Abstract
Background:
The variable response to fat-soluble vitamin supplementation in young children with CF, and factors contributing to this variability, remain under-investigated.
Objective:
To determine if recommended supplement doses normalize serum vitamin A (retinol), D (25-hydroxy-vitamin D, 25OHD) and E (α-tocopherol), and identify factors predictive of achieving sufficiency, in children with CF in the first 3 years of life.
Design:
We studied 144 infants born during 2012–2017 and diagnosed with CF through newborn screening. Serum retinol, 25OHD, α-tocopherol and plasma cytokines interleukin(IL)-6, IL-8, IL10 and tumor necrosis factor (TNF)-α were measured in early infancy and yearly thereafter. Vitamin supplement intakes and respiratory microbiology were assessed every 1–2 months in infancy and quarterly thereafter.
Results:
The prevalence of vitamin D insufficiency (<30 ng/mL) at all ages combined was significantly higher (22%) compared to vitamin A (<200 ng/mL, 3%) and vitamin E (<5 μg/mL, 5%). All children were vitamin A sufficient by age 2 years. Vitamin E insufficiency was rare. Only 42% were early responders of vitamin D and 17% remain insufficient despite high supplement intakes. IL-6 was positively correlated, while IL-8, IL-10 and TNF-α were negatively correlated, with retinol and 25OHD. Multiple regression analysis revealed that supplement dose, season, α-tocopherol, pancreatic insufficiency, respiratory infections and IL-10 were significant predictors of 25OHD.
Conclusion:
Diagnosis through newborn screening coupled with supplementation normalized serum retinol and α -tocopherol in almost all infants with CF by age 3 years. However, response to vitamin D supplements in young children with CF occurred later and variably despite early and sustained supplementation.
Keywords: cystic fibrosis, fat-soluble vitamins, vitamin D, 25-hydroxyvitamin D, vitamin D supplementation, vitamin A, vitamin E, infection, inflammation, children
INTRODUCTION
Cystic fibrosis (CF) is a relatively common autosomal recessive disorder caused by mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene. Exocrine pancreatic insufficiency (PI) due to CFTR dysfunction leads to malabsorption, particularly of fat, and nutrient deficiencies that typically begin during early infancy (1–3). Therefore, risks for fat-soluble vitamins A, D and E deficiencies in individuals with CF have been a longstanding concern (4–8) and have been implicated as playing a role in lung disease (9–12) along with macronutrient deficiencies (4–8). This has led to clinical care practices of routinely monitoring biomarkers of vitamins A (retinol), D (25-hydroxyvitamin D, 25OHD) and E (α-tocopherol), as well as prescribing large supplements. Specifically, the US Cystic Fibrosis Foundation (CFF) recommends daily supplementation of 1500 IU of vitamin A, 400–500 IU of vitamin D, and 40–50 IU of vitamin E for infants with CF (6–8). These dosages increase to 5000 IU of vitamin A, 800–1000 IU of vitamin D and 80–150 IU of vitamin E for children 1–10 y of age (6–8). Although deficits of vitamins A and vitamin E insufficiency appear to be largely corrected and their serum concentrations normalized (13–15), vitamin D has been an enigma, because variable responses to even very large daily doses of supplement have been observed (16–18).
Many studies have examined vitamin D status in older children and young adults with CF (9, 19–21). The reported prevalence of vitamin D insufficiency (serum 25OHD <30 ng/mL) ranged widely from 23% to 90% (20, 21), most likely due to differences in the study population, amount of vitamin D intake, as well as the methodology and season of 25OHD measurement. However, few studies have focused on vitamin D status in infants with CF primarily because this population was not available to study extensively until newborn screening (NBS) for CF was widely implemented (22). A 13-year prospective study conducted in infants diagnosed with CF via NBS found vitamin D deficiency (25OHD <14 ng/mL) in 23% of infants at 2 mo, 4% at 6 m, 2% at 12 mo, and 5% at 24 mo of age (23). A more recent study observed that 51% of infants diagnosed via NBS had serum 25OHD <30 ng/mL at 1 y of age (24). However, this study only reported a one-time assessment of vitamin D status and did not investigate other factors potentially contributing to hypovitaminosis D in CF such as decreased sun exposure and adherence to the prescribed vitamin D regimen. In addition, CF is characterized by lung disease that has been related to vitamin D status by some researchers (9–12), as well as neutrophil-dominated infiltration of the airways, even in infants as young as 3 mo of age (25, 26). However, no studies in young children with CF have examined the relationships between 25OHD and pulmonary infections and inflammations in CF, as pointed out in a recent, comprehensive review by Daley et al (9).
The present study aims to evaluate fat soluble vitamins A, D and E status in a multi-center prospective longitudinal study known as FIRST (Feeding Infants Right…from the STart). Specifically, we determined the prevalence of suboptimal vitamins A, D and E status, examined if supplementation according to CFF recommended doses (6–8) effectively normalized serum concentrations, and identified factors associated with vitamin D insufficiency including respiratory infections and inflammatory markers in children with CF in the first 3 y of life.
METERIALS AND METHODS
Study Design and Population
The FIRST cohort consists of 183 infants who were born during 2012–17, enrolled after NBS at age 1.8 ± 1.0 mo, and then are being followed at six CF Centers (Madison and Milwaukee, WI; Boston, MA; Indianapolis, IN; Salt Lake City, UT and Chicago, IL) until all children reach age 6 y in 2023. Study visits are conducted in conjunction with routine clinical care at these CF centers according to clinical practice guidelines for treatment and regular follow up evaluations (6, 8), i.e., monthly after diagnosis until age 6 mo, bi-monthly from 6 to 12 mo, and every 3 mo thereafter until 6 y of age. Blood specimens are also collected concurrently with clinical blood draws without additional phlebotomy at ~4 mo of age and annually thereafter. Serum and whole blood specimens from all study sites are sent to UW-Madison for biomarker measurements. The FIRST project was approved by the Institutional Review Boards at all participating institutions. Informed written consent was obtained from the parents/guardians of all participating infants.
As of 12/31/2020, the entire FIRST cohort had reached age 3 y of age; 172 (94%), 160 (87%) and 145 children (79%) completed follow-up to age 12, 24 and 36 mo, respectively. Among 145 children who completed follow-up to age 36 mo, 144 children totaling 518 measurements were included in the analyses.
Assessment of vitamins A, D and E status
Concentrations of retinol, 25OHD and α-tocopherol were measured in serum, or plasma when serum was not available, in the Clinical Laboratory Services of the University of Wisconsin Hospital using high-performance liquid chromatography. Vitamins A, D and E insufficiencies were defined as: retinol <200 ng/mL (4, 5), 25OHD <30 ng/mL (7), and α-tocopherol <5 μg/mL (13). The decision to use α-tocopherol rather than using the ratio of α-tocopherol to serum total lipids was based on two considerations. First, a series of studies (13, 27–32) by one of the co-authors (PMF) have demonstrated the validity of using α-tocopherol <5 μg/mL as the threshold value for defining vitamin E insufficiency in children with CF as compared to using the ratio of α-tocopherol to total serum lipids (threshold=0.8) based on biological antioxidant measures such as the erythrocyte hydrogen peroxide hemolysis test (29, 30). These studies also validated the use of normal vitamin E status in α-tocopherol supplemented patients as an indicator of adherence (28–32). Second, α-tocopherol concentration in μg/mL and a 3–5 μg/mL threshold are routinely used by CF centers as the indicator for vitamin E sufficiency in the care of patients and thus are commonly recognized (4–6).
Parent-reported intakes of vitamin supplements were obtained using a nutritional interval history questionnaire administered by trained research coordinator or clinical nutritionist at each CF center visit. Supplement intakes meeting the CFF guidelines (6–8) were defined as: ≥1500 IU in infancy and ≥5000 IU after 15 mo of age for vitamin A, ≥400 IU in infancy and ≥800 IU after 15 mo of age for vitamin D, and ≥40 IU in infancy and ≥80 IU after 15 mo of age for vitamin E.
