Abstract
Benign prostatic hyperplasia (BPH) is a feature of ageing males. Up to half demonstrate bladder outlet obstruction (BOO) with associated lower urinary tract symptoms (LUTS) including bladder overactivity. Current therapies to reduce obstruction, such as α1-adrenoceptor antagonists and 5α-reductase inhibitors, are not effective in all patients. The phosphodiesterase-5 inhibitor (PDE5I), tadalafil, is also approved to treat BPH and LUTS suggesting a role for nitric oxide (NO•), soluble guanylate cyclase (sGC), and cGMP signalling pathways. However, PDE5I refractoriness can develop for reasons including nitrergic nerve damage and decreased NO• production, or inflammation-related oxidation of the sGC haem group, normally maintained in a reduced state by the cofactor, cytochrome-b5-reductase 3 (CYB5R3). sGC activators, such as cinaciguat (BAY 58–2667), have been developed to enhance sGC activity in the absence of NO• or when sGC is oxidised. Accordingly, their effects on the prostate and LUT function of aged mice were evaluated. Aged mice (≥24 months) demonstrated a functional BPH/BOO phenotype, compared to adult animals (2–12 months), with low, delayed voiding responses and elevated intravesical pressures as measured by telemetric cystometry. This was consistent with outflow tract histological and molecular data that showed urethral constriction, increased prostate weight, greater collagen deposition and cellular hyperplasia. All changes in aged animals were attenuated by daily oral treatment with cinaciguat for two weeks, without effect on serum testosterone levels. Cinaciguat had only transient (1 h) cardiovascular effects with oral gavage suggesting a positive safety profile. The benefit of cinaciguat was suggested by its reversal of an overactive cystometric profile in CYB5R3 smooth muscle knock-out mice that mirrors a profile of oxidative dysfunction where PDE5I may not be effective. Thus, the aged male mouse is a suitable model for BPH-induced BOO and cinaciguat has a demonstrated ability to reduce prostate-induced obstruction and consequent effects on bladder function.
Keywords: Ageing, benign prostatic hyperplasia, bladder outlet obstruction, cGMP, cinaciguat, CYB5R3, lower urinary tract symptoms, nitric oxide, PDE5 inhibitors, sGC activators
Introduction
Prostatic enlargement due to benign prostatic hyperplasia (BPH) is among the ten most common clinical conditions in ageing men when by the age of 50 one-half, and by age 80 three-quarters of men will have this condition to some extent. Pathogenic mechanisms are probably multifactorial, but include altered androgen levels with ageing and related inflammation that contribute to the excess tissue proliferation and fibrosis [1]. A potential consequence of BPH is bladder outflow obstruction (BOO) and consequent development of storage, voiding and post-micturition lower urinary tract symptoms (LUTS) which are present in 40–50% of cases. Evidence of a direct link between BPH, BOO and LUTS is weak, with current work focused on improving voiding symptoms. However, clinical data link BOO with storage LUTS and with either overactive bladder (OAB) syndrome [2], or detrusor overactivity.
OAB is a filling disorder whereby abnormal sensations lead to urgency, frequency, and often incontinence. Afferent signalling pathways that regulate micturition play a central role in the pathogenesis of OAB, and thus represent important therapeutic targets. Several approved drugs used to treat OAB and male LUTS ultimately attenuate afferent signalling from the bladder as a shared therapeutic principle [3]. These act through different mechanisms to reflect the multifactorial nature of storage and voiding LUTS and include [4]: muscarinic receptor antagonists (antimuscarinics); β3-adrenoceptor agonists; α1-adrenoceptor antagonists; 5α-reductase inhibitors; and phosphodiesterase type 5 inhibitors (PDE5Is). The mechanisms of action for PDE5Is in LUTS and BPH have been extensively discussed [5].
PDEs are a family of hydrolytic enzymes that degrade cyclic nucleotides, cGMP and/or cyclic adenosine monophosphate (cAMP), key second messengers in several signalling pathways. Currently, 11 distinct PDE isoforms have been identified in various tissues, each with differing selectivity for cGMP (e.g., PDE5) or cAMP (e.g., PDE1 and PDE4) [6]. In the urinary bladder, cAMP pathways [7,8] have a greater role than those of cGMP [9,10] in regulating detrusor contractility. This is in contrast to penile erectile tissue where NO•-dependent cGMP production by sGC is crucial for relaxation and is the basis for the action of PDE5Is in managing erectile dysfunction (ED) [11].
The clinical efficacy of PDE5Is to ameliorate LUTS associated with BPH has been reported in multiple trials since 2002 and tadalafil (5 mg daily) is now licensed by the FDA to treat male LUTS with or without ED. PDE5Is enhance oxygenation, while concurrently reducing inflammation, tissue proliferation, and nerve-mediated activity in the LUT [5]. Recent findings from spinal cord-injured rats treated with another PDE5I, vardenafil, suggest that PDE5Is alleviate bladder sensory symptoms by reducing non-voiding contractions (NVCs) and afferent firing during filling [12], and suggest that an important effect of PDE5Is on the LUT is on sensory mechanisms. In addition, chronic treatment with PDE5Is inhibits and even reverses fibrosis and bladder wall remodelling following partial BOO [13] and increases contractile force generation in normal rat bladders [14]. Therefore, multiple lines of evidence underline the plausible role of NO•-cGMP signalling in the aetiology of BPH.
The NO• pathway is the key target of PDE5I therapy [15]. NO• binds to the haem group on the β-subunit of soluble guanylate cyclase (sGC), activating its catalytic domain to convert GTP to cGMP. This in turn activates protein kinase G (PKG), phosphorylating multiple downstream proteins. A prerequisite for NO•-induced cGMP production is reduced sGC haem, as NO• cannot bind when the haem is in an oxidised state (Figure 1A). CYB5R3 is a key enzyme that maintains sGC haem in the reduced state [16], but its activity can in turn be reduced by oxidative stress or inflammation [17] – both associated with ageing. This problem can be circumvented by small molecule sGC activators that are not structural analogues of cGMP, as are PDE5Is, but induce its production in the absence of NO• or when haem is oxidised or detached [18], and therefore may be effective for patients refractory to PDE5Is.
Figure 1. Regulation of sGC signalling and anatomical features of the mouse prostate.
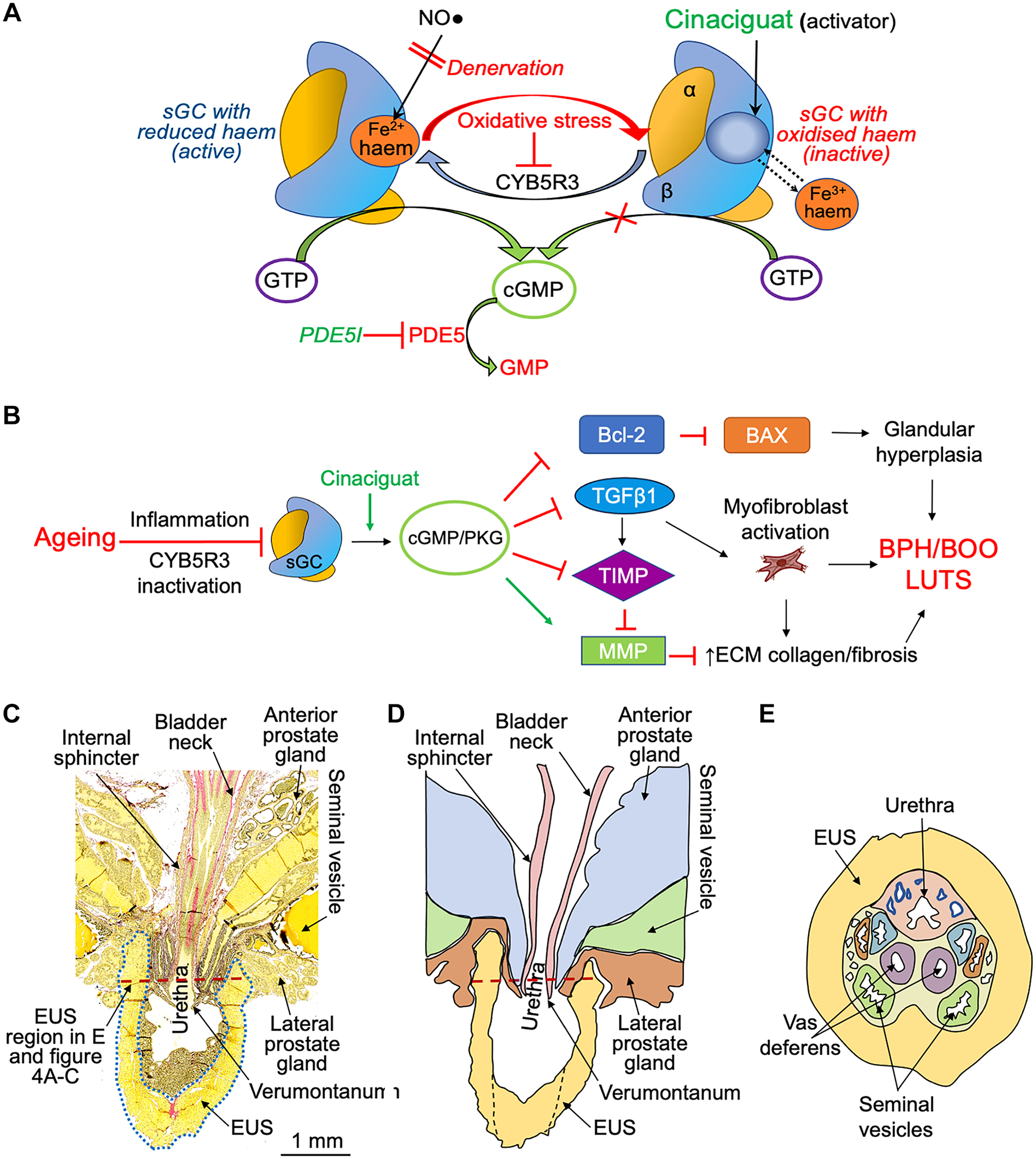
A) The reductase CYB5R3 maintains the active state of sGC with reduced haem (Fe2+) for NO• driven cGMP production. Oxidative stress can downregulate CYB5R3 activity resulting in haem oxidation and insensitivity of sGC to NO•. This can be reversed by sGC activators that do not require NO• or haem for cGMP production as they are haem analogues. PDE5Is are structural analogs of cGMP and competitive antagonists of PDE5 while cinaciguat is an allosteric activator of sGC. B) Hypothesized role for sGC inactivation in BPH/BOO pathophysiology and amelioration by cinaciguat through increased cGMP production and activation of protein kinase G (PKG). Cinaciguat reverses hyperplasia by normalising the Bcl-2:BAX ratio and inhibits TGFβ−1 to enhance MMP activity and increase ECM degradation. C) Longitudinal section of an adult mouse prostate; the dotted blue line outlines the upper EUS. D) Cartoon representing panel C for clarification. E) Cross-section of the prostate at the level of the red dashed lines in C) and D).
