Abstract
Background:
Extrinsic control of cardiomyocyte metabolism is poorly understood in heart failure. Fibroblast growth factor-21 (FGF21), a hormonal regulator of metabolism produced mainly in the liver and adipose tissue, is a prime candidate for such signaling.
Methods:
To investigate this further, we examined blood and tissue obtained from human subjects with end-stage heart failure with reduced ejection fraction (HFrEF) at the time of left ventricular assist device (LVAD) implantation, and correlated serum FGF21 levels with cardiac gene expression, immunohistochemistry, and clinical parameters.
Results:
Circulating FGF21 levels were substantially elevated in HFrEF, compared to healthy subjects (HFrEF: 834.4 [95% confidence interval: 628.4, 1040.3] pg/mL, n = 40; controls: 146.0 [86.3, 205.7] pg/mL, n = 20, p = 1.9 × 10−5). There was clear FGF21 staining in diseased cardiomyocytes, and circulating FGF21 levels negatively correlated with the expression of cardiac genes involved in ketone metabolism, consistent with cardiac FGF21 signaling. FGF21 gene expression was very low in failing and non-failing hearts, suggesting extracardiac production of the circulating hormone. Circulating FGF21 levels were correlated with BNP and total bilirubin, markers of chronic cardiac and hepatic congestion.
Conclusions:
Circulating FGF21 levels are elevated in HFrEF and appear to bind to the heart. The liver is likely the main extracardiac source. This supports a model of hepatic FGF21 communication to diseased cardiomyocytes, defining a potential cardio-hepatic signaling circuit in human heart failure.
Keywords: fibroblast growth factor, congestive hepatopathy, venous congestion, ketone metabolism, left ventricular assist device, BDH1, total bilirubin, B-type natriuretic peptide
Subject terms: Heart failure, cardiomyopathy
INTRODUCTION
A central feature of heart failure across different etiologies is a profound alteration in cardiomyocyte metabolism1. In heart failure with reduced ejection fraction (HFrEF), notable changes include increased reliance on glucose, ketones, and short-chain fatty acids, reduced pyruvate uptake by mitochondria and a consequent shunting of glycolysis towards the pentose-phosphate pathway2–5. Such adaptations are associated with altered expression of cardiomyocyte genes involved in the transport and metabolism of these different substrates. Some of these changes are dependent on cardiomyocyte-intrinsic signaling pathways triggered by cardiac dysfunction, but extrinsic control of cardiomyocyte metabolism is not well understood.
To explore extrinsic cardiometabolic signaling, we focused on fibroblast growth factor-21 (FGF21). This cytokine of the FGF19/21/23 family is produced primarily in the liver and adipose tissue, and is a potent regulator of fuel utilization and metabolism. Due to the absence of heparin-binding domains, secreted FGF21 can travel into the bloodstream and act as a hormone, binding to a receptor complex composed of a tyrosine kinase FGF receptor isoform and the β-Klotho (KLB) co-receptor. FGF21 regulates fatty acid oxidation in the liver, insulin sensitivity, glucose metabolism in adipose cells, and ketone usage6. In humans, FGF21 has been explored as a metabolic biomarker. In healthy subjects, it is induced late during the adaptive response to starvation7, but also during short-term carbohydrate overfeeding8, alcohol consumption9, and cold-induced thermogenesis8. Increased hepatic and adipose secretion is widely noted in diabetes and obesity, and skeletal muscle expression is noted after exercise or in hyperinsulemic states10–14.
FGF21 signaling appears to be protective in several animal models of cardiac disease, possibly by direct action on the heart15–17. However, FGF21 signaling in heart disease remains unresolved in humans. In particular, whereas FGF21 appears protective in most animal models of heart disease, elevated levels are poor prognostic indicators in humans. In two studies of diabetic patients with coronary disease, higher levels of FGF21 were predictive of poorer outcomes18, 19. In cardiomyopathies, FGF21 elevations predicted adverse events in both heart failure with reduced or preserved ejection fraction, though >40% of patients in both these studies had diabetes20, 21. Additionally, it is unclear whether the elevated blood FGF21 is synthesized in the heart itself or is produced in other organs. In mouse studies, FGF21 appears to be synthesized in diseased or metabolically-altered hearts, but not healthy cardiomyocytes, though cardiac expression was not seen in other studies15, 16, 22–24. Human data is scant, with one study showing an increase in FGF21 transcripts and two showing FGF21 staining in cardiomyocytes in alcoholic cardiomyopathy or hypertensive heart disease23, 25, 26. Thus, the extent of FGF21 elevation during HFrEF, what signals trigger this elevation, and whether it is active in the heart, remain open questions. Here, to investigate human cardiac FGF21 biology independent of its well-established elevation during diabetes, we took advantage of cardiac tissue collection during the implantation of left ventricular assist devices (LVAD) in non-diabetic patients with end-stage HFrEF.
METHODS
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Study Population
The HFrEF cohort was taken from a larger sample of patients enrolled at the time of LVAD implantation. Patients who required LVAD support due to acute heart failure (acute myocardial infarction, acute myocarditis, and others) were prospectively excluded. Patients (age ≥ 18-years) were consecutively enrolled in institutions comprising the Utah Transplantation Affiliated Hospitals (U.T.A.H.) Cardiac Transplant Program (University of Utah Health Science Center, Intermountain Medical Center, and the Veterans Administration Salt Lake City Health Care System) with clinical characteristics consistent with dilated cardiomyopathy and chronic advanced heart failure, who required circulatory support with continuous-flow LVAD. Non-failing donor hearts, not allocated for heart transplantation due to non-cardiac reasons (size, infection, and others) were used as controls. The study was approved by the institutional review board of the participating institutions, and informed consent was provided by all patients.
In the current study, we selected patients from the overall study who had blood samples available and who did not have diabetes (clinical diagnosis or hemoglobin A1c < 6.5%), overt renal failure (creatinine < 1.2 mg/dL), a clinical diagnosis of non-alcoholic fatty liver disease or viral hepatitis.
Blood samples used as the control reference were from healthy subjects recruited from the University of Utah and the surrounding Mountain West states. We obtained 20 samples of the same gender and within 2 years of the age to samples from our heart failure cohort. Healthy donors were medication free and without acute or chronic illnesses. All subjects provided written, informed consent and all study protocols were IRB approved.
Blood and Myocardial Tissue Acquisition
Myocardial tissue was prospectively collected from the LV apical core at the time of LVAD implantation and was frozen before storage at −80 °C. Control samples were acquired from hearts that were not transplanted due to non-cardiac reasons. Donor LV apical tissue was harvested and processed the same way as the failing hearts. For HFrEF and donor samples, blood was collected immediately prior to the beginning of the operation. For healthy control samples, blood was collected by venipuncture. Samples were centrifuged and serum or plasma collected and stored for later analysis.
Clinical Data Collection
Donor information like age, sex, and cause of death were collected with the help of DonorConnect. For HFrEF patients, clinical data including demographics, comorbidities, echocardiographic parameters, laboratory results, and other clinical data were collected within one week before LVAD implantation using our institutional research electronic data capture system (REDCap). We followed the echocardiographic data of each participant after surgery for up to 12 months. Based on left ventricular functional and structural changes following at least 3 months on LVAD support, patients were categorized as either responder or non-responder (see definitions in Results).
Blood FGF21 measurements
FGF21 levels were measured using the Quantikine ELISA Human FGF21 kit (R&D Systems, Minneapolis).
Immunohistochemistry
Formalin-fixed, paraffin-embedded cardiac tissue pieces were stained with anti-FGF21 antibody (Abcam, ab171941).
Cardiac FGF21 gene expression measurement
RNA isolation and cDNA synthesis from LV tissue samples were performed using commercially-available kits, and quantitative PCR was performed with Power SYBR Green PCR Master Mix (Thermo) and gene-specific primers.