Respiratory infections and inflammatory markers
At each FIRST study visit, a pulmonary interval history questionnaire is administered by trained research coordinators to collect data on respiratory symptoms, infections, and hospitalizations during the interval since the last visit. Data from 518 respiratory cultures obtained at the same as serum vitamin measurements were included in the analyses. Respiratory culture results were classified into 5 categories: normal flora, positive for Staphylococcus aureus (SA), positive for Pseudomonas aeruginosa (PA), positive for both SA and PA, and positive for organisms other than SA and PA (non-SAPA).
Four plasma cytokines, IL-6, IL-8, IL-10 and TNF-α, were quantified using the MILLIPLEX® MAP human cytokine/chemokine magnetic bead panel in the Luminex analyzer MAGPIX®, (EMD Millipore, Millipore Sigma, Merck KGaA, Darmstadt, Germany) according to the manufacturer’s instructions (33). Of the 518 blood samples with vitamin measurements, 385 had plasma available for cytokine measurements. Elevated cytokine levels were defined using values published from the Associated Regional and University Pathologists, Inc (ARUP) Laboratories (https://ltd.aruplab.com/Tests/Pub/0051394) as follows: IL-6 >2.5 pg/mL, IL-8 >9.4 pg/mL, IL-10 >5.3 pg/mL and TNF- α >14.5 pg/mL.
Other relevant covariates
Information on CF-causing variants in the CFTR gene, gestational age, birth weight and length, and the occurrence of meconium ileus (MI) were collected from electronic medical records. Pancreatic functional status was assessed at 2, 4, 6, 8, 12 mo of age and annually thereafter by fecal elastase-1 concentration. Children with CF were classified as pancreatic sufficient (PS) if their fecal elastase-1 were consistently >200 μg/g in the first 2 y of life (34).
Demographics were recorded by the research coordinators at enrollment. Race and ethnicity were termed recommended by the NIH, i.e., White, Black or African American, Asian, American Indian or Alaska Native, Native Hawaiian or other Pacific Islander, and More than One Race for race, and Hispanic or Latino and Not Hispanic or Latino for ethnicity. Socioeconomic status (SES) such as parent’s education, annual household income and health insurance coverage are being collected semi-annually via a parent questionnaire.
Statistical analysis
Analysis of variance and the median test were used to compare means and medians, respectively. Chi-square test and Fisher’s exact test (when sample size was <5 in any subgroup) were used to compare proportions. Correlations between serum vitamin and plasma cytokine concentrations were assessed by Pearson’s correlation coefficient.
To identify factors significantly associated longitudinal serum retinol, 25OHD and α-tocopherol through 3 y of age, multiple regression analyses using mixed effects with repeated measures and an autoregressive covariance were performed. To assess the likelihood of being a vitamin D responder, ordinal logistic regression analysis was performed using 5 levels of responder status in the following order: responders at age 4 mo, 1 y, 2 y, 3 y, respectively, and transient and non-responders. All analyses were performed by using SAS (version 9.4).
RESULTS
Characteristics of the study population (Table 1)
Table 1.
Characteristics of the study population* (N=144)
| CF baseline characteristics: | |
|---|---|
| CFTR genotype | |
| F508del/F508del | 74 (51%) |
| F508del/other | 59 (41%) |
| Other/other | 11 (8%) |
| Pancreatic phenotype | |
| Meconium ileus (MI)† | 23 (16%) |
| Pancreatic insufficiency (PI) | 103 (72%) |
| Pancreatic sufficiency (PS) | 18 (13%) |
| Birth weight (g) | 3236 ± 505 |
| Premature (gestational age <37 weeks) | 15 (10%) |
| Low birth weight (<2500 g) | 5 (3%) |
| Demographic, socioeconomic and family characteristics: | |
| Female sex | 66 (46%) |
| Race: Non-White | 4 (3%) |
| Ethnicity: Hispanic | 7 (5%) |
| Parental education: community college or above | |
| Mother & father | 90 (62%) |
| Mother | 11 (8%) |
| Father | 12 (8%) |
| Neither | 31 (22%) |
| Household annual income | |
| < $40,000 | 43 (30%) |
| $40,000-$79,000 | 48 (33%) |
| ≥$80,000 | 53 (37%) |
| Type of health Insurance | |
| Medicaid or other public insurance | 53 (37%) |
| Private health insurance | 91 (63%) |
Values are N(%) of subjects or mean ± SD
All MI subjects were PI as defined by fecal elastase-1 criterion as described in the METHODS section
CFTR genotypes classified by the predominant mutation F508del and pancreatic phenotypes resembled the national CF population as reported in the 2019 CFF Patient Registry (35). Compared to the PS phenotype, proportionately more infants with MI or PI had homozygous F508del (59% vs. 0%) and heterozygous F508del (35% vs. 83%) but less non-F508del genotype (6% vs. 17%), p<0.001. Our study cohort only had four Non-White subjects: one Black and three identifying as More Than One Race. About 60% of the subjects had at least one parent with an education of community college or above, and one-third had Medicaid or other public health insurance.
Serum concentrations and supplement intakes of vitamins A, D and E (Figure 1)
FIGURE 1.
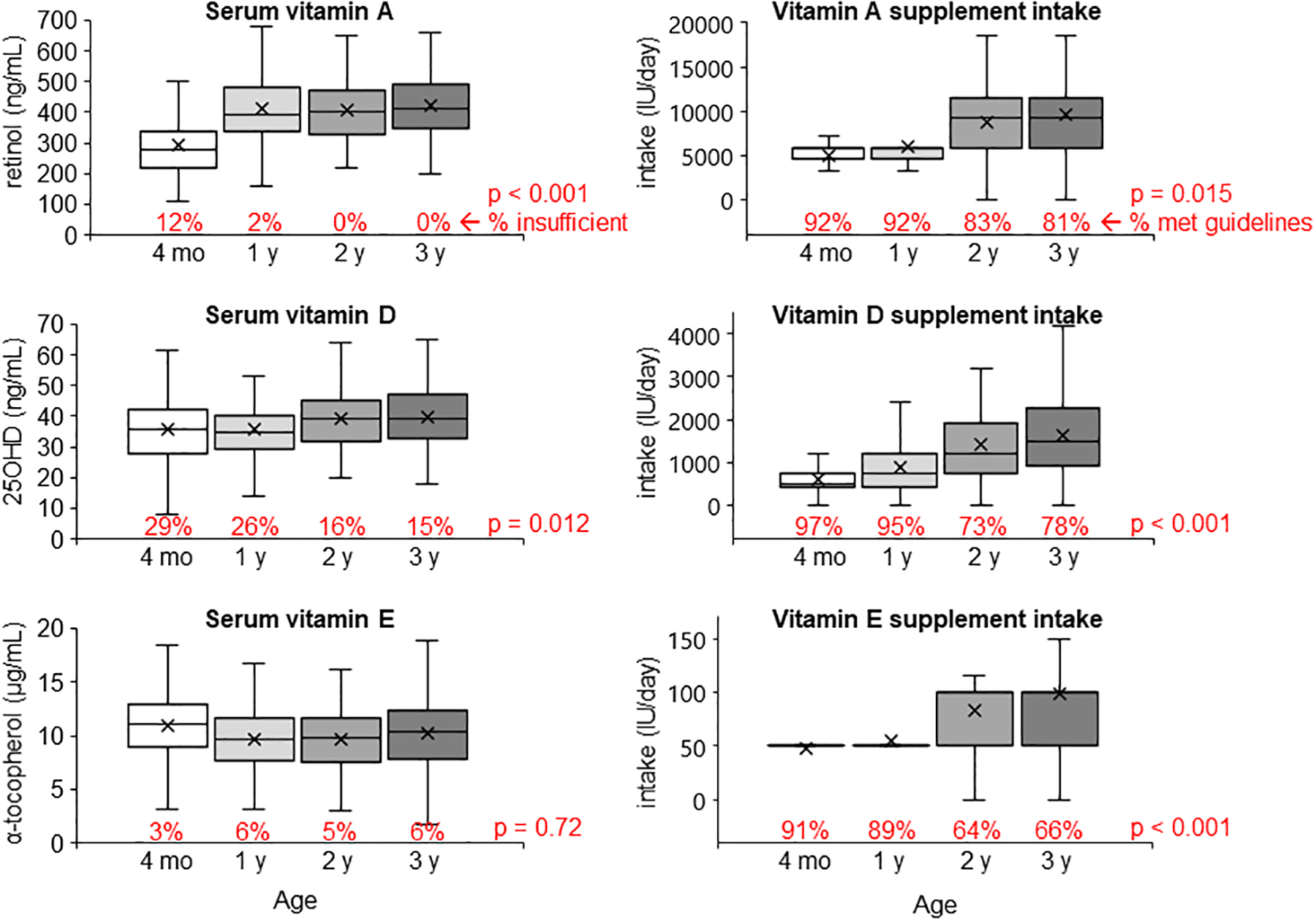
Boxplots of serum concentrations and supplement intakes of vitamins A, D and E at ages 4 mo, 1 y, 2 y and 3 y of age. The percentage under each bar indicates the prevalence of vitamin insufficiency (retinol <200 ng/mL; 25OHD <30 ng/mL; α-tocopherol <5 μg/mL) or supplement intake meeting the lower limit recommended in the CFF guidelines (vitamin A: ≥1500 IU for age ≤ 1 y and ≥5000 IU for age ≥2 y; vitamin D: ≥400 IU for age ≤ 1 y and ≥800 IU for age ≥2 y; vitamin E: ≥40 IU for age ≤ 1 y and ≥80 IU for age ≥2 y). P values indicate differences across age groups.
