Abstract
Importance:
Although typically impressive, objective responses to immune checkpoint inhibitors (ICIs) occur in only 12.5% of advanced cancer patients. The majority do not respond due to cell-intrinsic resistance mechanisms, including, but not restricted to HLA class-I antigen processing machinery (APM) defects. The latter, which have a negative impact on neoantigen presentation to cytotoxic T-lymphocytes (CTLs), are present in the majority of malignant tumors. They are caused by structural alterations in <25% of cases and by dysregulated signaling and/or epigenetic changes in the remaining cases, making them frequently correctable. In this review, we summarize the growing clinical evidence that chemotherapy, targeted therapies, and less so radiotherapy, can correct HLA class-I APM defects in cancer cells and improve responses to ICIs.
Observations:
Most chemotherapeutics enhance HLA class-I APM component expression/function in cancer cells, tumor CTL infiltration, and responses to ICIs in preclinical and clinical models. Despite preclinical evidence, radiotherapy does not appear to upregulate HLA class-I expression in patients and does not enhance the efficacy of ICIs in clinical settings. The latter findings underscore the need to optimize the dose/schedule of radiation and timing of ICI administration to maximize their immunogenic synergy. By increasing DNA/chromatin accessibility, epigenetic agents (histone deacetylase-, DNA methyltransferase-, and EZH2-inhibitors) upregulate HLA class-I APM component expression/function in many cancer types, a crucial contributor to their synergy with ICIs in patients. Furthermore, EGFR- and BRAF/MEK-inhibitors are effective at enhancing HLA class-I expression in EGFR- and BRAF-mutant tumors, respectively; these changes may contribute to the impressive clinical responses induced by these inhibitors in combination with ICIs.
Conclusions and Relevance:
Chemotherapy and targeted therapies are effective at enhancing HLA class-I APM component expression/function in cancer cells. Their resulting increased immunogenicity and recognition/elimination by cognate CTLs contributes to the anti-tumor activity of these therapies, as well as their synergy with ICIs.
Keywords: Antigen processing machinery, checkpoint blockade, chemotherapy, HLA class I, MHC class I, radiotherapy, targeted therapy
Introduction
The impressive clinical responses to immune checkpoint inhibitor (ICI)-based therapy have convincingly demonstrated that a patient’s immune system can recognize and eliminate malignant cells.1–3 However, only 12.5% of patients, on average, across several cancer types display objective responses to ICI-based therapy.4 A number of genomics studies, almost exclusively focusing on structural alterations within cancer cells, have identified subsets of patients who are resistant to ICI-based therapy. These patients include those with a low tumor mutation burden (reducing the likelihood cognate cytotoxic T lymphocytes [CTLs] recognize a suitable neoepitope target expressed by cancer cells), interferon-γ pathway abnormalities (resulting in impaired killing of cancer cells by cognate CTLs), or defects in human leukocyte antigen (HLA) class-I heavy chain or beta 2-microglobulin (β2m), the two HLA class-I subunits mediating tumor antigen presentation (resulting in escape of cancer cells from elimination by cognate CTLs).5 Because of their structural nature, the latter defects are only correctable via gene therapy, which cannot be routinely applied in a clinical setting. However, structural alterations in HLA class-I heavy chain, β2m, and other antigen processing machinery (APM) components represent at most 25% of the identified HLA class I APM defects.6 The majority of these defects which are frequently present in most, if not all cancer types (Figure 1, Table S1), are caused by dysregulated signaling within cancer cells and/or epigenetic mechanisms. Therefore, they are potentially correctable by clinically relevant strategies which counteract the underlying mechanism(s). HLA class-I APM component defects affect a key step in the sequence of events leading to the recognition and elimination of cancer cells by cognate CTLs and therefore are likely to play a major role in their resistance to T cell-mediated elimination.6 Growing evidence, summarized in this paper, suggests that correction of these defects can greatly enhance the efficacy of T cell-based immunotherapies, including ICI-based therapy.
Fig. 1 Legend. Frequency of HLA class-I APM Component Defects by Cancer Type.
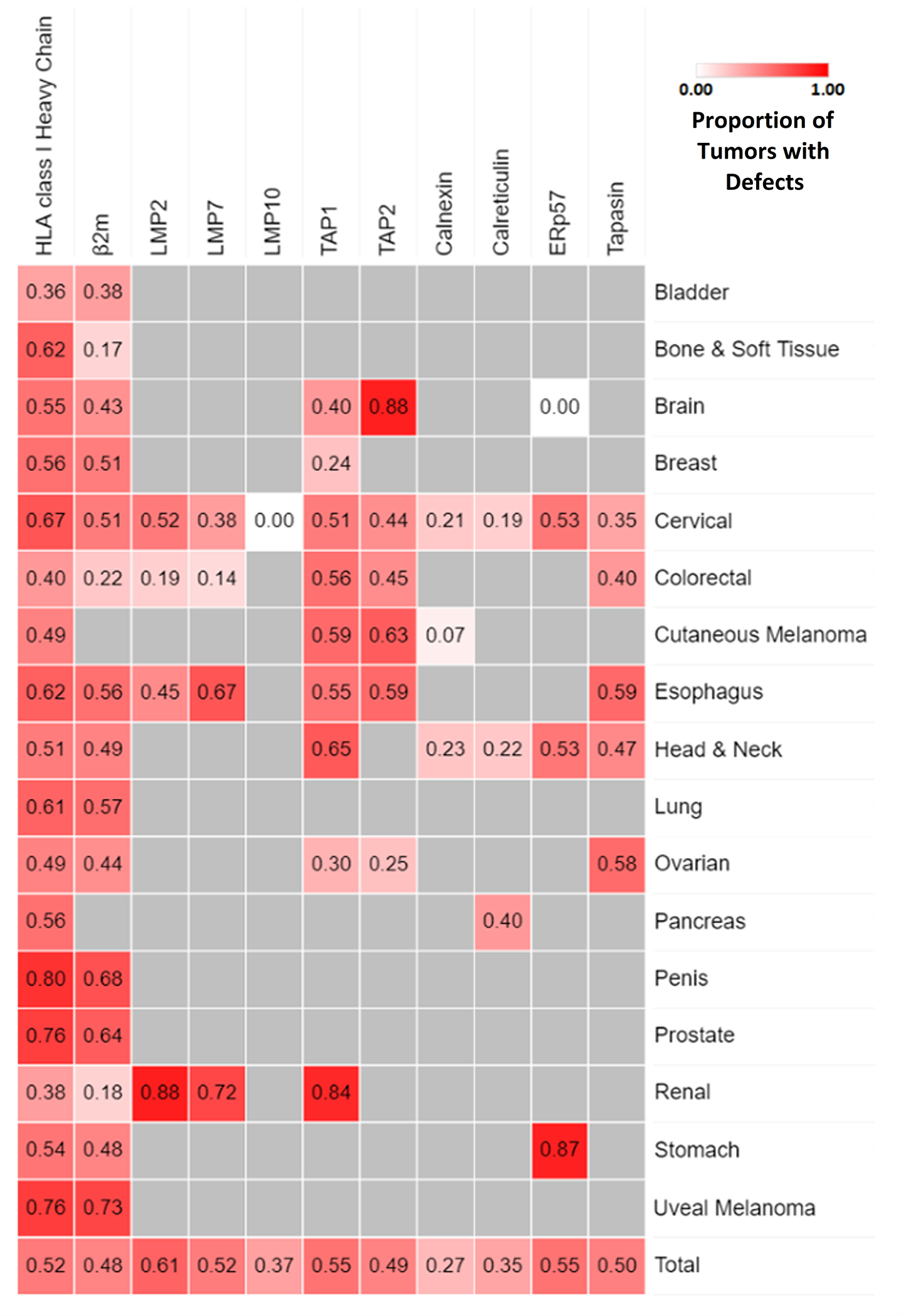
HLA class-I APM component defect frequency by cancer type was assessed via systematic review of the literature.6 A total of 237 papers were identified that analyzed immunohistochemical HLA class-I APM component expression in cancer tissues. Only cancer types in which HLA class-I APM component expression was assessed in at least 100 cases have been included in this heatmap. Combined defect frequency amongst all cancers is shown in the bottom row. Exact proportions of cancers with HLA class-I APM component defects are available in Supplementary Table 1.
