Abstract
The evolutionarily ancient methoxyindoleamine, melatonin, has long perplexed investigators by its versatility of functions and mechanisms of action, which include the regulation of vertebrate pigmentation. Although first discovered through its potent skin-lightening effects in amphibians, melatonin’s role in human skin and hair follicle pigmentation and its impact on melanocyte physiology remain unclear. Synthesizing our limited current understanding of this role, we specifically examine its impact on melanogenesis, oxidative biology, mitochondrial function, melanocyte senescence, and pigmentation-related clock gene activity, with emphasis on human skin, yet without ignoring instructive pointers from non-human species. Given the strict dependence of melanocyte functions on the epithelial microenvironment, we underscore that melanocyte responses to melatonin are best interrogated in a physiological tissue context.
Current evidence suggests that melatonin and some of its metabolites inhibit both, melanogenesis (via reducing tyrosinase activity) and melanocyte proliferation by stimulating melatonin membrane receptors (MT1, MT2). We discuss whether putative melanogenesis-inhibitory effects of melatonin may occur via activation of Nrf2-mediated PI3K/AKT signaling, estrogen receptor-mediated and/or melanocortin-1 receptor- and cAMP-dependent signaling, and/or via melatonin-regulated changes in peripheral clock genes that regulate human melanogenesis, namely Bmal1 and Per1. Melatonin and its metabolites also accumulate in melanocytes where they exert net cyto- and senescence-protective as well as anti-oxidative effects by operating as free radical scavengers, stimulating the synthesis and activity of ROS scavenging enzymes and other antioxidants, promoting DNA repair, and enhancing mitochondrial function.
We argue that it is clinically and biologically important to definitively clarify whether melanocyte cell culture-based observations translate into melatonin-induced pigmentary changes in a physiological tissue context, i.e., in human epidermis and hair follicles ex vivo, and are confirmed by clinical trial results. After defining major open questions in this field, we close by suggesting how to begin answering them in clinically relevant, currently available preclinical in situ research models.
INTRODUCTION
The ancient amphipathic indolamine, melatonin, which is believed to exist in all living organisms on Earth1,2, not only is the key neurohormone that regulates the circadian clock3 and antioxidant activities4,5, but also an astonishingly versatile molecule with a plethora of other complex biological functions6-8 (Table 1). Dermatologist Aaron B. Lerner discovered melatonin as the active molecule that exerts potent skin lightening effects on amphibian melanophores9. Since then, melatonin has long captured the attention of skin biologists and investigative dermatologists for its multitude of documented activities, many of which are relevant to human skin physiology and pathology, ranging from anti-aging10-13, UV-protection14-17, immunomodulation18,19, and anti-melanoma activity21-23 to potential hair growth-promoting24,25 and pigmentation-modulatory effects25-27.
Table 1.
A selection of recognized melatonin functions
| Melatonin functions: Examples | References |
|---|---|
| Aerobic glycolysis inhibition (glycolytic) | 202 |
| Anti-aging | 11,114,203 |
| Anti-inflammatory | 204-206 |
| Anti-melanoma: anti-proliferative and anti-invasive effects | 21,23,207,208 |
| Anti-neoplastic: anti-proliferative and cell cycle arrest | 209 |
| Antioxidant | 190,210 |
| Blood pressure regulation | 211 |
| Body mass regulation | 212 |
| Bone mass regulation | 213,214 |
| Cardioprotection | 211,215 |
| Circadian rhythm regulation | 3,6 |
| DNA repair | 15 |
| Dopaminergic neuron development in the substantia nigra | 216 |
| Estrogen receptor regulation | 49 |
| Gastrointestinal tract protection | 214 |
| Immune cell proliferation and cytokine release | 18,19 |
| Inner ear protection | 217 |
| Insulin secretion regulation | 218 |
| Liver disease protection | 219 |
| Mitochondrial function and biogenesis | 220 |
| Nephroprotection | 221 |
| Neuroprotection | 222 |
| Retina protection | 223,224 |
| Reproduction and sexual maturation regulation | 225,226 |
| Sensitization of cancers to radiation and chemotherapy | 227-231 |
| UV protection | 98,145,185,190,232,233 |
| Wound healing | 234 |
Yet, it remains unclear how exactly intracutaneously synthesized melatonin impacts on human skin and hair follicle pigmentation in situ and in vivo, how it affects other human melanocyte functions, and whether it protects these melanocytes from damage and/or senescence in situ. Even less is known about the relative contribution of insufficient melatonin synthesis and/or melatonin receptor expression in human skin in the context of melanocyte and skin pathology. Moreover, it is not yet entirely clear which of melatonin’s receptors or pathways mediate each of its functional effects in human skin, which receptors/pathways are involved in its modification of mitochondria and cellular metabolism, or which nuclear receptors are plausible candidates for its bioregulation. To a considerable extent, this may be owed to the fact that the bulk of published melatonin studies have utilized cell culture methodology or animal models, while human skin and hair follicle organ culture has been under-employed, even though these assays would have been most instructive from a physiological perspective. Additionally, melatonin’s mechanisms of actions in human skin are very complex and have not been dissected as systematically as desired, perhaps due to few investigative dermatologists conducting such research in the past decades, limited industry and NIH funding, and challenging requirements (e.g., ethical rules and IRB approval) for conducting experiments with humans.
Therefore, the current review re-explores the role of melatonin in human melanocyte physiology. We argue that it is timely and both clinically important and biologically instructive to now clarify definitively whether the previously reported observations regarding melatonin’s effects on isolated human melanocytes in vitro (see below) really translate to a human tissue context, i.e. where melanocyte activities are closely controlled by their intimate interactions with epidermal or hair follicle (HF) keratinocytes within the epidermal26,28,29 or HF pigmentary unit (EPU, HFPU)30-32.
This tissue context-perspective on human melanocyte biology is critically important, but too often ignored. Besides several pigmentation-regulatory growth factors, cytokines, and eicosanoids, keratinocytes produce and secrete major pigmentation-stimulatory neurohormones, such as α-melanocyte stimulating hormone (MSH), adrenocorticotropin (ACTH), corticotropin-releasing hormone (CRH) and thyroid-releasing hormone (TRH)18,19-23 and rigorously control melanocyte functions through interactions with corresponding G-protein coupled receptors26,39,40 and by regulating E- and P-cadherin expression on their cell surface41,42.
With recent insights into the impact of neurotransmitters (e.g. acetylcholine) released by sympathetic nerve fibers innervating the bulge, as in murine HF melanocyte stem cells43, a tissue context-dominated perspective on examining the role of melatonin in human pigmentation and melanocyte physiology has become even more important, but also more complex. Such a tissue context perspective must include the skin mesenchyme, since additional inputs on pigmentation originate from dermal fibroblasts in the human EPU39,40, inductive fibroblasts in the HF’s dermal papilla, and perifollicular dermal white adipose tissue which secrete HFPU- and melanogenesis-stimulatory hepatocyte growth factor46,47. These mesenchymal inputs rhythmically switch HF pigmentation on and off in a strictly hair cycle-dependent manner, with induction of HF melanocyte apoptosis during each phase of HF regression (catagen) and reconstruction of a new HFPU during each re-entry into the phase of active hair growth (anagen) from resident progenitor cells31,48. This dramatic remodeling of the HFPU, the cyclic mesodermal-neuroectodermal interactions that govern it, and the rhythmic extrapineal synthesis of melatonin within human HFs49 make the HFPU a fascinating and instructive model system for exploring the impact of melatonin on the complex controls of human hair pigmentation, which contrasts against the much less dynamic, constantly active EPU. This also illustrates why one cannot expect to recreate such a complex and dynamic cell-cell interaction system in melanocyte cell culture.
Importantly, melatonin and its precursors, serotonin and N-acetylserotonin (NAS), are synthesized within mammalian skin17,50-52, explicitly also in human skin, HFs, and resident cell populations of human epidermis or dermis52,53. Furthermore, the entire biochemical machinery necessary for transforming L-tryptophan into melatonin is expressed in all main tissue compartments of human skin, by epidermal and HF keratinocytes and melanocytes, dermal fibroblasts, and even mast cells17,53-55.
Since melatonin synthesis, metabolism, signal transduction (Figure 1), target genes, and mechanisms of action have been extensively reviewed elsewhere8,56-60, it suffice to summarize here some salient features. Melatonin is synthesized in a multistep process from tryptophan60 in the pineal gland and numerous “non-classical”, extrapineal tissues in the human body such as skin and HFs (see below)25,40, also in intact, wild-type rodent (including mice) skin14. While one key step in the classical (intrapineal) pathway of melatonin synthesis involves the conversion of serotonin to N-acetyl-serotonin (NAS, obligatory precursor to melatonin) by aralkylamine N-acetyltransferase (AANAT), there exists an alternative pathway operating in peripheral organs , such as that found in the AANAT-mutant C57BL/6 mouse strain61. Serotonin within C57BL/6 mice skin can instead be acetylated to NAS by arylamine N-acetyltransferase (NAT)61, which can then be transformed to melatonin by the enzyme common to both classical and alternative pathways, hydroxyindole-O-methyltransferase (HIOMT)52. Therefore, it is misleading to characterize C57BL/6 mice as a ‘natural melatonin knockdown’ species14,49,61.
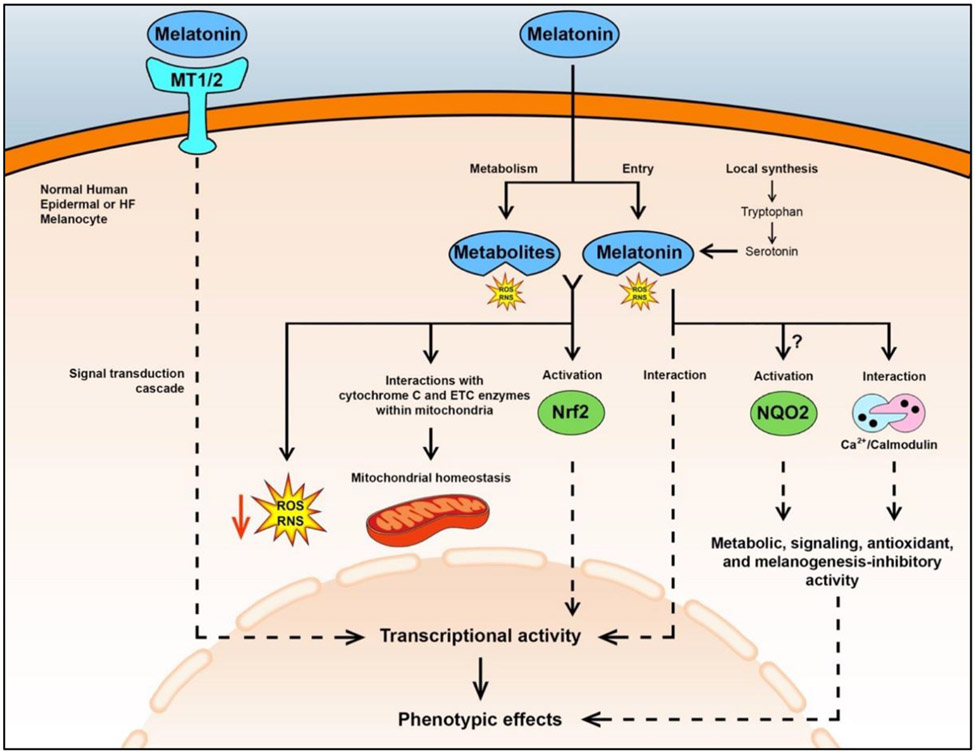
Figure 1. Schematic summary of melatonin’s effects in human epidermal and HF melanocytes.