A series of sGC activators, including cinaciguat, were developed as potential treatments for heart failure, peripheral arterial occlusive disease, and liver fibrosis [19]. However, they are also active on LUT tissues. sGC activators relax prostatic and urethral muscle [20,21] and ameliorate cyclophosphamide-induced bladder overactivity in mice with reduced sGC activity [22]. Thus, ageing related sGC inactivation may contribute to BPH pathology and associated LUTS. Enhancement of cGMP signalling has been demonstrated to have an antiproliferative effect in human prostatic epithelial cells [23]. Furthermore, the ability of the NO•-sGC-cGMP pathway to reduce TGFβ1-mediated fibrosis [24] allows generation of a hypothesis whereby cinaciguat reduces prostate fibrosis/cellular hyperplasia and age-associated BPH/BOO itself. The putative mechanism for sGC-cGMP signalling in BPH pathogenesis and therapeutic effect of sGC activator are summarised in Figure 1B.
Despite anatomical differences with humans, rodent models have been important to our understanding of key pathways in BPH pathogenesis. In ageing men, proliferating cells within the transitional zone cannot expand due to a fibrous capsule surrounding the prostate and the prostate enlargement compresses the urethra resulting in BOO [25]. While there is no capsule enclosing the lobes of the rodent prostate, the striated muscle external urethral sphincter (EUS) surrounds the prostatic urethra (see Figure 1C–F), and age-induced hyperplasia and inflammation within this region result in BOO, which justifies rodents as a research model for BPH/BOO pathology. Our aims were to: i) establish that age-related structural/functional changes to the prostate and outflow tract in male rodents are consistent with BOO; ii) describe changes to prostate structure and the function of the bladder and urine outflow tract in ageing mice; iii) generate empirical evidence using interdisciplinary experimental approaches to support the plausible role of NO•-cGMP signalling in BPH/BOO aetiology of aged mice; iv) measure the ability of cinaciguat to minimise age-related structural and functional LUT changes associated with BOO.
Materials and methods
Animals.
Male adult (2–12 months old) and aged (24–30 months old) C57Bl/6 mice were obtained from the National Institute of Aging colony or purchased from Jackson Laboratory (Bar Harbor, ME, USA). The 2–12 month old C57Bl/6 mice fall within the range of mature to middle aged mice, respectively [26] and have formed an homogeneous cohort in our studies. Male CYB5R3(flox/-flox/ +SMC-Cre) (CYB5R3−/−) and wildtype littermates (3–6 months old) [16] were obtained from Dr. Adam Straub, University of Pittsburgh. CYB5R3−/− and age-matched controls received daily intraperitoneal tamoxifen (10 days, 1 mg/day) and used for experiments one week after the final dose. Animals were maintained on a 12-hour light/dark cycle (7:00AM to 7:00PM), in a climate-controlled animal unit with food/water provided ad libitum. All procedures received approval from the Institutional Animal Care/Use Committee at the University of Pittsburgh. Dosing and formulation are based on previously reported methods (Table 1).
Table 1.
Different administration routes and formulations for cinaciguat and other sGC activators in preclinical studies.
| Route | Dose | Formulation | PMID/year |
|---|---|---|---|
| Oral | 10 mg/kg/d | Cinaciguat, 3%methylcellulose | 16391154, 2006 |
| Oral | 10 mg/kg | Cinaciguat, 1%methylcellulose | 19667237, 2009 |
| Oral | 0.03–3 mg/kg/d | BAY-60–4552, 0.5% methylcellulose, 5%DMSO, 0.1%Tween80 | 22783192, 2012 |
| Oral | 1 mg/kg | BAY 60–2770, Transcutol:Cremophor:water, ratio 1:2:7 | 24050894, 2014 |
| Oral | 1 mg/kg | Cinaciguat, Transcutol:Cremophor:water, ratio 1:2:7 | 27122537, 2016 |
| Oral | 1 mg/kg/d | Cinaciguat, 0.5%natrosol, 0.015%Tween80 | 32878579, 2020 |
| Oral | 10 mg/kg | Cinaciguat, 0.5% methylcellulose, 10% DMSO | Current Study |
| IV | 12.5 μg | Cinaciguat, 1:1 Transcutol and Cremophor | 19667237, 2009 |
| IV | 1–10 μg/kg | Cinaciguat, 10%DMSO | 22268103, 2012 |
| IV | 100 μg/kg | Cinaciguat, 20%Transcutol, 20%Cremophor EL, 60% PBS | 24015214, 2013 |
| IP | 0.04–0.4 mg/kg | Cinaciguat, 100%DMSO | 31487266, 2019 |
Cinaciguat.
Cinaciguat (BAY 58–2667; provided by Bayer AG) was dissolved in DMSO and made up in 0.5% methylcellulose with a final DMSO concentration of 10%. It was administered by oral gavage (10 mg/kg/day/14 days); control animals received an equivalent volume of vehicle.
Bladder pressure, urine flow, volume, frequency, and cardiovascular measurements in mobile mice.
Mice were anaesthetised with isoflurane (1–5%), and an HDX11 telemeter (Data Sciences International Inc., New Brighton, MN, USA; Figure 2A) implanted subcutaneously via a laparotomy with the pressure line inserted into the bladder and secured by a suture. Animals recovered over 7–10 days with prophylactic antibiotics and analgesics. After recovery, animals were housed in modified metabolic cages where telemeter recordings were obtained through receiving units (RSC-1, Data Sciences International Inc.). Load cells (5 μl/10 Hz sensitivity) below each cage measured the duration and volume of voids, and filter paper recorded voiding patterns. Data were recorded with 12-hour light/dark cycles using LabChart (ADInstruments, Colorado Springs, CO, USA) and OxyMax (Columbus Instruments, Columbus, OH, USA) software. Arterial blood pressure (BP) and heart rate (HR) were measured using a BP-2000 analysis system (Visitech Systems, Apex, NC, USA) before cinaciguat administration and then immediately, 1 h and 2 h after administration. Animals were acclimatised to the procedure for a week before cinaciguat treatment. Decerebrate CMG recordings were conducted as previously described [27].
Figure 2. BP/HR recordings and telemetric CMGs from adult and aged mice to assess physiological effects of cinaciguat.
