Abstract
COVID-19 is a severe acute respiratory syndrome. Recent reports showed that autoimmune thyroiditis might occur following COVID-19 infection. We aimed to review the literature to assess the prevalence, clinical features and outcome of autoimmune thyroid disorders triggered by COVID-19.
We reviewed case reports, case series, and observational studies of autoimmune thyroiditis including Graves’ disease, Hashimoto thyroiditis, and silent thyroiditis developed in COVID-19 patients by searching PubMed, SCOPUS and Web of Science and included in the systematic review.
Our search yielded no prevalence study. We noted 20 reported cases: Fourteen cases of Graves' disease, 5 cases of hypothyroidism due to Hashimoto's thyroiditis and one case of postpartum thyroiditis. The majority (16/20, 80%) were middle-aged (mean age: 40 years) female patients. Autoimmune thyroiditis was diagnosed either concomitantly or 7–90 days after the COVID-19 infection. Eight out of 14 cases with Graves' disease had a known thyroid disorder and they were stable in remission. One out of 5 cases with Hashimoto's thyroiditis had known prior hypothyroidism. The majority of the patients achieved remission within 3 months. One patient with thyroid storm due to Graves' disease and one patient with myxedema coma have died.
Current data suggest that COVID-19 may cause autoimmune thyroid disease or exacerbate the underlying thyroid disease in remission. It is reasonable to routinely assess the thyroid functions both in the acute phase and during the convalescence so as not to overlook a thyroid disorder and not to delay treatment especially in patients with preexisting autoimmune thyroid diseases.
Keywords: Thyroid, COVID-19, Graves' disease, Hashimoto's thyroiditis
1. Introduction
Coronavirus Disease 2019 (COVID-19) is a severe acute respiratory syndrome caused by SARS-CoV-2. After the first case reported in Wuhan, China, the number of cases increased rapidly and spread to all over the world [1]. The most common clinical manifestations of the disease are similar to those of other viral infections and it may cause impaired functions of several organs [2]. After SARS-CoV-2 enters the respiratory system, it binds to the angiotensin converting enzyme 2 (ACE2) receptors, which are present in many human cells including those in pancreas, thyroid, testis, ovary, adrenal glands and pituitary [3]. Thyroid gland can be affected from the SARS CoV-2 either directly (through the viral infection of the target cells) or indirectly (through the abnormal immune regulation) [4]. Destructive or inflammatory thyroiditis is a common form of thyroiditis which may be associated with increased pro-inflammatory cytokines, so-called “cytokine storm”. A retrospective study showed the relationship between thyrotoxicosis due to the systemic immune activation and increased IL-6 levels in 287 non-ICU COVID-19 patients during the hospitalization [5]. Subacute thyroiditis is strongly associated form of thyroiditis with viral infections [6]. Since COVID-19 outbreak, various case reports have been reported that SARS-CoV-2 is thought as a possible trigger [7,8]. Furthermore, recent reports showed that Graves' disease and Hashimoto's thyroiditis might occur following COVID-19 infection. Thus, we aimed to summarize current literature and perform a systematic review to assess the prevalence, clinical features and outcome of autoimmune thyroid disorders triggered by COVID-19.
2. Material and methods
The protocol for this review was published in PROSPERO (International Prospective Register of Systematic Reviews) under registration CRD42021269312.
