Abstract
Aim
To explore the association between the serum C1q/tumor necrosis factor-related protein 9 (CTRP9) and the formation of coronary collateral circulation in obstructive coronary artery disease (CAD).
Methods
A total of 206 patients who underwent coronary angiography at Beijing Anzhen Hospital and had epicardial arteries with at least 95% stenotic lesion were enrolled. Blood samples were taken after an overnight fasting before the coronary angiography. Serum CTRP9 level was measured using commercial enzyme linked immunosorbent assay (ELISA) kit. The development of coronary collateralization was determined according to the Rentrop classification system. Rentrop score 0-1 was graded as impaired or less-developed coronary collateralization (n = 54) while the Rentrop score 2-3 was defined as well-developed collateralization (n = 152).
Results
Serum CTRP9 level was significantly higher in well-developed collateralization and diabetes groups (P < 0.001). To further explore the association between the CTRP9 level and coronary collateralization, the enrolled participants were divided into 3 tertiles according to the serum CTRP9 level. The prevalence of impaired coronary collateralization decreased stepwise with the increasing CTRP9 tertiles (P for trend <0.001). Multivariate regression analysis showed that the serum CTRP9 is independently associated with well-developed collateralization, with an OR (95% CI) of 4.49 (1.75-11.55) and 8.98 (2.75-29.35) in the tertiles 2 and 3, respectively. The following subgroup and receiver-operating characteristic (ROC) analysis also indicated that the diagnostic value of serum CTRP9 level for detecting the formation of collateralization persisted only in nondiabetic participants. Lastly, adding the serum CTRP9 into the baseline model could increase the diagnostic value of established model consisting of relevant factor for the discrimination of well-developed collateralization only in the nondiabetic group (P = 0.046).
Conclusions
Serum CTRP9 reflects well-developed coronary collateralization in nondiabetic patients with obstructive CAD, and CTRP9 level ≥ 1.217 indicated a greater chance to forming well-developed coronary collaterals.
1. Introduction
CAD remained to be the leading cause of death worldwide [1]. Despite the great advance in the management and treatment of these patients, be it the optimal medical therapy (OMT) or invasive revascularization, there are still a certain number of patients suffering from recurrent refractory angina pectoris due to the diffuse coronary artery lesion, absent conduits used for coronary artery bypass grafting (CABG), or coronary distal vessel with small diameter that is ineligible for vascular revascularization [2]. Those patients continued to experience restricted physical capacity and declining quality of life. There are some alternative strategies for those patients, such as coronary sinus reducer implantation [3], spinal cord stimulation [4], or cardiac shock wave therapy [5]; yet, the treatment efficacy is still limited. Coronary collateralizations are networks of arterial-to-arterial anastomotic connections that have the potential to provide blood supply against the ischemia injury caused by severely stenotic or even occluded coronary vessels [6]. Well-developed coronary collateralization could exert myocardial salvaging effect, limit the ischemia territory, improve the cardiac function, and ameliorate the prognosis of patients [7]. Hence, therapies targeting at stimulating the coronary collateral growth (CCG) could be a promising strategy for those patients. However, a large proportion of patients in real clinical setting tended to develop impaired or less-developed coronary collateralization that limits the collateral capacity to provide sufficient blood flow in the event of coronary artery occlusion [8, 9]. The underlying mechanisms are still poorly understood.
CTRP9, defined as the closest paralog of adiponectin (APN) in the C1q/tumor necrosis factor-related protein (CTRPs) family, is highly expressed in the heart and the adipose tissue [10]. Similar to the APN not only in structure, CTRP9 has also been demonstrated to share high similarity in metabolic function with that of APN [11]. Previous studies have shown the strong association between the serum CTRP9 and the coronary atherosclerosis [12] and further elucidated the great potential of CTRP9 in cardiovascular protection via regulating endothelial dysfunction [11], modulating metabolic pathways [13, 14] and inhibiting vascular inflammation [15], which were thought as the important mechanisms influencing the process of CCG [6]. However, the association between the serum CTRP9 and the development of coronary collateralization has been largely unknown. Considering the great importance of well-developed coronary collateralization in alleviating symptoms and improving the quality of life for CAD patients not suitable for vascular revascularization, it is pertinent to examine the relationship between serum CTRP9 and the formation of collateralization in CAD patients and determine whether serum CTRP9 could be a promising therapeutic target for patients with impaired collateralization in different metabolic status.
2. Methods
2.1. Study Populations
This study retrospectively enrolled CAD patients who underwent coronary angiography between the 1st October 2020 and the 30th April 2021 at Beijing Anzhen Hospital. All participants had severe epicardial artery stenotic lesions defined as at least 95% luminal diameter stenosis. Exclusion criteria were as follows: (1) epicardial artery stenotic lesion < 95%, (2) history of coronary artery bridge grafting, (3) severe hepatic and renal dysfunction, (4) New York Heart Association (NYHA) III-IV or left ventricular ejection fraction (LVEF) < 30%, (5) type I diabetes mellitus, (6) chronic inflammatory or infectious disease, and (7) some basic demographic data or laboratory biomarkers were not available. Finally, a total of 206 participants were recruited into this study. Written informed consents were obtained from all participants, and all the clinical investigations were approved by the Ethics Committee of Beijing Anzhen Hospital and conducted under the principles of the Declaration of Helsinki.
The diagnosis of type II diabetes mellitus (T2DM), hyperlipidemia, and hypertension were based on the recommendations from American Diabetes Association [16], Third Report of the National Cholesterol Education Program [17], and the guideline from European Society of Hypertension/European society of Cardiology (ESC) [18]. Metabolic syndrome (MetS) was defined according to the Diabetes Branch of the Chinese Medical Association, using body mass index (BMI) as a replacement of the waist circumference, that included 3 or more metabolic abnormalities can be diagnosed [19]. The type of CAD was diagnosed according to the ESC guideline for the diagnosis and management of chronic coronary syndrome [1].
