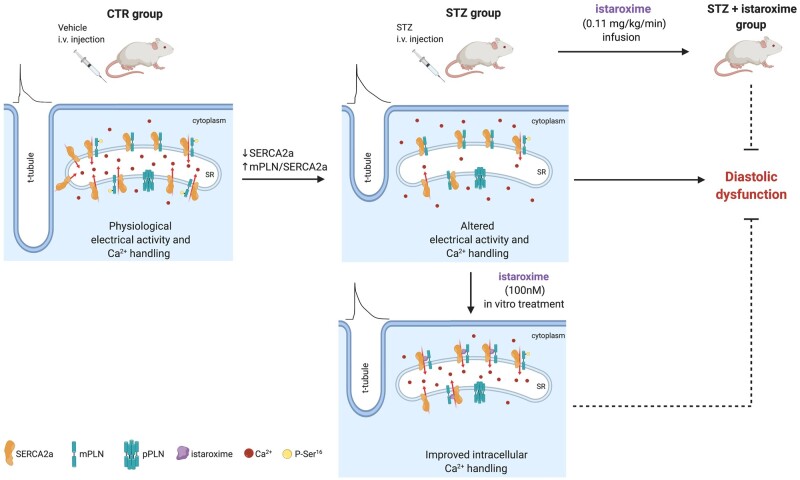
Abstract
Aims
Diabetic cardiomyopathy is a multifactorial disease characterized by an early onset of diastolic dysfunction (DD) that precedes the development of systolic impairment. Mechanisms that can restore cardiac relaxation improving intracellular Ca2+ dynamics represent a promising therapeutic approach for cardiovascular diseases associated to DD. Istaroxime has the dual properties to accelerate Ca2+ uptake into sarcoplasmic reticulum (SR) through the SR Ca2+ pump (SERCA2a) stimulation and to inhibit Na+/K+ ATPase (NKA). This project aims to characterize istaroxime effects at a concentration (100 nmol/L) marginally affecting NKA, in order to highlight its effects dependent on the stimulation of SERCA2a in an animal model of mild diabetes.
Methods and results
Streptozotocin (STZ) treated diabetic rats were studied at 9 weeks after STZ injection in comparison to controls (CTR). Istaroxime effects were evaluated in vivo and in left ventricular (LV) preparations. STZ animals showed (i) marked DD not associated to cardiac fibrosis, (ii) LV mass reduction associated to reduced LV cell dimension and T-tubules loss, (iii) reduced LV SERCA2 protein level and activity and (iv) slower SR Ca2+ uptake rate, (v) LV action potential (AP) prolongation and increased short-term variability (STV) of AP duration, (vi) increased diastolic Ca2+, and (vii) unaltered SR Ca2+ content and stability in intact cells. Acute istaroxime infusion (0.11 mg/kg/min for 15 min) reduced DD in STZ rats. Accordingly, in STZ myocytes istaroxime (100 nmol/L) stimulated SERCA2a activity and blunted STZ-induced abnormalities in LV Ca2+ dynamics. In CTR myocytes, istaroxime increased diastolic Ca2+ level due to NKA blockade albeit minimal, while its effects on SERCA2a were almost absent.
Conclusions
SERCA2a stimulation by istaroxime improved STZ-induced DD and intracellular Ca2+ handling anomalies. Thus, SERCA2a stimulation can be considered a promising therapeutic approach for DD treatment.
Keywords: SERCA, Istaroxime, Diastolic dysfunction, Streptozotocin, Calcium handling
Graphical Abstract
1. Introduction
Diabetes affects more than 300 million people globally and type 1 diabetes (T1D) accounts for up to 10% of cases.1 Heart failure (HF) is the predominant cardiovascular complication of diabetes and represents the leading cause of morbidity and mortality. Diabetic cardiomyopathy (DCM) is a complex and multifactorial disease characterized by an early onset of diastolic dysfunction (DD), which precedes the development of systolic impairment.2–5
The molecular and pathophysiological mechanisms underlying diabetes include abnormalities in the regulation of Ca2+ homeostasis in cardiomyocytes and the consequent alteration of ventricular excitation–contraction coupling. In the diabetic heart, a dysregulation of Ca2+ cycling includes a reduction of SERCA2 activity, which may be accompanied by a decreased SERCA2 protein expression (mostly SERCA2a isoform).6,7 A key role in the regulation of SERCA2a activity is played by phospholamban (PLN), a protein that behaves like its endogenous inhibitor when it is in its non-phosphorylated state.8 In most diabetic models, PLN expression level appears increased while its phosphorylation state is reduced, thus, contributing to the inhibition of SERCA2a function.6–8 This defect generates an impairment of sarcoplasmic reticulum (SR) Ca2+ refilling that results in slow diastolic relaxation. An abnormal Ca2+ distribution may facilitate cardiac arrhythmias appearance and myocyte apoptosis.9,10
Therefore, SERCA2a may represent a molecular target for a pharmacological intervention aimed at increasing the mechanical function and the energetic efficiency of the diabetic heart characterized by a defective SR Ca2+ loading. To date, the current medications have shown a limited efficacy in preventing the progression to HF in patients with DCM and diabetic complications.10–12 New hypotheses have been recently proposed in HF aimed at improving cardiac contractility,13–19 however, all these attempts are still far from being considered as beneficial treatment options available for clinicians and the treatment of HF and DCM remains an open field of research. The development of a small molecule as SERCA2a activator represents a promising strategy for HF and DCM treatment. Along this line, istaroxime is the first-in-class original luso-inotropic agent, shown to be highly effective and safe in patients.20 Istaroxime is endowed of a double mechanism of action that consists in the ability to inhibit Na+/K+ ATPase (NKA) and enhance SERCA2a ATPase activity,21 this last obtained through the relief of PLN inhibitory effect on SERCA2a,22 without inducing spontaneous Ca2+ release (SCR) from SR.21,23 In healthy and failing animal models and in patients with acute HF syndrome, istaroxime improves systolic and diastolic performance20,24–28 and efficiency of cardiac contraction with a low oxygen consumption,26 minimizing the risk of arrhythmias or ischaemia, without affecting other cardiovascular functions.29–32
In this study, we characterized the streptozotocin (STZ) model on different levels of biological organization, such as: (i) in vivo, to evaluate STZ-induced DD, (ii) in isolated left ventricular (LV) cardiomyocytes, to evaluate structure, intracellular Ca2+ () dynamics, electrical activity, and (iii) in LV and renal preparations (cell-free systems) to assess SERCA2a and NKA activity. We tested whether SERCA2a stimulation by a small molecule can improve the altered handling responsible for the DD in STZ-treated rats. To this end, istaroxime was tested (i) in vivo after iv infusion in STZ rats, (ii) in LV myocytes at a concentration marginally affecting NKA to highlight its effects mostly dependent on SERCA2a stimulation, and (iii) in the cell-free systems.
2. Methods
All experiments involving animals (methods detailed in the online Supplementary material) conformed to the guidelines for Animal Care endorsed by the University of Milano-Bicocca and to the Directive 2010/63/EU of the European Parliament on the protection of animals used for scientific purposes. Male Sprague Dawley rats (150–175 gr) were used to generate a STZ-induced T1D cardiomyopathy model according to the Health Minister of Italy permission.
2.1 STZ rat model
T1D was induced through a single STZ (Sigma-Aldrich, 50 mg/kg) injection into a rat-tail vein; littermate control (CTR) rats received only citrate buffer (vehicle). Overnight fasting or non-fasting glycaemia was measured after 1 week by Contour XT system (Bayer). Animals were considered diabetic with fasting glycaemia values >290 mg/dL.
2.2 Echocardiography
Eight weeks after vehicle/STZ injection, rats were submitted to a transthoracic echocardiographic and Tissue Doppler evaluation, performed under urethane anaesthesia (1.25 g/kg i.p.) (M9 Mindray Echographer equipped with a 10 MHz probe, P10-4s Transducer, Mindray, China). Systolic and diastolic parameters were measured in CTR and diabetic (STZ) animals by a blinded investigator. Details are shown in the online Supplementary material.
A group of STZ rats was subjected to istaroxime infusion at 0.11 mg/kg/min for 15 min accordingly to a previous study.25 Drug was infused through a polyethylene 50 cannula inserted into a jugular vein under urethane anaesthesia. Echocardiographic and Tissue Doppler parameters were measured under basal condition (before) and following 15 min istaroxime administration.
2.3 Morphometric parameters
Rats were euthanized by cervical dislocation under anaesthesia with ketamine-xylazine (130–7.5 mg/kg i.p) 9 weeks after STZ injection. Body weight (BW), heart weight (HW), LV weight (LVW), and kidney weight (KW) were measured. Body weight gain (BW gain) was obtained by subtracting the initial BW from the BW at sacrifice. HW and KW were normalized to tibia length (TL) to assess respectively cardiac and kidney indexes in CTR and STZ groups.
2.4 Myocyte dimensions and T-tubules (TT) analysis
Sarcolemmal membranes were stained by incubating isolated LV myocytes with 20 μmol/L di-3-ANEPPDHQ33 (Life Technologies, Carlsbad, United States) to measure cell dimensions and TT organization/periodicity by a method based on Fast Fourier Transform.34
2.5 SERCA2a and Na+/K+ pump (NKA) activity measurement
SERCA2a activity was measured in vitro as 32P-ATP hydrolysis at different Ca2+ concentrations (100–3000 nmol/L) in heart homogenates as previously described.25 Ca2+ concentration–response curves were fitted by using a logistic function to estimate SERCA2a Ca2+ affinity (Kd Ca2+) and Vmax.
NKA activity was assayed in vitro by measuring the release of 32P-ATP, as previously described.35 The concentration of compound causing 50% inhibition of the NKA activity (IC50) was calculated by using a logistic function.
