Abstract
Despite the unequal burden of environmental exposures borne by racially minoritized communities, these groups are often underrepresented in public health research. Here, we examined racial/ethnic disparities in exposure to metals among a multi-ethnic sample of pregnant women. Our objective was to examine what disparities, if any, exist in metal exposure among a sample of pregnant women. The sample included women enrolled in the PRogramming of Intergenerational Stress Mechanisms (PRISM) pregnancy cohort (N = 382). Urinary metal concentrations (arsenic [As], barium [Ba], cadmium [Cd], cesium [Cs], chromium [Cr], lead [Pb], antimony [Sb]) were measured during mid-pregnancy and information on individual- and neighborhood-level characteristics was ascertained during an in-person interview and from publicly available databases, respectively. Linear regression was used to examine individual and neighborhood characteristics in relation to metal concentrations.
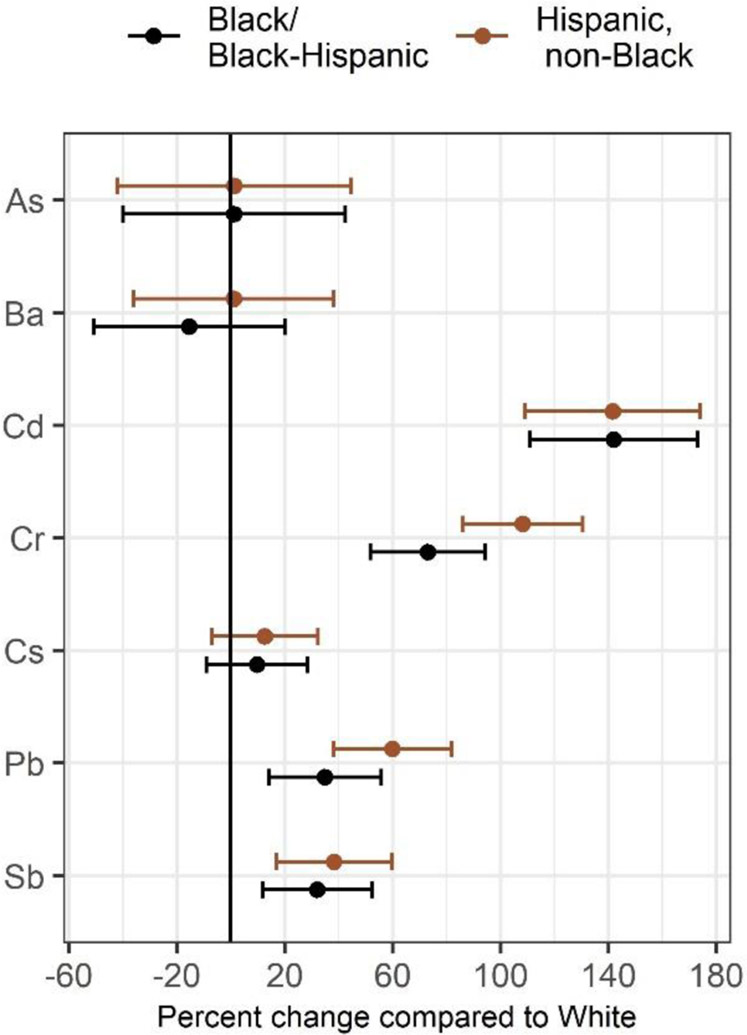
Black/Black-Hispanic women had Cd, Cr, Pb, and Sb levels that were 142.0%, 10.9%, 35.0%, and 32.1% higher than White, non-Hispanic women, respectively. Likewise, White-Hispanic women had corresponding levels that were 141.5%, 108.2%, 59.9%, and 38.3% higher. These same metals were also higher among women residing in areas with higher crime, higher diversity, lower educational attainment, lower household income, and higher poverty. Significant disparities in exposure to metals exist and may be driven by neighborhood-level factors. Exposure to metals for pregnant women can be especially harmful. Understanding exposure inequalities and identifying factors that increase risk can help inform targeted public health interventions.
Keywords: metals, exposure, race/ethnicity, disparities, environmental justice, pregnancy
Graphical Abstract
To be supplied with any subsequent revisions.
Introduction
In the United States, many toxic environmental exposures are unevenly distributed at the neighborhood-level, with lower socioeconomic status (SES), racially minoritized communities often bearing a disproportionate burden of environmental pollution1, 2. Lower SES neighborhoods often arose from redlining and other practices that promoted segregation and typically received fewer public works maintenance projects. These neighborhoods have older housing stock and are frequently located near highly trafficked roadways, transportation hubs, disposal and legacy contamination sites, and industrial plants3, 4. In turn, these neighborhoods have also been shown to experience higher rates of infectious disease, most notably COVID-19, and increased exposure to indoor pests, extreme heat, noise pollution, and air pollution2, 5-8. Growing research has also demonstrated disparities in exposure to lead (Pb), however, few studies have considered disparities in exposure to other toxic metals.
Metals are pervasive in our environment being commonly found in soil, water, air, dust, food, and consumer products 9-17. Human exposure occurs through a number of pathways including ingestion, inhalation, and dermal contact17, 18 and is associated with a range of adverse health outcomes across the neurological, cardiovascular, endocrine, pulmonary and renal systems17, 19-21. Exposure to metals during pregnancy is a particular concern given the high sensitivity of the developing fetus and the enduring consequences of early environmental insults across the life course. For example, prenatal cadmium (Cd) exposure has been shown to impair steroidogenesis and Pb exposure can interfere with calcium deposition in bone, both leading to suboptimal fetal growth22, 23. Prenatal exposure to metals has also been associated with adverse birth outcomes, including preterm birth and lower birthweight24, 25, as well as long-term consequences for neurodevelopment26. Expanding evidence further suggests that pregnancy may be a period of enhanced vulnerability for the mother, as the physiological shifts that occur to support the fetus may render the maternal metabolic system susceptible to dysregulation. For example, chromium (Cr) exposure during pregnancy has been linked to irregular blood sugar levels and antimony (Sb), along with other metals, has been associated with the development of gestational diabetes27, 28 and high blood pressure in women29. Exposure to metals, including during the vulnerable pregnancy period, is thus a salient public health concern.
