SUMMARY
Nanomaterials and targeted drug delivery vehicles improve the therapeutic index of drugs and permit greater control over their pharmacokinetics, biodistribution, and bioavailability. Here, nanotechnologies applied to cancer immunotherapy are discussed with a focus on current and next generation self-assembling drug delivery systems composed of lipids and/or polymers. Topics covered include the fundamental design, suitability, and inherent properties of nanomaterials that induce anti-tumor immune responses and support anti-cancer vaccination. Established active and passive targeting strategies as well as newer “indirect” methods are presented together with insights into how nanocarrier structure and surface chemistry can be leveraged for controlled delivery to the tumor microenvironment while minimizing off-target effects.
INTRODUCTION
While the biochemical mechanisms and routes of administration for chemotherapeutic and immunomodulatory drugs are both routinely optimized, a critical third step is usually overlooked: specifying the biodistribution (i.e., where an administered drug transports and accumulates within the body). Although therapeutic targets for cancer typically reside within the tumor microenvironment, the vast majority of administered agents accumulate elsewhere, resulting in what is often perceived to be unavoidable adverse events. In this regard, one could argue that many therapeutics that are currently approved remain as unfinished products, resulting in the use of less optimal dosing and the acceptance of severe side effects that arise from their nonspecific biodistributions. Immunotherapy presents a double-edged sword with the promise of a complete cure for a select few, but at the risk of severe and often life-altering side effects (Haratani et al., 2018; Sharma and Allison, 2015). With these considerations in mind, strategies for harnessing the host immune system to generate safe, efficacious, and sustained tumoricidal events have increasingly incorporated engineered systems for enhanced targeting of specific cells and tissues (Nam et al., 2019). Directing the biodistribution and kinetics of anti-cancer and/or immunomodulatory agents with high control and precision reduces dosage and minimizes adverse autoimmune events arising from nonspecific immunomodulation. Biomaterials and nanoscale science are critical to the design of these targeted delivery strategies and provide modular platforms for building precision cancer immunotherapies that have controllable cellular interactions and release profiles to meet diverse challenges in the clinic (Irvine and Dane, 2020). Of note, the immune system is particularly amenable to therapeutic targeting and modulation by nanoscale materials, as a diverse range of pro- and anti-tumor myeloid and lymphoid cells have evolved to collect, process, and respond to nanoscale pathogens and particulates.
Here, we examine how a subset of nanobiomaterials (i.e., nanostructured materials optimized for biomedical applications) have been employed as drug delivery vehicles, also known as nanocarriers, to address specific limitations of current regimens for cancer immunotherapy. Although nanobiomaterials can be fabricated from organic or inorganic components via a wide range of methods reviewed elsewhere (Mitchell et al., 2021), we focus on the unique applications of nanocarriers formed by the spontaneous self-assembly of amphiphilic lipids and polymers. We first present an overview of molecular self-assembly principles and how this information can be harnessed in the laboratory to construct nanocarriers with specific physical and chemical properties of interest. After discussing how the combined structure and surface chemistry influence the pharmacokinetics and cellular interactions of nanocarriers (Vincent et al., 2021b), we introduce ligand-mediated and ligand-free targeting strategies for enhancing their accumulation within cells of the tumor microenvironment (TME). Applications of these controlled delivery strategies for traditional chemotherapy, as well as immunogenic cell death, checkpoint blockade immunotherapies, and anti-cancer vaccines are presented. Our discussion of chemotherapeutic delivery focuses on strategies that enhance accumulation within tumor cells after systemic administration and further explores the role of nanobiomaterials in enhancing immunogenic cell death beyond what is achieved by administering the drugs in conventional forms. Finally, advancements in targeted anti-cancer vaccination are covered for systems administered via intramuscular, subcutaneous, and intravenous routes.
Self-assembled nanocarriers: customizable vehicles for enhancing treatment safety and efficacy
The field of nanotechnology broadly includes all materials and devices at the nanoscale (10−9 m). Among them are peptide therapeutics and peptide assemblies, recombinant proteins, proteins engineered by directed evolution and de novo techniques, and diverse nanocarrier types of organic and inorganic composition. “Nanocarriers” are defined as drug delivery vehicles with at least one dimension at the nanoscale. These nanotechnologies are versatile platforms for pharmaceutical development due to their ability to control the solubility, stability, transport, cellular entry, and accumulation of drugs, as well as their release from intracellular and extracellular environments. Depending on their chemical composition, nanocarriers can facilitate intracellular cargo release from endolysosomal compartments through a variety of mechanisms, which can include membrane fusion or disruption events, as well as pore formation and osmotic lysis (Xu et al., 2021). This capability of nanocarriers to facilitate selective intracellular drug delivery has proven to be highly advantageous for both chemotherapy and immunomodulation (Yao et al., 2020).
Nanocarriers that self-assemble from amphiphilic molecules (i.e., possessing both water-soluble and insoluble components) provide biocompatible and customizable systems (Figure 1; Box 1) that have been incorporated into a large number of Food and Drug Administration (FDA)-approved drugs and vaccines (Box 2) (Lammers and Ferrari, 2020; Mitchell et al., 2021). This class of materials includes lipid nanoparticles and liposomes, which have surged in popularity since being first applied to doxorubicin chemotherapy three decades ago (Doxil) (Barenholz, 2012) (Figures 1A and 1B). Most recently, self-assembling lipids were incorporated as key components of protein-based vaccines that protect against shingles (herpes zoster; Shingrix) (James et al., 2018) and the mRNA vaccines developed against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (Baden et al., 2021; Jackson et al., 2020; Polack et al., 2020). Lipids aside, there is also much interest in the development of self-assembling synthetic polymers. Synthetic polymers have several advantages over lipid systems, including their high physical and chemical customizability and greater control over vehicle shape/morphology (lipids are primarily limited to micelle and liposome morphologies) (Mitchell et al., 2021). Furthermore, these polymer systems are typically more stable than lipid systems in vivo, retain cargo more reliably and for longer periods of time, can avoid nonspecific lipase-mediated degradation, and offer greater control over chemistries that promote stimuli-responsive on-demand release of drug payloads (Bobbala et al., 2020; Mitchell et al., 2021).
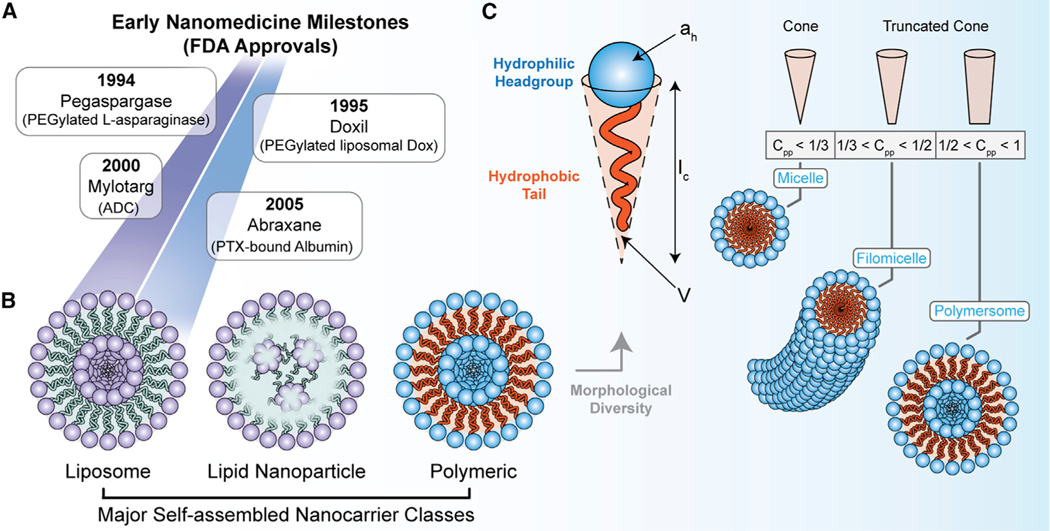
Figure 1. Summary of early nanomedicine milestones, self-assembling nanocarrier classes, and principles for controlling the assembled nanocarrier structure (i.e., morphology).
(A) Early milestones for the clinical adoption of anti-cancer nanotechnologies.
(B) The three major classes of self-assembling nanocarriers: liposomes, lipid nanoparticles (LNPs), and polymeric nanocarriers.
(C) The critical packing parameter for self-assembling polymer amphiphiles and its influence on nanocarrier morphology. The effective hydrophobic chain volume (V), critical chain length (lc), and effective surface area of the hydrophilic headgroup (ah) are presented from the equation for the critical packing parameter (Cpp). The Cpp for cone and truncated cone hydrophobic tail geometries is presented in the lower panel together with the favorable morphology for the self-assembled aggregate.
Box 1. Molecular self-assembly principles for nanocarrier development.
Molecular self-assembly is an equilibrium process that describes the spontaneous formation of organized aggregates of well-defined structure from smaller building blocks (polymers, lipids, protein subunits, etc.) and is driven by energy minimization (Whitesides et al., 1991). Non-covalent forces underlie self-assembly, including electrostatic interactions and hydrophobic interactions, as well as van der Waals interactions, hydrogen bonding, aromatic (p-p) stacking, and metal coordination. Self-assembled structures are ubiquitous in biology due to their key benefits for many processes and functions. All forms of life utilize self-assembly to wind up DNA into helical structures, fold polypeptides into functional three-dimensional shapes, and build phospholipid membranes that, together with other biomolecular components, are used for transport and to maintain concentration gradients of ions, nutrients, and waste products. Elsewhere in nature, this construction strategy is utilized extensively to create nanoscale compartments for various purposes, such as the 20- to 400-nm diameter icosahedral capsids of viruses that self-assemble from coat proteins and encapsulate viral genomes (Caspar and Klug, 1962; Losdorfer Bozic et al., 2013). These observations have inspired humans to harness molecular self-assembly principles in the laboratory to create designer drug delivery vehicles that improve modern medicine. This approach is advantageous for constructing drug-loaded nanobiomaterial delivery systems due to the speed of fabrication and loading of self-assembled nanocarriers, as well as the versatility of structures that can be stably formed in aqueous solutions. The microphase separation of diblock copolymer amphiphiles into thermodynamically stable morphologies depends on multiple factors. These include (1) the stretching of the hydrophobic blocks that comprise the aggregate core, (2) interfacial tension between the core and the outer solvent, and (3) repulsive interactions between chains within the hydrophilic corona (Mai and Eisenberg, 2012; Zhang and Eisenberg, 1995). The formation of different morphologies is governed by a competition between enthalpic contributions arising from interfacial energy (between the blocks) with entropic contributions from chain stretching (Mai and Eisenberg, 2012). The morphology that results from self-assembly of an amphiphilic polymer is related to the critical packing parameter (Cpp) (Lombardo et al., 2020), which is provided by Equation 1:
| (Equation 1) |
In Equation 1, V is the effective volume of the hydrophobic chains, Ic is the critical chain length, and a is the effective surface area of the hydrophilic head group. It is known that polymer amphiphiles having a Cpp < 1/3 self-assemble into micelles (Figure 1C). Polymer amphiphiles having a 1/3 < Cpp < 1/2 self-assemble into worm-like filamentous micelles (Geng et al., 2007; Karabin et al., 2018; Vincent et al., 2021b; Yi et al., 2016). Polymers with 1/2 < Cpp < 1 form vesicular structures or lamellae (Figure 1C). Planar lamellae and inverted structures can be observed at Cpp = 1 and Cpp > 1, respectively (Mai and Eisenberg, 2012).
Box 2. Summary of major self-assembling drug delivery platforms in clinical and late-stage preclinical development.
A variety of self-assembling nanobiomaterial platforms are available for cancer immunotherapy development, which can be divided into lipid and polymeric classes. Several lipid-based systems have been approved for clinical use, beginning with the FDA’s approval of Doxil in 1995 that consists of a PEGylated liposomal formulation of doxorubicin. New technologies continue to enter the clinic in various settings; most prominently in the development of mRNA vaccines against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which used pH-sensitive ionizable lipid nanoparticles to deliver nucleoside-substituted mRNA molecules encoding the SARS-CoV-2 spike (S) glycoprotein. Lipid systems bearing traditional anti-cancer agents, as well as novel anti-cancer vaccines delivering antigen-encoding nucleic acids, are likely to continue to emerge from the preclinical pipeline in the years to come (Guevara et al., 2020; Hou et al., 2021). The polymeric nanomaterial class is highly diverse due to the range of chemical modifications that can be used to finely tune the vehicle’s biological interactions and payload release to specific applications. The dominant polymeric materials under development include poly(lactic-co-glycolic acid) (PLGA), PEG-b-PLGA, and various Pluronic materials (Pluronic F-68, F-108, F-127; i.e., variants of the poly(ethylene oxide)-block-poly(propylene oxide)-block-poly(ethylene oxide) triblock copolymer). A broad range of polymeric materials are under preclinical investigation, including morphologically diverse polymeric delivery systems composed of poly(ethylene oxide)-block-polycaprolactone (PEO-b-PCL) (Geng and Discher, 2005; He et al., 2015) and the oxidation-responsive poly(ethylene glycol)-block-poly(propylene sulfide) (PEG-b-PPS) copolymers (Bobbala et al., 2020; Cerritelli et al., 2007; Scott et al., 2012; Vincent et al., 2021b; Yi et al., 2016), among others. Synthetic polymers have been introduced into the clinic as modifications to biologics (i.e., PEGylated biologics) and liposomal formulations (i.e., PEGylated liposomes such as Doxil). PLGA is perhaps the most well studied type of polymeric nanoparticle under clinical investigation, and has been applied as a carrier for chemotherapeutics and immunomodulatory agents (Rezvantalab et al., 2018). Regarding the latter, phase I dose escalation studies are currently under way for PRECIOUS-1 (trial ID = NCT04751786), a PLGA-based immunomodulatory nanoparticle encapsulating the invariant natural killer T cell (iNKT) threitolcermaide-6 and the New York Esophageal Squamous Cell Carcinoma-1 (NY-ESO-1) cancer-testis antigen peptides for treating patients with NY-ESO-1-positive tumors. For all nanobiomaterial platforms, the inherent toxicity and immunogenicity must be considered, together with extracellular and intracellular degradation mechanisms of candidate materials prior to pursuing more thorough formulation development. Other practical considerations include ease of manufacturing (fabrication methods, scalability, etc.), drug loading capacity and efficiency, drug release kinetics under physiological conditions, and formulation stability. Important design handles for targeting therapeutic delivery to specific cell types include the physicochemical properties (Benne et al., 2016; Bobbala et al., 2020; Vincent et al., 2021b; Wang et al., 2019; Yi et al., 2016; Yoon et al., 2018) of the “nanocarrier chassis” (Vincent et al., 2021b) and how these properties interact with biological environments prior to reaching cellular targets (Vincent et al., 2021a, 2021b), as well as the incorporation of passive targeting features (Geng et al., 2007) and/or surface-displayable active targeting moieties (Mai et al., 2009; Scodeller et al., 2017; Sugahara et al., 2009, 2010). Active targeting moieties, such as peptide ligands, can be either directly conjugated to nanobiomaterials or displayed on amphiphilic systems using lipid anchors. As of this writing, polymeric nanocarriers and lipid- and/or polymer-based delivery vehicles displaying active targeting moieties are not yet in clinical use.
Both natural and synthetic self-assembling macromolecules typically require amphiphilicity for controllable aggregation and therefore possess both a hydrophilic water-soluble (i.e., charged or polar head group or polymer block) and a hydrophobic insoluble (i.e., lipid tail or hydrophobic polymer block) component (Box 1; Figure 1C). Focus has been placed on the design of amphiphilic block (Mai and Eisenberg, 2012) or random (Li et al., 2014) copolymers wherein the hydrophobic/hydrophilic ratio specifies the morphology (shape) of the self-assembled nanostructures. Nanocarrier morphology is a major design choice due to its influence on cargo loading and release capabilities, as well as its role in governing multiscale interactions of the nanocarrier within physiological systems and its overall pharmacokinetics. Importantly, nanocarrier morphologies differ significantly in their size and solvent-exposed surface areas, which are critical properties for determining whether the vehicle will accumulate within tumors of different permeability (Cabral et al., 2011).
The most common nanocarrier morphologies include spherical micelles, cylindrical filomicelle, and vesicles. The micelle morphology describes structures having a hydrophobic core and a hydrophilic shell. Polymeric micelles are typically smaller structures, with a diameter of less than 100 nm. Filamentous micelles, also referred to as filomicelles, are cylindrical variants of the micelle that commonly have a nanoscale diameter and a length that either approaches or exceeds 1 μm (Geng et al., 2007; Karabin et al., 2018; Vincent et al., 2021b; Yi et al., 2016). The vesicle morphology consists of a hydrophilic shell, hydrophobic internal layer, and a hydrophilic lumen. Vesicles are referred to as liposomes for lipid amphiphiles and polymersomes for polymer amphiphiles, and commonly have a diameter of less than 200 nm (Discher et al., 1999). Cell-derived exosomes are another type of vesicular nanocarrier that is gaining interest in cancer immunotherapy. Exosomes are outside the scope of the present article but have been reviewed in detail elsewhere (Dai et al., 2020). Liposomes (Ashley et al., 2016) and polymersomes (Allen et al., 2017; Discher et al., 1999) have been of great interest due to their ability to load both hydrophilic and hydrophobic payloads in the bilayer shell or aqueous core, respectively. Recently, polymeric bicontinuous nanospheres (BCNs) (Bobbala et al., 2018) and lipid cubosomes (Barriga et al., 2019; Kim et al., 2018) have demonstrated the utility of an additional nanocarrier architecture. The BCN morphology consists of 200- to 400-nm spheres with an internal cubic lattice structure and has presented unique advantages for controlled drug delivery, particularly for multi-drug formulations and stimuli-responsive, sustained intracellular release of chemotherapeutics (Bobbala et al., 2020).