2.6 Intracellular Na+ and Ca2+ dynamics
Intracellular Na+ () and Ca2+ () dynamics were evaluated by incubating LV myocytes with the membrane-permeant form of the dyes Ion NaTRIUM Green-2 AM (5 µmol/L) and Fluo4-AM (10 μmol/L), respectively.
dynamics were monitored in I-clamp under physiological condition (Tyrode’s solution) and in V-clamp under modified Tyrode’s solution suitable to measure NKA current (INKA) at the same time.
dynamics were analysed in field stimulated (2 Hz) and in patch-clamped myocytes. In field stimulated cells, SR Ca2+ loading and stability were evaluated through a post-rest potentiation protocol (Supplementary material online, Figure S1). Ca2+ transient (CaT) parameters and SR Ca2+ content (CaSR) were estimated at steady state (2 Hz) and following caffeine (10 mmol/L) superfusion, respectively. Moreover, incidence of SCR events was evaluated in each group during resting pauses and diastole.
To better highlight changes in Ca2+ihandling not affected by modifications on electrical activity, dynamic was also evaluated in voltage-clamped cells. Firstly, action potential (AP) clamp experiments were performed to verify whether CaT amplitude and CaSR were dependent on AP durations (APDs). To this end, two AP waveforms were used to dynamic voltage clamp STZ myocytes: a ‘short AP’ and a ‘long AP’ representative of the CTR and STZ group in terms of AP characteristics, respectively. dynamics were then evaluated in voltage-clamped cells by standard V-clamp protocols.
Finally, to estimate SR uptake function in the absence of Na+/Ca2+ exchanger (NCX) and NKA function, SR reloading protocol was applied in V-clamped cells by removing Na+ from both sides of the sarcolemma (Supplementary material online, Figure S2).21 Kinetics of SR Ca2+ reloading was evaluated; in particular, we considered the time constant of CaT decay (τdecay) reflecting in this setting Ca2+ transport rate across the SR membrane, a functional index of SERCA2a activity.
2.7 Ca2+ sparks rate and characteristics
Spontaneous unitary Ca2+ release events (Ca2+ sparks) were recorded at room temperature in Fluo 4-AM (10 µmol/L) loaded myocytes at resting condition. Tyrode’s bath solution contained 1 mmol/L CaCl2.
2.8 AP rate-dependency and variability
APs were recorded in I-clamp condition by pacing myocytes at 1, 2, 4, and 7 Hz under Tyrode superfusion. Rate-dependency of APD at 50% (APD50) and 90% (APD90) of repolarization and diastolic potential (Ediast) were evaluated at steady state. Moreover, at each rate, a minimum of 30 APs were recorded at steady state to evaluate the short-term variability (STV) of APD90, a well-known pro-arrhythmic index,36 according to Eq. (1):
| (1) |
Incidence of delayed afterdepolarizations (DADs) was evaluated.
2.9 Statistical analysis
Normal distribution of the results was checked by using the Shapiro–Wilk test. Paired or unpaired Student’s t-test, one-way or two-way ANOVA were applied as appropriate test for significance between means. Post hoc comparison between individual means was performed by Tukey or Sidak multiple comparison tests. χ2 test was used for comparison of categorical variables. Results are expressed as mean ± SEM. A value of P < 0.05 was considered significant.
Except when specified, in vitro istaroxime effects were analysed by incubating cells with the drug for at least 30 min, thus group comparison analysis was performed. Number of animals (N) and cells (n) are shown in each figure legend.
3. Results
3.1 Morphometric parameters
Diabetic rats were obtained by a single injection of STZ (50 mg/kg) into a tail vein and were compared to CTR rats receiving only vehicle. Fasting and non-fasting glycaemia increased significantly 1 week after STZ administration (Table 1).
Table 1.
Glycaemia values, morphometric parameters, and LV cell dimensions
| CTR | STZ | P vs. CTR | |
|---|---|---|---|
| Fasting glycaemia (mg/dL) | 94±2 | 390±14 | * |
| Non-fasting glycaemia (mg/dL) | 126±4 | 560±8 | * |
| BW (g) | 400±7 | 202±6 | * |
| BW gain (g) | 230±14 | 26±8 | * |
| HW (g) | 1.65±0.08 | 1.03±0.03 | * |
| TL (cm) | 4.3±0.02 | 3.63±0.03 | * |
| HW/BW (g/kg) | 4.11±0.17 | 5.16±0.11 | * |
| HW/TL (g/cm) | 0.40±0.03 | 0.28±0.009 | * |
| LVW/HW (%) | 67.9±1.0 | 63.4±0.7 | * |
| KW (g) | 2.23±0.05 | 2.19±0.07 | NS |
| KW/TL (g/cm) | 0.52±0.01 | 0.6±0.02 | * |
| LV cell length (µm) | 136±2.8 | 120±2.1 | * |
| LV cell volume (103 µm3) | 65±1.9 | 37±1.03 | * |
| LV CSA (um2) | 482±13.8 | 309±7.5 | * |
| LV Cm (pF) | 179±6 | 136±4 | * |
BW, Body weight; HW, heart weight; KW, kidney weight; LVW, left ventricular weight; TL, tibia length.
Morphometric parameters: CTR N =15–21, STZ N =23–34. Cell dimensions (length, volume, and CSA): CTR N =4 (n =58), STZ N =6 (n =108). Cell membrane capacitance (Cm): CTR N =12 (n =75), STZ N =13 (n =83).
<0.05 vs. CTR (unpaired t-test).
At the time of STZ administration, BW was comparable among CTR and STZ groups (data not shown), while 9 weeks after STZ infusion, BW gain was largely different among groups because of a BW significantly lower in STZ than in CTR. TL was also measured as a rat growth index and resulted slightly reduced in STZ compared to CTR. HW was significantly lower in STZ than in CTR, even when HW was normalized to TL. Analogously, LVW normalized to HW, was significantly reduced in STZ in comparison to CTR. Likewise, LV cell length, volume, cross-sectional area (CSA), and cell membrane capacitance (Cm), a further index of cell dimension, were significantly reduced in STZ in comparison to CTR. Conversely, KW did not differ between the two groups, but KW/TL ratio resulted modestly increased in STZ rats vs. CTR, suggesting STZ-induced kidney hypertrophy (Table 1).
It was further investigated whether the decrease of cardiac weight/mass observed in STZ rats might be associated with cardiac fibrosis deposition. To this end, a western blot analysis for collagen type 1 and matrix metallopeptidase 9 (MMP-9) protein expression level was conducted on LV homogenates from CTR and STZ rats (Supplementary material online, Figure S3). The results indicate that any significant difference of collagen type 1 and MMP-9 protein content could be detected between the two rat groups.
3.2 STZ induces DD, reverted by acute istaroxime infusion
The echocardiographic parameters were measured in CTR and STZ rats 8 weeks after STZ injection (Table 2). Wall thickness for the interventricular septum (IVST) and posterior wall (PWT) both in diastole and systole did not differ between CTR and STZ rats. Analogously, LV end-diastolic and systolic diameter (LVEDD, LVESD) remained unchanged. The calculated fractional shortening (FS) did not differ while the TDI contraction velocity (s’) was reduced in STZ animals when compared to CTR, thus suggesting an overall systolic function only partially compromised in STZ rats at this stage (Table 2).
Table 2.
Echocardiographic and tissue Doppler parameters
| CTR | STZ basal |
STZ + istaroxime |
|
|---|---|---|---|
| IVSTd (mm) | 1.9±0.09 | 1.81±0.12 | 1.88±0.12 |
| PWTd (mm) | 1.71±0.17 | 1.45±0.08 | 1.47±0.07 |
| LVEDD (mm) | 6.6±0.35 | 7.08±0.32 | 7.27±0.23 |
| IVSTs (mm) | 2.6±0.22 | 2.54±0.18 | 2.57±0.19 |
| PWTs (mm) | 2.71±0.2 | 2.52±0.1 | 2.55±0.21 |
| LVESD (mm) | 3.07±0.39 | 3.11±0.28 | 3.1±0.34 |
| FS (%) | 53.8±5.66 | 56.2±2.4 | 57.7±3.7 |
| E (m/s) | 0.88±0.03 | 0.89±0.05 | 0.95±0.05 |
| A (m/s) | 0.52±0.07 | 0.7±0.03* | 0.81±0.05** |
| E/A | 1.82±0.21 | 1.26±0.03* | 1.18±0.05 |
| DT (ms) | 53.5±1.55 | 61±2.17* | 48.4±3.8** |
| DT/E (10−3 s2/m) | 61.3±1.43 | 69.3±4.5 | 52.2±5.6** |
| E/DT (103 m/s2) | 16.3±0.35 | 14.7±0.9 | 20.8±2.8** |
| s’ (mm/s) | 33.2±1.18 | 24.8±1.19* | 25.2±1.11 |
| e’ (mm/s) | 26.7±1.73 | 21.2±0.63* | 24.5±1.46** |
| a’ (mm/s) | 20.7±1.61 | 27.8±1.99* | 31.1±2 |
| e'/a’ | 1.31±0.063 | 0.77±0.03* | 0.79±0.02 |
| E/e' | 33.2±1.56 | 42.3±2.43* | 39.1±1.57 |
| HR (bpm) | 303±9.5 | 233±10* | 240±13 |
| SV (mL) | 0.59±0.1 | 0.73±0.08 | 0.78±0.05 |
| CO (mL/min) | 179.8±30.3 | 170.2±17 | 186.9±15 |
| EF (%) | 83.6±3.2 | 89.9±1.6 | 90.2±2.3 |
| N | 7 | 7 | 7 |
Average values in CTR and STZ animals before (basal) and after infusion with istaroxime at 0.11 mg/kg/min for 15 min.
