Abstract
Purpose:
The presence of non-obstructive coronary artery disease (CAD) on coronary computed tomography angiography (CTA) has been associated with the occurrence of major adverse cardiac events (MACE). However, factors associated with the development of MACE in women with non-obstructive CAD have not been fully elucidated. We sought to examine the influence of risk factors and coronary artery calcification on MACE in women with non-obstructive CAD on coronary CTA.
Methods and Results:
Women from PROMISE and SCOT-HEART trials with none or non-obstructive CAD on coronary CTA comprised the study cohort. Baseline characteristics and clinical presentation were assessed. Survival analysis using Kaplan-Meier curves was done to compare outcomes stratified by the atherosclerotic cardiovascular disease (ASCVD) risk score and the Agatston score. The primary endpoint was a composite of all-cause mortality, myocardial infarction, and revascularization. 2,597 women had non-obstructive CAD or normal coronary CTA, with a median follow-up of 32 months. Compared to women without MACE, women with MACE had lower high-density lipoprotein cholesterol (HDL-C) levels and higher mean ASCVD risk scores. Further, women with non-obstructive CAD and ASCVD ≥7.5% had higher risk of MACE than those with ASCVD < 7.5% [3.2 % vs. 1.1%, adjusted HR (aHR) of 3.1 (95% CI 1.32, 7.23), P-value 0.009]. The Agatston calcium score, on the other hand, was not independently associated with MACE among this population of symptomatic women.
Conclusions:
Symptomatic women with non-obstructive CAD on coronary CTA are at higher risk for MACE, with the ASCVD risk score being independently associated with the occurrence of adverse events.
Keywords: Coronary computed tomography angiography, coronary artery disease, myocardial infarction, coronary artery calcium score, atherosclerotic cardiovascular disease
INTRODUCTION
Coronary artery disease (CAD) accounts for substantial morbidity and mortality, despite persistent efforts aimed at enhancing diagnostic and therapeutic strategies.1 In the context of a low-intermediate risk presentation, the current diagnostic approach relies on identifying symptomatic individuals with functionally obstructive CAD.2 Yet, this paradigm has its intrinsic limitations, primarily due to the fact that the majority of acute coronary syndrome (ACS)-causing lesions are non-obstructive at baseline.3,4 For instance, serial angiographic examinations have revealed that 68% of myocardial infarction (MI) events are caused by lesions that are non-obstructive (diameter stenosis <50%) at baseline.5 With the advent of coronary computed tomography angiography (coronary CTA), noninvasive anatomical evaluation of the coronary vasculature for the detection and quantification of atherosclerotic plaque burden is becoming an integral part of clinical practice.6, 7 Coronary CTA allows for the detection of CAD across a wide range of clinical presentations, and findings on coronary CTA correlate with future clinical outcomes.8 Most notably, measures of plaque burden, even if non-obstructive, have been shown to be predictive of incident cardiac events.9–11 The ability to characterize the anatomical and functional footprint of coronary atherosclerosis has further enhanced the ability to understand the natural history of stable CAD.
In relation to sex disparity, CAD pathogenesis and clinical manifestations differ between women and men. For instance, ACS in women often occur in the presence of non-obstructive CAD as a result of smaller vessel size, increased vascular stiffness, less robust collateral circulation, lower coronary flow reserve, and differences in vascular remodeling.12, 13 Yet, even in the presence of obstructive CAD, the International Study of Comparative Health Effectiveness With Medical and Invasive Approaches (ISCHEMIA) trial showed that women had more frequent angina, independent of CAD extent and ischemia severity; this indicates that the relationship between angina, atherosclerosis, and ischemia in women is complex.14 Although women have a lower prevalence of coronary artery calcification (CAC) compared to similar-aged men, the presence of detectable CAC in women has been associated with a 1.3-higher hazard for cardiovascular death compared with men.15 The complex interaction between patient-level factors, as well as atherosclerotic plaque characteristics, accounts for persistent sex differences in cardiovascular outcomes. To date, no adequately sized study has examined the influence of risk factors, as well as coronary artery calcification, on the occurrence of major adverse cardiac events (MACE) in women with non-obstructive CAD. This study sought to define characteristics of women with non-obstructive CAD, and to determine factors associated with the occurrence of MACE, using a pooled analysis from the Scottish Computed Tomography of the HEART (SCOT-HEART) and the Prospective Multicenter Imaging Study for Evaluation of Chest Pain (PROMISE) randomized clinical trials.8,16
METHODS
Study Population
In this prospective cohort study, patient-level analysis was performed after combining publicly available data from two randomized clinical trials: PROMISE and SCOT-HEART using R software, version 4.0.2 (RStudio, Boston, MA). This analysis was approved by the local IRB committee at the University of Arkansas for Medical Sciences (UAMS) as IRB-exempt, since the data received from the PROMISE and SCOT-HEART investigators was de-identified (ClinicalTrials.gov identifier for PROMISE: NCT01174550; SCOT-HEART: NCT01149590). The PROMISE trial randomized 10,003 symptomatic outpatients without known CAD to an initial strategy of anatomical evaluation or functional testing with a median follow-up of 2 years, while the SCOT-HEART trial randomly assigned 4,146 patients with stable chest pain to standard care vs. standard care with coronary CTA, with median follow-up of 1.7 years. Data from the initial SCOT-HEART analysis were used to compare baseline characteristics.17 Female patients with no CAD and non-obstructive CAD on coronary CTA comprised the study cohort (n=2597). Subgroup analysis on women with non-obstructive CAD (n=1508) was done stratified by ASCVD risk score, coronary artery calcium score (CACS) and age. Female patients with uninterpretable coronary CTA scans, or those randomized to coronary CTA but who did not end up undergoing the scan were excluded. Two investigators independently merged data from both trials (MM & YL) to confirm accuracy, any discrepancy was addressed after mutual agreement between both investigators. Baseline characteristics from both trials were matched and merged using the tidyverse package in R18.
