Abstract
Background and aims
In this meta-analysis, we aimed to evaluate the prognostic properties of thyroid disorder during admission on poor prognosis and factors that may influence the relationship in patients with COVID-19.
Methods
A systematic literature search of PubMed, EBSCO, and CENTRAL was conducted from inception to August 27, 2021. The main exposure was unspecified and specified thyroid disorders–hypothyroidism or hypothyroidism. The outcome of interest was the COVID-19 composite poor outcome that comprises of severity, mortality, ICU admission, and hospitalization.
Results
There were 24,734 patients from 20 studies. Meta-analysis showed that thyroid disorder was associated with composite poor outcome (OR 2.87 (95% CI 2.04–4.04), p < 0.001; I2 = 62.4%, p < 0.001). Meta regression showed that age (p = 0.047) and hypertension (p = 0.01), but not gender (p = 0.15), DM (p = 0.10), CAD/CVD (p = 0.38), obesity (p = 0.84), and COPD (p = 0.07) affected the association. Subgroup analysis showed that thyroid disorder increased risk of severe COVID-19 (OR 5.13 (95% CI 3.22–8.17), p < 0.05; I2 = 0%, p = 0.70) and mortality (OR 2.78 (95%CI 1.31–5.90), p < 0.05; I2 = 80%, p < 0.01). Pooled diagnostic analysis of thyroid disorder yielded a sensitivity of 0.22 (0.13–0.35), specificity of 0.92 (0.87–0.95), and AUC of 0.72. The probability of poor outcome was 38% in patients with thyroid disorder and 15% in patients without thyroid abnormality.
Conclusion
On-admission thyroid disorder was associated with poor prognosis in COVID-19 patients.
Keywords: COVID-19, Hypothyroidism, Thyroid disorder, Prognosis
1. Introduction
To date, millions of people worldwide have coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). The evolving nature of virus subtype predisposes to the high prevalence followed by long incubation periods of the virus yields a long-lasting pandemic with an increasing number of cases across countries [1,2]. Multifaceted measures have been taken with various objectives–from prevention of viral transmission to prevent the complication of the disease.
The spectrum of clinical manifestation of COVID-19 is broad, ranging from asymptomatic patients to critically ill that requires hospitalization [1,3]. The disease progression has also been a concern due to its characteristics of wide-ranging and confounded by a multitude of factors. Early steps to mitigate disease progression by assessing risk stratification using a reliable predictive model are needed to prevent adverse outcomes. The relationship between comorbidities associated with metabolic disorders and COVID-19 outcomes has been extensively investigated. Utilizing patients’ clinical history and markers especially those that are regularly assessed is paramount to assess risks and allocate resources efficiently [4,5]. Previous studies have been conducted to evaluate the association between thyroid disorder and COVID-19 poor outcome [6,7]. However, the prognostic properties of thyroid disorder and whether its value is influenced by other comorbidities that may be present in COVID-19 patients remain unknown. Thus, this present systematic review and meta-analyses were aimed to investigate the prognostic value of preexisting thyroid disorder during admission for various COVID-19 poor outcomes and factors that may affect its relationship.
2. Material and methods
This systematic review and meta-analysis were written following the guideline of Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRIMSA) [8]. A detailed protocol has been previously registered in PROSPERO (CRD42021267278).
2.1. Eligibility criteria
The PECO (Population, Exposure, Comparison, Outcome) structure design was used to define the inclusion and exclusion criteria of exposure and outcomes. The inclusion criteria were research articles and letters that report COVID-19 patients with provided categorical data on thyroid disorder status during admission along with measurable outcomes–mortality, severity, ICU admission, and hospitalization.
Following types or articles were excluded: case report, non-research letter, editorial, invited commentary, review, abstract-only article, and preprint. Studies that included patients who have developed thyroid abnormalities secondary to the infection were excluded from further analysis. Also, studies reporting only continuous variables of thyroid serum level were excluded. There was no language restriction applied in this study.
2.2. Search strategy and study selection
A systematic search of PubMed, EBSCO, and Cochran Collaboration Central Register of Controlled Clinical Trials (CENTRAL) were initially performed from the inception to 27th of August 2021. An extended literature searching was then conducted by two independent investigators (NNMS and FAD) to pool articles until December 2nd, 2021. The following search terms were used: (("Thyroid Gland" [Mesh] OR "Thyroid (USP)" [Mesh] OR "Hyperthyroidism" [Mesh] OR "Hypothyroidism" [Mesh] OR "Thyroid Hormones" [Mesh] OR “thyroid disease” [tiab] OR “hyperthyroid” [tiab] OR “hypothyroid” [tiab] OR “thyroid” [tiab] OR thyroid OR “TSH” [tiab] OR “T3” [tiab] OR “T4” [tiab]) AND ("COVID-19" [Mesh] OR "SARS-CoV-2" [Mesh] OR COVID-19)) [7]. We used ‘related articles’ feature and hand searched the reference lists of the included articles to expand the search and obtain additional studies. Duplicate results were removed after the initial search.
2.3. Data extraction
Data extraction was conducted independently by two authors (NNMS and FAD). Included studies were summarized in a standardized form that comprised of the author, year of publication, study design, sample size, age, gender, and reported comorbidities–hypertension (HT), coronary artery disease/cerebrovascular disease (CAD/CVD). Diabetes mellitus (DM), obesity, and chronic pulmonary obstructive disease (COPD). Different opinions in extracting data or eliminating possible duplicates were resolved through discussion and consensus.
