Abstract
Programmed cell death (PCD) is an essential part of organismal development and plays fundamental roles in host defense against pathogens and the maintenance of homeostasis. However, excess activation of PCD pathways has proven to be detrimental and can drive disease. Additionally, resistance to PCD can also contribute to disease development. Modulation of PCD, therefore, has great therapeutic potential in a wide range of diseases, including infectious, neurodegenerative, autoinflammatory, and metabolic diseases and cancer. Nevertheless, manipulation of cell death and inflammation for therapeutic intervention is a delicate process, highly specific to the context of the disease of interest, making the selection of the appropriate target molecule crucially important. Several PCD pathways are associated with innate immunity, including pyroptosis, apoptosis, necroptosis, and PANoptosis, which is defined as an inflammatory PCD pathway with key features of pyroptosis, apoptosis, and/or necroptosis that cannot be accounted for by any of these three PCD pathways alone. All of these PCD pathways are regulated by upstream sensors and signaling cascades that assemble multimeric complexes to serve as activation platforms for downstream molecules; these sensors and signaling molecules provide attractive target points for therapeutic intervention. Here, we discuss the molecular mechanisms of innate cell death in health and disease, with a particular focus on the molecules putatively involved in the formation of the PANoptosome and the induction of inflammatory cell death. Further, we discuss the implications and feasibility of targeting these molecules to improve disease outcomes, as well as current clinical approaches.
Keywords: Pyroptosis, apoptosis, necroptosis, PANoptosis, PANoptosome, inflammation, RIPK1, RIPK3, MLKL, NLRP3, caspase-1, ASC, caspase-8, caspase-3, caspase-7, ZBP1, TNFα, IFNγ, IL-1, inflammasome, DISC, apoptosome, necrosome, ripoptosome, infection
1. INTRODUCTION: INFLAMMATORY CELL DEATH AND DISEASE
Cell death is vital to both normal organismal development and defense against invading pathogens. In the past, cell death was categorized as either programmed, immunologically silent apoptosis or unprogrammed, inflammatory necrosis. Based on that categorization, apoptosis is characterized by caspase activation, fragmentation of the nucleus, formation of apoptotic bodies, cell shrinkage, and phagocytosis of the cellular corpse to prevent the release of inflammatory stimuli (Elmore, 2007). The “quiet” nature of this cell death is beneficial, as it avoids activation of inflammatory immune reactions in response to the necessary death occurring during development or homeostatic cell turnover.
Unlike classically described apoptosis, necrosis or lytic cell death is immunologically active, characterized by damage or rupture of the cellular membrane and release of inflammatory stimuli into the surrounding environment. This inflammatory process was initially thought to be an unprogrammed, spontaneous event in response to injury or extremely unfavorable conditions (Kerr, Wyllie, & Currie, 1972), but it has now become clear that several host pathways function to regulate programmed necrosis (Galluzzi, Kepp, Krautwald, Kroemer, & Linkermann, 2014). Included among these inflammatory programmed cell death (PCD) pathways are pyroptosis and necroptosis, while emerging evidence suggests that caspase-mediated apoptosis can also be inflammatory (de Vasconcelos, et al., 2020; Gurung, et al., 2014; Lee, et al., 2018; Lukens, Gurung, et al., 2014; R. K. S. Malireddi, Gurung, et al., 2020; Sarhan, et al., 2018). Pyroptosis can serve as a host defense against pathogenic infection and is mediated by inflammasome formation and inflammatory caspase activation, leading to proinflammatory cytokine release through membrane pores formed by members of the gasdermin family (Aglietti, et al., 2016; S. Christgen, Place, & Kanneganti, 2020; W. T. He, et al., 2015; Kayagaki, et al., 2015; Sborgi, et al., 2016; J. Shi, Gao, & Shao, 2017; J. Shi, et al., 2015). In contrast, necroptosis is caspase-independent and involves activation of the receptorinteracting protein (RIP) kinases and phosphorylation of the pseudokinase mixed lineage-kinase domain-like (MLKL) (Degterev, et al., 2008; Degterev, et al., 2005; Sun, et al., 2012).
Dysregulation or aberration in the induction of cell death is closely linked to the development of several diseases, including neurological, metabolic, autoimmune, and infectious diseases and cancer (Fuchs & Steller, 2011; Gorman, 2008; Karki & Kanneganti, 2019; Kesavardhana, Malireddi, & Kanneganti, 2020; Van Opdenbosch & Lamkanfi, 2019). The regulation of PCD by defined cellular programs provides a number of checkpoints to guide the cell life or death decision process. As such, targeting these checkpoints has become an attractive strategy for the development of new therapeutics in a wide range of diseases. However, it is increasingly apparent that these molecular programs are deeply interwoven (Table 1). Studies probing cell death crosstalk have demonstrated numerous molecular interactions between pathways and shown that pathogens or sterile insults can induce multiple cell death pathways. For instance, apoptotic substrates can be activated by pyroptotic molecules and vice versa, while inhibition of one cell death pathway by a pathogen or other signaling defect can result in another pathway compensating (Gurung, et al., 2014; Gurung, Burton, & Kanneganti, 2016; Lamkanfi, et al., 2008; Lukens, Gross, et al., 2014; Lukens, Gurung, et al., 2014; R. K. Malireddi, Ippagunta, Lamkanfi, & Kanneganti, 2010; R. K. S. Malireddi, Gurung, et al., 2020; R. K. S. Malireddi, et al., 2018; Orning, et al., 2018; Sarhan, et al., 2018; M. Zheng, et al., 2020). Numerous observations of this crosstalk have led to the elucidation of PANoptosis, an inflammatory PCD pathway characterized by the engagement of key cell death molecules from pyroptosis, apoptosis, and/or necroptosis at a common scaffolding complex termed the PANoptosome (Banoth, et al., 2020; S. Christgen, Zheng, M., Kesavardhana, S., Karki, R., Malireddi, R.K.S., Banoth, B., Place, D.E., Briard, B., Sharma, B.R., Tuladhar, S., Samir, P., Burton, A., Kanneganti, T.-D., 2020; Gurung, et al., 2014; Gurung, et al., 2016; Karki, et al., 2020; Karki, et al., 2021; Kesavardhana, et al., 2017; Kesavardhana, Malireddi, Burton, et al., 2020; Kuriakose, et al., 2016; Lukens, Gurung, et al., 2014; R. K. Malireddi, et al., 2010; R. K. S. Malireddi, Gurung, et al., 2020; R. K. S. Malireddi, et al., 2021; R. K. S. Malireddi, Kesavardhana, et al., 2020; R. K. S. G. Malireddi, P.; Kesavardhana, S.; Samir, P.; Burton, A.; Mummareddy, H.; Vogel, P.; Pelletier, S.; Burgula, S.; Kanneganti, T.D., 2019; M. K. Zheng, R; Vogel, P; Kanneganti, TD 2020). Therefore, therapies to target PCD need to be designed carefully, as targeting a pathway at an improper juncture may lead to unintended consequences on other pathways. This makes systematic and in-depth study of the connections between PCD pathways imperative to the development of clinically successful therapeutics.
Table 1:
Cell death molecules
| Molecule | Host Domain architecture | Reported roles in PCD |
|---|---|---|
|
| ||
| Caspases | ||
|
| ||
| Caspase-1 |
|
Pyroptosis, PANoptosis: Cleaves and activates GSDMD, IL-1β, IL-18 |
| Caspase-3 |
|
Pyroptosis, apoptosis, PANoptosis: Cleaves and inactivates GSDMD, cleaves and activates GSDME, executes apoptosis through cleavage of other substrates |
| Caspase-4 |
|
Pyroptosis: Cleaves and activates GSDMD |
| Caspase-5 |
|
Pyroptosis: Cleaves and activates GSDMD |
| Caspase-6 |
|
Apoptosis, PANoptosis: Cleaves and activates caspase-3 and caspase-7, stabilizes PANoptosome complex |
| Caspase-7 |
|
Pyroptosis, apoptosis, PANoptosis: Cleaves and inactivates GSDMD, cleaves and activates GSDME, executes apoptosis through cleavage of other substrates |
| Caspase-8 |
|
Pyroptosis, apoptosis, necroptosis, PANoptosis: Capable of cleaving and activating GSDMD, GSDME, IL-1β, IL-18, caspase-3, caspase-7, caspase-9, and RIPK1 |
| Caspase-9 |
|
Apoptosis: Cleaves and activates caspase-3, caspase-7, caspase-8 |
| Caspase-10 |
|
Apoptosis: Cleaves and activates caspase-3 and caspase-7 |
| Caspase-11 |
|
Pyroptosis: Cleaves and activates GSDMD |
|
| ||
| Inflammasome sensors | ||
|
| ||
| NLRP1 |
|
Pyroptosis, apoptosis: Facilitates inflammasome formation after n-terminal cleavage |
| NLRP3 |
|
Pyroptosis, apoptosis, PANoptosis: Facilitates inflammasome formation downstream of a number of signals, including pore formation, trans-Golgi network disruption, K+ efflux, and TAK1 inhibition |
| NAIP |
|
Pyroptosis, apoptosis: Binds NLRC4-activating ligands, nucleates NLRC4 monomers to initiate inflammasome formation |
| NLRC4 |
|
Pyroptosis, apoptosis: Facilitates inflammasome formation after NAIP–ligand binding |
| AIM2 |
|
Pyroptosis, apoptosis: Facilitates inflammasome formation after nucleation on double-stranded DNA |
| Pyrin |
|
Pyroptosis: Facilitates inflammasome formation downstream of Rho GTPase inactivation |
|
| ||
| RHIM–containing molecules | ||
|
| ||
| RIPK1 |
|
Apoptosis, necroptosis, PANoptosis: Facilitates RIPK3 phosphorylation and activation |
| RIPK3 |
|
Necroptosis, PANoptosis: Phosphorylates MLKL after activation |
| ZBP1 |
|
Pyroptosis, apoptosis, necroptosis, PANoptosis: Senses Z-nucleic acids to induce inflammatory cell death |
|
| ||
| Adaptor molecules | ||
|
| ||
| ASC |
|
Pyroptosis, apoptosis, PANoptosis: Serves as a linker molecule between sensors and effector proteins (i.e., NLRP3 and caspase-1); recruits caspase-8 to the inflammasome |
| FADD |
|
Pyroptosis, apoptosis, PANoptosis: Serves as a bridge to link caspase-8 to death receptors; plays a role in inflammasome formation |
|
| ||
| Pore–forming molecules | ||
|
| ||
| GSDMD |
|
Pyroptosis, PANoptosis: N-terminal domain forms pores in cellular membrane after cleavage |
| GSDME |
|
Pyroptosis, PANoptosis: N-terminal domain forms pores in cellular membrane after cleavage |
| MLKL |
|
Necroptosis, PANoptosis: Forms pores in cellular membrane after phosphorylation |
Current therapeutic approaches to target cell death, such as inducing death through DNA damage or broadly inhibiting an enzyme’s activity, tend to have global impacts on cellular machinery beyond the PCD pathways. Further, this approach can contribute to off-target effects due to the multifaceted roles of many cell death molecules within the cell. A recurring theme among PCD pathways is regulation by the formation of multimeric protein complexes, which may serve as unique therapeutic targets. While understanding of these complexes has grown exponentially over recent years, many questions regarding the exact makeup and assembly mechanisms remain unanswered, and therapies designed to target assembly or disassembly of these complexes are underutilized in the clinic. In this review, we discuss the regulation of cell death by the formation of signaling complexes and current strategies to target molecules of these pathways at both the bench and the patient bedside, highlighting the potential benefits of newer, more targeted therapies to act on these pathways.
2. TARGETING CELL DEATH COMPLEXES IN INNATE IMMUNITY
The induction and execution of PCD is primarily mediated by the formation of large, signal-amplifying complexes assembled in response to sensor engagement by a sterile or pathogen-derived trigger. Often, assembly of these complexes is further controlled by upstream signaling pathways that regulate expression and conformation of core components of the complexes. Included among these death complexes are the apoptosis-associated death-inducing signaling complex (DISC) and apoptosome, the necroptosis-associated necrosome, the pyroptosis-associated inflammasome, and the PANoptosis-associated PANoptosome.