Clinical Variables & Coronary Calcium Score
Baseline demographics, risk factors and clinical presentation, electrocardiographic (ECG) findings, CAD risk estimates and events with time indicators for each event were collected for the study cohort. Clinical data and risk factor were previously defined by the PROMISE and SCOT-HEART trials.8,16 Non-obstructive CAD was defined as less than or equal to 49% diameter stenosis on coronary CTA. Further, sensitivity analysis with non-obstructive CAD defined as diameter stenosis less than 70% was performed. Patients with uninterpretable scans, those not undergoing the scan and those with obstructive CAD were excluded from the analysis (n=4,231). CACS was reported using the widely adopted Agatston method.19 The atherosclerotic cardiovascular disease (ASCVD) risk score was calculated using the 2013 ACC/AHA Guideline on the Assessment of Cardiovascular Risk.20 Race was not a reported clinical variable by the SCOT-HEART investigators, and as such the assumption was that the predominant race was Caucasian in order to compute the ASCVD score for all participants. The primary endpoint was a composite of MACE including all-cause mortality, myocardial infarction, and revascularization. Both PROMISE and SCOT-HEART used a universal definition for myocardial infarction (MI) and revascularization.21,22
Statistical Analysis
Baseline characteristics are presented as proportions for categorical variables and mean with standard deviation or median with interquartile range for continuous variables, as appropriate. Chi-square test was used to compare categorical variables. Survival analysis using Kaplan Meier curves was done to compare outcomes in female patients with non-obstructive CAD vs female patients with no CAD on coronary CTA. Additionally, we conducted subgroup analysis on women with non-obstructive CAD after excluding women with normal coronary CTA. Survival analysis using Kaplan Meier curves was done to compare outcomes in female patients with non-obstructive CAD stratified by age, ASCVD risk score and CACS. Log-rank test was used to assess for statistical significance between survival curves. Variables that were significant in log rank test, were further evaluated in adjusted analysis. We conducted cox proportional hazard models to estimate hazard ratios (HR) with 95% confidence interval. Calculated HR for women with no CAD (i.e., normal angiography) vs non-obstructive CAD was adjusted for age, hypertension (HTN), diabetes, smoking status, statin use and obesity (defined as body mass index (BMI) > 30 kg/m2). For the cox proportional hazard (CPH) model comparing women with non-obstructive CAD stratified by ASCVD, the calculated HR was adjusted for CACS, statin use and BMI (used as a continuous variable). Similarly, for the CPH model comparing women with non-obstructive CAD stratified by CACS, the calculated HR was adjusted for the ASCVD score. Schoenfeld test was used to confirm the proportionality assumption required for CPH modeling. All statistical analysis was performed using R version 4.0.2.
Patients with missing clinical variables or CACS were not included in the survival analysis (the proportion of missing variables was <7% for all variables (CACS missingness 7%, ASCVD missingness 0.4%), and missing variables were determined to be missing at random). Akaike information criterion (AIC) was used to help choose the best fitted CPH model and to avoid overfitting. A one-tailed p value less than 0.05 was considered significant. The Strengthening the Reporting of Observational studies in Epidemiology (STROBE) guidelines were used in the present analysis.
RESULTS
Study Population:
Our study population consisted of 2,597 female patients, (1,898 (73.1%) from PROMISE and 699 (26.9%) from SCOT-HEART) (Figure 1 supplement). 1089 (41.9%) women had normal coronary arteries, while 1,508 (58.1%) had non-obstructive CAD with diameter stenosis 1–49%. Median follow-up was 32 months (interquartile range, 23–45 months), with a maximum follow-up period of 86 months.
Baseline Characteristics:
Mean age of the study cohort was 59.8 years. In terms of baseline characteristics, 15.3% were current smokers, while 55.3% had hypertension, and 16.4% had diabetes mellitus (Table 1). In terms of pretest probability, 79.1% (2055) had an intermediate pretest probability (modified Diamond Forrester) while 13.1% (341) had low pretest probability. In terms of cardiovascular risk, 49% (1,273 out of 2,597) had a 10-year ASCVD risk of 7.5% or greater. From a clinical presentation perspective, 15.3% had typical chest pain, 64.4% had atypical chest pain and 20.2% had non-cardiac chest pain. All PROMISE patients were symptomatic (75.3% had chest pain while 13.5% had dyspnea), with the remaining 11.2% of patients experienced epigastric pain, shoulder pain, palpitations, syncope, lightheadedness, or weakness as a primary symptom. Statin therapy was present in 55.2% of the study cohort at baseline, 41% were on antiplatelet therapy, while 30.6% were on an angiotensin converting enzyme inhibitor or angiotensin receptor blocker.
Table 1.
Baseline characteristics of the study cohort.
| All participants (n=2,597) |
MACE (n=41) |
Without MACE (n=2,556) |
P value | |
|---|---|---|---|---|
| Age (yrs. ± SD) | 59.8 ± 8.3 | 62.7 ± 9.7 | 59.7 ± 8.4 | 0.057 |
| BMI (Mean, Kg/m2) | 30.3 ± 6.4 | 29.5 ± 7.1 | 30.3 ± 6.4 | 0.51 |
| History of CVD; n (%) | 106 (4.1) | 3 (7.3) | 103 (4.0) | 0.51 |
| Current smoker; n (%) | 398 (15.3) | 14 (34.1) | 384 (15.0) | 0.10 |
| Hypertension; n (%) | 1,436 (55.3) | 25 (61.0) | 1,411 (55.2) | 0.56 |
| Diabetes Mellitus; n (%) | 425 (16.4) | 10 (24.4) | 415 (16.2) | 0.23 |
| Serum total cholesterol, mg/dL | 208.0 ± 45.6 | 202.5 ± 55.2 | 208.1 ± 45.4 | 0.63 |
| HDL cholesterol, mg/dL | 57.8 ± 15.9 | 52.7 ± 10.7 | 57.9 ± 16.0 | 0.036 |
| Medical Therapy | ||||
| Any Statin therapy; n (%) | 1,728 (66.5) | 24 (58.5) | 1,704 (66.7) | 0.35 |
| Statin at baseline; n (%) | 1,433 (55.2) | 19 (46.3) | 1,414 (55.3) | 0.32 |
| Statin 6 to 8 weeks; n (%) | 1,499 (57.7) | 19 (46.3) | 1,480 (57.9) | 0.18 |
| Anti-platelet therapy; n (%) | 1,064 (41.0) | 13 (31.7) | 1,051 (41.1) | 0.21 |
| ACE-I or ARB; n (%) | 794 (30.6) | 13 (31.7) | 781 (30.6) | 0.23 |
| ASCVD risk | ||||
| ASCVD score, mean | 10.5 ± 9.6 | 16.5 ± 13.7 | 10.4 ± 9.5 | 0.007 |
| Low risk (<7.5%) | 1,306 (50.3) | 9 (21.9) | 1,297 (50.7) | 0.14 |
| Intermediate-high risk (≥7.5%) | 1,273 (49.0) | 32 (78.0) | 1,241 (48.6) | 0.26 |
| Calcium Score (n=2,389) | ||||
| Calcium score = 0 | 1,378 (53.1) | 17 (41.5) | 1,361 (53.2) | 0.08 |
| Calcium score 1 – 99 | 757 (29.1) | 15 (36.6) | 742 (29.0) | 0.36 |
| Calcium score 100 – 399 | 210 (8.1) | 6 (14.6) | 204 (8.0) | 0.21 |
| Calcium score > 400 | 45 (1.7) | 2 (4.9) | 43 (1.7) | 0.34 |
| Coronary Artery disease (CAD) | ||||
| No CAD; n (%) | 1089 (41.9) | 7 (17.1) | 1082 (42.3) | 0.002 |
| Non-obstructive CAD n (%) | 1508 (58.1) | 32 (78) | 1476 (57.7) | 0.014 |
| Chest pain Characteristics | ||||
| Typical; n (%) | 396 (15.2) | 8 (19.5) | 388 (15.2) | 0.6 |
| Atypical; n (%) | 1676 (64.5) | 23 (56.1) | 1653 (64.7) | 0.3 |
| Non Cardiac; n (%) | 525 (20.2) | 10 (24.4) | 515 (20.1) | 0.6 |
Abbreviations: SD: standard deviation; BMI: Body mass index; CVD: cardiovascular disease; HDL: High density lipoprotein; ACEI-I: angiotensin converting enzyme inhibitor; ARB: angiotensin receptor blocker.
