Summary
Arp2/3 complex nucleates branched actin filaments important for processes like DNA repair, endocytosis and cellular motility. Multiple factors are required to activate branching nucleation by Arp2/3 complex, including a WASP family protein and a pre-existing actin filament. Activation is achieved through two major conformational changes—subunit flattening and movement into the short pitch conformation—that allow the actin related proteins within the complex (Arp2 and Arp3) to mimic filamentous actin subunits, thereby templating new filament assembly. Some models suggest these changes are concerted and stimulated cooperatively by WASP and actin filaments, but how Arp2/3 complex integrates signals from multiple factors to drive switch-like activation of branching nucleation has been unknown. Here we use biochemical assays to show that instead of a concerted mechanism, signal integration by Arp2/3 complex occurs via distinct and unconcerted conformational changes; WASP stimulates the short pitch arrangement of Arp2 and Arp3 while actin filaments trigger a different activation step. An engineered Arp2/3 complex that bypasses the need for WASP but not actin filaments in activation potently assembles isotropic actin networks but fails to assemble sustained force-producing actin networks in bead motility assays. The engineered complex, which is crosslinked into the short pitch conformation, fails to target nucleation to the surface of the bead, creating unproductive branching events that deplete unpolymerized actin and halt assembly. Together our data demonstrate the requirement for multi-factor signal integration by Arp2/3 complex and highlight the importance of both the WASP- and actin filament-mediated activation steps in assembly of functional actin networks.
eTOC Blurb
Narvaez et al. show that the activating conformational changes in Arp2/3 complex triggered by WASP and actin filaments are distinct and unconcerted. By conformationally locking the complex into an intermediate state, they show that the WASP-mediated activation step is required for the assembly of sustained, force-producing actin networks.
Introduction
Arp2/3 complex is an important actin cytoskeleton regulator that nucleates new actin filaments in response to cellular signals, thus providing cells with spatial and temporal control over actin network assembly. The nucleation activity of Arp2/3 complex must be triggered by one of multiple classes of activator proteins called Nucleation Promoting Factors (NPFs)1. Multiple classes of NPFs have been reported, including the WASP family proteins (also called type I NPFs), cortactin and other type II NPFs, and the WISH/DIP/SPIN90 (WDS) family proteins2,3. WASP proteins represent the largest family of NPFs4, and are characterized by the presence of C-terminal actin monomer and Arp2/3 complex binding segments called V and CA, respectively5. Importantly, when Arp2/3 complex is activated by WASP it nucleates exclusively branched instead of linear actin filaments6,7. By regulating the branching nucleation activity of Arp2/3 complex, WASP family proteins control the assembly of dendritic actin networks critical for diverse cellular processes, ranging from DNA repair to endocytosis and cellular motility1,2,4,8.
Multiple factors are required to trigger WASP-mediated branching nucleation by Arp2/3 complex. In addition to WASP, which binds at two sites on Arp2/3 complex9-12, actin monomers must be directly tethered by WASP and recruited to the complex for activation13,14. An actin filament is also required for activation, because only once bound to the side of a preexisting actin filament can WASP-bound Arp2/3 complex nucleate a new filament7,15. Together, these activating signals make a logical AND gate, in which nucleation is triggered only when all activating factors are present. Integration of multiple signals by Arp2/3 complex—each required for activation—is thought to be critical for cells to properly regulate branched actin assembly. For instance, by responding to cellular signaling pathways, WASP provides temporal and spatial control over activation of Arp2/3 complex16. The requirement for a pre-existing actin filament ensures WASP-mediated activation of Arp2/3 complex creates branches7. Lastly, the requirement for WASP-recruited actin monomers means WASP-bound Arp2/3 complex competes with existing filament ends for free monomers; this competition is thought to regulate the density of new filament ends nucleated within assembling actin networks17.
Activation of Arp2/3 complex requires two major structural changes that allow the actin-related proteins in the complex, Arp2 and Arp3, to mimic a filamentous actin dimer, thereby templating polymerization of the new filament18-20,20-22. First, the structural clamp (subunits ARPC2 and ARPC4) that holds Arp2 and Arp3 twists, rotating a block of subunits—including Arp2—into a new position22. This rotation moves Arp2 and Arp3 into an arrangement that mimics an actin dimer along the short pitch helical axis of an actin filament. Second, an intrasubunit conformational change called flattening converts Arp2 and Arp3 from a conformation that mimics an unpolymerized actin monomer to one that mimics a filamentous actin subunit23-25. How each activating factor contributes to these structural changes to trigger switch-like activation of Arp2/3 complex has been an important open question. One model posits that subunit flattening and movement into the short pitch conformation are concerted structural changes cooperatively stimulated by WASP and actin filaments. In this model, inactive and fully active states are the only populated conformations (Figure 1). Activation via the concerted model would require high cooperativity between WASP and actin filaments26, because biochemical experiments show these factors predominantly turn on nucleation in an all or nothing (switch-like) fashion rather than incrementally in a rheostat-like fashion7. While some evidence suggests WASP and actin filaments bind cooperatively9, other experiments argue against a concerted model. For instance, FRET experiments suggested WASP and actin filaments may stimulate distinct structural changes in the complex19. Furthermore, engineered crosslinking experiments show that WASP and WASP-recruited actin monomers can stimulate the short pitch conformational change even without actin filaments12,18. These results suggest that the switch-like activation of Arp2/3 complex may not be the consequence of a highly cooperative set of concerted conformational changes, and leaves open the question of how Arp2/3 complex integrates activating signals from both WASP and actin filaments. Other models—including a unconcerted model in which actin filaments and WASP stimulate distinct conformational changes—have been proposed based on structural arguments27, but neither this nor the concerted model have been directly challenged.
Figure 1: Concerted model for activation of Arp2/3 complex by WASP and actin filaments.
In the concerted model, movement into the short pitch conformation (splayed → short pitch) and flattening of the actin related subunits (twisted → flattened) are concerted conformational changes. Both WASP and actin filaments stimulate movement into the active (short pitch, flattened) conformation, and switch like activity is driven by high cooperativity between WASP and filaments
Here we use crosslinking assays with a dual cysteine engineered Arp2/3 complex to test the concerted model for multi-factor signal integration by Arp2/3 complex. We demonstrate that unlike WASP, actin filaments do not stimulate the short pitch conformational switch, ruling out the concerted model for activation. The crosslinked Arp2/3 complex bypasses the requirement for WASP but not actin filaments in activation, showing that actin filaments trigger an activation step distinct from WASP. While the crosslinked complex potently nucleates isotropic actin networks, it fails to assemble sustained force-producing actin networks in a reconstituted motility assay. Therefore, the distinct activating steps stimulated by WASP versus actin filaments are both required to properly regulate Arp2/3 complex to assemble functional actin networks. These observations provide a foundation to understand how Arp2/3 complex integrates multiple activating signals to properly control the timing, localization and architectures of branched actin networks.
Results
Actin filaments stimulate the short pitch conformational change weakly or not at all
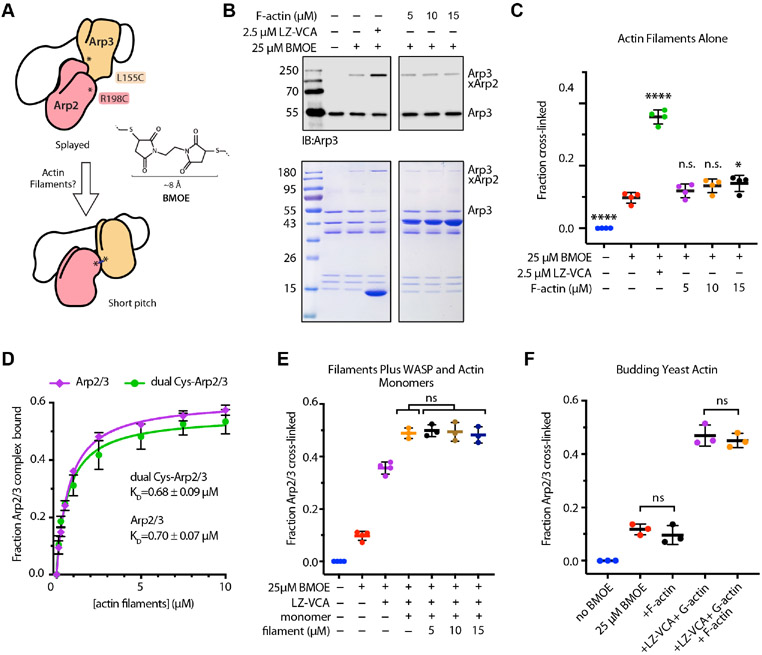
Biochemical tools to probe the two major activating conformational changes in Arp2/3 complex are critical to dissect the mechanism of multi-factor signal integration. While no tools currently exist to probe subunit flattening in Arp2 and Arp3, an assay was previously developed in which engineered cysteines on Arp2 and Arp3 can be crosslinked by a bis-maleimide crosslinker (BMOE) only when the complex is in the short pitch conformation (Figure 2A)18,28. Previous experiments used this assay to show that WASP on its own can stimulate the short pitch conformation12,18,28,29. However, a recent cryoEM structure suggests that even with bound WASP, the majority of Arp2/3 complexes adopt the inactive (splayed and twisted) conformation, indicating that WASP alone only weakly drives activating conformational changes12. Therefore, it is possible that switch-like activation of Arp2/3 complex is triggered by potent and concerted stimulation of both the short pitch conformation and subunit flattening driven by cooperative binding of WASP and actin filaments (Figure 1). To challenge this model, we used the short pitch crosslinking assay to investigate the role of actin filaments in stimulating the short pitch conformation. We first asked whether actin filaments alone influenced short pitch crosslinking. In these assays, incubation of the dual cysteine-engineered Saccharomyces cerevisiae Arp2/3 complex (ScArp2/3) with BMOE created short pitch crosslinks in ~9 % of the complex, consistent with previous results reported for the budding yeast complex18,29 (Figure 2B,C). These reactions lacked WASP, so ScArp2/3 complex at least weakly adopts the short pitch conformation even in the absence of WASP18,29. Adding a dimeric construct of the N-WASP VCA segment (LZ-VCA) increased formation of the short pitch crosslinks ~5 fold over reactions without LZ-VCA, as expected based on previous reports12,18,29. In contrast, incubation of Arp2/3 complex with preformed actin filaments showed an almost indetectable increase in the degree of short pitch crosslinking. This increase was statistically significant only at the highest concentration of actin filaments tested, 15 μM (Figure 2C). These data suggest that, at least on their own, actin filaments do not potently stimulate the short pitch conformation and in comparison to WASP, have a negligible influence on this conformational change.
Figure 2: Actin filaments stimulate the short pitch conformation weakly or not at all.
A. Cartoon depicting the short pitch crosslinking assay used to determine if actin filaments stimulate the short pitch conformation. BMOE (bismaleimidoethane) has a spacer arm of 8 Å. B. Analysis of crosslinking reactions by western blot with an anti-Arp3 antibody (top) or by Coomassie-staining of SDS-PAGE gels (bottom). Crosslinking reactions contained 1 μM ScArp2/3 dual cysteine complex with or without BMOE, LZ-VCA (leucine zipper N-WASP-VCA) or F-actin, as indicated. Reactions were quenched after 1 min for analysis. C. Quantification of the fraction of Arp2/3 complex with the short pitch crosslink for reaction conditions described in B. For all panels in this figure, bars represent the mean and standard deviation. n.s.: not significant, * p<0.05, ** p<0.005, **** p<0.0001 for sample compared with the reaction containing just BMOE. D. Binding isotherm showing fraction of Arp2/3 complex (45 nM total) bound versus concentration of actin filaments in copelleting assays. Each point indicates the mean (n = 5). Error bars: standard deviation. Data were fit as described in methods. We note that the affinity measured in these assays (~0.7 μM) is similar to affinities previously measured for S. cerevisiae, B. taurus and H. sapiens complexes using cosedimentation assays but ~30-400 fold tighter than affinities measured for S. cerevisiae or S. pombe complexes using fluorescence-based methods2,12,28,52. The source of these discrepancies is currently unknown. At saturation, only ~60% of the complex is bound to filaments. While we cannot currently explain this observation, both crosslinked and uncrosslinked complexes eluted from gel filtration columns after the void volume in single gaussian peaks (Figure S7), indicating that the complex is not significantly dissociated or aggregated. E. Quantification of crosslinking reactions containing 1 μM ScArp2/3 dual cysteine complex with or without BMOE, 2.5 μM LZ-VCA (leucine zipper N-WASP-VCA), 5 μM Latrunculin B bound actin monomers and F-actin, as indicated. Reactions were quenched after 1 min for analysis. F. Quantification of crosslinking reactions containing 1 μM ScArp2/3 dual cysteine complex with or without BMOE, 2.5 μM LZ-VCA (leucine zipper N-WASP-VCA), 5 μM Latrunculin B bound budding yeast actin monomers and 15 μM budding yeast F-actin, as indicated. Reactions were quenched after 1 min for analysis. See also Figures S1, S2 and S7.
