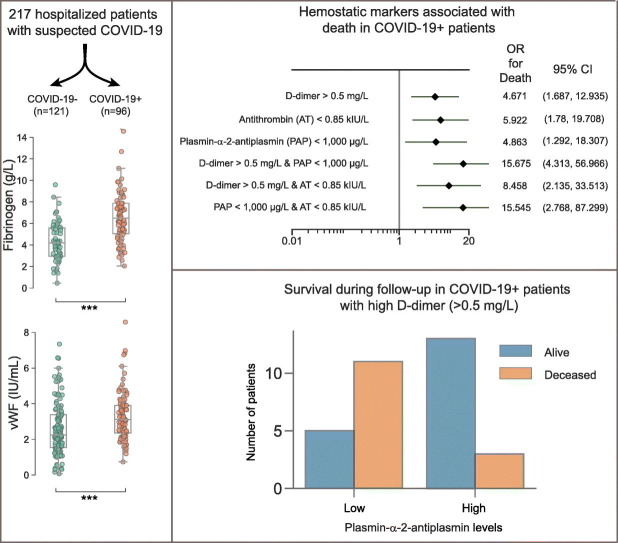
Abstract
In this single-center cohort study, we applied a panel of laboratory markers to characterize hemostatic function in 217 consecutive patients that underwent testing for COVID-19 as they were admitted to Linköping University Hospital between April and June 2020. In the 96 patients that tested positive for SARS-CoV-2 (COVID-19+), the cumulative incidences of death and venous thromboembolism were 24.0% and 19.8% as compared to 12.4% (p = 0.031) and 11.6% (p = 0.13) in the 121 patients that tested negative (COVID-19−). In COVID-19+ patients, we found pronounced increases in plasma levels of von Willebrand factor (vWF) and fibrinogen. Excess mortality was observed in COVID-19+ patients with the following aberrations in hemostatic markers: high D-dimer, low antithrombin or low plasmin-antiplasmin complex (PAP) formation, with Odds Ratios (OR) for death of 4.7 (95% confidence interval (CI95) 1.7–12.9; p = 0.003) for D-dimer >0.5 mg/L, 5.9 (CI95 1.8–19.7; p = 0.004) for antithrombin (AT) ˂0.85 kIU/l and 4.9 (CI95 1.3–18.3; p = 0.019) for PAP < 1000 μg/L. Compounding increases in mortality was observed in COVID-19+ patients with combined defects in markers of fibrinolysis and coagulation, with ORs for death of 15.7 (CI95 4.3–57; p < 0.001) for patients with PAP <1000 μg/L and D-dimer >0.5 mg/L and 15.5 (CI95 2.8–87, p = 0.002) for patients with PAP <1000 μg/L and AT ˂0.85 kIU/L. We observed an elevated fraction of incompletely degraded D-dimer fragments in COVID-19+ patients with low PAP, indicating impaired fibrinolytic breakdown of cross-linked fibrin.
Keywords: COVID-19, Plasmin-antiplasmin complex, Antithrombin, Fibrinolysis
Graphical abstract
1. Introduction
Since the outbreak of the COVID-19 pandemic, several studies have reported on associations between laboratory parameters of hemostasis and the clinical outcomes of hospitalized patients with COVID-19. One of the first publications from China reported an association between increased D-dimer-levels and mortality in COVID-19 [1]. Tang et al. reported that abnormal coagulation parameters were associated with poor prognosis in patients with COVID-19 [2]. Non-survivors displayed significantly higher levels of D-dimer and fibrin degradation products (FDP), longer prothrombin time and activated partial thromboplastin time compared to survivors. Other studies have shown that elevated D-dimer levels are associated with an increased risk of venous thromboembolism (VTE) and/or death in patients with COVID-19 [3], [4]. A high incidence (20–40%) of VTE have been observed despite the use of thromboprophylaxis with low-molecular weight heparins (LMH), especially in patients admitted to ICU [4], [5], [6], [7].
Different biomarkers have been evaluated to assess the thrombo-inflammatory phenotype associated with COVID-19 such as von Willebrand factor (VWF), soluble forms of thrombomodulin (sTM) and P-selectin [8], [9], [10], [11], [12], [13], [14]. Varga et al. demonstrated direct involvement of endothelial cells with SARS-CoV-2 viral elements in three patients with COVID-19 [15]. Autopsy findings from patients with COVID-19 have demonstrated the presence of both microthrombosis and endothelial cell damage [16]. An important role for endotheliopathy in the pathogenesis of COVID-19 has been suggested by data from a cross-sectional study wherein markers of endothelial cell and platelet activation were associated with the severity of COVID-19 [16], [17]. Furthermore, endothelial injury as a result of cell surface glycocalyx degradation was reported by Fraser et al. [18]. In this context it was shown that resting circulating platelets from patients with COVID-19 had a higher expression of P-selectin compared to healthy subjects. These platelets contributed to a procoagulant phenotype by forming aggregates with leukocytes and accelerating factor XII-dependent coagulation [19].
As suggested by Medcalf et al. [20], perturbations in the fibrinolytic system may also contribute to the disease phenotype. However, further clinical investigation of this issue in clinical studies is difficult due to the lack of any single established laboratory marker that can be used to characterize fibrinolytic capacity in COVID-19. Interpretation of the D-dimer test can be difficult, in part because of the lack of standardization of the test. Cross-linked fibrin degrades into a heterogeneous collection of fibrin fragments. Since the specificity for these fragments varies between different tests, direct comparisons can be misleading [21]. For this reason, the D-dimer test itself cannot be viewed as a marker of fibrinolytic activity. Since the size distribution of fibrin fragments to a large extent depends on the formation and elimination of plasmin, combined testing for plasmin-antiplasmin complexes (PAP) and D-dimer can provide additional information regarding fibrinolytic function in relation to coagulation activity. Interestingly, some studies have reported increased concentrations of PAP in patients with COVID-19 compared to healthy controls, indicating increased plasmin generation [14], [22], [23], [24].
In this study, we applied an extensive panel of laboratory markers on a cohort of patients that were hospitalized for symptoms consistent with COVID-19. We then compared the test results and clinical outcomes of patients testing positive and negative for the disease, enabling a thorough characterization of how COVID-19 related changes in hemostasis affect clinical outcomes.