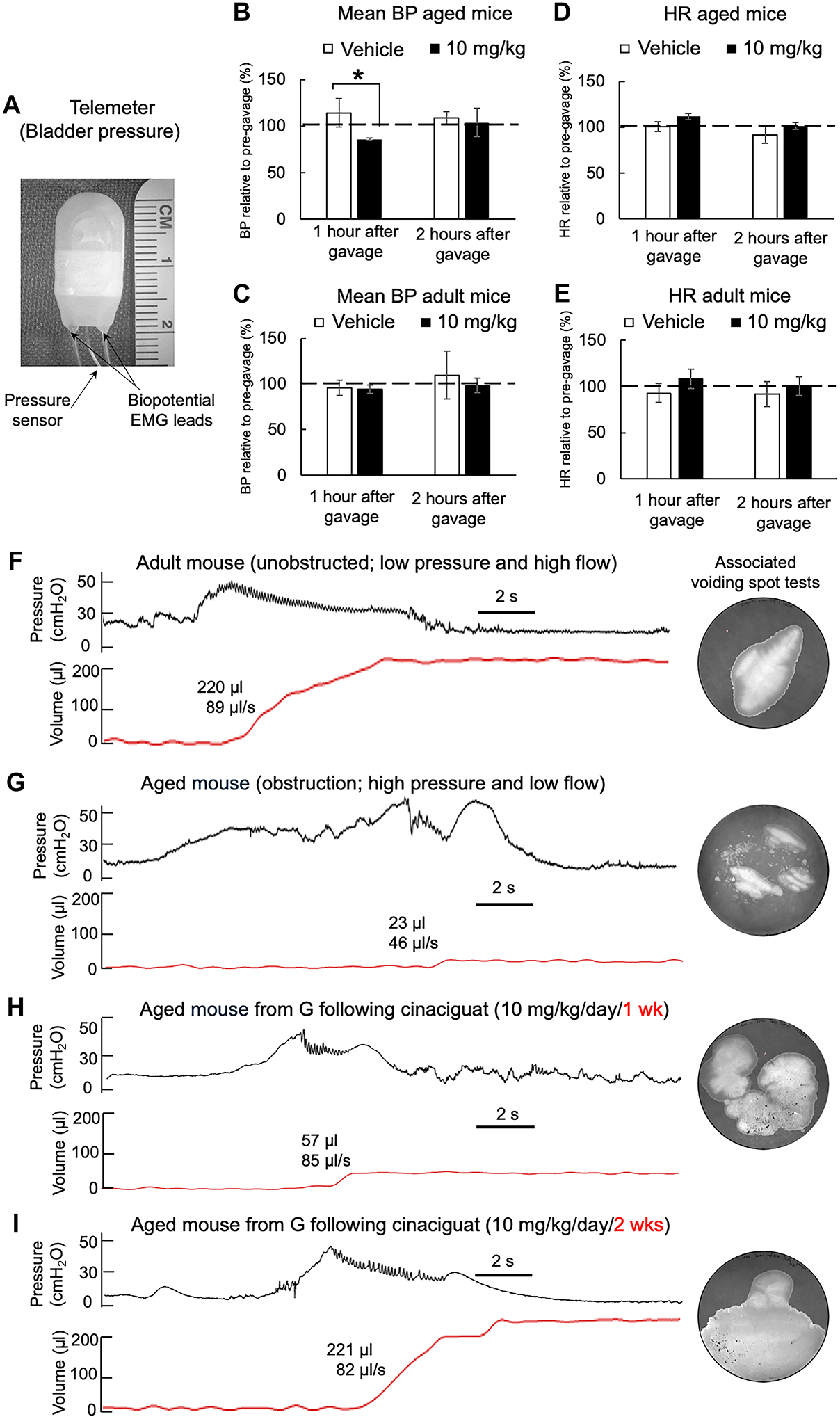
A) Image of a telemeter used in the study. B) and C) Mean arterial BP (percentage of baseline) from adult and aged mice gavaged with vehicle or 10 mg/kg cinaciguat, unpaired t-test, *p=0.01. D) and E) HR measurements from the same mice shown in B) and C (Mean±SD). Data were averaged over at least three separate recordings performed every other day. Measurements in B-E were taken before drug administration, and 1 and 2 hours after gavage (aged n=3, adult n=6). F-I) Telemetric bladder pressure, void (red trace) recordings with associated maximum flow rate at peak pressure, and respective voiding spot tests, from F) unobstructed adult mouse, G) severely obstructed aged mouse, H) and I) aged mouse after 1 week of cinaciguat treatment (10 mg/kg/day) and after 2 weeks of treatment. Values of voided volume and peak flow rate are shown by each void tracing.
Tissue preparation.
After in vivo experiments animals were humanely killed by CO2 asphyxiation in a closed chamber and tissues (bladder, prostate, and prostatic urethra) dissected for histological or molecular assays. Blood samples were obtained through cardiac punctures of mice under deep isoflurane anaesthesia using a 20-gauge needle and 3 ml syringe. Blood was allowed to coagulate in uncoated collection tubes at room temperature then centrifuged (1500 × g, 10 min, 4° C) to collect the serum. Tissue samples for molecular studies were snap-frozen on dry ice with isopropyl alcohol immediately following dissection. Protein lysates for western blot were obtained by bead homogenisation of tissue samples in Ca2+- and Mg2+-free Hank’s buffered saline solution with HaltTM protease/phosphatase inhibitor (87786, Thermofisher Scientific, Pittsburgh, PA, USA). Protein concentrations were determined by BCA protein assay (23252, Thermofisher) and lysates were snap-frozen until used for assays. The cGMP (ab65356, Abcam, Waltham, MA, USA) and serum testosterone (582701, Cayman Chemicals, Ann Arbor, MI, USA) ELISAs were performed following the manufacturers’ recommendations.
DNA extraction.
Left ventral and lateral prostatic lobes were homogenised using a FastPrep-24 bead homogeniser. Total DNA was extracted with the DNeasy Blood/Tissue Kit (69504, Qiagen LLC, Germantown, MA, USA). Concentrations, measured with a NanoDrop Microvolume Spectrophotometer (Thermofisher), were expressed as μg/mg of tissue.
Histology.
Prostate tissue was isolated, fixed overnight in 10 % buffered formalin, and paraffin sections (3 μm) cut on a motorised microtome (Microm HM360, ThermoFisher). Sections were stained with Verhoeff van Gieson for elastin/collagen (HT25A, Millipore-Sigma, Burlington, MA, USA) or acidified 1% Toluidine blue for mast cells. Images were recorded as bright-field 3D montages (Fluoview 3000/CellSense software, ≤15×15 frames, Olympus Corp, Center Valley, PA, USA) and analysed using NDP and HCImage software (Hamamatsu Photonics, Bridgewater, NJ, USA).
Immunofluorescence/immunohistochemistry.
Mouse prostate and bladder samples were processed for cryosectioning and immunofluorescence as described previously [27] with; sGC β1 subunit (160897, Cayman Chemical, 1:1,000 dilution), donkey anti-rabbit Alexaflor-488 (A-21206, ThermoFisher, 1:500 dilution), rhodamine-phalloidin (R415, ThermoFisher, 1:1,000 dilution) and DAPI (D1306, ThermoFisher, 1:5,000 dilution). Paraffin tissue sections were used for immunohistochemical detection of Bcl-2 (ab182858, Abcam, 1 μg/ml) and BAX (AF820, R&D Systems, Minneapolis, MN, USA; 1 μg/ml). Primary antibody binding was visualised by sequential incubation with biotinylated donkey anti-rabbit IgG (RPN1004V, Cytiva, Marlborough, MA, USA; 1:200 dilution) and streptavidin-peroxidase (E2886, Millipore-Sigma, 1:100 dilution) followed by diaminobenzidine (DAB) staining (8059, Cell Signaling Technology Inc., Danvers, MA, USA). Nuclei were counter-stained with haematoxylin. The Bcl-2:BAX ratio was calculated with ImageJ software (National Institutes of Health, Bethesda, MA, USA) [28].
Statistical analyses.
Continuous measures were summarised as mean ± SD or standard error of the mean (SEM), n=number of separate animals used. Data were fitted by ANOVA to each of the continuous measures with the groups defined by age and cinaciguat treatment combinations as the independent factor using Prism 8 software (GraphPad, San Diego, CA, USA). Tukey’s method was used to perform post hoc pairwise comparisons of interest between groups, with independent sample t-tests for two-group comparisons. Statistical significance was accepted at p<0.05.
Results
Effect of cinaciguat on blood pressure/heart rate (BP/HR) and bladder activity of adult/aged mice.
The BP/HR of adult and aged mice were recorded by photoplethysmography with an oral dose of vehicle or cinaciguat (10 mg/kg). There was a small transient reduction of BP, with a compensatory tachycardia, immediately after dosing, which returned to control levels within 2 h (Figure 2B, C). Significant changes to BP and HR following cinaciguat administration were not found in adult mice (Figure 2D, E).
Telemetric recordings of intravesical bladder pressure from adult mice show a transient rise (Figure 2F, black trace) followed by a single void after a 2 s delay (red trace). Treatment with cinaciguat (10 mg/kg/day) for two weeks had no effect on voiding characteristics or voiding frequency during the awake (night) period (Table 2). By contrast, eight of ten aged mice showed sustained increases of bladder pressure during more frequent voiding phases (Figure 2G), each followed by a longer delay (up to 20 s) for smaller void spots and at a reduced flow rate. Mean peak pressure at the start of flow was greater, but not significant, in aged animals with the data reflecting the larger variability in this cohort (Table 2). Overall, micturition characteristics recapitulate the symptoms of urinary hesitancy and frequency seen in BPH patients and the voided volume is indicative of incomplete bladder emptying. Daily cinaciguat treatment of aged mice for one or two weeks (Figure 2H, I) demonstrated a return to the adult voiding profile, with an eventual complete recovery of voided volume and flow rate, as well as maximum pressure at initiation of voiding, although voiding frequency remained elevated.