Serum retinol and 25OHD but not α-tocopherol improved significantly from infancy to 3 y of age (Figure 1, left panels). The prevalence of vitamin D insufficiency at all ages combined was 22%, which was significantly higher than vitamin A (3%) and vitamin E (5%). Regarding 25OHD, we observed the expected seasonal variation (36, 37), i.e., significantly higher in summer months (Jun-Aug, 39.1 ± 10.9) compared to winter months (Dec-Feb, 35.1 ± 9.9), p=0.005. Children with MI or PI had 2-fold higher prevalence of vitamin D insufficiency (23% vs. 11%, p=0.031) compared to those with PS, although their prevalence of vitamin D insufficiency did decrease by about half from 30% in infancy (30%) to 16% at 3 y of age (p=0.005).
Supplement doses increased with age for all 3 vitamins (Figure 1, right panels). The percentages of children who met the CFF guidelines for supplement doses were significantly higher during infancy than at 2–3 y of age, and in children with MI or PI compared to PS, for all 3 vitamins. Specifically, 91% and 88% of MI and PI children met the CFF guidelines for vitamin A and D supplement intakes, respectively, while in PS children, only half met the vitamin A supplement guidelines and about two-thirds met the vitamin D supplement guidelines. Additionally, we also found that vitamin supplement doses were significantly associated with parental education (higher among subjects who had one or both parents having community college or higher educational level compared to neither, p < 0.01 for all 3 vitamins) but not household income level or the type health insurance.
Individual response to vitamins A, D and E supplementation (Figure 2)
FIGURE 2.
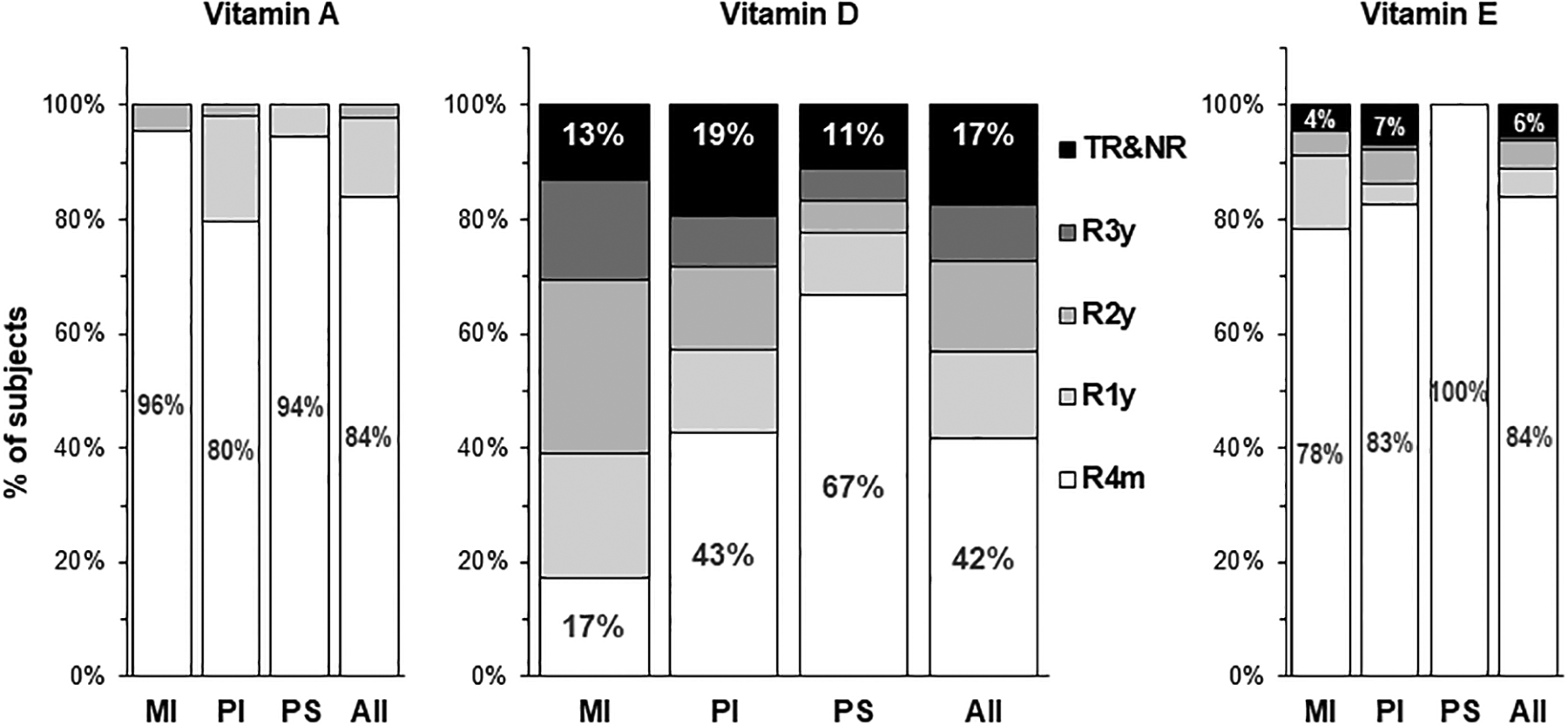
Distribution of vitamins A, D and E responder groups in the first 3 y of life by pancreatic phenotype, meconium ileus (MI), pancreatic insufficiency (PI) and pancreatic sufficiency (PS). R4m, R1y, R2y and R3y denote responders who achieved vitamin sufficiency (retinol ≥200 ng/mL; 25OHD ≥30 ng/mL; α-tocopherol ≥5 μg/mL) at age 4 mo, 1 y, 2 y and 3 y, respectively. TR&NR denotes transient responders and non-responders who temporarily or never achieved sufficiency in the first 3 y of life.
In conjunction with population level analyses, we also examined each subject’s serum retinol, 25OHD and α-tocopherol longitudinally over their first 3 y of life applying the responder/non-responder concept we pioneered (38–40) to determine if and at what age they achieved sufficiency (responders) or did not achieve sufficiency status (non-responders) for each vitamin. Greater than 80% of the FIRST cohort achieved vitamin A and E sufficiency in early infancy (the R4m group in Figure 2), while for vitamin D, only 42% were early responders. All children were vitamin A responders by 2 y of age, and almost all (94%) were vitamin E responders by 3 y of age.