Because adding actin increases the number of reactive cysteine residues in the reaction— which could sequester BMOE—we also tested the influence of actin filaments in reactions containing higher concentrations (75 μM) of BMOE. Like reactions containing lower concentrations of the crosslinker, these reactions showed that actin filaments only very weakly increase formation of the short pitch crosslinks (Figure S1A). As a second control for BMOE depletion by actin cysteines, we treated filaments with N-ethylmaleimide to block the cysteine that is reactive in folded actin30,31 (Figure S1B). Actin filaments treated with NEM did not stimulate short pitch crosslinking in dual cysteine engineered complex (Fig S1C). To ensure that the failure of actin filaments to stimulate the short pitch conformation was not due to an inability of the dual cysteine engineered Arp2/3 complex to bind filaments, we used an actin filament cosedimentation assay to measure filament binding. We found that the dual cysteine Arp2/3 complex bound actin filaments with an affinity indistinguishable from wild type Arp2/3 complex (~ 0.7 μM) (Figure 2D). In addition, dual Cys and wild-type Arp2/3 complexes had identical activity in pyrene-actin polymerization assays in the presence of the VCA segment of the Saccharomyces cerevisiae WASP protein, Las17, as previously reported (Figure S1D). Therefore, we conclude that actin filaments on their own influence movement of the complex into the short pitch conformation weakly or not at all.
We next asked if we could detect stimulation of the short pitch conformation by actin filaments when Arp2/3 complex is bound to WASP and WASP-recruited actin monomers. We reasoned that in a concerted model for activation, the presence of all activating factors would greatly increase short pitch crosslinking. To test this, we repeated the crosslinking assays, but added phalloidin-stabilized actin filaments to Arp2/3 complex in the presence of both LZ-VCA and actin monomers. While actin monomers and LZ-VCA stimulated short pitch crosslinking more than LZ-VCA alone, as previously reported18,28,29, addition of actin filaments did not further stimulate short pitch crosslinking in any of the conditions we tested (Figures 2E and S2). These data indicate that even in the presence of WASP and WASP-recruited actin monomers, actin filaments do not stimulate formation of the short pitch conformation. Importantly, the results were identical whether rabbit skeletal muscle actin or budding yeast actin was used in the assays (Figure 2F).
Evidence for a distinct activating step triggered by actin filaments
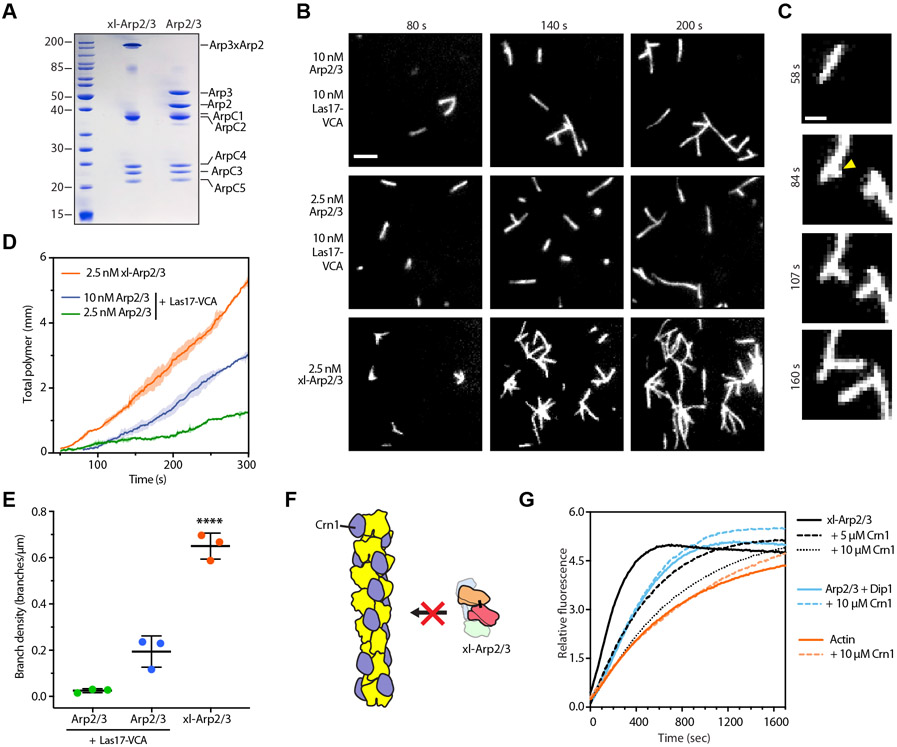
Our data suggest that actin filaments stimulate the short pitch conformation either weakly or not at all, even in the presence of WASP and actin monomers. These data argue against a model in which actin filaments and WASP cooperate to stimulate a concerted set of activating structural changes (Figure 1). Furthermore, these observations suggest that actin filaments trigger an activation step distinct from the stimulation of the short pitch conformation, the structural change triggered by WASP and WASP-recruited actin monomers12,18-20,28,29. To test this, we purified the short pitch crosslinked complex (xl-Arp2/3 complex, Figure 3A) and visualized its activity using total internal reflection fluorescence (TIRF) microscopy with Oregon green 488-labeled actin. We reasoned that if actin filaments trigger a distinct activation step, nucleation will require preformed actin filaments so, consequently, xl-Arp2/3 complex will create branches. Alternatively, if xl-Arp2/3 complex is not activated by preformed actin filaments, it will nucleate linear actin filaments.
Figure 3: Pre-existing actin filaments activate the short pitch crosslinked Arp2/3 complex.
A. Coomassie-stained SDS-PAGE gel of purified uncrosslinked and crosslinked Arp2/3 complexes. B. TIRF microscopy images showing polymerization of 1.5 μM actin (33% 488 Oregon green labeled) in the presence of 2.5 nM or 10 nM uncrosslinked Arp2/3 complex with 10 nM Las17-VCA or 2.5 nM purified crosslinked complex without Las17-VCA. Scale bar represents 3 μm. C. TIRF microscopy images with purified crosslinked complex showing branching from a pre-existing filament. Conditions are as described in panel B. Scale bar: 1 μm D. Plot of total polymer in field of view versus time for TIRF microscopy reactions represented in panel B. Shaded areas represent standard deviation (n=3 independent videos). E. Branch density vs time for TIRF data from panel B. Error bars represent the standard deviation of the mean from 3 separate reactions. F. Schematic showing Crn1 potentially inhibiting xl-Arp2/3 complex by preventing it from binding to actin filament sides. G. Polymerization time course of 2 μM actin monomers (15% pyrene-labeled) with 0.15 μM pre-polymerized actin (F-actin), 2.5 nM crosslinked Arp2/3 complex, and coronin (Crn1), as indicated. Control reactions contained actin monomers with 20 nM uncrosslinked Arp2/3 complex and 1 μM GST-Dip1 in absence or presence of Crn1 or actin monomers with 0.15 μM F-actin with or without Crn1. See also Video S1.
Examination of the TIRF microscopy videos showed that, as previously reported, xl-Arp2/3 complex has potent NPF-independent activity18 (Figure 3B-D). Importantly, new actin filaments nucleated from the side of pre-existing filaments, indicating that xl-Arp2/3 complex is activated by binding to actin filament sides (Figure 3B-C, Video S1). To ensure that branches did not arise from filaments nucleated in solution that rapidly attached to the side of a pre-existing filament, we tested the ability of the actin filament binding protein Crn1 to inhibit xl-Arp2/3 complex in a pyrene actin polymerization assay. Previous work showed that by binding to actin filament sides, Crn1 blocks Arp2/3 complex from interacting with filaments32. Therefore, we reasoned that if actin filaments are important for activation, Crn1 will inhibit xl-Arp2/3 complex (Figure 3F). Consistent with this prediction, Crn1 potently decreased actin filament assembly in pyrene actin assembly assays containing xl-Arp2/3 complex (Figure 3G). In contrast, Crn1 did not influence activation of (uncrosslinked) Arp2/3 complex by Dip1, an NPF that activates Arp2/3 complex without a pre-existing filament3.
Our data support the existence of two separate Arp2/3 complex activation steps that produce multiple unconcerted conformational changes along the activation pathway: (1) stimulation of the short pitch conformation by WASP and WASP-recruited monomers, and (2) stimulation of an additional distinct but required activating step by actin filaments (Figure 4A). We next sought to test predictions of this uncoupled activation model in the context of NPF proteins. Previous data showed that the WDS protein Dip1 activates Arp2/3 complex without pre-existing filaments, so it must stimulate both the short pitch conformation and the activating step(s) driven by actin filaments3 (Figure 4A). Therefore, we predicted that like actin filaments, Dip1 would activate xl-Arp2/3 complex. Consistent with this hypothesis, Dip1 weakly increased the actin polymerization rate in reactions containing xl-Arp2/3 complex (Figure 4B). This effect was small, likely because xl-Arp2/3 complex rapidly nucleates branches off of spontaneously nucleated actin filaments, creating a positive feedback loop that creates more actin filaments that further accelerate branching nucleation. To reduce activation of xl-Arp2/3 complex by actin filaments, we added Crn1 to block the complex from binding filament sides, as described above. The fluorescence time courses in these assays clearly showed potent activation of xl-Arp2/3 complex by Dip1 (Figure 4C), indicating that Dip1, like actin filaments, stimulates an activating conformational change other than the short pitch conformational switch.
Figure 4: Influence of Las17 and Dip1 on the activity of short pitch crosslinked Arp2/3 complex.
A. Schematic of an activation model that depends on multiple unconcerted conformational changes. The second activation step may involve subunit flattening in Arp2 and Arp3 (see discussion). Note that the depicted activation mechanism could also occur with the complex binding to actin filaments first. B. Time courses of pyrene actin polymerization for reactions containing 3 μM actin monomers (15% pyrene-labeled), 1 nM crosslinked ScArp2/3 complex and the indicated concentrations of Dip1. Inset: First 200 sec of the reaction. C. Time courses of pyrene actin polymerization for reactions containing 2 μM actin monomers (15% pyrene-labeled), 0.15 μM actin filaments, 2.5 nM crosslinked ScArp2/3 complex, 10 μM Crn1, and Dip1, as indicated or 250 nM Las17-VCA. D. Time courses of pyrene actin polymerization for reactions containing 3 μM actin monomers (15% pyrene-labeled), 1 nM crosslinked ScArp2/3 complex and the indicated concentrations of Las17-VCA. See also Figure S3.
We previously showed that Las17 stimulates the short pitch conformation, and driving this conformational change is its main role during activation of budding yeast Arp2/3 complex18,29. Here we show that even when locked into the short conformation, Arp2/3 complex requires preformed actin filaments for activation. Therefore, we predicted that addition of Las17-VCA to xl-Arp2/3 complex would not activate it. Consistent with this prediction, Las17-VCA concentrations up to 1 μM had no influence on the activity of the short pitch crosslinked complex (Figure 4D). Higher concentrations slowed actin polymerization, likely by either inhibiting spontaneous nucleation or by directly inhibiting the activity of the short pitch crosslinked complex18 (Figure S3). Together, our experiments confirm the existence of an activation step other than the short pitch conformational switch that can be triggered both by actin filaments and the NPF Dip1, but not by Las17.