Table 2.
Cystometric parameters of adult and aged mice.
| Voided vol (μl) | Max flow rate (μl/s) | Max voiding pressure when flow begins (cmH2O) | Voids/night | |
|---|---|---|---|---|
| Adult (n = 4) | 203 ± 83 | 127 ± 62 | 52 ± 6 | 5 ± 3 |
| Adult + 2 wks cinaciguat (n = 4) | 183 ± 82 | 119 ± 38 | 57 ± 7 | 6 ± 2 |
| Aged (n = 10) | 53 ± 23 * | 30 ± 9 * | 70 ± 16 | 17 ± 3 * |
| Aged + 1 wk cinaciguat (n = 4) | 99 ± 45 *§ | 61 ± 19§ | 57 ± 5§ | 14 ± 2 *§ |
| Aged + 2 wks cinaciguat (n = 4) | 231 ± 87§ | 82 ± 42§ | 45 ± 16§ | 12 ± 3§ |
p<0.05 versus adult,
p<0.05 versus aged.
ANOVA with Tukey’s method for post hoc pairwise comparisons, Mean±SD.
Since ambulatory cystometry suggested the presence of an obstructive pathology in the LUT of aged mice, we examined for compensatory phenotypic changes evoked in the urinary bladder. The bladder-to-body weight ratio was greater in aged animals (1.4±0.1 mg/g, n=6, versus 1.1±0.1 mg/g, n=4, old versus adult, p=0.003) and in aged animals was significantly reduced following cinaciguat treatment (1.1±0.1, p<0.05, n=5). These ambulatory cystometry and gross morphological data are consistent with the presence of BOO in aged rodent bladders without deficits in the functional capacity of the detrusor smooth muscle. In the context of this study, baseline recordings or lower urinary tract variables (LUTS) did not differ in mice aged between 2 and 12 months and formed a homogeneous cohort that has markedly different LUT properties.
Immunofluorescent localisation showed greater expression of sGC in the smooth muscle layers of the bladder neck and prostate compared to the dome (Figure 3A, B). In the bladder wall, sGC expression was more evident in fibroblasts/myofibroblasts, that are more abundant in the mucosa but also present around muscle bundles, compared to detrusor myocytes themselves (Figure 3C). This indicates that the outflow tract, including the bladder neck, is an important target for sGC activators. ELISA quantification of basal cGMP levels in prostate tissue showed significantly lower values in aged compared to adult mice (Figure 3D). By contrast, there was no corresponding decline in bladder tissue cGMP levels in aged mice (Figure 3E).
Figure 3. sGC expression in mouse bladders and prostates.
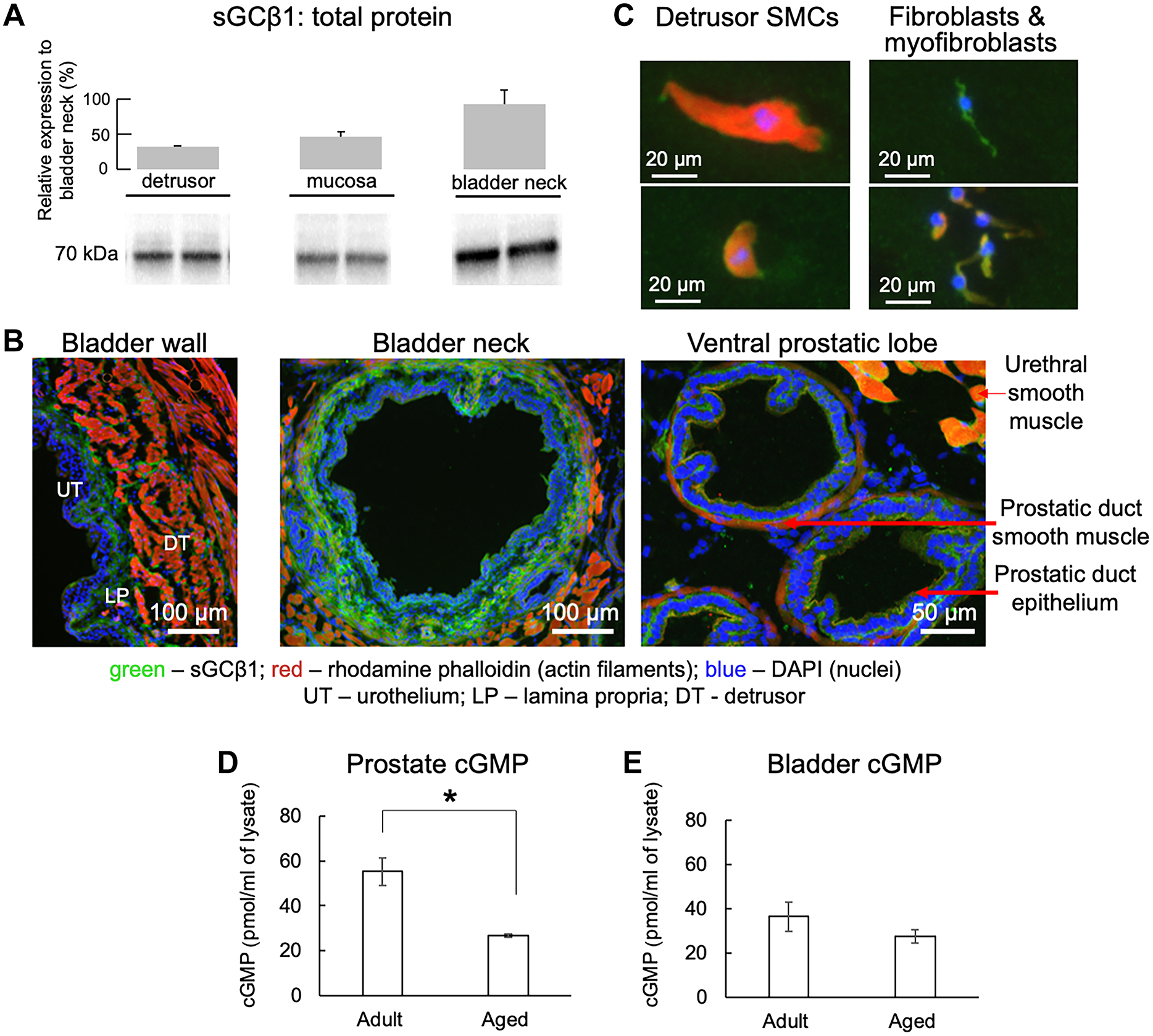
A) Western blot of sGCβ1 protein expression in detrusor, mucosa, and bladder neck of adult mice (n=4, mean±SD). The optical density of each sGCβ1 band was normalised to total protein. Values inside bars are percent expression compared to bladder neck. B) sGCβ1 localisation in the bladder wall, bladder neck and prostate (n=3). C) Immunofluorescence of sGCβ1 in dissociated bladder smooth muscle cells, fibroblasts and myofibroblasts (n=3). Green (Alexafluor 488) - sGCβ1; red (rhodamine phalloidin) – actin filaments; and blue (DAPI) – nuclei. D) prostate and E) bladder cGMP levels from adult and aged mice (n=4 each, mean±SEM, unpaired t-test *p=0.0008).
Cinaciguat reverses prostate/prostatic urethra hyperplasia.
In aged mice, constriction of the urethral lumen was coincident with hyperplasia of smooth muscle, stromal and epithelial cells within the prostatic urethra and prostatic lobes (Figure 4B). Cinaciguat treatment for two weeks (10 mg/kg/day) restored the constricted urethral opening of aged mice to that in adult animals (Figure 4C versus 4A; and 4D). This is consistent with the obstructed cystometry patterns in aged mice and reversed by cinaciguat. The collagen:tissue ratio within both prostate and urethra were significantly greater in aged animals than adults (Figure 4E). Mast cell numbers were also increased in aged mouse prostates compared to adults (Figure 4F). Oral cinaciguat treatment reversed these changes in aged animals without producing any significant effects in adults.
Figure 4. Age-related histological changes and effects of oral cinaciguat (10 mg/kg/day/2 wks) in mouse prostates.
