Abstract
Aims
Free fatty acid receptor 4 (Ffar4) is a G-protein-coupled receptor for endogenous medium-/long-chain fatty acids that attenuates metabolic disease and inflammation. However, the function of Ffar4 in the heart is unclear. Given its putative beneficial role, we hypothesized that Ffar4 would protect the heart from pathologic stress.
Methods and results
In mice lacking Ffar4 (Ffar4KO), we found that Ffar4 is required for an adaptive response to pressure overload induced by transverse aortic constriction (TAC), identifying a novel cardioprotective function for Ffar4. Following TAC, remodelling was worsened in Ffar4KO hearts, with greater hypertrophy and contractile dysfunction. Transcriptome analysis 3-day post-TAC identified transcriptional deficits in genes associated with cytoplasmic phospholipase A2α signalling and oxylipin synthesis and the reduction of oxidative stress in Ffar4KO myocytes. In cultured adult cardiac myocytes, Ffar4 induced the production of the eicosapentaenoic acid (EPA)-derived, pro-resolving oxylipin 18-hydroxyeicosapentaenoic acid (18-HEPE). Furthermore, the activation of Ffar4 attenuated cardiac myocyte death from oxidative stress, while 18-HEPE rescued Ffar4KO myocytes. Systemically, Ffar4 maintained pro-resolving oxylipins and attenuated autoxidation basally, and increased pro-inflammatory and pro-resolving oxylipins, including 18-HEPE, in high-density lipoproteins post-TAC. In humans, Ffar4 expression decreased in heart failure, while the signalling-deficient Ffar4 R270H polymorphism correlated with eccentric remodelling in a large clinical cohort paralleling changes observed in Ffar4KO mice post-TAC.
Conclusion
Our data indicate that Ffar4 in cardiac myocytes responds to endogenous fatty acids, reducing oxidative injury, and protecting the heart from pathologic stress, with significant translational implications for targeting Ffar4 in cardiovascular disease.
Keywords: Free fatty acid receptor 4 (Ffar4), GPR120, Heart failure, Cytoplasmic phospholipase A2α (cPLA2α), Eicosapentaenoic acid (EPA), 18-hydroxyeicosapentaenoic acid (18-HEPE)
Translational perspective
Here, we establish that free fatty acid receptor 4 (Ffar4, GPR120), a G-protein-coupled receptor for medium-/long-chain fatty acids, attenuates oxidative injury in cardiac myocytes and protects the heart from pathologic stress. This cardioprotective benefit of Ffar4 is observed in the absence of dietary intervention indicating that Ffar4 responds to endogenous fatty acids functioning as signalling molecules, not simply in their traditional role as an energy source. More broadly, these findings advance a novel paradigm whereby modulation of cellular/organ fatty acid composition could improve cardiovascular outcomes by the activation of receptor signalling, possibly through dietary supplementation with ω3-PUFAs. In humans, Ffar4 expression is down-regulated in heart failure (HF), while the signalling-deficient Ffar4 R270H polymorphism is associated with eccentric remodelling in a large clinical cohort. Together with our novel finding that Ffar4 is cardioprotective in mice, these concordant human data suggest a potential translational benefit to targeting Ffar4 in the clinical management of HF.
1. Introduction
Free fatty acid receptor 4 (Ffar4, GPR120) is a G-protein-coupled receptor (GPR) that functions as a nutrient sensor for fatty acids (FA) to regulate metabolism and attenuate inflammation.1,2 The endogenous ligands for Ffar4 include medium- and long-chain (C10–C22) saturated (SFA), mono-unsaturated (MUFA), and poly-unsaturated fatty acids (PUFA), which bind the receptor with affinities in the low µM range. However, PUFAs are generally full agonists, whereas SFAs are only partial agonists.3–5 Consistent with a primary role in regulating metabolism, Ffar4 is expressed in enteroendocrine cells in the GI tract,5 α, β, and δ-cells in the pancreas,5–7 and both white and brown adipose.8,9 However, Ffar4 is also highly expressed in the lung and the brain, with lower levels of expression in the heart, taste buds, and immune cells, including macrophages.4,10–12 Ffar4 signals through both Gq/11 and βarrestin-2 (βArr2)-mediated pathways, which can be cell-type specific.4,11,13,14 Interestingly, humans express two isoforms of Ffar4, short and long (Ffar4S, Ffar4L), differentiated by a 16 amino acid insertion in the third intracellular loop of Ffar4L, whereas other species express only one isoform, homologous to Ffar4S in humans.14 The Ffar4L isoform only signals through β-Arr2 and is unable to activate Gq/11-mediated signalling.14
Currently, nothing is known about Ffar4 function in the heart. Previous studies have indicated that ω3-polyunsaturated fatty acids (ω3-PUFAs) signalling through Ffar4 attenuate inflammation and obesity.4 Clinical studies indicate that ω3-PUFAs, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), improve outcomes in coronary heart disease and heart failure (HF),15 but the mechanisms underlying this benefit remain unclear. In mice, we were the first to demonstrate that EPA prevents fibrosis and contractile dysfunction in response to pathologic stress in the heart, but EPA was not incorporated into cardiac myocyte or fibroblast cellular membranes, the traditional mechanism of action for EPA.10,16 However, we found that Ffar4 is expressed in cardiac myocytes and fibroblasts, and that in cardiac fibroblasts, Ffar4 was sufficient and necessary to prevent TGFβ1-induced fibrosis.10 These findings suggest that Ffar4 might mediate ω3-PUFA cardioprotection, possibly by preventing fibrosis. However, to date, no studies have directly addressed the function of Ffar4 in the heart, nor provided any mechanistic link between Ffar4 and ω3-mediated cardioprotection.
Here, we hypothesized that Ffar4 functions as a nutrient sensor for endogenous medium- and long-chain fatty acids, protecting the heart from pathologic stress. To test this hypothesis, we employed mice with systemic deletion of Ffar4 (Ffar4KO mice) to determine if Ffar4 is necessary for an adaptive response to pathologic pressure overload induced by transverse aortic constriction (TAC). Briefly, our results indicated that TAC induced more profound pathologic remodelling in Ffar4KO mice, identifying a novel cardioprotective role for Ffar4. In cardiac myocytes, Ffar4 induced the production of the cardioprotective, pro-resolving oxylipin 18-hydroxyeicosapentaenoic acid (18-HEPE) and prevented cardiac myocyte cell death in response to oxidative stress. Systemically, Ffar4 was required to maintain pro-resolving oxylipins and suppress autoxidation basally, and to produce both pro-inflammatory and pro-resolving oxylipins in circulating high-density lipoproteins (HDL) following TAC. In humans, Ffar4 was expressed in the heart, and decreased in HF, while the signalling-deficient Ffar4 R270H polymorphism was correlated with eccentric remodelling in a large clinical cohort. In summary, our data suggest an entirely novel paradigm whereby FAs function as signalling molecules that activate receptor-mediated signalling through Ffar4 to attenuate oxidative stress and protect the heart from pathologic stress.
2. Methods
Detailed methods describing the mice, diet (Supplementary material online, Table S1 and S2), TAC surgery, measurement of cardiac function by echocardiography, measurement of erythrocyte FA composition, isolation and culture of adult mouse cardiac myocytes, RNA-Seq, measurement of oxylipins in myocytes and lipoproteins, measurement of cell death, measurement of Ffar4/1 RNA levels, analysis of R270H on cardiac function in humans (EchoWAS), and all statistical protocols are available in Supplementary material online.
2.1. Study approvals
2.1.1. Animal
All procedures on animals conformed to the NIH Guide for the Care and Use of Laboratory Animals and were reviewed and approved by the Institutional Animal Care and Use Committee at the University of Minnesota.
2.1.2. Human
All tissue samples and data used in this study were deidentified. Heart tissue was collected by and subsequently obtained from the Duke Human Heart Repository and approved by the Duke University Institutional Review Board. All analyses of human-derived echocardiographic data were approved by the Vanderbilt University Medical Center Institutional Review Board. The present study complies with the Declaration of Helsinki.
2.1.3. Additional compliance statements
For all surgeries, mice were anesthetized by induction with 3% isoflurane, and once anaesthesia was induced, mice were maintained at 1.5% isoflurane, and verified by toe-pinch. Post-surgery, buprenorphine (0.1 mg/kg IP) was administered for pain management during the first 24 h post-surgery and as needed thereafter. At the indicated time points post-surgery or for the isolation of cardiac myocytes, mice were anesthetized with 3% isoflurane, verified by toe-pinch, followed by removal of the heart in accordance with recommendations from the American Veterinary Medical Association.
