Introduction
Orthotopic liver transplantation (OLT) represents the only definitive cure for end-stage liver diseases. When the procedure is successful, patient outcome appears nearly miraculous with recovery of general well-being and physical performance (1); however, several short-and long-term complications may occur after transplant (2). Biliary tract complications are frequent after OLT, accounting approximately for 30% of cases, with leaks and anastomotic and non-anastomotic strictures (NAS) among the most frequent complications (3). While in the case of anastomotic complications a suboptimal surgical procedure may be generally advocated, the process occurring in NAS remains less clear and is a largely unpredictable condition that causes significant morbidity and graft loss. NAS is thought to occur mainly after enhanced ischemia-reperfusion injury (IRI); however, it is also associated to transplant in primary sclerosing cholangitis (PSC) and autoimmune hepatitis (AIH) patients (4). An accumulation of clinical evidence suggests that significant biliary loss is typically observed early after OLT because of IRI, nevertheless NAS develops in a minority of the cases (5).
Current Study and Findings
In order to clarify the possible path leading from OLT-associated biliary damage to NAS, de Jong, Overi and coauthors performed a detailed immune-histological, molecular and metabolomic study in explanted human NAS liver (6). In this elegant study several elements converge in identifying not only IRI, but also chronic ischemia and impaired biliary cell replacement as important elements in the onset of NAS. In fact, peribiliary glands (PBGs) (7), the vital reservoir for biliary cell (i.e., cholangiocyte) reproduction from biliary tree stem/progenitor cells (BTSCs), underwent a relevant disruption and depletion in patients with OLT-related NAS. Specifically, in eighteen livers from patients with OLT-related NAS, the PBG mass was reduced to one half in comparison with control and these glands exhibited cells with: i) decreased staining for proliferation markers and ii) increased apoptosis while the expression of cellular senescence markers remained unchanged. Characterization of BTSCs (Sox9 positive) in NAS livers, with regard to their evolution toward a cholangiocyte phenotype (evaluated by expression of secretin receptor and primary cilia formation), demonstrated an impaired progression to mature biliary cell compared to control. The NAS-affected biliary tract also exhibited increased wall thickness for collagen deposition that was associated to myofibroblast activation. Finally, the PVP (the delicate system of interconnecting small vessels surrounding the biliary tree originating from the hepatic artery and allowing for blood flow toward the sinusoidal space) representation was impaired and reduced in the NAS-affected biliary tract with increased apoptosis of endothelial cells and enhanced expression of ischemia markers such as hypoxia inducible factor 1α.