A, a’, late diastolic peak velocity; CO, cardiac output; DT, deceleration time; E, e’, early diastolic peak velocity; EF, ejection fraction; FS, fractional shortening; HR, heart rate; IVSTd, telediastolic interventricular septum thickness; IVSTs, telesystolic interventricular septum thickness; LVEDD, left ventricular early-diastolic diameter; LVESD, left ventricular early-systolic diameter; PWTd, telediastolic posterior wall thickness; PWTs, telesystolic posterior wall thickness; s’, systolic peak velocity; SV, stroke volume.
P<0.05 vs. CTR (unpaired t-test),
P<0.05 vs. STZ basal (paired t-test). CTR N=7, STZ N=7.
The transmitral Doppler parameters were altered in STZ rats indicating an impairment of diastolic function. In particular, in STZ rats, while early (E) peak diastolic velocity was unchanged, E wave deceleration time (DT) was prolonged, thus, the mitral deceleration index (DT/E) and the deceleration slope (E/DT) tended respectively to increase and decrease; late peak diastolic velocity (A) was significantly increased and thus, E/A ratio resulted significantly reduced. Tissue Doppler examination showed in STZ rats a significant reduction of early diastolic myocardial velocity (e’) and a significant increase of late diastolic myocardial velocity (a’), similarly to A wave. Thus, a significant reduction of e’/a’ ratio and increase of the E/e’ ratio was observed in STZ rats in comparison to CTR (Table 2).
The overall cardiac function indicated that stroke volume (SV), ejection fraction (EF), and cardiac output (CO) were not significantly affected in STZ rats although heart rate (HR) was reduced. Echocardiographic data mostly indicate that, at this time point, STZ induced a DCM characterized by DD and mostly preserved systolic function. Furthermore, the diastolic impairment observed in STZ rats at this early stage was not associated with cardiac fibrosis (Supplementary material online, Figure S3).
To analyse early in vivo effects of istaroxime in reducing STZ-induced DD, istaroxime was infused in STZ rats at 0.11 mg/kg/min25 and echocardiographic parameters were collected 15 min later. The results (Table 2) showed that the compound was able to revert the DD documented in STZ rats with a significant reduction of DT and DT/E and an increase of E/DT and e’. No effect on CO, SV, and HR was observed following istaroxime infusion at this early time point (Table 2). Moreover, to exclude changes due to time dependent effects of urethane, echocardiographic parameters were collected every 5 min in a set of animals not treated with the drug. Up to 20 min in urethane anaesthesia, diastolic and systolic parameters remained constant (Supplementary material online, Figure S4). It should be noted that in a parallel study, we estimated istaroxime plasma level in male rats after 1 hour infusion at 0.11 mg/kg/min, resulting 780 nmol/L (N = 3, unpublished data); this suggests that drug concentration at 15 min infusion should be reasonably around 200 nmol/L.
3.3 Istaroxime affinity for rat NKA
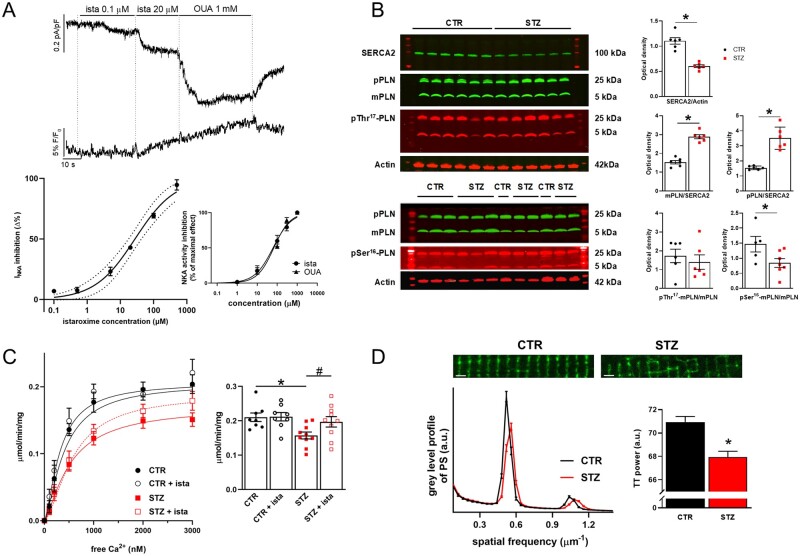
To identify the in vitro istaroxime concentration suitable to limit its effects dependent on NKA inhibition, INKA was isolated in CTR rat LV myocytes and the concentration–response curve for istaroxime was evaluated as previously shown for guinea-pig30 and mouse myocytes.23 A saturating concentration of ouabain (1 mmol/L) was used (Supplementary material online, Figure S5) to evaluate the INKA inhibition by istaroxime as percentage of the ouabain-induced change. Moreover, a subgroup of cells was incubated with Ion NaTRIUM Green-2 to monitor changes under istaroxime or ouabain superfusion. The estimated IC50 for INKA inhibition by istaroxime was 32 ± 4 µmol/L (Figure 1A); a similar value was detected in cardiac (84 ± 20 µmol/L) (inset Figure 1A) and renal preparations (55 ± 19 µmol/L, Supplementary material online, Figure S6). Moreover, while NKA inhibition by 100 nmol/L istaroxime was detectable by measuring INKA in isolated myocytes (-6.9 ± 1.2%, P < 0.05, N = 14), istaroxime effects on NKA were not detectable up to 1 µmol/L in cardiac and renal preparations.
Figure 1.
Istaroxime affinity for rat NKA. Changes in SERCA2, PLN levels, and TT expression in STZ vs. CTR rats. (A) Top: recordings of NKA current (INKA) and Ion NaTRIUM Green-2 fluorescence (Hp −40 mV) during exposure to increasing concentrations of istaroxime and, finally, to 1 mmol/L ouabain (OUA). Bottom: concentration-dependent INKA inhibition by istaroxime in isolated CTR LV myocytes (the best logistic fit and confidence intervals are shown, N = 5, n = 6–27). Concentration-dependent NKA activity inhibition by istaroxime and OUA in cardiac preparations is shown in the inset (N = 5). (B) Left: western blot for SERCA2, monomeric (m) and pentameric (p) PLN, pSer16-PLN and pThr17-PLN in STZ (N = 6,7) and CTR (N = 5,6) cardiac homogenates. Right: densitometric analysis; values are expressed as optical density in arbitrary units. *P<0.05 vs. CTR (unpaired t-test). (C) Left: Ca2+ activation curves of SERCA2a activity measured as cyclopiazonic acid sensitive component in cardiac SR homogenates from CTR (N = 8) and STZ (N = 10) rats with or w/o 500 nmol/L istaroxime. Right: statistics of the maximum velocity (Vmax) of the Ca2+ activation curves estimated by sigmoidal fitting. *P<0.05 vs. CTR (unpaired t-test), #P<0.05 vs. STZ (paired t-test). (D) Top: confocal images of di-3-ANEPPDHQ (20 µmol/L) loaded CTR and STZ myocytes (horizontal bars 2 µm). Bottom: mean power spectrum profile of TT in CTR (N = 5, n = 114) and STZ (N = 9, n = 181) group; average results of the power of the periodic component on the right. *P<0.05 vs. CTR (unpaired t-test).
In isolated rat ventricular myocytes increased slightly under cumulative istaroxime concentrations (20 µmol/L istaroxime +2.2 ± 0.7%, P < 0.05, N = 5), while it was evident under saturating ouabain concentration (+8 6 ± 1.4%, P < 0.05, N = 5) (Figure 1A).
Consistently with the aim of the study, istaroxime effects on STZ-induced changes were evaluated by testing the compound at concentrations marginally affecting NKA (100 or 500 nmol/L).
3.4 STZ induces SERCA2a down-regulation and TT loss
LV homogenates from CTR and STZ rats were used to measure SERCA2a and PLN protein level by western blot analysis. Representative western blots from CTR and STZ samples and the relative densitometric analysis indicized for actin content are shown in Figure 1B. SERCA2a protein expression resulted significantly reduced in STZ vs. CTR samples (-45%, P < 0.001); while monomeric (m) PLN levels were unchanged, pentameric (p) PLN levels were slightly increased (+22%, P < 0.05). As a consequence, both mPLN/SERCA2a and pPLN/SERCA2a ratio were significantly increased (+89% and +128%, respectively, P < 0.001), suggesting higher SERCA2a inhibitory activity by PLN in STZ group. Moreover, in STZ samples, while the fraction of phosphorylated Thr17-mPLN (pThr17-mPLN/mPLN) resulted unchanged, the fraction of phosphorylated Ser16-mPLN (pSer16-mPLN/mPLN) was reduced (-42%, P < 0.05), thus highlighting reduced PKA-dependent SERCA2a modulation in STZ. Most of these measurements were also performed in isolated LV myocytes showing comparable results as those shown in LV homogenates (Supplementary material online, Figure S7).
SERCA2a activity was measured in cardiac SR homogenates from CTR and STZ rats as 32P-ATP hydrolysis assay (Figure 1C). In comparison to CTR preparations, SERCA2a Vmax was significantly decreased (-25%, P < 0.05) in STZ, while the Kd Ca2+ did not differ (Supplementary material online, Figure S8). Overall, SERCA2a protein level and activity were reduced in STZ preparations, a result in line with echocardiographic parameters showing STZ-induced DD.
Disarray of the TT system has been described in several failure models and was generally characterized by loss of the transverse component. A sharp pattern of transverse striations was observed in CTR myocytes (Figure 1D); accordingly, in these myocytes, pixel variance was largely represented by the periodic component, whose period was consistent with transverse TT arrangement. LV disarray of the transverse TT was visually obvious in STZ myocytes, a result confirmed by the quantitative analysis of the power of the periodic component (Figure 1D).