The safety and efficacy of nanocarriers can be strongly impacted by their chemical composition. The chemical make-up of a nanocarrier determines its biodegradability and metabolism and contributes to its inherent immunomodulatory properties. Many lipid-based nanocarriers can be immunostimulatory, such as those incorporating dimethyldioctadecylammonium (DDA) (Gall, 1966; Korsholm et al., 2007), lipids derived from bacteria (Tandrup Schmidt et al., 2016), or modern ionizable cationic lipids (Ndeupen et al., 2021). The innate immunostimulation of certain nanocarrier platforms can prove beneficial when pro-inflammatory responses are desired to some extent, such as with lipid nanoparticles used for mRNA vaccination (Guevara et al., 2020; Hou et al., 2021). Furthermore, the intracellular presence alone of some nanomaterials can activate the NLRP3 inflammasome (Hornung et al., 2008; Zhu et al., 2020). In general, polymeric systems tend to have less background inflammatory responses, with some systems serving as “blank slates” for customizing immunostimulation based primarily on the encapsulated payloads (Burke et al., 2022; Dowling et al., 2017). In contrast to this generalization, several immunomodulatory responses of common synthetic polymers warrant mentioning. Poly(ethylene glycol) (PEG) is a common component of numerous consumer products and drugs and is used as a non-fouling (i.e., avoids nonspecific protein and cell interactions) outer layer for most lipid and polymer-based nanocarriers, resulting in an extensive prior exposure of this polymer to the public. Rare but notable anti-PEG antibody responses have been reported and linked to a range of mild to severe allergic responses upon nanoparticle and vaccine administration that remain highly controversial and critically understudied (Bigini et al., 2021; Freire Haddad et al., 2021). Lactate metabolic degradation products of poly(lactic-co-glycolic acid) (PLGA) have been shown to be highly immunosuppressive (Allen et al., 2018a; Sangsuwan et al., 2020), and notably PLGA is the basis of many synthetic drug delivery platforms undergoing clinical trials (Box 2). Recent findings show that lactic acid is key to the metabolic activity of immunosuppressive regulatory T cells within the tumor microenvironment necessitates further investigation of the utility and impact of PLGA-based nanocarriers for anti-tumor strategies (Watson et al., 2021).
Addressing limitations of cancer immunotherapy via nanocarrier-mediated controlled delivery
Strategies for therapeutic immunomodulation largely involve the induction or enhancement of T cell-mediated anti-tumor responses. Two clinically relevant examples that have been improved by controlled delivery strategies are immune checkpoint inhibitors (ICIs) and agents that exploit immunogenic cell death (ICD) (Zhou et al., 2019). Immune checkpoint receptors, such as the cytotoxic T-lymphocyte-associated protein 4 (CTLA-4/CD152) that is constitutively expressed by regulatory T cells (Takahashi et al., 2000), maintain self-tolerance and avoid autoimmunity. CTLA-4 inhibits CD28 co-stimulatory receptors, which are constitutively expressed on naive T cells and play critical roles in T cell activation, proliferation, cytokine production, anergy, as well as a variety of other functions (Harding et al., 1992; Mueller et al., 1989; Schwartz, 1992). Both CTLA-4 and CD28 bind competitively to CD80 (B7–1) and CD86 (B7–2), but the higher affinity interaction of CTLA-4 with these molecules diminishes CD28 signaling (Seidel et al., 2018). Similar negative regulation mechanisms are also known to influence the functions of other immune cell types aside from T cells (Oyewole-Said et al., 2020). For example, stimulating CTLA-4 receptors on B cells can inhibit their production of immunoglobulin (Ig)G1 and IgE (Pioli et al., 2000). Elsewhere, it has been shown that CTLA-4 is expressed by monocyte-derived dendritic cells, which restricts the maturation of these cells and downregulates their presentation of antigens (Wang et al., 2011).
CTLA-4 receptors and other negative regulators of T cell activation can also be exploited by tumors to restrict T cell activity. Pharmacological ICIs therefore “switch off” the molecular signals that would otherwise prevent T cell functions, thereby enabling the host to generate productive T cell-mediated anti-tumor responses (Wolchok and Saenger, 2008). The first approved CTLA-4 inhibitor was Ipilimumab (Yervoy), a monoclonal antibody for treating unresectable or metastatic melanoma and for adjuvant treatment of cutaneous melanoma with certain pathological features (Hodi et al., 2010; Robert et al., 2011), which has been followed by other targets that include programmed cell death ligand 1 (PD-L1) (Freeman et al., 2000) and programmed cell death protein 1 (PD-1/CD279) (Agata et al., 1996; Bennett et al., 2003; Ishida et al., 1992; Parry et al., 2005). Like CTLA-4, PD-1 is an inhibitory receptor that binds to PD-L1 to counteract activating signals from T cell receptors (TCRs) and CD28 (Sharpe and Pauken, 2018). Aside from their presence on T cells, PD-1 and PD-L1 are also expressed by a variety of other influential cell types (Vaddepally et al., 2020). Subsets of dendritic cells that express PD-1 and PD-L1 provide one such example and contribute to the negative regulation of T cells (Lim et al., 2016; Peng et al., 2020). Nivolumab (Opdivo) became the first FDA-approved human IgG4 monoclonal antibody against PD-1 in December of 2014, and is administered via intravenous infusion for the treatment of certain forms of melanoma, lung cancer, renal cell carcinoma, and Hodgkin lymphoma (Vaddepally et al., 2020). Pembrolizumab (Keytruda), an IgG4k monoclonal antibody against PD-1, was approved later that year for a broad range of cancers and was followed by Cemiplimab (Libtayo) in 2018 (Vaddepally et al., 2020). The FDA has approved three PD-L1 inhibitor antibodies for intravenous administration: avelumab (Bavencio), durvalumab (Imfinzi), and atezolizumab (Tecentriq) (Twomey and Zhang, 2021; Vaddepally et al., 2020). Avelumab is approved for certain populations of adult and pediatric patients with metastatic Merkel cell carcinoma. Durvalumab is available for certain forms of locally advanced or metastatic urothelial carcinoma, and for certain patients with unresectable stage III non-small cell lung cancer. Atezolizumab is approved for the treatment of urothelial carcinoma, and as adjuvant treatment, first-line treatment, or combined treatment of a variety of other cancers. While monotherapy blockades that individually target CTLA-4, PD-L1, and PD-1 have had success in some cases, combined ICI immunotherapies have also been developed to simultaneously target multiple checkpoint inhibitors for greater efficacy.
ICD includes chemotherapy, photodynamic therapy, and radiotherapy, and can increase tumor immunogenicity (Kroemer et al., 2013; Krysko et al., 2012). Tumor cells undergoing ICD produce potent distress signals called damage-associated molecular patterns (DAMPs) like ATP, calreticulin (CRT), and high-mobility group Box 1 (HMGB1). For example, DAMPs released by dying cells within the TME promote the maturation of dendritic cells (DCs), which activate CD8+ cytotoxic T lymphocytes (CTLs) to destroy tumor cells (Tuettenberg et al., 2007; Zhou et al., 2019). It is known that classic chemotherapeutics, such as oxaliplatin (OXA; ELOXATIN), doxorubicin (DOX; doxorubicin hydrochloride), idarubicin (IDR; Idamycin PFS), and mitoxantrone (MX; NOVANTRONE), evoke ICD responses (Chen et al., 2019; Koceva-Chyla et al., 2005; Menger et al., 2012; Minotti et al., 2004). These ICD agents, and others, are discussed in greater detail elsewhere (Fucikova et al., 2020; Sukkurwala et al., 2014).
Despite clinical success, modern ICI and ICD immunotherapies suffer from numerous issues. Many patients fail to respond to ICI immunotherapy due to resistance mechanisms, including mutations, immunoediting phenomena, metabolic alterations, immune cell subpopulation changes, and others (Liu et al., 2019a). These resistance mechanisms contribute to low objective response rates to ICI immunotherapy (Bol et al., 2019). Furthermore, ICIs commonly have toxicity issues arising from low treatment specificity, an issue that is frequently worse during combined ICI immunotherapy (Kennedy and Salama, 2020). Regarding ICD induction, these therapeutics are limited by circulation time, restricted tumor cellular uptake, and adverse events arising from nonspecific cytotoxicity (Gao et al., 2020). The reoccurring difficulties experienced by both ICI and ICD therapeutics result from suboptimal pharmacokinetics (PK) and pharmacodynamics (PD) (Centanni et al., 2019; Janicka and Gubernator, 2017). Improving the performance of therapeutics can be challenging, and often requires the formulation and route of administration to be optimized via trial and error or chemical modification based on structure-activity relationships. Efforts to improve therapeutic performance often comes at the cost of efficacy. The high customizability of nanocarrier platforms can be used to tune therapeutic performance without chemically modifying the encapsulated agents. The biodistribution, circulation time, and cellular uptake of a nanocarrier is passed on to the therapeutic payloads loaded within them, presenting a powerful platform for controlled delivery applications. Hydrogel-based systems and biomaterial scaffolds also provide control over cancer immunotherapy, and these technologies are reviewed elsewhere (Karabin et al., 2018; Li et al., 2020; Xie et al., 2021). We discuss how the shape, size, and surface chemistry of delivery vehicles formed from self-assembled nanobiomaterials can be customized for targeted biochemical and cellular interactions to enhance immunomodulatory strategies in the treatment of cancer.
Materials-based strategies for improving current cancer immunotherapies
Depending on the design and features of a nanocarrier, these technologies are capable of improving the efficacy of conventional cancer immunotherapies by increasing drug accumulation within the tumor, reducing systemic toxicity, and enabling the codelivery of multiple therapeutics (Peer et al., 2007). Nanobiomaterials can be engineered to stably transport therapeutics and diagnostics, either individually (Allen et al., 2017) or in combination (Yi et al., 2019), to specific biological targets. The diverse physicochemical properties of these payloads, such as nucleic acids, small molecule drugs, and proteins, pose great challenges for loading and encapsulation at optimal concentrations within nanocarriers. Approaches such as nanoprecipitation (Allen et al., 2018b; Markwalter et al., 2019) and microfluidics (Carugo et al., 2016) have improved the co-loading of diverse payloads into nanocarriers.
Work in the area of nanobiomaterial-mediated ICD is centered on applying nanocarriers to enhance chemotherapy, often with an emphasis on minimizing side effects that limit the maximum tolerable dose and by avoiding solubilization agents associated with toxicity concerns, such as cremophor EL (CREL). The continued development of nanocarrier chemotherapeutics is undoubtedly motivated by success of the PEGylated liposomal formulation of DOX, known as Doxil, with the objective to both build on its therapeutic index improvements as well as to address its flaws. Doxil has been proposed to achieve drug accumulation within tumors primarily via the enhanced permeability and retention (EPR) effect (the EPR effect is discussed in Box 3). Yet there is another facet to the story that extends beyond delivering chemotherapeutics at high concentration to its site of action. The efficacy of a given chemotherapy is defined not only by their own inherent ability to kill tumor cells, but also by their induction of anti-tumor immune responses that assist with this process (Zitvogel et al., 2008). In some cases, nanocarrier-enhanced chemotherapies stimulate anti-tumor responses while providing the patient with additional treatment benefits. One example is chimeric polypeptide DOX (CP-DOX) nanoparticles (MacKay et al., 2009), which induce cell death to a greater extent than free DOX in poorly immunogenic 4T1 mammary carcinoma tumors (Mastria et al., 2018). CP-DOX increased levels of leukocyte-recruiting chemokines (CCL2–5, CXCL2, and CXCL10) that increased tumor leukocyte infiltration such that >67% of live cells in a CP-DOX-treated tumor were leukocytes (Mastria et al., 2018), achieving robust mobilization of latent host immune responses. Importantly, poorly responsive 4T1 carcinoma cells obtained substantial immune sensitivity upon treatment with CP-DOX that was not observed for free DOX. In a separate study, OXA-encapsulated mPEG-PLGA nanoparticles (NP-OXA) increased HMGB1 release by 70% and ATP secretion by 48%, and further reported CRT increases of greater than 80%, compared with OXA treatment alone (Zhao et al., 2016). Furthermore, NP-OXA treatment promoted DC maturation, enhanced levels of interferon-γ positive CD8+ CTLs, and reduced tumor growth to greater extents in both immunocompetent and immunodeficient mice (Zhao et al., 2016). Collectively, these results provide good examples of how nanocarriers can enhance chemotherapy to actuate potent anti-tumor immune responses via the ICD pathway.
Box 3. Shortcomings of the enhanced permeability and retention (EPR) effect.
The enhanced permeability and retention (EPR) effect (Maeda, 2015) takes advantage of the leaky vasculature of tumors for targeted therapeutic delivery. Use of the EPR effect requires the nanomaterial-drug conjugates or nanocarrier-encapsulated drugs to be of a compatible size, and to remain stable in circulation for sufficiently long periods (often >6 h) to reach the target site (Maeda, 2015). Recent results suggest that ongoing debate controversy over the efficacy of this strategy may result from size-dependent restrictions that can differ between patients and tumors (Blanco et al., 2015; Dreaden et al., 2012; Matsumoto et al., 2016). For example, slow developing BRAF(V600E)/PTEN tumors have more developed vasculature relative to B16F10 melanomas, which instead rapidly develop leaky vasculature for a noted EPR effect (Maeda et al., 2000). Furthermore, variability in blood flow and coagulation, as well as the systolic blood pressure, also contribute to the heterogeneity of the EPR effect (Maeda, 2015). Limitations of the EPR effect have primarily manifested in applications that require highly efficient delivery to tumor cells (e.g., chemotherapy, siRNA for targeting oncogenes, etc.). These limitations may not be as detrimental for immunotherapy applications that only require the activation of a small subset of immune cells to catalyze a potent anti-tumor response. Functional synergy achieved by multi-component nanocarrier formulations illustrate this point. For example, a systemically administered nanocarrier dual-loaded with immunomodulators entered into the perivascular region of tumors to promote the rapid expansion of APCs and the infiltration of lymphocytes throughout pancreatic tumors (Lorkowski et al., 2021). The extent of tumor accumulation required for effective immunotherapy is variable due to tumor and patient heterogeneity, and the optimal half-life of immunotherapeutic agents is less well studied than for chemotherapeutics and other more traditional anti-cancer agents.
To mitigate the need for multiple injections and provide on-demand cytotoxicity, there is interest in delivering ICD agents using materials that release drug in response to an external stimulus, such as light. The synergistic controlled delivery of ICD-inducing chemotherapeutics and photosensitizers for photodynamic therapy (PDT) demonstrate the utility of this approach. In a recent study, a porphyrin photosensitizer, pheophorbide A (PhA), and a cytotoxic chemotherapeutic, camptothecin, were co-delivered using PEG17-b-PPS75 BCNs (Bobbala et al., 2020). Poly(ethylene glycol)-b-poly(propylene sulfide) (PEG-b-PPS) is a stimuli-responsive material that releases drug payloads upon oxidation within acidic lysosomal compartments. The polymer variant that self-assembles into the BCN morphology has a uniquely long hydrophobic block, PPS75 (i.e., 75 PPS units), that is exceptionally stable and permits the formation of long-lasting depots within lysosomes. Co-loading PhA photosensitizer with camptothecin yielded lysosomal depots that deliver chemotherapeutic intracellularly in response to stimulation with light (Bobbala et al., 2020). Under ambient conditions, the BCNs protected normal cells from cytotoxic payloads, whereas the light-triggered photo-oxidation of the PPS block produced a morphological transition from BCNs into micelles (Bobbala et al., 2020). The mechanism of this transition involves oxidation-mediated increases in polymer hydrophilicity that favor micellarization. Controlling BCN-to-micelle transitions using light allowed for the on-demand release of camptothecin inside of cells, highlighting its potential use of an off-on triggerable delivery platform as a novel technology for PDT- and ICD-induced immunogenicity (Bobbala et al., 2020). Aside from enhanced anti-tumor immunological responses and controlled release, nanobiomaterials also find utility in improving patient responses to ICI immunotherapies and increasing patient response rates (Goldberg, 2019).
Other opportunities exist for developing nanotechnologies that leverage synergy between ICI and ICD. A recent example is provided by nanoscale coordination polymer particles composed of an oxaliplatin (OxPt) prodrug core and a lipid-bilayer coating (Duan et al., 2019). The outer shell contained cholesterol conjugated to the ICD-inducing dihydroartemisinin (DHA) metabolite of artemisinin. These OxPt/DHA core-shell particles induced reactive oxygen species in cancer cells to elicit mitochondrial dysfunction and cell death. Furthermore, the core-shell particles enhanced anti-PD-L1 immunotherapy in a colorectal cancer mouse model (Duan et al., 2019). Synergy between OxPt/DHA core-shell particles and anti-PD-L1 treatments eliminated tumors after 40 to 50 days, led to the absence of tumor recurrence for 120 days, and produced a memory response capable of preventing tumor recurrence within a 1-month period after challenging mice with CT26 cell inoculation (Duan et al., 2019).
The need for targeted cancer immunotherapy
The primary objective of targeted drug delivery is to transport therapeutics directly to the site of action. Technologies that achieve this feat are sought after due to their potential to improve efficacy and to mitigate adverse effects arising from nonspecific drug accumulation at off-target locations. The latter consideration is particularly important for chemotherapeutics that can damage healthy cells and tissues. Furthermore, many immunotherapies employ potent immunomodulatory agents that can disrupt systemic immune homeostasis. For example, a host of adverse events commonly arise in response to ICB immunotherapy (Khalil et al., 2016), including autoimmune disorders and diabetes, often requiring the patient to discontinue treatment. Targeted nanobiomaterials can thus be leveraged to minimize or avoid many of these undesirable effects for a wide range of immunotherapeutic strategies (Figure 2).
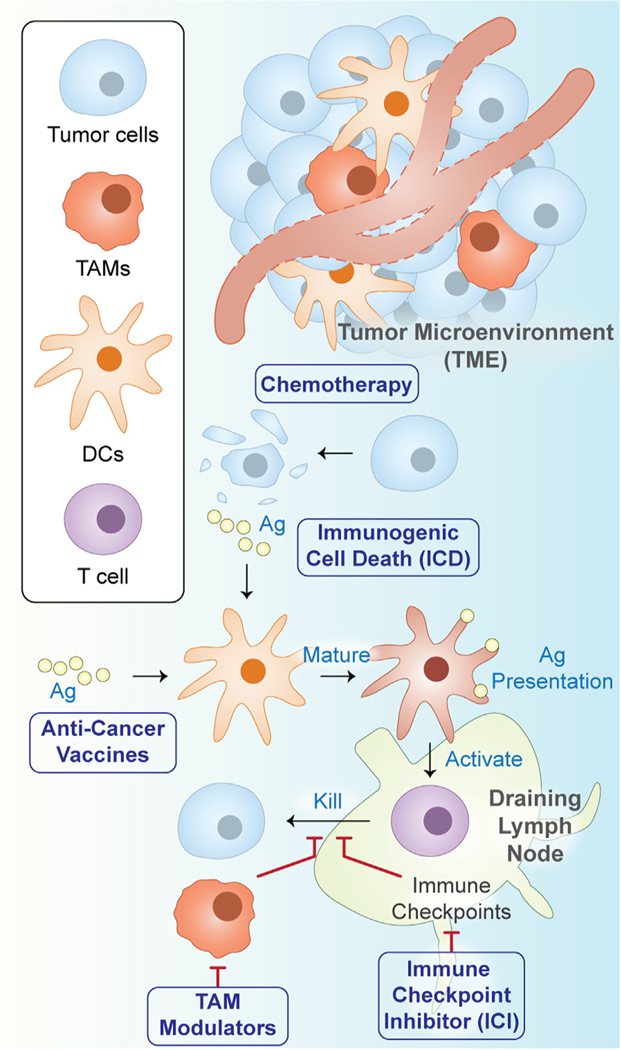
Figure 2. Overview of nanobiomaterial applications for targeted cancer immunotherapy.