2.2. Coronary Angiography and Rentrop Classification System
Coronary catheterization was routinely performed via the radial or femoral access using 6 or 7 Fr diagnostic catheters. Examination and evaluation of the angiograms were performed by two experienced interventional cardiologists who were blinded for the intention of this study. The complexity of CAD lesion was determined according to the SYNTAX scoring system [20]. Coronary chronic total occlusion (CTO) lesion was defined as a coronary lesion with thrombolysis in myocardial infarction flow grade 0 for at least 3 months [21]. The Rentrop classification system was used to evaluate the formation of the coronary collateralizations: 0, no visible filling of the coronary collaterals; 1, filling of the side branch but without the filling of the epicardial artery; 2, partial filling of the epicardial artery via the collateral branch; and 3, complete filling of the epicardial artery. In patients with one or more coronary collaterals, the highest grading of the collateral was selected for the analysis. Rentrop scores 2-3 were classified as well-developed coronary collateralization [22].
2.3. Demographic and Laboratory Measurements
Demographic data and medical history like age, BMI, current smoker, and alcohol use were all collected from the Beijing Anzhen Hospital medical information system. Blood samples were obtained at the day of patient admission after an overnight fasting. Routine laboratory technique was used to determine serum level of blood cell count, creatinine, blood urea, uric acid, high-sensitivity C-reactive protein (hs-CRP), and lipid and glucose profiles. The blood samples used to measure the level of CTRP9 were taken via venous puncture and placed into tubes coated with heparin as an anticoagulant. Centrifuge samples for 15 minutes at 3000 rpm at 5°C within 30 minutes, the layer of the supernatant was carefully transferred into the other tubes. These samples were stored at -80°C until the final measurements. Serum CTRP9 was assessed by commercially available ELISA kit (BLUE GENE, Shanghai, China) in accordance with the manufacturers' instructions. The assay procedure was as followed: (1) secure the desired numbers of coated wells in the holder then add 100 μL of samples to the appropriate well in the antibody pre-coated microtiter plate. (2) Add 100 μL of phosphate buffer saline (PBS) in the blank control well. Add 50 μL of conjugate to each well. (3) Cover and incubate the plate for 1 hour at 37°C. (4) Wash plate 5 times with diluted wash solution (350 μL/well/wash) using an auto water. (5) After washing, invert plate and blot by hitting the plate onto absorbent paper until no moisture appears. (6) Add 50 μL substrate A and 50 μL substrate B to each well including blank control well, subsequently. (7) Cover and incubate for 10 minutes at 37°C. (8) Add 50 μL of stop solution to each well including blank control well. (9) Determine the optical density (O.D.) at 450 nm using a microplate reader immediately. Estimated glomerular filtration rate (eGFR) was estimated using the creatinine-based Modification of Diet in Renal Disease (DMRD) formula [23].
2.4. Statistical Analysis
To explore the association between serum CTRP9 and the formation of coronary collateralizations, these participants were divided into 3 groups according to the CTRP9 tertiles. Continuous variables were expressed as the mean ± standard deviation (SD) for normally distributed continuous variables. Some laboratory measurements like fasting blood glucose, triglyceride, and hs-CRP were presented as median with interquartile for the distribution of these biomarkers that were highly skewed. The difference between tertiles was compared using the one-way ANOVA or Kruskal-Wallis H test for normally or nonnormally distributed continuous variables. The difference of categorical variables between tertiles was compared using the chi-square test or Fisher's exact test. Univariate logistic regression analysis was preliminarily performed to screen the potential factors correlated with the formation of coronary collateralization. P value <0.15 was considered clinically relevant. After adjustment for various confounding factors, the ORs and 95% CI of serum CTRP9 to discriminate well-developed coronary collaterals were calculated using weighted multivariate logistic regression analysis. Further subgroup analysis was constructed to evaluate the correlation between serum CTRP9 and the formation of coronary collateralization in different glycometabolic status. Lastly, ROC analysis was performed to compare the diagnostic value of serum CTRP9 for the well-developed coronary collateralization in those patients with diabetes or not. All statistical analyses were performed using SPSS 23.0 (SPSS, Inc. Chicago, IL, USA) and MedCalc 19.1 (MedCalc software, Belgium). Two-tailed P value <0.05 was considered statistically significant.
3. Results
3.1. Baseline Characteristic
The baseline characteristics of enrolled patients were shown in Table 1. The mean age of these participants was 57.24 ± 9.37 years old, and most of them were male (84.5%). The prevalence of current drinker, smoker, unstable angina pectoris (UAP), hypertension, T2DM, hyperlipidemia, and MetS was 23.3%, 40.3%, 65.5%, 64.1%, 17.0%, 83.0%, and 52.9%, respectively. Notably, the prevalence of T2DM and MetS raised stepwise with the increasing of CTRP9 tertiles (P < 0.05). No significant differences were found between tertiles in BMI (P = 0.384), heart rate (HR) (P = 0.702), systolic blood pressure (SBP) (P = 0.760), and diastolic blood pressure (DBP) (P = 0.990). As with the angiographic findings, the mean SYNTAX score was 17.91 ± 7.87. Most of participants came with multivessel disease (79.6%). About a half of the diseased arteries related to the formation of coronary collateralization were located in the right coronary artery (RCA) (47.5%). There is a trend that the proportion of CTO lesions increased stepwise from the lowest CTRP9 tertile to the highest one (P = 0.069).
Table 1.
Basic clinical characteristics of enrolled patients classified by serum CTRP9 level.