Statistics
Distributions were checked for normality visually, and skewed distributions were transformed by a base 10 logarithm to make them normal. Student’s t-test with unpaired, unequal variance samples were used for two-sample comparisons, assuming significance for p < 0.05. Correlations were performed using linear regression, with p-values for the coefficient of determination (r2) calculated from an analysis of variance using the F-statistic. For multiple hypothesis testing, a Benjamini-Hochberg procedure was used to derive corrected p-values (q-values) using a false discovery rate of 0.05. Analyses were performed in Excel and OriginPro 2020.
Additional methodological details are available in supplemental information.
RESULTS
FGF21 is elevated in end-stage HFrEF
We retrospectively analyzed serum samples, obtained at the time of left ventricular assist device (LVAD) implantation, in 40 patients with ischemic and nonischemic HFrEF. Because we specifically wanted to determine the effect of heart failure on FGF21 levels, none of these patients had diabetes, end-stage renal failure, viral hepatitis, or non-alcoholic fatty liver disease (NAFLD), conditions known to alter FGF21 levels11, 27–29. A description of patient characteristics is included in Table 1. For controls, we utilized plasma from 20 healthy controls.
Table 1.
Characteristics of study population
| Heart Failure | Controls | Donors | |
|---|---|---|---|
| N | 40 | 20 | 9 |
| Age (years) | 48 [38, 54] | 48 [39, 57] | 44 [38, 50] |
| Women | 15 (37.5%) | 11 (55%) | 5 (55.6%) |
| Hispanic/Black/White/Other | 3/2/33/2 | NA | 0/0/8/1 |
| Smoker | 17 (42.5%) | NA | NA |
| Body Mass Index (kg/m2) | 26 [22, 28] | NA | 25 [22, 28] |
| Left ventricular ejection fraction (%) | 19 [17, 21] | NA | 60 [55, 66] |
| Left ventricular end-diastolic diameter (cm) | 6.5 [6.3, 6.7] | NA | 4.1 [3.7, 4.4] |
| Ischemic HFrEF | 12 (30%) | 0 | 0 |
| Non-ischemic HFrEF: | 28 (70%) | 0 | 0 |
| B-type natriuretic peptide (pg/mL) | 1440 [1027, 1853] | NA | NA |
| Hemoglobin A1C | 5.7 [5.6, 5.8] | NA | 5.4 [5.1, 5.7] |
| Serum creatinine (mg/dL) | 0.9 [0.8, 0.9] | NA | 0.9 [0.6, 1.2] |
| Blood urea nitrogen (mg/dL) | 17.9 [15.7, 20.1] | NA | 16.3 [8.8, 23.9] |
| Aspartate aminotransferase (Units/L) | 45.6 [34.1, 57.0] | NA | 99.8 [34.8, 164.7] |
| Albumin (g/dL) | 3.7 [3.5, 3.9] | NA | 2.7 [2.4, 3.1] |
| Total bilirubin (mg/dL) | 1.2 [1.0, 1.5] | NA | 1.0 [0.7, 1.3] |
| Triglycerides (mg/dL) | 107.1 [91.3, 122.9] | NA | NA |
| Total Cholesterol (mg/dL) | 139.7 [123.7, 155.6] | NA | NA |
| Central venous pressure (mmHg) | 12 [10, 14] | NA | 9 [6, 11] |
| Mean pulmonary arterial pressure (mmHg) | 35 [32, 39] | NA | NA |
| Pulmonary capillary wedge pressure (mmHg) | 24 [21, 27] | NA | NA |
| Beta-blocker | 22 (55%) | NA | NA |
| ACE inhibitor | 20 (50%) | NA | NA |
| Angiotensin receptor blocker | 4 (10%) | NA | NA |
| Aldosterone Blocker | 19 (47.5%) | NA | NA |
| Diuretics | 37 (92.5%) | NA | NA |
| Aspirin | 17 (42.5%) | NA | NA |
| Clopidogrel | 3 (7.5%) | NA | NA |
| Anti-arrhythmics | 14 (35%) | NA | NA |
Values are counts or mean [95% confidence intervals]. NA: not available
Circulating FGF21 levels were more than fivefold higher in HFrEF patients compared to controls (834.4 [628.4, 1040.3] pg/mL vs. 146.0 [86.2, 205.7] pg/mL, mean [two-sided 95% confidence interval], Figure 1A). In prior studies of FGF21 in human cardiomyopathies, a substantial fraction of patients had diabetes, a comorbidity expected to raise FGF21. Our results here show the increase in FGF21 during HFrEF is not due only to concurrent diabetes or NAFLD. Within the HFrEF group, no significant difference was observed in serum FGF21 between females and males (822.7 [487.0, 1158.4] pg/mL vs. 841.4 [561.7, 1121.1] pg/mL, Figure 1B), or between ischemic and non-ischemic etiologies (691.1 [396.4, 985.9] pg/mL vs. 895.8 [622.1, 1169.4] pg/mL, Figure 1C). There was no correlation with the age of the subject (Figure 1D).
Figure 1. Circulating FGF21 levels are elevated in HFrEF.
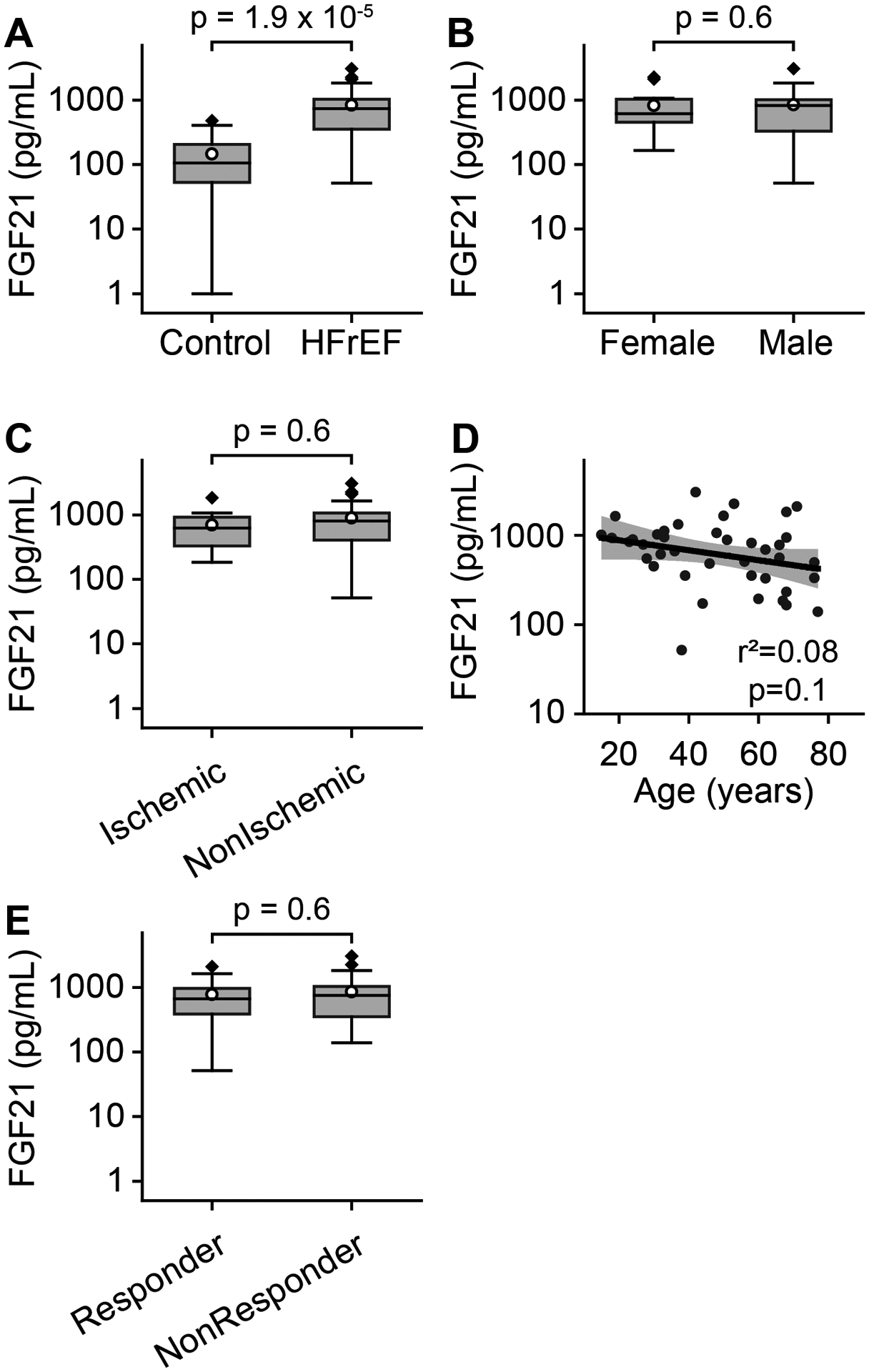
A. FGF21 levels in healthy controls (n=20) versus patients with heart failure with reduced ejection fraction (HFrEF, n=40) displayed as Tukey boxplots, with mean shown as white circle. Note logarithmic scales. Student’s t-test used for two-sample comparisons. B. No significant difference in FGF21 levels across gender in HFrEF patients (female = 15, male = 25). C. No significant difference in FGF21 levels between ischemic (n=12) and non-ischemic (n=28) HFrEF. D. No correlation with age of patient. Black line represents linear regression fit to the data, with shaded area representing 95% confidence bands. E. No significant difference in FGF21 between responders (n=18) and non-responders (n=22). Coefficient of determination (r2) and corresponding p value are shown.