Major applications for targeted nanobiomaterials include anti-cancer vaccines, immune checkpoint inhibition (ICI), immunogenic cell death (ICD), and chemotherapy. Abbreviations: tumor-associated macrophages (TAMs); dendritic cells (DCs); antigen (Ag).
Targeted nanocarriers are often employed to selectively modulate the TME (Figure 2), which consists of a complex milieu of tumor cells, immune cells, blood vessels, extracellular matrix, and biomolecules (Werb and Lu, 2015; Whiteside, 2008). Cancer therapy is frequently obfuscated by the role of the TME in suppressing local immune cells and hindering the elimination of cancer cells (Beatty and Gladney, 2015; Drake et al., 2006; Heinrich et al., 2012; Menter and Tzankov, 2018; North, 1982; Wang et al., 2017). Given its importance in cancer progression and drug resistance, various cell types within the TME have emerged as important targets for cancer immunotherapy (Tsai et al., 2014). Multiple paths exist for directing nanocarrier biodistribution and cellular interactions within the TME (Figure 2). These range from adjusting the physical and chemical properties of the delivery vehicle itself to surface functionalization with ligands to enhance desirable nanocarrier-cell interactions.
Types of targeting strategies
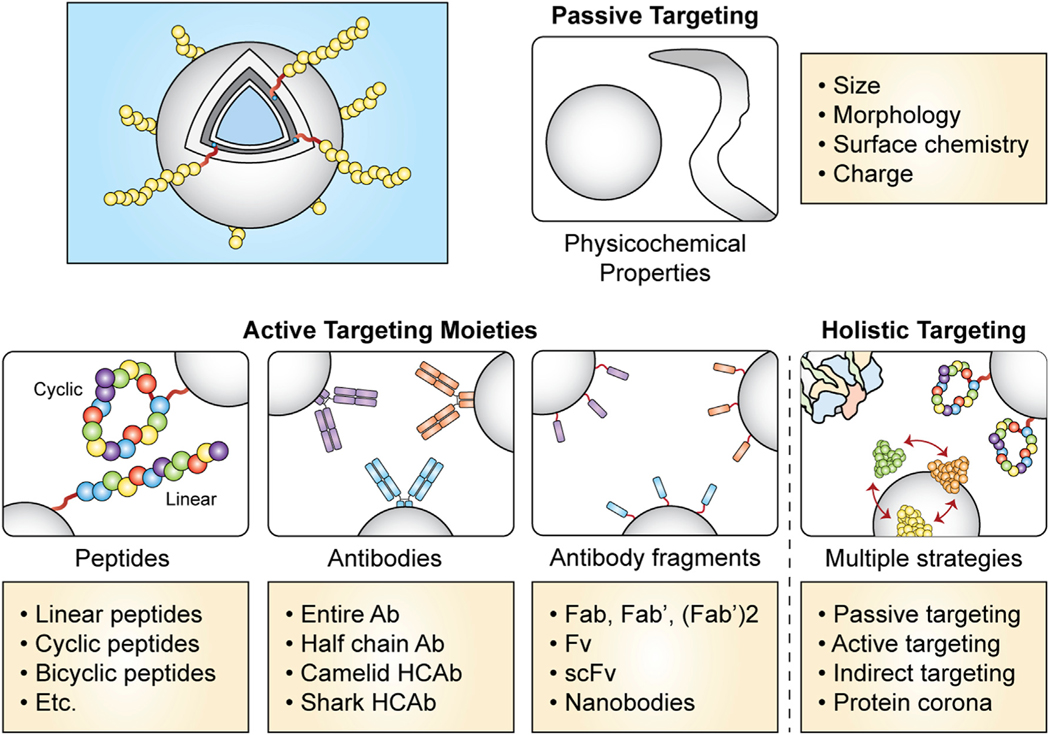
The important cellular targets for cancer immunotherapy include tumor cells and cells of tumor-draining lymph nodes, as well as a variety of TME constituents, including tumor-associated macrophages (TAMs), dendritic cells (DCs), T cells, and various suppressive myeloid cell populations. Targeting strategies are most commonly divided into one of two categories: passive targeting and active targeting. During passive targeting (Rosenblum et al., 2018), the physicochemical properties of the nanocarrier, such as size (Sykes et al., 2014; Zhang et al., 2009), shape (Champion and Mitragotri, 2006; Geng et al., 2007; Yi et al., 2016), and charge (Heuts et al., 2021; Wang and Mooney, 2018), all influence biodistribution in a holistic fashion by modulating the transport properties, adsorption of blood proteins (also known as protein corona formation), and interactions with cell membranes. Active targeting strategies employ native or engineered ligands to bind to specific components of cell membranes (Rosenblum et al., 2018). In this section, we begin by discussing antibody-based strategies for targeted cancer immunotherapy, highlighting antibody-drug conjugates as a more minimalistic delivery technology with that achieves high specificity and is in clinical use. Moving next into higher-order vehicles, we discuss the properties of nanocarriers that are known to provide ligand-free, passive targeting functionality. Afterward, we introduce the rational design of targeting ligands, such as peptides or aptamers, that render nanocarriers as active targeting vehicles. Numerous examples of targeted nanotechnologies engineered to direct cancer immunotherapy to specific cell types within the TME are presented. Finally, we introduce a third method of targeting that has recently received renewed attention, which we refer to as “indirect” targeting. This method enhances uptake within the TME and improves cell-selective delivery not by directly enhancing interactions with the target cells, but through indirect actions on nonspecific off-target cells that are responsible for rapid nanocarrier clearance from circulation.
Nanocarrier design principles for passive targeting
A critical step in designing a nanocarrier is selecting the structure (morphology/shape) of the vehicle and its surface chemistry, which should both possess suitable properties for the intended application. A useful analogy is to consider how the chassis of an automotive vehicle is optimized, where the shape and materials employed have direct implications on the utility. Similarly, the “nanocarrier chassis” (Vincent et al., 2021b) must be engineered to transport cargo, remain stable under in vivo conditions, interact with desired molecular targets, and degrade within specific biochemical environments (Figure 3). It is therefore important to note how distinct and unique different nanocarrier platforms can be despite common misconceptions that nanobiomaterials are interchangeable during drug delivery applications. The design space for these technologies is extensive, and even for material subsets that are similar, the nuances of their design can lead to a major divergence in performance. The chosen nanocarrier morphology must be capable of loading the selected cargo(es), often hydrophobic small molecules or hydrophilic biologics (proteins, nucleic acids, etc.) (Figure 3), at a quantity that is sufficient to achieve therapeutically relevant concentrations. The nanocarrier must also be designed to release drug at an appropriate rate, permit cargo escape from specific intracellular endolysosomal compartments, and should have physical dimensions that permit its transport across any biological barriers encountered in transit to the targeted site. While important, these considerations are covered at length elsewhere (Mitchell et al., 2021).
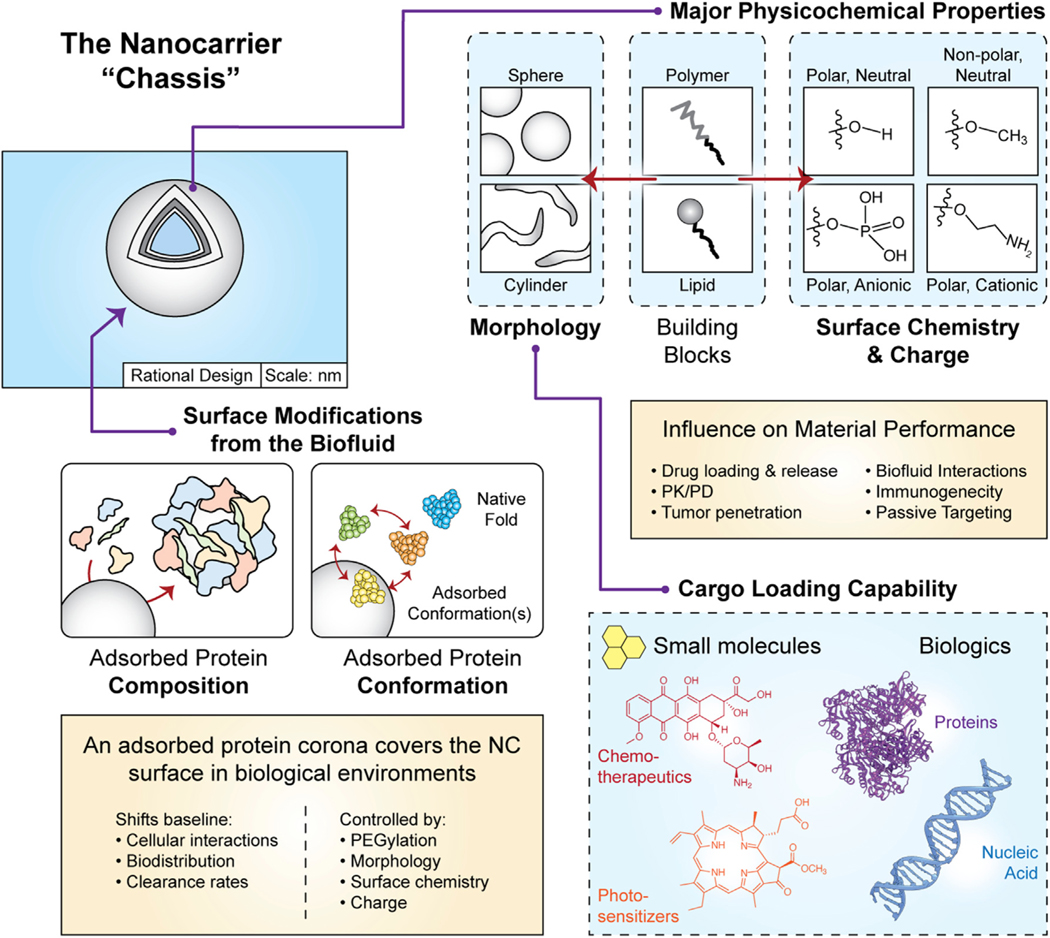
Figure 3. Overview of the nanocarrier “chassis” and surface biofouling.
The design of nanocarriers begins with the selection of a material type, or building block (here, polymers or lipids), which favorably self-assemble into the morphology/shape of interest. The cargo loading capability of the nanocarrier is largely determined by the chassis morphology. Potential cargoes include small molecules (traditional chemotherapeutics, photosensitizers, etc.) as well as biologics including various antigens, adjuvants, nucleic acids, etc. The ovalbumin protein (PDB ID: 1OVA) and the DNA molecule from PDB entry 4CJA are displayed for illustrative purposes. The building block should be engineered to contain terminal chemical groups of the desired atomic composition, polarity, and charge to yield the surface chemistry of interest. When designing the synthetic physicochemical properties, it is also important to understand how these design choices modulate nanocarrier interactions with biofluid proteins in a process referred to as biofouling. The formation of an adsorbed surface coating of proteins (i.e., protein corona) redefines the nanocarrier’s biochemical and cellular interactions. The identity of adsorbing protein species, their relative abundance, and the structural conformation(s) can be tuned using the physicochemical properties of the nanocarrier chassis. All surface features described here influence the pharmacokinetics, biodistribution, and inflammatory potential of the nanocarrier.
Here, our attention focuses on the surface properties of the nanocarrier chassis (Vincent et al., 2021b) that confer inherent targeting features. These surface engineering efforts differentially modulate the rate of nanocarrier uptake by diverse cell types and can therefore be customized for specific therapeutic applications. The surface chemistry of a nanocarrier modulates its baseline interactions with biological membranes and its rate of cellular internalization (Asati et al., 2010; Manzanares and Cena, 2020). Physicochemical properties further direct the composition (Vincent et al., 2021b; Walkey and Chan, 2012; Walkey et al., 2012), higher-order protein layer organization (Zhang et al., 2020a), and clearance-altering biophysical properties (Vincent et al., 2021a) of adsorbed protein layers formed on the nanocarrier surface in situ. These protein coatings redefine the synthetic surface with a biological identity that largely dictates nanocarrier cellular interactions and has significant consequences on drug biodistribution (Vincent et al., 2021a, 2021b). The combination of morphology and surface chemistry defines the nanocarrier biological identity to modulate its interactions with monocytes, macrophages, and dendritic cells (Vincent et al., 2021b), thereby providing another exploitable property for controlling nanocarrier interactions within the TME. Passive targeting by a nanocarrier is strongly influenced by its shape/morphology (Geng et al., 2007; Vincent et al., 2021b; Yi et al., 2016) and surface chemistry (Vincent et al., 2021a, 2021b). The morphology of a drug delivery vehicle influences many aspects of its performance including circulation time (Geng et al., 2007), biodistribution and preferential interactions with immune cell subsets (Vincent et al., 2021b), and route of internalization through different endocytosis pathways (Zhang et al., 2015).
Nanocarrier dimensions have been linked to the EPR effect for targeting solid tumors (Kang et al., 2020). The EPR effect primarily refers to the preferential accumulation of drug-bearing nanocarriers within tumors due to an increased leakiness of poorly developed tumor vasculature compared with normal, healthy tissues. For spherical systems, the diameter threshold has been shown to be below 200 nm to take advantage of the EPR effect (Peer et al., 2007). However, we note that while the EPR effect has drawn considerable excitement over the years, EPR-based targeting strategies have fallen short of expectations due to a variety of factors (see Box 3).
Spherical nanocarriers have been used in the clinic since the introduction of Doxil in 1995 (Barenholz, 2012) (Figure 1A). A range of spherical morphologies can be self-assembled from lipid and polymeric amphiphiles, including micelles, vesicle structures (lipid vesicles, polymersomes [Discher et al., 1999]), and BCNs (Angelov et al., 2015; Bobbala et al., 2018; La et al., 2014). These morphologies were introduced in depth earlier in this review. Micelles are primarily used in the delivery of small molecules that are sufficiently hydrophobic for encapsulation within their core. Hydrophilic payloads can also be delivered via micelles but typically require additional steps for stable complexation or conjugation respectively via electrostatic or covalent interactions. In contrast, vesicular structures can encapsulate biologics within their aqueous lumen without chemical modification. In addition, the hydrophobic component of vesicle bilayers permits the loading of lipophilic compounds using the same hydrophobic packing interactions observed for micellar nanostructures. The cubic structure of the BCN morphology provides a unique cage-like network of aqueous channels embedded within an extensive lipophilic volume, presenting an alternative to vesicles for facile loading and delivery of both hydrophilic and lipophilic payloads (Allen et al., 2018c). The BCN architecture possesses a remarkable stability that has proven useful in the development of photoresponsive and/or sustained anti-cancer therapies (Bobbala et al., 2020).
As described earlier, self-assembled filomicelles possess spherical cross sections of similar diameter as spherical micelles but are elongated in the third dimension to form a worm-like filamentous morphology (Figure 1C). The flexibility of these cylinders can be specified by their chemical composition (Li et al., 2021), allowing control over their circulation time and uptake by many myeloid cell populations (Geng et al., 2007). Of note, filomicelles of higher flexibility can circulate for extensive periods of time to avoid accumulation in off-target organs while accessing leaky vasculature (Christian et al., 2009; Yi et al., 2016), which has proven advantageous for targeting brain tumors (Baumann et al., 2013; Oltra et al., 2013). By mimicking the role of collagen fibers within the extracellular matrix, filomicelles can also serve as the basis of synthetic hydrogels for sustained delivery of therapeutics. In one example, crosslinked filomicelles composed of PEG-b-PPS diblock copolymers were employed as injectable hydrogels for sustained bioresponsive delivery of micelles containing hydrophobic payloads (Karabin et al., 2018). These oxidation-sensitive hydrogels continuously released therapeutic and diagnostic nanocarriers for up to 1 month by way of a unique cylinder-to-sphere transition mechanism. The ability to control transitions in vehicle morphology is an advantage of self-assembled systems. Many opportunities exist for harnessing morphological transitions and in situ gelation of nanomaterials to achieve sustained drug release strategies, such as vaccine implants (Bobbala and Hook, 2020) and intratumoral injections that locally stimulate immune responses against cancer antigens (Vohidov et al., 2020).
The role of nanocarrier morphology in modulating specific cellular interactions within the TME remains an open question. However, it is generally known that nanocarrier morphology has a profound effect on defining the baseline cellular uptake profile of a nanocarrier by various immune cell subsets. This a process that has been referred to as Nanostructure Enhanced Targeting (NSET) and has been extensively characterized on PEG-b-PPS nanocarriers (Yi et al., 2016). Yi et al. (2016) demonstrated that polymersomes achieve greater uptake by dendritic cells (DCs) in vivo compared with micelles and filomicelles of identical surface chemistry, suggesting the vesicular chassis was optimal for targeting DC subsets. Wilson and colleagues also employed the polymersome morphology to enhance delivery to DCs, specifically developing polymersome delivery systems for the stimulator of interferon genes (STING) agonist 2′3′ cyclic guanosine monophosphate–adenosine monophosphate (cGAMP) (Shae et al., 2019). Activation of the STING pathway can occur in response to cytosolic tumor-derived DNA, which elicits type I interferon (IFN-I) responses that promote DC maturation and improves the induction of anti-tumor T cell immunity (Nguyen et al., 2021). Polymersomes transporting STING-agonists improved vaccination against neoantigens (Shae et al., 2020), enhanced response to immune checkpoint blockade (Shae et al., 2019), and elicited ICD responses for anti-tumor immunity in neuroblastoma (Wang-Bishop et al., 2020).