Overall, baseline characteristics were not different amongst women experiencing a MACE event compared to those without an event. High-density lipoprotein cholesterol (HDL-C), on the other hand, was lower in women experiencing a MACE event (52.7 mg/dL vs. 57.9 mg/dL, p=0.036) while the mean ASCVD risk score was higher in women with a MACE event (16.5% vs. 10.4%, p=0.007). Although the prevalence of the distribution of higher CACS scores was greater among women with MACE vs no MACE, this did not meet statistical significance (Table 1). For reference, differences in patient-level characteristics between women with MACE versus men with MACE were compared in the present pooled analysis of SCOT-HEART and PROMISE (Table 1 supplement).
No CAD vs. Non-obstructive CAD:
In the setting of low-intermediate risk cohort with non-obstructive CAD, the primary endpoint event rate was 2.2% over the median follow-up interval of 32 months. Table 2 supplement shows differences in clinical characteristics in women with no CAD vs. non-obstructive CAD. Overall, women with non-obstructive CAD on coronary CTA were more likely to have the composite outcome of death/MI/revascularizations than those with no CAD on coronary CTA, adjusted HR [aHR] 2.51 95% CI (1.10–5.73); p=0.028, adjusted for age, hypertension, diabetes, obesity, smoking status and statin use at baseline (Figure 1). Findings did not change when non-obstructive CAD was defined as diameter stenosis less than 70% (similar findings were seen across subgroup analyses).
Figure 1.
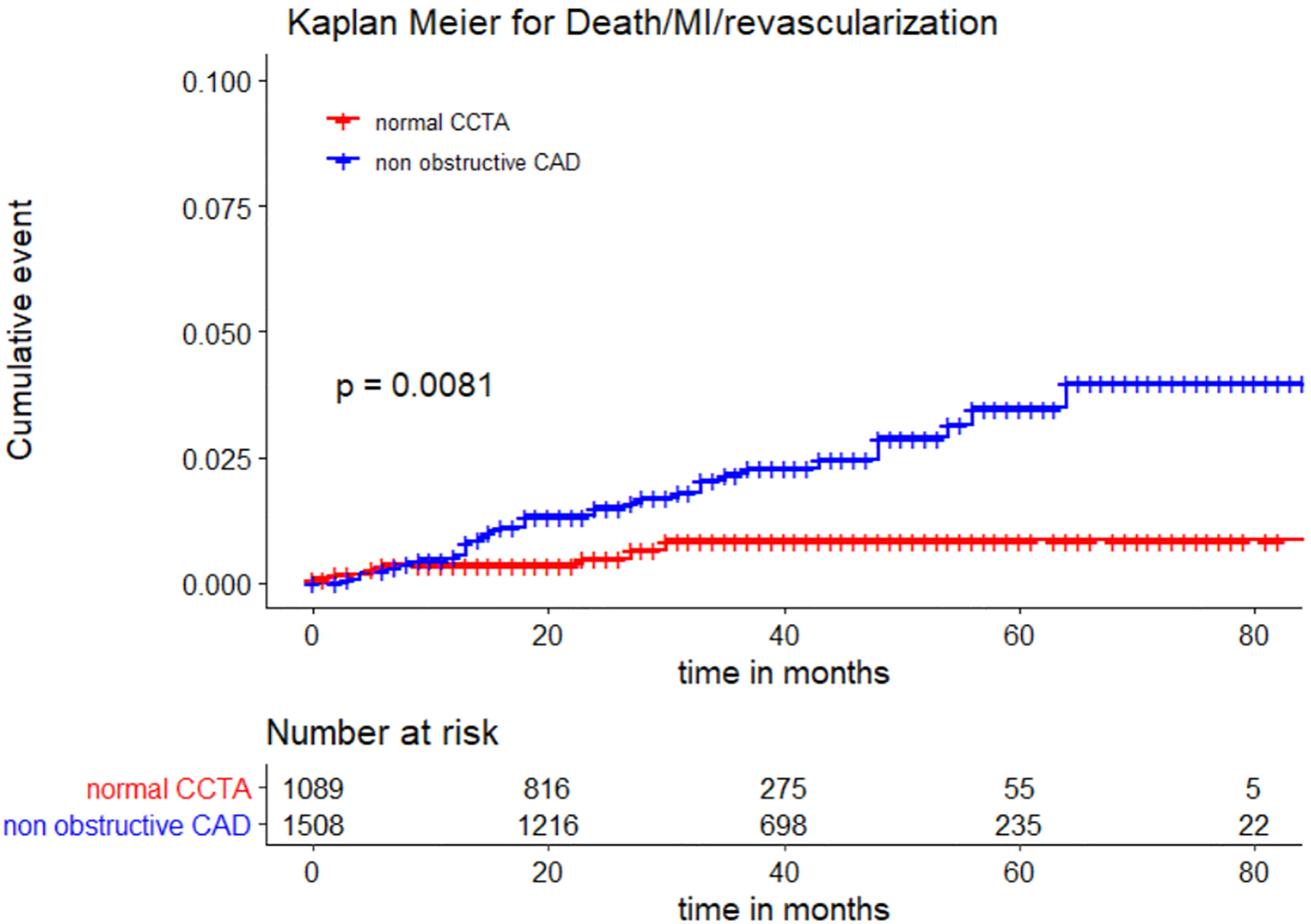
Kaplan-Meier (KM) curves with log rank p-value for women with non-obstructive CAD on coronary CTA compared to those with normal coronary arteries.