Exogenous or endogenously synthesized melatonin can regulate phenotype in these cells through interactions with membrane-bound MT1/2 receptors NQO2, and the calcium/calmodulin complex or through stimulation of Nrf2 (reviewed in56). However, it is not fully understood if melatonin activates NQO2, a detoxifying enzyme7,261,262 Noteworthy phenotypic effects of melatonin include melanogenesis inhibition, and stimulation of DNA repair, and expression and activity of antioxidant enzymes (e.g., superoxide dismutase and catalase) (reviewed in8). Melatonin may also be transported to different subcellular compartments, but the detailed mechanism is not fully understood25. Furthermore, melatonin can be synthesized within these melanocytes. Melatonin and its metabolites, such as cyclic-3-hydroxymelatonin (C-3HOM) and N-acetyl-5-methoxyknuramine (AMK), directly scavenge ROS/RNS125,126 and help maintain mitochondrial homeostasis through interactions with cytochrome C and enzymes of the electron transport chain. Specifically, cytochrome C within mitochondria is thought to be involved in the conversion of melatonin to its potent antioxidant metabolite, N(1)-acetyl-N(2)-formyl-5-methoxykynuramine (AFMK)158, and its secondary product, AMK, when in the presence of hydrogen peroxide101. Also, melatonin may interact with cytochrome C and electron transport chain (ETC) enzymes within mitochondria to promote mitochondrial homeostasis and decrease free radical formation160. Furthermore, melatonin may affect the transcription of peripheral clock genes Bmal1 and Per1 with alterations in melanogenesis and other melanocyte activities27,123,181. Direct effects are shown by solid lines and multiple reactions and signaling are shown by dashed lines. Melatonin receptors 1 and 2 (MT1/2); hair follicle (HF); reactive oxygen species (ROS); reactive nitrogen species (RNS); nuclear factor erythroid 2-related factor 2 (Nrf2); N-Ribosyldihydronicotinamide:Quinone Reductase 2 (NQO2).
Due to melatonin’s amphiphilic nature, it can readily penetrate any cell, tissue and cellular compartment62-65. Here, melatonin exerts its complex effects dependent on the expression, localization and types of melatonin receptors involved66,67, i.e. the cell membrane-bound, G-protein coupled MT1 and MT2 receptors, and on several membrane-bound receptor-independent mechanisms68 (Figure 1). It has been clarified that melatonin and its metabolites are not ligands for the nuclear receptor retinoid-related orphan receptor-α (ROR-α aka NR1F1), as shown by crystallography studies69,70, modeling and receptor functional assays71. However, melatonin may indirectly modulate ROR-α and other ROR activities70,72.
Moreover, facilitated by their dendritic morphology, which greatly augments their cell surface and thus contact area, melanocytes operate as multimodal sensory and stress-response cells73-77. Melanocytes also engage in bidirectional communication with their tissue-specific intraepithelial habitat73, for example, by secreting catecholamines, cytokines, eicosanoids, acetylcholine, melanocortins, ACTH, CRH, endorphins, enkephalins, nitric oxide, serotonin, and reactive oxygen species produced during melanogenesis17,36,51,78-90. Thus, melanocytes contribute actively to shaping the signaling and metabolic milieu they reside in74,76. Transfer of melanosomes into keratinocytes likely promotes keratinocyte terminal differentiation and other functions39,54,55. Recently, aging melanocytes have even been reported to act as drivers of epidermal senescence91.
Taken together, the crucial tissue context in which melanocytes operate renders it impossible to fully grasp how melatonin regulates human pigmentation under mere cell culture conditions, even when primary human melanocytes are co-cultured with selected isolated other cell populations, since even such co-culture system cannot recapitulate the complexity of physiological interactions between neural crest-derived, epithelial and mesenchymal cells that control pigmentation in situ73-75.
This review synthesizes the currently available evidence regarding melatonin’s effect on melanogenesis, oxidative biology and damage responses, senescence, and peripheral clock genes in the wider context of human melanocyte physiology within their cutaneous habitat. These include conditions of excessive oxidative stress, which underly melanocyte pathology, e.g. in vitiligo92,93 and hair graying30,94,95. We define major open questions, suggest how to answer them using currently available preclinical assay systems, and discuss the clinical relevance of systematically characterizing the role of melatonin in human melanocyte function in health and disease from a tissue context perspective.
SKIN AS A TARGET AND SOURCE OF MELATONIN BIOACTIVITY
Human skin possesses all key enzymes, substrates, and cofactors necessary for melatonin synthesis51,52, and melatonin synthesis in human scalp HFs ex vivo is stimulated by noradrenaline, just as in the pineal gland49. Given that this key psychoemotional stress-associated neurotransmitter can promote the depletion of melanocytes stem cells from their niche in murine HFs43, one wonders whether noradrenaline-induced up-regulation of HF melatonin synthesis simultaneously activates melatonin-dependent cytoprotective mechanisms (see below). Importantly, both keratinocytes and melanocytes of the EPU can also synthesize catecholamines96,97 and thereby could, in theory, augment their own melatonin synthesis in an autocrine and paracrine manner, possibly in response to local tissue stressors.
Human skin and HFs also are important targets of melatonin bioactivity and express melatonin receptors (MT1/2)35,58,81. Animal studies involving pinealectomy or melatonin administration have demonstrated changes in HF growth, cycling, and pigmentation (Table 2). The latter has raised the question how exactly melatonin affects human epidermal and HF melanocytes17,24,48 within their natural tissue habitat, rather than in culture isolated from their key communications with epidermal and HF keratinocytes, papillary dermal fibroblasts99, and HF dermal papilla fibroblasts30,31. Yet, dissecting how exactly endogenous melatonin alters human melanocyte biology in situ is challenged by melatonin’s complex interactions and rapid metabolism100,101, which make it exceptionally difficult to dissect precisely which phenotypic effects are regulated by melatonin itself versus its many metabolites, as well as by differential effects dependent on dose, cellular and hormonal environment, tissue, species, gender, age, race, and external (environmental) and internal stress levels24,98,102,103. Therefore, the results from in vitro and animal studies on the pigmentary impact of melatonin could be misleading as they cannot fully reflect the human in vivo condition. It is for this reason that we advocate the use of standardized, site- and gender-specific human skin and hair follicle organ culture models to definitively clarify the effects of melatonin on human skin pigmentation in UV-exposed versus non-exposed skin.
Table 2.
Effects of melatonin on hair growth and pigmentation
| Growth | ||
|---|---|---|
| Species | Effect | References |
| Mouse | Influence on the hair cycle by the pineal gland | 235 |
| Weasel | Induction of molt | 236 |
| Mink | Induction of autumn molt | 237 |
| Soay rams | Stimulation of molting | 238 |
| Limousine ram | Increased HF activity and reduced prolactin plasma levels | 239 |
| Mink | Induction of winter fur growth (supposedly by inhibition of prolactin) | 240 |
| Cashmere goat | Increase of growth initializing activity of secondary HFs in springtime | 241 |
| Red deer | Premature molting of summer pelage and reduced serum prolactin concentrations | 242 |
| Merino sheep | No influence of pinealectomy on wool growth and hair density | 243 |
| New Zealand goat | Induction of pro-anagen phase | 244 |
| Cashmere goat (cultured HFs) | Increase of hair shaft elongation and DNA-synthesis | 245 |
| Domestic pig | Increase of pelage development and cycle frequency | 246 |
| Ferret | Earlier change of winter and consecutive spring coat | 247 |
| Raccoon dogs | More rapid shedding of mature underfur hairs and growth of new underfur hairs; suppression of prolactin levels | 248 |
| Siberian Husky dogs | No change in hair growth or anagen rate (topical administration) | 249 |
| Rex Rabbit offspring | Maternal melatonin supplementation increased HF density, reduced hairiness, and improved fur quality of offspring | 250 |
| Cashmere goat | Continuous subcutaneous implantation of melatonin promoted cashmere to enter the anagen 2 months earlier and induce secondary hair follicle development. | 251 |
| Human (cultured HFs) | Increase of hair shaft elongation (30 μM); Decrease of hair shaft elongation (1–5 mM) | 252 |
| Human (cultured HFs) | No influence on hair shaft elongation, matrix keratinocyte proliferation/apoptosis and hair cycling (10−12–10−6 M) | 49 |
| Human (trichograms) | Slight increase of anagen hair rate in women with androgenetic and diffuse alopecia | 253 |
| Human (clinical assessment) | Topical melatonin loaded in antioxidant nanostructured lipid carriers significantly increased hair density and hair shaft diameter when compared to topical melatonin alone in men with androgenetic alopecia | 198 |
| Pigmentation | ||
| Species | Effect | References |
| Weasel | Induction of hair color change | 236 |
| Mammalians | Effects on hair color | 254 |
| Djungarian hamster | Pattern of melatonin release induced by experimentally induced photoperiods modifies molt into summer pelage | 255 |
| Siberian hamster (cultured HFs) | Post-tyrosinase inhibition of melanogenesis (10−10–10−6 M) | 256 |
| Yellow mice (C3H/He-A*vy) | Slight reduction of coat darkening | 257 |
| Mountain hares | Season-dependent effects of melatonin on fur color | 258 |
| Djungarian hamster | Induction of the winter molt and pelage color change | 259 |
| Djungarian hamster | Change of fur color | 260 |
| Mouse | Inhibition of melanogenesis | 117 |
| Human (cultured HFs) | No effect on pigmentation (10−12–10−6 M) | 49 |
Hair follicle (HF).
CLINICAL POINTERS
Clinical observations provide important pigmentary background information when interpreting in vitro and animal results under melatonin administration. Few studies have examined the effects of melatonin on pigmentation in humans without pigmentary disorders. In both former- and never-smoker postmenopausal women who received microdermabrasion, neither oral (2.5 mg/day) nor topical (0.5 mM) melatonin had significant effects on skin pigmentation104. Another pilot study saw no effects of oral melatonin on arm, leg, or back skin pigmentation of seven subjects105. This could have resulted from an insufficiently short observation period or the rapid metabolism of orally delivered melatonin upon liver passage.
There is an extreme scarcity of any documented potential cutaneous effects from the extensive ingestion of melatonin. This, in part, is likely best explained by ingested melatonin’s extensive and rapid metabolism during its first pass through the liver, where it is rapidly hydroxylated to 6-hydroxymelatonin with further sulfation or glucuronidation before reaching the skin. These biochemical modifications minimize the impact of orally administered melatonin on human skin function. Therefore, to see cutaneous effects of melatonin, it is best for it to be synthesized in situ or applied topically.
Very few studies have investigated melatonin effects on human pigmentation disorders. In one patient with adrenal hyperplasia-associated diffuse skin hyperpigmentation, a month of high-dose (1 g/day) oral melatonin decreased skin pigmentation, yet failed to alter skin pigmentation in three other patients with idiopathic hyperpigmentation and one patient with Addison’s disease106. In a small cohort of patients with acanthosis nigricans, oral melatonin (3 mg/day) reportedly reduced hyperpigmentation107. In patients with melasma, topical (5% cream) and oral (3 mg/day) melatonin reportedly showed significant skin-lightening effects108. Other studies also reported decreased skin pigmentation and enhanced protection against photoaging after topical melatonin15,25,109,110. These limited clinical observations suggest that melatonin may exert (direct or indirect) melanogenesis-inhibitory activities in human epidermis in vivo, yet conclusive evidence remains to be provided.
Interestingly, patients with vitiligo had significantly lower immunohistochemically-assessed melatonin-associated immunoreactivity in both lesioned and non-lesioned skin when compared to skin of heathy controls, suggesting a role for melatonin deficiency in the pathogenesis of vitiligo111. However, serotonin, 5-hydroxyindoleacetic acid (5-HIAA) and melatonin serum levels have been reported to be increased in a relatively small cohort of vitiligo patients112. Yet, the pathobiological significance and therapeutic potential of melatonin in vitiligo113 remains unexplored and requires systematic additional investigation.