The short pitch crosslinked complex fails to support sustained assembly of force-producing actin networks
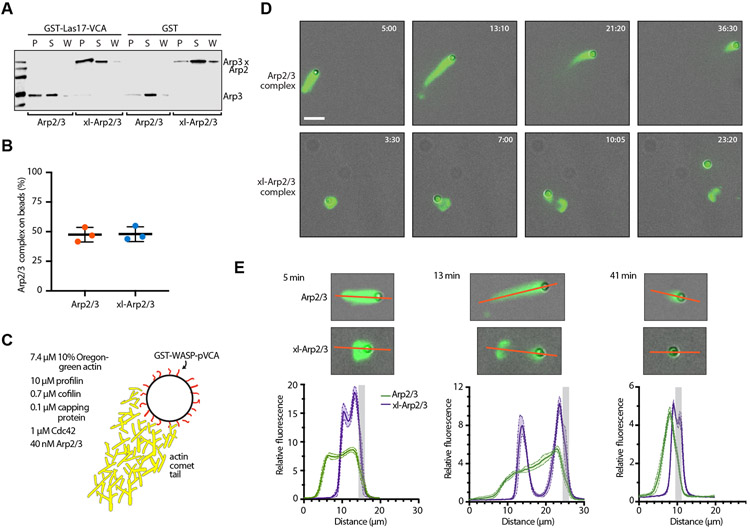
Our data show that crosslinking Arp2/3 complex into the short pitch conformation disrupts the multi-factor signal integration mechanism; specifically, it eliminates the requirement for WASP so that the nucleation activity of xl-Arp2/3 complex is controlled by actin filaments alone (Figure 4A, step 2). However, xl-Arp2/3 complex is still able to potently nucleate isotropic branched actin networks (Figure 3). Therefore, we wondered if the activation step triggered by actin filaments is also sufficient to allow xl-Arp2/3 complex to assemble a polarized force-producing actin network or, alternatively, if the WASP-mediated activation step (Figure 4A, step 1) is also required. Because WASP proteins are activated at membranes4, they help restrict nucleation to a region near the membrane surface. Limiting nucleation to the surface is thought to be important for assembling actin networks that effectively push against membranes, but this has not been directly tested33. Furthermore, it is possible that the WASP-mediated activation step is not absolutely required to restrict branching nucleation to the surface; WASP-mediated binding (Figure 5A,B) and recruitment of xl-Arp2/3 complex to a surface coupled with the filament-mediated activation step might be sufficient to localize most nucleation events to the surface. To address this question, we used a previously described reconstituted actin-based motility assay34. We coated polystyrene beads with GST-WASP-pVCA and added them to motility mixtures containing optimal concentrations of Oregon green 488 actin, profilin, cofilin, capping protein, Cdc42 and crosslinked or uncrosslinked Arp2/3 complex (Figures 5C and S4A).
Figure 5: ScArp2/3 complex crosslinked into the short pitch conformation cannot support sustained actin-based motility in vitro.
A. Anti-Arp3 western blot showing results of a GST pull-down assay. GST-Las17-VCA (330 μM) on glutathione agarose beads was used to pull down 1 μM crosslinked or uncrosslinked Arp2/3 complex. P: pellet, S: supernatant, W: wash. B. Quantification by enzyme-linked immunosorbent assay of GST pulldown assays described in panel A. C. Schematic of bead motility assay that lists optimal concentrations of proteins in motility mix. D. Time lapse images of bead motility assays with fluorescence and phase contrast imaging modes overlaid so both the GST-WASP-pVCA-coated bead and polymerized actin networks are visible. Scale bar: 10 μm E. Plots of fluorescence intensity of the Oregon green 488 labeled actin networks assembled by crosslinked and uncrosslinked Arp2/3 complex in the bead motility assays. Fluorescence intensity was measured along the red lines shown in the images and plotted as an arbitrary distance. An estimated bead position is indicated by light gray bands. See also Figure S4 and Video S2.
With optimal concentrations of motility proteins, actin initially polymerizes to form a shell around the beads. The shell then ruptures and a comet tail of actin forms that propels the bead35. Under optimal conditions with uncrosslinked Arp2/3 complex, shell formation and rupture were complete in less than 3 min (Figure 5D-E, Video S2), before we could begin imaging the reactions. The first frames in these videos showed long actin comet tails that propelled the bead forward and grew shorter over the course of the reaction, eventually reaching an average steady state length of 7.1 ± 1.5 μm. The average speed of the beads during the steady state phase was 2.2 ± 0.5 μm/min (Figure S4B). These results reveal a set of in vitro conditions under which the uncrosslinked budding yeast Arp2/3 complex can trigger assembly of force-producing actin comet tails.
When we repeated the assays with xl-Arp2/3 complex, we found that shell formation, rupture and assembly of the polarized comet tail occurred more slowly, and the bead could be observed attached to the ruptured shell in the first images taken (Figure 5D). A short tail grew and pushed the bead out from the shell, showing that the xl-Arp2/3 complex could—at least briefly—assembly a force-producing actin network (Figure 5D,E, Video S3). However, tail growth stopped after a few minutes, at which point the bead, which was still coated with actin, released from the shell (Figure 5D, Video S3). Unlike reactions with uncrosslinked Arp2/3 complex, the actin shell in the reactions containing xl-Arp2/3 complex failed to disassemble during the 1 hr imaging period. The signal of Oregon-green actin on the beads was more intense in the reactions with xl-Arp2/3 complex at early stages in the reaction, before the bead was released (Figure 5E). This suggests that xl-Arp2/3 complex initially assembles a denser actin network than uncrosslinked Arp2/3 complex. In contrast, the actin network nucleated by xl-Arp2/3 complex at late stages of the reaction, when the bead was released, had an intensity similar to the network assembled around beads by the uncrosslinked complex (Figure 5E). Together these observations indicate that the xl-Arp2/3 complex can initially assemble a force-producing actin network, but it fails to sustain comet tail assembly and motility. Therefore, the activating steps stimulated by WASP and actin filaments are both required for sustained assembly of a force-producing actin network.
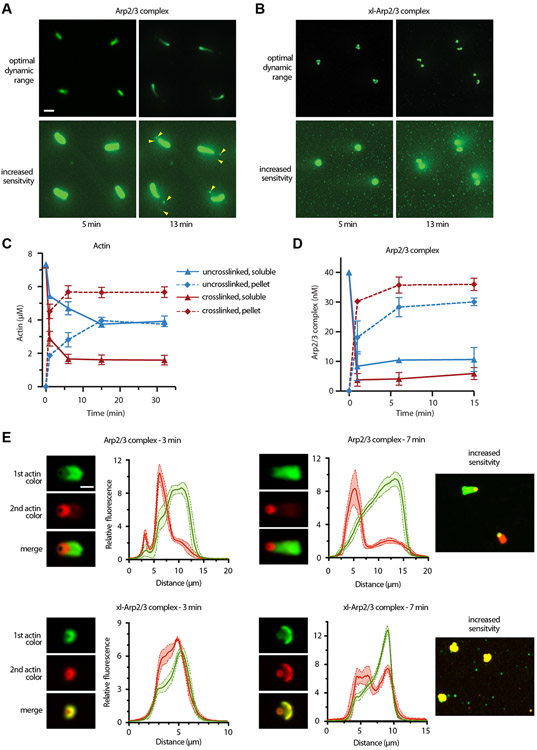
We next asked why short pitch crosslinked Arp2/3 complex could not assemble actin networks that supported sustained bead motility. Close examination of the videos provided a clue: reactions in the presence of xl-Arp2/3 complex contained many bright fluorescent puncta not connected to the beads (Figure 6A,B; Video S4). These puncta likely represent filamentous actin networks that nucleated and elongated in solution rather than on the surface of the bead, and were not present in reactions with uncrosslinked Arp2/3 complex (Figure 6A,B; Video S4). Incorporation of actin monomers and Arp2/3 complex into these detached actin networks could reduce the steady state concentration of these proteins in solution, and might explain why tail elongation eventually stops in reactions containing xl-Arp2/3 complex. To test this, we used a cocktail of phalloidin and Latrunculin B to arrest the motility reactions at various time points35, spun the reaction to pellet actin filaments, and compared the amount of soluble to pelleted actin. We found that in reactions with uncrosslinked complex, the concentration of soluble actin monomers decreased hyperbolically after the reaction was initiated, reaching a steady state of ~4 μM after about 15 min (Figure 6C). In reactions containing xl-Arp2/3 complex, the concentration of soluble actin monomers decreased more rapidly and reached a lower steady state concentration (1.8 μM) than the uncrosslinked complex (Figure 6C). Crosslinking Arp2/3 complex also increased its incorporation into actin networks; only 5 nM crosslinked Arp2/3 complex remained soluble at steady state, about half of the amount of uncrosslinked complex (Figure 6D).
Figure 6: Activation of Arp2/3 complex away from the bead surface decreases steady state soluble concentrations of actin monomers and Arp2/3 complex.
A. Time lapse images of bead motility assays with uncrosslinked Arp2/3 complex with fluorescence displayed at two different sensitivity levels. Yellow arrowheads show remnants of actin shell at 13 min. Scale bar: 15 μm. B. Time lapse images of bead motility assays with crosslinked Arp2/3 complex with fluorescence displayed at two different sensitivity levels. C. Quantification of soluble (monomeric) and pelleted (filamentous) actin versus time in the bead motility assays. Reactions were arrested by the addition of Latrunculin B and phalloidin and fractionated by centrifugation before analysis by immunoblot using anti-Actin antibodies. Data points show mean (n=3), error bars represent standard deviation. D. Quantification of soluble and pelleted Arp2/3 complex versus time in the bead motility assays as described in panel C. An anti-Arp3 antibody was used to detect Arp2/3 complex. E. Fluorescence images showing representative actin comet tails assembled on 2 μm GST-WASP-pVCA-coated beads in the presence of uncrosslinked or crosslinked Arp2/3 complex. Two-independent bead motility reactions were started at the same time, one containing 10% OG-labeled actin and the other containing 5% Alexa 647-labeled actin. After time indicated, a 1:1 mixture of the two reactions was prepared, incubated for 90 sec and quenched by the addition of Latrunculin B and phalloidin. Fluorescence intensity profiles of the Oregon green 488 and 647 labeled actin networks were obtained by drawing a horizontal line across the bead and tail. See also Figures S5 and S6 and Videos S2 and S3.
To provide additional evidence that depletion of actin and/or Arp2/3 complex stops motility, we ran a new set of bead motility assays with xl-Arp2/3, removing and fixing samples with phalloidin and Latrunculin B every 1 min for analysis. After 10 min, when motility was stopped and the bead was (typically) released from the comet tail, we spiked the reaction mix with fresh solution containing unpolymerized actin plus all other motility components (except for WASP-coated beads). Analysis of the reactions post-spiking showed that new actin assembled around the beads, a short tail grew, and motility was briefly restored (Figure S5). These results support a model in which actin and/or Arp2/3 complex depletion cause motility to stop in reactions with xl-Arp2/3 complex.
The presence of actin networks in solution (not attached to the bead) suggests that xl-Arp2/3 complex may also nucleate branches within the tail away from the surface of the bead, i.e., the activity of xl-Arp2/3 complex may not be restricted to the bead surface. To test this, we used a two color bead motility assay in which old and newly incorporated actin subunits were labeled with different fluorophores. Two identical reactions were initiated, each with a different label on actin (Oregon Green-488 or Alexa 647). After an incubation period the two reactions were mixed, and polymerization was allowed to proceed for an additional 90 seconds. In reactions with uncrosslinked Arp2/3 complex, new actin was incorporated near the surface of the bead (Figure 6E), as expected35, demonstrating spatial restriction of branching nucleation to the surface. In contrast, in reactions containing xl-Arp2/3 complex, new actin was incorporated throughout the broken actin shell and the nascent comet tail (Figure 6E). These data demonstrate that the WASP-mediated activation step is required to restrict branching nucleation near the bead surface.