Role of HLA Class-I APM Components in the Response to Immune Checkpoint Inhibitor-Based Therapy
Presentation of tumor antigen (TA)-derived peptides by class-I major histocompatibility complex (MHC) antigens is crucial for the recognition of malignant cells by cognate CTLs, which are the major players in immune editing.7 This process involves degradation of mostly, but not exclusively endogenous proteins by the proteasome or immunoproteasome, transport of the resulting peptides into the endoplasmic reticulum lumen through the heterodimeric TAP1/TAP2 complex and loading of the peptides onto MHC class-I heavy chain-β2m dimers. The last step involves chaperones calreticulin, tapasin, and ER resident protein 57 [ERp57]. The resulting MHC class-I heavy chain-β2m-peptide trimolecular complexes (MHC class-I in every species, HLA class-I in humans) travel to the cell membrane and are recognized by the T cell receptor (TCR) expressed by cognate CTLs (Figure 2).6
Fig. 2 Legend. Function of HLA Class-I Antigen Processing Machinery Components in Cancer Cells.
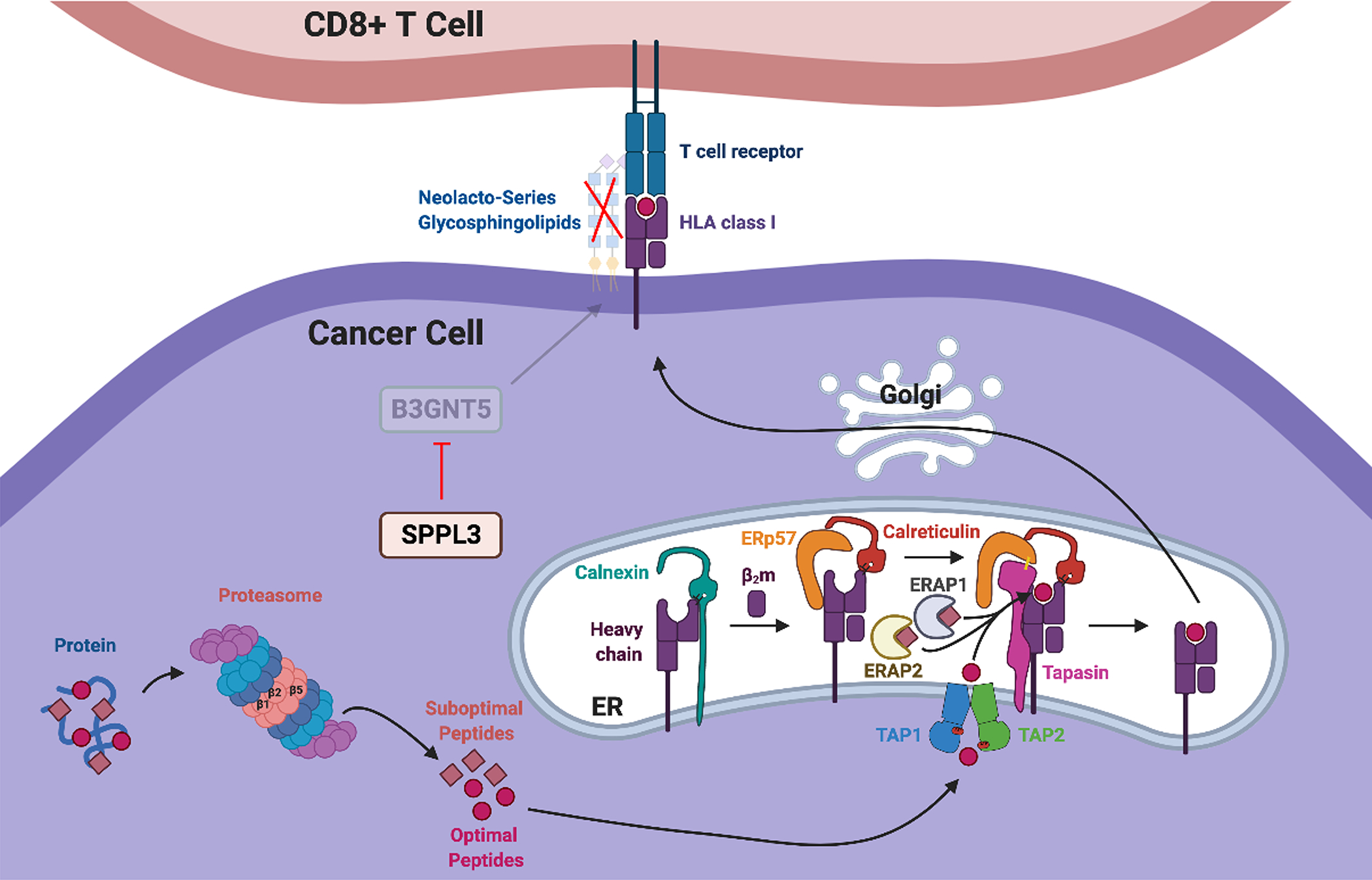
Proteins are degraded by the proteasome into peptides with the correct length and sequence to fit the HLA class-I heavy chain/β2m dimer groove. The catalytic β1 (Delta), β2 (Zeta) and β5 (MB1) proteasome subunits are involved in this process and are exchangeable with IFNγ-inducible LMP2, LMP10, LMP7 immunoproteasome subunits, respectively. The latter constitute the more efficient immunoproteasome. During degradation of proteins, suboptimal peptides are also generated. Both optimal and suboptimal peptides are shuttled into the endoplasmic reticulum lumen via the heterodimeric transporter associated with antigen processing complex consisting of TAP1 and TAP2. ER aminopeptidases ERAP1 and ERAP2 refine the length of the suboptimal peptides. Furthermore, newly synthesized HLA class-I heavy chain is stabilized by calnexin in the ER, associates with β2m, and then a processed peptide is loaded onto the dimer with the help of the chaperone molecules calreticulin, ER resident protein 57 (ERp57), and tapasin. The resulting trimolecular complexes are transported to the plasma membrane via the Golgi apparatus and are recognized by the T cell receptor of cognate cytotoxic T cells. Neolacto-series glycosphingolipids synthesized by glycosyltransferase B3GNT5 sterically impede the interactions of HLA class-I trimolecular complexes with the T cell receptor, impairing the recognition of cancer cells by cognate cytotoxic T lymphocytes. B3GNT5 activity is inhibited by protease SPPL3. Defects in HLA class-I antigen processing machinery component expression may compromise expression of HLA class-I antigen/tumor antigen-derived peptide complexes, negatively impacting recognition/elimination of cancer cells by cognate cytotoxic T lymphocytes, and potentially providing cancer cells with an escape mechanism from immune surveillance.
Collectively referred to as HLA class-I APM components, HLA class-I heavy chain, β2m, and proteins involved in the assembly and cell surface expression of HLA class-I heavy chain-β2m-peptide complexes play a crucial role in generating an optimal response to the T cell-based immunity triggered by therapies such as vaccines and ICIs. Indeed, B2m knockouts cause resistance to anti-PD-1 monoclonal antibody (mAb) therapy in mouse models of lung cancer. Moreover, in melanoma and lung cancer patients, mutations in β2m are associated with acquired resistance to ICIs.8,9 Lastly, non-structural alterations such as transcriptional repression of HLA-A or HLA-B are associated with acquired resistance to ICIs in Merkel cell carcinoma patients.10
In contrast, higher HLA class-I APM component expression levels may enhance TA-derived peptide presentation, improving immunogenicity as well as recognition and elimination of cancer cells by cognate CTLs. Consistent with this notion, high HLA class-I APM component expression in cancer cells is associated with improved prognosis, including longer disease-specific, progression-free, and overall survival in cancer patients (Table 1). Melanoma patients with >30% of malignant cells in their tumors stained by HLA class-I-specific mAbs, displayed a significantly lower frequency of progressive disease on week 13 of treatment with ipilimumab, compared to patients with <30% of malignant cells stained by HLA class-I-specific mAbs.11 Likewise, melanoma patients displaying high HLA class-I APM component mRNA expression in their tumors demonstrated prolonged overall- and progression free-survival in response to CTLA-4 blockade, compared to patients with low HLA class-I APM component mRNA expression.12
Table 1.