Serum melatonin levels decline with age10, which may contribute to the slow decline of organ function characteristic of aging114. Thus it is conceivable that a gradual loss of melatonin in aged or graying HFs along with an age-dependent accumulation of oxidative damage in the HFPU and correspondingly reduced oxidative damage protection of HF melanocytes by both systemic and intrafollicularly-produced melatonin levels contributes to hair graying30.
DIRECT IMPACT OF MELATONIN ON MELANOGENESIS
(1). Melatonin and its metabolites tend to inhibit melanogenesis, tyrosinase activity, and/or melanocyte proliferation in vitro
In normal human epidermis, melatonin and its metabolites, e.g., N1-Acetyl-5-Methoxykynuramine (AMK), N(1)-acetyl-N(2)-formyl-5-methoxykynuramine (AFMK), 6-hydroxymelatonin (6-OHM), and 5-methoxytryptamine (5-MT), accumulate in vivo103,115. In human epidermal melanocytes in vitro, melatonin and its metabolites inhibit melanocyte proliferation103 and some103 but not all115 metabolites inhibit melanogenesis by decreasing tyrosinase activity. Of all melatonin’s metabolites, 6-OHM showed the greatest inhibition (50%) of tyrosinase activity in normal human epidermal melanocytes in vitro103. In contrast, melatonin and its metabolites had no effect on melanogenesis in human SKMEL-188 melanoma cells, except for 5-MT (at 10 μM) which even stimulated melanogenesis103. Yet, in another human melanoma cell line, MNT-1, melatonin inhibited melanin production at high doses (1, 100, and 1000 μM)116.
Interestingly, in rodent melanoma cells, melatonin at low concentrations (0.1-10 nM) inhibited melanocyte proliferation but had no effect on melanogenesis, while at high concentrations (≥0.1 μM) it inhibited the induction of melanogenesis and tyrosinase activity but not proliferation23. Similarly high doses of melatonin were required to inhibit anagen-associated tyrosinase activity in histocultured skin from C57BL-6 mouse, and two high and low affinity binding sites were detected in crude skin extracts117. These are consistent with high doses of melatonin required for phenotypic effects in normal human epidermal melanocytes103.
Thus, melatonin’s effects on melanogenesis and melanocyte proliferation appear be to be rather variable, dependent on dose, tissue type, species, and signaling environment24,98. This further underscores that understanding the physiological and pharmacological responses of human melanocytes to melatonin stimulation is best studied in a full-thickness human skin or HF organ culture (ex vivo) or in vivo, rather than in cultured isolated melanocytes (in vitro).
(2). Mechanisms of melatonin regulation of human melanogenesis
Besides targeting its specific membrane receptors on melanocytes, in human skin, several indirect or non-classical mechanisms by which melatonin may regulate melanogenesis deserve consideration. For example, melatonin can downregulate estrogen receptor expression in mouse HFs49, which could antagonize 17-ß-estradiol’s stimulatory effects on melanogenesis118. However, at ≤1 μM melatonin did not inhibit intrafollicular melanin synthesis in organ-cultured human anagen VI scalp HFs49. Of note, anti-melanogenic activity of melatonin in rodent melanomas and murine skin organ culture required higher than 1 μM concentration to observe the phenotypic effect23,117.
Though ROR is no longer a credible direct nuclear hormone receptor for melatonin, an indirect modulation of ROR signaling activity by melatonin which could impact on melanogenesis remains theoretically conceivable70. Additional indirect mechanisms of action must also be considered. The observed melanogenesis inhibition by melatonin in Siberian hamster HF melanocytes appears to have antagonized the pigmentation-promoting effects of α-MSH119, likely by reducing expression of cognate α-MSH receptors (MC-1R), as described in mouse melanoma cells120, and/or counteracting the promotion of melanogenesis by α-MSH or L-tyrosine (based on in vitro data from hamster and murine melanoma cells)23. Since specific binding of tritiated-melatonin to purified membrane and nuclei melanocyte fractions were detected23,117, the anti-proliferative effect of melatonin was proposed to be mediated through interaction with MT receptors, while the melanogenesis-inhibitory effect might involve interaction with a putative nuclear receptor.
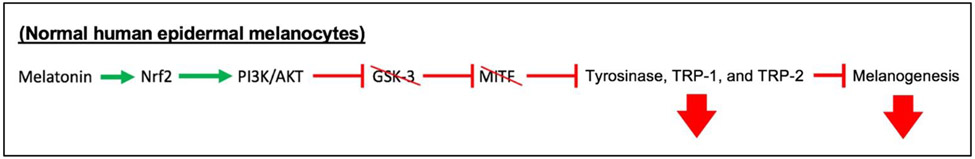
In normal human epidermal melanocytes, the central regulator of oxidative damage responses, nuclear factor erythroid 2-related factor 2 (Nrf2)121,122, is targeted by melatonin to upregulate antioxidant defenses15, but decreases melanogenesis through a pathway involving activation of PI3K/AKT. Activated PI3K/AKT signaling leads to inactivation of glycogen synthase kinase-3 (GSK-3) and microphthalmia-associated transcription factor (MITF), which inhibits the transcription of TYR, TRP-1, and TRP-2 (melanogenesis-associated enzymes) and decreases melanin synthesis123 (Figure 2). Thus, it is conceivable that melatonin may inhibit melanogenesis in normal human epidermal melanocytes also through activation of Nrf2 and subsequent activation of the PI3K/AKT pathway.
Figure 2. Schematic summary describing PI3K/AKT pathway modulation and its effects on melanogenesis.
In normal human melanocytes, melatonin stimulates Nrf215, which can activate the PI3K/AKT pathway to phosphorylate (i.e., inactivate) GSK-3. Without GSK-3, MITF remains unphosphorylated (i.e., inactive), leading to decreased transcription of tyrosinase, TRP-1, and TRP-2, thereby decreasing melanogenesis123.
MELATONIN, OXIDATIVE STRESS, AND THE MELANOCYTE ECOSYSTEM
(1). Melatonin and its metabolites regulate antioxidant enzyme expression and direct free radical scavenging
Melatonin and its metabolites [e.g., cyclic-3-hydroxymelatonin (C-3HOM) and AMK]124 are powerful direct scavengers of reactive oxygen (ROS) and nitrogen species (RNS)125,126 that protect human melanocytes from oxidative damage15. Moreover, once melatonin binds to MT1/2 receptors, the downstream signaling cascade stimulates expression of numerous antioxidant enzymes125,127 (Figure 3). These properties of melatonin may play a key role in maintaining skin15 and HF128 pigmentation, given that melanogenesis itself is a cytotoxic process that generates ROS and quinone and semiquinone compounds, which are buffered by melanin itself as well as by other mechanisms129-131.
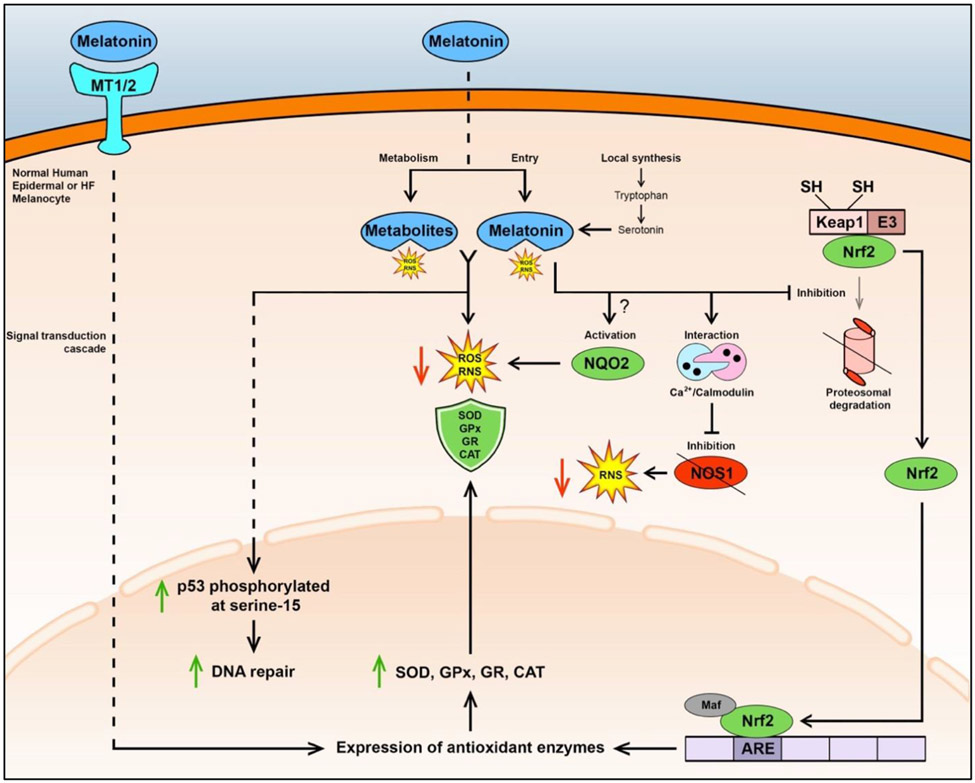
Figure 3. Schematic summary describing antioxidant defense mechanisms by melatonin and its metabolites in human melanocytes.
Melatonin can bind to MT1 and MT2 receptors on the cell membrane, triggering a signaling cascade that leads to expression of antioxidant enzymes (e.g., SOD, GPx, GR, and CAT) for defense against ROS and RNS125,127. Melatonin may also be transported to the cytoplasm, but the detailed mechanism is not fully understood25. Furthermore, melatonin can be synthesized within these melanocytes. Melatonin and its metabolites, such as C-3HOM and AMK, can directly scavenge ROS/RNS125,126. Furthermore, melatonin and its metabolites, including AFMK, 6-OHM, 5-MT, and NAS, protect human epidermal melanocytes from UV-B-induced damage/apoptosis by enhancing phosphorylation of p53 at Serine 15, thereby leading to activated p53 accumulation in the nucleus and stimulation of DNA repair15. Melatonin may activate NQO2, thereby reducing oxidative stress134,135, however this mechanism’s presence in these melanocytes is not fully understood7. Melatonin at concentrations higher than 1 nM within the cell can interact with the calcium/calmodulin complex leading to inhibition of NOS1-mediated generation of RNS, with potential reductions in RNS levels25. Melatonin may also inhibit the Keap1-E3 ligase complex and the ubiquitination and proteasomal degradation of Nrf2, thereby preserving high Nrf2 levels that translocate to the nucleus. In the nucleus, Nrf2 may couple with Maf, a transcription factor, allowing Nrf2 to bind ARE on the promoter region of genes encoding antioxidant enzymes (e.g., SOD and GPx), resulting in their increased expression and activity, which then convert ROS and RNS to unreactive products1. Direct effects are shown by solid lines and multiple reactions and signaling are shown by dashed lines. Reactive oxygen species (ROS); reactive nitrogen species (RNS); superoxide dismutase (SOD); glutathione peroxidase (GPx); glutathione reductase (GR); catalase (CAT).
Furthermore, melatonin can activate Nrf2, a transcription factor regarded as the master regulator of antioxidant defenses [e.g., defense against ultraviolet (UV) B radiation-induced oxidative skin damage]15, in part by upregulating its expression in human epidermal melanocytes. Nrf2 is also significantly up-regulated in response to oxidative stress in human anagen HFs, namely in the HFPU132. Interestingly, the melatonin-induced activation of Nrf2 in human epidermal melanocytes exposed to oxidative stress occurs independently of melatonin receptors15, possibly by regulating the Keap/Nrf2/ARE pathway and suppressing the ubiquitin/proteasome system, thereby increasing Nrf2-ARE activation and expression and activity of antioxidant enzymes1,114. Furthermore, Nrf2-ARE activation is necessary for protecting human epidermal melanocytes from hydrogen peroxide- (H2O2) induced oxidative stress133, either by metabolically eliminating ROS or by reducing their generation1, thereby preventing DNA damage and premature senescence11,15.