2.1. Search strategies and study selection
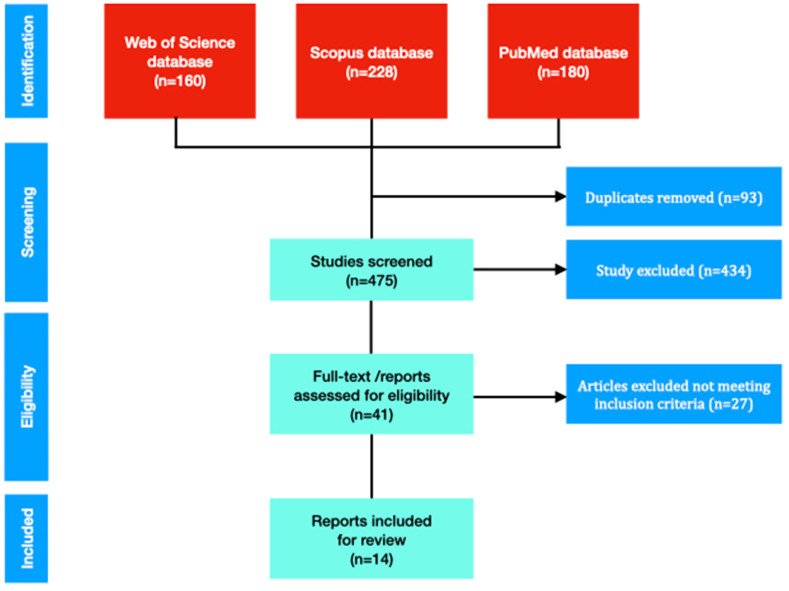
Literature search was performed in PubMed, PUBMED, Web of Science, and Scopus according to the PRISMA guidelines [9]. Papers published in English language between December 1, 2019, to October 28, 2021, were included. The literature was searched using keywords of (COVID-19 AND (autoimmune thyroiditis OR Graves’ disease OR Hashimoto thyroiditis)). The EndNote database was used from importing and managing abstracts and full texts. After first evaluation of the paper, duplicates were removed. Full text papers were evaluated and selected by two independent authors (Fig. 1 ). All the authors approved this selection process.
Fig. 1.
Flowchart of systematic review on COVID-19 and autoimmune thyroiditis.
2.2. Inclusion criteria
Case reports, case series, and observational studies describing the incidence, clinical features, and outcomes of autoimmune thyroiditis including Graves’ disease, Hashimoto thyroiditis, and silent thyroiditis developed after COVID-19 patients were included in the systematic review. Only adult cases (>18 years) were included. Case reports without clinical and laboratory features were excluded. Conference abstracts, reviews, and editorials were excluded from this review.
2.3. Case definition
COVID-19: Confirmed COVID-19 cases by COVID-19 PCR were included. Probable and possible COVID-19 cases were not included [10].
Autoimmune thyroiditis: Graves' disease was diagnosed with the combination of elevated free T4 and suppressed TSH levels in the presence of TSH receptor antibodies (TSH-R Ab) or thyroid-stimulating immunoglobulins (TSI) in addition to clinical symptoms and signs such as palpitations, nervousness, and weight loss. Thyroid ultrasound and radionuclide thyroid scan were also used for differential diagnosis in some patients. Hypothyroidism due to Hashimoto's thyroiditis was diagnosed with low serum free T4 and an elevated serum TSH levels in the presence of thioperoxides (TPO) autoantibodies and thyroglobulin (TG) autoantibodies. Thyroid ultrasonography was also used for making diagnosis. Postpartum thyroiditis is a variant of Hashimoto's thyroiditis generally begins 3 or 4 months after delivery. Diagnosis is made with increased T3, T4, and anti-TPO and decreased TSH and low radioiodine uptake with a normal erythrocyte sedimentation rate.
2.4. Data extraction
A standardized data extraction form was used. Demographic features, risk factors, co-morbidities, previous thyroid disease, clinical features, diagnostic methods, diagnosis, treatment, hospitalization, and outcome were analyzed by two authors.
2.5. Statistical analysis
Data were analyzed using SPSS software, version 20.0 (IBM Corp, Armonk, NY). Descriptive data were presented as mean ± standard deviation or percent. Continuous and categorical variables were compared by Student's t-test and chi-square respectively. p < 0.05 was taken statistically significant.
3. Results
Our literature search of databases (PUBMED, Web of Science, and Scopus) yielded 568 reports. Unrelated publications were omitted and remaining 14 publications were evaluated. (Fig. 1). All case reports have been reported after 2020 following COVID-19 pandemic.
No studies were found describing the prevalence. The papers reported 20 cases (Table 1 ) [[11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23]]. The majority (16/20, 80%) were female and mean age was 40 years. Fourteen cases of Graves' disease (autoimmune hyperthyroidism) [[11], [12], [13], [14], [15], [16], [17], [18], [19]], 5 cases of hypothyroidism due to Hashimoto's thyroiditis [11,18,[21], [22], [23]] and one case postpartum thyroiditis [24] have been described from several countries (Brazil, China, Egypt, Italy, Japan, Malta, USA, Iran, and Spain). All of the cases except four were diagnosed in females. Age range was 21–69 years. Autoimmune thyroiditis was diagnosed either concomitantly or 7–90 days after the COVID-19 infection.
