Abstract
Large conductance K+ channels, termed BK channels, regulate a variety of cellular and physiologic functions. Although universally activated by changes in voltage or [Ca2+]i, the threshold for BK channel activation varies among loci of expression, often arising from cell-specific regulatory subunits including a family of leucine-rich-repeat (LRR)-containing gamma subunits (LRRC26, LRRC52, LRRC55, and LRRC38). The “founding” member of this family, LRRC26, was originally identified as a tumor suppressor in various cancers. An LRRC26 KO mouse model recently revealed that LRRC26 is also highly expressed in secretory epithelial cells and partners with BK channels in the salivary gland and colonic goblet cells to promote sustained K+ fluxes likely essential for normal secretory function. To accomplish this, LRRC26 negatively shifts the range of BK channel activation such that channels contribute to K+ flux near typical epithelial cell resting conditions. In colon, the absence of LRRC26 increases vulnerability to colitis. LRRC26-containing BK channels are also likely important regulators of epithelial function in other loci, including airways, female reproductive tract, and mammary gland. Based on an LRRC52 KO mouse model, LRRC52 regulation of large conductance K+ channels plays a role both in sperm function and in cochlear inner hair cells. Although our understanding of LRRC-containing BK channels remains rudimentary, KO mouse models may help define other organs in which LRRC-containing channels support normal function. A key topic for future work concerns identification of endogenous mechanisms, whether posttranslational or via gene regulation, that may impact LRR-dependent pathologies.
Graphical Abstract
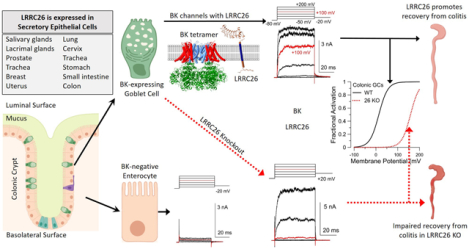
A major function of colonic crypts is the generation of a protective mucus layer separating gut contents from epithelial cells. Two major colonic epithelial cells are mucus-secreting goblet cells (GCs) and absorptive enterocytes. GCs specifically express BK channels containing the LRRC26 regulatory subunit, which shifts BK channel activation so that, at 0 cytosolic Ca2+, BK channels are activated at membrane potentials (Vm) as negative as −40 mV. In mice lacking LRRC26, GCs also express BK currents, but they not activated with 0 Ca2+ until Vm positive to +50 mV. Such LRRC26 KO mice exhibit enhanced sensitivity to colitis induced by dextran sodium sulfate. LRRC26 is expected to regulate BK-mediated K fluxes in a variety of secretory epithelial cells.
Introduction
Potassium channels of large conductance (BK or KCa1.1, encoded by the KCNMA1 gene (Butler et al., 1993)) are ubiquitously expressed and contribute to rapid repolarization in excitable cells or K+ fluxes in non-excitable cells (Contet et al., 2016; Bailey et al., 2019). A notable property of these channels is that they can be activated independently by two physiological signals: increases in submembrane Ca2+, even in the absence of depolarization, or depolarization in the absence of Ca2+. In a given cell type, it is the intrinsic properties of a BK channel, that is, the range of Vm and Ca2+ over which a particular channel is activated, that defines the circumstances under which it functions. Considering the variety of cell types in which BK channels is expressed and the functional roles they play, it is probably safe to assert that BK channels exhibit broader functional diversity than any other voltage-dependent K+ channel (Gonzalez-Perez & Lingle, 2019; Dudem et al., 2021).
Diversity in BK channel function arises from three general categories of mechanism: 1) alternative splice variants in the BK pore-forming α subunit (Shipston & Tian, 2016); 2) post-translational modifications of the BK α subunit (Shipston & Tian, 2016); and 3) three families of structurally distinct regulatory subunits, a β subunit family (β1−β4), a γ subunit family of leucine-rich-repeat containing (LRRC) proteins (γ1-γ4 with gene names, respectively, of LRRC26, LRRC52, LRRC55, LRRC38), and a recently described four member LINGO subfamily (LINGO1–4) (Dudem et al., 2020) of which the LINGO1 subunit has been shown to mediate very rapid BK current inactivation. Although alternative splicing and post-translational modifications are important contributors to BK regulation, the tissue-specific expression of regulatory subunits probably accounts for most of the distinctive functional diversity of BK channels in native cells, conferring inactivation behavior, affecting gating kinetics, and producing large gating shifts. The impact of β subunits on BK function and their potential physiological roles have been extensively investigated for over 35 years and recently reviewed (Li & Yan, 2016; Gonzalez-Perez & Lingle, 2019), while the LINGO subfamily is just beginning to be investigated. Here, the focus is progress that has been made pertinent to understanding physiological roles of LRRC/γ subunits and potential pathologies that may be associated with LRRC subunits. Specifically, the combination of biophysical studies of effects of LRRC subunits on BK channels together with development of animal models in which LRRC26 (γ1) and LRRC52 (γ2) have been knocked out suggest that LRRC subunits may play critical roles in maintaining normal function in a variety of epithelial tissues and glands and, when mis-regulation occurs, may contribute to tissue pathology.
This presentation is organized in three sections. First, we summarize the initial identification and description of functional properties of subunits containing LRR (Leucine-Rich-Repeat) domains that are pertinent to BK channels. Since LRRC subunits of BK channels have been included in other recent reviews (Zhang & Yan, 2014; Li & Yan, 2016; Gonzalez-Perez & Lingle, 2019; Dudem et al., 2021), here we highlight those properties of the subunits that we think are important for understanding their physiological roles and may provide clues to LRRC-dependent pathologies. Second, we summarize recent work with LRRC26 and LRRC52 that has addressed the role of LRRC proteins in native cells, with particular emphasis on what KO of LRRC subunits may be revealing about potential pathological consequences related to the LRRC subunits. Third, we consider how some of the functional results summarized in section I may point to processes that might underlie LRRC-dependent pathology highlighted in section II.
I. Identification and functional impact of the LRRC family of BK γ subunits
A. Overview of LRRC proteins.
LRRs (leucine-rich-repeats) are discrete structural domains found in an enormous number of proteins and thought to mediate protein-ligand or protein-protein interactions with functional roles ranging from innate immunity to nervous system development (Kobe & Kajava, 2001; Dolan et al., 2007; Ng et al., 2011). Whether as extracellular or cytosolic domains of proteins, the shared feature of LRR domains is that they contain some number of LRR repeats of 19–29 amino acids bracketed by characteristic N-terminal (LRRNT) and C-terminal (LRRCT) regions. The stacked arrangement of the LRR repeats generates a horseshoe-shaped structure, but the number of repeats in a given LRRC family of LRR-containing proteins, what other modules may be imbedded in the protein, e.g., immunoglobulin domains, and the topology in regard to transmembrane segments can vary.
The LRR-containing proteins under consideration here are termed LRRC subunits (Yan & Aldrich, 2012), reflecting their protein names (LRRC26, LRRC52, LRRC55, and LRRC38, given in order of their ability to influence BK channel activation, as summarized in Table 1). These four BK-related LRRC subunits consist sequentially of an extracellular N-terminal signal sequence followed by an LRRNT segment with four conserved cysteine residues, six LRR repeats with a consensus LxxLxLxxN sequence, and then a LRRCT segment also with four conserved cysteines ending the LRR domain. The LRR domain is followed by a linker that connects to the single transmembrane (TM) segment followed by a short cytosolic sequence of 17–42 residues that contains little sequence conservation among the 4 proteins. At present, there is no functional role that has been determined for the LRR domain of any LRRC subunit.
Table 1.
Potential loci of expression of BK LRRC subunits
| q-rtPCR; (human tissue) >10−3 RPLPO1; RT-PCR9; (mouse tissue) >10−2 β-actin3 | LRRC26 promoter activity (mouse)3 | Native Cell Recordings Appropriate gating shift (with KO confirmation in some cases) | Protein (confirmed by KO and deglycosylation)2 | ΔVh (Ca2+ free)1 Heterologous expression with BK α1 | |
|---|---|---|---|---|---|
| LRRC26 (γ1) | [Colon, aorta, trachea, prostate, salivary gland]1; [salivary gland, prostate, colon, pancreas, intestine]9; [lacrimal gland, salivary glands, vagina, prostate, trachea, glandular stomach, conjunctiva, lung, colon, spleen, mammary gland]3; | Secretory epithelium (lacrimal gland, salivary glands, colonic goblet cells, trachea epithelium, prostate, conjunctiva, uterine epithelium, mammary gland, vagina, oviduct. cervix) | Lacrimal gland3, parotid gland3,7, submandibular gland3, colonic goblet cells5, prostate6 | Lacrimal gland, parotid, submandibular, trachea, mammary gland lung, colon, glandular stomach. | −147 mV |
| LRRC52 (γ2) | [Testis, skeletal muscle]1; [testis, kidney]2 | n.a. | With Slo3 in sperm4; BK in IHCs8 | Testis and sperm4 | −101 mV |
| LRRC55 (γ3) | [Brain, fetal brain, thymus]1; [olfactory bulb, cortex spinal cord, hippocampus]3; [podocytes]10 | n.a. | Podocytes10 | n.d. | −51 mV |
| LRRC38 (γ4) | [Skeletal muscle, adrenal gland, thymus]1; | n.a. | n.d. | n.d. | −19 mV |
RPLPO (large ribosomal protein); n.a., not applicable; n.d, not determined
Potential loci of expression of each of the BK-related LRRC subunits surround BK and LRRC26 structures in Figure 1. Evidence in support of some loci remains weak, particularly for LRRC55 and LRRC38 (summarized in Table 1). The first LRRC protein to be associated with BK channels, LRRC26, was originally found to be enriched in several tumor cell lines including prostate, colon, and breast (Egland et al., 2006; Liu et al., 2012). A C-terminal myc tag attached to LRRC26, when expressed in tumor cells, localized in a fibrous network with cytokeratin just under the plasma membrane. This led to its designation as a Cytokeratin-Associated Protein in Cancer (CapC (Egland et al., 2006)). We will return to this topic below.
