Abstract
BACKGROUND:
Impulsivity is a defining characteristic of attention-deficit/hyperactivity disorder (ADHD), which has been associated with substance use disorders, higher accident rates, and lower educational and occupational outcomes. The meso- and nigrostriatal pathways of the dopamine system are hypothesized to be functionally heterogeneous, supporting diverse cognitive functions and impairments, including those associated with ADHD. We tested whether human midbrain pathways (where dopaminergic cell bodies originate) between the substantia nigra (SN) and ventral tegmental area (VTA) and the striatum differed between participants with ADHD and typically developing adolescent and young adult participants. We also assessed whether pathway connectivity predicted impulsivity regardless of diagnosis.
METHODS:
Diffusion tensor imaging data were used to predict impulsivity (parent and self-report ratings, task-based behavioral measures) from participants with ADHD and typically developing adolescent and young adult participants (n = 155; 86 male, 69 female). Using probabilistic tractography, we mapped these pathways and divided the tracts into limbic, executive, and sensorimotor based on frontostriatal connectivity. ADHD and typically developing participants differed on all behavioral measures of impulsivity. We used correlation and machine learning analyses to test for a relationship between tract probabilities and impulsivity regardless of diagnosis.
RESULTS:
Participants with ADHD had stronger structural connectivity between SN/VTA regions and the limbic striatum, weaker connectivity with the executive striatum, and no significant differences in sensorimotor tracts. Increased tract integrity between the limbic striatal and SN/VTA regions predicted greater impulsivity, while increased integrity between executive striatal and SN/VTA regions predicted reduced impulsivity.
CONCLUSIONS:
These findings support the theory that functional diversity in the dopamine system is an important consideration for understanding dysfunction in ADHD.
In the context of attention-deficit/hyperactivity disorder (ADHD), impulsivity is associated with higher rates of suicidality and substance use disorders. ADHD has been linked to dysfunction of the dopamine system. Animal studies have shown that meso- and nigrostriatal pathways are functionally heterogeneous. We report that tracts between the human limbic and executive striatum and the substantia nigra (SN) and ventral tegmental area (VTA) differed between adolescent and young adult individuals with and without ADHD and also predicted impulsivity regardless of diagnosis. Increased limbic connectivity was associated with greater impulsivity; the inverse relationship was found for executive connectivity. These results comport with hypotheses that SN/VTA-striatal circuits provide a neurobiological pathway for affective signals to influence executive control, and ultimately behavior, including the negative outcomes associated with impulsivity, particularly in adolescents and young adults with ADHD.
Limbic and Executive Meso- and Nigrostriatal Tracts Predict Impulsivity in ADHD
Individuals with extreme impulsivity, such as in ADHD combined presentation, are noted for elevated levels of self-harm behaviors (e.g., suicide attempts) (1,2), gambling and substance use disorders (3,4), higher rates of accidents (5), and premature death (6). The worldwide annual economic burden associated with ADHD is in the hundreds of billions of dollars (7). Impulsivity is particularly concerning during adolescence and young adulthood, as choices during this period can have enduring consequences.
The dopamine system has long been implicated in impulsivity and ADHD (8), in addition to other cognitive functions and behavioral processes (9–12). The diversity of dopamine-dependent cognitive effects is presumed to derive from processes supported by efferent targets of dopamine neurons (13).
Connectivity between the frontal cortex and striatum is defined by heterogeneous corticostriatal loops that subserve sensorimotor, executive, and limbic functions. These loops are arranged in a dorsoventral configuration, with each structural loop maintaining the functional characteristics of the target cortical region (14–16). The striatum has been divided into limbic, executive, and motor subdivisions (17) based on frontal connectivity (18). These subdivisions also likely reflect separable pathways from midbrain dopaminergic regions. The homogeneity of dopamine release measured with positron emission tomography in response to the administration of amphetamine (medication commonly used to treat ADHD) (19) is significantly higher within these functional subdivisions of the striatum compared to within anatomical subdivisions (i.e., putamen, caudate, and nucleus accumbens) (18).
Evidence for segregation in midbrain dopamine-related neuron pathways derives primarily from animal work. The primary anatomical subdivision within the SN/VTA divides neurons into dorsal and ventral tiers (20). The dorsal tier includes the VTA and the dorsal SN pars compacta (SNc), while the ventral tier includes the densocellular region and the cell columns that extend deep into the SN pars reticulata. Projections to the striatum have a medial/lateral and inverse ventral/dorsal topographical arrangement from the VTA, SNc, and SN pars reticulata (21–24). The sensorimotor and executive dorsal striatum receive inputs primarily from the ventral midbrain, and the ventral striatum (limbic) is innervated primarily by dorsal tier, the dorsal part of the ventral tier, and the dorsomedial SN pars reticulata (21,24–26). We used these findings as a priori predictions about the arrangement of tracts with end points in the human SN/VTA and striatum [c.f., (18)].
The anatomical subdivisions of the SN/VTA are hypothesized to retain the functional properties of their efferent projections. Limbic, executive, and motor functions are evident in the firing properties of neurons across functional subdivisions of SN/VTA. Single-unit recordings in animals have shown that cells in the VTA and medial SNc encode reward signals, characteristic of limbic processing. By contrast, neurons in more ventrolateral cells respond to salient events, which is expected for modulation of executive processes (27). Cells in the most ventrolateral regions of the SNc that innervate the sensorimotor putamen are crucial for motor control. A characteristic of Parkinson’s disease is preferential loss of these cells (28).
Increased impulsivity, a central behavioral manifestation of ADHD, is proposed to derive from dopaminergic deficits (8) that produce an imbalance in limbic and executive control systems [e.g., (29)]. In this dual pathway model, the reward system drives impulses while the executive control system evaluates and regulates these impulses (30–33). Identification of SN/VTA-striatum tracts allowed us to test two hypotheses about the dual pathway model of ADHD: 1) that individuals with ADHD and typically developing (TD) individuals differ in the structural integrity of limbic and executive tracts and 2) that the integrity of the SN/VTA-striatum tracts differentially predicts impulsivity, regardless of diagnosis. We used diffusion tensor imaging (DTI) combined with probabilistic tractography to assess the connectivity of midbrain pathways consistent with the dopamine signaling system in adolescent and young adult participants by defining streamlines with end points in regions of the SN/VTA and the limbic, executive, or sensorimotor striatum (18).
METHODS AND MATERIALS
Participants
We recruited 160 adolescent and young adult participants, 5 of whom (1 participant with ADHD and 4 TD participants) were dropped from analyses for not having any successful DTI streamlines reach to one or more striatal targets, leaving a final sample of 155 participants (12–24 years of age; mean age = 16.52 years, SD = 3.38 years). Of these, 74 were diagnosed with ADHD and significant impulsivity (50 male, 24 female). The remaining 81 were TD (36 male, 45 female). Detailed demographic information can be found in the Supplement. The number of subjects recruited for this study was determined by power calculations for a larger parent study examining the relationship between cognitive control, reward processing, and delay discounting in ADHD. This article is the result of a planned exploratory analysis.