Melatonin’s activation of NQO2, a detoxifying enzyme that directly reduces H2O2 and dangerous quinones, is another means of defense against oxidative stress134,135, which has not yet been identified in human HF melanocytes. However, NQO2 gene expression occurs widely in human skin7,98, including epidermal melanocytes15 and microarray data point towards its expression in murine HFs136. Also, intracellular melatonin at concentrations higher than 1 nM interacts with the calcium/calmodulin complex, which inhibits nitric oxide synthase 1 (NOS1 or nNOS) and its generation of RNS56,137-139. Furthermore, melatonin and its metabolites protect human epidermal melanocytes from UV-B-induced damage/apoptosis (see (3) below) by enhancing p53-stimulated DNA repair15. The discussed antioxidant mechanisms of melatonin and its metabolites are described in further detail in figure 3.
(2). Decreased melatonin levels related to ageing- or oxidative stress-related hair graying
Loss of human hair pigmentation (e.g., ageing- or stress-related graying) is thought to primarily result from oxidative damage that disrupts differentiated HF melanocytes of the HFPU and melanogenesis-related enzymes, with subsequent damage to HF melanocyte stem cells, eventually determining whether or not greying is reversible30,43,94,95.
A study using murine HFs demonstrated the protective effect that superoxide dismutase (SOD)140, an enzyme involved in melatonin’s antioxidant defense properties125,127, has against hair graying. Also, aged, gray HFs have increased reactivity to reducing and oxidizing agents when exposed to radiation-induced oxidative stress141, decreased antioxidant defense (e.g., decreased CAT activity and expression)142, and increased accumulation of tryptophan143. This invites two melatonin-related hypotheses: (a) decreased enzymatic conversion of tryptophan to melatonin in graying HFs, and/or (b) increased production of tryptophan used to enhance melatonin synthesis for combating oxidative stress within the HFPU.
The fine regulation of redox balance between free radicals and antioxidants is critical for maintaining normal functions in human epidermal129,131 and HF130 melanocytes. Without the melatonin-associated antioxidant defenses, it is possible that human epidermal and HF melanocytes may be substantially more susceptible to oxidative damage that results in cellular dysfunction, such as directly impaired tyrosinase activity by blunting methionine sulfoxide repair95, and apoptosis94,142,145 and. However, the potential association between reduced melatonin levels/expression in aging and graying HFs and other hypopigmentary conditions still needs to be clarified.
(3). Melatonin enhances protection of melanocytes against UV radiation
Oxidative stress generated by UV radiation (UVR) and visible light (VL) has the potential to induce cosmetically unappealing hyperpigmentation146. For this reason, the use of topical and oral antioxidants has become increasingly prevalent as therapy in adjunct to sun protection to prevent UVR- and VL-induced hyperpigmentation146,147. Pronounced photoprotective effects of “natural” and synthetic antioxidants were demonstrated in animal and human studies when applied topically before exposure to UVR, but no protective effects by antioxidants (e.g., melatonin, vitamins) were found by some authors when applied after exposure to UVR147. In contrast, others have demonstrated the protective action of melatonin and metabolites applied directly after UVB exposure15, such as their protection from and reversal of UVB-induced damage in cultured human epidermal melanocytes15. Similar effects were seen for vitamin D derivatives148-150. It must be noted, however, that active forms of vitamin D are more efficient in photoprotection than melatonin150,151.
(4). Melatonin regulates senescence progression and promotes mitochondrial homeostasis
Mitochondria play a vital role in skin and there exists increasing evidence that mitochondrial dysfunction and oxidative stress are key features in senescence and aging skin with direct links to skin and hair ageing phenotypes (e.g., uneven pigmentation and hair graying)30,152,153. Melatonin is found in especially high concentrations in mitochondria154,155, where it is transported to156, synthesized157, or metabolised101. Within mitochondria, cytochrome c converts melatonin to its potent antioxidant metabolite, AFMK158, and its secondary product, AMK in the presence of H2O2101.
The abundance of damaging free radicals generated by oxidative phosphorylation make the mitochondria an optimal location for such high concentrations of melatonin114. Various mechanisms have been proposed regarding melatonin’s ability to reduce mitochondrial oxidative stress and help maintain mitochondrial homeostasis, however, these remain to be fully studied in the context of human melanocytes
One proposed anti-aging mechanism that may be relevant in this context involves the stimulation of sirtuin3 (SIRT3) by melatonin in mitochondria, leading to the deacetylation and activation of superoxide dismutase-2 (SOD2), which enzymatically dismutates superoxide anion radicals159. Furthermore, melatonin can inhibit premature senescence by upregulating expression of sirtuin1 (SIRT1), which reduces oxidative stress, decreases expression and activation of p53, and inhibits NF-κB signaling11.
Melatonin may also act on mitochondrial uncoupling proteins to dissipate the proton gradient across the inner membrane to moderately reduce inner membrane potential, thereby increasing activities of complexes I and III, accelerating ETC electron transport, and decreasing electron leakage from the ETC; effects that reduce free radical formation160. Melatonin’s alleviation of oxidative damage in the mitochondrial matrix and intermembrane space2 has been proposed to decrease cardiolipin oxidation, mitochondrial permeability transition pore (MPTP) opening161, cytochrome c release, and mitochondria-related apoptosis, all of which are beneficial effects to slow aging and preserve cellular functioning114, likely also in human epidermal and HF melanocytes. Melatonin can also increase H2O2 scavenging114 and its metabolite 6-OHM can directly increase the electron flux through the respiratory chain and enhance ATP production by donating electrons162.
Finally, melatonin maintains the optimal mitochondrial membrane potential (Δψm)163 through its abilities to block the MPTP in conditions of stress and activate uncoupling proteins in normal conditions160.
(5). Melatonin regulates melanocyte autophagic flux
Autophagy is a critical cellular process that, in part, involves the removal of misfolded or aggregated proteins and clearance of damaged organelles, such as mitochondria (mitophagy), endoplasmic reticulum and peroxisomes164. Autophagy is activated in conditions of oxidative stress165, including aging166, and plays a key role in protecting normal human epidermal melanocytes from oxidative stress-induced apoptosis, loss of mitochondrial membrane potential, and intracellular ROS generation167,168, as well as in the regulation of melanogenesis, melanosome formation and maturation, and melanosome degradation in normal melanocytes and keratinocytes169-172.
The key role of melatonin in the regulation of autophagy has been documented in the context of various organ systems and pathologies173. Melatonin can help maintain cellular homeostasis either through autophagy promotion or suppression, depending on cellular requirements and oxidative stress levels174. Since autophagic flux is required for maintenance of anagen and thus pigment production in human HFs175, it is possible that the intrafollicular synthesis of melatonin49 contributes to adequate autophagy levels. However, it is unknown whether melatonin impacts on autophagy in human epidermal and HF melanocytes. Yet, since melatonin and autophagy are so closely related to oxidative stress in melanocytes, a relationship between them is anticipated166.
Melatonin prevents initiation of mitophagy through maintenance of the optimal Δψm176. Also, melatonin may reduce autophagy in epidermal and HF melanocytes indirectly by either reversing mitochondrial dysfunction through reduced oxidative stress or by improving endoplasmic reticulum efficiency, resulting in less misfolded proteins, which are effects exerted by melatonin in the context of other organs systems and pathologies173.
SIRT1 is an autophagy substrate and stimulator that works by preventing the acetylation of key autophagy proteins (via deacetylation) (e.g., ATG5, ATG7 and ATG8/LC3)177,178. SIRT1 levels are reduced through autophagic–lysosomal degradation in aging tissues, which could contribute to melanocyte cell cycle arrest and a pro-inflammatory senescence-associated secretory phenotype during skin aging179. Considering melatonin’s ability to upregulate SIRT1 expression11 (see (4) above), intracutaneously produced melatonin may thus positively regulate autophagy and exert anti-aging properties by stabilizing SIRT1 levels. The role of melatonin in skin aging has been recently extensively reviewed274.
MELATONIN AND PERIPHERAL CIRCADIAN CLOCK GENES
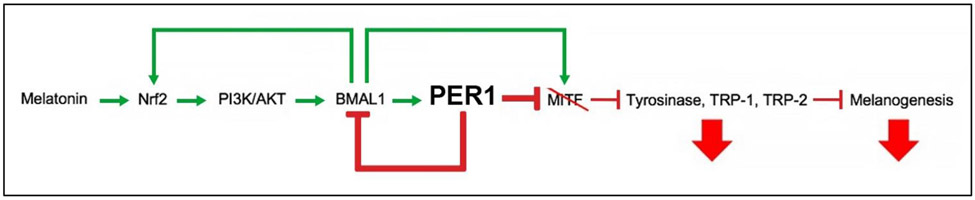
The peripheral clock genes Bmal1 and Per1 are known to control pigmentation in human epidermal and HF melanocytes while their silencing in human HFs ex vivo stimulates melanogenesis, tyrosinase expression and activity, TYRP1/2 expression, melanocyte dendricity121. It is unknown how melatonin, the key neuroendocrine regulator of the central circadian clock 6, impacts on the pigmentary activity of human epidermal and HF melanocytes through the peripheral clock. Yet, cell culture studies (e.g., human epidermal keratinocytes180 and mouse neuro2A cells181) provide instructive clues as follows: melatonin may regulate peripheral clock-associated pigmentary effects, probably through activation of Nrf2, which triggers PI3K/AKT signaling15. PI3K/AKT signaling leads to stimulation of both BMAL1181 and then PER1, which inhibits melanogenesis enzymes (tyrosinase, TRP-1, and TRP-2) and melanogenesis121,123, as hypothesized in figure 4 (for detailed discussion, see supplementary text 1).
Figure 4. Schematic summary describing hypothesized mechanisms by which melatonin may regulate melanogenesis in normal human epidermal and HF melanocytes.
Melatonin may activate the PI3K/AKT pathway, via Nrf2 activation15, to stimulate expression of Bmal1, thereby increasing BMAL1 levels181. BMAL1 may increase expression of Nrf2 to further stimulate this PI3K/AKT pathway263 and PER1 to inhibit MITF downstream121. PER1 also translocates to the nucleus and inhibits transcriptional activity of BMAL1264,265, thereby preventing BMAL1’s stimulation of MITF transcription266. Decreased MITF levels lead to decreased expression of melanogenesis enzymes tyrosinase, TRP-1, and TRP-2 and results in decreased melanogenesis123.
OPEN KEY QUESTIONS AND MODELS TO ANSWER THEM
To decisively advance the field, several open questions in addition to those already posed above must be clarified.
(1). Does exogenous melatonin robustly inhibit melanogenesis in human epidermis and/or HFs, and if so, by which mechanism(s)?
The majority of melatonin effects on mammalian melanogenesis have been observed in cell culture studies, even though – for the reasons discussed above – it is most meaningful to study melanocyte activities within their natural tissue habitat, rather than in isolation. Therefore, to best determine melatonin’s therapeutic potential in pigmentary disorders, and to clarify definitively whether it indeed robustly inhibits human melanogenesis in situ, it is critical to study melatonin in human epidermal and HF melanocytes in skin and HF organ culture. In these ex vivo assays, besides pharmacological antagonist and blocking-antibody studies, gene silencing can be performed for mechanistic research to elucidate the exact mechanisms by which melatonin alters key regulatory elements of melanogenesis along the lines synthesized in Figures 1-4. It is important to do this in a strictly hair cycle-standardized manner as HF pigmentation is active only during active hair growth (anagen) and HF cycling impacts substantially on extrafollicular skin physiology (e.g., by a maximal HF production of melanotropic neuropeptides and growth factors during anagen), which may in turn also affect the response of intraepidermal melanocytes to melatonin.