In a subset of patients with mechanically-unloaded failing hearts, cardiac structure and function improves to the point that some of these patients can be weaned from mechanical support, and we assessed whether FGF21 level might predict such myocardial function improvement. In our cohort, recovery was defined as an improvement in left ventricular ejection fraction to >40% and reduction in left ventricular end-diastolic diameter to ≤59 mm. However, there was no significant difference in serum FGF21 between patients who recovered left ventricular function during mechanical unloading (responder, 774.2 [395.8, 1152.5] pg/mL, Figure 1E) and those that did not (non-responder, 860.2 [599.5, 1120.9] pg/mL).
FGF21 present in the heart appears to have substantial extra-cardiac synthesis.
Next, we addressed whether FGF21 is found within heart tissue in HFrEF, and whether the elevated serum levels are due to cardiac FGF21 synthesis. To address the first question, we stained cardiac sections with anti-FGF21 antibodies in a subset of the HFrEF patients. As control, we examined cardiac sections in structurally intact hearts obtained from non-failing donors, but unused for human heart transplantation due to non-cardiac reasons. Although hearts in these donors were structurally and functionally normal, the donors themselves were deceased due to traumatic or anoxic brain injury, critical illnesses in which circulating FGF21 levels have been found to be elevated30–33. Our results replicated these findings, with donor serum FGF21 levels elevated (809.8 [185.9, 1433.7] pg/mL, n = 5) as in HFrEF serum. This set of conditions allows us to clearly identify if cardiac dysfunction leads to FGF21 signaling. In fact, whereas sections from donors showed essentially no cardiac FGF21 staining, sections obtained from HFrEF patients showed robust labeling throughout cardiomyocytes (Figure 2). This difference in cardiomyocyte staining is not due to altered levels of circulating FGF21, as serum levels were similarly elevated in both donor and HFrEF subjects. Rather, this result establishes that the failing heart is preferentially primed for FGF21-mediated metabolic signals.
Figure 2. FGF21 is present in cardiomyocytes from HFrEF patients.
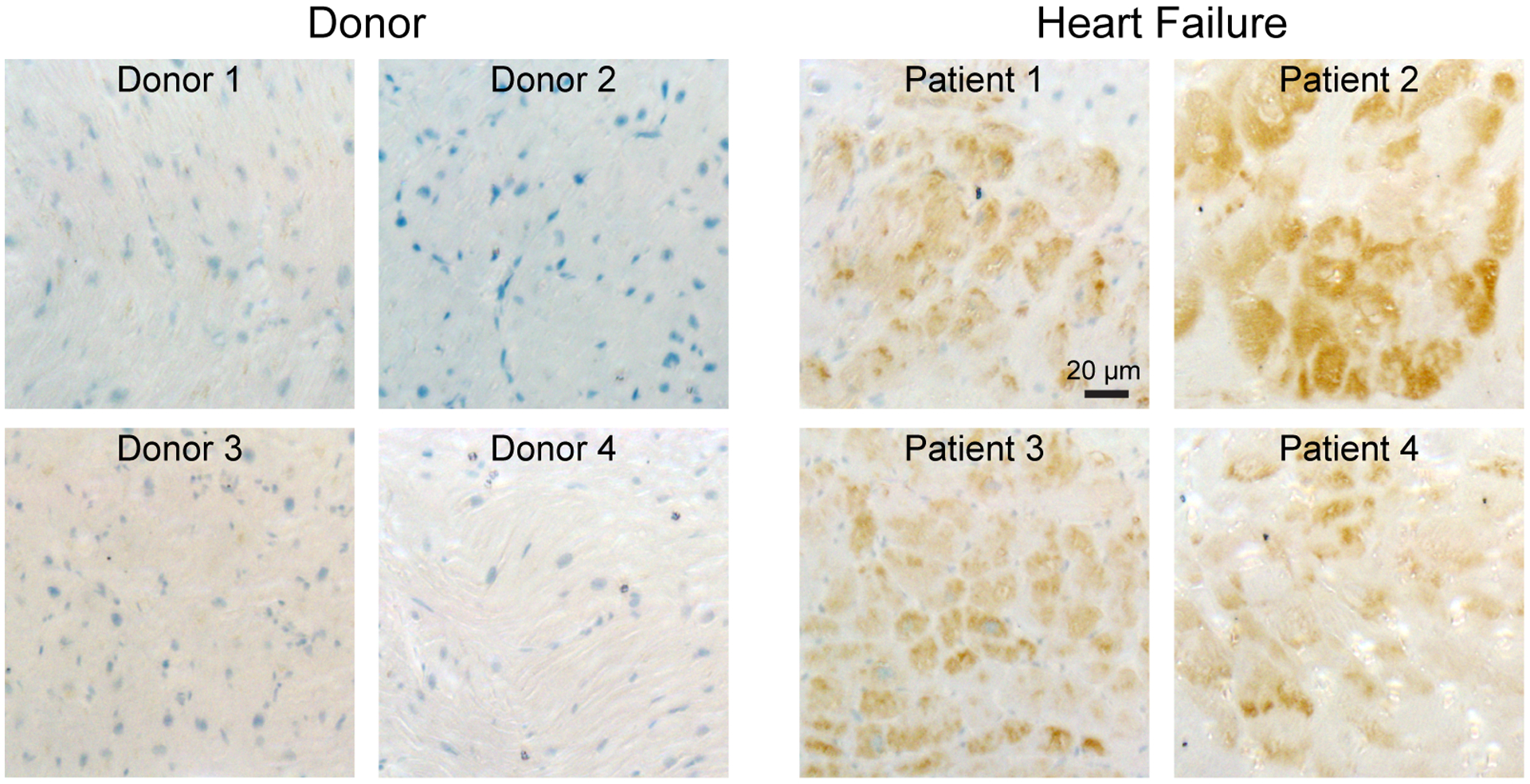
Immunostaining for FGF21 in human heart slices reveals little FGF21 present in donor hearts but robust staining in HFrEF hearts. Nuclei are labelled in blue and FGF21 immunostaining is brown. Each panel is from a different patient.
