Abstract
Because of profound effects observed in carcinogenesis, prostaglandins (PG), prostaglandin-endoperoxide synthases, and PG receptors are implicated in cancer development and progression. Understanding the molecular mechanisms of PG actions has potential clinical relevance for cancer prevention and therapy. This review focuses on the current status of PG signaling pathways in modulating cancer progression and aims to provide insights into the mechanistic actions of PGs and their receptors in influencing tumor progression. We also examine several small molecules identified as having anticancer activity that target prostaglandin receptors. The literature suggests that targeting PG pathways could provide opportunities for cancer prevention and therapy.
Introduction
The prostaglandins are physiologically active lipid compounds with diverse hormone-like effects. Prostaglandins (PG), prostaglandin-endoperoxide synthases (PTGS), and PG receptors are implicated in normal development, tissue homeostasis, inflammation, and cancer progression (1, 2). PGs are derived from the precursor 20-carbon chain fatty acid, arachidonic acid (AA), which is produced by membrane phospholipid enzymes, especially phospholipase A2. The cyclooxygenase (COX) enzymes convert AA to the precursor molecule prostaglandin H2 (PGH2; ref. 3). PGH2 is then converted to one of five primary prostanoids that include prostaglandin D2 (PGD2), prostaglandin E2 (PGE2), prostaglandin F2α (PGF2α), prostaglandin I2 (PGI2), and thromboxane A2 (TXA2). The conversion of PGH2 occurs through specific synthases, including prostaglandin D synthase (PGDS), prostaglandin E synthase (PGES), prostaglandin F synthase (PGFS), prostaglandin I synthase (PGIS), and thromboxane A synthase (TBXAS), respectively (1, 4, 5). After synthesis, PGs are rapidly transported into the extracellular microenvironment by the prostaglandin transporter (PGT), which is a protein belonging to a superfamily of 12-transmembrane anion-transporting polypeptides (6, 7). When exported to the microenvironment, the PGs bind to and activate G-protein-coupled receptors that include PGD2 receptor 1 (DP1), PGD2 receptor 2 (DP2), PGE2 receptor 1 (EP1), PGE2 receptor 2 (EP2), PGE2 receptor 3 (EP3), PGE2 receptor 4 (EP4), prostaglandin F receptor (FP), prostacyclin receptor (IP), and TXA2 receptor (TBXA2R). These receptors are classified according to their ligand specificity (8). PG binding to specific receptors leads to receptor activation and dissociation of the heterotrimeric G-protein complex. This action leads to the initiation of divergent signaling cascades (2) that mediate a variety of physiologic functions, including tumor progression.
Inhibiting COX enzymes reportedly could be effective in the prevention and treatment of multiple cancer types (9–11). However, inhibition of COX by NSAIDs or other COX inhibitors has seen limited use because these drugs are associated with various side effects, including gastrointestinal toxicity, increased risk of myocardial infarction, and cardiovascular disease (12–14). Thus, many studies have focused on the PGs downstream of COX, hoping to avoid the side effects associated with COX inhibition while retaining the potential for cancer prevention and treatment. Clarifying the function of individual receptors and development of specific inhibitors is critically important to improve the efficacy and avoid toxicity. Previously, researchers have mostly concentrated only on the EP receptors in various cancers (15). In this review, we comprehensively examine the role of PGs, their receptors, and signaling pathways involved in multiple types of cancer (Supplementary Table S1). We also discuss the potential of inhibitors of PG signaling pathways as well as their limitations and side effects in cancer treatment.
The Role and Mechanisms of PGE2 and PGE2 Receptors (EP) in Cancer
PGE2 is one of several major COX protein products detected in various physiological settings and mediates multiple functions such as inflammation, tumorigenesis, and immune responses (16, 17). PGE2 is synthesized by specific synthases. Three types of PGE synthases have been identified, including microsomal PGE synthase-1 (mPGES-1), microsomal PGES-2 (mPGES-2), and cytosolic PGE synthase (cPGES). The expression of mPGES-1 is inducible, whereas mPGES-2 and cPGES are constitutively expressed (18). 15-Hydroxyprostaglandin dehydrogenase (15-PGDH) is a prostaglandin-degrading enzyme that catalyzes oxidization of the 15(S)-hydroxyl group of PGE2 to yield an inactive 15-keto PGE2 (19). However, 15-keto-PGE2 might have an additional role as a biased and/or partial agonist capable of taking over the actions of PGE2 to gradually terminate reactions, which is significant for stopping PGE2-evoked inflammation and/or maintaining homeostasis of colorectal tissue functions (20). PGE2 exerts its cellular effects by binding to its cognate receptors (EP1, EP2, EP3, or EP4), which belong to the G-protein-coupled receptor (GPCR) family. Each of these receptors can enhance multiple physiologic functions such as carcinogenesis, inflammatory bowel disease, and Alzheimer disease (21–24). GPCRs are involved in aberrant intracellular signal transmission that is often associated with tumor growth and metastasis. Therefore, targeting the GPCRs contributing to oncogenic signaling provides a rational approach for the development of novel anticancer therapies for cancer (25). EPs are coupled with G proteins to perform their functions by mobilizing intracellular Ca2+ and mediating activation of adenylyl cyclase (AC) and different downstream signaling pathways, including the PI3K, MAPK, and β-catenin signaling pathways (26–30). These signaling pathways are known to be closely associated with cancer development. Most experiments regarding the role of EP receptors in cancer are still at the cellular or animal level or in initial clinical trials. But data thus far still provides hope for application in cancer prevention or treatment. Here, we examine the roles and mechanisms of EP1, EP2, EP3, and EP4 each in the development of cancer.
EP1 promotes cancer
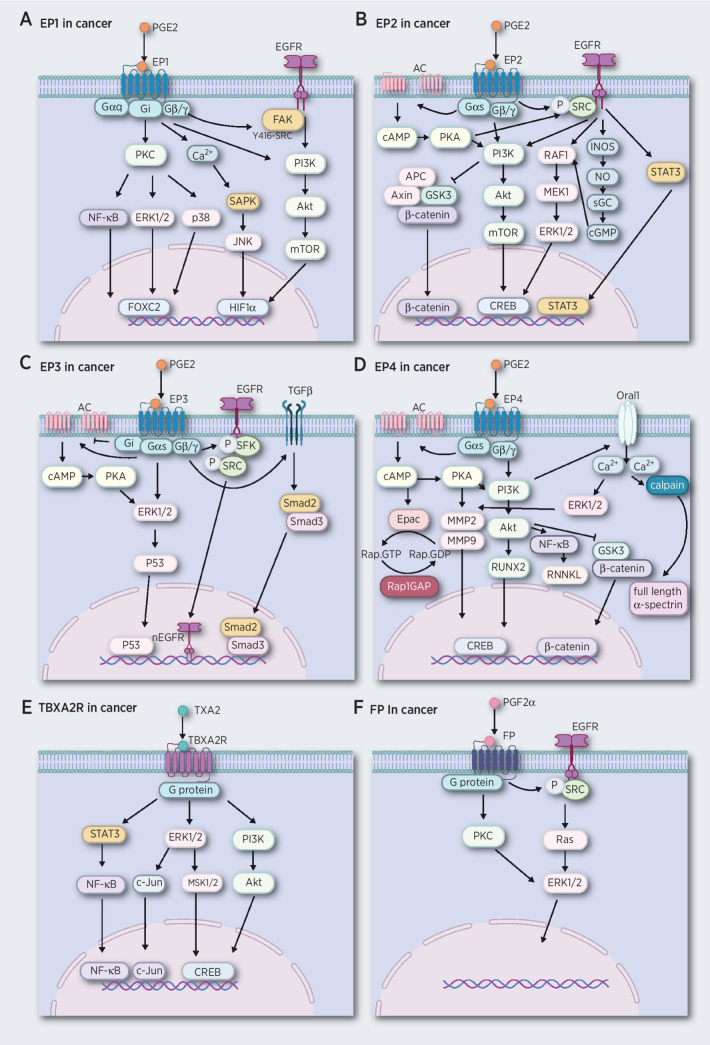
The EP1 receptor is highly expressed in various cancer tissues (31, 32), but shows the least affinity towards the PGE2 ligand compared with the other three EPs (33). These suggest that PGE2 must be at high levels to stimulate EP1 receptor activation, which is observed in multiple cancer types, including breast, ovarian, and colon (34–36). The EP1 receptor couples with G proteins and mediates cancer development by stimulating multiple signaling pathways in several cancer types. For example, PGE2 couples with EP1 enhancing cell growth, invasion, and migration in hepatocellular carcinoma cells (HCC) by activating the protein kinase C (PKC)/nuclear factor-kappa B (NF-κB)/Forkhead box protein C2 (FOXC2) and the EGFR/PI3K signaling pathways (37–39). Similarly, the EP1 receptor couples with Gi/o proteins promoting tumor growth and metastasis in osteoblastic (40, 41) and non–small cell lung cancer (NSCLC) cells (27) through the activation of PI3K and MAPK signaling pathways, resulting in activation of the E2F transcription factor 1 (E2F-1)/FOXC2 axis. In addition, the PGE2/EP1 signaling pathway enhances migration of oral cancer cells through intercellular adhesion molecule 1 (ICAM-1) production, which is a cell surface glycoprotein that plays an important role in tumor cell expansion or metastasis (42). Also, the upregulation of the EP1 receptor for PGE2 promotes skin tumor progression (43, 44). Collectively, PGE2 coupled with EP1 interacts with G proteins and triggers multiple signaling pathways, including EGFR, PI3K, MAPK, and NF-κB, that mediate the activation of transcription factors FOXC2 and HIF1α, resulting in enhanced cell growth and migration in cancer. (Fig. 1A).
Figure 1.
PG receptors mediate cancer development by activating multiple signaling pathways. A, The EP1 receptor links to G proteins and mediates signaling pathways by activation of EGFR, PKC, and increasing Ca2+ levels, leading to activation of the PI3K/AKT, MAPK, and NF-κB signaling pathways. B, The EP2 receptor couples to G proteins and performs its functions by inducing the AC system and enhancing the secondary messenger cAMP, leading to activation of PKA. The EP2 receptor also can activate EGFR, leading to activation of the PI3K/AKT, GSK3/β-catenin, RAF1/MEK1/ERK1/2, and STAT3 signaling pathways. C, The EP3 receptor couples to G proteins and performs its functions by inducing the AC/cAMP/PKA system and activation of EGFR and TGFβ receptors, leading to activation of ERK1/2/p53, Smad2/Smad3 activation, and nuclear translocation of EGFR. D, The EP4 receptors link to G proteins and activate the AC/cAMP/PKA system and Oral1, leading to activation of PI3K/AKT, NF-κB/RNNKL GSK3/β-catenin, ERKs, calpain, and Epac signaling pathways. E, The TBXA2R receptor couples to G proteins and performs its function by mediating PI3K/AKT, ERK1/2/MSK1/2/c-Jun, and STAT3/NF-κB signaling pathways. F, PGF2α links to G proteins and activates EGFR and leads to Ras/ERK1/2 signaling.
Several studies have investigated the role of EP1 in cancer by evaluating the effects of EP1 antagonists. For example, the EP1 antagonist SC-51089 inhibits growth and induces apoptosis in osteosarcoma and glioma cells (44–46). This evidence suggests that the EP1 receptor might be a potential target in some cancers. However, the current studies show the potential effectiveness only in in vitro studies or animal models. Thus, whether targeting the EP1 receptor will benefit human cancer is still not clear.
The role of EP2 in cancer progression
The PGE2 receptor 2 subtype (EP2) is a metabolite of AA that binds with PGE2 and regulates cellular responses to PGE2. Several studies show that the EP2 receptor protein level is abnormally expressed in various cancers, including prostate, breast, colon, and liver (45–49). Also the expression level of EP2 is substantially increased in patients with high-grade cervical intraepithelial neoplasia (50). These findings suggest that EP2 might have a role in the progression of cancer.