2. Material and methods
The study was performed in accordance with the Helsinki declaration and approved by the Regional Ethics Review Board, Dnr 2020-01908. 217 patients with at least one citrated blood sample available for later analysis were included in the study after being admitted to hospital with a clinical suspicion of COVID-19 from April 1st to June 22nd 2020. 96 out of 217 patients were diagnosed with COVID-19 (93 by PCR on nasal swabs, in three cases the diagnosis of COVID-19 was based on symptoms and the presence of antibodies to SARS CoV-2). In the remaining 121 patients, COVID-19 was ruled out due to negative test results. The clinical outcomes of patients included in the study were monitored until September 7th 2020, when all the surviving patients included in the study had been discharged from hospital.
VTE was diagnosed using ultrasound and/or CT pulmonary angiogram. Thromboprophylaxis with tinzaparin, either using standard (≤4500 IU/24 h, n = 62), intermediate (4500 IU–175 IU/kg/24 h. n = 51) or high dosing (≥175 IU/kg/24 h, n = 13) was administered to patients with COVID-19. Bleeding episodes were classified according to ISTH criteria [25].
Blood counts were analyzed on Cell-Dyn Saphire (Abbott Scandinavia AB, Stockholm, Sweden). Analysis of CRP, creatinine and ferritin was performed on Cobas c501/701 from Roche Diagnostics Scandinavia (Stockholm, Sweden). Procalcitonin measurements were performed on Cobas e 602 from Roche. Activated partial thromboplastin time (APTT), antithrombin, fibrinogen were analyzed on Sysmex C-5100 using reagents from Siemens (Marburg, Germany), and prothrombin time using the Owren method. D-dimer was analyzed on Sysmex using reagents from Medirox (Studsvik, Sweden). To complement routine testing, left-over citrated plasma were stored at −70 °C and then analyzed for von Willebrand factor (vWF), antithrombin, soluble endothelial protein C receptor (sEPCR), soluble thrombomodulin (sTM), soluble P-selectin (sP-selectin), fibrinogen, D-dimer, thrombin-antithrombin complex (TAT) and plasmin-antiplasmin complex (PAP). von Willebrand factor antigen was analyzed with an ELISA from Technoclone (Vienna, Austria), sP-selectin from Merck (Darmstadt, Germany), TAT from Siemens (Marburg, Germany), sTM from R&D Systems Europe (Abingdon, UK), sEPCR from antibodies-on-line (Aachen, Germany) and PAP from Technoclone.
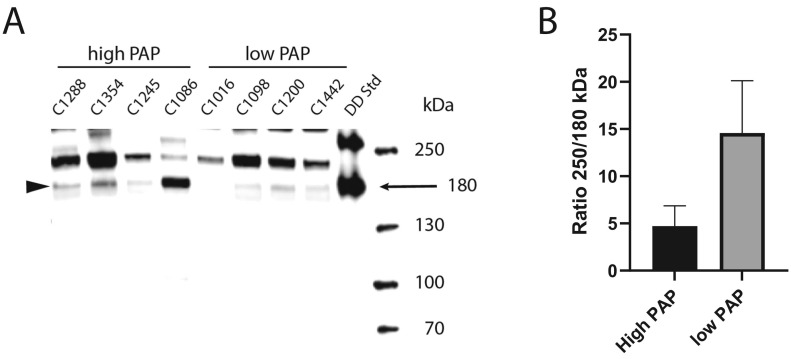
For markers without established reference intervals in our laboratory, plasma samples from 20 healthy volunteers (demographics: age range 18–65 years, sex: 10 men and 10 women, no medication allowed except contraceptives). Identical plasma volumes of 8 plasma samples from COVID-19+ patients with the highest (mean 2698 μg/L) and the lowest levels of PAP (mean 486 μg/L) were run in SDS-gel electrophoresis followed by Western blot using a D-dimer specific monoclonal antibody (AS27, Nordic biomarker, Umeå, Sweden). A D-dimer standard was made by limited plasmic digestion of a cross-linked fibrin clot, to obtain all fragment sizes [26].The intensity ratio of the 180 kDa D-dimer band relative to a 250 kDa band staining for D-dimer epitopes was quantified using Image Lab software, version 5.2.1 (Bio-Rad Laboratories).
Data analysis was performed in the Python programming language v. 3.6 (www.python.org). Missing data were not imputed. For statistical significance testing, the two-sided Mann-Whitney U test was used for comparisons of test results and the two-tailed Fischer's exact test was used for comparisons of categorical variables. Thresholds for statistical significance were set to P < 0.05 and were not adjusted for multiple comparisons.
3. Results
3.1. Baseline characteristics
Baseline patient characteristics are shown in Table 1 . The median age among COVID-19+ patients was significantly lower than that of COVID-19− patients; 63 vs. 72 years, respectively. The proportion of male patients was higher in the COVID-19+ group. Moreover, the median BMI was slightly higher in COVID-19+ patients, and the prevalence of smoking was lower. There were no significant differences in the prevalence of hypertension, cancer and diabetes mellitus between the two groups.
Table 1.
Baseline characteristics (median and range are shown).
| Covid-19− N = 121 |
Covid-19+ N = 96 |
P-value | |
|---|---|---|---|
| Age (median and range) | 72 (19–96) | 63 (25–94) | 0.006 |
| Sex (male/female) | 53.7%/46.3% | 70.8%/29.2% | 0.012 |
| BMI | 26.6 (17.2–60.2) | 27.8 (21.0–65.0) | 0.03 |
| Hypertension | 70.2% | 61.5% | 0.31 |
| Diabetes mellitus | 32 (26.4%) | 34 (35.4%) | 0.18 |
| Heart failure | 39 (32.2%) | 5 (5.2%) | <0.0001 |
| Atrial fibrillation | 35 (28.9%) | 15 (15.6%) | 0.024 |
| Previous venous thromboembolism | 16 (13.2%) | 12 (12.5%) | 1.0 |
| Previous arterial thromboembolism | 47 (38.8%) | 23 (24.0%) | 0.043 |
| Antithrombotic therapy on admission to hospital | 68 (56.2%) | 35 (36.5%) | 0.043 |
| Dyslipidemia | 49 (40.5%) | 35 (36.5%) | 0.58 |
| Cancer* | 29 (24.0%) | 14 (14.6%) | 0.12 |
| Chronic kidney disease | 62 (51.2%) | 34 (35.4%) | 0.027 |
| Smoking ongoing | 22 (18.2%) | 3 (3.1%) | 0.0004 |
However, the prevalence of heart failure, atrial fibrillation as well as previous arterial thromboembolism and chronic kidney disease was significantly higher among COVID-19− patients. A higher proportion of COVID-19− patients were treated with antithrombotic drugs at the time of hospital admission.