Association of Higher HLA Class-I APM Component Expression Level in Cancer Cells with Better Survival Outcomes.
| Cancer Type | Component | Survival Outcome | Reference1 |
|---|---|---|---|
|
| |||
| Bladder | Calreticulin | OS | Cathro et al. (2010) |
| Breast | HLA class-I | DSS | Sik Park et al. (2019) |
| Cervical | HLA-A, HLA class-I, LMP7, TAP1, ERAP1 | OS | Mehta et al. (2008) |
| Cervical | HLA-A, TAP1, ERAP1 | DFS | Mehta et al. (2008) |
| Clear Cell RCC | HLA class-I | OS, RFS | Sekar et al. (2016) |
| Colon | HLA-A, HLA-C | DFS | Benevolo et al. (2007) |
| Colon | HLA-C | OS | Benevolo et al. (2007) |
| Colon | Calreticulin | OS | Peng et al. (2010) |
| Colorectal | HLA class-I | DFS | Iwayama et al. (2015) |
| Colorectal | HLA-A | DFS | Sandel et al. (2005) |
| Colorectal | HLA class-I | DSS | Simpson et al. (2010) |
| Colorectal | Tapasin | OS | Sokol et al. (2015) |
| Colorectal | HLA-B/C+B2M co-expression | DSS | Watson et al. (2006) |
| Endometrial | HLA-B/C | PFS, OS | Yakabe et al. (2015) |
| ESFT | HLA class-I | OS | Yabe et al. (2011) |
| Esophageal | HLA class-I | RFS | Hosch et al. (1997) |
| Esophageal | HLA class-I, B2M, TAP1 | OS | Tanaka et al. (2012) |
| Esophageal SCC | HLA class-I | OS | Mizukami et al. (2008) |
| Esophageal SCC | HLA class-I | OS | Zhang et al. (2013) |
| Gastric | HLA class-I | OS | Ishigami et al. (2008) |
| Gastric | ERp57 | OS | Leys et al. (2007) |
| HCC | HLA-C | OS | Wang et al. (2019) |
| HNSCC | HLA-B/C | DFS | Bandoh et al. (2010) |
| HR-proficient ovarian | HLA class-I | PFS | Matsushita et al. (2020) |
| Laryngeal SCC | HLA-B/C, B2M, LMP2 | DFS, CSS | Ogino et al. (2006) |
| Maxillary Sinus SCC | Tapasin, HLA-B/C | OS | Ogino et al. (2003) |
| Melanoma | TAP1, TAP2, HLA-B/C | PFS, OS | Kageshita et al. (1999) |
| NSCLC | HLA class-I | OS | Ichinokawa et al. (2019) |
| NSCLC | HLA class-I | OS | Kikuchi et al. (2007) |
| Osteosarcoma | HLA class-I | OS, EFS | Tsukahara et al. (2006) |
| PDAC | HLA-B/C | OS | Michelakos et al. (2021) |
| Penile | HLA-A | OS | Djajadiningrat et al. (2015) |
| Prostate | HLA class-I | OS | Levin et al. (1994) |
| Rectal | HLA class-I | DFS | Reimers et al. (2014) |
| Rectal | HLA class-I | OS, DFS | Speetjens et al. (2008) |
Citations provided in Supplementary Information.
Abbreviations: B2M, beta 2-microglobulin; CSS, cause-specific survival; DFS, disease-free survival; DSS, disease-specific survival; EFS, event-free survival; ERAP, endoplasmic reticulum aminopeptidase; ERp57, endoplasmic reticulum resident protein 57; ESFT, Ewing sarcoma family of tumors; HCC, hepatocellular carcinoma; HLA, human leukocyte antigen; HNSCC, head and neck squamous cell carcinoma; HR, homologous recombination; NSCLC, non-small-cell lung cancer; OS, overall survival; PDAC, pancreatic ductal adenocarcinoma; PFS, progression-free survival; RCC, renal cell carcinoma; RFS, relapse-free survival; SCC, squamous cell carcinoma; TAP, transporter associated with antigen processing.
Thus, HLA class-I APM component upregulation in cancer cells may be a promising strategy to enhance the efficacy of ICI-based therapy. Herein, we present the growing clinical evidence that chemotherapy, certain targeted therapies, and less so radiotherapy can restore/enhance HLA class-I APM component expression in cancer cells. These changes lead to increased CD8+ T cell tumor infiltration and are associated with improved clinical responses to ICI-based therapy.
HLA Class-I APM Component Upregulation by Chemotherapy
Virtually all classes of chemotherapeutics upregulate HLA class-I APM component expression in various cancer cell lines, cultured in vitro and/or grafted in mice, and enhance their recognition/elimination by cognate CTLs.13–18 For many drugs, this evidence has been corroborated in the clinical setting. In ovarian cancer patients, both carboplatin and paclitaxel monotherapies significantly upregulate HLA-B expression in malignant cells. The functional implication of this change is indicated by its association with increased intratumoral CD8+ T cell infiltration in ovarian cancer patients treated with taxanes or platinum-based alkylating agents.19 Likewise, the 5-fluorouracil plus cisplatin combination enhances both HLA class-I expression and stromal CD8+ T cell infiltration in esophageal squamous cell carcinoma patients.20
In addition, chemotherapy-induced MHC class-I antigen upregulation is associated with better responses to ICI-based therapy. In a murine breast cancer cell line displaying MHC class-I antigen downregulation and poor response to anti-PD-L1 mAbs, irinotecan treatment significantly enhanced MHC class-I antigen expression and sensitized the breast cancer cells to PD-L1 inhibitors.21 These data have been corroborated by the results from clinical trials which also suggest an increased therapeutic efficacy of ICIs when combined with chemotherapeutics.22,23 Doxorubicin induction therapy doubled the objective response rate to nivolumab in a phase II trial of metastatic triple-negative breast cancer (TNBC) (35% ORR [6/17 patients] in the doxorubicin induction group vs. 17% ORR [2/12 patients] in the no induction group).22 Likewise, phase III trials in advanced non-small-cell lung cancer patients have shown that the chemotherapy plus ICI combination significantly prolongs patients’ survival compared to chemotherapy alone.24–26 Although the role of other variables cannot be excluded, chemotherapy-induced HLA class-I APM component upregulation in cancer cells may be an important contributor to the observed synergies.18,27–29
The mechanisms underlying chemotherapy-induced HLA class-I APM component upregulation in cancer cells include type I interferon (IFNα/β) secretion and subsequent autocrine/paracrine signaling, as well as NF-κB signaling activation (Figure S1, Figure S2).17,18 These processes are inducible by a number of agents that appear to synergize with ICIs in the clinical setting, possibly reflecting the important role played by HLA class-I expression in ICI response. In this regard, activation of stimulator of interferon genes (STING) by synthetic dinucleotides such as MK-1454 promotes type I interferon secretion by cancer cells. In phase I trials, the MK-1454 plus pembrolizumab combination yielded a 24% objective response rate (6/25 patients) in various advanced cancer types, while MK-1454 monotherapy induced no objective responses (0/26 patients).30 Moreover, retinoid-induced type I IFN secretion through the activation of retinoic-acid inducible gene I (RIG-I),31 can account for the MHC class-I antigen upregulation by all-trans retinoic acid on melanoma cells and enhancement of their eradication by CTLs both in vitro and in syngeneic mouse tumor models.32 An ongoing phase II trial investigating the efficacy of the all-trans retinoic acid and ipilimumab combination in stage III/IV melanoma patients (NCT02403778) will assess the clinical relevance of this combinatorial strategy. Furthermore, the RIG-I and STING signaling pathways are activated during the antiviral response33. Therefore, oncolytic viruses represent an attractive method to enhance HLA class-I APM component expression by cancer cells. Specifically, RIG-I- and STING-driven type I interferon secretion by infected cells and subsequent paracrine signaling are expected to enhance HLA class-I APM component expression in nearby cancer cells. Clinical evidence demonstrates a synergy of oncolytic viruses with ICIs. In a phase II trial of stage IIIB-IV melanoma patients, the talimogene laherparepvec plus ipilimumab combination induced a 39% objective response rate (38/98 patients), significantly higher than that induced by ipilimumab alone (ORR=18% [18/100 patients]).34