Subgroup Analysis by ASCVD:
For female patients with non-obstructive CAD, survival analysis was performed stratified by ASCVD risk score (Figure 2a). Female patients with non-obstructive CAD and ASCVD score ≥7.5% had higher risk of death/MI/revascularization than those with ASCVD score < 7.5% [3.2% vs. 1.1%, aHR 3.1 (95% CI 1.32 – 7.23), p=0.009 adjusted for statin use, CACS and BMI].
Figure 2.


(A) Kaplan-Meier (KM) curves with log rank p-value for women with non-obstructive CAD on coronary CTA stratified by ASCVD score (low risk vs. intermediate-high risk categories; n=1,497 since 11 participants did not have an ASCVD score). (B) KM curves for women with non-obstructive CAD stratified by CACS (CACS = 0 vs CACS > 0). (C) KM curves for women with non-obstructive CAD on coronary CTA stratified by age (> 60 vs < 60).
Subgroup Analysis by CACS:
When stratified by the Agatston CACS, female patients with non-obstructive CAD and CACS > 0 were more likely to experience the primary composite event of death/MI/revascularization than those with non-obstructive CAD and no CAC (CACS = 0) with a log rank P-value of 0.046 (Figure 2b). However, in a cox proportional hazard model adjusted for the ASCVD risk score, there was no statistical difference between CACS=0 and CACS>0, indicating that CACS is not an independent predictor [aHR 1.64 (95% CI 0.76 – 3.55), p=0.21].
Clinical Subgroups:
For female patients with non-obstructive CAD, survival analysis was performed stratified by age (Figure 2c). Among non-obstructive CAD cohort, women older than 60 years of age were more likely to experience major adverse cardiac events compared to younger female patients with non-obstructive CAD [2.86% vs. 1.5%, aHR 2.26 (95% CI 1.06–4.85), p= 0.035]. In addition, the risk of experiencing death/MI/revascularization for women with non-obstructive CAD increased by 5.7% for each one-year increase in age [aHR 1.057 (95% CI 1.016–1.1), p= 0.006].
Obesity, defined as BMI greater than 30 kg/m2, statin use, diabetes mellitus and hypercholesterolemia (defined as serum cholesterol levels greater than 200 mg/dL) were not independently associated with the occurrence of a MACE event in adjusted analyses (Figure 3).
Figure 3.

Adjusted hazard ratios (aHR) for subgroups of women with non-obstructive CAD on coronary CTA. Adjusted for age, HTN and smoking status.
DISCUSSION
Using pooled data from two clinical trials of women with non-obstructive CAD or normal coronary arteries and symptoms suggestive of stable angina or anginal equivalent, we found that age and the ASCVD risk score were both independently associated with future risk of MACE, but not CACS. The uptake of coronary CTA as a noninvasive anatomic imaging modality has provided an important avenue for the evaluation of the distribution, burden and characteristics of atherosclerotic plaque in low to intermediate risk symptomatic individuals. As a direct consequence of this approach, the presence of non-obstructive CAD has been shown to be associated with incident MACE in numerous cohorts. Further, the higher prevalence of non-obstructive CAD coupled with the presence of atypical symptoms account for the high burden of cardiovascular morbidity and mortality in women. Thus, the detection of non-obstructive CAD should prompt implementation of intensive lifestyle and pharmacologic therapies to lower ASCVD risk.
Our analysis focused on women with non-obstructive CAD, the findings are likely independent of gender as previous other analysis using SCOT-HEART and other cohorts did not find sex-specific differences in outcomes in patients undergoing coronary CTA.15,23 Nevertheless, there are limited data on the prognostic value of non-obstructive CAD in women on coronary CTA, and the importance of patient-level characteristics as well as coronary calcification on the occurrence of MACE. In this pooled analysis of the randomized multicenter trials of PROMISE and SCOT-HEART, we found that the presence of non-obstructive CAD was associated with the occurrence of MACE over a median follow up of 32 months. An intermediate-high ASCVD risk score, defined as risk greater than 7.5%, was an independent predictor of MACE, while the presence of coronary calcification (defined as CACS >0) was not an independent predictor after adjusting for ASCVD risk score. Such findings highlight the influence of patient-level characteristics on the development of MACE in women with non-obstructive CAD. As such, the present analysis sheds further insight on the importance of non-obstructive plaque in women, and stresses the need to recognize atherosclerotic plaque, even when non-obstructive, as a target for prevention especially in the setting of an ASCVD risk score > 7.5%.