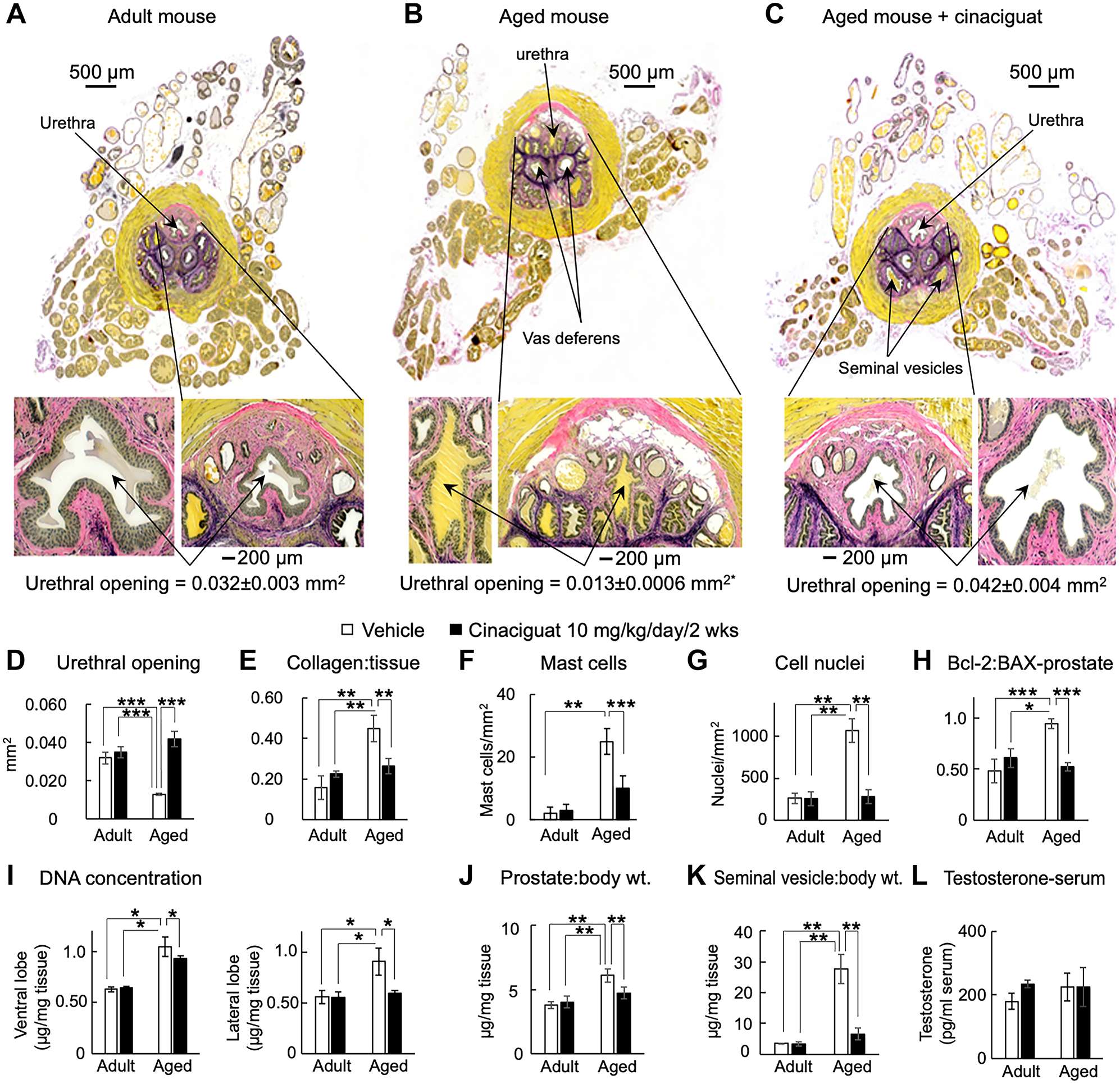
Verhoeff van Gieson stain to highlight the differences in urethral opening size: A) Adult mice (yellow inclusions in the lumen is trapped seminal fluid) versus B) Aged mice with hyperplasia and C) Aged mice treated with cinaciguat. Bottom panels are expanded views of prostatic urethras. Pink is collagen, yellow is muscle and black is elastin. D) Urethral cross-section area measured in at least four different sections from n≥3 mice in each group; p<0.0001 versus adult and aged treated with cinaciguat. E) Collagen:tissue ratio, F) Mast cell number per mm2 and G) cell nuclei count (n≥6 in each group). H) Bcl-2:BAX expression ratio from adult and aged mouse prostates with and without cinaciguat treatment (n=4 each). I) DNA quantification from left ventral and lateral lobes in aged animals, in the absence and presence of cinaciguat (n=3 each). J) Prostate to body weight ratio (n=10 each) and K) Seminal vesicle:body weight ratio (n=10 each) of adult and aged mice. *p≤0.05, **p≤0.01, ***p≤0.001, ANOVA with Tukey’s method for posthoc pairwise comparisons. All data are expressed as mean±SD. L) Serum testosterone in adult and aged mice, mean±SEM.
Compared to transgenic and surgical approaches used in the past to generate BOO, our data demonstrate that aged mice (≥24 months) exhibit pronounced cellular hyperplasia in the prostatic lobes as well as within the prostatic urethra. The latter is surrounded by the striated muscle of the EUS which restricts the anatomical volume, and the resulting tissue growth compresses the urethral lumen. These microscopic features and the associated physiologic effects mirror human BPH, where outward expansion is limited by a fibrous capsule. Moreover, while investigating the effects of ageing on LUTS in rodents, we discovered nodules on the enlarged ventrolateral prostate lobes in ~80% of aged males (≥24 mo, n = 15, see Figure 5), conspicuous by their absence in adults (9 months). Since the palpation of nodules on digital rectal examination is used to support the diagnosis of BPH in men, the existence of nodules in aged mice supports their use as preclinical models of BPH/BOO. We did not observe malignant nodules in aged mouse prostate.
Figure 5. Images of prostatic nodules in an aged rodent and corresponding histological sections.
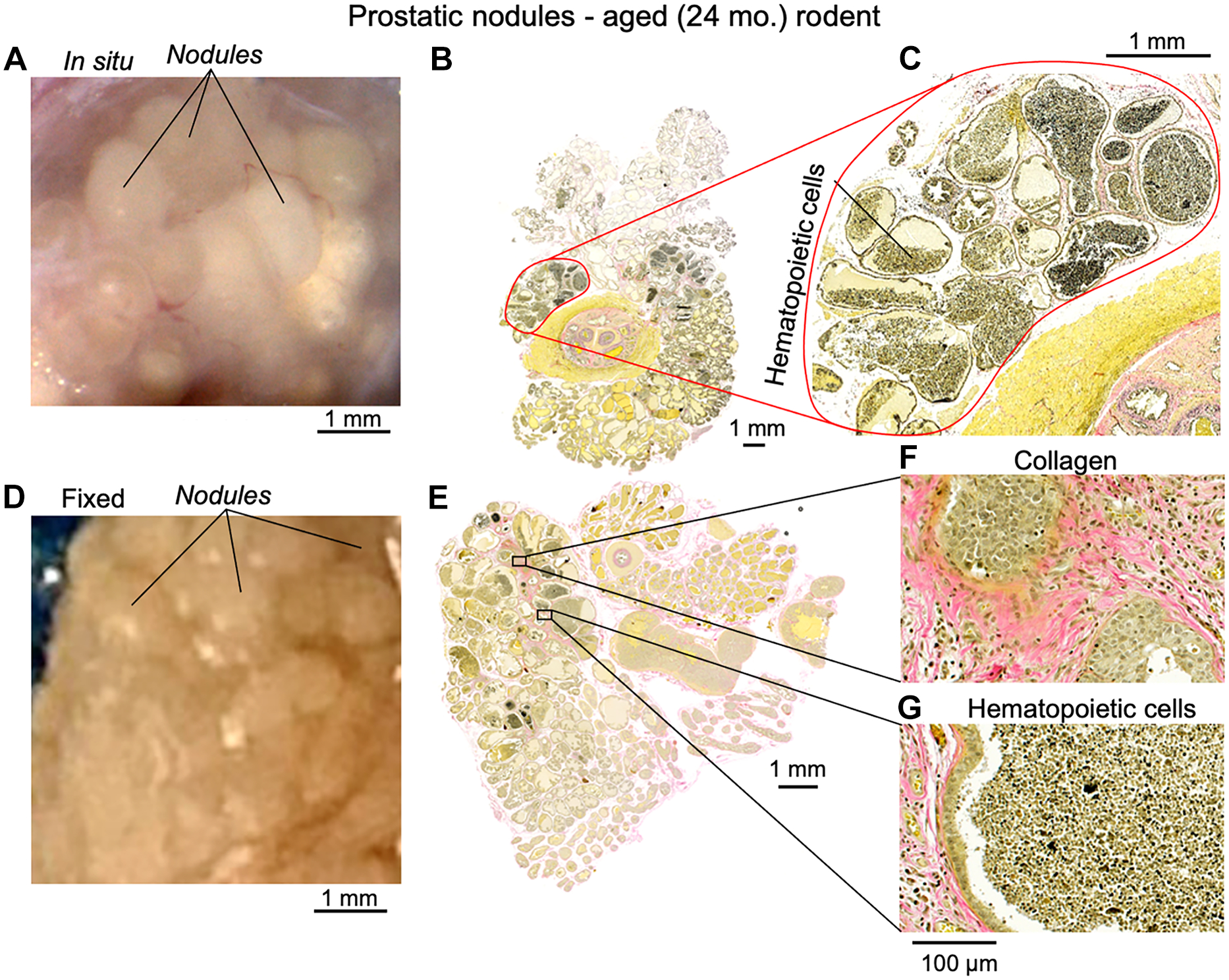
A) Image of the unfixed lateral prostate surface from an aged prostate. B) Corresponding histological section with Verhoeff van Gieson staining. C) Magnified image of a nodule from B), showing white blood cell infiltration into prostatic glands. D) Image of prostate nodules following fixation. E) Histological section from D) stained with Verhoeff van Gieson. F) Magnified region showing collagen deposition in prostate gland stroma and hyperplasia of glandular epithelium. G) Magnified region showing white blood cell infiltration.