3. Results
3.1. Ffar4 mitigates pathologic hypertrophy and attenuates contractile dysfunction induced by TAC
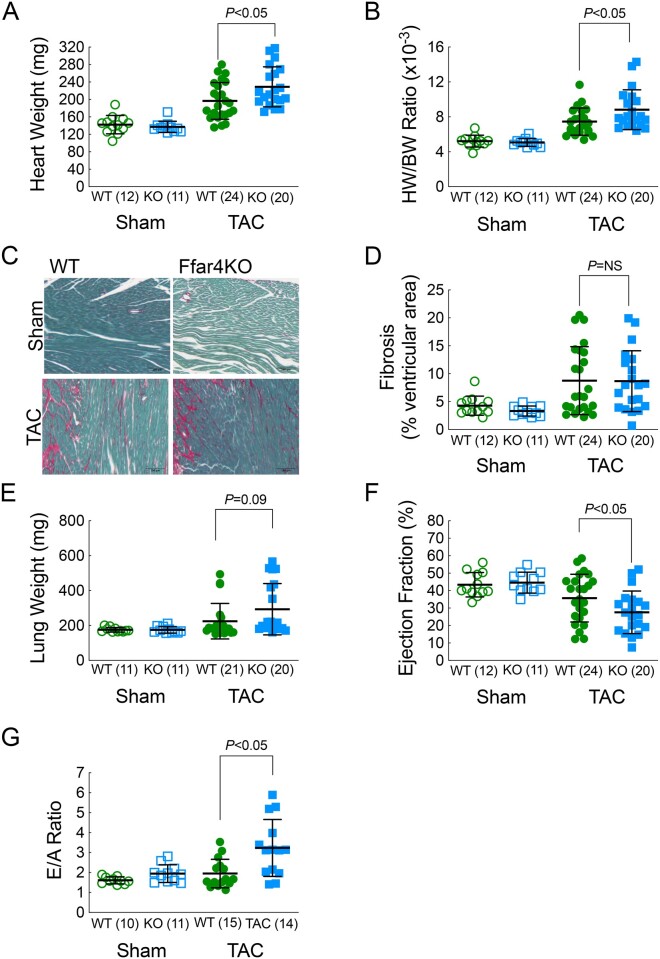
To test the hypothesis that Ffar4 is cardioprotective, wild-type (WT) and Ffar4KO mice were subjected to pathologic pressure overload by TAC. Based on our prior studies demonstrating that in cultured fibroblasts, Ffar4 is sufficient and necessary to prevent TGFβ1-induced fibrosis, we hypothesized that TAC would produce more fibrosis and diastolic dysfunction in the Ffar4KO. Therefore, we analysed the response to TAC at 4 weeks, a time point where the heart exhibits concentric remodelling with significant interstitial fibrosis prior to the transition to ventricular dilation and overt systolic failure. After 4 weeks, TAC induced an exaggerated hypertrophic response with a trend towards increased lung weights, but surprisingly not excessive fibrosis in male Ffar4KO mice (Figure 1A–E, Supplementary material online, Table S3A). Functionally, TAC induced significantly more systolic and diastolic dysfunction in male Ffar4KO mice (Figure 1F–G, Supplementary material online, Table S3B). In contrast, no significant differences were observed in female Ffar4KO mice (Supplementary material online, Figure 1). In summary, Ffar4 is necessary for an adaptive response to pathologic stress in the heart. The surprising lack of an exacerbated fibrotic response in the Ffar4KO, as we had hypothesized, would indicate that the more severe remodelling observed in the Ffar4KO heart was most likely due to the lack of cardioprotective Ffar4 signalling in cardiac myocytes.
Figure 1.
Four weeks following TAC, mice were euthanized and hearts were collected. (A) Heart weight (HW) and (B) heart weight-to-body weight ratio (HW/BW) of male WT and Ffar4KO mice. (C) Representative images of ventricular fibrosis quantified from ventricular cross sections from male WT and Ffar4KO mice stained with Sirius red/Fast Green. (D) Ventricular fibrosis quantified by fibrotic area (Sirius red)/total ventricular area (Fast green) from male WT and Ffar4KO mice. (E) Lung weight of male WT and Ffar4KO mice. Four weeks following TAC, cardiac function measured by echocardiography in male WT and Ffar4KO mice. (F) Ejection fraction (EF, %); (G) E/A ratio. Data were compared by a Welch’s two sample t test. Error bars represent the mean with SD.
3.2. Ffar4 induction of cytoplasmic phospholipase A2α (cPLA2α) signalling pathways in cardiac myocytes is essential for an adaptive response post-TAC
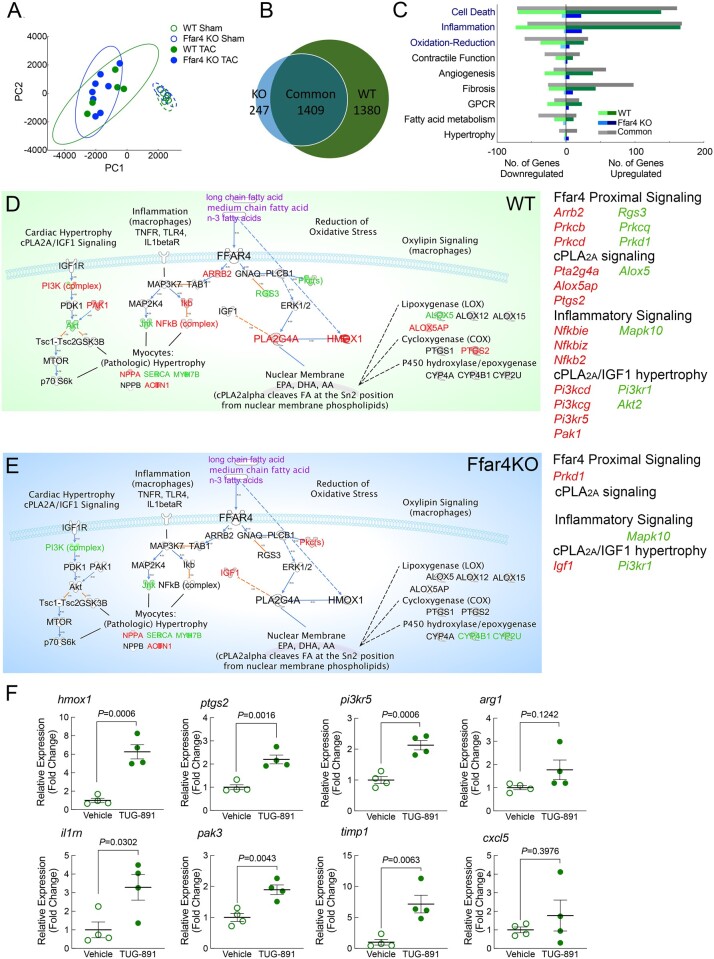
To define a potentially cardioprotective role for Ffar4 in cardiac myocytes in male mice (Figure 1), we used RNAseq to analyse transcriptomes of myocytes freshly isolated from male WT and Ffar4KO hearts 3 days post-TAC, an early time point when patterns of gene expression that will define the remodelling response show the greatest degree of regulation.17 While a principal component analysis (PCA) revealed only minor differences in the transcriptomes post-TAC, indicating a larger effect of surgery than genotype (Figure 2A), further analysis revealed a significant transcriptional deficit in male Ffar4KO myocytes post-TAC. By using a threshold of 1.7-fold change in expression, TAC altered the expression of 2789 genes in WT cardiac myocytes, but only 1656 genes in Ffar4KO myocytes. In total, 1409 genes were common between the two genotypes, whereas 1380 genes were unique to WT myocytes, but only 247 genes were unique to Ffar4KO myocytes (Figure 2B). Genes were further sorted by Gene Ontology terms for biological function for nine different categories that reflect cardiac myocyte biology (Figure 2C, Supplementary material online, Tables S5–S13). Genes for cell death, inflammation, and oxidation–reduction were the most divergent, with significantly more genes identified in WT cardiac myocytes (Figure 2C).
Figure 2.
Transcriptome analysis (RNA-seq) was performed on cardiac myocytes isolated from male WT and Ffar4KO mice 3-days post-TAC or sham surgery. (A) Principal component analysis of RNA transcriptomes from WT and Ffar4KO cardiac myocytes. (B) Venn diagram indicating genes differentially expressed ≥ 1.7-fold uniquely in WT cardiac myocytes post-TAC (1380 genes), upregulated ≥ 1.7-fold uniquely in Ffar4KO cardiac myocytes post-TAC (247 genes), or upregulated ≥ 1.7-fold in both (1409). (C) Differentially expressed genes identified in B were sorted based on gene ontology (GO) terms for biological function. Graphical representation of the number of genes up- or down-regulated in each category that were unique to the WT TAC (relative to sham, green), unique to Ffar4KO TAC (relative to sham, blue), and shared between WT and Ffar4KO (grey). (D, E) Data sets of gene expression from WT (D) and Ffar4KO (E) cardiac myocytes were analysed using Ingenuity Pathway Analysis Software (version 01-16). A custom pathway for known Ffar4 signalling pathways was generated, and expression data for WT and Ffar4KO were analysed (WT, green map, Ffar4KO blue map, as indicated). Genes upregulated (red) or down-regulated (green) for each group are indicated. (F) Induction of gene cardiac gene expression by TUG-891 (35 mg/kg/d, IP, for 3 days) in male WT mice. Each gene is indicated at the top of the graph. Data are mean ± SEM, n = 4 mice per group, data were analysed by Student’s t-test.