Behavioral Measures of Impulsivity
Impulsivity was measured using surveys and task-based measures. Participants completed the adolescent or adult version of the Barratt Impulsiveness Scale depending on age (34). The parents of the participants completed either the Conners Adult ADHD Rating Scales–Observer or the Conners Parent Rating Scale. Adult participants also completed the Conners Adult ADHD Rating Scales–Self-Report for supplemental information. The Zimbardo Time Perspective Inventory was administered, and we were particularly interested in two factors–present hedonism and future orientation–that have been shown to be associated with impulsivity (35). Finally, participants completed a delay discounting task in which they made a series of choices between smaller, immediate and delayed, larger monetary rewards (36). The percentage of “smaller sooner” preferences was used as an index of impulsivity.
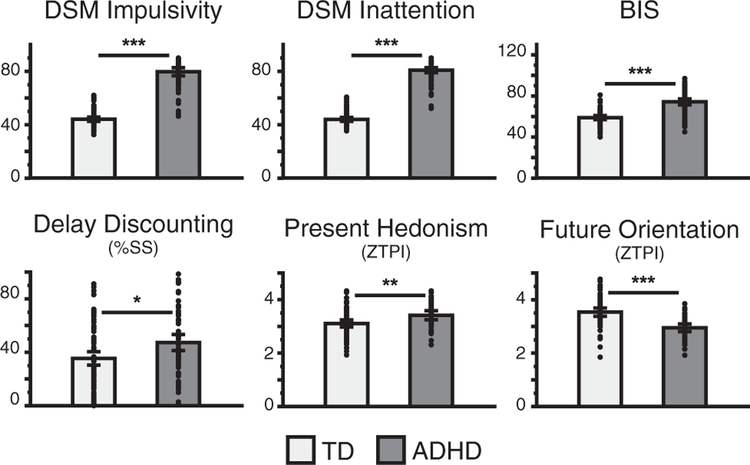
The differences in behavioral measures between the participants with ADHD and TD participants were assessed using separate independent-sample t tests with Bonferroni correction for multiple comparisons (Figure 1). Owing to the large number and diversity of behavioral assessments, impulsivity measures were aggregated into one latent factor using maximum likelihood factor analysis. Instead of principal component analysis, which extracts orthogonal components, we used maximum likelihood factor analysis, which accounts for covariance among variables. This method is more appropriate for estimating latent factors (37). Graphical examination of the scree plot using the scree test revealed one meaningful factor (Figure S1) (38). We interpreted the first factor as a latent construct representing impulsivity (see the Supplement for additional details). We used this factor score as our measure of impulsivity for correlational analyses with tract strength measures.
Figure 1.
Behavioral measures of impulsivity for participants with attention-deficit/hyperactivity disorder (ADHD) and typically developing (TD) participants. Individual points are subjects. Error bars represent 95% confidence interval. *p < .05, **p < .01, ***p < .001. %SS, percent of smaller sooner choices; BIS, Barratt Impulsiveness Scale; ZTPI, Zimbardo Time Perspective Inventory.
Magnetic Resonance Imaging Preprocessing and Analysis
Diffusion-Weighted Magnetic Resonance Imaging.
Diffusion-weighted data were processed using the FMRIB Software Library (www.fmrib.ox.ac.uk/fsl). Measures of tract strength were calculated using probabilistic tractography (39,40). Because previous studies have shown that the structural integrity of specific subsets of striatal tracts is related to different components of behavior (41,42), the goal of our structural connectivity analysis was to identify and quantify specific tracts with end points in the SN/VTA and striatum (limbic, executive, and motor regions). Because the dorsal and ventral tiers of the SN and VTA have not yet been resolved in humans with magnetic resonance imaging, we use the term “SN/VTA-striatum tracts” to describe meso- and nigrostriatal tracts. The use of this term is not meant to imply directionality, because diffusion-weighted imaging data do not include information about whether tracts are efferent or afferent. We tested whether the SN/VTA-striatum tracts differed among the participants with ADHD and TD participants. We also related integrity measures of these tracts to individual differences in impulsivity.
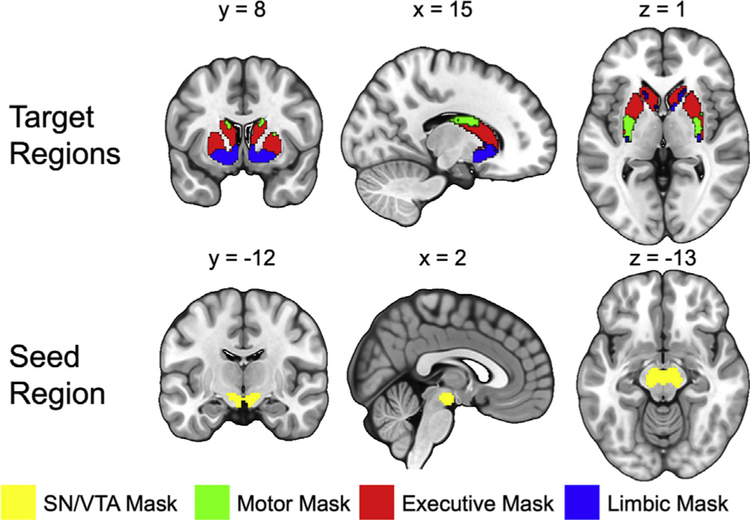
Fiber tracking was conducted in parallel for each voxel within a predefined SN/VTA seed mask. Target areas in the striatum were defined using a connectivity-based segmentation atlas with subdivisions for limbic, executive, and sensorimotor regions available in FSL (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/Atlases/striatumconn). Because amphetamine-induced dopamine release measured with positron emission tomography is significantly more homogeneous when the striatum is functionally parcellated based on cortical targets instead of anatomical regions like caudate, putamen, and nucleus accumbens, we used function-based regions of interest (ROIs) based on Tziortzi et al. (18). The seed ROI for the SN/VTA was defined using a probabilistic atlas of human SN/VTA (Figure 2) (43).
Figure 2.
Seed region and target regions for the tractography analysis. The target regions of the striatum were functionally segmented based on projections to motor, executive, and limbic cortices [from Tziortzi et al. (18)]. The seed region of the midbrain was generated from a probabilistic substantia nigra (SN)/ventral tegmental area (VTA) mask [from Murty et al. (43)]. The probabilistic mask was thresholded at 50% and binarized to create the seed mask for tractography analysis. These masks were then tailored to each subject’s T1-weighted anatomical scan.
Tractography was performed separately for the left and right striatum, and possible tracts were restricted to the hemisphere of origin using an exclusion mask of the contralateral hemisphere. This resulted in six value maps per participant, one for each target region and each hemisphere (3 striatal regions × 2 hemispheres). We used the mean of these value maps as the measure of SN/VTA-striatum tract strength.