Recent evidence may explain this morphology-dependent uptake of nanocarriers by specific immune cell subsets. Proteomic investigations using human blood and a PEG-b-PPS nanocarrier library that varied in morphology and surface chemistry found this passive targeting effect to be dependent on the biofouling of the nanocarrier surface by proteins within biological fluids (Figure 3). Protein adsorption is a biochemical process that occurs when nanocarriers are introduced into biological fluids and is defined by the adhesion of proteins to material surfaces, resulting in an outer layer proteins (Nel et al., 2009). This adsorbed coating of proteins is referred to as the “protein corona,” and its specific protein composition and biophysical properties are unique to the physicochemical properties of the material, resulting in nanocarrier-specific biochemical and cellular interactions and responses that often promote opsonization, clearance, and inflammation (Vincent et al., 2021a, 2021b). These properties are important because they influence pharmacokinetics through redefining nanocarrier interactions at the organ and cellular levels (Vincent et al., 2021a, 2021b). For example, higher uptake of methoxy-functionalized polymersomes by DCs was mediated by the unique fingerprint of proteins coating the nanocarrier surface (Vincent et al., 2021b).
In addition to the corona composition, the structure of denatured proteins within the adsorbed protein layer influences scavenging by innate immune cells. Specifically, the unfolded structure of albumin adsorbed onto nanocarriers within this same library was linked to scavenging by the SR-A1 receptor, resulting in distinct cellular biodistributions for polymersomes of identical size but possessing distinct surface chemistries (Vincent et al., 2021a). The multiscale mechanisms elucidated by this work hold promise for customizing nanocarrier morphology and surface chemistry to specify passive targeting across diverse myeloid populations within the TME for strategic and patient-specific immunotherapy. Of note, these results were specific to the PEG-b-PPS delivery system and could differ significantly across other platforms, highlighting both the need to extensively characterize and benchmark different nanocarrier platforms as well as the diversity in targeting capabilities that can be achieved when optimizing the nanocarrier chassis.
Antibody-mediated active targeting
A wide range of antibodies are readily available with high specificity and affinity for unique epitopes within the TME, making these technologies highly versatile options for precision medicine and enhancing nanocarrier targeting. These include monoclonal antibodies (mAbs), bispecific antibodies (bsAbs), and anti-body-drug conjugates (ADCs), each of which can achieve improved efficacy and versatility when incorporated into nanotherapies. For example, mAb monotherapies have similar shortcomings in pharmacokinetics and off-target effects as other cancer therapeutics, and suffer from efficacy limitations, as some patients do not respond to treatments or exhibit cancer recurrences over time (Gu et al., 2007). Conjugation to the surface of nanocarriers (Figure 4) can significantly modulate mAb biodistributions by enhancing their selectivity for the TME and limiting side effects resulting from accumulation in off-target locations. Furthermore, mAb monotherapies are often restricted to extracellular targets and struggle to achieve intracellular delivery when necessary (Imai and Takaoka, 2006). This issue can be addressed by encapsulating intracellular-acting mAbs into nanocarriers that house an aqueous lumen, like vesicles and BCNs. Tian et al. (2015) encapsulated IgG within PMPC-b-PDPA polymersomes that were coated with angiopep-2 peptide to target the low-density lipoprotein receptor-related protein 1 (LPR-1), a receptor associated with the transcytosis and endocytosis into cells of the CNS. The angiopep-2 decorated NPs efficiently crossed the blood-brain barrier (BBB), and co-localized with CNS cells such as astrocytes, neurons, and glial cells to deliver IgG intracellularly. Importantly, while further investigation is warranted for mAb encapsulation, these studies denote the potential for nanomaterials to overcome limitations of mAb therapies such as intracellular delivery and pharmacokinetics of delivery through biological barriers such as the BBB. Moreover, nanoparticles can be designed with surface coatings, notably PEGylation, or with morphologies or surface chemistry that bypass the mononuclear phagocyte system (MPS) to some extent and prolong blood circulation time (Geng et al., 2007; Owens and Peppas, 2006; Vincent et al., 2021a), which would further enhance the therapeutic index of mAbs.
Figure 4. Summary of common targeting strategies for directing nanocarrier interactions and payload delivery.
Targeting strategies include passive and active approaches, as well as holistic approaches that combine multiple targeting strategies in a single nanocarrier formulation to maximize control over drug interactions. Abbreviations: antibody (Ab); heavy chain antibody (HCAb); antigen-binding fragment (Fab); fragment variable (Fv); single-chain variable fragment (scFv).
Nanocarriers are excellent substrates for the multivalent display and crosslinking of multiple antibodies simultaneously to enhance bsAb strategies. For example, bispecific T cell engagers (BiTEs) are bsAbs designed to simultaneously target tumor-specific antigens and CD3 to enhance tumor cell killing by cytotoxic T cells. BiTEs can be used as an effective strategy for generating antigen-specific T cell immune responses toward tumors with low major histocompatibility complex I/antigen expression levels (Gong et al., 2021). Like mAbs, BiTEs are limited in their pharmacokinetics and require sustained exposure to achieve prolonged T cell activation, penetration, and expansion in target tissues (Goebeler et al., 2016). In addition, because of the monomeric nature of their binding arms, singular BiTEs may not establish strong linkages of T cells to tumor cells (Brischwein et al., 2007). Nanocarriers are able to overcome these limitations, and work in this area commonly simulates bsAbs-like interactions by displaying multiple functional ligands. The surface of nanocarriers can be modified to conjugate multivalent binding moieties that engage different targets. An additional benefit to NP-BiTE strategies is the ability to encapsulate and deliver payloads that may augment T cells to further amplify therapeutic performance and/or direct the clearance of tumor cells by professional APCs. A multivalent bispecific nanobioconjugate engager (mBiNE) was reported by Yuan et al. (2017) that used a carboxyl-functionalized nanoparticle core as a substrate to simultaneously display (1) trastuzumab mAb to target human epidermal growth factor receptor 2 (HER2) that is enriched on cancer cell membranes, and (2) calreticulin (CTR) to promote phagocytosis by APCs. This designed NP-BiTE promoted phagocytosis of HER2 E0771/E2 tumors by macrophages and enhanced T-lymphocyte activation and tumor accumulation, thereby producing a systematic and effective anti-tumor immune response (Yuan et al., 2017). Another study engineered HEK293 cells to produce exosomes that display two antibody types at their surface. This technology, referred to as SMART-Exos (synthetic multivalent antibodies retargeted exosomes), displayed mAbs against CD3 and EGFR to target T cells and epidermal growth factor receptor (EGFR)-positive triple-negative breast cancer cells (Cheng et al., 2018). The bispecific targeting SMART-Exos selectively recruited cytotoxic T cells to the tumor site and induced strong crosslinking between T cells and EGFR-expressing breast cancer cells to produce robust anti-tumor immunity (Cheng et al., 2018). These studies demonstrate that simulating bsAb BiTE functionality with engineered nanomaterials provides a novel class of immunotherapeutics for enhancing cellular anti-tumor responses.
The use of antibodies as targeting moieties is perhaps their most intriguing utility for developing the next wave of precision nanotherapies against cancer. ADCs are tripartite molecules that contain a cytotoxic agent stably linked to a tumor-specific mAb to target chemotherapeutics directly to cancer cells (Chau et al., 2019) and are among the fastest-growing classes of oncology therapeutics (Beck et al., 2010). Numerous ADCs are now approved for clinical use, and more than 100 ADC candidates are under clinical investigation (Chau et al., 2019). Immune-stimulating antibody conjugates (ISACs) are one such modern ADC technology that has performed at a high level (Ackerman et al., 2021). Ackerman et al. (2021) used HER2-targeting antibodies for the delivery of Toll-like receptor 7/8 (TLR7/8) dual agonists. The ISACs produced localized tumoricidal events mediated by myeloid cell populations and evoked T cell-mediated anti-tumor immunity (Ackerman et al., 2021). ADCs have also been applied to the delivery of novel bifunctional immunomodulatory agents (Wang et al., 2020). The agent in the cited study potentiates ICB therapies by upregulating PD-L1 expression through histone demethylase enzymes (i.e., an epigenetic regulation mechanism) and by activating TLR7/8 signaling but suffers from side effects arising from systemic exposure (He et al., 2021; Wang et al., 2020). Attaching these immunomodulatory agents to an anti-PD-L1 antibody created a unique therapeutic, referred to as an immune modulating antibody-drug conjugate (IM-ADC). IM-ADCs not only improved treatment specificity, but also enhanced ADC accumulation within tumor cells through a positive feedback loop that resulted from elevating PD-L1 expression levels (He et al., 2021).
The clinical success of ADCs has inspired the development of more sophisticated targeted drug delivery technologies that harness the benefits of nanocarrier platforms together with the cellular targeting specificity provided by antibodies. Nanomaterials with surface embedded mAbs are studied extensively, largely because of their ability to increase drug loading capacity and payload delivery by avoiding early metabolism and excretion. In one example, the FDA-approved anti-EGFR mAb Cetuximab (CTX) was applied as a targeting moiety at the surface of PLGA nanoparticles loaded with camptothecin (CPT) chemotherapeutic to direct their delivery to CTX-resistant pancreatic cells (McDaid et al., 2019). Compared with untargeted CPT-PLGA, the CTX-CPT-PLGA nanoparticles significantly reduced murine tumor growth, which was attributed to the specificity conferred by the CTX targeting moiety. Others have attempted to use mAb-targeting together with antibody-dependent cellular cytotoxicity (ADCC) in the TME through immune cell recruitment. Liu et al. (2019b) developed a multifunctional platform that (1) displays the anti-Claudin-4 mAb KM3934 on cell-membrane derived nanovesicles (NVs) to successfully deliver cytotoxic doxorubicin (DOX) to OV-CAR-3 human ovarian tumor cells, and (2) transfers KM3934 to cancer cell membranes upon delivery. Compared with free DOX alone, this targeted nanotechnology achieved greater reductions in tumor volume while improving treatment safety. Furthermore, the targeted therapeutic recruited natural killer cells to the TME and promoted tumor necrosis via ADCC (Liu et al., 2019b), show-casing its versatility in generating potent therapeutic outcomes. Overall, nanomaterial-based cancer therapies employing mAb surface functionalization continue to emerge from the preclinical pipeline, and these technologies show potential for improving the precision of cancer immunotherapy.
Peptide-mediated active targeting
Diverse classes of peptides are employed in targeted drug delivery applications. These include linear and cyclic peptides (Figure 4), as well as lipidated variants depending on the application and delivery system employed. While linear peptides are easier to synthesize and screen, cyclic peptides confer a variety of unique benefits in terms of greater binding affinity and stability. For example, whereas many linear peptides are disordered, the conformational freedom of cyclic peptides is more restricted. This commonly yields a more defined shape (Malde et al., 2019) that often achieves higher affinities for its binding partner (cell surface receptors, etc.) (Millward et al., 2007). Cyclic peptides are also less susceptible to proteolytic degradation (Millward et al., 2007), an event that would otherwise serve to strip the drug delivery vehicle of its active targeting moieties and associated specificity. Irrespective of the peptide class, these targeting ligands are displayable on nanocarrier surfaces through either direct conjugation (Petros and DeSimone, 2010) or through the use of a lipid anchor that can stably incorporate into the hydrophobic domains of self-assembled nanocarriers (Stack et al., 2020; Vincent et al., 2021c; Yi et al., 2019). Importantly, lipid-anchored targeting ligands must be rationally designed and optimized with respect to the surface properties of the nanocarrier chassis. For example, the PEG corona dimensions on a densely PEGylated vehicle will require a hydrophilic spacer, typically a PEG linker, between the lipid anchor and targeting peptide to allow accessibility of the peptide on the nanocarrier surface (Vincent et al., 2021c). Recent work has demonstrated that the spacer length of the linker is critical for tuning the biochemical access of the surface exposed targeting ligands to its molecular target, which has profound effects on receptor binding, nanocarrier engagement with the targeted cell type(s), and efficacy of the delivered drug payloads (Vincent et al., 2021c).
A variety of peptide ligands have been identified for engaging receptors that are enriched on various cell types in the tumor microenvironment (David, 2017). The most well-known examples are linear and cyclic peptide ligands containing the ArgGly-Asp (RGD) motif that bind to αν integrins expressed on many endothelial surfaces (Bogdanowich-Knipp et al., 1999; Pierschbacher and Ruoslahti, 1984; Ruoslahti and Pierschbacher, 1987) (Table 1). The direct conjugation of RGD peptide variants with higher-order functionality, such as the iRGD peptide (CRGDK/RGPD/EC) (Table 1), to drugs and imaging agents successfully targets their delivery to cancer cells while enhancing cargo penetration into tumors (Sugahara et al., 2009). The iRGD peptide first binds αν integrins on the tumor endothelium and yields CRGDK/R following proteolysis (Sugahara et al., 2009, 2010). This fragment is a C-end Rule (CendR) motif (R/KXXR/K) that binds to neutopilin-1 (NRP-1) (Teesalu et al., 2009), which enables tumor-specific tissue penetration (Sugahara et al., 2010). Pairing RGD binding peptide with other ligands has also been exploited to enhance targeting by binding multiple receptors at the surface of tumor cells. In one receptor example, combining the RGD sequence with folic acid (FA) enhanced the delivery of paclitaxel (PTX) to MCF-7 human breast cancer cells by mesoporous silica nanoparticles (MSN) (Yan et al., 2020). Cellular studies with PTX@MSNs-NH2-FA-RGD and control formulations provide further support for dual targeting strategies to enhance targeting specificity and improve efficacy of cytotoxic chemotherapeutics (Yan et al., 2020). Elsewhere, other studies have focused on targeting the delivery of cytotoxic agents to newly formed blood vessels at the tumor site. In one example, the PH1 peptide (Mai et al., 2009) was developed by phage displayed to bind Tie2 receptor tyrosine kinases that are overexpressed by the neovascular endothelium. Cisplatin-loaded liposomes displaying PH1 were significantly more cytotoxic to Tie2-positive cells than their untargeted counterparts that lacked peptide (Mai et al., 2009).
Table 1.
Selected peptide sequences for targeting the tumor microenvironment
| Peptide | Sequence | Molecular target | Cellular target | References |
|---|---|---|---|---|
| RGD | RGDa | Integrin αvβ3, αvβ5 | Tumor cells, tumor vasculature | (Ruoslahti and Pierschbacher, 1986) |
| iRGD | CRGDK/RGPD/EC | Integrin αvβ3, αvβ5 | Tumor cells, tumor vasculature | (Sugahara et al., 2009; Sugahara et al., 2010) |
| C7 peptide | MHTAPGWGYRLS | Folate receptor α | Tumor cells | (Xing et al., 2018) |
| PH1 | TMGFTAPRFPHY | Tie2 | Tumor neovascular endothelial cells | (Mai et al., 2009) |
| UNO | CSPGAKVRC | CD206/MRC1 | M2 tumor-associated macrophages | (Scodeller et al., 2017) |
| mUNO | CSPGAK | CD206/MRC1 | M2 tumor-associated macrophages | (Scodeller et al., 2017) |
| P-D2 | GGVTLTYQFAAGPRDK | CD11c | Dendritic cells | (Lewis et al., 2012) |
The main binding domain is displayed.
Aside from targeting endothelial cells within the tumor microenvironment, many peptide-mediated strategies have been developed to enhance the uptake of drugs and/or nanomaterials by various immune cell types. For example, the UNO peptide (Table 1) was developed by in vivo phage display using a 9-amino acid cyclic CX7C peptide library on peritoneal macrophages in mice bearing 4T1 metastatic breast tumors and binds to macrophage mannose receptor 1 (MRC1/CD206) (Scodeller et al., 2017). The surface display of the cyclic disulfide-bridged UNO peptide targeted PTX-loaded polyethylene glycol-polycaprolactone (PEG-PCL) polymersomes to CD206-expressing M2-skewed TAMs in vivo (Scodeller et al., 2017). The UNO peptide is redox responsive and required linearization within reducing environments to interact with CD206 (Lepland et al., 2020). Later studies focused on the development of a short linear variant of the UNO peptide, referred to as mUNO (CSPGAK; Table 1) (Lepland et al., 2020; Scodeller et al., 2017), and demonstrated its use in targeting M2 TAMs in primary breast tumors and metastatic lesions (Lepland et al., 2020).
Indirect targeting strategies
A critical strategy to enhance tumor targeting is to directly address the nonspecific clearance and scavenging of nanocarriers by innate immune cells. Despite the numerous advancements in strategies for targeting specific cells described above, much less progress has been made regarding the prevention of nonspecific clearance of nanomaterials by the MPS, which remains a key limitation of precision drug delivery (Figure 5A). This system of phagocytic cells indiscriminately clears nanomaterials from the circulation via receptor-dependent and independent mechanisms of endocytosis, inducing side effects and decreasing efficacy. A comprehensive survey of the literature reported that a median average of only 0.7% of administered nanoparticles successfully reaches solid tumors despite the use of surface-conjugated targeting moieties like antibodies, peptides, or aptamers (Wilhelm et al., 2016). The vast majority (>90%) of systemically administered nanoparticles is usually cleared by cells of the MPS (Albanese et al., 2012). The MPS consists of circulating and organ-resident phagocytic cells, which internalize nanoparticles and eventually clear them through the liver (Albanese et al., 2012). This issue was initially addressed in the design of the first commercially available nanocarrier therapeutic, liposomal doxorubicin (Doxil), wherein PEG was displayed at the liposome surface after clinical trials initially failed (Barenholz, 2012). PEGylation increased the circulation time of the liposomes to allow sufficient uptake by cancer cells and led to significant efficacy improvements. Yet PEGylation is not sufficient to inhibit the majority of clearance by the MPS, and a general failure to fully address this issue has limited the clinical translation of numerous nanotherapies (Park, 2013). Furthermore, new drug side effects can arise for nanocarrier-encapsulated formulations due to changes in drug biodistribution and exposure time. For example, it is generally known that liposomal DOX reduces the risk of adverse events such as cardiotoxicity and alopecia compared with conventional DOX treatment, but it is less commonly brought up that liposomal forms also increase certain mucocutaneous adverse events, such as palmar-plantar erythrodysesthesia (hand-foot syndrome) (Fukuda et al., 2017; Lorusso et al., 2007). It is known that the severity of this side effect is significantly correlated with the half-life of DOX (Lin et al., 2002). The negative consequences that can result from enhancements to drug circulation time and other performance metrics further motivate the development of strategies that enable greater control over nanocarrier biodistribution and stability in vivo.