3.2. Venous and arterial thrombosis
The incidence of thromboembolic complications during the follow-up period is shown in Table S-1 (Supplementary material). Symptomatic arterial and venous thrombosis occurred in 28.1% of COVID-19+ patients despite administration of thromboprophylaxis with low molecular weight heparin (LMWH). Among all patients with thrombotic complications, pulmonary embolism (PE) was the most common thromboembolic manifestation. The incidences of VTE were 19.8% in COVID-19+ and 11.6% COVID-19− patients (p = 0.127).
3.3. Bleeding
Major bleeding complications according to ISTH criteria [27] were reported in 14.6% and 5,8%, minor bleeding in 5.2% vs 3.3% and clinically relevant non-major bleeding in 9,4% vs 1.7% in the COVID-19+ and COVID-19− patient cohorts, respectively.
3.4. Mortality
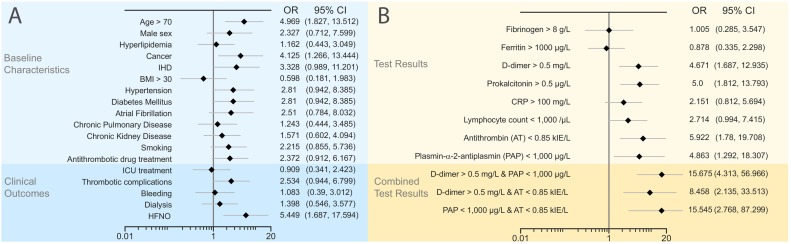
24,0% of COVID-19+ and 12,4% of COVID-19− patients died during the follow-up period (p = 0.0312). The mean age of surviving and deceased COVID-19+ patients was 61.5 and 73.2 years, respectively. Out of the clinical characteristics analyzed in this study, only advanced age (OR 4.97), cancer (OR 4.12) and treatment with high flow nasal oxygen therapy (OR 5.45) were significantly associated with mortality in the COVID-19+ patient cohort (Fig. 1 ).
Fig. 1.
Association of clinical and laboratory parameters with mortality in COVID-19+ patients. Univariate analysis of Odds Ratios for death in COVID-19+ patient subgroups. COVID-19+ patients were dichotomized according to (A) clinical or (B) laboratory parameters. Abbreviations: OR = Odds Ratio, CI = Confidence Interval, IHD = Ischemic heart disease, BMI = Body Mass Index, HFNO = High Flow Nasal Oxygen, CRP = C-reactive Protein.
3.5. Routine laboratory tests
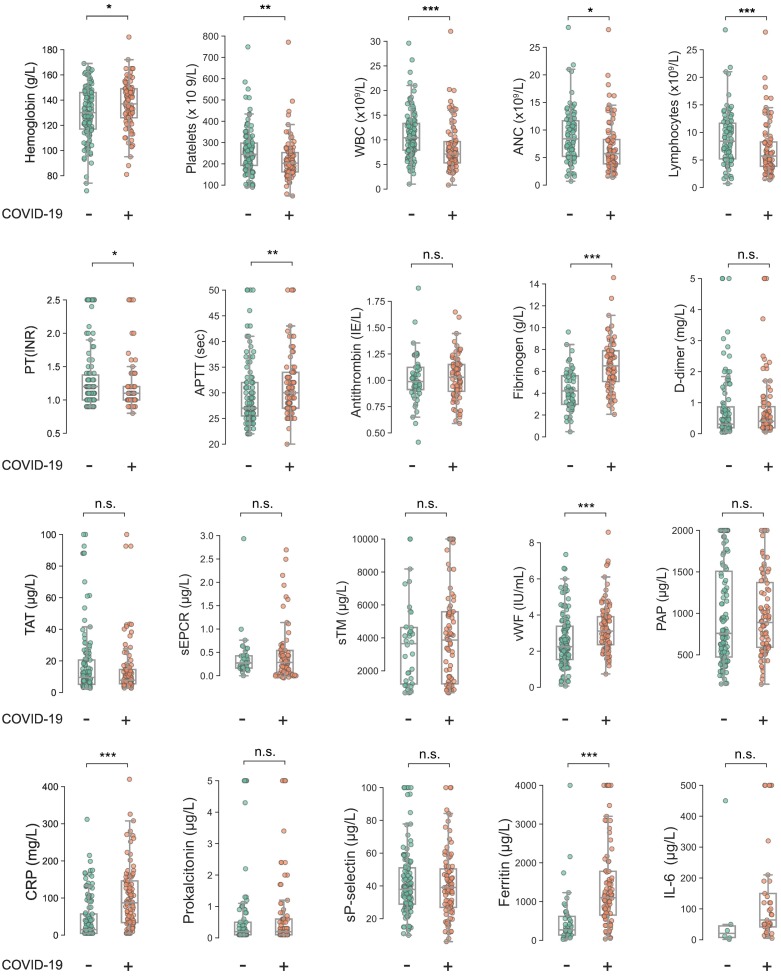
As shown in Fig. 2 and Supplemental Table 2, hemoglobin levels on admission to hospital were 137 g/L (IQR 126–149) and 130 g/L (IQR 117–146) in COVID-19+ and COVID-19− patients respectively (p = 0.018). Platelet counts were significantly lower in COVID-19+ patients:203 × 109/L (IQR 162–253) vs. 244 × 109/L (IQR 192–297) (p < 0.001). White blood cell counts (WBC) were significantly lower in the COVID-19+ group; 7.1 × 109/L (IQR 5.3–9.6) vs. 10.4 × 109/L (IQR 7.9–13.3) (p < 0.0001). Total lymphocyte count (TLC) was also significantly lower in COVID-19+ patients (p < 0.0001). Plasma ferritin levels were significantly higher in COVID-19+ patients: 1045 μg/L (IQR 539–1480) vs. 317 μg/L (IQR 140–598) (p < 0.001). There was no difference in procalcitonin between the groups at hospital admission; 0.2 μg/L (IQR 0.1–0.6) vs 0.2 μg/L (IQR 0.1–0.5) (ns). As shown in Fig. 2, COVID-19+ patients exhibited a slight but significant increase in median APTT (30 vs 27 s, p = 0.003) and PT(INR) (1.2 vs 1.1, p < 0.05) compared to COVID-19− patients. When excluding all patients treated with anticoagulants on admission, the difference in APTT between groups was still significant (p < 0.001), while no significant difference in PT(INR) could be demonstrated (p = 0.49). In the COVID-19+ cohort, patients admitted to ICU exhibited slightly higher PT(INR) and longer APTT compared to patients treated in the routine ward 1.1 (IQR 1.0–1.2) and 32 s (IQR 29–34) vs. 1.0 (IQR 1.0–1.2 ns) and 29 (IQR 26–34) (p = 0.02) sec. Of note, higher dosing regimens of LMH were more frequently used in ICU-patients. CRP levels on admission were significantly higher in COVID-19+ patients compared to COVID-19− patients 87 mg/L (IQR 34–146) vs 15 mg/L (IQR 5–57) (p < 0.001).