| CTRP9 | Total (n = 206) | T1 (n = 69) | T2 (n = 69) | T3 (n = 68) | P values |
|---|---|---|---|---|---|
| (T1 ≤ 1.23) | (1.23 < T2 < 1.36) | (T3 ≥ 1.36) | |||
| Demographic data | |||||
| Age (years) | 57.24 ± 9.37 | 55.87 ± 9.98 | 58.45 ± 9.24 | 57.41 ± 8.81 | 0.267 |
| Male sex, n (%) | 174 (84.5%) | 58 (84.1%) | 56 (81.2%) | 60 (88.2%) | 0.517 |
| BMI (kg/m2) | 26.55 ± 3.00 | 26.21 ± 2.95 | 26.49 ± 3.05 | 26.94 ± 3.00 | 0.384 |
| SBP (mmHg) | 137.64 ± 17.46 | 136.36 ± 16.72 | 138.25 ± 17.83 | 138.31 ± 18.00 | 0.760 |
| DBP (mmHg) | 80.78 ± 10.13 | 80.86 ± 9.43 | 80.64 ± 9.81 | 80.84 ± 11.23 | 0.990 |
| HR (bpm) | 72.59 ± 8.87 | 71.87 ± 7.94 | 72.84 ± 9.28 | 73.07 ± 9.40 | 0.702 |
| Alcohol use, n (%) | 48 (23.3%) | 19 (27.5%) | 14 (20.3%) | 15 (22.1%) | 0.577 |
| Current smokers, n (%) | 83 (40.3%) | 27 (39.1%) | 26 (37.7%) | 30 (44.1%) | 0.723 |
| Types of CAD | |||||
| UAP, n (%) | 135 (65.5%) | 52 (75.4%) | 42 (60.9%) | 41 (60.3%) | 0.108 |
| SAP, n (%) | 71 (34.5%) | 17 (24.6%) | 27 (39.1%) | 27 (39.7%) | 0.108 |
| Medical history | |||||
| Hypertension, n (%) | 132 (64.1%) | 47 (68.1%) | 44 (63.8%) | 41 (60.3%) | 0.633 |
| T2DM, n (%) | 35 (17.0%) | 1 (1.4%) | 10 (14.5%) | 24 (35.3%) | <0.001∗ |
| Hyperlipidemia, n (%) | 171 (83.0%) | 55 (79.7%) | 53 (76.8%) | 63 (92.6%) | 0.032∗ |
| MetS, n (%) | 109 (52.9%) | 30 (43.5%) | 35 (50.7%) | 44 (64.7%) | 0.041∗ |
| Laboratory measurement | |||||
| MONO | 0.38 ± 0.13 | 0.40 ± 0.13 | 0.38 ± 0.13 | 0.36 ± 0.11 | 0.226 |
| eGFR | 95.54 ± 14.88 | 95.65 ± 14.29 | 92.84 ± 16.56 | 98.18 ± 13.31 | 0.110 |
| CREA, mmol/L | 74.09 ± 15.29 | 74.88 ± 14.90 | 75.43 ± 17.13 | 71.94 ± 13.60 | 0.359 |
| UREA, mmol/L | 5.03 ± 1.31 | 5.03 ± 1.30 | 5.22 ± 1.22 | 4.85 ± 1.40 | 0.251 |
| UA, mmol/L | 353.32 ± 91.67 | 355.06 ± 97.98 | 364.23 ± 95.65 | 340.49 ± 79.95 | 0.313 |
| FBG, mmol/L | 5.36 (4.92-6.22) | 5.36 (5.01-6.09) | 5.49 (4.95-6.32) | 5.23 (4.77-6.35) | 0.684 |
| TC, mmol/L | 4.25 ± 1.18 | 4.21 ± 0.99 | 4.32 ± 1.27 | 4.23 ± 1.29 | 0.839 |
| TG, mmol/L | 1.52 (1.13-2.13) | 1.49 (1.07-2.19) | 1.53 (1.07-2.03) | 1.55 (1.18-2.21) | 0.738 |
| HDL-C, mmol/L | 1.01 ± 0.22 | 1.04 ± 0.23 | 1.05 ± 0.25 | 0.96 ± 0.16 | 0.030∗ |
| LDL-C, mmol/L | 2.46 ± 1.01 | 2.38 ± 0.82 | 2.49 ± 1.08 | 2.51 ± 1.12 | 0.703 |
| Non-HDL-C, mmol/L | 3.23 ± 1.14 | 3.17 ± 0.97 | 3.27 ± 1.21 | 3.25 ± 1.25 | 0.822 |
| Hs-CRP | 1.18 (0.65-2.47) | 1.29 (0.61-2.91) | 1.13 (0.71-2.52) | 1.12 (0.66-2.02) | 0.811 |
| Cardiovascular medications | |||||
| Antiplatelet agents, n (%) | 175 (85.0%) | 61 (88.4%) | 59 (85.5%) | 55 (80.9%) | 0.425 |
| ACEI/ARB, n (%) | 52 (25.2%) | 18 (26.1%) | 13 (18.8%) | 21 (30.9%) | 0.284 |
| CCB, n (%) | 64 (31.1%) | 26 (37.7%) | 21 (30.4%) | 17 (25.0%) | 0.277 |
| β receptor blocker, n (%) | 98 (47.6%) | 37 (53.6%) | 32 (46.4%) | 29 (42.6%) | 0.433 |
| Nitrates, n (%) | 106 (51.5%) | 43 (62.3%) | 33 (47.8%) | 30 (44.1%) | 0.084 |
| Statins, n (%) | 148 (71.8%) | 53 (76.8%) | 50 (72.5%) | 45 (66.2%) | 0.364 |
| Coronary angiography data | |||||
| SYNTAX score | 17.91 ± 7.87 | 19.18 ± 8.07 | 16.42 ± 6.91 | 17.99 ± 8.45 | 0.119 |
| Number of the diseased vessels | 0.644 | ||||
| One-vessel disease, n (%) | 42 (20.4%) | 12 (17.4%) | 13 (18.8%) | 17 (25.0%) | 0.503 |
| Two-vessel disease, n (%) | 62 (30.1%) | 19 (27.5%) | 24 (34.8%) | 19 (27.9%) | 0.581 |
| Three-vessel disease, n (%) | 102 (49.5%) | 38 (55.1%) | 32 (46.4%) | 32 (47.1%) | 0.525 |
| CTO lesions, n (%) | 104 (50.5%) | 27 (39.1%) | 39 (56.5%) | 38 (55.9%) | 0.069 |
| Location of the diseased artery | 0.353 | ||||
| RCA, n (%) | 98 (47.6%) | 32 (46.4%) | 29 (42.0%) | 37 (54.4%) | 0.339 |
| LCX, n (%) | 35 (17.0%) | 9 (13.0%) | 13 (18.8%) | 13 (19.1%) | 0.563 |
| LAD, n (%) | 73 (35.4%) | 28 (40.6%) | 27 (39.1%) | 18 (26.5%) | 0.165 |
∗ indicated the differences between groups were statistically significant. Abbreviations: CTRP9: C1q/tumor necrosis factor-related protein 9; BMI: body mass index; SBP: systolic blood pressure; DBP: diastolic blood pressure; HR: heart rate; CAD: coronary artery disease; UAP: unstable angina pectoris; SAP: stable angina pectoris; T2DM: type II diabetes mellitus; MetS: metabolic syndrome; MONO: monocyte count; eGFR: estimated glomerular filtration rate; CREA: creatinine; UREA: urea; UA: uric acid; FBG: fasting blood glucose; TC: total cholesterol; TG: triglyceride; HDL-C: high-density lipoprotein cholesterol; LDL-C: low-density lipoprotein cholesterol; Hs-CRP: high sensitivity C-reactive protein; ACEI: angiotensin-converting enzyme inhibitor; ARB: angiotensin receptor blocker; CCB: calcium channel blocker; CTO: chronic total occlusion; RCA: right coronary artery; LCX: left circumflex artery; LAD: left anterior descending artery.