The primary exposure was preexisting thyroid disease from baseline characteristics or a group of patients with lower- or higher-than-normal measured thyroid hormone levels during admission. The type of thyroid measurement during admission to categorize the patient's type of thyroid level abnormality was thyroid-stimulating hormone (TSH), free triiodothyronine (fT3), or free thyroxine (fT4). The patient was considered hypothyroidism if the level of thyroid hormone was lower than normal reference according to the specified cut-off defined by each study. Conversely, the patient was considered hypothyroidism if the level of measured thyroid hormone was higher than the normal range. Furthermore, we also performed subgroup analyses on each type of thyroid abnormalities–undefined, hypothyroidism, and hypothyroidism. The primary measured outcome of interest in this present study was the composite poor outcome that comprised of COVID-19 mortality, severity, ICU admission, and hospitalization.
We used Newcastle-Ottawa Scale (NOS) to assess the quality of included studies. The following aspects were taken into consideration in the assessment: cohort selection, the comparability of the cohort based on the design or analysis, the way exposure is determined, and the way of outcomes of interest are evaluated. Discrepancies of perception were resolved by discussion.
2.4. Statistical analysis
All statistical analysis was performed using R (version 4.0.4, The R Foundation, Vienna, Austria). The incidence of poor composite outcome and thyroid disorder was pooled using meta-analysis of proportion. Also, the diagnostic odds ratio (DOR) was calculated using DerSimonian and Laird method random-effects model. The inconsistency index (I 2) and subgroup analysis using the Chi-square test was used to explore potential sources of heterogeneity. An I 2 of more than 50% and a p-value of less than 0.05 were considered significant for heterogeneity [9]. Random-effects model was used regardless of the heterogeneity value. Sensitivity analysis was conducted by using leave-one-out analysis to detect studies that significantly influence the pooled estimate. Meta-regression was also conducted with restricted-maximum likelihood random-effects with age, gender, and comorbidities (hypertension, DM, CAD/CVD, obesity, and COPD).
Diagnostic meta-analysis was done by calculating pooled sensitivity, specificity, positive likelihood ratio (PLR), and negative likelihood ratio (NLR). Pooled sensitivity and specificity were displayed using a forest plot and summary receiver operating characteristics (SROC) curve [10]. Fagan's nomogram was also used to present the prediction of post-test probability from pre-test probability. We also performed Deek's funnel plot asymmetry test to identify publication bias and used Egger's test to ascertain the findings [11].
3. Results
3.1. Study selection
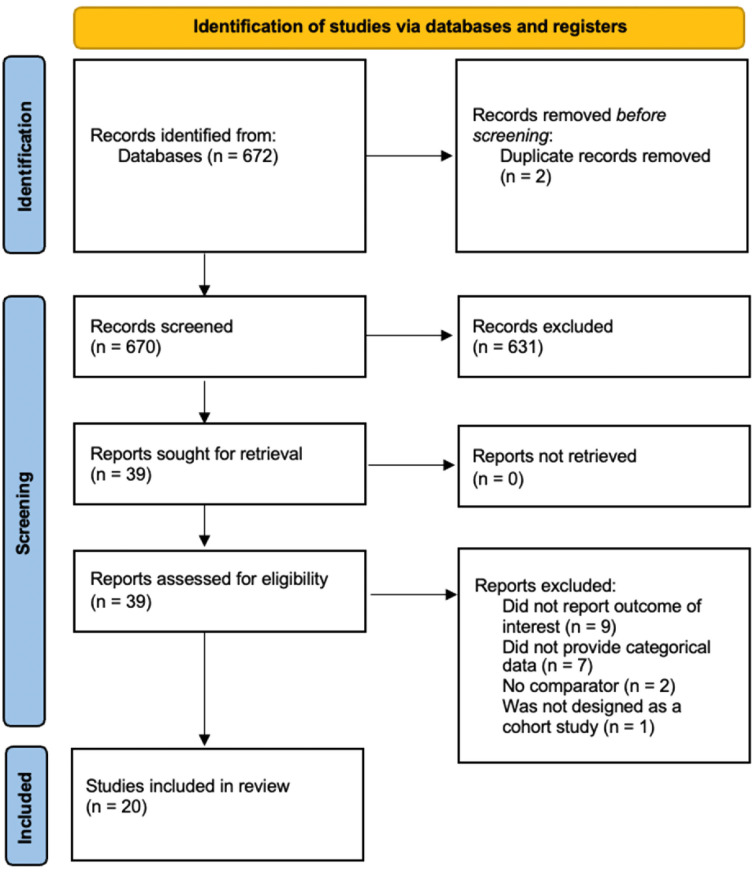
The selection to obtain included studies in the systematic review is shown in (Fig. 1 ). The initial search yielded 672 articles from PubMed, EBSCO, and CENTRAL.
Fig. 1.
PRISMA literature search flowchart.
