor humoral responses after standard vaccination regimens, and a third vaccination increased seroconversion for those taking mycophenolate mofetil combination treatments, whereas effects for those on anti-CD20 therapy and S1P modulators were limited. Collectively, these results could inform physicians and policy makers about decisions on additional vaccinations in a very broad range of patients with immune-mediated inflammatory disorders using immunosuppressants.
Data sharing
After publication, anonymised individual participant data and a data dictionary will be made available upon request to the corresponding author to researchers who provide a methodologically sound proposal. Data will be shared through a secure online platform.
Declaration of interests
FE and TWK report (governmental) grants from ZonMw to study immune response after SARS-Cov-2 vaccination in autoimmune diseases. FE also reports grants from Prinses Beatrix Spierfonds, CSL Behring, Kedrion, Terumo BCT, Grifols, Takeda Pharmaceutical Company, and GBS-CIDP Foundation; consulting fees from UCB Pharma and CSL Behring; and honoraria from Grifols. AJvdK reports grants from CSL Behring and participation on an advisory board for Argen-X. ML reports a grant from Galapagos not related to this study, and honoraria from Bristol Myers Squibb, Pfizer, Takeda, and Tillotts. PIS is involved in clinical trials with many pharmaceutical industries that manufacture drugs used for the treatment of, for example, psoriasis and atopic dermatitis, for which financial compensation is paid to the department or hospital, and is a chief investigator of the TREAT NL registry taskforce and SECURE-AD registry. MWB is a secretary for the Dutch Experimental Dermatology Board; head of the pigmentary disorders group within the Dutch Dermatology Board; and reports honoraria from Pfizer, Sanofi, Novartis, and Fondation René Touraine. JK has speaking relationships with Merck Serono, Biogen Idec, TEVA, Sanofi, Genzyme, Roche, and Novartis; received financial support to his institution for research activities from Merck Serono, Bayer Shcering Pharma, Biogen Idec, GlaxoSmithKline (GSK), Roche, Teva, Sanofi, Genzyme, and Novartis. BH reports unpaid positions as a medical adviser for several patient groups, a board position for ERN-SKIN, and associate editor for The British Journal of Dermatology; reports grants from AbbVie, Akari Therapeutics, Celgene, and Novartis; consulting fees from UCB Pharma, Novartis, and Janssen; and honoraria from AbbVie. JJGMV reports consulting fees from Argenx, Alexion, and NMD Pharma, and is a co-inventor on patent applications based on MuSK protein-related research. DJH reports grants from AbbVie, AstraZeneca, Janssen, LEO Pharma, and UCB; honoraria from AbbVie, Galderma, Janssen, Lilly, Pfizer, Sanofi, and UCB; and a paid position on an advisory board for BIOMAP IMI. PAvD participated on an advisory board for Octapharma. PvP reports grants from Alexion Pharma and GSK, and participation on advisory boards for GSK and Vifor Pharma. GRAMD'H reports consulting fees from AbbVie, Agomab, AstraZeneca, AM Pharma, AMT, Arena Pharmaceuticals, Bristol Myers Squibb, Boehringer Ingelheim, Celltrion, Eli Lilly, Exeliom Biosciences, Exo Biologics, Galapagos, Index Pharmaceuticals, Kaleido, Roche, Gilead, GSK, Gossamerbio, Pfizer, Immunic, Johnson and Johnson, Origo, Polpharma, Procise Diagnostics, Prometheus Laboratories, Prometheus Biosciences, Progenity, and Protagonist; honoraria from AbbVie, Arena, Galapagos, Gilead, Pfizer, Bristol Myers Squibb, and Takeda; and participation on advisory boards for AbbVie, Seres Health, Galapagos, and AstraZeneca. RBT reports honoraria from Sobi and Norgine, and participation on an advisory board for Norgine. HSG is a board member of the Dutch Society of Clinical Neurophysiology (unpaid), reports grants from Prinses Beatrix Spierfonds, and received speaker fees from Shire/Takeda. KAHZ reports paid data safety monitoring board positions for Torrent and Foresee. All other authors declare no competing interests.
Acknowledgments
Acknowledgments
We thank ZonMw (The Netherlands Organization for Health Research and Development, grant 10430072010007) for the funding of the study and the T2B partners, including the patient groups, and Health Holland for the support in this study. This collaboration project is financed by the PPP Allowance made available by Top Sector Life Sciences & Health to Samenwerkende Gezondheidsfondsen (SGF) under project number LSHM18055-SGF to stimulate public–private partnerships and co-financing by health foundations that are part of the SGF. We also thank E P Moll van Charante (Department of Public and Occupational Health and Department of General Practice, Amsterdam UMC, University of Amsterdam; and Amsterdam Public Health Research Institute, Amsterdam, Netherlands), J A Bogaards (Department of Epidemiology and Data Science, Amsterdam UMC), and R A Scholte (Clinical Research Unit, Amsterdam UMC, University of Amsterdam) for their guidance in the data safety monitoring board.
