Abstract
Parenteral Nutrition (PN) is a therapy that delivers essential nutrients intravenously to patients that are unable to meet their nutritional requirements via standard enteral feeding. This methodology is often referred to as Parenteral Nutrition (PN) when accompanied by minimal or no enteral nutrition. While PN is lifesaving, significant complications can arise such as intestinal failure associated liver disease (IFALD) and gut-mucosal atrophy. The exact mechanism of injury remains ill defined.
This review was designed to explore available literature related to the drivers of injury mechanisms.
The Farnesoid X Receptor (FXR) and Fibroblast Growth Factor-19 (FGF19) signaling pathway seems to play an important role in gut-systemic signaling and its alteration during PN provides insights into mechanistic links. Central line infections also play a key role in mediating PN associated injury. While lipid reduction strategies as well as the use of multicomponent lipid emulsions and vitamin E have shown promise, the cornerstone of preventing injury is early establishment of enteral nutrition.
Keywords: intestinal failure, parenteral nutrition, liver disease, Farnesoid X receptor, fibroblast growth factor-19, enteral nutrition
Graphical Abstract
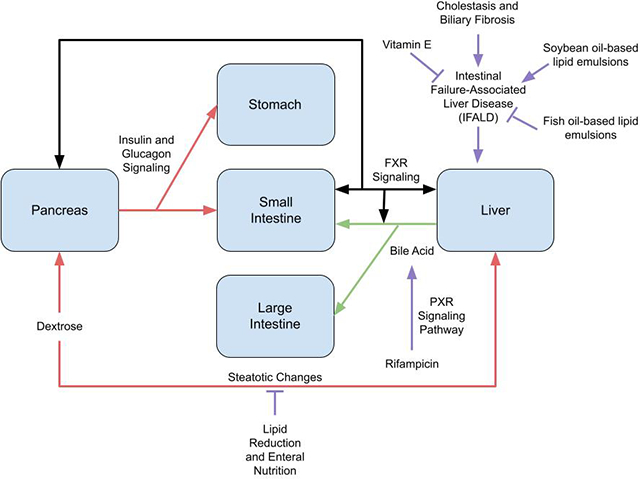
Introduction
Parenteral Nutrition (PN) is a therapy that delivers nutrients intravenously to patients who are unable to meet their nutritional needs via standard enteral feeding routes. This requires that fats, carbohydrates, proteins, essential vitamins, minerals, and micronutrients are all provided directly into the bloodstream intravenously via an infusion pump, bypassing the alimentary system.1,2 When a patient receives all nutrients using this process, it is referred to as total parenteral nutrition (TPN). A patient might require TPN as a result of intestinal failure, where there is a lack of functional gut to absorb the nutrients required to support life, or secondary to short bowel syndrome (SBS) from bowel resection or trauma to the gastrointestinal tract.1 TPN is a life-saving therapy for these individuals, who include neonatal, pediatric, and adult patients.3,4 While TPN has become a gold-standard treatment for diseases such as SBS, this critical modality for nutrient delivery does not come without potential complications.5–9
Unfortunately, while a necessity for these patients, PN is associated with significant gut and liver disease, leading to gut-mucosal atrophy and progressive liver injury culminating in liver failure often initially characterized by high serum bilirubin levels.10–13 PN-related complications such as steatosis, cholestasis, development of biliary sludge, cholecystitis, etc. is variable depending upon age and disease state.14 Evidence of steatosis and elevations in hepatic enzymes can occur within 2 weeks of starting PN. Cholestasis is also known to occur as early as within 2 weeks of starting PN.14 Indeed, a large majority of patients on chronic PN develop cholestatic injury resulting in mortality rates near 20%.9,15 The mechanisms are elusive and remain an active area of research.16 Additionally, there are limited therapeutics, with many patients progressing to multi-visceral organ transplant with advanced disease stages.17,18
In this review, we will evaluate the current known mechanisms behind the development of parenteral nutrition associated injury and review how intravenous lipid emulsions can modulate the condition.
Intestinal Failure Associated Liver Disease (IFALD)
Although PN has been successfully used for many years as a lifesaving method of nutrition, prolonged use of PN is known to cause parenteral nutrition associated liver disease (PNALD).
Recent research has facilitated the development of a working understanding of the importance of the gut with respect to its role in liver injury and the term intestinal failure associated liver disease (IFALD) is often used instead of PNALD. IFALD presents itself as a19,20 spectrum ranging from relatively minor liver dysfunction such as mildly elevated serum liver enzymes to progressive cholestasis and eventual liver fibrosis/cirrhosis.16,21,22 IFALD also includes intra hepatic bile acid (BA) accumulation, steatosis, inflammation, glucose intolerance and dyslipidemia.
There is evidence that with just a small amount of enteral nutrition (EN), one can minimize TPN-associated side effects, validating the importance of gut-liver signaling in maintaining hepatic homeostasis.12
Enteral bile acid mediated FXR induction of FGF19 during PN
In clinical settings, liver injury is reduced in patients on PN if some EN is provided5, though such EN is not possible in many patients due to a lack of remaining bowel length, especially in those with significant bowel resection, requiring lifelong PN.
Recent studies, demonstrate that during EN, as part of normal enterohepatic circulation, bile acid mediated gut Farnesoid X Receptor (FXR) activation stimulates the production of a growth factor, Fibroblast Growth Factor – 19 (FGF19).3,6,7,23 FGF19 functions as a major secretory signal to the liver, regulating hepatic bile acid synthesis via repression of CYP7A1 (Cholesterol 7 alpha-hydroxylase – rate limiting step).24 FGF19 also modulates hepatic cholestasis, glycemic and lipid control.9,11,25
Indeed, data suggests that during EN, hepatic bile acid synthesis, lipid and glucose metabolism is modulated via intestinal FXR-FGF19 signaling.3,18,26,27 In studies using a novel SBS model with 75% bowel resection (and no EN), it has been demonstrated that there is inadequate gut FXR activation and liver injury.1,3,15,17,18 Using a piglet model, hepatic protection with the enteral FXR agonists, has been established.3 These results provide strong evidence that in the absence of EN, liver disease during PN results from inadequate gut FXR activation impairing the FXR-FGF19 signaling axis.
