The purpose of this article is to emphasize exciting new interpretations we have concluded about the circulation as reported recently.1
In exercise, up to moderately high levels, the rate of arterial oxygen delivery (DO2) to skeletal muscle increases directly in proportion to the rate of oxygen consumption (VO2). This physiological, evolutionary, feature avoids ischaemia of the exercising muscle. Oxygen extraction is precisely controlled.2 Exercising muscle DO2 and hence blood flow control is an example of near universal control of individual tissue blood supply, with appropriate individual tissue DO2.1 Venous return to the heart, and hence cardiac output (CO), is made up of the total of all tissue regulated blood flows. ‘The heart puts out what it receives’.3 Hence, the tissues control CO.
DO2 to individual tissues is scaled relative to VO2; DO2/VO2 is around 1.5 for skeletal muscle, oxygen extraction of 2/3, or 0.67. Similarly, for the brain, DO2/VO2 is close to 3, oxygen extraction 1/3.
Under resting conditions the oxygen extraction values, for the brain, are also sustained during changes in arterial blood pressure, over a fairly wide range—this is the well-known phenomenon—‘auto-regulation’ of cerebral blood flow (CBF). Adjustment of cerebral arteriolar resistance sustains correct DO2 in the face of arterial pressure change.
The current assumption that the systemic vascular resistance (SVR) controls arterial blood pressure in health is incorrect, because:
Each tissue regulates its own blood flow sustaining an appropriate DO2 by means of appropriate adjustment of its arteriolar input resistance. Hence, SVR is the net effect of multiple individually regulated arteriolar resistances;
Arterial blood pressure depends on arterial volume; indeed, it is the relationship between pressure and volume which defines arterial wall compliance. Arterial compliance is low relative to venous compliance, which determines the relatively smaller arterial volume. Changes in arterial volume result in changes in arterial blood pressure. So, making a modest shift in blood volume from veins to arteries, or arteries to veins, causes immediate pressure change, without a need for change in total blood volume.
Venous compliance can also, usefully, be expressed as the inverse—venous tone. Venous tone is largely controlled by the sympathetic nervous system. Venous/arterial volume distribution is sustained by ongoing venous sympathetic activity. An increase in arterial pressure will result from an increase in venous wall muscle tension, due to increased sympathetic stimulation. This is veno-constriction, an important distinction from ‘vaso-constriction’.
In our paper,1 we suggested that the arteriolar sympathetic supply sustains a constant, tonic, state in health, except where there is a gross increase in arterial pressure, when an abrupt protective effect, a sympathetic surge, constricts cerebral arterioles. This prevents excess intracerebral pressure and cerebral damage.4 However, during experimental increases in arterial pressure, the autoregulation range of pressures with sustained CBF can be extended to higher pressure levels by the addition of sympathetic stimulation. Hence, during the auto-regulation range, extra sympathetic stimulation still allows the compensatory cerebral arteriolar resistance adjustment. The apparent co-activation, where arterial pressure and SVR change together in experimental work either results from the compensatory tissue response to raised arterial blood pressure or is an artefact from sustaining an imposed constant CO.1 Furthermore, we may need to revise the site of action of drugs raising arterial pressure as they may well act on venous tone rather than on arteriolar resistance. These medications may also interfere with in-tissue regulation of arteriolar resistance, preventing adequate oxygenation.
So, tissues are responsible for modification of input resistance, either specifically for their own need to alter DO2 in parallel with VO2 change, or to resist blood flow change in the face of alterations in arterial blood pressure.
Hence, the two fundamental properties of the circulation are1:
Figure 1.