Contributors
All authors met the criteria for authorship set by the International Committee of Medical Journal Editors. TR, MS, SK, JBDK, AB, and OC did the serological assays; all other authors contributed in data acquisition. LW, KPJvD, and FE wrote the first draft of the manuscript. LW and KAHZ did the data analyses. LW, KPJvD, MS, EWS, and LYLK had full access to and verified the underlying data. All authors helped to revise the manuscript for important intellectual content and had final responsibility for the decision to submit for publication.
Contributor Information
T2B! Immunity against SARS-CoV-2 study group:
R. de Jongh, C.E. van de Sandt, L. Kuijper, M. Duurland, R.R. Hagen, J. van den Dijssel, C. Kreher, A. Bos, V. Palomares Cabeza, V.A.L. Konijn, G. Elias, J.G. Vallejo, M.J. van Gils, T.M. Ashhurst, S. Nejentsev, and E.S. Mirfazeli
Supplementary Material
References
- 1.Furer V, Eviatar T, Zisman D, et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in adult patients with autoimmune inflammatory rheumatic diseases and in the general population: a multicentre study. Ann Rheum Dis. 2021;80:1330–1338. doi: 10.1136/annrheumdis-2021-220647. [DOI] [PubMed] [Google Scholar]
- 2.Braun-Moscovici Y, Kaplan M, Braun M, et al. Disease activity and humoral response in patients with inflammatory rheumatic diseases after two doses of the Pfizer mRNA vaccine against SARS-CoV-2. Ann Rheum Dis. 2021;80:1317–1321. doi: 10.1136/annrheumdis-2021-220503. [DOI] [PubMed] [Google Scholar]
- 3.Moor MB, Suter-Riniker F, Horn MP, et al. Humoral and cellular responses to mRNA vaccines against SARS-CoV-2 in patients with a history of CD20 B-cell-depleting therapy (RituxiVac): an investigator-initiated, single-centre, open-label study. Lancet Rheumatol. 2021;3:e789–e797. doi: 10.1016/S2665-9913(21)00251-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tallantyre EC, Vickaryous N, Anderson V, et al. COVID-19 vaccine response in people with multiple sclerosis. Ann Neurol. 2022;91:89–100. doi: 10.1002/ana.26251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Milne G, Hames T, Scotton C, et al. Does infection with or vaccination against SARS-CoV-2 lead to lasting immunity? Lancet Respir Med. 2021;9:1450–1466. doi: 10.1016/S2213-2600(21)00407-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kantauskaite M, Müller L, Kolb T, et al. Intensity of mycophenolate mofetil treatment is associated with an impaired immune response to SARS-CoV-2 vaccination in kidney transplant recipients. Am J Transplant. 2022;22:634–639. doi: 10.1111/ajt.16851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moyon Q, Sterlin D, Miyara M, et al. BNT162b2 vaccine-induced humoral and cellular responses against SARS-CoV-2 variants in systemic lupus erythematosus. Ann Rheum Dis. 2021 doi: 10.1136/annrheumdis-2021-221097. published online Oct 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hod T, Ben-David A, Olmer L, et al. Humoral response of renal transplant recipients to the BNT162b2 SARS-CoV-2 mRNA vaccine using both RBD IgG and neutralizing antibodies. Transplantation. 2021;105:e234–e243. doi: 10.1097/TP.0000000000003889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mahil SK, Bechman K, Raharja A, et al. The effect of methotrexate and targeted immunosuppression on humoral and cellular immune responses to the COVID-19 vaccine BNT162b2: a cohort study. Lancet Rheumatol. 2021;3:e627–e637. doi: 10.1016/S2665-9913(21)00212-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goel RR, Apostolidis SA, Painter MM, et al. Distinct antibody and memory B cell responses in SARS-CoV-2 naïve and recovered individuals following mRNA vaccination. Sci Immunol. 2021;6 doi: 10.1126/sciimmunol.abi6950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Z, Muecksch F, Schaefer-Babajew D, et al. Naturally enhanced neutralizing breadth against SARS-CoV-2 one year after infection. Nature. 2021;595:426–431. doi: 10.1038/s41586-021-03696-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boekel L, Steenhuis M, Hooijberg F, et al. Antibody development after COVID-19 vaccination in patients with autoimmune diseases in the Netherlands: a substudy of data from two prospective cohort studies. Lancet Rheumatol. 2021;3:e778–e788. doi: 10.1016/S2665-9913(21)00222-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Kempen ZLE, Strijbis EMM, Al MMCT, et al. SARS-CoV-2 antibodies in adult patients with multiple sclerosis in the Amsterdam MS cohort. JAMA Neurol. 2021;78:880–882. doi: 10.1001/jamaneurol.2021.1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vogelzang EH, Loeff FC, Derksen NIL, et al. Development of a SARS-CoV-2 total antibody assay and the dynamics of antibody response over time in hospitalized and nonhospitalized patients with COVID-19. J Immunol. 2020;205:3491–3499. doi: 10.4049/jimmunol.2000767. [DOI] [PubMed] [Google Scholar]