Figure 1. A family of four LRRC subunits contribute to tissue-specific regulation of BK channels and related family members.
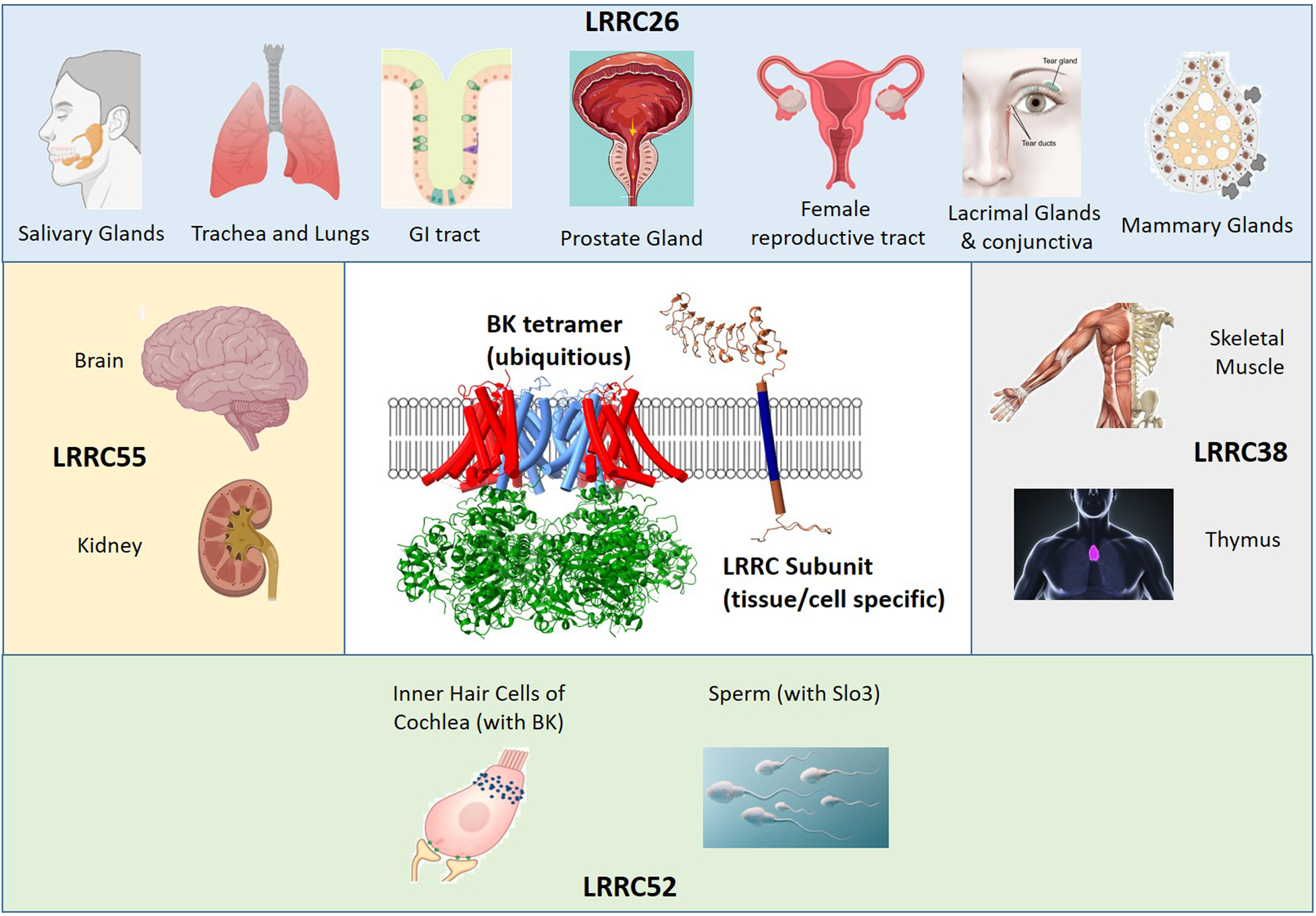
In the center, structures are shown of a human Ca2+-liganded BK channel (PDB 6V38, (Tao & MacKinnon, 2019)) along with an isolated LRRC26 subunit from Google Alpha Fold (Uniprot Q2I0M4, (Jumper et al., 2021)). For the BK structure, red highlights the voltage-sensor domain (VSD), green is the ligand-sensing domain or so-called gating ring, while blue corresponds to pore-gate-domain (PGD). Around the center, for each of the four LRRC subunits, some of the potential loci of expression are displayed, which are summarized in Table 1.
At present, it is unknown where LRRC subunits bind within the BK channel complex. For illustrative purposes, the center of Figure 1 displays the structure of the Ca2+ liganded human BK channel (Tao & MacKinnon, 2019) and an LRRC26 structure defined by AlphaFold (Jumper et al., 2021). Two bits of functional data suggest some constraints on where LRRC26 may be positioned in a BK channel complex. First, LRRC26 and BK β subunits can coassemble within single BK channels (Gonzalez-Perez et al., 2015) and, second, LRRC26 is known to influence voltage-sensor domain (VSD)/pore-gate domain (PGD) coupling (Yan & Aldrich, 2010). This suggests that LRRC binding is not occluded by simultaneous occupancy by β subunits, and may also interact with some elements in both the VSD and PGD.
B. LRRC26 and BK channels.
The existence of BK channels with unusually leftward shifted activation ranges was initially reported in prostate tumor cells (Gessner et al., 2005) and inner hair cells (IHCs) of the cochlea (Thurm et al., 2005). In such cases, even with nominally Ca2+-free cytosolic solutions, BK currents were activated at membrane voltages much more negative than could be accounted for by any combination of heterologously expressed BK α and β subunits. Taking advantage of prostate tumor cell lines, Yan & Aldrich undertook a proteomic screen of BK-associated peptides that led to identification of a protein, LRRC26, with a predicted single pass transmembrane (TM) segment with an extracellular LRR domain (Yan & Aldrich, 2010). In comparison to BK β regulatory subunits which can also produce negative shifts in BK gating of about −80 mV but only with elevated cytosolic Ca2+ (Cox & Aldrich, 2000), LRRC26, when coexpressed with BK α subunits, produces a shift in the voltage of half activation (Vh) of the BK current conductance/voltage (G/V) relationship of about −140 mV (Fig. 2A) (Yan & Aldrich, 2010). Most importantly, this gating shift also occurs at low cytosolic Ca2+, a property distinct from effects of β subunits (Yan & Aldrich, 2010). LRRC26 essentially converts BK channels from a high-voltage activated (HVA) K+ channel to a low voltage-activated (LVA) K+ channel, even in the absence of Ca2+. This provides a potential explanation for how BK channels might contribute to K+ fluxes under physiological conditions, i.e., negative membrane potentials and low resting intracellular Ca2+, in many non-excitable cells. Confirming that LRRC26 was likely responsible for gating shifts in native cells, knockdown of LRRC26 in LNCaP prostate tumor cells abolishes the gating shift (Fig. 2A) (Yan & Aldrich, 2010). Similarly, knockout of LRRC26 in mice abolishes the gating shift in salivary gland acinar cells (Fig. 2B (Yang et al., 2017)) and colonic goblet cells (Fig. 2C (Gonzalez-Perez et al., 2021)). Subsequently, heterologous expression of other LRR subunits with BK subunits revealed that LRRC52 produces substantive shifts in BK gating (up to about −90 mV), LRRC55 produces a modest negative shift (about −50 mV) at 0 Ca2+, but no shift at elevated Ca2+, while effects of LRRC38 are less clear (Yan & Aldrich, 2012). It was proposed that LRRC26, LRRC52, LRRC55, and LRRC38 be considered a BK γ subunit family, γ1 to γ4, respectively, in order of their respective abilities to shift BK gating leftward (Yan & Aldrich, 2012). Here, we primarily use the gene names. We now review several studies that have probed functional aspects of LRRC26-mediated gating shifts, which may be informative about pathological changes in regulation by LRRC26.
Figure 2. LRRC26 produces robust leftward BK gating shifts explaining low-voltage activation in prostate gland, lacrimal gland, parotid gland, and colonic goblet cells.
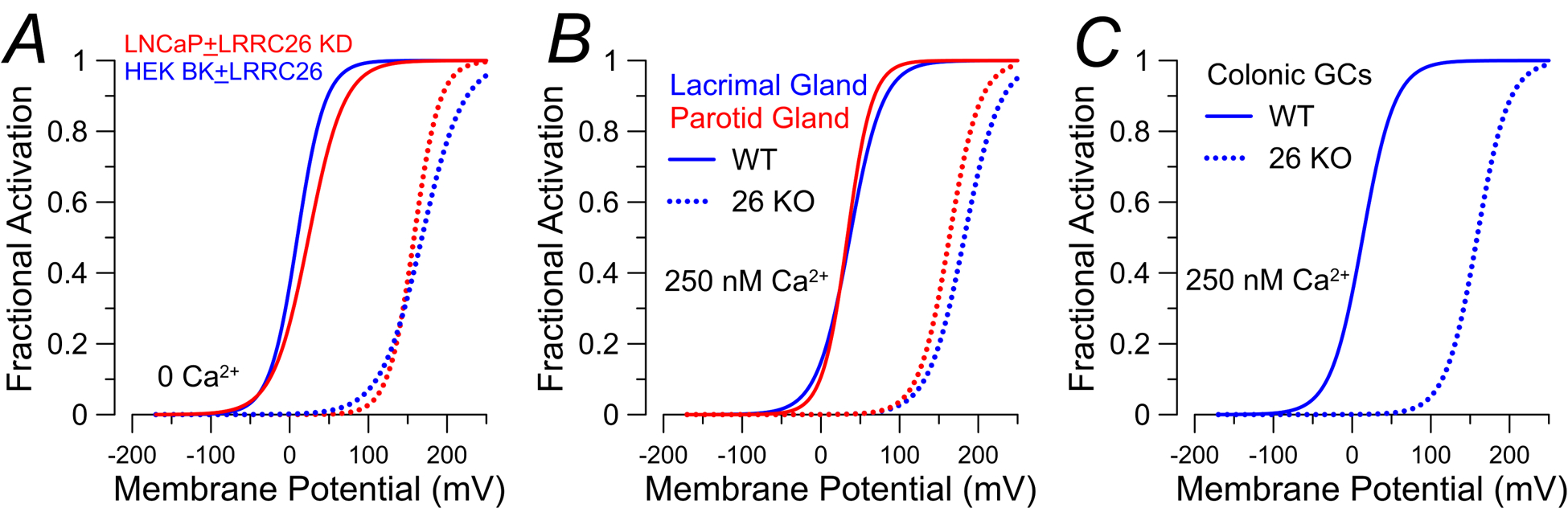
G/Vs here and in Figure 3 were generated from published Boltzmann fit values from papers cited in the text. Dotted lines correspond to G/V curves generated under conditions where LRRC26 is absent, while solid lines represent G/V curves for BKα with LRRC26. All G/Vs were generated under low Ca2+ conditions (either 0 (A) or 250 nM (B,C) Ca2+). A, G/V curves for BK current activated at 0 Ca2+ in LNCaP prostate tumor cells (red lines) without (solid red line) or with knockdown (KD, dotted red) of LRRC26. KD of LRRC26 shifts gating rightward making it indistinguishable from BKα currents alone. Expression of BK alone in HEK cells (dotted blue) is similar to KD of LRRC26 in LNCaP cells, while BK+LRRC26 expression in HEK cells (solid blue) shifts gating leftward (Yan & Aldrich, 2010). B, Native BK currents from acinar epithelial cells from lacrimal gland and parotid gland result in G/V curves similar to BK+LRRC26 (solid lines). In the LRRC26 KO mouse, the gating shift is abolished (dotted lines) (Yang et al., 2017). C, in identified colonic GCs, WT cells exhibit LVA BK currents (solid line), but GCs from LRRC26 KO mice exhibit HVA BK currents (dotted line) (Gonzalez-Perez et al., 2021).