In the present paper, racial and ethnic disparities in exposure to metals were examined among a multi-ethnic sample of pregnant women living in two major metropolitan areas in the northeastern United States (U.S.). Other sociodemographic and lifestyle factors that may contribute to differential exposure were also explored and variability in biomonitoring data by neighborhood-level characteristics was examined.
Methods
The PRISM cohort
The study sample included a subset of women enrolled in the PRogramming of Intergenerational Stress Mechanisms (PRISM) pregnancy cohort, which recruited women from prenatal clinics in Boston and New York City beginning in 2011. Recruitment remains ongoing and at the time of this study, 936 eligible participants had been enrolled. Women are considered ineligible if they are less than 18 years old, HIV positive, drink any alcohol during pregnancy or more than seven alcoholic drinks per week before pregnancy recognition, do not speak English or Spanish fluently, or have a multiple gestation pregnancy. Urinary metals and creatinine were measured in N = 409 participants during pregnancy (mean ± sd: 31±6 weeks, range: 11-40 weeks). An additional 27 participants missing information on key determinants of interest were also excluded, resulting in a final analytic samples of 382 women.
Urinary metals and creatinine
Participants collected a clean catch, spot urine sample in their home on the morning of a scheduled study visit. Samples were kept cool in the participant’s home freezer until transport to the PRISM laboratory on the day of collection. Upon arrival, urine samples were immediately thawed, aliquoted, and stored at −80°C. Samples (200 μL) were diluted to 10 mL with a solution containing 0.05% Triton X-100, 0.5% nitric acid, and appropriate internal standard for each element (As and Sb: tellurium; Ba, C, Pb: lutetium; Cd: rhodium; Cr: yttrium). Samples were then analyzed for metals using an inductively coupled plasma-mass spectrometer-triple quadrupole (ICP-MS) instrument (Agilent 8900-QQQ)30, 31. Urine creatinine was measured using a well-established colorimetric method (limit of detection [LOD]: 0.3125 mg/dL). Quality control measures for metals and creatinine analysis have been previously described in detail 32, 33. The current analysis included seven metals with established toxicity, including: arsenic (As), barium (Ba), cadmium (Cd), chromium (Cr), cesium (Cs), lead (Pb), and antimony (Sb). The number of samples with non-detectable values were as follows: As (n = 1, 0.0%), Ba (n = 6, 1.6%), Cd (n = 53, 13.9%), Cr (n = 43, 11.3%), Cs (n = 0, 0.0%), Pb (n = 2, 0.5%), Sb (n = 77, 20.16%). Metal concentrations below the limit of detection (LOD) were replaced with the LOD divided by the square root of two34. Creatinine-standardized metal concentrations (μg/g creatinine) were derived by dividing metal levels (μg/L) by creatinine (mg/dL) and applying a conversion factor of 100.
Covariates
Information on race/ethnicity (White, non-Hispanic vs. White-Hispanic vs. Black/Black-Hispanic), education (less than high school vs. high school degree vs. college degree or more), age (in years), financial strain (low vs. moderate vs. high), and smoking during pregnancy (ever vs. never) was collected from participants via self-report using a standardized questionnaire administered during an in-person study visit during pregnancy. Financial strain was assessed using the question “In general, how do your family’s finances usually work out at the end of the month?" Responses were classified into low, moderate, and high corresponding with self-report of ‘extra money at the end of the month’, ‘just enough money at the end of the month’, or ‘not enough money at the end of the month’, respectively. Pre-pregnancy body mass index (BMI) was calculated as self-reported pre-pregnancy weight divided by height squared and categorized women as healthy weight (<25 kg/m2), overweight (25 - 29.9 kg/m2), or obese (≥30 kg/m2) based on standard guidelines.
Neighborhood-level characteristics
For each participant, residential address during pregnancy was geocoded using ArcGIS Esri World Geocoder technology as previously described35. Initial completeness was approximately 90% and the few erroneous or unmatched addresses were then geocoded manually. Geocoding was validated by visually examining the locations on a map using established map services like the Environmental Systems Research Institute (ESRI) ArcGIS street datasets for 15% of the sample. Neighborhood-level characteristics spanning the 2019-2020 period were ascertained from ArcGIS at the census tract-level and included the following variables: median household income, percent of households below the poverty level, Diversity Index, Crime Index, and percent of the population age 25 or older with a high school degree (or equivalent) or less. The Total Crime Index provides an assessment of the relative risk of seven major crime types: murder, rape, robbery, assault, burglary, larceny, and motor vehicle theft. It is modeled using data from the FBI Uniform Crime Report and demographic data from the Census and Applied Geographic Solutions (AGS). Median household income refers to current-year estimate of median household income as defined by the Census Bureau. Percent of households below the poverty level is derived from the American Community Survey (ACS) with period estimates based on a rolling sample survey spanning a 60-month period. The Diversity Index shows the likelihood that two persons chosen at random from the same area belong to different race or ethnic groups. The index ranges from 0 (no diversity) to 100 (complete diversity). These neighborhood-level characteristics were available for a subsample of 359 (94% of the 382) participants included in this analysis.