Previous work has established that functional coronary evaluation in symptomatic women at low to intermediate pretest probability can be less accurate as a result of limited sensitivity and specificity.24–28 In a published meta-analysis that included 19 ECG treadmill testing studies with a total of 3,721 women, sensitivity and specificity were 61% and 70%, respectively.29 Similarly, myocardial perfusion imaging using single photon emission computed tomography (SPECT) has been known to have limited diagnostic performance in women, including false-positive results due to breast attenuation and false-negative results due to smaller left ventricular dimensions.27, 30 In fact, incident adverse events are prevalent in women even after a negative functional evaluation.28, 31 This is likely explained by the fact that functional evaluation lacks the ability to detect non-obstructive CAD. In fact, in the large, multicenter, SCOT-HEART trial, an anatomical approach using coronary CTA was found to reduce the occurrence of death from coronary heart disease or nonfatal MI at 5 years (2.3% vs. 3.9%; hazard ratio, 0.59; 95% CI, 0.41 to 0.84; p=0.004). This can be partially attributable to the fact that coronary CTA can detect non-obstructive CAD, leading to incremental use of targeted medical therapy.8 While previously considered benign, numerous analyses have held that patients with non-obstructive CAD on coronary CTA have higher incidence of MACE. The fact that a predominance of precursor lesions in the setting of ACS tend to be non-obstructive, coupled with the fact that women are more likely to have non-obstructive CAD than men, augments the significance of establishing the presence of non-obstructive CAD in women.8, 23, 32 Although non-obstructive CAD is more common in women, large scale prospective studies investigating the prognostic value in women with non-obstructive CAD are sporadic.12, 33
The use of coronary CTA to diagnose the full spectrum of CAD has expanded in the last two decades, unfolding a large amount of data on plaque characteristics and calcium deposits within atherosclerotic plaques.7 This has resulted in an ongoing debate and research on the interaction between patient-level characteristics and atherosclerotic plaque features on the occurrence of cardiac events. For instance, the occurrence of high risk plaque stipulated by the presence of positive remodeling, spotty calcification, low attenuation plaque and the napkin ring sign, as well as overall calcified plaque burden, have been shown to confer an increased risk of coronary heart disease death or nonfatal MI in a subsequent analysis of the SCOT-HEART and PROMISE cohorts.34–36 In addition, numerous studies have revealed that plaques with high risk features on coronary CTA are strong predictors of MACE in patients with CAD, which confers greater relative risk when present in women than in men.34, 37, 38 In subgroup analysis of SCOT-HEART, the presence of high-risk plaque was associated with a higher risk of adverse events in women, with a non-significant P value that is likely a result of an under-powered analysis (HR 3.27; 95% CI 1.00 – 10.71; p=0.051).34 In PROMISE, on the other hand, high-risk plaque was a stronger predictor of MACE in women (aHR, 2.41; 95% CI, 1.25–4.64) vs. men (aHR, 1.40; 95% CI, 0.81–2.39). While the present analysis did not include an assessment of high-risk plaque, we demonstrate that a global measure of cardiovascular risk, defined as an ASCVD risk score > 7.5%, is an independent predictor of adverse events in symptomatic women with non-obstructive CAD. This suggests that adverse events in lower risk symptomatic women can be better assessed using patient-level characteristics, with an adjusted hazard ratio of 3.1 (95% CI 1.32 – 7.23; p=0.009) in a pooled analysis of PROMISE and SCOT-HEART. Accordingly, combining coronary CTA findings with the commonly used ASCVD risk score can help identify symptomatic women with non-obstructive CAD who are at higher risk for adverse events and who might benefit from even more intensive preventive therapies.
Although the prognostic value of CACS is best established in asymptomatic populations, it also has strong prognostic value in patients with stable chest pain. Multiple studies demonstrated that higher CACS is associated with adverse cardiac outcomes in a graded fashion. In women, a CACS >100 identifies individuals at elevated risk of adverse events, while women with CACS >300 have similar event rate when compared to those with known stable CAD.39 On the other hand, data from a recent meta-analysis revealed a potentially discrepant relationship between coronary calcification and adverse cardiac event.37 The analysis suggested that calcified plaques have the weakest association with MACE, whereas the risk of future events was increased when a lesion displayed evidence of spotty calcification suggesting that the pattern and extent of intimal calcification may be an important predictor of cardiac outcomes.37 Clearly, understanding the characteristics of calcium deposition is crucial to guide risk stratification in women with non-obstructive CAD. Nevertheless, our results are congruent with previously published analysis, as Otaki et al. had shown that an increasing number of cardiovascular risk factors in women were associated with a significant increase in non-calcified plaques only.40 Importantly, in an analysis of quantitative plaque measures in the SCOT-HEART cohort, low-attenuation plaque burden was found to be the strongest predictor of fatal or nonfatal myocardial infarction.9
Our study is not without limitations. First, as expected from a prevention cohort in whom obstructive CAD was excluded, the number of events was low (2.2% over 32 months). Nevertheless, this is the largest study to date examining the prognostic value of non-obstructive CAD on coronary CTA in women. Second, neither race nor ASCVD score were reported in SCOT-HEART. To calculate the ASCVD score, the assumption was made that the predominant race was Caucasian. However, considering the official racial distribution in Scotland in 2018, we expect that this will unlikely alter the study results. Third, CACS has been extensively validated as a powerful tool for the prognostication of adverse events within asymptomatic primary prevention cohorts, while the present analysis found that CACS>0 was not an independent predictor of MACE among symptomatic women. In addition, 55.2% of the cohort had already been on Statin therapy, which could have attenuated the relationship between coronary calcification and the occurrence of MACE. Further, plaque characteristics such as non-calcified plaque burden or high-risk plaque burden might be better than CAC for risk stratification among symptomatic women but could not be assessed in present analysis since it was not available for both PROMISE and SCOT-HEART in the publicly available dataset. Given the study design which selected for low risk women with non-obstructive CAD, it is not surprising that most patients had CACS <100, while only 7 patients had a CACS >1000. Combined with a low primary event rate, it is possible that the present analysis was underpowered to detect an association between coronary calcification and MACE. Finally, the follow up period in SCOT-HEART and PROMISE was different. Nevertheless, sensitivity analysis comparing outcomes during a 2 year follow up interval was performed without a change in the results.
In conclusion, symptomatic women with non-obstructive CAD on coronary CTA are at higher risk for MACE compared to women with normal coronaries on coronary CTA. In women with non-obstructive CAD, an ASCVD rise score >7.5% was independently associated with MACE, while CACS >0 was not an independent predictor of MACE in this symptomatic cohort. These findings highlight the importance of non-obstructive plaque in women, and stress the need to recognize atherosclerotic plaque, even when non-obstructive, as a target for prevention especially in the setting of an ASCVD score >7.5%.