A single LRRC26 subunit produces a full LRRC26-mediated gating shift, although four LRRC26 subunits can assemble in a BK channel.
An usual aspect of LRRC26-produced shifts in BK gating is that, as one increases the fractional occupancy of BKα channels with LRRC26 subunits, the BK currents appear composed of two populations, one exhibiting the expected −120 mV gating shift and another behaving like BK channels without any LRRC26 subunits (Gonzalez-Perez et al., 2014) (Fig. 3A). Thus, as the fraction of injected LRRC26 cRNA is increased relative to that for BKα, the fraction of channels exhibiting the full gating shift increases, until essentially all appear fully shifted. Thus, LRRC26-mediated gating shifts occur in an all-or-none fashion, being either shifted or unshifted. This behavior differs markedly from that observed with BK β subunits for which, as one increases the number of β subunits in a BK channel from 0 up to 4, each β-subunit incrementally shifts BK gating leftward (Wang et al., 2002). For BK β2 subunits, the stoichiometry was revealed by examination at the single channel level of gating shifts as a function of inactivation rate. The cytosolic N-terminus of a β2 subunit behaves as a mobile peptide segment that blocks the channel, independent of other β2 N-termini. Since inactivation rate varies linearly as a function of the number of inactivation domains in a channel (1–4), the distribution of single channel inactivation rates into four components unambiguously revealed that 1, 2, 3, or 4 subunits could be present in a β2+BKα channel (Wang et al., 2002). The LRRC26 stoichiometric problem is more complex. The unusual all-or-none behavior might occur from a number of potential LRRC26/BK assemblies, e.g., all BK channels might only contain a single unit of LRRC26 subunits (either 1, 2 or 4) or, alternatively, perhaps multiple LRRC26 subunits can assemble in a channel, but only one LRRC26 subunit is necessary for the full functional effect. By putting lanthanide tags on LRRC26 subunits and an emission acceptor on the BK blocker, iberiotoxin, it was shown that LRRC26 can associate with BKα in a maximal stoichiometry of 1:1 (Carrasquel-Ursulaez et al., 2018). Similarly, Forster Resonance Energy Transfer between fluorescently tagged LRRC26 and BKα subunits led to a similar conclusion (Gonzalez-Perez et al., 2018). However, to address the basis for the all-or-none behavior, a chimeric β2/LRRC26 construct with a β2 inactivation domain as a functional reporter was utilized to count number of inactivation domains in a single channel while also measuring the LRRC26-mediated gating shift (Gonzalez-Perez et al., 2018). It had been previously shown that a similar β1/LRRC26 construct retained the LRRC26-like ability to shift BK gating (Li et al., 2016). Use of the β2/LRRC26 construct revealed that single channels containing this chimeric subunit exhibited one of four linearly related inactivation rates, but all such channels exhibit the identical full gating shift expected with intact LRRC26. Thus, one LRRC26 subunit appears sufficient for a full gating shift. The mechanistic basis for this all-or-none shift remains undefined, as is the question of whether the unique stoichiometric properties of LRRC26:BKα assembly may have physiological implications.
Figure 3. Factors affecting LRRC26 regulatory effects on BK G/V curves.
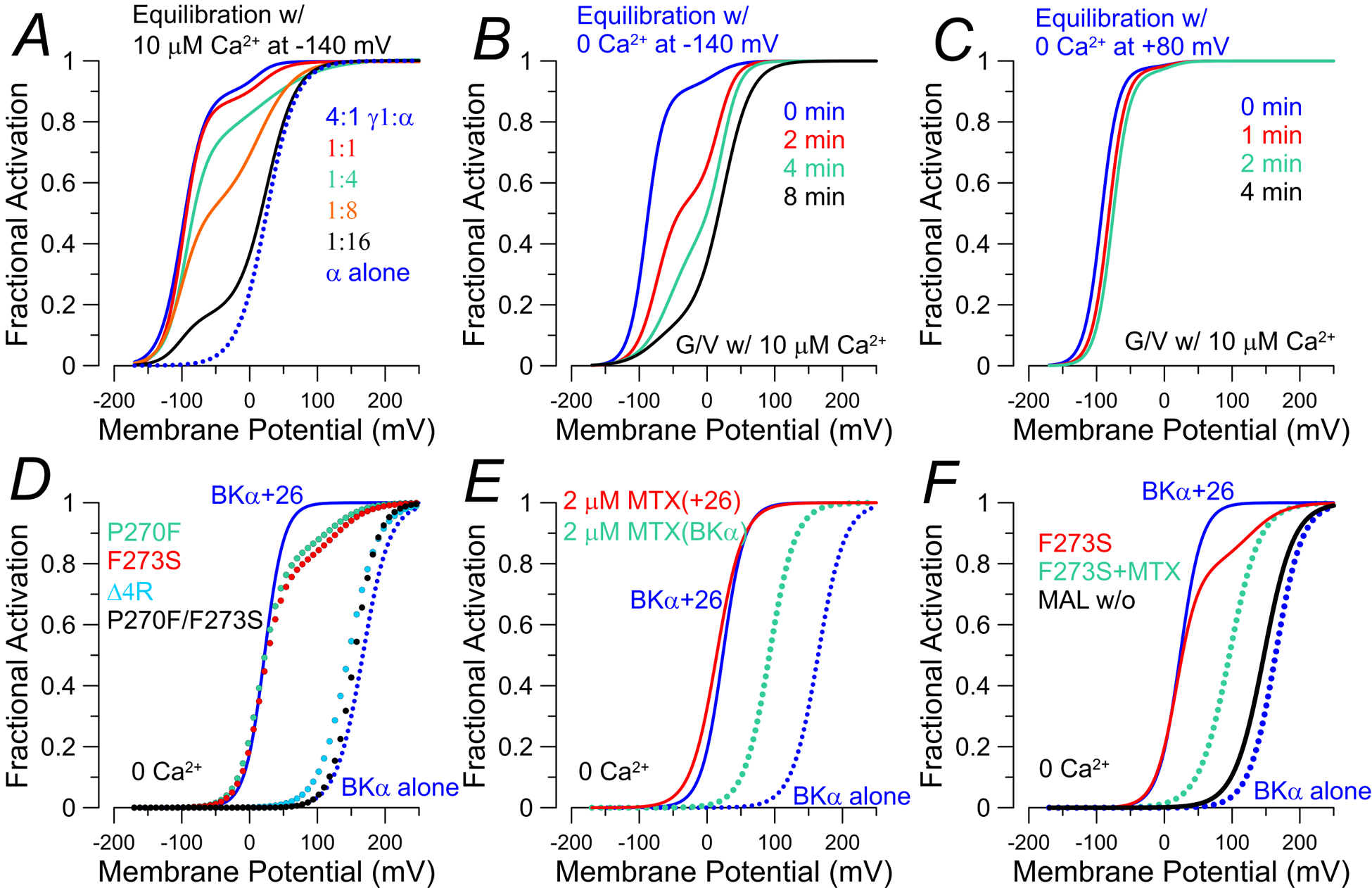
G/V curves were constructed from published values in the cited papers. For panels A-C, G/V curves were generated with 10 μM. A, BK G/V curves show loss of shifted (LVA) fraction as injected ratio of LRRC26:BKα is reduced from 4:1 to BKα alone (Gonzalez-Perez et al., 2014). B, for BK+LRRC26 channels expressed in oocytes, exposure of patches to 0 Ca2+ for the indicated times between acquisition of G/Vs with 10 μM Ca2+ results in gradual loss of shifted fraction of channels (Gonzalez-Perez et al., 2014). C, LVA behavior of BK+LRRC26 channels does not disappear if channels are held under conditions that promote channel activation (0 Ca2+, +80 mV, or 10 μM Ca2+, 0 mV) (Gonzalez-Perez et al., 2014). In D-F, G/V curves were generated with 0 Ca2+ with expression in HEK cells (solid blue lines: BKα+LRRC26; dotted blue: BKα alone). D, G/V curves for mutations in LRRC26 S6 TM segment that result in reductions or complete loss of LVA component of BK gating (Li et al., 2016). P270F and F273S result in loss of roughly over 80% of Co-IP with BKα, while Δ4R produces minimal effect on assembly with BKα, E, The BK activator, mallotoxin (MTX), shifts gating of BKα alone channels (dotted cyan), but not BKα+LRRC26 channels (dotted red) (Guan et al., 2017). F, for LRRC26-F273S (red line), following application of MTX the LVA component of BK current is abolished (dotted cyan), while still exhibiting a shift comparable to effect of MTX on BKα alone. Following washout of MTX (dotted red), the G/V becomes like BKα alone (Guan et al., 2017).