Statistical analysis
First, the frequency and percent of participants within categories of sociodemographic and lifestyle characteristics was calculated. Then the distribution of each urinary metal was visualized using boxplots and histograms, descriptive statistics for each metal were calculated, and Spearman correlations between metals were examined. Linear regression was used to examine associations between gestational age at sample collection, in weeks, and urinary metal concentrations additionally adjusting for urinary creatinine, an indicator of urine dilution; these relationships were visualized using scatter plots. Next, linear regression was used to examine associations between metals and a series of individual-level (race/ethnicity, education, financial strain, age, smoking, BMI) and neighborhood-level (median household income, percent of households below the poverty level, Diversity Index, Crime Index, percent of the population age 25 or older with a high school degree or less) characteristics, each in a separate model. These models were adjusted for urinary creatinine and gestational week of urine collection, which were considered precision variables that may influence urinary metal concentrations. In secondary analyses, mutually adjusted multivariable models that included all individual-level covariates (urinary creatinine, gestational week at urine collection, race/ethnicity, education, financial strain, age, smoking, BMI) were explored. In all regression models, metal concentrations were natural-log transformed to normalize the distribution and each metal was considered separately. Because metal concentrations are log-transformed, effect estimates are interpreted in terms of percent change.
Results
The sample is racially/ethnically diverse, with approximately 14% of participants self-identifying as White, non-Hispanic, 32% as White-Hispanic, and 54% as Black/Black-Hispanic. Table 1 provides individual- and neighborhood-level characteristics for participants by race/ethnicity. Approximately 20% of women had less than a high school education and 20% reported a high degree of financial strain. On average, women were 28 years old and 13% reported smoking during pregnancy. In this sample, White, non-Hispanic women were older, more highly educated, less likely to experience financial strain, less likely to smoke during pregnancy and less likely to be overweight or obese, compared to White-Hispanic and Black/Black-Hispanic women. With regards to neighborhood-level factors, compared to White, non-Hispanic women, racially minoritized women were more likely to live in census tracts with lower median incomes, a higher percent of households below the poverty level, and a higher percent of the population with a high school degree or less. White-Hispanic and Black/Black-Hispanic women were also more likely to live in areas with higher crime and higher diversity (Table 1). A map of participant residential location by race/ethnicity is provided in Supplemental Figure S1.
Table 1.
Participant- and neighborhood-level characteristics stratified by race/ethnicity. Values are N (%) unless otherwise noted.
| All (n = 382, 100%) |
White, non-Hispanic (n = 52, 14%) |
White-Hispanic (n = 123, 32%) |
Black/ Black-Hispanic (n = 207, 54%) |
|
|---|---|---|---|---|
| Individual-level factors | ||||
| Education | ||||
| < High school | 75 (20) | 1 (1.9) | 31 (25) | 43 (21) |
| High school degree | 213 (56) | 6 (12) | 76 (62) | 131 (63) |
| College degree or more | 94 (25) | 45 (87) | 16 (13) | 33 (16) |
| Financial strain | ||||
| Low | 148 (39) | 44 (85) | 38 (31) | 66 (32) |
| Moderate | 162 (42) | 6 (12) | 57 (46) | 99 (48) |
| High | 72 (19) | 2 (3.8) | 28 (23) | 42 (20) |
| Age (years) | ||||
| 18 - 28 | 210 (55) | 8 (15) | 73 (59) | 129 (62) |
| >28 | 172 (45) | 44 (85) | 50 (41) | 78 (38) |
| Smoking during pregnancy | ||||
| Never | 331 (87) | 46 (89) | 103 (84) | 182 (88) |
| Ever | 51 (13) | 6 (11) | 20 (16) | 25 (12) |
| Pre-pregnancy BMI | ||||
| Healthy weight | 181 (47) | 39 (75) | 53 (43) | 89 (43) |
| Overweight | 96 (25) | 9 (17) | 31 (25) | 56 (27) |
| Obese | 105 (28) | 4 (7.7) | 39 (32) | 62 (30) |
| Neighborhood-level factors a | ||||
| Median household income ($) | 36,600 (30,400) | 104,000 (45,600) | 32,000 (18,200) | 36,000 (24,700) |
| Percent below poverty levelc | 29 (21) | 9.5 (9.8) | 31 (15) | 30 (19) |
| Diversity Index | 87 (21) | 55 (25) | 89 (5) | 87 (15) |
| Crime Index | 104 (48) | 68 (52) | 102 (52) | 110 (42) |
| Percent with HS degree or lessd | 26 (9.9) | 10 (14) | 25 (8.3) | 27 (7.4) |
N = 23 missing. Vales are median (interquartile range)
Percent of households below the poverty level
Value is the median of the percent of the population age ≥25 with a high school degree (or equivalent) or less
Metal concentrations for the sample overall and by race/ethnicity are provided in Table 2. Metal concentrations were log-normally distributed (Supplemental Figure S2) and moderately to highly correlated with each other, with the strongest positive correlations observed for Pb, Cr, and Sb (Supplemental Figure S3). On average, As and Ba increased over the course of gestation, whereas the other metals decreased, with significant negative associations observed for Cd (percent change per week: - 2.30%, 95% CI: - 3.60, - 0.98), Cr ( - 1.58%, 95% CI: - 0.63, - 2.52), Cs (- 1.15%, 95% CI: - 0.38, - 1.92), Pb ( - 1.48%, 95% CI: - 0.56, - 2.39) and Sb ( - 1.61%, 95% CI: - 0.71, - 2.49) (Supplemental Figure S4).
Table 2.