To confirm that prostate enlargement was due to hyperplasia, and not hypertrophy, the number of nuclei per area (Figure 4G), the expression ratio of anti-apoptotic Bcl-2 to pro-apoptotic BAX proteins (Figure 4H) and total DNA content in mouse ventral and lateral prostatic lobe tissues was measured (Figure 4I). All variables were significantly elevated in aged prostates compared to adult and were wholly or partially reversed with cinaciguat treatment.
Treatment with 10 mg/kg cinaciguat caused significant reduction of prostate- (Figure 4J) and seminal vesicle-to-body weight ratios (Figure 4K). Since hormonal imbalance contributes to BPH pathology, the effect of cinaciguat on serum testosterone was evaluated, but there were no significant alterations to adult or aged mice levels after treatment (Figure 4L).
Smooth muscle CYB5R3 deficient mice exhibit bladder overactivity reversed by cinaciguat demonstrating the pathological role of oxidised sGC.
A key test of the hypothesis that cinaciguat exerts its actions through direct activation of oxidised sGC was to measure cystometric activity in adult mice where the CYB5R3 gene has been conditionally deleted in smooth muscle. Cystometry of such knockouts showed NVCs, shortened intercontractile intervals (ICIs) and reduced filling compliance (Figure 6B versus control Figure 6A, see Table 3 for values). CYB5R3−/− mice also exhibited refractoriness to the PDE5I, sildenafil (Figure 6C), consistent with reduced ability to synthesise cGMP. However, cinaciguat was able to restore a wild-type cystometric profile in CYB5R3−/− mice (Figure 6D).
Figure 6. Effect of acute cinaciguat administration on CMG recordings from adult CYB5R3SMC−/− mice.
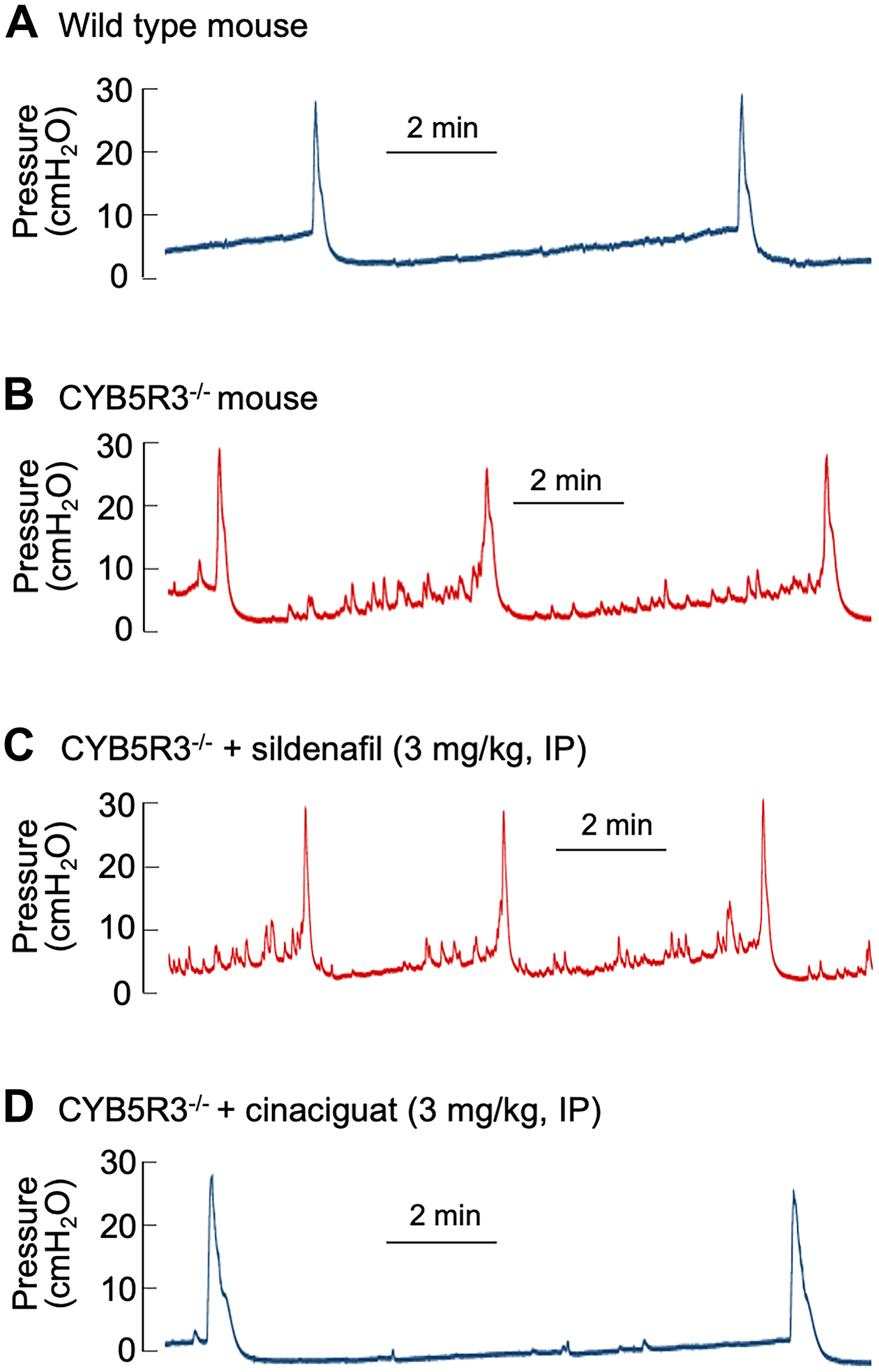
Decerebrate CMG recordings from: A) Control; and B) CYB5R3SMC−/− mice. Effects of acute intraperitoneal injections of C) sildenafil (3 mg/kg) and D) cinaciguat (3 mg/kg) in CYB5R3SMC−/− mice.
Table 3.
Cystometric parameters of CYB5R3−/− mice.
| BP | MVP | NVC | ICI | BC | |
|---|---|---|---|---|---|
| control (n = 6) | 4.9 ± 0.6 | 22.2 ± 1.3 | 0.2 ± 0.4 | 544 ± 44 | 19.7 ± 5.5 |
| CYB5R3−/− (n = 4) | 5.5 ± 1.9 | 28.4 ± 2.4 | 11 ± 3 * | 320 ± 107 * | 8.9 ± 2.2 * |
| CYB5R3−/− + sildenafil (n = 4) | 5.1 ± 0.9 | 29.3 ± 1.9 | 10 ± 4 * | 270 ± 21 * | 10.3 ± 1.4 * |
| CYB5R3−/− + cinaciguat (n = 4) | 5.0 ± 0.7 | 24.2 ± 1.9 | 0.7 ± 0.8 ** | 589 ± 23‡ § | 21.0 ± 6.8‡ § |
BP (baseline pressure), MVP (maximal voiding pressure), NVC (non-voiding contractions), ICI (intercontractile interval), BC (bladder compliance).
p<0.0001 vs control,
p<0.0002 versus CYB5R3−/−,
p<0.0001 versus CYB5R3−/−,
p≤0.0003 versus CYB5R3−/− + sildenafil, one-way ANOVA with Tukey’s multiple comparison test. Mean±SD.
Discussion
A broad consensus now exists on the multifactorial pathophysiology of male LUTS [5,29–31] and conventional wisdom focuses on age-related changes in prostate and urethra for the pathogenesis of LUTS. BPH is a progressive disease where persistent prostatic enlargement can produce BOO with additional changes to bladder afferent nerve activity. A clinical study suggested sensory stimuli from an anatomic alteration in the prostatic urethra could induce detrusor overactivity [32]. Dichotomising afferent projections innervating bladder and prostate [33] also suggest bladder overactivity symptoms may be provoked by prostatic inflammation [34]. Thus, BPH/BOO patients with LUTS could benefit from attenuation of both bladder and prostate sensory stimuli [32]. Findings from our conditional smooth muscle specific CYB5R3−/− mice (Figure 6A–D) suggest that oxidation-induced sGC inactivation may contribute to the development of BPH/BOO/LUTS. Most importantly, deletion of CYB5R3 in smooth muscle recapitulated the refractoriness of LUTS to PDE5I, sildenafil (Figure 6C) in the clinic which was surmountable by cinaciguat (Figure 6D). Our findings support an important role for cGMP signalling in bladder function and suggest the effectiveness of cinaciguat in raising the cellular cGMP levels in patients refractory to PDE5I.