Gating shifts produced by LRRC26 can be labile.
Co-expression of LRRC26 with BKα causes a −120 mV shift in the GV curve compared to BK alone, whether G/V curves are compared at 0 or 10 μM cytosolic Ca2+. However, when excised patches are held at −140 mV while bathed in zero Ca2+ between generation of G/V curves (at 10 μM Ca2+), the G/V curve undergoes a rightward shift with time (Fig. 3B). Returning to elevated Ca2+ for extended periods of time does not reverse the gating shift. When patches were held at +80 mV, a condition which favors channel activation despite the 0 Ca2+, this gradual shift did not occur (Fig. 3C), suggesting a state-dependence to the loss of the gating shift (Gonzalez-Perez et al., 2014). At intermediate times during the shift, G/V curves for BK channel activation were described by double Boltzmann’s, with one component corresponding to shifted, LVA LRRC26-containing BK channels and the other behaving like HVA BKα alone channels (Fig. 3B). On average, the transition to HVA gating behavior occurred with a time constant of about 7.5 min. Although it is possible LRRC26 remains associated with BKα but has become inactive, another potential explanation for this phenomenon is that LRRC26 dissociates from BK channels. However, to date this phenomenon has not been noted in recordings of LRRC26-containing BK channels in excised inside-out patches from prostate tumor lines or with overexpression in HEK cells (Yan & Aldrich, 2010, 2012). Similarly, whole-cell recordings from parotid gland cells dialyzed with cytosolic 250 nM Ca2+ (Yang et al., 2017) do not reveal any time-dependent shifts in G/V curves. Yet, channels expressed in oocytes arising from coassembly of LRRC26 and β2 subunits with BKα also do not exhibit the loss of LVA gating behavior when maintained in 0 Ca2+ (Gonzalez-Perez et al., 2015), perhaps suggesting that cell-specific components may impact on the maintenance of the LRRC26 regulatory effect.
The LRRC26 TM segment and cytosolic tail contribute to gating shifts and may also influence affinity between LRRC26 and BK α subunits.
Potential determinants of the ability of LRRC26 and other LRRC subunits to produce gating shifts have been functionally and biochemically probed in three papers: 1. a series of deletion mutants through the entire LRRC26 subunit (Yan & Aldrich, 2010); 2. chimeras generated from different segments of the four LRRC subunts (Li et al., 2015); and 3. a β1/LRRC26 chimeric construct with only TM and cytosolic tail from LRRC26, along with mutational analysis of key residues through TM and the tail (Li et al., 2016). Overall, the results indicate that the TM segment and a set of 4 basic residues just at the beginning of the LRRC26 cytosolic tail contain key determinants of the ability of a LRRC subunit to produce gating shifts. However, a complexity in the interpretation of the loss of gating shifts is whether a mutated construct is altering the functional effect, or disrupting the ability of a construct to coassemble properly with BK. Some features of the results suggest that, although some mutations may specifically alter the ability of LRRC26 to shift gating while still being associated with the channels, some mutations may affect the affinity of interaction of LRRC26 with BKα. For the first case, mutation of 4 (Δ4R) basic residues at the beginning of the cytosolic tail of LRRC26 tail markedly reduces the gating shift, while coimmunoprecipation of Δ4R with BKα yields bands in western blots similar to WT LRRC26 (Yan & Aldrich, 2010). In contrast, two mutations in the LRRC26 TM segment may reflect weakened affinity of interaction. For these two mutations, P270F and F273S, G/V currents are best described by two components (Fig. 3D), an LVA population of about 75% with the remainder being HVA (Li et al., 2016). Yet, despite the fact that about 75% of channels exhibit the full gating shift, co-immunoprecipitation of these mutated constructs from the BK channel complexes is reduced to much less than half of that of WT LRRC26 coassembly with BKα. Although densitometry was not used to assess this quantitatively, the reductions appear close to less than 10–20% of WT controls. In later work, densitometric measurements confirmed that association of F273S-LRRC26 with BKα is reduced to 20% of that for WT LRRC26 (Guan et al., 2017). Mutation of these residues together fully abolishes the LRRC26-induced gating shift. On balance, the Co-IP experiments strongly support the idea that the affinity of interaction between LRRC26 and BK has been weakened by some TM mutations. Yan and colleagues suggest that their results are consistent with the all-or-none gating shift model in which a substantially reduced occupancy of BK channels by LRRC26 is still sufficient to permit most channels to exhibit LVA gating behavior.
The idea that double Boltzmann’s in G/V curves can arise from altered affinity of interaction between LRRC26 and BK is also supported by results with the BK activator, mallotoxin, a potent BK activator (Zakharov et al., 2005; Almassy & Begenisich, 2012). Mallotoxin (MTX) shifts BK gating about −120 mV at 0 cytosolic Ca2+ (Zakharov et al., 2005; Almassy & Begenisich, 2012). Intriguingly, LRRC26 abolishes the ability of MTX to shift BK gating (Fig. 3E, (Guan et al., 2017)). This has been interpreted to indicate that the presence of LRRC26 occludes the ability of MTX to reach its binding site. However, for the LRRC26-F273S TM mutation, which results in a double Boltzmann behavior, in the presence of MTX the LVA component of BK gating disappears and the resulting G/V is comparable to that arising from MTX effects on BKα alone (Guan et al., 2017). With washout of MTX, the G/V then becomes similar to BKα alone. This has been interpreted to indicate that weakened binding of F273S-LRRC26 subunit to BK may not completely occlude the ability of MTX to reach its site (Fig. 3F), such that once MTX is bound it further destabilizes interaction of F273S-LRRC26 with the BKα subunit, promoting dissociation of the F273S-LRRC26 subunit from the channel. Supporting this view, MTX reduces F273S-LRRC26 co-IP with BK channels, while having no effect on WT LRRC26 in BK channels (Guan et al., 2017).
Together these results, while pointing out the importance of particular LRRC26 residues in defining the gating shift, also support the idea that the intrinsic binding affinity that underlies LRRC26 subunit interaction with BK subunits may be poised to be more readily disrupted, potentially allowing down (or up) regulation of the fraction of LRRC26 subunits in the BK population. Clearly, additional work is necessary to evaluate this premise, but the set of results raises the possibility that association of LRRC26 with BKα may be labile and perhaps subject to dynamic regulation, at least in some cellular environments.
C. LRRC52 (γ2): a regulatory subunit of both Slo3 and BK channels.
Channel regulation by LRRC52 also reveals some interesting insights into LRRCs. The closest homolog of the BK channel is a voltage- and pH-regulated K+ channel, termed Slo3 (encoded by KCNU1), which is abundantly expressed in testis (Schreiber et al., 1998). As expected, a voltage- and alkalization-activated K+ current was later identified in native mouse sperm (Navarro et al., 2007), but the native sperm current was activated at more negative voltages than currents from heterologously expressed Slo3 (Zhang et al., 2006). Yet, KO of Slo3 abolished all voltage- and alkalization-activated K+ current in mature sperm (Zeng et al., 2011).Subsequently, LRRC52 was then shown to be present in mammalian sperm and to shift Slo3 gating to more negative voltages, potentially accounting for the differences between heterologously expressed Slo3 and native sperm K+ current (Yang et al., 2011). Supporting a critical role of LRRC52 in sperm K+ function, genetic KO of LRRC52 produced fertility deficits in mouse sperm while also shifting sperm K currents to be more comparable to properties of heterologously expressed Slo3 alone (Zeng et al., 2015).
Surprisingly, although initially considered to be sperm-specific, LRRC52 was subsequently found to be critical to defining the functional properties of BK channels in inner hair cells (IHCs) of the mouse cochlea (Lingle et al., 2019). Remarkably, KO of LRRC52 produced a positive 210 mV gating shift of BK channels in IHCs, shifting the native IHC G/V curve to be similar to that of BK channels lacking regulatory subunits. The fact that heterologously expressed LRRC52 shifts BK gating about −90 mV suggests that LRRC52 may help stabilize some other as-yet-unidentified factor that contributes to the enormously shifted IHC BK current. Furthermore, LRRC52 KO disrupts the high-density clustering of BK channels between the stereocilia and above the nuclei, suggesting that LRRC proteins may help stabilize macromolecular BK ion channel complexes.
The discovery that LRRC52 can partner either with Slo3 or with BKα raises the question, to what extent are LRRC subunits promiscuous in their ability to assemble and regulate Slo family channels? In the initial characterization of the ability of LRRC52 to alter Slo3 function, Slo3 was also shown to coassemble with LRRC26, but only weakly with LRRC38 and LRRC55, although even for LRRC26 functional effects on Slo3 were minimal at elevated pH (Yang et al., 2011). There are no reports of whether any LRRC subunits may interact with and regulate either of the two Na-activated Slo2 subunits, KCNT1 and KCNT2 (Salkoff et al., 2006). The coupling of LRRC52 and BKα in IHCs also points out two challenges that are faced in attempts to understand the roles of LRRC proteins. First, because of the large single channel currents associated with BK channels, only a few channels need to be activated or even expressed in a cell in order to produce important physiological effects. Second, LRRCs may partner in very circumscribed cellular loci where the total message and protein abundance might not be readily revealed in some screening methods. Relevant to that possibility, message (Chen et al., 2021a, b) and protein (Limbutara et al., 2020) for LRRC52 has been recently identified in distal collecting tubule segments and cells of the kidney. Yet, message or protein for BKα was not observed in the same segments, although BK channels have been unambiguously recorded from other locations in the kidney, including from intercalated cells of collecting tubule and cortical collecting duct cells (Palmer & Frindt, 2007). It is possible that levels of both message and protein for BKα are too low for the methodologies employed.