Apoptosis, DISC, and the Apoptosome
Apoptosis is generally divided into an intrinsic pathway, dependent on cytosolic apoptotic protease activating factor-1 (APAF-1), and an extrinsic pathway, dependent on membrane-bound death receptors (Figure 1). Both pathways converge on the activation of executioner caspases, which contain a long and short subunit, by upstream initiator caspases, comprised of a recruitment domain and long and short subunit (Table 1) (Strasser, Harris, Huang, Krammer, & Cory, 1995).
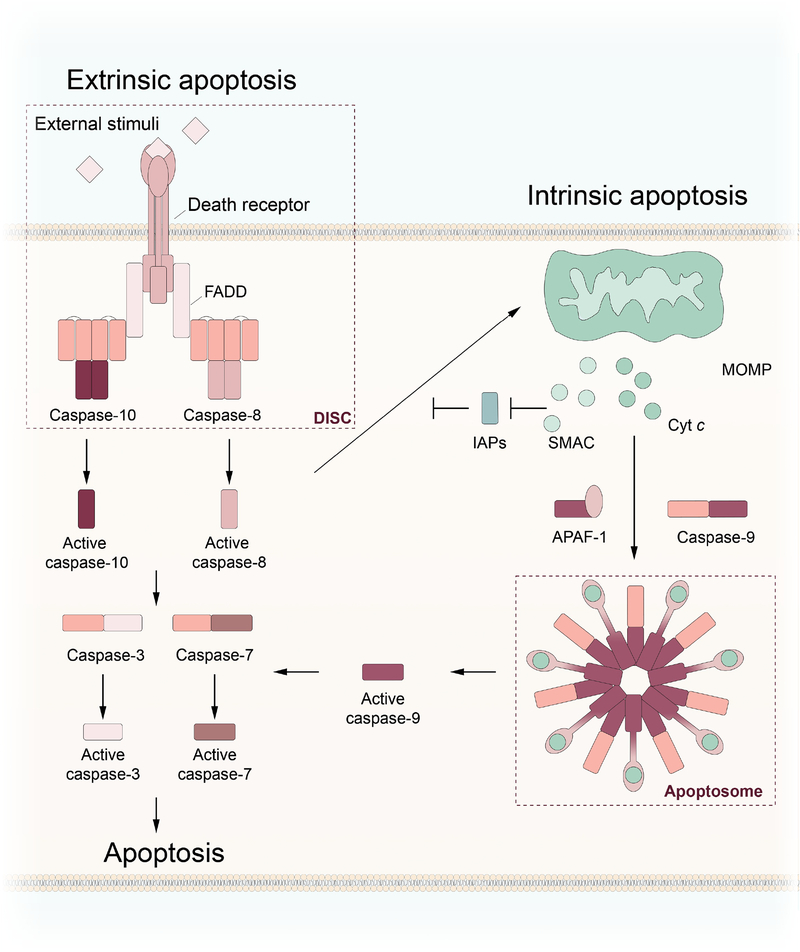
Figure 1: Regulation of apoptosis by the apoptosome and DISC.
Extrinsic apoptosis: Activation of caspase-8 and caspase-10 is regulated by the death-inducing signaling complex (DISC). Following external stimulation of death receptors, FADD recruits caspase-8 or caspase-10 to the DISC through homotypic interactions. Intrinsic apoptosis: Internal signals, including BID cleavage and DNA damage, induce mitochondrial outer membrane permeabilization (MOMP), leading to the release of cytochrome c (Cyt c). Cyt c binds to APAF-1, triggering formation of the apoptosome and recruitment and subsequent activation of caspase-9. The two pathways converge on the activation of executioner caspases, such as caspase-3 and caspase-7 to execute apoptotic cell death.
In the intrinsic pathway of apoptosis, cell death is primarily governed by pro- and anti-apoptotic members of the Bcl-2 (B-cell lymphoma 2) family of proteins. In response to pro-apoptotic stimuli, activation of pro-apoptotic Bcl-2 family proteins, including BAX, BAK, and BID, triggers mitochondrial outer membrane permeabilization (MOMP), resulting in leakage of cytochrome c (Cyt c) into the cytosol (Kalkavan & Green, 2018). There, Cyt c binds to monomers of APAF-1, leading to the multi-step, nucleotide-dependent oligomerization of APAF-1 and the formation of the apoptosome (Figure 1). Binding of Cyt c to APAF-1 triggers a conformational rearrangement that makes nucleotide exchange of ADP to ATP, a process required for full apoptosome assembly, more favorable (Chinnaiyan, O’Rourke, Lane, & Dixit, 1997; Y. Hu, Benedict, Ding, & Nunez, 1999; P. Li, et al., 1997). The coupled events of Cyt c binding and nucleotide exchange result in the formation of a death platform comprised of a heptameric central hub with an auxiliary, acentric caspase activation and recruitment domain (CARD) wheel extending outwards. The intrinsic initiator caspase, caspase-9, is recruited to the extended surface via homotypic CARD-CARD interactions, resulting in its activation (Acehan, et al., 2002). Caspase-9 then cleaves and activates the executioner caspases caspase-3 and caspase-7, resulting in apoptosis. In addition to Cyt c, other proteins normally found within the inner membrane of mitochondria, including pro-apoptotic SMAC, also known as DIABLO, are released into the cytosol. Once in the cytosol, SMAC inhibits anti-apoptotic members of the IAP (inhibitors of apoptosis) family, which help regulate the extrinsic pathway of apoptosis.
In contrast to intrinsic apoptosis, extrinsic apoptosis is regulated by cell-surface death receptor signaling, formation of the death-inducing signaling complex (DISC), and activation of caspase-8 or caspase-10. Activation of death receptors such as tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) family receptors by ligand binding results in the recruitment of adaptor proteins, including Fas-associated death domain (FADD), through death domain (DD) interactions. Helical oligomerization of the adaptor proteins through homotypic DD interactions is followed by recruitment of caspase-8/10, mediated by the formation of death effector domain (DED) filaments extending from the DISC (Fox, et al., 2021; Fu, et al., 2016; Schleich, et al., 2016). Active caspase-8/10 are then free to cleave and activate their substrates, including executioner caspases caspase-3, caspase-7, and caspase-6. Caspase-8 has also been shown to cleave and activate BID, driving the intrinsic pathway of apoptosis downstream of extrinsic activation. Extrinsic apoptosis can be blocked at the DISC by the binding of cellular or viral FLICE-like inhibitory protein (cFLIP, vFLIP) (Schleich, et al., 2016). Humans possess three isoforms of cFLIP that can inhibit apoptosis and promote cellular survival by either completely blocking caspase-8 activation and activity (cFLIPS and cFLIPR) or by severely limiting caspase-8 activity (cFLIPL) through the formation of caspase-8/cFLIP heterodimers (Oberst, et al., 2011; Safa, 2012). A recent structural study into the interactions between caspase-8 and FADD showed the formation of a tandem DED repeating triple strand helix (Fox, et al., 2021). Binding of cFLIP to FADD results in disruption of the triple strand helix, leading to a misalignment of the caspase-8 molecules and decreased catalytic activity.
Resistance to apoptosis is one of the hallmarks of cancer development. Excessive growth and proliferation without an apoptotic brake contributes to tumor growth and development, while evasion of apoptosis further results in resistance to host immunosurveillance and limits the efficacy of many cancer treatments (Igney & Krammer, 2002). In cancer cells, this manifests by a pathological upregulation of anti-apoptotic molecules and inhibition or downregulation of pro-apoptotic molecules. As such, molecules targeting the induction of apoptosis, such as SMAC mimetics and inhibitors of members of the IAP family, have gained popularity as anti-cancer therapies to sensitize tumors to cell death. Currently, IAP inhibitors, like venetoclax, are used in conjunction with inducers of apoptosis, such as rituximab, to improve outcomes in patients with cancer (Seymour, et al., 2018) (Table 2). However, further improvements in cancer therapy could potentially be made through strategies that induce additional cell death effectors (R. K. S. Malireddi, et al., 2021), as discussed in detail later.
Table 2:
Compounds targeting programmed cell death
| Compound | Target | Clinical Stage | FDA-approved indications | References |
|---|---|---|---|---|
|
| ||||
| Inhibitors of cell death | ||||
|
| ||||
| MCC950 | NLRP3 | N/A | N/A | (Coll, et al., 2015) |
| INF39 | NLRP3 | N/A | N/A | (Cocco, et al., 2017) |
| Oridonin | NLRP3 | N/A | N/A | (H. He, et al., 2018) |
| Glyburide | NLRP3 | Phase 4; earlier phases for other clinical indications | Type 2 diabetes | (Lamkanfi, et al., 2009) |
| Dapansutrile (OLT1177) | NLRP3 | Phase 2 enrollment | N/A | (Klück, et al., 2020; Wohlford, et al., 2020) |
| Colchicine | NLRP3 | Phase 4; earlier phases for other clinical indications | Gout and FMF | |
| Necrostatin-1 | RIPK1 | N/A | N/A | (Degterev, et al., 2008) |
| GSK2982772 | RIPK1 | Phase 1* | ||
| DNL-747 | RIPK1 | Development paused in Phase 1b | ||
| GSK2399872A (GSK872) | RIPK3 | N/A | N/A | (Kaiser, et al., 2013) |
| Disulfiram | GSDMD | Phase 4; earlier phases for other clinical indications | Alcohol dependence | (J. J. Hu, et al., 2020) |
| Dimethyl fumarate | GSDMD | Phase 4; earlier phases for other clinical indications | Relapsing multiple sclerosis | (Humphries, et al., 2020) |
| Necrosulfonamide | MLKL/GSDMD | N/A | N/A | (Rathkey, et al., 2018; Ueda, et al., 2021; Y. Wang, Wang, et al., 2018) |
|
| ||||
| Inhibitors of cytokine signaling | ||||
|
| ||||
| Rilonacept | IL-1 | Phase 4; earlier phases for other clinical indications | CAPS, DIRA, recurrent pericarditis | |
| Anakinra | IL-1R | Phase 4; earlier phases for other clinical indications | Rheumatoid arthritis, NOMID; and DIRA | |
| Canakinumab | IL-1β | Phase 3; earlier phases for other clinical indications | CAPS, TNFR-associated periodic syndrome, hyperimmunoglobulin D syndrome, FMF, adult-onset still’s disease, systemic juvenile idiopathic arthritis | (Ridker, Everett, et al., 2017; Ridker, MacFadyen, et al., 2017; Ridker, et al., 2011) |
|
| ||||
| Inducers of cell death | ||||
|
| ||||
| Navitoclax | Bcl-2 | Phase 3; earlier phases for other clinical indications | N/A | |
| Venetoclax | Bcl-2 | Phase 4; earlier phases for other clinical indications | Chronic lymphocytic leukemia, small lymphocytic lymphoma, acute myeloid leukemia | |
| Doxorubicin | Topoisomerase | Phase 4; earlier phases for other clinical indications | Ovarian cancer, AIDS-related Kaposi’s sarcoma, multiple myeloma | |
| Cyclophosphamide | DNA alkylation | Phase 4 | Malignant lymphomas, multiple myeloma, leukemias, mycosis fungoides, neuroblastoma, adenocarcinoma of the ovary, retinoblastoma, breast carcinoma | |
| Oxaliplatin | DNA crosslinking | Phase 4 | Colorectal carcinoma | |
| TNFα + IFNγ | JAK/STAT signaling | Phase 3 (in combination with melphalan in HILP) | N/A | |
removed from Phase 2 clinical trials and reintroduced at a higher dosage in Phase 1
In contrast to cancer where apoptosis is often intrinsically inhibited, excessive induction of apoptosis contributes to the pathology of other diseases, such as neurodegenerative conditions like Alzheimer’s disease (Yuan & Yankner, 2000). However, strategies to inhibit pathological apoptosis for therapeutic approaches is complicated by molecular overlap with the activation of other PCD pathways.
Necroptosis, the Ripoptosome, and the Necrosome
Activation of necroptosis is mediated through the activity of the RIP kinases RIPK1 and RIPK3, along with the pore-forming pseudokinase MLKL, downstream of necrosome formation (Cai, et al., 2014; Cho, et al., 2009; S. He, et al., 2009; Sun, et al., 2012; H. Wang, et al., 2014; D. W. Zhang, et al., 2009; Zhao, et al., 2012). Necroptosis is sometimes referred to as a “back-up” form of cell death, as many reported instances of necroptosis result from the initiation, but failed execution, of apoptosis. As such, necroptosis can be activated by pathogens that block the induction of apoptosis, resulting in the lytic nature of this pathway alerting the immune system to the potential invading threat. Because this cell death pathway is primarily activated in pathological contexts, there is growing interest in necroptosis-targeting therapeutics.