Cancer development is known to be closely associated with inflammation (51, 52). The binding of PGE2 with EP2 is one of several major inflammatory mediators driven by COX2 that can induce several proinflammatory factors (53). For example, activation of EP2 can markedly enhance the expression of IL1β and IL6, which can increase growth, invasion, and angiogenesis in several cancer types (48, 54–56). Indeed, the activation of EP2 is critical for cancer development. PGE2 binds with EP2 to activate AC, leading to increases in cAMP levels and protein kinase A (PKA), which subsequently triggers the activation of various downstream transcription factors, including cAMP response element-binding protein (CREB; refs. 57, 58). The EP2 receptor plays a major role in skin tumor development by mediating cAMP levels (59) and also facilitates activity of the EGFR in numerous cancers (28, 60–62). For example, EP2 transactivates the EGFR to regulate the proliferation and invasion capability of esophageal squamous cell carcinoma (ESCC) cells (60). EP2 enhances the tumor promotor, survivin, through the EGFR/STAT3 pathway in UVB-exposed mouse skin (61). It can also mediate IL1α expression through the EGFR/PI3K/protein kinase B (AKT) pathway in neoplastic cervical cells (28). EP2 promotes squamous cell carcinoma growth through EGFR transactivation and the inducible nitric oxide synthase (iNOS) and the extracellular-signal-regulated kinase 1/2 (ERK1/2) pathways (62). Besides the EGFR-associated pathways, PGE2 increases EP2 receptor expression, which triggers β-arrestin 1/JNK/profilin-1 activation and induces cell migration and proliferation in human mesenchymal stem cells (63). In addition, PGE2/EP2 mediates cancer development through the β-catenin signaling pathway and PGE2 stimulates colon cancer cell growth through the G proteins/axin/β-catenin signaling axis (29). The β-catenin signaling pathway is known to be one of the most significant pathways that enhances the progression of various cancers, including colon, breast, and ovarian (64–66). EP2 can activate PI3K/AKT signaling that results in activation of the glycogen synthase kinase 3 beta (GSK3β) and β-catenin pathways, which enhances the transcription of several genes including c-myc and cyclin D1 in cancer, resulting in enhanced tumorigenesis (67–71). On the basis of this evidence, the EP2 receptor can activate multiple oncogenic signaling pathways to perform its tumorigenic function in multiple cancer types.
Overall, PGE2 binding with EP2 interacts with G proteins and activates AC and EGFR, which triggers multiple signaling pathways. These pathways include β-catenin, PI3K, ERKs, and STAT3, which markedly influence inflammation, tumor growth, migration, and apoptosis (Fig. 1B). Compared with EP1, EP2 performs its functions through multiple signaling pathways in some cancer types. Targeting EP2 with antagonists like TG4–155, or TG6–129 inhibits cell growth and metastasis in multiple cancer types, including colorectal, prostate, and neuroblastoma (72, 73). A phase Ia/Ib study of the EP2/EP4 antagonist, TPST-1495, is being conducted in solid tumors, including colorectal, lung, head and neck, urothelial, endometrial, gastroesophageal, and gastric cancers (NCT04344795) and could provide much needed evidence for potential clinical use.
EP3 exerts duality in cancer
The function of EP3 in tumorigenesis appears to be a double-edged sword because some studies suggest that the EP3 receptor plays a critical role in tumor development (74–81) and others suggest no role for EP3. For example, as a factor in tumor development, EP3 might facilitate the migration of cervical cancer cells by activating the phosphorylated ERK1/2 and p53 signaling pathways, which modulate the expression of plasminogen activator inhibitor type 1 (PAI-1) and urokinase-type plasminogen activator receptor (uPAR). PAI-1 has a protumorigenic role in cancer enhancing angiogenesis and tumor cell survival (82). uPAR expression enhances invasion and metastasis in tumors (83). The EP3 receptor antagonist, L798,106, can reduce proliferation and migration of SK-BR-3 breast cancer cells (75) and inhibition of EP3 attenuates migration and promotes apoptosis of NSCLC cells through the TGFβ/Smad signaling pathway (76). In addition, deletion of the EP3 receptor suppresses tumor-associated angiogenesis in the stroma by reducing VEGF and matrix metalloproteinase-9 (MMP9) expression (77–79), both of which are angiogenic activators. PGE2/EP3/c-SRC signaling regulates tumor growth and malignant progression by inducing EGFR nuclear translocation. Notably, nuclear EGFR is a hallmark of cancer aggressiveness (81). Collectively, PGE2 binds with EP3 then activates AC, EGFR, and TGFβ. The activation of these signaling pathways leads to p53, EFGR, and Smad2/Smad3 nuclear translocation enhancing tumor growth and malignant progression (Fig. 1C).
In contrast to these studies, some have shown that the EP3 receptor has no effect on tumorigenesis. EP1, EP2, and EP4 are highly expressed in breast cancer tissue compared with normal mammary tissue, whereas EP3 receptor expression appears to be downregulated in these cancer tissues (84). For instance, the expression level of EP3 is decreased in late-stage colon cancer compared with earlier stages (85). Similar results were observed in breast and skin cancers (84, 86, 87). These findings indicate that EP3 might exert negative or no obvious effects in mediating tumorigenesis of these cancers. Indeed, EP3 receptor expression does not appear to affect skin tumor development (59) and deletion of this receptor has no effect on colon tumor development in APCmin mice (88).
Notably, various isoforms of EP3 are generated by alternative mRNA splicing (89), which could account for the distinct effects of EP3 observed in different tumor types. The EP3 receptor has multiple splice variants differing in their C-terminal tails. In human tissue, nine mRNA and eight isoforms (EP3I, EP3II, EP3III, EP3IV, EP3V, EP3IV, EP3e, and EP3f) have been described (90–92). EP3 receptor variants are mostly expressed in clusters of multiple isoforms. For example, the human uterus expresses mRNA for the EP3V and EP3VI receptor isoforms, whereas in primary keratinocytes, the EP3I, EP3II, and EP3IV splice variants are expressed (93). These suggest that the role of the EP3 receptor is likely tumor- and cell-type specific and the different roles of the EP3 receptor in cancer might be due to the various isoforms. On the basis of these reports, the EP3 receptor does not appear to be a promising target for cancer treatment.
Targeting EP4 in cancer
The EP4 receptor is the most widely studied EP receptor in terms of its involvement in cancer development and is one of the most promising targets for the prevention and treatment of cancer. This receptor is upregulated in numerous cancers, including breast, colon, prostate, ovary, lung, and skin (89, 90, 94, 95, 96). The EP4 receptor is associated with multiple signaling pathways that are involved in cancer development and progression, including those governing proliferation, apoptosis, migration, and metastasis. EP4 plays a role in cell proliferation or apoptosis in several cancer types. EP4 can trigger the PKA/PI3K/AKT signaling pathway increasing proliferation in cells and xenograft prostate cancer models (91, 92). Inhibition of EP4 receptor-mediated signaling suppresses IGF1 and PKA-induced proliferation of pancreatic cancer cells (93, 97). EP4 antagonists hinder proliferation and induce apoptosis in cervical, colon, and oral squamous carcinoma cells by inhibiting the activation of the cyclic adenosine monophosphate/PKA/CREB axis as well as pathways involving the EGFR/Ras/MAPK/CREB and AKT/GSK3β/β-catenin pathways in human cancer cell lines (98–100). EP4 is also associated with increased migration in several cancer types by activation of the PI3K, ERKs, Rap GTPase, and arrestin1/c-Src pathways. For example, the EP4 receptor mediates migration through the activation of PI3K/Orai1/ERKs signaling in oral cancer cells (101). Activation of the EP4 receptor promotes renal cell invasion and metastasis through the Rap GTPase signaling pathway (102–105). PGE2 binding with EP4 can promote lung cancer cell migration through the EP4/β-arrestin1/c-Src pathway, which activates invasion and metastasis (106, 107). The EP4 receptor facilitates invasion of prostate cancer by mediating the cAMP/PKA/PI3K/AKT signaling pathway (92). Importantly, antagonists of EP4 suppress invasion, migration, and metastasis of prostate, breast, and colon cancer cells (96, 108–110). All these preclinical studies suggest that targeting the EP4 receptor should be one potential way to suppress cancer progression by inhibiting cell proliferation, apoptosis, invasion, and migration.
In addition, EP4 contributes to the activity of the immune system, enhancing the efficacy of immunotherapy. PGE2 activation of EP4 in natural killer (NK) cells can regulate cytokines that promote the replication and activation of cytotoxic T lymphocytes. The critical interactions between NK and dendric cells further drive both innate and adaptive antitumor responses. Each of these functions is regulated by PGE2 acting on EP4 expressed on NK cells (95, 111–113). In particular, antagonism of EP4 inhibits metastasis and enhances NK function and this inhibitory function is lost in mice lacking NK cells (111). Activation of the EP4 receptor on myeloid-derived suppressor cells mediates the p38/ERKs pathway, leading to secretion of TGFβ, which inhibits proliferation of NK cells (114). As indicated earlier, EP4 receptor antagonists are critical in cancer immunotherapy (95, 115, 116). For example, a combination of a PD-L1 antibody and an EP4 antagonist enhances immunotherapeutic efficacy, suggesting that inhibiting EP4 expression might offer a potential strategy for increasing the efficacy of immunotherapy (117, 118).
EP4 may also influence or be influenced by miRNA, which are a family of small noncoding RNAs that regulate a wide array of biological processes, including carcinogenesis (119). In cancer, some miRNAs inhibit or enhance carcinogenesis by mediating the EP4 receptor. For example, miR-92 has been shown to suppress gastric cancer cell proliferation and induce apoptosis by targeting the EP4/Notch1 axis, which plays important roles in biological processes that include proliferation and apoptosis (120, 121). Therapeutic strategies against miR-101 act by posttranscriptionally regulating the expression of the EP4 receptor in colon cancers (122). miR-155 is involved in immunomodulatory therapies through its modulation of macrophages' responses to nontuberculous mycobacteria through COX2/PGE2 signaling. Blocking EP4 can reduce bacterial survival in macrophages (123). In contrast, EP4 mediates cancer progression by targeting certain miRNAs. For example, the activation of EP4 can elevate oncogenic miR-526, which promotes breast cancer progression (124). EP4 siRNA or antagonists can impair NF-κB activity and upregulate miR-16 and miR-21 and cell proliferation in gastric cancer (125). This evidence suggests that the EP4 receptor is closely associated with some miRNAs in mediating carcinogenesis. This cumulative evidence indicates that EP4 is a potential preventive or therapeutic target, which is associated with multiple signaling pathways that include the Rap GTPase, PKA/MMPs, PI3K, ERK1/2, NF-κB, and β-catenin conduits (Fig. 1D). Importantly, several clinical trials are being undertaken to study the effect of EP4 antagonists in advanced cancer and metastatic tumors. For example, a phase Ib clinical study is being conducted to evaluate the safety and preliminary efficacy of E7046 in combination with pembrolizumab in patients with locally advanced/metastatic tumors (NCT04432857). Clinical studies are also evaluating the safety, tolerability, pharmacokinetics, efficacy, and biomarker potential of ONO-4578 in subjects with advanced or metastatic solid tumors and subjects with unresectable, advanced or recurrent gastric or colorectal cancer (NCT03661632; NCT03155061). In addition, a phase I clinical study to assess safety and tolerability of AAT-007 is being performed in patients with microsatellite stable colorectal cancer (NCT03658772). Although these clinical trials are still in their infant phase I stage, they could provide us with hope for effective targeting of EP4 in cancer therapy.
TXA2 and TBXA2R Are Potential Targets in Cancer
TXA2 synthesis is driven by the COXs and these enzymes convert AA to the precursor molecule PGH2, which is then transformed to TXA2 through the TXA2 synthase pathway and exerts its biological activities through the TBXA2 receptor (TBXA2R; refs. 1, 3). TBXA2R has two separate isoforms that are termed TBXA2Rα and TBXA2Rβ. TBXA2Rα is broadly expressed in numerous tissues whereas the splice variant, TBXA2Rβ, may have a more limited tissue distribution. The first 328 amino acids are the same for both TBXA2Rα and TBXA2Rβ isoforms, but the β isoform exhibits an extended C-terminal cytoplasmic domain. Both isoforms stimulate cells in part by activating the G proteins (126). Accumulating evidence demonstrates that TXA2-related molecules are actively involved in tumor progression (127–130).
Notably, solid evidence suggests that circulating levels of TXB2, which is the stable metabolite of TXA2, could serve as a potential biomarker for early detection of Barrett esophagus (BE) and esophageal adenocarcinoma (EAC; ref. 127). In addition, one report indicated that colorectal cancer progression is associated with higher circulating levels of TXB2 (128). Also, another group reported that TBXA2R may have significant clinical potential as a diagnostic biomarker and predictor of prostate cancer disease recurrence (131). High levels of TXA2 are associated with enhanced proliferation of various cancer cells, including esophageal, breast, lung, and colon cancer (127, 128, 129, 132). Consistent with a role in cancer progression, TXAS and TBXA2R overexpression has been observed in many tumor tissues, including breast, prostate, lung, colon, bladder, and esophageal (119–122, 127, 128, 130, 132, 133). Several studies have already studied the function of TXA2 pathways in breast, prostate, lung, and gastrointestinal cancers.
The TXA2 pathway plays a critical role in breast cancer development because TXAS or the TXA2R is required for breast cancer cell growth and invasion capabilities. The expression level of TBXA2R negatively correlates with disease-free and overall survival in human breast cancer (130). The TXA2R enhances triple negative breast cancer cell migration and invasion and activates the Rho signaling pathway, which mediates cell death in tumors (123, 124). Switching off TXA2 biosynthesis has been reported to effectively suppress either MMTV-HER-2-driven mammary tumorigenesis or breast cancer metastasis in preclinical animal models (129).
