Abstract
Eighty-one clinical isolates of Helicobacter pylori showed no resistance to rifampin (MIC range, 0.032 to 2 μg/ml; MIC at which 50% of isolates are inhibited [MIC50], 0.25 μg/ml). The MIC50 of rifabutin was 0.008 μg/ml (n = 16). All resistant laboratory mutants of H. pylori ATCC 43504 showed amino acid exchanges in codons 524 to 545 or codon 585 of the rpoB gene, corresponding to the gene sequences from Mycobacterium tuberculosis and Escherichia coli.
Rifabutin (RBU) is a spiro-piperidyl-rifamycin derived from rifamycin-S. It is structurally related to rifampin (RFP) and shares many of its properties. RBU has a broad spectrum of antimicrobial activity. It is considerably more active than RFP in vitro against mycobacteria and is active against most gram-positive and many gram-negative bacteria, including Helicobacter pylori (4). RBU is inhibitory in vitro to H. pylori at very low concentrations and is a possible candidate for second- or third-line eradication combination therapy. There are no data available about the resistance rate and resistance mechanisms in H. pylori.
The target of all rifamycins is the DNA-directed RNA polymerase, mostly the β-subunit encoded by the rpoB gene (4). An amino acid exchange encoded in a 69-bp region (codons 511 to 533) of the rpoB gene in mycobacteria (7, 12) or in codons 507 to 533, 563 to 572, and 687 in Escherichia coli (9) induces resistance. For different bacteria, rpoB mutations have been described (1–3, 6, 11, 13). It was shown that a triple therapy containing RBU is effective in the eradication of H. pylori after the failure of other therapies and in spite of resistance to other antibiotics (8). The success rate of a combination of 40 mg of pantoprazole twice daily, 1 g of amoxicillin twice daily, and 400 mg of RBU once daily was 78.6%. Because no resistant clinical isolates in Germany were identified (n = 81), resistant mutants were selected by serial passage in vitro of H. pylori ATCC 43504 and analyzed for mutations in the rpoB gene in this study.
RBU was provided by Pharmacia & Upjohn (Erlangen, Germany). RFP was obtained from Sigma (Deisenhofen, Germany). Susceptibility testing was performed by E-test assay (Biodisk) for RFP and by agar dilution assay (Multipoint AD; Mast) on Wilkins-Chalgren agar with 8% lysed horse blood for both drugs. For the E-test assay, a dense suspension was streaked out with sterile cotton swabs and an E-test strip was placed on the dried plate. The air was evacuated from anaerobe jars (Schütt, Göttingen, Germany) to 4 × 104 Pa with a diaphragm pump and then replaced by a gas mix of 5% O2, 15% CO2, and 80% N2 (Messer, Griessheim, Germany), resulting in an atmosphere of 9% CO2, 11% O2, and 80% N2. The jars were incubated at 36°C for 48 to 72 h.
The MICs of RBU in agar dilution assays ranged from 0.004 to 0.016 μg/ml (MIC at which 90% of the isolates are inhibited [MIC90] = 0.008 μg/ml). The MICs of RFP ranged from 0.064 to 2 μg/ml (MIC90 = 1 μg/ml). In E-test assays the MICs of RFP ranged from 0.032 to 2 μg/ml (MIC90 = 2 μg/ml) (Table 1).
TABLE 1.
Distribution of MICs of RFP for unrelated clinical H. pylori isolates (n = 81), mostly after failure of multiple therapies
| MIC (μg/ml) (E-test) | No. of isolates |
|---|---|
| 0.032 | 4 |
| 0.064 | 5 |
| 0.125 | 9 |
| 0.250 | 23 |
| 0.500 | 14 |
| 1.000 | 15 |
| 2.000 | 11 |
Resistant mutants were generated by placing an RFP disc (RA30; bioMérieux) on an H. pylori culture grown for at least 24 h under optimal conditions. After another 24 to 48 h, colonies in and around the original inhibition zone were placed on selective agar containing 0.032 μg of RBU per ml, or the procedure was repeated. Resistant mutants were usually obtained after the fifth passage. Mutation after one passage was very rare; we estimate that the frequency of resistance is lower than 1 in 109. Mutants of independent passages were analyzed for their resistance level and rpoB sequence alterations.
The MICs of RFP for the resistant mutants ranged from 32 to 64 μg/ml in the agar dilution assay and were higher in the E-test (Table 2). While all mutants were resistant to RFP, the MICs of RBU ranged from 0.064 to 64 μg/ml, suggesting different mutations.
TABLE 2.