3.5 Istaroxime effects on STZ-induced changes in Ca2+ dynamics
Istaroxime (500 nmol/L) stimulated SERCA2a activity in cardiac SR homogenates from STZ diabetic rats by increasing SERCA2a Vmax (+25%, P < 0.01) to a value similar to CTR rats (Figure 1C) without affecting the Kd Ca2+ affinity (575 ± 98 nmol/L vs. 450 ± 51 nmol/L, NS, Supplementary material online, Figure S8). Conversely, in CTR rat preparations, Vmax (Figure 1C) and Kd Ca2+(Supplementary material online, Figure S8) parameters were unchanged in the presence of istaroxime.
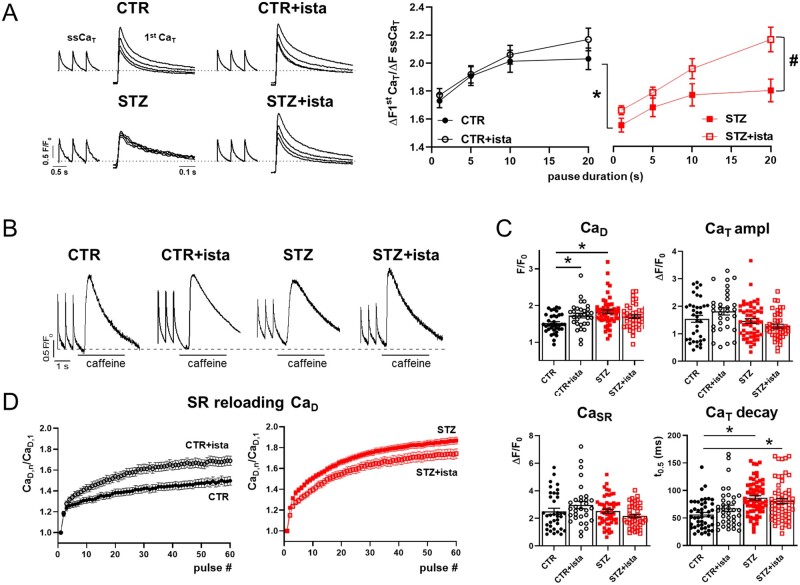
Istaroxime effects on STZ-induced DD were then evaluated at the cellular level by measuring the SR ability to accumulate resting Ca2+ through a post-rest potentiation protocol in field stimulated myocytes. As shown in Figure 2A, following increasing resting pauses, the amplitude of the first CaT increased progressively in CTR myocytes; according to STZ-induced SERCA2a down-regulation, post-rest potentiation was reduced in STZ myocytes at all resting intervals. Istaroxime at 100 nmol/L failed to affect post-rest potentiation in CTR myocytes, while it improved the ability of SR to accumulate Ca2+ especially at long resting pauses in STZ myocytes, in agreement with its stimulatory action on SERCA2a.
Figure 2.
STZ-induced changes in dynamics in field stimulated myocytes. Analysis of istaroxime effects. (A) Left: post-rest potentiation protocol in Fluo4 field stimulated (2 Hz) myocytes; steady state Ca2+ transients (ssCaT) and superimposed first Ca2+ transients (1st CaT) following increasing resting pauses (1–5–10–20 s) are reported in CTR and STZ myocytes, with or w/o 100 nmol/L istaroxime. Traces were normalized to own diastolic Ca2+ (CaD) level (dotted lines). Right: analysis of the 1st CaT amplitude normalized to the amplitude of the pre-pause ssCaT and its pause-dependency. CTR N = 5 (w/o istaroxime n = 44, with istaroxime n = 34), STZ N = 3 (w/o istaroxime n = 35, with istaroxime n = 23). *P<0.05 vs. CTR w/o istaroxime; #P<0.05 vs. STZ w/o istaroxime (two-way ANOVA plus post hoc Sidak’s multiple comparisons). (B) ssCaT and caffeine-induced CaT evocated in field stimulated CTR and STZ myocytes with or w/o 100 nmol/L istaroxime (the dotted line indicates the CaD in CTR w/o istaroxime). (C) Statistics for ssCaD, ssCaT amplitude, caffeine-induced CaT (named CaSR), and ssCaT half decay time (t0.5). CTR N = 5 (n = 36 w/o istaroxime, n = 31 with istaroxime), STZ N = 7 (n = 52 w/o istaroxime, n = 42 with istaroxime). *P<0.05 vs. CTR w/o istaroxime (one-way ANOVA plus post hoc Tukey’s multiple comparisons). (D) Changes in CaD during the reloading process after caffeine-induced SR depletion. CaD values (CaD,n) were normalized to the 1st pulse CaD (CaD,1). CTR N = 5 (n = 36 w/o istaroxime, n = 29 with istaroxime), STZ N = 7 (n = 49 w/o istaroxime, n = 43 with istaroxime).
At steady-state, STZ increased diastolic Ca2+ (CaD) and CaT decay time (t0.5), while leaving unchanged CaT amplitude and CaSR (Figure 2B and C). Istaroxime (100 nmol/L) significantly increased CaD in CTR myocytes, while blunted STZ-induced CaD enhancement in STZ myocytes. This was furtherly appreciable monitoring the time course of CaD enhancement during the SR reloading process following caffeine superfusion (Figure 2D). On the other hand, CaT amplitude, decay kinetics, and CaSR were not significantly affected by istaroxime in both CTR and STZ myocytes. Overall, STZ-induced SERCA2a down-regulation resulted in cytosolic CaD enhancement probably due to a reduced ability of SR to compartmentalize Ca2+ into the SR; however, SR Ca2+ content was preserved. In parallel, the effect of istaroxime on CaD in CTR myocytes was likely attributable to a partial NKA blockade, that was blunted in STZ myocytes by the simultaneous action on SERCA2a.
SCR events were evaluated in CTR and STZ cells. SCR events were absent in CTR while a not significant number of events occurred in STZ myocytes; istaroxime not affected their incidence in both CTR and STZ myocytes.
3.6 STZ induces changes in electrical activity affecting dynamics. Analysis of istaroxime effects
Potential changes in electrical activity in STZ myocytes might mask expected changes directly resulting from SERCA2a down-regulation (e.g. changes in CaSR).
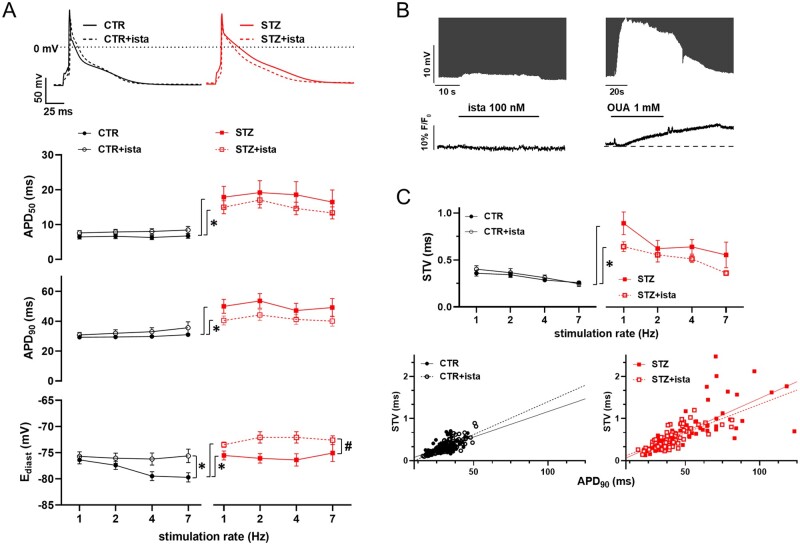
Thus, to verify STZ-induced changes in electrical activity, AP rate-dependency was evaluated in STZ myocytes in comparison to CTR. STZ induced a significant APD prolongation at all stimulation rates (Figure 3A), accordingly to voltage-dependent K+ channels down-regulation.37,38 Moreover, rate-dependency of Ediast observed in CTR myocytes was absent in STZ myocytes, probably due to STZ-induced NKA down-regulation.39 In both CTR and STZ myocytes, istaroxime at 100 nmol/L not affected APD, while slightly depolarized Ediast especially in STZ myocytes (Figure 3A).
Figure 3.
STZ-induced changes in electrical activity. Analysis of istaroxime effects. (A) Top: representative AP recorded at 1 Hz in CTR and STZ myocytes with or w/o 100 nmol/L istaroxime. Bottom: rate-dependency of AP parameters (APD50, APD90, Ediast) in CTR and STZ myocytes with or w/o 100 nM istaroxime. CTR N = 4 (n = 29 w/o istaroxime, n = 25 with istaroxime), STZ N = 3 (n = 24 w/o istaroxime, n = 19 with istaroxime). *P<0.05 vs. CTR w/o istaroxime, #P<0.05 vs. STZ w/o istaroxime (two-way ANOVA plus post hoc Sidak’s multiple comparisons). (B) Effects of 100 nmol/L istaroxime superfusion on Ediast (top, AP y axis zoomed to highlight changes) and (bottom) in comparison to the effect of 1 mmol/L OUA in CTR myocytes loaded with Ion Natrium Green-2 and stimulated at 7 Hz. (C) Top: rate-dependency of APD90 STV in each experimental group. CTR N = 4 (n = 27 w/o istaroxime, n = 21 with istaroxime), STZ N = 3 (n = 24 w/o istaroxime, n = 20 with istaroxime). *P<0.05 vs. CTR w/o istaroxime (two-way ANOVA plus post hoc Sidak’s multiple comparisons). Bottom: linear correlation between STV of APD90 and APD90 values in CTR and STZ groups; data from all stimulation rates were pooled.