Downstream of TNFR signaling, the cell fate decision between survival, apoptotic cell death, and necroptotic cell death is primarily guided by caspase-8, in conjunction with the kinase RIPK1, through modulation of a ripoptosome complex (Amin, et al., 2018; Dillon, et al., 2014; Geng, et al., 2017; Kaiser, et al., 2014; Lin, et al., 2016; R. K. S. Malireddi, Gurung, et al., 2020; R. K. S. Malireddi, Kesavardhana, et al., 2020; Newton, et al., 2019; Newton, et al., 2016) (Figure 2). When TNF binds TNFR1, complex I is formed, containing TRADD (TNFR type 1-associated DEATH domain), TRAFs (TNFR-associated factors), cIAP1/2, RIPK1, and other proteins. In a pro-survival scenario, RIPK1 is ubiquitinated by E3 ubiquitin ligases (cIAP1/2), resulting in activation of transforming growth factor β-activated kinase 1 (TAK1) through TAK1-binding proteins (TABs), degradation of RIPK1, and promotion of NF-κB signaling (Dondelinger, et al., 2013; Peltzer, Darding, & Walczak, 2016; J. Zhang, et al., 2019). In the event of a checkpoint failure through loss of activity of one of the numerous components of complex I, the signaling complex can become a pro-death platform as either apoptotic complex IIa (ripoptosome), in the presence of active caspase-8, or necroptotic complex IIb (necrosome), in the absence of caspase-8 activity (Figure 2). While the full details of the molecular makeup of these complexes, including the components that differentiate one from the other, is not entirely clear and requires further study (Wegner, Saleh, & Degterev, 2017), caspase-8 activity is generally the factor that controls the cell death or survival outcome.
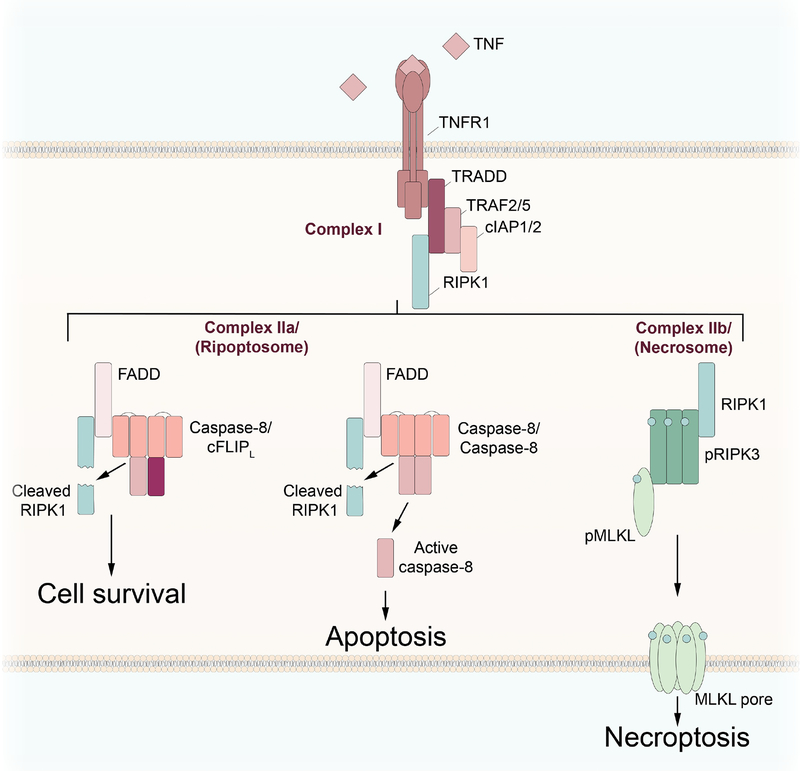
Figure 2: Regulation of necroptosis by the ripoptosome and necrosome.
Loss of checkpoint inhibition downstream of TNFR1 signaling leads to RIPK1 activation, causing complex I to shift to complex II. In the presence of limited caspase-8 activity, mediated by caspase-8/cFLIPL heterodimers, complex IIa, or the ripoptosome, facilitates cell survival through RIPK1 cleavage. Full activation of caspase-8 by the formation of caspase-8 homodimers leads to RIPK1 cleavage and ripoptosome-mediated apoptosis. Loss or inhibition of caspase-8 activity leads to the formation of the necrosome, phosphorylation of RIPK3 and MLKL, and the induction of necroptosis.
When the ripoptosome, comprised of death molecules including caspase-8, FADD, and RIPK1, is assembled (Tenev, et al., 2011), monomers of caspase-8 dimerize, leading to activation. Active caspase-8 cleaves RIPK1 before directing the cell to die through apoptosis. However, induction of ripoptosome-mediated apoptosis can be subverted by the expression of cFLIPL, resulting in the formation of caspase-8/cFLIPL heterodimers and restricted caspase-8 activation. These heterodimers are capable of cleaving RIPK1, but not caspase-8, leading to RIPK1 inactivation without subsequent apoptosis activation and maintaining cell survival (Oberst, et al., 2011). Absence or inhibition of caspase-8 results in RIPK1 activation and the transition of the ripoptosome to complex IIb, or the necrosome (Zhao, et al., 2012). Active RIPK1 induces phosphorylation and activation of RIPK3, which in turn phosphorylates the pseudokinase MLKL. Phosphorylated MLKL translocates to the cellular membrane and forms pores through oligomerization of helical N-terminal domains, culminating in the lytic cell death known as necroptosis (Cai, et al., 2014; Sun, et al., 2012; H. Wang, et al., 2014; Zhao, et al., 2012). Cellular lysis resulting from this form of cell death leads to the release of intracellular contents, including damage-associated molecular patterns (DAMPs), that trigger inflammatory responses. In addition to the RIPK1-dependent mechanism, necroptosis can also occur in a RIPK1-independent manner, through RIP homotypic interaction motif (RHIM)-RHIM interactions between RIPK3 and other RHIM-containing proteins, such as TIR domain-containing adapterinducing interferon-β (TRIF) (Kaiser, et al., 2013) and Z-DNA binding protein 1 (ZBP1), also known as DAI (Kesavardhana, et al., 2017; Kuriakose, et al., 2016; Lin, et al., 2016; Newton, et al., 2016).
The programmed nature of necroptosis was first identified when it was discovered that small molecule inhibitors could block TNFR-mediated necrosis; follow-up studies clarified that RIPK1 was the target of these inhibitors (Degterev, et al., 2008; Degterev, et al., 2005). Due to the high frequency of necroptosis in pathological conditions, interest has grown in refining necroptotic inhibitors into effective pharmacological therapies, particularly for neurodegenerative diseases. Within the context of the central nervous system, the ability to use TNF-neutralizing therapies is severely limited due to the role of TNFR2 signaling in remyelination and oligodendrocyte proliferation (Arnett, et al., 2001), making the identification of an alternative target regulating TNF-induced necroptosis of vital interest. As such, a number of RIPK1 inhibitors have been evaluated in clinical trials. Some, including GSK2982772 and DNL747, have passed Phase 1 safety trials. However, GSK2982772 was later removed from a Phase 2 study and sent back into research development, while development of DNL747 was paused in favor of clinical development of a more effective version of this compound. A new Phase 1 clinical trial examining the safety and efficacy of higher daily doses of GSK2982772 during psoriasis treatment is currently ongoing. The results of these clinical studies strongly suggest that further research into the role of RIPK1 in cell death and disease is required to safely and effectively target this molecule. The elucidation of kinase-independent roles of RIPK1 in cell death, particularly in pyroptosis (R. K. S. Malireddi, Gurung, et al., 2020), may help explain why kinase-only inhibitors have not realized their expected potential.
Pyroptosis and the Inflammasome
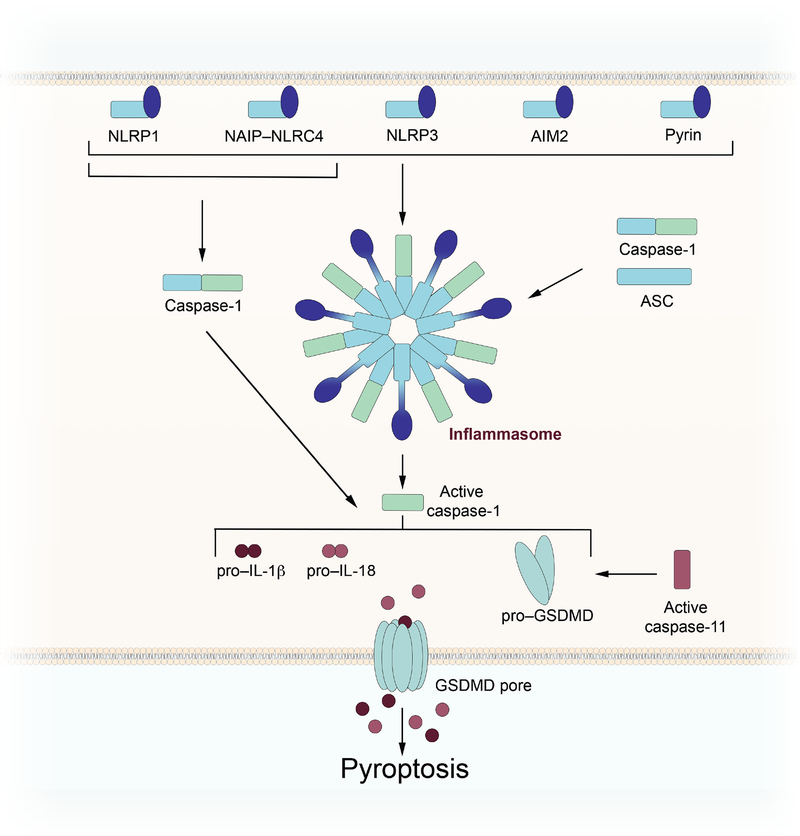
Another form of programmed lytic cell death is pyroptosis. Pyroptosis is characterized by the activation of inflammatory caspases, including caspase-1 and caspase-4/5 (caspase-11 in mice), and release of pro-inflammatory cytokines, including mature interleukin (IL)-1β and IL-18. DAMPs and pathogen-associated molecular patterns (PAMPs) are sensed by cytosolic pattern recognition receptors (PRRs), including NLRP1 (nucleotide-binding oligomerization domain-like receptor (NLR)-family, pyrin domain (PYD)-containing 1), NLRP3 (NLR-family, PYD-containing 3), NAIP–NLRC4 (NLR-family apoptosis inhibitory protein–NLR-family, CARD-containing 4), AIM2 (absent in melanoma 2), and pyrin. Activation of these sensors results in oligomerization, often followed by recruitment of ASC (apoptosis-associated speck-like protein containing a CARD) through homotypic PYD-PYD or CARD-CARD interactions, resulting in the formation of an inflammasome complex (Figure 3). Caspase-1 is then recruited to the complex through homotypic CARD-CARD interactions. Some inflammasome sensors, namely NLRP1 and NAIP/NLRC4, contain CARD domains, which could allow for direct interaction between the multimerized inflammasome sensor and caspase-1 (S. Christgen & Kanneganti, 2020). Following recruitment of caspase-1 to the inflammasome complex, the protease becomes activated through a proximity-based mechanism of self-cleavage. After activation, caspase-1 is free to act upon its other substrates and process the immature forms of IL-1β and IL-18 to their mature signaling forms. In addition, active caspase-1 cleaves the executioner of pyroptotic cell death, gasdermin D (GSDMD). Cleavage of GSDMD results in the formation of membrane pores by the N-terminus of the protein, thereby allowing for the release of IL-1β and IL-18, as well as the execution of pyroptosis. GSDMD can also be cleaved by caspase-11 in mice and caspase-4/5 in humans (W. T. He, et al., 2015; Kayagaki, et al., 2015; Sborgi, et al., 2016), though inflammatory cytokines are not known to be processed to their mature forms by these caspases. In this non-canonical model of inflammasome activation, binding of bacterial lipopolysaccharide (LPS) to mouse caspase-11 or human caspase-4/5 triggers caspase activation and subsequent GSDMD cleavage (W. T. He, et al., 2015; Kayagaki, et al., 2015; Sborgi, et al., 2016). Pores formed by active GSDMD and subsequent ionic flux lead to the activation of the NLRP3 inflammasome, followed by caspase-1 activation and cytokine release.