Gut TGR5 Signaling and Glucagon Like Peptides during PN
Several studies have demonstrated that gut injury with gut mucosal atrophy and increased gut permeability occurs with PN1,3,13,21, though the mechanisms remain ill-defined.1,22 Data also suggests that luminal EN can modulate gut mucosal proliferation28,29, gut permeability30,31 and enhance serum Glucagon Like Peptide 2 (GLP-2).1,3,22
GLP-2 is one of the most critical gut-trophic factors22,29 and modulates the gut barrier.32,33 Emerging data shows that GLP-2 secretion by enteroendocrine cells is under regulation of the bile acid activated G protein-coupled receptor TGR5.13,34,35 Indeed, TGR5 is highly localized in crypts and is known to modulate systemic effects via GLP-2.36,37
In studies using a SBS model it has been shown that there is inadequate gut TGR5 activation.1,2,36,37 Additionally, a significantly enhanced expression of GLP-2 with bile acid treatment in animals on PN has been demonstrated.3 Thus, there is an impairment in TGR5-GLP axis signaling during PN delivery.
Gut Microbiota Alterations
Due to a lack of luminal nutrition, it has been published that significant alterations occur in gut microbiota as well as gut derived signaling in animal models.3,21,23,38 While there is dominance of the Firmicutes phylum in normal EN fed animals, in animals on PN there is a proliferation of the Bacteroidetes phylum.21,38 Using multilevel logistic regression, significant sub-phylum changes in microbial community composition has been shown in PN vs EN. Data suggest that such microbial shifts promote intestinal inflammation39,40 and increase intestinal permeability.21,41,42. In a study examining the stool samples of infants with IFALD, significant alterations in the gut microbiome were observed.30
Indeed, it has been hypothesized that gut microbial alterations enable bacterial flux across the mucosa which also results in cytokine mediated hepatocellular injury.43–45 Upon initiation of enterally administered antibiotics, a significant reduction in liver injury has been reported.46–48 Additionally, significantly elevated Interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) have been noted during PN.49–51
Novel Mechanisms for Bacterial Modulation of Bile Acid Driven Signaling:
During normal enterohepatic circulation, primary bile acids (BA), synthesized by the liver undergo transformation to secondary BA by the gut microbiota.52,53 Since the primary BA are preferential ligands for FXR and secondary BA are preferential TGR5 ligands, this highlights an important and novel mechanism by which gut microbes can modulate BA signaling properties.54–56
During PN, changes in microbial communities thus drive variable gut FXR and gut TGR5 activation and alter the course of injury. This aspect of microbial regulation of gut derived signaling, is highly unique. Notably, the microbial changes during PN were accompanied by decreased stool BA and altered gut FXR and TGR5 activation.1,21,38,54,55,57
Recent data also shows that both FXR and TGR5 modulate the intestinal barrier, permeability, and immune responses30,31,58,59 and gut TGR5/FXR ligands lower IL-1β and TNF-α.20,31,60–62 While there has been recent use of omega-3 based lipid emulsions22,38, such do not resolve liver injury1,63–65 and have associated complications.65,66 Of note, soy based, ‘omega-6’ emulsions contain phytosterols, which are known FXR antagonists. 19,67
Gut-Systemic signaling in overlapping disease processes:
An interesting observation worth an additional note is that while hepatic steatosis, inflammation, glucose intolerance, and dyslipidemia are noted in IFALD68,69, intriguingly these phenotypes have an overlap with Nonalcoholic Steatohepatitis (NASH).70,71 Indeed, there is increasing evidence of gut-derived signaling in modulating liver pathology in NASH.72 In fact, FXR ligands and FGF-19 variants are being evaluated as treatments for NASH.73,74 Thus, the existence of gut derived signaling modulating liver disease with overlapping phenotypes, across varying model systems, provides additional justification to the role of such mechanism in driving PN associated injury.
Dextrose
Dextrose is the major energy source in parenteral nutrition, it also supplies carbon skeletons for tissue growth and repair.36 Tolerance to dextrose depends on rate of infusion and underlying patient conditions.36 The most common complication associated with excess dextrose is hyperglycemia.
An increased dextrose infusion has been linked with hepatic steatosis.75 Dextrose changes the levels of insulin and glucagon. Indeed, the insulin-glucagon molar ratio plays a critical role in the development of PN-associated hepatic steatosis.21
Central Line Infections
The use of central lines during PN delivery can increase the risk of central-line-associated bloodstream infections (CLABSI).52,53 Endotoxin mediated liver injury is a well-known cause of cholestasis and strategies mitigating CLABSI remain forefront in the care of patients on PN.
Lipid and Hepatic Steatosis
Enterally given lipids are acted upon via lipoprotein lipase38 and are then absorbed by the enterocyte as a micelle. Lipids are then packaged into chylomicrons for the liver to metabolize.38 Parenterally given lipids are envisioned to mimic chylomicrons however they undergo a differing metabolic pathway.39 Such has been proposed to result in the accumulation of lipid particles in hepatocytes.40 This was reported by Ekelund et al in rats where animals on TPN had increased liver triglycerides (p < 0.001) in association with lower HDL cholesterol (p < 0.001) and phospholipid levels (p < 0.05).
Interestingly, hepatic lipase was also shown to have decreased activity in the TPN group compared to the control (p < 0.001), possibly due to the triglyceride accumulation in hepatic tissue.38 In heart tissue, lipoprotein lipase activity was increased for the TPN group as compared to the control (p < 0.001), indicating the differential role of lipids across many tissue types.38 Several studies have noted that parenteral lipids can lead to severe and persistent steatosis42, further attesting to the need of rapid transition to EN.