Our data indicate that actin assembly away from the bead surface (either in solution or along the actin comet tail) depletes actin monomers to slow or halt motility. However, decreased rates of filament disassembly in reactions with xl-Arp2/3 complex may also contribute to the reduced steady state concentration of actin monomers. To limit the effect of Arp2/3 complex crosslinking to assembly only, we repeated the assays without cofilin, a protein that promotes actin network disassembly by severing filaments and stimulating dissociation of branches36,37. In reactions with uncrosslinked Arp2/3 complex and no cofilin, comet tails grew approximately 22 μm long over approximately 60 min, with growth slowing almost to a halt by the end of the 60 min imaging period (Figure S6). Consistent with previous studies, the bead-associated actin networks showed no evidence of disassembly; the actin shell remained intact and connected to the bead, with no tail shortening or thinning38. In these reactions, failure to disassemble actin decreases the concentration of actin monomers in solution, eventually causing motility to halt39. The crosslinked Arp2/3 complex showed significant defects compared to the uncrosslinked complex; comet tails grew from beads but extended very slowly, stopping when the average comet tail (plus shell) length was only ~4.5 μm (Figure S6). Careful examination of the videos revealed fluorescent puncta away from the surface of the bead, indicating xl-Arp2/3 fails to target assembly/nucleation to the bead surface. Importantly, motility stopped sooner in reactions with the crosslinked Arp2/3 complex than with the uncrosslinked complex, consistent with more rapid monomer depletion due to untargeted Arp2/3 complex activity (Figure S6). These experiments indicate that even in the absence of actin filament disassembly, the xl-Arp2/3 complex shows major defects in building force-producing actin networks.
Discussion
We show here that actin filaments and WASP stimulate distinct activating steps during branching nucleation, arguing against a concerted model in which switch-like activation is driven by cooperativity between WASP and actin filaments (Figure 1). Instead, our results support a model in which WASP and actin filaments stimulate distinct and unconcerted conformational changes in Arp2/3 complex, each required for activation (Figure 4A). A key advantage of an unconcerted mechanism of multi-factor signal integration is that it can exhibit switch-like behavior across a broad range of concentrations of activating signals26. In contrast, a concerted mechanism would exhibit switch-like behavior only at low concentrations of both activators26. Therefore, the unconcerted mechanism may ensure that Arp2/3 complex activation occurs via a strict “AND gate” mechanism across a broad range of WASP and actin filament concentrations. One disadvantage of the unconcerted mechanism is that full activation is difficult to achieve without high input concentrations26. However, WASP proteins are thought to cluster at high concentrations on membranes (~10,000 molecules/μm2), so are unlikely to limit activation40,41. Similarly, once actin network assembly begins, a high local concentration of actin filaments accumulate near the membrane42, driving activation of membrane-tethered Arp2/3 complexes. It is important to note that the unconcerted model presented here is simplified to address how signals from WASP and actin filaments are integrated by Arp2/3 complex. A complete understanding of the activation process will require biochemical dissection of the multiple levels of signal integration by Arp2/3 complex. This includes not only WASP binding to each of its two sites9,10,12,43, actin monomer recruitment by each WASP molecule5,14, and actin filament binding7, but also ATP binding to the actin-related subunits and in some cases, phosphorylation of Arp2/3 complex44,45.
Our data indicate that the activating conformational changes stimulated by actin filaments and WASP are distinct and unconcerted, but they do not formally rule out the possibility of coupling between these activation steps. If coupling exists, it must be weak because moderate or strong coupling would allow significant activation of the complex in the presence of only one of the activators. Some of our short pitch crosslinking assays showed weak stimulation of the short pitch conformation at the highest concentrations of actin filaments. This trend was not consistent under all assay conditions, but it leaves open the possibility of weak conformational coupling. Weak conformational coupling could also explain previously reported evidence for positive cooperativity between WASP and actin filaments in binding to Arp2/3 complex9,46, though it should be noted that not all measurements support the existence of positive cooperativity9,47. Finally, while the WDS family of NPFs can directly stimulate adoption of the short pitch conformation3, structural data suggests they may rely on weak coupling between the short pitch conformation and the actin filament-stimulated activation step to trigger nucleation without preformed actin filaments22. Determining if these activation steps are coupled will be important for understanding regulation of both branching and linear filament nucleation by Arp2/3 complex and is an important future direction.
The activation step stimulated by actin filaments is critical for the function of Arp2/3 complex because it ensures WASP-mediated activation of Arp2/3 complex creates branches. While additional corroboration is required to verify the nature of this activation step, recent data suggest that actin filaments trigger subunit flattening in the Arps. For instance, recent 9.0 Å cryo-EM structure of Arp2/3 complex at a branch junction reveals contacts between ARPC3 and the mother actin filament that might depend on Arp3 flattening21. In addition, recent Forster Resonance Energy Transfer (FRET) experiments showed actin filaments (but not WASP) increased FRET between probes on ARPC1 and ARPC3, suggesting filaments could stimulate movement the ARPC3 subunit toward ARPC119. Flattening of Arp3 would be expected to cause this movement, because ARPC3 is bound to Arp3, and its relative position within the complex is sensitive to Arp3 flattening48.
Our experiments focused on the budding yeast Arp2/3 complex, but we expect that the unconcerted multi-factor signal integration mechanism—in which actin filaments stimulate a distinct activation step from WASP—in conserved across diverse species. This hypothesis is supported by the high sequence conservation of the surface of actin filaments and of the actin filament binding site on Arp2/3 complex27,49. Furthermore, recent experiments show that WASP on its own (or bound to actin monomers) can trigger population of the short pitch conformation in human Arp2/3 complex12. This suggests that, as in the budding yeast system, cooperative stimulation of the short pitch conformational change by WASP and actin filaments may not be responsible for switch-like activation of human Arp2/3 complex. However, recent data showed potentially important differences among Arp2/3 complexes from different species. For instance, crosslinking the budding yeast complex into the short pitch conformation eliminates the requirement for WASP in activation, indicating that stimulating this conformational change is the sole function of WASP18. In contrast, short pitch crosslinking experiments with human Arp2/3 complex indicate that WASP and WASP-recruited actin monomers must bind to stimulate activating steps other than adoption of the short pitch conformation12. Understanding these mechanistic differences and how they impact signal integration by the complex in distinct cellular contexts is an important future direction.
Here we found that the activation steps triggered by WASP and actin filaments are both required for Arp2/3 complex to generate a sustained force-producing actin network. Specifically, we showed that xl-Arp2/3 complex, which bypasses the need for the WASP-mediated activation step18, nucleates new actin filaments both along the length of actin comet tails and in solution rather than just at the bead surface, thereby depleting actin monomers and stopping bead motility. Unrestricted xl-Arp2/3 complex activity could also more directly influence motility by changing the orientation of branches at the bead surface50. However, if branch orientation defects exist, they slow but do not stop motility, since xl-Arp2/3 supports motility at the start of each reaction (Figure 5), and after spiking in fresh actin and Arp2/3 complex (Figure S5).
Our data indicate that decreased filament depolymerization/disassembly may contribute to the reduced steady state concentration of actin monomers in bead motility assays with cofilin (Figure 6). In reactions containing uncrosslinked Arp2/3 complex, the broken actin shell is rapidly disassembled via the actin severing activity of cofilin. However, the shell remains throughout the entirety of the imaging period (~1 hr) in reactions containing the short pitch crosslinked complex. By continuously nucleating filaments from pre-existing filaments within the shell (Figure 6E,F), the xl-Arp2/3 complex provides a continuous source of branches loaded with ATP or ADP-Pi actin subunits. ATP/ADP-Pi loaded actin filaments are less susceptible to the severing activity of cofilin than ADP bound filaments, explaining the stability of the xl-Arp2/3-nucelated shells51. Hence, by restricting nucleation to the bead surface, the activation step stimulated by WASP (and WASP-recruited actin monomers) permits efficient network recycling by allowing actin networks to segregate based on their age. We note that it is also possible that crosslinking the complex into the short pitch conformation may stabilize branch junctions, providing an additional perturbation in the balance of actin network assembly/disassembly.
STAR Methods
RESOURCE AVAILABILITY
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Brad Nolen (bnolen@uoregon.edu).
Materials availability
This study did not generate new unique reagents.
Data and code availability
Data reported in this paper will be shared by the lead contact upon request.
This paper does not report original code.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Yeast strains
Strains were kept as glycerol frozen stocks (stored at 80 °C). Cells used to express and purify Arp2/3 complex were plated on SD -Leu -Lys plates and grown for 3 days at 30 °C. A single colony was picked to inoculate 5 mL liquid YPAD before growing overnight at 30 °C with shaking. A small sample (1 or 2 mL) of the overnight culture was used to inoculate 100 mL of liquid SD -Leu -Lys media and grown for 4 – 5hrs at 30 °C with shaking to be used as starting culture.
METHOD DETAILS
Protein preparation
Actin was purified from rabbit skeletal muscle and labeled with pyrene iodoacetamide or 488 Oregon green maleimide as previously described53-55. Budding yeast actin was purified using a DNaseI column as described with minor modifications56. Briefly, the strain ScBN283 (pep4::KAN), kindly provided by Tom Stevens, was grown to saturation in YPAD media. Cultures were supplemented with 1 mM PMSF and 2 mM EDTA before harvesting the cells by centrifugation. Cells were homogenized in extraction buffer (10 mM Tris-HCl, pH 7.5, 0.2 mM CaCl2, 0.5 mM ATP, 1 mM DTT) containing a protease inhibitor tablet (complete, Roche) per 50 mL of cell suspension and disrupted in a microfluidizer (Microfluidics Model M-110EH-30 Microfluidizer Processor) at 23 kPSI for 8 to 10 passes. Cell lysate was spun in JLA14 at 13,000 rpm for 25 min, clarified by ultracentrifugation at 215,000 ×g for 2 hrs and filtered through cheesecloth. Clarified lysate was loaded onto a DNaseI affinity column previously assembled by coupling 200 mg of DNaseI (Worthington) with 10 mL Affi-Gel 10X resin (Biorad) and rocked for 2 hrs. The DNaseI affinity column was washed sequentially with G-buffer, G-buffer containing 0.4 M ammonium chloride and G-buffer containing 1M NaCl. Yeast actin was eluted using G-buffer plus 50%formamide and injected on a 6 mL Source30Q column immediately. Protein was eluted with a 50-300 mM gradient of KCl and pooled fractions were dialyzed against G-buffer overnight. Protein was concentrated using a Vivaspin 10K MWCO (Sartorious), quantified by absorbance at 290 (ε290 = 0.63 mL mg−1 cm−1)57 and flash frozen.
Saccharomyces cerevisiae Arp2/3 complex was purified from the ScBN020 and ScBN021 strains using a WASP affinity column, ion exchange, and gel filtration columns. Each liter of YPAD media in a 2.8 L flask was inoculated to OD600 of 0.02 and incubated at 30 °C with shaking to an OD600 of 10 measured in an Agilent 8453 UV-Vis spectrophotometer (Agilent technologies). Cultures were then spiked with 25 g/L fresh YPAD media and grown for 2 additional hours. To prevent protein degradation, 1 mM PMSF and 5 mM EDTA were added before harvesting the cells by centrifugation. All subsequent steps were carried out at 4 °C. Cell pellet was resuspended in lysis buffer (20 mM Tris pH 8.0, 50 mM NaCl, 1 mM EDTA, 1 mM DTT containing a protease inhibitor tablet (complete, Roche) per 50 mL of cell suspension and disrupted in a microfluidizer (Microfluidics Model M-110EH-30 Microfluidizer Processor) at 23 kPSI for 5 to 6 passes. Cell lysate was supplemented with 0.5 mM PMSF and spun down in a JA-10 (Beckman) rotor at 9,000 rpm for 25 minutes. Supernatant was clarified by ultracentrifugation at 185,500 ×g for 75 minutes and filtered through cheesecloth. Under heavy stirring, 313 g of ammonium sulfate per liter of supernatant was added and stirred for 30 minutes. The ammonium sulfate pellet was separated by ultracentrifugation at 167,000 ×g for 45 minutes, resuspended in PKME buffer (25 mM PIPES-KOH pH 7.0, 50 mM KCl, 1 mM EGTA, 3 mM MgCl2, 1 mM DTT and 0.1 mM ATP) and dialyzed against PKME overnight in 50,000 MWCO dialysis tubing. The dialysate was spun down at 185,000 ×g for 1 hour and loaded on a glutathione sepharose column charged with GST-N-WASP-VCA, which was purified as previously described32. The GST-N-WASP-VCA affinity column was washed with 7 CV of GST binding buffer (20 mM Tris pH 8.0, 140 mM NaCl, 1 mM EDTA, and 1 mM DTT) followed by GST binding buffer containing 200 mM NaCl until no protein was detectable in the flow through by Bradford assay. The column was then equilibrated with PKME buffer before loading the supernatant at 1 mL/min. The column was then washed with 10-15 column volumes of PKME, followed by 10-15 column volumes of PKME + 150 mM KCl and PKME + 200 mM KCl. ScArp2/3 complex was eluted with a 0.2 – 1.0 M NaCl gradient in buffer with 20 mM Tris pH 8.0 and 1 mM DTT. NaCl concentration was decreased to 100 mM NaCl in the fractions containing the protein by the addition of 20 mM Tris pH 8.0 and 1 mM DTT, before loading onto a 6 mL Resource Q anion exchange column (GE Healthcare). Protein was eluted with a NaCl gradient (100-500 mM) over 20 column volumes. Pooled fractions from the ion exchange column were concentrated to 1-2 mL and loaded onto a Superdex200 gel filtration column (GE Healthcare) and eluted over a column volume of 20 mM Tris pH 8.0, 600 mM NaCl and 1 mM DTT. Purified protein was dialyzed against 20 mM Tris pH 8.0, 100 mM NaCl, concentrated, quantified by measuring the absorbance at 290 nm (ε290 = 154,000 M−1 cm−1) and flash frozen.