In contrast, vitamin D responder status varied significantly by pancreatic phenotype – children with PS had the highest percent of responders and achieved it at earlier ages, followed by children with PI, and lowest and latest in children with MI. In addition, 17% were transient or non-responders who only temporarily or never achieved vitamin D sufficiency by 3 y of age. Among them, 56% always met or exceeded the CFF guidelines for vitamin D supplement intakes (400–1350 IU in infancy and 800–4200 IU at age 1–3 y), the other 44% had vitamin D supplement intakes occasionally below the recommended lower limit of 400 IU in infancy (range: 200–1075 IU) and 800 IU after 1 y of age (range: 400–1800 IU at age 1–3 y).
Taken together, results from Figures 1 and 2 indicated that, in our study cohort, all children were vitamin A sufficient by 2 y of age. Vitamin E insufficiency was rare, while vitamin D insufficiency was prevalent despite high vitamin D supplement intakes.
Respiratory infections, inflammations, and serum vitamins A, D and E status
We next examined if vitamins A, D and E status varied according to culture positivity for SA, PA or non-SAPA organisms as indicative of respiratory infections and four cytokines best characterized in the CF population, namely, IL-6, IL-8, IL-10, and TNF-α (41).
As shown in the top left panel of Figure 3, in the first 3 y of life, 196 (38%) of the cultures were normal flora, among them, the prevalence of vitamin A insufficiency was significantly lower in respiratory cultures with normal flora (1%) in comparison with the other 322 (62%) cultures that were positive for any organisms (5%), p=0.023. The same association was noted regarding vitamin D (top panel of Figure 4, 16% vs. 25%, p=0.022). Subsequent analyses revealed that these associations were primarily attributable to non-PA organisms (Figures 3 and 4, top right panels). No significant association was found between respiratory infections and vitamin E insufficiency
FIGURE 3.
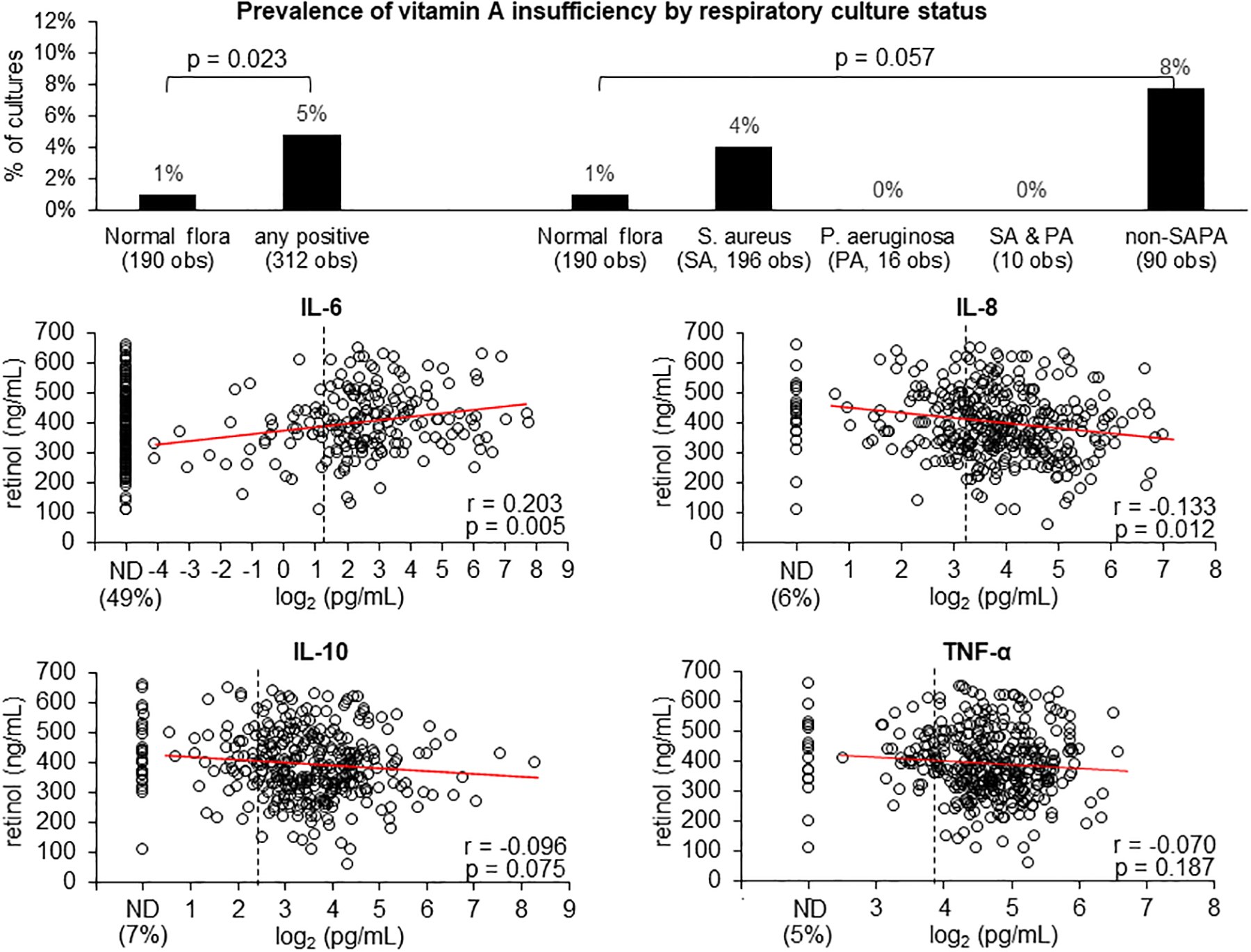
Top panel shows the prevalence of vitamin A insufficiency (serum retinol <200 ng/mL) by respiratory cultures classified into 5 categories: normal flora, positive for Staphylococcus aureus (SA), positive for Pseudomonas aeruginosa (PA), positive for both SA and PA, and positive for organisms other than SA and PA (non-SAPA). Bottom 4 panels show the correlations between serum retinol and 4 plasma cytokines with corresponding Pearson’s correlation coefficients (r) and p values. The percentages of samples with not-detectable (ND) values are shown on the left of the x-axis. Elevated cytokine levels (pg/mL) are defined as IL-6 >2.5, IL-8 >9.4, IL-10 >5.3 and TNF- α >14.5 and shown by the vertical dashed lines.
FIGURE 4.