Fig. 2.
Distribution of test results in COVID-19+ and COVID-19− patients at hospital admission.*P < 0.05; **P < 0.01; ***P < 0.001; not significant (n.s.).
3.6. Markers of coagulation, fibrinolysis and endothelial activation
At the time of hospital admission, fibrinogen and VWF-antigen levels were significantly higher in the COVID-19+ patient cohort compared to healthy controls (Fig. 2 & Table 2 ). The proportion of results above the upper limit of the reference range was higher than 50% for most variables. The endothelial markers sTM and sEPCR did not differ markedly from the healthy control group, but their distributions were wider and many patients had lower or higher values compared to the healthy controls. The COVID-19− patient cohort displayed a similar pattern of elevated marker levels, although not as pronounced as for the COVID-19+ patients. In particular, fibrinogen was more modestly elevated in COVID-19− patients, almost all results in the 25–75 percentile were within the established reference interval, whereas all of the COVID-19+ patients with fibrinogen in the same IQR had their values well above 4.0 g/L, the upper limit of the normal reference range.
Table 2.
Biomarkers of coagulation, fibrinolysis and endothelial activation in patient cohorts and healthy controls.
| Biomarker | Normal range for healthy controls⁎ | COVID-19− |
COVID-19+ |
p-Value | ||
|---|---|---|---|---|---|---|
| Median value (IQR) | Above/under reference interval (%) | Median value (IQR) | Above/under reference interval (%) | |||
| D-dimer (mg/L) | <0.25 | 0.30 (0.19–0.86) | 57.8/42.2 | 0.40 (0.19–0.87) | 61.3/38.7 | Ns |
| Fibrinogen (g/L) | 2.0–4.0 | 4.2 (3.0–5.6) | 53.3/10.0 | 6.6 (5.1–7.9) | 84.1./0.0 | <0.001 |
| VWF (IU/mL) | 0.45–1.69 | 2.25 (1.54–3.38) | 71.1/5.0 | 3.11 (2.36–3.90) | 92.7/0.0 | <0.001 |
| TAT (μg/L) | 1.33–5.38 | 9.56 (5.22–20.59) | 73.6/0.0 | 7.77 (5.59–14.64) | 77.1/0.0 | ns |
| PAP (μg/L) | 200–583 | 759 (475–1506) | 66.4/2.5 | 890 (591–1371) | 76.1/1.2 | ns |
| sTM (μg/L) | 3.30–5.77 | 3.65 (1.20–4.63) | 16.7/47.2 | 3.87 (1.21–5.58) | 24.4/43.9 | ns |
| sEPCR (μg/L) | 0.20–0.46 | 0.27 (0.16–0.43) | 22.9/37.1 | 0.28 (0.08–0.54) | 31.7/37.8 | ns |
| sP-selectin (μg/L) | 14.3–33.3 | 37.9 (28.9–51.1) | 63.6/2.5 | 39.1 (26.9–50.4) | 62.5/6.2 | ns |
Normal ranges (reference interval) are 2.5–97.5 percentiles of healthy controls. as median and interquartile range (IQR), P: significance values. For D-dimer a decision limit of <0.25 mg/L is used to differentiate between healthy and controls. For fibrinogen it is the reference interval for the hospital laboratory.
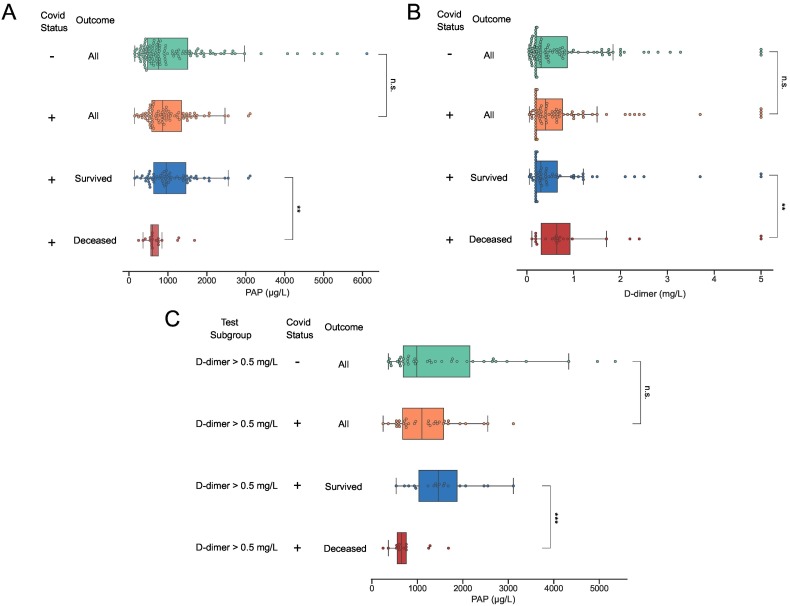
In a subgroup analysis of the COVID19+ patient cohort, some notable differences in the distributions of test results were observed (Table 3 ). COVID-19+ patients admitted to ICU had higher D-dimer (median 0.43 IQR 0.30–1.3 vs 0.26 IQR 0.19–0.66 mg/L, p = 0.013) and vWF levels (median 3.53 IQR 3.06–4.17 vs 2.75 IQR 2.16–3.89 kIU/L, p = 0.008) compared to patients that were treated in a regular hospital ward (Table 3). Significantly higher D-dimer levels on admission were observed in the COVID19+ patient subgroups with thrombotic events (median 0.87 IQR 0.44–1.9 and 0.29 0.19–0.60 mg/L p < 0.001) and in patients that died during follow-up (median 0.64 mg/L IQR 0.31–0.92 and 0.31 mg/L 0.19–0.70; p = 0.040). In addition, baseline CRP and PAP levels differed significantly between patients who died compared to survivors. Median CRP was 141 mg/L (IQR 84–252) in non-survivors compared to 92 mg/L (IQR 30–158) in survivors (p = 0.009). Median PAP concentrations were 604 μg/L (IQR 563–755) in non-survivors and 960 μg/L (IQR 635–1481) in survivors, respectively (p = 0.009). In the COVID-19+ cohort, patients with D-dimer >0.5 mg/L had an OR for death of 4.7 (CI95 1.7–12.9, p = 0.003), and for patients with a level of PAP <1000 μg/L the corresponding OR was 4.9 (CI95 1.3–18.3, p = 0.02) (Fig. 2). For patients with a combination of low PAP and high D-dimer (>0.50 mg/L) the OR for death was 15.7 (CI95 4.3–57; p < 0.001). A similar additive effect on the risk of fatal outcome was seen in patients with low PAP and antithrombin levels below 0.85 kIU/L (OR 15.5 Cl 95 2.8–87.3 p = 0.002) (Fig. 3 ).