Figure 3: Regulation of pyroptosis by the inflammasome.
Activation of an inflammasome sensor by damage- or pathogen-associated molecular patterns results in the formation of an inflammasome complex and the activation of caspase-1. Active caspase-1 cleaves pro–IL-1β, pro–IL-18, and full length gasdermin D (GSDMD) to their active forms, resulting in pyroptotic cell death and inflammatory signaling. Caspase-11, activated by lipopolysaccharide, triggers non-canonical inflammasome activation by directly cleaving GSDMD.
The line between inflammasome activation as a defense or a pathological mechanism is very fine. Mutations within NLRP3 result in the development of several autoinflammatory diseases driven by systemic inflammation, collectively referred to as CAPS (cryopyrin-associated periodic syndromes), including familial cold autoinflammatory syndrome (FCAS), Muckle-Wells syndrome, and neonatal-onset multi-system inflammatory disease (NOMID). Aberrant activation of the pyrin inflammasome due to gain of function mutations results in the development of familial Mediterranean fever (FMF), while overactive NLRC4 mutations have been linked to other inflammasomopathies (Canna, et al., 2014; French, 1997; Romberg, et al., 2014). As such, there is a high level of interest in the development of therapeutics to block improper inflammasome activation. Further, reports of pyroptosis contributing to disease development in a number of pathological contexts, including neurological diseases, such as Alzheimer’s disease and multiple sclerosis, metabolic syndromes, and cancer (Karki & Kanneganti, 2019), have deepened interest in inflammasome-targeting therapies. However, significant care needs to be taken in determining therapeutic targets, as loss of inflammasome activity impairs host responses to pathogens, potentially rendering patients more susceptible to infections.
Although activation of each inflammasome converges on the induction of pyroptotic cell death, domain organization and the mechanisms inducing inflammasome formation are unique to the apical sensor. Some inflammasome complexes form as a result of direct binding of a warning substrate to the sensor, such as DNA to the HIN-200 domain of AIM2 and flagellin or other bacterial proteins to NAIP–NLRC4; in contrast, other sensors, such as NLRP1b, NLRP3, and pyrin, respond to an indirect sensing of a perturbation in the normal cell environment (S. Christgen, et al., 2020). The diversity in activation mechanisms of different inflammasomes presents useful therapeutic opportunities, as it may be possible to selectively block one pathway while leaving others unaltered and able to respond to their respective pathogenic insults. Further, the expression of some sensors, such as NLRP3 or NAIPs, are controlled by upstream, innate signaling pathways, providing additional therapeutic targets (Bauernfeind, et al., 2009; Karki, et al., 2018).
Specifically, the development of targeted therapeutics to prevent inflammatory cell death driven by the NLRP3 inflammasome has been of high research interest for a number of years. The current lack of understanding of the specific activation mechanism of NLRP3 complicates the development of targeted therapeutics, but a number of compounds have been shown to have inhibitory effects on NLRP3, including glyburide (Lamkanfi, et al., 2009), which is approved for the treatment of type 2 diabetes, and colchicine (Martinon, Petrilli, Mayor, Tardivel, & Tschopp, 2006), which is approved for treatment of gout and FMF, along with the more selective targeted inhibitors MCC950 (Coll, et al., 2015), IFN39 (Cocco, et al., 2017), and dapansutrile (OLT1177). Dapansutrile has advanced to clinical trials for gout, osteoarthritis, and heart failure, where it has been generally well-tolerated and shown early signs of potential efficacy (Klück, et al., 2020; Wohlford, et al., 2020). This compound has also recently been investigated in SARS-CoV-2 infection. Furthermore, recent structural and mechanistic insights into the NLRP3 inflammasome have identified new pharmacological strategies, including disruption of the interactions between NLRP3 and NEK7, a molecule reported to have a crucial role in NLRP3 activation (Y. He, Zeng, Yang, Motro, & Nunez, 2016; Sharif, et al., 2019; H. Shi, et al., 2016), by the compound oridonin (H. He, et al., 2018). Therapies disrupting the interactions holding the inflammasome complex together may prove more effective than enzymatic inhibition, as the loss of some molecules reported to be involved in NLRP3 binding and activation are embryonically or perinatally lethal, including NEK7 and DDX3X (DEAD-box helicase 3 X-linked) (Q. Li, et al., 2014; Salem, et al., 2010; Samir, et al., 2019). It should further be noted that some molecules reported to bind to inflammasome sensors help to sequester and inactivate the sensors rather than activate them (Hollingsworth, et al., 2021; Huang, et al., 2021). Small molecules targeting these binding partners would therefore result in the induction of pyroptosis, further highlighting the need for thorough and systematic study of potential druggable targets.
3. PANOPTOSIS AND THE PANOPTOSOME
While the concept of regulated, controlled cell death is one that has been established for many years, with the delineation between “programmed” and “accidental” cell death being discussed as early as 1972 (Kerr, et al., 1972), numerous findings over the years have highlighted that some of these base assumptions need to be revisited, particularly the molecular delineations between PCD pathways. Treatment with inflammasome-activating stimuli in the absence of caspase-1 or GSDMD results in recruitment and activation of caspase-8 by the inflammasome complex, leading to caspase-8–mediated inflammatory cell death (de Vasconcelos, et al., 2020; Mascarenhas, et al., 2017; Motani, et al., 2011; Pierini, et al., 2012; Sagulenko, et al., 2013; Tsuchiya, et al., 2019; Van Opdenbosch, et al., 2017). Additionally, apoptotic executioner caspases, caspase-3 and caspase-7, are reported to inactivate pyroptotic GSDMD through N-terminal cleavage (Taabazuing, Okondo, & Bachovchin, 2017). However, these caspases are also reported shift the morphology of cell death from apoptotic to pyroptotic through cleavage of another member of the gasdermin family, gasdermin E (GSDME), also known as DFNA5 (Lu, et al., 2018; Y. Wang, Yin, et al., 2018; Yu, 2019; C. C. L. Zhang, C.G.; Wang, Y.F.; Xu, L.H.; He, X.H.; Zeng, Q.Z.; Zeng, C.Y.; Mai, F.Y.; Hu, B.; Ouyang, D.Y., 2019). Recent evidence also suggests that GSDME can serve as a conduit for IL-1β release (Zhou & Abbott, 2021). It is also clear that other pyroptotic molecules, such as caspase-1, can regulate apoptosis and the cleavage of apoptotic substrates, while apoptotic molecules caspase-8 and FADD can regulate pyroptosis activation (Gurung, et al., 2014; Lamkanfi, et al., 2008; R. K. Malireddi, et al., 2010). Execution of necroptosis and the formation of MLKL pores can lead to the activation of pyroptosis through membrane damage (Conos, et al., 2017; Gutierrez, et al., 2017). Additionally, RIPK1 and caspase-8 function as rheostats to guide the cell fate decision between survival signaling, apoptosis, and necroptosis (R. K. S. Malireddi, Gurung, et al., 2020; R. K. S. Malireddi, et al., 2018; R. K. S. Malireddi, Kesavardhana, et al., 2020; Newton, et al., 2019). These and other observations make it clear that there are numerous molecular connections between the cell death pathways that may complicate the development of therapeutics.
Furthermore, live pathogens and individual PAMPs have been documented to contextually induce multiple PCD pathways through innate sensors. For example, ZBP1, which is upregulated in response to interferon (IFN) signaling through IFN regulatory factor 1 (IRF1), acts as a sensor of viral ribonucleoproteins during influenza A virus infection to activate pyroptosis, apoptosis, and necroptosis in macrophages (Kesavardhana, et al., 2017; Kesavardhana, Malireddi, Burton, et al., 2020; Kuriakose, et al., 2016; Kuriakose, Zheng, Neale, & Kanneganti, 2018). Additionally, while RIPK1 regulates cell death through kinase activity, a scaffolding role of RIPK1 in multiple PCD pathways has also been observed (R. K. S. Malireddi, Gurung, et al., 2020; R. K. S. Malireddi, Kesavardhana, et al., 2020; Newton, et al., 2019). The crosstalk between pathways has therefore led to the establishment of the concept of PANoptosis, defined as an inflammatory PCD pathway with key features of pyroptosis, apoptosis, and/or necroptosis that cannot be accounted for by any of these three PCD pathways alone. PANoptosis is regulated by the PANoptosome complex, a molecular scaffold for the contemporaneous engagement of key pyroptotic, apoptotic, and necroptotic machinery (Banoth, et al., 2020; S. Christgen, Zheng, M., Kesavardhana, S., Karki, R., Malireddi, R.K.S., Banoth, B., Place, D.E., Briard, B., Sharma, B.R., Tuladhar, S., Samir, P., Burton, A., Kanneganti, T.-D., 2020; Gurung, et al., 2014; Gurung, et al., 2016; Karki, et al., 2020; Karki, et al., 2021; Kesavardhana, Malireddi, Burton, et al., 2020; Kuriakose, et al., 2016; Lamkanfi, et al., 2008; Lukens, Gross, et al., 2014; Lukens, Gurung, et al., 2014; R. K. Malireddi, et al., 2010; R. K. S. Malireddi, Gurung, et al., 2020; R. K. S. Malireddi, et al., 2018; R. K. S. Malireddi, et al., 2021; R. K. S. Malireddi, Kesavardhana, et al., 2020). The ability of death molecules from each of these pathways to interact allows for an intricate coregulation of cell death, with the phenotypic contribution of each arm of PANoptosis varying based on the context of the stimulus provided.
The composition of the PANoptosome scaffold, interaction kinetics of these components, and identity of both upstream sensors and regulatory signaling pathways is still an area of active research, and it is likely that the specific repertoire of molecules involved depends on the initial trigger, similar to that of inflammasomes. Reported PANoptosome components include RIPK3, caspase-8, caspase-6, ZBP1, ASC, and NLRP3 (S. Christgen, Zheng, M., Kesavardhana, S., Karki, R., Malireddi, R.K.S., Banoth, B., Place, D.E., Briard, B., Sharma, B.R., Tuladhar, S., Samir, P., Burton, A., Kanneganti, T.-D., 2020; R. K. S. Malireddi, Gurung, et al., 2020; R. K. S. Malireddi, Kesavardhana, et al., 2020; M. K. Zheng, R; Vogel, P; Kanneganti, TD 2020). Downstream of the PANoptosome, pyroptotic, apoptotic, and/or necroptotic executioners can be activated, with the molecular details of cell death execution likely dependent on the trigger and cell type. Recent studies have implicated activation of PANoptosis in response to viral, bacterial, and fungal triggers as well as in the pathogenesis of autoinflammatory diseases, cytokine storm, and cancer (Banoth, et al., 2020; S. Christgen, Zheng, M., Kesavardhana, S., Karki, R., Malireddi, R.K.S., Banoth, B., Place, D.E., Briard, B., Sharma, B.R., Tuladhar, S., Samir, P., Burton, A., Kanneganti, T.-D., 2020; Gurung, et al., 2014; Gurung, et al., 2016; Karki, et al., 2020; Karki, et al., 2021; Kesavardhana, et al., 2017; Kesavardhana, Malireddi, Burton, et al., 2020; Kuriakose, et al., 2016; Lukens, Gross, et al., 2014; R. K. S. Malireddi, Gurung, et al., 2020; R. K. S. Malireddi, et al., 2018; R. K. S. Malireddi, et al., 2021; R. K. S. Malireddi, Kesavardhana, et al., 2020; M. Zheng, et al., 2020).
The conceptualization of PANoptosis highlights the importance of molecule selection in the development of therapeutics (Figure 4). As may be expected due to the induction of PANoptosis, pharmacological or therapeutic inhibition of one arm of the pathway may result in compensatory cell death and inflammation mediated by the activation or enhancement of other arms (de Vasconcelos, et al., 2020; Gurung, et al., 2016; Lukens, Gross, et al., 2014; Lukens, Gurung, et al., 2014; Place, Christgen, et al., 2021; M. Zheng, et al., 2020). Therefore, targeting a single pathway of cell death, without consideration of additional pathways functioning as redundant deadly backups, can lead to initially promising therapeutics that eventually fail as the lines between individual cell death pathways become molecularly blurred. Current inhibitory approaches include targeting sensor molecules, such as NLRP3, inhibiting enzymatic targets, such as RIPK1, and neutralizing downstream cytokine signaling, such as IL-1 signaling. While these inhibitors were designed with pyroptosis, apoptosis, and necroptosis specifically in mind, it is critical to assess how they will be effective in the context of PANoptosis. Upstream targeting of inflammatory signaling pathways, sensors, or scaffolding molecules could effectively block the induction of all three arms of this pathway, while targeting specific downstream executioner molecules can still allow cell death to occur through alternate executioners, driving the selection of cell death into a different direction.
