Abstract
Purpose:
To describe breast cancer treatment patterns among premenopausal women by age and time since last pregnancy.
Methods:
Data were analyzed from 1,179 women diagnosed with premenopausal breast cancer in the Carolina Breast Cancer Study. Of these, 160 had a recent pregnancy (within 5 years of cancer diagnosis). Relative frequency differences (RFDs) and 95% confidence intervals (CIs) were used to compare cancer stage, treatment modality received, treatment initiation delay (>30 days), and prolonged treatment duration (>2 to >8 months depending on the treatment received) by age and recency of pregnancy.
Results:
Recently postpartum women were significantly more likely to have stage III disease [RFD (95% CI): 12.2% (3.6%, 20.8%)] and to receive more aggressive treatment compared to nulliparous women. After adjustment for age, race and standard clinical tumor characteristics, recently postpartum women were significantly less likely to have delayed treatment initiation [RFD (95% CI): −11.2% (−21.4%, −1.0%)] and prolonged treatment duration [RFD (95% CI): −17.5% (−28.0%, −7.1%)], and were more likely to have mastectomy [RFD (95% CI): 14.9% (4.8%, 25.0%)] compared to nulliparous. Similarly, younger women (<40 years of age) were significantly less likely to experience prolonged treatment duration [RFD (95% CI): −5.6% (−11.1%, −0.0%)] and more likely to undergo mastectomy [RFD (95% CI): 10.6% (5.2%, 16.0%)] compared to the study population as a whole.
Conclusion:
These results suggest that recently postpartum and younger women often received prompt and aggressive breast cancer treatment. Higher mortality and recurrence among recently pregnant women are unlikely to be related to undertreatment.
Keywords: Breast cancer, premenopausal women, treatment initiation delay, prolonged treatment duration, treatment modalities
Background
Younger (<40 years of age) and recently postpartum (within 5 years of cancer diagnosis) women have been found to have worse breast cancer outcomes and higher mortality compared to other women with breast cancer [1–23]. Previous studies have hypothesized that tumor biology, delayed diagnosis, or treatment delay and variation contribute to poorer disease outcomes for recently postpartum women [1–7, 24–41]. Our recent findings, in the Carolina Breast Cancer Study (CBCS), suggest that breast tumors of recently postpartum women were more frequently node positive and had unique immune microenvironments, but it is unknown how common treatment delay is among these women [42]. Previous analyses from the CBCS indicated that younger (<50 years of age) women had fewer treatment delays compared to older (50–74 years of age) women [43, 44], but comparisons in that study were not restricted to premenopausal women or to those with recent pregnancy. It is important to understand differences in treatment patterns for women with higher risk of aggressive cancers because delays and undertreatment are linked to worse overall and breast cancer-specific survival [45].
Using data from participants diagnosed with premenopausal breast cancers in CBCS Phase III, we hypothesized that stage at diagnosis, treatment initiation delay, prolonged treatment duration, and treatment modality would vary according to time since last childbirth and age at diagnosis. Recently postpartum women were defined as those diagnosed up to 5 years after their last full-term (≥7 months) pregnancy. Young-onset breast cancers were defined as cancers diagnosed at <40 years of age.
Methods
Study population
The CBCS phase III is a population-based study of women diagnosed with breast cancer in 44 counties of central and eastern North Carolina (2008–2013, N=2998); study details have been described previously [46–49]. Written informed consent was obtained at baseline (approximately 5 months after diagnosis) prior to data collection. All study protocols were approved by the Office of Human Research Ethics, Institutional Review Board at the University of North Carolina at Chapel Hill (UNC). Briefly, the primary study enrolled 20–74-year-old women with first primary invasive breast cancer and oversampled for black and younger women (< 50 years of age) using randomized recruitment. The current analysis examined treatment time-related factors and treatment modality for premenopausal women under 50 years of age (N=1179). Only participants with stage I-III breast cancers were included, as treatment pathways for metastatic disease are distinct from those for localized disease. Additionally, we excluded participants who did not elect surgical treatment (N=5). Cases with missing data for last full-term birth were excluded (N=2). Breast cancers diagnosed during pregnancy were excluded (N=7). Figure 1 depicts participant numbers according to inclusion/exclusion criteria. In order to examine the effect of age on treatment patterns, we also considered the CBCS phase III population as a whole (including women 20–74 years of age at diagnosis).
Figure 1:
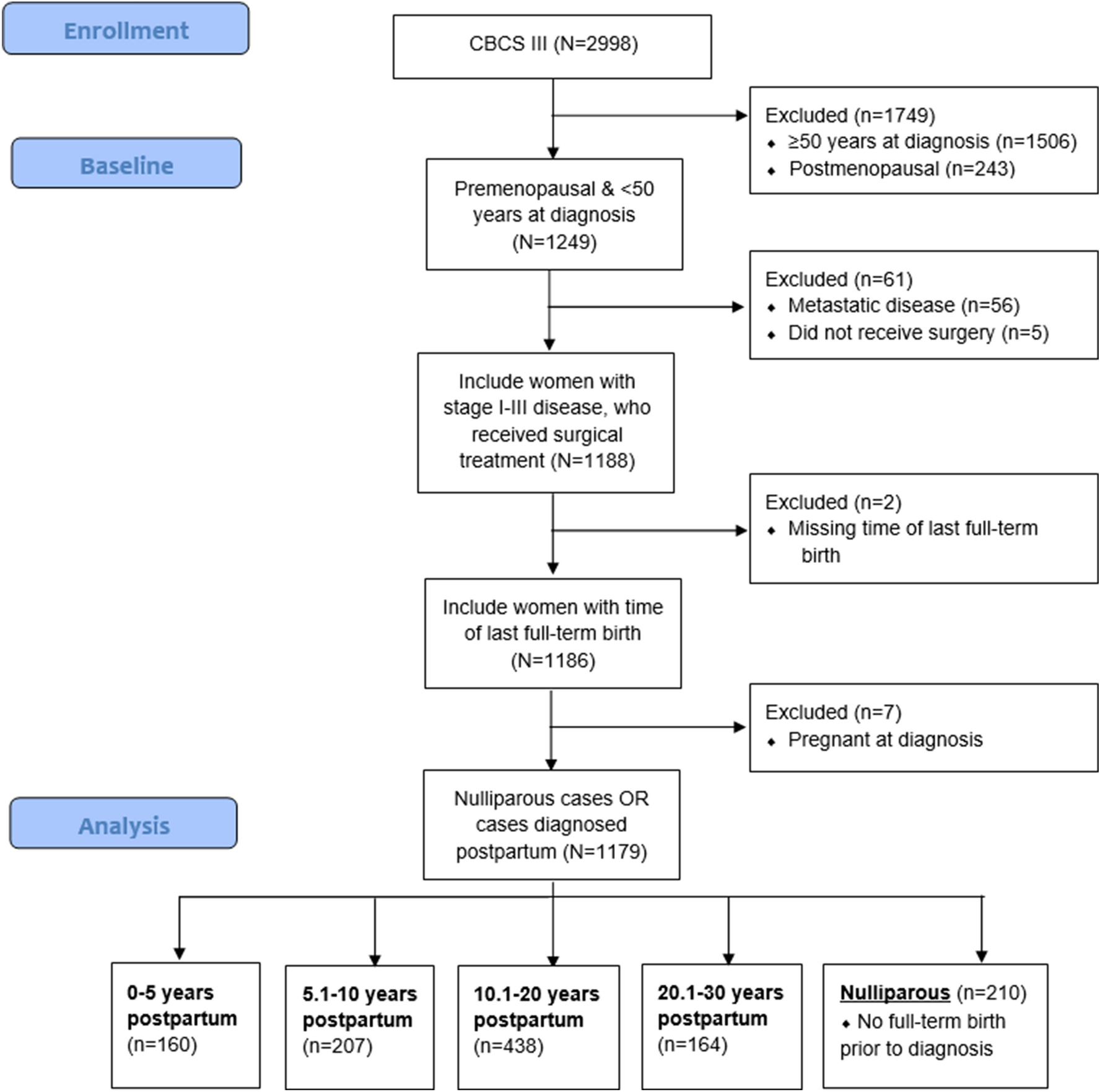
Study population inclusion/exclusion criteria flowchart
Recency of last childbirth
In-person interviews were conducted by trained nurses to collect medical history including detailed information on pregnancy history. Date of breast cancer diagnosis was collected by medical record abstraction. Time since last full-term birth was calculated by subtracting date of last full-term (≥7 months pregnancy) birth from date of breast cancer diagnosis. Women were grouped according to their time since last full-term birth: 0–5 years postpartum (N=160); 5.1–10 years postpartum (N=207); 10.1–20 years postpartum (N=438); 20.1–30 years postpartum (N=164). Women who never had a full-term (≥7 months) pregnancy prior to their diagnosis were assigned to the “nulliparous” group (N=210). Women who were up to 5 years postpartum were referred to as recently postpartum. Women who were 10.1–20 years postpartum were referred to as remotely postpartum.