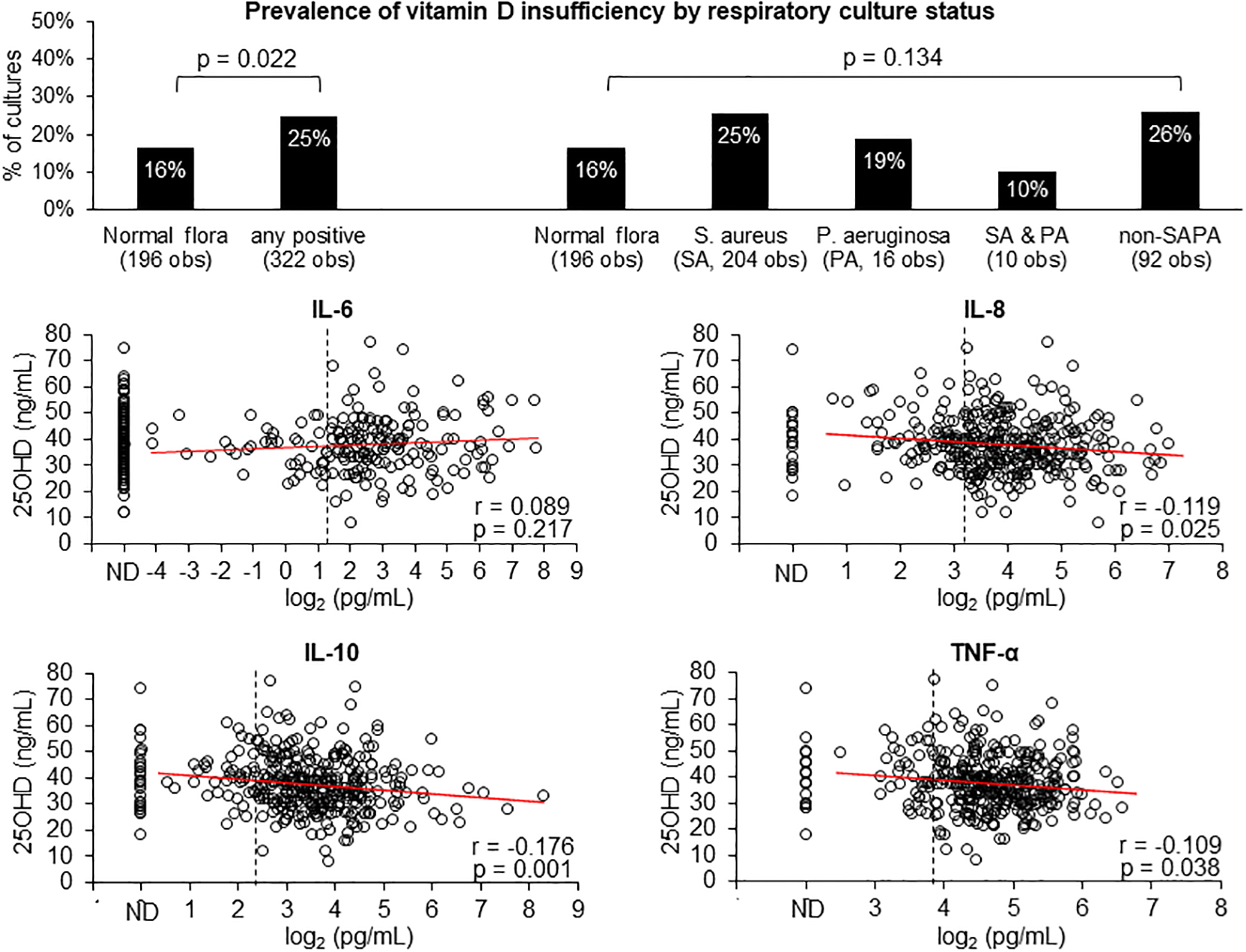
Top panel shows the prevalence of vitamin D insufficiency (serum 25OHD <30 ng/mL) by respiratory cultures classified into 5 categories: normal flora, positive for Staphylococcus aureus (SA), positive for Pseudomonas aeruginosa (PA), positive for both SA and PA, and positive for organisms other than SA and PA (non-SAPA). Bottom 4 panels show the correlations between serum 25OHD and 4 plasma cytokines with corresponding Pearson’s correlation coefficients (r) and p values. The percentages of samples with not-detectable (ND) values are shown on the left of the x-axis. Elevated cytokine levels (pg/mL) are defined as IL-6 >2.5, IL-8 >9.4, IL-10 >5.3 and TNF- α >14.5 and shown by the vertical dashed lines.
The prevalence of elevated plasma cytokines in the FIRST cohort in their first 3 y of life was high: 41%, 72%, 82%, and 85% for IL-6, IL-8, IL-10 and TNF-α, respectively. Among samples with detectible values, IL-6 was positively correlated, while IL-8, IL-10 and TNF-α were negatively correlated, with serum retinol (Figure 3) and 25OHD (Figure 4). Most of these correlations were statistically significant. For vitamin E, only IL-10 was found to be negatively correlated with α-tocopherol (r=−0.117 and p=0.029).
Predictors of 25OHD and vitamin D responder status by multiple regression analysis
Our final analysis used multiple regression models to identify factors predictive of vitamin D status. Because IL-8, IL-10 and TNF-α were highly correlated (r>0.56 for all pairwise correlations), we chose to include IL-10 (lowest p-value in the correlation analysis shown in Figure 3) and IL-6 (low correlations with all other 3 cytokines, r<0.20 for all pairwise correlations) in the multiple regression models.
Longitudinal mixed effects model showed that vitamin D supplement dose, season, serum α-tocopherol, pancreatic phenotype, respiratory infections, and plasma IL-10 were significant predictors of serum 25OHD in children with CF in their first 3 y of life (Table 2). Additionally, ordinal logistic regression model showed that, at the subject level, the likelihood of being an early vitamin D responder was significantly higher with having a sufficient vitamin E status (i.e., serum α-tocopherol always >5 μg/mL), PS rather than MI or PI, normal flora in all respiratory cultures, and non-elevated plasma IL-10.
Table 2.
Factors associated with vitamin A and D status in children with CF in their first 3 years of life
| Longitudinal Mixed Effects Model: | Coefficient | p value |
|---|---|---|
| Outcome: 25OHD concentration (ng/mL) | ||
| Predictors of interest*: | ||
| Vitamin D supplement dose (unit: 400 IU) | 1.3 | <0.001 |
| Serum α-tocopherol | 0.8 | <0.001 |
| Season at 25OHD measurement (ref=Winter, Dec-Feb) | ||
| Fall (Sep-Nov) | 2.8 | 0.016 |
| Spring (Mar-May) | 3.4 | 0.003 |
| Summer (Jun-Aug) | 3.8 | 0.002 |
| Age (years) at 25OHD measurement | 1.6 | 0.002 |
| Meconium ileus (MI) vs. pancreatic sufficiency (PS) | −6.0 | 0.033 |
| Pancreatic insufficiency (PI) vs. pancreatic sufficiency (PS) | −5.1 | 0.023 |
| Respiratory cultures (ref=normal flora) | ||
| Staphylococcus aureus (SA) | −1.2 | 0.202 |
| Pseudomonas aeruginosa (PA) | −1.3 | 0.494 |
| SA and PA | 8.4 | 0.006 |
| Non-PASA | 1.2 | 0.252 |
| Plasma interleukin (IL)-6 (ref=not detectible) | ||
| normal | 0.9 | 0.487 |
| elevated‡ | −0.4 | 0.645 |
| Plasma IL-10 (ref=not detectible) | ||
| normal | 4.0 | 0.028 |
| elevated‡ | 3.7 | 0.017 |
| Ordinal Logistic Regression Model: | Odds Ratio (95% CI) | p value |
| Outcome: vitamin D responder groups† | ||
| Predictors of interest: | ||
| Average vitamin D supplement dose (unit: 400 IU) | 1.00 (0.99 – 1.01) | 0.828 |
| Serum α-tocopherol always >5 μg/mL | 4.12 (1.53 – 11.06) | 0.005 |
| MI vs. PS | 0.18 (0.05 – 0.71) | 0.021 |
| PI vs. PS | 0.31 (0.10 – 0.95) | 0.034 |
| % cultures with any positive organisms | 0.35 (0.12 – 1.00) | 0.051 |
| % plasma with elevated IL-6 | 1.58 (0.64 – 3.86) | 0.321 |
| % plasma with elevated IL-10 | 0.14 (0.04 – 0.51) | 0.003 |
Additional covariates adjusted in the model: sex, race, ethnicity, parental education, household annual income level, type of health insurance.
5 groups in the following order: responders at 4 mo, 1 y, 2 y, 3 y, respectively, and transient and non-responders. Probabilities modeled were cumulated over the lower ordered values.
Based on the reference values published by the ARUP Laboratories (https://ltd.aruplab.com/Tests/Pub/3002601); IL-6: >2.5 pg/mL, IL-10: >5.3 pg/mL).