To gain insight into cellular functions altered in Ffar4KO cardiac myocytes post-TAC, we employed a custom Ffar4 signalling map designed with the Ingenuity Pathway Analysis Software (Figure 2D,E). We designated five gene categories: Ffar4 proximal signalling, inflammatory signalling,4 cPLA2α-oxylipin production,18 cPLA2α-IGF hypertrophy,19 and oxidation–reduction,20 and Table 1 indicates specific genes altered in each category. Most striking, WT myocytes increased genes associated with cytoplasmic phospholipase A2α signalling (cPLA2α, Pla2g4a, up 2.8-fold)) and oxylipin synthesis, including Cyclooxygenase 2 (Cox-2, Ptgs2, a known cardioprotective gene,21 up 7.2-fold) and arachidonate 5-lipoxygenase activating protein (Alox5ap), with decreased arachidonate 5-lipoxygenase (Alox5) (Figure 2D and Table 1). Conversely, in Ffar4KO myocytes, two cytochrome P450 isoforms were down-regulated (CYP4B1 and CYP2U) (Figure 2E and Table 1). Additionally, WT myocytes increased genes associated with the reduction of oxidative stress, most notably heme-oxygenase-1 (Hmox1, a known cardioprotective gene,22 up 24-fold) (Figure 2D and E and Table 1). Alternatively, to validate specific cardiac gene regulation by Ffar4, WT mice were dosed with the Ffar4 agonist TUG-891 (35 mg/kg/d),23 and cardiac gene expression was measured after 3 days. TUG-891 infusion specifically increased the expression of three genes from Table 1, hmox, ptgs2, and pi3kr5, as well as three of five genes selected from Supplementary material online, Tables S5–S13, il1rn, pak3, and timp1, in the hearts from WT mice (Figure 2F). The concordance between the two models (75%), TAC vs. Ffar4 agonist infusion, supports our original findings with RNAseq, and improves the rigor of the findings. In summary, the transcriptome analysis indicated specific transcriptional deficits in Ffar4KO myocytes related to the synthesis of biologically active oxygenated fatty acids (oxylipins) or cytoprotection through induction of haeme oxygenase-1 expression and reduction of oxidative stress.
Table 1.
Ffar4 signalling pathway gene expression in WT and Ffar4KO cardiac myocytes 3-day post-TAC
| Gene | Gene name | WT | Ffar4KO |
|---|---|---|---|
| Proximal signalling | Fold change | ||
| Arrb2 | βArestin2 | 2.48 | |
| Rgs3 | Regulator of G-protein signalling 3 | −1.77 | |
| Prkcb | Protein kinase Cb | 2.11 | |
| Prkcd | Protein kinase Cd | 2.08 | 2.79 |
| Prkcq | Protein kinase Cq | −3.79 | |
| Prkd1 | Protein kinase D1 | −1.97 | |
| Inflammatory signalling | |||
| Nfkb2 | NFkB2 | 1.87 | |
| Nfkbie | Ikb-e (NFkb inhibitor, epsilon) | 5.08 | |
| Nfkbiz | Ikb-z (NFkb inhibitor, zeta) | 3.25 | |
| Mapk10 | Jnk3 (mitogen activated protein kinase 10) | −1.83 | −1.81 |
| cPLA2a-oxylipin production | |||
| Pla2g4a | Cytoplasmic phospholipase A2a | 2.79 | |
| Alox5 | Arachidonate 5-lipoxygenase | −1.77 | |
| Alox5ap | Arachidonate 5-lipoxygenase activating protein | 4.29 | |
| Ptgs2 | Prostaglandin-endoperoxide synthase 2 (cyclooxygenase 2) | 7.15 | |
| Cyp2u1 | Cytochrome P450, family 2, subfamily u, polypeptide 1 | −1.86 | |
| Cyp4b1 | Cytochrome P450, family 4, subfamily b, polypeptide 1 | −1.80 | |
| cPLA2α/IGF hypertrophy | |||
| Akt2 | Akt2, PKBb | −1.85 | |
| Igf1 | Insulin-like growth factor 1 | 2.79 | |
| Pak1 | PAK1 (p21 (RAC1) activated kinase 1) | 6.59 | |
| Pi3kcd | p110d (phospatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit delta) | 2.34 | |
| Pi3kcg | p110g (phospatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit gamma) | 3.24 | |
| Pi3kr1 | p85a (phospatidylinositol-4,5-bisphosphate 3-kinase regulatory subunit alpha) | −7.46 | |
| Pi3kr5 | Phospatidylinositol-4,5-bisphosphate 3-kinase regulatory subunit 5 | 10.22 | |
| Oxidation-reduction | |||
| Hmox1 | Haeme oxygenase-1 | 24.04 | |
| Pla2g4a | Cytoplasmic phospholipase A2a | 2.79 | |
3.3. Ffar4 increases production of 18-HEPE in adult cardiac myocytes
To define the functional impact of Ffar4 in cardiac myocytes, we focused on Ffar4-cPLA2α signalling. Upon activation, cPLA2α can translocate to the nuclear membrane and cleave PUFAs from the sn2-acyl bond in membrane phospholipids, traditionally arachidonic acid (AA), but also EPA, DHA, or other PUFAs such as linoleic acid (LA).24 Cleaved FAs are subsequently metabolized by lipoxygenases (LOX), cyclooxygenases (COX), and CYPhydroxylases and CYPepoxygenases to produce biologically active oxylipins (Supplementary material online, Figure S2, outlines this mechanism).
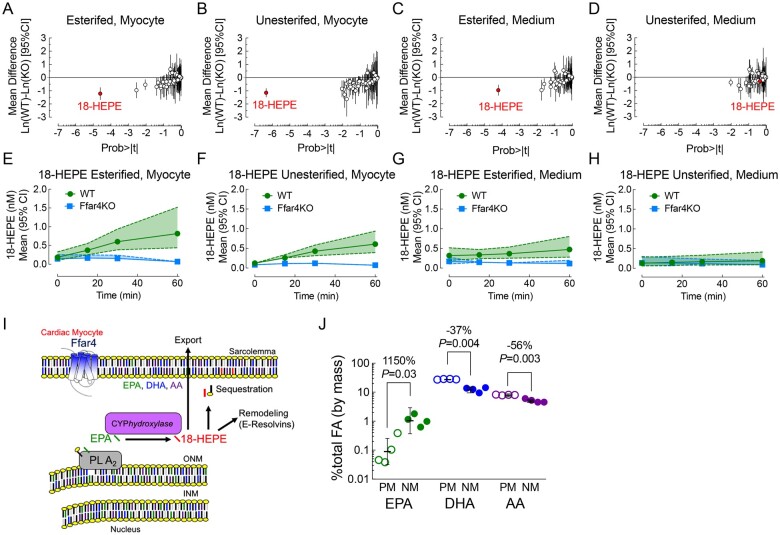
In adult cardiac myocytes treated with the Ffar4 agonist TUG-891, which was used to avoid confounding effects of exogenous FAs, we employed UPLC/MS/MS to measure oxylipin production as an indirect measurement of cPLA2α activity. In total, we were able to identify 57 different oxylipins produced following TUG-891 treatment (Figure 3A–H, Supplementary material online, Tables S14–S15). Surprisingly, we found that TUG-891 selectively increased the intracellular production of one oxylipin almost to the exclusion of all of the other 56 oxylipins. Ffar4 specifically increased the EPA-derived oxylipin 18-hydroxyeicosapentaenoic acid (18-HEPE) in WT but not Ffar4KO cardiac myocytes (esterified 18-HEPE, Figure 3A and unesterified 3B) as well as 18-HEPE exported from myocytes (esterified 18-HEPE, Figure 3C). Interestingly, EPA shows preferential enrichment in the nuclear membrane, the site of cPLA2α activity (Figure 3I), in adult cardiac myocytes (Figure 3J).
Figure 3.