Next, we addressed whether the FGF21 staining seen in heart sections represented a fraction bound from the elevated circulating levels or protein synthesized within cardiomyocytes. To address this issue, we assessed the expression of cardiac genes related to FGF21 signaling (Figure 3). Because the limited amounts of tissue obtained per patient are used in various assays across multiple studies, precluding Western blot analyses of protein levels, we restricted our analysis to measuring transcript levels via quantitative reverse-transcriptase polymerase chain reaction (qPCR). When we examined FGF21 transcripts with qPCR, we found expression was low, near the limits of detection. Moreover, there was no clear change in expression between donor and HFrEF patients, nor any correlation with serum FGF21 levels (Figure 3), suggesting the FGF21 gene may have low cardiac expression. To further assess cardiac FGF21 transcription, we examined 7 published RNA-seq datasets obtained from cardiac tissue in human patients with ischemic, non-ischemic, restrictive, and hypertrophic cardiomyopathies (Table 2)34–40. FGF21 transcripts were detectable in only 14 out of 167 samples, primarily in cardiomyopathy samples, and in most of these cases corresponded to 1–2 reads. Given limited cardiac FGF21 synthesis, it appears elevated circulating FGF21 during HFrEF may have a primarily extracardiac source. However, though FGF21 is synthesized elsewhere, it clearly signals to the heart, given the robust FGF21 staining we found in cardiac tissue in HFrEF, but not donors. Taken together, these results reveal an unexpected metabolic signaling axis to the heart from organs synthesizing FGF21.
Figure 3. Correlations between serum FGF21 and cardiac gene expression.
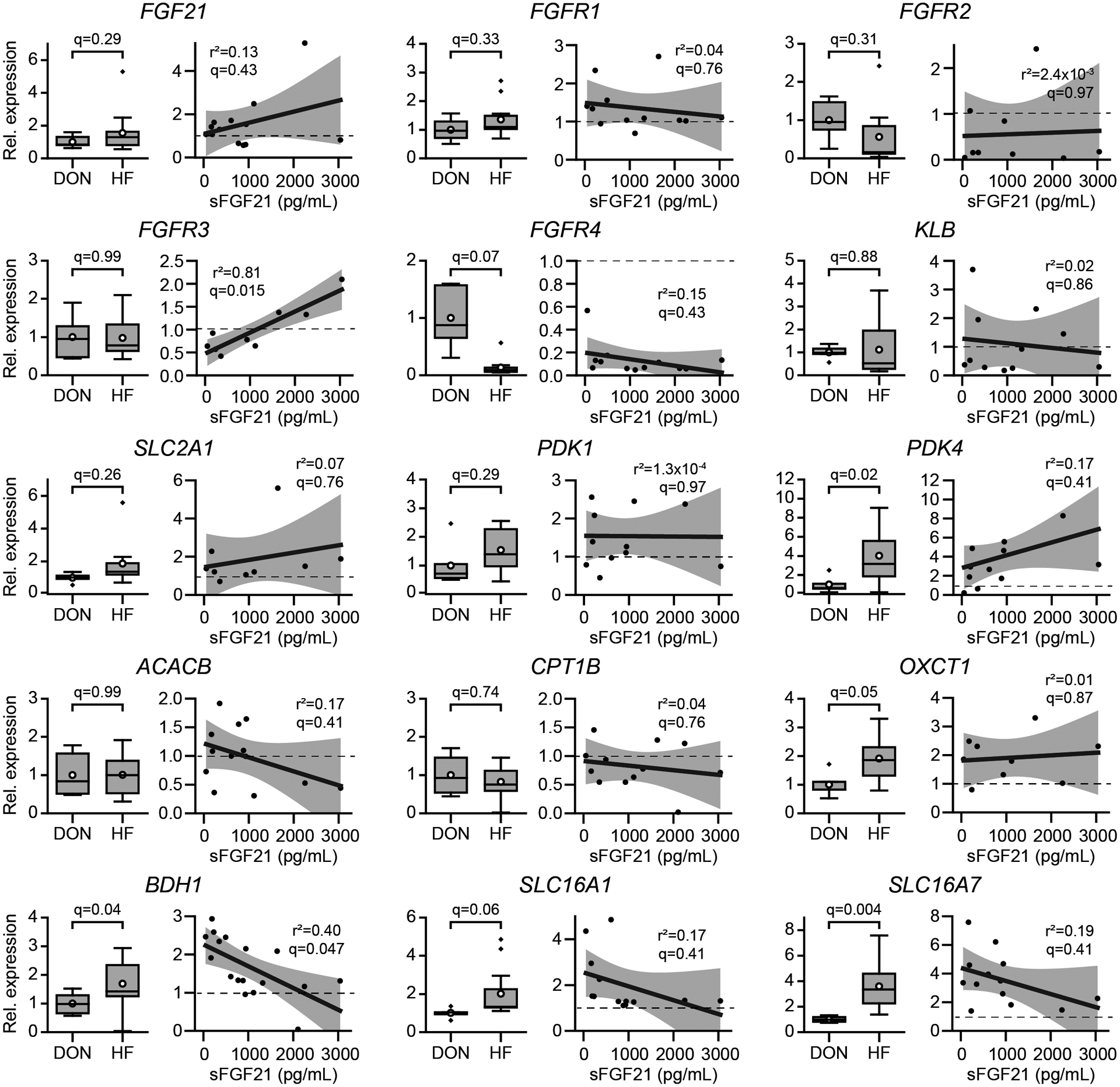
For each gene listed above, the left panel is the relative gene expression between normal donor (DON, n=4–6) and HFrEF hearts (HF, n=9–17). The right panel for each gene shows the linear regression for the HFrEF hearts against serum FGF21 (sFGF21) levels. The black line is the linear regression, with shaded area corresponding to 95% confidence bands. Coefficient of determination (r2) and corresponding Benjamini-Hochberg corrected p value (q) for a false discovery rate of 0.05 are shown.
Table 2.
Human cardiomyopathy RNA-Seq datasets
| Dataset (Ref.) | Cardiomyopathy Type | Total Samples | Detectable FGF21 transcripts – Non-failing (positive/total) | Detectable FGF21 transcripts – Cardiomyopathy (positive/total) |
|---|---|---|---|---|
| GSE135055 (34) | HFrEF | 30 | 0/7 | 0/23 |
| GSE71613 (35) | HFrEF, Restrictive | 8 | 0/4 | 2/4 |
| GSE57344 (36) | HFrEF | 6 | 1/3 | 1/3 |
| GSE46224 (37) | HFrEF | 40 | 0/8 | 1/32 |
| GSE160997 (38) | Hypertrophic | 23 | 0/5 | 2/18 |
| GSE133054 (39) | HFrEF, Hypertrophic | 23 | 0/8 | 5/15 |
| GSE130036 (40) | Hypertrophic | 37 | 0/9 | 2/28 |
| 167 | 1/44 | 13/123 |
Correlation of circulating FGF21 with cardiac metabolic gene expression.
As a first step towards examining cardiac FGF21 signaling in humans, we also assayed genes involved in the FGF response and fuel metabolism via qPCR (Figure 3). FGF21 exerts its effects primarily by binding to a receptor complex composed of one of four tyrosine kinase FGF receptor isoforms, typically FGFR1, and the β-Klotho (KLB) co-receptor41. In our hands, there was no evidence for a net increase in gene expression of FGF receptors nor the co-receptor β-Klotho between HFrEF versus donor samples. Intriguingly, however, there was a strong positive correlation between serum FGF21 and cardiac FGFR3 expression. In contrast, most samples had suppressed FGFR4 expression. We then turned to genes involved in the metabolism of glucose, fatty acids, and ketones. Compared to donor heart tissue, HFrEF cardiac samples had increased levels of pyruvate dehydrogenase kinase 4 (PDK4), which inhibits the conversion of pyruvate into acetyl-CoA, as well as several genes involved in the transport and metabolism of ketones, including solute carrier family 16 member 7 (SLC16A7 or MCT2), a monocarboxylate transporter responsible for ketone uptake, 3-hydroxybutyrate dehydrogenase (BDH1), which catalyzes the interconversion between the ketones β-hydroxybutyrate and acetoacetate, and 3-oxoacid-CoA transferase (OXCT1 or SCOT), which transfers the CoA group to the ketone acetoacetate. Unexpectedly, we found a negative correlation between serum FGF21 levels and BDH1 transcripts, and similar but much weaker trends with the SLC16A1 and SLC16A7 transporters. This result was intriguing, as prior mouse studies revealed that inhibition of FGF21 reduced Bdh1 transcripts, opposite to the correlation found here42. Although these analyses of correlations do not establish a particular mechanism for cardiac FGF21 activity, they do lend support to the hypothesis that FGF21 may act as a hormonal regulator of cardiac metabolism.