DISCUSSION
To our knowledge, this study provides the first longitudinal, comprehensive analysis of fat-soluble vitamins A, D and E status, as well as their associations with respiratory infections and inflammatory markers in very young children with CF. The design of the FIRST Project with enrollment in early infancy after NBS-facilitated diagnosis enabled us to address a longstanding enigma about vitamin D insufficiency as we focused on its onset, persistence, and some potential explanatory factors. Most importantly, the data reported here were collected prospectively and systematically at frequent intervals, which is critical during early life when growth and development occur at the fastest rate of all life stages. Our study demonstrated that infants with CF diagnosed through NBS responded to vitamins A and E supplementation quickly and almost universally (>90% achieved sufficiency status by 1 y of age), while response to vitamin D supplementation occurred more slowly (<50% in infancy) and variably (17% failed to respond by age 3 y) despite early and sustained supplementation. Although the prevalence of vitamin D insufficiency in the CF population has often been reported in older patients (16–25), our study is the first to evaluate individual response longitudinally from early infancy to 3 y of age. This allows us to conclude that vitamin D insufficiency is a relatively common feature in the early phases of this disease.
For late, transient, and non-responders of vitamin D, there are several clinically relevant questions. First, what are the determinants of delayed or lack of response? Second, does prolonged vitamin D insufficiency in very young children with CF leads to poor bone mineral density later in life, or predispose them to other adverse health outcomes shown to be associated with suboptimal vitamin D such as increased risks/susceptibility to infections and/or dysregulated immune system (36)? Third, how should persistent hypovitaminosis D in young children with CF be managed in routine clinical care?
Our data reveal that predictors of 25OHD concentrations and responder status include the vitamin D supplement dose, serum α-tocopherol, season, pancreatic functional status, and respiratory infections. It was surprising to us that at 2 and 3 y of age, 27% and 22% of the patients, respectively, were not meeting the 800 IU/day guideline of the CFF for supplementation (Figure 1). The most obvious explanation for vitamin D non-responsiveness one would suspect is lack of adherence to supplementation. Among transient and non-responders of vitamin D in our study cohort, 24% also had low vitamin E (α-tocopherol <5 μg/mL), an indirect indicator of non-adherence we established previously (13, 28–32). The remaining 75% of transient and non-responders of vitamin D had normal serum α-tocopherol and retinol concentrations, therefore, factors other than adherence are more likely to explain their inadequate response to vitamin D supplements. For example, SES factors such as health insurance coverage may affect access to vitamin supplements, although our analysis revealed that, parental education (i.e., at least one parent completing community college or above) but not health insurance or household income were associated with higher intakes of vitamin D supplement doses.
Not surprisingly, season was found to have a significant impact on 25OHD as others have reported (36, 37), although not in a cohort as young as the children we studied. Our finding underscores the special need for recommended vitamin D supplements during December through February. Reduced sunlight exposure during winters creates unique challenges for parents to maintain doses of fat-soluble vitamin supplements just as summer months require more attention to salt supplements.
Genetic variation is another probable factor influencing vitamin D responsiveness observed in our study cohort. Twin and family studies have reported a wide range of heritability estimates on 25OHD concentrations, ranging from 0% (40) to 90% (37, 42). Genome-wide association studies have identified common single-nucleotide polymorphisms located in biologically plausible genes and more than 100 loci in regions (43) that were associated with 25OHD concentrations. Investigating the genetic contribution to vitamin D response in the CF population will provide new insight for developing personalized treatment strategies to help each individual CF patient achieve success in vitamin D supplementation.
The traditional concern for children with CF exposed to prolonged vitamin D insufficiency is impaired bone health. Current CF guidelines recommend screening bone disease with dual energy x-ray absorptiometry starting at the age of 8–10 y (44). As bone accretion accumulates over time and is crucial during early childhood, we recommend monitoring bone mineral density earlier than 8 y of age for vitamin D non-responders.
Besides bone health, anti-bacterial and anti-inflammatory properties have been attributed to vitamin D (10). In CF, pulmonary exacerbations (11) and dysfunction (12) have been reported to be associated with vitamin D deficiency. In support of these findings, we also observed that the prevalence of vitamin A and D insufficiencies were significantly higher in respiratory cultures with non-normal flora compared to those with normal flora. However, our data also revealed that these associations were primarily attributable to non-PA infections. This observation is surprising and the underlying explanations are uncertain; however, one possibility is that there were few positive PA cultures (only 26 out of 518) in this young cohort of CF children (age ≤3 y) and that these early PA colonizations were eradiated successfully with treatment protocols used in the CF centers. The difference in vitamin D insufficiency prevalence between the PA and non-SAPA groups were in the same trend but not as large as in vitamin A.
With respect to inflammation, vitamin D has been shown to down-regulate proinflammatory cytokines IL-6, IL8 and TNF-α and upregulate anti-inflammatory cytokine IL-10 (45). However, other investigators dispute these conclusions (9, 46). In our study, we also did not observe significant associations between IL-6 and 25OHD, and results in IL-10 were inconsistent (higher IL-10 associated with higher 25OHD in the mixed effects model but less likely to be an early responder in the logistic regression model). Taken together, these findings indicate that the interrelationships between respiratory infections, cytokine disturbances, and vitamin D status in the CF population are complex, especially during early childhood when the immune system is still being developed. Therefore, longer periods of time are needed to characterize their temporal relationships.
The limitations of our study include a paucity of African-American and Hispanic CF patients and the use of α-tocopherol concentrations in μg/mL rather than adjusted with a serum lipid denominator. Although CF is traditionally been considered a genetic disease of Caucasians, patients diagnosed through newborn screening during the past decade have been recently shown to include 20% nonwhite children (47). We made every effort to recruit minorities, particularly by expanding enrollment opportunities to include the Chicago area and approaching all eligible families for consent.
In conclusion, vitamin D insufficiency in CF is unique compared to fat-soluble vitamins A and E because it develops early but is resistant to correction with sustained supplementation. Elucidation of the causes of this abnormality and its impact will require further longitudinal studies. In the meantime, for transient or non-responders to standard vitamin D supplementation, increased efforts should be made to rule out/minimize other factors that may reduce vitamin D bioavailability; namely, repeating assessment of PI, optimizing pancreatic enzyme replacement therapy, and adjusting adjuvant therapy such as acid blockers.
ACKNOWLEDGEMENTS
We thank the following faculty members at the six participating CF centers that assumed the leadership role in the Feeding Infants Right… From the Start (FIRST) Project: Michael Rock, MD (University of Wisconsin – Madison and American Family Children’s Hospital, Madison, WI), Nick Antos, MD and Hara Levy, MD (Medical College of Wisconsin and Children’s Hospital of Wisconsin, Milwaukee, WI), Jon Gaffin, MD and Henry Dorkin, MD (Harvard University and Children’s Hospital Boston, Boston, MA), Michelle Howenstine, MD and Clement Ren, MD (Indiana University and Riley Children’s Hospital, Indianapolis, IN), Fadi Asfour, MD and Barbara Chatfield, MD (University of Utah and Intermountain Primary Children’s Hospital, Salt Lake City, UT), and Suzanna McColley, MD and Hara Levy, MD (Northwestern University and Lurie Children’s Hospital, Chicago, IL).
We are most grateful for the following research coordinators for their superb management of the FIRST study activities on a day-to-day basis at each study site: Danielle Sander, Taiya Bach and Anita Laxova (Madison, WI), Laura Roth, Danielle Graf, Theresa Kump, Briana Horn, and Rachel Bersie (Milwaukee, WI), Olivia Killilea, Maggie Hui, Rachel Gross, Kayla Regan, Sean Ruvolo, Kathy Doan, Kelsey Hill, Audrey Petteruti, Olyn Andrade (Boston, MA), Misty Thompson and Lisa Bendy (Indianapolis, IN), Jane Vroom, Heather Oldroyd (Salt Lake City, UT), and Rashika Rangaraj and Zainub Ashrafi (Chicago, IL).
We are deeply indebted to the following Registered Dietitian Nutritionists (RDNs) for their commitment and laborious collection of nutritional data: Erin Seffrood and Mary Marcus (Madison, WI), Olivia Lampone and Tami Miller (Milwaukee, WI), Laura Jay, Jessica Leonard, Sharon Silverman, Mollie Studley (Boston, MA), Karen Maguiness (Indianapolis, IN), Catherine McDonald (Salt Lake City, UT) and Eileen Potter (Chicago, IL).