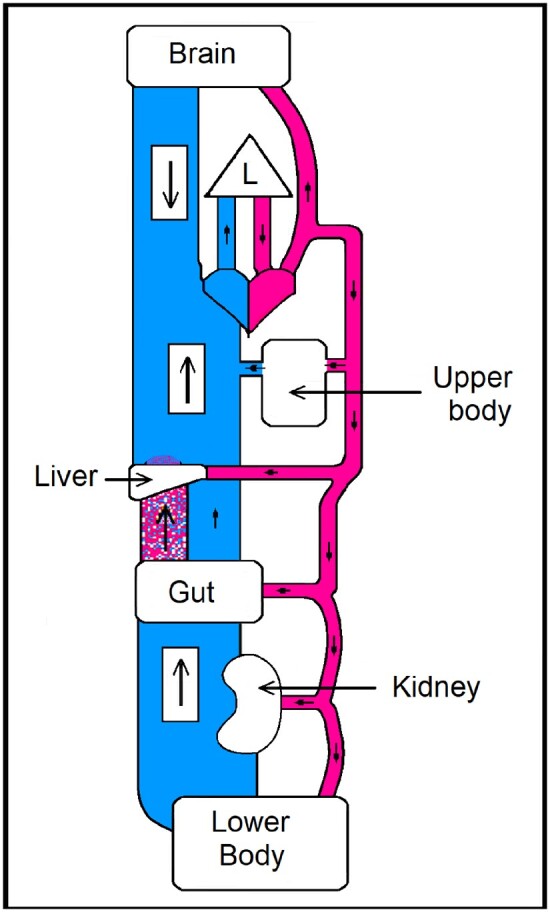
Illustration of the circulation, with the venous system depicted as having a much larger capacity and compliance than arterial. Arterial blood pressure is intimately related to the arterial blood volume. Veins blue: capacity 5 × that of arteries. Arteries red: A small change in venous volume causes a large change in arterial volume—and hence pressure. Arterial volume and hence pressure are sustained by the level of venous tone. Venous tone depends mainly on the intensity of the sympathetic outflow from the brain to venous wall musculature. The portal blood flow is shown stippled red and blue, an intermediate drainage from gut to liver. Apart from the emphasis on the different venous and arterial volumes, the tissues, heart, and lung (L) dimensions are arbitrary.
Individual tissues control local blood flow such that they regulate their own rate of DO2. In the normal range, this amounts to maintenance of constant oxygen extraction even when VO2 varies over a considerable range. The total of all individual tissue blood flows becomes venous return and hence CO.
Arterial blood pressure is proportionately related to the arterial volume. It is not determined by SVR. The multiple tissue arteriolar resistance values each normally sustain constant individual DO2 to VO2 ratios (and hence constant oxygen extraction values). These are sustained both with changes in VO2, as in exercise, and during arterial pressure change. Hence, SVR is affected by both tissue metabolism and arterial blood pressure. Arterial blood pressure changes are caused by changes in venous volume, not by SVR change.
Areas for further research
Re-examination of arterial blood pressure regulation, in view of the presence of intracerebral baro-receptors.5
The higher venous tone sustaining hypertension, but with no increase in total blood volume.
Sites of action of vaso-active drugs (an increase in emphasis on veins).
Effects of hormones on venous tone (e.g. endothelin, NO, angiotensin II).
Redox chemistry and regulation of within-organ/tissue control of oxygen delivery.
Mechanisms which underlay accurate cellular oxygen extraction: including cellular oxygen recognition6 and arteriolar resistance control.
Role of neuro-humoral and immunological interaction and arterial blood pressure.7
Potential for intensive care prioritization of whole body DO2 optimization.
Therapeutic dividends
Maintaining DO2 limits the buildup of oxygen debt, potential organ dysfunction and perhaps cognitive impairment.8
Removal of partial obstructions to CBF; e.g. carotid endarterectomy with lowering of arterial blood pressure.1
Potential treatment for ischaemic heart disease.1
Potential for lower doses of existing drugs, or different drugs acting on veins to lower blood pressure.1
This revision of circulatory mechanisms constitutes a paradigm shift and upsets reliance on previous long-term interpretations. It is hoped our account1 can facilitate transition to a new approach.
Conflict of interest: none declared.
Authors

Biography: Christopher Bancroft Wolff, MB ChB, PhD, FRCP, was born on 22 January 1938 in Oxford, UK and he lives in Cambridge, UK. He studied at the Sheffield University Medical School in 1956–1962, MRCP from London, FRCP in 1995, and PhD Physiology in 1972; ‘Dynamic sinus nerve firing response to respiratory arterial CO2 oscillations’. Further areas of study: papers on respiration, a monograph on respiratory control (1992); high altitude (over 5000 m—16400 ft) respiratory physiology papers with Dr Collier. Papers on Acclimatization, cerebral oxygen extraction at altitude, and arterial respiratory pH oscillation correlation with periodic breathing despite steady PACO2. Constant oxygen extraction found for skeletal muscle, heart, and brain in normal subjects. Circulatory pathophysiology of anaesthesia published with Dr Green. Recent collaborative studies of CO, BP, and SVR with parallel autonomic nervous system testing in patients with hypertension, chronic fatigue, and postural intolerance. Mild exercise in normal subjects showed CO and conductance correlated and BP unchanged, whereas correlation failed with the patients and BP rose.