D. LRRC55 (γ3) and LRRC38 (γ4)
LRRC55 is intriguing because of robust expression of LRRC55 message in brain (Yan & Aldrich, 2012); in situ hybridization has demonstrated elevated LRRC55 expression in adult brain in medial habenula nucleus, cerebellum, and pons (Zhang et al., 2018). Despite the apparent absence of gating shifts at elevated Ca2+, the ability of LRRC55 to produce a −50 mV gating shift at 0 Ca2+ might potentially have unique effects on neuronal excitability (Yan & Aldrich, 2012). As yet, there is no information about LRRC55-containing BK channels in native neurons. Recently, evidence supporting the idea that LRRC55 can impact on properties of BK channels in native tissues appeared in a study of podocytes in kidney (Hu et al., 2021). Specifically, Lrrc55 message and LRRC55 protein was found to be present in glomerular tissues and expression was increased in podocytes of patients with focal segmental glomerulosclerosis, diabetic nephropathy, and membranous nephropathy. Similarly, Lrrc55 expression was increased in podocytes during angiotensin II induced podocytes injury in mice (Hu et al., 2021). Knockout of LRRC55 ameliorated the pathology. Podocytes were shown to express a BK current, which was enhanced by angiotensin II, while LRRC55 KO abolished the angiotensin II-induced enhancement of BK current. Although these results do not reveal a specific effect of LRRC55 on BK gating properties, LRRC55 dramatically increases total BK channel current density in podocytes, with resulting pathological consequences.
Effects of LRRC38 on BK channels are quite modest (Yan & Aldrich, 2012; Kshatri et al., 2017) and LRRC38 also has no effect on Slo3 gating (Yang et al., 2011). As yet, whether LRRC38 can even coassemble with BK channels has probably not been satisfactorily addressed. Any role of LRRC38 on BK or other Slo family channels remains to be revealed.
II. LRR subunit regulation of channels in native cells, physiological roles, and potential implications for pathology.
A. A general role for LRRC26-containing BK channels in secretory epithelial cells.
Secretory epithelial cells provide a supportive barrier to mucosal surfaces through the production and secretion of peptides, mucus, fluid, bicarbonate, and/or salts. Regardless of location or secretory constituents, ion channels and transporters regulate the function of epithelial cells. An important gap in knowledge remains the full identification of all the sets of ion channels and transporters and their specific membrane localization (apical vs. basolateral) that work together to fulfill the particular requirements of a given epithelial layer or gland. A possible contribution of BK channels to K+ conductance in epithelial cells has been known for some time (Lomax et al., 1996; Butterfield et al., 1997; Linley et al., 2014). However, at membrane potentials and cytosolic Ca2+ concentrations thought to be typical of inexcitable cells, channels arising from known BK splice variants and β regulatory subunits would not be expected to contribute to resting K+ fluxes in such cells. This conundrum was resolved by the discovery of LRRC26 and its ability to shift BK activation leftward by over 120 mV even at 0 Ca2+.
With the identification of LRRC26 as a BK regulatory subunit, potential contributions of LRRC26-containing BK channels were quickly established in salivary gland acinar epithelial cells (Almassy & Begenisich, 2012) and airway epithelium (Manzanares et al., 2014; Manzanares et al., 2015). Parotid gland cells express BK currents with G/V properties similar to heterologously expressed LRRC26-containing BK channels and also exhibit resistance to stimulation by mallotoxin (Almassy & Begenisich, 2012). In airway epithelial cells, an interferon-γ (IFN-γ)-stimulated reduction of BK channels expression has been implicated in mucociliary dysfunction. Stimulation by IFN-γ is also associated with reduction in LRRC26 expression and the appearance of mallotoxin-activated transepithelial currents, which are absent in untreated tissue (Manzanares et al., 2014), supporting the idea that LRRC26-containing BK channels are present in airway epithelium. Negative effects of transforming growth factor beta1 on airway function have also been shown to be associated with reduced epithelial cell BK activity arising from reduction of LRRC26 expression (Manzanares et al., 2015). The latter results are intriguing because they point out that normally occurring signaling pathways can regulate LRRC26 expression.
A primary role of LRRC26-containing BK channels in essentially all secretory epithelial cells was given support with the development of a LRRC26 general KO mouse in which the Lrrc26 promoter controlled expression of LacZ (Yang et al., 2017). Essentially all secretory epithelia that were examined express Lrrc26 message and/or exhibit Bluo-Gal staining indicative of Lrrc26-promoter activity. Furthermore, in lacrimal glands and various salivary glands (parotid and submandibular gland), comparison of currents in cells from WT and LRRC26 KO animals directly demonstrated that KO of LRRC26 shifted BK gating in such cells from low-voltage activated to the high-voltage activated currents typical of BK channels without regulatory subunits (Fig. 3B), consistent with the effect of LRRC26 observed when coexpressed with BK α subunits.
For salivary glands, the involvement of BK channels in K secretion is well-established (Catalan et al., 2014). The concentration of K+ in saliva is typically 5–10 fold that in plasma, but KO of BK channels markedly reduces K+ secretion in saliva. In contrast, KO of the KCa3.1 (IK1) Ca2+-activated, voltage-independent K channel was without effect on K+ secretion (Nakamoto et al., 2008). Consistent with the importance of LRRC26 in defining properties of salivary gland BK channels, LRRC26 KO produces a reduction of K+ content in saliva (Yang et al., 2017) similar to that occurring with full BK KO (Nakamoto et al., 2008).
B. LRRC26-containing BK channels and colitis.
Recently, the just-described LRRC26 KO mice were used to probe the potential role of LRRC26-containing BK channels in the colonic epithelium (Gonzalez-Perez et al., 2021). The single layer of colonic epithelial cells organize in crypts to form a physical and biochemical barrier between the host and the environment while promoting mucosal homeostasis (Peterson & Artis, 2014). Colonic epithelium consists of multiple cell types, with the primary cell types being 1. absorptive enterocytes comprising more than 75% of the cells, 2. mucin2-secreting goblet cells, which are responsible for formation of the protective mucus layer, and 3. enteroendocrine cells. Although BK channels have been known to be present in colonic epithelium and BK channels are thought to be associated with colonic K+ loss during secretory diarrhea (Sandle & Hunter, 2010), the precise cellular membranes, the cellular loci, and circumstances of activation of BK channels in colonic epithelium have remained incompletely resolved. Somewhat surprisingly, genetic deletion of LRRC26 renders mice sensitive to colitis induced by dextran sodium sulfate (DSS) (Gonzalez-Perez et al., 2021), a standard model of colitis primarily involving the distal colon. In response to DSS, LRRC26 KO mice exhibited irreversible loss of body weight, high disease severity indices, and examination of distal colon revealed loss of crypts and GCs. By crossing LRRC26 KO mice with mice that express a transgene encoding mCherry attached to Mucin2 under control of the Mucin2 promoter (Birchenough et al., 2016), direct visualization of mCherry fluorescence in GCs allowed electrophysiological recordings from unambiguously identified fluorescent GCs and adjacent non-fluorescent enterocytes from both WT and LRRC26 KO mice. This showed that LRRC26-containing BK channels were only present in the mCherry-identified GCs, while the non-fluorescent enterocytes exhibited no BK current at all. The specific mechanism underlying the LRRC26 KO-induced colitis, i.e., whether the loss of BK function leads to increased sensitivity to colitis via a deficit in mucus formation, deficient stem cell proliferation or growth, or deficient healing and recovery, remains to be determined.
Given the critical role of GCs in maintaining the protective mucus layer of the colon (Nystrom et al., 2021), these results point to a potential role of BK channels in helping to sustain the generation of normal mucus layer properties. However, a number of ion channels of the colonic epithelium, including CFTR (Gustafsson et al., 2012) and bestrophin2 (Yu et al., 2010), are also known to be important. The present results support the idea that BK channels on the luminal side of some epithelial cells (Sandle & Rajendran, 2012) may be critical for supporting anion fluxes essential for mucus secretion and maturation. The recent work (Gonzalez-Perez et al., 2021) now shows clearly that dysfunction in BK channel properties may also increase susceptibility or perpetuation of colonic disease. Given the presence of LRRC26-containing BK channels in most other secretory epithelial cells (Yang et al., 2017), LRRC26-containing BK channels may play a general role in a number of tissues in helping maintain normal secretory function, whether fluid, peptides, or mucus.
Although LRRC26-containing BK channels are suited to play a prominent role in secretory epithelial cells, our understanding of how such fluxes are critical to normal physiology remains incomplete. One issue is that in the recent work a specific colitis-inducing stimulus, DSS, was required to unmask a critical role of LRRC26-containing channels. However, the idea that some naturally occurring diminution in LRRC26 expression might be a contributing factor to development of pathology is worth consideration. Related to this possibility, analysis of gene-expression profiles of colon from IBD patients has revealed a reduced expression of LRRC26 in patients with active ulcerative colitis (UC) in comparison to patients with inactive disease (Gonzalez-Perez et al., 2021). Yet, somewhat contrary to this idea, it has also been reported that colonic BKα subunit message is increased in UC patients (Sandle et al., 2007). However, it is unknown whether this is a compensatory change to the underlying pathology or causative. Given that loss of BK function predisposes to colitis sensitivity, we would suggest that the increase in BK subunit expression is part of a compensatory process.
C. LRRC26 and smooth muscle
A potential role of LRRC26-containing BK channels has also been proposed for rat cerebral arteries (Evanson et al., 2014), mouse uterine artery (Lorca et al., 2018), and mouse bronchiole smooth muscle cells (mBSMCs) (Noda et al., 2020). A role of LRRC26-containing BK channels in smooth muscle would be of high impact because of expected profound effect on vascular tone (Dopico et al., 2018). As highlighted, the primary distinguishing feature of LRRC26-containing BK channels is their marked −120 to −140 mV leftward gating shift, which is clearly apparent both from heterologously expressed channels and also native cells for which WT and LRRC26 KO animals are compared. Unfortunately, for some native cells, whole-cell measurements of G/V relationships can be difficult to define. Furthermore, as yet, there is no demonstration of a useful antibody for LRRC26 immunohistochemistry that meets the criteria of differential staining between WT and KO tissues. However, a recent paper that compares BK currents and the resulting G/V curves in mBSMCs to those in mouse aortic SMCs (mASMCs) provides some good support for a role of LRRC26-containing BK channels in some vascular myocytes (Noda et al., 2020). With 10 nM cytosolic Ca2+, the Vh for activation of currents from BSMCs was similar to that (Vh ~ −15 to −25 mV) for BKα+LRRC26 expressed by the same authors in HEK cells (Noda et al., 2020). In contrast, BK currents from mASMCs exhibited a Vh similar to that of either BK α expressed alone or BKα+β1 in HEK cells (Vh ~ +130-+150 mV).