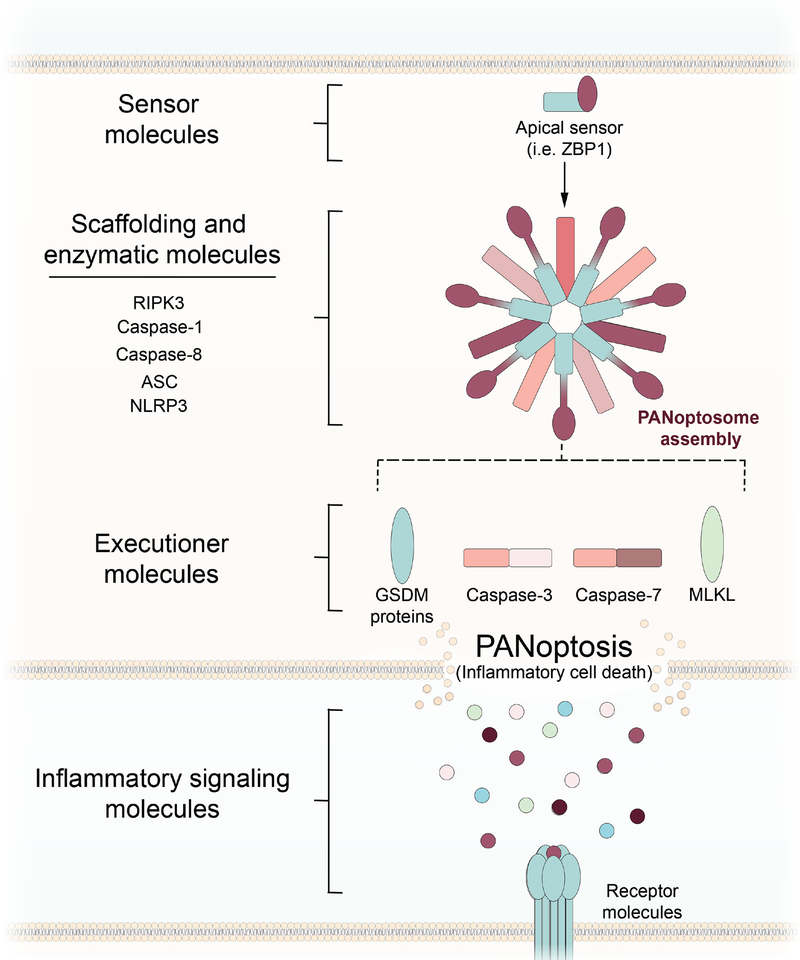
Figure 4: Targeting PANoptotic regulation of inflammatory cell death.
Activation of an apical sensor, such as Z-DNA-binding protein 1 (ZBP1), induces formation of a PANoptosome complex containing key pyroptotic, apoptotic, and necroptotic molecules. Assembly of the PANoptosome leads to the induction of three arms of cell death, executed by members of the gasdermin (GSDM) family of proteins, caspase-3/7, and MLKL. Inhibition of PANoptosis or PANoptosis-induced inflammation can occur at a number of levels. Direct blockade of sensor molecules, such as ZBP1, could block formation of the PANoptosome and induction of PANoptosis. Inhibition of scaffolding or enzymatic molecules could alter the activity and composition of the PANoptosome, while inhibition of specific executioners of one arm of cell death can drive death into a different arm of PANoptosis. An alternative approach is the blockade of downstream inflammatory signaling molecules, such as IL-1.
4. THERAPEUTIC BLOCKADE OF CYTOKINE SIGNALING
Targeting innate immune-mediated cell death and inflammation for therapeutic intervention without producing unwanted, off-target effects is a delicate process that is highly specific to the context of the disease of interest. As discussed in previous sections, numerous compounds are currently in use to inhibit cell death at different regulatory levels (Table 2; Figure 4). While some of these are currently under clinical study, others have mainly been utilized in the lab and will require significant refinement to yield viable therapeutics.
An alternative clinical approach to targeting upstream signaling pathways, sensors, PANoptosome components, and other regulators of inflammatory cell death is to instead block the associated inflammatory cytokine signaling. This strategy could help mitigate pathological impacts of inflammation while simultaneously preventing a PANoptosis-associated cytokine storm, which is defined as a life-threatening condition caused by excessive production of cytokines mediated by inflammatory cell death (Karki & Kanneganti, 2021). For example, TNF-α and IFN-γ together induce PANoptosis, and blocking these cytokines with a combinatorial treatment of anti-TNF-α and anti-IFN-γ neutralizing antibodies significantly improves survival in experimental models of diseases characterized by cytokine storms, including SARS-CoV-2 infection, hemophagocytic lymphohistiocytosis, and sepsis (Karki, et al., 2021). In addition, targeting inflammatory signaling could minimize upregulation of innate immune sensors involved in the induction of inflammatory cell death.
Furthermore, inflammatory cell death is also directly associated with the release of other DAMPs, such as HMGB1 (Scaffidi, Misteli, & Bianchi, 2002), and cytokines, particularly the inflammasome-dependent cytokines IL-1β and IL-18, that can induce pathology. Ablation or blockade of cytokine signaling has been shown to have beneficial effects in a number of disease models in mice, including models of infection, autoinflammatory diseases, and cancer (Aggen, et al., 2021; Lukens, Gurung, et al., 2014; Lukens, et al., 2013; Winchell, et al., 2020). Clinically, success has been seen with a recombinant IL-1R receptor antagonist known as anakinra. Anakinra is FDA approved for treatment of rheumatoid arthritis, CAPS, and NOMID, and is being investigated as a treatment in other diseases, including diabetes, dermatitis, and cancer. Similarly, rilonacept, a recombinant fusion protein containing IL-1R components, has been approved in the treatment of CAPS. Additionally, a Phase 3 study of the monoclonal antibody canakinumab found that IL-1β blockade is associated with reduced recurrent incidences of myocardial infarction and lung cancer (Ridker, Everett, et al., 2017; Ridker, MacFadyen, et al., 2017; Ridker, Thuren, Zalewski, & Libby, 2011). Overall, blockade of inflammatory cytokine signaling has shown promise in several diseases associated with aberrant cell death.
5. INDUCTION OF INFLAMMATORY CELL DEATH IN CANCER TREATMENT
In addition to inhibiting cell death and inflammatory signaling, the induction and modulation of inflammatory cell death is utilized as an approach in anticancer therapy. Activation of inflammatory cell death in the context of cancer is advantageous over inducing silent death as it triggers an immune response. The process of activating the immune system through cell death, also referred to as immunogenic cell death, is used to train the immune system to identify malignant host cells and facilitate the clearance of tumor cells, similar to immune recognition of an invading pathogen. This therapeutic strategy relies on both the ability of the induced cell death to stimulate the immune system, referred to as adjuvanticity, and the ability of the immune system to distinguish the malignant cell from a healthy cell, referred to as antigenicity. A number of molecules capable of inducing immunogenic cell death have been approved for use by the FDA, including doxorubicin, oxaliplatin, and cyclophosphamide. However, these therapeutics are not specifically targeted at cell death regulation and instead induce general DNA damage or protein synthesis inhibition to cause cell death, resulting in their association with adverse events in patients. Cancer cell resistance to cell death induction further restricts the therapeutic window of these drugs with broad mechanisms of action. Other more targeted compounds are currently under study in clinical trials, including tolinapant, which received FDA orphan drug designation, DEBIO 1143, which received FDA breakthrough therapy designation, and LCL-161, which has passed Phase 2 clinical trials. Other immunotherapies paired with death-inducing agents to mobilize the immune system to target tumors have seen marked success in some patients, such as that of programmed death ligand 1 (PD-L1) inhibitors (Gou, et al., 2020), suggesting that the further development of cell death-inducing therapies will be advantageous for patients. Molecules involved in the regulation of PCD pathways, such as PANoptosis, and cytokine signaling are frequently dysregulated in different cancers (Karki & Kanneganti, 2019; Karki, et al., 2020; Landskron, De la Fuente, Thuwajit, Thuwajit, & Hermoso, 2014; R. K. S. Malireddi, et al., 2021). Further, the mechanism of PANoptotic cell death induced by TNF-α and IFN-γ is different in human cancer cell lines than in healthy murine BMDMs, with nitric oxide being required in BMDMs but dispensable in human cancer cells; these findings suggest that there is potential for a targeted therapeutic to induce inflammatory cell death in tumors specifically (Karki, et al., 2021; R. K. S. Malireddi, et al., 2021). Indeed, combinational intratumoral delivery of TNF-α and IFN-γ reduces tumor weight and volume in transplanted xenograft tumors in NSG mice (R. K. S. Malireddi, et al., 2021). Currently, combinational treatment of TNF-α and IFN-γ along with hyperthermic isolated limb perfusion (HILP) is under study in a Phase 3 clinical trial of patients with locally advanced melanoma. Further studies into the regulation of molecules involved in PANoptosis and the formation of the PANoptosome in cancer models may identify additional molecular targets to boost tailored anti-cancer immunotherapies.
6. SUMMARY AND FUTURE PERSPECTIVES
The study of PCD and inflammation has expanded rapidly, with new mechanistic details about regulation and molecular crosstalk between pathways previously viewed as independent emerging as topics of particular interest. The role of these pathways during development, infection, and disease pathogenesis has prompted interest in the clinical use of molecules to block or induce different pathways of cell death. Reliance of these pathways on signal-amplifying complexes provides a number of potential therapeutic targets. Particular care must be taken in the development of cell death inhibitors, so as to prevent aberrant activation of an alternative PCD pathway or executioner. This is highlighted in the context of PANoptosis, whereby molecules involved in pyroptosis, apoptosis, and/or necroptosis can be activated together in response to specific stimuli. Further, inhibition of inflammatory cell death can leave the host susceptible to infection, while overactivation of inflammatory cell death can induce a systemic inflammatory state with pathological consequences. In the arms race between hosts and pathogens, some pathogens have evolved to subvert certain PCD pathways, leading to redundancy between pathways (Place, Lee, & Kanneganti, 2021). Understanding the optimal juncture at which to target inflammation during disease is crucial to the development of effective therapeutics, which requires thorough, mechanistic dissection of individual pathways of cell death, as well as identification of the overlaps and points of communication between them. For instance, targeting executioners may not protect a cell from death, but instead trigger an alternative arm of PCD. The growing mechanistic understanding of cell death crosstalk in innate immunity and the complexes that regulate these processes provides critical insight to refine the selection of specific target molecules and minimize the nonspecific effects of previous attempts to modulate the interface between cell survival and death. Understanding the molecular decisions underpinning how a cell chooses to die, and the players involved in those cell death decisions, is as important as understanding the pathways in isolation during the development of safe and effective therapies.