Among 672 studies, two articles were removed automatically due to duplication, yielding the remaining 670 articles that were eligible to be screened and sought for retrieval. From all 670 screened articles, 631 irrelevant studies were excluded, resulting in the remaining 39 studies to be assessed for each eligibility. From 39 studies, a total of 19 full-text articles were excluded from further analysis in which the studies did not present the outcome of interest and did not categorize patients into different thyroid statuses, the reported thyroid disorder was continuous data, and no comparator was reported. Thereby, 20 studies were included in the systematic review and meta-analysis [[12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31]].
3.2. Characteristics of included studies
The characteristics of the included studies are available in Table 1 . There are 24,734 patients from 20 studies included in this present systematic review and meta-analysis. All patients were adult confirmed COVID-19 cases. There was a variability of cut-offs used within studies in which patients were grouped as hypothyroidism or hypothyroidism. However, the amount of study using each of the different cut-offs was inadequate to perform diagnostic meta-analysis with different cut points to generate optimal cut-off value. In addition, we extracted the number of comorbidities reported in the included studies cumulatively.
Table 1.
Characteristics of thyroid disorders and COVID-19 outcomes related studies.
| Authors | Study Design | Samples | Male (%) | Age (years) | Hypertension (%) | CAD/CVD (%) | DM (%) | Obesity (%) | COPD (%) | Outcome | NOS |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Alqahtani et al., 2020 (12) | Retrospective cohort | 458 | 86.9 | N/A | 10.94 | 1.97 | 13.6 | 57.1 | N/A | Severity | 8 |
| Almazeedi et al., 2020 (13) | Retrospective cohort | 1096 | 81 | 41 | 16.1 | 4.3 | 14.1 | 21.8 | 0.5 | Severity | 6 |
| Baldelli et al., 2021 (14) | Retrospective cohort | 46 | 69.5 | 59.6 | N/A | N/A | N/A | N/A | N/A | ICU | 7 |
| Brix et al., 2021 (15) | Retrospective cohort | 16,502 | 45.6 | 57.3 | 26.73 | 17.35 | 11.96 | 10.9 | 5.8 | Mortality | 7 |
| Cao et al., 2020 (16) | Retrospective cohort | 198 | 51 | 50.1 | 21.2 | 6 | 7.6 | N/A | N/A | ICU | 7 |
| Daraei et al., 2020 (17) | Retrospective cohort | 390 | 67.7 | 58.1 | N/A | N/A | N/A | N/A | N/A | Mortality | 6 |
| Gao et al., 2020 (18) | Retrospective cohort | 100 | 52 | 62.3 | N/A | N/A | N/A | N/A | N/A | Severity and Mortality | 9 |
| Gong et al., 2021 (19) | Retrospective cohort | 150 | 54 | 69.8 | N/A | 11.33 | N/A | N/A | N/A | Mortality | 9 |
| Güven et al., 2021 (20) | Prospective cohort | 250 | 63 | 68 | N/A | N/A | N/A | N/A | N/A | ICU | 8 |
| Khoo et al., 2020 (21) | Prospective cohort | 334 | 60.8 | 66.1 | 48.5 | 23.7 | 39.5 | N/A | 17.4 | Mortality and ICU | 9 |
| Lang et al., 2021 (22) | Retrospective cohort | 127 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | Mortality | 7 |
| Lui et al., 2020 (23) | Prospective cohort | 191 | 51.8 | 53.5 | 27.2 | 6.3 | 13.1 | N/A | 3.1 | Severity | 8 |
| Muller et al., 2020 (24) | Prospective cohort | 145 | 61.3 | 66.9 | N/A | N/A | N/A | N/A | N/A | ICU | 6 |
| Sen et al., 2021 (25) | Prospective cohort | 60 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | Severity | 6 |
| Shabrawishi et al., 2020 (26) | Retrospective cohort | 150 | 60 | 46.1 | 28.8 | 8 | 26 | N/A | 0.7 | Severity | 6 |
| Sisó-Almirall et al., 2020 (27) | Retrospective cohort | 322 | 50 | 56.7 | 33.9 | 7.8 | 14.3 | 14.3 | 5.9 | Hospitalization | 7 |
| van Gerwen et al., 2020 (28) | Retrospective cohort | 3703 | 55.3 | 56,9 | N/A | N/A | N/A | 57.4 | N/A | Hospitalization | 8 |
| Wang et al., 2020 (29) | Retrospective cohort | 84 | 63.1 | 57.3 | N/A | N/A | N/A | N/A | N/A | Severity | 9 |
| Zhang et al., 2020 (30) | Retrospective cohort | 140 | 50.7 | 56.3 | 30 | 7.1 | 12.1 | N/A | 1.4 | Severity | 7 |
| Zhang et al., 2020 (31) | Retrospective cohort | 71 | 56.3 | 62.7 | 28.2 | 21.1 | 18.3 | N/A | N/A | Severity and Mortality | 7 |
CAD/CVD: Coronary artery disease/cardiovascular disease; COPD: Chronic Obstructive Pulmonary Disease; DM: Diabetes Mellitus; HTN: Hypertension; N/A: Not applicable; NOS: Newcastle-Ottawa Scale.