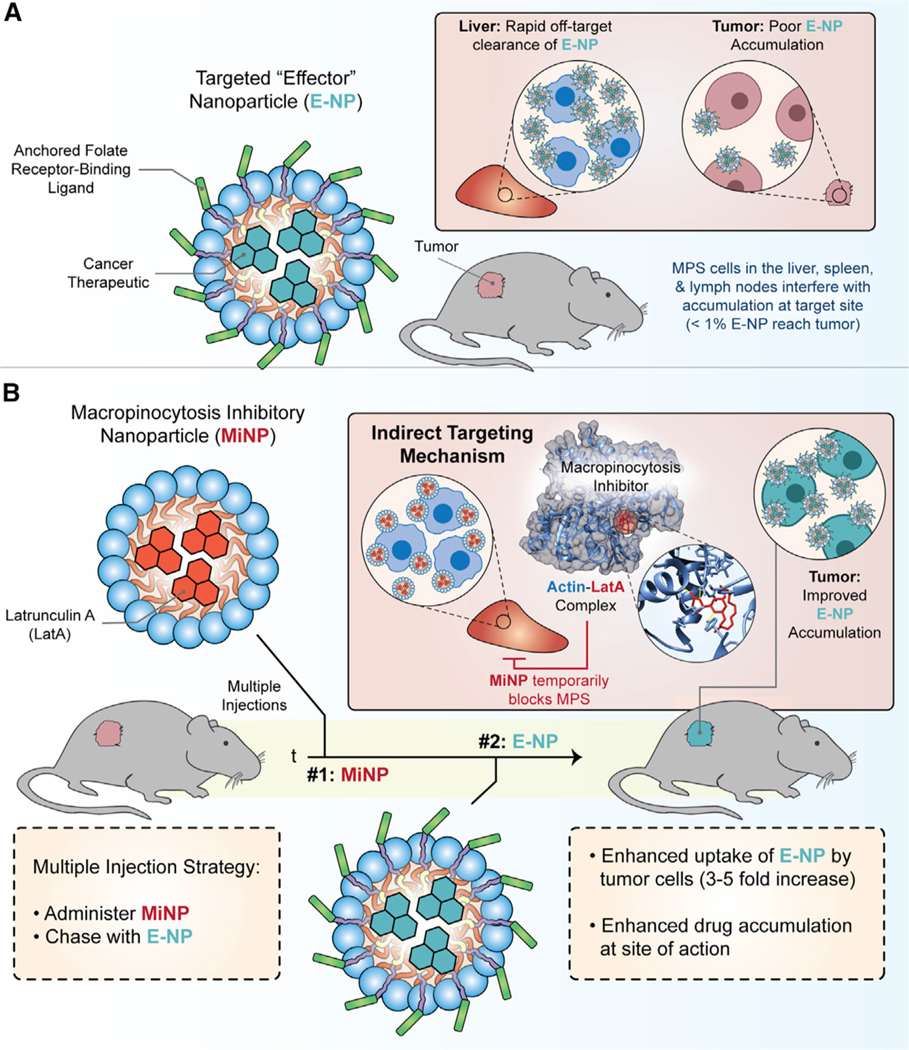
Figure 5. “Indirect” targeting via macropinocytosis inhibitory nanoparticles (MiNP).
(A) Example targeted “effector” nanoparticles (E-NP) bearing cancer therapeutic cargo and displaying a folate receptor binding ligand. E-NP commonly accumulate within tumor cells at low levels due to high uptake by cells of the mononuclear phagocyte system (MPS).
(B) Indirect targeting strategies provide one solution to this issue via silencing MPS uptake with micropinocytosis inhibitory nanoparticles (MiNP). The macropinocytosis inhibitor Latrunculin A (LatA) is depicted here. LatA is a 16-membered macrolide that depolymerizes actin in the cytoskeleton and blocks the incorporation of actin monomers into actin filaments (LatA-actin complex is displayed from PDB ID: 1ESV). The pre-injection of MiNP results in the safe and transient inhibition of MPS cells that are responsible for scavenging and clearing the majority of administered nanocarriers and biologics. The subsequent administration of E-NPs achieves enhanced accumulation within the TME. Aspects of this figure were adapted from Stack et al., 2021. Nanoscale Horiz. 6, 393–400.
Clearance by the MPS occurs primarily in the liver, spleen, lymph nodes, and blood through several endocytic pathways, including clathrin-mediated and clathrin-independent pathways, macropinocytosis, and phagocytosis (Rennick et al., 2021). Macropinocytosis is a process by which membranes extend to indiscriminately engulf extracellular fluid for internalization, while phagocytosis is usually receptor-initiated and internalizes with or without extension of plasma membranes via membrane invaginations (Rennick et al., 2021). If these pathways are temporarily inhibited in MPS cells prior to or in conjunction with the introduction of therapeutic nanocarriers that target the TME, the bioavailability and therapeutic efficacy of these nanocarriers can increase significantly (Stack et al., 2021). Developing nanoparticles with “stealth” properties to avoid this nonspecific uptake remains a critical objective for nanomedicine and many different strategies such as PEGylation, sialic acid modifications, and CD47 “don’t eat me” peptides, have been tried with variable levels of effectiveness. CD47 functions as a “marker of self” that is expressed at the surface of all cells in humans, as well as a variety of mice and other preclinical mammalian animal models. CD47 binds to signal regulatory protein-a (SIRPa), transducing a signal that prevents phagocytes from clearing host cells. The computationally designed CD47-binding peptides developed by the Discher group fall in this category (Rodriguez et al., 2013). Notably, nanocarriers displaying CD47 peptides enhanced drug delivery to tumors and improved circulation time by reducing clearance by macrophages (Rodriguez et al., 2013).
Alternatively, cells of the MPS can be directly depleted to avoid their interference during a study, which has most often been achieved using clodronate liposomes. A variant of this strategy was recently employed by Chan and colleagues to investigate the role of subcapsular macrophages during nanovaccination (Zhang et al., 2020b). Immunizing mice after depleting these cells resulted in a 30-fold increase in antigen-specific antibody production compared with the nanovaccine alone. In a separate study, Chan’s team found that instead of depleting MPS cells, the use of a high dose of nanocarriers to overload these cells could overcome their scavenging effects (Ouyang et al., 2020). A threshold dose of 1 trillion nanoparticles was identified, where going above this amount significantly increased the tumor delivery efficiency from 0.03% of the injected dose to 12%, with up to 93% of cells in tumors being targeted. Recently, a less heavy-handed and potentially more clinically translatable strategy was demonstrated by Stack et al. (2021), where macropinocytosis inhibitory nanoparticles (MiNP) were directly engineered to safely and temporarily inhibit cells of the MPS as a pre-injection prior to administration of a therapeutic or diagnostic “effector” nanocarrier (Figures 5A and 5B). Subcutaneously pre-injected MiNP safely and temporarily disrupted nonspecific systemic clearance by MPS cells without impeding receptor-mediated endocytosis, allowing an up to 8-fold increase in uptake of folate-targeted effector nanocarriers within the TME. Interestingly, MiNP inhibition of phagocytes within subcutaneous tissue and lymphatics allowed subsequent subcutaneous administration of effector nanocarriers to achieve serum concentrations on par with standard intravenous injections. Strategies within this third category of targeting that indirectly enhance uptake within the TME by modulating off-target cells, instead of the nanocarrier itself, can therefore synergize well with standard active and passive targeting strategies.
Targeted anti-cancer vaccines
Nanocarriers have become critical components of vaccine formulations in general, largely owing to their ability to transport lipophilic molecules and protein antigens and combinations thereof for enhanced adjuvancy and targeting of innate immune cells (Soni et al., 2021). Important for cancer vaccination, nanocarriers can stimulate effective CTL responses to combat tumors by efficiently delivering tumor-specific antigens and adjuvants to antigen-presenting cell (APC) subsets (Fan and Moon, 2015; Wang and Mooney, 2018). To date, numerous controlled drug delivery platforms have been employed to induce both prophylactic and therapeutic anti-tumor immune responses. The nanocarriers within these formulations are often composed of biodegradable polymers, lipids, and inorganic materials, as well as virus-derived and virus-inspired self-assembling materials (Caldeira et al., 2020; Yoon et al., 2018). A key advantage of these delivery systems is the ability to target multiple payloads, such as both adjuvant and antigen, simultaneously to APCs in a similar fashion as pathogens, and this has led to nanocarriers being commonly referred to as “pathogen-like-particles” (Soni et al., 2021). For example, self-assembled nanocomplexes using synthetic mRNA antigen, cationic lipid, and an adjuvant have been developed as prophylactic and therapeutic cancer vaccines (Islam et al., 2021). Nanocomplexes made up of ovalbumin (OVA) mRNA and a lipidated derivative of the adjuvant resiquimod (TLR7/8 agonist) improved the transfection efficiency of mRNA by 95% and induced potent OVA-specific CTL responses in vivo as compared with OVA mRNA nanoparticles without adjuvant. Peptide-adjuvant constructs have also been designed to enhance CD8 T cell immunity to tumor antigens (Baharom et al., 2021; Lynn et al., 2020). These constructs made up of Reps1, an MC38 murine colon carcinoma neoantigen and imidazoquinoline-based TLR-7/8 agonists self-assembled to form 20-nm nanoparticles, which stimulated strong tumor-specific CD8+ T cell responses following intravenous administration (Baharom et al., 2021).
Various nanocarrier characteristics, such as size, shape, and surface charge, play a vital role in dictating the fate of elicited immune responses (Vincent et al., 2021a, 2021b; Wang et al., 2019). Nanocarriers having a diameter smaller than 30 nm efficiently drain to lymph nodes following subcutaneous administration for internalization by APCs compared with nanocarriers with a size exceeding 100 nm (Benne et al., 2016; Schudel et al., 2020). For example, polymeric hybrid micelles (<30 nm) encapsulated with melanoma antigen peptide, Trp2 and TLR-9 agonist CpG oligodeoxynucleotide showed efficient internalization by DCs and stimulated strong antigen-specific CTL responses in vivo (Zeng et al., 2017). Large nanocarriers (>100 nm) can exhibit longer retention both at the injection site and in lymph nodes as compared with smaller-sized nanocarriers (Benne et al., 2016). Regarding surface charge, a positive surface charge can improve immunostimulatory properties of nanocarriers by enhancing their uptake by APCs due to favorable electrostatic interactions with negatively charged cell membranes (Heuts et al., 2021). Morphological influences have been examined to a lesser extent, but while spherical-shaped nanocarriers are widely studied as vaccine delivery vehicles, recent studies demonstrate that short aspect ratio rod-shaped particles are readily phagocytosed by APCs (Wang et al., 2019). Furthermore, nanocarrier physicochemical properties can be engineered for on-demand intracellular release of vaccine components upon physiological (oxidation, pH, enzymatic degradation) or external stimuli (light, ultrasound) to boost immune responses (Pacifici et al., 2020).
Considered to be the most critical and potent APCs during vaccination, DCs have become a major focus and target of nanotherapy for cancer vaccination. In this area, DC targeting has been achieved using both ligand-free and active approaches. An example of the former strategy was recently provided by Kranz et al. (2016), in the development of RNA-lipoplexes (RNA-LPX) by adjusting the net negative charge of the lipid carrier. Systemically administered RNA-LPX bearing a negative charge targeted the delivery of RNA-encoded antigens to lymphoid-resident DCs, induced the TLR-mediated release of IFN-a to promote IFN-a-dependent tumor rejection, and resulted in the expansion of antigen-specific T cells. Results from preclinical animal models matched well with performance in humans, as RNA-LPX induced IFN-a and antigen-specific T cell responses in a small number of melanoma patients in phase I dose escalation studies. The platform is amenable to the development of personalized RNA vaccines against cancer that can be tailored to the needs of individual patients (Sahin et al., 2017). In later clinical studies, the intravenous administration of RNA-LPX vaccines produced strong CD4+ and CD8+ T cell immunity in patients with advanced melanoma (Sahin et al., 2020). This strategy of optimizing and tuning the surface charge was compatible with common lipid nanocarriers (1,2-di-O-octadecenyl-3-trimethylammonium propane [DOTMA], cationic lipid 1,2-dioleoyl-3-trimethylammonium-propane [DOTAP], dioleoylphosphatidylethanolamine [DOPE], cholesterol, etc.) (Kranz et al., 2016). Charge tuning has also proven effective in enhancing APC uptake of personalized self-assembling peptide-TLR7/8a conjugate vaccines to promote T cell-mediated immunity (Baharom et al., 2021; Lynn et al., 2020). It is possible that charge tuning can also be applied in the development of ligand-free targeted polymeric nanovaccines. Furthermore, the work by Sahin et al. (2017, 2020), also demonstrates that targeting MPS subsets can be leveraged to reprogram important phagocyte populations with anti-tumor function.
Active targeting strategies specific for DC receptors such as mannose, DEC-205, CD40, Fc-receptor, CD11c, clec-9a, and mucin have been studied (Hossain and Wall, 2019; Wculek et al., 2020). Of note, the mannose receptor is highly enriched on the surface of APCs and has been frequently employed to enhance nanocarrier uptake by DCs. For example, Shi et al. (2017) designed mannose-decorated chitosan nanoparticles (MNS-CTS NP) for targeted delivery of tumor antigens to DCs. In this study, tumor cell lysates were prepared from B16 melanoma cells and encapsulated into MNS-CTS NP, which significantly enhanced uptake by endogenous DCs and increased the production of antigen-specific CTL responses following a subcutaneous administration in mice as compared with chitosan nanoparticles without mannose decoration. Interestingly, the enhanced CTL responses generated by MNS-CTS NP conferred both prophylactic and therapeutic protection in B16 F10 melanoma-induced mouse models.
A combinatorial therapeutic cancer vaccine strategy consisting of both active targeting of DCs as well as immune checkpoint blockade has been explored recently. Zhang et al. (2019) developed an effective DC-targeted strategy for cancer vaccination using lipid-polymer hybrid nanoparticles co-delivering an antigen and two adjuvants. These nanocarriers consisted of a biodegradable polymer polyethylene glycol-polycaprolactone-polyethylene glycol (PEG-PCL-PEG), DOTAP, and 1, 2-Distearoyl-sn-glycero-3-phosphoethanolamine (DSPE)-PEG-Mannose. The adjuvants imiquimod (TLR7/8 agonist) and monophosphoryl lipid A (TLR4 agonist) were encapsulated within the lipid bilayer whereas model antigen OVA was adsorbed onto the nanoparticle surface due to electrostatic interactions with the cationic lipid. When administered subcutaneously into mice, the mannose-decorated lipid-hybrid nanoparticles showed greater retention in the lymphoid organs with enhanced cellular uptake and significantly increased the production of antigen-specific CTL responses as compared with nanoparticles without mannose decoration. In combination with an immune point checkpoint inhibitor (anti-PD-1), anti-tumor responses were further enhanced and prolonged the survival rate in an E.G7-OVA tumor model.
Several nanocarrier-based cancer vaccines are currently in different phases of clinical trials (Roy et al., 2020). Of note, the majority of these trials involve liposome-based delivery systems, including Lipo-MERIT (RNA antigens; advanced melanoma), DPX-Survivac (Survivin antigen; advanced stage ovarian, fallopian, or peritoneal cancer), DPX-0907 (peptide antigens and polynucleotide adjuvant; advanced stage ovarian, breast and prostate cancer), and ONT-10 (glycopeptide antigen and PET lipid A adjuvant; advanced ovarian or breast cancer). Due to their prior clinical usage, long history of development, and familiar composition, liposomes continue to dominate as the nanocarrier of choice for clinical applications. The more recently developed polymeric nanocarriers are likely to eventually gain clinical adoption due to their enhanced stability, customization, and versatility. Importantly, polymeric technologies provide these benefits while simultaneously solving numerous practical issues associated with nanocarrier-based vaccine formulations. It was recently shown that mixtures of self-assembling polymer amphiphiles, biologic payloads, and lipid-anchored targeting ligands can be formulated as carbohydrate-based powders that rapidly self-assemble into targeted nanocarriers upon hydration with water or saline (Bobbala et al., 2021). Powdered forms are stable in storage at room temperature for more than 6 months; are processed using a hydration procedure that can be completed within minutes to prepare monodisperse polymeric nanocarriers for on-demand clinical use; and are compatible with passive, active, and indirect targeting strategies (Bobbala et al., 2021). Thus, formulating targeted polymeric nanocarriers using these pharmaceutical engineering strategies may provide scalable, storage-stable platforms for anti-cancer vaccine development that can avoid cold chain requirements and have less manufacturing complexity.
Future avenues for developing holistic targeting strategies to improve the safety and efficacy of cancer immunotherapies
Future cancer immunotherapies can be improved by leveraging multiple targeting strategies together in a single formulation to achieve more precise and specific engagement with key cell subpopulations, potentially including subsets that have been neglected by existing anti-cancer drugs and technologies. For example, higher-performing targeting strategies may emerge through using a suitable drug delivery vehicle chassis together with engineered targeting ligands and inhibition of relevant off-target MPS cells. This would represent a holistic targeting strategy that employs a combination of passive, active, and indirect targeting methods and takes advantage of our improved understanding of the synthetic properties needed to direct how the nanocarrier surface is inevitably modified within biological environments. The bulk physicochemical properties of the delivery vehicle (size, shape, charge, and surface chemistry) can thus be tailored to the desired therapeutic objective and to bias baseline cellular interactions in a particular direction. The nanocarrier surface can be further engineered to display rationally designed active targeting moieties that enhance uptake by the cell type(s) of interest as well as direct delivery to specific intracellular compartments. And finally, decreasing nonspecific interactions with the MPS via incorporation of indirect targeted methods would increase circulation time and allow more opportunities for nanocarriers to find their targets.
One holistic pairing of targeting strategies with potential benefits includes the use of nanocarrier surface chemistries that modulate interactions with TAMs. Recently, it was demonstrated that the surface chemistry of a nanocarrier can be engineered to control the three-dimensional structure of adsorbed albumin (Vincent et al., 2021a). Albumin is the most abundant blood protein (40–50 mg/mL) and inevitably adsorbs to nanocarrier surfaces in biological fluids having a protein composition derived from blood. The conformation of adsorbed albumin is a molecular determinant of nanocarrier clearance by the innate immune system with particularly important implications for controlling scavenging by monocyte and macrophage subsets (Vincent et al., 2021a), suggesting a potential future utility for controlling interactions with TAMs. Anionic nanocarrier surfaces displaying biomimetic phosphate groups stabilized adsorbed albumin through electrostatic repulsion to minimize macrophage uptake through the evasion of class A1 scavenger receptors (SR-A1; CD204), whereas common neutral hydroxyl- and methoxy-groups denatured adsorbed albumin to enhance nanocarrier clearance by various macrophage subsets in vivo (Vincent et al., 2021a). An immunohistochemical analysis of 136 human glioma samples reported high SR-A1 expression by TAMs derived from circulating monocytes (Zhang et al., 2016). Collectively, these relationships suggest nanocarrier surface chemistry can be specified to control the structure of albumin coatings, applied prior to administration or formed in situ, to selectively tune interactions with TAMs. Since SR-A1 expression increases with the glioma grade (Zhang et al., 2016), the selectivity of this targeting strategy is expected to increase with cancer severity.