The TXA2 pathway appears to play an important role in prostate cancer because tumor suppressor genes like Wilms's tumor (WT)1, Forkhead box P1 (FOXP1), and NK3 Homeobox 1 (NKX3.1) transcriptionally decrease TBXA2R expression in prostate cancer (125, 134, 135). For example, the transcriptional regulator WT1 is expressed and implicated in prostate and breast cancers and transcriptionally represses TBXA2Rα expression through GC-enrich WT1 cis-elements (135). TBXA2R promotes proliferation and migration in prostate cancer by activating protein kinase C-related kinase (PRK) signaling, which is associated with many physiological functions, including tumor growth and metastasis (136–139). TBXA2R is also reported to facilitate prostate cancer cell motility and cytoskeleton reorganization through the activation of Rho kinase (ROCK), which is one of the best characterized effectors of the small GTPase RhoA-regulating tumor progression (119).
The TXA2 pathway also enhances lung cancer development. 4-(Methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) is potent carcinogen that has been found to effectively induce various types of lung cancer in mouse models (140). NNK treatment can enhance the level of TXA2, which binds with the TBXA2R to enhance cell proliferation through the activation of CREB. CREB is downstream of the PI3K/AKT and COX2/ERKs/NF-κB pathways. The TXA2 pathway also plays a critical role in increasing gastrointestinal cancer growth as observed by TBXA2R facilitation of colorectal cancer cell growth through the activation of the cAMP and Gαq/PLC signaling pathways (128, 133, 141, 142). In addition, the COX1/2-driven TBXA2 pathway facilitates BE and EAC through the activation of the ERKs/mitogen- and stress-activated protein kinases (MSK)/CREB/c-Jun and STAT3/NF-κB pathways (127).
Agonists and antagonists of TBXA2 have also been shown to influence tumor growth in various cancers. For instance, U46619 and (Z)-7-[(1S,2R,3R,4R)-3-[(E,3R)-3-hydroxy- 4-(4-iodophenoxy)but-1-enyl]-7-oxabicyclo[2.2.1]heptan-2-yl]hept-5-enoic acid (I-BOP) are TBXA2R agonists that stimulate cell proliferation in a PKA/CREB-dependent manner (132, 143, 144). In contract, the TBXA2R antagonist, SQ29548, can effectively inhibit prostate and lung cancer cell growth (119, 121). Overall, the TBXA2R is a potential target for cancer treatment through the mediation of multiple signaling pathways, such as NF-κB, ERKs, and PI3K (Fig. 1E). Several studies demonstrate the concept of targeting the TBXA2 signaling pathway in cancer treatment. Increasing attention focuses on the potential of targeting TBXA2R. A phase II clinical trial is being conducted to study the TBXA2R antagonist ifetroban in treating patients with malignant solid tumors at high risk of metastatic recurrence (NCT03694249). This study could provide us with the needed information to effectively use a TBXA2R antagonist for cancer treatment in humans.
The Role of PGI2 and I-prostanoid in Cancer
PGI2, also called prostacyclin, is produced by PGI synthase (PGIS) and has a single I-prostanoid (IP) receptor (145). PGIS encodes a member of the cytochrome P450 superfamily that catalyzes the metabolism of many drugs and the synthesis of lipids, such as cholesterol and various steroids. In addition, PGIS might be involved in oxidative stress, xenobiotic and drug metabolism, and prostaglandin metabolism (146, 147). The PGI2 product acts as a vasodilator and anticoagulant to maintain vascular homeostasis and is involved in inflammatory responses and activation of CD4+ T cells during numerous physiologic processes such as allergic diseases and asthma (148).
PGI2 and TXA2 are known to exert opposing effects on vasculature. PGI2 is a vasodilator, which inactivates platelets and inhibits their aggregation, whereas TXA2 has opposite effects as a vasoconstrictor. In cancer, PGI2 and TXA2 and their corresponding enzymes, PGIS and TXAS, respectively, have been shown to exert markedly contrasting effects (149, 150). The PGIS/PGI2 signaling pathway acts as a tumor suppressor in cancer development. The expression of PGIS is lower in bladder, cervical, colorectal, head and neck, leukemia, lung, ovarian, and prostate cancers compared with normal tissues. Kaplan–Meier plots have shown that low PGIS is associated with poor overall and shorter progression-free survival in patients with lung, ovarian, and gastric cancer (151). Hypermethylation of the PGIS promoter has been reported to be associated with its diminished gene expression in colorectal carcinogenesis (152). Deletion of PGIS increases the number of azoxymethane (AOM)-induced colon polyps and enhances colon carcinogenesis in mice (153). Its product, PGI2, has been implicated as a potential marker, potent antimitogenic, antimetastatic agent, or chemopreventive agent in cancer (154–157). A PGI2 analog has been reported to suppress lung cancer metastasis in a mouse lung metastasis model by recruiting pericytes in tumor angiogenesis (158). PGI2 analogues can also activate PPARδ and PPARγ and have been shown to inhibit the growth of lung cancer cells (159, 160).
Collectively, the mechanisms involving the PGIS/PGI2 signaling pathway in cancer cells are still uncertain. Current information suggests that the PGI2 signaling pathway might function as a tumor suppressor. The studies focusing on PGI2 and IP in cancer are still in the early stages and further studies are still needed to clarify the functions of this signaling pathway.
PGD2 and DP1/2 Act as Tumor Suppressors
PGD2 is produced from PGH2 by lipocalin-type PGD synthase (L-PGDS) or hematopoietic PGD synthase (H-PGDS). It performs its biological functions by binding with D prostanoid receptor 1 (DP1) and chemoattractant receptor-homologous molecule expressed on Th2 cells (CRTH2; alternative name DP2; ref. 161). L-PGDS is mainly expressed in central nerves and is known to be associated with sleep (162) and H-PGDS has been identified as a possible drug target, due to its involvement in asthma and inflammation (163).
The PGD2 pathway reportedly acts as a tumor suppressor and gene deficiency of the DP receptor enhances angiogenesis and tumor growth in a mouse tumor transplantation model (164). H-PGDS gene deficiency significantly decreases the production of PGD2 and strongly accelerates the growth of implanted Lewis lung carcinoma cells (165). H-PGDS deficiency exacerbates colitis and enhances the number of adenomas in an AOM/dextran sulfate sodium-induced colon cancer model. Moreover, deletion of H-PGDS also increases intestinal polyposis in an APC mutation mouse model. Furthermore, deletion of H-PGDS can enhance inflammatory cytokine expression, especially TNFα in mouse models (166, 167). Inhibition of L-PGDS and DP2 by Yes-associated protein 1 is associated with carcinogenesis in various organs and promotes self-renewal of gastric cancer cells (168, 169). Finally, deletion of L-PGDS accelerates the growth of melanoma in mice, whereas treatment with an agonist of the DP1 receptor attenuates growth (170). This evidence demonstrates the potential of targeting PGD2 in cancer treatment. However, more clinically relevant studies should be conducted to further evaluate the function of PGD2.
The Function of PGF2α and FP in Cancer
PGF2α is produced by PGFS and binds to a cognate G-protein-coupled receptor, FP, to perform its function in biological processes as diverse as smooth muscle contraction, regulation of intraocular pressure, renal filtration, inhibition of adipocyte differentiation, and hair growth stimulation (Fig. 1F; ref. 171). FP is expressed as two alternatively spliced transcript variants encoding different isoforms, FPA and FPB, which have varying C-terminal lengths. FPA and FPB receptor isoforms have similar pharmacologic properties. Both isoforms are able to stimulate IP accumulation to the same degree, but the basal level of hydrolysis is 130% higher for the FPB isoform than for the FPA isoform (172). The PGF2α signaling pathway is also involved in mediating cancer development because PGF2α has been shown to promote carcinogen-induced malignant transformation (173). Studies of prostaglandin production in colon cancer have shown that the production levels of PGF2α are 30-fold higher than for PGE2 in intestinal tissue from patients with familial adenomatous polyposis (FAP; ref. 174). PGF2α might have a critical role in FAP patients. Actually, studies demonstrate that PGF2α increases chloride secretion in a cAMP-dependent manner. This observation may have implications for a variety of inflammatory disorders, including infectious colitis (i.e., clostridium difficile) as well as inflammatory bowel disease (175). PGF2α also stimulate motility and invasion of colorectal tumor cells in an animal invasion assay model (176, 177). In addition, the PGF2α-FP receptor is reported to activate EGFR and then the subsequent phosphorylation of ERK1/2 mediates angiogenesis in endometrial adenocarcinoma (178). These data show that PGF2α may play a promoting role in the malignant progression of tumors. Although the FP antagonist AL8810 can suppress prostate cancer cell proliferation, direct evidence supporting inhibition of tumor development in humans by targeting FP is still very limited. Further studies are clearly needed to understand the biological role of PGF2 in cancer development.
Inhibition of PG Pathways Provides a Potential Strategy for Attenuating Cancer Development
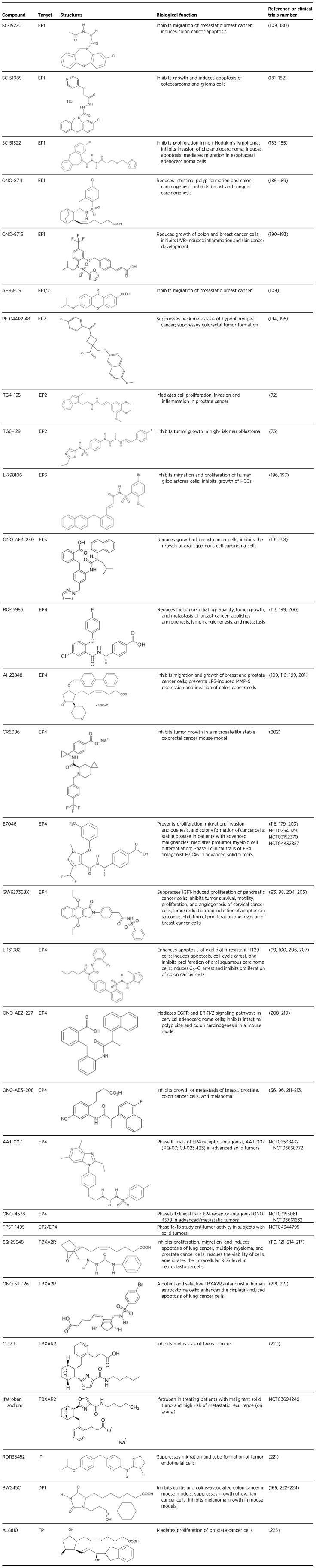
This review thus far has examined the role of PG pathways in tumorigenesis. The accumulated findings demonstrate that PG receptors could be potential targets for cancer prevention and therapy. In recent years, several small molecules with anticancer activity that target PG receptors have been identified in preclinical and clinical studies focusing on multiple cancer types. The effects of various antagonists and agonists of PG receptors in cancer are summarized in Table 1. A number of extensive studies are focusing on the signaling pathways of PG receptors and several clinical studies are being conducted to determine the effectiveness of antagonists of EP and TXA2R against cancer development. As we partly mentioned previously, the EP receptor antagonists E7046 (NCT02540291; NCT03152370; NCT04432857), ONO-4578 (NCT03661632; NCT03155061), AAT-007 (NCT02538432; NCT03658772), and TPST-1495(NCT04344795) are being used to evaluate their inhibitory effects in advanced cancers and metastatic tumors. In addition, a clinical trial with the TBXA2 receptor antagonist, ifetroban, is being conducted in the treatment of patients with malignant solid tumors at high risk of metastatic recurrence (NCT03694249). All these studies should provide evidence as to whether targeting the PG receptors could be a promising strategy for cancer treatment.
Table 1.
Targeting prostaglandin signaling pathways in cancer.
Limitations and Adverse Effects Associated with Targeting PG Pathways
Preclinical, epidemiologic, and clinical studies have demonstrated that targeting the COX pathway could be effective in the prevention and treatment of multiple cancer types (9–11). However, suppression of COX signaling by the use of NSAIDs, or other inhibitors has been limited because the use of these drugs is associated with various side effects, including gastrointestinal toxicity and increased risk of myocardial infarction (12, 13). Extensive studies were conducted to determine the downstream signaling pathways of the PG receptors (Fig. 1), with the hope that targeting PG receptors might circumvent the toxic effects associated with COX inhibition while still exerting anticancer effects. Thus, several small molecules have been demonstrated to exert anticancer effects in multiple cancer types by targeting PG receptors (Table 1). However, whether these small molecules are effective clinically with less toxicity is still not clear although several clinical studies targeting EP receptors or the TBXA2 receptor have been conducted or are ongoing. Further studies will be needed to evaluate the effectiveness versus the unwanted side effects that have been associated with targeting PG receptors downstream of COX. Another limitation is that directly inhibiting individual PG receptors may not be as efficient as targeting COX with NSAIDS, which likely suppresses the majority of the PG pathways. Bioavailability has always been an issue with drug treatments, and thus, to improve bioavailability, multiple inhibitors might be required to attenuate cancer development. In fact, smaller doses of multiple inhibitors might be less toxic overall. For example, the TXA2:PGI2 balance is known to be critical for maintaining a healthy cardiovascular system (179). Targeting COX cannot specifically mediate the TXA2, PGI2, or other downstream signaling pathways. Thus, a combination of PG inhibitors that maintains a proper balance of TXA2 and PGI2 might have greater potential to suppress cancer development and avoid cardiovascular toxicity. Unfortunately, targeting the PG receptors has not yet made it past the stage of biological tools and future clinical studies are absolutely required to determine their potential in cancer prevention and treatment.