Clinical parameters suggest elevated circulating FGF21 may be due to hepatic congestion.
To investigate the source of FGF21 production, we correlated serum FGF21 levels with the available clinical data on adiposity and cardiac, hepatic, and renal function (Figure 4). Notably, advanced HFrEF is associated with a cachectic phenotype, and elevated FGF21 levels have been attributed to muscle wasting during cardiac cachexia or prolonged fasting7, 43. In our cohort, however, we found no correlation with body-mass index (BMI) or aspartate aminotransferase (AST), a liver marker also released during muscle breakdown in cachectic states. Intriguingly, we observed a significant correlation with B-type natriuretic peptide (BNP) and total bilirubin, a marker of hepatic dysfunction. Moreover, we observed strong negative correlations with triglycerides and total cholesterol, which also partly reflect hepatic synthesis. We did not pursue correlations with invasive hemodynamic data because of confounding factors. Hemodynamic values are instantaneous measures, but in our study cohort were obtained on average 10 days apart from blood samples. Moreover, hemodynamic values were often targeted for optimization in the immediate preoperative period prior to LVAD implantation. Taken together, our data here supports the hypothesis that one main source of elevated circulating FGF21 observed in HFrEF is due to hepatic release.
Figure 4. Correlations between serum FGF21 and clinical parameters.
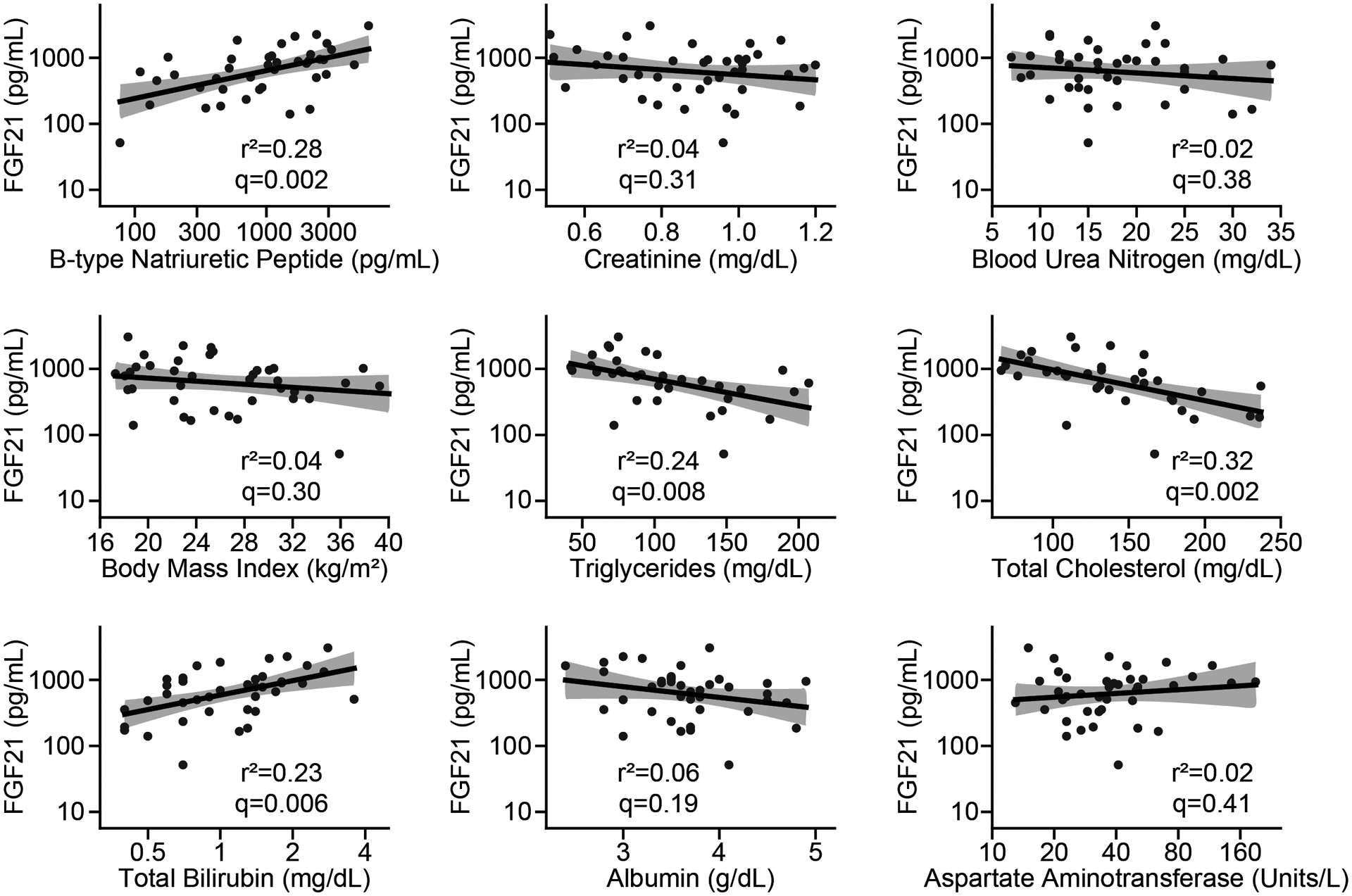
Correlation of serum FGF21 values with clinical index via linear regression (n=34–40). The black line is the linear regression, with shaded area corresponding to 95% confidence bands. Coefficient of determination (r2) and corresponding Benjamini-Hochberg corrected p value (q) for a false discovery rate of 0.05 are shown. Note logarithmic scale for FGF21, BNP, AST, and total bilirubin.
DISCUSSION
This study describes three major findings defining human cardiac FGF21 biology. First, serum levels of FGF21 are elevated in the setting of HFrEF independent of other comorbidities, such as diabetes, that can raise hormone concentration. Second, FGF21 is present in cardiomyocytes from failing but not donor hearts, suggesting that it may activate downstream cardiac signals. Third, the origin of the elevated FGF21 appears to be partly extracardiac, with the liver as the most likely extracardiac source.
Elevated serum FGF21 levels have been seen in prior studies of heart failure biomarkers with either reduced or preserved ejection fraction20, 21, 43. In two studies, diabetes was a significant comorbidity in >40% of subjects, confounding the ability to interpret the basis for elevated FGF2120, 21. Notably, the HFrEF study by Shen et. al. was powered to detect cardiovascular outcomes, and elevated FGF21 levels predicted cardiac death independently of N-terminal pro-BNP levels21. More broadly, elevated FGF21 levels have been associated with adverse cardiac events in individuals with coronary disease and/or diabetes18, 19, 44, 45. Thus, elevated FGF21 is a potential biomarker for severity in cardiac disease, raising the question of what derangements are causing this elevation, and whether the elevated FGF21 is an intrinsic or hormonal signal for the diseased heart.
The source of FGF21 signaling to the heart has been difficult to resolve. Though some studies suggested minimal expression in the mouse heart, others found upregulation during cardiac hypertrophy15, 16, 46. In the only study of human cardiac gene expression, transcriptomic analysis revealed somewhat increased FGF21 expression during end-stage HFrEF23. In our qPCR data, whereas elevated transcription was seen in particular HFrEF samples, a uniform increase was not detected, corroborated by analysis of previously published transcriptomic datasets. Taken together, this suggests that cardiac FGF21 gene expression is low at baseline, with perhaps a slight, heterogeneous increase in cardiomyopathies.