3.2. Association between the Serum CTRP9 and Coronary Collateralization
The comparison of serum CTRP9 level between different groups and the prevalence of well-developed coronary collateralization stratified by the serum CTRP9 value were shown in Figure 1. The level of serum CTRP9 was significantly higher in patients with well-developed coronary collateralization (Figure 1(a)) and diabetes (Figure 1(b)) (P < 0.001). The prevalence of well-developed collateral increased from the lowest CTRP9 tertile to the highest one (tertile1 56.5% vs. tertile2 81.2% vs. tertile3 83.6%, P for trend <0.001) (Figure 1(c)). The results of the association between the serum CTRP9 and coronary collateralization after adjusting for various confounding factors were shown in Figure 2. The serum CTRP9 was modified as a categorical variable, the tertile 1 group was used as the reference, and the serum CTRP9 was independently associated with the well-developed coronary collateralization in the tertile 2 group (OR 4.49, 95% CI 1.75-11.55, P = 0.002) and tertile 3 group (OR 8.98, 95% CI 2.75-29.35, P < 0.001). Figure 3 and Table 2 show that the diagnostic value of serum CTRP9 continued to persist in the nondiabetic group (tertile 2: OR 4.08, 95% CI 1.52-10.98, P = 0.005; tertile 3: OR 6.35, 95% CI 2.07-19.48, P = 0.001) but failed to substantiate that serum CTRP9 could independently discriminate the well-developed coronary collaterals in those with diabetes (tertile 2: OR 1.24, 95% CI 0.10-15.01, P = 0.865; tertile 3: OR 1.05, 95% CI 0.07-16.50, P = 0.975). The ROC curve in Figure 4(a) revealed the diagnostic values of serum CTRP9 to discriminate the formation of coronary collaterals in different glycometabolic status. A significant improvement in area under the curve (AUC) for serum CTRP9 to discern the well-developed collaterals can be found in the nondiabetic group (AUC in nondiabetic group 0.728 vs. AUC in overall participants 0.652, P < 0.05). Besides, Figure 4(b) shows that adding the serum CTRP9 to the established model consisting of HR, type of CAD, hyperlipidemia, MetS, diseased artery associated with the collateralization, CTO lesion, eGFR, high-density lipoprotein cholesterol (HDL-C), and Log hs-CRP can significantly the C-statistics for the discrimination of the coronary collateral growth (P = 0.046).
Figure 1.
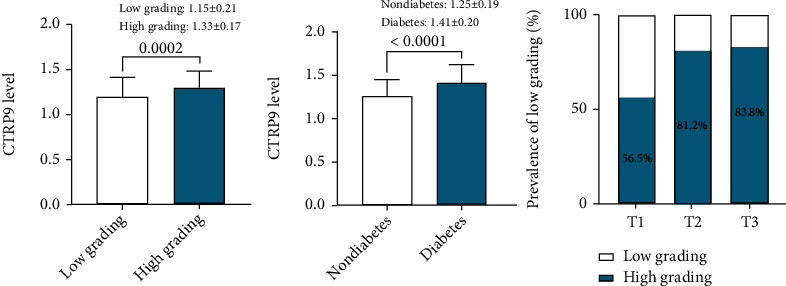
Comparison between the serum CTRP9 level in groups with different collateral grading; CTRP9high grading vs. CTRP9Low grading: 1.33 ± 0.17 vs. 1.15 ± 0.21, with 95% CI of 0.05 to 0.17 (a) and glycometabolic status (b). The prevalence of low collateralization among patients stratified by serum CTRP9 tertiles (c). Abbreviation: CTRP9: C1q/tumor necrosis factor-related protein 9.
Figure 2.
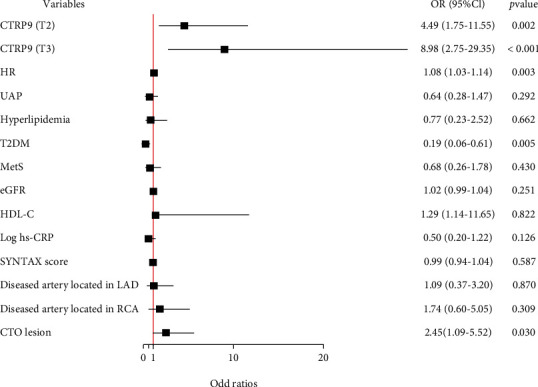
Forest plot of the results of multivariate logistic regression model exploring the association between serum CTRP9 and the formation of coronary collateralization in overall population. Abbreviations: CTRP9: C1q/tumor necrosis factor-related protein 9; HR: heart rate; UAP: unstable angina pectoris; T2DM: type II diabetes mellitus; MetS: metabolic syndrome; eGFR: estimated glomerular filtration rate; HDL-C: high-density lipoprotein cholesterol; Hs-CRP: high-sensitivity C-reactive protein; LAD: left anterior descending artery; RCA: right coronary artery; CTO: chronic total occlusion; OR: odd ratio; CI: confidence interval.
Figure 3.