Summary
In this review, we examined the role the PG pathways in cancer development. Generally, PG pathways contribute to tumorigenesis by mediating cell proliferation, growth, apoptosis, invasion, migration, metastasis, and angiogenesis. PGs are critical mediators in cancer and efforts have focused on identifying the signaling pathways activated by PG receptors in multiple cancer types (Fig. 1; Supplementary Table S1). The PGE2/EPs, TXA2/TBXA2R, and PGF2α/FP signaling pathways mainly act as cancer promoters, whereas the PGI2/IP and PGD2/DP axes appear to primarily act as cancer suppressors. The accumulating evidence provides hope for targeting PG receptors, including EPs and TBXA2R to suppress tumorigenesis. In the extracellular microenvironment, these PGs bind with G protein receptors including EP1–4, TBXA2R, IP, DP, or FP to influence multiple cancer types. PG antagonists or antagonists interrupt binding between the PGs and receptors to suppress the development and growth of tumors (Supplementary Fig. S2). PG antagonists or agonists, in particular those targeting the EPs and TBXA2R receptors, have been successful in preclinical studies in the inhibition of tumorigenesis or metastasis (Table 1). Many studies have focused on the role of EPs, especially EP4, in the development of cancer. Indeed, EPs have shown their potential as biological targets in cancer. Notably, TXA2 and TBXA2R are also very promising targets for esophageal and colon cancer prevention and treatment because of their high expression in patients with BE, EAC, and colorectal cancer compared with normal subjects. Notably, the absolute change in TXA2 in these cancers is markedly higher compared to changes in PGE2 (127, 128). These results suggest that the TXA2 pathway might be more important in gastrointestinal cancers compared with PGE2 and other PGs. On the basis of preclinical and clinical studies, targeting EP4 and TBXA2R might be a productive and effective way to treat cancer. However, as the many studies demonstrated in this review, most evidence for the role of PG receptors has been generated from in vitro or animal studies. In particular, whether targeting EP2 can bring benefits in the clinical treatment of cancer still remains elusive. Overall, this review examined the function of PG signaling pathways in cancer and suggest that targeting PG pathways might provide opportunities for cancer prevention and therapy.
Authors' Disclosures
No disclosures were reported.
Supplementary Material
Acknowledgments
This work was supported by The Hormel Foundation and NIH grant 1P01CA229112-01A1.
Footnotes
Note: Supplementary data for this article are available at Cancer Research Online (http://cancerres.aacrjournals.org/).
References
- 1. Wang D, DuBois RN. Eicosanoids and cancer. Nat Rev Cancer 2010;10:181–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jabbour H, Sales K. Prostaglandin receptor signalling and function in human endometrial pathology. Trends Endocrinol Metab 2004;15:398–404. [DOI] [PubMed] [Google Scholar]
- 3. Vane J, Bakhle Y, Botting R. Cyclooxygenases 1 and 2. Annu Rev Pharmacol Toxicol 1998;38:97–120. [DOI] [PubMed] [Google Scholar]
- 4. Wang M-T, Honn KV, Nie D. Cyclooxygenases, prostanoids, and tumor progression. Cancer Metastasis Rev 2007;26:525. [DOI] [PubMed] [Google Scholar]
- 5. Menter DG, Schilsky RL, DuBois RN. Cyclooxygenase-2 and cancer treatment: understanding the risk should be worth the reward. Clin Cancer Res 2010:1078–0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schuster VL. Molecular mechanisms of prostaglandin transport. Annu Rev Physiol 1998;60:221–42. [DOI] [PubMed] [Google Scholar]
- 7. Schuster VL. Prostaglandin transport. Prostaglandins Other Lipid Mediat 2002;68:633–47. [DOI] [PubMed] [Google Scholar]
- 8. Takeuchi K, Kato S, Amagase K. Prostaglandin EP receptors involved in modulating gastrointestinal mucosal integrity. J Pharmacol Sci 2010;114:248–61. [DOI] [PubMed] [Google Scholar]
- 9. Ghosh N, Chaki R, Mandal V, Mandal SC. COX-2 as a target for cancer chemotherapy. Pharmacol Rep 2010;62:233–44. [DOI] [PubMed] [Google Scholar]
- 10. Khan Z, Khan N, P Tiwari R, K Sah N, Prasad G, S Bisen P. Biology of Cox-2: an application in cancer therapeutics. Curr Drug Targets 2011;12:1082–93. [DOI] [PubMed] [Google Scholar]
- 11. Mantovani G, Macciò A, Madeddu C, Serpe R, Antoni G, Massa E, et al. Phase II nonrandomized study of the efficacy and safety of COX-2 inhibitor celecoxib on patients with cancer cachexia. J Mol Med 2010;88:85–92. [DOI] [PubMed] [Google Scholar]
- 12. Lanas A, Wu P, Medin J, Mills EJ. Low doses of acetylsalicylic acid increase risk of gastrointestinal bleeding in a meta-analysis. Clin Gastroenterol Hepatol 2011;9:762–8. [DOI] [PubMed] [Google Scholar]
- 13. Trelle S, Reichenbach S, Wandel S, Hildebrand P, Tschannen B, Villiger PM, et al. Cardiovascular safety of non-steroidal anti-inflammatory drugs: network meta-analysis. BMJ 2011;342:c7086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. FitzGerald GA. Coxibs and cardiovascular disease. N Engl J Med 2004;351:1709–11. [DOI] [PubMed] [Google Scholar]
- 15. Sun X, Li Q. Prostaglandin EP2 receptor: novel therapeutic target for human cancers. Int J Mol Med 2018;42:1203–14. [DOI] [PubMed] [Google Scholar]
- 16. Ricciotti E, FitzGerald GA. Prostaglandins and inflammation. Arterioscler Thromb Vasc Biol 2011;31:986–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kalinski P. Regulation of immune responses by prostaglandin E2. J Immunol 2012;188:21–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gudis K, Tatsuguchi A, Wada K, Futagami S, Nagata K, Hiratsuka T, et al. Microsomal prostaglandin E synthase (mPGES)-1, mPGES-2 and cytosolic PGES expression in human gastritis and gastric ulcer tissue. Lab Invest 2005;85:225–36. [DOI] [PubMed] [Google Scholar]
- 19. Tai H-H, Ensor CM, Tong M, Zhou H, Yan F. Prostaglandin catabolizing enzymes. Prostaglandins Other Lipid Mediat 2002;68:483–93. [DOI] [PubMed] [Google Scholar]
- 20. Endo S, Suganami A, Fukushima K, Senoo K, Araki Y, Regan JW, et al. 15-Keto-PGE2 acts as a biased/partial agonist to terminate PGE2-evoked signaling. J Biol Chem 2020;295:13338–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Narumiya S, Sugimoto Y, Ushikubi F. Prostanoid receptors: structures, properties, and functions. Physiol Rev 1999;79:1193–226. [DOI] [PubMed] [Google Scholar]
- 22. Sugimoto Y, Narumiya S. Prostaglandin E receptors. J Biol Chem 2007;282:11613–7. [DOI] [PubMed] [Google Scholar]
- 23. Maingret V, Barthet G, Deforges S, Jiang N, Mulle C, Amédée T. PGE2-EP3 signaling pathway impairs hippocampal presynaptic long-term plasticity in a mouse model of Alzheimer's disease. Neurobiol Aging 2017;50:13–24. [DOI] [PubMed] [Google Scholar]
- 24. Takeuchi K, Amagase K. Roles of cyclooxygenase, prostaglandin E2 and EP receptors in mucosal protection and ulcer healing in the gastrointestinal tract. Curr Pharm Des 2018;24:2002–11. [DOI] [PubMed] [Google Scholar]
- 25. Dorsam RT, Gutkind JS. G-protein-coupled receptors and cancer. Nat Rev Cancer 2007;7:79. [DOI] [PubMed] [Google Scholar]
- 26. Sokolowska M, Chen L-Y, Liu Y, Martinez-Anton A, Qi H-Y, Logun C, et al. Prostaglandin E2 inhibits NLRP3 inflammasome activation through EP4 receptor and intracellular cyclic AMP in human macrophages. J Immunol 2015;194:5472–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pan J, Yang Q, Shao J, Zhang L, Ma J, Wang Y, et al. Cyclooxygenase-2 induced β1-integrin expression in NSCLC and promoted cell invasion via the EP1/MAPK/E2F-1/FoxC2 signal pathway. Sci Rep 2016;6:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Adefuye AO, Sales KJ, Katz AA. Seminal plasma induces the expression of IL-1α in normal and neoplastic cervical cells via EP2/EGFR/PI3K/AKT pathway. J Mol Signaling 2014;9:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Castellone MD, Teramoto H, Williams BO, Druey KM, Gutkind JS. Prostaglandin E2 promotes colon cancer cell growth through a Gs-axin-ß-catenin signaling axis. Science 2005;310:1504–10. [DOI] [PubMed] [Google Scholar]
- 30. O'callaghan G, Houston A. Prostaglandin E2 and the EP receptors in malignancy: possible therapeutic targets? Br J Pharmacol 2015;172:5239–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. O'callaghan G, Kelly J, Shanahan F, Houston A. Prostaglandin E 2 stimulates Fas ligand expression via the EP1 receptor in colon cancer cells. Br J Cancer 2008;99:502–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ma X, Kundu N, Ioffe OB, Goloubeva O, Konger R, Baquet C, et al. Prostaglandin E receptor EP1 suppresses breast cancer metastasis and is linked to survival differences and cancer disparities. Mol Cancer Res 2010;8:1310–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dey I, Lejeune M, Chadee K. Prostaglandin E2 receptor distribution and function in the gastrointestinal tract. Br J Pharmacol 2006;149:611–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bilal M, Al-Saleh J, Fakher FA. Serum levels of prostaglandin E2 (PGE2) and interleukin 17 (IL-17) are associated with angiogenesis and metastasis in breast cancer patients. Angiogenesis 2021;23:56.1. [Google Scholar]
- 35. Nagaraja AS, Dorniak PL, Sadaoui NC, Kang Y, Lin T, Armaiz-Pena G, et al. Sustained adrenergic signaling leads to increased metastasis in ovarian cancer via increased PGE2 synthesis. Oncogene 2016;35:2390–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wang D, Fu L, Sun H, Guo L, DuBois RN. Prostaglandin E2 promotes colorectal cancer stem cell expansion and metastasis in mice. Gastroenterology 2015;149:1884–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bai X, Wang J, Guo Y, Pan J, Yang Q, Zhang M, et al. Prostaglandin E 2 stimulates β1-integrin expression in hepatocellular carcinoma through the EP1 receptor/PKC/NF-κB pathway. Sci Rep 2014;4:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bai X, Wang J, Zhang L, Ma J, Zhang H, Xia S, et al. Prostaglandin E2 receptor EP1-mediated phosphorylation of focal adhesion kinase enhances cell adhesion and migration in hepatocellular carcinoma cells. Int J Oncol 2013;42:1833–41. [DOI] [PubMed] [Google Scholar]
- 39. Bai X-M, Jiang H, Ding J-X, Peng T, Ma J, Wang Y-H, et al. Prostaglandin E2 upregulates survivin expression via the EP1 receptor in hepatocellular carcinoma cells. Life Sci 2010;86:214–23. [DOI] [PubMed] [Google Scholar]
- 40. Ji R, Chou C-L, Xu W, Chen X-B, Woodward DF, Regan JW. EP1 prostanoid receptor coupling to Gi/o up-regulates the expression of hypoxia-inducible factor-1α through activation of a phosphoinositide-3 kinase signaling pathway. Mol Pharmacol 2010;77:1025–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Genetos DC, Lee CM, Wong A, Yellowley CE. HIF-1α regulates hypoxia-induced EP1 expression in osteoblastic cells. J Cell Biochem 2009;107:233–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yang S-F, Chen M-K, Hsieh Y-S, Chung T-T, Hsieh Y-H, Lin C-W, et al. Prostaglandin E2/EP1 signaling pathway enhances intercellular adhesion molecule 1 (ICAM-1) expression and cell motility in oral cancer cells. J Biol Chem 2010;285:29808–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Surh I, Rundhaug J, Pavone A, Mikulec C, Abel E, Fischer SM. Upregulation of the EP1 receptor for prostaglandin E2 promotes skin tumor progression. Mol Carcinog 2011;50:458–68. [DOI] [PubMed] [Google Scholar]