HLA Class-I APM Component Upregulation by Radiation Therapy
Radiation upregulates HLA class-I APM component expression in cancer cells both in vitro and in animal models utilizing the same molecular mechanisms as chemotherapy (Figure S1).17,35–38 At variance with chemotherapy, radiation-induced HLA class-I upregulation has rarely been described in patients. Compared to uveal melanoma patients receiving no neoadjuvant therapy, those receiving neoadjuvant radiotherapy did not display any significant change in either HLA class-I or β2m expression level.39 Similarly, HLA-A and HLA-B/C expression levels in irradiated pancreatic ductal adenocarcinoma (PDAC) patients were not significantly different from patients receiving no neoadjuvant therapy.40 Likewise, HLA class-I expression level on rectal cancer cells was not significantly different between 476 irradiated and 532 non-irradiated patients.41 It is unclear whether the lack of effect of radiation reflects differences between preclinical systems and clinical settings or flaws in the analysis of the clinical data. We believe the latter to be the case. The conclusions of the clinical studies are not derived from analyses of sequential samples obtained from the same patient but are instead derived from comparisons among tumors surgically removed from irradiated and non-irradiated patients. Patients receiving neoadjuvant radiotherapy are likely to harbor more advanced cancers with greater HLA class-I APM component downregulation, due to selective pressure acting over a longer timeframe.42 Therefore, it is possible that radiation-induced HLA class-I upregulation may not be appreciated, since the patients used for comparison are in an earlier stage of the disease and therefore, display a lower frequency of HLA class-I defects. Our hypothesis is supported by the results obtained in sarcoma patients for whom sequential biopsies could be analyzed; in 60% (9/15) of the patients receiving radiation, HLA class-I expression level was significantly higher post-treatment.43 However, other factors including differences in the dose/schedule of radiation among studies cannot be excluded.
Many trials combining radiation with ICIs are ongoing. Results from a phase II trial in metastatic head and neck squamous cell carcinoma patients testing the stereotactic body radiotherapy (SBRT) plus nivolumab combination versus nivolumab alone indicate no significant differences in the objective response rate between the two groups (nivolumab plus SBRT ORR: 29.0% [9/31 patients; 95% CI, 16.1% to 46.6%], nivolumab ORR: 34.5% [10/29 patients; 95% CI, 19.9% to 52.7%], p = 0.86).44 Likewise, metastatic TNBC patients receiving nivolumab plus induction radiotherapy in a phase II trial displayed a slightly lower objective response rate than patients receiving nivolumab alone (8% ORR [1/12 patients] in the induction radiotherapy group vs. 17% ORR [2/12 patients] in the no induction group).22 These findings emphasize the need to optimize the dose/schedule of radiation when it is used as an immunological adjuvant, perhaps not with the primary goal of maximizing radiation-induced cytotoxicity and with greater emphasis on its potential impact on a patient’s immune system.45–47 In the context of antigen presentation, this optimization is especially important as radiation and chemotherapy transiently induce the release from cancer cells of antigens which are loaded on tumor stromal cells and sensitize them to elimination by cognate CTLs. The time-dependence of this process stresses the importance of dose/schedule optimization of anti-cancer therapies in the adjuvant setting.48
HLA Class-I APM Component Upregulation by Targeted Therapy
Correcting Epigenetic Repression of HLA Class-I APM Component Transcription
Several epigenetic regulators of HLA class-I APM component expression have been identified including DNA methyltransferases (DNMTs), histone deacetylases (HDACs), and polycomb repressive complex 2 (PRC2) enzymatic component enhancer of zeste homolog 2 (EZH2) (Figure S1). They operate by reducing transcription factor accessibility of HLA class-I APM component chromatin/DNA, thereby resulting in their downregulation.49–52 The reduced HLA class-I APM component transcription caused by high DNA methylation levels in melanoma cells53 has provided the rationale for a phase II clinical trial testing the DNA methyltransferase inhibitor guadecitabine plus ipilimumab combination; in stage III/IV melanoma patients, a 26% objective response rate was reported (5/19 patients).54 DNMT inhibition may also activate endogenous retroviral genes (ERVs) and trigger production of type III interferons.55 This mechanism underlies MHC class-I APM component upregulation by CDK4/6 inhibitors, which downregulate DNMT1 expression, and account for the synergy between CDK4/6 inhibitors and anti-PD-L1 mAbs described in murine breast cancer models.56 Moreover, HDACs have been shown to repress HLA class-I APM component transcription in Merkel cell carcinoma cells in vitro,57 providing the rationale to combine the HDAC inhibitor panobinostat with avelumab in the treatment of a metastatic Merkel cell carcinoma patient refractory to ICI-based therapy due to lack of HLA class-I expression. Panobinostat treatment restored HLA class-I expression by malignant cells, increased intratumoral CD8+ T cell infiltration, and in combination with avelumab, stabilized the disease for 3 months.58 Lastly, murine oral squamous cell carcinoma (OSCC) cells have been shown to overexpress EZH2 and downregulate MHC class-I antigens. The restoration of their expression by the EZH2 inhibitor GSK126 sensitizes the OSCC cells to anti-PD-1 mAbs.59 A similar finding has been reported in murine malignant prostate tumors.60 Overall, DNMT, HDAC, and EZH2 inhibitors are effective at enhancing HLA class-I expression across a wide range of malignancies (Table 2). These inhibitors have the potential to improve the efficacy of ICI-based therapy in cancer types which display HLA class-I APM component expression defects caused by epigenetic mechanisms.
Table 2.
Upregulation of MHC Class-I APM Component Expression by Targeted Therapies in Distinct Cancer Types.