Route of administration
The primary treatment for IFALD is the elimination of PN and the establishment of enteral nutrition (EN).16,38 However, for children and infants who cannot be tapered from PN due to insufficient bowel function or length alternative strategies could be used. These include, lipid-dose reduction, fish oil-based lipid emulsions, multicomponent lipid emulsions, and possibly Vitamin E.
EN leads to the best outcomes in patients on PN. 12 infants who were found to have evidence of cholestasis while on parenteral nutrition (PN) were transitioned to full enteral nutrition with discontinuation of PN; following initiation of enteral nutrition (EN), all infants showed normalization of bilirubin levels and improvement of alkaline phosphatase levels.42 In a similar study conducted by Guzman et al, 16 neonatal pigs were either provided EN or TPN for 3 weeks. Pigs receiving TPN demonstrated multiple alterations in hepato-biliary markers suggesting deprivation of EN significantly alters hepatobiliary receptors that drive gut-systemic signaling.
Lipid-Dose Reduction
In patients who are unable to establish enteral nutrition, lipid-dose reduction has been used to reduce liver damage and sometimes reverse damage, however, the impact on growth and brain development needs continued evaluation in children.45,46
Soybean Oil
In the United States, soybean oil-based lipid emulsions are the predominant available intravenous lipid emulsion.47 As one of the first generation lipid emulsions, SOLEs provided a concentrated source of energy.
There are several components of soybean lipid emulsions that have been linked to the development of hepatic injury. Soybean emulsions are known to contain high concentrations of Omega-6 polyunsaturated fatty acids (PUFA) such as linoleic acid whose peroxidation can lead to hepatocyte damage. The conversion of linoleic acid to arachidonic acid, which is a precursor to several inflammatory molecules like thromboxanes, leukotrienes, and prostaglandins, may cause the persistence of an inflammatory state which may drive hepatocyte damage and cholestasis. Further, soybean oil emulsions have a high concentration of phytosterols which have been correlated with liver fibrosis in PN-dependent children with intestinal failure. The suggested mechanism of phytosterol leading hepatic damage is through the inhibition of the transcription of bile transport proteins by antagonism of FXR. Lastly, a low concentration of α-Tocopherol often seen in soybean emulsions could lead to further decreased protection from oxidative stress.49
Fish Oil
An alternative treatment to lipid restriction is the replacement of SOLE, with Fish-Oil Lipid Emulsion (FOLE). FOLEs have been shown to significantly decrease cholestasis compared to SOLE in children and also in adults with intestinal failure associated with liver disease.51,52 FOLEs generate eicosanoids that are generally less inflammatory because they are derived from omega-3 fatty acids instead of the omega-6 fatty acids.47 Fish oil also includes increased concentration of Vitamin E, an antioxidant, discussed below.52,53 Fish oil-based injectable lipid emulsions have been shown to improve IFALD by providing fatty acids (particularly omega-3) (FAs) and Vitamin E.53
FOLEs were approved for compassionate use in patients with IFALD in 2004.48 In a study performed by Gura et al, 4% of FOLE patients (n = 189) underwent liver transplantation as an end result compared to 12% of SOLE patients (n = 73) (P = 0.0245). Patients receiving FOLEs demonstrated improved aspartate aminotransferase to platelet ratio index (APRI) scores while SOLE recipients demonstrated worsened APRI scores.48 The results noted a resolution of cholestasis in 65% of patients given FOLE compared to only 16% of SOLE patients. This showed an effective decrease in cholestasis with the administration of FOLE compared to SOLE, however, currently there are a limited number of studies evaluating the long-term outcomes of children on FOLE monotherapy for PNALD. Additionally, there may be an increased bleeding risk due to FOLE therapy in pediatric patients and this would also need to be further assessed.46
In case reports by Fuchs et al, two short bowel syndrome patients were placed on FOLE due to IFALD. In both patients, liver injury markers improved. One patient was able to be removed from the liver transplant list and the other resolved their cholestasis.55 In another study, the median time to reversal of cholestasis was 9.4 weeks in patients switched to FOLEs in contrast to the 44.1 weeks in a historical group on SOLEs.57 In a study by Puder et al, there was a statistically significant reduction in risk of death and liver transplantation with FOLE at 9.5% in the FOLE group vs 34.7% in the control group.54 Reversal of cholestasis and decrease of direct bilirubin and ALT were observed in the FOLE group.54 FOLEs were also found to be safer than the control group.54
Multicomponent Lipid Emulsions
As previously discussed, the composition of lipid emulsions is suspected to play a role in the development of IFALD. This has led to the release of several newer lipid emulsions.61 Multicomponent lipid emulsion generally consist of 30% soybean Oil, 30% medium chain triglycerides, 25% olive oil, as well as 15% fish oil.62 Because they are derived from multiple sources, multicomponent lipid emulsions offer a more balanced composition of lipids and could be optimal in preventing IFALD in infants.61
Several studies have compared the hepatoprotective effects of multicomponent lipid emulsions to standard lipid profiles. A study done by Jackson et al evaluated 136 neonates who received PN for more than 14 days. Out of 81 patients receiving multicomponent lipid emulsions, only 2 developed cholestasis (2.5%), and out of 55 patients receiving SOLE, 9 developed cholestasis (16.4%), indicating preventative effects of multicomponent lipid emulsions in the development of PN-associated Cholestasis.76 Another study compared multicomponent lipid emulsions with SOLE in PN-dependent infants who showed evidence of early IFALD. Out of the 24 infants who participated in the trial, those receiving multicomponent lipid emulsions noted a decline in serum conjugated bilirubin, whereas those receiving SOLE noted an increase in conjugated bilirubin. This demonstrated that multicomponent lipid emulsions could not only mitigate PN-associated cholestasis in preterm infants, but may also prevent the progression of IFALD in infants with existing intestinal failure.63