Nevertheless, our data here is consistent with mouse and human data showing direct activity of circulating FGF21 on the heart15, 24–26. Robust cardiac FGF21 staining was seen in all the HFrEF samples but none of the non-failing donors, suggesting that elevated circulating FGF21 binds to the failing heart. In terms of cardiac regulation, exogenous FGF21 reduced fibrosis, inflammation, apoptosis, and maladaptive changes in cardiac energy metabolism15–17. In this report, we queried a range of genes involved in cardiac metabolism. Surprisingly, we found an overall negative correlation between FGF21 and genes involved in the synthesis and transport of ketones. Whereas some prior data has suggested a direct relationship between FGF21 and fasting-induced ketogenesis, in humans the ketogenic response seems to precede the release of FGF21, suggesting a more circuitous relationship7, 42. Since our data is entirely correlational, we can only speculate that FGF21 acting on the heart may be a compensatory signal to preserve energetic homeostasis during progressive contractile failure. Intriguingly, there must definitely be changes in the response or susceptibility to circulating FGF21 during cardiomyopathies, as we saw no significant FGF21 staining in functionally normal hearts collected from donors, despite these individuals having similarly elevated serum FGF21 levels. In examining the FGF receptor family, we saw no obvious transcriptional upregulation that would indicate how FGF21 binds cardiomyocytes during HFrEF, though there was a strong positive correlation with FGFR3, and possibly transcriptional suppression of FGFR4.
Finally, we investigated the pathology responsible for elevating blood FGF21. The liver is the primary source of FGF21, though adipose tissue also produces it. It is likely the elevated FGF21 in HFrEF derives from the liver rather than adipose tissue, as we saw no relationship with BMI, and, when increased FGF21 levels are due to adipose release, it shows a positive correlation with lipid profile11, 47. Instead, here we find higher FGF21 associated with a lower lipid levels, which may reflect the triglyceride-lowering effect of hepatic FGF2148, 49. In regards to the mechanism activating hepatic FGF21 secretion, our data revealed a strong correlation between FGF21 and BNP levels, a sensitive marker of chronic pathological myocardial stretch and vascular congestion. We also showed a correlation between FGF21 and elevated bilirubin, but not elevated liver function enzymes (AST). This pattern is most consistent with congestive hepatopathy, which is also due to chronic vascular congestion. This suggests that chronic venous congestion may be a proximal signal for hepatic FGF21 secretion. In summary, pathological hepatic venous congestion in HFrEF may cause FGF21 release, with elevated FGF21 feeding back on the heart to regulate its metabolism. Thus, FGF21 defines a potential cardio-hepatic metabolic signaling loop in HFrEF.
LIMITATIONS
First, although we assay a variety of clinical indexes, cardiac gene expression, and cardiac FGF21 protein, our analysis is based on correlations between these parameters, and a causal pathway has not been established. Without corresponding liver samples from these patients, we cannot confirm that elevated circulating FGF21 is due to hepatic congestion. Second, we did not study clinical outcomes. Our study was not powered or designed for clinical outcomes, as HFrEF patients went on to LVAD implantation and, for a substantial number of individuals, cardiac transplants. Third, we studied a subset of patients without diabetes, end-stage renal failure, or several other forms of liver disease. The biological activity of FGF21 may be more complex when these comorbidities are present. Finally, our study population consisted of patients with end-stage HFrEF. Whether FGF21 alters cardiac metabolism during earlier periods in HFrEF with relatively preserved cardiac output has not been established.
Supplementary Material
What is new?
There are pronounced changes in metabolism during the progression of heart failure with reduced ejection fraction (HFrEF), including in the fuels the heart uses. We investigated if levels of a signaling molecule, fibroblast growth factor 21 (FGF21), which is known to alter fuel utilization, were altered in end-stage HFrEF. We found large increases in circulating FGF21 in HFrEF.
In addition, though FGF21 protein was present in failing but not structurally normal hearts, there appeared to be a lack of cardiac FGF21 gene expression in either condition, suggesting FGF21 acts as a hormonal signal from another organ, most likely the liver.
What are the clinical implications?
Our results expand the idea that HFrEF is in part a metabolic disease. Subclinical alterations in the behavior of other organs, such as the liver, may be leading to changes in cardiac fuel metabolism and impacting HFrEF progression, possibly defining different HFrEF subgroups.
FGF21 analogs are also in development as therapies for metabolic diseases, and these may alter cardiac function in patients with cardiomyopathies.
SOURCES OF FUNDING
This work was supported in part by the Nora Eccles Treadwell Foundation; the NIH Heart, Lung, and Blood Institute under awards R01HL141353 (D.C.), R01HL135121 (S.G.D.), R01HL132067 (S.G.D.), and T32HL007576 (S.S.); the American Heart Association under award 16SFRN29020000 (S.G.D.); and the Department of Veterans Affairs under award Merit I01 CX002291 (S.G.D.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.
Footnotes
REFERENCES
- 1.Lopaschuk GD, Karwi QG, Tian R, Wende AR and Abel ED. Cardiac Energy Metabolism in Heart Failure. Circ Res. 2021; 128:1487–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carley AN, Maurya SK, Fasano M, Wang Y, Selzman CH, Drakos SG and Lewandowski ED. Short-Chain Fatty Acids Outpace Ketone Oxidation in the Failing Heart. Circulation. 2021; 143:1797–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cluntun AA, Badolia R, Lettlova S, Parnell KM, Shankar TS, Diakos NA, Olson KA, Taleb I, Tatum SM, Berg JA, Cunningham CN, Van Ry T, Bott AJ, Krokidi AT, Fogarty S, Skedros S, Swiatek WI, Yu X, Luo B, Merx S, Navankasattusas S, Cox JE, Ducker GS, Holland WL, McKellar SH, Rutter J and Drakos SG. The pyruvate-lactate axis modulates cardiac hypertrophy and heart failure. Cell Metab. 2021; 33:629–648.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Badolia R, Ramadurai DKA, Abel ED, Ferrin P, Taleb I, Shankar TS, Krokidi AT, Navankasattusas S, McKellar SH, Yin M, Kfoury AG, Wever-Pinzon O, Fang JC, Selzman CH, Chaudhuri D, Rutter J and Drakos SG. The Role of Nonglycolytic Glucose Metabolism in Myocardial Recovery Upon Mechanical Unloading and Circulatory Support in Chronic Heart Failure. Circulation. 2020; 142:259–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Diakos NA, Navankasattusas S, Abel ED, Rutter J, McCreath L, Ferrin P, McKellar SH, Miller DV, Park SY, Richardson RS, Deberardinis R, Cox JE, Kfoury AG, Selzman CH, Stehlik J, Fang JC, Li DY and Drakos SG. Evidence of Glycolysis Up-Regulation and Pyruvate Mitochondrial Oxidation Mismatch During Mechanical Unloading of the Failing Human Heart: Implications for Cardiac Reloading and Conditioning. JACC Basic Transl Sci. 2016; 1:432–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kliewer SA and Mangelsdorf DJ. A Dozen Years of Discovery: Insights into the Physiology and Pharmacology of FGF21. Cell Metab. 