| Targeted Molecule/Pathway | Cancer Type | References1 |
|---|---|---|
|
| ||
| Histone deacetylases (HDACs) | Merkel cell carcinoma2 | Ugurel et al. (2019), Ritter et al. (2017) |
| Breast cancer | Gameiro et al. (2016) | |
| Cervical cancer | Mora-García et al. (2006) | |
| Colon cancer | Khan et al. (2008) | |
| Gastric cancer | Shan et al. (2011) | |
| Glioma | Yang et al. (2020) | |
| Melanoma | Komatsu et al. (1998), Khan et al. (2007), Setiadi et al. (2008), Woan et al. (2015) | |
| Prostate cancer | Kortenhorst et al. (2013), Kitamura et al. (2007), Setiadi et al. (2008) | |
| DNA methyltransferases (DNMTs) | Melanoma2 | Fonsatti et al. (2007), Fazio et al. (2018), Rodríguez et al. (2007), Coral et al. (1999), Fonsatti et al. (2003), Coral et al. (2006), Gasparollo et al. (2001), Serrano et al. (2001), Coral et al. (2013) |
| Bladder cancer | Santamaria et al. (1988) | |
| Breast cancer | Carlow et al. (1985), Sultan et al. (2018) | |
| Cervical cancer | Ye et al. (2010), Jiang et al. (2010) | |
| Esophageal SCC | Nie et al. (2001) | |
| Fibrosarcoma | Bonal et al. (1986), Aboud et al. (1991), Ananthaswamy (1988) | |
| Glioma | Natsume et al. (2008), Konkankit et al. (2011) | |
| Non-small-cell lung cancer | Tripathi et al. (2016) | |
| Oral SCC | Jiang et al. (2010) | |
| Ovarian cancer | Adair et al. (2009) | |
| Teratoma | Cremisi (1983) | |
| Enhancer of zeste homolog 2 (EZH2) | Oral SCC3 | Zhou et al. (2020) |
| Merkel cell carcinoma | Burr et al. (2019) | |
| Neuroblastoma | Burr et al. (2019) | |
| Small cell lung cancer | Burr et al. (2019) | |
| Epidermal growth factor receptor (EGFR) | Head and neck cancer4 | Srivastava et al. (2015) |
| Lung cancer4 | Watanabe et al. (2019), He et al. (2013), Garrido et al. (2017) | |
| Breast cancer | Garrido et al. (2017) | |
| Colon cancer | Garrido et al. (2017) | |
| Epidermoid carcinoma | Garrido et al. (2017), Pollack et al. (2011) | |
| Esophageal SCC | Mimura et al. (2013) | |
| Gastric cancer | Mimura et al. (2013) | |
| Melanoma | Garrido et al. (2017) | |
| BRAF | Melanoma4 | Sabbatino et al. (2016), Frazao et al. (2017), Sottile et al. (2016), Kakavand et al. (2017) |
| MEK | Breast cancer | Inoue et al. (2012) |
| Colorectal cancer | Gravett et al. (2018), Sers et al. (2009) | |
| Esophageal SCC | Mimura et al. (2013) | |
| Gastric cancer | Mimura et al. (2013) | |
| Non-small-cell lung cancer | Watanabe et al. (2019) | |
| Papillary thyroid cancer | Angell et al. (2014) | |
| SHP2 | Colorectal cancer5 | Quintana et al. (2020) |
| Hedgehog signaling pathway | EBV-associated gastric cancer | Deb Pal et al. (2015) |
| Lysosome | PDAC5 | Yamamoto et al. (2020) |
| Lung cancer | Yamamoto et al. (2020) | |
| Melanoma | Li et al. (2010) | |
| Autophagy | Melanoma | Li et al. (2010) |
Citations provided in Supplementary Information
Evidence in patients in combination with checkpoint inhibitors
Evidence in syngeneic mouse tumor models in combination with checkpoint inhibitors
Evidence in patients as monotherapy
Evidence in syngeneic mouse tumor models as monotherapy.
Abbreviations: EBV, Epstein-Barr virus; PDAC, pancreatic ductal adenocarcinoma; SCC, squamous cell carcinoma.
Correcting HLA Class-I APM Component Downregulation Caused by Dysregulated Signaling Pathways
Dysregulated signaling, including activation of the hedgehog signaling pathway and the receptor-tyrosine kinase (RTK)/mitogen-activated protein kinase (MAPK) pathway, may also downregulate HLA class-I APM component expression in cancer cells (Figure S1).61–63 Hedgehog-mediated HLA class-I downregulation appears to be restricted to Epstein-Barr virus (EBV)-associated cancers that express latent membrane protein 2A (Table 2).61 On the other hand, activating mutations or amplifications in the RTK/MAPK pathway (e.g. alterations in EGFR, RAF, RAS, MEK, and/or SHP2) result in HLA class-I APM component downregulation in many cancer types. This effect is mediated by MAPK-driven inactivation of STAT1 and STAT3, which play a major role in HLA class-I APM component transcription.64,65 In these instances, HLA class-I APM component expression is usually restored by MAPK inhibitors (Table 2). In the clinical setting, treatment of EGFR-mutated non-small-cell lung cancer patients with EGFR inhibitors markedly increased HLA class-I expression by malignant cells.66 Moreover, in head and neck cancer patients treated with cetuximab, both HLA-A and HLA-B/C were significantly upregulated in the responding tumors as determined by a volume reduction.67 Similarly, in BRAF-mutated melanoma patients treated with BRAF inhibitors ± MEK inhibitors, HLA-A expression post-therapy was enhanced or stable in cases of tumor shrinkage, but was selectively downregulated in progressing tumors.68
Synergy has been observed between RTK/MAPK inhibitors and ICIs in preclinical and clinical settings. In mice grafted with syngeneic head and neck squamous cell carcinoma cells displaying high levels of MAPK activation, the MEK inhibitor trametinib inhibited ERK phosphorylation, enhanced MHC class-I antigen expression, and in combination with anti-PD-L1 mAbs, overcame resistance to monotherapy and significantly controlled tumor growth.65 In phase I trials, EGFR tyrosine kinase inhibitor plus ICI combinations induced objective response rates of up to 73% in advanced renal cell carcinoma patients (38/52 patients treated with the axitinib plus pembrolizumab combination) and up to 80% in EGFR-mutant non-small-cell lung cancer patients (8/10 patients treated with the gefitinib plus durvalumab combination).69,70 Furthermore, the cobimetinib, vemurafenib, and atezolizumab combination induced a 93% objective response rate (13/14 patients) in BRAF-mutant melanoma patients.71 HLA class I upregulation is likely to play an important role in the described exceptional clinical responses.62,63,66,67,72,73
Correcting Abnormalities in HLA Class-I Trafficking
Abnormalities in trafficking of MHC class-I heavy chain-β2m-peptide trimolecular complexes may cause MHC class-I downregulation in cancer cells (Figure S1). This mechanism underlies HLA class I defects in pancreatic cancer cells, which are characterized by elevated autophagic flux: the autophagy receptor NBR1 mediates the trafficking of HLA class-I trimolecular complexes to the lysosomes. Treatment of cells with a lysosome inhibitor such as chloroquine prevents HLA class-I trimolecular complex degradation and restores their expression on the cell surface.74 Guided by this finding, chloroquine was shown to enhance MHC class-I antigen expression on murine PDAC tumors, sensitizing them to dual immune checkpoint inhibition with anti-PD-1 and anti-CTLA4 mAbs. A concomitant increase in intratumoral CD8+ T cells and PD1+TIM3- (non-exhausted) T cell infiltration was observed.74 Interestingly, in a phase II trial in PDAC patients, the hydroxychloroquine plus gemcitabine/nab-paclitaxel combination induced a significant increase in tumor immune cell infiltration compared to patients treated with chemotherapy-only.75 This finding is consistent with the ability of lysosome inhibitors to induce HLA class-I upregulation on PDAC cells and supports the notion that they will synergize with ICIs in patients. Autophagy-lysosomal pathway-dependent degradation of MHC class-I trimolecular complexes is not restricted to PDAC cells (Table 2). Autophagy inhibition with 3-methyladenine or wortmannin, and lysosome inhibition with chloroquine or bafilomycin A1 in murine melanoma cells upregulated MHC class-I antigen expression in vitro.76
Lastly, proprotein convertase subtilisin/kexin type 9 (PCSK9), a protein involved in cholesterol metabolism, can also promote lysosomal trafficking of MHC class-I antigens (Table 2).77 Specifically, PCSK9 is secreted by cancer cells and binds to the extracellular α1 region of murine H2-K1 (ortholog of HLA class-I heavy chain). The resulting PCSK9-heavy chain-β2m-peptide complex is endocytosed and degraded through the endosomal-lysosomal pathway.77 Two inhibitors binding to extracellular PCSK9 (alirocumab, evolocumab) have already been approved for the treatment of hypercholesterolemia. These inhibitors enhance MHC class-I antigen expression and show synergy with ICIs in the treatment of murine colorectal cancer cells grafted in syngeneic, immunocompetent mice.77 Clinical trials testing anti-PCSK9 mAb plus ICI combinations are warranted.