(A–H) Cultured adult cardiac myocytes from WT (green) and Ffar4KO (blue) male mice were treated with the Ffar4 agonist, TUG-891 (50 µM) for 0, 15, 30, and 60 min. Oxylipins were detected by mass spectrometry from cardiac myocyte membranes (esterified) or cytosolic fractions [non-esterified, or from the culture medium in lipoproteins (esterified) or free (non-esterified)]. Probability plots for oxylipins detected from cardiac myocytes in the (A) esterified or (B) unesterified fractions after 60 min, or in the culture medium in the (C) esterified or (D) unesterifed fractions, or time course of 18-HEPE production in myocytes in the (E) esterified or (F) unesterifed fractions, or in the medium in the (G) esterified or (H) unesterified fractions (dashed lines represent the 95% CI). (I) Cytoplasmic phospholipase A2α (cPLA2α) mediated cleavage of EPA from membrane phospholipids and production of 18-HEPE by CYPhydroxylase. 18-HEPE is re-acylated into membrane phospholipids (sequestration), remains free in the cell and is further metabolized (potentially E-Resolvins), or exported, esterified in a lipoprotein. (J) EPA, DHA, and AA concentration in cardiac myocyte nuclear membrane (NM) or sarcolemma/plasma membrane (PM) fractions. Data are mean ± SD, n = 3 separate preparations, fold differences indicated.
3.4. Ffar4 prevents cell death from oxidative stress
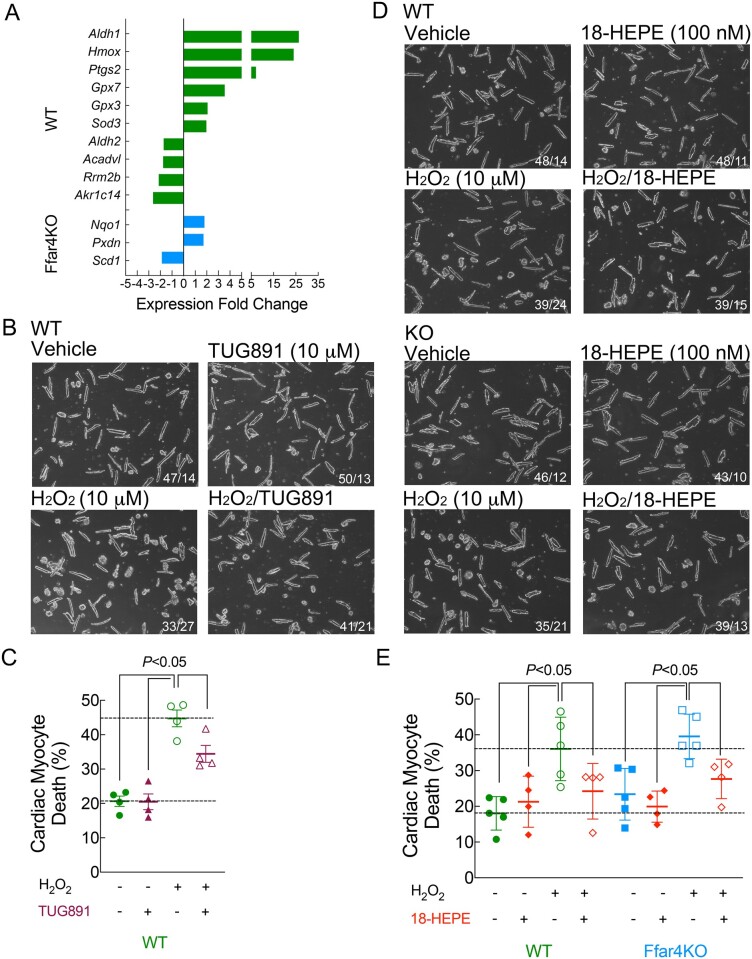
Results from the transcriptome analysis indicated that Ffar4KO cardiac myocytes failed to increase genes associated with cell death and oxidation–reduction in Ffar4KO myocytes (Figure 2C), suggesting a loss of cytoprotective signalling. Examination of the oxidation–reduction genes identified several cytoprotective genes uniquely up-regulated in WT myocytes (Figure 4A), most notably, haeme-oxygenase-1 (Hmox1). Therefore, the inability to produce 18-HEPE or up-regulate Hmox1 in Ffar4KO myocytes might increase their susceptibility to oxidative stress post-TAC. To investigate this more directly, we quantified the ability of TUG-891 and 18-HEPE to prevent cell death induced by oxidative stress (H2O2) in cultured myocytes. In WT but not Ffar4KO myocytes, TUG-891 pre-treatment overnight, but not for 2 h, attenuated cell death induced by H2O2 (Figure 4B and C). The requirement for a longer pre-treatment might indicate that time is needed to mount a cardioprotective effect, possibly through production/accumulation of 18-HEPE or up-regulation of cytoprotective genes such as Hmox1. Interestingly, 18-HEPE pre-treatment for 2 h attenuated cell death induced by H2O2 in both WT and Ffar4KO cardiac myocytes (Figure 4D and E). In summary, we suggest that Ffar4 reduction of oxidative stress provides one potential mechanistic explanation for the worse outcomes in Ffar4KO mice post-TAC.
Figure 4.
(A) Genes identified by the oxidation–reduction gene ontology sorting that could specifically affect the redox state of cardiac myocytes post-TAC. Data are n = 4–6, as in Figure 3, and fold change in expression for WT (green) and Ffar4KO (blue) are shown. (B) Cultured adult cardiac myocytes from WT mice were treated for 24 h with vehicle or 10 µM TUG-891, and for the final 2 h, myocytes were treated with vehicle or 10 µM H2O2 to induce oxidative damage and cell death. For all panels, images were captured using a ×10 objective, the entire field is shown, and cell death was indicated by round-shaped myocytes. The number of rod-shaped/round myocytes is in the inset. (C) Cell morphology was recorded, with at least 150 cells from 5 different fields measured per condition. Data are mean ± SEM, n = 4 separate cultures, data were analysed by two-way ANOVA, with Sidek post-test. (D) Cultured adult cardiac myocytes from WT (top) and Ffar4KO (bottom) mice were treated for 4 h with vehicle or 100 nM 18-HEPE and for the final 2 h, myocytes were treated with vehicle or 10 µM H2O2. The number of rod-shaped/round myocytes is in the inset. (E) Cell morphology was assessed as above. Data are mean ± SEM, n = 4 separate cultures, data were analysed by three-way ANOVA, with Sidek post-test. In this case, the primary interaction was not significant, but the post-test indicated a significant effect of both 18-HEPE and H2O2, indicating no difference between WT and Ffar4KO.
3.5. Loss of Ffar4 changes circulating HDL oxylipins consistent with autoxidation and prevents initiation of both mediators of inflammation and resolution following TAC
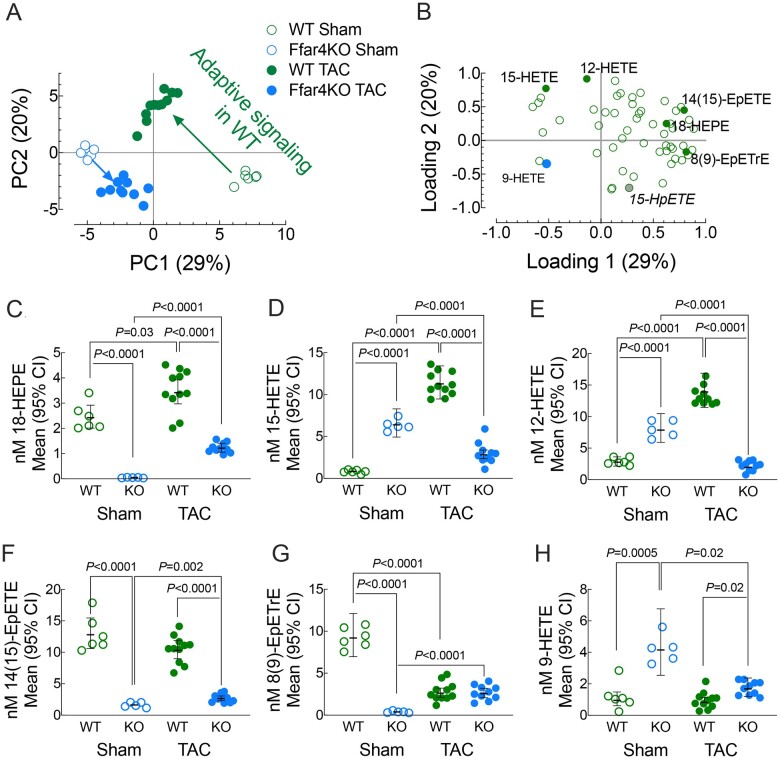
AA, EPA, DHA, and LA oxylipins are produced intracellularly but are exported and trafficked in plasma HDL. HDL are the largest reservoir of plasma oxylipins and the likely destination for 18-HEPE produced in and exported from cardiac myocytes (Figure 3C and G).25–27 Therefore, we quantified changes in HDL oxylipin content in Ffar4KO mice post-TAC. Across all groups, we identified 56 oxylipins in HDL and they were analysed by wide PCA to compare oxylipin content in HDL amongst groups (Figures 5A and B and Supplementary material online, Tables S16 and S17). In WT mice, TAC had a profound effect on HDL oxylipins, as evidenced by the TAC-dependent shift in location from the lower right quadrant upwards towards the centre relative to sham mice (Figure 5A, green circles). HDL from WT sham mice were enriched in e.g. 8(9)-epoxyeicosatrienoic acid (8(9)-EpETrE) and 15-hydroxyeicosatetraenoic acid (15-HpETE), but low levels of 12-hydroxyeiscosatetraenoic acid (12-HETE) and 15-HETE. Post-TAC, HDL oxylipin content in WT mice reflected an increase in pro-inflammatory (12-HETE and 15-HETE) and pro-resolving (18-HEPE) oxylipins. In contrast, TAC had a minimal adaptive effect on HDL–oxylipin content in Ffar4KO mice. Ffar4KO sham mice loaded oppositely of most oxylipins except 9-HETE, which is a unique marker of autoxidative oxylipin generation, and displayed a minimal post- TAC-dependent shift in oxylipin composition (Figure 5A). Changes in specific, influential oxylipins most explained by principal components 1–3; 18-HEPE, 15-HETE, 12-HETE, 14(15)-epoxyeicosatetraenoic acid (14(15)-EpETE), and 8(9)-EpETrE, as well as 9-HETE, were further examined (Figure 5C and H). Of note, 18-HEPE, which is a metabolite of CYPhydroxylase, was nearly undetected in Ffar4KO HDL (Figure 5C), suggesting that Ffar4 is required for basal production of 18-HEPE. However, both WT and Ffar4KO mice could increase 18-HEPE, presumably through a Ffar4-independent mechanism, but levels were still low in the Ffar4KO. Also of note, was the increase in pro-inflammatory oxylipins 15-HETE and 12-HETE post-TAC in WT HDL (Figure 5D and E). In summary, the adaptive response in WT mice post-TAC was characterized by increased pro-inflammatory LA and AA 15-LOX metabolites as well as increased pro-resolving EPA metabolite, 18-HEPE. We also confirmed that the production of 18-HEPE is impaired in Ffar4KO mice. Finally, the Ffar4KO mice, especially sham mice, were characterized by an autoxidative condition suggesting that activities regulated by Ffar4 prevent autoxidation.