Interestingly, authors from the same group, employing bile duct slices of human liver discarded from OLT, previously demonstrated that enhanced proliferative activities in PBGs were effective in restoring the denudated biliary tract with normal cholangiocytes (8). A schematic provided in Figure 1 depicts the fate of peribiliary glands following IRI and peribiliary vascular plexus (PVP) damage.
Figure 1:
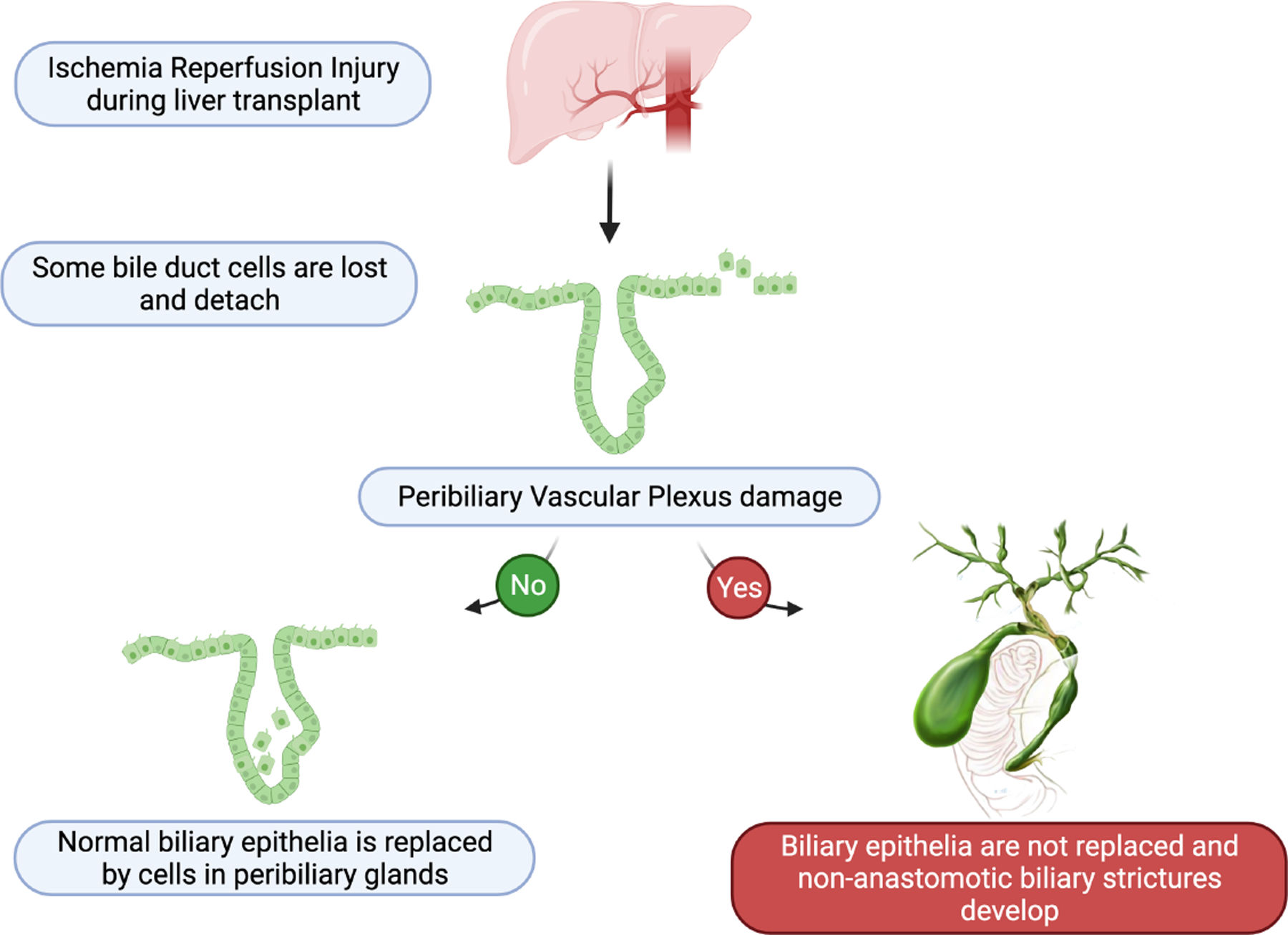
Schematic representation of the suggested pathological model leading to non-anastomotic biliary stricture after liver transplant.
In support of these findings, PBG alterations are also present in patients with PSC, a well-known ductopenic, scarring disorder of the biliary tract (9). Also in PSC, enhanced PBG cells hyperplasia was observed supporting the possible evolution toward dysplastic transformation and epithelial to-mesenchymal transition (EMT), thereby sustaining the fibrotic process in the biliary system (9). Taken together these studies on PBGs shed the light on the important role of these glands in human cholangiopathies. Research focusing on this aspect will likely provide important findings in the future.
Limitations
Despite these findings, several areas remain elusive including the possible differential role of toxic, inflammatory, hypoxic, and other components related to PBGs activity, as well as the possibility to modulate PBG function and BTSC homeostasis. With regard to NAS and OLT, it is also important to consider that several clinical variables aside from IRI may impact PVP integrity and PBGs function including rejection, immunosuppressive regimen, and the inflammatory background that is demonstrated by the association of NAS with OLT in PSC and AIH patients. Moreover, possible irreversible damage to the PVP during surgical manipulation should not be excluded. Finally, regenerative and proliferative processes of biliary epithelium are complex activities, finely tuned in the course of normal and pathological conditions by an intricate network of autocrine and paracrine signals coming from gastrointestinal hormones, angiogenic factors, neurotransmitters, and other molecules (10). In this perspective, possible PBG isolation and culture would be [as also recognized by de Jong, Overi and co-authors (6)] a formidable system to assess the determinants leading to BSTC proliferation and differentiation in cholangiocytes or toward apoptosis, senescence, and also EMT.