3.3. Thyroid disorders and poor outcomes
The preexisting of thyroid disorder was associated with higher odds of COVID-19 composite poor outcome (OR 2.87 (95% CI 2.04–4.04), p < 0.001; I 2 = 62.4%, p < 0.001). Subgroup analysis showed that thyroid disorder was associated with higher risks of increased COVID-19 severity (OR 5.13 (95% CI 3.22–8.17), p < 0.05; I 2 = 0%, p = 0.70), ICU admission (OR 2.14 (95%CI 0.87–15.30), p > 0.05; I 2 = 53%, p = 0.07), mortality (OR 2.78 (95%CI 1.31–5.90), p < 0.05; I 2 = 80%, p < 0.01), and hospitalization (OR 1.84 (95%CI 1.40–2.40), p < 0.05; I 2 = 0%, p = 0.61) (Fig. 2 ). Publication bias was evaluated qualitatively and quantitatively using funnel-plot analysis and Egger's test, respectively indicating a small study effect demonstrated in an asymmetrical shape (p < 0.001). Trim and fill analysis with imputation of 5 studies yielded an OR of (2.24 (95%. CI 1.57–3.18) for poor outcomes. Sensitivity analysis by omitting individual study did not affect pooled estimate significantly. Meta regression showed that the incidence was vary by age (p = 0.047) and hypertension (p = 0.014), but not gender (p = 0.148), DM (p = 0.102), CAD/CVD (p = 0.383), obesity (p = 0.835), and COPD (p = 0.069). The incidence of composite poor outcome was 17%.
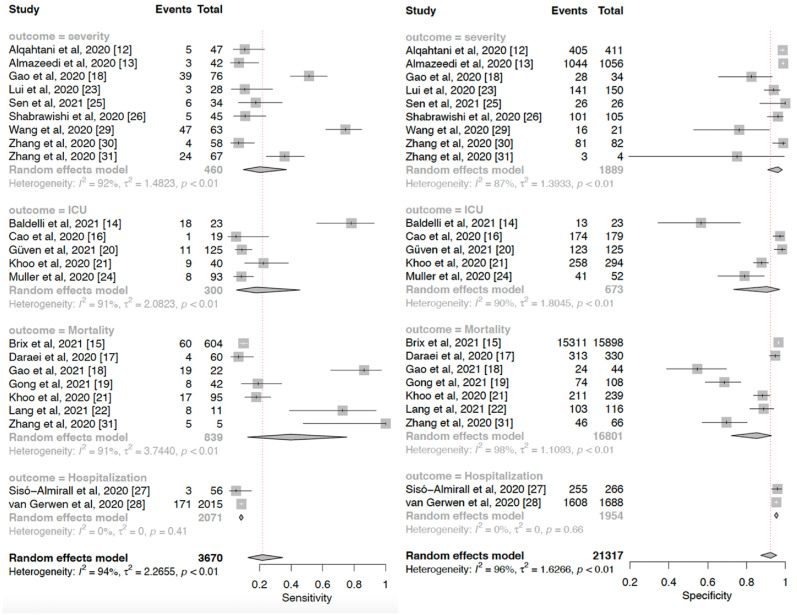
Fig. 2.
Pooled sensitivity and specificity of thyroid disorders for COVID-19 poor outcomes.
3.4. Diagnostic meta-analysis
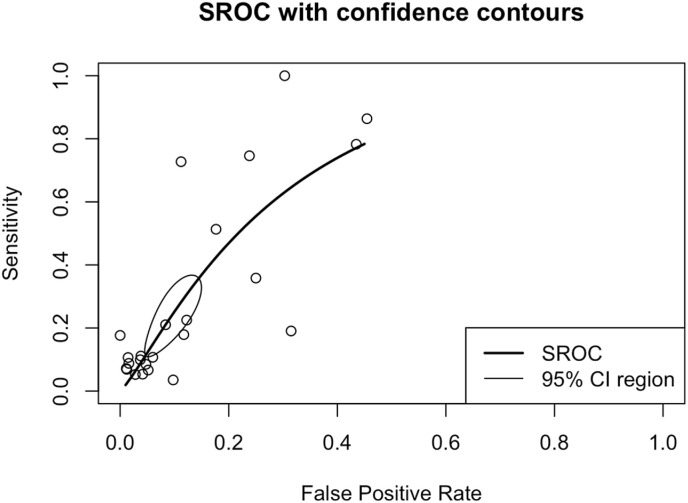
Thyroid disorder had a sensitivity of 0.22 (0.13–0.35), specificity of 0.93 (0.88–0.96), DOR of 2.87 (2.04–4.04), and Area under curve (AUC) of 0.72 in predicting COVID-19 poor outcomes. Pooled sensitivity and specificity to predict poor outcomes are shown in Fig. 4. A subgroup analysis of sensitivity showed the sensitivity of thyroid disorder to predict severe COVID-19 was 0.20 (95% CI 0.10–0.37), ICU admission was 0.18 (95% CI 0.05–0.46), mortality was 0.40 (95% CI 0.13–0.75), and hospitalization was 0.08 (0.07–0.10). Subsequently, subgroup analyses of the specificity of thyroid disorder to predict severe COVID-19 was 0.96 (95% CI 0.91–0.98), ICU admission was 0.90 (95% CI 0.73–0.97), mortality was 0.85 (95% CI 0.72–0.93), and hospitalization was 0.95 (0.94–0.96). Also, a summary receiver operating characteristics curve to predict composite poor outcome was conducted (Fig. 3 ).