Figure 5.
Plasma was collected 4 weeks following TAC or sham surgery, and oxylipins in HDL were detected by liquid chromatography/mass spectrometry. To discriminate changes in oxylipin content between WT and Ffar4KO in sham and TAC operated mice, wide principal component analysis was performed (A). Scores plot of group locations by PC Scores 1 (x-axis) and PC Scores 2 (y-axis). (B) PCA loadings with the identification and location of selected oxylipins. To exemplify changes, five oxylipins selected according to total variance explained in the first three-principle components were selected along with 9-HETE, an autoxidative marker. Important fold-differences (95%CIs) are summarized in each graph. (C) 18-HEPE, EPA metabolite of CYPhydroxylase. (D) 15-HETE, AA-metabolite of 12/15-LOX. (E) 12-HETE, AA-metabolite of 12/15-LOX. (F) 14(15)-EpETE, EPA metabolite of CYPepoxygenase. (G) 8(9)-EpETrE, AA metabolite of CYPepoxygenase and (H) 9-HETE, auto-oxidatively derived from AA.
3.6. Ffar4 is expressed in the human heart and down-regulated in HF
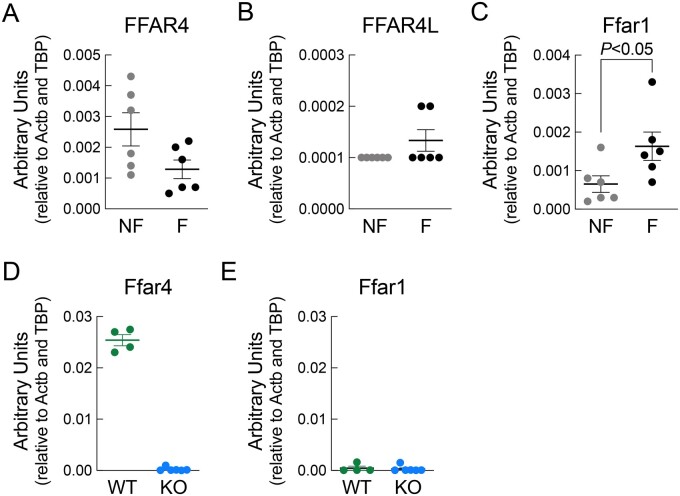
Similar to mice, nothing is known about Ffar4 in the human heart. Measuring Ffar4 levels in samples from both healthy donors (non-failing) and HF patients indicated that human hearts do express Ffar4 (Ffar4S + Ffar4L) and that Ffar4 expression is reduced in failing hearts (Figure 6A and B). Unexpectedly, the expression of Ffar1, the only other known GPR for medium- and long-chain fatty acids, was reciprocally increased in failing hearts relative to healthy controls (Figure 6C), which is not observed in mice (Figure 6D and E).10
Figure 6.
RT-PCR to detect the expression of human, (A) total Ffar4 (both short and long isoforms) (B) Ffar4L only, and (C) Ffar1 in cardiac tissue obtained from non-failing (NF) and failing (F) human hearts. RT-PCR to detect the expression of mouse (D) Ffar4 and (E) Ffar1 in wild-type (WT) and Ffar4KO (KO) mouse heart.
3.7. Genetic inhibition of FFAR4 is associated with eccentric remodelling in humans
To determine whether the propensity towards eccentric remodelling observed in Ffar4KO mice post-TAC was present in humans, we assessed for association between genetic inhibition of Ffar4 signalling and echocardiographic indices of LV mass and diastolic dimension in a cohort of 7140 genotyped subjects (median age 65.0 [53.0,74.9] years, 48% female) who underwent clinically indicated transthoracic echocardiographic examination. We found that that a genetic proxy for Ffar4 inhibition, the loss of function FFAR4 R270H variant (minor allele frequency 1.8%),28 was associated with an increase in maximum LV mass and maximum LV end-diastolic diameter (Table 2). In secondary analyses, R270H was also associated with increased maximum left atrial size (P = 0.004) and a non-significant trend towards lower minimum LV ejection fraction (Table 2). In sex stratified analyses, R270H was associated with a similar pattern of remodelling in males but not females, with an increase in LV mass, end-diastolic diameter, and maximal left atrial diameter observed only in males (Table 2). These findings identify an interesting parallel between humans with the signalling-deficient FFAR4 R270H polymorphism and the worse outcomes in Ffar4KO mice post-TAC, including differences by sex, and suggest that impaired Ffar4 signalling in humans may be associated with predilection towards eccentric left ventricular remodelling.
Table 2.
Genetic inhibition of FFAR4 (FFAR4 R270H) is associated with eccentric remodelling in humans
| Overall |
Males |
Females |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Phenotype | Beta [95% CI] | n | P value | Beta [95% CI] | n | P value | Beta [95% CI] | n | P value |
| LV mass max (g) | 10.9 [1.9, 19.9] | 6402 | 0.02 | 20.4 [5.2, 35.6] | 3182 | 0.009 | 3.0 [−7.2, 13.2] | 3220 | 0.56 |
| LVIDd max (mm) | 1.25 [1.2, 0.5] | 6969 | 0.01 | 2.8 [1.2, 4.5] | 3357 | <0.001 | −0.0004 [−1.2, 1.1] | 3612 | 0.99 |
| LA diam max (mm) | 1.48 [0.5, 2.5] | 6894 | 0.004 | 2.4 [0.74, 4.1] | 3337 | 0.005 | 0.80 [−2.0, 0.4] | 3557 | 0.20 |
| EF min (%) | −0.96 [−2.4, 0.5] | 6964 | 0.19 | −2.3 [−0.17, 4.8] | 3559 | 0.07 | 0.13 [−1.5, 1.7] | 3605 | 0.87 |
EF min, ejection fraction minimum; LA diam, left atrial diameter; LV mass max, left ventricular mass maximum; LVIDd, left ventricular internal dimension during diastole. Bold values indicate statistical significance.