(2). Does melatonin regulate human melanocyte proliferation, survival, and/or senescence under physiological circumstances and via which receptor or pathway?
Similarly, whether melatonin regulates the proliferation, survival and/or senescence of human epidermal and HF melanocytes under physiological conditions remains unclear. There is sufficient evidence that melatonin can affect these phenotypic traits in vitro. However, it remains to be established where melatonin ranks in the hierarchy of other local regulators of these melanocyte activities. Again, this is best studied in human skin and HF organ culture assays ex vivo. Ideally, this is complemented by studying human skin xenotransplants on SCID mice for long-term preclinical in vivo studies directly in the human target organ, and by knocking out or overexpressing cell type-dependent local production of melatonin, individual receptors (MT1 vs MT2), or different signaling pathways in defined human skin cell populations that are co-cultured under 3D conditions in human skin “equivalents”. This will also require the development of MT1-selective agents to match the abundance of available MT2-selective agents182.
(3). Can melatonin prevent and/or treat pigmentation disorders?
Ultimately, we need definitive answers to this question regarding which human pigmentary disorders can effectively be prevented or managed by melatonin administration, either topically or systemically. Above, we have delineated the rationale and preliminary clinical observations that encourage one to explore melatonin treatment in the pathophysiology and/or management of, for example, vitiligo, melasma, hair greying, and solar-related hyperpigmentation. However, more rigorous, well-controlled, prospective, randomized clinical trials are needed to determine utility and mode of application (systemic or topical) of melatonin, its metabolites, and its chemically synthesized derivatives, using optimally standardized and sensitive methods for recording changes in human skin/hair pigmentation. Also, skin or HF tissue samples from patients with such pigmentary disorders (perhaps beginning with vitiligo, melasma and hair greying) should be systematically screened for abnormalities in the cutaneous melatonin system, then organ-cultured, exposed to melatonin of varying concentrations, and analyzed for changes in key melanocyte biology read-outs in situ (e.g., melanin production, tyrosinase activity, expression of c-kit, gp100, MITF, TRP-1, TRP-2, Ki-67, senescence markers).
THERAPEUTIC PERSPECTIVES
The multiple levels at which melatonin and its metabolites could intervene with human skin pigmentation invite therapeutic applications. In addition, melatonin’s safety, lack of or very low toxicity, and pleiotropic effects (e.g., UV protection, potent antioxidant activity, DNA repair, anti-aging, anti-inflammation, and melanogenesis inhibition) make melatonin an attractive therapeutic candidate for treatment of pigmentary disorders, such as melasma108 and acanthosis nigrans-associated hyperpigmentation107.
Its photoprotective16,17, anti-photoaging11,110,183-186, anti-oxidative damage-protective5,15,56,101,125,127,134 and DNA damage-repair15 properties also raise the possibility that melatonin may be useful to slow intrinsic and extrinsic skin aging and may exert melanocyte-protective properties in vitiligo and perhaps even aging-associated hair graying resulting from oxidative damage to the HFPU30. In fact, melatonin and its metabolites (e.g., AFMK) protect melanocytes in vitro15 from UV-induced DNA damage and apoptosis185,187 when applied both before188 and immediately after UVB exposure15. This renders melatonin an effective therapeutic candidate for the prevention and management of solar radiation-induced pigmentation disorders16,189-191. Finally, melatonin’s regulation of autophagy192 (see above) might be exploited to treat pigmentary disorders with recognized autophagic defects such as vitiligo165,197, tuberous sclerosis193, and Cockayne syndrome194.
Due to its ability to penetrate the stratum corneum195 and to thus evade prominent first-pass metabolism of oral melatonin by the liver12, topical administration of melatonin may be superior to the oral route, and permits administration of high melatonin doses directly to human skin target cells, namely epidermal and HF melanocytes and their keratinocyte environment in the EPU and HFPU. Indeed, the use of topical sunscreen fortified with melatonin offers superior sun protection and the ability to counteract UV radiation-induced oxidative stress187. A topical sunscreen formulation fortified with melatonin and pumpkin seed oil reportedly had enhanced photoprotective effects196. Also, the application of 12.5% melatonin cream protects skin from natural sunlight-induced erythema197. New topical formulations such as nanostructured lipid carriers198,199 and ethosomes200 promise optimized melatonin delivery in future clinical trials.
Given that theophylline (which is licensed for topical application as a cosmetic agent) can increase melatonin levels released by organ-cultured human skin into the medium201 while noradrenaline stimulates melatonin synthesis within human scalp HFs ex vivo49, it is also possible that the intracutaneous synthesis of melatonin can be stimulated by topically applied agents that increase intracellular cAMP levels and thus intracutaneous production of endogenous melatonin.
To our knowledge, genetic disorders associated with melatonin deficiency and its receptor deficiencies have not yet been described but may well have been missed. In addition, melatonin’s nuclear receptors, as opposed to its membrane receptors (MT1 and MT2), still must be definitively identified. Of note, many of melatonin’s protective effects in melanocytes described above, such as melatonin’s role as a free radical scavenger125,126 and stimulator of DNA repair15 and antioxidant enzyme expression and activity1 are independent of MT1 and MT2 signaling. Therefore, MT1 and/or MT2 genetic disorders would not directly alter receptor-independent protective effects in melanocytes. While deficiencies in melatonin synthesis or receptor expression levels in the human system, namely in human skin, clearly await more systematic scrutiny, this limits what can be deduced from the study of dysfunctional MT1/2, even if patients become identified, for example, with receptor mutations. Furthermore, no genetically mutant mice are currently known that have substantial melatonin synthesis or MT receptor deficiencies in their skin.
CONCLUSIONS
In normal human epidermal melanocytes, melatonin and its metabolites, such as AFMK, 6-OHM, and 5-MT, inhibit melanogenesis, tyrosinase activity, and melanocyte proliferation in vitro. Yet, it is unclear how robustly this translates to the physiological tissue context in human epidermis and HFs. Instructive organ culture assays are readily available to clarify this.
Melatonin may inhibit melanogenesis not only by stimulation of MT receptors (MT1/2), but also indirectly by cell desensitization to estrogens, reducing skin sensitivity to α-MSH stimulation, and activation of Nrf2 and PI3k/AKT pathways and/or MAPK signaling. Additional indirect mechanisms/targets by which melatonin may regulate human melanocyte physiology include calcium-calmodulin complex, NOS1, p53, cytochrome c, ETC enzymes, SIRT3/SOD2, and possibly NQO2 (see Figures 1 and 2).
Besides accounting for dose-, application mode-, species-, gender-, age-, and ethnicity-dependent differences in the melatonin response of melanocytes in a given tissue location, much greater attention must be paid to the tissue context in which melatonin affects human melanocyte physiology, such as the specific hormonal tissue environment, internal and external stressors, and local determinants of melatonin metabolism through indolic and kynuric pathways.
There is good in vitro evidence that melatonin can unfold powerful oxidative damage-limiting effects on melanocytes, namely under skin photodamage conditions, through MT1/2 activation, direct ROS scavenging, Nrf2 activation, promotion of mitochondrial homeostasis, calcium/calmodulin complex-induced inhibition of NOS1, and possible action on NQO2. Yet, whether melatonin really does so under physiological conditions and inhibits melanocyte senescence in human epidermis and HFs in situ, remains to be conclusively demonstrated.
Dysfunctional mitochondria and inadequate autophagy may also contribute to premature senescence and accelerated aging in human epidermal and HF melanocytes. Melatonin’s high concentrations in mitochondria and ability to help maintain mitochondrial homeostasis and modulate mitophagy justify the expectation that melatonin will become useful not only in limiting melanocyte senescence, a potential driver overall skin aging, but also invites clinical melatonin applications in the emerging field of “mitochondrial dermatology”.
Since silencing of the core peripheral clock genes, Clock, Bmal1, and Per1 stimulates melanogenesis in human epidermis and HFs in situ, melatonin may regulate peripheral clock-controlled pigmentary effects. One conceivable pathway is the activation of Nrf2 and PI3K/AKT signaling, which is expected to promote BMAL1 and PER1’s downstream melanogenesis-inhibitory effects, e.g., on tyrosinase activity.
The field is challenged to now move from in vitro to ex vivo and preclinical in vivo studies, using available human skin and HF organ culture assays as well as human skin xenotransplants on immunocompromised mice, to definitively clarify the relevance of melatonin in human melanocyte physiology and to more rigorously probe how therapeutically useful melatonin really is in selected human pigmentary disorders, ranging from hair greying to melasma and vitiligo.
Supplementary Material
Acknowledgement
RP was supported by a Frost Endowed Scholarship. Additional support for review writing was provided by NIH grants 1R01AR073004-01A1, R01AR071189-01A1 and VA merit grant (no. 1I01BX004293-01A1) to A.T.S.
Footnotes
The paper is dedicated to Aaron B. Lerner, a discoverer of melatonin, and mentor of one of the co-authors (A. T. Slominski).