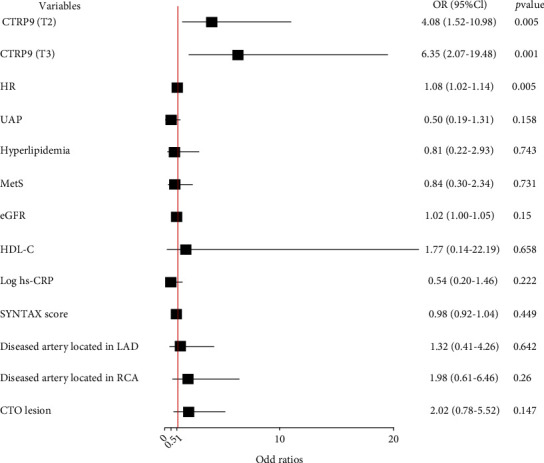
Forest plot of the multivariate logistic regression analysis model in nondiabetic patients with obstructive CAD exploring the association between serum CTRP9 level and the formation of collateralization. Abbreviations: CTRP9: C1q/tumor necrosis factor-related protein 9; HR: heart rate; UAP: unstable angina pectoris; MetS: metabolic syndrome; eGFR: estimated glomerular filtration rate; HDL-C: high-density lipoprotein cholesterol; hs-CRP: high sensitivity C-reactive protein; LAD: left anterior descending artery; RCA: right coronary artery; CTO: chronic total lesion; OR: odd ratio; CI: confidence interval.
Table 2.
Multivariate logistic regression analysis for the discrimination of impaired collateralization in the diabetic group.
| Variables | Univariate | Multivariate | ||
|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |
| UAP | 1.42 (0.31-6.47) | 0.653 | 0.58 (0.05-7.06) | 0.671 |
| HR | 1.08 (0.97-1.20) | 0.155 | 1.18 (0.95-1.46) | 0.134 |
| Hyperlipidemia | 0.61 (0.06-6.55) | 0.680 | 0.21 (0.01-55.82) | 0.206 |
| MetS | 0.33 (0.03-3.18) | 0.336 | 0.90 (0.02-47.66) | 0.895 |
| eGFR | 1.00 (0.94-1.06) | 0.908 | 0.95 (0.86-1.06) | 0.355 |
| HDL-C | 0.64 (0.02-26.97) | 0.816 | 0.07 (0.01-136.32) | 0.483 |
| Log hs-CRP | 0.55 (0.10-3.02) | 0.488 | 0.31 (0.02-4.84) | 0.404 |
| SYNTAX score | 1.01 (0.93-1.11) | 0.758 | 1.04 (0.90-1.19) | 0.623 |
| Diseased artery in LAD | 0.39 (0.09-1.77) | 0.222 | 0.07 (0.01-2.30) | 0.136 |
| Diseased artery in RCA | 1.56 (0.38-6.36) | 0.538 | 0.14 (0.01-3.60) | 0.237 |
| CTRP9 (T2) | 1.67 (0.29-9.45) | 0.564 | 1.24 (0.10-15.01) | 0.865 |
| CTRP9 (T3) | 1.09 (0.21-5.76) | 0.916 | 1.05 (0.07-16.50) | 0.975 |
Abbreviations: UAP: unstable angina pectoris; HR: heart rate; MetS: metabolic syndrome; eGFR: estimated glomerular filtration rate; HDL-C: high-density lipoprotein cholesterol; Hs-CRP: high-sensitivity C-reactive protein; LAD: left anterior descending artery; RCA: right coronary artery; CTRP9: C1q/tumor necrosis factor-related protein 9.
Figure 4.
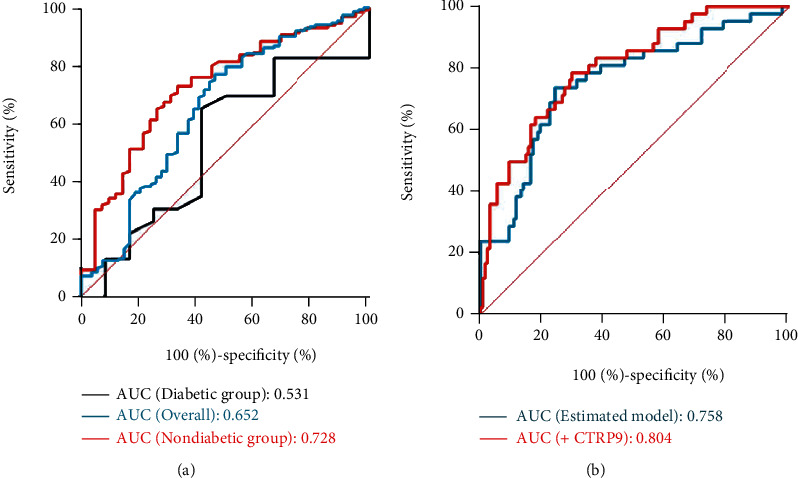
The diagnostic value of serum CTRP9 level in discriminating well-developed coronary collateralization in patients with different glycometabolic status (a). Different models for evaluating the formation of well-developed collateralization in nondiabetic patients (b). Established model include HR, type of CAD, hyperlipidemia, T2DM, MetS, eGFR, HDL-C, Log hs-CRP, diseased artery associated with the collateralization, SYNTAX score, and CTO lesion. Abbreviations: CTRP9: C1q/tumor necrosis factor-related protein 9; AUC: area under the curve; HR: heart rate; CAD: coronary artery disease; T2DM: type II diabetes mellitus; MetS: metabolic syndrome; eGFR: estimated glomerular filtration rate; HDL-C: high density lipoprotein cholesterol; hs-CRP: high-sensitivity C-reactive protein; CTO: chronic total occlusion.
4. Discussion
The findings of this study are as follows: (1) the serum CTRP9 level is significantly higher in CAD patients with diabetes and well-developed coronary collateralization. (2) Serum CTRP9 is associated with well-developed coronary collaterals only in nondiabetic participants but not in those with diabetes. (3) Adding the serum CTRP9 into the baseline model consisting of various factors affecting the coronary collaterals could enhance the diagnostic value of discriminating well-developed collateralization in nondiabetic CAD patients.