All these measurements were done following istaroxime incubation for at least 30 min to allow drug accumulation inside the cell and stimulate SERCA2a. On the other hand, to better understand drug effects on Ediast, likely attributable to NKA inhibition, a group of CTR myocytes were loaded with Ion NaTRIUM Green-2 and membrane potential plus were simultaneously recorded at 7 Hz (to highlight the contribution of NKA to Ediast) under basal condition and following istaroxime (100 nmol/L) superfusion; ouabain at saturating concentration was also tested as reference compound inhibiting NKA (Figure 3B). Istaroxime at 100 nmol/L slightly depolarized Ediast (Δ-0.58 ± 0.1 mV, n = 8, P < 0.05) in comparison to ouabain superfusion (Δ-13.5 ± 1.2 mV, n = 13, P < 0.05); in parallel, a significant enhancement was detectable during ouabain only (+3 ± 0.5% n = 13, P < 0.05).
Overall, as expected, STZ treatment largely affects ion channels and pumps resulting in AP shape changes; istaroxime at 100 nmol/L substantially leaved unchanged STZ-induced AP changes and further slightly depolarized Ediast, resulting from a minimal (about -7%) NKA inhibition.
The STV of APD was evaluated in all groups, as a well-known pro-arrhythmic index. In comparison to CTR, STZ increased STV of APD at all pacing rates (Figure 3C); in both CTR and STZ myocytes, STV was not significantly affected by istaroxime, even though tended to be reduced in STZ myocytes. As expected, STV was directly correlated to APD90 in all groups; the slope of this correlation tended to increase in STZ group without reaching statistical significance (0.016 vs. 0.012, NS) and it was not significantly affected by istaroxime in both groups. These results suggest the absence of major mechanisms other than APD prolongation significantly affecting STV in all groups.37
Likewise to SCR incidence, DADs were completely absent in CTR myocytes and were present only in few cells in STZ groups (data not shown).
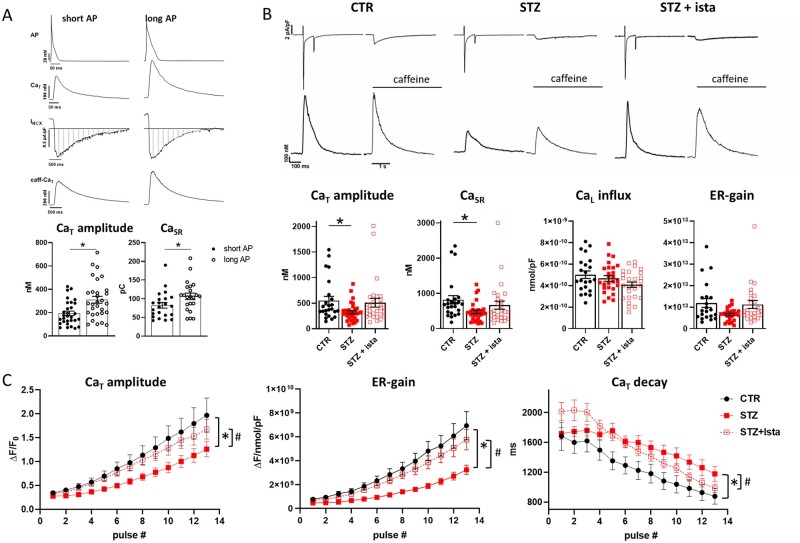
Given the STZ-induced APD prolongation, we verified if APD could effectively affect handling in STZ myocytes. To this end, AP-clamp measurements were performed (Figure 4A). CaT were evocated in the same cell by using as voltage commands waveforms named ‘short’ AP (CTR AP) and ‘long’ AP (STZ AP) (see Section 2). In comparison to the short AP waveform, the long AP one caused a huge increase in CaT amplitude (+66 ± 9.4%, P < 0.05) and CaSR (+36 ± 9.8%, P < 0.05), confirming the hypothesis that the prolonged AP in STZ myocytes affected handling.
Figure 4.
STZ-induced handling changes under control of membrane potential. Analysis of istaroxime effects. (A) Top: APs waveforms (CTR and STZ APs named short and long APs, respectively) and corresponding CaT evocated in V-clamped STZ myocyte through AP-clamp experiments (2 Hz). Caffeine-induced CaT (caff-CaT) and the corresponding NCX current (INCX) were recorded at −80 mV following steady state stimulation with short and long AP to estimate changes in SR Ca2+ content (CaSR). Bottom: statistics of CaT amplitude (N = 5, n = 30) and CaSR (integral of inward INCX, marked as striped area) (N = 5, n = 22) under short and long AP stimulation. Fluorescence signals were converted to free Ca2+ estimating Fmax in each cell. *P<0.05 vs. short AP (paired t-test). (B) Top: transmembrane currents and CaT simultaneously recorded in voltage-clamped cells (Hp −35 mV) from CTR and STZ myocytes with or w/o 100 nmol/L istaroxime. Bottom: statistics of CaT amplitude, CaSR, Ca2+ influx through L-type Ca2+ channel (CaL influx), and ER gain. CTR N = 3 (n = 22–24), STZ N = 5 (w/o istaroxime n = 26–33, with istaroxime n = 28). Fluorescence signals were converted to free Ca2+ estimating Fmax in each cell. *P<0.05 vs. CTR (one-way ANOVA plus Tukey’s multiple comparison). (C) Statistics of CaT parameters (CaT amplitude, ER-gain, and CaT decay time constant) measured during each pulse after SR depletion under NCX blockade (see Supplementary material online, Figure S2) in CTR and STZ myocytes with or w/o 100 nmol/L istaroxime. *P<0.05 vs. CTR; #P<0.05 vs. STZ w/o istaroxime (two-way ANOVA); CTR N = 5 (n = 13–21), STZ N = 4 (w/o istaroxime n = 13–28, with istaroxime n = 19–24).
3.7 STZ-induced handling changes under control of membrane potential are reverted by istaroxime SERCA2a stimulation
To clarify direct effects of SERCA2a down-regulation and its stimulation by istaroxime on handling, analysis on voltage-clamped myocytes was performed (Figure 4B) through a standard V-clamp protocol. Cells were superfused with Tyrode’s solution to allow evaluation of both SR and NCX function. As shown in Figure 4B, STZ induced CaT and CaSR amplitude reduction, leaving unchanged fractional release. Influx through L-type Ca2+ channels (CaL influx) was not affected in STZ group, leading to an excitation–release (ER) gain that tended to be reduced in comparison to CTR. Moreover, in STZ myocytes, ICaL peak density at 0 mV was significantly reduced, but the current decay tended to be slower; in particular, the fast decay time constant (τfast), reflecting Ca2+-dependent inactivation, tended to increase in comparison to CTR myocytes (Supplementary material online, Figure S9). Thus, all ICaL changes justify a global unaltered Ca2+ influx in STZ myocytes under these settings. Finally, the slope of the linear correlation between NCX current (INCX) and the CaSR (ΔINCX/ΔCaSR) was similar in CTR and STZ myocytes (Supplementary material online, Figure S10), suggesting that SERCA2a down-regulation was not associated to changes in NCX activity in STZ myocytes. Treatment of STZ myocytes with istaroxime blunted differences between CTR and STZ.
Lastly, to estimate SR Ca2+ uptake function in the absence of NCX and NKA function, SR reloading protocol was applied in V-clamped cells by removing Na+ from both sides of the sarcolemma as previously described.21 As shown in Figure 4C, after SR depletion by caffeine superfusion, in comparison to CTR myocytes, the SR reloading process was slower in STZ myocytes, clearly confirming the SERCA2a down-regulation. In particular, in STZ myocytes, the rate of CaT increment was reduced and this was associated with a slower enhancement of the ER-gain. Moreover, the decay time constant, mostly representing SR Ca2+ uptake function, increased at each pulse, accordingly to a reduced SERCA2a function in STZ myocytes. Stimulation of SERCA2a by istaroxime caused faster SR reloading and all parameters were restored to CTR condition.
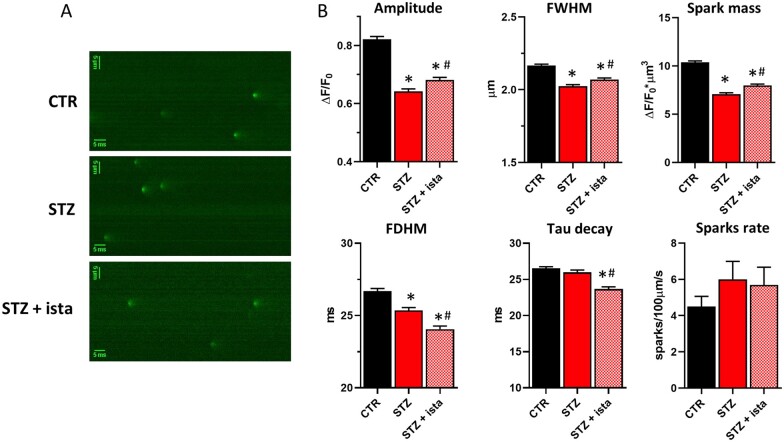
3.8 SERCA2a activity affects Ca2+ sparks characteristics
As shown before, both DADs and SCR events were detected only in few STZ myocytes, suggesting that SR stability is mostly preserved in this DCM model. To further analyse this point, Ca2+ sparks rate and characteristics were evaluated in all groups (Figure 5). Compared to CTR, STZ myocytes showed Ca2+ sparks with reduced amplitude, width, duration, and spark mass (Figure 5B), in agreement with a reduced SR Ca2+ content at resting. Istaroxime, by stimulating SERCA2a, partially restored Ca2+ sparks characteristics in STZ myocytes. In particular, istaroxime-induced SERCA2a stimulation emerged also by the analysis of Ca2+ sparks decay that significantly became faster in the presence of the compound. Sparks rate was not significantly affected by STZ and istaroxime.
Figure 5.