In addition, a study done by Kasirer et al assessed whether the administration of multicomponent lipid emulsions to preterm neonates with birth weight lower than 1,500 g prevents or reduces cholestasis. They compared the incidence as well as severity of direct hyperbilirubinemia during two consecutive 20-month periods, the first in which preterm infants on TPN received a medium chain triglyceride/soybean oil emulsion, and the second in which preterm infants on TPN received another multicomponent lipid emulsion. Infants receiving multicomponent lipid emulsions experienced a lower incidence of TPN-Associated Cholestasis (6% compared to 13%) and lower peak direct bilirubin levels (3.2 vs. 7.1 mg/dL), displaying hepatoprotective effects of multicomponent lipid emulsions.61
Alternative Treatments
Rifampicin
Rifampicin is an antibiotic often used for the treatment of tuberculosis. However, it is also a potent agonist of the pregnane X- receptor (PXR) mediated pathway involved in the metabolism of bile acids which may make it useful in helping patients suffering from the adverse effects of TPN. In the study done by Guthrie et al, rifampicin was one of the supplements included with soy-based lipid emulsions at 10 mg/kg/day for term-born piglets given daily through the same jugular catheter as PN. The study showed an increase in CYP3A29 and UGT1A6 genes which are targets of PXR, along with an increase in the metabolism of chenodeoxycholic acid to hyocholic acid.77 The rifampicin treatment group showed an increased metabolism of bile acids with a reduction in serum bile acid. Such was hypothesized to result from activation of the PXR-mediated drug metabolism. The effect on the PXR mediated pathway and its target genes could be further studied for possible treatment options for IFALD.
Vitamin E
α-tocopherol (vitamin E) is a fat-soluble antioxidant that is abundant in fish oil and could have protective effects.49,52,58 Vitamin E in PN is especially useful in cases of oxidative stress like that seen in chronic inflammation, sepsis, and organ failure, however, its dosage should be carefully monitored using plasma levels.
α-tocopherol has been studied for its potential anti-inflammatory properties. Using a murine model of hepatosteatosis, a study by Baker et al compared the effects of Fish oil (FO), Soybean oil (SO), and different ratios of FO/SO with medium chain triglycerides (MCTs) and α-tocopherol (AT) all given intravenously. Mice received an unpurified diet (UPD), PN+saline and PN + soybean as controls. The results showed mice that received SO developed hepatosteatosis, while mice that received 30:70 FO:MCT developed minimal hepatosteatosis. Additionally, mice receiving FO or SO showed significantly higher inflammatory markers compared to the UPD controls. However, mice fed 50:50 FO:MCT, 30:70 FO:MCT, FO + AT and 50:50 FO + MCT + AT showed significantly lower inflammatory markers, which were similar to what was seen by the UPD fed controls. Serum IL-6 and TNF-α were used as markers of systemic inflammation. The study concluded that the addition of AT and mixed FO/MCT improved the systemic inflammatory response and prevented PN induced liver injury, but AT did not produce a synergistic effect with MCT. The anti-inflammatory effects seen here are particular to a murine model and to assess the protective effect of α-tocopherol, further human studies may be required.53
As such, a study conducted by Ng et al with 14 preterm piglets further evaluated the advantage of including vitamin E in TPN. The piglets received PN that was either 100% soybean oil (IL), 100% soybean oil + vitamin E (ILE), 100% fish oil or 100% fish oil + phytosterols.78 The study by Ng et al showed significantly lower serum levels of direct bilirubin, serum triglycerides, gamma glutamyl transferase, LDL and hepatic triglyceride contents in the groups who received ILE, 100% fish oil or 100% fish oil + phytosterols compared to IL (p < 0.05).78 The study concluded that α-tocopherol in fish oil and when added to IL prevented an increase in lipidemic markers of IFALD in preterm piglets.60
In a follow up study done by the same group, the beneficial effects of α-tocopherol were not as apparent. Neonatal piglets received PN for 14 days with soy-based emulsion (IL), IL supplemented with vitamin E (VITE) at 12.6 mg/kg/day or IL. Unlike the study by Ng et al, in this study vitamin E supplementation with IL (VITE) did not prevent IFALD and paradoxically a rise in direct bilirubin and bile acids was observed. However, this study had a higher lipid dose of 10 g/kg/day than what was given in the previous study. Indeed, this higher lipid load caused a fourfold increase in direct bilirubin. The disparities in these studies show more work needs to be done to standardize the effectiveness of the use of α-tocopherol with PN. An appropriate recommendation would need to be created that can carefully instruct for when and how vitamin E supplementation with lipid emulsions would be the most beneficial.
Conclusion
Parenteral Nutrition is a life-saving therapy for those who cannot acquire the necessary nutrients enterally. Despite the obvious benefits, there are significant side effects of this therapy. While multiple potential mechanisms for IFALD have been evaluated the exact mechanism remains unclear. An altered bile acid signaling, altered hepatobiliary receptors and the role of lipids remains at the forefront of mechanistic probabilities. Continued work into modulation of gut derived signaling seems promising and could be a focus of ongoing clinical trials and bench research. While several therapeutic strategies are being trialed, establishing enteral nutrition remains the priority, providing the best chance of mitigating PN associated complications.
Funding:
The work was supported by the National Institutes of Health [grant numbers R03EB015955-01 and NIH-1R01DK131136-01] and internal funding through Saint Louis Liver Center seed grant.