We greatly appreciate the assistance from the following nurses for facilitating subject enrollment, data and biological specimen collections: Darci Pfeil (Madison, WI), Nicole Brueck (Milwaukee, WI), Monica Ulles and Chelsey Cheng (Boston, MA), Jennifer Hamilton (Salt Lake City, UT), Stacey Bichl (Chicago, IL).
In addition, the FIRST project includes an outstanding team of researchers in the Madison Data Coordinating Center that are responsible for validating and compiling the data (Danielle Sander, Taiya Bach, Lyanne Chin, Suzanne Shoff and Makayla Schuchardt), food record analysis (Rachel Fenske and Lisa Davis), biological specimen management and biomarker analysis (Sangita Murali and Lyanne Chin) as well as statistical analysis (Zhumin Zhang and Lyanne Chin).
Lastly, Frank Greer, MD (neonatologist infant nutrition consultant) and Philip M Farrell, MD, PhD (multi-site advisor and Co-PI on some grants) are acknowledged with gratitude for their essential contributions to the startup phase and project operations, respectively.
Funding:
R01 DK072126, R01 DK109692, CF Foundation grants LAI14A0 and LAI15A0.
Abbreviations:
- CF
cystic fibrosis
- 25OHD
25-hydroxyvtamin D
- FIRST
Feeding Infants Right…from the Start
- MI
meconium ileus
- PI
pancreatic insufficiency
- PS
pancreatic sufficiency
- PA
Pseudomonas aeruginosa
- SA
Staphylococcus aureus
- IL-6
Interleukin-6
- TNF-α
Tumor necrosis factor α
Footnotes
Conflict of Interest/Disclosures: The authors have no conflict of interest to disclose
REFERENCES
- 1.Marcus MS, Sondel SA, Farrell PM, Laxova A, Carey PM, Langhough R, Mischler EH. Nutritional status of infants with cystic fibrosis associated with early diagnosis and intervention. Am J Clin Nutr. 1991;54(3):578–585. [DOI] [PubMed] [Google Scholar]
- 2.Bronstein MN, Sokol RJ, Abman SH, Chatfield BA, Hammond KB, Hambidge KM, Stall CD, Accurso FJ. Pancreatic insufficiency, growth, and nutrition in infants identified by newborn screening as having cystic fibrosis. J Pediatr. 1992;120(4):533–540. [DOI] [PubMed] [Google Scholar]
- 3.Wilfond BS, Farrell PM, Laxova A, Mischler E. Severe hemolytic anemia associated with vitamin E deficiency in infants with cystic fibrosis. Implications for neonatal screening. Clin Pediatr (Phila). 1994;33(1):2–7. [DOI] [PubMed] [Google Scholar]
- 4.Borowitz D, Baker RD, Stallings V. Consensus report on nutrition for pediatric patients with cystic fibrosis. J Pediatr Gastroenterol Nutr. 2002;35(3):246–259. [DOI] [PubMed] [Google Scholar]
- 5.Stallings VA, Stark LJ, Robinson KA, Feranchak AP, Quinton H; Clinical Practice Guidelines on Growth and Nutrition Subcommittee; Ad Hoc Working Group. Evidence-based practice recommendations for nutrition-related management of children and adults with cystic fibrosis and pancreatic insufficiency: results of a systematic review. J Am Diet Assoc. 2008;108(5):832–839. [DOI] [PubMed] [Google Scholar]
- 6.Borowitz D, Robinson KA, Rosenfeld M, Davis SD, Sabadosa KA, Spear SL, Michel SH, Parad RB, White TB, Farrell PM, et al. Cystic Fibrosis Foundation Evidence-Based Guidelines for Management of Infants with Cystic Fibrosis. J Pediatr. 2009;155(6 Suppl):S73–S93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tangpricha V, Kelly A, Stephenson A, Maguiness K, Enders J, Robinson KA, Marshall BC, Borowitz D, Cystic Fibrosis Foundation Vitamin D Evidence-Based Review Committee. An Update on the Screening, Diagnosis, Management, and Treatment of Vitamin D Deficiency in Individuals with Cystic Fibrosis: Evidence-Based Recommendations from the Cystic Fibrosis Foundation. J Clin Endocrinol Metab. 2012;97(4):1082–1093. [DOI] [PubMed] [Google Scholar]
- 8.Lahiri T, Hempstead SE, Brady C, Cannon CL, Clark K, Condren ME, Gull MF, Guillerman RP, Leone CG, Maguiness K, et al. Clinical practice guidelines from the Cystic Fibrosis Foundation for preschoolers with cystic fibrosis. Pediatrics 2016;137(4):1–26. 10.1542/peds.2015-1784. [DOI] [PubMed] [Google Scholar]
- 9.Daley T, Hughan K, Rayas M, Kelly A, Tangpricha V. Vitamin D deficiency and its treatment in cystic fibrosis. J Cyst Fibros. 2019;18 Suppl 2:S66–S73. [DOI] [PubMed] [Google Scholar]
- 10.McCauley LA, Thomas W, Laguna TA, Regelmann WE, Moran A, Polgreen LE. Vitamin D deficiency is associated with pulmonary exacerbations in children with cystic fibrosis. Ann Am Thorac Soc. 2014;11(2):198–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sexauer WP, Hadeh A, Ohman-Strickland PA, Zanni RL, Varlotta L, Holsclaw D, Fiel S, Graff GR, Atlas A, Bisberg D, et al. Vitamin D deficiency is associated with pulmonary dysfunction in cystic fibrosis. J Cyst Fibros. 2015;14(4):497–506. [DOI] [PubMed] [Google Scholar]
- 12.Bhimavarapu A, Deng Q, Bean M, et al. Factors Contributing to Vitamin D Status at Hospital Admission for Pulmonary Exacerbation in Adults With Cystic Fibrosis. Am J Med Sci. 2021;361(1):75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Farrell PM, Bieri JG, Fratantoni JF, Wood RE, di Sant’Agnese PA. The occurrence and effects of human vitamin E deficiency. A study in patients with cystic fibrosis. J Clin Invest. 1977;60(1):233–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Vries JJ, Chang AB, Bonifant CM, Shevill E, Marchant JM. Vitamin A and Beta (β)-carotene supplementation for cystic fibrosis. Cochrane Database of Syst Rev. 2018;(8): CD006751. doi: 10.1002/14651858.cd006751.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Okebukola PO, Kansra S, Barrett J. Vitamin E supplementation in people with cystic fibrosis. Cochrane Database Syst Rev. 2017;(3):CD00942. doi: 10.1002/14651858.cd009422.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hubbard VS, Farrell PM, di Sant’Agnese PA. 25-hydroxycholecalciferol levels in patients with cystic fibrosis. J Pediatr. 1979;94(1):84–86. [DOI] [PubMed] [Google Scholar]
- 17.Green DM, Leonard AR, Paranjape SM, Rosenstein BJ, Zeitlin PL, Mogayzel PJ Jr. Transient effectiveness of vitamin D2 therapy in pediatric cystic fibrosis patients. J Cyst Fibros. 2010;9(2):143–149. [DOI] [PubMed] [Google Scholar]
- 18.Green D, Carson K, Leonard A, Davis JE, Rosenstein B, Zeitlin P, Mogayzel P Jr. Current treatment recommendations for correcting vitamin D deficiency in pediatric patients with cystic fibrosis are inadequate. J Pediatr. 2008;153(4):554–559. [DOI] [PubMed] [Google Scholar]
- 19.Chesdachai S, Tangpricha V. Treatment of vitamin D deficiency in cystic fibrosis. J Steroid Biochem Mol Biol. 2016;164:36–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rovner AJ, Stallings VA, Schall JI, Leonard MB, Zemel BS. Vitamin D insufficiency in children, adolescents, and young adults with cystic fibrosis despite routine oral supplementation. Am J Clin Nutr. 2007;86(6):1694–1699. [DOI] [PubMed] [Google Scholar]