4. Discussion
Here, we identify an entirely novel cardioprotective role for Ffar4 in the heart with significant translational potential. In cardiac myocytes, Ffar4 specifically induced production of the cardioprotective oxylipin 18-HEPE and attenuated oxidative stress, while the loss of these cardioprotective signals resulted in worse pathologic remodelling in Ffar4KO mice post-TAC (Figure 1). Furthermore, Ffar4-mediated cardioprotection occurred independent of any dietary intervention implying that Ffar4 senses and responds to endogenous fatty acids to protect the heart. Mechanistically, transcriptome analysis of cardiac myocytes from Ffar4KO hearts 3 days post-TAC identified significant deficits in the regulation of genes associated with cell death, inflammation, and oxidation-reduction, and specific deficits in genes associated with cytoplasmic phospholipase A2α (cPLA2α) signalling and reduction of oxidative stress (Figure 2). In cardiac myocytes, Ffar4 specifically induced the production of the EPA-derived, cardioprotective, and pro-resolving oxylipin 18-HEPE (Figure 3). Furthermore, Ffar4 signalling protected WT cardiac myocytes from oxidative stress-induced cell death, while 18-HEPE protected both WT and Ffar4KO cardiac myocytes (Figure 4). Systemically, the loss of Ffar4 was associated with lower overall oxylipin content in HDL, the presence of an autoxidative oxylipin (9-HETE), and the inability to increase pro-inflammatory oxylipins in lipoxygenase pathways and EPA-derived pro-resolving oxylipins (18-HEPE) following TAC (Figure 5). Together, these results suggest that Ffar4 cardioprotection depends upon induction of pro-resolving oxylipins that reduce oxidative stress in cardiac myocytes directly, and through indirectly effects in balancing pro-inflammatory and pro-resolving oxylipin composition systemically. In the human heart, Ffar4 expression was evident, and decreased in HF, while the signalling-deficient Ffar4 R270H polymorphism was correlated with eccentric remodelling in a large clinical cohort (Figure 6 and Table 2). In summary, our results indicate that Ffar4 is a cardioprotective nutrient sensor for endogenous fatty acids that reduces oxidative stress to protect the heart from pathologic stress.
Transcriptome analysis of cardiac myocytes performed 3 days post-TAC suggested a deficit in cPLA2α signalling in Ffar4 cardiac myocytes (Figure 2).24 There are six cPLA2 family members (α, β, γ, δ, ε, and ζ), which show only roughly 30% sequence homology, but have different enzymatic properties, tissue expression, and subcellular localizations.24 cPLA2α is widely expressed and has biologic functions in a multitude of cell types,24 and upon activation, it localizes to the nuclear membrane.29 Functionally, cPLA2α cleaves PUFAs from the sn2-acyl bond in membrane phospholipids. AA is the most common substrate, but DHA and EPA are also well-known substrates, leading to the production of downstream oxylipins.24 Initially, cleaved FAs (AA, EPA, DHA, LA) are metabolized by lipoxygenases (5-LOX, 12/15-LOX), cyclooxygenases (COX1/2), or CYPhydroxylases and CYPepoxygenases to generate oxygenated signalling mediators. These modified fatty acids, including leukotrienes and prostaglandins, are collectively known as oxylipins, many of which mediate pro- or anti-inflammatory responses, or initiate resolution of inflammation.30 Here, we found that in cardiac myocytes, the Ffar4 agonist TUG-891 induced the production of a specific EPA-derived oxylipin, 18-HEPE (Figure 3). Interestingly, we also found that EPA levels in red blood cells were low, 0.1–0.2% of total membrane FAs, whereas DHA was 4% and AA was 13% (Supplementary material online, Tables S2A and S2B), similar to our previous studies.10 Furthermore, we previously found that in cardiac myocytes, EPA was 0.5% of total membrane FAs, whereas DHA was 10% and AA was about 12%.10 Therefore, Ffar4 displays surprising and unprecedented degree of specificity for the production of 18-HEPE, largely to the exclusion of other EPA, DHA, AA, or LA-derived oxylipins despite the low levels of EPA in cardiac myocyte membranes.
While there are no previous studies regarding the direct effects of 18-HEPE in cardiac myocytes, macrophages from fat-1 mice, which have high endogenous levels of ω3-PUFAs, particularly EPA, due to overexpression of the Caenorhabditis elegans fat-1 gene, produced high levels of 18-HEPE that were associated with cardioprotection in fat-1 mice post-TAC.31 The high levels of EPA that Fat-1 transgenic mice produce can override canonical signalling mechanisms to produce 18-HEPE, whereas our study identifies Ffar4 signalling as an important contributor of 18-HEPE at physiological levels of EPA. Furthermore, direct injection of 18-HEPE attenuated remodelling post-TAC.31 Interestingly, 18-HEPE is also the precursor for E-series resolvins (RvE1, RvE2, and RvE3), a class of pro-resolving oxylipins.32 E-resolvins signal through ERV1/ChemR23, an orphan GPR that binds to and is activated by both E-resolvins and the endogenous peptide chemerin, suggesting a potential mechanism to explain EPA-mediated inflammation resolving effects.33 ERV1/ChemR23 is expressed in the heart, and preconditioning with RvE1 reduces ischemia/reperfusion injury,34 while RvE1 infusion 1-week following coronary artery ligation attenuates post-MI inflammatory response.35 Here, we show for the first time that the activation of Ffar4 prevents cell death from oxidative stress (Figure 4), and that 18-HEPE, the downstream product of Ffar4-cPLA2α signalling in cardiac myocytes, protected both WT and Ffar4KO cardiac myocytes (Figure 4). In summary, our studies demonstrate that Ffar4-cPLA2α-mediated production of 18-HEPE is cytoprotective, either through a direct effect or possibly effects via production of E-resolvins and activation of ERV1/ChemR23.
Both quantitative and qualitative deficits were observed in circulating HDL oxylipin profiles in Ffar4KO mice; first, Ffar4KO mice lacked the capacity to produce oxylipins in response to TAC consistent with impairment of cPLA2α and, second, they lacked the capacity to modify the types of oxylipins produced in response to TAC. Surprisingly, WT TAC mice did not suppress the activation of inflammatory oxylipin production. This is consistent with previous reports that 15-LOX activity36 and CYPhydroxylase-mediated production of unesterified HETEs37 remains high in HF, and may mediate cardiac myocyte hypertrophy by MAPK- and NF-κB-dependent mechanisms.38 We add further evidence by demonstrating successful adaptation post-TAC in WT mice required concurrent activation of pro-inflammatory, anti-inflammatory, and pro-resolving signalling pathways. In mice, the overexpression of 12/15-LOX is associated with increased levels of unesterified 12-HETE, 15-HETE, and HF.36 We found that despite this pro-inflammatory insult, WT mice were protected through their capacity to co-produce the pro-resolving 18-HEPE, and the anti-inflammatory 14(15)-EpETE, which was lacking in Ffar4KO mice. This suggests that the capacity to produce 12/15-LOX metabolites is beneficial, provided this can be moderated by simultaneously producing anti-inflammatory/pro-resolving oxylipins.
Interestingly, Ffar4-mediated cardioprotection was sex-specific, and loss of Ffar4 signalling in both mice (Ffar4KO) and humans (FFAR4 R270H) was associated with worse cardiac outcomes in males (Figure 1 and Table 2). To our knowledge, this is the first report of an Ffar4 sex-based phenotype. In humans, where sex-based differences in the prevalence of cardiovascular disease are well noted,39 the association between FFAR4 R270H and eccentric left ventricular remodelling in males is particularly noteworthy, but somewhat surprising given that prior studies reported no sex-based association between FFAR4 R270H and obesity.28 In humans, pathologic ventricular remodelling in women is generally more favourable, potentially due to estrogen.40 Mechanistic studies in mice indicate that oestrogen attenuates ventricular remodelling post-TAC,41 and requires oestrogen receptor-β and non-nuclear ERα signaling.42,43 However, oestrogen inhibits Ffar4 expression in the pituitary,44 which would not necessarily support a protective role of oestrogen in our experiments. Alternatively, we considered that 18-HEPE levels might be increased in HDL from female vs. male Ffar4KO mice and contribute to cardioprotection in females. While 18-HEPE levels were increased in HDL from female Ffar4KO relative to males in both sham and TAC mice, the absolute levels were still significantly less than observed in WT mice post-TAC and unlikely to explain the sex-based difference (Supplementary material online, Figure S3). In summary, although the mechanistic basis for the sex-based difference in Ffar4-dependent ventricular remodelling remains unclear, our data identify a clear cardioprotective function of Ffar4 in males.
