Abstract
Nucleoside analogues with a Z- or an E-methylenecyclopropane moiety were synthesized and examined for activity against human immunodeficiency virus type 1 (HIV-1) in vitro. The addition of a methyl phenyl phosphoro-l-alaninate moiety to modestly active analogues resulted in potentiation of their anti-HIV-1 activity. Two such compounds, designated QYL-685 (with 2,6-diaminopurine) and QYL-609 (with adenine), were most potent against HIV-1 in vitro, with 50% inhibitory concentrations of 0.034 and 0.0026 μM, respectively, in MT-2 cell-based assays. Both compounds were active against zidovudine-resistant, didanosine-resistant, and multi-dideoxynucleoside-resistant infectious clones in vitro. Further development of these analogues as potential therapies for HIV-1 infection is warranted.
The emergence of drug-resistant human immunodeficiency virus type 1 (HIV-1) variants during antiviral chemotherapy with nucleoside reverse transcriptase inhibitors (NRTIs) has limited their efficacy and complicated the strategy for controlling HIV-1-associated diseases (15, 16). Nevertheless, NRTIs continue to play an important role in the therapy of AIDS. A number of novel NRTIs are currently under preclinical or clinical development (8, 10, 11, 14, 25).
Recently, the replacement of the ribofuranose backbone on certain nucleoside analogues with a Z-methylenecyclopropane moiety (designated QYL compounds) was reported to confer potent antiviral properties (18, 19). Some of these QYL compounds, such as guanine and adenine analogues QYL-438 and QYL-284A, respectively (their structures are shown in Table 1 and Fig. 1), displayed a broad array of antiviral activity in vitro (18). A 2,6-diaminopurine derivative, QYL-546, had exhibited potent antiviral activity against human and murine cytomegalovirus (HCMV and MCMV), human hepatitis B virus (HBV), and Epstein-Barr virus (EBV). A cytosine analogue, QYL-468 has also been found to inhibit EBV, varicella zoster virus as well as HBV (19).
TABLE 1.
Antiviral activity of QYL compounds against HIV-1LAIa
| Structure of the compound
|
Inhibition of CPE and toxicity
|
Inhibition of p24 (IC50 [μM]) | |||||
|---|---|---|---|---|---|---|---|
| Compound | Configuration | Base | PPA | IC50 (μM) | CC50 (μM) | SIb | |
| QYL-284A | Z | Adenine | − | 0.75 ± 0.35 | 32 ± 11 | 42 | 0.54 ± 0.13 |
| QYL-284B | E | Adenine | − | >100 | >100 | NDc | |
| QYL-546 | Z | da-Purined | − | 12 ± 1.5 | >100 | >8.7 | 4.1 ± 2.0 |
| QYL-658 | E | da-Purine | − | >100 | ND | ND | |
| QYL-609 | Z | Adenine | + | 0.0026 ± 0.0016 | 0.24 ± 0.06 | 92 | 0.0022 ± 0.001 |
| QYL-600 | E | Adenine | + | 0.66 ± 0.15 | 24 ± 3.2 | 36 | 0.51 ± 0.12 |
| QYL-685 | Z | da-Purine | + | 0.034 ± 0.007 | 21 ± 4.7 | 615 | 0.038 ± 0.018 |
| QYL-675 | E | da-Purine | + | 5.3 ± 2.3 | 44 ± 11 | 8.4 | 2.4 ± 1.2 |
| QYL-438 | Z | Guanine | − | >70 | 70 ± 10 | ND | |
| QYL-442 | E | Guanine | − | >100 | >100 | ND | |
| QYL-678 | Z | Guanine | + | >76 | 76 ± 5.1 | ND | |
| QYL-673 | E | Guanine | + | >100 | >100 | ND | |
| QYL-468 | Z | Cytosine | − | 64 ± 18 | >100 | >1.6 | 38 ± 9.3 |
| QYL-466 | E | Cytosine | − | >100 | >100 | ND | |
| QYL-692 | Z | Thymine | − | >100 | >100 | ND | |
| QYL-693 | E | Thymine | − | >100 | >100 | ND | |
| AZT | Thymine | − | 0.019 ± 0.006 | >100 | >5,263 | 0.020 ± 0.008 | |
| ddIe | Hypoxanthine | − | 2.5 ± 1.5 | >100 | >40 | 2.0 ± 1.0 | |
MT-2 cells were exposed to 100 50% tissue culture infective doses of HIV-1LAI plus various concentrations of each QYL compound, and the cells were cultured for 7 days (13, 17, 20, 21). Data shown represent the mean values of triplicate determinations. More than two independent experiments were conducted for each drug.