Financial support for manuscript preparation: None
Footnotes
Conflicts of Interest:
None
References
- 1.Guzman M, Manithody C, Krebs J, et al. Impaired gut–systemic signaling drives total parenteral nutrition-associated injury. Nutrients. 2020;12(5). doi: 10.3390/nu12051493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Price A, Blomenkamp K, Manithody C, et al. Developing a Novel Ambulatory Total Parenteral Nutrition-Dependent Short Bowel Syndrome Animal Model. J Surg Res. 2019;234:13–19. doi: 10.1016/j.jss.2018.08.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jain AK, Stoll B, Burrin DG, Holst JJ, Moore DD. Enteral bile acid treatment improves parenteral nutrition-related liver disease and intestinal mucosal atrophy in neonatal pigs. Am J Physiol - Gastrointest Liver Physiol. 2012;302(2):2021. doi: 10.1152/ajpgi.00280.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dudrick SJ, Palesty JA. Historical Highlights of the Development of Total Parenteral Nutrition. Surg Clin North Am. 2011;91(3):693–717. doi: 10.1016/j.suc.2011.02.009 [DOI] [PubMed] [Google Scholar]
- 5.Javid PJ, Greene AK, Garza J, et al. The route of lipid administration affects parenteral nutrition-induced hepatic steatosis in a mouse model. J Pediatr Surg. 2005;40(9):1446–1453. doi: 10.1016/j.jpedsurg.2005.05.045 [DOI] [PubMed] [Google Scholar]
- 6.Inagaki T, Choi M, Moschetta A, et al. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab. 2005;2(4):217–225. doi: 10.1016/j.cmet.2005.09.001 [DOI] [PubMed] [Google Scholar]
- 7.Wang L, Lee YK, Bundman D, et al. Redundant pathways for negative feedback regulation of bile acid production. Dev Cell. 2002;2(6):721–731. [DOI] [PubMed] [Google Scholar]
- 8.Li J, Pircher PC, Schulman IG, Westin SK. Regulation of Complement C3 Expression by the Bile Acid Receptor FXR. J Biol Chem. 2005;280(9):7427–7434. doi: 10.1074/jbc.M411473200 [DOI] [PubMed] [Google Scholar]
- 9.Tomlinson E, Fu L, John L, et al. Transgenic Mice Expressing Human Fibroblast Growth Factor-19 Display Increased Metabolic Rate and Decreased Adiposity. Endocrinology. 2002;143(5):1741–1747. doi: 10.1210/endo.143.5.8850 [DOI] [PubMed] [Google Scholar]
- 10.Fu L, John LM, Adams SH, et al. Fibroblast Growth Factor 19 Increases Metabolic Rate and Reverses Dietary and Leptin-Deficient Diabetes. Endocrinology. 2004;145(6):2594–2603. doi: 10.1210/en.2003-1671 [DOI] [PubMed] [Google Scholar]
- 11.Huang X, Yang C, Luo Y, Jin C, Wang F, McKeehan WL. FGFR4 Prevents Hyperlipidemia and Insulin Resistance but Underlies High-Fat Diet Induced Fatty Liver. Diabetes. 2007;56(10):2501–2510. doi: 10.2337/db07-0648 [DOI] [PubMed] [Google Scholar]
- 12.Denton C, Price A, Friend J, et al. Role of the Gut–Liver Axis in Driving Parenteral Nutrition-Associated Injury. Children. 2018;5(10):136. doi: 10.3390/children5100136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jain AK, Sharma A, Arora S, et al. Preserved Gut Microbial Diversity Accompanies Upregulation of TGR5 and Hepatobiliary Transporters in Bile Acid-Treated Animals Receiving Parenteral Nutrition. JPEN J Parenter Enteral Nutr 2017;41(2):198–207. doi: 10.1177/0148607116661838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hartl WH, Jauch KW, Parhofer K, Rittler P. Complications and Monitoring -- Guidelines on Parenteral Nutrition. Ger Med Sci. 2009;7:1–12. doi: 10.3205/000076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parks DJ. Bile Acids: Natural Ligands for an Orphan Nuclear Receptor. Science (80- ). 1999;284(5418):1365–1368. doi: 10.1126/science.284.5418.1365 [DOI] [PubMed] [Google Scholar]
- 16.Manithody C, Denton C, Price A, et al. Development and validation of an ambulatory piglet model for short bowel syndrome with ileo-colonic anastomosis. Exp Biol Med. 2020;245(12):1049–1057. doi: 10.1177/1535370220915881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gälman C, Arvidsson I, Angelin B, Rudling M. Monitoring hepatic cholesterol 7α-hydroxylase activity by assay of the stable bile acid intermediate 7α-hydroxy-4-cholesten-3-one in peripheral blood. J Lipid Res. 2003;44(4):859–866. doi: 10.1194/jlr.D200043-JLR200 [DOI] [PubMed] [Google Scholar]
- 18.Lu TT, Makishima M, Repa JJ, et al. Molecular Basis for Feedback Regulation of Bile Acid Synthesis by Nuclear Receptors. Mol Cell. 2000;6(3):507–515. doi: 10.1016/S1097-2765(00)00050-2 [DOI] [PubMed] [Google Scholar]
- 19.Carter BA, Taylor OA, Prendergast DR, et al. Stigmasterol, a Soy Lipid–Derived Phytosterol, Is an Antagonist of the Bile Acid Nuclear Receptor FXR. Pediatr Res. 2007;62(3):301–306. doi: 10.1203/PDR.0b013e3181256492 [DOI] [PubMed] [Google Scholar]
- 20.Ceulemans LJ, Verbeke L, Decuypere J-P, et al. Farnesoid X Receptor Activation Attenuates Intestinal Ischemia Reperfusion Injury in Rats. Gracia-Sancho J, ed. PLoS One. 2017;12(1):e0169331. doi: 10.1371/journal.pone.0169331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jain AK, Wen JX, Blomenkamp KS, et al. Oleanolic Acid Improves Gut Atrophy Induced by Parenteral Nutrition. J Parenter Enter Nutr. 2016;40(1):67–72. doi: 10.1177/0148607115583536 [DOI] [PubMed] [Google Scholar]