MICs of RFP and RBU for a representative selection of resistant mutants of H. pylori type strain ATCC 43504 with codon substitutions in the rpoB gene, clinical isolates, and quality control strains
| Strain | No. of isolates (frequency) | Amino acid positiona and substitution | MIC (μg/ml)b
|
||
|---|---|---|---|---|---|
| RFP
|
RBU (AD) | ||||
| E-test | AD | ||||
| H. pylori | |||||
| ATCC 43504 | None | 0.094 | 0.25 | 0.008 | |
| IV/8 | 1 | L525P | >256 | 64 | 0.25 |
| IV/3 | 2 | Q527K | >256 | 64 | 32 |
| V/11 | 2 | Q527R | >256 | 64 | 64 |
| V/3 | 6 | D530V | >256 | 32 | 16 |
| V/19 | 1 | D530N | >256 | 32 | 8 |
| III/2 | 2 | H540Y | >256 | 32 | 32 |
| VII/4 | 5 | H540N | >256 | 32 | 0.5 |
| V/5 | 5 | S545L | >256 | 32 | 64 |
| II/4 | 2 | I586N | >256 | 32 | 0.25 |
| IV/9 | 1 | I586L | 96 | 32 | 0.064 |
| E. coli ATCC 25922 | 32 | 16 | 8 | ||
| Staphylococcus aureus ATCC 29213 | 0.016 | 0.008 | 0.004 | ||
| Enterococcus faecalis ATCC 29212 | 2 | 4 | 4 | ||
| Clinical isolates | 16 | None | 0.032–2 | 0.064–2 | 0.004–0.016 |
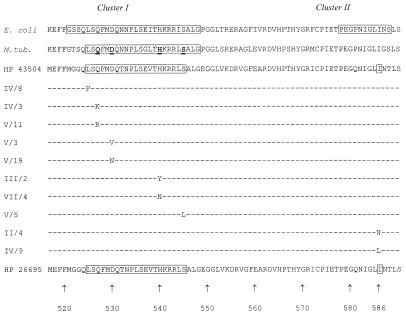
Parts of the rpoB gene product deduced from the published H. pylori genome sequence exhibit amino acid similarity to the homologous resistance-determining regions in E. coli and Mycobacterium tuberculosis (Fig. 1). We amplified a 300-bp sequence containing this region by PCR (forward primer, AAATGATCACAAGCACCATC; reverse primer, ACCTTGCCATCCACAACC). H. pylori ATCC 43504 was used as the wild type, and samples from 16 German and 10 Asian clinical isolates were sequenced to confirm the wild-type sequence. Sequencing was done with an ABI Prism 377 (Perkin-Elmer Applied Biosystems). The entire amino acid sequence of the partial rpoB gene product in H. pylori was conserved in all clinical strains. The observed amino acid substitutions in resistant mutants are shown in Table 2 and Fig. 1.
FIG. 1.
Codon substitutions in resistant mutants of H. pylori type strain ATCC 43504 and correlation to the rpoB resistance-determining regions in E. coli (9) and M. tuberculosis (12). E. coli clusters I and II are resistance-determining regions (9) (GenBank accession no. U77436). For M. tuberculosis (M.tub), bold letters indicate the most frequent in vivo mutations (GenBank accession no. AF060282). HP, H. pylori. The amino acid sequence of the H. pylori 26695 rpoB gene product has been published (10) (GenBank accession no. AE000625). The corresponding sequences from 26 clinical isolates and H. pylori ATCC 43504 were identical to that of H. pylori 26695. Strain designations for laboratory mutants of H. pylori ATCC 43504 are as in Table 2.
There was no RFP resistance detectable by the E-test among the 81 clinical isolates (Table 1). Fifty of these isolates came from patients with one or more therapy failures (metronidazole resistance, 75%; clarithromycin resistance, 62%), and the other 31 isolates were from patients with recent, untreated infections. No subpopulations with different resistance profiles could be identified.
We were able to show that mutational RBU resistance is associated with high-level RFP resistance and with amino acid substitutions between codons 525 and 544 or in codon 585. These regions show high homology to the resistance-determining region in E. coli and mycobacteria. Certain mutations (L525P, H540N, I586N, and I586L) induced a 10- to 50-fold elevation of the RBU MIC only but induced high-level RFP resistance. Which mutations occur in vivo will be determined during clinical trials. The four residues (codons 527, 530, 540, 545) which harbor more than 80% of all resistant clinical isolates in M. tuberculosis (12) also showed the most frequent mutations in our passages of H. pylori. These identical amino acids (Fig. 1) are often replaced by the amino acids that confer resistance in both organisms, as determined by the RFP E-test for H. pylori. As there is no RBU E-test available, we suggest testing RFP to discriminate between susceptibility and emerging resistance to rifamycins. The knowledge of the correlation between the RFP and RBU MICs, and the replacement amino acids at the respective codon positions, might lead to the development of a DNA-based detection system for resistance.