2019; 29:246–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fazeli PK, Lun M, Kim SM, Bredella MA, Wright S, Zhang Y, Lee H, Catana C, Klibanski A, Patwari P and Steinhauser ML. FGF21 and the late adaptive response to starvation in humans. J Clin Invest. 2015; 125:4601–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lundsgaard AM, Fritzen AM, Sjoberg KA, Myrmel LS, Madsen L, Wojtaszewski JFP, Richter EA and Kiens B. Circulating FGF21 in humans is potently induced by short term overfeeding of carbohydrates. Mol Metab. 2017; 6:22–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Desai BN, Singhal G, Watanabe M, Stevanovic D, Lundasen T, Fisher FM, Mather ML, Vardeh HG, Douris N, Adams AC, Nasser IA, FitzGerald GA, Flier JS, Skarke C and Maratos-Flier E. Fibroblast growth factor 21 (FGF21) is robustly induced by ethanol and has a protective role in ethanol associated liver injury. Mol Metab. 2017; 6:1395–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hong ES, Lim C, Choi HY, Lee YK, Ku EJ, Moon JH, Park KS, Jang HC and Choi SH. Plasma fibroblast growth factor 21 levels increase with ectopic fat accumulation and its receptor levels are decreased in the visceral fat of patients with type 2 diabetes. BMJ Open Diabetes Research & Care. 2019; 7:e000776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Y-S, Ye J, Cao Y-H, Zhang R, Liu Y, Zhang S-W, Dai W and Zhang Q. Increased serum/plasma fibroblast growth factor 21 in type 2 diabetes mellitus: a systematic review and meta-analysis. Postgraduate Medical Journal. 2019; 95:134–139. [DOI] [PubMed] [Google Scholar]
- 12.Chavez AO, Molina-Carrion M, Abdul-Ghani MA, Folli F, Defronzo RA and Tripathy D. Circulating Fibroblast Growth Factor-21 Is Elevated in Impaired Glucose Tolerance and Type 2 Diabetes and Correlates With Muscle and Hepatic Insulin Resistance. Diabetes Care. 2009; 32:1542–1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hojman P, Pedersen M, Nielsen AR, Krogh-Madsen R, Yfanti C, Akerstrom T, Nielsen S and Pedersen BK. Fibroblast growth factor-21 is induced in human skeletal muscles by hyperinsulinemia. Diabetes. 2009; 58:2797–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kruse R, Vienberg SG, Vind BF, Andersen B and Hojlund K. Effects of insulin and exercise training on FGF21, its receptors and target genes in obesity and type 2 diabetes. Diabetologia. 2017; 60:2042–2051. [DOI] [PubMed] [Google Scholar]
- 15.Planavila A, Redondo I, Hondares E, Vinciguerra M, Munts C, Iglesias R, Gabrielli LA, Sitges M, Giralt M, van Bilsen M and Villarroya F. Fibroblast growth factor 21 protects against cardiac hypertrophy in mice. Nat Commun. 2013; 4:2019. [DOI] [PubMed] [Google Scholar]
- 16.Ruan CC, Kong LR, Chen XH, Ma Y, Pan XX, Zhang ZB and Gao PJ. A2A Receptor Activation Attenuates Hypertensive Cardiac Remodeling via Promoting Brown Adipose Tissue-Derived FGF21. Cell Metab. 2018; 28:476–489 e5. [DOI] [PubMed] [Google Scholar]
- 17.Joki Y, Ohashi K, Yuasa D, Shibata R, Ito M, Matsuo K, Kambara T, Uemura Y, Hayakawa S, Hiramatsu-Ito M, Kanemura N, Ogawa H, Daida H, Murohara T and Ouchi N. FGF21 attenuates pathological myocardial remodeling following myocardial infarction through the adiponectin-dependent mechanism. Biochem Biophys Res Commun. 2015; 459:124–30. [DOI] [PubMed] [Google Scholar]
- 18.Gan F, Huang J, Dai T, Li M and Liu J. Serum level of fibroblast growth factor 21 predicts long-term prognosis in patients with both diabetes mellitus and coronary artery calcification. Ann Palliat Med. 2020; 9:368–374. [DOI] [PubMed] [Google Scholar]
- 19.Ong KL, Januszewski AS, O’Connell R, Jenkins AJ, Xu A, Sullivan DR, Barter PJ, Hung WT, Scott RS, Taskinen MR, Keech AC and Rye KA. The relationship of fibroblast growth factor 21 with cardiovascular outcome events in the Fenofibrate Intervention and Event Lowering in Diabetes study. Diabetologia. 2015; 58:464–73. [DOI] [PubMed] [Google Scholar]
- 20.Chou RH, Huang PH, Hsu CY, Chang CC, Leu HB, Huang CC, Chen JW and Lin SJ. Circulating Fibroblast Growth Factor 21 is Associated with Diastolic Dysfunction in Heart Failure Patients with Preserved Ejection Fraction. Sci Rep. 2016; 6:33953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shen Y, Zhang X, Pan X, Xu Y, Xiong Q, Lu Z, Ma X, Bao Y and Jia W. Contribution of serum FGF21 level to the identification of left ventricular systolic dysfunction and cardiac death. Cardiovasc Diabetol. 2017; 16:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Redondo-Angulo I, Mas-Stachurska A, Sitges M, Tinahones FJ, Giralt M, Villarroya F and Planavila A. Fgf21 is required for cardiac remodeling in pregnancy. Cardiovasc Res. 2017; 113:1574–1584. [DOI] [PubMed] [Google Scholar]
- 23.Planavila A, Redondo-Angulo I, Ribas F, Garrabou G, Casademont J, Giralt M and Villarroya F. Fibroblast growth factor 21 protects the heart from oxidative stress. Cardiovasc Res. 2015; 106:19–31. [DOI] [PubMed] [Google Scholar]
- 24.Pol CJ, Pollak NM, Jurczak MJ, Zacharia E, Karagiannides I, Kyriazis ID, Ntziachristos P, Scerbo DA, Brown BR, Aifantis I, Shulman GI, Goldberg IJ and Drosatos K. Cardiac myocyte KLF5 regulates body weight via alteration of cardiac FGF21. Biochim Biophys Acta Mol Basis Dis. 2019; 1865:2125–2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferrer-Curriu G, Guitart-Mampel M, Rupérez C, Zamora M, Crispi F, Villarroya F, Fernández-Solà J, Garrabou G and Planavila A. The protective effect of fibroblast growth factor-21 in alcoholic cardiomyopathy: a role in protecting cardiac mitochondrial function. J Pathol. 2021; 253:198–208. [DOI] [PubMed] [Google Scholar]
- 26.Ferrer-Curriu G, Redondo-Angulo I, Guitart-Mampel M, Ruperez C, Mas-Stachurska A, Sitges M, Garrabou G, Villarroya F, Fernández-Solà J and Planavila A. Fibroblast growth factor-21 protects against fibrosis in hypertensive heart disease. J Pathol. 2019; 248:30–40. [DOI] [PubMed] [Google Scholar]
- 27.Barb D, Bril F, Kalavalapalli S and Cusi K. Plasma Fibroblast Growth Factor 21 Is Associated With Severity of Nonalcoholic Steatohepatitis in Patients With Obesity and Type 2 Diabetes. J Clin Endocrinol Metab. 2019; 104:3327–3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kohara M, Masuda T, Shiizaki K, Akimoto T, Watanabe Y, Honma S, Sekiguchi C, Miyazawa Y, Kusano E, Kanda Y, Asano Y, Kuro OM and Nagata D. Association between circulating fibroblast growth factor 21 and mortality in end-stage renal disease. PLoS One. 2017; 12:e0178971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu L, Pan Q, Wu G, Qian L, Zhang J, Zhang L, Fang Q, Zang G, Wang Y, Lau G, Li H and Jia W. Diverse Changes of Circulating Fibroblast Growth Factor 21 Levels in Hepatitis B Virus-Related Diseases. Sci Rep. 2017; 7:16482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pekkarinen PT, Skrifvars MB, Lievonen V, Jakkula P, Albrecht L, Loisa P, Tiainen M, Pettilä V, Reinikainen M and Hästbacka J. Serum fibroblast growth factor 21 levels after out of hospital cardiac arrest are associated with neurological outcome. Sci Rep. 2021; 11:690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ruiz-Margáin A, Pohlmann A, Ryan P, Schierwagen R, Chi-Cervera LA, Jansen C, Mendez-Guerrero O, Flores-García NC, Lehmann J, Torre A, Macías-Rodríguez RU and Trebicka J. Fibroblast growth factor 21 is an early predictor of acute-on-chronic liver failure in critically ill patients with cirrhosis. Liver Transpl. 2018; 24:595–605. [DOI] [PubMed] [Google Scholar]