Biography: David W. Green, MB BS (Hons) FRCA MBA, was born on 5 March 1950. He graduated MB BS with honours from Kings College Hospital (KCH) Medical School, London in 1973. He undertook training in anaesthesia, intensive care, and pain relief in London and, following 1 year as Assistant Professor in the University of Texas South-Western Medical School in Dallas, he was appointed consultant in anaesthesia and intensive care at KCH in 1980. A keen interest in IT led him to obtaining an MBA in technology management from the Open University in 1999. His main interest has been in multimodal monitoring of high-risk surgical patients undergoing major surgery.

Biography: Professor Julian F.R. Paton, BSc(Hon) PhD FRSNZ, was born on 21 June 1962. He graduated from the University of Birmingham in 1984 (Biological Sciences, Physiology). His PhD was obtained from the University of London (Royal Free Hospital, London) in 1987. After a post-doc in London, he trained at Du Pont de Nemours, Delaware and the University of Washington in Seattle, USA (1989–1991). He was awarded an Alexander von Humboldt Fellowship (1992–1994) to train at the University of Goettingen and subsequently a British Heart Foundation Fellowship (1994–2004), which allowed him to initiate his independent career at the University of Bristol. After 24 years, he transferred to the University of Auckland, New Zealand (2017) and became a Fellow of the Royal Society of New Zealand in 2021. His research has centred around neural coupling of the cardiovascular-respiratory system in health and disease resulting in putative new ways to treat hypertension and a novel pacemaker for heart failure.

Biography: Dr David J. Collier, MBBS PhD (Barts) was born on 16 December 1964. Infected, during his BSc year (UMDS Guys/StThomas'), with enthusiasm for cardiorespiratory control physiology (David Band, Chris Wolff, and Jennifer Angell-James), he won one of the first Wellcome Prize PhD studentships which delayed clinical graduation until 1992 (PhD examined by K.M. Spyer and P. Sleight). He led research teams on altitude physiology in Nepal on Chamlang (1991) and the British Mount Everest Medical Expedition in 1994 with Dr Andrew Pollard. He set up the William Harvey Clinical Research Centre for Sir Mark Caulfield and led top-recruitment contributions to landmark cardiovascular outcome trials such as ASCOT and BHF PATHWAY 2 and lesser roles in ILLUMINATE, FOURIER, SPIRE, and CANTOS. He is currently running a remote care trial PERSONAL-COVIDBP and is Deputy Director of Barts Clinical Trials Unit (UKCRC registered 04).
References
- 1. Wolff CB, Green DW, Paton JFR, Collier DJ.. A new radically improved model of the circulation with important clinical implications. Am J Surg Clin Case Rep 2020;2:1–25. [Google Scholar]
- 2. Wolff CB. Cardiac output, oxygen consumption and muscle oxygen delivery in submaximal exercise: normal and low O2 states. Adv Exp Med Biol 2003;510:279–284. [DOI] [PubMed] [Google Scholar]
- 3. Starling EH. The wisdom of the body: the Harveian oration. Brit Med J 1923;2:685–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Harper AM. Physiological control of the cerebral circulation. In: Harper AM, Jennett S (eds). Cerebral Blood Flow and Metabolism. Physiology Society Study Guides—Number 5. Manchester and New York: Manchester University Press; 1990. p4–26. [Google Scholar]
- 5. Nephtali M, Christie IN, Korsak A, Doronin M, Brazhe A, Patrick S, Hosford PS, Wells JA, Sheikhbahaei S, Humoud I, Paton JFR, Lythgoe MF, Semyanov A, Kasparov S, Gourine AV.. Astrocytes monitor cerebral perfusion and control systemic circulation to maintain brain blood flow. Nat Commun 2020;11:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kaelin WG, Ratcliffe PJ, Semenza GL.. Pathways for oxygen regulation and homeostasis: the 2016 Albert Lasker Basic Medical Research Award. JAMA 2016;316:1252–1253. [DOI] [PubMed] [Google Scholar]
- 7. Lembo G, Perrotta M.. The neurology of hypertension:merging academic specialties to connect heart and brain pathophysiology. Cardiovasc Res 2021;117:e70–e72. [DOI] [PubMed] [Google Scholar]
- 8. Ballard C, Jones E, Gauge N, Aarsland D, Nilsen OB, Saxby BK, Lowery D, Corbett A, Wesnes K, Katsaiti E, Arden J, Amaoko D, Prophet N, Purushothaman B, Green D.. Optimised anaesthesia to reduce post operative cognitive decline (POCD) in older patients undergoing elective surgery, a randomised controlled trial. PLoS One 2012;7:e37410. [DOI] [PMC free article] [PubMed] [Google Scholar]