The crosslinked complex was purified from the ScBN021 strain as described above with additional steps. After gel filtration, fractions containing Arp2/3 complex were pooled, concentrated to 1 μM and the buffer was changed to crosslinking buffer (10 mM Imidazole pH 7.0, 50 mM KCl, 1 mM MgCl2, 1 mM EGTA, and 0.1 mM ATP). Under stirring at 4 °C, ATP and BMOE were added to 0.5 mM and 5 μM, respectively. After 4 hours, crosslinking was quenched with 1 mM DTT and the protein was dialyzed against GST binding buffer in 50 MWCO dialysis tubing overnight. Twenty milliliters of GS4B beads equilibrated in GST binding buffer were charged with 15 mg of GST-Dip1 to make a GST-Dip1 affinity column. Additional binding buffer was added until no protein was detectable in the flow through by Bradford assay. The column was then equilibrated in crosslinking buffer and then the dialysate was loaded at 0.5 mL per min before washing the column with additional crosslinking buffer. The crosslinked complex was eluted by running a gradient from 0.025 – 1 M KCl in 20 mM Tris pH 8.0, 1mM MgCl2, and 1 mM DTT. Eluted fractions containing pure crosslinked Arp2/3 complex were concentrated to 1.5 mL and flowed over a Superdex 200 size exclusion column equilibrated in 20 mM Tris pH 8.0, 600 mM NaCl, and 1 mM DTT. Pooled fractions were diluted to 20 mM Tris pH 8.0, 100 mM NaCl, 1 mM DTT and concentrated. Protein concentration was determined by measuring the absorbance at 290 nm (ε290 = 154,000 M−1 cm−1) before flash freezing.
Mus musculus capping protein was expressed in E. coli by transforming BL21(DE3)-RIL cells with the pRSF Duet 1 (MmCPα1β2) vector58. Cultures were grown at 37 °C to an OD600 of 0.7, then induced with 0.5 mM IPTG for 16 hrs at 16 °C. Cells were pelleted, washed with cold 1x PBS, and resuspended in extraction buffer (50 mM NaH2PO4 pH 8.0, 500 mM NaCl, 10% glycerol, 10 mM imidazole, 10 mM β-mercaptoethanol, 0.5 mM PMSF and 2 protease inhibitor tablets (complete, Roche)). Cells were lysed by sonication and the lysate was spun down at 100,000 × g for 45 min before loading onto a Talon metal affinity column (Clontech) and eluting with extraction buffer containing 250 mM imidazole. Eluted fraction was dialyzed overnight against QA buffer (20 mM Tris pH 8.5, 50 mM NaCl, 5% glycerol, 0.01% NaN3, 1 mM DTT) in 3.5 MWCO dialysis tubing, then loaded on a Resource Q column using a gradient of 50 to 500 mM. Peak fractions were dialyzed in storage buffer (20 mM HEPES pH 8.0, 1 mM EDTA, 200 mM KCl, 0.01% NaN3, 10% glycerol, 1 mM DTT) and flash frozen.
The Q61L constitutively active mutant of the Cdc42 protein was expressed as His-tagged fusion protein in E. coli by transforming BL21(DE3)-RIL cells with the pBH_Cdc42_Q61L vector (a gift from Ken Prehoda). Bacteria were grown at 37 °C to OD600 of 0.6, induced with 0.4 mM IPTG and grown overnight at 22°C. Cultures were supplemented with 0.5 mM PMSF and 2 mM EDTA before harvesting the cells. Cells were resuspended in lysis buffer (40 mM Sodium phosphate buffer pH 7.8, 0.3 M NaCl and 10 mM Imidazole), and lysed by sonication. The lysate, cleared by centrifugation at 15,000 in a JA-20 rotor for 25 min, was incubated with Ni-sepharose 6 Fast flow resin (GE) for 1 hr at 4 °C. Mixture was poured into an empty column and the resin was washed with lysis buffer containing 20 mM imidazole, Cdc42_Q61L protein was eluted with 300 mM imidazole and dialyzed against 20 mM Tris-HCl pH 8, 20 mM NaCl, 1 mM DTT. Dialyzed protein was concentrated in a Vivaspin Turbo 15 (10K MWCO), centrifuged at 20,800 × g for 15 min, and flash-frozen in liquid nitrogen.
Homo sapiens Profilin 1, was purified as described in Ezezika et al.59 To purify Homo sapiens cofilin, 5 mL of LB plus 100 mg/mL ampicillin and 35 mg/mL chloramphenicol was inoculated with BL21(DE3)-RIL cells transformed with a pMW172-HsCofilin1 plasmid and grown overnight at 37 °C. One milliliter of this culture was used to inoculate 50 mL of LB plus ampicillin and chloramphenicol. The 50 mL culture was grown at 37 °C with shaking until turbid and was then used to inoculate several 1L cultures of LB plus ampicillin and chloramphenicol. These cultures were grown at 37 °C to an OD600 of 0.6 and induced with 0.4 mM IPTG. Protein expression was carried out at 22 °C for 12-14 hr. Before collecting the cells by centrifugation, EDTA and PMSF were added to 2 mM and 0.5 mM, respectively. The pellet was resuspended in lysis buffer (10 mM Tris pH 7.5, 50 mM NaCl, 1mM EDTA, 1% Triton X100, 5% glycerol, 1 mM DTT, 0.5 mM PMSF) plus 2 protease inhibitor tablets. Cells were lysed by sonication on ice and the lysate was spun down for 45 min at 19,000 rpm in a JA-20 rotor at 4 °C. Supernatant was further clarified by ultracentrifugation at 133,000 ×g for 30 min. The clarified lysate was loaded onto a 10 mL DEAE column equilibrated in DEAE buffer (10 mM Tris pH 7.5, 50 mM NaCl, 1 mM EDTA, 1 mM DTT). HsCofilin1 eluted in the flow-through, which was concentrated to 10 mL and dialyzed against 2 L of SA buffer (10 mM PIPES pH 6.5, 25 mM NaCl, 0.2 mM EGTA, 1 mM DTT) in 12-14 MWCO dialysis tubing overnight. The dialyzed protein was loaded on a Source 15S ion-exchange column washed and equilibrated in SA. The bound protein was then eluted by running a gradient from SA to SB buffer (same as SA but with 500 mM NaCl) and the fractions containing the protein were concentrated to 2-3 mL using Vivaspin Turbo15, 10K MWCO. Concentrated protein was dialyzed against 1L of storage buffer (20 mM Tris pH 7.5, 25 mM NaCl, 1 mM DTT) overnight. Before flash freezing, the protein was concentrated, and its final concentration was determined by measuring the absorbance at 280 nm (ε280 =14,440 M−1cm−1).
Yeast Crn1-WD-CC (residues 1 to 410 and 594 to 651) was produced as a recombinant protein in BL21(DE3)RIL E. coli as previously described32. This construct lacks the unique region (which binds Arp2/3 complex so would complicate interpretation of the experiments) but binds actin filaments and inhibits branching nucleation by Arp2/3 complex identically to full length coronin32.
GST-WASP-pVCA was purified from Rosetta 2(DE3)pLysS E. coli cells transformed with the pGV67-Hs-WASP-pVCA vector (residues 150 – 502). Cultures were grown at 37 °C to OD600 of 0.6, then protein expression was induced overnight at 20 °C by adding 0.5 mM IPTG. The cell pellet was resuspended in lysis buffer (20 mM Tris-HCl pH8, 200 mM NaCl, 1 mM EDTA, 0,1% Triton X-100, 5% glycerol, 1 mM DTT, 2 mM PMSF and a protease inhibitor tablet), sonicated and clarified by centrifugation at 16,000 rpm in a JA–20 rotor for 30 min at 4 °C. Supernatant was loaded into a glutathione sepharose column, washed and eluted using lysis buffer containing 100 mM reduced L-glutathione. Fractions containing GST-N-WAS-pVCA were pooled, dialyzed overnight against 20 mM Tris-HCl pH 8.0, 100 mM NaCl, 1 mM EDTA, 1 mM DTT and 5% glycerol, injected into a 6 mL Resource 30Q, and eluted with a gradient from 0.1 to 1 M NaCl. Purified protein was concentrated using a Vivaspin Turbo 30K MWCO and flash frozen in liquid nitrogen.
Las17-VCA was purified as previously described32. Briefly, BL21(DE3)RIL E. coli competent cells transformed with the pGV67-GST-Las17-VCA (residues 529-633) expression vector were grown to an OD600 of 0.6 at 37 °C, and then induced with 0.4 mM IPTG overnight. Cells were disrupted by sonication on ice and the lysate was clarified by centrifugation at 19,000 rpm in a JA-20 rotor at 4 °C for 40 min. The clarified lysate was loaded on a glutathione sepharose affinity column, washed and eluted using 50 mM reduced glutathione and dialyzed against 2 L QA buffer (20 mM Tris pH 8.0, 100 mM NaCl, and 1 mM DTT) overnight. The protein was then loaded on a 6mL Resource Q column and eluted over a gradient of 100 to 600 mM NaCl. Peak fractions were pooled, concentrated, and buffer exchanged to 20 mM Tris pH 8.0, 50 mM NaCl, and 1 mM DTT. Protein was concentrated, concentration was measured (ε280 =5500 M−1cm−1) and flash frozen in liquid nitrogen.
Schizosaccharomyces pombe Dip1 and GST-Dip1 were prepared as previously described60. Briefly, BL21(DE3)RIL E. coli transformed with a pGV67-SpDip1 expression vector was grown to an O.D600 of 0.6, induced with 0.4 mM IPTG, and grown overnight at 22 °C. Cells were lysed by sonication and clarified by centrifugation, and the soluble fraction was loaded on a glutathione sepharose column and eluted with 20 mM Tris pH 8.0, 140 mM NaCl and 50 mM glutathione reduced. Protein was dialyzed overnight against QA buffer (20 mM Tris pH 8.0, 50 mM NaCl, 1 mM DTT) before loading onto a 6ml Resource Q column at pH 8.0 and eluted with a gradient of 50 mM to 500 mM NaCl. Fractions containing GST-Dip1 were concentrated before flash freezing.
Actin polymerization and filament binding assays
Twenty microliters of 15% pyrene labeled rabbit skeletal muscle actin in 1x G buffer (5 mM Tris pH 8.0, 0.2 mM CaCl2, 0.2 mM ATP, 0.2 mM DTT) was aliquoted into a 96-well flat bottom black polystyrene assay plate (Corning). Actin was mixed with 2 μL 10x M/E buffer (5 mM MgCl2, 20 mM EGTA) and incubated for 2 min before adding 78 μL of a mixture of other proteins (Arp2/3 complex, NPFs) in 1X KMEI buffer (10 mM Imidazole pH 7.0, 50 mM KCl, 1 mM EGTA, 1 mM MgCl2, 0.2 mM ATP and 1 mM DTT). The fluorescence of pyrene-labeled actin was measured at room temperature using a TECAN Safire 2 plate reader by exciting at 365 nm and monitoring the emission at 407 nm. For reactions shown in Figures 3G and 4C, in which Crn1(WD-CC) was used to block filament binding by Arp2/3 complex, we added 0.15 μM preformed actin filaments to prevent Crn1 from inhibiting spontaneous nucleation. Experiments were performed at 25 °C in a 60 μL cuvette using an ISS PC1 Photon counting Spectrofluorometer with excitation and emission wavelengths set at 365 and 407 nm, respectively.