STZ-induced changes in Ca2+ sparks rate and characteristics. Analysis of istaroxime effects. (A) Representative xt images showing Ca2+ sparks at resting in CTR and STZ myocytes with or w/o 100 nmol/L istaroxime. (B) Statistics of Ca2+ sparks characteristics and rate for each group. *P<0.05 vs. CTR; #P<0.05 vs. STZ w/o istarpoxime (one-way ANOVA plus Tukey’s multiple comparison); CTR N = 7 (n = 62, sparks # = 2789), STZ N = 5 (w/o istaroxime n = 53, sparks # = 2019, with istaroxime n = 47, sparks # = 1940). FWHM, full width at half maximum; FDHM, full duration at half maximum. Spark mass (spark amplitude*1.206* FWHM3).
4. Discussion
Aim of this study was to assess the effect of SERCA2a stimulation mediated by istaroxime in improving dynamics in a diabetic rat model characterized by impaired diastolic function.
Several therapeutic approaches that increase SERCA2a function have been recently investigated.18,40–43 However, despite of the intense research in discovering small molecules or gene therapy aimed at selectively activating SERCA2a, no promising clinical outcomes have been reached so far.
Istaroxime is the first-in-class original luso-inotropic agent targeting SERCA2a in addition to NKA inhibition, that has shown efficacy and safety in clinical trials on patients with acute HF syndrome.20,28 In the past, in vitro istaroxime effects were largely characterized at concentrations showing dual mechanism of action.21,23,27,29,30 In this study, lusitropic SERCA2a-dependent istaroxime effects were evaluated by testing istaroxime both in vitro and in vivo at concentrations marginally affecting NKA. Estimated drug plasma level at 15 min infusion and drug concentrations adopted for in vitro assays were largely comparable.
To our knowledge, no other small molecules active on SERCA2a at submicromolar concentration are available.
4.1 STZ-induced DCM. DD is associated to down-regulated SERCA2a expression and activity and is improved by istaroxime infusion
STZ rats showed a clear DD highlighted by changes in mitral inflow, in line with published results, reporting that DCM often manifests first as DD (Table 2). Our echo measurements evidenced marked alterations on DD indexes in STZ rats. In particular, we showed a significant transmitral Doppler flow enhancement of E wave DT and reduced E/A ratio in STZ rats. Analogously, TDI parameters, relatively unaffected by load, indicated a significant reduction of early diastolic myocardial velocity (e’) and e’/a’ with an increase of E/e’ ratio in STZ rats. Systolic function appeared almost unaffected in STZ as compared to CTR rats, as indicated by FS and CO values. Moreover, we observed a marked bradycardia, consistent with the impaired autonomic function and down-regulation of the expression of the pacemaker channel HCN4.44
Consistently with STZ-induced DD, in heart preparations and in cardiomyocytes from STZ rats, we observed a clear reduction of SERCA2 protein expression level, an increase of mPLN/SERCA2 ratio and a reduction of Ser16 phosphorylated mPLN (Figure 1 and Supplementary material online, Figure S7). Conversely, CaMKII-dependent Thr17 phosphorylation of mPLN was similar between STZ and CTR rats (Figure 1). These biochemical alterations were associated with the reduction of SERCA2a ATPase activity observed in heart preparations from STZ compared to CTR rats (Figure 1) and indicate that these may translate into the impairment of diastolic function seen by the echocardiographic examination.
DCM is reported to be associated with cardiac fibrosis, which is responsible for increased LV stiffness and decreased ventricular wall compliance resulting in systolic and, in particular, DD.45 However, in this study, no change of collagen type 1 and MMP-9 protein expression has been observed in LV from CTR and STZ rats, indicating that 8 weeks after STZ injection may be a time not long enough to develop this alteration. Moreover, several indexes indicated the absence of a concrete LV hypertrophy in STZ rats, because the increase in HW/BW was strictly dependent on BW loss. Otherwise, we observed reduced HW/TL and LV/HW ratios, results confirmed at the cellular level with reduced Cm, CSA, cell volume, and TT organization. These results are supported by a recent study showing reduced sinoatrial Cm in STZ-treated mice.44 Loss of viable cardiomyocytes in STZ rats is also a possibility as previously shown.46
Collectively, these results indicate that STZ-induced DCM is characterized by impaired diastolic function associated with the down-regulation of SERCA2a expression and activity. This model is therefore suitable for testing the cardiac effects of SERCA2a stimulation by istaroxime. Istaroxime infused at 0.11 mg/kg/min for 15 min in STZ rats reverted the DD, inducing a significant reduction of DT and DT/E and an increase of e’ (Table 2). The favourable mechanistic profile of istaroxime action is once again corroborated by our results in ameliorating DD in a DCM model.
4.2 STZ-induced changes in dynamics and electrical activity. Istaroxime effects at a concentration marginally affecting NKA
Consequences of STZ-induced SERCA2a down-regulation were functionally analysed in isolated LV myocytes. In particular, the post-rest potentiation protocol clearly highlighted the reduced ability of SR to accumulate Ca2+ at resting in STZ myocytes in comparison to CTR ones. This resulted in CaD enhancement when pacing cells at 2 Hz (Figure 2); in spite of this, CaSR left unchanged, probably as a consequence of STZ-induced changes in electrical activity. Indeed, STZ induced marked APD prolongation at all stimulation rates (Figure 3A), according to voltage-dependent K+ channels down-regulation.37 Moreover, the lack of Ediast rate dependent hyperpolarization in STZ myocytes is in agreement with STZ-induced NKA down-regulation.39
AP-clamp experiments clearly demonstrated the relevance of AP waveform in controlling dynamics (Figure 4A). Indeed, AP prolongation caused a sharp loading. Thus, STZ-induced changes in electrical activity might indirectly affect dynamics. In agreement with this, following the control of membrane potential (Figure 4B), direct effects of STZ-induced SERCA2a down-regulation were detected on handling. In particular, by clamping myocytes at -35 mV, STZ induced CaSR and CaT amplitude reduction, effects that were unseen in intact field stimulated cells. Moreover, incubating myocytes in extracellular and intracellular free solutions to remove NCX and NKA contribution (Figure 4C), SR Ca2+ uptake reloading kinetic following caffeine-induced SR depletion was clearly depressed in STZ myocytes.
Istaroxime stimulated SERCA2a in cardiac preparations from STZ rats by re-establishing the STZ-induced reduction of its maximal activity (Vmax) without affecting its affinity for Ca2+ (Kd). Moreover, no effects on SERCA2a activity were detected in CTR heart preparation (Figure 1), indicating that the stimulatory action on SERCA2a is more remarkable when a pathological alteration (i.e. STZ-induced SERCA2a down-regulation) is present. Analogously, in dog cardiac SR vesicles, the stimulatory effect of istaroxime prevailed in the failing vs. healthy dog.22 However, in healthy guinea pig cardiac microsomes, istaroxime stimulated SERCA2a by reducing the Kd Ca2+.21 The different effect of the compound on SERCA2a kinetic parameters in rat and dog (Vmax enhancement) vs. guinea pig (Kd Ca2+ reduction) may not exclude species-specific differences in SERCA2a-PLN functional complex formation along the heart preparation, affecting istaroxime interaction. Furthermore, these kinetic changes across species might depend on how the compound interferes with species-specific SERCA2a-PLN complex domains. Although Ferrandi et al.22 has already shown that istaroxime stimulates SERCA2a activity through a direct interaction with SERCA2a/PLN complex, favouring a partial dissociation of PLN from SERCA2a, further structural studies are still necessary to full understand istaroxime molecular mechanism of action.
At the cellular level, istaroxime stimulated SR Ca2+ uptake as clearly shown by applying the post-rest potentiation protocol to STZ myocytes (Figure 2). Moreover, as explain above, SERCA2a stimulation by the drug was fully remarkable by controlling membrane potential changes in voltage-clamped myocytes (Figure 4). Indeed, istaroxime, by stimulating SERCA2a, mostly restored STZ-induced changes in CaSR and CaT amplitude and it accelerated the SR uptake function, effects all compatible with a sharp enhancement of Ca2+ uptake by the SR, as expected from stimulation of SERCA2a activity.
STZ-induced CaD enhancement was blunted by istaroxime in paced STZ myocytes; by contrast, CaD was significantly increased by the drug in CTR myocytes. Moreover, istaroxime slightly depolarized Ediast in both CTR and STZ myocytes, as a result of a partial NKA blockade. Overall, the modulation of CaD by 100 nmol/L istaroxime might be the consequence of the balance between effects depending on SERCA2a stimulation and NKA inhibition, although negligible.
Abnormalities of the SR uptake function can be due to reduced SERCA2a activity or to increased Ca2+ leak through ryanodine receptor (RyR) channels. While functional and structural SERCA2a down-regulation (increased inhibition by PLN and reduced SERCA2a protein level) was observed, RyR open probability was not significantly changed in STZ myocytes. Indeed, Ca2+ sparks frequency (Figure 5), the incidence of Ca2+ waves and the related DADs were not significantly increased in STZ myocytes, thus suggesting the absence of a sharp SR instability at this stage of STZ-induced DCM. These findings lead to limit the detection of potential anti-arrhythmic effect of istaroxime as a direct consequence of SERCA2a stimulation.
Moreover, STZ-induced changes in Ca2+ sparks characteristics are a mirror image of the reduced SR Ca2+ content in STZ myocytes (Figure 5). Indeed, in comparison to CTR myocytes, Ca2+ sparks became smaller in amplitude, spatial and time duration, resulting in a smaller spark mass. Istaroxime, by stimulating SERCA2a, blunted these changes and even markedly accelerated Ca2+ sparks decay. The last event is relevant for the potential anti-arrhythmic efficacy of istaroxime because of a faster Ca2+ release unit switch off, that can limit Ca2+ waves genesis. Moreover, the acceleration of Ca2+ spark decay induced by istaroxime seems independent on STZ-induced changes; thus, we cannot exclude direct effects of the drug on Ca2+ spark termination mechanisms.