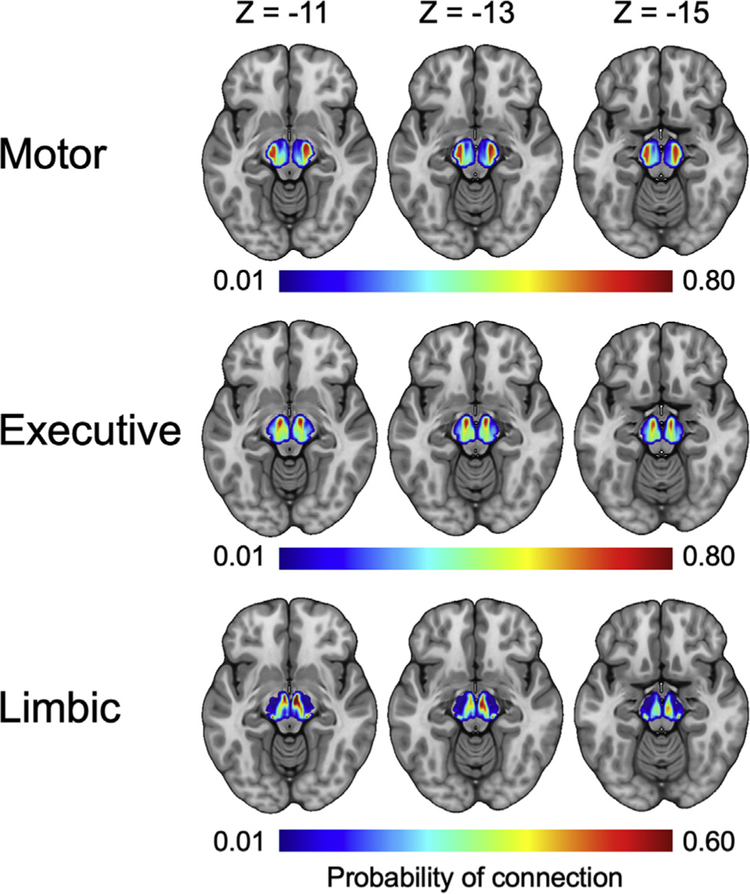
To determine how the SN/VTA-striatum tracts were organized across participants, individual probability maps were normalized into Montreal Neurological Institute space. Each map was then thresholded so that only voxels that had at least 50 samples reaching any target region were included. The resulting maps were averaged to create six value maps for the entire sample of participants (Figure 3). A detailed description of the imaging acquisition, procedure, and analysis can be found in the Supplement.
Figure 3.
Group average projections from the three functional striatum targets (motor, executive, limbic) to the substantia nigra/ventral tegmental area. Each column corresponds to a different axial plane. A threshold of 50 samples was applied to each participant’s image prior to averaging.
Tract Strength Differences Between Participants With ADHD and TD Participants.
To test for differences in tract strength between the participants with ADHD and TD participants, we calculated the mean of the tract probability within each individually determined SN/VTA-striatum segment (three per hemisphere, six in total). We used multivariate analysis of covariance for this calculation. Age and intracranial volume were included as covariates. The six tract strength measures were the dependent variables, and a positive ADHD diagnosis was the independent variable.
The ultimate goal of cognitive neuroscience is to be able to predict important aspects of behavior from brain structure and function. Prediction is difficult with generalized linear model approaches due to well-known overfitting problems. Therefore, machine learning (i.e., least absolute shrinkage and selection operator [LASSO] regression) (44) was used to predict ADHD diagnosis from the six SN/VTA-striatum tract strength measures. We used LASSO instead of other possible techniques because it is well suited to magnetic resonance imaging data (45). The measures were controlled for age and intracranial volume by regressing each measure (impulsivity, motor, executive, and limbic tract strength in each hemisphere) onto age and intracranial volume. The residuals from the seven regressions were used for the LASSO regression. We implemented a leave-one-subject-out cross-validation loop (46) in R version 3.6.2 (R Foundation for Statistical Computing) with the glmnet package using a binomial response type for the binary dependent variable (i.e., positive ADHD diagnosis) (47). Mean LASSO regression coefficients and confidence intervals (CIs) were calculated as the mean of the coefficients across each of the leave-one-out fits (i.e., jackknife resampling). Coefficients were considered significantly different from zero if the 95% CIs did not contain zero.
Tract Strength and Impulsivity.
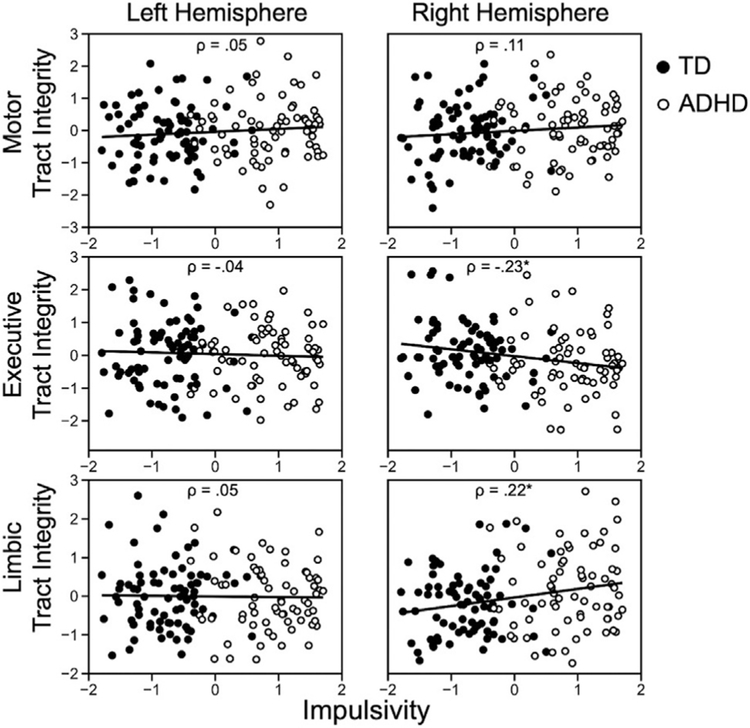
In addition to testing for tract strength differences categorically (between participants with ADHD and TD participants), we also tested where tract strength measures were correlated with behavioral measures of impulsivity regardless of diagnosis. There is increasing effort to consider disorders like ADHD to be reflective of extremes of a continuous underlying cognitive difference (e.g., impulsivity). To test whether individual differences in impulsivity were related to SN/VTA-striatum tract strength, Spearman’s rank-order correlations were calculated between tract strength measures and the behavioral factor score of impulsivity (the tract strength values are not normally distributed, so nonparametric correlations are appropriate) (Figure 4). Age and intracranial volume were included as covariates. Bonferroni correction was applied for all six comparisons (3 targets × 2 hemispheres). Results were considered statistically significant if they were less than the Bonferroni corrected value of .008. Machine learning (i.e., LASSO regression) (44) was used to predict impulsivity from the six SN/VTA-striatum tract strength measures. This analysis mirrored the LASSO regression predicting ADHD diagnosis, except that the model now implemented a Gaussian response type for the continuous dependent variable.