D. LRRC26 and cancer.
The LRRC26 gene was originally identified from a cDNA library prepared from human breast and prostate cancer cell lines (Egland et al., 2006). The abundance of this message in a number of different cancer cell lines and its cellular localization in close juxtaposition with sub-plasma membrane cytokeratin led to its designation as CAPC (Cytokeratin-Associated Protein in Cancer) (Egland et al., 2006). In general, both message and protein for LRRC26 are elevated in cancer cell lines or cancer specimens in comparison to normal tissue, leading to the proposal that LRRC26 may serve as a tumor marker and may influence cancer cell behavior. Although evaluation of the role of LRRC26 in tumor initiation and development is beyond the scope of this commentary, some results are worth mentioning. In both prostate and breast cancer cell lines, knockdown of LRRC26 expression by siRNA enhances tumor growth, while overexpression negatively regulates NF-κB activation and suppresses tumor growth and metastasis This parallels the observation that triple negative breast cancer (TNBC) is associated with downregulation of LRRC26 expression associated with epigenetic hyper-methylation of LRRC26 promoter sequence upstream of the LRRC26 open reading frame (Miyagawa et al., 2018). The specific molecular cascade by which LRRC26 may impact on tumor cell growth remains unknown.
Are the effects of LRRC26 in tumor cells specifically linked to BK channels? The negative impact of LRRC26 overexpression on NF-κB activation may be independent of BK channels, since knockdown of BKα by siRNA did not abolish or reduce the LRRC26-mediated reduction in NF-κB activation in MCF7 cells nor in LNCaP cells which abundantly express LRRC26 (Liu et al., 2012). Furthermore, if the impact of LRRC26 on tumor growth was associated with BK channels, one might expect that the consequences of manipulations of BK expression might parallel those of LRRC26. Yet, reduction of BK expression is associated with increased tumor free survival in mice while both in breast cancer and prostate cancer cell lines increases in BK expression lead to more metastatic tumor growth (Bloch et al., 2007; Khaitan et al., 2009; Oeggerli et al., 2012). Although the impacts of various ion channels on tumor growth remain inadequately understood (Lang & Stournaras, 2014; Prevarskaya et al., 2018), both the failure of BK knockdown to remove the LRRC26-mediated effects on NF-κB activation and the dissociation of BK channel effects on tumor growth from LRRC26 effects would seem to suggest LRRC26 may have functions independent of its role as a BK regulatory subunit. Clearly, this requires additional consideration. Furthermore, irrespective of the underlying mechanism by which LRRC26 impacts on tumor growth, the pronounced suppression of breast tumor metastasis by LRRC26 overexpression (Liu et al., 2012) certainly warrants additional investigation, particularly in regards to tumors recalcitrant to other treatment strategies, e.g., the TNBCs.
E. Summary
The above sections highlight the prominent contributions of LRRC26-containing BK channels in both glandular acinar epithelium and layered epithelium, and point out the likely role of such channels in essentially all secretory epithelium, and perhaps in some vascular smooth muscle cells. For salivary glands, airways, and colon, loss of LRRC26 or downregulation by naturally occurring signals leads to or is associated with profound pathological changes in those tissues, presumably in some way related to changes in K+ flux or other aspects of secretion. At present, we are really in the early stages of unveiling the potential roles this category of BK channel can play in different tissues. With appropriate tests, similar pathological consequences of LRRC26 loss might be expected in other tissues. A number of questions must be addressed. Why do LRRC26 KO mice not exhibit clear spontaneous phenotypic deficits? Full KO’s grow and breed normally as far as has been observed. In the absence of the DSS manipulation, no obvious colitis phenotype has been noted out to 12 weeks. In other systems for which LRRC26-containing secretory cells play a central role, might a particular assault, e.g., a uterine infection, an airway irritant, or other xenobiotic factor that enters the lumen surrounded by the vulnerable epithelium, trigger pathological consequences?
III. Possible underpinnings of LRR-associated pathology
We now turn to a consideration of potential mechanisms that might underlie naturally occurring LRR-associated disease. Results cited above indicate clearly that loss of LRRC26 from specific epithelial tissues can result in pathological function. Although gene KO may point to a potential role of a protein in particular pathologies, it does not reveal how changes in expression occur normally. Here we consider two kinds of mechanism that might influence the availability of LRRC26 for assembly in BK channels: first, factors that may influence the affinity of the LRRC26-BKα interaction and, second, regulatory mechanisms that may alter LRRC26 expression.
A. Implications of low affinity interactions of LRRC26 with BK channels.
We have highlighted several results suggesting that the intrinsic affinity of the LRRC26-BK interaction may be poised such that subtle changes in that affinity may shift BK channels to high voltage-activated. First, the unusual stoichiometric relationship in which a single LRRC26 subunit is sufficient to produce a full gating shift permits a complete shift from LVA to HVA gating by loss of a single subunit (Gonzalez-Perez et al., 2014; Gonzalez-Perez et al., 2018). This would be most impactful in cases where the average LRRC26 occupancy in a channel population is low. It will be interesting to see whether the double Boltzmann behavior observed with partial LRRC26 occupancy may be a feature of currents in some native cells or in disease states. Second, the shift from LVA to HVA behavior observed for LRRC26-containing BK channels expressed in oocytes when patches are exposed continuously to 0 Ca2+ under conditions that favor closed channels suggests (Gonzalez-Perez et al., 2014) that Ca2+ or state-dependent mechanisms can influence LRRC26-BK affinity. Third, point mutations in the LRRC26 TM produce gating shifts consistent with weakened affinity, supporting the idea that the native affinity may be poised at a level permissive for regulation (Li et al., 2016). Fourth, mallotoxin can irreversibly drive the gating of the F273S-LRRC26 construct to become fully HVA with associated biochemical evidence that mallotoxin reduces F273S-LRRC26 coassembly with BK channels. (Guan et al., 2017).
Together these results suggest that the physical interaction between LRRC26 and BKα, might be subject to modulation, perhaps by cell-specific factors, that may alter the fractional occupancy of BK channels by LRRC26. At present, no such regulatory components are known, although the LRRC26 cytosolic C-terminus contains both consensus phosphorylation sites and a cluster of basic residues near the membrane that might be a target for phospholipid interaction. A labile LRRC26-BK interaction would not only be physiologically important, but also might lead to pathological consequences, as illustrated by the impact of LRRC26 KO on colitis. It must be kept in mind that, in order for a loss of LRRC26 to be physiologically impactful in regards to association with BK, it probably requires that almost all channels lose LRRC26 subunits, since even a small fraction of LVA channels might be expected to dominate physiologically.
B. Potential mechanisms of down-regulation of expression of LRRC26
Results cited above implicate two distinct signaling pathways in the down-regulation of LRRC26 message and protein in airway epithelial. In one case, the cytokine, IFN-γ, reduces BK function in airway epithelial via downregulation of LRRC26 expression (Manzanares et al., 2014). In the other case, in cystic fibrosis, transforming growth factor β1 (TFG-β1) is upregulated and induces down-regulation of LRRC26 and reduction in BK function underlying deficits in mucociliary clearance (Manzanares et al., 2015). Presumably these effects arise from regulation of transcription.
An additional mechanism suggested above that may underlie down regulation of LRRC26 expression is suggested from an RNA-sequencing analysis of triple-negative breast cancer (TNBC) specimens along with analysis of The Cancer Genome Atlas TNBC dataset (Miyagawa et al., 2018). The LRRC26 sequence is remarkable in the extent to which it contains GpC islands that are the candidate loci for methylation. This analysis identified frequent downregulation of LRRC26 expression in TNBC tissues (Miyagawa et al., 2018). Further evaluation revealed the LRRC26 was frequently silenced in both TNBC tissues and cell lines as a consequence of promoter methylation. Intriguingly, in a TNBC cell line that lacks LRRC26 expression, LRRC26 expression was restored by an inhibitor of methylation. Although the analysis just summarized focused on the potential importance of LRRC26 expression in tumor growth regulation, that LRRC26 expression can be influenced by epigenetic methylation of the LRRC26 promoter region (Miyagawa et al., 2018) indicates that mechanisms are available that could lead to pathologies similar to those that may be associated with LRRC26 KO. Methylation and demethylation are cell and tissue specific processes (Lanata et al., 2018). Furthermore, although it typical to think of methylation as irreversible, this is not uniformly the case (Lanata et al., 2018). Although a detailed examination of methylation pertinent to LRRC26 is beyond the summary here, such a mechanism has two key features that might merit its consideration as a contributor to LRRC26 based pathologies, namely tissue specificity and reversibility. Although identification of genes that show up- or down-regulation by methylation in response to different diseases and xenobiotic agents is a major cottage industry that largely just identifies associations, it is interesting to note LRRC26 is one of a set of 22 methylated CpGs in 21 gene loci associated with Ischemic Stroke (Soriano-Tarraga et al., 2020).
Overview.
Since recognition almost 12 years ago that a family of homologous LRRC proteins are potential regulators of BK channel function, we are really only at the beginning stages of understanding how such subunits may impact on normal physiological function. Yet, KO animal models are now revealing that, in the absence of particular LRRC subunits, major alterations in BK channel function and/or expression can occur that can result in major pathological consequences, e.g., colitis for LRRC26, male infertility for LRRC52, and perhaps nephropathy for LRRC55. Given the pervasiveness of LRRC26 in a variety of critical organ systems, there are probably many more pathological consequences to be discovered. Yet, despite what can be learned from KO models, they are only useful in pointing out potential consequences of changes in subunit expression, but fail to identify mechanisms that might contribute to disease. Here, particularly in the case of LRRC26, we have highlighted aspects of the regulation of BK channels by LRRC26 that may eventually help identify the molecular underpinnings of disease that may arise from mis-regulation or altered expression of LRRC subunits.