- 44. Surh I, Rundhaug JE, Pavone A, Mikulec C, Abel E, Simper M, et al. The EP1 receptor for prostaglandin E2 promotes the development and progression of malignant murine skin tumors. Mol Carcinog 2012;51:553–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hsu H-H, Lin Y-M, Shen C-Y, Shibu MA, Li S-Y, Chang S-H, et al. Prostaglandin E2-induced COX-2 expressions via EP2 and EP4 signaling pathways in human LoVo colon cancer cells. Int J Mol Sci 2017;18:1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hsu H-H, Chen M-C, Day CH, Lin Y-M, Li S-Y, Tu C-C, et al. Thymoquinone suppresses migration of LoVo human colon cancer cells by reducing prostaglandin E2 induced COX-2 activation. World J Gastroenterol 2017;23:1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lian S, Xia Y, Ung TT, Khoi PN, Yoon HJ, Lee SG, et al. Prostaglandin E2 stimulates urokinase-type plasminogen activator receptor via EP2 receptor-dependent signaling pathways in human AGS gastric cancer cells. Mol Carcinog 2017;56:664–80. [DOI] [PubMed] [Google Scholar]
- 48. Merz C, von Mässenhausen A, Queisser A, Vogel W, Andrén O, Kirfel J, et al. IL-6 overexpression in ERG-positive prostate cancer is mediated by prostaglandin receptor EP2. Am J Pathol 2016;186:974–84. [DOI] [PubMed] [Google Scholar]
- 49. Zang S, Ma X, Wu Y, Liu W, Cheng H, Li J, et al. PGE2 synthesis and signaling in malignant transformation and progression of human hepatocellular carcinoma. Hum Pathol 2017;63:120–7. [DOI] [PubMed] [Google Scholar]
- 50. Schmoeckel E, Fraungruber P, Kuhn C, Jeschke U, Mahner S, Kolben TM, et al. The role of EP-2 receptor expression in cervical intraepithelial neoplasia. Histochem Cell Biol 2020;154:655–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Coussens LM, Werb Z. Inflammation and cancer. Nature 2002;420:860–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Singh N, Baby D, Rajguru JP, Patil PB, Thakkannavar SS, Pujari VB. Inflammation and cancer. Ann Afr Med 2019;18:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Liu Y, Fang S, Li X, Feng J, Du J, Guo L, et al. Aspirin inhibits LPS-induced macrophage activation via the NF-κB pathway. Sci Rep 2017;7:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Gill SK, Yao Y, Kay LJ, Bewley MA, Marriott HM, Peachell PT. The anti-inflammatory effects of PGE2 on human lung macrophages are mediated by the EP4 receptor. Br J Pharmacol 2016;173:3099–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kawahara K, Hohjoh H, Inazumi T, Tsuchiya S, Sugimoto Y. Prostaglandin E2-induced inflammation: relevance of prostaglandin E receptors. Biochim Biophys Acta 2015;1851:414–21. [DOI] [PubMed] [Google Scholar]
- 56. Han IH, Kim JH, Jang KS, Ryu JS. Inflammatory mediators of prostate epithelial cells stimulated with Trichomonas vaginalis promote proliferative and invasive properties of prostate cancer cells. Prostate 2019;79:1133–46. [DOI] [PubMed] [Google Scholar]
- 57. Woo SM, Min KJ, Chae IG, Chun KS, Kwon TK. Silymarin suppresses the PGE2-induced cell migration through inhibition of EP2 activation; G protein-dependent PKA-CREB and G protein-independent Src-STAT3 signal pathways. Mol Carcinog 2015;54:216–28. [DOI] [PubMed] [Google Scholar]
- 58. Zhao J, Liu K, Lu J, Ma J, Zhang X, Jiang Y, et al. Alternariol induces DNA polymerase β expression through the PKA-CREB signaling pathway. Int J Oncol 2012;40:1923–8. [DOI] [PubMed] [Google Scholar]
- 59. Sung YM, He G, Fischer SM. Lack of expression of the EP2 but not EP3 receptor for prostaglandin E2 results in suppression of skin tumor development. Cancer Res 2005;65:9304–11. [DOI] [PubMed] [Google Scholar]
- 60. Cui F, Huang D, Zhang F, Gao E, Zhang Y, Cao Y, et al. Investigation on the regulatory effect of PGE2 on ESCC cells through the trans-activation of EGFR by EP2 and the relevant mechanism. Eur Rev Med Pharmacol Sci 2017;21:5668–76. [DOI] [PubMed] [Google Scholar]
- 61. Chun KS, Langenbach R. The prostaglandin E2 receptor, EP2, regulates survivin expression via an EGFR/STAT3 pathway in UVB-exposed mouse skin. Mol Carcinog 2011;50:439–48. [DOI] [PubMed] [Google Scholar]
- 62. Donnini S, Finetti F, Solito R, Terzuoli E, Sacchetti A, Morbidelli L, et al. EP2 prostanoid receptor promotes squamous cell carcinoma growth through epidermal growth factor receptor transactivation and iNOS and ERK1/2 pathways. FASEB J 2007;21:2418–30. [DOI] [PubMed] [Google Scholar]
- 63. Yun SP, Ryu JM, Jang MW, Han HJ. Interaction of profilin-1 and F-actin via a β-arrestin-1/JNK signaling pathway involved in prostaglandin E2-induced human mesenchymal stem cells migration and proliferation. J Cell Physiol 2011;226:559–71. [DOI] [PubMed] [Google Scholar]
- 64. Nguyen VHL, Hough R, Bernaudo S, Peng C. Wnt/β-catenin signalling in ovarian cancer: insights into its hyperactivation and function in tumorigenesis. J Ovarian Res 2019;12:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Ren L, Chen H, Song J, Chen X, Lin C, Zhang X, et al. MiR-454–3p-mediated Wnt/β-catenin signaling antagonists suppression promotes breast cancer metastasis. Theranostics 2019;9:449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Rubinstein MR, Baik JE, Lagana SM, Han RP, Raab WJ, Sahoo D, et al. Fusobacterium nucleatum promotes colorectal cancer by inducing Wnt/β-catenin modulator Annexin A1. EMBO Rep 2019;20:e47638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Fujino H, West KA, Regan JW. Phosphorylation of glycogen synthase kinase-3 and stimulation of T-cell factor signaling following activation of EP2 and EP4 prostanoid receptors by prostaglandin E2. J Biol Chem 2002;277:2614–9. [DOI] [PubMed] [Google Scholar]
- 68. Yang T-W, Gao Y-H, Ma S-Y, Wu Q, Li Z-F. Low-grade slightly elevated and polypoid colorectal adenomas display differential β-catenin-TCF/LEF activity, c-Myc, and cyclin D1 expression. World J Gastroenterol 2017;23:3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Prasad R, Katiyar SK. Ultraviolet radiation-induced inflammation activates β-catenin signaling in mouse skin and skin tumors. Int J Oncol 2014;44:1199–206. [DOI] [PubMed] [Google Scholar]
- 70. Vaid M, Singh T, Prasad R, Kappes JC, Katiyar SK. Therapeutic intervention of proanthocyanidins on the migration capacity of melanoma cells is mediated through PGE2 receptors and β-catenin signaling molecules. Am J Cancer Res 2015;5:3325. [PMC free article] [PubMed] [Google Scholar]
- 71. Chang H-H, Young SH, Sinnett-Smith J, Chou CEN, Moro A, Hertzer KM, et al. Prostaglandin E2 activates the mTORC1 pathway through an EP4/cAMP/PKA-and EP1/Ca2+-mediated mechanism in the human pancreatic carcinoma cell line PANC-1. Am J Physiol Cell Physiol 2015;309:C639–C49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Jiang J, Dingledine R. Role of prostaglandin receptor EP2 in the regulations of cancer cell proliferation, invasion, and inflammation. J Pharmacol Exp Ther 2013;344:360–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Hou R, Yu Y, Nguyen DT, Sluter MN, Li L, Yang J, et al. Prostaglandin receptor EP2 is a novel molecular target for high-risk neuroblastoma. BioRxiv 2020. [Google Scholar]
- 74. Ye Y, Peng L, Vattai A, Deuster E, Kuhn C, Dannecker C, et al. Prostaglandin E2 receptor 3 (EP3) signaling promotes migration of cervical cancer via urokinase-type plasminogen activator receptor (uPAR). J Cancer Res Clin Oncol 2020;146:2189–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Hester A, Salzmann B, Rahmeh M, Kolben T, Czogalla B, Ditsch N, et al. EP3 receptor antagonist L798, 106 reduces proliferation and migration of SK-BR-3 breast cancer cells. OncoTargets Ther 2019;12:6053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Li L, Lv Y, Yan D. Inhibition of Ep3 attenuates migration and promotes apoptosis of non- small cell lung cancer cells via suppression of TGF-β/Smad signaling. Oncol Lett 2018;16:5645–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Amano H, Hayashi I, Endo H, Kitasato H, Yamashina S, Maruyama T, et al. Host prostaglandin E2-EP3 signaling regulates tumor-associated angiogenesis and tumor growth. J Exp Med 2003;197:221–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Amano H, Ito Y, Suzuki T, Kato S, Matsui Y, Ogawa F, et al. Roles of a prostaglandin E-type receptor, EP3, in upregulation of matrix metalloproteinase-9 and vascular endothelial growth factor during enhancement of tumor metastasis. Cancer Sci 2009;100:2318–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Ogawa Y, Suzuki T, Oikawa A, Hosono K, Kubo H, Amano H, et al. Bone marrow-derived EP3-expressing stromal cells enhance tumor-associated angiogenesis and tumor growth. Biochem Biophys Res Commun 2009;382:720–5. [DOI] [PubMed] [Google Scholar]
- 80. Kubo H, Hosono K, Suzuki T, Ogawa Y, Kato H, Kamata H, et al. Host prostaglandin EP3 receptor signaling relevant to tumor-associated lymphangiogenesis. Biomed Pharmacother 2010;64:101–6. [DOI] [PubMed] [Google Scholar]
- 81. Bazzani L, Donnini S, Finetti F, Christofori G, Ziche M. PGE2/EP3/SRC signaling induces EGFR nuclear translocation and growth through EGFR ligands release in lung adenocarcinoma cells. Oncotarget 2017;8:31270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Placencio VR, DeClerck YA. Plasminogen activator inhibitor-1 in cancer: rationale and insight for future therapeutic testing. Cancer Res 2015;75:2969–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Di Mauro C, Pesapane A, Formisano L, Rosa R, D’Amato V, Ciciola P, et al. Urokinase-type plasminogen activator receptor (uPAR) expression enhances invasion and metastasis in RAS mutated tumors. Sci Rep 2017;7:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Chang S-H, Liu CH, Conway R, Han DK, Nithipatikom K, Trifan OC, et al. Role of prostaglandin E2-dependent angiogenic switch in cyclooxygenase 2-induced breast cancer progression. Proc Natl Acad Sci 2004;101:591–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Shoji Y, Takahashi M, Kitamura T, Watanabe K, Kawamori T, Maruyama T, et al. Downregulation of prostaglandin E receptor subtype EP3 during colon cancer development. Gut 2004;53:1151–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Semmlinger A, von Schoenfeldt V, Wolf V, Meuter A, Kolben TM, Kolben T, et al. EP3 (prostaglandin E2 receptor 3) expression is a prognostic factor for progression-free and overall survival in sporadic breast cancer. BMC Cancer 2018;18:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Lee JL, Kim A, Kopelovich L, Bickers DR, Athar M. Differential expression of E prostanoid receptors in murine and human non-melanoma skin cancer. J Invest Dermatol 2005;125:818–25. [DOI] [PubMed] [Google Scholar]
- 88. Sonoshita M, Takaku K, Sasaki N, Sugimoto Y, Ushikubi F, Narumiya S, et al. Acceleration of intestinal polyposis through prostaglandin receptor EP2 in Apc Δ716 knockout mice. Nat Med 2001;7:1048–51. [DOI] [PubMed] [Google Scholar]
- 89. Chell SD, Witherden IR, Dobson RR, Moorghen M, Herman AA, Qualtrough D, et al. Increased EP4 receptor expression in colorectal cancer progression promotes cell growth and anchorage independence. Cancer Res 2006;66:3106–13. [DOI] [PubMed] [Google Scholar]
- 90. Rundhaug J, Simper M, Surh I, Fischer S. The role of the EP receptors for prostaglandin E 2 in skin and skin cancer. Cancer Metastasis Rev 2011;30:465–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Terada N, Shimizu Y, Kamba T, Inoue T, Maeno A, Kobayashi T, et al. Identification of EP4 as a potential target for the treatment of castration-resistant prostate cancer using a novel xenograft model. Cancer Res 2010;70:1606–15. [DOI] [PubMed] [Google Scholar]
- 92. Xu S, Zhou W, Ge J, Zhang Z. Prostaglandin E2 receptor EP4 is involved in the cell growth and invasion of prostate cancer via the cAMP-PKA/PI3K-Akt signaling pathway. Mol Med Rep 2018;17:4702–12. [DOI] [PubMed] [Google Scholar]