REFERENCES:
- 1.Manchester LC, Coto-Montes A, Boga JA, et al. Melatonin: an ancient molecule that makes oxygen metabolically tolerable. Journal of Pineal Research. 2015;59(4):403–419. doi: 10.1111/jpi.12267 [DOI] [PubMed] [Google Scholar]
- 2.Reiter RJ, Rosales-Corral S, Tan DX, Jou MJ, Galano A, Xu B. Melatonin as a mitochondria-targeted antioxidant: one of evolution’s best ideas. Cell Mol Life Sci. 2017;74(21):3863–3881. doi: 10.1007/s00018-017-2609-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dubocovich ML. Melatonin receptors: Role on sleep and circadian rhythm regulation. Sleep Medicine. 2007;8:34–42. doi: 10.1016/j.sleep.2007.10.007 [DOI] [PubMed] [Google Scholar]
- 4.Reiter RJ, Tan DX, Rosales-Corral S, Manchester LC. The universal nature, unequal distribution and antioxidant functions of melatonin and its derivatives. Mini Rev Med Chem. 2013;13(3):373–384. doi: 10.2174/1389557511313030006 [DOI] [PubMed] [Google Scholar]
- 5.Tan DX, Manchester LC, Terron MP, Flores LJ, Reiter RJ. One molecule, many derivatives: a never-ending interaction of melatonin with reactive oxygen and nitrogen species? J Pineal Res. 2007;42(1):28–42. doi: 10.1111/j.1600-079X.2006.00407.x [DOI] [PubMed] [Google Scholar]
- 6.Pandi-Perumal SR, Srinivasan V, Maestroni GJM, Cardinali DP, Poeggeler B, Hardeland R. Melatonin.: Nature’s most versatile biological signal? FEBS Journal. 2006;273(13):2813–2838. doi: 10.1111/j.1742-4658.2006.05322.x [DOI] [PubMed] [Google Scholar]
- 7.Acuña-Castroviejo D, Escames G, Venegas C, et al. Extrapineal melatonin: sources, regulation, and potential functions. Cell Mol Life Sci. 2014;71(16):2997–3025. doi: 10.1007/s00018-014-1579-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Slominski A, Tobin DJ, Zmijewski MA, Wortsman J, Paus R. Melatonin in the skin: synthesis, metabolism and functions. Trends in Endocrinology & Metabolism. 2008;19(1):17–24. doi: 10.1016/j.tem.2007.10.007 [DOI] [PubMed] [Google Scholar]
- 9.Lerner AB, Case JD, Takahashi Y. Isolation of melatonin and 5-methoxyindole-3-acetic acid from bovine pineal glands. J Biol Chem. 1960;235:1992–1997. [PubMed] [Google Scholar]
- 10.Karasek M. Melatonin, human aging, and age-related diseases. Exp Gerontol. 2004;39(11-12):1723–1729. doi: 10.1016/j.exger.2004.04.012 [DOI] [PubMed] [Google Scholar]
- 11.Ma L, Liu Q, Tian M, Tian X, Gao L. Mechanisms of melatonin in anti-aging and its regulation effects in radiation-induced premature senescence. Radiation Medicine and Protection. 2021;2(1):33–37. doi: 10.1016/j.radmp.2021.01.003 [DOI] [Google Scholar]
- 12.Milani M, Sparavigna A. Antiaging efficacy of melatonin-based day and night creams: a randomized, split-face, assessor-blinded proof-of-concept trial. CCID. 2018;Volume 11:51–57. doi: 10.2147/CCID.S153905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poeggeler B, Reiter RJ, Tan DX, Chen LD, Manchester LC. Melatonin, hydroxyl radical-mediated oxidative damage, and aging: a hypothesis. J Pineal Res. 1993;14(4):151–168. doi: 10.1111/j.1600-079x.1993.tb00498.x [DOI] [PubMed] [Google Scholar]
- 14.Fischer TW, Slominski A, Zmijewski MA, Reiter RJ, Paus R. Melatonin as a major skin protectant: from free radical scavenging to DNA damage repair. Exp Dermatol. 2008;17(9):713–730. doi: 10.1111/j.1600-0625.2008.00767.x [DOI] [PubMed] [Google Scholar]
- 15.Janjetovic Z, Jarrett SG, Lee EF, Duprey C, Reiter RJ, Slominski AT. Melatonin and its metabolites protect human melanocytes against UVB-induced damage: Involvement of NRF2-mediated pathways. Sci Rep. 2017;7(1):1274. doi: 10.1038/s41598-017-01305-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Slominski AT, Kleszczyński K, Semak I, et al. Local melatoninergic system as the protector of skin integrity. Int J Mol Sci. 2014;15(10):17705–17732. doi: 10.3390/ijms151017705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Slominski A, Wortsman J, Tobin DJ. The cutaneous serotoninergic/melatoninergic system: securing a place under the sun. FASEB J. 2005;19(2):176–194. doi: 10.1096/fj.04-2079rev [DOI] [PubMed] [Google Scholar]
- 18.Ma N, Zhang J, Reiter RJ, Ma X. Melatonin mediates mucosal immune cells, microbial metabolism, and rhythm crosstalk: A therapeutic target to reduce intestinal inflammation. Medicinal Research Reviews. 2020;40(2):606–632. doi: 10.1002/med.21628 [DOI] [PubMed] [Google Scholar]
- 19.Moradkhani F, Moloudizargari M, Fallah M, Asghari N, Khoei HH, Asghari MH. Immunoregulatory role of melatonin in cancer. Journal of Cellular Physiology. 2020;235(2):745–757. doi: 10.1002/jcp.29036 [DOI] [PubMed] [Google Scholar]
- 20.Carrillo-Vico A, Lardone P, Álvarez-Sánchez N, Rodríguez-Rodríguez A, Guerrero J. Melatonin: Buffering the Immune System. IJMS. 2013;14(4):8638–8683. doi: 10.3390/ijms14048638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fischer TW, Zmijewski MA, Zbytek B, et al. Oncostatic effects of the indole melatonin and expression of its cytosolic and nuclear receptors in cultured human melanoma cell lines. Int J Oncol. 2006;29(3):665–672. doi: 10.3892/ijo.29.3.665 [DOI] [PubMed] [Google Scholar]
- 22.Pourhanifeh MH, Mahdavinia M, Reiter RJ, Asemi Z. Potential use of melatonin in skin cancer treatment: A review of current biological evidence. J Cell Physiol. 2019;234(8):12142–12148. doi: 10.1002/jcp.28129 [DOI] [PubMed] [Google Scholar]
- 23.Slominski A, Pruski D. Melatonin inhibits proliferation and melanogenesis in rodent melanoma cells. Exp Cell Res. 1993;206(2):189–194. doi: 10.1006/excr.1993.1137 [DOI] [PubMed] [Google Scholar]
- 24.Fischer TW, Slominski A, Tobin DJ, Paus R. Melatonin and the hair follicle. J Pineal Res. 2007;0(0):071027134919001-??? doi: 10.1111/j.1600-079X.2007.00512.x [DOI] [PubMed] [Google Scholar]
- 25.Slominski AT, Hardeland R, Zmijewski MA, Slominski RM, Reiter RJ, Paus R. Melatonin: A Cutaneous Perspective on its Production, Metabolism, and Functions. Journal of Investigative Dermatology. 2018;138(3):490–499. doi: 10.1016/j.jid.2017.10.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Slominski A, Tobin DJ, Shibahara S, Wortsman J. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol Rev. 2004;84(4):1155–1228. doi: 10.1152/physrev.00044.2003 [DOI] [PubMed] [Google Scholar]
- 27.Slominski AT, Hardeland R, Reiter RJ. When the Circadian Clock Meets the Melanin Pigmentary System. Journal of Investigative Dermatology. 2015;135(4):943–945. doi: 10.1038/jid.2014.553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moreiras H, Seabra MC, Barral DC. Melanin Transfer in the Epidermis: The Pursuit of Skin Pigmentation Control Mechanisms. International Journal of Molecular Sciences. 2021;22(9):4466. doi: 10.3390/ijms22094466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tadokoro R, Takahashi Y. Intercellular transfer of organelles during body pigmentation. Current Opinion in Genetics & Development. 2017;45:132–138. doi: 10.1016/j.gde.2017.05.001 [DOI] [PubMed] [Google Scholar]
- 30.O’Sullivan JDB, Nicu C, Picard M, et al. The biology of human hair greying. Biological Reviews. 2021;96(1):107–128. doi: 10.1111/brv.12648 [DOI] [PubMed] [Google Scholar]
- 31.Slominski A, Wortsman J, Plonka PM, Schallreuter KU, Paus R, Tobin DJ. Hair Follicle Pigmentation. Journal of Investigative Dermatology. 2005;124(1):13–21. doi: 10.1111/j.0022-202X.2004.23528.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tobin DJ. The cell biology of human hair follicle pigmentation. Pigment Cell & Melanoma Research. 2011;24(1):75–88. doi: 10.1111/j.1755-148X.2010.00803.x [DOI] [PubMed] [Google Scholar]
- 33.Paus R. A neuroendocrinological perspective on human hair follicle pigmentation. Pigment Cell & Melanoma Research. 2011;24(1):89–106. doi: 10.1111/j.1755-148X.2010.00808.x [DOI] [PubMed] [Google Scholar]
- 34.Paus R, Langan EA, Vidali S, Ramot Y, Andersen B. Neuroendocrinology of the hair follicle: principles and clinical perspectives. Trends in Molecular Medicine. 2014;20(10):559–570. doi: 10.1016/j.molmed.2014.06.002 [DOI] [PubMed] [Google Scholar]
- 35.Ramot Y, Böhm M, Paus R. Translational Neuroendocrinology of Human Skin: Concepts and Perspectives. Trends in Molecular Medicine. 2021;27(1):60–74. doi: 10.1016/j.molmed.2020.09.002 [DOI] [PubMed] [Google Scholar]
- 36.Slominski A, Wortsman J, Luger T, Paus R, Solomon S. Corticotropin releasing hormone and proopiomelanocortin involvement in the cutaneous response to stress. Physiol Rev. 2000;80(3):979–1020. doi: 10.1152/physrev.2000.80.3.979 [DOI] [PubMed] [Google Scholar]
- 37.Slominski A, Wortsman J, Kohn L, et al. Expression of hypothalamic-pituitary-thyroid axis related genes in the human skin. J Invest Dermatol. 2002;119(6):1449–1455. doi: 10.1046/j.1523-1747.2002.19617.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Slominski AT, Zmijewski MA, Zbytek B, Tobin DJ, Theoharides TC, Rivier J. Key Role of CRF in the Skin Stress Response System. Endocrine Reviews. 2013;34(6):827–884. doi: 10.1210/er.2012-1092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Slominski A, Zbytek B, Pisarchik A, Slominski RM, Zmijewski MA, Wortsman J. CRH functions as a growth factor/cytokine in the skin. J Cell Physiol. 2006;206(3):780–791. doi: 10.1002/jcp.20530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.At S, Ma Z, C S, B Z, Rm S, Jd S. Sensing the environment: regulation of local and global homeostasis by the skin’s neuroendocrine system. Adv Anat Embryol Cell Biol. 2012;212:v, vii, 1–115. doi: 10.1007/978-3-642-19683-6_1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Samuelov L, Sprecher E, Sugawara K, et al. Topobiology of human pigmentation: P-cadherin selectively stimulates hair follicle melanogenesis. J Invest Dermatol. 2013;133(6):1591–1600. doi: 10.1038/jid.2013.18 [DOI] [PubMed] [Google Scholar]
- 42.Sobiepanek A, Baran J, Milner-Krawczyk M, Kobiela T. Different Types of Surface Modification used for Improving the Adhesion and Interactions of Skin Cells. 2020;2:275–278. doi: 10.38125/OAJBS.000161 [DOI] [Google Scholar]
- 43.Zhang B, Ma S, Rachmin I, et al. Hyperactivation of sympathetic nerves drives depletion of melanocyte stem cells. Nature. 2020;577(7792):676–681. doi: 10.1038/s41586-020-1935-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Upadhyay PR, Ho T, Abdel-Malek ZA. Participation of keratinocyte- and fibroblast-derived factors in melanocyte homeostasis, the response to UV, and pigmentary disorders. Pigment Cell & Melanoma Research. 2021;34(4):762–776. doi: 10.1111/pcmr.12985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang Y, Viennet C, Robin S, Berthon JY, He L, Humbert P. Precise role of dermal fibroblasts on melanocyte pigmentation. Journal of Dermatological Science. 2017;88(2):159–166. doi: 10.1016/j.jdermsci.2017.06.018 [DOI] [PubMed] [Google Scholar]