Ageing and inflammation are intercalated in affecting multiple signalling pathways contributing to decreased cellular functionality. Evidence supports a hypothesis for the reduced activation of NO•-sGC-cGMP signalling in aged rodents [35]. This was further supported in the present study where lower basal cGMP levels were measured in aged versus adult mouse prostate tissue (Figure 3D). There are several enzymatic determinants of cGMP levels in this pathway including NOS, CYB5R3, PDE5 and sGC. However, it is still unclear if the decreased signalling through the NO•-sGC pathway contributes to BPH pathogenesis as there are no reports of any alterations in prostatic tissue from several studies examining LUTS in sGC [36] or PKG type 1 [37] knockout mice.
Compared to the transgenic and surgical approaches used in the past to generate BOO, our data demonstrate that aged mice (≥24 months) exhibit pronounced cellular hyperplasia in the prostatic lobes, as well as within the prostatic urethra, surrounded by the striated muscle of the EUS where the resulting tissue growth ends up compressing the urethral lumen. The histologic features of aged mouse prostates are similar to those observed in a majority of human males with BPH. A relatively small percentage (20%) of human BPH cases show either stromal-dominant or epithelial-dominant nodules and growth patterns; but most show a mixed epithelial-stromal nodular phenotype, as we observed with the aged rodent model (Figure 5). An increased prostate weight of aged animals (Figure 4J) was associated with hyperplasia of epithelial, stromal, and smooth muscle cells along with increased collagen deposition (Figure 4E) that would contribute to obstruction of the prostatic urethra bounded by the EUS [31]. Hyperplasia rather than cellular hypertrophy was supported by increases of both DNA content and the Bcl-2:BAX expression ratio in prostatic tissue (Figure 4G–I). Awake cystometry recordings (Figure 2F–I and Table 2) confirmed that a naturally occurring obstructed phenotype is a consequence of an enlarged prostate in aged mice. The histological changes correlated with the raised bladder pressures, reduced voided volumes and behaviour changes indicative of urgency and overflow incontinence. The obstructive consequence of prostate hyperplasia is further supported by enlargement of the seminal vesicles in aged mice (Figure 4K), as the seminal ducts converge at the level of the proximal urethra in rodents (Figure 1C–F and Figure 4B). Taken together, the histologic features and the associated physiologic effects mirror human BPH, where the outward expansion is limited by the fibrous capsule. Our study underlines that age-related alteration of the NO•-sGC-cGMP pathway [35] exert a plausible role in the BPH/BOO etiology and pathology which can be targeted by the sGC activator, cinaciguat for therapeutic effect. Using interdisciplinary experimental approaches and cinaciguat, we probed the role of this signalling pathway in the naturally exhibited phenotype of BPH/BOO in aged mice. These findings on age-related prostatic growth, without the intervention of exogenous hormonal treatments underlines the utility of aged rodents as preclinical models for understanding the pathophysiology of BPH/BOO.
Decreased cGMP signalling has also been proposed to be a direct contributor to inflammation in the prostate and associated LUTS [38]. Our data support that prostatic cGMP signalling could be affected in aged mice (Figure 3D) which correlated with elevated mast cell numbers, indicating ongoing inflammation (Figure 4F). Previous reports support the observed anti-inflammatory effect of cinaciguat, whereby increasing sGC-cGMP signalling can suppress the release of inflammatory chemokines that in turn promote BPH epithelial cell proliferation [39,40]. Moreover, increasing cGMP levels alone may prevent cell proliferation in the prostate [23]. Thus, cinaciguat has the capacity to inhibits both inflammatory and proliferative activity in the aged mouse prostate by respectively reducing mast cell numbers and hyperplasia.
Cinaciguat administration over two weeks diminished prostate collagen content (Figure 4E) to alleviate outlet obstruction and improve voiding function. The beneficial effect of promoting sGC-cGMP signalling on tissue fibrosis has been widely reported [41]. PDE5I [42] and sGC activators [43] prevent fibroblast to myofibroblast conversion in the prostate, which would prevent the further deposition of ECM. Enhanced expression of MMP [44] with a concurrent decrease in TIMP [43] can also be promoted by cGMP signalling. These reported findings support mechanisms by which cinaciguat could reduce collagen content through the prostate and tissue surrounding the urethra in aged mice.
Outlet obstruction can have adverse effects on bladder function due to chronic urinary retention and overdistention, with further risk of urinary tract infections and even renal impairment. Surgical models of BOO demonstrate significant bladder hypertrophy and diminished detrusor contractile activity due to decompensation [45]. This suggests prostatic enlargement in aged rodents does not result in a severe BOO phenotype that can detrimentally affect detrusor contractility. This concept is further supported by the small but significant increase in bladder weights in aged rodents but much less than those reported from surgical BOO models [45].
Hormonal dysregulation is believed to contribute to the development of BPH, accordingly, modulation of the androgen pathway (e.g., finasteride) is utilised as a treatment for BPH/BOO. A potential interaction between cGMP and androgen signalling pathways in the ageing prostate was suggested by the association of altered serum testosterone with the induction of prostatic hyperplasia and fibrosis in conjunction with the increase in PDE5 expression in rats [46]. Furthermore, cell-based assays showed small elevations in cGMP enhancing testosterone production while high elevations had an inhibitory effect [47] but our in vivo studies did not find a significant effect of cinaciguat-mediated increases in cGMP on serum testosterone levels in mice (Figure 4L). Based on our findings, we can rule out the effect of androgen status for eliciting the therapeutic effects of cinaciguat on prostatic hyperplasia.
In summary, these data support that a single daily dose of cinaciguat over two weeks improves bladder function without adversely affecting BP. Since sGC activators can override the oxidative stress mediated inactivation of sGC and deficit in cGMP production, they may be a potential therapeutic option for BPH/BOO patients refractory to standard treatments including inhibitors of PDE5.
Acknowledgments
This research was funded by grants from: National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) R01DK098361 to A. Kanai, C. Fry, M. Drake; R01DK071085 to A. Kanai; National Institute on Aging (NIA) R01AG056944 to L. Birder; NIA R21AG062971 to P. Tyagi. National Heart Lung, and Blood Institute (NHLBI) R01HL128304, R01HL133864 and American Heart Association (AHA) Established Investigator Grant 19E1A34770095 to A. Straub.
Footnotes
Conflicts of Interest: M. Drake (speaker engagements for Astellas, Ferring and Sun Pharma). A. Straub (grant recipient, Bayer AG). P. Sandner (employee, Bayer AG). No other conflicts of interest were declared
References
- 1.Rodriguez-Nieves JA, Macoska JA. Prostatic fibrosis, lower urinary tract symptoms, and BPH. Nat Rev Urol 2013; 10: 546–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Osman N, Chapple C. In: Overactive bladder: practical management, Chapter 19: Bladder outlet obstruction and the overactive bladder. Crocos J, MacDiarmid S, Heesakkers J, (ed)^(eds). John Wiley & Sons, 2015; 222–232. [Google Scholar]
- 3.Hood B, Andersson KE. Common theme for drugs effective in overactive bladder treatment: inhibition of afferent signaling from the bladder. Int J Urol 2013; 20: 21–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Serati M, Andersson KE, Dmochowski R, et al. Systematic Review of Combination Drug Therapy for Non-neurogenic Lower Urinary Tract Symptoms. Eur Urol 2019; 75: 129–168. [DOI] [PubMed] [Google Scholar]
- 5.Gacci M, Andersson KE, Chapple C, et al. Latest Evidence on the Use of Phosphodiesterase Type 5 Inhibitors for the Treatment of Lower Urinary Tract Symptoms Secondary to Benign Prostatic Hyperplasia. Eur Urol 2016; 70: 124–133. [DOI] [PubMed] [Google Scholar]
- 6.Andersson KE, Uckert S, Stief C, et al. Phosphodiesterases (PDEs) and PDE inhibitors for treatment of LUTS. Neurourol Urodyn 2007; 26: 928–933. [DOI] [PubMed] [Google Scholar]
- 7.Truss MC, Stief CG, Uckert S, et al. Phosphodiesterase 1 inhibition in the treatment of lower urinary tract dysfunction: from bench to bedside. World J Urol 2001; 19: 344–350. [DOI] [PubMed] [Google Scholar]