Supplementary Material
Funding.
No funding was received in order to prepare this article, but the authors’ research is supported by NIH grant GM-081748 (to C.J.L.).
Abbreviations
- BK
large conductance, Ca and voltage-activated K channel
- LRRC
leucine-rich-repeat-containing
- GCs
goblet cells
- Vm
membrane potential
- G/V
conductance/voltage
- MTX
mallotoxin
- HVA
high-voltage-activated
- LVA
low-voltage-activated
- Vh
voltage of half activation
Author profiles

Vivian Gonzalez-Perez is currently an Instructor of Anesthesiology at Washington University in St. Louis. During Ph.D. work, she explored nuances of K+ channel voltage-sensor movement. Subsequently, she has studied regulation of BK-type K+ channels by regulatory subunits and seeks to define physiological roles of BK channels in native cells. Yu Zhou, currently an Instructor of Anesthesiology, studied AMPA receptors as a grad student, while more recently probing structure-function of BK pore elements and the mechanistic and structural basis of fungal alkaloid inhibition of BK channels. Matt Ciorba, a physician scientist, directs the Washington University Inflammatory Bowel Disease Center and leads a group focused on solving challenges of intestinal disease. Matt began his career studying regulation of voltage-dependent K+ channels. Chris Lingle is Professor of Anesthesiology at Washington University. His group has explored Ca2+-dependent regulation of BK channels, the pH-regulated Slo3 K+ channel, and properties of regulatory subunits.
Footnotes
Competing interests
The authors declare that they have no competing financial interests.
References
- Almassy J & Begenisich T. (2012). The LRRC26 protein selectively alters the efficacy of BK channel activators. Molecular pharmacology 81, 21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey CS, Moldenhauer HJ, Park SM, Keros S & Meredith AL. (2019). KCNMA1-linked channelopathy. Journal of General Physiology 151, 1173–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birchenough GM, Nystrom EE, Johansson ME & Hansson GC. (2016). A sentinel goblet cell guards the colonic crypt by triggering Nlrp6-dependent Muc2 secretion. Science 352, 1535–1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch M, Ousingsawat J, Simon R, Schraml P, Gasser TC, Mihatsch MJ, Kunzelmann K & Bubendorf L. (2007). KCNMA1 gene amplification promotes tumor cell proliferation in human prostate cancer. Oncogene 26, 2525–2534. [DOI] [PubMed] [Google Scholar]
- Butler A, Tsunoda S, McCobb DP, Wei A & Salkoff L. (1993). mSlo, a complex mouse gene encoding “maxi” calcium-activated potassium channels. Science 261, 221–224. [DOI] [PubMed] [Google Scholar]
- Butterfield I, Warhurst G, Jones MN & Sandle GI. (1997). Characterization of apical potassium channels induced in rat distal colon during potassium adaptation. The Journal of physiology 501 (Pt 3), 537–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasquel-Ursulaez W, Alvarez O, Bezanilla F & Latorre R. (2018). Determination of the Stoichiometry between alpha- and gamma1 Subunits of the BK Channel Using LRET. Biophys J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalan MA, Pena-Munzenmayer G & Melvin JE. (2014). Ca(2)(+)-dependent K(+) channels in exocrine salivary glands. Cell calcium 55, 362–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Chou CL & Knepper MA. (2021a). A Comprehensive Map of mRNAs and Their Isoforms across All 14 Renal Tubule Segments of Mouse. Journal of American Society of Nephrology 32, 897–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Chou CL & Knepper MA. (2021b). Targeted Single-Cell RNA-seq Identifies Minority Cell Types of Kidney Distal Nephron. Journal of the American Society of Nephrology 32, 886–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contet C, Goulding SP, Kuljis DA & Barth AL. (2016). BK Channels in the Central Nervous System. Int Rev Neurobiol 128, 281–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox D & Aldrich R. (2000). Role of the β1 subunit in large-conductance Ca2+-activated K+ channel gating energetics. Mechanisms of enhanced Ca2+ sensitivity. Journal of General Physiology 116, 411–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan J, Walshe K, Alsbury S, Hokamp K, O’Keeffe S, Okafuji T, Miller SF, Tear G & Mitchell KJ. (2007). The extracellular leucine-rich repeat superfamily; a comparative survey and analysis of evolutionary relationships and expression patterns. BMC Genomics 8, 320–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dopico AM, Bukiya AN & Jaggar JH. (2018). Calcium- and voltage-gated BK channels in vascular smooth muscle. Pflügers Archiv - European Journal of Physiology 470, 1271–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudem S, Large RJ, Kulkarni S, McClafferty H, Tikhonova IG, Sergeant GP, Thornbury KD, Shipston MJ, Perrino BA & Hollywood MA. (2020). LINGO1 is a regulatory subunit of large conductance, Ca(2+)-activated potassium channels. Proceedings of the National Academy of Sciences of the United States of America 117, 2194–2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudem S, Sergeant GP, Thornbury KD & Hollywood MA. (2021). Calcium-Activated K(+) Channels (KCa) and Therapeutic Implications. Handbook of experimental pharmacology 267, 379–416. [DOI] [PubMed] [Google Scholar]
- Egland KA, Liu XF, Squires S, Nagata S, Man YG, Bera TK, Onda M, Vincent JJ, Strausberg RL, Lee B & Pastan I. (2006). High expression of a cytokeratin-associated protein in many cancers. Proceedings of the National Academy of Sciences of the United States of America 103, 5929–5934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evanson KW, Bannister JP, Leo MD & Jaggar JH. (2014). LRRC26 Is a Functional BK Channel Auxiliary gamma Subunit in Arterial Smooth Muscle Cells. Circulation research 115, 423–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gessner G, Schonherr K, Soom M, Hansel A, Asim M, Baniahmad A, Derst C, Hoshi T & Heinemann SH. (2005). BKCa channels activating at resting potential without calcium in LNCaP prostate cancer cells. The Journal of membrane biology 208, 229–240. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Perez V, Ben Johny M, Xia XM & Lingle CJ. (2018). Regulatory gamma1 subunits defy symmetry in functional modulation of BK channels. Proceedings of the National Academy of Sciences of the United States of America 115, 9923–9928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Perez V & Lingle CJ. (2019). Regulation of BK Channels by Beta and Gamma Subunits. Annual review of physiology 81, 113–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Perez V, Martinez-Espinosa PL, Sala-Rabanal M, Bharadwaj N, Xia XM, Chen AC, Alvarado D, Gustafsson JK, Hu H, Ciorba MA & Lingle CJ. (2021). Goblet cell LRRC26 regulates BK channel activation and protects against colitis in mice. Proceedings of the National Academy of Sciences of the United States of America 118, e2019149118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Perez V, Xia XM & Lingle CJ. (2014). Functional regulation of BK potassium channels by gamma1 auxiliary subunits. Proceedings of the National Academy of Sciences of the United States of America 111, 4868–4873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Perez V, Xia XM & Lingle CJ. (2015). Two classes of regulatory subunits coassemble in the same BK channel and independently regulate gating. Nature communications 6, 8341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan X, Li Q & Yan J. (2017). Relationship between auxiliary gamma subunits and mallotoxin on BK channel modulation. Scientific reports 7, 42240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson JK, Ermund A, Ambort D, Johansson ME, Nilsson HE, Thorell K, Hebert H, Sjovall H & Hansson GC. (2012). Bicarbonate and functional CFTR channel are required for proper mucin secretion and link cystic fibrosis with its mucus phenotype. J Exp Med 209, 1263–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S, Han R, Chen L, Qin W, Xu X, Shi J, Zhu X, Zhang M, Zeng C, Tang Z, Bao H & Liu Z. (2021). Upregulated LRRC55 promotes BK channel activation and aggravates cell injury in podocytes. J Exp Med 218, e20192373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jumper J, Evans R, Pritzel A, Green T, Figurnov M, Ronneberger O, Tunyasuvunakool K, Bates R, Zidek A, Potapenko A, Bridgland A, Meyer C, Kohl SAA, Ballard AJ, Cowie A, Romera-Paredes B, Nikolov S, Jain R, Adler J, Back T, Petersen S, Reiman D, Clancy E, Zielinski M, Steinegger M, Pacholska M, Berghammer T, Bodenstein S, Silver D, Vinyals O, Senior AW, Kavukcuoglu K, Kohli P & Hassabis D. (2021). Highly accurate protein structure prediction with AlphaFold. Nature 596, 583–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaitan D, Sankpal UT, Weksler B, Meister EA, Romero IA, Couraud PO & Ningaraj NS. (2009). Role of KCNMA1 gene in breast cancer invasion and metastasis to brain. BMC cancer 9, 258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobe B & Kajava AV. (2001). The leucine-rich repeat as a protein recognition motif. Curr Opin Struct Biol 11, 725–732. [DOI] [PubMed] [Google Scholar]
- Kshatri AS, Li Q, Yan J, Large RJ, Sergeant GP, McHale NG, Thornbury KD & Hollywood MA. (2017). Differential efficacy of GoSlo-SR compounds on BKalpha and BKalphagamma1–4 channels. Channels 11, 66–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanata CM, Chung SA & Criswell LA. (2018). DNA methylation 101: what is important to know about DNA methylation and its role in SLE risk and disease heterogeneity. Lupus Sci Med 5, e000285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang F & Stournaras C. (2014). Ion channels in cancer: future perspectives and clinical potential. Philosophical transactions of the Royal Society of London Series B, Biological sciences 369, 20130108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Fan F, Kwak HR & Yan J. (2015). Molecular basis for differential modulation of BK channel voltage-dependent gating by auxiliary gamma subunits. The Journal of general physiology 145, 543–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Guan X, Yen K, Zhang J & Yan J. (2016). The single transmembrane segment determines the modulatory function of the BK channel auxiliary gamma subunit. The Journal of general physiology 147, 337–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q & Yan J. (2016). Modulation of BK Channel Function by Auxiliary Beta and Gamma Subunits. Int Rev Neurobiol 128, 51–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limbutara K, Chou CL & Knepper MA. (2020). Quantitative Proteomics of All 14 Renal Tubule Segments in Rat. Journal of the American Society of Nephrology : JASN 31, 1255–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingle CJ, Martinez-Espinosa PL, Yang-Hood A, Boero LE, Payne S, Persic D, B VG, Xiao M, Zhou Y, Xia XM, Pyott SJ & Rutherford MA. (2019). LRRC52 regulates BK channel function and localization in mouse cochlear inner hair cells. Proceedings of the National Academy of Sciences of the United States of America 116, 18397–18403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linley J, Loganathan A, Kopanati S, Sandle GI & Hunter M. (2014). Evidence that two distinct crypt cell types secrete chloride and potassium in human colon. Gut 63, 472–479. [DOI] [PubMed] [Google Scholar]