Metal concentrations stratified by race/ethnicity. Values are median (interquartile range).
| All (n = 382, 100%) |
White, non-Hispanic (n = 52, 13.6%) |
White- Hispanic (n = 123, 32.2%) |
Black/ Black-Hispanic (n = 207, 54.2%) |
P- valuea |
|
|---|---|---|---|---|---|
| Metals (μg/g creatinine) | |||||
| Arsenic (As) | 8.77 (12.7) | 9.60 (25.9) | 8.75 (10.9) | 8.76 (12.7) | 0.18 |
| Barium (Ba) | 2.63 (3.25) | 3.46 (4.67) | 2.99 (3.41) | 2.25 (2.92) | 0.01 |
| Cadmium (Cd) | 0.195 (0.18) | 0.136 (0.14) | 0.223 (0.17) | 0.195 (0.18) | <0.001 |
| Chromium (Cr) | 0.540 (0.53) | 0.434 (0.33) | 0.675 (0.75) | 0.538 (0.47) | <0.001 |
| Cesium (Cs) | 4.30 (2.27) | 4.62 (2.56) | 4.35 (2.27) | 4.15 (2.23) | 0.44 |
| Lead (Pb) | 0.645 (0.46) | 0.475 (0.49) | 0.767 (0.48) | 0.603 (0.44) | <0.001 |
| Antimony (Sb) | 0.111 (0.08) | 0.0864 (0.08) | 0.114(0.10) | 0.112 (0.08) | 0.01 |
P-values are from Kruskal-Wallis ANOVA test for the difference in metals concentration by race/ethnicity.
Figure 1 presents the percent change in urinary metal concentrations across individual-level participant characteristics, adjusting for urine creatinine and gestational week of urine collection. A majority of the metals significantly varied by race/ethnicity, with racially minoritized women having higher levels of Cd, Cr, Pb and Sb. In addition, these four metals showed a similar pattern across the other characteristics considered, and were generally associated with younger age, lower education, higher financial strain, smoking and obesity, with the exception that Cd was associated with older age. As and Cs were also associated with older age. In multivariable linear regression models mutually adjusting for all individual-level characteristics, minority race/ethnicity remained strongly associated with metals exposure. Compared to White, non-Hispanic women, Black/Black-Hispanic women had Cd, Cr, Pb, and Sb levels that were 142.0% (95% CI: 111.1, 173.0), 73.0% (95% CI: 51.7, 94.3), 35.0% (95% CI: 14.1, 55.8), and 32.1% (95% CI: 11.7, 52.5%) higher, respectively. Likewise, White-Hispanic women had corresponding levels that were 141.5% (95% CI: 109.1, 174.1), 108.2% (95% CI: 85.8, 130.5), 59.9% (95% CI: 38.0, 81.8), and 38.3% (95% CI: 16.9, 59.7) higher (Figure 2). When considering neighborhood-level factors in separate models, urinary levels of Cd, Cr, Pb, and Sb were higher among women residing in areas with higher crime, higher diversity, lower educational attainment, lower household income, and higher poverty; these associations were significant with the exception of Cd and lower educational attainment (Figure 3). A model mutually adjusted for all neighborhood-level factors was not considered given a high degree of spatial overlap across several of the characteristics considered.
Figure 1.
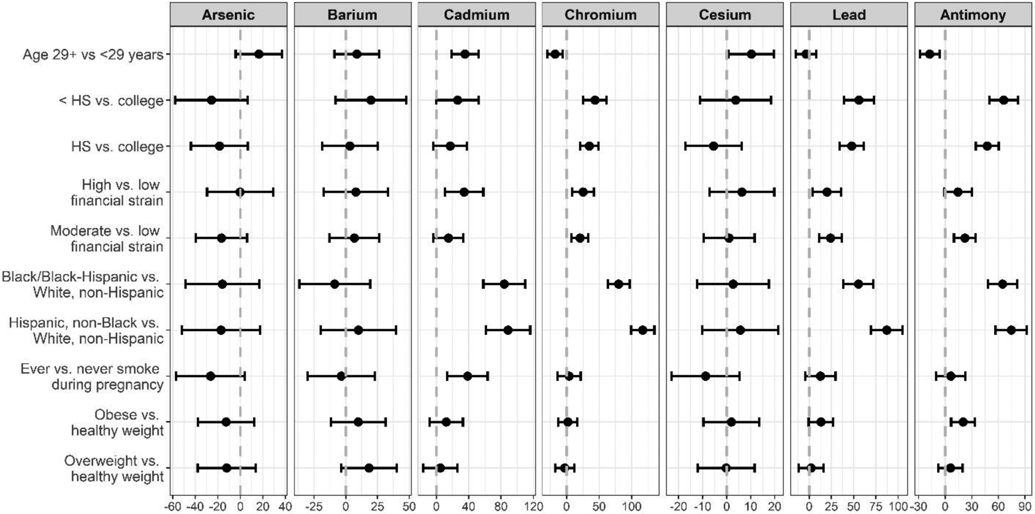
Percent change in urinary metal concentrations by individual-level characteristics (N = 382).
Note: Points indicate percent change and bars indicate 95% confidence intervals. Each characteristic (age, education, financial strain, race/ethnicity, smoking, BMI) is considered in a separate model adjusted for urinary creatinine and gestational week at urine collection.
Figure 2.
Percent change in urinary metal concentrations during pregnancy among Black/Black-Hispanic and White-Hispanic participants compared to White, non-Hispanic participants from models adjusted for urinary creatinine, gestational week at sample collection, education, financial strain, age, pre-pregnancy BMI and smoking during pregnancy.
Figure 3.