- 93. Takahashi T, Ichikawa H, Morimoto Y, Tsuneyama K, Hijikata T. Inhibition of EP2/EP4 prostanoid receptor-mediated signaling suppresses IGF-1-induced proliferation of pancreatic cancer BxPC-3 cells via upregulating γ-glutamyl cyclotransferase expression. Biochem Biophys Res Commun 2019;516:388–96. [DOI] [PubMed] [Google Scholar]
- 94. Majumder M, Nandi P, Omar A, Ugwuagbo KC, Lala PK. EP4 as a therapeutic target for aggressive human breast cancer. Int J Mol Sci 2018;19:1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Mc Millan Ching JR, Fulton AM. Eicosanoids in cancer: prostaglandin E2 receptor 4 in cancer therapeutics and immunotherapy. Front Pharmacol 2020;11:819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Xu S, Zhang Z, Ogawa O, Yoshikawa T, Sakamoto H, Shibasaki N, et al. An EP4 antagonist ONO-AE3–208 suppresses cell invasion, migration, and metastasis of prostate cancer. Cell Biochem Biophys 2014;70:521–7. [DOI] [PubMed] [Google Scholar]
- 97. Schmidt A, Sinnett-Smith J, Young S, Chang H-H, Hines OJ, Dawson DW, et al. Direct growth-inhibitory effects of prostaglandin E2 in pancreatic cancer cells in vitro through an EP4/PKA-mediated mechanism. Surgery 2017;161:1570–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Parida S, Pal I, Parekh A, Thakur B, Bharti R, Das S, et al. GW627368X inhibits proliferation and induces apoptosis in cervical cancer by interfering with EP4/EGFR interactive signaling. Cell Death Dis 2016;7:e2154- e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Cherukuri DP, Chen XB, Goulet A-C, Young RN, Han Y, Heimark RL, et al. The EP4 receptor antagonist, L-161,982, blocks prostaglandin E2-induced signal transduction and cell proliferation in HCA-7 colon cancer cells. Exp Cell Res 2007;313:2969–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Li X, Yang B, Han G, Li W. The EP 4 antagonist, L-161,982, induces apoptosis, cell cycle arrest, and inhibits prostaglandin E2-induced proliferation in oral squamous carcinoma Tca8113 cells. J Oral Pathol Med 2017;46:991–7. [DOI] [PubMed] [Google Scholar]
- 101. Osawa K, Umemura M, Nakakaji R, Tanaka R, Islam RM, Nagasako A, et al. Prostaglandin E2 receptor EP4 regulates cell migration through Orai1. Cancer Sci 2020;111:160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Zhang Y, Purayil HT, Black JB, Fetto F, Lynch LD, Masannat JN, et al. Prostaglandin E2 receptor 4 mediates renal cell carcinoma intravasation and metastasis. Cancer Lett 2017;391:50–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Wu J, Zhang Y, Frilot N, Kim JI, Kim W-J, Daaka Y. Prostaglandin E2 regulates renal cell carcinoma invasion through the EP4 receptor-Rap GTPase signal transduction pathway. J Biol Chem 2011;286:33954–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Li Z, Zhang Y, Kim W, Daaka Y. PGE2 promotes renal carcinoma cell invasion through activated RalA. Oncogene 2013;32:1408–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Gopal Krishnan PD, Golden E, Woodward EA, Pavlos NJ, Blancafort P. Rab GTPases: emerging oncogenes and tumor suppressive regulators for the editing of survival pathways in cancer. Cancers 2020;12:259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Kim JI, Lakshmikanthan V, Frilot N, Daaka Y. Prostaglandin E2 promotes lung cancer cell migration via EP4-βArrestin1-c-Src signalsome. Mol Cancer Res 2010;8:569–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Song Q, Ji Q, Li Q. The role and mechanism of β-arrestins in cancer invasion and metastasis. Int J Mol Med 2018;41:631–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Xin X, Majumder M, Girish GV, Mohindra V, Maruyama T, Lala PK. Targeting COX-2 and EP4 to control tumor growth, angiogenesis, lymphangiogenesis and metastasis to the lungs and lymph nodes in a breast cancer model. Lab Invest 2012;92:1115–28. [DOI] [PubMed] [Google Scholar]
- 109. Timoshenko AV, Xu G, Chakrabarti S, Lala PK, Chakraborty C. Role of prostaglandin E2 receptors in migration of murine and human breast cancer cells. Exp Cell Res 2003;289:265–74. [DOI] [PubMed] [Google Scholar]
- 110. Jeong J-W, Park C, Cha H-J, Hong SH, Park S-H, Kim G-Y, et al. Cordycepin inhibits lipopolysaccharide-induced cell migration and invasion in human colorectal carcinoma HCT-116 cells through down-regulation of prostaglandin E2 receptor EP4. BMB Rep 2018;51:532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Kundu N, Ma X, Holt D, Goloubeva O, Ostrand-Rosenberg S, Fulton AM. Antagonism of the prostaglandin E receptor EP4 inhibits metastasis and enhances NK function. Breast Cancer Res Treat 2009;117:235–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Holt D, Ma X, Kundu N, Collin PD, Fulton AM. Modulation of host natural killer cell functions in breast cancer via prostaglandin E2 receptors EP2 and EP4. J Immunother 2012;35:179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Ma X, Holt D, Kundu N, Reader J, Goloubeva O, Take Y, et al. A prostaglandin E (PGE) receptor EP4 antagonist protects natural killer cells from PGE2-mediated immunosuppression and inhibits breast cancer metastasis. Oncoimmunology 2013;2:e22647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Mao Y, Sarhan D, Steven A, Seliger B, Kiessling R, Lundqvist A. Inhibition of tumor-derived prostaglandin-e2 blocks the induction of myeloid-derived suppressor cells and recovers natural killer cell activity. Clin Cancer Res 2014;20:4096–106. [DOI] [PubMed] [Google Scholar]
- 115. Yang J-J, Yu W-W, Hu L-L, Liu W-J, Lin X-H, Wang W, et al. Discovery and characterization of 1 H-1, 2, 3-triazole derivatives as novel prostanoid EP4 receptor antagonists for cancer immunotherapy. J Med Chem 2019;63:569–90. [DOI] [PubMed] [Google Scholar]
- 116. Albu DI, Wang Z, Huang K-C, Wu J, Twine N, Leacu S, et al. EP4 Antagonism by E7046 diminishes Myeloid immunosuppression and synergizes with Treg-reducing IL-2-Diphtheria toxin fusion protein in restoring anti-tumor immunity. Oncoimmunology 2017;6:e1338239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Sajiki Y, Konnai S, Cai Z, Takada K, Okagawa T, Maekawa N, et al. Enhanced immunotherapeutic efficacy of anti–PD-L1 antibody in combination with an EP4 antagonist. ImmunoHorizons 2020;4:837–50. [DOI] [PubMed] [Google Scholar]
- 118. Lu W, Yu W, He J, Liu W, Yang J, Lin X, et al. Reprogramming immunosuppressive myeloid cells facilitates immunotherapy for colorectal cancer. EMBO Mol Med 2021;13:e12798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Nie D, Guo Y, Yang D, Tang Y, Chen Y, Wang M-T, et al. Thromboxane A2 receptors in prostate carcinoma: expression and its role in regulating cell motility via small GTPase Rho. Cancer Res 2008;68:115–21. [DOI] [PubMed] [Google Scholar]
- 120. Cathcart M-C, Gately K, Cummins R, Kay E, O'Byrne KJ, Pidgeon GP. Examination of thromboxane synthase as a prognostic factor and therapeutic target in non-small cell lung cancer. Mol Cancer 2011;10:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Huang R, Li M, Hsin M, Underwood M, Ma L, Mok T, et al. 4-Methylnitrosamino-1–3-pyridyl-1-butanone (NNK) promotes lung cancer cell survival by stimulating thromboxane A 2 and its receptor. Oncogene 2011;30:106. [DOI] [PubMed] [Google Scholar]
- 122. Moussa O, Ashton AW, Fraig M, Garrett-Mayer E, Ghoneim MA, Halushka PV, et al. Novel role of thromboxane receptors β isoform in bladder cancer pathogenesis. Cancer Res 2008;68:4097–104. [DOI] [PubMed] [Google Scholar]
- 123. Orr K, Buckley NE, Haddock P, James C, Parent J-L, McQuaid S, et al. Thromboxane A2 receptor (TBXA2R) is a potent survival factor for triple negative breast cancers (TNBCs). Oncotarget 2016;7:55458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Zeng C, Zeng B, Dong C, Liu J, Xing F. Rho-ROCK signaling mediates entotic cell death in tumor. Cell death discovery 2020;6:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Mulvaney EP, O'Sullivan ÁG, Eivers SB, Reid HM, Kinsella BT. Differential expression of the TPα and TPβ isoforms of the human T prostanoid receptor during chronic inflammation of the prostate: role for FOXP1 in the transcriptional regulation of TPβ during monocyte-macrophage differentiation. Exp Mol Pathol 2019;110:104277. [DOI] [PubMed] [Google Scholar]
- 126. Huang J. Ramamurthy SK, Lin X, and Le Breton GC. Cell signalling through thromboxane A 2004;2:521–33. [DOI] [PubMed] [Google Scholar]
- 127. Zhang T, Wang Q, Ma W-Y, Wang K, Chang X, Johnson ML, et al. Targeting the COX1/2-Driven thromboxane A2 pathway suppresses Barrett's esophagus and esophageal adenocarcinoma development. EBioMedicine 2019;49:145–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Li H, Liu K, Boardman LA, Zhao Y, Wang L, Sheng Y, et al. Circulating prostaglandin biosynthesis in colorectal cancer and potential clinical significance. EBioMedicine 2015;2:165–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Li H, Lee M-H, Liu K, Wang T, Song M, Han Y, et al. Inhibiting breast cancer by targeting the thromboxane A 2 pathway. NPJ Precis Oncol 2017;1:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Watkins G, Douglas-Jones A, Mansel RE, Jiang WG. Expression of thromboxane synthase, TBXAS1 and the thromboxane A2 receptor, TBXA2R, in human breast cancer. 2005. Springer. p 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Mulvaney EP, Shilling C, Eivers SB, Perry AS, Bjartell A, Kay EW, et al. Expression of the TPα and TPβ isoforms of the thromboxane prostanoid receptor (TP) in prostate cancer: clinical significance and diagnostic potential. Oncotarget 2016;7:73171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Li X, Tai H-H. Activation of thromboxane A 2 receptors induces orphan nuclear receptor Nurr1 expression and stimulates cell proliferation in human lung cancer cells. Carcinogenesis 2009;30:1606–13. [DOI] [PubMed] [Google Scholar]
- 133. Sakai H, Suzuki T, Takahashi Y, Ukai M, Tauchi K, Fujii T, et al. Upregulation of thromboxane synthase in human colorectal carcinoma and the cancer cell proliferation by thromboxane A2. FEBS Lett 2006;580:3368–74. [DOI] [PubMed] [Google Scholar]
- 134. O'Sullivan AG, Eivers SB, Mulvaney EP, Kinsella BT. Regulated expression of the TPβ isoform of the human T prostanoid receptor by the tumour suppressors FOXP1 and NKX3. 1: Implications for the role of thromboxane in prostate cancer. Biochim Biophys Acta 2017;1863:3153–69. [DOI] [PubMed] [Google Scholar]
- 135. Keating GL, Reid HM, Eivers SB, Mulvaney EP, Kinsella BT. Transcriptional regulation of the human thromboxane A2 receptor gene by Wilms' tumor (WT) 1 and hypermethylated in cancer (HIC) 1 in prostate and breast cancers. Biochim Biophys Acta 2014;1839:476–92. [DOI] [PubMed] [Google Scholar]
- 136. O'Sullivan AG, Mulvaney EP, Kinsella BT. Regulation of protein kinase C-related kinase (PRK) signalling by the TPα and TPβ isoforms of the human thromboxane A2 receptor: implications for thromboxane-and androgen-dependent neoplastic and epigenetic responses in prostate cancer. Biochim Biophys Acta 2017;1863:838–56. [DOI] [PubMed] [Google Scholar]