- 8.Qiu Y, Kraft P, Craig EC, et al. Cyclic nucleotide phosphodiesterases in rabbit detrusor smooth muscle. Urology 2002; 59: 145–149. [DOI] [PubMed] [Google Scholar]
- 9.Fujiwara M, Andersson K, Persson K. Nitric oxide-induced cGMP accumulation in the mouse bladder is not related to smooth muscle relaxation. Eur J Pharmacol 2000; 401: 241–250. [DOI] [PubMed] [Google Scholar]
- 10.Moon A Influence of nitric oxide signalling pathways on pre-contracted human detrusor smooth muscle in vitro. BJU Int 2002; 89: 942–949. [DOI] [PubMed] [Google Scholar]
- 11.Andersson KE. PDE5 inhibitors - pharmacology and clinical applications 20 years after sildenafil discovery. Br J Pharmacol 2018; 175: 2554–2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Behr-Roussel D, Oger S, Caisey S, et al. Vardenafil decreases bladder afferent nerve activity in unanesthetized, decerebrate, spinal cord-injured rats. Eur Urol 2011; 59: 272–279. [DOI] [PubMed] [Google Scholar]
- 13.Beamon CR, Mazar C, Salkini MW, et al. The effect of sildenafil citrate on bladder outlet obstruction: a mouse model. BJU Int 2009; 104: 252–256. [DOI] [PubMed] [Google Scholar]
- 14.Matsumoto S, Hanai T, Uemura H. Chronic treatment with a PDE5 inhibitor increases contractile force of normal bladder in rats. Int Urol Nephrol 2010; 42: 53–56. [DOI] [PubMed] [Google Scholar]
- 15.De Nunzio C, Roehrborn CG, Andersson KE, et al. Erectile Dysfunction and Lower Urinary Tract Symptoms. Eur Urol Focus 2017; 3: 352–363. [DOI] [PubMed] [Google Scholar]
- 16.Durgin BG, Hahn SA, Schmidt HM, et al. Loss of smooth muscle CYB5R3 amplifies angiotensin II-induced hypertension by increasing sGC heme oxidation. JCI Insight 2019; 4: e129183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fan J, Du W, Kim-Muller JY, et al. Cyb5r3 links FoxO1-dependent mitochondrial dysfunction with beta-cell failure. Mol Metab 2020; 34: 97–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schmidt HH, Schmidt PM, Stasch JP. NO- and haem-independent soluble guanylate cyclase activators. Handb Exp Pharmacol 2009: 309–339. [DOI] [PubMed] [Google Scholar]
- 19.Knorr A, Hirth-Dietrich C, Alonso-Alija C, et al. Nitric oxide-independent activation of soluble guanylate cyclase by BAY 60–2770 in experimental liver fibrosis. Arzneimittelforschung 2008; 58: 71–80. [DOI] [PubMed] [Google Scholar]
- 20.Calmasini FB, Alexandre EC, Silva FH, et al. Soluble Guanylate Cyclase Modulators, BAY 41–2272 and BAY 60–2770, Inhibit Human and Rabbit Prostate Contractility. Urology 2016; 94: 312 e319–312 e315. [DOI] [PubMed] [Google Scholar]
- 21.Fullhase C, Hennenberg M, Sandner P, et al. Reduction of obstruction related bladder overactivity by the guanylyl cyclase modulators BAY 41–2272 and BAY 60–2770 alone or in combination with a phosphodiesterase type 5 inhibitor. Neurourol Urodyn 2015; 34: 787–793. [DOI] [PubMed] [Google Scholar]
- 22.de Oliveira MG, Calmasini FB, Alexandre EC, et al. Activation of soluble guanylyl cyclase by BAY 58–2667 improves bladder function in cyclophosphamide-induced cystitis in mice. Am J Physiol Renal Physiol 2016; 311: F85–93. [DOI] [PubMed] [Google Scholar]
- 23.Liu CM, Lo YC, Wu BN, et al. cGMP-enhancing- and alpha1A/alpha1D-adrenoceptor blockade-derived inhibition of Rho-kinase by KMUP-1 provides optimal prostate relaxation and epithelial cell anti-proliferation efficacy. Prostate 2007; 67: 1397–1410. [DOI] [PubMed] [Google Scholar]
- 24.Sandner P, Stasch JP. Anti-fibrotic effects of soluble guanylate cyclase stimulators and activators: A review of the preclinical evidence. Respir Med 2017; 122 Suppl 1: S1–S9. [DOI] [PubMed] [Google Scholar]
- 25.Chughtai B, Forde JC, Thomas DD, et al. Benign prostatic hyperplasia. Nat Rev Dis Primers 2016; 2: 16031. [DOI] [PubMed] [Google Scholar]
- 26.Flurkey K, Currer J, Harrison D. The mouse in aging research. In: The mouse in biomedical research, American College of Laboratory Animal Medicine series. 2nd ed. Fox JG, (ed)^(eds). Elsevier, AP: Amsterdam; Boston, 2007; 637–672. [Google Scholar]
- 27.Ikeda Y, Zabbarova IV, Birder LA, et al. Relaxin-2 therapy reverses radiation-induced fibrosis and restores bladder function in mice. Neurourol Urodyn 2018; 37: 2441–2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Crowe AR, Yue W. Semi-quantitative Determination of Protein Expression using Immunohistochemistry Staining and Analysis: An Integrated Protocol. Bio Protoc 2019; 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Andersson KE. Storage and voiding symptoms: pathophysiologic aspects. Urology 2003; 62: 3–10. [DOI] [PubMed] [Google Scholar]
- 30.Chapple CR, Wein AJ, Abrams P, et al. Lower urinary tract symptoms revisited: a broader clinical perspective. Eur Urol 2008; 54: 563–569. [DOI] [PubMed] [Google Scholar]
- 31.Roosen A, Chapple CR, Dmochowski RR, et al. A refocus on the bladder as the originator of storage lower urinary tract symptoms: a systematic review of the latest literature. Eur Urol 2009; 56: 810–819. [DOI] [PubMed] [Google Scholar]
- 32.Chalfin SA, Bradley WE. The etiology of detrusor hyperreflexia in patients with infravesical obstruction. J Urol 1982; 127: 938–942. [DOI] [PubMed] [Google Scholar]
- 33.Lee S, Yang G, Xiang W, et al. Retrograde double-labeling demonstrates convergent afferent innervation of the prostate and bladder. Prostate 2016; 76: 767–775. [DOI] [PubMed] [Google Scholar]
- 34.Funahashi Y, Takahashi R, Mizoguchi S, et al. Bladder overactivity and afferent hyperexcitability induced by prostate-to-bladder cross-sensitization in rats with prostatic inflammation. J Physiol 2019; 597: 2063–2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Monica FZ, Antunes E. Stimulators and activators of soluble guanylate cyclase for urogenital disorders. Nat Rev Urol 2018; 15: 42–54. [DOI] [PubMed] [Google Scholar]
- 36.Lies B, Groneberg D, Friebe A. Correlation of cellular expression with function of NO-sensitive guanylyl cyclase in the murine lower urinary tract. J Physiol 2013; 591: 5365–5375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Persson K, Pandita RK, Aszodi A, et al. Functional characteristics of urinary tract smooth muscles in mice lacking cGMP protein kinase type I. Am J Physiol Regul Integr Comp Physiol 2000; 279: R1112–1120. [DOI] [PubMed] [Google Scholar]
- 38.Vignozzi L, Gacci M, Cellai I, et al. PDE5 inhibitors blunt inflammation in human BPH: a potential mechanism of action for PDE5 inhibitors in LUTS. Prostate 2013; 73: 1391–1402. [DOI] [PubMed] [Google Scholar]
- 39.Jin S, Xiang P, Liu J, et al. Activation of cGMP/PKG/p65 signaling associated with PDE5-Is downregulates CCL5 secretion by CD8 (+) T cells in benign prostatic hyperplasia. Prostate 2019; 79: 909–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang Y, Hu S, Liu J, et al. CD8+ T cells promote proliferation of benign prostatic hyperplasia epithelial cells under low androgen level via modulation of CCL5/STAT5/CCND1 signaling pathway. Sci Rep 2017; 7: 42893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sandner P, Berger P, Zenzmaier C. The Potential of sGC Modulators for the Treatment of Age-Related Fibrosis: A Mini-Review. Gerontology 2017; 63: 216–227. [DOI] [PubMed] [Google Scholar]
- 42.Zenzmaier C, Sampson N, Pernkopf D, et al. Attenuated proliferation and trans-differentiation of prostatic stromal cells indicate suitability of phosphodiesterase type 5 inhibitors for prevention and treatment of benign prostatic hyperplasia. Endocrinology 2010; 151: 3975–3984. [DOI] [PubMed] [Google Scholar]
- 43.Zenzmaier C, Kern J, Heitz M, et al. Activators and stimulators of soluble guanylate cyclase counteract myofibroblast differentiation of prostatic and dermal stromal cells. Exp Cell Res 2015; 338: 162–169. [DOI] [PubMed] [Google Scholar]
- 44.Wang C, Kemp-Harper BK, Kocan M, et al. The Anti-fibrotic Actions of Relaxin Are Mediated Through a NO-sGC-cGMP-Dependent Pathway in Renal Myofibroblasts In Vitro and Enhanced by the NO Donor, Diethylamine NONOate. Front Pharmacol 2016; 7: 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pereira ML, D’Ancona CA, Rojas-Moscoso JA, et al. Effects of nitric oxide inhibitors in mice with bladder outlet obstruction. Int Braz J Urol 2017; 43: 356–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang W, Zang N, Jiang Y, et al. Upregulation of Phosphodiesterase type 5 in the Hyperplastic Prostate. Sci Rep 2015; 5: 17888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Valenti S, Cuttica CM, Fazzuoli L, et al. Biphasic effect of nitric oxide on testosterone and cyclic GMP production by purified rat Leydig cells cultured in vitro. Int J Androl 1999; 22: 336–341. [DOI] [PubMed] [Google Scholar]