Table 3.
Biomarkers in COVID-19+ patients in relation to mortality and thrombosis.
| Biomarker | Non-thrombosis | Thrombosis | P | Non-ICU | ICU | P | Survivors | Non-survivors | P |
|---|---|---|---|---|---|---|---|---|---|
| D-dimer (μg/L) | 0.29 (0.19–0.60) |
0.87 (0.44–1.9) |
<0.001 | 0.26 (0.19–0.66) |
0.43 (0.30–1.30) |
0.013 | 0.31 (0.19–0.70) |
0.64 (0.31–0.92) |
0.040 |
| Fibrinogen (g/L) | 6.5 (5.0–7.9) |
6.8 (5.4–8.0) |
ns | 6.3 (4.6–7.9) |
6.7 (5.7–7.5) |
ns | 6.7 (5.2–7.9) |
5.9 (4.5–7.7) |
Ns |
| VWF (IU/mL) | 3.07 (2.21–3.90) |
3.51 (2.47–4.05) |
ns | 2.75 (2.16–3.89) |
3.53 (3.06–4.17) |
0.008 | 3.07 (2.27–3.90) |
3.47 (2.69–4.10) |
Ns |
| TAT (μg/L) | 7.07 (5.23–14.37) |
9.28 (6.73–16.49) |
ns | 7.30 (5.18–13.81) |
8.34 (6.43–21.18) |
ns | 7.34 (5.23–14.65) |
9.48 (6.59–13.61) |
Ns |
| PAP (μg/L) | 851 (553–1190) |
963 (605–1461) |
ns | 847 (564–1121) |
1023 (606–1533) |
ns | 960 (635–1481) |
604 (563–755) |
0.009 |
| sTM (μg/L) | 4.00 (1.63–5.53) |
1.66 (1.21–6.28) |
ns | 4.08 (1.21–5.61) |
2.20 (1.24–5.58) |
ns | 4.00 (1.18–5.54) |
2.05 (1.28–5.87) |
Ns |
| sEPCR (μg/L) | 0.27 (0.07–0.50) |
0.31 (0.27–0.55) |
ns | 0.27 (0.08–0.47) |
0.37 (0.10–0.54) |
ns | 0.28 (0.07–0.54) |
0.31 (0.08–0.52) |
Ns |
| sP-Selectin (μg/L) | 36.8 (25.9–46.3) |
44.8 (33.3–61.4) |
ns | 36.4 (25.3–46.3) |
44.0 (34.2–52.0) |
ns | 37.4 (25.9–48.3) |
44.4 (32.7–52.0) |
Ns |
| Plt (×10^9/L) | 270 (199–356) |
340 (226–442) |
ns | 262 (197–360) |
330 (208–436) |
ns | 274 (200–416) |
259 (194–316) |
Ns |
| CRP (mg/L) | 100 (32–170) |
105 (54–186) |
ns | 90 (30–161) |
113 (60–176) |
ns | 92 (30–158) |
141 (84–252) |
0.009 |
Results are expressed as median and interquartile range (IQR), P: significance values.
Fig. 3.
Low PAP and high D-dimer at hospital admission in COVID-19 patients who died during follow-up. Distribution of PAP (A & C) and D-dimer (B) at admission in the following patient subgroups: all COVID-19− patients, all COVID-19+ patients, COVID-19+ patients that were still alive at follow-up, and COVID-19+ patients who died during the follow-up period. In (C) only results from patients with D-dimer levels >0.5 mg/L at baseline are included. Boxes indicate the interquartile range with vertical lines denoting median test results. COVID-19 status is indicated with “−” or “+”. *P < 0.05; **P < 0.01; ***P < 0.001; not significant (n.s.).
Plasma samples from COVID-19+ patients with PAP content in each of the extremes (n = 4 for each group) were analyzed on SDS-gel electrophoresis and Western blot for proteins with D-dimer epitopes (Fig. 4 ). To estimate the extent of fibrinolytic activity in the samples, we calculated a ratio between a 250 kDa band representing incompletely degraded cross-linked fibrin, and the 180 kDa band, representing the end product of fibrinolysis. As shown in Fig. 4B, the mean ratio was approximately three times higher in patients with the lowest PAP content than in patients with high PAP (14.6 vs 4.7), indicating incomplete degradation of cross-linked fibrin in these patients. Although this difference was not statistically significant (p = 0.15), it is interesting to note that all four patients with the lowest PAP levels died during hospitalization, whereas all four patients with the highest levels survived during follow-up.
Fig. 4.
Levels of D-Dimer in COVID-19+ patients. Western blotting analysis of D-dimer fragments in plasma samples of COVID-19+ patients. Equal volume of plasma was loaded onto each lane. (A) The D-dimer standard band at 180kDA is indicated by arrow and the corresponding bands in patients with low and high PAP is indicated by arrowhead. (B) Quantification of intensity ratios of the 250kDA/180 kDa band in patients with low and high PAP (+SEM). n = 4 for each group. The difference between groups is not significant.
4. Discussion
In this study, we used a panel of biomarkers to investigate the hemostatic abnormalities associated with infection with SARS-CoV-2. 217 consecutive patients admitted to Linköping University Hospital after initial work-up including testing for COVID-19 were included during the spring of 2020. Due to the scarcity of test kits during the inclusion period, testing was not routinely performed on admission in patients without clinical suspicion of COVID-19. Despite this, a diagnosis of COVID-19 could only be made in 96 patients (COVID-19+), with the remaining 121 patients (COVID-19−) ultimately receiving hospital care for a variety of other conditions. Naturally, a small proportion of these COVID-19− patients may still have been suffering from COVID-19, albeit with a sufficiently low viral load in the nasopharynx to test negative for the virus. Nevertheless, due to the high sensitivity of PCR testing for SARS-CoV-2, we believe that the COVID-19− patient cohort in our study constitute a valuable control group, allowing us to identify laboratory findings that are specific for COVID-19 and not a general feature of patients hospitalized with respiratory and/or infectious conditions.