Resistance to RFP and RBU seems to be rare in patient isolates in Germany up until now but can be induced in H. pylori in vitro. We expect that after therapy there will be an increased rate of resistance to rifamycins, as is observed for clarithromycin. Whether this will be higher than with clarithromycin or dependent on different combination partners, e.g. metronidazole, requires further studies.
RBU might be a potent alternative for second- or third-line H. pylori therapy because of its high activity against susceptible bacteria, but clinical trials are warranted. RBU is of great importance for the successful therapy of patients with mycobacteria; however, a broader use of this substance in populations with a high prevalence of tuberculosis might lead to the development of resistance in M. tuberculosis when H. pylori infections are treated in the presence of unrecognized, active tuberculosis. The prevalence of RBU and RFP resistance in H. pylori might also be higher in populations treated for tubercular infections. Therefore, careful monitoring of both agents for resistance development is necessary.
Acknowledgments
We thank R. Birngruber, T. Grundler, and P. Neumann for their greatly appreciated, excellent technical assistance.
REFERENCES
- 1.Abadi F J R, Carter P E, Cash P, Pennington T H. Rifampin resistance in Neisseria meningitidis due to alterations in membrane permeability. Antimicrob Agents Chemother. 1996;40:646–651. doi: 10.1128/aac.40.3.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blanc-Potard A B, Gari E, Spirito F, Figueroa-Bossi N, Bossi L. RNA polymerase (rpoB) mutants selected for increased resistance to gyrase inhibitors in Salmonella typhimurium. Mol Gen Genet. 1995;247:680–692. doi: 10.1007/BF00290399. [DOI] [PubMed] [Google Scholar]
- 3.Enright M, Zawadski P, Pickerill P, Dowson C G. Molecular evolution of rifampicin resistance in Streptococcus pneumoniae. Microb Drug Resist. 1998;4:65–70. doi: 10.1089/mdr.1998.4.65. [DOI] [PubMed] [Google Scholar]
- 4.Kunin C M. Antimicrobial activity of rifabutin. Clin Infect Dis. 1996;22(Suppl. 1):S3–S13. doi: 10.1093/clinids/22.supplement_1.s3. [DOI] [PubMed] [Google Scholar]
- 5.National Committee for Clinical Laboratory Standards. Approved standard M7-A4. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 6.Nolte O. Rifampicin resistance in Neisseria meningitidis: evidence from a study of sibling strains, description of new mutations and notes on population genetics. J Antimicrob Chemother. 1997;39:747–755. doi: 10.1093/jac/39.6.747. [DOI] [PubMed] [Google Scholar]
- 7.Ohno H, Koga H, Kohno S, Tashiro T, Hara K. Relationship between rifampin MICs for and rpoB mutations of Mycobacterium tuberculosis strains isolated in Japan. Antimicrob Agents Chemother. 1996;40:1053–1056. doi: 10.1128/aac.40.4.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perri F, Festa V, Andriulli A. Treatment of antibiotic-resistant Helicobacter pylori infection. N Engl J Med. 1998;339:53. doi: 10.1056/NEJM199807023390116. [DOI] [PubMed] [Google Scholar]
- 9.Severinov K, Soushko M, Goldfarb A, Nikiforov V. Rifampicin region revisited. New rifampicin-resistant and streptolydigin-resistant mutants in the beta subunit of Escherichia coli RNA polymerase. J Biol Chem. 1993;268:14820–14825. [PubMed] [Google Scholar]
- 10.Tomb J F, White O, Kerlavage A R, Clayton R A, Sutton G G, Fleischmann R D, Ketchum K A, Klenk H P, Gill S, Dougherty B A, Nelson K, Quackenbush J, Zhou L, Kirkness E F, Peterson S, Loftus B, Richardson D, Dodson R, Khalak H G, Glodek A, McKenney K, Fitzegerald L M, Lee N, Adams M D, Venter J C. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature. 1997;388:539–547. doi: 10.1038/41483. [DOI] [PubMed] [Google Scholar]
- 11.Troyer J M, Radulovic S, Andersson S G E, Azad A F. Detection of point mutations in rpoB gene of rifampin-resistant Rickettsia typhi. Antimicrob Agents Chemother. 1998;42:1845–1846. doi: 10.1128/aac.42.7.1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williams D L, Spring L, Collins L, Miller L P, Heifets L B, Gangadharam P R J, Gillis T P. Contribution of rpoB mutations to development of rifamycin cross-resistance in Mycobacterium tuberculosis. Antimicrob Agents Chemother. 1998;42:1853–1857. doi: 10.1128/aac.42.7.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yee Y C, Kisslinger B, Yu V L, Jin D J. A mechanism of rifamycin inhibition and resistance in Pseudomonas aeruginosa. J Antimicrob Chemother. 1996;38:133–137. doi: 10.1093/jac/38.1.133. [DOI] [PubMed] [Google Scholar]