- 32.Thiessen SE, Vanhorebeek I, Derese I, Gunst J and Van den Berghe G. FGF21 Response to Critical Illness: Effect of Blood Glucose Control and Relation With Cellular Stress and Survival. J Clin Endocrinol Metab. 2015; 100:E1319–27. [DOI] [PubMed] [Google Scholar]
- 33.Zheng W, Matei N, Pang J, Luo X, Song Z, Tang J and Zhang JH. Delayed recanalization at 3 days after permanent MCAO attenuates neuronal apoptosis through FGF21/FGFR1/PI3K/Caspase-3 pathway in rats. Exp Neurol. 2019; 320:113007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hua X, Wang YY, Jia P, Xiong Q, Hu Y, Chang Y, Lai S, Xu Y, Zhao Z and Song J. Multi-level transcriptome sequencing identifies COL1A1 as a candidate marker in human heart failure progression. BMC Med. 2020; 18:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schiano C, Costa V, Aprile M, Grimaldi V, Maiello C, Esposito R, Soricelli A, Colantuoni V, Donatelli F, Ciccodicola A and Napoli C. Heart failure: Pilot transcriptomic analysis of cardiac tissue by RNA-sequencing. Cardiol J. 2017; 24:539–553. [DOI] [PubMed] [Google Scholar]
- 36.Liu Y, Morley M, Brandimarto J, Hannenhalli S, Hu Y, Ashley EA, Tang WH, Moravec CS, Margulies KB, Cappola TP and Li M. RNA-Seq identifies novel myocardial gene expression signatures of heart failure. Genomics. 2015; 105:83–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang KC, Yamada KA, Patel AY, Topkara VK, George I, Cheema FH, Ewald GA, Mann DL and Nerbonne JM. Deep RNA sequencing reveals dynamic regulation of myocardial noncoding RNAs in failing human heart and remodeling with mechanical circulatory support. Circulation. 2014; 129:1009–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maron BA, Wang RS, Shevtsov S, Drakos SG, Arons E, Wever-Pinzon O, Huggins GS, Samokhin AO, Oldham WM, Aguib Y, Yacoub MH, Rowin EJ, Maron BJ, Maron MS and Loscalzo J. Individualized interactomes for network-based precision medicine in hypertrophic cardiomyopathy with implications for other clinical pathophenotypes. Nat Commun. 2021; 12:873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ren Z, Yu P, Li D, Li Z, Liao Y, Wang Y, Zhou B and Wang L. Single-Cell Reconstruction of Progression Trajectory Reveals Intervention Principles in Pathological Cardiac Hypertrophy. Circulation. 2020; 141:1704–1719. [DOI] [PubMed] [Google Scholar]
- 40.Liu X, Ma Y, Yin K, Li W, Chen W, Zhang Y, Zhu C, Li T, Han B, Liu X, Wang S and Zhou Z. Long non-coding and coding RNA profiling using strand-specific RNA-seq in human hypertrophic cardiomyopathy. Sci Data. 2019; 6:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kurosu H, Choi M, Ogawa Y, Dickson AS, Goetz R, Eliseenkova AV, Mohammadi M, Rosenblatt KP, Kliewer SA and Kuro OM. Tissue-specific expression of betaKlotho and fibroblast growth factor (FGF) receptor isoforms determines metabolic activity of FGF19 and FGF21. J Biol Chem. 2007; 282:26687–26695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Badman MK, Pissios P, Kennedy AR, Koukos G, Flier JS and Maratos-Flier E. Hepatic fibroblast growth factor 21 is regulated by PPARalpha and is a key mediator of hepatic lipid metabolism in ketotic states. Cell Metab. 2007; 5:426–37. [DOI] [PubMed] [Google Scholar]
- 43.Refsgaard Holm M, Christensen H, Rasmussen J, Johansen ML, Schou M, Faber J and Kistorp C. Fibroblast growth factor 21 in patients with cardiac cachexia: a possible role of chronic inflammation. ESC Heart Fail. 2019; 6:983–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ong KL, Campbell S, Wu BJ, McClelland RL, Kokkinos J, Szklo M, Polak JF, Allison MA and Rye KA. Relationship of fibroblast growth factor 21 with subclinical atherosclerosis and cardiovascular events: Multi-Ethnic Study of Atherosclerosis. Atherosclerosis. 2019; 287:46–53. [DOI] [PubMed] [Google Scholar]
- 45.Li Q, Zhang Y, Ding D, Yang Y, Chen Q, Su D, Chen X, Yang W, Qiu J and Ling W. Association Between Serum Fibroblast Growth Factor 21 and Mortality Among Patients With Coronary Artery Disease. J Clin Endocrinol Metab. 2016; 101:4886–4894. [DOI] [PubMed] [Google Scholar]
- 46.Fon Tacer K, Bookout AL, Ding X, Kurosu H, John GB, Wang L, Goetz R, Mohammadi M, Kuro-o M, Mangelsdorf DJ and Kliewer SA. Research resource: Comprehensive expression atlas of the fibroblast growth factor system in adult mouse. Mol Endocrinol. 2010; 24:2050–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang X, Yeung DC, Karpisek M, Stejskal D, Zhou ZG, Liu F, Wong RL, Chow WS, Tso AW, Lam KS and Xu A. Serum FGF21 levels are increased in obesity and are independently associated with the metabolic syndrome in humans. Diabetes. 2008; 57:1246–53. [DOI] [PubMed] [Google Scholar]
- 48.Schlein C, Talukdar S, Heine M, Fischer AW, Krott LM, Nilsson SK, Brenner MB, Heeren J and Scheja L. FGF21 Lowers Plasma Triglycerides by Accelerating Lipoprotein Catabolism in White and Brown Adipose Tissues. Cell Metab. 2016; 23:441–53. [DOI] [PubMed] [Google Scholar]
- 49.Kaufman A, Abuqayyas L, Denney WS, Tillman EJ and Rolph T. AKR-001, an Fc-FGF21 Analog, Showed Sustained Pharmacodynamic Effects on Insulin Sensitivity and Lipid Metabolism in Type 2 Diabetes Patients. Cell Rep Med. 2020; 1:100057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sarabhai T, Kahl S, Szendroedi J, Markgraf DF, Zaharia OP, Barosa C, Herder C, Wickrath F, Bobrov P, Hwang JH, Jones JG and Roden M. Monounsaturated fat rapidly induces hepatic gluconeogenesis and whole-body insulin resistance. JCI Insight. 2020;5: e134520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jaworska J, Micielska K, Kozłowska M, Wnorowski K, Skrobecki J, Radzimiński L, Babińska A, Rodziewicz E, Lombardi G and Ziemann E. A 2-Week Specific Volleyball Training Supported by the Whole Body Cryostimulation Protocol Induced an Increase of Growth Factors and Counteracted Deterioration of Physical Performance. Front Physiol. 2018;9:1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Foo JP, Aronis KN, Chamberland JP and Mantzoros CS. Lack of Day/Night variation in fibroblast growth factor 21 levels in young healthy men. Int J Obes (Lond). 2015;39:945–948. [DOI] [PubMed] [Google Scholar]
- 53.Besse-Patin A, Montastier E, Vinel C, Castan-Laurell I, Louche K, Dray C, Daviaud D, Mir L, Marques MA, Thalamas C, Valet P, Langin D, Moro C and Viguerie N. Effect of endurance training on skeletal muscle myokine expression in obese men: identification of apelin as a novel myokine. Int J Obes (Lond). 2014;38:707–713. [DOI] [PubMed] [Google Scholar]
- 54.Søberg S, Andersen ES, Dalsgaard NB, Jarlhelt I, Hansen NL, Hoffmann N, Vilsbøll T, Chenchar A, Jensen M, Grevengoed TJ, Trammell SAJ, Knop FK and Gillum MP. FGF21, a liver hormone that inhibits alcohol intake in mice, increases in human circulation after acute alcohol ingestion and sustained binge drinking at Oktoberfest. Mol Metab. 2018;11:96–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Toloza FJK, Mantilla-Rivas JO, Pérez-Matos MC, Ricardo-Silgado ML, Morales-Alvarez MC, Pinzón-Cortés JA, Pérez-Mayorga M, Arévalo-Garcia ML, Tolosa-González G and Mendivil CO. Plasma Levels of Myonectin But Not Myostatin or Fibroblast-Derived Growth Factor 21 Are Associated with Insulin Resistance in Adult Humans without Diabetes Mellitus. Front Endocrinol (Lausanne). 2018;9:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