Figure 4.
Spearman’s rank-order correlations between impulsivity and substantia nigra/ventral tegmental area-striatum tract strength measure from the three functional striatum targets (motor, executive, limbic), controlling for age and intracranial volume. ADHD, attention-deficit/hyperactivity disorder; TD, typically developing.
RESULTS
Participants with ADHD and TD participants differed across all behavioral and rating scale measures of impulsivity (Figure 1, Table 1); thus, we calculated an aggregate impulsivity score using maximum likelihood factor analysis to extract the shared variance among the behavioral measures. Table 2 displays the factor loadings and communalities for each of the six behavioral measures. One primary factor emerged that had an eigenvalue of 2.84 and explained 47.40% of the variance. All measures that correlated positively with impulsivity had positive factor loadings, and the one measure that correlated negatively with impulsivity (Zimbardo Time Perspective Inventory future orientation) had a negative factor loading, as expected. We interpreted this factor to represent impulsivity, with greater scores reflecting more impulsive decision making, and used the factor score for further analysis.
Table 1.
Descriptive Statistics and Independent-Samples t Tests for the Behavioral Measures
| Behavioral Measure | ADHD Group | TD Group | t | df | Cohen’s d | ||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||||
| DSM Impulsivity | 79.66 | 12.45 | 43.95 | 7.07 | 21.45a | 142 | 3.53 |
| DSM Inattention | 80.83 | 9.53 | 43.81 | 6.35 | 27.68a | 142 | 4.57 |
| BIS | 74.27 | 11.82 | 58.68 | 8.45 | 9.26a | 144 | 1.52 |
| Delay Discounting | 47.26 | 27.06 | 35.70 | 24.04 | 2.82b | 153 | 0.45 |
| Present Hedonism | 3.42 | 0.54 | 3.10 | 0.53 | 2.95b | 101 | 0.60 |
| Future Orientation | 2.95 | 0.46 | 3.54 | 0.61 | −5.36a | 101 | 1.09 |
DSM impulsivity and DSM inattention are derived from the Conners Parent Rating Scale or Conners Adult ADHD Rating Scales subscales. Delay discounting is derived from the percent of smaller sooner choices. Present hedonism is derived from the Zimbardo Time Perspective Inventory present hedonism, and future orientation is derived from the Zimbardo Time Perspective Inventory future orientation. Differences in degrees of freedom across measures reflect missing data.
ADHD, attention-deficit/hyperactivity disorder; BIS, Barratt Impulsiveness Scale; TD, typically developing.
p < .001.
p < .01.
Table 2.
Factor Loadings and Communalities Based on Maximum Likelihood Estimation Factor Analysis
| Impulsivity (Factor 1) | Factor 2 | Communality | |
|---|---|---|---|
| DSM Impulsivity | 0.909 | 0.034 | 0.817 |
| DSM Inattention | 0.991 | −0.021 | 0.831 |
| BIS | 0.599 | 0.287 | 0.422 |
| Delay Discounting | 0.145 | −0.167 | 0.061 |
| Present Hedonism | 0.255 | 0.787 | 0.215 |
| Future Orientation | −0.422 | −0.325 | 0.258 |
DSM impulsivity and DSM inattention are derived from the Conners Parent Rating Scale or Conners Adult ADHD Rating Scales subscales. Delay discounting is derived from the percent of smaller sooner choices. Present hedonism is derived from the Zimbardo Time Perspective Inventory present hedonism, and future orientation is derived from the Zimbardo Time Perspective Inventory future orientation. Differences in degrees of freedom across measures reflect missing data.
ADHD, attention-deficit/hyperactivity disorder; BIS, Barratt Impulsiveness Scale.
Functional Organization of the SN/VTA
When tracts with end points in the midbrain SN/VTA and functionally parcellated striatal regions were classified, three subdivisions emerged within the midbrain SN/VTA (Figure 3). The organization of these subdivisions was consistent with known anatomy in nonhuman primates (13).
We performed two parallel analyses to test for differences in SN/VTA-striatum tracts based on group (ADHD vs. TD) and based on a continuous relationship with our aggregate measure of impulsivity. These analyses are not independent, but instead are separate ways to test for differences in SN/VTA-striatum connectivity related to ADHD. We conducted the two parallel sets of analyses for completeness.
First, to test for group differences in tract strength between the participants with ADHD and TD participants, we calculated the mean of the tract probabilities within each individually determined SN/VTA-striatum tract segment (three per hemisphere, six in total). We implemented multivariate analysis of covariance, with age and intracranial volume as covariates, the six tract strength measures as dependent variables, and a positive ADHD diagnosis as the independent variable. When controlling for age and intracranial volume, the executive and limbic SN/VTA-striatum tract strength measures differed between participants with ADHD and TD participants, albeit in opposite directions (Table 3). In the right hemisphere, participants with ADHD had greater structural connectivity in the limbic SN/VTA-striatum tract compared with TD participants (F1,154 = 8.860, p < .01 Bonferroni corrected, ηp2 = 0.056) but decreased structural connectivity in the executive tract (F1,154 = 4.292, p < .05 Bonferroni corrected, ηp2 = 0.028). This difference was only significant in the right hemisphere. No differences were found in sensorimotor tracts across the two groups (Table 3).
Table 3.
Group Comparisons of Tract Integrity Controlling for Age and Intracranial Volume
| TD | ADHD | Difference (TD-ADHD) | F | Partial η2 | |||
|---|---|---|---|---|---|---|---|
| EMM | SEM | EMM | SEM | ||||
| Left Motor | 0.429 | 0.010 | 0.439 | 0.011 | −0.011 | 0.473 | 0.003 |
| Left Executive | 0.470 | 0.011 | 0.457 | 0.012 | 0.013 | 0.592 | 0.004 |
| Left Limbic | 0.139 | 0.008 | 0.145 | 0.009 | −0.006 | 0.234 | 0.002 |
| Right Motor | 0.443 | 0.009 | 0.455 | 0.009 | −0.012 | 0.837 | 0.006 |
| Right Executive | 0.444 | 0.010 | 0.414 | 0.010 | 0.030a | 4.292 | 0.028 |
| Right Limbic | 0.165 | 0.007 | 0.196 | 0.007 | −0.031b | 8.860 | 0.056 |
ADHD, attention-deficit/hyperactivity disorder; EMM, estimated marginal mean; SEM, standard error of the mean; TD, typically developing.
p < .01 (Bonferroni corrected).
p < .001 (Bonferroni corrected).
To test whether tract strength predicted ADHD diagnosis, we used machine learning techniques (binomial LASSO) to predict ADHD diagnosis in individual subjects. The leave-one-subject-out cross-validation analysis showed that SN/VTA-striatum tract strengths measures significantly predicted ADHD diagnosis (Spearman’s ρ = 0.22, p < .01, 2-tailed). We calculated 95% CIs on the LASSO regression coefficients using jackknife resampling. CIs did not include zero only for the right limbic (β = 0.21; 95% CI, 0.14 to 0.27) and right executive (β = –0.17; 95% CI, –0.23 to –0.08) tracts.