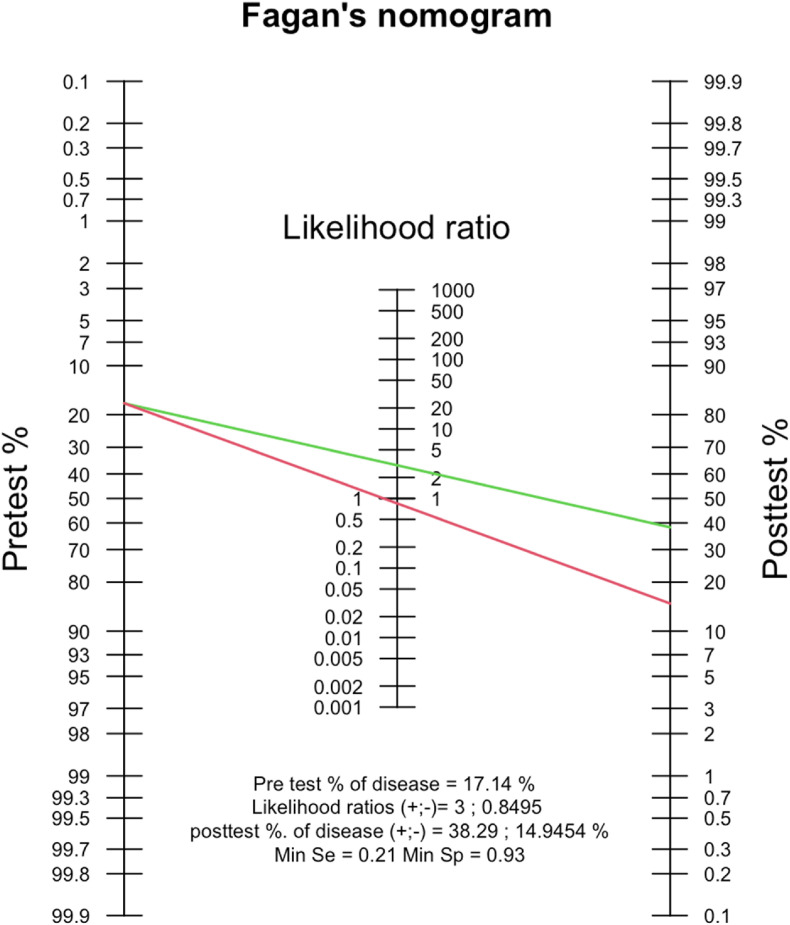
Fig. 4.
Fagan's nomogram analysis elucidating the post-test probability of the presence of thyroid disorders in predicting COVID-19 poor outcomes.
Fig. 3.
Summary receiver operating characteristics (SROC) of thyroid disorders for COVID-19 composite poor outcome.
Fagan's nomogram showed that the post-test probability of poor outcome was 38% in patients with a thyroid disorder and the post-test probability of a poor outcome in a group with normal thyroid was 15% (Fig. 4). The positive likelihood ratio (PLR) was 3 and the negative likelihood ratio (NLR) was 0.85. Deek's funnel plot showed asymmetry with respect to the regression line and the asymmetry test showed significant results (p = 0.01).
4. Discussion
The presence of thyroid disorder was associated with an almost three-fold increase in composite poor outcomes compared to patients with a sensitivity of 22% and specificity of 93%. To date, this is the first study to exhibit pooled analyses from the existing evidence to describe the prognostic properties of thyroid disorder to the outcome of COVID-19.
Meta-regression analyses indicated that the association between thyroid disorder and poor COVID-19 outcomes vary by age and hypertension. It is postulated that physiological responses have deteriorated in the elderly population and this will further also lead to decreased levels of TSH and thyroxine [[32], [33], [34], [35]]. The iodine nutritional status of the populations may also result in different thyroid function patterns during aging [33,34]. Previous studies also found the association between hypertension and poor COVID-19 outcomes [7,36]. In addition, it is also found that both types of thyroid disorders–hypothyroidism and hypothyroidism can lead to hypertension. Although these disorders influenced the cardiovascular system through distinct mechanisms, these relationships may explain the influence of hypertension on the findings [37,38]. Furthermore, our results showed that the association between thyroid disorders and poor outcomes did not vary with other comorbidities–gender, DM, CAD/CVD. Obesity, and COPD.
Thyroid disorder has 93% of specificity with a 38% of positive probability of poor outcome and 17% of pre-test poor outcome probability. The sensitivity of thyroid disorder was relatively low (21%) and therefore, thyroid disorder in COVID-19 is better to be implemented to rule in the risk of poor outcomes rather than ruling it out. Among different types of poor outcomes defined in this study, a relatively stronger association was found between thyroid disorder and disease severity. The diagnostic analysis also showed that the sensitivity of thyroid disorder and death outcome was found comparably higher than other poor outcomes, although the finding posed a relatively higher heterogeneity.
Overall, our results showed a moderate level of heterogeneity. It is likely due to unspecified types of thyroid disorders included in the analysis. In addition, studies that included hypothyroidism or hypothyroidism populations potentially used different cut-off points, diagnostic tools, and quantification methods used among studies that further explain the relatively high heterogeneity of the results [39]. However, sensitivity analysis through leave-one-out analyses indicated that retracting a single study did not significantly alter the effect estimate and heterogeneity of the association between thyroid disorders and poor outcomes. Subsequently, publication bias was also noted in this study as the funnel plot showed a slightly asymmetrical. Trim-and-fill analysis was performed, and it showed a reduced effect estimate without altering its significance after five imputed studies were included. Therefore, the prognostic performance will not be affected by the inclusion of additional studies.