- 46.Nicu C, O’Sullivan JDB, Ramos R, et al. Dermal Adipose Tissue Secretes HGF to Promote Human Hair Growth and Pigmentation. Journal of Investigative Dermatology. 2021;141(7):1633–1645.e13. doi: 10.1016/j.jid.2020.12.019 [DOI] [PubMed] [Google Scholar]
- 47.Lindner G, Menrad A, Gherardi E, et al. Involvement of hepatocyte growth factor/scatter factor and Met receptor signaling in hair follicle morphogenesis and cycling. The FASEB Journal. 2000;14(2):319–332. doi: 10.1096/fasebj.14.2.319 [DOI] [PubMed] [Google Scholar]
- 48.Slominski A, Paus R, Plonka P, et al. Melanogenesis during the anagen-catagen-telogen transformation of the murine hair cycle. J Invest Dermatol. 1994;102(6):862–869. doi: 10.1111/1523-1747.ep12382606 [DOI] [PubMed] [Google Scholar]
- 49.Kobayashi H, Kromminga A, Dunlop TW, et al. A role of melatonin in neuroectodermal-mesodermal interactions: the hair follicle synthesizes melatonin and expresses functional melatonin receptors. FASEB j. 2005;19(12):1710–1712. doi: 10.1096/fj.04-2293fje [DOI] [PubMed] [Google Scholar]
- 50.Semak I, Korik E, Naumova M, Wortsman J, Slominski A. Serotonin metabolism in rat skin: characterization by liquid chromatography-mass spectrometry. Arch Biochem Biophys. 2004;421(1):61–66. doi: 10.1016/j.abb.2003.08.036 [DOI] [PubMed] [Google Scholar]
- 51.Slominski A, Baker J, Rosano TG, et al. Metabolism of Serotonin to N-Acetylserotonin, Melatonin, and 5-Methoxytryptamine in Hamster Skin Culture. Journal of Biological Chemistry. 1996;271(21):12281–12286. doi: 10.1074/jbc.271.21.12281 [DOI] [PubMed] [Google Scholar]
- 52.Slominski A, Pisarchik A, Semak I, et al. Serotoninergic and melatoninergic systems are fully expressed in human skin. The FASEB Journal. 2002;16(8):896–898. doi: 10.1096/fj.01-0952fje [DOI] [PubMed] [Google Scholar]
- 53.Slominski AT, Kim TK, Kleszczyński K, et al. Characterization of serotonin and N-acetylserotonin systems in the human epidermis and skin cells. J Pineal Res. 2020;68(2):e12626. doi: 10.1111/jpi.12626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Slominski A, Semak I, Pisarchik A, Sweatman T, Szczesniewski A, Wortsman J. Conversion of L-tryptophan to serotonin and melatonin in human melanoma cells. FEBS Lett. 2002;511(1–3):102–106. doi: 10.1016/s0014-5793(01)03319-1 [DOI] [PubMed] [Google Scholar]
- 55.Slominski A, Pisarchik A, Johansson O, et al. Tryptophan hydroxylase expression in human skin cells. Biochim Biophys Acta. 2003;1639(2):80–86. doi: 10.1016/s0925-4439(03)00124-8 [DOI] [PubMed] [Google Scholar]
- 56.Slominski AT, Zmijewski MA, Semak I, et al. Melatonin, mitochondria, and the skin. Cell Mol Life Sci. 2017;74(21):3913–3925. doi: 10.1007/s00018-017-2617-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mannino G, Pernici C, Serio G, Gentile C, Bertea CM. Melatonin and Phytomelatonin: Chemistry, Biosynthesis, Metabolism, Distribution and Bioactivity in Plants and Animals-An Overview. Int J Mol Sci. 2021;22(18):9996. doi: 10.3390/ijms22189996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hardeland R. Melatonin, Its Metabolites and Their Interference with Reactive Nitrogen Compounds. Molecules. 2021;26(13):4105. doi: 10.3390/molecules26134105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Oishi A, Gbahou F, Jockers R. Melatonin receptors, brain functions, and therapies. Handb Clin Neurol. 2021;179:345–356. doi: 10.1016/B978-0-12-819975-6.00022-4 [DOI] [PubMed] [Google Scholar]
- 60.Fiore A, Murray PJ. Tryptophan and indole metabolism in immune regulation. Curr Opin Immunol. 2021;70:7–14. doi: 10.1016/j.coi.2020.12.001 [DOI] [PubMed] [Google Scholar]
- 61.Slominski A, Pisarchik A, Semak I, Sweatman T, Wortsman J. Characterization of the serotoninergic system in the C57BL/6 mouse skin. Eur J Biochem. 2003;270(16):3335–3344. doi: 10.1046/j.1432-1033.2003.03708.x [DOI] [PubMed] [Google Scholar]
- 62.Jou MJ, Peng TI, Yu PZ, et al. Melatonin protects against common deletion of mitochondrial DNA-augmented mitochondrial oxidative stress and apoptosis. J Pineal Res. 2007;43(4):389–403. doi: 10.1111/j.1600-079X.2007.00490.x [DOI] [PubMed] [Google Scholar]
- 63.Menendez-Pelaez A, Poeggeler B, Reiter RJ, Barlow-Walden L, Pablos MI, Tan DX. Nuclear localization of melatonin in different mammalian tissues: immunocytochemical and radioimmunoassay evidence. J Cell Biochem. 1993;53(4):373–382. doi: 10.1002/jcb.240530415 [DOI] [PubMed] [Google Scholar]
- 64.Rodríguez MI, Escames G, López LC, et al. Improved mitochondrial function and increased life span after chronic melatonin treatment in senescent prone mice. Exp Gerontol. 2008;43(8):749–756. doi: 10.1016/j.exger.2008.04.003 [DOI] [PubMed] [Google Scholar]
- 65.Yu H, Dickson EJ, Jung SR, Koh DS, Hille B. High membrane permeability for melatonin. J Gen Physiol. 2016;147(1):63–76. doi: 10.1085/jgp.201511526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hacışevki A, Baba B. An Overview of Melatonin as an Antioxidant Molecule: A Biochemical Approach. In: Manuela Drăgoi C, Crenguţa Nicolae A, eds. Melatonin - Molecular Biology, Clinical and Pharmaceutical Approaches. IntechOpen; 2018. doi: 10.5772/intechopen.79421 [DOI] [Google Scholar]
- 67.Tordjman S, Chokron S, Delorme R, et al. Melatonin: Pharmacology, Functions and Therapeutic Benefits. CN. 2017;15(3):434–443. doi: 10.2174/1570159X14666161228122115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Slominski RM, Reiter RJ, Schlabritz-Loutsevitch N, Ostrom RS, Slominski AT. Melatonin membrane receptors in peripheral tissues: Distribution and functions. Molecular and Cellular Endocrinology. 2012;351(2):152–166. doi: 10.1016/j.mce.2012.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kallen J, Schlaeppi JM, Bitsch F, Delhon I, Fournier B. Crystal structure of the human RORalpha Ligand binding domain in complex with cholesterol sulfate at 2.2 A. J Biol Chem. 2004;279(14):14033–14038. doi: 10.1074/jbc.M400302200 [DOI] [PubMed] [Google Scholar]
- 70.Slominski AT, Zmijewski MA, Jetten AM. RORα is not a receptor for melatonin. Bioessays. 2016;38(12):1193–1194. doi: 10.1002/bies.201600204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Slominski AT, Kim TK, Takeda Y, et al. RORα and ROR γ are expressed in human skin and serve as receptors for endogenously produced noncalcemic 20-hydroxy- and 20,23-dihydroxyvitamin D. The FASEB Journal. 2014;28(7):2775–2789. doi: 10.1096/fj.13-242040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ma H, Kang J, Fan W, He H, Huang F. ROR: Nuclear Receptor for Melatonin or Not? Molecules. 2021;26(9):2693. doi: 10.3390/molecules26092693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Slominski A, Paus R, Schadendorf D. Melanocytes as “Sensory” and Regulatory Cells in the Epidermis. Journal of Theoretical Biology. 1993;164(1):103–120. doi: 10.1006/jtbi.1993.1142 [DOI] [PubMed] [Google Scholar]
- 74.Slominski A, Paus R. Melanogenesis is coupled to murine anagen: toward new concepts for the role of melanocytes and the regulation of melanogenesis in hair growth. J Invest Dermatol. 1993;101(1 Suppl):90S–97S. doi: 10.1111/1523-1747.ep12362991 [DOI] [PubMed] [Google Scholar]
- 75.Slominski AT, Zmijewski MA, Plonka PM, Szaflarski JP, Paus R. How UV Light Touches the Brain and Endocrine System Through Skin, and Why. Endocrinology. 2018;159(5):1992–2007. doi: 10.1210/en.2017-03230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Slominski A Neuroendocrine activity of the melanocyte. Exp Dermatol. 2009;18(9):760–763. doi: 10.1111/j.1600-0625.2009.00892.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Choi HI, Sohn KC, Hong DK, et al. Melanosome uptake is associated with the proliferation and differentiation of keratinocytes. Arch Dermatol Res. 2014;306(1):59–66. doi: 10.1007/s00403-013-1422-x [DOI] [PubMed] [Google Scholar]
- 78.Böhm M, Luger TA, Tobin DJ, García-Borrón JC. Melanocortin receptor ligands: new horizons for skin biology and clinical dermatology. J Invest Dermatol. 2006;126(9):1966–1975. doi: 10.1038/sj.jid.5700421 [DOI] [PubMed] [Google Scholar]
- 79.Chakraborty AK, Funasaka Y, Slominski A, et al. UV light and MSH receptors. Ann N Y Acad Sci. 1999;885:100–116. doi: 10.1111/j.1749-6632.1999.tb08668.x [DOI] [PubMed] [Google Scholar]
- 80.Domingues L, Hurbain I, Gilles-Marsens F, et al. Coupling of melanocyte signaling and mechanics by caveolae is required for human skin pigmentation. Nat Commun. 2020;11(1):2988. doi: 10.1038/s41467-020-16738-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Grando SA. Cholinergic control of epidermal cohesion. Exp Dermatol. 2006;15(4):265–282. doi: 10.1111/j.0906-6705.2006.00410.x [DOI] [PubMed] [Google Scholar]
- 82.Grando SA, Pittelkow MR, Schallreuter KU. Adrenergic and Cholinergic Control in the Biology of Epidermis: Physiological and Clinical Significance. Journal of Investigative Dermatology. 2006;126(9):1948–1965. doi: 10.1038/sj.jid.5700151 [DOI] [PubMed] [Google Scholar]
- 83.Li M, Knapp SK, Iden S. Mechanisms of melanocyte polarity and differentiation: What can we learn from other neuroectoderm-derived lineages? Current Opinion in Cell Biology. 2020;67:99–108. doi: 10.1016/j.ceb.2020.09.001 [DOI] [PubMed] [Google Scholar]
- 84.Pelle E, Mammone T, Maes D, Frenkel K. Keratinocytes Act as a Source of Reactive Oxygen Species by Transferring Hydrogen Peroxide to Melanocytes. Journal of Investigative Dermatology. 2005;124(4):793–797. doi: 10.1111/j.0022-202X.2005.23661.x [DOI] [PubMed] [Google Scholar]
- 85.Sarkar S, Gaddameedhi S. Solar ultraviolet-induced DNA damage response: Melanocytes story in transformation to environmental melanomagenesis. Environmental and Molecular Mutagenesis. 2020;61(7):736–751. doi: 10.1002/em.22370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Slominski A, Wortsman J, Pisarchik A, et al. Cutaneous expression of corticotropin-releasing hormone (CRH), urocortin, and CRH receptors. FASEB J. 2001;15(10):1678–1693. doi: 10.1096/fj.00-0850rev [DOI] [PubMed] [Google Scholar]
- 87.Slominski A, Paus R. Are L-tyrosine and L-dopa hormone-like bioregulators? J Theor Biol. 1990;143(1):123–138. doi: 10.1016/s0022-5193(05)80292-9 [DOI] [PubMed] [Google Scholar]
- 88.Slominski AT, Zmijewski MA, Zbytek B, et al. Regulated proenkephalin expression in human skin and cultured skin cells. J Invest Dermatol. 2011;131(3):613–622. doi: 10.1038/jid.2010.376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tobin DJ, Kauser S. Beta-endorphin: the forgotten hair follicle melanotropin. J Investig Dermatol Symp Proc. 2005;10(3):212–216. doi: 10.1111/j.1087-0024.2005.10108.x [DOI] [PubMed] [Google Scholar]
- 90.Yuan XH, Jin ZH. Paracrine regulation of melanogenesis. British Journal of Dermatology. 2018;178(3):632–639. doi: 10.1111/bjd.15651 [DOI] [PubMed] [Google Scholar]