SI, selectivity index (CC50/IC50).
ND, not determined.
da-Purine, 2,6-diaminopurine.
ddI, dideoxyinosine.
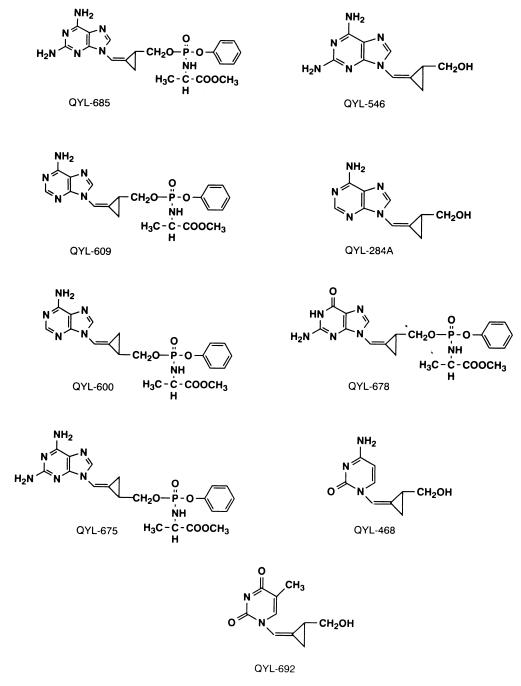
FIG. 1.
Structures of various QYL compounds with or without the PPA moiety.
Sixteen Z- or E-2-[(hydroxymethyl)cyclopropylidene]methyladenine and -guanine analogues with or without a methyl phenyl phosphoro-l-alaninate (PPA) moiety were designed and synthesized as previously described (18, 19, 19a). Structures of nine major analogues are illustrated in Fig. 1. 3′-Azido-2′,3′-dideoxythymidine (AZT, or zidovudine) and 2′,3′-dideoxyinosine (ddI, or didanosine) were purchased from Sigma (St. Louis, Mo.) and CalBioChem (La Jolla, Calif.), respectively.
We first examined a panel of nucleoside analogues containing a methylenecyclopropane moiety for anti-HIV-1 activity and cytotoxicity in vitro in the MT-2-based assays as previously described (20, 21). As shown in Table 1, compared to the antiviral potency of zidovudine and didanosine, five (QYL-284A, -609, -600, -685, and -675) of 16 compounds assessed showed comparable activities against HIV-1LAI in MT-2 cells assessed by the MTT assay. Assays for p24 Gag protein production with MT-2 cells corroborated the data with the MTT assay. We subsequently examined the possible structure-activity relationship among these compounds (Fig. 1 and Table 1). QYL-546, which has 2,6-diaminopurine and a Z-(cis)-methylenecyclopropane group, was moderately active against the virus, with a 50% inhibitory concentration (IC50) of 12 ± 1.5 μM (means ± standard deviations) (Table 1). The addition of the PPA moiety to QYL-546 produced QYL-685, which was quite potent against the virus, with an IC50 of 0.034 ± 0.007 μM. QYL-284A, adenine and a Z-(cis)-methylenecyclopropane group, was active against the virus, with an IC50 of 0.75 ± 0.35 μM (Table 1). The addition of the PPA moiety to QYL-284A produced QYL-609, which proved to be the most potent moiety against the virus (IC50 of 0.0026 ± 0.0016 μM) but also the most toxic among the compounds tested. QYL-675, unlike QYL-685, has an E (trans)-methylenecyclopropane with the PPA moiety and showed no significant antiviral activity. QYL-678 has a guanine and a Z-(cis)-methylenecyclopropane group with the PPA moiety and exerted no significant antiviral activity either. These data suggest that methylenecyclopropane analogues with a Z configuration and the PPA moiety have optimal antiviral activity against HIV-1.