ADHD may be considered an extreme of variation in a behavioral phenotype presenting on a continuum that varies dimensionally across all people. One candidate behavioral measure that may partially define ADHD is impulsivity. We conducted a second set of analyses to determine whether individual differences in impulsivity were related to SN/VTA-striatum tract strength, regardless of diagnosis. We computed Spearman’s rank-order correlations between the tract strength measures and aggregate impulsivity score. Age and intracranial volume were included as covariates. Individual differences in impulsivity were negatively associated with tract strength between the SN/VTA and executive striatum (Spearman’s ρ = –0.23, p < .05, Bonferroni corrected). Conversely, individual differences in impulsivity were positively associated with tract strength between the SN/VTA and limbic striatum (Spearman’s ρ = 0.22, p < .05, Bonferroni corrected). These results were again significant in the right hemisphere only.
While the correlations revealed distinct relationships between SN/VTA-striatum tracts and impulsivity, correlations lack predictive power. To resolve this, we used LASSO to predict impulsivity in individual subjects. The results from the leave-one-subject-out cross-validation analysis revealed that SN/VTA-striatum tract strength measures significantly predict impulsivity (Spearman’s ρ = 0.17, p < .05, 2-tailed). We calculated 95% CIs on the LASSO regression coefficients using jackknife resampling. CIs did not include zero only for the right limbic (β = 0.07; 95% CI, 0.05 to 0.10) and right executive (β = –0.13; 95% CI, –0.16 to –0.11) tracts.
Overall, our results suggest that strength of limbic SN/VTA-striatum tracts in the participants with ADHD was greater than in the TD group, and executive SN/VTA-striatum tracts in the participants with ADHD were less than in the TD group. The strengths of these tracts were predictive of impulsivity, suggesting that observed anatomical differences contribute to the observed differences in impulsivity between the ADHD and TD groups.
DISCUSSION
The dopamine system has long been implicated in impulsivity and ADHD [e.g., (48,49)]. This association derives in large part from the fact that stimulant medications selective for dopamine receptors are effective and commonly used treatments for ADHD (50). Data also show that dopamine agonists acutely increase impulsivity (51). However, details about how the dopamine system relates to impulsivity and symptoms of ADHD is still actively being investigated. This study provides a new contribution to this field, demonstrating that dopamine-related limbic and executive tracts from midbrain nuclei (SN/VTA) differentially predict impulsivity and ADHD.
The anatomy of human midbrain neurons and their afferent and efferent projections remains understudied. Confirmation of connectivity patterns requires study of postmortem tissue. However, anatomy in nonhuman primates indicates that dopamine neurons can be partitioned into dorsal and ventral tiers based on cell morphology and connectivity patterns (26,52). There is an overall connectivity pattern such that medial regions of SN/VTA are interconnected with ventral regions of striatum. This pattern of connectivity was shown in our tractography using DTI, giving us confidence in the projection patterns estimated in our dataset. However, it is important to note that human DTI is too coarse-grained of a technique to distinguish dopaminergic SN/VTA projections from other proximal, and often intermingled, pathways.
The functional organization of SN/VTA neurons has been argued to derive from striatal connectivity, which in turn depends on corticostriatal connectivity (13). We characterized the organization of the human midbrain SN/VTA in terms of its connections to limbic, executive, and motor striatal regions (18). It is unclear whether these are appropriate categorical subdivisions, or whether the striatum includes more or even continuous distributions of function. The limbic, executive, and motor distinctions fit with common distinctions of frontal cortex function [e.g., (53)] and have been shown to have greater homogeneity of dopamine release than structural subdivisions (18). We feel that this separation of functions is a reasonable current approximation and may be particularly appropriate when considering relationships between brain networks and behavior such as impulsivity. Impulsivity has long been believed to depend on interactions between affective drive (limbic) and controlled regulation of those drives (executive) (54,55).
We used these tract integrity measures to test whether connections of the limbic and executive striatal circuits to SN/VTA regions differed between individuals with ADHD and TD control subjects. Compared with the control group, the ADHD group had lower tract integrity values between SN/VTA and executive striatum. They also had greater tract integrity values between SN/VTA and limbic striatum. The tract integrity values associated with tracts connecting the executive and limbic striatum with midbrain SN/VTA regions were also predictive of individual differences in impulsivity. Individuals with higher connectivity in the executive circuit had lower impulsivity scores. Conversely, individuals with higher connectivity in the limbic circuit had higher impulsivity scores. These results are in line with hypotheses that the spiraling SN/VTA-striatum circuits provide a neurobiological pathway for affective signals to influence executive control signals and ultimately behavior (13). They also support the Research Domain Criteria framework that clinically important phenotypes such as impulsivity may exist on a spectrum with common biological substrates, and that clinical diagnoses like ADHD reflect extremes of these phenotypes.
Previous studies have used DTI to show that corticostriatal circuits are differentially related to impulsivity (35,42,56). It is intriguing to consider that the true physiological basis of impulsivity may be large-scale networks that span numerous brain regions. This would fit with findings from whole-brain functional connectivity analyses showing long-range networks (57). It remains a future objective to determine the full extent of brain networks related to impulsivity. Relatedly, we conducted exploratory meditation analyses to determine whether tract strength accounted for the relationship between ADHD and impulsivity. These analyses were not significant and were not reported in the Results. We suspect that ADHD and impulsivity is mediated by brain connectivity but that the SN/VTA-striatum tracts that we studied are only a part of the patterns of connectivity that underlie this relationship.
Our study found significant effects between SN/VTA-striatum connectivity and impulsivity in the right hemisphere only. Although we did not have a priori hypotheses regarding lateralization of effects, previous studies have also found significant effects in only the right hemisphere of the brain in studies of ADHD (58–60). Some researchers have hypothesized that ADHD is primarily a right hemisphere syndrome (61) or is an imbalance between right and left hemispheres (62). The localization of effects to the right hemisphere in this study are consistent with this lateralization hypothesis.
In addition to striatal regions, midbrain dopamine neurons have diverse projections to frontal, parietal, occipital, and temporal cortices (63,64). Midbrain dopamine neurons also project to many midline structures such as the hippocampus, amygdala, hypothalamus, and periaqueductal gray. These projections might have functionally distinct roles in regulating a plethora of cognitive functions (for example, the VTA-hippocampal circuit is hypothesized to control the encoding of information into long-term memory) (10,65,66). We expect that other cognitive processes with known dependence on dopamine function specifically depend on some of these other projection pathways.
One limitation of this study is that a subset of the ADHD group included participants with a history of medication prescribed for their ADHD. Although participants were free of ADHD medication for five half-lives before the imaging data were acquired, the effects of prior medication use are unknown. Given that stimulant medication in individuals with ADHD has been shown to reduce behavioral symptomatology and might be associated with normalization of brain structure abnormalities (67), the heterogeneity in medication history could have affected the findings or added noise to the results. The addition of a drug-naive group would mitigate this concern but is challenging in practice.