Temporal dispersion of repolarization, quantified as STV of APD, is a well-known pro-arrhythmic index because plays an important role in the initiation of ventricular arrhythmias like torsade de point.47 STV was significantly increased in STZ myocytes and this was mainly associated to APD prolongation (Figure 3C); istaroxime did not significantly affect STV.
4.3 Study limitation
The aim of the study was to test the effect of SERCA2a stimulation on DD in a DCM model. The study spreads from in vivo to in vitro effects of istaroxime at a concentration marginally affecting NKA. We would like to stress that even though effects dependent on NKA inhibition were detected, the general findings of the study are largely dependent on SERCA2a stimulation by the drug.
5. Conclusions and clinical implications
SERCA2a stimulation by istaroxime improves DD in diabetic rats, by controlling compartmentalization. Thus, SERCA2a stimulation can be considered a promising therapeutic approach for DCM treatment. Even though the translation of drug effects from animal models to patients must take into account differences in the pathophysiological mechanisms/picture between animals and patients, STZ model was useful for studying the cardiac mechanical improvement produced by a drug endowed with a SERCA2a stimulatory activity. Accordingly, a recent phase II randomized clinical study in patients hospitalized for acute HF28 showed that a 24 h infusion of istaroxime at 0.5 and 1 µg/kg/min improved cardiac function without major cardiac adverse effects. This is a proof-of-concept that SERCA2a stimulation is a novel and valid target for the treatment of high risk patients with reduced LVEF. Therefore, the development of small molecules active on SERCA2a only (‘pure SERCA2a activators’) might be clinically relevant to treat targeted patients with unfavourable cardiovascular outcomes with traditional therapies.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Authors’ contributions
E.T. performed electrophysiological studies and drafted the manuscript; M.A. performed Ca2+ handling experiments; A.M.L. analysed data; E.S. and S.V. measured TT distribution and cell dimensions; M.F. and P.B. performed biochemical measurements; S.-C.H. and G.-J.C. performed in vivo measurements; E.B. and C.B. contributed to echocardiographic evaluations; C.A. analysed Ca2+ sparks; G.M. contributed with high-level technical assistance; P.F. and G.B. critically supervised the study; M.R. coordinated the study and wrote the manuscript.
Funding
This work was supported by CVie Therapeutics Limited (Taipei, Taiwan), Windtree Therapeutics (Warrington, USA), and FAR2019 of the University of Milano-Bicocca.
Conflict of interest: M.F. and P.B. are Windtree employees, P.F. and G.B. are Windtree consultants, S.-C.H. is employee of CVie Therapeutics Limited.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
Translational perspective
Deficient SR Ca2+ uptake has been identified in cardiomyocytes from failing human hearts with impaired diastolic relaxation (e.g. diabetic hearts) and has been associated with a decreased SERCA2a expression and activity and/or with a higher SERCA2a inhibition by PLN. Thus, SERCA2a may represent a pharmacological target for interventions aimed at improving cytosolic Ca2+ compartmentalization into the SR to limit diastolic dysfunction pathologies. In this context, istaroxime is the first-in-class luso-inotropic agent targeting SERCA2a that has already demonstrated its efficacy in clinical trials and may be useful to clarify the relevance of SERCA2a stimulation in controlling cytosolic Ca2+ level.
Supplementary Material
References
- 1. Sherwin R, Jastreboff AM.. Year in diabetes 2012: the diabetes tsunami. J Clin Endocrinol Metab 2012;97:4293–4301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schannwell CM, Schneppenheim M, Perings S, Plehn G, Strauer BE.. Left ventricular diastolic dysfunction as an early manifestation of diabetic cardiomyopathy. Cardiology 2002;98:33–39. [DOI] [PubMed] [Google Scholar]
- 3. Belke DD, Dillmann WH.. Altered cardiac calcium handling in diabetes. Curr Hypertens Rep 2004;6:424–429. [DOI] [PubMed] [Google Scholar]
- 4. Boudina S, Abel ED.. Diabetic cardiomyopathy revisited. Circulation 2007;115:3213–3223. [DOI] [PubMed] [Google Scholar]
- 5. Lebeche D, Davidoff AJ, Hajjar RJ.. Interplay between impaired calcium regulation and insulin signaling abnormalities in diabetic cardiomyopathy. Nat Clin Pract Cardiovasc Med 2008;5:715–724. [DOI] [PubMed] [Google Scholar]
- 6. Choi KM, Zhong Y, Hoit BD, Grupp IL, Hahn H, Dilly KW, Guatimosim S, Jonathan Lederer W, Matlib MA.. Defective intracellular Ca2+ signaling contributes to cardiomyopathy in type 1 diabetic rats. Am J Physiol Heart Circ Physiol 2002;283:H1398–H1408. [DOI] [PubMed] [Google Scholar]
- 7. Vasanji Z, Dhalla NS, Netticadan T.. Increased inhibition of SERCA2 by phospholamban in the type I diabetic heart. Mol Cell Biochem 2004;261:245–249. [DOI] [PubMed] [Google Scholar]
- 8. Kranias EG, Hajjar RJ.. Modulation of cardiac contractility by the phopholamban/SERCA2a regulatome. Circ Res 2012;110:1646–1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zaza A, Rocchetti M.. Calcium store stability as an antiarrhythmic endpoint. Curr Pharm Des 2015;21:1053–1061. [DOI] [PubMed] [Google Scholar]
- 10. Malek V, Gaikwad AB.. Telmisartan and thiorphan combination treatment attenuates fibrosis and apoptosis in preventing diabetic cardiomyopathy. Cardiovasc Res 2019;115:373–384. [DOI] [PubMed] [Google Scholar]
- 11. Dobrin JS, Lebeche D.. Diabetic cardiomyopathy: signaling defects and therapeutic approaches. Expert Rev Cardiovasc Ther 2010;8:373–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ng HH, Leo CH, Parry LJ, Ritchie RH.. Relaxin as a therapeutic target for the cardiovascular complications of diabetes. Front Pharmacol 2018;9:501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jaski BE, Jessup ML, Mancini DM, Cappola TP, Pauly DF, Greenberg B, Borow K, Dittrich H, Zsebo KM, Hajjar RJ.. Calcium upregulation by percutaneous administration of gene therapy in cardiac disease (CUPID Trial), a first-in-human phase 1/2 clinical trial. J Card Fail 2009;15:171–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Clark RJ, McDonough PM, Swanson E, Trost SU, Suzuki M, Fukuda M, Dillmann WH.. Diabetes and the accompanying hyperglycemia impairs cardiomyocyte calcium cycling through increased nuclear O-GlcNAcylation. J Biol Chem 2003;278:44230–44237. [DOI] [PubMed] [Google Scholar]
- 15. Shao CH, Capek HL, Patel KP, Wang M, Tang K, DeSouza C, Nagai R, Mayhan W, Periasamy M, Bidasee KR.. Carbonylation contributes to SERCA2a activity loss and diastolic dysfunction in a rat model of type 1 diabetes. Diabetes 2011;60:947–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kho C, Lee A, Jeong D, Oh JG, Gorski PA, Fish K, Sanchez R, Devita RJ, Christensen G, Dahl R, Hajjar RJ.. Small-molecule activation of SERCA2a SUMOylation for the treatment of heart failure. Nat Commun 2015;6:7229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Peng BY, Dubey NK, Mishra VK, Tsai FC, Dubey R, Deng WP, Wei HJ.. Addressing stem cell therapeutic approaches in pathobiology of diabetes and its complications. J Diabetes Res 2018;2018:7806435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kaneko M, Yamamoto H, Sakai H, Kamada Y, Tanaka T, Fujiwara S, Yamamoto S, Takahagi H, Igawa H, Kasai S, Noda M, Inui M, Nishimoto T.. A pyridone derivative activates SERCA2a by attenuating the inhibitory effect of phospholamban. Eur J Pharmacol 2017;814:1–8. [DOI] [PubMed] [Google Scholar]
- 19. Bidasee KR, Zhang Y, Shao CH, Wang M, Patel KP, Dincer ÜD, Besch HR.. Diabetes increases formation of advanced glycation end products on sarco(endo)plasmic reticulum Ca2+-ATPase. Diabetes 2004;53:463–473. [DOI] [PubMed] [Google Scholar]
- 20. Shah SJ, Blair JEA, Filippatos GS, MacArie C, Ruzyllo W, Korewicki J, Bubenek-Turconi SI, Ceracchi M, Bianchetti M, Carminati P, Kremastinos D, Grzybowski J, Valentini G, Sabbah HN, Gheorghiade M.. Effects of istaroxime on diastolic stiffness in acute heart failure syndromes: results from the Hemodynamic, Echocardiographic, and Neurohormonal Effects of Istaroxime, a Novel Intravenous Inotropic and Lusitropic Agent: a Randomized Controlled Trial in Patients Hospitalized with Heart Failure (HORIZON-HF) trial. Am Heart J 2009;157:1035–1041. [DOI] [PubMed] [Google Scholar]