QYL-685 and QYL-609 were further examined in PHA-PBM exposed to a clinical HIV-1 isolate, HIV-1ERS104pre, by using p24 protein production as an endpoint as previously described (23). Both compounds were potent against HIV-1ERS104pre in phytohemagglutinin-activated peripheral blood mononuclear cells, with IC50s of QYL-685 and QYL-609 of 0.21 ± 0.05 and 0.013 ± 0.001 μM, respectively. The IC90s of QYL-685 and QYL-609 were 1.6 ± 0.2 and 0.03 ± 0.008 μM, respectively. The 50% cytotoxic concentrations (CC50s) determined as previously described (20, 21) for QYL-685 and QYL-609 were 13 ± 1.4 μM and 0.35 ± 0.04 μM, respectively.
Certain permuted versions of nucleoside analogues such as 1-[(2-hydroxyethoxymethyl]-6-(phenylthio)thymine (HEPT) compounds selectively inhibit reverse transcriptase (RT) from HIV-1 but do not inhibit HIV-2 RT, thus falling within the group of nonnucleoside RT inhibitors (3, 24). We, therefore, tested QYL-685 and QYL-609 for activity against HIV-2ROD (7) in MT-2 cells by using the p24 Gag protein production assay. Both compounds were active against HIV-2 (IC50s, 0.38 ± 0.13 μM and 0.03 ± 0.01 μM, respectively) but apparently with a moderately reduced potency compared to their potency against HIV-1.
We also asked whether QYL-685 and QYL-609 were active against three major drug-resistant HIV-1 variants: a zidovudine-resistant HIV-1215, a didanosine-resistant HIV-174, and a multi-dideoxynucleoside-resistant infectious clone carrying five amino acid substitutions, HIV-162/75/77/116/151 (22, 25). In agreement with previous reports (9, 22, 23), HIV-1215 was resistant to zidovudine, HIV-174 was resistant to didanosine, and HIV-162/75/77/116/151 was highly resistant to both zidovudine and didanosine (Table 2). QYL-685 and QYL-609 were, however, active against all these drug-resistant HIV-1 variants examined.
TABLE 2.
Antiviral activity of QYL-685 and QYL-609 against wild-type and drug-resistant infectious HIV-1
| Compound | IC50 (μM)a for:
|
|||
|---|---|---|---|---|
| HIV-1wtb | HIV-1215 | HIV-174 | HIV-162/75/77/116/151 | |
| AZT | 0.017 ± 0.004 | 0.14 ± 0.031 (10×)c | 0.023 ± 0.005 (1×) | 11 ± 5.2 (630×) |
| ddId | 3.7 ± 1.8 | 4.2 ± 1.1 (1×) | 25.4 ± 8.9 (7×) | 47 ± 8.3 (13×) |
| QYL-685 | 0.36 ± 0.054 | 0.30 ± 0.051 (1×) | 0.39 ± 0.25 (1×) | 0.39 ± 0.06 (1×) |
| QYL-609 | 0.029 ± 0.011 | 0.03 ± 0.01 (0.8×) | 0.013 ± 0.01 (0.5×) | 0.037 ± 0.02 (1×) |
MT-2 cells were exposed to 100 50% tissue culture infective doses of each infectious clone, the amounts of p24 Gag protein produced were determined by radioimmunoassay, and IC50s were computed (13, 17, 20, 21). Data shown represent the mean values (with standard deviations) derived from three independent experiments conducted in triplicate. All HIV-1 strains used were infectious clones as previously reported (22, 25).