Although participants were excluded from the study if they did not achieve a Full Scale IQ score >80 (see the Supplement for more information), an independent-samples t test revealed a significant difference in IQ scores between participants with ADHD (mean = 106.68, SD = 13.22) and TD participants (mean = 114.67, SD = 11.34) (t150 = –4.01, p < .001, Cohen’s d = 12.26 [3 participants with ADHD were missing scores]). Although differences in IQ have been previously observed in the literature (68), this observed difference is a limitation in the current study.
Another limitation is potential sex differences between groups. Although the current study aimed to recruit close to an equal number of males and females per group as possible, this is difficult in practice given that males are about three times more likely to be diagnosed with ADHD than females (69). Our sample included 68% males in the ADHD group and 44% males in the TD group. This is an area of growing interest, as there are known differences in ADHD symptoms between males and females as well (e.g., males typically show externalized symptoms, whereas females show internalized symptoms) (70). Future research should investigate neuroanatomical differences related to sex in ADHD.
Impulsivity is a central component of ADHD but does not capture the full manifestation of the disorder. We took these issues into account in developing the inclusion and exclusion criteria and during the subject recruitment and evaluation process to mitigate potential confounding clinical differences. Additionally, we acknowledge that impulsivity may be an aggregate construct [e.g., (71)] with multiple underlying behavioral and neural constructs. Our data indicate that a single factor best described variability across participants on our numerous measures. We also note that impulsivity is a common focus for the etiology of ADHD. Even if a construct different from impulsivity truly defined ADHD, or if a subcomponent of impulsivity is most relevant to ADHD, we believe that our results contribute significantly to understanding of the disorder.
This work provides novel insight into the architecture of connections between the dopamine-related human midbrain pathways and striatum. The strength of the connections between the limbic and executive striatum and midbrain SN/VTA differed between the ADHD and TD groups and also predicted individual differences in impulsive behavior. These results provide evidence for differential neuroanatomical circuits that manifest impulsive behavior and ADHD symptomatology. Neuroimaging techniques could potentially be used in the future to identify neuroanatomical subtypes of ADHD. Because previous studies have shown that the reward system develops earlier than the executive control system (32,72–75), investigating the neuroanatomy of this system across development as it relates to ADHD symptomatology will be an exciting future direction of research.
Supplementary Material
ACKNOWLEDGMENTS AND DISCLOSURES
This work was supported by the National Science Foundation Graduate Research Fellowships Program (to BLE), National Science Foundation Grant No. 1634179 (to SMM), and National Institute of Mental Health Grant No. 2 R01 MH091068 (to JBS and SMM).
The authors thank J. Faye Dixon, Ph.D., for assistance with the diagnostic procedure and Murat Pakyurek for screening for medical concerns. The authors acknowledge the kind support of all their research participants, as well as of Catherine Fassbender, Ph.D.; Tadeus Arthur Hartanto, M.B.A.; Catrina A. Calub, M.A.; Amrita Ramakrishnan; Erin Calfee, M.A.; Lauren Boyle, Ph.D.; Laurel Cavallo; Maria B.E. Menor; Jessica Nguyen; and Steven J. Riley, M.A.
Footnotes
The authors report no biomedical financial interests or potential conflicts of interest.
Supplementary material cited in this article is available online at https://doi.org/10.1016/j.bpsc.2021.05.002.
Contributor Information
Blake L. Elliott, Department of Psychology, Arizona State University, Tempe, Arizona
Kimberlee D’Ardenne, Department of Psychology, Arizona State University, Tempe, Arizona.
Prerona Mukherjee, Department of Psychiatry and Behavioral Sciences, University of California, Davis, Sacramento, California; MIND Institute, University of California, Davis, Sacramento, California.
Julie B. Schweitzer, Department of Psychiatry and Behavioral Sciences, University of California, Davis, Sacramento, California MIND Institute, University of California, Davis, Sacramento, California.
Samuel M. McClure, Department of Psychology, Arizona State University, Tempe, Arizona
REFERENCES
- 1.Hinshaw SP, Owens EB, Zalecki C, Huggins SP, Montenegro-Nevado AJ, Schrodek E, et al. (2012): Prospective follow-up of girls with attention-deficit/hyperactivity disorder into early adulthood: Continuing impairment includes elevated risk for suicide attempts and self-injury. J Consult Clin Psychol 80:1041–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang K-L, Wei H-T, Hsu J-W, Bai Y-M, Su T-P, Li C-T, et al. (2018): Risk of suicide attempts in adolescents and young adults with attention-deficit hyperactivity disorder: A nationwide longitudinal study. Br J Psychiatry 212:234–238. [DOI] [PubMed] [Google Scholar]
- 3.Breyer JL, Botzet AM, Winters KC, Stinchfield RD, August G, Realmuto G (2009): Young adult gambling behaviors and their relationship with the persistence of ADHD. J Gambl Stud 25:227–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kalbag AS, Levin FR (2005): Adult ADHD and substance abuse: Diagnostic and treatment issues. Subst Use Misuse 40:1955–1981. [DOI] [PubMed] [Google Scholar]
- 5.Ruiz-Goikoetxea M, Cortese S, Aznarez-Sanado M, Magallón S, Zallo NA, Luis EO, et al. (2018): Risk of unintentional injuries in children and adolescents with ADHD and the impact of ADHD medications: A systematic review and meta-analysis. Neurosci Biobehav Rev 84:63–71. [DOI] [PubMed] [Google Scholar]
- 6.Dalsgaard S, Østergaard SD, Leckman JF, Mortensen PB, Pedersen MG (2015): Mortality in children, adolescents, and adults with attention deficit hyperactivity disorder: A nationwide cohort study. Lancet 385:2190–2196. [DOI] [PubMed] [Google Scholar]
- 7.Doshi JA, Hodgkins P, Kahle J, Sikirica V, Cangelosi MJ, Setyawan J, et al. (2012): Economic impact of childhood and adult attention-deficit/hyperactivity disorder in the United States. J Am Acad Child Adolesc Psychiatry 51:990–1002. e1002. [DOI] [PubMed] [Google Scholar]
- 8.Swanson JM, Kinsbourne M, Nigg J, Lanphear B, Stefanatos GA, Volkow N, et al. (2007): Etiologic subtypes of attention-deficit/hyperactivity disorder: Brain imaging, molecular genetic and environmental factors and the dopamine hypothesis. Neuropsychol Rev 17:39–59. [DOI] [PubMed] [Google Scholar]