Acknowledgements
We apologize to our colleagues in the field whose work could not be cited owing to space limitations. We thank all the members of the Kanneganti laboratory for their comments and suggestions. Work from our laboratory is supported by the US National Institutes of Health (AI101935, AI124346, AI160179, AR056296 and CA253095 to T.-D.K.) and the American Lebanese Syrian Associated Charities (to T.-D.K.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of interest statement
The authors declare that they have no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acehan D, Jiang X, Morgan DG, Heuser JE, Wang X, & Akey CW (2002). Three-dimensional structure of the apoptosome: implications for assembly, procaspase-9 binding, and activation. Mol Cell, 9, 423–432. [DOI] [PubMed] [Google Scholar]
- Aggen DH, Ager CR, Obradovic AZ, Chowdhury N, Ghasemzadeh A, Mao W, Chaimowitz MG, Lopez-Bujanda ZA, Spina CS, Hawley JE, Dallos MC, Zhang C, Wang V, Li H, Guo XV, & Drake CG (2021). Blocking IL1 Beta Promotes Tumor Regression and Remodeling of the Myeloid Compartment in a Renal Cell Carcinoma Model: Multidimensional Analyses. Clin Cancer Res, 27, 608–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aglietti RA, Estevez A, Gupta A, Ramirez MG, Liu PS, Kayagaki N, Ciferri C, Dixit VM, & Dueber EC (2016). GsdmD p30 elicited by caspase-11 during pyroptosis forms pores in membranes. Proc Natl Acad Sci U S A, 113, 7858–7863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin P, Florez M, Najafov A, Pan H, Geng J, Ofengeim D, Dziedzic SA, Wang H, Barrett VJ, Ito Y, LaVoie MJ, & Yuan J (2018). Regulation of a distinct activated RIPK1 intermediate bridging complex I and complex II in TNFalpha-mediated apoptosis. Proc Natl Acad Sci U S A, 115, E5944–E5953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnett HA, Mason J, Marino M, Suzuki K, Matsushima GK, & Ting JP (2001). TNF alpha promotes proliferation of oligodendrocyte progenitors and remyelination. Nat Neurosci, 4, 1116–1122. [DOI] [PubMed] [Google Scholar]
- Banoth B, Tuladhar S, Karki R, Sharma BR, Briard B, Kesavardhana S, Burton A, & Kanneganti TD (2020). ZBP1 promotes fungi-induced inflammasome activation and pyroptosis, apoptosis, and necroptosis (PANoptosis). J Biol Chem, 295, 18276–18283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauernfeind FG, Horvath G, Stutz A, Alnemri ES, MacDonald K, Speert D, Fernandes-Alnemri T, Wu J, Monks BG, Fitzgerald KA, Hornung V, & Latz E (2009). Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J Immunol, 183, 787–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Z, Jitkaew S, Zhao J, Chiang HC, Choksi S, Liu J, Ward Y, Wu LG, & Liu ZG (2014). Plasma membrane translocation of trimerized MLKL protein is required for TNF-induced necroptosis. Nat Cell Biol, 16, 55–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canna SW, de Jesus AA, Gouni S, Brooks SR, Marrero B, Liu Y, DiMattia MA, Zaal KJ, Sanchez GA, Kim H, Chapelle D, Plass N, Huang Y, Villarino AV, Biancotto A, Fleisher TA, Duncan JA, O’Shea JJ, Benseler S, Grom A, Deng Z, Laxer RM, & Goldbach-Mansky R (2014). An activating NLRC4 inflammasome mutation causes autoinflammation with recurrent macrophage activation syndrome. Nat Genet, 46, 1140–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinnaiyan AM, O’Rourke K, Lane BR, & Dixit VM (1997). Interaction of CED-4 with CED-3 and CED-9: a molecular framework for cell death. Science, 275, 1122–1126. [DOI] [PubMed] [Google Scholar]
- Cho YS, Challa S, Moquin D, Genga R, Ray TD, Guildford M, & Chan FK (2009). Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell, 137, 1112–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christgen S, & Kanneganti TD (2020). Inflammasomes and the fine line between defense and disease. Curr Opin Immunol, 62, 39–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christgen S, Place DE, & Kanneganti TD (2020). Toward targeting inflammasomes: insights into their regulation and activation. Cell Res, 30, 315–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christgen S, Zheng M, Kesavardhana S, Karki R, Malireddi RKS, Banoth B, Place DE, Briard B, Sharma BR, Tuladhar S, Samir P, Burton A, Kanneganti T-D (2020). Identification of the PANoptosome: A molecular platform triggering pyroptosis, apoptosis, and necroptosis (PANoptosis). Front Cell Infect Microbiol, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocco M, Pellegrini C, Martinez-Banaclocha H, Giorgis M, Marini E, Costale A, Miglio G, Fornai M, Antonioli L, Lopez-Castejon G, Tapia-Abellan A, Angosto D, Hafner-Bratkovic I, Regazzoni L, Blandizzi C, Pelegrin P, & Bertinaria M (2017). Development of an Acrylate Derivative Targeting the NLRP3 Inflammasome for the Treatment of Inflammatory Bowel Disease. J Med Chem, 60, 3656–3671. [DOI] [PubMed] [Google Scholar]
- Coll RC, Robertson AA, Chae JJ, Higgins SC, Munoz-Planillo R, Inserra MC, Vetter I, Dungan LS, Monks BG, Stutz A, Croker DE, Butler MS, Haneklaus M, Sutton CE, Nunez G, Latz E, Kastner DL, Mills KH, Masters SL, Schroder K, Cooper MA, & O’Neill LA (2015). A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat Med, 21, 248–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conos SA, Chen KW, De Nardo D, Hara H, Whitehead L, Nunez G, Masters SL, Murphy JM, Schroder K, Vaux DL, Lawlor KE, Lindqvist LM, & Vince JE (2017). Active MLKL triggers the NLRP3 inflammasome in a cell-intrinsic manner. Proc Natl Acad Sci U S A, 114, E961–E969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vasconcelos NM, Van Opdenbosch N, Van Gorp H, Martin-Perez R, Zecchin A, Vandenabeele P, & Lamkanfi M (2020). An Apoptotic Caspase Network Safeguards Cell Death Induction in Pyroptotic Macrophages. Cell Rep, 32, 107959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degterev A, Hitomi J, Germscheid M, Ch’en IL, Korkina O, Teng X, Abbott D, Cuny GD, Yuan C, Wagner G, Hedrick SM, Gerber SA, Lugovskoy A, & Yuan J (2008). Identification of RIP1 kinase as a specific cellular target of necrostatins. Nat Chem Biol, 4, 313–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degterev A, Huang Z, Boyce M, Li Y, Jagtap P, Mizushima N, Cuny GD, Mitchison TJ, Moskowitz MA, & Yuan J (2005). Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol, 1, 112–119. [DOI] [PubMed] [Google Scholar]
- Dillon CP, Weinlich R, Rodriguez DA, Cripps JG, Quarato G, Gurung P, Verbist KC, Brewer TL, Llambi F, Gong YN, Janke LJ, Kelliher MA, Kanneganti TD, & Green DR (2014). RIPK1 blocks early postnatal lethality mediated by caspase-8 and RIPK3. Cell, 157, 1189–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dondelinger Y, Aguileta MA, Goossens V, Dubuisson C, Grootjans S, Dejardin E, Vandenabeele P, & Bertrand MJ (2013). RIPK3 contributes to TNFR1-mediated RIPK1 kinase-dependent apoptosis in conditions of cIAP1/2 depletion or TAK1 kinase inhibition. Cell Death Differ, 20, 1381–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmore S (2007). Apoptosis: a review of programmed cell death. Toxicol Pathol, 35, 495–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox JL, Hughes MA, Meng X, Sarnowska NA, Powley IR, Jukes-Jones R, Dinsdale D, Ragan TJ, Fairall L, Schwabe JWR, Morone N, Cain K, & MacFarlane M (2021). Cryo-EM structural analysis of FADD:Caspase-8 complexes defines the catalytic dimer architecture for co-ordinated control of cell fate. Nat Commun, 12, 819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French FMFC (1997). A candidate gene for familial Mediterranean fever. Nat Genet, 17, 25–31. [DOI] [PubMed] [Google Scholar]
- Fu TM, Li Y, Lu A, Li Z, Vajjhala PR, Cruz AC, Srivastava DB, DiMaio F, Penczek PA, Siegel RM, Stacey KJ, Egelman EH, & Wu H (2016). Cryo-EM Structure of Caspase-8 Tandem DED Filament Reveals Assembly and Regulation Mechanisms of the Death-Inducing Signaling Complex. Mol Cell, 64, 236–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs Y, & Steller H (2011). Programmed cell death in animal development and disease. Cell, 147, 742–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galluzzi L, Kepp O, Krautwald S, Kroemer G, & Linkermann A (2014). Molecular mechanisms of regulated necrosis. Semin Cell Dev Biol, 35, 24–32. [DOI] [PubMed] [Google Scholar]
- Geng J, Ito Y, Shi L, Amin P, Chu J, Ouchida AT, Mookhtiar AK, Zhao H, Xu D, Shan B, Najafov A, Gao G, Akira S, & Yuan J (2017). Regulation of RIPK1 activation by TAK1-mediated phosphorylation dictates apoptosis and necroptosis. Nat Commun, 8, 359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman AM (2008). Neuronal cell death in neurodegenerative diseases: recurring themes around protein handling. J Cell Mol Med, 12, 2263–2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gou Q, Dong C, Xu H, Khan B, Jin J, Liu Q, Shi J, & Hou Y (2020). PD-L1 degradation pathway and immunotherapy for cancer. Cell Death Dis, 11, 955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurung P, Anand PK, Malireddi RK, Vande Walle L, Van Opdenbosch N, Dillon CP, Weinlich R, Green DR, Lamkanfi M, & Kanneganti TD (2014). FADD and caspase-8 mediate priming and activation of the canonical and noncanonical Nlrp3 inflammasomes. J Immunol, 192, 1835–1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurung P, Burton A, & Kanneganti TD (2016). NLRP3 inflammasome plays a redundant role with caspase 8 to promote IL-1beta-mediated osteomyelitis. Proc Natl Acad Sci U S A, 113, 4452–4457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez KD, Davis MA, Daniels BP, Olsen TM, Ralli-Jain P, Tait SW, Gale M Jr., & Oberst A (2017). MLKL Activation Triggers NLRP3-Mediated Processing and Release of IL-1beta Independently of Gasdermin-D. J Immunol, 198, 2156–2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He H, Jiang H, Chen Y, Ye J, Wang A, Wang C, Liu Q, Liang G, Deng X, Jiang W, & Zhou R (2018). Oridonin is a covalent NLRP3 inhibitor with strong anti-inflammasome activity. Nat Commun, 9, 2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He S, Wang L, Miao L, Wang T, Du F, Zhao L, & Wang X (2009). Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-alpha. Cell, 137, 1100–1111. [DOI] [PubMed] [Google Scholar]
- He WT, Wan H, Hu L, Chen P, Wang X, Huang Z, Yang ZH, Zhong CQ, & Han J (2015). Gasdermin D is an executor of pyroptosis and required for interleukin-1beta secretion. Cell Res, 25, 1285–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Zeng MY, Yang D, Motro B, & Nunez G (2016). NEK7 is an essential mediator of NLRP3 activation downstream of potassium efflux. Nature, 530, 354–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingsworth LR, Sharif H, Griswold AR, Fontana P, Mintseris J, Dagbay KB, Paulo JA, Gygi SP, Bachovchin DA, & Wu H (2021). DPP9 sequesters the C terminus of NLRP1 to repress inflammasome activation. Nature. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu JJ, Liu X, Xia S, Zhang Z, Zhang Y, Zhao J, Ruan J, Luo X, Lou X, Bai Y, Wang J, Hollingsworth LR, Magupalli VG, Zhao L, Luo HR, Kim J, Lieberman J, & Wu H (2020). FDA-approved disulfiram inhibits pyroptosis by blocking gasdermin D pore formation. Nat Immunol, 21, 736–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Benedict MA, Ding L, & Nunez G (1999). Role of cytochrome c and dATP/ATP hydrolysis in Apaf-1-mediated caspase-9 activation and apoptosis. EMBO J, 18, 3586–3595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang M, Zhang X, Toh GA, Gong Q, Wang J, Han Z, Wu B, Zhong F, & Chai J (2021). Structural and biochemical mechanisms of NLRP1 inhibition by DPP9. Nature. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries F, Shmuel-Galia L, Ketelut-Carneiro N, Li S, Wang B, Nemmara VV, Wilson R, Jiang Z, Khalighinejad F, Muneeruddin K, Shaffer SA, Dutta R, Ionete C, Pesiridis S, Yang S, Thompson PR, & Fitzgerald KA (2020). Succination inactivates gasdermin D and blocks pyroptosis. Science, 369, 1633–1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igney FH, & Krammer PH (2002). Death and anti-death: tumour resistance to apoptosis. Nat Rev Cancer, 2, 277–288. [DOI] [PubMed] [Google Scholar]