Supplementary Material
Disclosure:
Subhi J. Al’Aref is supported by NIH 2R01 HL12766105 and receives royalty fees from Elsevier. Michelle C. Williams (FS/ICRF/20/26002) is supported by the British Heart Foundation. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
Publisher's Disclaimer: This AM is a PDF file of the manuscript accepted for publication after peer review, when applicable, but does not reflect post-acceptance improvements, or any corrections. Use of this AM is subject to the publisher’s embargo period and AM terms of use. Under no circumstances may this AM be shared or distributed under a Creative Commons or other form of open access license, nor may it be reformatted or enhanced, whether by the Author or third parties. See here for Springer Nature’s terms of use for AM versions of subscription articles: https://www.springernature.com/gp/open-research/policies/accepted-manuscript-terms
REFERENCES
- 1.Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Delling FN, Djousse L, Elkind MSV, Ferguson JF, Fornage M, Khan SS, Kissela BM, Knutson KL, Kwan TW, Lackland DT, Lewis TT, Lichtman JH, Longenecker CT, Loop MS, Lutsey PL, Martin SS, Matsushita K, Moran AE, Mussolino ME, Perak AM, Rosamond WD, Roth GA, Sampson UKA, Satou GM, Schroeder EB, Shah SH, Shay CM, Spartano NL, Stokes A, Tirschwell DL, VanWagner LB, Tsao CW, American Heart Association Council on E, Prevention Statistics C, Stroke Statistics S. Heart Disease and Stroke Statistics-2020 Update: A Report From the American Heart Association. Circulation 2020;141(9):e139–e596. [DOI] [PubMed] [Google Scholar]
- 2.Fihn SD, Blankenship JC, Alexander KP, Bittl JA, Byrne JG, Fletcher BJ, Fonarow GC, Lange RA, Levine GN, Maddox TM, Naidu SS, Ohman EM, Smith PK, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Guyton RA, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, American College of Cardiology/Americal Heart Association Task Force on Practice G, American Association for Thoracic S, Preventive Cardiovascular Nurses A, Society for Cardiovascular A, Interventions, Society of Thoracic S. 2014 ACC/AHA/AATS/PCNA/SCAI/STS focused update of the guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines, and the American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Thorac Cardiovasc Surg 2015;149(3):e5–23. [DOI] [PubMed] [Google Scholar]
- 3.Stone GW, Maehara A, Lansky AJ, de Bruyne B, Cristea E, Mintz GS, Mehran R, McPherson J, Farhat N, Marso SP, Parise H, Templin B, White R, Zhang Z, Serruys PW, Investigators P. A prospective natural-history study of coronary atherosclerosis. N Engl J Med 2011;364(3):226–35. [DOI] [PubMed] [Google Scholar]
- 4.Ambrose JA, Tannenbaum MA, Alexopoulos D, Hjemdahl-Monsen CE, Leavy J, Weiss M, Borrico S, Gorlin R, Fuster V. Angiographic progression of coronary artery disease and the development of myocardial infarction. J Am Coll Cardiol 1988;12(1):56–62. [DOI] [PubMed] [Google Scholar]
- 5.Falk E, Shah PK, Fuster V. Coronary plaque disruption. Circulation 1995;92(3):657–71. [DOI] [PubMed] [Google Scholar]
- 6.Shaw LJ, Blankstein R, Bax JJ, Ferencik M, Bittencourt MS, Min JK, Berman DS, Leipsic J, Villines TC, Dey D, Al’Aref S, Williams MC, Lin F, Baskaran L, Litt H, Litmanovich D, Cury R, Gianni U, van den Hoogen I, A RvR, Budoff M, Chang HJ, H EH, Feuchtner G, Ahmadi A, Ghoshajra BB, Newby D, Chandrashekhar YS, Narula J. Society of Cardiovascular Computed Tomography / North American Society of Cardiovascular Imaging - Expert Consensus Document on Coronary CT Imaging of Atherosclerotic Plaque. J Cardiovasc Comput Tomogr 2020. [DOI] [PubMed] [Google Scholar]
- 7.Al’Aref SJ, Min JK. Cardiac CT: current practice and emerging applications. Heart 2019;105(20):1597–1605. [DOI] [PubMed] [Google Scholar]
- 8.Investigators S-H, Newby DE, Adamson PD, Berry C, Boon NA, Dweck MR, Flather M, Forbes J, Hunter A, Lewis S, MacLean S, Mills NL, Norrie J, Roditi G, Shah ASV, Timmis AD, van Beek EJR, Williams MC. Coronary CT Angiography and 5-Year Risk of Myocardial Infarction. N Engl J Med 2018;379(10):924–933. [DOI] [PubMed] [Google Scholar]
- 9.Williams MC, Kwiecinski J, Doris M, McElhinney P, D’Souza MS, Cadet S, Adamson PD, Moss AJ, Alam S, Hunter A, Shah ASV, Mills NL, Pawade T, Wang C, Weir McCall J, Bonnici-Mallia M, Murrills C, Roditi G, van Beek EJR, Shaw LJ, Nicol ED, Berman DS, Slomka PJ, Newby DE, Dweck MR, Dey D. Low-Attenuation Noncalcified Plaque on Coronary Computed Tomography Angiography Predicts Myocardial Infarction: Results From the Multicenter SCOT-HEART Trial (Scottish Computed Tomography of the HEART). Circulation 2020;141(18):1452–1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jespersen L, Hvelplund A, Abildstrom SZ, Pedersen F, Galatius S, Madsen JK, Jorgensen E, Kelbaek H, Prescott E. Stable angina pectoris with no obstructive coronary artery disease is associated with increased risks of major adverse cardiovascular events. Eur Heart J 2012;33(6):734–44. [DOI] [PubMed] [Google Scholar]
- 11.Lin FY, Shaw LJ, Dunning AM, Labounty TM, Choi JH, Weinsaft JW, Koduru S, Gomez MJ, Delago AJ, Callister TQ, Berman DS, Min JK. Mortality risk in symptomatic patients with nonobstructive coronary artery disease: a prospective 2-center study of 2,583 patients undergoing 64-detector row coronary computed tomographic angiography. J Am Coll Cardiol 2011;58(5):510–9. [DOI] [PubMed] [Google Scholar]
- 12.Shaw LJ, Bugiardini R, Merz CN. Women and ischemic heart disease: evolving knowledge. J Am Coll Cardiol 2009;54(17):1561–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pepine CJ, Kerensky RA, Lambert CR, Smith KM, von Mering GO, Sopko G, Bairey Merz CN. Some thoughts on the vasculopathy of women with ischemic heart disease. J Am Coll Cardiol 2006;47(3 Suppl):S30–5. [DOI] [PubMed] [Google Scholar]