- 137. O'Sullivan AG, Mulvaney EP, Hyland PB, Kinsella BT. Protein kinase C-related kinase 1 and 2 play an essential role in thromboxane-mediated neoplastic responses in prostate cancer. Oncotarget 2015;6:26437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Turner EC, Kavanagh DJ, Mulvaney EP, McLean C, Wikström K, Reid HM, et al. Identification of an interaction between the TPα and TPβ isoforms of the human thromboxane A2 receptor with protein kinase C-related kinase (PRK) 1 implications for prostate cancer. J Biol Chem 2011;286:15440–57. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 139. Sophocleous G, Owen D, Mott HR. The structure and function of protein kinase C-related kinases (PRKs). Biochem Soc Trans 2021;49:217–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Ge G-Z, Xu T-R, Chen C. Tobacco carcinogen NNK-induced lung cancer animal models and associated carcinogenic mechanisms. Acta Biochim Biophys Sin 2015;47:477–87. [DOI] [PubMed] [Google Scholar]
- 141. Shimizu T, Fujii T, Takahashi Y, Takahashi Y, Suzuki T, Ukai M, et al. Up-regulation of Kv7. 1 channels in thromboxane A 2-induced colonic cancer cell proliferation. Pflügers Arch 2014;466:541–8. [DOI] [PubMed] [Google Scholar]
- 142. Rodrigues S, Nguyen Q-D, Faivre S, Bruyneel E, Thim L, Westley B, et al. Activation of cellular invasion by trefoil peptides and SRC is mediated by cyclooxygenase-and thromboxane A2 receptor-dependent signaling pathways. FASEB J 2001;15:1517–28. [DOI] [PubMed] [Google Scholar]
- 143. Nie D, Che M, Zacharek A, Qiao Y, Li L, Li X, et al. Differential expression of thromboxane synthase in prostate carcinoma: role in tumor cell motility. Am J Pathol 2004;164:429–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Li X, Wei J, Tai H-H. Activation of extracellular signal-regulated kinase by 12-hydroxyheptadecatrienoic acid in prostate cancer PC3 cells. Arch Biochem Biophys 2007;467:20–30. [DOI] [PubMed] [Google Scholar]
- 145. Wu KK, Liou J-Y. Cellular and molecular biology of prostacyclin synthase. Biochem Biophys Res Commun 2005;338:45–52. [DOI] [PubMed] [Google Scholar]
- 146. Gnedenko O, Yablokov E, Ershov P, Svirid A, Shkel T, Haidukevich I, et al. Interaction of prostacyclin synthase with cytochromes P450. Biomed Khim 2019;65:63–6. [DOI] [PubMed] [Google Scholar]
- 147. Lin Y-z, Wu KK, Ruan K-H. Characterization of the secondary structure and membrane interaction of the putative membrane anchor domains of Prostaglandin I2Synthase and cytochrome P450 2C1. Arch Biochem Biophys 1998;352:78–84. [DOI] [PubMed] [Google Scholar]
- 148. Zhou W, Zhang J, Goleniewska K, Dulek DE, Toki S, Newcomb DC, et al. Prostaglandin I2 suppresses proinflammatory chemokine expression, CD4 T cell activation, and STAT6-independent allergic lung inflammation. J Immunol 2016;197:1577–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Kij A, Kus K, Smeda M, Zakrzewska A, Proniewski B, Matyjaszczyk K, et al. Differential effects of nitric oxide deficiency on primary tumour growth, pulmonary metastasis and prostacyclin/thromboxane A2 balance in orthotopic and intravenous murine models of 4T1 breast cancer. J Physiol Pharmacol 2018;69:911–9. [DOI] [PubMed] [Google Scholar]
- 150. Cathcart M-C, Reynolds JV, O'Byrne KJ, Pidgeon GP. The role of prostacyclin synthase and thromboxane synthase signaling in the development and progression of cancer. Biochim Biophys Acta 2010;1805:153–66. [DOI] [PubMed] [Google Scholar]
- 151. Dai D, Chen B, Feng Y, Wang W, Jiang Y, Huang H, et al. Prognostic value of prostaglandin I2 synthase and its correlation with tumor-infiltrating immune cells in lung cancer, ovarian cancer, and gastric cancer. Aging 2020;12:9658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Frigola J, Munoz M, Clark SJ, Moreno V, Capella G, Peinado MA. Hypermethylation of the prostacyclin synthase (PTGIS) promoter is a frequent event in colorectal cancer and associated with aneuploidy. Oncogene 2005;24:7320–6. [DOI] [PubMed] [Google Scholar]
- 153. Sasaki Y, Kamiyama S, Kamiyama A, Matsumoto K, Akatsu M, Nakatani Y, et al. Genetic-deletion of cyclooxygenase-2 downstream prostacyclin synthase suppresses inflammatory reactions but facilitates carcinogenesis, unlike deletion of microsomal prostaglandin E synthase-1. Sci Rep 2015;5:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Honn KV, Cicone B, Skoff A. Prostacyclin: a potent antimetastatic agent. Science 1981;212:1270–2. [DOI] [PubMed] [Google Scholar]
- 155. New ML, White CM, McGonigle P, McArthur DG, Dwyer-Nield LD, Merrick DT, et al. Prostacyclin and EMT pathway markers for monitoring response to lung cancer chemoprevention. Cancer Prev Res 2018;11:643–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Tennis MA, Vanscoyk M, Keith RL, Winn RA. The role of prostacyclin in lung cancer. Transl Res 2010;155:57–61. [DOI] [PubMed] [Google Scholar]
- 157. Geraci MW. Targeting the prostacyclin/peroxisome proliferator-activated receptor gamma axis in lung cancer chemoprevention. Trans Am Clin Climatol Assoc 2018;129:48. [PMC free article] [PubMed] [Google Scholar]
- 158. Minami Y, Sasaki T, Bochimoto H, Kawabe JI, Endo S, Hira Y, et al. Prostaglandin I2 analog suppresses lung metastasis by recruiting pericytes in tumor angiogenesis. Int J Oncol 2015;46:548–54. [DOI] [PubMed] [Google Scholar]
- 159. Nemenoff R, Meyer AM, Hudish TM, Mozer AB, Snee A, Narumiya S, et al. Prostacyclin prevents murine lung cancer independent of the membrane receptor by activation of peroxisomal proliferator–activated receptor γ. Cancer Prev Res 2008;1:349–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Ahn J-H, Lee K-T, Choi YS, Choi J-H. Iloprost, a prostacyclin analog, inhibits the invasion of ovarian cancer cells by downregulating matrix metallopeptidase-2 (MMP-2) through the IP-dependent pathway. Prostaglandins Other Lipid Mediat 2018;134:47–56. [DOI] [PubMed] [Google Scholar]
- 161. Kostenis E, Ulven T. Emerging roles of DP and CRTH2 in allergic inflammation. Trends Mol Med 2006;12:148–58. [DOI] [PubMed] [Google Scholar]
- 162. Ueno R, Honda K, Inoue S, Hayaishi O. Prostaglandin D2, a cerebral sleep-inducing substance in rats. Proc Natl Acad Sci 1983;80:1735–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Inoue T, Irikura D, Okazaki N, Kinugasa S, Matsumura H, Uodome N, et al. Mechanism of metal activation of human hematopoietic prostaglandin D synthase. Nat Struct Mol Biol 2003;10:291–6. [DOI] [PubMed] [Google Scholar]
- 164. Murata T, Lin MI, Aritake K, Matsumoto S, Narumiya S, Ozaki H, et al. Role of prostaglandin D2 receptor DP as a suppressor of tumor hyperpermeability and angiogenesis in vivo. Proc Natl Acad Sci 2008;105:20009–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Murata T, Aritake K, Matsumoto S, Kamauchi S, Nakagawa T, Hori M, et al. Prostagladin D2 is a mast cell-derived antiangiogenic factor in lung carcinoma. Proc Natl Acad Sci 2011;108:19802–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Iwanaga K, Nakamura T, Maeda S, Aritake K, Hori M, Urade Y, et al. Mast cell–derived prostaglandin D2 inhibits colitis and colitis-associated colon cancer in mice. Cancer Res 2014;74:3011–9. [DOI] [PubMed] [Google Scholar]
- 167. Park JM, Kanaoka Y, Eguchi N, Aritake K, Grujic S, Materi AM, et al. Hematopoietic prostaglandin D synthase suppresses intestinal adenomas in ApcMin/+ mice. Cancer Res 2007;67:881–9. [DOI] [PubMed] [Google Scholar]
- 168. Zhang B, Bie Q, Wu P, Zhang J, You B, Shi H, et al. PGD2/PTGDR2 signaling restricts the self-renewal and tumorigenesis of gastric cancer. Stem Cells 2018;36:990–1003. [DOI] [PubMed] [Google Scholar]
- 169. Bie Q, Li X, Liu S, Yang X, Qian Z, Zhao R, et al. YAP promotes self-renewal of gastric cancer cells by inhibiting expression of L-PTGDS and PTGDR2. Int J Clin Oncol 2020;25:2055–65. [DOI] [PubMed] [Google Scholar]
- 170. Omori K, Morikawa T, Kunita A, Nakamura T, Aritake K, Urade Y, et al. Lipocalin-type prostaglandin D synthase-derived PGD2 attenuates malignant properties of tumor endothelial cells. J Pathol 2018;244:84–96. [DOI] [PubMed] [Google Scholar]
- 171. Kabututu Z, Manin M, Pointud J-C, Maruyama T, Nagata N, Lambert S, et al. Prostaglandin f2α synthase activities of aldo–keto reductase 1b1, 1b3 and 1b7. J Biochem 2009;145:161–8. [DOI] [PubMed] [Google Scholar]
- 172. Pierce KL, Bailey TJ, Hoyer PB, Gil DW, Woodward DF, Regan JW. Cloning of a carboxyl-terminal isoform of the prostanoid FP receptor. J Biol Chem 1997;272:883–7. [DOI] [PubMed] [Google Scholar]
- 173. Wölfle D. Enhancement of carcinogen-induced malignant cell transformation by prostaglandin F2α. Toxicology 2003;188:139–47. [DOI] [PubMed] [Google Scholar]
- 174. Nugent K, Spigelman A, Phillips R. Tissue prostaglandin levels in familial adenomatous polyposis patients treated with sulindac. Dis Colon Rectum 1996;39:659–62. [DOI] [PubMed] [Google Scholar]
- 175. Collins D, Hogan A, Skelly M, Baird A, Winter D. Cyclic AMP-mediated chloride secretion is induced by prostaglandin F2α in human isolated colon. Br J Pharmacol 2009;158:1771–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Qualtrough D, Kaidi A, Chell S, Jabbour HN, Williams AC, Paraskeva C. Prostaglandin F2α stimulates motility and invasion in colorectal tumor cells. Int J Cancer 2007;121:734–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Tsai C-SS, Luo S-F, Ning C-C, Lin C-L, Jiang M-C, Liao C-F. Acetylsalicylic acid regulates MMP-2 activity and inhibits colorectal invasion of murine B16F0 melanoma cells in C57BL/6J mice: effects of prostaglandin F2α. Biomed Pharmacother 2009;63:522–7. [DOI] [PubMed] [Google Scholar]
- 178. Goupil E, Wisehart V, Khoury E, Zimmerman B, Jaffal S, Hébert TE, et al. Biasing the prostaglandin F2α receptor responses toward EGFR-dependent transactivation of MAPK. Mol Endocrinol 2012;26:1189–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Karpisheh V, Afjadi JF, Afjadi MN, Haeri MS, Sough TSA, Asl SH, et al. Inhibition of HIF-1α/EP4 axis by hyaluronate-trimethyl chitosan-SPION nanoparticles markedly suppresses the growth and development of cancer cells. Int J Biol Macromol 2021;167:1006–19. [DOI] [PubMed] [Google Scholar]
- 180. Chien CC, Ko CH, Shen SC, Yang LY, Chen YC. The role of COX-2/PGE2 in gossypol-induced apoptosis of colorectal carcinoma cells. J Cell Physiol 2012;227:3128–37. [DOI] [PubMed] [Google Scholar]
- 181. Niu J-c, Ma N, Liu W, Wang P-j. EP1 receptor is involved in prostaglandin E2-induced osteosarcoma growth. Bosn J Basic Med Sci 2019;19:265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Matsuo M, Yoshida N, Zaitsu M, Ishii K, Hamasaki Y. Inhibition of human glioma cell growth by a PHS-2 inhibitor, NS398, and a prostaglandin E receptor subtype EP1-selective antagonist, SC51089. J Neurooncol 2004;66:285–92. [DOI] [PubMed] [Google Scholar]
- 183. Paul AG, Chandran B, Sharma-Walia N. Concurrent targeting of eicosanoid receptor 1/eicosanoid receptor 4 receptors and COX-2 induces synergistic apoptosis in Kaposi's sarcoma-associated herpesvirus and Epstein-Barr virus associated non-Hodgkin lymphoma cell lines. Transl Res 2013;161:447–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Sun B, Rong R, Jiang H, Zhang H, Wang Y, Bai X, et al. Prostaglandin E 2 receptor EP1 phosphorylate CREB and mediates MMP2 expression in human cholangiocarcinoma cells. Mol Cell Biochem 2013;378:195–203. [DOI] [PubMed] [Google Scholar]
- 185. Piazuelo E, Jiménez P, Strunk M, Santander S, García A, Esteva F, et al. Effects of selective PGE2 receptor antagonists in esophageal adenocarcinoma cells derived from Barrett's esophagus. Prostaglandins Other Lipid Mediat 2006;81:150–61. [DOI] [PubMed] [Google Scholar]
- 186. Watanabe K, Kawamori T, Nakatsugi S, Ohta T, Ohuchida S, Yamamoto H, et al. Role of the prostaglandin E receptor subtype EP1 in colon carcinogenesis. Cancer Res 1999;59:5093–6. [PubMed] [Google Scholar]
- 187. Kawamori T, Uchiya N, Nakatsugi S, Watanabe K, Ohuchida S, Yamamoto H, et al. Chemopreventive effects of ONO-8711, a selective prostaglandin E receptor EP1 antagonist, on breast cancer development. Carcinogenesis 2001;22:2001–4. [DOI] [PubMed] [Google Scholar]