A wide range of incidences of thromboembolic complications to COVID-19 have been reported, with estimates ranging from 5 to 40% [3], [4], [6], [7], [28], [29]. In our study, symptomatic VTE was diagnosed in 19.8% in patients with COVID-19 during follow-up despite routine administration of thromboprophylaxis with LMWH. In the COVID-19− group, symptomatic VTE was diagnosed in 11.2%. Two potential explanations for the surprisingly high incidence of VTE in the COVID-19− group are that the clinical presentation of pulmonary embolism can be hard to distinguish from that of COVID-19 and that thromboprophylaxis was not routinely administered to these patients during hospitalization,
D-dimer has previously been identified as a valuable biomarker for risk stratification of patients with COVID-19, with prognostic value for several important clinical outcomes such as death [1], ICU treatment and thromboembolic disease [3]. In our cohort, median D-dimer levels in COVID-19+ patients were more than twofold higher in non-survivors compared to survivors (Table 3). When applying a similar cut-off for the D-dimer as in [1], in our case >0.5 mg/L (as this corresponds to 1.0 mg/L with D-dimer values expressed in fibrinogen equivalent units), the OR for death in our study was estimated to 4.7 (1.7–12.9; p = 0.04) for COVID-19+ patients.
It has been shown by others that the fibrinolytic system is affected by COVID-19. In a small study with 23 Spanish patients, of whom 12 were admitted to ICU and 11 to general wards, plasma clot lysis time was longer in the patients than in a control group comprising 20 healthy individuals [12]. Blasi et al. also found that levels of TAT and PAP-complexes were strongly elevated, with median levels that were approximately 5 times higher than those of the healthy controls. We found similar results in COVID-19+ patients, with median TAT- and PAP values of 7.77 μg/mL and 890 μg/L, respectively. These values were 2.9 and 3.2 times higher than the median TAT and PAP values for healthy controls, respectively. However, as we found similar trends in the COVID-19− patient cohort, our results indicate that this increase is not specific for COVID-19, but rather a general feature of patients with acute respiratory and/or infectious disease.
A more important finding in this study is perhaps the association between mortality and low levels of plasmin-antiplasmin (PAP) complexes in COVID-19+ patients. A similar trend was noted by Blasi et al. in a small cohort study of COVID-19 patients [12], reporting a non-insignificant two-fold reduction in median PAP-levels in non-survivors compared to survivors. In contrast, Jin. et al. reported PAP levels that were significantly higher in non-survivors compared to survivors with COVID-19 [22]. Other attempts have also been made to quantify TAT and PAP levels in patients with COVID-19 [14], [17], [22], [23], [24]. Most studies report increased levels of TAT and PAP. Conflicting data have been reported; two studies reported that increased TAT levels that were associated with the severity of COVID-19 [22], [24]. In contrast, two other studies did not report significant differences between groups with different COVID-19 severities [12], [30]. However, direct comparisons between these conflicting results are made difficult by a lack of information on the timing of sample collection and the reagents that were used in the study by Jin et al. [22]. In our study, the negative association found between PAP levels and mortality in COVID-19+ patients was found to be even more pronounced in patients with high levels of D-dimer and/or low levels of antithrombin, as demonstrated by a mortality of 11/16 (69%) in COVID+ patients with low PAP and high D-dimer and 6/8 (75%) in patients with low antithrombin and low PAP during follow up, as compared to 23/96 (24%) in all COVID-19+ patients. This finding indicates that hypercoagulation, combined with reduced plasminogen activity, may have compounding effects on the organ damage caused by dysregulated hemostasis in COVID-19. To investigate the consequences of this combination on the composition of fibrin degradation products, we performed SDS-gel electrophoresis and Western blotting for the D-dimer epitope, finding reduced fractions of the final fibrin degradation product D-dimer in COVID-19+ patients with low PAP.
There is increasing evidence suggesting that endothelial cell injury may play a key role in the COVID-19 associated coagulopathy [31], [32]. A massive elevation of VWF, a factor that is released upon endothelial cell activation, has been reported by several studies [9], [12], [17], [18], [19], [33]. A high VWF activity, combined with a decreased ADAMTS-13, has also been suggested as a contributing mechanism for development of thrombotic complications and as possible predictors of mortality in COVID-19 patients [12], [13], [34]. Our data confirm a massive increase of VWF in COVID-19+ compared to COVID-19− patients (p < 0.001). In our subgroup analysis, we found higher levels of VWF in ICU patients compared to patients treated at routine wards (p = 0.010). We also examined the plasma levels of the soluble endothelial cell receptors sTM and sEPCR, both important players in the natural protein C anticoagulant pathway [35], [36], [37]. Elevated concentrations of sTM and sEPCR are an effect of increased shedding of these receptors from injured endothelial cells as a response to an inflammatory stimulus.
So far, there have been few reports about the role of sTM, and none about sEPCR, in the context of COVID-19 induced endotheliopathy. Cugno et al., demonstrated in a small study in 50 patients with COVID-19 that the sTM concentrations were significantly elevated compared to healthy controls [18]. In their subgroup analysis no significant difference was found between the patients with mild, moderate or severe COVID-19 [33]. Another study by Goshua G et al., comprising 68 COVID-19 patients, reported elevated sTM concentrations compared to a small group of healthy controls but the difference was not statistically significant [17]. However, the authors found an association between the sTM concentration, and the duration of hospital stay. Patients that had sTM concentration below the median value (3.26 ng/mL) were more often discharged from hospital. In our cohort we found that sTM values were moderately higher in COVID-19 patients compared to healthy controls (Table 2) but did not differ in comparison to patients without SARS-CoV-2 infection. To our knowledge, there has been no report on COVID-19 and its effects on the levels of sEPCR. sEPCR is reported to be rapidly increased in inflammatory conditions such as sepsis, but there are also data that indicates that sTM and sEPCR may be differently expressed as they are not always correlated [37], [38]. In our study, we found no significant difference in sEPCR levels between COVID-19+ and COVID-19− patients, and the sEPCR levels in both patient groups were only moderately increased compared to healthy controls.
In a study on patients with Lassa fever [39], a significant correlation was found between sTM and sEPCR in the subgroup with fatal Lassa fever. We could not identify a similar correlation between the sTM and sEPCR in our study, indicating that the endotheliopathy may have a different pathology for different viruses. There are a few, but contradictory, reports about sP-selectin as a marker of COVID-19 and disease severity. Fraser et al. could not find any difference in sP-selectin between patients and healthy controls at admission but they could observe that sP-selectin significantly increased at later stage (day 3) compared to patients negative for the SARS-Cov-2 virus [18]. In another report by Goshua et al., it was shown that sP-selectin was significantly higher in ICU-patients compared with non-ICU patients or controls [17]. A third study reported that sP-selectin was significantly lower in the COVID-19 patients compared to healthy controls [40]. Venter et al. concluded that their reported mass concentration of sP-selectin for the patients with COVID 19 was comparable to the reported values by Goshua et al. but the sP-selectin concentrations in their healthy control population was much higher. In our cohort, we could not find any significant differences in the concentrations of sP-selectin comparing patients with or without COVID-19 or between the different COVID-19 subgroups.