The maturation of coronary collateralization into functional vessels has great clinical significance to preserving the normal cardiac function for its potency to provide an alternative source of blood supply to the ischemic myocardial territory supplied by the stenotic or even occluded vessels, especially present for at least 3 months [6]. Arteriogenesis, refers to the remodeling of pre-existing arterioles through wall thickening and diameter expanding into functional branches, is the main mechanism of collateral maturation in the presence of CAD [24]. The current study found a strong correlation between the serum CTRP9 level and well-developed coronary collateralization in the nondiabetic CAD patients, indicating that CTRP9 may involve in the process of arteriogenesis. The mechanism underlying could be explained by at least three aspects: mediating vascular inflammation, ameliorating endothelial dysfunction, and regulating oxidative stress [11, 15, 25–27]. In vivo studies have ensured the potential role of CTRP9 in inhibiting the expression of proinflammatory cytokines in the vascular endothelium [15, 25]. Jung et al. for the first time found that CTRP9 could decrease the expression of NF-κB-mediated proinflammatory genes and attenuate the cytokine-induced vascular inflammation via the AMP-activated protein kinase (AMPK) way [15]. Studies about whether CTRP9 could promote endothelial function have also delivered positive results [11, 27]. Yamaguchi et al. [27] found that CTRP9-knockout mice are more likely to experience impaired blood flow recovery and decreased capillary density after the unilateral hindlimb ischemic surgery, and recombinant CTRP9 treatment could increase the capillary networks and migration of endothelial cells via the AMPK way, indicating the potential of CTRP9 in promoting vascular revascularization in the event of ischemic events. Zheng et al. [11] further elucidated CTRP9 showed the strongest function of mediating vascular relaxation among the CTRPs family via adiponectin receptor-1/AMPK/eNOS/nitric oxide signaling pathway. Excessive oxidative stress induced by abnormal lipid and glucose metabolism is another reason for less-developed collateral branch [13, 26]. CTRP9 could attenuate high glucose-induced oxidative stress by decreasing the expression of reactive oxygen species (ROS) through the activation of AMPK/Nrf2 signaling pathway [13]. Oxidized low-density lipoprotein (ox-LDL) accumulation in vascular endothelium plays a pivotal role in endothelial dysfunction and coronary atherosclerosis. CTRP9 can ameliorate ox LDL-induced endothelial injury by activating antioxidant enzyme via the peroxisome proliferator-activated receptor γ co-activator 1α/AMPK pathway [26]. Besides, recent study has also found cholesterol efflux capacity, characterized by well-functioning HDL is also a determinant of forming well-developed collateral branch [28]. Notably, this study failed to find the association between HDL and CCG, which is in accordance with our findings in current study (Figure 2). Lei et al. substantiated that CTRP9 could upregulated the expression of proteins important for cholesterol efflux and thus promoting the process of CCG [14].
In current study, we found that the serum CTRP9 level is significantly higher in patients with diabetes (Figure 1(b)). Previous studies have shown the important role of CTRP9 in glucose metabolism and mediating insulin resistance (IR) [25, 29–32]. In animal studies, CTRP9 was found to have the potential to inhibit vascular inflammation, protect the integrity of vascular endothelium, and exert cardioprotective effect via attenuating the endoplasmic reticulum stress in diabetic mice [25, 29]. Conversely, targeted deletion of CTRP9 in mice would lead to an increase in food take and body weight and also result in peripheral IR [32]. However, the relationship between serum CTRP9 and the presence of diabetes and IR in human have always been controversial [30, 31]. Jia et al. [31] found that serum CTRP9 was significantly higher in those diabetic and obese patients and positively correlated with metabolic indicators, which is also reflected in current study. The mechanism of the increased CTRP9 expression in diabetic patients could be attributable to the compensatory response to the IR or hyperglycemia or defensive response to the metabolic stress [33]. However, another study conducted by Hwang et al. [30] showed serum CTRP9 was inversely associated with fasting glucose and homeostasis model assessment for IR (HOMA-IR), indicating increased CTRP9 could reflect favorable glucose metabolic status. Besides, we also found that serum CTRP9 failed to be a determinant of well-developed coronary collateral branch in the diabetic group (Table 2). The angiogenic growth factor therapy failed to achieve the same results animal studies have delivered in human [34]. One likely explanation could be that CAD patients in clinical settings are generally older and are more likely to have multiple complications like T2DM and MetS compared with animal models, and previous studies have shown that patients with metabolic disorders tended to have complicated coronary lesions and form impaired collateralizations [8, 9]. Whether CTRP9 could be a promising therapeutic target for diffuse CAD patients not suitable for vascular revascularization especially for those with diabetes still needed to be further investigated.
Several limitations should be acknowledged: (1) the study design can only allow us to detect the association but incapable of exploring whether there exists a causal relationship between the serum CTRP9 and the development of coronary collateralization. (2) Although relevant factors influencing the development of coronary collateralization were incorporated into the multivariate logistic regression analysis, the current study did not include other possible factors like inflammatory cytokines and duration of stenotic or occluded coronary lesion. (3) While the formation of coronary collateralization could be more accurately assessed by coronary flow index [35], the Rentrop score system is relatively easy to use in clinical practice. (4) The findings of the association between CTRP9 and development of collateralization in diabetic group may be easily affected by the relatively small samples (n = 35). (5) While the Rentrop score is easy to use in current clinical practice, it is limited to the angiographic findings. A functional examination, such as cardiac magnetic resonance (CMR), to evaluate the perfusion and myocardial vitality could add additional value in understanding the cardioprotective role of CTRP9.
5. Conclusion
In conclusion, this study for the first time showed that serum CTRP9 is independently associated with the formation of well-developed coronary collateralization in nondiabetic CAD patients. Therapies targeting at serum CTRP9 may be a promising strategy for diffuse CAD patients without diabetes not suitable for vascular revascularization.
Acknowledgments
This work was supported by the grant from the National Key Research and Development Program of China (2017YFC0908800), the “Beijing Municipal Administration of Hospitals” Ascent Plan (DFL20150601) and “Beijing Municipal Administration of Hospitals” Mission Plan (SML20180601), and Beijing Municipal Health Commission “Project of Science and Technology Innovation Center” (PXM2019_026272_000006) (PXM2019_026272_000005).