- Kaiser WJ, Daley-Bauer LP, Thapa RJ, Mandal P, Berger SB, Huang C, Sundararajan A, Guo H, Roback L, Speck SH, Bertin J, Gough PJ, Balachandran S, & Mocarski ES (2014). RIP1 suppresses innate immune necrotic as well as apoptotic cell death during mammalian parturition. Proc Natl Acad Sci U S A, 111, 7753–7758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser WJ, Sridharan H, Huang C, Mandal P, Upton JW, Gough PJ, Sehon CA, Marquis RW, Bertin J, & Mocarski ES (2013). Toll-like receptor 3-mediated necrosis via TRIF, RIP3, and MLKL. J Biol Chem, 288, 31268–31279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalkavan H, & Green DR (2018). MOMP, cell suicide as a BCL-2 family business. Cell Death Differ, 25, 46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karki R, & Kanneganti TD (2019). Diverging inflammasome signals in tumorigenesis and potential targeting. Nat Rev Cancer, 19, 197–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karki R, & Kanneganti TD (2021). The ‘cytokine storm’: molecular mechanisms and therapeutic prospects. Trends Immunol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karki R, Lee E, Place D, Samir P, Mavuluri J, Sharma BR, Balakrishnan A, Malireddi RKS, Geiger R, Zhu Q, Neale G, & Kanneganti TD (2018). IRF8 Regulates Transcription of Naips for NLRC4 Inflammasome Activation. Cell, 173, 920–933 e913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karki R, Sharma BR, Lee E, Banoth B, Malireddi RKS, Samir P, Tuladhar S, Mummareddy H, Burton AR, Vogel P, & Kanneganti TD (2020). Interferon regulatory factor 1 regulates PANoptosis to prevent colorectal cancer.. JCI Insight, 5, 136720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karki R, Sharma BR, Tuladhar S, Williams EP, Zalduondo L, Samir P, Zheng M, Sundaram B, Banoth B, Malireddi RKS, Schreiner P, Neale G, Vogel P, Webby R, Jonsson CB, & Kanneganti TD (2021). Synergism of TNF-alpha and IFN-gamma Triggers Inflammatory Cell Death, Tissue Damage, and Mortality in SARS-CoV-2 Infection and Cytokine Shock Syndromes. Cell, 184, 149–168 e117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayagaki N, Stowe IB, Lee BL, O’Rourke K, Anderson K, Warming S, Cuellar T, Haley B, Roose-Girma M, Phung QT, Liu PS, Lill JR, Li H, Wu J, Kummerfeld S, Zhang J, Lee WP, Snipas SJ, Salvesen GS, Morris LX, Fitzgerald L, Zhang Y, Bertram EM, Goodnow CC, & Dixit VM (2015). Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature, 526, 666–671. [DOI] [PubMed] [Google Scholar]
- Kerr JF, Wyllie AH, & Currie AR (1972). Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer, 26, 239–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesavardhana S, Kuriakose T, Guy CS, Samir P, Malireddi RKS, Mishra A, & Kanneganti TD (2017). ZBP1/DAI ubiquitination and sensing of influenza vRNPs activate programmed cell death. J Exp Med, 214, 2217–2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesavardhana S, Malireddi RKS, Burton AR, Porter SN, Vogel P, Pruett-Miller SM, & Kanneganti TD (2020). The Zα2 domain of ZBP1 is a molecular switch regulating influenza-induced PANoptosis and perinatal lethality during development. J Biol Chem, 295, 8325–8330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesavardhana S, Malireddi RKS, & Kanneganti TD (2020). Caspases in Cell Death, Inflammation, and Pyroptosis. Annu Rev Immunol, 38, 567–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klück V, Jansen T, Janssen M, Comarniceanu A, Efdé M, Tengesdal IW, Schraa K, Cleophas MCP, Scribner CL, Skouras DB, Marchetti C, Dinarello CA, & Joosten LAB (2020). Dapansutrile, an oral selective NLRP3 inflammasome inhibitor, for treatment of gout flares: an open-label, dose-adaptive, proof-of-concept, phase 2a trial. Lancet Rheumatol, 2, e270–e280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuriakose T, Man SM, Malireddi RK, Karki R, Kesavardhana S, Place DE, Neale G, Vogel P, & Kanneganti TD (2016). ZBP1/DAI is an innate sensor of influenza virus triggering the NLRP3 inflammasome and programmed cell death pathways. Sci Immunol, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuriakose T, Zheng M, Neale G, & Kanneganti TD (2018). IRF1 Is a Transcriptional Regulator of ZBP1 Promoting NLRP3 Inflammasome Activation and Cell Death during Influenza Virus Infection. J Immunol, 200, 1489–1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamkanfi M, Kanneganti TD, Van Damme P, Vanden Berghe T, Vanoverberghe I, Vandekerckhove J, Vandenabeele P, Gevaert K, & Nunez G (2008). Targeted peptidecentric proteomics reveals caspase-7 as a substrate of the caspase-1 inflammasomes. Mol Cell Proteomics, 7, 2350–2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamkanfi M, Mueller JL, Vitari AC, Misaghi S, Fedorova A, Deshayes K, Lee WP, Hoffman HM, & Dixit VM (2009). Glyburide inhibits the Cryopyrin/Nalp3 inflammasome. J Cell Biol, 187, 61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landskron G, De la Fuente M, Thuwajit P, Thuwajit C, & Hermoso MA (2014). Chronic inflammation and cytokines in the tumor microenvironment. J Immunol Res, 2014, 149185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BL, Mirrashidi KM, Stowe IB, Kummerfeld SK, Watanabe C, Haley B, Cuellar TL, Reichelt M, & Kayagaki N (2018). ASC- and caspase-8-dependent apoptotic pathway diverges from the NLRC4 inflammasome in macrophages. Sci Rep, 8, 3788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES, & Wang X (1997). Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell, 91, 479–489. [DOI] [PubMed] [Google Scholar]
- Li Q, Zhang P, Zhang C, Wang Y, Wan R, Yang Y, Guo X, Huo R, Lin M, Zhou Z, & Sha J (2014). DDX3X regulates cell survival and cell cycle during mouse early embryonic development. J Biomed Res, 28, 282–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J, Kumari S, Kim C, Van TM, Wachsmuth L, Polykratis A, & Pasparakis M (2016). RIPK1 counteracts ZBP1-mediated necroptosis to inhibit inflammation. Nature, 540, 124–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Zhang S, Wu J, Chen M, Cai MC, Fu Y, Li W, Wang J, Zhao X, Yu Z, Ma P, & Zhuang G (2018). Molecular Targeted Therapies Elicit Concurrent Apoptotic and GSDME-Dependent Pyroptotic Tumor Cell Death. Clin Cancer Res, 24, 6066–6077. [DOI] [PubMed] [Google Scholar]
- Lukens JR, Gross JM, Calabrese C, Iwakura Y, Lamkanfi M, Vogel P, & Kanneganti TD (2014). Critical role for inflammasome-independent IL-1beta production in osteomyelitis. Proc Natl Acad Sci U S A, 111, 1066–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukens JR, Gurung P, Vogel P, Johnson GR, Carter RA, McGoldrick DJ, Bandi SR, Calabrese CR, Vande Walle L, Lamkanfi M, & Kanneganti TD (2014). Dietary modulation of the microbiome affects autoinflammatory disease. Nature, 516, 246–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukens JR, Vogel P, Johnson GR, Kelliher MA, Iwakura Y, Lamkanfi M, & Kanneganti TD (2013). RIP1-driven autoinflammation targets IL-1alpha independently of inflammasomes and RIP3. Nature, 498, 224–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malireddi RK, Ippagunta S, Lamkanfi M, & Kanneganti TD (2010). Cutting edge: proteolytic inactivation of poly(ADP-ribose) polymerase 1 by the Nlrp3 and Nlrc4 inflammasomes. J Immunol, 185, 3127–3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malireddi RKS, Gurung P, Kesavardhana S, Samir P, Burton A, Mummareddy H, Vogel P, Pelletier S, Burgula S, & Kanneganti TD (2020). Innate immune priming in the absence of TAK1 drives RIPK1 kinase activity-independent pyroptosis, apoptosis, necroptosis, and inflammatory disease. J Exp Med, 217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malireddi RKS, Gurung P, Mavuluri J, Dasari TK, Klco JM, Chi H, & Kanneganti TD (2018). TAK1 restricts spontaneous NLRP3 activation and cell death to control myeloid proliferation. J Exp Med, 215, 1023–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malireddi RKS, Karki R, Sundaram B, Kancharana B, Lee S, Samir P, & Kanneganti TD (2021). Inflammatory Cell Death, PANoptosis, Mediated by Cytokines in Diverse Cancer Lineages Inhibits Tumor Growth. Immunohorizons, 5, 568–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malireddi RKS, Kesavardhana S, Karki R, Kancharana B, Burton AR, & Kanneganti TD (2020). RIPK1 Distinctly Regulates Yersinia-Induced Inflammatory Cell Death, PANoptosis. Immunohorizons, 4, 789–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malireddi RKS Gurung P; Kesavardhana S; Samir P; Burton A; Mummareddy H; Vogel P; Pelletier S; Burgula S; Kanneganti TD (2019). Innate immune priming in the absence of TAK1 drives RIPK1 kinase activity-independent pyroptosis, apoptosis, necroptosis, and inflammatory disease. J Exp Med, In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinon F, Petrilli V, Mayor A, Tardivel A, & Tschopp J (2006). Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature, 440, 237–241. [DOI] [PubMed] [Google Scholar]
- Mascarenhas DPA, Cerqueira DM, Pereira MSF, Castanheira FVS, Fernandes TD, Manin GZ, Cunha LD, & Zamboni DS (2017). Inhibition of caspase-1 or gasdermin-D enable caspase-8 activation in the Naip5/NLRC4/ASC inflammasome. PLoS Pathog, 13, e1006502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motani K, Kushiyama H, Imamura R, Kinoshita T, Nishiuchi T, & Suda T (2011). Caspase-1 protein induces apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC)-mediated necrosis independently of its catalytic activity. J Biol Chem, 286, 33963–33972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton K, Wickliffe KE, Dugger DL, Maltzman A, Roose-Girma M, Dohse M, Kőműves L, Webster JD, & Dixit VM (2019). Cleavage of RIPK1 by caspase-8 is crucial for limiting apoptosis and necroptosis. Nature, 574, 428–431. [DOI] [PubMed] [Google Scholar]
- Newton K, Wickliffe KE, Maltzman A, Dugger DL, Strasser A, Pham VC, Lill JR, Roose-Girma M, Warming S, Solon M, Ngu H, Webster JD, & Dixit VM (2016). RIPK1 inhibits ZBP1-driven necroptosis during development. Nature, 540, 129–133. [DOI] [PubMed] [Google Scholar]
- Oberst A, Dillon CP, Weinlich R, McCormick LL, Fitzgerald P, Pop C, Hakem R, Salvesen GS, & Green DR (2011). Catalytic activity of the caspase-8-FLIP(L) complex inhibits RIPK3-dependent necrosis. Nature, 471, 363–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orning P, Weng D, Starheim K, Ratner D, Best Z, Lee B, Brooks A, Xia S, Wu H, Kelliher MA, Berger SB, Gough PJ, Bertin J, Proulx MM, Goguen JD, Kayagaki N, Fitzgerald KA, & Lien E (2018). Pathogen blockade of TAK1 triggers caspase-8-dependent cleavage of gasdermin D and cell death. Science, 362, 1064–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltzer N, Darding M, & Walczak H (2016). Holding RIPK1 on the Ubiquitin Leash in TNFR1 Signaling. Trends Cell Biol, 26, 445–461. [DOI] [PubMed] [Google Scholar]