Treatment initiation, treatment modalities and prolonged treatment duration
Time to treatment initiation (in days) was defined as the time between breast cancer diagnosis and first treatment (defined as surgery, adjuvant or neoadjuvant chemotherapy, or radiation); this information was abstracted from medical records. Treatment initiation was categorized as occurring ≤ 30 vs. > 30 days after diagnosis, based on a recent publication by Bleicher et al. that reported better overall-survival among invasive non-metastatic breast cancer patients who received treatment within 30 days of diagnosis compared to longer wait to treatment initiation [50]. Information on treatment type, including type of surgery (mastectomy vs. breast-conserving surgery), chemotherapy receipt (yes vs. no), radiation therapy receipt (yes vs. no), and hormone therapy (yes vs. no), was abstracted from medical records. For prolonged treatment duration, participants were sorted in four treatment groups: surgery only, surgery and radiation, surgery and chemotherapy, and surgery, chemotherapy and radiation. Treatment duration was categorized within each treatment group by subtracting the date of last treatment from date of first treatment. Prolonged treatment duration (yes vs. no) was defined using American Cancer Society [51, 52] treatment recommendations within strata of treatment modality as follows: (1) surgery only, prolonged treatment duration was “yes” if surgery was performed ≥ 30 days after diagnosis, in-line with treatment initiation delay; (2) surgery and radiation, prolonged treatment duration was “yes” if treatment duration > 2 months [52]; (3) surgery and chemotherapy, prolonged treatment duration was “ yes” if treatment duration > 6 months [51]; (4) surgery, radiation and chemotherapy, prolonged treatment duration was “yes” if treatment duration > 8 months [51, 52]. Information on breast cancer stage at diagnosis was abstracted from medical records.
Covariate assessment
Race was determined by self-report and categorized as Black or non-Black. Less than 2% of non-Black participants self-identified as multiracial, Hispanic, or other race/ethnicities. Age at diagnosis was obtained from the baseline survey and used as a continuous variable in models. Information on parity was obtained from baseline survey and categories as nulliparous, or 1, 2 and ≥ 3 full-term (≥7 months pregnancy) births. Self-reported income (USD < $20K, $20K-$50K, and >$50K), education (≤ high school education/GED, some college education/college degree, and post-graduate/professional degree), marital status (married vs. not married), and health insurance status (yes vs. no) were obtained from the baseline survey.
Statistical analyses
Descriptive statistics for patient sociodemographic and tumor characteristics of recently postpartum (0–5 years postpartum) and 5.1–10 years postpartum women were compared with nulliparous or remotely postpartum (10.1–20 years postpartum) women using chi-square tests or Fisher’s exact tests when cell count <5. Generalized linear models were used to estimate relative frequency differences (RFDs) and 95% CIs as a measure of association for recency of last childbirth and age at diagnosis with respect to treatment initiation delay, prolonged treatment duration, and treatment modalities (type of surgery, chemotherapy receipt, radiation therapy receipt and hormone therapy receipt) [53]. Models were adjusted for age, race, tumor stage, size, grade, lymph node status, hormone receptor status and human epidermal growth factor receptor 2 status. Due to the limited cell size for recently (0–5 years) postpartum women without chemotherapy (n=20) and hormone therapy (n=14) treatment groups, these models were only adjusted for age and race. We minimally adjusted stage models for age, race, income, education, marital status, and health insurance status at baseline. All analyses were conducted in SAS version 9.4 (SAS Institute, Cary, NC). P-values were two-sided with an alpha of 0.05 for statistical significance.
Results
Patient and Tumor characteristics
Among women with premenopausal breast cancer, 160 women were recently postpartum (0–5 years postpartum), 207 women were 5.1–10 years postpartum, 438 women were remotely postpartum (10.1–20 years postpartum), 164 women were 20.1–30 years postpartum women, and 210 women were nulliparous. Table 1 shows the distribution of age at diagnosis, race, parity, income, education, marital status, and health insurance at baseline according to time intervals from most recent childbirth. Recently postpartum women had a significantly younger age at diagnosis (median = 37 years) compared to those who were remotely postpartum (median = 44 years) or were nulliparous (median = 42 years). Although no significant difference in race was observed between recently postpartum and nulliparous group, the 20.1–30 years postpartum group had a higher frequency of Black compared to non-Black participants, consistent with a trend toward younger age at first birth among Black women in this study population. Recently postpartum women were significantly more likely to have health insurance at baseline (94.4% vs. 88.6%) and were more commonly married (73.1% vs. 40.0%) compared to nulliparous women. Compared to remotely postpartum women, recently postpartum women had higher income and education, and were more likely to be primiparous. There were no differences in income and education between recently postpartum and nulliparous women.