Thyroid hormones are known to have bidirectional interaction between hormonal regulation and immune response. Studies have investigated these interactions in both pathological and physiological settings. Thyroid hormones influence both innate and adaptive immune responses [40,41]. Both are affected through cytokine release that is modulated by intracellular signaling as the response of thyroid hormone bindings [42,43]. Several studies have shown that in the acute phase of critically ill patients, the concentration of T3 was markedly decreased [12,13,18]. Hence, in the presence of thyroid disorder, the robustness of the immune response may be reduced as the regulation of hormonal release has obliterated. Another mechanism that could contribute to the worsening of the outcomes is the impairment of renin-angiotensinogen system (RAS) regulation [44,45]. Patients with thyroid abnormalities are potent to have an increased angiotensin-converting enzyme 2 (ACE2) expression [46]. This may increase a greater risk of severe infection as ACE2 plays an essential role in SARS-CoV-2 infection as the key receptor of the virus to enter the host [[47], [48], [49], [50]]. Taken together, the presence of thyroid disorder may worsen COVID-19 infection through immune response dysregulation or viral entry modulation that both increase the risk of poor outcomes.
The main limitation of this present study is due to retrospective studies that have a higher risk of bias. In addition, this study involved all baseline thyroid disorders that may contribute to a greater rate of heterogeneity of the results. Importantly, the paucity of reported data of other comorbidities on each study makes further study with complete information on baseline characteristics to reassure the relationship of contributing factors is still required.
5. Conclusion
This study shows that on-admission thyroid disorders were associated with poor outcomes in patients with COVID-19 and was vary with age and hypertension, but not gender, DM, CAD/CVD, obesity, and COPD. Pooled diagnostic analysis indicates the sensitivity of 22% and specificity of 92%.
Source of financial support
None.
Declaration of competing interest
The authors declare that there is no conflict of interest.
References
- 1.Gao Z., Xu Y., Sun C., Wang X., Guo Y., Qiu S., et al. A systematic review of asymptomatic infections with COVID-19. J Microbiol Immunol Infect. 2021;54(1):12–16. doi: 10.1016/j.jmii.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chung J.Y., Thone M.N., Kwon Y.J. COVID-19 vaccines: the status and perspectives in delivery points of view. Adv Drug Deliv Rev. 2021;170:1–25. doi: 10.1016/j.addr.2020.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hu B., Guo H., Zhou P., Shi Z.L. Characteristics of SARS-CoV-2 and COVID-19. Nat Rev Microbiol. 2021;19(3):141–154. doi: 10.1038/s41579-020-00459-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Malik P., Patel U., Mehta D., Patel N., Kelkar R., Akrmah M., et al. Biomarkers and outcomes of COVID-19 hospitalisations: systematic review and meta-analysis. BMJ Evid Based Med. 2020 doi: 10.1136/bmjebm-2020-111536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soetedjo N.N.M., Iryaningrum M.R., Damara F.A., Permadhi I., Sutanto L.B., Hartono H., et al. Clinical Nutrition ESPEN; 2021. Prognostic properties of hypoalbuminemia in COVID-19 patients: a systematic review and diagnostic meta-analysis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hariyanto T.I., Kurniawan A. Thyroid disease is associated with severe coronavirus disease 2019 (COVID-19) infection. Diabetes Metab Syndr. 2020;14(5):1429–1430. doi: 10.1016/j.dsx.2020.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Damara F.A., Muchamad G.R., Ikhsani R., Hendro, Syafiyah A.H., Bashari M.H. Thyroid disease and hypothyroidism are associated with poor COVID-19 outcomes: a systematic review, meta-analysis, and meta-regression. Diabetes & Metabolic Syndrome: Clin Res Rev. 2021;15(6):102312. doi: 10.1016/j.dsx.2021.102312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339 [PMC free article] [PubMed] [Google Scholar]
- 9.Higgins J.P., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moses L.E., Shapiro D., Littenberg B. Combining independent studies of a diagnostic test into a summary ROC curve: data-analytic approaches and some additional considerations. Stat Med. 1993;12(14):1293–1316. doi: 10.1002/sim.4780121403. [DOI] [PubMed] [Google Scholar]
- 11.Begg C.B., Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–1101. [PubMed] [Google Scholar]