- 22.Madnawat H, Welu AL, Gilbert EJ, et al. Mechanisms of Parenteral Nutrition–Associated Liver and Gut Injury. Nutr Clin Pract. 2020;35(1):63–71. doi: 10.1002/ncp.10461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Manithody CS, Van Nispen J, Murali V, et al. Role of Bile Acids and Gut Microbiota in Parenteral Nutrition Associated Injury. J Hum Nutr (Carson City, Nev). 2020;4(1). doi: 10.36959/487/286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ayloo S, Pentakota SR, Molinari M. Trends of characteristics and outcomes of donors and recipients of deceased donor liver transplantation in the United States: 1990 to 2013. World J Transplant. 2018;8(5):167–177. doi: 10.5500/wjt.v8.i5.167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Niinikoski H, Stoll B, Guan X, et al. Onset of Small Intestinal Atrophy Is Associated with Reduced Intestinal Blood Flow in TPN-Fed Neonatal Piglets. J Nutr. 2004;134(6):1467–1474. doi: 10.1093/jn/134.6.1467 [DOI] [PubMed] [Google Scholar]
- 26.Galman C, Arvidsson I, Angelin B, Rudling M. Monitoring hepatic cholesterol 7alpha-hydroxylase activity by assay of the stable bile acid intermediate 7alpha-hydroxy-4-cholesten-3-one in peripheral blood. J Lipid Res. 2003;44(4):859–866. doi: 10.1194/jlr.D200043-JLR200 [DOI] [PubMed] [Google Scholar]
- 27.Parks DJ, Blanchard SG, Bledsoe RK, et al. Bile acids: natural ligands for an orphan nuclear receptor. Science (80- ). 1999;284(5418):1365–1368. http://www.ncbi.nlm.nih.gov/pubmed/10334993. [DOI] [PubMed] [Google Scholar]
- 28.Ekelund M, Kristensson E, Ekelund M, Ekblad E. Total Parenteral Nutrition Causes Circumferential Intestinal Atrophy, Remodeling of the Intestinal Wall, and Redistribution of Eosinophils in the Rat Gastrointestinal Tract. Dig Dis Sci. 2007;52(8):1833–1839. doi: 10.1007/s10620-006-9678-z [DOI] [PubMed] [Google Scholar]
- 29.Burrin D, Stoll B, Moore D. DIGESTIVE PHYSIOLOGY OF THE PIG SYMPOSIUM: Intestinal bile acid sensing is linked to key endocrine and metabolic signaling pathways12. J Anim Sci. 2013;91(5):1991–2000. doi: 10.2527/jas.2013-6331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Inagaki T, Moschetta A, Lee YK, et al. Regulation of antibacterial defense in the small intestine by the nuclear bile acid receptor. Proc Natl Acad Sci U S A. 2006;103(10):3920–3925. doi: 10.1073/pnas.0509592103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Verbeke L, Farre R, Verbinnen B, et al. The FXR Agonist Obeticholic Acid Prevents Gut Barrier Dysfunction and Bacterial Translocation in Cholestatic Rats. Am J Pathol. 2015;185(2):409–419. doi: 10.1016/j.ajpath.2014.10.009 [DOI] [PubMed] [Google Scholar]
- 32.Maruta K, Takajo T, Akiba Y, et al. GLP-2 Acutely Prevents Endotoxin-Related Increased Intestinal Paracellular Permeability in Rats. Dig Dis Sci. 2020;65(9):2605–2618. doi: 10.1007/s10620-020-06097-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Banerjee M, Robbins D, Chen T. Targeting xenobiotic receptors PXR and CAR in human diseases. Drug Discov Today. 2015;20(5):618–628. doi: 10.1016/j.drudis.2014.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martínez-Augustin O, Medina FS de. Intestinal bile acid physiology and pathophysiology. World J Gastroenterol. 2008;14(37):5630. doi: 10.3748/wjg.14.5630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boesjes M, Brufau G. Metabolic Effects of Bile Acids in the Gut in Health and Disease. Curr Med Chem. 2014;21(24):2822–2829. doi: 10.2174/0929867321666140303142053 [DOI] [PubMed] [Google Scholar]
- 36.Yasuda H, Hirata S, Inoue K, Mashima H, Ohnishi H, Yoshiba M. Involvement of membrane-type bile acid receptor M-BAR/TGR5 in bile acid-induced activation of epidermal growth factor receptor and mitogen-activated protein kinases in gastric carcinoma cells. Biochem Biophys Res Commun. 2007;354(1):154–159. doi: 10.1016/j.bbrc.2006.12.168 [DOI] [PubMed] [Google Scholar]
- 37.Pellicciari R, Sato H, Gioiello A, et al. Nongenomic Actions of Bile Acids. Synthesis and Preliminary Characterization of 23- and 6,23-Alkyl-Substituted Bile Acid Derivatives as Selective Modulators for the G-Protein Coupled Receptor TGR5. J Med Chem. 2007;50(18):4265–4268. doi: 10.1021/jm070633p [DOI] [PubMed] [Google Scholar]
- 38.Jain AK, Wen JX, Arora S, et al. Validating hyperbilirubinemia and gut mucosal atrophy with a novel ultramobile ambulatory total parenteral nutrition piglet model. Nutr Res. 2015;35(2):169–174. doi: 10.1016/j.nutres.2014.12.004 [DOI] [PubMed] [Google Scholar]