Table 1.
Studies and clinical characteristics, treatment and outcomes in patients with COVID-19-associated autoimmune thyroid disorders.
| Author(s) | References | Country | Age (years) | Gender | Previous thyroid disease | Pulmonary involvement of COVID-19 | Hospitalization for COVID 19 | Diagnosis | Imaging study | Time from COVID-19 | TSH level (mIU/L) | TRab (N:<0.9 U/L) | Anti-TPO (N < 60 IU/mL) | Anti-TG (N < 60 IU/mL) | Associated disease | Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Allam et al. | [10] | Egypt | 33 | F | Graves' disease, 9 months ago | No | No | Graves' disease with Graves' ophtalmopathy | US showed enlarged gland with a relative diffuse reduction in vascularity and heterogeneous parenchyma | Concomitantly | 0.01 | 11.2 (<1.8) | Graves' ophthalmopathy | Carbimazole, selenium, prednisolone 20 mg/day | Euthyroidism was achieved 3 months later | ||
| Harris et al. | [11] | USA | 21 | F | No | No | No | Graves' disease | NA | 16 days | <0.01 | 17 | Methimazole | Clinical euthyroidism was achieved 3 months later | |||
| Jimenez – Blanco et al. | [12] | Spain | 45 | F | GD and Graves' orbitopathy of 25 years | Yes | NA | Graves' disease | US showed hypervascularization | Concomitantly | <0.005 | 28 | Methimazole | Euthyroidism was achived 3 months later | |||
| 61 | F | GD diagnosed in 2004 | NA | NA | Graves' disease relapse | Radionuclide thyroid scanning showed increased uniform tracer uptake and thyroid US showed hypervascularization | 1 month later | <0.01 | 1.31 | Methimazole | Euthyroidism was achived 3 months later | ||||||
| Lanzolla et al. | [13] | Italy | 33 | F | No | NA | NA | Graves' disease with mild Graves' orbitopathy | US revealed a diffuse hypoechoic pattern of the thyroid | 2 months | ↓ | + | A mild, inactive GO was diagnosed | Methimazole | Euthyroidism achieved | ||
| Mateu et al. | [14] | Spain | 60 | F | GD was diagnosed at age 23 and normal thyroid function since age 25 | Yes | NA | Graves' disease | Radio iodine uptake was increased to 30% and 45.7% at 2 and 24 h after administration of 100 μCi of iodine | 1 month | <0.01 | 2.13 | 1343 | 199 | Thiamazole and propranolol | Symptoms and thyroid functions improved after treatment | |
| 53 | F | No | Yes | No | Graves' disease | Iodine-uptake was increased to 61 and 62% at 2 and 24 h respectively | 2 months | <0.01 | 6.07 | 3239 | 1617 | Thiamazole and propranolol | Symptoms and thyroid functions improved after treatment | ||||
| Pastor et al. | [15] | Spain | 45 | F | Graves disease in stable remission | No | No | Thyrotoxic crisis | NA | Concomitantly | <0.01 | NA | Thiamazole, atenolol and hydrocortisone | Good response to treatment | |||
| Montebello et al. | [16] | Malta | 22 | F | Graves' disease in stable remission since 2018 | No | No | Graves' disease | US showed normal sized thyroid gland with heterogeneous texture | 2 months | 0.009 | 6.9 | <10 IU/mL (0–50) | Carbimazole 40 mg | A significant improvement was achieved after 1 month of treatment | ||
| Feghali et al. | [17] | USA | 33 | F | No | NA | No | Graves' disease | US revealed mild thyromegaly with heterogeneous and hyperrvascular appearance | 7 weeks | <0.01 | TSI: 309 (n<140%) TRab: <1 |
14 IU/mL (<1) | Methimazole and propranolol | Symptoms relieved two weeks later | ||
| Milani et al. | [18] | Iran | 39 | M | Graves' disease for three years | Yes | Yes | Thyroid storm due to Graves' disease | NA | 2 weeks | <0.05 | Eye examination indicated proptosis | Methimazole, hydrocortisone, popranolol, iodine | Symptoms improved after three days and discharged after 8 days | |||
| 50 | M | A history of hyperthyroidism for ten years | No | Yes | Thyroid storm due to Graves' disease | NA | Concomitantly | <0.01 | ARDS on the seventh day of hospitalization | Methimazole, hydrocortisone, popranolol, iodine | The patient died on the 8th day | ||||||