Table 1:
Distribution of select characteristics by time since last childbirth among premenopausal women <50 years of age in Carolina Breast Cancer Study - Phase III, 2008–2013 (N=1179)
| Nulliparous (N=210) | 0–5 postpartum (N=160) | 5.1–10 postpartum (N=207) | 10.1–20 postpartum (N=438) | 20.1–30 postpartum (N=164) | |
|---|---|---|---|---|---|
| Age at diagnosis (years) | |||||
| Median (Range) | 42 (23, 49) | 37 (24, 49) | 41 (26, 49) | 44 (30, 49) | 47 (38, 49) |
| <30 | 14 (6.7) | 17 (10.6) | 4 (1.9) | 0 (0.0) | 0 (0.0) |
| 30–39 | 52 (24.8) | 96 (60.0) | 78 (37.7) | 60 (13.7) | 1 (0.61) |
| 40–49 | 144 (68.6) | 47 (29.4) | 125 (60.4) | 378 (86.3) | 163 (99.4) |
| p-valuea | <0.0001 | ||||
| Race | |||||
| Black | 97 (46.2) | 77 (48.1) | 92 (44.4) | 209 (47.7) | 108 (65.9) |
| Non-Black | 113 (53.8) | 83 (51.9) | 115 (55.6) | 229 (52.3) | 56 (34.2) |
| p-valuea | 0.7 | ||||
| Parity | |||||
| 1 | - | 47 (29.4) | 47 (22.7) | 94 (21.5) | 64 (39.0) |
| 2 | - | 57 (35.6) | 86 (41.6) | 199 (45.4) | 61 (37.2) |
| ≥3 | - | 56 (35.0) | 74 (35.8) | 145 (33.1) | 39 (23.8) |
| p-valueb | 0.05 | ||||
| Income | |||||
| <$20,000 | 34 (16.8) | 19 (12.0) | 24 (11.9) | 80 (19.1) | 46 (29.3) |
| $20,000–50,000 | 64 (31.5) | 44 (27.9) | 52 (25.7) | 134 (32.0) | 47 (29.9) |
| >$50,000 | 105 (51.7) | 95 (60.1) | 126 (62.4) | 205 (48.9) | 64 (40.8) |
| Missing | 7 | 2 | 5 | 19 | 7 |
| p-valuea | 0.2 | ||||
| Education | |||||
| ≤High school graduate/GED | 45 (21.4) | 26 (16.2) | 50 (24.1) | 140 (32.0) | 81 (49.4) |
| Some college/College graduate | 121 (57.6) | 99 (61.9) | 119 (57.5) | 249 (57.0) | 80 (48.8) |
| Post-graduate/Professional degree | 44 (21.0) | 35 (21.9) | 38 (18.4) | 48 (11.0) | 3 (1.8) |
| Missing | 0 | 0 | 0 | 1 | 0 |
| p-valuea | 0.5 | ||||
| Married | |||||
| No | 126 (60.0) | 43 (26.9) | 63 (30.4) | 164 (37.5) | 72 (43.9) |
| Yes | 84 (40.0) | 117 (73.1) | 144 (69.6) | 273 (62.5) | 92 (56.1) |
| Missing | 0 | 0 | 0 | 1 | 0 |
| p-valuea | <0.0001 | ||||
| Health Insurance at Baseline | |||||
| Yes | 186 (88.6) | 151 (94.4) | 198 (96.1) | 412 (94.3) | 150 (91.5) |
| No | 24 (11.4) | 9 (5.6) | 8 (3.9) | 25 (5.7) | 14 (8.5) |
| Missing | 0 | 0 | 1 | 1 | 0 |
| p-valuea | 0.05 |
P-values generated by chi-square test between nulliparous and >0–5 years postpartum women, except when expected cell count <5, they were calculated by Fisher’s exact test.
P-value for chi-square test between women >0–5 years vs. >10–20 years postpartum. Missing values were excluded from percentage calculations. Percentages may not add up to 100 due to rounding.
Tumor stage at diagnosis & Treatment time-related factors
Recently postpartum women were significantly more likely to have stage III disease [RFD (95% CI): 12.2% (3.6%, 20.8%)] compared to nulliparous women, and these differences remained significant even after adjustment for age, race and socioeconomic factors including income, education, health insurance status and marital status. The median time to treatment initiation was 31 days (interquartile range, 20–44). Approximately 60% of participants had treatment initiation > 30 days after diagnosis. After adjustment for age, race and standard clinical tumor characteristics, recently postpartum women were significantly less likely to have delayed treatment initiation and prolonged treatment duration compared to nulliparous women and compared to remotely postpartum (Table 2 & Figure 2). Similarly, younger women (<40 years) were significantly less likely to experience prolonged treatment duration compared to older women (≥40 years), but no significant difference was observed with respect to tumor stage and delayed treatment initiation (Table 3 & Figure 3).