- Liu XF, Xiang L, Zhang Y, Becker KG, Bera TK & Pastan I. (2012). CAPC negatively regulates NF-kappaB activation and suppresses tumor growth and metastasis. Oncogene 31, 1673–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomax RB, Warhurst G & Sandle GI. (1996). Characteristics of two basolateral potassium channel populations in human colonic crypts. Gut 38, 243–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorca RA, Wakle-Prabagaran M, Freeman WE, Pillai MK & England SK. (2018). The large-conductance voltage- and Ca(2+) -activated K(+) channel and its gamma1 subunit modulate mouse uterine artery function during pregnancy. The Journal of physiology 596, 1019–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzanares D, Krick S, Baumlin N, Dennis JS, Tyrrell J, Tarran R & Salathe M. (2015). Airway Surface Dehydration by Transforming Growth Factor beta (TGF-beta) in Cystic Fibrosis Is Due to Decreased Function of a Voltage-dependent Potassium Channel and Can Be Rescued by the Drug Pirfenidone. The Journal of biological chemistry 290, 25710–25716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzanares D, Srinivasan M, Salathe ST, Ivonnet P, Baumlin N, Dennis JS, Conner GE & Salathe M. (2014). IFN-gamma-mediated reduction of large-conductance, Ca2+-activated, voltage-dependent K+ (BK) channel activity in airway epithelial cells leads to mucociliary dysfunction. American journal of physiology Lung cellular and molecular physiology 306, L453–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyagawa Y, Matsushita Y, Suzuki H, Komatsu M, Yoshimaru T, Kimura R, Yanai A, Honda J, Tangoku A, Sasa M, Miyoshi Y & Katagiri T. (2018). Frequent downregulation of LRRC26 by epigenetic alterations is involved in the malignant progression of triple-negative breast cancer. Int J Oncol 52, 1539–1558. [DOI] [PubMed] [Google Scholar]
- Nakamoto T, Romanenko VG, Takahashi A, Begenisich T & Melvin JE. (2008). Apical maxi-K (KCa1.1) channels mediate K+ secretion by the mouse submandibular exocrine gland. American journal of physiology Cell physiology 294, C810–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro B, Kirichok Y & Clapham DE. (2007). KSper, a pH-sensitive K+ current that controls sperm membrane potential. Proceedings of the National Academy of Sciences of the United States of America 104, 7688–7692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng AC, Eisenberg JM, Heath RJ, Huett A, Robinson CM, Nau GJ & Xavier RJ. (2011). Human leucine-rich repeat proteins: a genome-wide bioinformatic categorization and functional analysis in innate immunity. Proceedings of the National Academy of Sciences of the United States of America 108 Suppl 1, 4631–4638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda S, Suzuki Y, Yamamura H, Giles WR & Imaizumi Y. (2020). Roles of LRRC26 as an auxiliary gamma1-subunit of large-conductance Ca(2+)-activated K(+) channels in bronchial smooth muscle cells. American journal of physiology Lung cellular and molecular physiology 318, L366–L375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nystrom EEL, Martinez-Abad B, Arike L, Birchenough GMH, Nonnecke EB, Castillo PA, Svensson F, Bevins CL, Hansson GC & Johansson MEV. (2021). An intercrypt subpopulation of goblet cells is essential for colonic mucus barrier function. Science 372, eabb1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oeggerli M, Tian Y, Ruiz C, Wijker B, Sauter G, Obermann E, Guth U, Zlobec I, Sausbier M, Kunzelmann K & Bubendorf L. (2012). Role of KCNMA1 in breast cancer. PloS one 7, e41664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer LG & Frindt G. (2007). High-conductance K channels in intercalated cells of the rat distal nephron. American journal of physiology Renal physiology 292, F966–973. [DOI] [PubMed] [Google Scholar]
- Peterson LW & Artis D. (2014). Intestinal epithelial cells: regulators of barrier function and immune homeostasis. Nat Rev Immunol 14, 141–153. [DOI] [PubMed] [Google Scholar]
- Prevarskaya N, Skryma R & Shuba Y. (2018). Ion Channels in Cancer: Are Cancer Hallmarks Oncochannelopathies? Physiological reviews 98, 559–621. [DOI] [PubMed] [Google Scholar]
- Salkoff L, Butler A, Ferreira G, Santi C & Wei A. (2006). High-conductance potassium channels of the SLO family. Nature Reviews Neuroscience 7, 921–931. [DOI] [PubMed] [Google Scholar]
- Sandle GI & Hunter M. (2010). Apical potassium (BK) channels and enhanced potassium secretion in human colon. QJM : monthly journal of the Association of Physicians 103, 85–89. [DOI] [PubMed] [Google Scholar]
- Sandle GI, Perry MD, Mathialahan T, Linley JE, Robinson P, Hunter M & MacLennan KA. (2007). Altered cryptal expression of luminal potassium (BK) channels in ulcerative colitis. J Pathol 212, 66–73. [DOI] [PubMed] [Google Scholar]
- Sandle GI & Rajendran VM. (2012). Cyclic AMP-induced K+ secretion occurs independently of Cl-secretion in rat distal colon. American journal of physiology Cell physiology 303, C328–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber M, Wei A, Yuan A, Gaut J, Saito M & Salkoff L. (1998). Slo3, a novel pH-sensitive K+ channel from mammalian spermatocytes. Journal of Biological Chemistry 273, 3509–3516. [DOI] [PubMed] [Google Scholar]
- Shipston MJ & Tian L. (2016). Posttranscriptional and Posttranslational Regulation of BK Channels. Int Rev Neurobiol 128, 91–126. [DOI] [PubMed] [Google Scholar]
- Soriano-Tarraga C, Lazcano U, Giralt-Steinhauer E, Avellaneda-Gomez C, Ois A, Rodriguez-Campello A, Cuadrado-Godia E, Gomez-Gonzalez A, Fernandez-Sanles A, Elosua R, Fernandez-Cadenas I, Cullell N, Montaner J, Moran S, Esteller M, Jimenez-Conde J & Roquer J. (2020). Identification of 20 novel loci associated with ischaemic stroke. Epigenome-wide association study. Epigenetics 15, 988–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao X & MacKinnon R. (2019). Molecular structures of the human Slo1 K(+) channel in complex with beta4. eLife 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurm H, Fakler B & Oliver D. (2005). Ca2+-independent activation of BKCa channels at negative potentials in mammalian inner hair cells. The Journal of physiology 569, 137–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y-W, Ding JP, Xia X-M & Lingle CJ. (2002). Consequences of the stoichiometry of Slo1 α and auxiliary β subunits on functional properties of BK-type Ca2+-activated K+ channels. Journal of Neuroscience 22, 1550–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J & Aldrich RW. (2010). LRRC26 auxiliary protein allows BK channel activation at resting voltage without calcium. Nature 466, 513–516. [DOI] [PubMed] [Google Scholar]
- Yan J & Aldrich RW. (2012). BK potassium channel modulation by leucine-rich repeat-containing proteins. Proceedings of the National Academy of Sciences of the United States of America 109, 7917–7922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Gonzalez-Perez V, Mukaibo T, Melvin JE, Xia XM & Lingle CJ. (2017). Knockout of the LRRC26 subunit reveals a primary role of LRRC26-containing BK channels in secretory epithelial cells. Proceedings of the National Academy of Sciences of the United States of America 114, E3739–E3747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Zeng XH, Zhou Y, Xia XM & Lingle CJ. (2011). LRRC52 (leucine-rich-repeat-containing protein 52), a testis-specific auxiliary subunit of the alkalization-activated Slo3 channel. Proceedings of the National Academy of Sciences of the United States of America 108, 19419–19424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu K, Lujan R, Marmorstein A, Gabriel S & Hartzell HC. (2010). Bestrophin-2 mediates bicarbonate transport by goblet cells in mouse colon. The Journal of clinical investigation 120, 1722–1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakharov SI, Morrow JP, Liu G, Yang L & Marx SO. (2005). Activation of the BK (SLO1) potassium channel by mallotoxin. The Journal of biological chemistry 280, 30882–30887. [DOI] [PubMed] [Google Scholar]
- Zeng XH, Yang C, Kim ST, Lingle CJ & Xia XM. (2011). Deletion of the Slo3 gene abolishes alkalization-activated K+ current in mouse spermatozoa. Proceedings of the National Academy of Sciences of the United States of America 108, 5879–5884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng XH, Yang C, Xia XM, Liu M & Lingle CJ. (2015). SLO3 auxiliary subunit LRRC52 controls gating of sperm KSPER currents and is critical for normal fertility. Proceedings of the National Academy of Sciences of the United States of America 112, 2599–2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J & Yan J. (2014). Regulation of BK channels by auxiliary gamma subunits. Frontiers in physiology 5, 401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Zeng X-H & Lingle CJ. (2006). Slo3 K+ channels: voltage and pH dependence of macroscopic currents. Journal of General Physiology 128, 317–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YY, Han X, Liu Y, Chen J, Hua L, Ma Q, Huang YY, Tang QY & Zhang Z. (2018). +mRNA expression of LRRC55 protein (leucine-rich repeat-containing protein 55) in the adult mouse brain. PloS one 13, e0191749. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