| Edwards et al. | [19] | USA | 27 | M | No | No | No | Thyroid storm due to Graves' Disease | US showed a diffusely enlarged, heterogenous, hypervascular gland | Concomitantly | <0.01 | TRAb 9.4 TSI:8.43 IU/L (≤0.54) | The patients was admitted to ICU and hospitalized for 8 days | Methimazole, potassium iodide, esmolol, propranolol, cholestyramine | Partial biochemical improvement was achieved after t2 weeks and methimazole dose was increased | ||
| 21 | F | No | No | No | Impending storm | NA | Concomitantly | <0.01 | TSI: 7.77 (≤0.54IU/L) | The patient was admitted to hospital for impending thyroid storm | Methimazole, hydrocortisone, propranolol | Partial biochemical improvement was achieved after 2 weeks and methimazole dose was increased | |||||
| Allam et al. | [10] | Egypt | 42 | F | Hashimoto's thyroiditis for 10 years | No | No | Hypothyroidisim due to Hashimoto's thyroiditis | USG showed a homogenous thyroid gland with streaks and relative hypervascularity | 81 days | 25.46 | <35 | Levothyroxin | Euthyroidism was achived 2 months later | |||
| Feghali et al. | [17] | USA | 38 | F | No | NA | No | Hypothyroidisim due to Hashimoto's thyroiditis | US revealed thyromegaly with a heterogenous and hypoechoic sonographic appearance | 6 weeks | 136 | <1 IU/L | >900 | >1000 | Levothyroxin | Euthyroidism was achived 1 month later | |
| Dixit et al. | [20] | USA | 69 | F | Not documented before admission | Yes | Yes | Myxedema coma with concomitant COVID-19 | NA | Concomitantly | 61.3 | 33.4 | Myxedema coma and sudden cardiac arrest | Levothyroxin | Died | ||
| Tee et al. | [21] | China | 45 | M | No | No | No | Hypothyroidisim due to Hashimoto's thyroiditis | NA | 7 days | 6.49 | >2000IU/mL | levothyroxine | Symptom relief two weeks later | |||
| Knack et al. | [22] | Brazil | 33 | F | No | No | no | Hypothyroidisim due to Hashimoto's thyroiditis | US revealed diffusely hypoechoic and heterogeneous thyroid glands | 20 days | 8 | 115 | 252 | Levothyroxine | Euthyroidism was achived 4 months later | ||
| Mizuno et al. | [23] | Japan | 29 | F | Hashimoto's thyroiditis for 5 years | NA | Yes | Postpartum thyroiditis | US revealed coarse echotexture | 36 days | 0.02 | <0.9 | <3 | 12.2 | None | Euthyroidism was achived 69 days later |
TSH: thyroid stimulating hormone, TRab: anti-TSH receptor antibody, anti-TPO: anti-thyroperoxidase autoantibody, anti-TG: anti-thyroglobulin antibody, F: female, M: male, GD: Graves' disease, GO: Graves' ophthalmopathy, NA: not available, US: ultrasound, TSI: Thyroid stimulating immunoglobulin, ARDS: acute respiratory distress syndrome, ICU: intensive care unit.
Eight cases with Graves' disease had a known thyroid disorder (Graves' disease) and one had Graves' ophthalmopathy required glucocorticoid therapy for 3 months. All cases with known Graves' disease were stable in remission without any medical treatment except two patients. Five cases were diagnosed thyrotoxic crisis and two needed intensive care unit until symptoms become stable [16,19,20]. One of them with thyrotoxic crisis developed respiratory distress and was connected to mechanical ventilator. He died on the eighth day of hospital admission [19]. Mild Graves' ophthalmopathy was detected in four cases, which did not require an additional therapy [11,13,14,19]. Laboratory investigation revealed suppressed serum TSH and increased fT4 and fT3. Also, anti-TSH receptor antibody (anti-TSHR-Ab) was detected positive. Thyroid ultrasound showed heterogeneous echotexture and increased vascularity. Along with increased radiotracer uptake, all patients were assessed as Graves’ disease (an autoimmune thyrotoxicosis) and treated with methimazole. After reaching euthyroid state, daily maintenance dose was continued.