- 39.Wu S, Powell J, Mathioudakis N, Kane S, Fernandez E, Sears CL. Bacteroides fragilis Enterotoxin Induces Intestinal Epithelial Cell Secretion of Interleukin-8 through Mitogen-Activated Protein Kinases and a Tyrosine Kinase-Regulated Nuclear Factor-κB Pathway. Infect Immun. 2004;72(10):5832–5839. doi: 10.1128/IAI.72.10.5832-5839.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yoon YM, Lee JY, Yoo D, et al. Bacteroides fragilis Enterotoxin Induces Human β-Defensin-2 Expression in Intestinal Epithelial Cells via a Mitogen-Activated Protein Kinase/IκB Kinase/NF-κB-Dependent Pathway. Infect Immun. 2010;78(5):2024–2033. doi: 10.1128/IAI.00118-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu S, Lim K-C, Huang J, Saidi RF, Sears CL. Bacteroides fragilis enterotoxin cleaves the zonula adherens protein, E-cadherin. Proc Natl Acad Sci. 1998;95(25):14979–14984. doi: 10.1073/pnas.95.25.14979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Remacle AG, Shiryaev SA, Strongin AY. Distinct Interactions with Cellular E-Cadherin of the Two Virulent Metalloproteinases Encoded by a Bacteroides fragilis Pathogenicity Island. McDowell A, ed. PLoS One. 2014;9(11):e113896. doi: 10.1371/journal.pone.0113896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Green R, Beier D, Gollan J. Regulation of hepatocyte bile salt transporters by endotoxin and inflammatory cytokines in rodents. Gastroenterology. 1996;111(1):193–198. doi: 10.1053/gast.1996.v111.pm8698199 [DOI] [PubMed] [Google Scholar]
- 44.Alrefai WA, Gill RK. Bile Acid Transporters: Structure, Function, Regulation and Pathophysiological Implications. Pharm Res. 2007;24(10):1803–1823. doi: 10.1007/s11095-007-9289-1 [DOI] [PubMed] [Google Scholar]
- 45.Trauner M, Arrese M, Lee H, Boyer JL, Karpen SJ. Endotoxin downregulates rat hepatic ntcp gene expression via decreased activity of critical transcription factors. J Clin Invest. 1998;101(10):2092–2100. doi: 10.1172/JCI1680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lichtman SN, Keku J, Schwab JH, Sartor RB. Hepatic injury associated with small bowel bacterial overgrowth in rats is prevented by metronidazole and tetracycline. Gastroenterology. 1991;100(2):513–519. doi: 10.1016/0016-5085(91)90224-9 [DOI] [PubMed] [Google Scholar]
- 47.Freund HR, Muggia-Sullam M, LaFrance R, Enrione EB, Popp MB, Bjornson HS. A possible beneficial effect of metronidazole in reducing TPN-associated liver function derangements. J Surg Res. 1985;38(4):356–363. doi: 10.1016/0022-4804(85)90049-6 [DOI] [PubMed] [Google Scholar]
- 48.Koga H, Sakisaka S, Yoshitake M, et al. Abnormal accumulation in lipopolysaccharide in biliary epithelial cells of rats with self-filling blind loop. Int J Mol Med. 2002;9(6):621–626. http://www.ncbi.nlm.nih.gov/pubmed/12011979. [PubMed] [Google Scholar]
- 49.Whiting J Tumor necrosis factor-alpha decreases hepatocyte bile salt uptake and mediates endotoxin-induced cholestasis*1, *2. Hepatology. 1995;22(4):1273–1278. doi: 10.1016/0270-9139(95)90639-8 [DOI] [PubMed] [Google Scholar]
- 50.Zheng Y, Tam Y, Coutts R. Endotoxin and cytokine released during parenteral nutrition. J Parenter Enter Nutr. 2004;28(3):163–168. doi: 10.1177/0148607104028003163 [DOI] [PubMed] [Google Scholar]
- 51.Lehmann GL, Carreras FI, Soria LR, Gradilone SA, Marinelli RA. LPS induces the TNF-α-mediated downregulation of rat liver aquaporin-8: role in sepsis-associated cholestasis. Am J Physiol Liver Physiol. 2008;294(2):G567–G575. doi: 10.1152/ajpgi.00232.2007 [DOI] [PubMed] [Google Scholar]
- 52.Ridlon JM, Kang D-J, Hylemon PB. Bile salt biotransformations by human intestinal bacteria. J Lipid Res. 2006;47(2):241–259. doi: 10.1194/jlr.R500013-JLR200 [DOI] [PubMed] [Google Scholar]
- 53.Park M-Y, Kim SJ, Ko EK, Ahn S-H, Seo H, Sung M-K. Gut microbiota-associated bile acid deconjugation accelerates hepatic steatosis in ob/ob mice. J Appl Microbiol. 2016;121(3):800–810. doi: 10.1111/jam.13158 [DOI] [PubMed] [Google Scholar]
- 54.Ridlon JM, Kang DJ, Hylemon PB, Najaj JS. Bile Acids and the Gut Microbiome. Curr Opin Gastroenterol. 2014;30(3):332–338. doi: 10.1097/MOG.0000000000000057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ramirez-Perez O, Cruz-Ramon V, Chinchilla-Lopez P, Mendez-Sanchez N. The Role of the Gut Microbiota in Bile Acid Metabolism. Ann Hepatol. 2017;16(Suppl. 1):s15–s20. [DOI] [PubMed] [Google Scholar]
- 56.Fiorucci S, Di Giorgio C, Distrutti E. Obeticholic Acid: An Update of Its Pharmacological Activities in Liver Disorders. Handb Exp Pharmacol. 2019;(256):283–295. doi: 10.1007/164_2019_227 [DOI] [PubMed] [Google Scholar]
- 57.Fiorucci S, Distrutti E. Bile Acid-Activated Receptors, Intestinal Microbiota, and the Treatment of Metabolic Disorders. Trends Mol Med. 2015;21(11):702–714. doi: 10.1016/j.molmed.2015.09.001 [DOI] [PubMed] [Google Scholar]