In conclusion, our results indicate that Ffar4, a GPR for medium and long-chain fatty acids, is cardioprotective. In cardiac myocytes, Ffar4 activation of cPLA2α and production of the cardioprotective, EPA-derived oxylipin 18-HEPE as well as upregulation of heme-oxygenase-1 protected cardiac myocytes from oxidative stress. Systemically, Ffar4 was required to maintain pro-resolving oxylipins and suppress autoxidation basally, and to produce both pro-inflammatory and pro-resolving oxylipins following TAC in circulating HDL. We suggest that these results establish an entirely novel paradigm in the heart whereby Ffar4 functions as a nutrient sensor responding to endogenous fatty acids functioning as signalling molecules, not simply an energy source, to activate a GPR and protect the heart from pathologic stress. Interestingly, Ffar4 displayed remarkable and previously unsuspected specificity to produce EPA-derived oxylipins, even in basal conditions where EPA levels are generally much lower than DHA or AA levels. This implies that EPA is not only a ligand for Ffar4 but also a substrate for Ffar-cPLA2α-mediated oxylipin production, functioning in a feed-forward cardioprotective mechanism. Finally, Ffar4-mediated production of anti-inflammatory and pro-resolving oxylipins has broader mechanistic implications for attenuating systemic inflammation associated with ischemic HF, atherosclerosis, and metabolic syndrome.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Authors’ contributions
Conceptualization: K.A.M., B.A.H., B.C.J., G.C.S., and T.D.O. Methodology: K.A.M., B.A.H., C.L.H., S.S.J., W.H., C.D.W., Q.S.W., G.C.S., and T.D.O. Formal analysis: K.A.M., B.A.H., C.L.H., S.S.J., S.H., R.E.W., B.M.W., K.M.E., W.H., N.T., B.C.J., Q.S.W., G.C.S., and T.D.O. Investigation: K.A.M., B.A.H., C.L.H., S.S.J., B.M.W., K.M.E., W.H., C.D.W., G.C.S., and T.D.O. Writing-Original Draft: K.A.M., Q.S.W., G.C.S., and T.D.O. Writing-Review & Editing: K.A.M., B.A.H., C.L.H., R.C.B., N.T., B.C.J, Q.S.W., G.C.S., and T.D.O. Visualization: K.A.M., B.A.H., B.C.J, Q.S.W., G.C.S., and T.D.O. Supervision: G.C.S. and T.D.O. Funding Acquisition: G.C.S. and T.D.O.
Supplementary Material
Acknowledgements
The authors acknowledge the Genomics Core at the University of Minnesota for technical expertise on all data acquisition and assistance with analysis of transcriptome phenotypes, and the University Imaging Centers for support on echocardiography.
Funding
This work was supported by NIH HLR01130099 (T.D.O. and G.C.S.) and HLR01152215 (T.D.O. and G.C.S.), Minnesota Obesity Prevention Training Program T32 NIH Grant 1T32DK083250-01A1 (K.M.), and a grant from Amarin Corporation (T.D.O.).
Conflict of interest: none declared.
Data availability
The data underlying this article are available in the article and in its Supplementary material online.
References
- 1. Alvarez-Curto E, Milligan G.. Metabolism meets immunity: the role of free fatty acid receptors in the immune system. Biochem Pharmacol 2016;114:3–13. [DOI] [PubMed] [Google Scholar]
- 2. Ichimura A, Hasegawa S, Kasubuchi M, Kimura I.. Free fatty acid receptors as therapeutic targets for the treatment of diabetes. Front Pharmacol 2014;5:236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Christiansen E, Watterson KR, Stocker CJ, Sokol E, Jenkins L, Simon K, Grundmann M, Petersen RK, Wargent ET, Hudson BD, Kostenis E, Ejsing CS, Cawthorne MA, Milligan G, Ulven T.. Activity of dietary fatty acids on FFA1 and FFA4 and characterisation of pinolenic acid as a dual FFA1/FFA4 agonist with potential effect against metabolic diseases. Br J Nutr 2015;113:1677–1688. [DOI] [PubMed] [Google Scholar]
- 4. Oh DY, Talukdar S, Bae EJ, Imamura T, Morinaga H, Fan W, Li P, Lu WJ, Watkins SM, Olefsky JM.. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell 2010;142:687–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Suckow AT, Polidori D, Yan W, Chon S, Ma JY, Leonard J, Briscoe CP.. Alteration of the glucagon axis in GPR120 (FFAR4) knockout mice: a role for GPR120 in glucagon secretion. J Biol Chem 2014;289:15751–15763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Moran BM, Abdel-Wahab YH, Flatt PR, McKillop AM.. Evaluation of the insulin-releasing and glucose-lowering effects of GPR120 activation in pancreatic beta-cells. Diabetes Obes Metab 2014;16:1128–1139. [DOI] [PubMed] [Google Scholar]
- 7. Stone VM, Dhayal S, Brocklehurst KJ, Lenaghan C, Sorhede WM, Hammar M, Xu X, Smith DM, Morgan NG.. GPR120 (FFAR4) is preferentially expressed in pancreatic delta cells and regulates somatostatin secretion from murine islets of Langerhans. Diabetologia 2014;57:1182–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gotoh C, Hong YH, Iga T, Hishikawa D, Suzuki Y, Song SH, Choi KC, Adachi T, Hirasawa A, Tsujimoto G, Sasaki S, Roh SG.. The regulation of adipogenesis through GPR120. Biochem Biophys Res Commun 2007;354:591–597. [DOI] [PubMed] [Google Scholar]
- 9. Quesada-Lopez T, Cereijo R, Turatsinze JV, Planavila A, Cairo M, Gavalda-Navarro A, Peyrou M, Moure R, Iglesias R, Giralt M, Eizirik DL, Villarroya F.. The lipid sensor GPR120 promotes brown fat activation and FGF21 release from adipocytes. Nat Commun 2016;7:13479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Eclov JA, Qian Q, Redetzke R, Chen Q, Wu SC, Healy CL, Ortmeier SB, Harmon E, Shearer GC, O'Connell TD.. EPA, not DHA, prevents fibrosis in pressure overload induced heart failure; potential role of free fatty acid receptor 4. J Lipid Res 2015;56:2297–2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hirasawa A, Tsumaya K, Awaji T, Katsuma S, Adachi T, Yamada M, Sugimoto Y, Miyazaki S, Tsujimoto G.. Free fatty acids regulate gut incretin glucagon-like peptide-1 secretion through GPR120. Nat Med 2005;11:90–94. [DOI] [PubMed] [Google Scholar]
- 12. Tanaka T, Yano T, Adachi T, Koshimizu TA, Hirasawa A, Tsujimoto G.. Cloning and characterization of the rat free fatty acid receptor GPR120: in vivo effect of the natural ligand on GLP-1 secretion and proliferation of pancreatic beta cells. Naunyn Schmiedebergs Arch Pharmacol 2008;377:515–522. [DOI] [PubMed] [Google Scholar]
- 13. Hudson BD, Shimpukade B, Mackenzie AE, Butcher AJ, Pediani JD, Christiansen E, Heathcote H, Tobin AB, Ulven T, Milligan G.. The pharmacology of TUG-891, a potent and selective agonist of the free fatty acid receptor 4 (FFA4/GPR120), demonstrates both potential opportunity and possible challenges to therapeutic agonism. Mol Pharmacol 2013;84:710–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Watson SJ, Brown AJ, Holliday ND.. Differential signaling by splice variants of the human free fatty acid receptor GPR120. Mol Pharmacol 2012;81:631–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Siscovick DS, Barringer TA, Fretts AM, Wu JH, Lichtenstein AH, Costello RB, Kris-Etherton PM, Jacobson TA, Engler MB, Alger HM, Appel LJ, Mozaffarian D; American Heart Association Nutrition Committee of the Council on Lifestyle and Cardiometabolic Health; Council on Epidemiology and Prevention; Council on Cardiovascular Disease in the Young; Council on Cardiovascular and Stroke Nursing; and Council on Clinical Cardiology. Omega-3 polyunsaturated fatty acid (fish oil) supplementation and the prevention of clinical cardiovascular disease: a Science Advisory From the American Heart Association. Circulation 2017;135:e867–e884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chen J, Shearer GC, Chen Q, Healy CL, Beyer AJ, Nareddy VB, Gerdes AM, Harris WS, O'Connell TD, Wang D.. Omega-3 fatty acids prevent pressure overload-induced cardiac fibrosis through activation of cyclic GMP/protein kinase G signaling in cardiac fibroblasts. Circulation 2011;123:584–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yu P, Zhang B, Liu M, Yu Y, Zhao J, Zhang C, Li Y, Zhang L, Yang X, Jiang H, Zou Y, Ge J.. Transcriptome analysis of hypertrophic heart tissues from murine transverse aortic constriction and human aortic stenosis reveals key genes and transcription factors involved in cardiac remodeling induced by mechanical stress. Dis Markers 2019;2019:5058313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liu Y, Chen LY, Sokolowska M, Eberlein M, Alsaaty S, Martinez-Anton A, Logun C, Qi HY, Shelhamer JH.. The fish oil ingredient, docosahexaenoic acid, activates cytosolic phospholipase A(2) via GPR120 receptor to produce prostaglandin E(2) and plays an anti-inflammatory role in macrophages. Immunology 2014;143:81–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Haq S, Kilter H, Michael A, Tao J, O'Leary E, Sun XM, Walters B, Bhattacharya K, Chen X, Cui L, Andreucci M, Rosenzweig A, Guerrero JL, Patten R, Liao R, Molkentin J, Picard M, Bonventre JV, Force T.. Deletion of cytosolic phospholipase A2 promotes striated muscle growth. Nat Med 2003;9:944–951. [DOI] [PubMed] [Google Scholar]