Among patients with Hashimoto's thyroiditis, only one of the cases had known prior hypothyroidism [21]. A 69-year-old woman with small cell lung cancer has been reported to develop myxedema coma precipitated by COVID-19. She was presented with hypothermia, hypotension, and hypoventilation with a Glasgow Coma score of 5. A diagnosis of myxedema coma was made with increased TSH (61.3 mIU/L) and decreased fT4 (0.2 ng/dL) levels. The patient died on the third day of hospital admission. Decompensated hypothyroidism precipitated COVID-19 in this patient was attributed to preexisting immunotherapy induced autoimmune thyroiditis. Two patients with mild hypothyroidism (TSH <10 mIU/L) were reported while three had profound hypothyroidism [11,18,21]. In addition, anti-TPO antibody and anti-TG antibody levels were detected positive. Thyroid ultrasound showed heterogeneous and hypoechoic glands consistent with thyroiditis. All patients were treated with levothyroxine.
Only one case report of postpartum thyroiditis has been reported after COVID-19 [24]. She had a history of painless thyroiditis under the background of Hashimoto's thyroiditis five years ago with a positive anti-TG antibody. Her thyroid functions returned to normal without any specific treatment.
4. Discussion
Our literature search found 20 patients with COVID-19-associated autoimmune thyroid diseases. The vast majority were middle-aged female patients. All forms of thyroid disorders showed a mild course and responded to medical treatment. COVID-19 is thought as a causative factor for Graves' disease and Hashimoto's thyroiditis either as a new onset or as a flare-up the disease in remission in the reported cases.
Graves' disease is an autoimmune thyroid disorder that is triggered by different environmental factors such as viruses in susceptible subjects. Molecular mimicry is thought the one of the possible mechanisms. The breakdown of immune tolerance against the TSH-R, TPO and TG starts the disease. Various infectious agents have been investigated for their possible role in the pathogenesis of Graves' disease. Foamy viruses, Parvovirus B19, Epstein-Barr virus and hepatitis C virus are well known etiological agents [25]. Similarly, various viruses including hepatitis C, parvovirus B19 is thought to play a role in the pathogenesis of Hashimoto's thyroiditis although not fully elucidated as in Graves' disease [26]. Thus COVID-19 may act as another causative role in the pathogenesis of autoimmune thyroiditis as in these cases. However although case studies raise this concern of COVID-19 and thyroiditis association, they are not the proofs of a causal relationship.
Many autoimmune diseases other than autoimmune thyroid disease have been described associated with COVID 19 since the pandemic outbreak in December 2019 such as autoimmune hemolytic anemia, Guillain–Barre' syndrome and systemic lupus erythematosus. As in autoimmune diseases, increased production of pro-inflammatory cytokines in COVID-19 can cause organ damage in some patients [26]. Antibodies against SARS-CoV-2 were shown to react with several human tissues including the thyroid [27]. They also showed a similarity and homology between spike, nucleoprotein, and many other SARS-CoV-2 proteins with the human tissue antigens mitochondria M2, F-actin and TPO. This immune cross reactivity may precipitate the onset of a new autoimmune disease and also exacerbate autoimmunity in susceptible subjects as in the patients in this paper [27].
Altered thyroid functions commonly known as “non thyroidal illness syndrome” (or low T3 syndrome, or euthyroid sick syndrome) can be seen during the severe acute or chronic illness (trauma, sepsis, malnutrition, hepatic diseases, major systemic illness). Among COVID-19 patients, free T3, TSH and FT3/FT4 were significantly lower in severe or critically ill patients than in non-critical ones [28]. Another study evaluating thyroid status in 287 patients in non-intensive care unit demonstrated that COVID 19 presented with overt (10.8%) and subclinical (14.6%) thyrotoxicosis. Serum IL-6 levels were detected significantly correlated with TSH values due to systemic immune activation induced by SARS-Cov-2 infection [5]. Both negative TRab, anti-TG and anti-TPO levels in nine patients and improvement spontaneously during follow up, it may hypothesize that possible mechanism was destructive thyroiditis in these patients. Among the reviewed cases, pulmonary involvement, as an indicator of severity of the disease, was reported in 5. On the other hand, associated thyroid dysfunctions were severe in some patients and two patients died: one patient with Graves’ disease and thyroid storm due to adult respiratory distress syndrome and another patient with hypothyroidism due to myxedema coma and sudden cardiac arrest.