- 58.Cipriani S, Mencarelli A, Chini MG, et al. The Bile Acid Receptor GPBAR-1 (TGR5) Modulates Integrity of Intestinal Barrier and Immune Response to Experimental Colitis. Ryffel B, ed. PLoS One. 2011;6(10):e25637. doi: 10.1371/journal.pone.0025637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Duboc H, Taché Y, Hofmann AF. The bile acid TGR5 membrane receptor: From basic research to clinical application. Dig Liver Dis. 2014;46(4):302–312. doi: 10.1016/j.dld.2013.10.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Keitel V, Donner M, Winandy S, Kubitz R, Häussinger D. Expression and function of the bile acid receptor TGR5 in Kupffer cells. Biochem Biophys Res Commun. 2008;372(1):78–84. doi: 10.1016/j.bbrc.2008.04.171 [DOI] [PubMed] [Google Scholar]
- 61.Huang H, Lei H, Yang F, Fan X, Dang Q, Li Y. Activation of the bile acid receptor GPBAR1 (TGR5) ameliorates interleukin-1β (IL-1β)- induced chondrocytes senescence. Biomed Pharmacother. 2018;106:1713–1719. doi: 10.1016/j.biopha.2018.06.154 [DOI] [PubMed] [Google Scholar]
- 62.Guo C, Chen W-D, Wang Y-D. TGR5, Not Only a Metabolic Regulator. Front Physiol. 2016;7. doi: 10.3389/fphys.2016.00646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pradelli L, Mayer K, Klek S, et al. ω‐3 Fatty-Acid Enriched Parenteral Nutrition in Hospitalized Patients: Systematic Review With Meta-Analysis and Trial Sequential Analysis. J Parenter Enter Nutr. 2020;44(1):44–57. doi: 10.1002/jpen.1672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Al-Goblan A, Alalfi M, Khan M. Mechanism linking diabetes mellitus and obesity. Diabetes, Metab Syndr Obes Targets Ther. 2014;4(7):587–591. doi: 10.2147/DMSO.S67400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lim DW, Turner JM, Wales PW. Emerging Piglet Models of Neonatal Short Bowel Syndrome. J Parenter Enter Nutr. 2015;39(6):636–643. doi: 10.1177/0148607114554621 [DOI] [PubMed] [Google Scholar]
- 66.Costa S, Iannotta R, Maggio L, Barone G, Serrao F, Vento G. Fish oil-based lipid emulsion in the treatment of parenteral nutrition-associated cholestasis. Ital J Pediatr. 2018;44(1):101. doi: 10.1186/s13052-018-0539-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Moreau RA, Nyström L, Whitaker BD, et al. Phytosterols and their derivatives: Structural diversity, distribution, metabolism, analysis, and health-promoting uses. Prog Lipid Res. 2018;70:35–61. doi: 10.1016/j.plipres.2018.04.001 [DOI] [PubMed] [Google Scholar]
- 68.Buchman A Total Parenteral Nutrition-Associated Liver Disease. J Parenter Enter Nutr. 2002;26(5_suppl):S43–S48. doi: 10.1177/014860710202600512 [DOI] [PubMed] [Google Scholar]
- 69.Högler W, Baumann U, Kelly D. Growth and bone health in chronic liver disease and following liver transplantation in children. Pediatr Endocrinol Rev. 2010;7(3):266–274. http://www.ncbi.nlm.nih.gov/pubmed/20526240. [PubMed] [Google Scholar]
- 70.Brunt EM. Histopathology of nonalcoholic fatty liver disease. World J Gastroenterol. 2010;16(42):5286. doi: 10.3748/wjg.v16.i42.5286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Singer C, Stancu P, Coşoveanu S, Botu A. Non-alcoholic Fatty liver disease in children. Curr Heal Sci J. 2014;40(3):170–176. doi: 10.12865/CHSJ.40.03.03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schwimmer JB, Johnson JS, Angeles JE, et al. Microbiome Signatures Associated With Steatohepatitis and Moderate to Severe Fibrosis in Children With Nonalcoholic Fatty Liver Disease. Gastroenterology. 2019;157(4):1109–1122. doi: 10.1053/j.gastro.2019.06.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Noureddin M, Zhang A, Loomba R. Promising therapies for treatment of nonalcoholic steatohepatitis. Expert Opin Emerg Drugs. 2016;21(3):343–357. doi: 10.1080/14728214.2016.1220533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Konerman MA, Jones JC, Harrison SA. Pharmacotherapy for NASH: Current and emerging. J Hepatol. 2018;68(2):362–375. doi: 10.1016/j.jhep.2017.10.015 [DOI] [PubMed] [Google Scholar]
- 75.Villalona G, Price A, Blomenkamp K, et al. No Gut No Gain! Enteral Bile Acid Treatment Preserves Gut Growth but Not Parenteral Nutrition-Associated Liver Injury in a Novel Extensive Short Bowel Animal Model. J Parenter Enter Nutr. 2018;42(8):1238–1251. doi: 10.1002/jpen.1167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kumar J, Teckman J. Controversies in the Mechanism of Total Parenteral Nutrition Induced Pathology. Children. 2015;2(3):358–370. doi: 10.3390/children2030358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Guthrie G, Stoll B, Chacko S, Lauridsen C, Plat J, Burrin D. Rifampicin, not vitamin E, suppresses parenteral nutrition-associated liver disease development through the pregnane X receptor pathway in piglets. Am J Physiol - Gastrointest Liver Physiol. 2020;318(1):G41–G52. doi: 10.1152/AJPGI.00193.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ng K, Stoll B, Chacko S, et al. Vitamin E in New-Generation Lipid Emulsions Protects Against Parenteral Nutrition–Associated Liver Disease in Parenteral Nutrition–Fed Preterm Pigs. J Parenter Enter Nutr. 2016;40(5):656–671. doi: 10.1177/0148607114567900 [DOI] [PMC free article] [PubMed] [Google Scholar]