HIV-1wt, wild-type HIV-1.
Numbers in parentheses indicate fold changes compared to the IC50 against HIV-1wt.
ddI, dideoxyinosine.
The introduction of a Z-methylenecyclopropane moiety in place of the ribofuranose portion of nucleoside analogues achieves two goals that could promote antiviral properties. One obvious advantage is the removal of the 3′-OH group necessary for phosphodiester linkages between nucleosides, resulting in a DNA chain termination. The other consequence of this structural change could be a conformational constraint (18). It has been shown that the introduction of a rigid structure into nucleosides can promote antiviral properties in certain compounds (1, 2, 5, 18). For example, a conformationally constrained analogue of acyclovir was found to have potent antiherpetic properties (herpes simplex virus type 1 [HSV-1] and HSV-2) comparable to those of the nonconstrained acyclovir (2). The structural rigidity of stavudine (D4T) also appears to play an important role in its antiviral activity (4, 5). For these reasons, QYL compounds have become increasingly attractive as potential antiviral agents (18, 19). The addition of a PPA moiety to Z-methylenecyclopropane nucleoside analogues (QYL compounds) often either enhances existing antiviral activity or renders inert compounds active against certain viruses (19a). This effect is presumably due to the lipophilicity of the PPA moiety facilitating cellular entry and effective intracellular di- and triphosphorylation of the monophosphate generated inside the cells.
In the present study of structure-activity relationships, it was found that adenine and 2,6-diaminopurine Z-methylenecyclopropanes (QYL-284A and QYL-546A, respectively) were moderately active against HIV-1 in vitro. Several hydroxymethylcyclopropylidine-methyladenines without the PPA moiety (e.g., QYL-284A but not QYL-546) have been recently described by Cheng et al. (6). However, the addition of the PPA moiety, producing QYL-685 and QYL-609, significantly increased the antiviral activity against HIV-1 (Table 1). It is presumed that the agents possessing the highly lipophilic PPA moiety enter cells relatively quickly. This property of the PPA moiety may bring about an enhancement of the agent’s activity, in particular, when it is added to agents which hardly penetrate the cellular membrane or to unstable agents that tend to deteriorate before cellular entry. Furthermore, it should be noted that methylenecyclopropanes with the PPA moiety are already monophosphorylated with the phosphate group within the PPA moiety. Although the question of which cellular enzyme(s) is responsible for the monophosphorylation of methylenecyclopropane analogues QYL-284A and QYL-546 has not yet been answered, it is likely that these compounds are only moderately (or poorly) monophosphorylated in HIV-1-exposed cells. Thus, the introduction of the PPA moiety effectively bypassed the intracellular monophosphorylation step for the intracellular delivery of QYL-546- and QYL-284A-monophosphates, which, presumably, were ultimately converted to triphosphates to exert their potent anti-HIV-1 activity.
The moderate level of anti-HIV-1 activity of the 2,6-diaminopurine E analogue QYL-675 implies that geometric isomerism of QYL compounds is of great importance. It is not known, however, whether or not RT or intracellular kinases can effectively operate on E-isomers of these compounds. This question could be answered by synthesizing a triphosphate form of this compound and subjecting it to an RT assay, in a future investigation. Two pyrimidine QYL compounds, QYL-692 and QYL-468, failed to exhibit significant anti-HIV-1 activity. It is important to note, however, that these drugs do not have the PPA moiety. Until pyrimidine analogues with the PPA moiety are tested for anti-HIV-1 activity, conclusions cannot be made as to whether pyrimidine QYL compounds could be active against HIV-1.