Abbreviations
- CTRP9:
.C1q/tumor necrosis factor-related protein 9
- CAD:
Coronary artery disease
- ELISA:
Enzyme linked immunosorbent assay
- ROC:
Receiver-operating characteristics
- AUC:
Area under the curve
- OR:
Odds ratio
- CI:
Confidence interval
- SD:
Standard deviation
- OMT:
Optimal drug therapy
- CABG:
Coronary artery bridge grafting
- CCG:
Coronary collateral growth
- APN:
Adiponectin
- NYHA:
New York Heart Association
- LVEF:
Left ventricular ejection fraction
- T2DM:
Type II diabetes mellitus
- eGFR:
Estimated glomerular filtration rate
- DMRD:
Modification of Diet in Renal Disease
- ESC:
European Society of Cardiology
- MetS:
Metabolic syndrome
- BMI:
Body mass index
- CTO:
Chronic total occlusion
- Hs-CRP:
High-sensitivity C-reactive protein
- UAP:
Unstable angina pectoris
- HR:
Heart rate
- SBP:
Systolic blood pressure
- DBP:
Diastolic blood pressure
- RCA:
Right coronary artery
- IR:
Insulin resistance
- HOMA-IR:
Homeostasis model assessment for IR
- HDL-C:
High-density lipoprotein cholesterol
- ox-LDL:
Oxidized low-density lipoprotein
- ROS:
Reactive oxygen species
- AMPK:
AMP-activated protein kinase
- CMR:
Cardiac magnetic resonance
- PBS:
Phosphate buffer saline.
Contributor Information
Hongya Han, Email: hhy123100@163.com.
Yingxin Zhao, Email: zyingxinmi@163.com.
Data Availability
The dataset and materials mentioned above are available from the authors.
Ethical Approval
The current study was conducted in accordance with Declaration of Helsinki and was approved by the Ethics Committee of Beijing Anzhen Hospital, Capital Medical University.
Conflicts of Interest
The authors declare that they have no competing interests.
Authors' Contributions
YZ and AG contributed to the conception and design of this study and AG wrote this article. All authors made contribution to collect and analyze the data. All authors read and approved the final manuscript.
References
- 1.Knuuti J., Wijns W., Saraste A., et al. 2019 ESC guidelines for the diagnosis and management of chronic coronary syndromes. European Heart Journal . 2020;41(3):407–477. doi: 10.1093/eurheartj/ehz425. [DOI] [PubMed] [Google Scholar]
- 2.Brown R. A., Shantsila E., Varma C., Lip G. Y. Epidemiology and pathogenesis of diffuse obstructive coronary artery disease: the role of arterial stiffness, shear stress, monocyte subsets and circulating microparticles. Annals of Medicine . 2016;48(6):444–455. doi: 10.1080/07853890.2016.1190861. [DOI] [PubMed] [Google Scholar]
- 3.Ponticelli F., Tzanis G., Gallone G., et al. Safety and efficacy of coronary sinus reducer implantation at 2-year follow-up. International Journal of Cardiology . 2019;292:87–90. doi: 10.1016/j.ijcard.2019.05.026. [DOI] [PubMed] [Google Scholar]
- 4.Imran T. F., Malapero R., Qavi A. H., et al. Efficacy of spinal cord stimulation as an adjunct therapy for chronic refractory angina pectoris. International Journal of Cardiology . 2017;227:535–542. doi: 10.1016/j.ijcard.2016.10.105. [DOI] [PubMed] [Google Scholar]
- 5.Jia N., Zhang R., Liu B., et al. Efficacy and safety of cardiac shock wave therapy for patients with severe coronary artery disease: a randomized, double-blind control study. Journal of Nuclear Cardiology . 2021 doi: 10.1007/s12350-021-02768-7. [DOI] [PubMed] [Google Scholar]
- 6.Allahwala U. K., Khachigian L. M., Nour D., et al. Recruitment and maturation of the coronary collateral circulation: current understanding and perspectives in arteriogenesis. Microvascular Research . 2020;132:p. 104058. doi: 10.1016/j.mvr.2020.104058. [DOI] [PubMed] [Google Scholar]
- 7.Seiler C., Stoller M., Pitt B., Meier P. The human coronary collateral circulation: development and clinical importance. European Heart Journal . 2013;34(34):2674–2682. doi: 10.1093/eurheartj/eht195. [DOI] [PubMed] [Google Scholar]
- 8.Liu T., Wu Z., Liu J., Lv Y., Li W. Metabolic syndrome and its components reduce coronary collateralization in chronic total occlusion: an observational study. Cardiovascular Diabetology . 2021;20(1):p. 104. doi: 10.1186/s12933-021-01297-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shen Y., Ding F. H., Dai Y., et al. Reduced coronary collateralization in type 2 diabetic patients with chronic total occlusion. Cardiovascular Diabetology . 2018;17(1):p. 26. doi: 10.1186/s12933-018-0671-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wong G. W., Krawczyk S. A., Kitidis-Mitrokostas C., et al. Identification and characterization of CTRP9, a novel secreted glycoprotein, from adipose tissue that reduces serum glucose in mice and forms heterotrimers with adiponectin. The FASEB Journal . 2009;23(1):241–258. doi: 10.1096/fj.08-114991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zheng Q., Yuan Y., Yi W., et al. C1q/TNF-related proteins, a family of novel adipokines, induce vascular relaxation through the adiponectin receptor-1/AMPK/eNOS/nitric oxide signaling pathway. Arteriosclerosis, Thrombosis, and Vascular Biology . 2011;31(11):2616–2623. doi: 10.1161/ATVBAHA.111.231050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang J., Hang T., Cheng X. M., et al. Associations of C1q/TNF-related protein-9 levels in serum and epicardial adipose tissue with coronary atherosclerosis in humans. BioMed Research International . 2015;2015:1–971686. doi: 10.1155/2015/971683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheng Y., Qi Y., Liu S., et al. C1q/TNF-related protein 9 inhibits high glucose-induced oxidative stress and apoptosis in retinal pigment epithelial cells through the activation of AMPK/Nrf2 signaling pathway. Cell Transplantation . 2020;29:p. 096368972096205. doi: 10.1177/0963689720962052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lei S., Chen J., Song C., et al. CTRP9 alleviates foam cells apoptosis by enhancing cholesterol efflux. Molecular and Cellular Endocrinology . 2021;522:p. 111138. doi: 10.1016/j.mce.2020.111138. [DOI] [PubMed] [Google Scholar]