Conventional ICB strategies have focused on disrupting negative regulatory mechanisms to harness T cell-mediated anti-tumor responses, yet other immune cell subsets are gaining attention for their role in this process (Sarvaria et al., 2017; Tsou et al., 2016). Tumor-infiltrating B cells are one such lesser understood cell type, which emerged from a recent multi-omic study that examined differential B cell contributions in patients that respond to ICB treatment versus non-responding patients (Helmink et al., 2020). This study identified class-switched memory B cells that might be responsible for producing anti-tumor antibody responses in treatment-responsive patients, and further noted that antigen presentation and/or cytokine responses associated with these B cell subpopulations may be important for driving effective tumor-associated T cell responses (Helmink et al., 2020). Utilizing targeted nanomaterials to generate or promote B cells with these functional characteristics could prove beneficial for either direct anti-tumor responses or more effective T cell-mediated responses to improve patient outcomes during ICB cancer immunotherapy.
CONCLUDING REMARKS
In closing, self-assembled nanobiomaterials are highly customizable and scalable platforms that will facilitate the development of the next wave of potent cancer immunotherapies. These technologies are compatible with a variety of therapeutic strategies, including traditional chemotherapy, ICI, ICD, and vaccination. Furthermore, the specificity and safety of cancer treatment can be improved using passive, active, and indirect targeting strategies. The greatest control over the pharmacokinetics, biodistribution, and bioavailability of encapsulated therapeutics likely will be achieved via holistic approaches that rationally incorporate multiple targeting strategies simultaneously to promote cell-specific interactions, account for fate-altering biofouling of the nanocarrier surface, and avoid off-target MPS clearance within physiological environments.
ACKNOWLEDGMENTS
M.P.V. gratefully acknowledges support from the Ryan Fellowship, the International Institute for Nanotechnology at Northwestern University, and the Northwestern University Multidisciplinary Visual Sciences Training Program (T32 Fellowship funded by National Eye Institute (NEI) Award 2T32EY025202-06). This work was supported by the Director’s New Innovator Award (grant no. 1DP2HL132390-01) and the National Science Foundation (CBET-1806007 and CAREER Award no. 1453576).
REFERENCES
- Ackerman SE, Pearson CI, Gregorio JD, Gonzalez JC, Kenkel JA, Hartmann FJ, Luo A, Ho PY, LeBlanc H, Blum LK, et al. (2021). Immune-stimulating antibody conjugates elicit robust myeloid activation and durable antitumor immunity. Nat. Cancer 2, 18–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agata Y, Kawasaki A, Nishimura H, Ishida Y, Tsubata T, Yagita H, and Honjo T. (1996). Expression of the PD-1 antigen on the surface of stimulated mouse T and B lymphocytes. Int. Immunol 8, 765–772. [DOI] [PubMed] [Google Scholar]
- Albanese A, Tang PS, and Chan WC (2012). The effect of nanoparticle size, shape, and surface chemistry on biological systems. Annu. Rev. Biomed. Eng 14, 1–16. [DOI] [PubMed] [Google Scholar]
- Allen RP, Bolandparvaz A, Ma JA, Manickam VA, and Lewis JS (2018a). Latent, immunosuppressive nature of poly(lactic-co-glycolic acid) microparticles. ACS Biomater. Sci. Eng 4, 900–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen S, Osorio O, Liu Y-G, and Scott E. (2017). Facile assembly and loading of theranostic polymersomes via multi-impingement flash nanoprecipitation. J. Control. Release 262, 91–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen S, Vincent M, and Scott E. (2018b). Rapid, scalable assembly and loading of bioactive proteins and immunostimulants into diverse synthetic nanocarriers via flash nanoprecipitation. J. Vis. Exp 57793. 10.3791/57793. [DOI] [PMC free article] [PubMed]
- Allen SD, Bobbala S, Karabin NB, Modak M, and Scott EA (2018c). Benchmarking bicontinuous nanospheres against polymersomes for in vivo biodistribution and dual intracellular delivery of lipophilic and water-soluble payloads. ACS Appl. Mater. Interfaces 10, 33857–33866. [DOI] [PubMed] [Google Scholar]
- Angelov B, Angelova A, Drechsler M, Garamus VM, Mutafchieva R, and Lesieur S. (2015). Identification of large channels in cationic PEGylated cubosome nanoparticles by synchrotron radiation SAXS and Cryo-TEM imaging. Soft Matter 11, 3686–3692. [DOI] [PubMed] [Google Scholar]
- Asati A, Santra S, Kaittanis C, and Perez JM (2010). Surface-charge-dependent cell localization and cytotoxicity of cerium oxide nanoparticles. ACS Nano 4, 5321–5331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashley JD, Quinlan CJ, Schroeder VA, Suckow MA, Pizzuti VJ, Kiziltepe T, and Bilgicer B. (2016). Dual carfilzomib and doxorubicin–loaded liposomal nanoparticles for synergistic efficacy in multiple myeloma. Mol. Cancer Ther 15, 1452–1459. [DOI] [PubMed] [Google Scholar]
- Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, Diemert D, Spector SA, Rouphael N, Creech CB, et al. (2021). Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med 384, 403–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baharom F, Ramirez-Valdez RA, Tobin KKS, Yamane H, Dutertre CA, Khalilnezhad A, Reynoso GV, Coble VL, Lynn GM, Mule MP, et al. (2021). Intravenous nanoparticle vaccination generates stem-like TCF1(+) neoantigen-specific CD8(+) T cells. Nat. Immunol 22, 41–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barenholz Y. (2012). Doxil(R)–the first FDA-approved nano-drug: lessons learned. J. Control. Release 160, 117–134. [DOI] [PubMed] [Google Scholar]
- Barriga HMG, Holme MN, and Stevens MM (2019). Cubosomes: the next generation of smart lipid nanoparticles? Angew. Chem. Int. Ed 58, 2958–2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann BC, Kao GD, Mahmud A, Harada T, Swift J, Chapman C, Xu X, Discher DE, and Dorsey JF (2013). Enhancing the efficacy of drug-loaded nanocarriers against brain tumors by targeted radiation therapy. Oncotarget 4, 64–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatty GL, and Gladney WL (2015). Immune escape mechanisms as a guide for cancer immunotherapy. Clin. Cancer Res 21, 687–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck A, Wurch T, Bailly C, and Corvaia N. (2010). Strategies and challenges for the next generation of therapeutic antibodies. Nat. Rev. Immunol 10, 345–352. [DOI] [PubMed] [Google Scholar]
- Benne N, van Duijn J, Kuiper J, Jiskoot W, and Slutter B. (2016). Orchestrating immune responses: how size, shape and rigidity affect the immunogenicity of particulate vaccines. J. Control. Release 234, 124–134. [DOI] [PubMed] [Google Scholar]
- Bennett F, Luxenberg D, Ling V, Wang IM, Marquette K, Lowe D, Khan N, Veldman G, Jacobs KA, Valge-Archer VE, et al. (2003). Program death-1 engagement upon TCR activation has distinct effects on costimulation and cytokine-driven proliferation: attenuation of ICOS, IL-4, and IL-21, but not CD28, IL-7, and IL-15 responses. J. Immunol 170, 711–718. [DOI] [PubMed] [Google Scholar]
- Bigini P, Gobbi M, Bonati M, Clavenna A, Zucchetti M, Garattini S, and Pasut G. (2021). The role and impact of polyethylene glycol on anaphylactic reactions to COVID-19 nano-vaccines. Nat. Nanotechnol 16, 1169–1171. [DOI] [PubMed] [Google Scholar]
- Blanco E, Shen H, and Ferrari M. (2015). Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol 33, 941–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobbala S, Allen SD, and Scott EA (2018). Flash nanoprecipitation permits versatile assembly and loading of polymeric bicontinuous cubic nanospheres. Nanoscale 10, 5078–5088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobbala S, Allen SD, Yi S, Vincent M, Frey M, Karabin NB, and Scott EA (2020). Employing bicontinuous-to-micellar transitions in nanostructure morphology for on-demand photo-oxidation responsive cytosolic delivery and off-on cytotoxicity. Nanoscale 12, 5332–5340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobbala S, and Hook S. (2020). Vaccine implants: current status and recent advancements. Emerg. Top Life Sci 4, 319–330. [DOI] [PubMed] [Google Scholar]
- Bobbala S, Vincent MP, and Scott EA (2021). Just add water: hydratable, morphologically diverse nanocarrier powders for targeted delivery. Nanoscale 13, 11349–11359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdanowich-Knipp SJ, Chakrabarti S, Williams TD, Dillman RK, and Siahaan TJ (1999). Solution stability of linear vs. cyclic RGD peptides. J. Pept. Res 53, 530–541. [DOI] [PubMed] [Google Scholar]
- Bol KF, Ellebaek E, Hoejberg L, Bagger MM, Larsen MS, Klausen TW, Kohler UH, Schmidt H, Bastholt L, Kiilgaard JF, et al. (2019). Real-world impact of immune checkpoint inhibitors in metastatic uveal melanoma. Cancers 11, 1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brischwein K, Parr L, Pflanz S, Volkland J, Lumsden J, Klinger M, Locher M, Hammond SA, Kiener P, Kufer P, et al. (2007). Strictly target cell-dependent activation of T cells by bispecific single-chain antibody constructs of the BiTE class. J. Immunother 30, 798–807. [DOI] [PubMed] [Google Scholar]
- Burke JA, Zhang X, Bobbala S, Frey MA, Furentes CB, Haddad HF, Allen SD, Richardson R, Ameer GA, and Scott EA (2022). Subcutaneous nanotherapy repurposes the immunosuppressive mechanism of rapamycin to enhance allogeneic islet graft viability. Nat. Nanotechnol 10.1038/s41565-021-01048-2. [DOI] [PMC free article] [PubMed]
- Cabral H, Matsumoto Y, Mizuno K, Chen Q, Murakami M, Kimura M, Terada Y, Kano MR, Miyazono K, Uesaka M, et al. (2011). Accumulation of sub-100 nm polymeric micelles in poorly permeable tumours depends on size. Nat. Nanotechnol 6, 815–823. [DOI] [PubMed] [Google Scholar]
- Caldeira JC, Perrine M, Pericle F, and Cavallo F. (2020). Virus-like particles as an immunogenic platform for cancer vaccines. Viruses 12, 488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carugo D, Bottaro E, Owen J, Stride E, and Nastruzzi C. (2016). Liposome production by microfluidics: potential and limiting factors. Sci. Rep 6, 25876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspar DL, and Klug A. (1962). Physical principles in the construction of regular viruses. Cold Spring Harb. Symp. Quant. Biol 27, 1–24. [DOI] [PubMed] [Google Scholar]
- Centanni M, Moes D, Troconiz IF, Ciccolini J, and van Hasselt JGC (2019). Clinical pharmacokinetics and pharmacodynamics of immune checkpoint inhibitors. Clin. Pharmacokinet 58, 835–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerritelli S, Velluto D, and Hubbell JA (2007). PEG-SS-PPS: reduction-sensitive disulfide block copolymer vesicles for intracellular drug delivery. Biomacromolecules 8, 1966–1972. [DOI] [PubMed] [Google Scholar]
- Champion JA, and Mitragotri S. (2006). Role of target geometry in phagocytosis. Proc. Natl. Acad. Sci. U S A 103, 4930–4934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chau CH, Steeg PS, and Figg WD (2019). Antibody-drug conjugates for cancer. Lancet 394, 793–804. [DOI] [PubMed] [Google Scholar]
- Chen Q, Liu L, Lu Y, Chen X, Zhang Y, Zhou W, Guo Q, Li C, Zhang Y, Zhang Y, et al. (2019). Tumor microenvironment-triggered aggregated magnetic nanoparticles for reinforced image-guided immunogenic chemotherapy. Adv. Sci 6, 1802134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Q., Shi X., Han M., Smbatyan G., Lenz HJ., and Zhang Y. (2018). Reprogramming exosomes as nanoscale controllers of cellular immunity. J. Am. Chem. Soc 140, 16413–16417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian DA, Cai S, Garbuzenko OB, Harada T, Zajac AL, Minko T, and Discher DE (2009). Flexible filaments for in vivo imaging and delivery: persistent circulation of filomicelles opens the dosage window for sustained tumor shrinkage. Mol. Pharm 6, 1343–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai J, Su Y, Zhong S, Cong L, Liu B, Yang J, Tao Y, He Z, Chen C, and Jiang Y. (2020). Exosomes: key players in cancer and potential therapeutic strategy. Signal Transduct Target Ther. 5, 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David A. (2017). Peptide ligand-modified nanomedicines for targeting cells at the tumor microenvironment. Adv. Drug Deliv. Rev 119, 120–142. [DOI] [PubMed] [Google Scholar]
- Discher BM, Won YY, Ege DS, Lee JC, Bates FS, Discher DE, and Hammer DA (1999). Polymersomes: tough vesicles made from diblock copolymers. Science 284, 1143–1146. [DOI] [PubMed] [Google Scholar]
- Dowling DJ, Scott EA, Scheid A, Bergelson I, Joshi S, Pietrasanta C, Brightman S, Sanchez-Schmitz G, Van Haren SD, Ninkovic J, et al. (2017). Toll-like receptor 8 agonist nanoparticles mimic immunomodulating effects of the live BCG vaccine and enhance neonatal innate and adaptive immune responses. J. Allergy Clin. Immunol 140, 1339–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake CG, Jaffee E, and Pardoll DM (2006). Mechanisms of immune evasion by tumors. Adv. Immunol 90, 51–81. [DOI] [PubMed] [Google Scholar]
- Dreaden EC, Austin LA, Mackey MA, and El-Sayed MA (2012). Size matters: gold nanoparticles in targeted cancer drug delivery. Ther. Deliv 3, 457–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan X, Chan C, Han W, Guo N, Weichselbaum R, and Lin W. (2019). Immunostimulatory nanomedicines synergize with checkpoint blockade immunotherapy to eradicate colorectal tumors. Nat. Commun 10, 1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y, and Moon JJ (2015). Nanoparticle drug delivery systems designed to improve cancer vaccines and immunotherapy. Vaccines 3, 662–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne MC, et al. (2000). Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J. Exp. Med 192, 1027–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freire Haddad H, Burke JA, Scott EA, and Ameer GA (2021). Clinical relevance of pre-existing and treatment-induced anti-poly(ethylene glycol) antibodies. Regen. Eng. Transl. Med 10.1007/s40883-021-00198-y. [DOI] [PMC free article] [PubMed]
- Fucikova J, Kepp O, Kasikova L, Petroni G, Yamazaki T, Liu P, Zhao L, Spisek R, Kroemer G, and Galluzzi L. (2020). Detection of immunogenic cell death and its relevance for cancer therapy. Cell Death Dis. 11, 1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda A, Tahara K, Hane Y, Matsui T, Sasaoka S, Hatahira H, Motooka Y, Hasegawa S, Naganuma M, Abe J, et al. (2017). Comparison of the adverse event profiles of conventional and liposomal formulations of doxorubicin using the FDA adverse event reporting system. PLoS ONE 12, e0185654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gall D. (1966). The adjuvant activity of aliphatic nitrogenous bases. Immunology 11, 369–386. [PMC free article] [PubMed] [Google Scholar]
- Gao J, Wang WQ, Pei Q, Lord MS, and Yu HJ (2020). Engineering nanomedicines through boosting immunogenic cell death for improved cancer immunotherapy. Acta Pharmacol. Sin 41, 986–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng Y, Dalhaimer P, Cai S, Tsai R, Tewari M, Minko T, and Discher DE (2007). Shape effects of filaments versus spherical particles in flow and drug delivery. Nat. Nanotechnol 2, 249–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng Y, and Discher DE (2005). Hydrolytic degradation of poly(ethylene oxide)-block-polycaprolactone worm micelles. J. Am. Chem. Soc 127, 12780–12781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goebeler ME, Knop S, Viardot A, Kufer P, Topp MS, Einsele H, Noppeney R, Hess G, Kallert S, Mackensen A, et al. (2016). Bispecific T-cell engager (BiTE) antibody construct blinatumomab for the treatment of patients with relapsed/refractory non-hodgkin lymphoma: final results from a phase I study. J. Clin. Oncol 34, 1104–1111. [DOI] [PubMed] [Google Scholar]
- Goldberg MS (2019). Improving cancer immunotherapy through nanotechnology. Nat. Rev. Cancer 19, 587–602. [DOI] [PubMed] [Google Scholar]
- Gong N, Sheppard NC, Billingsley MM, June CH, and Mitchell MJ (2021). Nanomaterials for T-cell cancer immunotherapy. Nat. Nanotechnol 16, 25–36. [DOI] [PubMed] [Google Scholar]
- Gu FX, Karnik R, Wang AZ, Frank A, Levy-Nissenbaum E, Hong S, Langer R, and Farokhzad OC (2007). Targeted nanoparticles for cancer therapy. Nano Today 2, 14–21. [Google Scholar]
- Guevara ML, Persano F, and Persano S. (2020). Advances in lipid nanoparticles for mRNA-based cancer immunotherapy. Front. Chem 8, 589959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haratani K, Hayashi H, Chiba Y, Kudo K, Yonesaka K, Kato R, Kaneda H, Hasegawa Y, Tanaka K, Takeda M, and Nakagawa K. (2018). Association of immune-related adverse events with nivolumab efficacy in non-small-cell lung cancer. JAMA Oncol. 4, 374–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding FA, McArthur JG, Gross JA, Raulet DH, and Allison JP (1992). CD28-mediated signalling co-stimulates murine T cells and prevents induction of anergy in T-cell clones. Nature 356, 607–609. [DOI] [PubMed] [Google Scholar]
- He L, Wang L, Wang Z, Li T, Chen H, Zhang Y, Hu Z, Dimitrov DS, Du J, and Liao X. (2021). Immune modulating antibody-drug conjugate (IM-ADC) for cancer immunotherapy. J. Med. Chem 64, 15716–15726. [DOI] [PubMed] [Google Scholar]