Conclusions and Future Directions
In conclusion, the study by de Jong and Overi et al. provides key data and information regarding the loss of microvasculature during NAS after OLT. Further, the authors have demonstrated that PBG preservation is key to proper regeneration and bile duct integrity. Extension of research regarding the interplay between PVP and PBGs will likely improve our comprehension of other human biliary diseases, in the future.
Acknowledgments
This work was supported by Indiana University Health, Indiana University School of Medicine Strategic Research Initiative, the VA Merit award to Dr. Francis (1I01BX003031) from the United States Department of Veteran’s Affairs, Biomedical Laboratory Research and Development Service and NIH grants DK108959 and DK119421 (Dr. Francis). This material results from work partially supported by resources at Richard L. Roudebush VA Medical Center, Indianapolis, IN. The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs.
Abbreviations:
- AIH
autoimmune hepatitis
- BTSC
biliary tree stem/progenitor cell
- EMT
epithelial-mesenchymal transition
- IRI
ischemic reperfusion injury
- NAS
non-anastomotic strictures
- OLT
orthotopic liver transplant/transplantation
- PBG
peribiliary gland
- PSC
primary sclerosing cholangitis
- PVP
peribiliary vascular plexus
References:
- 1.Zarrinpar A, Busuttil RW. Liver transplantation: past, present and future. Nat Rev Gastroenterol Hepatol 2013;10:434–440. [DOI] [PubMed] [Google Scholar]
- 2.Neuberger J. An update on liver transplantation: A critical review. J Autoimmun 2016;66:51–59. [DOI] [PubMed] [Google Scholar]
- 3.Seehofer D, Eurich D, Veltzke-Schlieker W, Neuhaus P. Biliary complications after liver transplantation: old problems and new challenges. Am J Transplant 2013;13:253–265. [DOI] [PubMed] [Google Scholar]
- 4.Nims RW, Beebe LE, Dragnev KH, Thomas PE, Fox SD, Issaq HJ, Jones CR, et al. Induction of hepatic CYP1A in male F344/NCr rats by dietary exposure to Aroclor 1254: examination of immunochemical, RNA, catalytic, and pharmacokinetic endpoints. Environ Res 1992;59:447–466. [DOI] [PubMed] [Google Scholar]
- 5.de Vries Y, von Meijenfeldt FA, Porte RJ. Post-transplant cholangiopathy: Classification, pathogenesis, and preventive strategies. Biochim Biophys Acta Mol Basis Dis 2018;1864:1507–1515. [DOI] [PubMed] [Google Scholar]
- 6.de Jong IEM, Overi D, Carpino G, Gouw ASH, van den Heuvel MC, van Kempen LC, Mancone C, et al. Persistent biliary hypoxia and lack of regeneration are key mechanisms in the pathogenesis of posttransplant nonanastomotic strictures. Hepatology 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matsubara T, Kozaka K, Matsui O, Nakanuma Y, Uesaka K, Inoue D, Yoneda N, et al. Peribiliary glands: development, dysfunction, related conditions and imaging findings. Abdom Radiol (NY) 2020;45:416–436. [DOI] [PubMed] [Google Scholar]
- 8.de Jong IEM, Matton APM, van Praagh JB, van Haaften WT, Wiersema-Buist J, van Wijk LA, Oosterhuis D, et al. Peribiliary Glands Are Key in Regeneration of the Human Biliary Epithelium After Severe Bile Duct Injury. Hepatology 2019;69:1719–1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carpino G, Cardinale V, Renzi A, Hov JR, Berloco PB, Rossi M, Karlsen TH, et al. Activation of biliary tree stem cells within peribiliary glands in primary sclerosing cholangitis. J Hepatol 2015;63:1220–1228. [DOI] [PubMed] [Google Scholar]
- 10.Hall C, Sato K, Wu N, Zhou T, Kyritsi K, Meng F, Glaser S, et al. Regulators of Cholangiocyte Proliferation. Gene Expr 2017;17:155–171. [DOI] [PMC free article] [PubMed] [Google Scholar]