- 14.Reynolds HR, Shaw LJ, Min JK, Spertus JA, Chaitman BR, Berman DS, Picard MH, Kwong RY, Bairey-Merz CN, Cyr DD, Lopes RD, Lopez-Sendon JL, Held C, Szwed H, Senior R, Gosselin G, Nair RG, Elghamaz A, Bockeria O, Chen J, Chernyavskiy AM, Bhargava B, Newman JD, Hinic SB, Jaroch J, Hoye A, Berger J, Boden WE, O’Brien SM, Maron DJ, Hochman JS, Group IR. Association of Sex With Severity of Coronary Artery Disease, Ischemia, and Symptom Burden in Patients With Moderate or Severe Ischemia: Secondary Analysis of the ISCHEMIA Randomized Clinical Trial. JAMA Cardiol 2020;5(7):773–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shaw LJ, Min JK, Nasir K, Xie JX, Berman DS, Miedema MD, Whelton SP, Dardari ZA, Rozanski A, Rumberger J, Bairey Merz CN, Al-Mallah MH, Budoff MJ, Blaha MJ. Sex differences in calcified plaque and long-term cardiovascular mortality: observations from the CAC Consortium. Eur Heart J 2018;39(41):3727–3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Douglas PS, Hoffmann U, Patel MR, Mark DB, Al-Khalidi HR, Cavanaugh B, Cole J, Dolor RJ, Fordyce CB, Huang M, Khan MA, Kosinski AS, Krucoff MW, Malhotra V, Picard MH, Udelson JE, Velazquez EJ, Yow E, Cooper LS, Lee KL, Investigators P. Outcomes of anatomical versus functional testing for coronary artery disease. N Engl J Med 2015;372(14):1291–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.investigators S-H. CT coronary angiography in patients with suspected angina due to coronary heart disease (SCOT-HEART): an open-label, parallel-group, multicentre trial. Lancet 2015;385(9985):2383–91. [DOI] [PubMed] [Google Scholar]
- 18.Wickham H, A M, B J, C W, M LDA, F R, G G, H A, Henry Lionel, H J, K M, L T, M E, Bache Stephan Milton, M K, O J, Robinson David, S DP, Spinu Vitalie, T K, V D, W C, W K, Y H. Welcome to the tidyverse. Journal of Open Source Software 2019(4(43)):1686. [Google Scholar]
- 19.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M Jr., Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol 1990;15(4):827–32. [DOI] [PubMed] [Google Scholar]
- 20.Eckel RH, Jakicic JM, Ard JD, de Jesus JM, Houston Miller N, Hubbard VS, Lee IM, Lichtenstein AH, Loria CM, Millen BE, Nonas CA, Sacks FM, Smith SC Jr., Svetkey LP, Wadden TA, Yanovski SZ, Kendall KA, Morgan LC, Trisolini MG, Velasco G, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Smith SC Jr., Tomaselli GF, American College of Cardiology/American Heart Association Task Force on Practice G. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014;129(25 Suppl 2):S76–99. [DOI] [PubMed] [Google Scholar]
- 21.Newby DE, Williams MC, Flapan AD, Forbes JF, Hargreaves AD, Leslie SJ, Lewis SC, McKillop G, McLean S, Reid JH, Sprat JC, Uren NG, van Beek EJ, Boon NA, Clark L, Craig P, Flather MD, McCormack C, Roditi G, Timmis AD, Krishan A, Donaldson G, Fotheringham M, Hall FJ, Neary P, Cram L, Perkins S, Taylor F, Eteiba H, Rae AP, Robb K, Barrie D, Bissett K, Dawson A, Dundas S, Fogarty Y, Ramkumar PG, Houston GJ, Letham D, O’Neill L, Pringle SD, Ritchie V, Sudarshan T, Weir-McCall J, Cormack A, Findlay IN, Hood S, Murphy C, Peat E, Allen B, Baird A, Bertram D, Brian D, Cowan A, Cruden NL, Dweck MR, Flint L, Fyfe S, Keanie C, MacGillivray TJ, Maclachlan DS, MacLeod M, Mirsadraee S, Morrison A, Mills NL, Minns FC, Phillips A, Queripel LJ, Weir NW, Bett F, Divers F, Fairley K, Jacob AJ, Keegan E, White T, Gemmill J, Henry M, McGowan J, Dinnel L, Francis CM, Sandeman D, Yerramasu A, Berry C, Boylan H, Brown A, Duffy K, Frood A, Johnstone J, Lanaghan K, MacDuff R, MacLeod M, McGlynn D, McMillan N, Murdoch L, Noble C, Paterson V, Steedman T, Tzemos N. Role of multidetector computed tomography in the diagnosis and management of patients attending the rapid access chest pain clinic, The Scottish computed tomography of the heart (SCOT-HEART) trial: study protocol for randomized controlled trial. Trials 2012;13:184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Douglas PS, Hoffmann U, Lee KL, Mark DB, Al-Khalidi HR, Anstrom K, Dolor RJ, Kosinski A, Krucoff MW, Mudrick DW, Patel MR, Picard MH, Udelson JE, Velazquez EJ, Cooper L, investigators P. PROspective Multicenter Imaging Study for Evaluation of chest pain: rationale and design of the PROMISE trial. Am Heart J 2014;167(6):796–803 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Min JK, Dunning A, Lin FY, Achenbach S, Al-Mallah M, Budoff MJ, Cademartiri F, Callister TQ, Chang HJ, Cheng V, Chinnaiyan K, Chow BJ, Delago A, Hadamitzky M, Hausleiter J, Kaufmann P, Maffei E, Raff G, Shaw LJ, Villines T, Berman DS, Investigators C. Age- and sex-related differences in all-cause mortality risk based on coronary computed tomography angiography findings results from the International Multicenter CONFIRM (Coronary CT Angiography Evaluation for Clinical Outcomes: An International Multicenter Registry) of 23,854 patients without known coronary artery disease. J Am Coll Cardiol 2011;58(8):849–60. [DOI] [PubMed] [Google Scholar]
- 24.Williams MJ, Marwick TH, O’Gorman D, Foale RA. Comparison of exercise echocardiography with an exercise score to diagnose coronary artery disease in women. Am J Cardiol 1994;74(5):435–8. [DOI] [PubMed] [Google Scholar]
- 25.Kohli P, Gulati M. Exercise stress testing in women: going back to the basics. Circulation 2010;122(24):2570–80. [DOI] [PubMed] [Google Scholar]
- 26.Cortigiani L, Gigli G, Vallebona A, Mariani PR, Bigi R, Desideri A. The stress echo prognostic gender gap. Eur J Echocardiogr 2001;2(2):132–8. [DOI] [PubMed] [Google Scholar]