- Pierini R, Juruj C, Perret M, Jones CL, Mangeot P, Weiss DS, & Henry T (2012). AIM2/ASC triggers caspase-8-dependent apoptosis in Francisella-infected caspase-1-deficient macrophages. Cell Death Differ, 19, 1709–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Place DE, Christgen S, Tuladhar S, Vogel P, Malireddi RKS, & Kanneganti TD (2021). Hierarchical Cell Death Program Disrupts the Intracellular Niche Required for Burkholderia thailandensis Pathogenesis. MBio, e0105921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Place DE, Lee S, & Kanneganti TD (2021). PANoptosis in microbial infection. Curr Opin Microbiol, 59, 42–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathkey JK, Zhao J, Liu Z, Chen Y, Yang J, Kondolf HC, Benson BL, Chirieleison SM, Huang AY, Dubyak GR, Xiao TS, Li X, & Abbott DW (2018). Chemical disruption of the pyroptotic pore-forming protein gasdermin D inhibits inflammatory cell death and sepsis. Sci Immunol, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, Kastelein JJP, Cornel JH, Pais P, Pella D, Genest J, Cifkova R, Lorenzatti A, Forster T, Kobalava Z, Vida-Simiti L, Flather M, Shimokawa H, Ogawa H, Dellborg M, Rossi PRF, Troquay RPT, Libby P, Glynn RJ, & Group CT (2017). Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N Engl J Med, 377, 1119–1131. [DOI] [PubMed] [Google Scholar]
- Ridker PM, MacFadyen JG, Thuren T, Everett BM, Libby P, Glynn RJ, & Group CT (2017). Effect of interleukin-1beta inhibition with canakinumab on incident lung cancer in patients with atherosclerosis: exploratory results from a randomised, double-blind, placebo-controlled trial. Lancet, 390, 1833–1842. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Thuren T, Zalewski A, & Libby P (2011). Interleukin-1beta inhibition and the prevention of recurrent cardiovascular events: rationale and design of the Canakinumab Anti-inflammatory Thrombosis Outcomes Study (CANTOS). Am Heart J, 162, 597–605. [DOI] [PubMed] [Google Scholar]
- Romberg N, Al Moussawi K, Nelson-Williams C, Stiegler AL, Loring E, Choi M, Overton J, Meffre E, Khokha MK, Huttner AJ, West B, Podoltsev NA, Boggon TJ, Kazmierczak BI, & Lifton RP (2014). Mutation of NLRC4 causes a syndrome of enterocolitis and autoinflammation. Nat Genet, 46, 1135–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safa AR (2012). c-FLIP, a master anti-apoptotic regulator. Exp Oncol, 34, 176–184. [PMC free article] [PubMed] [Google Scholar]
- Sagulenko V, Thygesen SJ, Sester DP, Idris A, Cridland JA, Vajjhala PR, Roberts TL, Schroder K, Vince JE, Hill JM, Silke J, & Stacey KJ (2013). AIM2 and NLRP3 inflammasomes activate both apoptotic and pyroptotic death pathways via ASC. Cell Death Differ, 20, 1149–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salem H, Rachmin I, Yissachar N, Cohen S, Amiel A, Haffner R, Lavi L, & Motro B (2010). Nek7 kinase targeting leads to early mortality, cytokinesis disturbance and polyploidy. Oncogene, 29, 4046–4057. [DOI] [PubMed] [Google Scholar]
- Samir P, Kesavardhana S, Patmore DM, Gingras S, Malireddi RKS, Karki R, Guy CS, Briard B, Place DE, Bhattacharya A, Sharma BR, Nourse A, King SV, Pitre A, Burton AR, Pelletier S, Gilbertson RJ, & Kanneganti TD (2019). DDX3X acts as a live-or-die checkpoint in stressed cells by regulating NLRP3 inflammasome. Nature, 573, 590–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarhan J, Liu BC, Muendlein HI, Li P, Nilson R, Tang AY, Rongvaux A, Bunnell SC, Shao F, Green DR, & Poltorak A (2018). Caspase-8 induces cleavage of gasdermin D to elicit pyroptosis during Yersinia infection. Proc Natl Acad Sci U S A, 115, E10888–E10897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sborgi L, Ruhl S, Mulvihill E, Pipercevic J, Heilig R, Stahlberg H, Farady CJ, Muller DJ, Broz P, & Hiller S (2016). GSDMD membrane pore formation constitutes the mechanism of pyroptotic cell death. EMBO J, 35, 1766–1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaffidi P, Misteli T, & Bianchi ME (2002). Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature, 418, 191–195. [DOI] [PubMed] [Google Scholar]
- Schleich K, Buchbinder JH, Pietkiewicz S, Kahne T, Warnken U, Ozturk S, Schnolzer M, Naumann M, Krammer PH, & Lavrik IN (2016). Molecular architecture of the DED chains at the DISC: regulation of procaspase-8 activation by short DED proteins c-FLIP and procaspase-8 prodomain. Cell Death Differ, 23, 681–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seymour JF, Kipps TJ, Eichhorst B, Hillmen P, D’Rozario J, Assouline S, Owen C, Gerecitano J, Robak T, De la Serna J, Jaeger U, Cartron G, Montillo M, Humerickhouse R, Punnoose EA, Li Y, Boyer M, Humphrey K, Mobasher M, & Kater AP (2018). Venetoclax-Rituximab in Relapsed or Refractory Chronic Lymphocytic Leukemia. N Engl J Med, 378, 1107–1120. [DOI] [PubMed] [Google Scholar]
- Sharif H, Wang L, Wang WL, Magupalli VG, Andreeva L, Qiao Q, Hauenstein AV, Wu Z, Núñez G, Mao Y, & Wu H (2019). Structural mechanism for NEK7-licensed activation of NLRP3 inflammasome. Nature. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H, Wang Y, Li X, Zhan X, Tang M, Fina M, Su L, Pratt D, Bu CH, Hildebrand S, Lyon S, Scott L, Quan J, Sun Q, Russell J, Arnett S, Jurek P, Chen D, Kravchenko VV, Mathison JC, Moresco EM, Monson NL, Ulevitch RJ, & Beutler B (2016). NLRP3 activation and mitosis are mutually exclusive events coordinated by NEK7, a new inflammasome component. Nat Immunol, 17, 250–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Gao W, & Shao F (2017). Pyroptosis: gasdermin-mediated programmed necrotic cell death. Trends Biochem Sci, 42, 245–254. [DOI] [PubMed] [Google Scholar]
- Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H, Zhuang Y, Cai T, Wang F, & Shao F (2015). Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature, 526, 660–665. [DOI] [PubMed] [Google Scholar]
- Strasser A, Harris AW, Huang DC, Krammer PH, & Cory S (1995). Bcl-2 and Fas/APO-1 regulate distinct pathways to lymphocyte apoptosis. EMBO J, 14, 6136–6147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L, Wang H, Wang Z, He S, Chen S, Liao D, Wang L, Yan J, Liu W, Lei X, & Wang X (2012). Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell, 148, 213–227. [DOI] [PubMed] [Google Scholar]
- Taabazuing CY, Okondo MC, & Bachovchin DA (2017). Pyroptosis and Apoptosis Pathways Engage in Bidirectional Crosstalk in Monocytes and Macrophages. Cell Chem Biol, 24, 507–514 e504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenev T, Bianchi K, Darding M, Broemer M, Langlais C, Wallberg F, Zachariou A, Lopez J, MacFarlane M, Cain K, & Meier P (2011). The Ripoptosome, a signaling platform that assembles in response to genotoxic stress and loss of IAPs. Mol Cell, 43, 432–448. [DOI] [PubMed] [Google Scholar]
- Tsuchiya K, Nakajima S, Hosojima S, Thi Nguyen D, Hattori T, Manh Le T, Hori O, Mahib MR, Yamaguchi Y, Miura M, Kinoshita T, Kushiyama H, Sakurai M, Shiroishi T, & Suda T (2019). Caspase-1 initiates apoptosis in the absence of gasdermin D. Nat Commun, 10, 2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda S, Chen-Yoshikawa TF, Tanaka S, Yamada Y, Nakajima D, Ohsumi A, & Date H (2021). Protective effect of necrosulfonamide on rat pulmonary ischemia-reperfusion injury via inhibition of necroptosis. J Thorac Cardiovasc Surg. [DOI] [PubMed] [Google Scholar]
- Van Opdenbosch N, & Lamkanfi M (2019). Caspases in Cell Death, Inflammation, and Disease. Immunity, 50, 1352–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Opdenbosch N, Van Gorp H, Verdonckt M, Saavedra PHV, de Vasconcelos NM, Goncalves A, Vande Walle L, Demon D, Matusiak M, Van Hauwermeiren F, D’Hont J, Hochepied T, Krautwald S, Kanneganti TD, & Lamkanfi M (2017). Caspase-1 Engagement and TLR-Induced c-FLIP Expression Suppress ASC/Caspase-8-Dependent Apoptosis by Inflammasome Sensors NLRP1b and NLRC4. Cell Rep, 21, 3427–3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Sun L, Su L, Rizo J, Liu L, Wang LF, Wang FS, & Wang X (2014). Mixed lineage kinase domain-like protein MLKL causes necrotic membrane disruption upon phosphorylation by RIP3. Mol Cell, 54, 133–146. [DOI] [PubMed] [Google Scholar]
- Wang Y, Wang J, Wang H, Feng X, Tao Y, Yang J, & Cai J (2018). Necrosulfonamide Attenuates Spinal Cord Injury via Necroptosis Inhibition. World Neurosurg, 114, e1186–e1191. [DOI] [PubMed] [Google Scholar]
- Wang Y, Yin B, Li D, Wang G, Han X, & Sun X (2018). GSDME mediates caspase-3-dependent pyroptosis in gastric cancer. Biochem Biophys Res Commun, 495, 1418–1425. [DOI] [PubMed] [Google Scholar]
- Wegner KW, Saleh D, & Degterev A (2017). Complex Pathologic Roles of RIPK1 and RIPK3: Moving Beyond Necroptosis. Trends Pharmacol Sci, 38, 202–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winchell CG, Mishra BB, Phuah JY, Saqib M, Nelson SJ, Maiello P, Causgrove CM, Ameel CL, Stein B, Borish HJ, White AG, Klein EC, Zimmerman MD, Dartois V, Lin PL, Sassetti CM, & Flynn JL (2020). Evaluation of IL-1 Blockade as an Adjunct to Linezolid Therapy for Tuberculosis in Mice and Macaques. Front Immunol, 11, 891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohlford GF, Van Tassell BW, Billingsley HE, Kadariya D, Canada JM, Carbone S, Mihalick VL, Bonaventura A, Vecchié A, Chiabrando JG, Bressi E, Thomas G, Ho AC, Marawan AA, Dell M, Trankle CR, Turlington J, Markley R, & Abbate A (2020). Phase 1B, Randomized, Double-Blinded, Dose Escalation, Single-Center, Repeat Dose Safety and Pharmacodynamics Study of the Oral NLRP3 Inhibitor Dapansutrile in Subjects With NYHA II-III Systolic Heart Failure. J Cardiovasc Pharmacol, 77, 49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu JLS; Qi J; Chen Z; Wu Y; Guo J; Wang K; Sun X; Zheng J (2019). Cleavage of GSDME by caspase-3 determines lobaplatin-induced pyroptosis in colon cancer cells. Cell Death Dis, 10, 193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan J, & Yankner BA (2000). Apoptosis in the nervous system. Nature, 407, 802–809. [DOI] [PubMed] [Google Scholar]
- Zhang CCLCG; Wang YF; Xu LH; He XH; Zeng QZ; Zeng CY; Mai FY; Hu B; Ouyang DY (2019). Chemotherapeutic paclitaxel and cisplatin differentially induce pyroptosis in A549 lung cancer cells via caspase-3/GSDME activation. Apoptosis, 24, 312–325. [DOI] [PubMed] [Google Scholar]
- Zhang DW, Shao J, Lin J, Zhang N, Lu BJ, Lin SC, Dong MQ, & Han J (2009). RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science, 325, 332–336. [DOI] [PubMed] [Google Scholar]
- Zhang J, Webster JD, Dugger DL, Goncharov T, Roose-Girma M, Hung J, Kwon YC, Vucic D, Newton K, & Dixit VM (2019). Ubiquitin Ligases cIAP1 and cIAP2 Limit Cell Death to Prevent Inflammation. Cell Rep, 27, 2679–2689.e2673. [DOI] [PubMed] [Google Scholar]
- Zhao J, Jitkaew S, Cai Z, Choksi S, Li Q, Luo J, & Liu ZG (2012). Mixed lineage kinase domain-like is a key receptor interacting protein 3 downstream component of TNF-induced necrosis. Proc Natl Acad Sci U S A, 109, 5322–5327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng M, Williams EP, Malireddi RKS, Karki R, Banoth B, Burton A, Webby R, Channappanavar R, Jonsson CB, & Kanneganti TD (2020). Impaired NLRP3 inflammasome activation/pyroptosis leads to robust inflammatory cell death via caspase-8/RIPK3 during coronavirus infection. J Biol Chem. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng M Karki R; Vogel P; Kanneganti TD (2020). Caspase-6 is a key regulator of innate immunity, inflammasome activation and host defense. Cell, 181, 674–687.e613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B, & Abbott DW (2021). Gasdermin E permits interleukin-1 beta release in distinct sublytic and pyroptotic phases. Cell Rep, 35, 108998. [DOI] [PMC free article] [PubMed] [Google Scholar]