- 9.Braver TS, Cohen JD (2000): On the control of control: The role of dopamine in regulating prefrontal function and working memory. In: Monsell S, Driver J, editors. Control of Cognitive Processes: Attention and Performance XVIII. Cambridge, MA: MIT Press, 713–737. [Google Scholar]
- 10.Lisman JE, Grace AA (2005): The hippocampal-VTA loop: Controlling the entry of information into long-term memory. Neuron 46:703–713. [DOI] [PubMed] [Google Scholar]
- 11.McClure SM, Berns GS, Montague PR (2003): Temporal prediction errors in a passive learning task activate human striatum. Neuron 38:339–346. [DOI] [PubMed] [Google Scholar]
- 12.Schultz W, Dayan P, Montague PR (1997): A neural substrate of prediction and reward. Science 275:1593–1599. [DOI] [PubMed] [Google Scholar]
- 13.Haber SN, Knutson B (2010): The reward circuit: Linking primate anatomy and human imaging. Neuropsychopharmacology 35:4–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haber SN (2003): The primate basal ganglia: Parallel and integrative networks. J Chem Neuroanat 26:317–330. [DOI] [PubMed] [Google Scholar]
- 15.Haber SN, Kim KS, Mailly P, Calzavara R (2006): Reward-related cortical inputs define a large striatal region in primates that interface with associative cortical connections, providing a substrate for incentive-based learning. J Neurosci 26:8368–8376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parent A, Hazrati L-N (1995): Functional anatomy of the basal ganglia. I. The cortico-basal ganglia-thalamo-cortical loop. Brain Res Rev 20:91–127. [DOI] [PubMed] [Google Scholar]
- 17.Piray P, den Ouden HE, van der Schaaf ME, Toni I, Cools R (2017): Dopaminergic modulation of the functional ventrodorsal architecture of the human striatum. Cereb Cortex 27:485–495. [DOI] [PubMed] [Google Scholar]
- 18.Tziortzi AC, Haber SN, Searle GE, Tsoumpas C, Long CJ, Shotbolt P, et al. (2014): Connectivity-based functional analysis of dopamine release in the striatum using diffusion-weighted MRI and positron emission tomography. Cereb Cortex 24:1165–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Faraone SV, Biederman J, Roe C (2002): Comparative efficacy of Adderall and methylphenidate in attention-deficit/hyperactivity disorder: A meta-analysis. J Clin Psychopharmacol 22:468–473. [DOI] [PubMed] [Google Scholar]
- 20.Haber S, Ryoo H, Cox C, Lu W (1995): Subsets of midbrain dopaminergic neurons in monkeys are distinguished by different levels of mRNA for the dopamine transporter: Comparison with the mRNA for the D2 receptor, tyrosine hydroxylase and calbindin immunoreactivity. J Comp Neurol 362:400–410. [DOI] [PubMed] [Google Scholar]
- 21.Hedreen JC, Delong MR (1991): Organization of striatopallidal, striatonigral, and nigrostriatal projections in the macaque. J Comp Neurol 304:569–595. [DOI] [PubMed] [Google Scholar]
- 22.Lynd-Balta E, Haber S (1994): The organization of midbrain projections to the striatum in the primate: Sensorimotor-related striatum versus ventral striatum. Neuroscience 59:625–640. [DOI] [PubMed] [Google Scholar]
- 23.Selemon L, Goldman-Rakic P (1990): Topographic intermingling of striatonigral and striatopallidal neurons in the rhesus monkey. J Comp Neurol 297:359–376. [DOI] [PubMed] [Google Scholar]
- 24.Szabó J (1979): Strionigral and nigrostriatal connections. Stereotact Funct Neurosurg 42:9–12. [DOI] [PubMed] [Google Scholar]
- 25.Haber SN, Fudge JL, McFarland NR (2000): Striatonigrostriatal pathways in primates form an ascending spiral from the shell to the dorsolateral striatum. J Neurosci 20:2369–2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lynd-Balta E, Haber S (1994): Primate striatonigral projections: A comparison of the sensorimotor-related striatum and the ventral striatum. J Comp Neurol 345:562–578. [DOI] [PubMed] [Google Scholar]
- 27.Bromberg-Martin ES, Matsumoto M, Hikosaka O (2010): Dopamine in motivational control: Rewarding, aversive, and alerting. Neuron 68:815–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moore RY (2003): Organization of midbrain dopamine systems and the pathophysiology of Parkinson’s disease. Parkinsonism Relat Disord 9:65–71. [DOI] [PubMed] [Google Scholar]
- 29.Sonuga-Barke EJ (2002): Psychological heterogeneity in AD/HD—A dual pathway model of behaviour and cognition. Behav Brain Res 130:29–36. [DOI] [PubMed] [Google Scholar]
- 30.Barkley RA (1997): Behavioral inhibition, sustained attention, and executive functions: Constructing a unifying theory of ADHD. Psychol Bull 121:65–94. [DOI] [PubMed] [Google Scholar]
- 31.Castellanos FX, Sonuga-Barke EJ, Milham MP, Tannock R (2006): Characterizing cognition in ADHD: Beyond executive dysfunction. Trends Cogn Sci 10:117–123. [DOI] [PubMed] [Google Scholar]
- 32.Shulman EP, Smith AR, Silva K, Icenogle G, Duell N, Chein J, et al. (2016): The dual systems model: Review, reappraisal, and reaffirmation. Dev Cogn Neurosci 17:103–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sonuga-Barke EJ (2003): The dual pathway model of AD/HD: An elaboration of neuro-developmental characteristics. Neurosci Biobehav Rev 27:593–604. [DOI] [PubMed] [Google Scholar]
- 34.Barratt ES (1965): Factor analysis of some psychometric measures of impulsiveness and anxiety. Psychol Rep 16:547–554. [DOI] [PubMed] [Google Scholar]
- 35.Van Den Bos W, Rodriguez CA, Schweitzer JB, McClure SM (2015): Adolescent impatience decreases with increased frontostriatal connectivity. Proc Natl Acad Sci U S A 112:E3765–E3774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kirby KN (1997): Bidding on the future: Evidence against normative discounting of delayed rewards. J Exp Psychol Gen 126:54–70. [Google Scholar]
- 37.Preacher KJ, MacCallum RC (2003): Repairing Tom Swift’s electric factor analysis machine. Understand Stat 2:13–43. [Google Scholar]
- 38.Cattell RB (1966): The scree test for the number of factors. Multivariate Behav Res 1:245–276. [DOI] [PubMed] [Google Scholar]
- 39.Behrens TE, Berg HJ, Jbabdi S, Rushworth MF, Woolrich MW (2007): Probabilistic diffusion tractography with multiple fibre orientations: What can we gain? Neuroimage 34:144–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Behrens TE, Woolrich MW, Jenkinson M, Johansen-Berg H, Nunes RG, Clare S, et al. (2003): Characterization and propagation of uncertainty in diffusion-weighted MR imaging. Magn Reson Med 50:1077–1088. [DOI] [PubMed] [Google Scholar]
- 41.Cohen MX, Schoene-Bake J-C, Elger CE, Weber B (2009): Connectivity-based segregation of the human striatum predicts personality characteristics. Nat Neurosci 12:32–34. [DOI] [PubMed] [Google Scholar]