Graves' disease and Hashimoto's thyroiditis may occur a few months after subacute thyroiditis, which is thought a kind of viral originated destructive thyroiditis [29]. Long term follow up of these COVID-19 related thyrotoxicosis patients may be valuable to evaluate further development of an autoimmune thyroid disease. In the current cases, most of the cases occurred after a time interval that is after possible cytokine increase. We do not know whether a destructive thyroiditis occurred in the acute phase of the infection in these patients.
Autoimmunity was evaluated in the sera of 120 hospitalized patients with COVID-19 by using a panel of thyroid, rheumatic and antiphospholipid antibodies and compared with prepandemic healthy controls. TPO, β2-glycopotein 1, cardiolipin antibodies, rheumatoid factor, anti-nuclear antibody and cyclic citrullinated peptide 3 antibodies were more frequently detected in COVID-19 patients than those in prepandemic controls. High titers of anti-cardiolipin antibodies were associated with a worse outcome (mechanical ventilation and death) [30]. It may suggest that more severe COVID-19 disease causes more autoimmunity via inflammatory response. One study investigated the effect of COVID-19 disease on thyroid function and thyroid autoimmunity among COVID-19 survivors [31]. A total of 122 non-critically ill patients assessed at baseline and after a median interval of 90 days from COVID-19. They have found that 20 (16.4%) patients had abnormal thyroid function test at baseline (five of subclinical thyrotoxicosis, fifteen non-thyroidal illness). All returned to normal range except two patients (one had T3-toxicosis, one had NTIS). Anti-TPO titers and anti-TG titers increased significantly on reassessment and four patients became positive for anti-TPO. In the current review none of the patients required hospitalization except four patients (one myxedema coma, one for the severity of COVID-19 disease and two thyroid storm). The reason of the hospitalization was the thyroid storm in two of them rather than COVID-19. These two studies that evaluated autoimmunity in COVID-19 patients were performed on hospitalized patients and mostly in men. The patients with COVID-19-associated autoimmune thyroid disease reported to date are mostly women with a mild disease as in classical course of this diseases. Further follow-up studies in larger population are needed to clarify thyroid disfunction among COVID-19 survivors.
Beside COVID-19 disease itself, COVID-19 vaccines may trigger emergence of autoimmune thyroiditis. BNT162b2 mRNA vaccine contains a nanoparticle encoding a modified SARS-CoV-2 spike protein [32]. The vaccine mimics the infection and autoimmune reactions may be induced by the similar mechanisms in the infection. Additionally, the adjuvants in the vaccines have been postulated for the occurrence of thyroiditis contributing to the entity “autoimmune/inflammatory syndrome induced by adjuvants” [33].
One drawback of the study is that beside COVID-19, there may be co-infection of other viruses, which have been reported to trigger autoimmune thyroiditis. COVID-19 was confirmed by PCR study, however the other viruses were not excluded in all.
5. Conclusion
COVID-19 may cause autoimmune thyroid disease in susceptible subjects. It is reasonable to routinely assess the thyroid functions in both in the acute phase and during the convalescence so as not to overlook a thyroid disorder and not to delay treatment especially in patients with preexisting autoimmune thyroid diseases. Future prospective studies may clarify the relationship between SARS-COV-2 and thyroid autoimmunity.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CRediT authorship contribution statement
Esra Tutal: Screened all papers, compiled the tables, writing - original draft, revising the manuscript critically for important intellectual content. All co-authors contributed to, and endorsed, the final version of the manuscript.
Resat Ozaras: Revising the manuscript critically for important intellectual content. All co-authors contributed to, and endorsed, the final version of the manuscript.
Hakan Leblebicioglu: Senior author. Writing - original draft, designed the study, conducted the literature searches, revising the manuscript critically for important intellectual content All co-authors contributed to, and endorsed, the final version of the manuscript.