Conclusion
HLA class-I APM component expression/function are frequently defective in cancer cells; in most cases, expression/function of these components can be restored or enhanced by chemotherapy, targeted therapies, and less so by radiotherapy. The correction of these defects appears to have clinical relevance, as it is associated with increased CD8+ T cell tumor infiltration, enhanced susceptibility of cancer cells to T cell-mediated immunity triggered by ICI-based therapy, and an improved clinical course of the disease. These results provide a convincing rationale to combine chemo-, radio-, and targeted therapies with ICI-based therapy. This strategy is likely to require changes in the dosing and timing of chemo-, radio-, and targeted therapies to maximize their immunogenic effects. Lastly, as HLA class-I APM component defects are associated with acquired resistance to ICI-based therapy, it will be critical to assess their value as biomarkers of ICI response, as well as identify the mechanisms underlying their downregulation. The latter information will be crucial for the rational design of individualized strategies to counteract these defects.
Supplementary Material
Funding:
This work was supported by the National Institute of Health (grants R01DE028172, R01CA230275, R03CA219603 and R03CA253319, +DOD grant – already in Luke’s).
Footnotes
Conflicts of Interest: The authors declare no potential conflicts of interest.
References
- 1.Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus Ipilimumab in Advanced Melanoma. New England Journal of Medicine. 2013;369(2):122–133. doi: 10.1056/nejmoa1302369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, Activity, and Immune Correlates of Anti–PD-1 Antibody in Cancer. New England Journal of Medicine. 2012;366(26):2443–2454. doi: 10.1056/nejmoa1200690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hodi FS, O’Day SJ, McDermott DF, et al. Improved Survival with Ipilimumab in Patients with Metastatic Melanoma. New England Journal of Medicine. 2010;363(8):711–723. doi: 10.1056/nejmoa1003466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haslam A, Prasad V. Estimation of the Percentage of US Patients With Cancer Who Are Eligible for and Respond to Checkpoint Inhibitor Immunotherapy Drugs. JAMA network open. 2019;2(5):e192535. doi: 10.1001/jamanetworkopen.2019.2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Keenan TE, Burke KP, van Allen EM. Genomic correlates of response to immune checkpoint blockade. Nature Medicine. 2019;25(3):389–402. doi: 10.1038/s41591-019-0382-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cai L, Michelakos T, Yamada T, et al. Structural and Functional Defects in HLA class I Antigen Processing Machinery in Cancer Cells: Molecular Mechanisms and Clinical Relevance. In: Butterfield L, Kaufman H, Marincola F, eds. Cancer Immunotherapy Principles and Practice. 2nd Edition. Springer Publishing Co.; 2021. [Google Scholar]
- 7.Cai L, Michelakos T, Yamada T, et al. Defective HLA class I antigen processing machinery in cancer. Cancer Immunology, Immunotherapy. 2018;67(6):999–1009. doi: 10.1007/s00262-018-2131-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gettinger S, Choi J, Hastings K, et al. Impaired HLA class I antigen processing and presentation as a mechanism of acquired resistance to immune checkpoint inhibitors in lung cancer. Cancer Discovery. 2017;7(12):1420–1435. doi: 10.1158/2159-8290.CD-17-0593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zaretsky JM, Garcia-Diaz A, Shin DS, et al. Mutations Associated with Acquired Resistance to PD-1 Blockade in Melanoma. New England Journal of Medicine. 2016;375(9):819–829. doi: 10.1056/nejmoa1604958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paulson KG, Voillet V, McAfee MS, et al. Acquired cancer resistance to combination immunotherapy from transcriptional loss of class I HLA. Nature Communications. 2018;9(1). doi: 10.1038/s41467-018-06300-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rodig SJ, Gusenleitner D, Jackson DG, et al. MHC proteins confer differential sensitivity to CTLA-4 and PD-1 blockade in untreated metastatic melanoma. Science Translational Medicine. 2018;10(450). doi: 10.1126/scitranslmed.aar3342 [DOI] [PubMed] [Google Scholar]
- 12.Such L, Zhao F, Liu D, et al. Targeting the innate immunoreceptor RIG-I overcomes melanoma-intrinsic resistance to T cell immunotherapy. Journal of Clinical Investigation. 2020;140(8):4266–4281. doi: 10.1172/JCI131572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Z, Chen J, Hu J, et al. CGAS/STING axis mediates a topoisomerase II inhibitor–induced tumor immunogenicity. Journal of Clinical Investigation. 2019;129(11):4850–4862. doi: 10.1172/JCI127471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gravett AM, Trautwein N, Stevanović S, Dalgleish AG, Copier J. Gemcitabine alters the proteasome composition and immunopeptidome of tumour cells. OncoImmunology. 2018;7(6). doi: 10.1080/2162402X.2018.1438107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamamura Y, Tsuchikawa T, Miyauchi K, et al. The key role of calreticulin in immunomodulation induced by chemotherapeutic agents. International Journal of Clinical Oncology. 2015;20(2):386–394. doi: 10.1007/s10147-014-0719-x [DOI] [PubMed] [Google Scholar]
- 16.Hodge JW, Garnett CT, Farsaci B, et al. Chemotherapy-induced immunogenic modulation of tumor cells enhances killing by cytotoxic T lymphocytes and is distinct from immunogenic cell death. International Journal of Cancer. 2013;133(3):624–636. doi: 10.1002/ijc.28070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wan S, Pestka S, Jubin RG, Lyu YL, Tsai YC, Liu LF. Chemotherapeutics and radiation stimulate MHC class i expression through elevated interferon-beta signaling in breast cancer cells. PLoS ONE. 2012;7(3). doi: 10.1371/journal.pone.0032542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pellicciotta I, Yang CPH, Goldberg GL, Shahabi S. Epothilone B enhances Class i HLA and HLA-A2 surface molecule expression in ovarian cancer cells. Gynecologic Oncology. 2011;122(3):625–631. doi: 10.1016/j.ygyno.2011.05.007 [DOI] [PubMed] [Google Scholar]
- 19.Peng J, Hamanishi J, Matsumura N, et al. Chemotherapy induces programmed cell death-ligand 1 overexpression via the nuclear factor-κBto foster an immunosuppressive tumor microenvironment in Ovarian Cancer. Cancer Research. 2015;75(23):5034–5045. doi: 10.1158/0008-5472.CAN-14-3098 [DOI] [PubMed] [Google Scholar]
- 20.Tsuchikawa T, Miyamoto M, Yamamura Y, Shichinohe T, Hirano S, Kondo S. The immunological impact of neoadjuvant chemotherapy on the tumor microenvironment of esophageal squamous cell carcinoma. Annals of Surgical Oncology. 2012;19(5):1713–1719. doi: 10.1245/s10434-011-1906-x [DOI] [PubMed] [Google Scholar]
- 21.Iwai T, Sugimoto M, Wakita D, Yorozu K, Kurasawa M, Yamamoto K. Topoisomerase I inhibitor, irinotecan, depletes regulatory T cells and up-regulates MHC class I and PD-L1 expression, resulting in a supra-additive antitumor effect when combined with anti- PD-L1 antibodies. Oncotarget. 2018;9(59):31411–31421. doi: 10.18632/oncotarget.25830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Voorwerk L, Slagter M, Horlings HM, et al. Immune induction strategies in metastatic triple-negative breast cancer to enhance the sensitivity to PD-1 blockade: the TONIC trial. Nature Medicine. 2019;25(6):920–928. doi: 10.1038/s41591-019-0432-4 [DOI] [PubMed] [Google Scholar]