The budding yeast actin pyrene assays were performed in a cuvette using an ISS PC1 Photon counting Spectrofluorometer. Polymerization was carried out at 25 °C by mixing 2 μM of actin (1.8 μM budding yeast actin plus 0.2 μM pyrene labeled rabbit skeletal muscle actin) with 10X M/E buffer 1 min before adding a mixture of the other proteins in 1X KMEI.
For actin copelleting assays, 45 nM Arp2/3 complexes were incubated with the indicated concentrations of pre-polymerized actin in 1X KMEI for 15 min at room temperature. The reactions were then centrifuged for 30 min at 310,000 × g, and the supernatants were analyzed by ELISA as follows. Samples were prepared by mixing each supernatant with 50 mM Carbonate-bicarbonate buffer pH 9.4, then triplicate 50 μL aliquots were added to wells of a flat bottom UltraCruz ELISA plate (Santa Cruz) and incubated for 1 hr at room temperature. Plate was washed twice with 1X TBST (20 mM Tris pH 7.4, 150 mM NaCl, 0.1% Tween20) followed by the addition of blocking solution (5% non-fat milk in 1X PBS), incubation for 1-2 hrs at room temperature, and addition of Anti-Arp3 antibodies (1:2000 dilution in 5% non-fat milk/1X PBS Santa Cruz sc-11973). The primary antibody was incubated for 20 min, then washed out (4 times with 1X TBST). The plate was incubated for further 20 min at room temperature with anti-goat horseradish peroxidase antibodies (1:10,000 in 5% non-fat milk/1X PBS), washed for four times with 1X TBST before adding 100 μL of TMB substrate (ThermoFisher) per well to allow development of color for 15-30 min, and then quenched with 100 μL 0.2 N H2SO4. Absorbances were read with a 450 nm filter using a TECAN Safire 2 microplate reader. An eight-point standard curve of ScArp2/3 complex was included with each plate. Curve fitting and KD determination was carried out by fitting the data to the ligand binding equation show below using Prism software (GraphPad Software).
To fit the binding isotherms, the maximum fraction of Arp2/3 complex bound was allowed to float because not all Arp2/3 complex was bound at saturation, indicating that a fraction of the complex is unable to bind to filaments. This does not appear to be due to misfolding or aggregation of Arp2/3 complex since it eluted after the void volume as a single, gaussian peak during gel filtration (see main text). We note that incomplete binding of Arp2/3 complex at saturating actin filaments has been observed previously in filament copelleting assays with human, bovine and S. cerevisiae Arp2/3 complex2,12,28,52.
Crosslinking assays
To exclude DTT from the crosslinking reactions, actin monomers were dialyzed against G buffer without DTT and LZ-VCA was dialyzed against 1X KMEI buffer without DTT using 3.5K slide-A-Lyzer mini dialysis units (ThermoFisher) for at least 4 hrs at 4 °C. Then, a 2:1 molar ratio of Latrunculin B was added to actin monomers to inhibit spontaneous nucleation. A standard reaction was set up by mixing 1 μM dual cys Arp2/3 complex to a solution of the other components, such as LZ-VCA, LatB-actin monomers and/or preformed actin filaments in 1X KMEI buffer and incubated for 15 min at room temperature. The crosslinking reaction was initiated by adding BMOE and quenched after 60 sec with SDS loading buffer containing 50 mM DTT. Note that the incubation time and concentration of BMOE was previously standardized18. Samples were resolved by SDS-PAGE, followed by western blot analysis using goat anti-Arp3 (Santa Cruz, sc-11973, 1:1000 dilution) and donkey anti-goat IRDye 680RD (LICOR, 1:10000 dilution). The blots shown in the figures are representative of three independent experiments. Samples were not boiled before running on SDS-PAGE to prevent reduction of the maleimide bond, causing some of the filamentous actin to stay in the stacking portion of the gel (Figure S2A). Band intensities were determined using the ImageStudioLite software (LI-COR Biosciences), and p values were calculated by a one-way ANOVA including a Tukey’s multiple comparisons test in GraphPad Prism 9.
For the crosslinking experiments shown in Figures 2F and S1C, actin filaments were pre-treated with N-Ethylmaleimide (NEM, Sigma), as follows. Actin monomers were dialyzed against G buffer without DTT using 3.5K slide-A-Lyzer mini dialysis units (ThermoFisher) for at least 4 hrs at 4°C. Actin was then polymerized for 45 min at room temperature by adding 1X M/E and 1X KMEI buffer, followed by the addition of 10x molar excess of NEM and incubation for 1 hr at room temperature. Incorporation of one NEM molecule per actin subunit was verified by MALDI mass spectrometry using Sinapinic acid (SA) as a MALDI matrix in a Microflex Smart LS instrument (Bruker) (Figure S1B).
GST-pulldown assays
For each binding reaction, 10 μL of Glutathione agarose beads (GoldBio) were incubated in GST-Binding buffer (20 mM Tris-HCl, pH 8.0, 140 mM NaCl, 2 mM EGTA, and 1 mM DTT) with 80 μg of the purified GST-Las17-VCA or GST alone for 1 hr at 4°C. After washing four times with GST-Binding buffer and twice with 1x KMEI buffer, the GST-Las17-VCA/GST beads were incubated with 2 μM ScArp2/3 uncrosslinked or crosslinked complex for 1 hr at 4 °C in 1x KMEI buffer. Beads were collected by centrifugation, supernatant was removed and the beads were washed three times with 1x KMEI, and boiled at 95 °C for 5 min in 100 μL of 2X SDS-PAGE reducing buffer to elute proteins. Protein bound and unbound to the beads was analyzed by 10-20% gradient SDS–PAGE followed by immunoblotting with anti-Arp3 antibody (Santa Cruz, sc-11973, 1:1000). Experiments were conducted by triplicate. Band intensities were determined using the ImageStudioLite software (LI-COR Biosciences). The amount of protein, which was found to be non-specific absorbed to the beads, was subtracted from the signal of the pellet fraction.
TIRF microscopy and Image analysis
TIRF flow chambers were built as previously described61. Glass microscope sides and 24 x 60 #1.5 coverslips were soaked in acetone, 1 M KOH and water, then, aminosilanized in 1% APTES, 5% acetic acid in methanol. Chambers were assembled by putting double-sided tape between a glass microscope slide and a coverslip (24 360 # 1.5) to create a ~14 μL chamber. A mixture of methoxy PEG succinimidyl succinate, MW5000 (JenKem) and 1%–3% biotin-PEG NHS ester, MW5000 (JenKem) dissolved in 0.1 M NaHCO3 pH 8.3 was added to the chamber and incubated for 4 hours. Chambers were then flushed sequentially with 1 μM NeutrAvidin (ThermoFisher), 50 nM biotin inactivated myosin (Cytoskeleton, Inc), and twice with 20 mg/mL BSA in 50 mM Tris pH 7.5, 600 mM NaCl. The chamber was then rinsed with TIRF buffer (10 mM Imidazole pH 7.0, 1 mM MgCl2, 1 mM EGTA, 50 mM KCl, 100 mM DTT, 0.2 mM ATP, 25 mM glucose, 0.5% metylcellulose (400cP), 0.02 mg/mL catalase and 0.1 mg/mL glucose oxidase) and the reaction mixture was set up by mixing 33% Oregon-green actin (1.5 μM final concentration) with 10X M/E buffer just before adding the protein mixture (ScArp2/3 crosslinked or uncrosslinked complex plus Las17-VCA) in TIRF buffer. The reaction was imaged on a Nikon TE2000-E inverted microscope equipped with a 100x 1.49 NA TIRF objective, an Andor iXon3 EM-CCD (DU-897-CS0) camera, and a TIRF Quad filter cube (Chroma C141789). Reactions were imaged for 10 min with the 488 nm laser (Coherent) at 10 mW, 100 ms exposure times at 1 sec intervals.
Images and videos were prepared in Image J. Background signal was subtracted with a 10-pixel rolling ball radius. The total actin polymer was calculated using a custom image processing script run in MATLAB, where for each frame, pixels corresponding to filament fluorescence were identified using image segmentation followed by morphological area opening to remove non-filament small fluorescent objects. The final pixel number value was converted to micrometers (1px = 106.7 nm) to yield the total length of actin filaments in the image frame.
To calculate the branch densities, the lengths of mother actin filaments were measured using an Image J plugin, courtesy of Jeff Kuhn. The number of branches was counted manually and divided by the length of the mother filaments when the total polymer length in each video was approximately 1200 mm.
In vitro actin-based motility assays
Polystyrene carboxylated microspheres (2 μm, Polysciences) were incubated with 2 μM GST-WAS-pVCA under rotation for 2 hrs at 4 °C, washed twice, stored at 4 °C in 2 mM Tris pH 8 containing 1 mg/mL BSA and used for up to 4 days. Functionalized beads were then diluted 1:20 in motility medium, which included Xb buffer (10 mM HEPES pH 7.0, 50 mM KCl, 1 mM MgCl2, 1.5 mM NaN3, 0.1 mM CaCl2), 1 mM ATP, 5 mg/mL BSA, 0.25% Methylcellulose (1500 cP), 15 mM TCEP-KOH and 1 mM DABCO. Proteins were added to the motility medium at 10 μM profilin, 0.1 μM capping, 1 μM Cdc42(Q61L), 0.7 μM cofilin and 40 nM ScArp2/3 uncrosslinked or crosslinked complex. We included Cdc42(Q61L) in the reaction mix because the GST-WASP-pVCA construct includes the autoinhibitory sequence that decreases its activity in solution. While the GST-WASP-pVCA construct is active when clustered on beads, we found that the addition of Cdc42(Q61L) increased comet tail assembly rates, so we added Cdc42(Q61L) to all of the motility reactions. Each motility reaction was initiated by mixing with 7.4 μM Oregon-green actin (10% labeled), then 2-3 μL was pressed between a pre-cleaned microscope slide and 22-mm-square glass coverslip and sealed with Valap (vaseline:lanolin:paraffin, 1:1:1). The reaction was visualized in a DeltaVision widefield fluorescence inverted microscope equipped with a motorized stage and using a 20X or 40X objective and a sCMOS camera. Videos were typically recorded for 1 to 2 hours, taking images at 70 sec intervals. Relative quantification of actin intensity around the bead was performed in ImageJ by drawing a line across the bead and generating its plot profile. The speed of the beads was calculated only in the reactions containing the uncrosslinked ScArp2/3 complex using the Filament Tracer module of Imaris 9.6 software.
For experiments in which actin dynamics were arrested at specific time points, 40 μM Latrunculin B and 40 μM Phalloidin in 1X KMEI were added at the indicated time points. Beads in the arrested reactions were ultracentrifuged at 91,000 rpm using a TLA100 rotor for 20 minutes. The amount of actin and Arp2/3 complex in each fraction (high speed pellets and supernatants) was calculated by immunoblot using anti-actin clone C4 (Millipore, MAB1501, 1:1000) and anti-Arp3 (Santa Cruz, sc-11973, 1:1000 dilution).
Dual color bead motility assays in Figure 6 were performed by setting up two reactions, one with 10%-labeled Oregon-green actin and the other with 5%-labeled Alexa647 actin, simultaneously. Reactions were individually incubated for 3 or 7 minutes, then a 1:1 mixture of the 2 reactions was pooled in a different tube, incubated for 90 seconds and quenched by the addition of 40 μM Latrunculin B and 40 μM phalloidin.
QUANTIFICATION AND STATISTICAL ANALYSIS:
For the crosslinking assays shown in Figure 2C,E,F; Figure S1A,C and S2B each reaction was run at least three times. The bars represent the mean and standard deviation, p values were calculated by a one-way ANOVA including a Tukey’s multiple comparisons test in GraphPad Prism 9. n.s.: not significant, * p<0.05, ** p<0.005, **** p<0.0001.