- 12.Alqahtani A.M., AlMalki Z.S., Alalweet R.M., Almazrou S.H., Alanazi A.S., Alanazi M.A., et al. Assessing the severity of illness in patients with coronavirus disease in Saudi arabia: a retrospective descriptive cross-sectional study. Front Public Health. 2020;8:593256. doi: 10.3389/fpubh.2020.593256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Almazeedi S., Al-Youha S., Jamal M.H., Al-Haddad M., Al-Muhaini A., Al-Ghimlas F., et al. Characteristics, risk factors and outcomes among the first consecutive 1096 patients diagnosed with COVID-19 in Kuwait. EClinicalMedicine. 2020;24:100448. doi: 10.1016/j.eclinm.2020.100448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baldelli R., Nicastri E., Petrosillo N., Marchioni L., Gubbiotti A., Sperduti I., et al. Thyroid dysfunction in COVID-19 patients. J Endocrinol Invest. 2021:1–5. doi: 10.1007/s40618-021-01599-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brix T.H., Hegedüs L., Hallas J., Lund L.C. Risk and course of SARS-CoV-2 infection in patients treated for hypothyroidism and hyperthyroidism. Lancet Diabetes Endocrinol. 2021;9(4):197–199. doi: 10.1016/S2213-8587(21)00028-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cao M., Zhang D., Wang Y., Lu Y., Zhu X., Li Y., et al. 2020. Clinical features of patients infected with the 2019 novel coronavirus (COVID-19) in Shanghai, China. medRxiv. [Google Scholar]
- 17.Daraei M., Hasibi M., Abdollahi H., Mirabdolhagh Hazaveh M., Zebaradst J., Hajinoori M., et al. Possible role of hypothyroidism in the prognosis of COVID-19. Intern Med J. 2020;50(11):1410–1412. doi: 10.1111/imj.15000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao W., Guo W., Guo Y., Shi M., Dong G., Wang G., et al. Thyroid hormone concentrations in severely or critically ill patients with COVID-19. J Endocrinol Invest. 2021;44(5):1031–1040. doi: 10.1007/s40618-020-01460-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gong J., Wang D.K., Dong H., Xia Q.S., Huang Z.Y., Zhao Y., et al. Prognostic significance of low TSH concentration in patients with COVID-19 presenting with non-thyroidal illness syndrome. BMC Endocr Disord. 2021;21(1):111. doi: 10.1186/s12902-021-00766-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Güven M., Gültekin H. The prognostic impact of thyroid disorders on the clinical severity of COVID-19: results of single-centre pandemic hospital. Int J Clin Pract. 2021;75(6) doi: 10.1111/ijcp.14129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khoo B., Tan T., Clarke S.A., Mills E.G., Patel B., Modi M., et al. Thyroid function before, during, and after COVID-19. J Clin Endocrinol Metab. 2021;106(2):e803–e811. doi: 10.1210/clinem/dgaa830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lang S., Liu Y., Qu X., Lu R., Fu W., Zhang W., et al. Association between thyroid function and prognosis of COVID-19: a retrospective observational study. Endocr Res. 2021:1–8. doi: 10.1080/07435800.2021.1924770. [DOI] [PubMed] [Google Scholar]
- 23.Lui D.T.W., Lee C.H., Chow W.S., Lee A.C.H., Tam A.R., Fong C.H.Y., et al. Thyroid dysfunction in relation to immune profile, disease status, and outcome in 191 patients with COVID-19. J Clin Endocrinol Metab. 2021;106(2):e926–e935. doi: 10.1210/clinem/dgaa813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muller I., Cannavaro D., Dazzi D., Covelli D., Mantovani G., Muscatello A., et al. SARS-CoV-2-related atypical thyroiditis. Lancet Diabetes Endocrinol. 2020;8(9):739–741. doi: 10.1016/S2213-8587(20)30266-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sen K., Sinha A., Sen S., Chakraborty S., Alam M.S. Thyroid function test in COVID-19 patients: a cross-sectional study in a tertiary care hospital. Indian J Endocrinol Metab. 2020;24(6):532–536. doi: 10.4103/ijem.IJEM_779_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shabrawishi M., Al-Gethamy M.M., Naser A.Y., Ghazawi M.A., Alsharif G.F., Obaid E.F., et al. Clinical, radiological and therapeutic characteristics of patients with COVID-19 in Saudi Arabia. PLoS One. 2020;15(8) doi: 10.1371/journal.pone.0237130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Siso-Almirall A., Kostov B., Mas-Heredia M., Vilanova-Rotllan S., Sequeira-Aymar E., Sans-Corrales M., et al. Prognostic factors in Spanish COVID-19 patients: a case series from Barcelona. PLoS One. 2020;15(8) doi: 10.1371/journal.pone.0237960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Gerwen M., Alsen M., Little C., Barlow J., Naymagon L., Tremblay D., et al. Outcomes of patients with hypothyroidism and COVID-19: a retrospective cohort study. Front Endocrinol (Lausanne). 2020;11:565. doi: 10.3389/fendo.2020.00565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang W., Su X., Ding Y., Fan W., Zhou W., Su J., et al. Thyroid function abnormalities in COVID-19 patients. Front Endocrinol (Lausanne) 2020;11:623792. doi: 10.3389/fendo.2020.623792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang J.J., Dong X., Cao Y.Y., Yuan Y.D., Yang Y.B., Yan Y.Q., et al. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020;75(7):1730–1741. doi: 10.1111/all.14238. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Y., Lin F., Tu W., Zhang J., Choudhry A.A., Ahmed O., et al. Thyroid dysfunction may be associated with poor outcomes in patients with COVID-19. Mol Cell Endocrinol. 2021;521:111097. doi: 10.1016/j.mce.2020.111097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aggarwal N., Razvi S. Thyroid and aging or the aging thyroid? An evidence-based analysis of the literature. J Thyroid Res. 2013;2013:481287. doi: 10.1155/2013/481287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barbesino G. Thyroid function changes in the elderly and their relationship to cardiovascular health: a mini-review. Gerontology. 2019;65(1):1–8. doi: 10.1159/000490911. [DOI] [PubMed] [Google Scholar]