- 23.Kuo CHS, Wang CC, Huang YC, et al. Comparison of a combination of chemotherapy and immune checkpoint inhibitors and immune checkpoint inhibitors alone for the treatment of advanced and metastatic non-small cell lung cancer. Thoracic Cancer. 2019;10(5):1158–1166. doi: 10.1111/1759-7714.13057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.West H, McCleod M, Hussein M, et al. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): a multicentre, randomised, open-label, phase 3 trial. The Lancet Oncology. 2019;20(7):924–937. doi: 10.1016/S1470-2045(19)30167-6 [DOI] [PubMed] [Google Scholar]
- 25.Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus Chemotherapy in Metastatic Non–Small-Cell Lung Cancer. New England Journal of Medicine. 2018;378(22):2078–2092. doi: 10.1056/nejmoa1801005 [DOI] [PubMed] [Google Scholar]
- 26.Paz-Ares L, Luft A, Vicente D, et al. Pembrolizumab plus Chemotherapy for Squamous Non–Small-Cell Lung Cancer. New England Journal of Medicine. 2018;379(21):2040–2051. doi: 10.1056/nejmoa1810865 [DOI] [PubMed] [Google Scholar]
- 27.Wang WJ, Qin SH, Zhang JW, Jiang YY, Zhang JN, Zhao L. Combination doxorubicin and interferon-α therapy stimulates immunogenicity of murine pancreatic cancer Panc02 cells via up-regulation of NKG2D ligands and MHC class I. Asian Pacific Journal of Cancer Prevention. 2014;15(22):9667–9672. doi: 10.7314/APJCP.2014.15.22.9667 [DOI] [PubMed] [Google Scholar]
- 28.de Biasi AR, Villena-Vargas J, Adusumilli PS. Cisplatin-induced antitumor immunomodulation: A review of preclinical and clinical evidence. Clinical Cancer Research. 2014;20(21):5384–5391. doi: 10.1158/1078-0432.CCR-14-1298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matsuoka H, Eura M, Chikamatsu K, et al. Low doses of anticancer drugs increase susceptibility of tumor cells to lysis by autologous killer cells. Anticancer Research. 1995;15(1). doi: 10.1080/2162402X.2017.1316438 [DOI] [PubMed] [Google Scholar]
- 30.Harrington KJ, Brody J, Ingham M, et al. Preliminary results of the first-in-human (FIH) study of MK-1454, an agonist of stimulator of interferon genes (STING), as monotherapy or in combination with pembrolizumab (pembro) in patients with advanced solid tumors or lymphomas. Annals of Oncology. 2018;29:viii712. doi: 10.1093/annonc/mdy424.015 [DOI] [Google Scholar]
- 31.Matsumiya T, Stafforini DM. Function and regulation of retinoic acid-inducible gene-I. Critical Reviews in Immunology. 2010;30(6):489–513. doi: 10.1615/critrevimmunol.v30.i6.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yin W, Song Y, Liu Q, Wu Y, He R. Topical treatment of all-trans retinoic acid inhibits murine melanoma partly by promoting CD8+ T-cell immunity. Immunology. 2017;152(2):287–297. doi: 10.1111/imm.12768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zevini A, Olagnier D, Hiscott J. Crosstalk between Cytoplasmic RIG-I and STING Sensing Pathways. Trends in Immunology. 2017;38(3):194–205. doi: 10.1016/j.it.2016.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chesney J, Puzanov I, Collichio F, et al. Randomized, open-label phase II study evaluating the efficacy and safety of talimogene laherparepvec in combination with ipilimumab versus ipilimumab alone in patients with advanced, unresectable melanoma. Journal of Clinical Oncology. 2018;36(17):1658–1667. doi: 10.1200/JCO.2017.73.7379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ranoa DRE, Parekh AD, Pitroda SP, et al. Cancer therapies activate RIG-I-like receptor pathway through endogenous non-coding RNAs. Oncotarget. 2016;7(18):26496–26515. doi: 10.18632/oncotarget.8420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Santin AD, Hiserodt JC, Fruehauf J, DiSaia PJ, Pecorelli S, Granger GA. Effects of irradiation on the expression of surface antigens in human ovarian cancer. Gynecologic Oncology. 1996;60(3):468–474. doi: 10.1006/gyno.1996.0075 [DOI] [PubMed] [Google Scholar]
- 37.Gameiro SR, Jammeh ML, Wattenberg MM, Tsang KY, Ferrone S, Hodge JW. Radiation-induced immunogenic modulation of tumor enhances antigen processing and calreticulin exposure, resulting in enhanced T-cell killign. Oncotarget. 2014;5(2):403–416. doi: 10.18632/oncotarget.1719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gameiro SR, Malamas AS, Bernstein MB, et al. Tumor Cells Surviving Exposure to Proton or Photon Radiation Share a Common Immunogenic Modulation Signature, Rendering Them More Sensitive to T Cell-Mediated Killing. International Journal of Radiation Oncology Biology Physics. 2016;95(1):120–130. doi: 10.1016/j.ijrobp.2016.02.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jager MJ, van der Pol JP, de Wolff-Rouendaal D, de Jong PTVM, Ruiter DJ. Decreased expression of HLA class II antigens on human uveal melanoma cells after in vivo X-ray irradiation. American Journal of Ophthalmology. 1988;105(1):78–86. doi: 10.1016/0002-9394(88)90125-0 [DOI] [PubMed] [Google Scholar]
- 40.Michelakos T, Cai L, Villani V, et al. Tumor Microenvironment Immune Response in Pancreatic Ductal Adenocarcinoma Patients Treated With Neoadjuvant Therapy. JNCI: Journal of the National Cancer Institute. 2021;113(2):182–191. doi: 10.1093/jnci/djaa073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Speetjens FM, de Bruin EC, Morreau H, et al. Clinical impact of HLA class I expression in rectal cancer. Cancer Immunology, Immunotherapy. 2008;57(5):601–609. doi: 10.1007/s00262-007-0396-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chang CC, Ferrone S. Immune selective pressure and HLA class I antigen defects in malignant lesions. Cancer Immunology, Immunotherapy. 2007;56(2):227–236. doi: 10.1007/s00262-006-0183-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sharma A, Bode B, Wenger RH, et al. Γ-Radiation promotes immunological recognition of cancer cells through increased expression of cancer-testis antigens in vitro and in vivo. PLoS ONE. 2011;6(11). doi: 10.1371/journal.pone.0028217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McBride S, Sherman E, Jillian Tsai C, et al. Randomized phase II trial of nivolumab with stereotactic body radiotherapy versus nivolumab alone in metastatic head and neck squamous cell carcinoma. Journal of Clinical Oncology. 2021;39(1):30–37. doi: 10.1200/JCO.20.00290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Seung Lisa P., Weichselbaum Ralph R., Toledano Alicia, Schreiber Karin, Schreiber Hans. Radiation can inhibit tumor growth indirectly while depleting circulating leukocytes. Radiation Research. 1996;146(6):612–618. [PubMed] [Google Scholar]
- 46.Formenti SC, Demaria S. Systemic effects of local radiotherapy. The Lancet Oncology. 2009;10(7):718–726. doi: 10.1016/S1470-2045(09)70082-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Grassberger C, Ellsworth SG, Wilks MQ, Keane FK, Loeffler JS. Assessing the interactions between radiotherapy and antitumour immunity. Nature Reviews Clinical Oncology. 2019;16(12):729–745. doi: 10.1038/s41571-019-0238-9 [DOI] [PubMed] [Google Scholar]
- 48.Zhang B, Bowerman NA, Salama JK, et al. Induced sensitization of tumor stroma leads to eradication of established cancer by T cells. Journal of Experimental Medicine. 2007;204(1):49–55. doi: 10.1084/jem.20062056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Burr ML, Sparbier CE, Chan KL, et al. An Evolutionarily Conserved Function of Polycomb Silences the MHC Class I Antigen Presentation Pathway and Enables Immune Evasion in Cancer. Cancer Cell. 2019;36(4):385–401.e8. doi: 10.1016/j.ccell.2019.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ye Q, Shen Y, Wang X, et al. Hypermethylation of HLA class i gene is associated with HLA class i down-regulation in human gastric cancer. Tissue Antigens. 2010;75(1):30–39. doi: 10.1111/j.1399-0039.2009.01390.x [DOI] [PubMed] [Google Scholar]