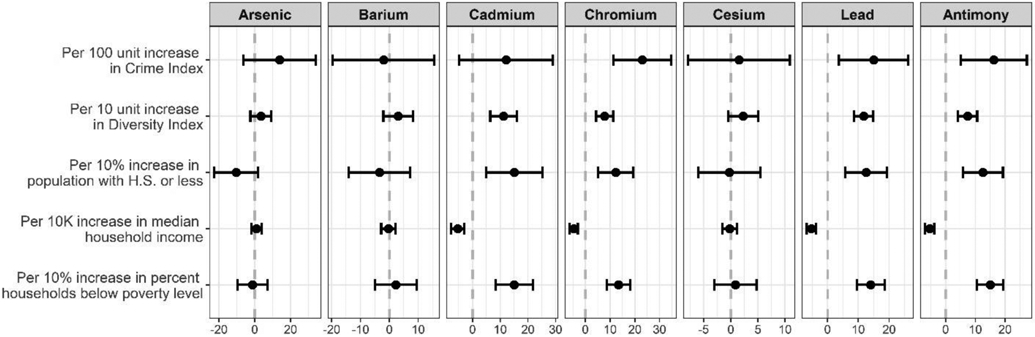
Percent change in urinary metal concentrations by census tract-level characteristics (N = 359).
Note: Points indicate percent change and bars indicate 95% confidence intervals. Each characteristic (age, education, financial strain, race/ethnicity, smoking, BMI) is considered in a separate model adjusted for urinary creatinine and gestational week at urine collection.
Discussion
In this study, racial/ethnic disparities in exposure to metals was evaluated among a multi-ethnic sample of pregnant women living in two major metropolitan areas in the Northeastern United States. Compared to White, non-Hispanic women, Black/Black-Hispanic women and White-Hispanic women had significantly increased urinary levels of Cd, Cr, Pb and Sb. In addition to race/ethnicity and other individual-level characteristics, urinary metals were also explored in relation to neighborhood-level factors, which revealed that urinary levels of the same four metals (Cd, Cr, Pb, and Sb) were higher among women residing in areas with higher crime, higher diversity, lower educational attainment, lower household income, and higher poverty. These findings suggest that social drivers of residential location may contribute to an individual’s propensity for exposure to metals in the environment.
Compared to pregnant women enrolled in the National Health and Nutrition Examination Survey (NHANES, cycles 1999-2016), women in this study had higher geometric mean urinary levels of all metals considered except for Cs36. This could reflect different sources of metals contamination by region, as NHANES enrolls participants from across the U.S., whereas this study enrolled from two large urban areas on the northeast coast. Our findings are also consistent with a 2017 analysis of socioeconomically disadvantaged black pregnant women in Houston, TX that found elevated levels of urinary Cd and Sb compared to a nationally representative sample of adult women in the US (NHANES) 37. In this prior study, exploratory factor analysis revealed that potential sources of metals exposure may include the home environment, diet, industrial and natural sources, or traffic37.
Our finding of higher Pb levels among racial/ethnic minorities and among lower-income, higher poverty households is aligned with decades of research documenting racial and socioeconomic disparities in Pb exposure. For example, a New York City-based study conducted as far back as the early 1970s found blood Pb levels (BLLs) varied by race and ethnicity, with minorities having higher body burdens38. In other urban areas such as Chicago, it has also been shown that neighborhoods with the highest prevalence of elevated BLLs are predominately occupied by people of color39. These neighborhoods often have older housing stock that release ingestible or inhalable fine Pb paint dust into the home environment40. Pb is also present in neighborhood soils and community gardens due to decades of emissions from car exhaust prior to the ban of leaded gasoline41. Furthermore, studies examining the distribution of air Pb levels suggest that it is not only the home environment that presents a risk, but also the air quality around the home42. For example, a positive relationship has been demonstrated between the percentage of Black children residing in a county and the average air Pb concentration42. NHANES has likewise documented persistent disparities in exposure to Pb, with Hispanic and Black children consistently having higher BLLs compared to White children 43. Findings from these previous studies in conjunction with our research reflects a form of biosocial stratification not only present in the northeastern U.S. where our study focuses, but across the country44, 45 and exposes neighborhood-level factors46, 47 that contribute to environmental injustices and reinforce racial inequality 48.
With regard to Cd, the racial and ethnic differences that were observed may in part reflect differences in smoking. Tobacco plants are known to take up soil cadmium49 and in our sample, 16.3% of White-Hispanic women and 12.1% of Black/Black-Hispanic women reported smoking during pregnancy compared to 11.5% of White, non-Hispanic women. While this finding is consistent with prior studies documenting racial/ethnic disparities in Cd body burden attributable to smoking behavior50, previous research among non-smokers has also reported higher levels of Cd among Black and Hispanic individuals compared to White individuals51. Possible explanations could relate to differences in diet and cultural practices. For example, foods including shellfish and several common vegetables such as potatoes and onions are high in Cd and the intake of these items has been shown to vary across racial and ethnic groups 52-54. Furthermore, disparities in essential micronutrient intake may also in part explain these results, as human and animal research has shown increased Cd absorption when intake of calcium, iron, or zinc is deficient55, 56. Notably, inadequate micronutrient intakes during pregnancy that varied based on sociodemographic factors and race/ethnicity have been previously described among women enrolled in the PRISM cohort57. While it was beyond the scope of this analysis to examine dietary drivers of the observed disparities, future research that considers how food security, availability and choice influences environment exposures is needed to more comprehensively understand potentially modifiable exposure pathways.
Consistent with this study, prior research has also documented associations between Sb and both race/ethnicity and neighborhood deprivation 58-60. Sb is a metalloid with properties similar to arsenic that is used in a range of industrial products, including household paint, sheet metal, ammunition and storage batteries 61. It is also released by several anthropogenic sources, including power plants, tobacco smoke and traffic emissions (from brake dust)61. Despite repeated findings of higher levels of Sb among racially minoritized community samples, the specific determinants underlying this unequal exposure pattern are poorly understood. It is also worth highlighting that Sb and Cr were highly correlated with Pb, suggesting possible shared exposure sources and/or pathways. For example, lead chromate (PbCrO4) is a vivid yellow color that has been used for various applications, including in printing inks and traffic paint. PbCrO4 particles are detectable in the atmosphere and could be one possible combined source of both Pb and Cr exposure 62.