A limitation of our study is the lack of established reference ranges for some of the markers, (i.e. PAP, sEPCR, sP-selectin, TAT and TM) used in our analysis. To compensate for this weakness, we computed provisional reference ranges using samples from healthy controls. However, due to the small sample size and demographic differences between patients and the reference group, these values have to be interpreted cautiously.
In conclusion, our study confirms that many biomarkers of coagulation, endothelial activation and fibrinolysis, are affected by COVID-19. However, our study also shows that many of these biomarkers are similarly altered in hospitalized patients without detectable COVID-19 infection, albeit with some notable differences as the increase in fibrinogen and VWF levels were much more pronounced in our COVID-19+ cohort. From a pathophysiological perspective, our finding that reduced PAP formation together with hyperactive or dysregulated coagulation is strongly predictive of mortality in COVID-19 is especially noteworthy. This finding lends support to the hypothesis that reduced plasmin activity, caused either by diminished release of tPA due to direct viral injury to the endothelium or by exhaustion of plasminogen levels, may render the fibrinolytic system incapable of processing fibrin polymers that are released into the circulation during severe COVID-19 infection. This notion is also supported by our finding that low PAP is associated with decreased levels of fibrinolytic end products in patient plasma, even in the presence of high D-dimer levels. Consequently, the resulting incomplete breakdown of soluble fibrin polymers could contribute to the micro-thrombotic lesions that are characteristic features of severe COVID-19 disease. These findings thus strengthen the rationale for the continued evaluation of treatments that increase fibrinolytic activity in hospitalized patients with COVID-19.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We express our gratitude to the patients and the healthy blood donors and the personal at the department of Clinical Chemistry for saving plasma samples. We are thankful to Maria Wallstedt for help with the analysis of stored samples This work was supported by the Swedish Heart-Lung Foundation (2019-0370 to TL), and Region Östergötland to NB, TL, and MH. The authors have no potential conflicts of interest to declare.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.thromres.2022.03.013.
Appendix A. Supplementary data
Supplementary Information
References
- 1.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb. Haemost. 2020;18(4):844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Middeldorp S., Coppens M., van Haaps T.F., Foppen M., Vlaar A.P., Müller M.C.A., et al. Incidence of venous thromboembolism in hospitalized patients with COVID-19. J. Thromb. Haemost. 2020;18(8):1995–2002. doi: 10.1111/jth.14888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al-Samkari H., Karp Leaf R.S., Dzik W.H., Carlson J.C.T., Fogerty A.E., Waheed A., et al. COVID-19 and coagulation: bleeding and thrombotic manifestations of SARS-CoV-2 infection. Blood. 2020;136(4):489–500. doi: 10.1182/blood.2020006520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Helms J., Tacquard C., Severac F., Leonard-Lorant I., Ohana M., Delabranche X., et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46(6):1089–1098. doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klok F.A., Kruip M., van der Meer N.J.M., Arbous M.S., Gommers D., Kant K.M., et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb. Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jonmarker S., Hollenberg J., Dahlberg M., Stackelberg O., Litorell J., Everhov Å.H., et al. Dosing of thromboprophylaxis and mortality in critically ill COVID-19 patients. Crit. Care. 2020;24(1):653. doi: 10.1186/s13054-020-03375-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bazzan M., Montaruli B., Sciascia S., Cosseddu D., Norbiato C., Roccatello D. Low ADAMTS 13 plasma levels are predictors of mortality in COVID-19 patients. Intern. Emerg. Med. 2020;15(5):861–863. doi: 10.1007/s11739-020-02394-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ladikou E.E., Sivaloganathan H., Milne K.M., Arter W.E., Ramasamy R., Saad R., et al. Von willebrand factor (vWF): marker of endothelial damage and thrombotic risk in COVID-19? Clin Med (Lond). 2020;20(5):e178–e182. doi: 10.7861/clinmed.2020-0346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Escher R., Breakey N., Lämmle B. Severe COVID-19 infection associated with endothelial activation. Thromb. Res. 2020;190:62. doi: 10.1016/j.thromres.2020.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.White D., MacDonald S., Edwards T., Bridgeman C., Hayman M., Sharp M., et al. Evaluation of COVID-19 coagulopathy; laboratory characterization using thrombin generation and nonconventional haemostasis assays. Int. J. Lab. Hematol. 2021;43(1):123–130. doi: 10.1111/ijlh.13329. [DOI] [PubMed] [Google Scholar]
- 12.Blasi A., von Meijenfeldt F.A., Adelmeijer J., Calvo A., Ibañez C., Perdomo J., et al. In vitro hypercoagulability and ongoing in vivo activation of coagulation and fibrinolysis in COVID-19 patients on anticoagulation. J. Thromb. Haemost. 2020;18(10):2646–2653. doi: 10.1111/jth.15043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huisman A., Beun R., Sikma M., Westerink J., Kusadasi N. Involvement of ADAMTS13 and von willebrand factor in thromboembolic events in patients infected with SARS-CoV-2. Int. J. Lab. Hematol. 2020;42(5):e211–e212. doi: 10.1111/ijlh.13244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bouck E.G., Denorme F., Holle L.A., Middelton E.A., Blair A.M., de Laat B., et al. COVID-19 and sepsis are associated with different abnormalities in plasma procoagulant and fibrinolytic activity. Arterioscler. Thromb. Vasc. Biol. 2021;41(1):401–414. doi: 10.1161/ATVBAHA.120.315338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Varga Z., Flammer A.J., Steiger P., Haberecker M., Andermatt R., Zinkernagel A.S., et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395(10234):1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ackermann M., Verleden S.E., Kuehnel M., Haverich A., Welte T., Laenger F., et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N. Engl. J. Med. 2020;383(2):120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goshua G., Pine A.B., Meizlish M.L., Chang C.H., Zhang H., Bahel P., et al. Endotheliopathy in COVID-19-associated coagulopathy: evidence from a single-Centre, cross-sectional study. Lancet Haematol. 