- He X, Li L, Su H, Zhou D, Song H, Wang L, and Jiang X. (2015). Poly(ethylene glycol)-block-poly(epsilon-caprolactone)-and phospholipid-based stealth nanoparticles with enhanced therapeutic efficacy on murine breast cancer by improved intracellular drug delivery. Int. J. Nanomed 10, 1791–1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrich EL, Walser TC, Krysan K, Liclican EL, Grant JL, Rodriguez NL, and Dubinett SM (2012). The inflammatory tumor microenvironment, epithelial mesenchymal transition and lung carcinogenesis. Cancer Microenviron. 5, 5–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmink BA, Reddy SM, Gao J, Zhang S, Basar R, Thakur R, Yizhak K, Sade-Feldman M, Blando J, Han G, et al. (2020). B cells and tertiary lymphoid structures promote immunotherapy response. Nature 577, 549–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuts J, Jiskoot W, Ossendorp F, and van der Maaden K. (2021). Cationic nanoparticle-based cancer vaccines. Pharmaceutics 13, 596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, et al. (2010). Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med 363, 711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornung V, Bauernfeind F, Halle A, Samstad EO, Kono H, Rock KL, Fitzgerald KA, and Latz E. (2008). Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat. Immunol 9, 847–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain MK, and Wall KA (2019). Use of dendritic cell receptors as targets for enhancing anti-cancer immune responses. Cancers 11, 418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou X, Zaks T, Langer R, and Dong Y. (2021). Lipid nanoparticles for mRNA delivery. Nat. Rev. Mater 6, 1078–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai K, and Takaoka A. (2006). Comparing antibody and small-molecule therapies for cancer. Nat. Rev. Cancer 6, 714–727. [DOI] [PubMed] [Google Scholar]
- Irvine DJ, and Dane EL (2020). Enhancing cancer immunotherapy with nanomedicine. Nat. Rev. Immunol 20, 321–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida Y, Agata Y, Shibahara K, and Honjo T. (1992). Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 11, 3887–3895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam MA, Rice J, Reesor E, Zope H, Tao W, Lim M, Ding J, Chen Y, Aduluso D, Zetter BR, et al. (2021). Adjuvant-pulsed mRNA vaccine nanoparticle for immunoprophylactic and therapeutic tumor suppression in mice. Biomaterials 266, 120431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson LA, Anderson EJ, Rouphael NG, Roberts PC, Makhene M, Coler RN, McCullough MP, Chappell JD, Denison MR, Stevens LJ, et al. (2020). An mRNA vaccine against SARS-CoV-2 - preliminary report. N. Engl. J. Med 383, 1920–1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James SF, Chahine EB, Sucher AJ, and Hanna C. (2018). Shingrix: the new adjuvanted recombinant herpes zoster vaccine. Ann. Pharmacother 52, 673–680. [DOI] [PubMed] [Google Scholar]
- Janicka M., and Gubernator J. (2017). Use of nanotechnology for improved pharmacokinetics and activity of immunogenic cell death inducers used in cancer chemotherapy. Expert Opin. Drug Deliv 14, 1059–1075. [DOI] [PubMed] [Google Scholar]
- Kang H, Rho S, Stiles WR, Hu S, Baek Y, Hwang DW, Kashiwagi S, Kim MS, and Choi HS (2020). Size-dependent EPR effect of polymeric nanoparticles on tumor targeting. Adv. Healthc. Mater 9, e1901223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karabin NB, Allen S, Kwon HK, Bobbala S, Firlar E, Shokuhfar T, Shull KR, and Scott EA (2018). Sustained micellar delivery via inducible transitions in nanostructure morphology. Nat. Commun 9, 624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy LB, and Salama AKS (2020). A review of cancer immunotherapy toxicity. CA Cancer J. Clin 70, 86–104. [DOI] [PubMed] [Google Scholar]
- Khalil DN, Smith EL, Brentjens RJ, and Wolchok JD (2016). The future of cancer treatment: immunomodulation, CARs and combination immunotherapy. Nat. Rev. Clin. Oncol 13, 273–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Sung J, Chang Y, Alfeche A, and Leal C. (2018). Microfluidics synthesis of gene silencing cubosomes. ACS Nano 12, 9196–9205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koceva-Chyla A, Jedrzejczak M, Skierski J, Kania K, and Jozwiak Z. (2005). Mechanisms of induction of apoptosis by anthraquinone anticancer drugs aclarubicin and mitoxantrone in comparison with doxorubicin: relation to drug cytotoxicity and caspase-3 activation. Apoptosis 10, 1497–1514. [DOI] [PubMed] [Google Scholar]
- Korsholm KS, Agger EM, Foged C, Christensen D, Dietrich J, Andersen CS, Geisler C, and Andersen P. (2007). The adjuvant mechanism of cationic dimethyldioctadecylammonium liposomes. Immunology 121, 216–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranz LM, Diken M, Haas H, Kreiter S, Loquai C, Reuter KC, Meng M, Fritz D, Vascotto F, Hefesha H, et al. (2016). Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy. Nature 534, 396–401. [DOI] [PubMed] [Google Scholar]
- Kroemer G, Galluzzi L, Kepp O, and Zitvogel L. (2013). Immunogenic cell death in cancer therapy. Annu. Rev. Immunol 31, 51–72. [DOI] [PubMed] [Google Scholar]
- Krysko DV, Garg AD, Kaczmarek A, Krysko O, Agostinis P, and Vandenabeele P. (2012). Immunogenic cell death and DAMPs in cancer therapy. Nat. Rev. Cancer 12, 860–875. [DOI] [PubMed] [Google Scholar]
- La Y, Park C, Shin TJ, Joo SH, Kang S, and Kim KT (2014). Colloidal inverse bicontinuous cubic membranes of block copolymers with tunable surface functional groups. Nat. Chem 6, 534–541. [DOI] [PubMed] [Google Scholar]
- Lammers T, and Ferrari M. (2020). The success of nanomedicine. Nano Today 31, 100853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepland A, Asciutto EK, Malfanti A, Simon-Gracia L, Sidorenko V, Vicent MJ, Teesalu T, and Scodeller P. (2020). Targeting pro-tumoral macrophages in early primary and metastatic breast tumors with the CD206-binding mUNO peptide. Mol. Pharm 17, 2518–2531. [DOI] [PubMed] [Google Scholar]
- Lewis JS, Zaveri TD, Crooks CP 2nd, and Keselowsky BG (2012). Microparticle surface modifications targeting dendritic cells for non-activating applications. Biomaterials 33, 7221–7232. 10.1016/j.biomaterials.2012.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Luo Y, Li B, Xia Y, Wang H, and Fu C. (2020). Implantable and injectable biomaterial scaffolds for cancer immunotherapy. Front. Bioeng. Biotechnol 8, 612950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Raghupathi K, Song C, Prasad P, and Thayumanavan S. (2014). Self-assembly of random copolymers. Chem. Commun 50, 13417–13432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Bobbala S, Vincent MP, Modak M, Liu Y, and Scott EA (2021). Pistacking enhances stability, scalability of formation, control over flexibility, and circulation time of polymeric filamentous nanocarriers. Adv. NanoBiomed Res 1, 2100063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim TS, Chew V, Sieow JL, Goh S, Yeong JP, Soon AL, and Ricciardi-Castagnoli P. (2016). PD-1 expression on dendritic cells suppresses CD8(+) T cell function and antitumor immunity. Oncoimmunology 5, e1085146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin E, Morris JS, and Ayers GD (2002). Effect of celecoxib on capecitabine-induced hand-foot syndrome and antitumor activity. Oncology 16, 31–37. [PubMed] [Google Scholar]
- Liu D, Jenkins RW, and Sullivan RJ (2019a). Mechanisms of resistance to immune checkpoint blockade. Am. J. Clin. Dermatol 20, 41–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Liu C, Zheng Z, Chen S, Pang X, Xiang X, Tang J, Ren E, Chen Y, You M, et al. (2019b). Vesicular antibodies: a bioactive multifunctional combination platform for targeted therapeutic delivery and cancer immunotherapy. Adv. Mater 31, e1808294. [DOI] [PubMed] [Google Scholar]
- Lombardo D, Calandra P, Pasqua L, and Magazu S. (2020). Self-assembly of organic nanomaterials and biomaterials: the bottom-up approach for functional nanostructures formation and advanced applications. Materials 13, 1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorkowski ME, Atukorale PU, Bielecki PA, Tong KH, Covarrubias G, Zhang Y, Loutrianakis G, Moon TJ, Santulli AR, Becicka WM, and Karathanasis E. (2021). Immunostimulatory nanoparticle incorporating two immune agonists for the treatment of pancreatic tumors. J. Control. Release 330, 1095–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorusso D, Di Stefano A, Carone V, Fagotti A, Pisconti S, and Scambia G. (2007). Pegylated liposomal doxorubicin-related palmar-plantar erythrodysesthesia (‘hand-foot’ syndrome). Ann. Oncol 18, 1159–1164. [DOI] [PubMed] [Google Scholar]
- Losdorfer Bozic A, Siber A, and Podgornik R. (2013). Statistical analysis of sizes and shapes of virus capsids and their resulting elastic properties. J. Biol. Phys 39, 215–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynn GM, Sedlik C, Baharom F, Zhu Y, Ramirez-Valdez RA, Coble VL, Tobin K, Nichols SR, Itzkowitz Y, Zaidi N, et al. (2020). Peptide-TLR-7/8a conjugate vaccines chemically programmed for nanoparticle self-assembly enhance CD8 T-cell immunity to tumor antigens. Nat. Biotechnol 38, 320–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKay JA, Chen M, McDaniel JR, Liu W, Simnick AJ, and Chilkoti A. (2009). Self-assembling chimeric polypeptide-doxorubicin conjugate nanoparticles that abolish tumours after a single injection. Nat. Mater 8, 993–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda H. (2015). Toward a full understanding of the EPR effect in primary and metastatic tumors as well as issues related to its heterogeneity. Adv. Drug Deliv. Rev 91, 3–6. [DOI] [PubMed] [Google Scholar]
- Maeda H, Wu J, Sawa T, Matsumura Y, and Hori K. (2000). Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J. Control. Release 65, 271–284. [DOI] [PubMed] [Google Scholar]
- Mai J, Song S, Rui M, Liu D, Ding Q, Peng J, and Xu Y. (2009). A synthetic peptide mediated active targeting of cisplatin liposomes to Tie2 expressing cells. J. Control. Release 139, 174–181. [DOI] [PubMed] [Google Scholar]
- Mai Y, and Eisenberg A. (2012). Self-assembly of block copolymers. Chem. Soc. Rev 41, 5969–5985. [DOI] [PubMed] [Google Scholar]
- Malde AK, Hill TA, Iyer A, and Fairlie DP (2019). Crystal structures of protein-bound cyclic peptides. Chem. Rev 119, 9861–9914. [DOI] [PubMed] [Google Scholar]
- Markwalter CE, Pagels RF, Wilson BK, Ristroph KD, and Prud’homme RK (2019). Flash nanoprecipitation for the encapsulation of hydrophobic and hydrophilic compounds in polymeric nanoparticles. JoVE, e58757. 10.3791/58757. [DOI] [PubMed]
- Manzanares D, and Cena V. (2020). Endocytosis: the nanoparticle and submicron nanocompounds gateway into the cell. Pharmaceutics 12, 371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastria EM, Cai LY, Kan MJ, Li X, Schaal JL, Fiering S, Gunn MD, Dewhirst MW, Nair SK, and Chilkoti A. (2018). Nanoparticle formulation improves doxorubicin efficacy by enhancing host antitumor immunity. J. Control. Release 269, 364–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto Y, Nichols JW, Toh K, Nomoto T, Cabral H, Miura Y, Christie RJ, Yamada N, Ogura T, Kano MR, et al. (2016). Vascular bursts enhance permeability of tumour blood vessels and improve nanoparticle delivery. Nat. Nanotechnol 11, 533–538. [DOI] [PubMed] [Google Scholar]
- McDaid WJ, Greene MK, Johnston MC, Pollheimer E, Smyth P, McLaughlin K, Van Schaeybroeck S, Straubinger RM, Longley DB, and Scott CJ (2019). Repurposing of cetuximab in antibody-directed chemotherapy-loaded nanoparticles in EGFR therapy-resistant pancreatic tumours. Nanoscale 11, 20261–20273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menger L, Vacchelli E, Adjemian S, Martins I, Ma Y, Shen S, Yamazaki T, Sukkurwala AQ, Michaud M, Mignot G, et al. (2012). Cardiac glycosides exert anticancer effects by inducing immunogenic cell death. Sci. Transl. Med 4, 143ra199. [DOI] [PubMed] [Google Scholar]
- Menter T, and Tzankov A. (2018). Mechanisms of immune evasion and immune modulation by lymphoma cells. Front. Oncol 8, 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millward SW., Fiacco S., Austin RJ., and Roberts RW. (2007). Design of cyclic peptides that bind protein surfaces with antibody-like affinity. ACS Chem. Biol 2, 625–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minotti G, Menna P, Salvatorelli E, Cairo G, and Gianni L. (2004). Anthracyclines: molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol. Rev 56, 185–229. [DOI] [PubMed] [Google Scholar]
- Mitchell MJ, Billingsley MM, Haley RM, Wechsler ME, Peppas NA, and Langer R. (2021). Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov 20, 101–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller DL, Jenkins MK, and Schwartz RH (1989). Clonal expansion versus functional clonal inactivation: a costimulatory signalling pathway determines the outcome of T cell antigen receptor occupancy. Annu. Rev. Immunol 7, 445–480. [DOI] [PubMed] [Google Scholar]
- Nam J, Son S, Park KS, Zou W, Shea LD, and Moon JJ (2019). Cancer nanomedicine for combination cancer immunotherapy. Nat. Rev. Mater 4, 398–414. [Google Scholar]
- Ndeupen S, Qin Z, Jacobsen S, Bouteau A, Estanbouli H, and Igyarto BZ (2021). The mRNA-LNP platform’s lipid nanoparticle component used in preclinical vaccine studies is highly inflammatory. iScience 24, 103479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nel AE, Madler L, Velegol D, Xia T, Hoek EM, Somasundaran P, Klaessig F, Castranova V, and Thompson M. (2009). Understanding biophysicochemical interactions at the nano-bio interface. Nat. Mater 8, 543–557. [DOI] [PubMed] [Google Scholar]
- Nguyen DC, Shae D, Pagendarm HM, Becker KW, Wehbe M, Kilchrist KV, Pastora LE, Palmer CR, Seber P, Christov PP, et al. (2021). Amphiphilic polyelectrolyte graft copolymers enhance the activity of cyclic dinucleotide STING agonists. Adv. Healthc. Mater 10, e2001056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North RJ (1982). Cyclophosphamide-facilitated adoptive immunotherapy of an established tumor depends on elimination of tumor-induced suppressor T cells. J. Exp. Med 155, 1063–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oltra NS, Swift J, Mahmud A, Rajagopal K, Loverde SM, and Discher DE (2013). Filomicelles in nanomedicine – from flexible, fragmentable, and ligand-targetable drug carrier designs to combination therapy for brain tumors. J. Mater. Chem B 1, 5177–5185. [DOI] [PubMed] [Google Scholar]
- Ouyang B, Poon W, Zhang YN, Lin ZP, Kingston BR, Tavares AJ, Zhang Y, Chen J, Valic M, Syed AM, et al. (2020). The dose threshold for nanoparticle tumour delivery. Nat. Mater 19, 1362–1371. [DOI] [PubMed] [Google Scholar]
- Owens DE 3rd, and Peppas NA (2006). Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Int. J. Pharm 307, 93–102. [DOI] [PubMed] [Google Scholar]
- Oyewole-Said D, Konduri V, Vazquez-Perez J, Weldon SA, Levitt JM, and Decker WK (2020). Beyond T-cells: functional characterization of CTLA-4 expression in immune and non-immune cell types. Front Immunol. 11, 608024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacifici N, Bolandparvaz A, and Lewis JS (2020). Stimuli-responsive biomaterials for vaccines and immunotherapeutic applications. Adv. Ther 3, 2000129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park K. (2013). Facing the truth about nanotechnology in drug delivery. ACS Nano 7, 7442–7447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry RV, Chemnitz JM, Frauwirth KA, Lanfranco AR, Braunstein I, Kobayashi SV, Linsley PS, Thompson CB, and Riley JL (2005). CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol. Cell Biol 25, 9543–9553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, and Langer R. (2007). Nanocarriers as an emerging platform for cancer therapy. Nat. Nanotechnol 2, 751–760. [DOI] [PubMed] [Google Scholar]
- Peng Q, Qiu X, Zhang Z, Zhang S, Zhang Y, Liang Y, Guo J, Peng H, Chen M, Fu YX, and Tang H. (2020). PD-L1 on dendritic cells attenuates T cell activation and regulates response to immune checkpoint blockade. Nat. Commun 11, 4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petros RA, and DeSimone JM (2010). Strategies in the design of nanoparticles for therapeutic applications. Nat. Rev. Drug Discov 9, 615–627. [DOI] [PubMed] [Google Scholar]
- Pierschbacher MD, and Ruoslahti E. (1984). Cell attachment activity of fibronectin can be duplicated by small synthetic fragments of the molecule. Nature 309, 30–33. [DOI] [PubMed] [Google Scholar]
- Pioli C, Gatta L, Ubaldi V, and Doria G. (2000). Inhibition of IgG1 and IgE production by stimulation of the B cell CTLA-4 receptor. J. Immunol 165, 5530–5536. [DOI] [PubMed] [Google Scholar]
- Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Perez Marc G, Moreira ED, Zerbini C, et al. (2020). Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med 383, 2603–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennick JJ, Johnston APR, and Parton RG (2021). Key principles and methods for studying the endocytosis of biological and nanoparticle therapeutics. Nat. Nanotechnol 16, 266–276. [DOI] [PubMed] [Google Scholar]
- Rezvantalab S, Drude NI, Moraveji MK, Guvener N, Koons EK, Shi Y, Lammers T, and Kiessling F. (2018). PLGA-based nanoparticles in cancer treatment. Front. Pharmacol 9, 1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert C, Thomas L, Bondarenko I, O’Day S, Weber J, Garbe C, Lebbe C, Baurain JF, Testori A, Grob JJ, et al. (2011). Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N. Engl. J. Med 364, 2517–2526. [DOI] [PubMed] [Google Scholar]