Table 2:
Association between breast cancer tumor stage & treatment time-related factors and recency of last childbirth among premenopausal women <50 years of age in the Carolina Breast Cancer Study, 2008–2013 (N=1179)
| Nulliparous (N=210) | 0–5 postpartum (N=160) | 5.1–10 postpartum (N=207) | 10.1–20 postpartum (N=438) | 20.1–30 postpartum (N=164) | |
|---|---|---|---|---|---|
| Stage | |||||
| I-II | 181 (86.2) | 119 (74.4) | 175 (84.5) | 365 (83.3) | 135 (82.3) |
| III | 29 (13.8) | 41 (25.6) | 32 (15.5) | 73 (16.7) | 29 (17.7) |
| III vs. I-II, Age & Race-Adjusted RFD (95% CI) | Ref. | 12.6% (4.0%, 21.1%) | 2.6% (−4.2%, 9.3%) | 3.4% (−2.4%, 9.1%) | 2.8% (−4.7%, 10.4%) |
| Fully Adjusted RFD (95% CI)a | Ref. | 12.2% (3.6%, 20.8%) | 1.8% (−4.8%, 8.5%) | 2.3% (−3.5%, 8.1%) | 1.1% (−6.5%, 8.7%) |
| Delayed Initiation | |||||
| ≤30 days | 122 (58.1) | 115 (71.9) | 130 (62.8) | 284 (64.8) | 91 (55.5) |
| >30 days | 88 (41.9) | 45 (28.1) | 77 (37.2) | 154 (35.2) | 73 (44.5) |
| >30 vs. ≤30 days, Age & Race-Adjusted RFD (95% CI) | Ref. | −14.6% (−25.1%, −4.2%) | −5.0% (−14.8%, 4.8%) | −8.4% (−16.9%, 0.1%) | −0.2% (−11.2%, 10.9%) |
| Fully Adjusted RFD (95% CI)b | Ref. | −11.2% (−21.4%, −1.0%) | −3.2% (−13.0%, 6.5%) | −7.1% (−15.4%, 1.3%) | 1.0% (−10.2%, 12.1%) |
| Prolonged Treatment Duration | |||||
| No | 113 (53.8) | 112 (70.0) | 104 (50.2) | 259 (59.1) | 88 (53.7) |
| Yes | 97 (46.2) | 48 (30.0) | 103 (49.8) | 179 (40.9) | 76 (46.3) |
| Yes vs. No, Age & Race-Adjusted RFD (95% CI) | Ref. | −17.2% (−27.8%, −6.6%) | 4.8% (−5.3%, 14.8%) | −6.0% (−14.7%, 2.6%) | −3.1% (−14.1%, 7.9%) |
| Fully Adjusted RFD (95% CI) b | Ref. | −17.5% (−28.0%, −7.1%) | 4.6% (−5.4%, 14.6%) | −5.8% (−14.5%, 2.8%) | −1.1% (−12.3%, 10.1%) |
Abbreviations: RFD, relative frequency difference; CI, confidence interval
Adjusted for age, race, income, education, marital status, and health insurance status at baseline
Adjusted for age, race, tumor stage, size, grade, lymph node status, hormone receptor status and human epidermal growth factor receptor 2 status
Figure 2: Association between treatment timing and recency of last childbirth.
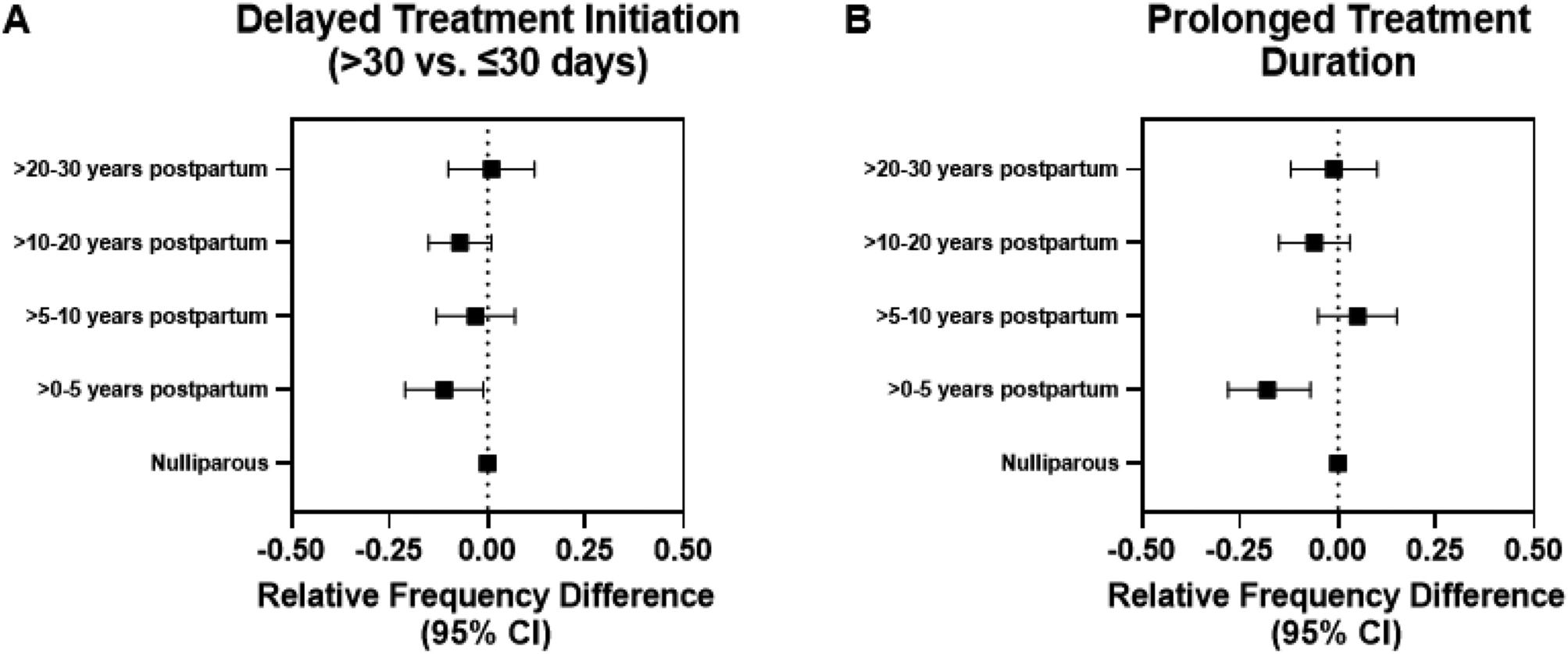
(A & B) Forest plots of relative frequency difference for treatment time-related factors by time since last childbirth, adjusted for age, race, tumor stage, size, grade, lymph node status, hormone receptor status and human epidermal growth factor receptor 2 status. CI: confidence interval.