Declaration of competing interest
The authors declare they have no conflict of interest.
References
- 1.Ahn D.G., Shin H.J., Kim M.H., Lee S., Kim H.S., Myoung J., et al. Current status of epidemiology, diagnosis, therapeutics, and vaccines for novel Coronavirus disease 2019 (COVID-19) J Microbiol Biotechnol. 2020;30(3):313–324. doi: 10.4014/jmb.2003.03011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wiersinga W.J., Rhodes A., Cheng A.C., Peacock S.J., Prescott H.C. Pathophysiology, transmission, diagnosis, and treatment of Coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;324(8):782–793. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- 3.Garg M.K., Gopalakrishnan M., Yadav P., Misra S. Endocrine involvement in COVID-19: mechanisms, clinical features, and implications for care. Indian J Endocrinol Metab. 2020;24(5):381–386. doi: 10.4103/ijem.IJEM_440_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scappaticcio L., Pitoia F., Esposito K., Piccardo A., Trimboli P. Impact of COVID-19 on the thyroid gland: an update. Rev Endocr Metab Disord. 2020;22(4):803–815. doi: 10.1007/s11154-020-09615-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lania A., Sandri M.T., Cellini M., Mirani M., Lavezzi E., Mazziotti G. Thyrotoxicosis in patients with COVID-19: the THYRCOV study. Eur J Endocrinol. 2020;183(4):381–387. doi: 10.1530/EJE-20-0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Desailloud R., Hober D. Viruses and thyroiditis: an update. Virol J. 2009;6:5. doi: 10.1186/1743-422X-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aemaz Ur Rehman M., Farooq H., Ali M.M., Ebaad Ur Rehman M., Dar Q.A., Hussain A. The association of subacute thyroiditis with COVID-19: a systematic review. SN Compr Clin Med. 2021:1–13. doi: 10.1007/s42399-021-00912-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khatri A., Charlap E., Kim A. Subacute thyroiditis from COVID-19 infection: a case report and review of literature. Eur Thyroid J. 2021;9(6):324–328. doi: 10.1159/000511872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.WHO WHO COVID-19 Case definition. 2020. https://www.who.int/publications/i/item/WHO-2019-nCoV-Surveillance_Case_Definition-2020.2 [cited October 5, 2021]; Available from:
- 11.Allam M.M., El-Zawawy H.T., Ahmed S.M., Aly Abdelhamid M. Thyroid disease and covid-19 infection: case series. Clin Case Rep. 2021;9(6) doi: 10.1002/ccr3.4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harris A., Al Mushref M. Graves' thyrotoxicosis following SARS-CoV-2 infection. AACE Clin Case Rep. 2021;7(1):14–16. doi: 10.1016/j.aace.2020.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jimenez-Blanco S., Pla-Peris B., Marazuela M. COVID-19: a cause of recurrent Graves' hyperthyroidism? J Endocrinol Invest. 2021;44(2):387–388. doi: 10.1007/s40618-020-01440-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lanzolla G., Marcocci C., Marino M. Graves' disease and Graves' orbitopathy following COVID-19. J Endocrinol Invest. 2021;44(9):2011–2012. doi: 10.1007/s40618-021-01576-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mateu-Salat M., Urgell E., Chico A. SARS-COV-2 as a trigger for autoimmune disease: report of two cases of Graves' disease after COVID-19. J Endocrinol Invest. 2020;43(10):1527–1528. doi: 10.1007/s40618-020-01366-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pastor S., Molina A., Sr, De Celis E. Thyrotoxic crisis and COVID-19 infection: an extraordinary case and literature review. Cureus. 