- 20. Yang YC, Lii CK, Wei YL, Li CC, Lu CY, Liu KL, Chen HW.. Docosahexaenoic acid inhibition of inflammation is partially via cross-talk between Nrf2/heme oxygenase 1 and IKK/NF-kappaB pathways. J Nutr Biochem 2013;24:204–212. [DOI] [PubMed] [Google Scholar]
- 21. Mitchell JA, Kirkby NS.. Eicosanoids, prostacyclin and cyclooxygenase in the cardiovascular system. Br J Pharmacol 2019;176:1038–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Otterbein LE, Foresti R, Motterlini R.. Heme oxygenase-1 and carbon monoxide in the heart: the balancing act between danger signaling and pro-survival. Circ Res 2016;118:1940–1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schilperoort M, Dam AD, Hoeke G, Shabalina IG, Okolo A, Hanyaloglu AC, Dib LH, Mol IM, Caengprasath N, Chan Y‐W, Damak S, Miller AR, Coskun T, Shimpukade B, Ulven T, Kooijman S, Rensen PC, Christian M.. The GPR120 agonist TUG-891 promotes metabolic health by stimulating mitochondrial respiration in brown fat. EMBO Mol Med 2018;10:e8047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Leslie CC. Cytosolic phospholipase A(2): physiological function and role in disease. J Lipid Res 2015;56:1386–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Newman JW, Pedersen TL, Brandenburg VR, Harris WS, Shearer GC.. Effect of omega-3 fatty acid ethyl esters on the oxylipin composition of lipoproteins in hypertriglyceridemic, statin-treated subjects. PLoS One 2014;9:e111471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shearer GC, Newman JW.. Lipoprotein lipase releases esterified oxylipins from very low-density lipoproteins. Prostaglandins Leukot Essent Fatty Acids 2008;79:215–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rodriguez A, Trigatti BL, Mineo C, Knaack D, Wilkins JT, Sahoo D, Asztalos BF, Mora S, Cuchel M, Pownall HJ, Rosales C, Bernatchez P, Ribeiro Martins da Silva A, Getz GS, Barber JL, Shearer GC, Zivkovic AM, Tietge UJF, Sacks FM, Connelly MA, Oda MN, Davidson WS, Sorci-Thomas MG, Vaisar T, Ruotolo G, Vickers KC, Martel C.. Proceedings of the Ninth HDL (High-Density Lipoprotein) Workshop: focus on cardiovascular disease. Arterioscler Thromb Vasc Biol 2019;39:2457–2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ichimura A, Hirasawa A, Poulain-Godefroy O, Bonnefond A, Hara T, Yengo L, Kimura I, Leloire A, Liu N, Iida K, Choquet H, Besnard P, Lecoeur C, Vivequin S, Ayukawa K, Takeuchi M, Ozawa K, Tauber M, Maffeis C, Morandi A, Buzzetti R, Elliott P, Pouta A, Jarvelin MR, Korner A, Kiess W, Pigeyre M, Caiazzo R, Van Hul W, Van Gaal L, Horber F, Balkau B, Levy-Marchal C, Rouskas K, Kouvatsi A, Hebebrand J, Hinney A, Scherag A, Pattou F, Meyre D, Koshimizu TA, Wolowczuk I, Tsujimoto G, Froguel P.. Dysfunction of lipid sensor GPR120 leads to obesity in both mouse and human. Nature 2012;483:350–354. [DOI] [PubMed] [Google Scholar]
- 29. Peters-Golden M, Song K, Marshall T, Brock T.. Translocation of cytosolic phospholipase A2 to the nuclear envelope elicits topographically localized phospholipid hydrolysis. Biochem J 1996;318:797–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shearer GC, Walker RE.. An overview of the biologic effects of omega-6 oxylipins in humans. Prostaglandins Leukot Essent Fatty Acids 2018;137:26–38. [DOI] [PubMed] [Google Scholar]
- 31. Endo J, Sano M, Isobe Y, Fukuda K, Kang JX, Arai H, Arita M.. 18-HEPE, an n-3 fatty acid metabolite released by macrophages, prevents pressure overload-induced maladaptive cardiac remodeling. J Exp Med 2014;211:1673–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dalli J, Serhan CN.. Identification and structure elucidation of the pro-resolving mediators provides novel leads for resolution pharmacology. Br J Pharmacol 2019;176:1024–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Arita M, Bianchini F, Aliberti J, Sher A, Chiang N, Hong S, Yang R, Petasis NA, Serhan CN.. Stereochemical assignment, antiinflammatory properties, and receptor for the omega-3 lipid mediator resolvin E1. J Exp Med 2005;201:713–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Keyes KT, Ye Y, Lin Y, Zhang C, Perez-Polo JR, Gjorstrup P, Birnbaum Y.. Resolvin E1 protects the rat heart against reperfusion injury. Am J Physiol Heart Circ Physiol 2010;299:H153–H164. [DOI] [PubMed] [Google Scholar]
- 35. Liu G, Liu Q, Shen Y, Kong D, Gong Y, Tao B, Chen G, Guo S, Li J, Zuo S, Yu Y, Yin H, Zhang L, Zhou B, Funk CD, Zhang J, Yu Y.. Early treatment with Resolvin E1 facilitates myocardial recovery from ischaemia in mice. Br J Pharmacol 2018;175:1205–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kayama Y, Minamino T, Toko H, Sakamoto M, Shimizu I, Takahashi H, Okada S, Tateno K, Moriya J, Yokoyama M, Nojima A, Yoshimura M, Egashira K, Aburatani H, Komuro I.. Cardiac 12/15 lipoxygenase-induced inflammation is involved in heart failure. J Exp Med 2009;206:1565–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Maayah ZH, Althurwi HN, El-Sherbeni AA, Abdelhamid G, Siraki AG, El-Kadi AO.. The role of cytochrome P450 1B1 and its associated mid-chain hydroxyeicosatetraenoic acid metabolites in the development of cardiac hypertrophy induced by isoproterenol. Mol Cell Biochem 2017;429:151–165. [DOI] [PubMed] [Google Scholar]
- 38. Maayah ZH, El-Kadi AO.. 5-, 12- and 15-Hydroxyeicosatetraenoic acids induce cellular hypertrophy in the human ventricular cardiomyocyte, RL-14 cell line, through MAPK- and NF-kappaB-dependent mechanism. Arch Toxicol 2016;90:359–373. [DOI] [PubMed] [Google Scholar]
- 39. Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, Chiuve SE, Cushman M, Delling FN, Deo R, de Ferranti SD, Ferguson JF, Fornage M, Gillespie C, Isasi CR, Jiménez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Lutsey PL, Mackey JS, Matchar DB, Matsushita K, Mussolino ME, Nasir K, O’Flaherty M, Palaniappan LP, Pandey A, Pandey DK, Reeves MJ, Ritchey MD, Rodriguez CJ, Roth GA, Rosamond WD, Sampson UKA, Satou GM, Shah SH, Spartano NL, Tirschwell DL, Tsao CW, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P.. American Heart Association Council on E, Prevention Statistics C, Stroke Statistics S. Heart Disease and Stroke Statistics-2018 Update: a Report From the American Heart Association. Circulation 2018;137:e67–e492. [DOI] [PubMed] [Google Scholar]
- 40. Piro M, Della Bona R, Abbate A, Biasucci LM, Crea F.. Sex-related differences in myocardial remodeling. J Am Coll Cardiol 2010;55:1057–1065. [DOI] [PubMed] [Google Scholar]
- 41. Donaldson C, Eder S, Baker C, Aronovitz MJ, Weiss AD, Hall-Porter M, Wang F, Ackerman A, Karas RH, Molkentin JD, Patten RD.. Estrogen attenuates left ventricular and cardiomyocyte hypertrophy by an estrogen receptor-dependent pathway that increases calcineurin degradation. Circ Res 2009;104:265–275, 11p following 275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Babiker FA, Lips D, Meyer R, Delvaux E, Zandberg P, Janssen B, van Eys G, Grohe C, Doevendans PA.. Estrogen receptor beta protects the murine heart against left ventricular hypertrophy. Arterioscler Thromb Vasc Biol 2006;26:1524–1530. [DOI] [PubMed] [Google Scholar]
- 43. Fukuma N, Takimoto E, Ueda K, Liu P, Tajima M, Otsu Y, Kariya T, Harada M, Toko H, Koga K, Blanton RM Jr, Karas RH, Komuro I.. Estrogen receptor-alpha non-nuclear signaling confers cardioprotection and is essential to cGMP-PDE5 inhibition efficacy. JACC Basic Transl Sci 2020;5:282–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Moriyama R, Ueda K, Deura C.. Effects of ovarian hormones on GPR120 mRNA expression in mouse pituitary gonadotrophs. Endocr J 2017;64:1055–1061. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in the article and in its Supplementary material online.