- 15.Jung C. H., Lee M. J., Kang Y. M., et al. C1q/TNF-related protein-9 inhibits cytokine-induced vascular inflammation and leukocyte adhesiveness via AMP-activated protein kinase activation in endothelial cells. Molecular and Cellular Endocrinology . 2016;419:235–243. doi: 10.1016/j.mce.2015.10.023. [DOI] [PubMed] [Google Scholar]
- 16.American Diabetes A. 2. Classification and diagnosis of diabetes:standards of medical care in diabetes-2018. Diabetes Care . 2018;41(Supplement_1):S13–S27. doi: 10.2337/dc18-S002. [DOI] [PubMed] [Google Scholar]
- 17.Third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III) final report. Circulation . 2002;106(25):3143–3421. doi: 10.1161/circ.106.25.3143. [DOI] [PubMed] [Google Scholar]
- 18.Williams B., Mancia G., Spiering W., et al. 2018 ESC/ESH guidelines for the management of arterial hypertension. European Heart Journal . 2018;39(33):3021–3104. doi: 10.1093/eurheartj/ehy339. [DOI] [PubMed] [Google Scholar]
- 19.Fan L., Hao Z., Gao L., Qi M., Feng S., Zhou G. Non-linear relationship between sleep duration and metabolic syndrome: a population-based study. Medicine (Baltimore) . 2020;99(2, article e18753) doi: 10.1097/MD.0000000000018753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kappetein A. P., Dawkins K. D., Mohr F. W., et al. Current Percutaneous Coronary Intervention and Coronary Artery Bypass Grafting Practices for Three-Vessel and Left Main Coronary Artery Disease. Insights from the SYNTAX Run-in Phase. European Journal of Cardio-Thoracic Surgery . 2006;29(4):486–491. doi: 10.1016/j.ejcts.2006.01.047. [DOI] [PubMed] [Google Scholar]
- 21.Brilakis E. S., Mashayekhi K., Tsuchikane E., et al. Guiding principles for chronic total occlusion percutaneous coronary intervention. Circulation . 2019;140(5):420–433. doi: 10.1161/CIRCULATIONAHA.119.039797. [DOI] [PubMed] [Google Scholar]
- 22.Rentrop K. P., Cohen M., Blanke H., Phillips R. A. Changes in collateral channel filling immediately after controlled coronary artery occlusion by an angioplasty balloon in human subjects. Journal of the American College of Cardiology . 1985;5(3):587–592. doi: 10.1016/S0735-1097(85)80380-6. [DOI] [PubMed] [Google Scholar]
- 23.Ma Y. C., Zuo L., Chen J. H., et al. Modified glomerular filtration rate estimating equation for Chinese patients with chronic kidney disease. Journal of the American Society of Nephrology: JASN . 2006;17(10):2937–2944. doi: 10.1681/ASN.2006040368. [DOI] [PubMed] [Google Scholar]
- 24.Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nature Medicine . 2000;6(4):389–395. doi: 10.1038/74651. [DOI] [PubMed] [Google Scholar]
- 25.Li W., Ma N., Liu M. X., et al. C1q/TNF-related protein-9 attenuates retinal inflammation and protects blood-retinal barrier in db/db mice. European Journal of Pharmacology . 2019;853:289–298. doi: 10.1016/j.ejphar.2019.04.012. [DOI] [PubMed] [Google Scholar]
- 26.Sun H., Zhu X., Zhou Y., Cai W., Qiu L. C1q/TNF-related Protein-9 ameliorates ox-LDL-induced endothelial dysfunction via PGC-1α/AMPK-mediated antioxidant enzyme induction. International Journal of Molecular Sciences . 2017;18(6):p. 1097. doi: 10.3390/ijms18061097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamaguchi S., Shibata R., Ohashi K., et al. C1q/TNF-related protein 9 promotes revascularization in response to ischemia via an eNOS-dependent manner. Frontiers in Pharmacology . 2020;11:p. 1313. doi: 10.3389/fphar.2020.01313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee S., Park J. M., Ann S. J., et al. Cholesterol efflux and collateral circulation in chronic total coronary occlusion: effect-Circ study. Journal of the American Heart Association . 2021;10(5, article e019060) doi: 10.1161/JAHA.120.019060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bai S., Cheng L., Yang Y., et al. C1q/TNF-related protein 9 protects diabetic rat heart against ischemia reperfusion injury: role of endoplasmic reticulum stress. Oxidative Medicine and Cellular Longevity . 2016;2016:14. doi: 10.1155/2016/1902025.1902025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hwang Y. C., Woo O. S., Park S. W., Park C. Y. Association of serum C1q/TNF-related Protein-9 (CTRP9) concentration with visceral adiposity and metabolic syndrome in humans. International Journal of Obesity (2005) . 2014;38(9):1207–1212. doi: 10.1038/ijo.2013.242. [DOI] [PubMed] [Google Scholar]
- 31.Jia Y., Luo X., Ji Y., et al. Circulating CTRP9 levels are increased in patients with newly diagnosed type 2 diabetes and correlated with insulin resistance. Diabetes Research and Clinical Practice . 2017;131:116–123. doi: 10.1016/j.diabres.2017.07.003. [DOI] [PubMed] [Google Scholar]
- 32.Wei Z., Lei X., Petersen P. S., Aja S., Wong G. W. Targeted deletion of C1q/TNF-related protein 9 increases food intake, decreases insulin sensitivity, and promotes hepatic steatosis in mice. Endocrinology and Metabolism . 2014;306(7):E779–E790. doi: 10.1152/ajpendo.00593.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seldin M. M., Tan S. Y., Wong G. W. Metabolic function of the CTRP family of hormones. Reviews in Endocrine & Metabolic Disorders . 2014;15(2):111–123. doi: 10.1007/s11154-013-9255-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rubanyi G. M. Mechanistic, technical, and clinical perspectives in therapeutic stimulation of coronary collateral development by angiogenic growth factors. Molecular Therapy . 2013;21(4):725–738. doi: 10.1038/mt.2013.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pijls N. H., Bech G. J., el Gamal M. I., et al. Quantification of recruitable coronary collateral blood flow in conscious humans and its potential to predict future ischemic events. Journal of the American College of Cardiology . 1995;25(7):1522–1528. doi: 10.1016/0735-1097(95)00111-G. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The dataset and materials mentioned above are available from the authors.