QYL-685 and QYL-609 were active against HIV-2 but with a moderately reduced potency. Since no triphosphate forms of QYL-685 or QYL-609 are presently available (we have tried unsuccessfully to radiolabel QYL-685 and QYL-609 multiple times, using two different procedures [16a]), it is not possible to examine the enzymatic properties of these compounds in the enzymatic assays. However, for the following two reasons, we assume that these two compounds fall within a category of NRTIs. First, all the known non-NRTIs are absolutely ineffective against HIV-2 in in vitro cell-based assays, whereas they are very effective against HIV-1 (3, 9, 10, 15). Thus, our observations that PPA derivatives are about 10-fold less potent against HIV-2 than against HIV-1 make the possibility that PPA derivatives are non-NRTIs less likely. Second, we performed RT assays with HIV-1 RT and found that PPA derivatives failed to block HIV-1 RT’s enzymatic activity (data not shown), meaning that without further phosphorylation, PPA derivatives do not inhibit HIV-1 RT. This result also strongly suggests that the QYL compounds are NRTIs.
It is worth noting that allene analogues (12) related to methylenecyclopropane compounds have a narrower range of antiviral activity (cytallene has activity against HIV and HBV), whereas methylenecyclopropanes have a broader antiviral spectrum without the PPA group (19a). However, QYL-685 and QYL-609 have proved to be active against a variety of viral pathogens, including human and murine cytomegaloviruses, human hepatitis B virus, Epstein-Barr virus, varicella zoster virus, and herpes simplex virus-1 and -2 (19a).
It should be stressed that QYL-685 and QYL-609 were active against three major drug-resistant HIV-1 variants, HIV-1215, HIV-174, and HIV-162/75/77/116/151; this is quite interesting, since these compounds may be potentially clinically useful in the treatment of individuals harboring such drug-resistant HIV-1 variants. Further development of methylenecyclopropane nucleoside analogues with the PPA moiety as potential therapies for HIV-1 infection is thus warranted.
Acknowledgments
The work at the Karmanos Cancer Institute was supported in part by research grant CA32779 from the National Cancer Institute. H.M. received grants from the Ministry of Health and Welfare of Japan (Promotion of AIDS Research) and the Japan Society for the Promotion of Science.
REFERENCES
- 1.Ashton W T, Canning L F, Reynolds G F, Tolman R L, Karkas J D, Liou R, Davies M E, DeWitt C M, Perry H C, Field A K. Synthesis and antiherpetic activity of (S)-, (R)-, and (+/−)-9-[(2,3-dihydroxy-1-propoxy)methyl]guanine, linear isomers of 2′-nor-2′-deoxyguanosine. J Med Chem. 1985;28:926–933. doi: 10.1021/jm00145a014. [DOI] [PubMed] [Google Scholar]
- 2.Ashton W T, Meurer L C, Cantone C L, Field A K, Hannah J, Karkas J D, Liou R, Patel G F, Perry H C, Wagner A F. Synthesis and antiherpetic activity of (+/−)-9-[[(Z)-2-(hydroxymethyl)cyclopropyl]methyl]guanine and related compounds. J Med Chem. 1988;31:2304–2315. doi: 10.1021/jm00120a010. [DOI] [PubMed] [Google Scholar]