In Figure 3E, the branch densities were calculated from 3 independent videos per condition. Each branch density value is the result of the analysis of ≥50 mother filaments. the lengths of mother actin filaments were measured using an Image J plugin, courtesy of Jeff Kuhn. The number of branches was counted manually and divided by the length of the mother filaments when the total polymer length in each video was approximately 1200 mm. p values were calculated by a one-way ANOVA including a Tukey’s multiple comparisons test in GraphPad Prism 9.
Supplementary Material
Video S1: TIRF microscopy videos showing actin assembly in the presence of crosslinked or uncrosslinked Arp2/3 complex, Related to Figure 3.
TIRF microscopy videos showing polymerization of 1.5 uM 33% Oregon-Green actin with uncrosslinked or crosslinked (xl) Arp2/3 complex with or without Las17-VCA, as indicated. Images were acquired at 100x magnification at a rate of 1 frame/sec. Movie playback is 15 frames per second (fps).
Video S2: Fluorescence microscopy videos of reconstituted actin assembly assays containing crosslinked or uncrosslinked Arp2/3 complex, Related to Figure 5.
Videos of reconstituted bead motility assay in the presence of 40 nM uncrosslinked or crosslinked Arp2/3 complex. The video corresponds to the time-lapse series in Figure 5D. Total imaging time of ~45 min was accelerated ~550 fold to create the 5 s video.
Video S3: Brightness and contrast adjusted videos of reconstituted actin assembly assays containing crosslinked or uncrosslinked Arp2/3 complex, Related to Figure 6.
Videos of reconstituted bead motility assay in the presence of 40 nM uncrosslinked or crosslinked Arp2/3 complex. The video corresponds to the time-lapse series in Figure 5D, with the brightness and contrast adjusted to show actin networks disconnected from bead. Total imaging time of ~45 min was accelerated ~550 fold to create the 5 s video.
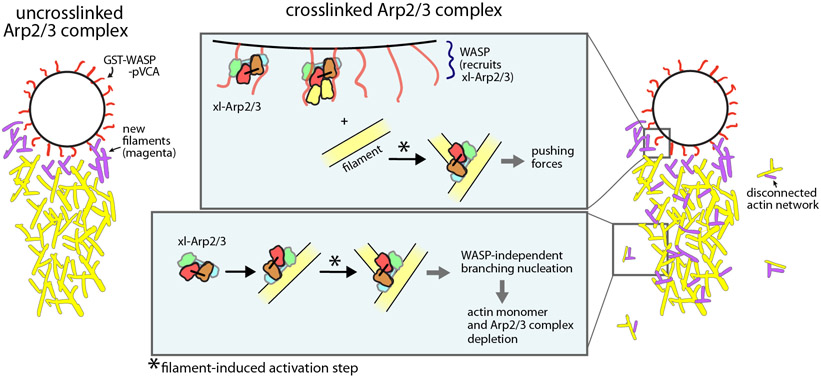
Figure 7: Schematic showing influence of bypassing the WASP-mediated activation step on the assembly of force-producing actin networks.
With the uncrosslinked Arp2/3 complex, the requirement for the WASP-mediated activation step ensures that new actin assembly is restricted to the surface of the bead. With xl-Arp2/3 complex, WASP recruits the complex to the surface of the beads, where it is activated by filaments to nucleate branches that push the bead forward. However, xl-Arp2/3 also binds to the comet tail away from the surface of the bead, nucleating new branches that don’t contribute to bead motility. It also creates actin networks that are disconnected from the bead by binding to and branching from spontaneously nucleated actin filaments in solution. The activity of the complex away from the surface of the bead depletes it and actin monomers from solution.
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Goat polyclonal Anti-Arp3 | Santa Cruz Biotechnology | sc-11973 |
| Mouse monoclonal anti-actin clone C4 | Sigma-Aldrich /Millipore | MAB1501 |
| Bacterial and virus strains | ||
| BL21-CodonPlus(DE3)-RIL | Agilent | Cat. #230245 |
| Biological samples | ||
| Acetone powder of rabbit skeletal muscle | Pel-Freeze Biologicals | Cat. #41995-2 |
| Chemicals, peptides, and recombinant proteins | ||
| Alexa Fluor 647 C2 maleimide | ThermoFisher Scientific | Cat. # A20347 |
| Oregon Green 488 maleimide | ThermoFisher Scientific | Cat. # O6034 |
| N-(1-pyrene)iodoacetamide | ThermoFisher Scientific | Cat. #P29 |
| PEG succinimidyl succinate, MW5000 | JenKem | Cat. # A3011-1 |
| EZ-Link-NHS-PEG12-biotin | ThermoFisher Scientific | Cat. # 21312 |
| biotin-PEG NHS ester, MW5000 | JenKem | A5027-1 |
| NeutrAvidin | ThermoFisher Scientific | Cat.# 31000 |
| cOmplete, EDTA-free Protease Inhibitor Cocktail tablets | Sigma | Cat.# 11873580001 |
| Catalase | Sigma | Cat.# C3515 |
| Glucose Oxidase | MP Biomedicals | Cat.#195196 |
| Myosin II, rabbit skeletal muscle | Cytoskeleton | Cat # MYO2 |
| Albumin, from bovine serum | Sigma | Cat # A2153 |
| Critical commercial assays | ||
| Deposited data | ||
| Experimental models: Cell lines | ||
| Experimental models: Organisms/strains | ||
| S. cerevisiae: BN020, Genotype: MAT?, ura3-52, his3-Δ200, leu2-3, lys2-801, trp1-1, Δarp2::TRP1, Δarp3::HIS3 pBN002(ARP3)::LEU2 pBN001(ARP2)::LYS2 | 28 | N/A |
| S. cerevisiae: BN021, Genotype: MAT?, ura3-52, his3-Δ200, leu2-3, lys2-801, trp1-901, Δarp2::TRP1 Δarp3::HIS3 pBN009(arp3 L155C)::LEU2 pBN003(arp2 R198C)::LYS2 | 28 | N/A |
| S. cerevisiae: BN283, Genotype: pep4::kan | Gift from Tom Stevens | N/A |
| Oligonucleotides | ||
| Recombinant DNA | ||
| pGEX-N-WASp-WA | 52 | Plasmid #22 |
| pGV67_SpDip1 | 3 | Plasmid #174 |
| pRSF Duet 1 (MmCPα1β2) | 58 | Plasmid #147 |
| pMW172-HsCofilin | gift from David Kovar | Plasmid #371 |
| pBH_Cdc42_Q61L | gift from Ken Prehoda | Plasmid #350 |
| pGV67_Crn-WD-CC | 32 | Plasmid #90 |
| pGV67-Hs-WASP-pVCA | gift from Rajaa Boujemaa-Paterski, recloned into pGV67 | Plasmid #335 |
| pGV67-GST-Las17-VCA | 32 | Plasmid #73 |
| pMW Human PRF1 | gift from David Kovar32 | Plasmid #359 |
| Software and algorithms | ||
| ImageJ | NIH, version 1.53c, open-source software | https://imagej.nih.gov/ij/; RRID:SCR_002285 |
| ImageJ filament tracking plugin | gift from Jeff Kuhn | N/A |
| Imaris v9.7.2 | Bitplane | http://www.bitplane.com/imaris/imaris;RRID:SCR_007370 |
| ImageStudioLite software | LI-COR Biosciences | https://www.licor.com/bio/image-studio-lite/; RRID:SCR_013715 |
| GraphPad Prism v9.2.0 | GraphPad Software | RRID:SCR_002798 |
| MATLAB | Mathworks | RRID:SCR_001622 |
| Other | ||
Highlights.
Actin filaments stimulate the short pitch conformational change weakly or not at all
Actin filaments stimulate an activating step distinct from WASP
The WASP- and actin filament-mediated Arp2/3 activation steps are unconcerted
Both steps are required to create sustained force-producing branched actin networks
Acknowledgements
Research reported in this publication was supported by the National Institute of General Medical Sciences of the NIH under award number R35GM136319 (B.J.N.) We thank Raaja Boujemaa-Paterski for the providing the GST-WASP-pVCA vector and Raaja Boujemaa-Paterski and Orkun Akin for advice on the bead motility assays. We thank Adam Fries for assistance with imaging and analysis of bead motility assays. We thank Mike Lynch and Ken Prehoda for comments on the manuscript. We thank Zachary Corbin for purifying profilin. We thank David Kovar for profilin, cofilin, and capping protein expression plasmids. We thank Jeff Kuhn for sharing the Fiji filament tracking plugin.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare no competing interests.