Table 3:
Association between breast cancer stage at diagnosis & treatment-related factors and younger age at diagnosis among women 20–74 years of age in the Carolina Breast Cancer Study, 2008–2013 (N=2842)
| <40 years (N=351) | ≥40 years (N=2491) | <40 vs. ≥40 Race-Adjusted RFD (95% CI) | <40 vs. ≥40 Model 1* RFD (95% CI) | <40 vs. ≥40 Full Modela RFD (95% CI) | |
|---|---|---|---|---|---|
| Stage b | |||||
| I-II | 285 (81.2) | 2134 (85.7) | Ref. | - | Ref. |
| III | 66 (18.8) | 357 (14.3) | 3.5% (−1.0%, 7.9%) | - | 3.5% (−1.1%, 8.1%) |
| Delayed Initiation | |||||
| ≤30 days | 232 (66.1) | 1615 (64.8) | Ref. | Ref. | Ref. |
| >30 days | 119 (33.9) | 876 (35.2) | −1.2% (−6.7%, 4.3%) | −2.0% (−7.5%, 3.6%) | −1.2% (−6.8%, 4.3%) |
| Prolonged Treatment Duration | |||||
| No | 216 (61.5) | 1306 (52.4) | Ref. | Ref. | Ref. |
| Yes | 135 (38.5) | 1185 (47.6) | −8.9% (−14.6%, −3.3%) | −7.7% (−13.3%, −2.0%) | −5.6% (−11.1%, −0.0%) |
| Type of Surgery | |||||
| Mastectomy | 204 (58.3) | 1077 (43.2) | 15.1% (9.4%, 20.8%) | 10.7% (5.3%, 16.2%) | 10.6% (5.2%, 16.0%) |
| Breast-conserving surgery | 146 (41.7) | 1414 (56.8) | Ref. | Ref. | Ref. |
| Received Chemotherapy c | |||||
| Yes | 299 (85.2) | 1516 (60.9) | 22.2% (18.2%, 26.2%) | 9.1% (6.6%, 11.6%) | - |
| No | 52 (14.8) | 975 (39.1) | Ref. | Ref. | - |
| Received Radiation | |||||
| Yes | 241 (68.7) | 1835 (73.7) | −4.7% (−9.9%, 0.6%) | −5.2% (−10.4%, 0.0%) | −6.0% (−11.2%, −0.8%) |
| No | 110 (31.3) | 656 (26.3) | Ref. | Ref. | Ref. |
| Received Hormone therapy c, Φ | |||||
| Yes | 194 (85.1) | 1672 (90.4) | −5.4% (−10.4%, −0.4%) | −5.9% (−10.9%, −0.9%) | - |
| No | 34 (14.9) | 178 (9.6) | Ref. | Ref. | - |
Abbreviations: RFD, relative frequency difference; CI, confidence interval
Model 1 – Adjusted for race, tumor stage, and lymph node status
Full Model – Adjusted for race, tumor stage, size, grade, lymph node status, hormone receptor status and human epidermal growth factor receptor 2 status
Full Model for Stage – Adjusted for race, income, education, marital status, and health insurance status at baseline
Fully Adjusted model not possible due to limited cell size
Includes only ER positive and borderline cases (≥1% cell positivity)
Figure 3: Associations between tumor stage at diagnosis & treatment-related factors and young age at diagnosis.
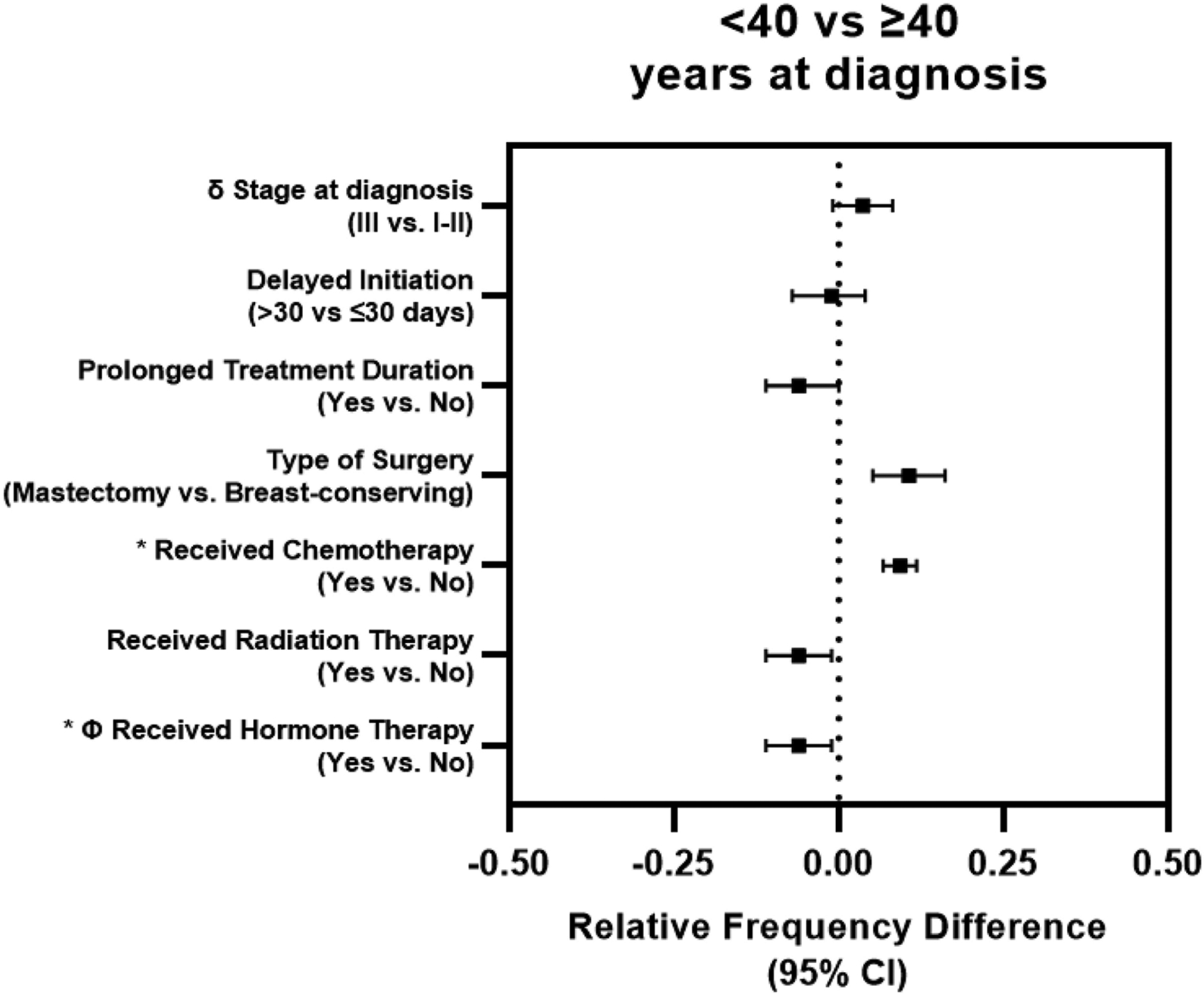
Forest plot of relative frequency difference for tumor stage at diagnosis, treatment time-related factors and treatment received between cases diagnosed at <40 vs. ≥40 years of age. δ Stage model adjusted for age, race, income, education, marital status and health insurance status at baseline. Treatment time-related factors and treatment received adjusted for race, tumor stage, size, grade, lymph node status, hormone receptor status and human epidermal growth factor receptor 2 status; * only adjusted for race, tumor stage and lymph node status due to small number of untreated cases. Φ Includes only ER positive and borderline cases (≥1% cell positivity). CI: confidence interval.