- 3.Baba M, Shigeta S, Yuasa S, Takashima H, Sekiya K, Ubasawa M, Tanaka H, Miyasaka T, Walker R T, De Clercq E. Preclinical evaluation of MKC-442, a highly potent and specific inhibitor of human immunodeficiency virus type 1 in vitro. Antimicrob Agents Chemother. 1994;38:688–692. doi: 10.1128/aac.38.4.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balzarini J, Karlsson A, Aquaro S, Perno C F, Cahard D, Naesens L, De Clercq E, McGuigan C. Mechanism of anti-HIV action of masked alaninyl d4T-MP derivatives. Proc Natl Acad Sci USA. 1996;93:7295–7299. doi: 10.1073/pnas.93.14.7295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barrish J C, Zahler R. Antiviral agents. Annu Rep Med Chem. 1993;28:131–140. [Google Scholar]
- 6.Cheng C, Shimo T, Somekawa K, Baba M. 9-Hydroxymethyl-cyclopropylidenemethylenyladenine: the design, facile synthesis, isomer separation and anti-HIV-1 activities. Tetrahedron. 1998;54:2031–2040. [Google Scholar]
- 7.Clavel F, Guetard D, Brun-Vezinet F, Chamaret S, Rey M A, Santos-Ferreira M O, Laurent A G, Dauguet C, Katlama C, Rouzious C, Klatzmann D, Champalimaud J L, Montagnier L. Isolation of a new human retrovirus from West African patients with AIDS. Science. 1986;233:343–346. doi: 10.1126/science.2425430. [DOI] [PubMed] [Google Scholar]
- 8.Daluge S M, Good S S, Faletto M B, Miller W H, St. Clair M H, Boone L R, Tisdale M, Parry N R, Reardon J E, Dornsife R E, Averett D R, Krenitsky T A. 1592U89, a novel carbocyclic nucleoside analog with potent, selective anti-human immunodeficiency virus activity. Antimicrob Agents Chemother. 1997;41:1082–1093. doi: 10.1128/aac.41.5.1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Clercq E. HIV resistance to reverse transcriptase inhibitors. Biochem Pharmacol. 1994;47:155–169. doi: 10.1016/0006-2952(94)90001-9. [DOI] [PubMed] [Google Scholar]
- 10.De Clercq E. In search of a selective antiviral chemotherapy. Clin Microbiol Rev. 1997;10:674–693. doi: 10.1128/cmr.10.4.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deeks S G, Collier A, Lalezari J, Pavia A, Rodrigue D, Drew W L, Toole J, Jaffe H S, Mulato A S, Lamy P D, Li W, Cherrington J M, Hellmann N, Kahn J. The safety and efficacy of adefovir dipivoxil, a novel anti-human immunodeficiency virus (HIV) therapy, in HIV-infected adults: a randomized, double-blind, placebo-controlled trial. J Infect Dis. 1997;176:1517–1523. doi: 10.1086/514150. [DOI] [PubMed] [Google Scholar]
- 12.Hayashi S, Phadtare S, Zemlicka J, Matsukura M, Mitsuya H, Broder S. Adenallene and cytallene: acyclic nucleosides that inhibit replication and cytopathic effect of human immunodeficiency virus (HIV) in vitro. Proc Natl Acad Sci USA. 1988;85:6127–6131. doi: 10.1073/pnas.85.16.6127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kodama E, Shigeta S, Suzuki T, De Clercq E. Application of a gastric cancer cell line (MKN-28) for anti-adenovirus screening using the MTT method. Antivir Res. 1996;31:159–164. doi: 10.1016/0166-3542(96)06966-5. [DOI] [PubMed] [Google Scholar]
- 14.Marquez V E, Tseng C K-H, Mitsuya H, Aoki S, Kelley J A, Ford H J, Roth J S, Broder S, Johns D G, Driscoll J S. Acid-stable 2′-β-fluoro purine dideoxynucleosides as active agents against HIV. J Med Chem. 1990;33:978–985. doi: 10.1021/jm00165a015. [DOI] [PubMed] [Google Scholar]
- 15.Mitsuya H. Nucleoside analogues and their antiretroviral activity. In: Mitsuya H, editor. Anti-HIV nucleosides: past, present and future. Austin, Tex: R. G. Langes Company; 1997. pp. 3–22. [Google Scholar]
- 16.Mitsuya H, Erickson J. Discovery and development of antiretroviral therapeutics for HIV infection. In: Merigan T C, editor. Textbook of AIDS medicine. Baltimore, Md: Williams & Wilkins; 1998. pp. 751–780. [Google Scholar]
- 16a.Moravek, J., and H. Mitsuya. Unpublished data.