- 51.Gameiro SR, Malamas AS, Tsang KY, Ferrone S, Hodge JW. Inhibitors of histone deacetylase 1 reverse the immune evasion phenotype to enhance T-cell mediated lysis of prostate and breast carcinoma cells. Oncotarget. 2016;7(7):7390–7402. doi: 10.18632/oncotarget.7180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Khan ANH, Gregorie CJ, Tomasi TB. Histone deacetylase inhibitors induce TAP, LMP, Tapasin genes and MHC class I antigen presentation by melanoma cells. Cancer Immunology, Immunotherapy. 2008;57(5):647–654. doi: 10.1007/s00262-007-0402-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fazio C, Covre A, Cutaia O, et al. Immunomodulatory properties of DNA hypomethylating agents: Selecting the optimal epigenetic partner for cancer immunotherapy. Frontiers in Pharmacology. 2018;9. doi: 10.3389/fphar.2018.01443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.di Giacomo AM, Covre A, Finotello F, et al. Guadecitabine plus ipilimumab in unresectable melanoma: The NIBIT-M4 clinical trial. Clinical Cancer Research. 2019;25(24):7351–7362. doi: 10.1158/1078-0432.CCR-19-1335 [DOI] [PubMed] [Google Scholar]
- 55.Roulois D, Loo Yau H, Singhania R, et al. DNA-Demethylating Agents Target Colorectal Cancer Cells by Inducing Viral Mimicry by Endogenous Transcripts. Cell. 2015;162(5):961–973. doi: 10.1016/j.cell.2015.07.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Goel S, Decristo MJ, Watt AC, et al. CDK4/6 inhibition triggers anti-tumour immunity. Nature. 2017;548(7668):471–475. doi: 10.1038/nature23465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ritter C, Fan K, Paschen A, et al. Epigenetic priming restores the HLA class-I antigen processing machinery expression in Merkel cell carcinoma. Scientific Reports. 2017;7(1). doi: 10.1038/s41598-017-02608-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ugurel S, Spassova I, Wohlfarth J, et al. MHC class-I downregulation in PD-1/PD-L1 inhibitor refractory Merkel cell carcinoma and its potential reversal by histone deacetylase inhibition: a case series. Cancer Immunology, Immunotherapy. 2019;68(6):983–990. doi: 10.1007/s00262-019-02341-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhou L, Mudianto T, Ma X, Riley R, Uppaluri R. Targeting EZH2 enhances antigen presentation, antitumor immunity, and circumvents anti–PD-1 resistance in head and neck cancer. Clinical Cancer Research. 2020;26(1):290–300. doi: 10.1158/1078-0432.CCR-19-1351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sheahan A v., Morel KL, Burkhart DL, et al. Targeting EZH2 Increases Therapeutic Efficacy of Check-Point Blockade in Models of Prostate Cancer. bioRxiv. Published online August 8, 2019. doi: 10.1101/730135 [DOI] [Google Scholar]
- 61.Deb Pal A, Banerjee S. Epstein-Barr virus latent membrane protein 2A mediated activation of Sonic Hedgehog pathway induces HLA class Ia downregulation in gastric cancer cells. Virology. 2015;484:22–32. doi: 10.1016/j.virol.2015.05.007 [DOI] [PubMed] [Google Scholar]
- 62.Garrido G, Rabasa A, Garrido C, et al. Upregulation of HLA Class I Expression on Tumor Cells by the Anti-EGFR Antibody Nimotuzumab. Frontiers in Pharmacology. 2017;8(OCT). doi: 10.3389/fphar.2017.00595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mimura K, Shiraishi K, Mueller A, et al. The MAPK Pathway Is a Predominant Regulator of HLA-A Expression in Esophageal and Gastric Cancer. The Journal of Immunology. 2013;191(12):6261–6272. doi: 10.4049/jimmunol.1301597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Brea EJ, Oh CY, Manchado E, et al. Kinase regulation of human MHC class i molecule expression on cancer cells. Cancer Immunology Research. 2016;4(11):936–947. doi: 10.1158/2326-6066.CIR-16-0177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kang SH, Keam B, Ahn YO, et al. Inhibition of MEK with trametinib enhances the efficacy of anti-PD-L1 inhibitor by regulating anti-tumor immunity in head and neck squamous cell carcinoma. OncoImmunology. 2019;8(1). doi: 10.1080/2162402X.2018.1515057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Watanabe S, Hayashi H, Haratani K, et al. Mutational activation of the epidermal growth factor receptor down-regulates major histocompatibility complex class I expression via the extracellular signal-regulated kinase in non–small cell lung cancer. Cancer Science. 2019;110(1):52–60. doi: 10.1111/cas.13860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Srivastava RM, Trivedi S, Concha-Benavente F, et al. Stat1-induced HLA class i upregulation enhances immunogenicity and clinical response to anti-EGFR mab cetuximab therapy in HNC patients. Cancer Immunology Research. 2015;3(8):936–945. doi: 10.1158/2326-6066.CIR-15-0053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kakavand H, Rawson R v., Pupo GM, et al. PD-L1 expression and immune escape in melanoma resistance to MAPK inhibitors. Clinical Cancer Research. 2017;23(20):6054–6061. doi: 10.1158/1078-0432.CCR-16-1688 [DOI] [PubMed] [Google Scholar]
- 69.Gibbons DL, Chow LQ, Kim DW, et al. 57O Efficacy, safety and tolerability of MEDI4736 (durvalumab [D]), a human IgG1 anti-programmed cell death-ligand-1 (PD-L1) antibody, combined with gefitinib (G): A phase I expansion in TKI-naïve patients (pts) with EGFR mutant NSCLC. Journal of Thoracic Oncology. 2016;11(4):S79. doi: 10.1016/S1556-0864(16)30171-X [DOI] [Google Scholar]
- 70.Atkins MB, Plimack ER, Puzanov I, et al. Axitinib in combination with pembrolizumab in patients with advanced renal cell cancer: a non-randomised, open-label, dose-finding, and dose-expansion phase 1b trial. The Lancet Oncology. 2018;19(3):405–415. doi: 10.1016/S1470-2045(18)30081-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hwu P, Hamid O, Gonzalez R, et al. Preliminary safety and clinical activity of atezolizumab combined with cobimetinib and vemurafenib in BRAF V600-mutant metastatic melanoma. Annals of Oncology. 2016;27:vi380. doi: 10.1093/annonc/mdw379.05 [DOI] [Google Scholar]
- 72.Sabbatino F, Wang Y, Scognamiglio G, et al. Antitumor activity of BRAF inhibitor and IFN×alpha; Combination in BRAF-mutant melanoma. Journal of the National Cancer Institute. 2016;108(7). doi: 10.1093/jnci/djv435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sottile R, Pangigadde PN, Tan T, et al. HLA class I downregulation is associated with enhanced NK-cell killing of melanoma cells with acquired drug resistance to BRAF inhibitors. European Journal of Immunology. 2016;46(2):409–419. doi: 10.1002/eji.201445289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yamamoto K, Venida A, Yano J, et al. Autophagy promotes immune evasion of pancreatic cancer by degrading MHC-I. Nature. 2020;581(7806):100–105. doi: 10.1038/s41586-020-2229-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zeh HJ, Bahary N, Boone BA, et al. A Randomized Phase II Preoperative Study of Autophagy Inhibition with High-Dose Hydroxychloroquine and Gemcitabine/Nab-Paclitaxel in Pancreatic Cancer Patients. Clinical cancer research : an official journal of the American Association for Cancer Research. 2020;26(13):3126–3134. doi: 10.1158/1078-0432.CCR-19-4042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li B, Lei Z, Lichty BD, et al. Autophagy facilitates major histocompatibility complex class I expression induced by IFN-γ in B16 melanoma cells. Cancer Immunology, Immunotherapy. 2010;59(2):313–321. doi: 10.1007/s00262-009-0752-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liu X, Bao X, Hu M, et al. Inhibition of PCSK9 potentiates immune checkpoint therapy for cancer. Nature. 2020;588(7839):693–698. doi: 10.1038/s41586-020-2911-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