- 15.Steenhuis M, van Mierlo G, Derksen NIL, et al. Dynamics of antibodies to SARS-CoV-2 in convalescent plasma donors. Clin Transl Immunology. 2021;10 doi: 10.1002/cti2.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nguyen DL, Nguyen ET, Bechtold ML. Effect of Immunosuppressive therapies for the treatment of inflammatory bowel disease on response to routine vaccinations: a meta-analysis. Dig Dis Sci. 2015;60:2446–2453. doi: 10.1007/s10620-015-3631-y. [DOI] [PubMed] [Google Scholar]
- 17.Mrak D, Tobudic S, Koblischke M, et al. SARS-CoV-2 vaccination in rituximab-treated patients: B cells promote humoral immune responses in the presence of T-cell-mediated immunity. Ann Rheum Dis. 2021;80:1345–1350. doi: 10.1136/annrheumdis-2021-220781. [DOI] [PubMed] [Google Scholar]
- 18.Novak F, Nilsson AC, Nielsen C, et al. Humoral immune response following SARS-CoV-2 mRNA vaccination concomitant to anti-CD20 therapy in multiple sclerosis. Mult Scler Relat Disord. 2021;56 doi: 10.1016/j.msard.2021.103251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stumpf J, Siepmann T, Lindner T, et al. Humoral and cellular immunity to SARS-CoV-2 vaccination in renal transplant versus dialysis patients: a prospective, multicenter observational study using mRNA-1273 or BNT162b2 mRNA vaccine. Lancet Reg Health Eur. 2021;9 doi: 10.1016/j.lanepe.2021.100178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hall VG, Ferreira VH, Ku T, et al. Randomized trial of a third dose of mRNA-1273 vaccine in transplant recipients. N Engl J Med. 2021;385:1244–1246. doi: 10.1056/NEJMc2111462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tortorella C, Aiello A, Gasperini C, et al. Humoral- and T-cell-specific immune responses to SARS-CoV-2 mRNA vaccination in patients with MS using different disease-modifying therapies. Neurology. 2022;98:e541–e554. doi: 10.1212/WNL.0000000000013108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Andersen KM, Mehta HB, Palamuttam N, et al. Association between chronic use of immunosuppresive drugs and clinical outcomes from coronavirus disease 2019 (COVID-19) hospitalization: a retrospective cohort study in a large US health system. Clin Infect Dis. 2021;73:e4124–e4130. doi: 10.1093/cid/ciaa1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Simpson-Yap S, De Brouwer E, Kalincik T, et al. Associations of disease-modifying therapies with COVID-19 severity in multiple sclerosis. Neurology. 2021;97:e1870–e1885. doi: 10.1212/WNL.0000000000012753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weinreich DM, Sivapalasingam S, Norton T, et al. REGEN-COV antibody combination and outcomes in outpatients with Covid-19. N Engl J Med. 2021;385:e81. doi: 10.1056/NEJMoa2108163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Willyard C. How antiviral pill molnupiravir shot ahead in the COVID drug hunt. Nature. 2021 doi: 10.1038/d41586-021-02783-1. published online Oct 8. [DOI] [PubMed] [Google Scholar]
- 26.Khoury DS, Cromer D, Reynaldi A, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27:1205–1211. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- 27.Levin EG, Lustig Y, Cohen C, et al. Waning immune humoral response to BNT162b2 Covid-19 vaccine over 6 months. N Engl J Med. 2021;385:e84. doi: 10.1056/NEJMoa2114583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krammer F, Srivastava K, Alshammary H, et al. Antibody responses in seropositive persons after a single dose of SARS-CoV-2 mRNA vaccine. N Engl J Med. 2021;384:1372–1374. doi: 10.1056/NEJMc2101667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rijkers G, Murk JL, Wintermans B, et al. Differences in antibody kinetics and functionality between severe and mild severe acute respiratory syndrome coronavirus 2 infections. J Infect Dis. 2020;222:1265–1269. doi: 10.1093/infdis/jiaa463. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
After publication, anonymised individual participant data and a data dictionary will be made available upon request to the corresponding author to researchers who provide a methodologically sound proposal. Data will be shared through a secure online platform.