References
- 1.Rotty JD, Wu C, and Bear JE (2013). New insights into the regulation and cellular functions of the ARP2/3 complex. Nat. Rev. Mol. Cell Biol 14, 7–12. [DOI] [PubMed] [Google Scholar]
- 2.Goley ED, and Welch MD (2006). The ARP2/3 complex: an actin nucleator comes of age. Nat. Rev. Mol. Cell Biol 7, 713–726. [DOI] [PubMed] [Google Scholar]
- 3.Wagner AR, Luan Q, Liu S-L, and Nolen BJ (2013). Dip1 defines a class of Arp2/3 complex activators that function without preformed actin filaments. Curr. Biol 23, 1990–1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alekhina O, Burstein E, and Billadeau DD (2017). Cellular functions of WASP family proteins at a glance. J. Cell. Sci 130, 2235–2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marchand JB, Kaiser DA, Pollard TD, and Higgs HN (2001). Interaction of WASP/Scar proteins with actin and vertebrate Arp2/3 complex. Nat. Cell Biol 3, 76–82. [DOI] [PubMed] [Google Scholar]
- 6.Amann KJ, and Pollard TD (2001). The Arp2/3 complex nucleates actin filament branches from the sides of pre-existing filaments. Nat. Cell Biol 3, 306–310. [DOI] [PubMed] [Google Scholar]
- 7.Achard V, Martiel J-L, Michelot A, Guérin C, Reymann A-C, Blanchoin L, and Boujemaa-Paterski R (2010). A “primer”-based mechanism underlies branched actin filament network formation and motility. Curr. Biol 20, 423–428. [DOI] [PubMed] [Google Scholar]
- 8.Schrank BR, Aparicio T, Li Y, Chang W, Chait BT, Gundersen GG, Gottesman ME, and Gautier J (2018). Nuclear ARP2/3 drives DNA break clustering for homology-directed repair. Nature 559, 61–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ti S-C, Jurgenson CT, Nolen BJ, and Pollard TD (2011). Structural and biochemical characterization of two binding sites for nucleation-promoting factor WASp-VCA on Arp2/3 complex. Proc. Natl. Acad. Sci. U.S.A 108, E463–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Padrick SB, Doolittle LK, Brautigam CA, King DS, and Rosen MK (2011). Arp2/3 complex is bound and activated by two WASP proteins. Proc. Natl. Acad. Sci. U.S.A 108, E472–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boczkowska M, Rebowski G, Kast DJ, and Dominguez R (2014). Structural analysis of the transitional state of Arp2/3 complex activation by two actin-bound WCAs. Nat Commun 5, 3308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zimmet A, Van Eeuwen T, Boczkowska M, Rebowski G, Murakami K, and Dominguez R (2020). Cryo-EM structure of NPF-bound human Arp2/3 complex and activation mechanism. Sci Adv 6, eaaz7651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marchand J-B, Kaiser DA, Pollard TD, and Higgs HN (2001). Interaction of WASP/Scar proteins with actin and vertebrate Arp2/3 complex. Nature Cell Biology 3, 76–82. [DOI] [PubMed] [Google Scholar]
- 14.Rohatgi R, Ma L, Miki H, Lopez M, Kirchhausen T, Takenawa T, and Kirschner MW (1999). The interaction between N-WASP and the Arp2/3 complex links Cdc42-dependent signals to actin assembly. Cell 97, 221–231. [DOI] [PubMed] [Google Scholar]
- 15.Machesky LM, Mullins RD, Higgs HN, Kaiser DA, Blanchoin L, May RC, Hall ME, and Pollard TD (1999). Scar, a WASp-related protein, activates nucleation of actin filaments by the Arp2/3 complex. Proc. Natl. Acad. Sci. U.S.A 96, 3739–3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burianek LE, and Soderling SH (2013). Under lock and key: spatiotemporal regulation of WASP family proteins coordinates separate dynamic cellular processes. Semin. Cell Dev. Biol 24, 258–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mullins RD, Bieling P, and Fletcher DA (2018). From solution to surface to filament: actin flux into branched networks. Biophys Rev 10, 1537–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rodnick-Smith M, Luan Q, Liu S-L, and Nolen BJ (2016). Role and structural mechanism of WASP-triggered conformational changes in branched actin filament nucleation by Arp2/3 complex. Proc. Natl. Acad. Sci. U.S.A 113, E3834–3843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sanchez Espinoza, Sofia Metskas, Ann Lauren, Chou Steven Z., Rhoades Elizabeth, and Pollard Thomas D. (2018). Conformational changes in Arp2/3 complex induced by ATP, WASp-VCA and actin filaments. PNAS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu X-P, Rouiller I, Slaughter BD, Egile C, Kim E, Unruh JR, Fan X, Pollard TD, Li R, Hanein D, et al. (2012). Three-dimensional reconstructions of Arp2/3 complex with bound nucleation promoting factors. EMBO J. 31, 236–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fäßler F, Dimchev G, Hodirnau V-V, Wan W, and Schur FK (2020). Novel cryo-electron tomography structure of Arp2/3 complex in cells reveals mechanisms of branch formation. bioRxiv, 2020.08.25.266262. [Google Scholar]
- 22.Shaaban M, Chowdhury S, and Nolen BJ (2020). Cryo-EM reveals the transition of Arp2/3 complex from inactive to nucleation-competent state. Nat. Struct. Mol. Biol [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chou SZ, and Pollard TD (2019). Mechanism of actin polymerization revealed by cryo-EM structures of actin filaments with three different bound nucleotides. Proc. Natl. Acad. Sci. U.S.A 116, 4265–4274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Merino F, Pospich S, Funk J, Wagner T, Küllmer F, Arndt H-D, Bieling P, and Raunser S (2018). Structural transitions of F-actin upon ATP hydrolysis at near-atomic resolution revealed by cryo-EM. Nat. Struct. Mol. Biol 25, 528–537. [DOI] [PubMed] [Google Scholar]
- 25.Oda T, Iwasa M, Aihara T, Maéda Y, and Narita A (2009). The nature of the globular- to fibrous-actin transition. Nature 457, 441–445. [DOI] [PubMed] [Google Scholar]
- 26.Prehoda KE, and Lim WA (2002). How signaling proteins integrate multiple inputs: a comparison of N-WASP and Cdk2. Curr. Opin. Cell Biol 14, 149–154. [DOI] [PubMed] [Google Scholar]
- 27.Luan Q, Liu S-L, Helgeson LA, and Nolen BJ (2018). Structure of the nucleation-promoting factor SPIN90 bound to the actin filament nucleator Arp2/3 complex. EMBO J. 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hetrick B, Han MS, Helgeson LA, and Nolen BJ (2013). Small molecules CK-666 and CK-869 inhibit actin-related protein 2/3 complex by blocking an activating conformational change. Chem. Biol 20, 701–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rodnick-Smith M, Liu S-L, Balzer CJ, Luan Q, and Nolen BJ (2016). Identification of an ATP-controlled allosteric switch that controls actin filament nucleation by Arp2/3 complex. Nat Commun 7, 12226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bender N, Fasold H, Kenmoku A, Middelhoff G, and Volk KE (1976). The selective blocking of the polymerization reaction of striated muscle actin leading to a derivative suitable for crystallization. Modification of Tyr-53 by 5-diazonium-(1H)tetrazole. Eur. J. Biochem 64, 215–218. [DOI] [PubMed] [Google Scholar]
- 31.Drewes G, and Faulstich H (1991). A reversible conformational transition in muscle actin is caused by nucleotide exchange and uncovers cysteine in position 10. J. Biol. Chem 266, 5508–5513. [PubMed] [Google Scholar]
- 32.Liu S-L, Needham KM, May JR, and Nolen BJ (2011). Mechanism of a concentration-dependent switch between activation and inhibition of Arp2/3 complex by coronin. J. Biol. Chem 286, 17039–17046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Atilgan E, Wirtz D, and Sun SX (2005). Morphology of the lamellipodium and organization of actin filaments at the leading edge of crawling cells. Biophys. J 89, 3589–3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Le Clainche C, and Carlier M-F (2004). Actin-based motility assay. Curr Protoc Cell Biol Chapter 12, Unit 12.7. [DOI] [PubMed] [Google Scholar]
- 35.Akin O, and Mullins RD (2008). Capping protein increases the rate of actin-based motility by promoting filament nucleation by the Arp2/3 complex. Cell 133, 841–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chan C, Beltzner CC, and Pollard TD (2009). Cofilin Dissociates Arp2/3 Complex and Branches from Actin Filaments. Curr Biol 19, 537–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Andrianantoandro E, and Pollard TD (2006). Mechanism of actin filament turnover by severing and nucleation at different concentrations of ADF/cofilin. Mol Cell 24, 13–23. [DOI] [PubMed] [Google Scholar]
- 38.Reymann A-C, Suarez C, Guérin C, Martiel J-L, Staiger CJ, Blanchoin L, and Boujemaa-Paterski R (2011). Turnover of branched actin filament networks by stochastic fragmentation with ADF/cofilin. Mol Biol Cell 22, 2541–2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carlier MF, Ressad F, and Pantaloni D (1999). Control of actin dynamics in cell motility. Role of ADF/cofilin. J Biol Chem 274, 33827–33830. [DOI] [PubMed] [Google Scholar]
- 40.Footer MJ, Lyo JK, and Theriot JA (2008). Close packing of Listeria monocytogenes ActA, a natively unfolded protein, enhances F-actin assembly without dimerization. J. Biol. Chem 283, 23852–23862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ditlev JA, Michalski PJ, Huber G, Rivera GM, Mohler WA, Loew LM, and Mayer BJ (2012). Stoichiometry of Nck-dependent actin polymerization in living cells. J. Cell Biol 197, 643–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sirotkin V, Berro J, Macmillan K, Zhao L, and Pollard TD (2010). Quantitative Analysis of the Mechanism of Endocytic Actin Patch Assembly and Disassembly in Fission Yeast. Mol Biol Cell 21, 2894–2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Luan Q, Zelter A, MacCoss MJ, Davis TN, and Nolen BJ (2018). Identification of Wiskott-Aldrich syndrome protein (WASP) binding sites on the branched actin filament nucleator Arp2/3 complex. Proc. Natl. Acad. Sci. U.S.A 115, E1409–E1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.LeClaire LL, Rana M, Baumgartner M, and Barber DL (2015). The Nck-interacting kinase NIK increases Arp2/3 complex activity by phosphorylating the Arp2 subunit. J. Cell Biol 208, 161–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.LeClaire LL, Baumgartner M, Iwasa JH, Mullins RD, and Barber DL (2008). Phosphorylation of the Arp2/3 complex is necessary to nucleate actin filaments. J. Cell Biol 182, 647–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smith BA, Daugherty-Clarke K, Goode BL, and Gelles J (2013). Pathway of actin filament branch formation by Arp2/3 complex revealed by single-molecule imaging. Proc. Natl. Acad. Sci. U.S.A 110, 1285–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Smith BA, Padrick SB, Doolittle LK, Daugherty-Clarke K, Corrêa IR, Xu M-Q, Goode BL, Rosen MK, and Gelles J (2013). Three-color single molecule imaging shows WASP detachment from Arp2/3 complex triggers actin filament branch formation. Elife 2, e01008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Robinson RC, Turbedsky K, Kaiser DA, Marchand JB, Higgs HN, Choe S, and Pollard TD (2001). Crystal structure of Arp2/3 complex. Science 294, 1679–1684. [DOI] [PubMed] [Google Scholar]
- 49.Gunning PW, Ghoshdastider U, Whitaker S, Popp D, and Robinson RC (2015). The evolution of compositionally and functionally distinct actin filaments. J Cell Sci 128, 2009–2019. [DOI] [PubMed] [Google Scholar]
- 50.Atilgan E, Wirtz D, and Sun SX (2005). Morphology of the lamellipodium and organization of actin filaments at the leading edge of crawling cells. Biophys. J 89, 3589–3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Michelot A, Berro J, Guérin C, Boujemaa-Paterski R, Staiger CJ, Martiel J-L, and Blanchoin L (2007). Actin-Filament Stochastic Dynamics Mediated by ADF/Cofilin. Current Biology 17, 825–833. [DOI] [PubMed] [Google Scholar]
- 52.Liu S-L, May JR, Helgeson LA, and Nolen BJ (2013). Insertions within the actin core of actin-related protein 3 (Arp3) modulate branching nucleation by Arp2/3 complex. J. Biol. Chem 288, 487–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kuhn JR, and Pollard TD (2005). Real-time measurements of actin filament polymerization by total internal reflection fluorescence microscopy. Biophysical Journal 88, 1387–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.MacLean-Fletcher S, and Pollard TD (1980). Identification of a factor in conventional muscle actin preparations which inhibits actin filament self-association. Topics in Catalysis 96, 18–27. [DOI] [PubMed] [Google Scholar]
- 55.Pollard TD (1984). Polymerization of ADP-Actin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Goode BL (2002). Purification of yeast actin and actin-associated proteins. Methods Enzymol 351, 433–441. [DOI] [PubMed] [Google Scholar]
- 57.Houk TW, and Ue K (1974). The measurement of actin concentration in solution: a comparison of methods. Anal Biochem 62, 66–74. [DOI] [PubMed] [Google Scholar]
- 58.Palmgren S, Ojala PJ, Wear MA, Cooper JA, and Lappalainen P (2001). Interactions with PIP2, ADP-actin monomers, and capping protein regulate the activity and localization of yeast twinfilin. Journal of Cell Biology 155, 251–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ezezika OC, Younger NS, Lu J, Kaiser DA, Corbin ZA, Nolen BJ, Kovar DR, and Pollard TD (2009). Incompatibility with Formin Cdc12p Prevents Human Profilin from Substituting for Fission Yeast Profilin: INSIGHTS FROM CRYSTAL STRUCTURES OF FISSION YEAST PROFILIN*. Journal of Biological Chemistry 284, 2088–2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wagner AR, Luan Q, Liu SL, and Nolen BJ (2013). Dip1 defines a class of Arp2/3 complex activators that function without preformed actin filaments. Current Biology 23, 1990–1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Balzer CJ, Wagner AR, Helgeson LA, and Nolen BJ (2018). Dip1 Co-opts Features of Branching Nucleation to Create Linear Actin Filaments that Activate WASP-Bound Arp2/3 Complex. Current Biology 28, 3886–3891.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video S1: TIRF microscopy videos showing actin assembly in the presence of crosslinked or uncrosslinked Arp2/3 complex, Related to Figure 3.
TIRF microscopy videos showing polymerization of 1.5 uM 33% Oregon-Green actin with uncrosslinked or crosslinked (xl) Arp2/3 complex with or without Las17-VCA, as indicated. Images were acquired at 100x magnification at a rate of 1 frame/sec. Movie playback is 15 frames per second (fps).
Video S2: Fluorescence microscopy videos of reconstituted actin assembly assays containing crosslinked or uncrosslinked Arp2/3 complex, Related to Figure 5.
Videos of reconstituted bead motility assay in the presence of 40 nM uncrosslinked or crosslinked Arp2/3 complex. The video corresponds to the time-lapse series in Figure 5D. Total imaging time of ~45 min was accelerated ~550 fold to create the 5 s video.
Video S3: Brightness and contrast adjusted videos of reconstituted actin assembly assays containing crosslinked or uncrosslinked Arp2/3 complex, Related to Figure 6.
Videos of reconstituted bead motility assay in the presence of 40 nM uncrosslinked or crosslinked Arp2/3 complex. The video corresponds to the time-lapse series in Figure 5D, with the brightness and contrast adjusted to show actin networks disconnected from bead. Total imaging time of ~45 min was accelerated ~550 fold to create the 5 s video.
Data Availability Statement
Data reported in this paper will be shared by the lead contact upon request.
This paper does not report original code.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.