Treatment modalities
Recently postpartum women were more likely to receive more aggressive treatments compared to nulliparous women (Table 4). Considering type of surgery, the relative frequency difference for recency of birth was attenuated after adjusting for age, race, and standard clinical tumor characteristics, but women who were up to 10 years postpartum remained significantly more likely to get mastectomy (vs. breast-conserving surgery) compared to nulliparous women. Considering chemotherapy, associations with recency of birth were strongly related to tumor stage; we were unable to adjust for clinical tumor characteristics due to positivity violations, with all stage III cases in the recently pregnant group receiving chemotherapy. Thus, the association between postpartum status and chemotherapy is not independent of tumor characteristics. With respect to radiation therapy and hormone therapy (among ER positive and borderline cases only), no significant differences were observed between the recently postpartum and nulliparous group. Similarly, treatment patterns among younger vs. older women were strongly related to tumor clinical characteristics (especially, stage at diagnosis and lymph node status), with younger women being significantly more likely to received mastectomy, chemotherapy and less likely to receive radiation and hormone therapy compared to older women (Table 3 & Figure 3). Supplemental Tables 1 and 2 show the distribution of tumor characteristics by postpartum status and age, respectively. Sensitivity analyses adjusting for Area Health Education Center (AHEC) region or including cases diagnosed during pregnancy (n=7) in the recently postpartum group (n=160) did not substantially alter these results.
Table 4:
Distribution of treatment received and recency of last childbirth among premenopausal women <50 years of age in the Carolina Breast Cancer Study, 2008–2013 (N=1179)
| Nulliparous (N=210) | 0–5 postpartum (N=160) | 5.1–10 postpartum (N=207) | 10.1–20 postpartum (N=438) | 20.1–30 postpartum (N=164) | |
|---|---|---|---|---|---|
| Type of Surgery | |||||
| Mastectomy | 99 (47.1) | 106 (66.7) | 127 (61.4) | 219 (50.0) | 83 (50.6) |
| Breast-conserving surgery | 111 (52.9) | 53 (33.3) | 80 (38.6) | 219 (50.0) | 81 (49.4) |
| Mastectomy vs. Breast-conserving surgery, Age & Race-Adjusted RFD (95% CI) | Ref. | 18.2% (7.5%, 29.0%) | 14.3% (4.5%, 24.1%) | 4.5% (−4.1%, 13.1%) | 8.8% (−2.1%, 19.7%) |
| Fully Adjusted RFD (95% CI)a | Ref. | 14.9% (4.8%, 25.0%) | 15.0% (5.4%, 24.5%) | 4.1% (−4.4%, 12.5%) | 9.8% (−1.0%, 20.6%) |
| Received Chemotherapy | |||||
| Yes | 147 (70.0) | 140 (87.5) | 145 (70.0) | 333 (76.0) | 129 (78.7) |
| No | 63 (30.0) | 20 (12.5) | 62 (30.0) | 105 (24.0) | 35 (21.3) |
| Yes vs. No, Age & Race-Adjusted RFD (95% CI) | Ref. | 10.1% (1.0%, 19.3%) | 0.1% (−9.1%, 9.3%) | 6.4% (−1.3%, 14.1%) | 9.3% (−0.2%, 18.9%) |
| Received Radiation | |||||
| Yes | 146 (69.5) | 110 (68.8) | 137 (66.2) | 327 (74.7) | 118 (72.0) |
| No | 64 (30.5) | 50 (31.2) | 70 (33.8) | 111 (25.3) | 46 (28.0) |
| Yes vs. No, Age & Race-Adjusted RFD (95% CI) | Ref. | −1.5% (−11.4%, 8.3%) | −2.9% (−12.1%, 6.3%) | 2.8% (−4.7%, 10.4%) | −2.5% (−12.3%, 7.2%) |
| Fully Adjusted RFD (95% CI) a | Ref. | −3.4% (−13.6%, 6.8%) | −4.9% (−13.4%, 3.7%) | −0.9% (−8.0%, 6.3%) | −5.8% (−14.8%, 3.1%) |
| Received Hormone therapy Φ | |||||
| Yes | 143 (87.2) | 94 (87.0) | 138 (89.0) | 279 (87.7) | 84 (87.5) |
| No | 21 (12.8) | 14 (13.0) | 17 (11.0) | 39 (12.3) | 12 (12.5) |
| Yes vs. No, Age & Race-Adjusted RFD (95% CI) | Ref. | 1.6% (−6.4%, 9.6%) | 3.3% (−3.8%, 10.4%) | 1.2% (−4.9%, 7.4%) | 1.3% (−7.2%, 9.7%) |
Abbreviations: RFD, relative frequency difference; CI, confidence interval
Adjusted for age, race, tumor stage, size, grade, lymph node status, hormone receptor status and human epidermal growth factor receptor 2 status
Includes only ER positive and borderline cases (≥1% cell positivity)
Discussion
In the Carolina Breast Cancer Study Phase III, conducted between 2008–2013, recently postpartum women had prompt and aggressive post-diagnostic treatment, at least in part due to more aggressive clinical tumor characteristics (i.e., later stage and lymph node positivity). Recently pregnant women tended to have later stage at diagnosis, prompt treatment initiation, shorter treatment duration, and were more likely to receive mastectomy (vs. breast-conserving surgery) and chemotherapy. These trends were mirrored by patterns in young-onset (<40 years of age) breast cancer cases. These findings suggest that patterns of poorer outcomes for recently pregnant and younger women are not driven by undertreatment or treatment delays.
Only one previous study has examined treatment initiation delay with respect to recency of pregnancy; however, that study compared pregnant vs. postpartum breast cancer cases and reported delayed initiation among pregnant women [3]. No previous studies have evaluated treatment timelines among postpartum women or comparing postpartum women to nulliparous women. Similarly, few studies have investigated treatment modality among recently pregnant women [1–4]. The majority of these studies found no significant association between treatment modality such as receipt of chemotherapy [1–3], radiation [1, 4] and surgery [1–4], and recency of pregnancy. However, previous studies included pregnant women[54, 55], for whom treatment plans must address risk to both mother and child, and therefore are difficult to interpret relative to our analysis of premenopausal breast cancer among postpartum and nulliparous women. The current findings separating the postpartum period add resolution to the unique experience of this group.
Recent pregnancy and younger age both were associated with treatment timeliness and modality, but the higher incidence of more aggressive tumors appeared to drive the shift in treatment patterns for both groups. We were not able to examine these relationships separately by race due to limited number of recently postpartum cases. We were also not able to evaluate differences in specific chemotherapy regimens. Future research should address whether social support, childcare needs, or other treatment-related “workload” experiences by younger women influence their reported quality of life and stress levels. Our results show that worse breast cancer outcomes among younger and recently postpartum women are unlikely to be related to post-diagnostic undertreatment.