- 188. Le Leu RK, Brown IL, Hu Y, Esterman A, Young GP. Suppression of azoxymethane-induced colon cancer development in rats by dietary resistant starch. Cancer Biol Ther 2007;6:1621–6. [DOI] [PubMed] [Google Scholar]
- 189. Makita H, Mutoh M, Maruyama T, Yonemoto K, Kobayashi A, Fujitsuka H, et al. A prostaglandin E 2 receptor subtype EP 1-selective antagonist, ONO-8711, suppresses 4-nitroquinoline 1-oxide-induced rat tongue carcinogenesis. Carcinogenesis 2007;28:677–84. [DOI] [PubMed] [Google Scholar]
- 190. O'Callaghan G, Ryan A, Neary P, O'Mahony C, Shanahan F, Houston A. Targeting the EP1 receptor reduces Fas ligand expression and increases the antitumor immune response in an in vivo model of colon cancer. Int J Cancer 2013;133:825–34. [DOI] [PubMed] [Google Scholar]
- 191. Baryawno N, Sveinbjörnsson B, Eksborg S, Orrego A, Segerström L, Oqvist CO, et al. Tumor-growth–promoting cyclooxygenase-2 prostaglandin E2 pathway provides medulloblastoma therapeutic targets. Neuro-oncol 2008;10:661–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Tober KL, Wilgus TA, Kusewitt DF, Thomas-Ahner JM, Maruyama T, Oberyszyn TM. Importance of the EP1 receptor in cutaneous UVB-induced inflammation and tumor development. J Invest Dermatol 2006;126:205–11. [DOI] [PubMed] [Google Scholar]
- 193. Watanabe K, Kawamori T, Nakatsugi S, Ohta T, Ohuchida S, Yamamoto H, et al. Inhibitory effect of a prostaglandin E receptor subtype EP1 selective antagonist, ONO-8713, on development of azoxymethane-induced aberrant crypt foci in mice. Cancer Lett 2000;156:57–61. [DOI] [PubMed] [Google Scholar]
- 194. Watanabe Y, Imanishi Y, Ozawa H, Sakamoto K, Fujii R, Shigetomi S, et al. Selective EP2 and Cox-2 inhibition suppresses cell migration by reversing epithelial-to-mesenchymal transition and Cox-2 overexpression and E-cadherin downregulation are implicated in neck metastasis of hypopharyngeal cancer. Am J Transl Res 2020;12:1096. [PMC free article] [PubMed] [Google Scholar]
- 195. Aoki T, Narumiya S. Prostaglandin E 2-EP2 signaling as a node of chronic inflammation in the colon tumor microenvironment. Inflamm Regen 2017;37:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Tian Y, Yang T, Yu S, Liu C, He M, Hu C. Prostaglandin E2 increases migration and proliferation of human glioblastoma cells by activating transient receptor potential melastatin 7 channels. J Cell Mol Med 2018;22:6327–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Fang T, Hou J, He M, Wang L, Zheng M, Wang X, et al. Actinidia chinensis Planch root extract (acRoots) inhibits hepatocellular carcinoma progression by inhibiting EP3 expression. Cell Biol Toxicol 2016;32:499–511. [DOI] [PubMed] [Google Scholar]
- 198. Hoshikawa H, Goto R, Mori T, Mitani T, Mori N. Expression of prostaglandin E2 receptors in oral squamous cell carcinomas and growth inhibitory effects of an EP3 selective antagonist, ONO-AE3–240. Int J Oncol 2009;34:847–52. [DOI] [PubMed] [Google Scholar]
- 199. Kundu N, Ma X, Kochel T, Goloubeva O, Staats P, Thompson K, et al. Prostaglandin E receptor EP4 is a therapeutic target in breast cancer cells with stem-like properties. Breast Cancer Res Treat 2014;143:19–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Majumder M, Xin X, Liu L, Girish GV, Lala PK. Prostaglandin E2 receptor EP 4 as the common target on cancer cells and macrophages to abolish angiogenesis, lymphangiogenesis, metastasis, and stem-like cell functions. Cancer Sci 2014;105:1142–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Vo BT, Morton D Jr, Komaragiri S, Millena AC, Leath C, Khan SA. TGF-β effects on prostate cancer cell migration and invasion are mediated by PGE2 through activation of PI3K/AKT/mTOR pathway. Endocrinology 2013;154:1768–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Karpisheh V, Joshi N, Zekiy AO, Beyzai B, Hojjat-Farsangi M, Namdar A, et al. EP4 receptor as a novel promising therapeutic target in colon cancer: EP4 receptor in colon cancer. Pathol Res Practice 2020:153247. [DOI] [PubMed] [Google Scholar]
- 203. Hong DS, Parikh A, Shapiro GI, Varga A, Naing A, Meric-Bernstam F, et al. First-in-human phase I study of immunomodulatory E7046, an antagonist of PGE2-receptor E-type 4 (EP4), in patients with advanced cancers. J Immunother Cancer 2020;8:e000222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Parida S, Parekh A, Dey G, Ghosh SC, Mandal M. Molecular inhibition of prostaglandin E2 with GW627368X: therapeutic potential and preclinical safety assessment in mouse sarcoma model. Cancer Biol Ther 2015;16:922–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Robertson FM, Simeone A-M, Mazumdar A, Shah AH, McMurray JS, Ghosh S, et al. Molecular and pharmacological blockade of the EP4 receptor selectively inhibits both proliferation and invasion of human inflammatory breast cancer cells. J Exp Ther Oncol 2008;7:299–312. [PubMed] [Google Scholar]
- 206. Huang H, Aladelokun O, Ideta T, Giardina C, Ellis LM, Rosenberg DW. Inhibition of PGE 2/EP4 receptor signaling enhances oxaliplatin efficacy in resistant colon cancer cells through modulation of oxidative stress. Sci Rep 2019;9:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Doherty GA, Byrne SM, Molloy ES, Malhotra V, Austin SC, Kay EW, et al. Proneoplastic effects of PGE 2 mediated by EP4 receptor in colorectal cancer. BMC Cancer 2009;9:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Muller M, Sales KJ, Katz AA, Jabbour HN. Seminal plasma promotes the expression of tumorigenic and angiogenic genes in cervical adenocarcinoma cells via the E-series prostanoid 4 receptor. Endocrinology 2006;147:3356–65. [DOI] [PubMed] [Google Scholar]
- 209. Kitamura T, Itoh M, Noda T, Tani K, Kobayashi M, Maruyama T, et al. Combined effects of prostaglandin E receptor subtype EP1 and subtype EP4 antagonists on intestinal tumorigenesis in adenomatous polyposis coli gene knockout mice. Cancer Sci 2003;94:618–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Mutoh M, Watanabe K, Kitamura T, Shoji Y, Takahashi M, Kawamori T, et al. Involvement of prostaglandin E receptor subtype EP4 in colon carcinogenesis. Cancer Res 2002;62:28–32. [PubMed] [Google Scholar]
- 211. Ma X, Kundu N, Rifat S, Walser T, Fulton AM. Prostaglandin E receptor EP4 antagonism inhibits breast cancer metastasis. Cancer Res 2006;66:2923–7. [DOI] [PubMed] [Google Scholar]
- 212. Gervin E, Shin B, Opperman R, Cullen M, Feser R, Maiti S, et al. Chemically induced hypoxia enhances miRNA functions in breast cancer. Cancers 2020;12:2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Takita M, Inada M, Maruyama T, Miyaura C. Prostaglandin E receptor EP4 antagonist suppresses osteolysis due to bone metastasis of mouse malignant melanoma cells. FEBS Lett 2007;581:565–71. [DOI] [PubMed] [Google Scholar]
- 214. Cai G, Yan A, Fu N, Fu Y. Thromboxane A2 receptor antagonist SQ29548 attenuates SH-SY5Y neuroblastoma cell impairments induced by oxidative stress. Int J Mol Med 2018;42:479–88. [DOI] [PubMed] [Google Scholar]
- 215. Liu Q, Tao B, Liu G, Chen G, Zhu Q, Yu Y, et al. Thromboxane A2 receptor inhibition suppresses multiple myeloma cell proliferation by inducing p38/c-Jun N-terminal kinase (JNK) mitogen-activated protein kinase (MAPK)-mediated G2–M progression delay and cell apoptosis. J Biol Chem 2016;291:4779–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Huang R-Y, Li S-S, Guo H-Z, Huang Y, Zhang X, Li M-Y, et al. Thromboxane A2 exerts promoting effects on cell proliferation through mediating cyclooxygenase-2 signal in lung adenocarcinoma cells. J Cancer Res Clin Oncol 2014;140:375–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Nie D, Lamberti M, Zacharek A, Li L, Szekeres K, Tang K, et al. Thromboxane A2 regulation of endothelial cell migration, angiogenesis, and tumor metastasis. Biochem Biophys Res Commun 2000;267:245–51. [DOI] [PubMed] [Google Scholar]
- 218. Nakahata N, Sato K, Marilene TA, Nakanishi H. ONO NT-126 is a potent and selective thromboxane A2 antagonist in human astrocytoma cells. Eur J Pharmacol 1990;184:233–8. [DOI] [PubMed] [Google Scholar]
- 219. Fujimura M, Kasahara K, Shirasaki H, Heki U, Iwasa K, Ueda A, et al. Up-regulation of ICH-1 L protein by thromboxane A 2 antagonists enhances cisplatin-induced apoptosis in non-small-cell lung-cancer cell lines. J Cancer Res Clin Oncol 1999;125:389–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Werfel TA, Hicks DJ, Rahman B, Bendeman WE, Duvernay MT, Maeng JG, et al. Repurposing of a thromboxane receptor inhibitor based on a novel role in metastasis identified by phenome-wide association study. Mol Cancer Ther 2020;19:2454–64. [DOI] [PubMed] [Google Scholar]
- 221. Osawa T, Ohga N, Hida Y, Kitayama K, Akiyama K, Onodera Y, et al. Prostacyclin receptor in tumor endothelial cells promotes angiogenesis in an autocrine manner. Cancer Sci 2012;103:1038–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Malki S, Bibeau F, Notarnicola C, Roques S, Berta P, Poulat F, et al. Expression and biological role of the prostaglandin D synthase/SOX9 pathway in human ovarian cancer cells. Cancer Lett 2007;255:182–93. [DOI] [PubMed] [Google Scholar]
- 223. Passeron T, Valencia JC, Namiki T, Vieira WD, Passeron H, Miyamura Y, et al. Upregulation of SOX9 inhibits the growth of human and mouse melanomas and restores their sensitivity to retinoic acid. J Clin Invest 2009;119:954–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Torres D, Paget C, Fontaine J, Mallevaey T, Matsuoka T, Maruyama T, et al. Prostaglandin D2 inhibits the production of IFN-γ by invariant NK T cells: consequences in the control of B16 melanoma. J Immunol 2008;180:783–92. [DOI] [PubMed] [Google Scholar]
- 225. Wang S, Yang Q, Fung K-M, Lin H-K. AKR1C2 and AKR1C3 mediated prostaglandin D2 metabolism augments the PI3K/Akt proliferative signaling pathway in human prostate cancer cells. Mol Cell Endocrinol 2008;289:60–6. [DOI] [PubMed] [Google Scholar]
- 226. Shin VY, Siu M-T, Liu X, Ng EK, Kwong A, Chu K-M. MiR-92 suppresses proliferation and induces apoptosis by targeting EP4/Notch1 axis in gastric cancer. Oncotarget 2018;9:24209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Li X, Tai HH. Thromboxane A2 receptor-mediated release of matrix metalloproteinase-1 (MMP-1) induces expression of monocyte chemoattractant protein-1 (MCP-1) by activation of protease-activated receptor 2 (PAR2) in A549 human lung adenocarcinoma cells. Mol Carcinog 2014;53:659–66. [DOI] [PubMed] [Google Scholar]
- 228. Li X, Tai H-H. Activation of Thromboxane A 2 Receptor (TP) increases the expression of monocyte chemoattractant protein-1 (MCP-1)/Chemokine (CC motif) ligand 2 (CCL2) and recruits macrophages to promote invasion of lung cancer cells. PLoS One 2013;8:e54073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Sobolesky PM, Halushka PV, Garrett-Mayer E, Smith MT, Moussa O. Regulation of the tumor suppressor FOXO3 by the thromboxane-A 2 receptors in urothelial cancer. PLoS One 2014;9:e107530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Yokoyama I, Hayashi S, Kobayashi T, Negita M, Yasutomi M, Uchida K, et al. Prevention of experimental hepatic metastasis with thromboxne synthase inhibitor. Res Exp Med 1995;195:209–15. [DOI] [PubMed] [Google Scholar]
- 231. Lichao S, Liang P, Chunguang G, Fang L, Zhihua Y, Yuliang R. Overexpression of PTGIS could predict liver metastasis and is correlated with poor prognosis in colon cancer patients. Pathol Oncol Res 2012;18:563–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.