2020;7(8):e575–e582. doi: 10.1016/S2352-3026(20)30216-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fraser D.D., Patterson E.K., Slessarev M., Gill S.E., Martin C., Daley M., et al. Endothelial injury and glycocalyx degradation in critically ill coronavirus disease 2019 patients: implications for microvascular platelet aggregation. Crit Care Explor. 2020;2(9) doi: 10.1097/CCE.0000000000000194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taus F., Salvagno G., Canè S., Fava C., Mazzaferri F., Carrara E., et al. Platelets promote thromboinflammation in SARS-CoV-2 pneumonia. Arterioscler. Thromb. Vasc. Biol. 2020;40(12):2975–2989. doi: 10.1161/ATVBAHA.120.315175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Medcalf R.L., Keragala C.B., Myles P.S. Fibrinolysis and COVID-19: a plasmin paradox. J. Thromb. Haemost. 2020;18(9):2118–2122. doi: 10.1111/jth.14960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dempfle C.E. Validation, calibration, and specificity of quantitative D-dimer assays. Semin. Vasc. Med. 2005;5(4):315–320. doi: 10.1055/s-2005-922476. [DOI] [PubMed] [Google Scholar]
- 22.Jin X., Duan Y., Bao T., Gu J., Chen Y., Li Y., et al. The values of coagulation function in COVID-19 patients. PLoS One. 2020;15(10) doi: 10.1371/journal.pone.0241329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ranucci M., Sitzia C., Baryshnikova E., Di Dedda U., Cardani R., Martelli F., et al. Covid-19-associated coagulopathy: biomarkers of thrombin generation and fibrinolysis leading the outcome. J. Clin. Med. 2020;9(11) doi: 10.3390/jcm9113487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Umemura Y., Yamakawa K., Kiguchi T., Nishida T., Kawada M., Fujimi S. Hematological phenotype of COVID-19-induced coagulopathy: far from typical sepsis-induced coagulopathy. J. Clin. Med. 2020;9(9) doi: 10.3390/jcm9092875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaatz S., Ahmad D., Spyropoulos A.C., Schulman S. Definition of clinically relevant non-major bleeding in studies of anticoagulants in atrial fibrillation and venous thromboembolic disease in non-surgical patients: communication from the SSC of the ISTH. J. Thromb. Haemost. 2015;13(11):2119–2126. doi: 10.1111/jth.13140. [DOI] [PubMed] [Google Scholar]
- 26.Francis C.W.M., Bralow G.H. Plasmic degradation of crosslinked fibrin. J. Clin. Invest. 1980;66:1033–1043. doi: 10.1172/JCI109931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schulman S., Kearon C. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J. Thromb. Haemost. 2005;3(4):692–694. doi: 10.1111/j.1538-7836.2005.01204.x. [DOI] [PubMed] [Google Scholar]
- 28.Sjöström A., Wersäll J.D., Warnqvist A., Farm M., Magnusson M., Oldner A., et al. Platelet count rose while D-dimer levels dropped as deaths and thrombosis declined-an observational study on anticoagulation shift in COVID-19. Thromb. Haemost. 2021;121(12):1610–1621. doi: 10.1055/a-1477-3829. [DOI] [PubMed] [Google Scholar]
- 29.Lodigiani C., Iapichino G., Carenzo L., Cecconi M., Ferrazzi P., Sebastian T., et al. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb. Res. 2020;191:9–14. doi: 10.1016/j.thromres.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.White D., MacDonald S., Bull T., Hayman M., de Monteverde-Robb R., Sapsford D., et al. Heparin resistance in COVID-19 patients in the intensive care unit. J. Thromb. Thrombolysis. 2020;50(2):287–291. doi: 10.1007/s11239-020-02145-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Katneni U.K., Alexaki A., Hunt R.C., Schiller T., DiCuccio M., Buehler P.W., et al. Coagulopathy and thrombosis as a result of severe COVID-19 infection: a microvascular focus. Thromb. Haemost. 2020;120(12):1668–1679. doi: 10.1055/s-0040-1715841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sardu C., Gambardella J., Morelli M.B., Wang X., Marfella R., Santulli G. Hypertension, thrombosis, kidney failure, and diabetes: is COVID-19 an endothelial Disease? A comprehensive evaluation of clinical and basic evidence. J. Clin. Med. 2020;9(5) doi: 10.3390/jcm9051417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cugno M., Meroni P.L., Gualtierotti R., Griffini S., Grovetti E., Torri A., et al. Complement activation and endothelial perturbation parallel COVID-19 severity and activity. J. Autoimmun. 2021;116 doi: 10.1016/j.jaut.2020.102560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krishnamachary B., Cook C., Spikes L., Chalise P., Dhillon N.K. The potential role of extracellular vesicles in COVID-19 associated endothelial injury and pro-inflammation. medRxiv. 2020 doi: 10.1101/2020.08.27.20182808. [DOI] [Google Scholar]
- 35.Dahlbäck B., Villoutreix B.O. The anticoagulant protein C pathway. FEBS Lett. 2005;579(15):3310–3316. doi: 10.1016/j.febslet.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 36.Orfanos S.E., Hirsch A.M., Giovinazzo M., Armaganidis A., Catravas J.D., Langleben D. Pulmonary capillary endothelial metabolic function in chronic thromboembolic pulmonary hypertension. J. Thromb. Haemost. 2008;6(8):1275–1280. doi: 10.1111/j.1538-7836.2008.03046.x. [DOI] [PubMed] [Google Scholar]
- 37.Kurosawa S., Stearns-Kurosawa D.J., Carson C.W., D'Angelo A., Della Valle P., Esmon C.T. Plasma levels of endothelial cell protein C receptor are elevated in patients with sepsis and systemic lupus erythematosus: lack of correlation with thrombomodulin suggests involvement of different pathological processes. Blood. 1998;91(2):725–727. [PubMed] [Google Scholar]
- 38.Boomsma M.M., Stearns-Kurosawa D.J., Stegeman C.A., Raschi E., Meroni P.L., Kurosawa S., et al. Plasma levels of soluble endothelial cell protein C receptor in patients with Wegener's granulomatosis. Clin. Exp. Immunol. 2002;128(1):187–194. doi: 10.1046/j.1365-2249.2002.01803.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Horton L.E., Cross R.W., Hartnett J.N., Engel E.J., Sakabe S., Goba A., et al. Endotheliopathy and platelet dysfunction as hallmarks of fatal Lassa fever. Emerg. Infect. Dis. 2020;26(11):2625–2637. doi: 10.3201/eid2611.191694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Venter C., Bezuidenhout J.A., Laubscher G.J., Lourens P.J., Steenkamp J., Kell D.B., et al. Erythrocyte, platelet, serum ferritin, and P-selectin pathophysiology implicated in severe hypercoagulation and vascular complications in COVID-19. Int. J. Mol. Sci. 2020;21(21) doi: 10.3390/ijms21218234. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information