Supplementary Material
ACKNOWLEDGEMENTS
This research was supported by a grant from UNC Lineberger Comprehensive Cancer Center, which is funded by the University Cancer Research Fund of North Carolina, the Susan G. Komen Foundation (OGUNC1202), the Komen Graduate Training in Disparities Research Grant (GTDR16381071), the National Cancer Institute of the National Institutes of Health (P01CA151135), and the National Cancer Institute Specialized Program of Research Excellence (SPORE) in Breast Cancer (NIH/NCI P50-CA58223). This research recruited participants &/or obtained data with the assistance of Rapid Case Ascertainment, a collaboration between the North Carolina Central Cancer Registry and UNC Lineberger. RCA is supported by a grant from the National Cancer Institute of the National Institutes of Health (P30CA016086). We are grateful to CBCS participants and study staff. We also acknowledge the late Robert C. Millikan, founder of the CBCS Phase III.
Funding:
Sanah N. Vohra was supported by the University Cancer Research Fund of North Carolina, the Komen Graduate Training in Disparities Research Grant (GTDR16381071), the Doctoral Degree Advancement Award by the University of North Carolina (UNC) - Initiative for Minority Excellence, and the UNC Cancer Control Education Program (T32CA057726). Melissa A. Troester was supported by the National Cancer Institute of the National Institutes of Health (P01CA151135), and the National Cancer Institute Specialized Program of Research Excellence (SPORE) in Breast Cancer (NIH/NCI P50-CA58223). The Carolina Breast Cancer Study was funded by the University Cancer Research Fund of North Carolina and Susan G. Komen for the Cure (OGUNC1202).
Footnotes
Publisher's Disclaimer: This AM is a PDF file of the manuscript accepted for publication after peer review, when applicable, but does not reflect post-acceptance improvements, or any corrections. Use of this AM is subject to the publisher’s embargo period and AM terms of use. Under no circumstances may this AM be shared or distributed under a Creative Commons or other form of open access license, nor may it be reformatted or enhanced, whether by the Author or third parties. See here for Springer Nature’s terms of use for AM versions of subscription articles: https://www.springernature.com/gp/open-research/policies/accepted-manuscript-terms
Conflicts of interest/Competing interests: The authors declare that they have no conflict of interest.
Ethics approval: All study protocols were approved by the Office of Human Research Ethics, Institutional Review Board at the University of North Carolina at Chapel Hill (UNC).
Consent to participate: Written informed consent was obtained from all individual participants included in the study prior to data collection.
Consent for publication: All participants consented to the submission of study results for journal publication.
Availability of data and material:
For participant confidentiality and due to ethical restrictions, data are available upon request and are subject to data use agreements and other stipulations. Permission to access data from the Carolina Breast Cancer Study may be obtained online (https://unclineberger.org/cbcs/) or by contacting the authors.
References
- 1.Azim HA Jr., et al. , The biological features and prognosis of breast cancer diagnosed during pregnancy: a case-control study. Acta Oncol, 2012. 51(5): p. 653–61. [DOI] [PubMed] [Google Scholar]
- 2.Madaras L, et al. , Clinicopathological features and prognosis of pregnancy associated breast cancer - a matched case control study. Pathol Oncol Res, 2014. 20(3): p. 581–90. [DOI] [PubMed] [Google Scholar]
- 3.Mathelin C, et al. , Pregnancy and post-partum breast cancer: a prospective study. Anticancer Res, 2008. 28(4c): p. 2447–52. [PubMed] [Google Scholar]
- 4.Rodriguez AO, et al. , Evidence of poorer survival in pregnancy-associated breast cancer. Obstet Gynecol, 2008. 112(1): p. 71–8. [DOI] [PubMed] [Google Scholar]
- 5.Callihan EB, et al. , Postpartum diagnosis demonstrates a high risk for metastasis and merits an expanded definition of pregnancy-associated breast cancer. Breast Cancer Res Treat, 2013. 138(2): p. 549–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dimitrakakis C, et al. , Does pregnancy-associated breast cancer imply a worse prognosis? A matched case-case study. Breast Care (Basel), 2013. 8(3): p. 203–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ali SA, et al. , Survival outcomes in pregnancy associated breast cancer: a retrospective case control study. Breast J, 2012. 18(2): p. 139–44. [DOI] [PubMed] [Google Scholar]
- 8.Johansson AL, et al. , Increased mortality in women with breast cancer detected during pregnancy and different periods postpartum. Cancer Epidemiol Biomarkers Prev, 2011. 20(9): p. 1865–72. [DOI] [PubMed] [Google Scholar]
- 9.Moreira WB, et al. , Prognosis for patients diagnosed with pregnancy-associated breast cancer: a paired case-control study. Sao Paulo Med J, 2010. 128(3): p. 119–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Azim HA Jr., et al. , Prognosis of pregnancy-associated breast cancer: a meta-analysis of 30 studies. Cancer Treat Rev, 2012. 38(7): p. 834–42. [DOI] [PubMed] [Google Scholar]
- 11.Dodds L, et al. , Relationship of time since childbirth and other pregnancy factors to premenopausal breast cancer prognosis. Obstet Gynecol, 2008. 111(5): p. 1167–73. [DOI] [PubMed] [Google Scholar]
- 12.Trivers KF, et al. , Association between reproductive factors and breast cancer survival in younger women. Breast Cancer Res Treat, 2007. 103(1): p. 93–102. [DOI] [PubMed] [Google Scholar]
- 13.Bladström A, Anderson H, and Olsson H, Worse survival in breast cancer among women with recent childbirth: results from a Swedish population-based register study. Clin Breast Cancer, 2003. 4(4): p. 280–5. [DOI] [PubMed] [Google Scholar]
- 14.Perou CM, et al. , Molecular portraits of human breast tumours. Nature, 2000. 406(6797): p. 747–752. [DOI] [PubMed] [Google Scholar]
- 15.Schedin P, Pregnancy-associated breast cancer and metastasis. Nat Rev Cancer, 2006. 6(4): p. 281–91. [DOI] [PubMed] [Google Scholar]
- 16.Maggard MA, et al. , Do young breast cancer patients have worse outcomes? J Surg Res, 2003. 113(1): p. 109–13. [DOI] [PubMed] [Google Scholar]
- 17.Han W, et al. , Young age: an independent risk factor for disease-free survival in women with operable breast cancer. BMC Cancer, 2004. 4: p. 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.El Saghir NS, et al. , Effects of young age at presentation on survival in breast cancer. BMC Cancer, 2006. 6: p. 194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anderson WF, et al. , Qualitative age interactions (or effect modification) suggest different cancer pathways for early-onset and late-onset breast cancers. Cancer Causes Control, 2007. 18(10): p. 1187–98. [DOI] [PubMed] [Google Scholar]