- 42.van den Bos W, Rodriguez CA, Schweitzer JB, McClure SM (2014): Connectivity strength of dissociable striatal tracts predict individual differences in temporal discounting. J Neurosci 34:10298–10310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Murty VP, Shermohammed M, Smith DV, Carter RM, Huettel SA, Adcock RA (2014): Resting state networks distinguish human ventral tegmental area from substantia nigra. Neuroimage 100:580–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tibshirani R (1996): Regression shrinkage and selection via the lasso. J R Stat Soc Series B Stat Methodol 58:267–288. [Google Scholar]
- 45.Wager TD, Atlas LY, Leotti LA, Rilling JK (2011): Predicting individual differences in placebo analgesia: Contributions of brain activity during anticipation and pain experience. J Neurosci 31:439–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hastie T, Tibshirani R, Friedman J (2009): The Elements of Statistical Learning: Data Mining, Inference, and Prediction. New York: Springer Science & Business Media. [Google Scholar]
- 47.Friedman J, Hastie T, Tibshirani R (2010): Regularization paths for generalized linear models via coordinate descent. J Stat Softw 33:1. [PMC free article] [PubMed] [Google Scholar]
- 48.Castellanos FX, Swanson J (2002): Biological underpinnings of ADHD. Hyperact Atten Disord Childhood 2:336–366. [Google Scholar]
- 49.Volkow ND, Wang G-J, Newcorn J, Fowler JS, Telang F, Solanto MV, et al. (2007): Brain dopamine transporter levels in treatment and drug naive adults with ADHD. Neuroimage 34:1182–1190. [DOI] [PubMed] [Google Scholar]
- 50.Barkley RA (2015): Attention Deficit Hyperactivity Disorder: A Handbook for Diagnosis and Treatment – IV Edition. New York: Guilford Press. [Google Scholar]
- 51.Pine A, Shiner T, Seymour B, Dolan RJ (2010): Dopamine, time, and impulsivity in humans. J Neurosci 30:8888–8896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fallon JH, Moore RY (1978): Catecholamine innervation of the basal forebrain IV. Topography of the dopamine projection to the basal forebrain and neostriatum. J Comp Neurol 180:545–579. [DOI] [PubMed] [Google Scholar]
- 53.Szczepanski SM, Knight RT (2014): Insights into human behavior from lesions to the prefrontal cortex. Neuron 83:1002–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Loewenstein G (1996): Out of control: Visceral influences on behavior. Organ Behav Hum Decis Process 65:272–292. [Google Scholar]
- 55.McClure SM, Laibson DI, Loewenstein G, Cohen JD (2004): Separate neural systems value immediate and delayed monetary rewards. Science 306:503–507. [DOI] [PubMed] [Google Scholar]
- 56.Peper JS, Mandl RC, Braams BR, De Water E, Heijboer AC, Koolschijn PCM, et al. (2013): Delay discounting and frontostriatal fiber tracts: A combined DTI and MTR study on impulsive choices in healthy young adults. Cereb Cortex 23:1695–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yeo BT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, et al. (2011): The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol 106:1125–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ashtari M, Kumra S, Bhaskar SL, Clarke T, Thaden E, Cervellione KL, et al. (2005): Attention-deficit/hyperactivity disorder: A preliminary diffusion tensor imaging study. Biol Psychiatry 57:448–455. [DOI] [PubMed] [Google Scholar]
- 59.Geeraerts S, Lafosse C, Vaes N, Vandenbussche E, Verfaillie K (2008): Dysfunction of right-hemisphere attentional networks in attention deficit hyperactivity disorder. J Clin Exp Neuropsychol 30:42–52. [DOI] [PubMed] [Google Scholar]
- 60.Sandson TA, Bachna KJ, Morin MD (2000): Right hemisphere dysfunction in ADHD: Visual hemispatial inattention and clinical subtype. J Learn Disabil 33:83–90. [DOI] [PubMed] [Google Scholar]
- 61.Stefanatos GA, Wasserstein J (2001): Attention deficit/hyperactivity disorder as a right hemisphere syndrome: Selective literature review and detailed neuropsychological case studies. Ann N Y Acad Sci 931:172–195. [PubMed] [Google Scholar]
- 62.Hale TS, Loo SK, Zaidel E, Hanada G, Macion J, Smalley SL (2009): Rethinking a right hemisphere deficit in ADHD. J Atten Disord 13:3–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gaspar P, Stepniewska I, Kaas J (1992): Topography and collateralization of the dopaminergic projections to motor and lateral prefrontal cortex in owl monkeys. J Comp Neurol 325:1–21. [DOI] [PubMed] [Google Scholar]
- 64.Lidow MS, Goldman-Rakic PS, Gallager D, Rakic P (1991): Distribution of dopaminergic receptors in the primate cerebral cortex: Quantitative autoradiographic analysis using [3H] raclopride,[3H] spiperone and [3H] SCH23390. Neuroscience 40:657–671. [DOI] [PubMed] [Google Scholar]
- 65.Lisman J, Grace AA, Duzel E (2011): A neoHebbian framework for episodic memory; role of dopamine-dependent late LTP. Trends Neurosci 34:536–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rutishauser U (2019): Testing models of human declarative memory at the single-neuron level. Trends Cogn Sci 23:510–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nakao T, Radua J, Rubia K, Mataix-Cols D (2011): Gray matter volume abnormalities in ADHD: Voxel-based meta-analysis exploring the effects of age and stimulant medication. Am J Psychiatry 168:1154–1163.. [DOI] [PubMed] [Google Scholar]
- 68.Kuntsi J, Eley T, Taylor A, Hughes C, Asherson P, Caspi A, et al. (2004): Co-occurrence of ADHD and low IQ has genetic origins. Am J Med Genet Part B Neuropsychiat Genet 124:41–47. [DOI] [PubMed] [Google Scholar]
- 69.Danielson ML, Visser SN, Chronis-Tuscano A, DuPaul GJ (2018): A national description of treatment among United States children and adolescents with attention-deficit/hyperactivity disorder. J Pediatr 192:240–246.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rucklidge JJ (2010): Gender differences in attention-deficit/hyperactivity disorder. Psychiatr Clin North Am 33:357–373. [DOI] [PubMed] [Google Scholar]
- 71.Caswell AJ, Bond R, Duka T, Morgan MJ (2015): Further evidence of the heterogeneous nature of impulsivity. Pers Individ Diff 76:68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Casey BJ (2015): Beyond simple models of self-control to circuit-based accounts of adolescent behavior. Annu Rev Psychol 66:295–319.. [DOI] [PubMed] [Google Scholar]
- 73.Casey BJ, Jones RM, Hare TA (2008): The adolescent brain. Ann N Y Acad Sci 1124:111–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Steinberg L (2008): A social neuroscience perspective on adolescent risk-taking. Dev Rev 28:78–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.van Duijvenvoorde ACK, Achterberg M, Braams BR, Peters S, Crone EA (2016): Testing a dual-systems model of adolescent brain development using resting-state connectivity analyses. Neuroimage 124:409–420. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.