- 27.Mieres JH, Shaw LJ, Arai A, Budoff MJ, Flamm SD, Hundley WG, Marwick TH, Mosca L, Patel AR, Quinones MA, Redberg RF, Taubert KA, Taylor AJ, Thomas GS, Wenger NK, Cardiac Imaging Committee CoCC, the Cardiovascular I, Intervention Committee CoCR, Intervention AHA. Role of noninvasive testing in the clinical evaluation of women with suspected coronary artery disease: Consensus statement from the Cardiac Imaging Committee, Council on Clinical Cardiology, and the Cardiovascular Imaging and Intervention Committee, Council on Cardiovascular Radiology and Intervention, American Heart Association. Circulation 2005;111(5):682–96. [DOI] [PubMed] [Google Scholar]
- 28.Grady D, Chaput L, Kristof M. Diagnosis and treatment of coronary heart disease in women: systematic reviews of evidence on selected topics. Evid Rep Technol Assess (Summ) 2003(81):1–4. [PMC free article] [PubMed] [Google Scholar]
- 29.Kwok Y, Kim C, Grady D, Segal M, Redberg R. Meta-analysis of exercise testing to detect coronary artery disease in women. Am J Cardiol 1999;83(5):660–6. [DOI] [PubMed] [Google Scholar]
- 30.Mieres JH, Shaw LJ, Hendel RC, Miller DD, Bonow RO, Berman DS, Heller GV, Mieres JH, Bairey-Merz CN, Berman DS, Bonow RO, Cacciabaudo JM, Heller GV, Hendel RC, Kiess MC, Miller DD, Polk DM, Shaw LJ, Smanio PE, Walsh MN, Writing Group on Perfusion Imaging in W. American Society of Nuclear Cardiology consensus statement: Task Force on Women and Coronary Artery Disease--the role of myocardial perfusion imaging in the clinical evaluation of coronary artery disease in women [correction]. J Nucl Cardiol 2003;10(1):95–101. [DOI] [PubMed] [Google Scholar]
- 31.Samiei N, Parsaee M, Pourafkari L, Tajlil A, Pasbani Y, Rafati A, Nader ND. The value of negative stress echocardiography in predicting cardiovascular events among adults with no known coronary disease. J Cardiovasc Thorac Res 2019;11(2):85–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maddox TM, Stanislawski MA, Grunwald GK, Bradley SM, Ho PM, Tsai TT, Patel MR, Sandhu A, Valle J, Magid DJ, Leon B, Bhatt DL, Fihn SD, Rumsfeld JS. Nonobstructive coronary artery disease and risk of myocardial infarction. JAMA 2014;312(17):1754–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patel MB, Bui LP, Kirkeeide RL, Gould KL. Imaging Microvascular Dysfunction and Mechanisms for Female-Male Differences in CAD. JACC Cardiovasc Imaging 2016;9(4):465–82. [DOI] [PubMed] [Google Scholar]
- 34.Williams MC, Moss AJ, Dweck M, Adamson PD, Alam S, Hunter A, Shah ASV, Pawade T, Weir-McCall JR, Roditi G, van Beek EJR, Newby DE, Nicol ED. Coronary Artery Plaque Characteristics Associated With Adverse Outcomes in the SCOT-HEART Study. J Am Coll Cardiol 2019;73(3):291–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ferencik M, Mayrhofer T, Bittner DO, Emami H, Puchner SB, Lu MT, Meyersohn NM, Ivanov AV, Adami EC, Patel MR, Mark DB, Udelson JE, Lee KL, Douglas PS, Hoffmann U. Use of High-Risk Coronary Atherosclerotic Plaque Detection for Risk Stratification of Patients With Stable Chest Pain: A Secondary Analysis of the PROMISE Randomized Clinical Trial. JAMA Cardiol 2018;3(2):144–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xie JX, Eshtehardi P, Varghese T, Goyal A, Mehta PK, Kang W, Leipsic J, B OH, Bairey Merz CN, Berman DS, Gransar H, Budoff MJ, Achenbach S, Callister TQ, Marques H, Rubinshtein R, Al-Mallah MH, Andreini D, Pontone G, Cademartiri F, Maffei E, Chinnaiyan K, Raff G, Hadamitzky M, Hausleiter J, Feuchtner G, Kaufmann PA, Villines TC, Chow BJW, Min JK, Shaw LJ. Prognostic Significance of Nonobstructive Left Main Coronary Artery Disease in Women Versus Men: Long-Term Outcomes From the CONFIRM (Coronary CT Angiography Evaluation For Clinical Outcomes: An International Multicenter) Registry. Circ Cardiovasc Imaging 2017;10(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nerlekar N, Ha FJ, Cheshire C, Rashid H, Cameron JD, Wong DT, Seneviratne S, Brown AJ. Computed Tomographic Coronary Angiography-Derived Plaque Characteristics Predict Major Adverse Cardiovascular Events: A Systematic Review and Meta-Analysis. Circ Cardiovasc Imaging 2018;11(1):e006973. [DOI] [PubMed] [Google Scholar]
- 38.Conte E, Annoni A, Pontone G, Mushtaq S, Guglielmo M, Baggiano A, Volpato V, Agalbato C, Bonomi A, Veglia F, Formenti A, Fiorentini C, Bartorelli AL, Pepi M, Andreini D. Evaluation of coronary plaque characteristics with coronary computed tomography angiography in patients with non-obstructive coronary artery disease: a long-term follow-up study. Eur Heart J Cardiovasc Imaging 2017;18(10):1170–1178. [DOI] [PubMed] [Google Scholar]
- 39.Kalra DK, Heo R, Valenti V, Nakazato R, Min JK. Role of computed tomography for diagnosis and risk stratification of patients with suspected or known coronary artery disease. Arterioscler Thromb Vasc Biol 2014;34(6):1144–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Otaki Y, Gransar H, Cheng VY, Dey D, Labounty T, Lin FY, Achenbach S, Al-Mallah M, Budoff MJ, Cademartiri F, Callister TQ, Chang HJ, Chinnaiyan K, Chow BJ, Delago A, Hadamitzky M, Hausleiter J, Kaufmann P, Maffei E, Raff G, Shaw LJ, Villines TC, Dunning A, Cury RC, Feuchtner G, Kim YJ, Leipsic J, Berman DS, Min JK. Gender differences in the prevalence, severity, and composition of coronary artery disease in the young: a study of 1635 individuals undergoing coronary CT angiography from the prospective, multinational confirm registry. Eur Heart J Cardiovasc Imaging 2015;16(5):490–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