Strengths of this study include the inclusion of a racially and ethnically diverse sample, which is a traditionally understudied demographic and which allowed us to examine disparities in exposure. This research was focused on pregnant women, who may be particularly sensitive to metals exposure and who may respond differently to metals due to pregnancy-related physiological and metabolic shifts. This study also examined how multiple metals varied across a wide range of socioeconomic and lifestyle characteristics, including at both the individual- and neighborhood-levels. Unfortunately, there were a number of factors that we were not able to consider here that would be interesting to examine in future research, such as diet, acculturation and household properties. With the exception of Cd and Cs, the metals we measured have short urinary half-lives, thus concentrations detected in our spot urine samples may not reflect long-term exposure. However, many of the characteristics that were associated with metal exposure are likely stable over time, especially the neighborhood-level factors. Nonetheless, the potential for misclassification exists if the sociodemographic characteristics of neighborhoods, which were ascertained for the 2019-2010 period, changed across the birth cohort enrollment period from 2011-2020. Additionally, As was not speciated and therefore represents total levels, including largely non-toxic forms such as arsenobetaine. It is likely that dietary sources contributed to measured variability in As levels between participants, potentially masking our ability to identify other individual and neighborhood-level determinants.
In summary, this research supports that both individual- and community-level factors are associated with differential distributions of metal exposures by race/ethnicity, which may translate to disparities in health outcomes. Despite the unequal burden of environmental exposures borne by lower-SES communities and communities of color, these groups are often underrepresented in public health research. Our findings emphasize the importance of conducting environmental health research across socioeconomic and demographic strata and can help guide public health interventions to reduce environmental and health disparities.
Supplementary Material
Highlights for Review.
Despite the unequal burden of environmental exposures borne by racially minoritized communities, these groups are often underrepresented in public health research.
Our objective was to examine what disparities, if any, exist in metal exposure among a sample of pregnant women.
Black/Black-Hispanic women had Cd, Cr, Pb, and Sb levels higher than White, non-Hispanic women, respectively.
White-Hispanic women also had increased levels of Cd, Cr, Pb, and Sb.
These metals were also higher among women residing in areas with higher crime, higher diversity, lower educational attainment, lower household income, and higher poverty.
Acknowledgments:
The Mount Sinai HHEAR laboratory hub acknowledges Shirisha Yelamanchili who performed the measurements of creatinine in urine and Malgorzata Knap and Mahmoud Awawda for metal analysis.
Funding:
This work was supported by NIH grants: R01ES030302, P30ES023515, UG3OD023337, and K99ES032029.
Footnotes
Conflicts of interest: None to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data Availability
The data analyzed in this paper were collected as part of the PRogramming of Intergenerational Stress Mechanisms (PRISM) pregnancy cohort study at the Icahn School of Medicine at Mount Sinai. Due to human subjects confidentiality, data are not public available; however, a limited dataset may be obtained from the corresponding author upon reasonable request.
References
- 1.Apelberg BJ, Buckley TJ, White RH Socioeconomic and racial disparities in cancer risk from air toxics in Maryland. Environ Health Perspect 2005; 113: 693–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hajat A, Hsia C, O'Neill MS Socioeconomic Disparities and Air Pollution Exposure: a Global Review. Curr Environ Health Rep 2015; 2: 440–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pratt GC, Vadali ML, Kvale DL, Ellickson KM Traffic, air pollution, minority and socio-economic status: addressing inequities in exposure and risk. Int J Environ Res Public Health 2015; 12: 5355–5372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tyrrell J, Melzer D, Henley W, Galloway TS, Osborne NJ Associations between socioeconomic status and environmental toxicant concentrations in adults in the USA: NHANES 2001-2010. Environ Int 2013; 59: 328–335. [DOI] [PubMed] [Google Scholar]
- 5.Casey JA, Morello-Frosch R, Mennitt DJ, Fristrup K, Ogburn EL, James P Race/Ethnicity, Socioeconomic Status, Residential Segregation, and Spatial Variation in Noise Exposure in the Contiguous United States. Environ Health Perspect 2017; 125: 077017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lewis NM, Friedrichs M, Wagstaff S, Sage K, LaCross N, Bui D et al. Disparities in COVID-19 Incidence, Hospitalizations, and Testing, by Area-Level Deprivation - Utah, March 3-July 9, 2020. MMWR Morb Mortal Wkly Rep 2020; 69: 1369–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dialesandro J, Brazil N, Wheeler S, Abunnasr Y Dimensions of Thermal Inequity: Neighborhood Social Demographics and Urban Heat in the Southwestern U.S. Int J Environ Res Public Health 2021; 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walsh MG Rat sightings in New York City are associated with neighborhood sociodemographics, housing characteristics, and proximity to open public space. PeerJ 2014; 2: e533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Toxicological profile for antimony and compounds. Agency for Toxic Substances and Disease Registry, U.S. Public Health Service: Atlanta, Ga., 1992. [PubMed] [Google Scholar]