- 21.Norton L, Page S, Sheehan M, Mazurak V, Brunet-Wood K, Larsen B. Prevalence of inadequate vitamin D status and associated factors in children with cystic fibrosis. Nutr Clin Pract. 2015;30(1):111–116. [DOI] [PubMed] [Google Scholar]
- 22.Farrell PM, Rosenstein BJ, White TB, Accurso FJ, Castellani C, Cutting GR, Durie PR, LeGrys VA, Massie J, Parad RB, et al. Guidelines for diagnosis of cystic fibrosis in newborns through older adults: Cystic Fibrosis Foundation consensus report. J Pediatr. 2008;153(2):S4–S14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feranchak AP, Sontag MK, Wagener JS, Hammond KB, Accurso FJ, Sokol RJ. Prospective, long-term study of fat-soluble vitamin status in children with cystic fibrosis identified by newborn screen. J Pediatr. 1999;135(5):601–610. [DOI] [PubMed] [Google Scholar]
- 24.Leung DH, Heltshe SL, Borowitz D, Gelfond D, Kloster M, Heubi JE, Stalvey M, Ramsey BW, and of the Baby Observational and Nutrition Study (BONUS) Investigators of the Cystic Fibrosis Foundation Therapeutics Development Network. Effects of diagnosis by newborn screening for cystic fibrosis on weight and length in the first year of life. JAMA Pediatr. 2017;171(6):546–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Balough K, McCubbin M, Weinberger M, Smits W, Ahrens R, Fick R. The relationship between infection and inflammation in the early stages of lung disease from cystic fibrosis. Pediatr Pulmonol. 1995;20(2):63–70. [DOI] [PubMed] [Google Scholar]
- 26.Khan TZ, Wagener JS, Bost T, Martinez J, Accurso FJ, Riches DW. Early pulmonary inflammation in infants with cystic fibrosis. Am J Respir Crit Care Med. 1995;151(4):1075–1082. [DOI] [PubMed] [Google Scholar]
- 27.Farrell PM, Bieri JG. Megavitamin E supplementation in man. Am J Clin Nutr 1975;28(12):1381–6. [DOI] [PubMed] [Google Scholar]
- 28.Farrell PM. Vitamin E in human health and disease, In Machlin LJ, Ed. Vitamin E. New York, Marcel Dekker, pp. 519–620,1976. [Google Scholar]
- 29.Farrell PM, Levine SL, Murphy MD, Adams AJ. Plasma tocopherol levels and tocopherol-lipid relationships in a normal population of children as compared to healthy adults. Am J Clin Nutr 1978;31(10):1720–6. [DOI] [PubMed] [Google Scholar]
- 30.Farrell PM, Mischler EH, Gutcher GR. Evaluation of vitamin E deficiency in children with lung disease. Ann N Y Acad Sci 1982;393:96–108. [DOI] [PubMed] [Google Scholar]
- 31.Gutcher GR, Raynor WJ, Farrell PM. An evaluation of vitamin E status in premature infants. Am J Clin Nutr 1984;40(5):1078–89. [DOI] [PubMed] [Google Scholar]
- 32.Farrell PM, Mischler EH, Engle MJ, Brown DJ, Lau SM. Fatty acid abnormalities in cystic fibrosis. Pediatr Res 1985;19(1):104–9. [DOI] [PubMed] [Google Scholar]
- 33.Giavedoni LD. Simultaneous detection of multiple cytokines and chemokines from nonhuman primates using luminex technology. J Immunol Methods. 2005;301(1–2):89–101. 10.1016/j.jim.2005.03.015 [DOI] [PubMed] [Google Scholar]
- 34.Borowitz D, Baker SS, Duffy L, Baker RD, Fitzpatrick L, Gyamfi J, Jarembek K. Use of fecal elastase-1 to classify pancreatic status in patients with cystic fibrosis. J Pediatr. 2004;145(3):322–326. [DOI] [PubMed] [Google Scholar]
- 35.Cystic Fibrosis Foundation Patient Registry. 2019 Annual Data Report. Bethesda, Maryland: 2020. Cystic Fibrosis Foundation; https://www.cff.org/Research/Researcher-Resources/Patient-Registry/2019-Patient-Registry-Annual-Data-Report.pdf. [accessed 2021 Apr 21] [Google Scholar]
- 36.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266–281. doi: 10.1056/NEJMra070553 [DOI] [PubMed] [Google Scholar]
- 37.Karohl C, Su S, Kumari M, Tangpricha V, Veledar E, Vaccarino V, Raggi P. Heritability and seasonal variability of vitamin D concentrations in male twins. Am J Clin Nutr. 2010;92(6):1393–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shoff SM, Ahn H, Davis LA, Lai HJ; Wisconsin CF Neonatal Screening Group. Temporal associations between energy intake, plasma linoleic acid and growth improvement in response to treatment initiation after diagnosis of cystic fibrosis. Pediatrics. 2006;117(2):391–400. [DOI] [PubMed] [Google Scholar]
- 39.Lai HJ, Shoff SM, Farrell PM; Wisconsin Cystic Fibrosis Neonatal Screening Group. Recovery of birth weight z score within 2 years of diagnosis is positively associated with pulmonary status at 6 years of age in children with cystic fibrosis. Pediatrics. 2009;123(2):714–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sanders DB, Zhang Z, Farrell PM, Lai HJ; Wisconsin CF Neonatal Screening Group. Early life growth patterns persist for 12 years and impact pulmonary outcomes in cystic fibrosis. J Cyst Fibros. 2018;17(4):528–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sagel SD, Accurso FJ. Monitoring inflammation in CF. Cytokines. Clin Rev Allergy Immunol. 2002;23(1):41–57. [DOI] [PubMed] [Google Scholar]
- 42.Mills NT, Wright MJ, Henders AK, Eyles DW, Baune BT, McGrath JJ, Byrne EM, Hansell NK, Birosova E, Scott JG, et al. Heritability of transforming growth factor-β1 and tumor necrosis factor-receptor type 1 expression and vitamin D levels in healthy adolescent twins. Twin Res Hum Genet. 2015;18(1):28–35. [DOI] [PubMed] [Google Scholar]
- 43.Revez JA, Lin T, Qiao Z, Xue A, Holtz Y, Zhu Z, Zeng J, Wang H, Sidorenko J, Kemper KE, et al. Genome-wide association study identifies 143 loci associated with 25 hydroxyvitamin D concentration. Nature Communications 2020;11:1647. doi: 10.1038/s41467-020-15421-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Anabtawi A, Le T, Putman M, Tangpricha V, Bianchi ML. Cystic fibrosis bone disease: Pathophysiology, assessment and prognostic implications. J Cyst Fibros. 2019;18 Suppl 2:S48–S55. [DOI] [PubMed] [Google Scholar]
- 45.Moustaki M, Loukou I, Priftis KN, Douros. Role of vitamin D in cystic fibrosis and non-cystic fibrosis bronchiectasis. World J Clin Pediatr 2017; 6(3): 132–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thursfield RM, Khayam Naderi K, Leaver N, Rosenthal M, Alton EWFW, Andrew Bush A, Davies JC. Children with cystic fibrosis demonstrate no respiratory immunological, infective or physiological, consequences of vitamin D deficiency. J Cyst Fibros. 2018;17(5):657–665. [DOI] [PubMed] [Google Scholar]
- 47.Martiniano SL, Elbert AA, Farrell PM, Ren CL, Sontag MK, Wu R, McColley SA. Outcomes of infants born during the first 9 years of CF newborn screening in the United States: A retrospective Cystic Fibrosis Foundation Patient Registry cohort study. Pediatr Pulmonol. 2021;56(12):3758–3767. doi: 10.1002/ppul.25658. Epub 2021 Sep 13. [DOI] [PubMed] [Google Scholar]