- 20.Anders CK, et al. , Young age at diagnosis correlates with worse prognosis and defines a subset of breast cancers with shared patterns of gene expression. J Clin Oncol, 2008. 26(20): p. 3324–30. [DOI] [PubMed] [Google Scholar]
- 21.Anders CK, et al. , Breast cancer before age 40 years. Semin Oncol, 2009. 36(3): p. 237–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fredholm H, et al. , Breast cancer in young women: poor survival despite intensive treatment. PLoS One, 2009. 4(11): p. e7695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chollet-Hinton L, et al. , Breast cancer biologic and etiologic heterogeneity by young age and menopausal status in the Carolina Breast Cancer Study: a case-control study. Breast Cancer Res, 2016. 18(1): p. 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goddard ET, et al. , Association Between Postpartum Breast Cancer Diagnosis and Metastasis and the Clinical Features Underlying Risk. JAMA Netw Open, 2019. 2(1): p. e186997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beadle BM, et al. , The impact of pregnancy on breast cancer outcomes in women<or=35 years. Cancer, 2009. 115(6): p. 1174–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Halaska MJ, et al. , Presentation, management and outcome of 32 patients with pregnancy-associated breast cancer: a matched controlled study. Breast J, 2009. 15(5): p. 461–7. [DOI] [PubMed] [Google Scholar]
- 27.Pilewskie M, et al. , Association between recency of last pregnancy and biologic subtype of breast cancer. Ann Surg Oncol, 2012. 19(4): p. 1167–73. [DOI] [PubMed] [Google Scholar]
- 28.Asztalos S, et al. , High incidence of triple negative breast cancers following pregnancy and an associated gene expression signature. Springerplus, 2015. 4: p. 710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Collins LC, et al. , Molecular Phenotype of Breast Cancer According to Time Since Last Pregnancy in a Large Cohort of Young Women. Oncologist, 2015. 20(7): p. 713–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Genin AS, et al. , Pregnancy-associated breast cancers: do they differ from other breast cancers in young women? Breast, 2012. 21(4): p. 550–5. [DOI] [PubMed] [Google Scholar]
- 31.Nagatsuma AK, et al. , Impact of recent parity on histopathological tumor features and breast cancer outcome in premenopausal Japanese women. Breast Cancer Res Treat, 2013. 138(3): p. 941–50. [DOI] [PubMed] [Google Scholar]
- 32.Polyak K, Pregnancy and breast cancer: The other side of the coin. Cancer Cell, 2006. 9(3): p. 151–153. [DOI] [PubMed] [Google Scholar]
- 33.Petrek JA, Dukoff R, and Rogatko A, Prognosis of pregnancy-associated breast cancer. Cancer, 1991. 67(4): p. 869–72. [DOI] [PubMed] [Google Scholar]
- 34.Ishida T, et al. , Clinicopathologic characteristics and prognosis of breast cancer patients associated with pregnancy and lactation: analysis of case-control study in Japan. Jpn J Cancer Res, 1992. 83(11): p. 1143–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Petrek JA, Breast cancer during pregnancy. Cancer, 1994. 74(S1): p. 518–527. [DOI] [PubMed] [Google Scholar]
- 36.Lambe M and Ekbom A, Cancers coinciding with childbearing: delayed diagnosis during pregnancy? Bmj, 1995. 311(7020): p. 1607–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.DiFronzo LA and O’Connell TX, Breast cancer in pregnancy and lactation. Surg Clin North Am, 1996. 76(2): p. 267–78. [DOI] [PubMed] [Google Scholar]
- 38.Puckridge PJ, et al. , Breast cancer and pregnancy: a diagnostic and management dilemma. ANZ J Surg, 2003. 73(7): p. 500–3. [DOI] [PubMed] [Google Scholar]
- 39.Woo JC, Yu T, and Hurd TC, Breast cancer in pregnancy: a literature review. Arch Surg, 2003. 138(1): p. 91–8; discussion 99. [DOI] [PubMed] [Google Scholar]
- 40.Son EJ, Oh KK, and Kim EK, Pregnancy-associated breast disease: radiologic features and diagnostic dilemmas. Yonsei Med J, 2006. 47(1): p. 34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Beyer I, et al. , Breast Lesions during Pregnancy - a Diagnostic Challenge: Case Report. Breast Care (Basel), 2015. 10(3): p. 207–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vohra SN, et al. , Molecular and clinical characterization of postpartum-associated breast cancer in the Carolina Breast Cancer Study Phase I-III, 1993–2013. Cancer Epidemiol Biomarkers Prev, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Emerson MA, et al. , Integrating access to care and tumor patterns by race and age in the Carolina Breast Cancer Study, 2008–2013. Cancer Causes Control, 2020. 31(3): p. 221–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Emerson MA, et al. , Breast cancer treatment delays by socioeconomic and health care access latent classes in Black and White women. Cancer, 2020. 126(22): p. 4957–4966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ho PJ, et al. , Impact of delayed treatment in women diagnosed with breast cancer: A population-based study. Cancer Med, 2020. 9(7): p. 2435–2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Millikan RC, et al. , Epidemiology of basal-like breast cancer. Breast Cancer Res Treat, 2008. 109(1): p. 123–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Newman B, et al. , The Carolina Breast Cancer Study: integrating population-based epidemiology and molecular biology. Breast Cancer Res Treat, 1995. 35(1): p. 51–60. [DOI] [PubMed] [Google Scholar]
- 48.Hair BY, et al. , Racial differences in physical activity among breast cancer survivors: implications for breast cancer care. Cancer, 2014. 120(14): p. 2174–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McGee SA, et al. , Determinants of breast cancer treatment delay differ for African American and White women. Cancer Epidemiol Biomarkers Prev, 2013. 22(7): p. 1227–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bleicher RJ, et al. , Time to Surgery and Breast Cancer Survival in the United States. JAMA Oncology, 2016. 2(3): p. 330–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Society, A.C. Chemotherapy for Breast Cancer. February 2nd, 2021]; Available from: https://www.cancer.org/cancer/breast-cancer/treatment/chemotherapy-for-breast-cancer.html.
- 52.Society, A.C., Radiation for Breast Cancer.
- 53.Spiegelman D and Hertzmark E, Easy SAS calculations for risk or prevalence ratios and differences. Am J Epidemiol, 2005. 162(3): p. 199–200. [DOI] [PubMed] [Google Scholar]
- 54.Antonelli NM, et al. , Cancer in pregnancy: a review of the literature. Part I. Obstet Gynecol Surv, 1996. 51(2): p. 125–34. [DOI] [PubMed] [Google Scholar]
- 55.Helewa M, et al. , Breast cancer, pregnancy, and breastfeeding. J Obstet Gynaecol Can, 2002. 24(2): p. 164–80; quiz 181–4. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
For participant confidentiality and due to ethical restrictions, data are available upon request and are subject to data use agreements and other stipulations. Permission to access data from the Carolina Breast Cancer Study may be obtained online (https://unclineberger.org/cbcs/) or by contacting the authors.