- 10.Toxicological profile for chromium. U.S. Dept. of Health and Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry: Atlanta, Ga., 2000. [Google Scholar]
- 11.Toxicological profile for cesium. U.S. Dept. of Health and Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry: Atlanta, Ga., 2004. [PubMed] [Google Scholar]
- 12.Toxicological profile for arsenic. U.S. Dept. of Health and Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry: Atlanta, Ga, 2007. [PubMed] [Google Scholar]
- 13.Toxicological profile for barium and barium compounds. Agency for Toxic Substances and Disease Registry: Atlanta, Ga, 2007. [PubMed] [Google Scholar]
- 14.Abadin H, United States. Agency for Toxic S, Disease R, Syracuse Research C. Toxicological profile for lead. U.S. Department of Health and Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry: Atlanta, Georgia, 2007. [PubMed] [Google Scholar]
- 15.Faroon O, United States. Agency for Toxic S, Disease R. Toxicological profile for cadmium. U.S. Department of Health and Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry: Atlanta, Georgia, 2012. [PubMed] [Google Scholar]
- 16.Briffa J, Sinagra E, Blundell R Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 2020; 6: e04691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tchounwou PB, Yedjou CG, Patlolla AK, Sutton DJ Heavy metal toxicity and the environment. Exp Suppl 2012; 101: 133–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Al Osman M, Yang F, Massey IY Exposure routes and health effects of heavy metals on children. Biometals 2019; 32: 563–573. [DOI] [PubMed] [Google Scholar]
- 19.Barbier O, Jacquillet G, Tauc M, Cougnon M, Poujeol P Effect of heavy metals on, and handling by, the kidney. Nephron Physiol 2005; 99: p105–110. [DOI] [PubMed] [Google Scholar]
- 20.Lin C, Kim R, Tsaih SW, Sparrow D, Hu H Determinants of bone and blood lead levels among minorities living in the Boston area. Environ Health Perspect 2004; 112: 1147–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Solenkova NV, Newman JD, Berger JS, Thurston G, Hochman JS, Lamas GA Metal pollutants and cardiovascular disease: mechanisms and consequences of exposure. Am Heart J 2014; 168: 812–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rodriguez J, Mandalunis PM A Review of Metal Exposure and Its Effects on Bone Health. J Toxicol 2018; 2018: 4854152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Samuel JB, Stanley JA, Princess RA, Shanthi P, Sebastian MS Gestational cadmium exposure-induced ovotoxicity delays puberty through oxidative stress and impaired steroid hormone levels. J Med Toxicol 2011; 7: 195–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wai KM, Mar O, Kosaka S, Umemura M, Watanabe C Prenatal Heavy Metal Exposure and Adverse Birth Outcomes in Myanmar: A Birth-Cohort Study. Int J Environ Res Public Health 2017; 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Quansah R, Armah FA, Essumang DK, Luginaah I, Clarke E, Marfoh K et al. Association of arsenic with adverse pregnancy outcomes/infant mortality: a systematic review and meta-analysis. Environ Health Perspect 2015; 123: 412–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shah-Kulkarni S, Lee S, Jeong KS, Hong YC, Park H, Ha M et al. Prenatal exposure to mixtures of heavy metals and neurodevelopment in infants at 6 months. Environ Res 2020; 182: 109122. [DOI] [PubMed] [Google Scholar]
- 27.Wang X, Gao D, Zhang G, Zhang X, Li Q, Gao Q et al. Exposure to multiple metals in early pregnancy and gestational diabetes mellitus: A prospective cohort study. Environ Int 2020; 135: 105370. [DOI] [PubMed] [Google Scholar]
- 28.Ettinger AS, Zota AR, Amarasiriwardena CJ, Hopkins MR, Schwartz J, Hu H et al. Maternal arsenic exposure and impaired glucose tolerance during pregnancy. Environ Health Perspect 2009; 117: 1059–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Farzan SF, Chen Y, Wu F, Jiang J, Liu M, Baker E et al. Blood Pressure Changes in Relation to Arsenic Exposure in a U.S. Pregnancy Cohort. Environ Health Perspect 2015; 123: 999–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goen T, Schaller KH, Drexler H External quality assessment of human biomonitoring in the range of environmental exposure levels. Int J Hyg Environ Health 2012; 215: 229–232. [DOI] [PubMed] [Google Scholar]
- 31.Taussky HH A microcolorimetric determination of creatine in urine by the Jaffe reaction. J Biol Chem 1954; 208: 853–861. [PubMed] [Google Scholar]
- 32.Cowell W, Colicino E, Tanner E, Amarasiriwardena C, Andra SS, Bollati V et al. Prenatal toxic metal mixture exposure and newborn telomere length: Modification by maternal antioxidant intake. Environ Res 2020; 190: 110009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee AG, Cowell W, Kannan S, Ganguri HB, Nentin F, Wilson A et al. Prenatal particulate air pollution and newborn telomere length: Effect modification by maternal antioxidant intakes and infant sex. Environ Res 2020; 187: 109707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.CDC. Fourth Report on Human Exposure to Environmental Chemicals. In. Atlanta, GA.: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, 2009. [Google Scholar]
- 35.Brunst KJ, Sanchez-Guerra M, Chiu YM, Wilson A, Coull BA, Kloog I et al. Prenatal particulate matter exposure and mitochondrial dysfunction at the maternal-fetal interface: Effect modification by maternal lifetime trauma and child sex. Environ Int 2018; 112: 49–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Watson CV, Lewin M, Ragin-Wilson A, Jones R, Jarrett JM, Wallon K et al. Characterization of trace elements exposure in pregnant women in the United States, NHANES 1999-2016. Environ Res 2020; 183: 109208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Han I, Whitworth KW, Zhang X, Afshar M, Berens PD, Symanski E Characterization of urinary concentrations of heavy metals among socioeconomically disadvantaged black pregnant women. Environ Monit Assess 2020; 192: 200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Billick IH, Curran AS, Shier DR Analysis of pediatric blood lead levels in New York City for 1970-1976. Environ Health Perspect 1979; 31: 183–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oyana TJ, Margai FM Geographic analysis of health risks of pediatric lead exposure: a golden opportunity to promote healthy neighborhoods. Arch Environ Occup Health 2007; 62: 93–104. [DOI] [PubMed] [Google Scholar]