2020;12(11) doi: 10.7759/cureus.11305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Montebello A. Recurrent Graves' disease post SARS-CoV-2 infection. BMJ Case Rep. 2021;14(8) doi: 10.1136/bcr-2021-244714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feghali K., Atallah J., Norman C. Manifestations of thyroid disease post COVID-19 illness: report of Hashimoto thyroiditis, Graves' disease, and subacute thyroiditis. J Clin Transl Endocrinol Case Rep. 2021;22:100094. doi: 10.1016/j.jecr.2021.100094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Milani N., Najafpour M., Mohebbi M. Case series: rare cases of thyroid storm in COVID-19 patients. Clin. Case Rep. 2021;9(9) doi: 10.1002/ccr3.4772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Edwards K., Hussain I. Two cases of severe autoimmune thyrotoxicosis following SARS-CoV-2 infection. J Invest. Med High Impact Case Rep. 2021;9 doi: 10.1177/23247096211056497. 23247096211056497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dixit N.M., Truong K.P., Rabadia S.V., Li D., Srivastava P.K., Mosaferi T., et al. Sudden cardiac arrest in a patient with myxedema coma and COVID-19. J Endocr Soc. 2020;4(10):bvaa130. doi: 10.1210/jendso/bvaa130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tee L.Y., Harjanto S., Rosario B.H. COVID-19 complicated by Hashimoto's thyroiditis. Singap Med J. 2021;62(5):265. doi: 10.11622/smedj.2020106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Knack R.S., Hanada T., Knack R.S., Mayr K. Hashimoto's thyroiditis following SARS-CoV-2 infection. BMJ Case Rep. 2021;14(8) doi: 10.1136/bcr-2021-244909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mizuno S., Inaba H., Kobayashi K.I., Kubo K., Ito S., Hirobata T., et al. A case of postpartum thyroiditis following SARS-CoV-2 infection. Endocr J. 2021;68(3):371–374. doi: 10.1507/endocrj.EJ20-0553. [DOI] [PubMed] [Google Scholar]
- 25.Antonelli A., Fallahi P., Elia G., Ragusa F., Paparo S.R., Ruffilli I., et al. Graves' disease: clinical manifestations, immune pathogenesis (cytokines and chemokines) and therapy. Best Pract Res Clin Endocrinol Metab. 2020;34(1):101388. doi: 10.1016/j.beem.2020.101388. [DOI] [PubMed] [Google Scholar]
- 26.Mori K., Yoshida K. Viral infection in induction of Hashimoto's thyroiditis: a key player or just a bystander? Curr Opin Endocrinol Diabetes Obes. 2010;17(5):418–424. doi: 10.1097/MED.0b013e32833cf518. [DOI] [PubMed] [Google Scholar]
- 27.Vojdani A., Vojdani E., Kharrazian D. Reaction of human monoclonal antibodies to SARS-CoV-2 proteins with tissue antigens: implications for autoimmune diseases. Front Immunol. 2020;11:617089. doi: 10.3389/fimmu.2020.617089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gao W., Guo W., Guo Y., Shi M., Dong G., Wang G., et al. Thyroid hormone concentrations in severely or critically ill patients with COVID-19. J Endocrinol Invest. 2021;44(5):1031–1040. doi: 10.1007/s40618-020-01460-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakano Y., Kurihara H., Sasaki J. Graves' disease following subacute thyroiditis. Tohoku J Exp Med. 2011;225(4):301–309. doi: 10.1620/tjem.225.301. [DOI] [PubMed] [Google Scholar]
- 30.Anaya J.M., Monsalve D.M., Rojas M., Rodriguez Y., Montoya-Garcia N., Mancera-Navarro L.M., et al. Latent rheumatic, thyroid and phospholipid autoimmunity in hospitalized patients with COVID-19. J Translat Autoimmun. 2021;4:100091. doi: 10.1016/j.jtauto.2021.100091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lui D.T.W., Lee C.H., Chow W.S., Lee A.C.H., Tam A.R., Fong C.H.Y., et al. Insights from a prospective follow-up of thyroid function and autoimmunity among COVID-19 survivors. Endocrinol Metab. 2021;36(3):582–589. doi: 10.3803/EnM.2021.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.WHO. mRNA vaccines against COVID-19: pfizer-BioNTech COVID-19 vaccine BNT162b2: prepared by the Strategic Advisory Group of Experts (SAGE) on immunization working group on COVID-19 vaccines. 2020. [cited March 12, 2022]; Available from: [Google Scholar]
- 33.Watad A., David P., Brown S., Shoenfeld Y. Autoimmune/inflammatory syndrome induced by adjuvants and thyroid autoimmunity. Front Endocrinol. 2016;7:150. doi: 10.3389/fendo.2016.00150. [DOI] [PMC free article] [PubMed] [Google Scholar]