- Rodriguez PL, Harada T, Christian DA, Pantano DA, Tsai RK, and Discher DE (2013). Minimal “Self” peptides that inhibit phagocytic clearance and enhance delivery of nanoparticles. Science 339, 971–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblum D, Joshi N, Tao W, Karp JM, and Peer D. (2018). Progress and challenges towards targeted delivery of cancer therapeutics. Nat. Commun 9, 1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S, Sethi TK, Taylor D, Kim YJ, and Johnson DB (2020). Break-through concepts in immune-oncology: cancer vaccines at the bedside. J. Leukoc. Biol 108, 1455–1489. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E, and Pierschbacher MD (1986). Arg-Gly-Asp: a versatile cell recognition signal. Cell 44, 517–518. 10.1016/0092-8674(86)90259-x. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E, and Pierschbacher MD (1987). New perspectives in cell adhesion: RGD and integrins. Science 238, 491–497. [DOI] [PubMed] [Google Scholar]
- Sahin U, Derhovanessian E, Miller M, Kloke BP, Simon P, Lower M, Bukur V, Tadmor AD, Luxemburger U, Schrors B, et al. (2017). Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature 547, 222–226. [DOI] [PubMed] [Google Scholar]
- Sahin U, Oehm P, Derhovanessian E, Jabulowsky RA, Vormehr M, Gold M, Maurus D, Schwarck-Kokarakis D, Kuhn AN, Omokoko T, et al. (2020). An RNA vaccine drives immunity in checkpoint-inhibitor-treated melanoma. Nature 585, 107–112. [DOI] [PubMed] [Google Scholar]
- Sangsuwan R, Thuamsang B, Pacifici N, Allen R, Han H, Miakicheva S, and Lewis JS (2020). Lactate exposure promotes immunosuppressive phenotypes in innate immune cells. Cell Mol. Bioeng 13, 541–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarvaria A, Madrigal JA, and Saudemont A. (2017). B cell regulation in cancer and anti-tumor immunity. Cell Mol. Immunol 14, 662–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schudel A, Chapman AP, Yau MK, Higginson CJ, Francis DM, Manspeaker MP, Avecilla ARC, Rohner NA, Finn MG, and Thomas SN (2020). Programmable multistage drug delivery to lymph nodes. Nat. Nanotechnol 15, 491–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz RH (1992). Costimulation of T lymphocytes: the role of CD28, CTLA-4, and B7/BB1 in interleukin-2 production and immunotherapy. Cell 71, 1065–1068. [DOI] [PubMed] [Google Scholar]
- Scodeller P, Simon-Gracia L, Kopanchuk S, Tobi A, Kilk K, Saalik P, Kurm K, Squadrito ML, Kotamraju VR, Rinken A, et al. (2017). Precision targeting of tumor macrophages with a CD206 binding peptide. Sci. Rep 7, 14655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott EA, Stano A, Gillard M, Maio-Liu AC, Swartz MA, and Hubbell JA (2012). Dendritic cell activation and T cell priming with adjuvant- and antigen-loaded oxidation-sensitive polymersomes. Biomaterials 33, 6211–6219. [DOI] [PubMed] [Google Scholar]
- Seidel JA, Otsuka A, and Kabashima K. (2018). Anti-PD-1 and anti-CTLA-4 therapies in cancer: mechanisms of action, efficacy, and limitations. Front. Oncol 8, 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shae D, Baljon JJ, Wehbe M, Christov PP, Becker KW, Kumar A, Suryadevara N, Carson CS, Palmer CR, Knight FC, et al. (2020). Co-delivery of peptide neoantigens and stimulator of interferon genes agonists enhances response to cancer vaccines. ACS Nano 14, 9904–9916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shae D, Becker KW, Christov P, Yun DS, Lytton-Jean AKR, Sevimli S, Ascano M, Kelley M, Johnson DB, Balko JM, and Wilson JT (2019). Endosomolytic polymersomes increase the activity of cyclic dinucleotide STING agonists to enhance cancer immunotherapy. Nat. Nanotechnol 14, 269–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma P, and Allison JP (2015). Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell 161, 205–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe AH, and Pauken KE (2018). The diverse functions of the PD1 inhibitory pathway. Nat. Rev. Immunol 18, 153–167. [DOI] [PubMed] [Google Scholar]
- Shi GN, Zhang CN, Xu R, Niu JF, Song HJ, Zhang XY, Wang WW, Wang YM, Li C, Wei XQ, and Kong DL (2017). Enhanced antitumor immunity by targeting dendritic cells with tumor cell lysate-loaded chitosan nanoparticles vaccine. Biomaterials 113, 191–202. [DOI] [PubMed] [Google Scholar]
- Soni D, Bobbala S, Li S, Scott EA, and Dowling DJ (2021). The sixth revolution in pediatric vaccinology: immunoengineering and delivery systems. Pediatr. Res 89, 1364–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stack T, Liu Y, Frey M, Bobbala S, Vincent M, and Scott E. (2021). Enhancing subcutaneous injection and target tissue accumulation of nanoparticles via co-administration with macropinocytosis inhibitory nanoparticles (MiNP). Nanoscale Horiz. 6, 393–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stack T, Vincent M, Vahabikashi A, Li G, Perkumas KM, Stamer WD, Johnson M, and Scott E. (2020). Targeted delivery of cell softening micelles to Schlemm’s canal endothelial cells for treatment of glaucoma. Small 16, e2004205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugahara KN, Teesalu T, Karmali PP, Kotamraju VR, Agemy L, Girard OM, Hanahan D, Mattrey RF, and Ruoslahti E. (2009). Tissue-penetrating delivery of compounds and nanoparticles into tumors. Cancer Cell 16, 510–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugahara KN, Teesalu T, Karmali PP, Kotamraju VR, Agemy L, Greenwald DR, and Ruoslahti E. (2010). Coadministration of a tumor-penetrating peptide enhances the efficacy of cancer drugs. Science 328, 1031–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukkurwala AQ, Adjemian S, Senovilla L, Michaud M, Spaggiari S, Vacchelli E, Baracco EE, Galluzzi L, Zitvogel L, Kepp O, and Kroemer G. (2014). Screening of novel immunogenic cell death inducers within the NCI mechanistic diversity set. Oncoimmunology 3, e28473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykes EA, Chen J, Zheng G, and Chan WC (2014). Investigating the impact of nanoparticle size on active and passive tumor targeting efficiency. ACS Nano 8, 5696–5706. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Tagami T, Yamazaki S, Uede T, Shimizu J, Sakaguchi N, Mak TW, and Sakaguchi S. (2000). Immunologic self-tolerance maintained by CD25(+)CD4(+) regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J. Exp. Med 192, 303–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tandrup Schmidt S, Foged C, Korsholm KS, Rades T, and Christensen D. (2016). Liposome-based adjuvants for subunit vaccines: formulation strategies for subunit antigens and immunostimulators. Pharmaceutics 8, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teesalu T, Sugahara KN, Kotamraju VR, and Ruoslahti E. (2009). C-end rule peptides mediate neuropilin-1-dependent cell, vascular, and tissue penetration. Proc. Natl. Acad. Sci. U S A 106, 16157–16162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian X, Nyberg S, Sharp PS, Madsen J, Daneshpour N, Armes SP, Berwick J, Azzouz M, Shaw P, Abbott NJ, and Battaglia G. (2015). LRP-1-mediated intracellular antibody delivery to the Central Nervous System. Sci. Rep 5, 11990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai MJ, Chang WA, Huang MS, and Kuo PL (2014). Tumor microenvironment: a new treatment target for cancer. ISRN Biochem. 2014, 351959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsou P, Katayama H, Ostrin EJ, and Hanash SM (2016). The emerging role of B cells in tumor immunity. Cancer Res. 76, 5597–5601. [DOI] [PubMed] [Google Scholar]
- Tuettenberg A, Schmitt E, Knop J, and Jonuleit H. (2007). Dendritic cell-based immunotherapy of malignant melanoma: success and limitations. J. Dtsch Dermatol. Ges 5, 190–196. [DOI] [PubMed] [Google Scholar]
- Twomey JD, and Zhang B. (2021). Cancer immunotherapy update: FDA-approved checkpoint inhibitors and companion diagnostics. AAPS J. 23, 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaddepally RK, Kharel P, Pandey R, Garje R, and Chandra AB (2020). Review of indications of FDA-approved immune checkpoint inhibitors per NCCN guidelines with the level of evidence. Cancers 12, 738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent MP, Bobbala S, Karabin NB, Frey M, Liu Y, Navidzadeh JO, Stack T, and Scott EA (2021a). Surface chemistry-mediated modulation of adsorbed albumin folding state specifies nanocarrier clearance by distinct macrophage subsets. Nat. Commun 12, 648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent MP, Karabin NB, Allen SD, Bobbala S, Frey MA, Yi S, Yang Y, and Scott EA (2021b). The combination of morphology and surface chemistry defines the immunological identity of nanocarriers in human blood. Adv Ther. 4, 2100062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent MP, Stack T, Vahabikashi A, Li G, Perkumas KM, Ren R, Gong H, Stamer WD, Johnson M, and Scott EA (2021c). Surface engineering of FLT4-targeted nanocarriers enhances cell-softening glaucoma therapy. ACS Appl. Mater. Interfaces 13, 32823–32836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vohidov F, Milling LE, Chen Q, Zhang W, Bhagchandani S, Nguyen HVT, Irvine DJ, and Johnson JA (2020). ABC triblock bottlebrush copolymer-based injectable hydrogels: design, synthesis, and application to expanding the therapeutic index of cancer immunochemotherapy. Chem. Sci 11, 5974–5986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walkey CD, and Chan WC (2012). Understanding and controlling the interaction of nanomaterials with proteins in a physiological environment. Chem. Soc. Rev 41, 2780–2799. [DOI] [PubMed] [Google Scholar]
- Walkey CD, Olsen JB, Guo H, Emili A, and Chan WC (2012). Nanoparticle size and surface chemistry determine serum protein adsorption and macrophage uptake. J. Am. Chem. Soc 134, 2139–2147. [DOI] [PubMed] [Google Scholar]
- Wang H, and Mooney DJ (2018). Biomaterial-assisted targeted modulation of immune cells in cancer treatment. Nat. Mater 17, 761–772. [DOI] [PubMed] [Google Scholar]
- Wang L, Gao Y, Zhang G, Li D, Wang Z, Zhang J, Hermida LC, He L, Wang Z, Si J, et al. (2020). Enhancing KDM5A and TLR activity improves the response to immune checkpoint blockade. Sci. Transl. Med 12, eaax2282. [DOI] [PubMed] [Google Scholar]
- Wang M, Zhang C, Song Y, Wang Z, Wang Y, Luo F, Xu Y, Zhao Y, Wu Z, and Xu Y. (2017). Mechanism of immune evasion in breast cancer. Onco Targets Ther. 10, 1561–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Ihara S, Li X, Ito A, Sogo Y, Watanabe Y, Yamazaki A, Tsuji NM, and Ohno T. (2019). Rod-scale design strategies for immune-targeted delivery system toward cancer immunotherapy. ACS Nano 13, 7705–7715. [DOI] [PubMed] [Google Scholar]
- Wang XB, Fan ZZ, Anton D, Vollenhoven AV, Ni ZH, Chen XF, and Lefvert AK (2011). CTLA4 is expressed on mature dendritic cells derived from human monocytes and influences their maturation and antigen presentation. BMC Immunol. 12, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang-Bishop L, Wehbe M, Shae D, James J, Hacker BC, Garland K, Chistov PP, Rafat M, Balko JM, and Wilson JT (2020). Potent STING activation stimulates immunogenic cell death to enhance antitumor immunity in neuroblastoma. J. Immunother. Cancer 8, e000282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson MJ, Vignali PDA, Mullett SJ, Overacre-Delgoffe AE, Peralta RM, Grebinoski S, Menk AV, Rittenhouse NL, DePeaux K, Whetstone RD, et al. (2021). Metabolic support of tumour-infiltrating regulatory T cells by lactic acid. Nature 591, 645–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wculek SK, Cueto FJ, Mujal AM, Melero I, Krummel MF, and Sancho D. (2020). Dendritic cells in cancer immunology and immunotherapy. Nat. Rev. Immunol 20, 7–24. [DOI] [PubMed] [Google Scholar]
- Werb Z, and Lu P. (2015). The role of stroma in tumor development. Cancer J. 21, 250–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteside TL (2008). The tumor microenvironment and its role in promoting tumor growth. Oncogene 27, 5904–5912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitesides GM, Mathias JP, and Seto CT (1991). Molecular self-assembly and nanochemistry: a chemical strategy for the synthesis of nanostructures. Science 254, 1312–1319. [DOI] [PubMed] [Google Scholar]
- Wilhelm S, Tavares AJ, Dai Q, Ohta S, Audet J, Dvorak HF, and Chan WCW (2016). Analysis of nanoparticle delivery to tumours. Nat. Rev. Mater 1, 16014. [Google Scholar]
- Wolchok JD, and Saenger Y. (2008). The mechanism of anti-CTLA-4 activity and the negative regulation of T-cell activation. Oncologist 13, 2–9. [DOI] [PubMed] [Google Scholar]
- Xie Z., Shen J., Sun H., Li J., and Wang X. (2021). Polymer-based hydrogels with local drug release for cancer immunotherapy. Biomed. Pharmacother 137, 111333. [DOI] [PubMed] [Google Scholar]
- Xing L, Xu Y, Sun K, Wang H, Zhang F, Zhou Z, Zhang J, Zhang F, Caliskan B, Qiu Z, and Wang M. (2018). Identification of a peptide for folate receptor alpha by phage display and its tumor targeting activity in ovary cancer xenograft. Sci Rep 8, 8426. 10.1038/s41598-018-26683-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu E, Saltzman WM, and Piotrowski-Daspit AS (2021). Escaping the endosome: assessing cellular trafficking mechanisms of non-viral vehicles. J. Control. Release 335, 465–480. [DOI] [PubMed] [Google Scholar]
- Yan H, You Y, Li X, Liu L, Guo F, Zhang Q, Liu D, Tong Y, Ding S, and Wang J. (2020). Preparation of RGD peptide/folate acid double-targeted mesoporous silica nanoparticles and its application in human breast cancer MCF-7 cells. Front. Pharmacol 11, 898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Y, Zhou Y, Liu L, Xu Y, Chen Q, Wang Y, Wu S, Deng Y, Zhang J, and Shao A. (2020). Nanoparticle-based drug delivery in cancer therapy and its role in overcoming drug resistance. Front. Mol. Biosci 7, 193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi S, Allen SD, Liu Y-G, Ouyang BZ, Li X, Augsornworawat P, Thorp EB, and Scott EA (2016). Tailoring nanostructure morphology for enhanced targeting of dendritic cells in atherosclerosis. ACS Nano 10, 11290–11303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi S, Zhang X, Sangji M, Liu YG, Allen S, Xiao B, Bobbala S, Braverman C, Cai L, Hecker P, et al. (2019). Surface engineered polymersomes for enhanced modulation of dendritic cells during cardiovascular immunotherapy. Adv. Funct. Mater 29, 1904399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon HY, Selvan ST, Yang Y, Kim MJ, Yi DK, Kwon IC, and Kim K. (2018). Engineering nanoparticle strategies for effective cancer immunotherapy. Biomaterials 178, 597–607. [DOI] [PubMed] [Google Scholar]
- Yuan H, Jiang W, von Roemeling CA, Qie Y, Liu X, Chen Y, Wang Y, Wharen RE, Yun K, Bu G, et al. (2017). Multivalent bi-specific nanobioconjugate engager for targeted cancer immunotherapy. Nat. Nanotechnol 12, 763–769. [DOI] [PubMed] [Google Scholar]
- Zeng Q, Li H, Jiang H, Yu J, Wang Y, Ke H, Gong T, Zhang Z, and Sun X. (2017). Tailoring polymeric hybrid micelles with lymph node targeting ability to improve the potency of cancer vaccines. Biomaterials 122, 105–113. [DOI] [PubMed] [Google Scholar]
- Zhang G, Yang Z, Lu W, Zhang R, Huang Q, Tian M, Li L, Liang D, and Li C. (2009). Influence of anchoring ligands and particle size on the colloidal stability and in vivo biodistribution of polyethylene glycol-coated gold nanoparticles in tumor-xenografted mice. Biomaterials 30, 1928–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Zhang W, Sun X, Dang R, Zhou R, Bai H, Ben J, Zhu X, Zhang Y, Yang Q, et al. (2016). Class A1 scavenger receptor modulates glioma progression by regulating M2-like tumor-associated macrophage polarization. Oncotarget 7, 50099–50116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, and Eisenberg A. (1995). Multiple morphologies of “Crew-Cut” aggregates of polystyrene-b-poly(acrylic acid) block copolymers. Science 268, 1728–1731. [DOI] [PubMed] [Google Scholar]
- Zhang L, Wu S, Qin Y, Fan F, Zhang Z, Huang C, Ji W, Lu L, Wang C, Sun H, et al. (2019). Targeted codelivery of an antigen and dual agonists by hybrid nanoparticles for enhanced cancer immunotherapy. Nano Lett. 19, 4237–4249. [DOI] [PubMed] [Google Scholar]
- Zhang S, Gao H, and Bao G. (2015). Physical principles of nanoparticle cellular endocytosis. ACS Nano 9, 8655–8671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Wu JLY, Lazarovits J, and Chan WCW (2020a). An analysis of the binding function and structural organization of the protein corona. J. Am. Chem. Soc 142, 8827–8836. [DOI] [PubMed] [Google Scholar]
- Zhang YN, Poon W, Sefton E, and Chan WCW (2020b). Suppressing subcapsular sinus macrophages enhances transport of nanovaccines to lymph node follicles for robust humoral immunity. ACS Nano 14, 9478–9490. [DOI] [PubMed] [Google Scholar]
- Zhao X, Yang K, Zhao R, Ji T, Wang X, Yang X, Zhang Y, Cheng K, Liu S, Hao J, et al. (2016). Inducing enhanced immunogenic cell death with nanocarrier-based drug delivery systems for pancreatic cancer therapy. Biomaterials 102, 187–197. [DOI] [PubMed] [Google Scholar]
- Zhou J, Wang G, Chen Y, Wang H, Hua Y, and Cai Z. (2019). Immunogenic cell death in cancer therapy: present and emerging inducers. J. Cell Mol. Med 23, 4854–4865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu M, Du L, Zhao R, Wang HY, Zhao Y, Nie G, and Wang R-F (2020). Cell-Penetrating nanoparticles activate the inflammasome to enhance antibody production by targeting microtubule-associated protein 1-light chain 3 for degradation. ACS Nano 14, 3703–3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zitvogel L, Apetoh L, Ghiringhelli F, and Kroemer G. (2008). Immunological aspects of cancer chemotherapy. Nat. Rev. Immunol 8, 59–73. [DOI] [PubMed] [Google Scholar]