- 40.Meyer PA, Pivetz T, Dignam TA, Homa DM, Schoonover J, Brody D et al. Surveillance for elevated blood lead levels among children--United States, 1997-2001. MMWR Surveill Summ 2003; 52: 1–21. [PubMed] [Google Scholar]
- 41.Ge Y, Murray P, Hendershot WH Trace metal speciation and bioavailability in urban soils. Environ Pollut 2000; 107: 137–144. [DOI] [PubMed] [Google Scholar]
- 42.Stretesky PB The Distribution of Air Lead Levels across U.S. Counties: Implications for the Production of Racial Inequality Sociological Spectrum 2003; 23: 91–118. [Google Scholar]
- 43.Teye SO, Yanosky JD, Cuffee Y, Weng X, Luquis R, Farace E et al. Exploring persistent racial/ethnic disparities in lead exposure among American children aged 1-5 years: results from NHANES 1999-2016. Int Arch Occup Environ Health 2021; 94: 723–730. [DOI] [PubMed] [Google Scholar]
- 44.Massey DS Segregation and Stratification: A Biosocial Perspective. Du Bois Review: Social Science Research on Race 2004; 1: 7–25. [Google Scholar]
- 45.Massey D, Wagner B Segregation, Stigma, and Stratification: A Biosocial Model. The Oxford Handbook of Stigma, Discrimination, and Health 2016. [Google Scholar]
- 46.Perkins KL, Sampson RJ Compounded Deprivation in the Transition to Adulthood: The Intersection of Racial and Economic Inequality Among Chicagoans, 1995–2013. RSF: The Russell Sage Foundation Journal of the Social Sciences 2015; 1: 35–54. [Google Scholar]
- 47.Sampson RJ. Great American city : Chicago and the enduring neighborhood effect. The University of Chicago Press: Chicago ; London, 2012. [Google Scholar]
- 48.Sampson RJ, Winter AS The Racial Ecology Of Lead Poisoning: Toxic Inequality in Chicago Neighborhoods, 1995-2013. . Du Bois Review: Social Science Research on Race 2016; 13: 261–283. [Google Scholar]
- 49.Li X, Li Y, Zhu X, Gui X, Ma C, Peng W et al. Evaluation of the cadmium phytoextraction potential of tobacco (Nicotiana tabacum) and rhizosphere micro-characteristics under different cadmium levels. Chemosphere 2021; 286: 131714. [DOI] [PubMed] [Google Scholar]
- 50.Ashrap P, Watkins DJ, Mukherjee B, Boss J, Richards MJ, Rosario Z et al. Predictors of urinary and blood Metal(loid) concentrations among pregnant women in Northern Puerto Rico. Environ Res 2020; 183: 109178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mijal RS, Holzman CB Blood cadmium levels in women of childbearing age vary by race/ethnicity. Environ Res 2010; 110: 505–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Satarug S, Moore MR Adverse health effects of chronic exposure to low-level cadmium in foodstuffs and cigarette smoke. Environ Health Perspect 2004; 112: 1099–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vahter M, Akesson A, Liden C, Ceccatelli S, Berglund M Gender differences in the disposition and toxicity of metals. Environ Res 2007; 104: 85–95. [DOI] [PubMed] [Google Scholar]
- 54.Yi Z, Lehto NJ, Robinson BH, Cavanagh JE Environmental and edaphic factors affecting soil cadmium uptake by spinach, potatoes, onion and wheat. Sci Total Environ 2020; 713: 136694. [DOI] [PubMed] [Google Scholar]
- 55.Reeves PG, Chaney RL Bioavailability as an issue in risk assessment and management of food cadmium: a review. Sci Total Environ 2008; 398: 13–19. [DOI] [PubMed] [Google Scholar]
- 56.Nawrot TS, Staessen JA, Roels HA, Munters E, Cuypers A, Richart T et al. Cadmium exposure in the population: from health risks to strategies of prevention. Biometals 2010; 23: 769–782. [DOI] [PubMed] [Google Scholar]
- 57.Brunst KJ, Wright RO, DiGioia K, Enlow MB, Fernandez H, Wright RJ et al. Racial/ethnic and sociodemographic factors associated with micronutrient intakes and inadequacies among pregnant women in an urban US population. Public Health Nutr 2014; 17: 1960–1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Belova A, Greco SL, Riederer AM, Olsho LE, Corrales MA A method to screen U.S. environmental biomonitoring data for race/ethnicity and income-related disparity. Environ Health 2013; 12: 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gonzales FA, Jones RR, Deardorff J, Windham GC, Hiatt RA, Kushi LH Neighborhood deprivation, race/ethnicity, and urinary metal concentrations among young girls in California. Environ Int 2016; 91: 29–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kresovich JK, Erdal S, Chen HY, Gann PH, Argos M, Rauscher GH Metallic air pollutants and breast cancer heterogeneity. Environ Res 2019; 177: 108639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cooper RG, Harrison AP The exposure to and health effects of antimony. Indian J Occup Environ Med 2009; 13: 3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee PK, Yu S, Chang HJ, Cho HY, Kang MJ, Chae BG Lead chromate detected as a source of atmospheric Pb and Cr (VI) pollution. Sci Rep 2016; 6: 36088. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data analyzed in this paper were collected as part of the PRogramming of Intergenerational Stress Mechanisms (PRISM) pregnancy cohort study at the Icahn School of Medicine at Mount Sinai. Due to human subjects confidentiality, data are not public available; however, a limited dataset may be obtained from the corresponding author upon reasonable request.