- 34.Bremner A.P., Feddema P., Leedman P.J., Brown S.J., Beilby J.P., Lim E.M., et al. Age-related changes in thyroid function: a longitudinal study of a community-based cohort. J Clin Endocrinol Metab. 2012;97(5):1554–1562. doi: 10.1210/jc.2011-3020. [DOI] [PubMed] [Google Scholar]
- 35.Li X., Marmar T., Xu Q., Tu J., Yin Y., Tao Q., et al. Predictive indicators of severe COVID-19 independent of comorbidities and advanced age: a nested case-control study. Epidemiol Infect. 2020;148:e255. doi: 10.1017/S0950268820002502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pranata R., Lim M.A., Huang I., Raharjo S.B., Lukito A.A. Hypertension is associated with increased mortality and severity of disease in COVID-19 pneumonia: a systematic review, meta-analysis and meta-regression. J Renin-Angiotensin-Aldosterone Syst JRAAS. 2020;21(2) doi: 10.1177/1470320320926899. 1470320320926899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Berta E., Lengyel I., Halmi S., Zrinyi M., Erdei A., Harangi M., et al. Hypertension in thyroid disorders. Front Endocrinol (Lausanne) 2019;10:482. doi: 10.3389/fendo.2019.00482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saito I., Saruta T. Hypertension in thyroid disorders. Endocrinol Metab Clin N Am. 1994;23(2):379–386. [PubMed] [Google Scholar]
- 39.Infusino I., Panteghini M. Serum albumin: accuracy and clinical use. Clin Chim Acta. 2013;419:15–18. doi: 10.1016/j.cca.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 40.Kumari K., Gbn Chainy, Subudhi U. Prospective role of thyroid disorders in monitoring COVID-19 pandemic. Heliyon. 2020;6(12) doi: 10.1016/j.heliyon.2020.e05712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rubingh J., van der Spek A., Fliers E., Boelen A. The role of thyroid hormone in the innate and adaptive immune response during infection. Compr Physiol. 2020;10(4):1277–1287. doi: 10.1002/cphy.c200003. [DOI] [PubMed] [Google Scholar]
- 42.Montesinos M.D.M., Pellizas C.G. Thyroid hormone action on innate immunity. Front Endocrinol (Lausanne). 2019;10:350. doi: 10.3389/fendo.2019.00350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.De Luca R., Davis P.J., Lin H.Y., Gionfra F., Percario Z.A., Affabris E., et al. Thyroid hormones interaction with immune response, inflammation and non-thyroidal illness syndrome. Front Cell Dev Biol. 2020;8:614030. doi: 10.3389/fcell.2020.614030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Park C.W., Shin Y.S., Ahn S.J., Kim S.Y., Choi E.J., Chang Y.S., et al. Thyroxine treatment induces upregulation of renin-angiotensin-aldosterone system due to decreasing effective plasma volume in patients with primary myxoedema. Nephrol Dial Transplant. 2001;16(9):1799–1806. doi: 10.1093/ndt/16.9.1799. [DOI] [PubMed] [Google Scholar]
- 45.Vargas F., Rodriguez-Gomez I., Vargas-Tendero P., Jimenez E., Montiel M. The renin-angiotensin system in thyroid disorders and its role in cardiovascular and renal manifestations. J Endocrinol. 2012;213(1):25–36. doi: 10.1530/JOE-11-0349. [DOI] [PubMed] [Google Scholar]
- 46.Diniz G.P., Senger N., Carneiro-Ramos M.S., Santos R.A., Barreto-Chaves M.L. Cardiac ACE2/angiotensin 1-7/Mas receptor axis is activated in thyroid hormone-induced cardiac hypertrophy. Ther Adv Cardiovasc Dis. 2016;10(4):192–202. doi: 10.1177/1753944715623228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vieira C., Nery L., Martins L., Jabour L., Dias R., Simoes E.S.A.C. Downregulation of membrane-bound angiotensin converting enzyme 2 (ACE2) receptor has a pivotal role in COVID-19 immunopathology. Curr Drug Targets. 2021;22(3):254–281. doi: 10.2174/1389450121666201020154033. [DOI] [PubMed] [Google Scholar]
- 48.Aleksova A., Ferro F., Gagno G., Cappelletto C., Santon D., Rossi M., et al. COVID-19 and renin-angiotensin system inhibition: role of angiotensin converting enzyme 2 (ACE2) - is there any scientific evidence for controversy? J Intern Med. 2020;288(4):410–421. doi: 10.1111/joim.13101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ni W., Yang X., Yang D., Bao J., Li R., Xiao Y., et al. Role of angiotensin-converting enzyme 2 (ACE2) in COVID-19. Crit Care. 2020;24(1):422. doi: 10.1186/s13054-020-03120-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kragstrup T.W., Singh H.S., Grundberg I., Nielsen A.L., Rivellese F., Mehta A., et al. Plasma ACE2 predicts outcome of COVID-19 in hospitalized patients. PLoS One. 2021;16(6) doi: 10.1371/journal.pone.0252799. [DOI] [PMC free article] [PubMed] [Google Scholar]