- 21. Rocchetti M, Besana A, Mostacciuolo G, Micheletti R, Ferrari P, Sarkozi S, Szegedi C, Jona I, Zaza A.. Modulation of sarcoplasmic reticulum function by Na+/K + pump inhibitors with different toxicity: digoxin and PST2744 [(E,Z)-3-((2-aminoethoxy)imino)androstane-6,17-dione hydrochloride]. J Pharmacol Exp Ther 2005;313:207–215. [DOI] [PubMed] [Google Scholar]
- 22. Ferrandi M, Barassi P, Tadini-Buoninsegni F, Bartolommei G, Molinari I, Tripodi MG, Reina C, Moncelli MR, Bianchi G, Ferrari P.. Istaroxime stimulates SERCA2a and accelerates calcium cycling in heart failure by relieving phospholamban inhibition. Br J Pharmacol 2013;169:1849–1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Alemanni M, Rocchetti M, Re D, Zaza A.. Role and mechanism of subcellular Ca2+ distribution in the action of two inotropic agents with different toxicity. J Mol Cell Cardiol 2011;50:910–918. [DOI] [PubMed] [Google Scholar]
- 24. Adamson PB, Vanoli E, Mattera GG, Germany R, Gagnol JP, Carminati P, Schwartz PJ.. Hemodynamic effects of a new inotropic compound, PST-2744, in dogs with chronic ischemic heart failure. J Cardiovasc Pharmacol 2003;42:169–173. [DOI] [PubMed] [Google Scholar]
- 25. Micheletti R, Palazzo F, Barassi P, Giacalone G, Ferrandi M, Schiavone A, Moro B, Parodi O, Ferrari P, Bianchi G.. Istaroxime, a stimulator of sarcoplasmic reticulum calcium adenosine triphosphatase isoform 2a activity, as a novel therapeutic approach to heart failure. Am J Cardiol 2007;99:24A–32A. [DOI] [PubMed] [Google Scholar]
- 26. Sabbah HN, Imai M, Cowart D, Amato A, Carminati P, Gheorghiade M.. Hemodynamic properties of a new-generation positive luso-inotropic agent for the acute treatment of advanced heart failure. Am J Cardiol 2007;99:41A–46A. [DOI] [PubMed] [Google Scholar]
- 27. Rocchetti M, Alemanni M, Mostacciuolo G, Barassi P, Altomare C, Chisci R, Micheletti R, Ferrari P, Zaza A.. Modulation of sarcoplasmic reticulum function by PST2744 [Istaroxime; (E,Z)-3-((2-aminoethoxy)imino) androstane-6,17-dione hydrochloride] in a pressure-overload heart failure model. J Pharmacol Exp Ther 2008;326:957–965. [DOI] [PubMed] [Google Scholar]
- 28. Carubelli V, Zhang Y, Metra M, Lombardi C, Felker GM, Filippatos G, O'Connor CM, Teerlink JR, Simmons P, Segal R, Malfatto G, La Rovere MT, Li D, Han X, Yuan Z, Yao Y, Li B, Lau LF, Bianchi G, Zhang J, Istaroxime ADHF Trial Group. Treatment with 24 hour istaroxime infusion in patients hospitalised for acute heart failure: a randomised, placebo-controlled trial. Eur J Heart Fail 2020;22:1684–1693. [DOI] [PubMed] [Google Scholar]
- 29. Micheletti R, Mattera GG, Rocchetti M, Schiavone A, Loi MF, Zaza A, Gagnol RJP, De Munari S, Melloni P, Carminati P, Bianchi G, Ferrari P.. Pharmacological profile of the novel inotropic agent (E,Z)-3-((2-aminoethoxy)imino)androstane-6,17-dione hydrochloride (PST2744). J Pharmacol Exp Ther 2002;303:592–600. [DOI] [PubMed] [Google Scholar]
- 30. Rocchetti M, Besana A, Mostacciuolo G, Ferrari P, Micheletti R, Zaza A.. Diverse toxicity associated with cardiac Na+/K+ pump inhibition: evaluation of electrophysiological mechanisms. J Pharmacol Exp Ther 2003;305:765–771. [DOI] [PubMed] [Google Scholar]
- 31. Gheorghiade M, Ambrosy AP, Ferrandi M, Ferrari P.. Combining SERCA2a activation and Na-K ATPase inhibition: a promising new approach to managing acute heart failure syndromes with low cardiac output. Discov Med 2011;12:141–151. [PubMed] [Google Scholar]
- 32. Bossu A, Kostense A, Beekman HDM, Houtman MJC, van der Heyden MAG, Vos MA.. Istaroxime, a positive inotropic agent devoid of proarrhythmic properties in sensitive chronic atrioventricular block dogs. Pharmacol Res 2018;133:132–140. [DOI] [PubMed] [Google Scholar]
- 33. Rocchetti M, Sala L, Rizzetto R, Irene Staszewsky L, Alemanni M, Zambelli V, Russo I, Barile L, Cornaghi L, Altomare C, Ronchi C, Mostacciuolo G, Lucchetti J, Gobbi M, Latini R, Zaza A.. Ranolazine prevents INaL enhancement and blunts myocardial remodelling in a model of pulmonary hypertension. Cardiovasc Res 2014;104:37–48. [DOI] [PubMed] [Google Scholar]
- 34. Pasqualin C, Gannier F, Malécot CO, Bredeloux P, Maupoil V.. Automatic quantitative analysis of t-tubule organization in cardiac myocytes using ImageJ. Am J Physiol Cell Physiol 2015;308:C237–C245. [DOI] [PubMed] [Google Scholar]
- 35. Ferrandi M, Tripodi G, Salardi S, Florio M, Modica R, Barassi P, Parenti P, Shainskaya A, Karlish S, Bianchi G, Ferrari P.. Renal Na,K-ATPase in genetic hypertension. Hypertension 1996;28:1018–1025. [DOI] [PubMed] [Google Scholar]
- 36. Altomare C, Bartolucci C, Sala L, Bernardi J, Mostacciuolo G, Rocchetti M, Severi S, Zaza A.. IKr impact on repolarization and its variability assessed by dynamic clamp. Circ Arrhythm Electrophysiol 2015;8:1265–1275. [DOI] [PubMed] [Google Scholar]
- 37. Meo M, Meste O, Signore S, Sorrentino A, Cannata A, Zhou Y, Matsuda A, Luciani M, Kannappan R, Goichberg P, Leri A, Anversa P, Rota M.. Reduction in Kv current enhances the temporal dispersion of the action potential in diabetic myocytes: insights from a novel repolarization algorithm. J Am Heart Assoc 2016;5:e003078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Howarth FC, Jacobson M, Qureshi MA, Shafiullah M, Hameed RS, Zilahi E, Al Haj A, Nowotny N, Adeghate E.. Altered gene expression may underlie prolonged duration of the QT interval and ventricular action potential in streptozotocin-induced diabetic rat heart. Mol Cell Biochem 2009;328:57–65. [DOI] [PubMed] [Google Scholar]
- 39. Ku DD, Sellers BM.. Effects of streptozotocin diabetes and insulin treatment on myocardial sodium pump and contractility of the rat heart. J Pharmacol Exp Ther 1982;222:395–400. [PubMed] [Google Scholar]
- 40. Hoshijima M, Ikeda Y, Iwanaga Y, Minamisawa S, Date MO, Gu Y, Iwatate M, Li M, Wang L, Wilson JM, Wang Y, Ross J, Chien KR.. Chronic suppression of heart-failure progression by a pseudophosphorylated mutant of phospholamban via in vivo cardiac rAAV gene delivery. Nat Med 2002;8:864–871. [DOI] [PubMed] [Google Scholar]
- 41. Suckau L, Fechner H, Chemaly E, Krohn S, Hadri L, Kockskamper J, Westermann D, Bisping E, Ly H, Wang X, Kawase Y, Chen J, Liang L, Sipo I, Vetter R, Weger S, Kurreck J, Erdmann V, Tschope C, Pieske B, Lebeche D, Schultheiss HP, Hajjar RJ, Poller WC.. Long-term cardiac-targeted RNA interference for the treatment of heart failure restores cardiac function and reduces pathological hypertrophy. Circulation 2009;119:1241–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Watanabe A, Arai M, Yamazaki M, Koitabashi N, Wuytack F, Kurabayashi M.. Phospholamban ablation by RNA interference increases Ca2+uptake into rat cardiac myocyte sarcoplasmic reticulum. J Mol Cell Cardiol 2004;37:691–698. [DOI] [PubMed] [Google Scholar]
- 43. Suzuki T, Wang JH.. Stimulation of bovine cardiac sarcoplasmic reticulum Ca2+ pump and blocking of phospholamban phosphorylation and dephosphorylation by a phospholamban monoclonal antibody. J Biol Chem 1986;261:7018–7023. [PubMed] [Google Scholar]
- 44. Zhang Y, Wang Y, Yanni J, Qureshi MA, Logantha SJRJ, Kassab S, Boyett MR, Gardiner NJ, Sun H, Howarth FC, Dobrzynski H.. Electrical conduction system remodeling in streptozotocin-induced diabetes mellitus rat heart. Front Physiol 2019;10:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mihm MJ, Seifert JL, Coyle CM, Bauer JA.. Diabetes related cardiomyopathy time dependent echocardiographic evaluation in an experimental rat model. Life Sci 2001;69:527–542. [DOI] [PubMed] [Google Scholar]
- 46. Wu W, Liu X, Han L.. Apoptosis of cardiomyocytes in diabetic cardiomyopathy involves overexpression of glycogen synthase kinase-3β. Biosci Rep 2019;39:BSR20171307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Smoczynska A, Beekman HDM, Vos MA.. The increment of short-term variability of repolarisation determines the severity of the imminent arrhythmic outcome. Arrhythm Electrophysiol Rev 2019;8:166–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.
Translational perspective
Deficient SR Ca2+ uptake has been identified in cardiomyocytes from failing human hearts with impaired diastolic relaxation (e.g. diabetic hearts) and has been associated with a decreased SERCA2a expression and activity and/or with a higher SERCA2a inhibition by PLN. Thus, SERCA2a may represent a pharmacological target for interventions aimed at improving cytosolic Ca2+ compartmentalization into the SR to limit diastolic dysfunction pathologies. In this context, istaroxime is the first-in-class luso-inotropic agent targeting SERCA2a that has already demonstrated its efficacy in clinical trials and may be useful to clarify the relevance of SERCA2a stimulation in controlling cytosolic Ca2+ level.