- 17.Pauwels R, Balzarini J, Baba M, Snoeck R, Schols D, Herdewijn P, Desmyter J, De Clercq E. Rapid and automated tetrazolium-based colorimetric assay for the detection of anti-HIV compounds. J Virol Methods. 1988;20:309–321. doi: 10.1016/0166-0934(88)90134-6. [DOI] [PubMed] [Google Scholar]
- 18.Qiu Y L, Ksebati M B, Ptak R G, Fan B Y, Breitenbach J M, Lin J-S, Cheng Y C, Kern E R, Drach J C, Zemlicka J. (Z)- and (E)-2-[(hydroxymethyl)cyclopropylidene]methyladenine and -guanine. New nucleoside analogues with a broad-spectrum antiviral activity. J Med Chem. 1998;41:10–23. doi: 10.1021/jm9705723. [DOI] [PubMed] [Google Scholar]
- 19.Qiu Y L, Ptak R G, Breitenbach J M, Lin J-S, Cheng Y-C, Kern E R, Drach J C, Zemlicka J. (Z)- and (E)-2-[(hydroxymethyl)cyclopropylidene]methylpurines and pyrimidines as antiviral agents. Antivir Chem Chemother. 1998;9:341–352. [PubMed] [Google Scholar]
- 19a.Qiu, Y.-L., R. G. Ptak, J. M. Breitenbach, J.-S. Lin, Y.-C. Cheng, J. C. Drach, E. R. Kern, and J. Zemlicka. Synthesis and antiviral activity of phosphoralaninate derivatives of methylenecyclopropane analogues of nucleosides. Antivir. Res., in press. [DOI] [PubMed]
- 20.Richman D D, Johnson V A, Mayers D L, Shirasaka T, O’Brien M C, Mitsuya H. In vitro evaluation of experimental agents for anti-HIV activity. In: Coligan J E, Kruisbeek A M, Margulies D H, Shevach E M, Strober W, editors. Current protocols in immunology. New York, N.Y: John Wiley & Sons, Inc.; 1993. pp. 12.9.1–12.9.21. [DOI] [PubMed] [Google Scholar]
- 21.Shirasaka T, Chokekijchai S, Yamada A, Gosselin G, Imbach J-L, Mitsuya H. Comparative analysis of anti-human immunodeficiency virus type 1 activities of dideoxynucleoside analogs in resting and activated peripheral blood mononuclear cells. Antimicrob Agents Chemother. 1995;39:2555–2559. doi: 10.1128/aac.39.11.2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shirasaka T, Kavlick M F, Ueno T, Gao W-Y, Kojima E, Alcaide M L, Chokekijchai S, Roy B M, Arnold E, Yarchoan R, Mitsuya H. Emergence of human immunodeficiency virus type-1 variants with resistance to multiple dideoxynucleosides in patients receiving therapy with dideoxynucleosides. Proc Natl Acad Sci USA. 1995;92:2398–2402. doi: 10.1073/pnas.92.6.2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shirasaka T, Yarchoan R, O’Brien M C, Husson R N, Anderson B D, Kojima E, Shimada T, Broder S, Mitsuya H. Changes in drug sensitivity of human immunodeficiency virus type 1 during therapy with azidothymidine, dideoxycytidine, and dideoxyinosine: an in vitro comparative study. Proc Natl Acad Sci USA. 1993;90:562–566. doi: 10.1073/pnas.90.2.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tanaka H, Baba M, Saito S, Miyasaka T, Takasha H, Sekiya K, Ubasawa M, Nitta I, Walker R T, Nakashima H, De Clercq E. Specific anti-HIV-1 “acyclonucleosides” which cannot be phosphorylated: synthesis of some deoxy analogues of 1-[(2-hydroxyethoxy)methyl]-6-(phenylthio)thymine. J Med Chem. 1991;34:1508–1511. doi: 10.1021/jm00108a041. [DOI] [PubMed] [Google Scholar]
- 25.Tanaka M, Srinivas R V, Ueno T, Kavlick M F, Hui F K, Fridland A, Driscoll J S, Mitsuya H. In vitro induction of human immunodeficiency virus type 1 (HIV-1) variants resistant to 2′-β-fluoro-2′,3′-dideoxyadenosine (F-ddA) Antimicrob Agents Chemother. 1997;41:1313–1318. doi: 10.1128/aac.41.6.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]