- 91.Lee AY. Skin Pigmentation Abnormalities and Their Possible Relationship with Skin Aging. International Journal of Molecular Sciences. 2021;22(7):3727. doi: 10.3390/ijms22073727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Schallreuter KU, Salem MAEL, Holtz S, Panske A. Basic evidence for epidermal H 2 O 2 /ONOO− -mediated oxidation/nitration in segmental vitiligo is supported by repigmentation of skin and eyelashes after reduction of epidermal H 2 O 2 with topical NB-UVB-activated pseudocatalase PC-KUS. FASEB j. 2013;27(8):3113–3122. doi: 10.1096/fj.12-226779 [DOI] [PubMed] [Google Scholar]
- 93.Speeckaert R, Dugardin J, Lambert J, et al. Critical appraisal of the oxidative stress pathway in vitiligo: a systematic review and meta-analysis. J Eur Acad Dermatol Venereol. 2018;32(7):1089–1098. doi: 10.1111/jdv.14792 [DOI] [PubMed] [Google Scholar]
- 94.Arck PC, Overall R, Spatz K, et al. Towards a “free radical theory of graying”: melanocyte apoptosis in the aging human hair follicle is an indicator of oxidative stress induced tissue damage. FASEB j. 2006;20(9):1567–1569. doi: 10.1096/fj.05-4039fje [DOI] [PubMed] [Google Scholar]
- 95.Wood JM, Decker H, Hartmann H, et al. Senile hair graying: H2O2-mediated oxidative stress affects human hair color by blunting methionine sulfoxide repair. FASEB J. 2009;23(7):2065–2075. doi: 10.1096/fj.08-125435 [DOI] [PubMed] [Google Scholar]
- 96.Gillbro JM, Marles LK, Hibberts NA, Schallreuter KU. Autocrine Catecholamine Biosynthesis and the β2-Adrenoceptor Signal Promote Pigmentation in Human Epidermal Melanocytes. Journal of Investigative Dermatology. 2004;123(2):346–353. doi: 10.1111/j.0022-202X.2004.23210.x [DOI] [PubMed] [Google Scholar]
- 97.Schallreuter KU, Wood JM, Lemke R, et al. Production of catecholamines in the human epidermis. Biochemical and Biophysical Research Communications. 1992;189(1):72–78. doi: 10.1016/0006-291X(92)91527-W [DOI] [PubMed] [Google Scholar]
- 98.Slominski A, Fischer TW, Zmijewski MA, et al. On the Role of Melatonin in Skin Physiology and Pathology. ENDO. 2005;27(2):137–148. doi: 10.1385/ENDO:27:2:137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cichorek M, Wachulska M, Stasiewicz A, Tymińska A. Skin melanocytes: biology and development. Postepy Dermatol Alergol. 2013;30(1):30–41. doi: 10.5114/pdia.2013.33376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kim TK, Kleszczyński K, Janjetovic Z, et al. Metabolism of melatonin and biological activity of intermediates of melatoninergic pathway in human skin cells. The FASEB Journal. 2013;27(7):2742–2755. doi: 10.1096/fj.12-224691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Slominski AT, Semak I, Fischer TW, et al. Metabolism of melatonin in the skin: Why is it important? Experimental Dermatology. 2017;26(7):563–568. doi: 10.1111/exd.13208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hardeland R. Melatonin in Aging and Disease —Multiple Consequences of Reduced Secretion, Options and Limits of Treatment. Aging Dis. 2011;3(2):194–225. [PMC free article] [PubMed] [Google Scholar]
- 103.Kim TK, Lin Z, Tidwell WJ, Li W, Slominski AT. Melatonin and its metabolites accumulate in the human epidermis in vivo and inhibit proliferation and tyrosinase activity in epidermal melanocytes in vitro. Mol Cell Endocrinol. 2015;404:1–8. doi: 10.1016/j.mce.2014.07.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sagan D, Stepniak J, Gesing A, Lewinski A, Karbownik-Lewinska M. Melatonin reverses the enhanced oxidative damage to membrane lipids and improves skin biophysical characteristics in former-smokers - A study in postmenopausal women. Ann Agric Environ Med. 2017;24(4):659–666. doi: 10.5604/12321966.1235174 [DOI] [PubMed] [Google Scholar]
- 105.McElhinney DB, Hoffman SJ, Robinson WA, Ferguson J. Effect of melatonin on human skin color. J Invest Dermatol. 1994;102(2):258–259. doi: 10.1111/1523-1747.ep12371773 [DOI] [PubMed] [Google Scholar]
- 106.Nordlund JJ, Lerner AB. The Effects of Oral Melatonin on Skin Color and on the Release of Pituitary Hormones. The Journal of Clinical Endocrinology & Metabolism. 1977;45(4):768–774. doi: 10.1210/jcem-45-4-768 [DOI] [PubMed] [Google Scholar]
- 107.Sun H, Wang X, Chen J, et al. Melatonin Treatment Improves Insulin Resistance and Pigmentation in Obese Patients with Acanthosis Nigricans. International Journal of Endocrinology. 2018;2018:1–7. doi: 10.1155/2018/2304746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hamadi SA, Mohammed MM, Aljaf AN, Abdulrazak A. The role of topical and oral melatonin in management of melasma patients. J Arab Univ Basic Appl Sci. 2009;8:30–42. [Google Scholar]
- 109.Milani M, Puviani M. Anti-Aging Efficacy of Melatonin-Based Cream: Clinical and Instrumental Skin Evaluation. Cosmetics. 2018;5(4):60. doi: 10.3390/cosmetics5040060 [DOI] [Google Scholar]
- 110.Granger C, Brown A, Aladren S, Narda M. Night Cream Containing Melatonin, Carnosine and Helichrysum italicum Extract Helps Reduce Skin Reactivity and Signs of Photodamage: Ex Vivo and Clinical Studies. Dermatol Ther (Heidelb). 2020;10(6):1315–1329. doi: 10.1007/s13555-020-00443-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Schallreuter KU, Salem MAEL, Gibbons NCJ, et al. Blunted epidermal L-tryptophan metabolism in vitiligo affects immune response and ROS scavenging by Fenton chemistry, part 1: Epidermal H2O2/ONOO(−)-mediated stress abrogates tryptophan hydroxylase and dopa decarboxylase activities, leading to low serotonin and melatonin levels. FASEB J. 2012;26(6):2457–2470. doi: 10.1096/fj.11-197137 [DOI] [PubMed] [Google Scholar]
- 112.Kotb El-Sayed MI, Abd El-Ghany AA, Mohamed RR. Neural and Endocrinal Pathobiochemistry of Vitiligo: Comparative Study for a Hypothesized Mechanism. Front Endocrinol (Lausanne). 2018;9:197. doi: 10.3389/fendo.2018.00197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Slominski A, Paus R, Bomirski A. Hypothesis: possible role for the melatonin receptor in vitiligo: discussion paper. J R Soc Med. 1989;82(9):539–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Reiter RJ, Tan DX, Rosales-Corral S, Galano A, Zhou XJ, Xu B. Mitochondria: Central Organelles for Melatonin′s Antioxidant and Anti-Aging Actions. Molecules. 2018;23(2):509. doi: 10.3390/molecules23020509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kim TK, Lin Z, Li W, Reiter RJ, Slominski AT. N1-Acetyl-5-Methoxykynuramine (AMK) is produced in the human epidermis and shows antiproliferative effects. Endocrinology. 2015;156(5):1630–1636. doi: 10.1210/en.2014-1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kleszczyński K, Kim TK, Bilska B, et al. Melatonin exerts oncostatic capacity and decreases melanogenesis in human MNT-1 melanoma cells. Journal of Pineal Research. 2019;67(4):e12610. doi: 10.1111/jpi.12610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Slominski A, Chassalevris N, Mazurkiewicz J, Maurer M, Paus R. Murine skin as a target for melatonin bioregulation. Exp Dermatol. 1994;3(1):45–50. doi: 10.1111/j.1600-0625.1994.tb00265.x [DOI] [PubMed] [Google Scholar]
- 118.Sun M, Xie H fu, Tang Y, et al. G protein-coupled estrogen receptor enhances melanogenesis via cAMP-protein kinase (PKA) by upregulating microphthalmia-related transcription factor-tyrosinase in melanoma. The Journal of Steroid Biochemistry and Molecular Biology. 2017;165:236–246. doi: 10.1016/j.jsbmb.2016.06.012 [DOI] [PubMed] [Google Scholar]
- 119.Weatherhead B, Logan A. INTERACTION OF α-MELANOCYTE-STIMULATING HORMONE, MELATONIN, CYCLIC AMP AND CYCLIC GMP IN THE CONTROL OF MELANOGENESIS IN HAIR FOLLICLE MELANOCYTES IN VITRO. Journal of Endocrinology. 1981;90(1):89–96. doi: 10.1677/joe.0.0900089 [DOI] [PubMed] [Google Scholar]
- 120.Valverde P, Benedito E, Solano F, Oaknin S, Lozano JA, García-Borrón JC. Melatonin antagonizes alpha-melanocyte-stimulating hormone enhancement of melanogenesis in mouse melanoma cells by blocking the hormone-induced accumulation of the c locus tyrosinase. Eur J Biochem. 1995;232(1):257–263. doi: 10.1111/j.1432-1033.1995.tb20807.x [DOI] [PubMed] [Google Scholar]
- 121.Hardman JA, Tobin DJ, Haslam IS, et al. The peripheral clock regulates human pigmentation. J Invest Dermatol. 2015;135(4):1053–1064. doi: 10.1038/jid.2014.442 [DOI] [PubMed] [Google Scholar]
- 122.Lin X, Meng X, Song Z, Lin J. Nuclear factor erythroid 2-related factor 2 (Nrf2) as a potential therapeutic target for vitiligo. Arch Biochem Biophys. 2020;696:108670. doi: 10.1016/j.abb.2020.108670 [DOI] [PubMed] [Google Scholar]
- 123.Shin JM, Kim MY, Sohn KC, et al. Nrf2 Negatively Regulates Melanogenesis by Modulating PI3K/Akt Signaling. Berdeaux R, ed. PLoS ONE. 2014;9(4):e96035. doi: 10.1371/journal.pone.0096035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Galano A, Reiter RJ. Melatonin and its metabolites vs oxidative stress: From individual actions to collective protection. Journal of Pineal Research. 2018;65(1):e12514. doi: 10.1111/jpi.12514 [DOI] [PubMed] [Google Scholar]
- 125.Jockers R, Delagrange P, Dubocovich ML, et al. Update on melatonin receptors: IUPHAR Review 20. Br J Pharmacol. 2016;173(18):2702–2725. doi: 10.1111/bph.13536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mayo JC, Sainz RM, González-Menéndez P, Hevia D, Cernuda-Cernuda R. Melatonin transport into mitochondria. Cell Mol Life Sci. 2017;74(21):3927–3940. doi: 10.1007/s00018-017-2616-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sharafati-Chaleshtori R, Shirzad H, Rafieian-Kopaei M, Soltani A. Melatonin and human mitochondrial diseases. J Res Med Sci. 2017;22:2. doi: 10.4103/1735-1995.199092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Trueb R. Oxidative stress in ageing of hair. Int J Trichol. 2009;1(1):6. doi: 10.4103/0974-7753.51923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Campisi J, Kapahi P, Lithgow GJ, Melov S, Newman JC, Verdin E. From discoveries in ageing research to therapeutics for healthy ageing. Nature. 2019;571(7764):183–192. doi: 10.1038/s41586-019-1365-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.De Tollenaere M, Chapuis E, Auriol P, Auriol D, Scandolera A, Reynaud R. Global Repigmentation Strategy of Grey Hair Follicles by Targeting Oxidative Stress and Stem Cells Protection. Applied Sciences. 2021;11(4):1533. doi: 10.3390/app11041533 [DOI] [Google Scholar]
- 131.Victorelli S, Lagnado A, Halim J, et al. Senescent human melanocytes drive skin ageing via paracrine telomere dysfunction. The EMBO Journal. 2019;38(23):e101982. doi: 10.15252/embj.2019101982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Haslam IS, Jadkauskaite L, Szabó IL, et al. Oxidative Damage Control in a Human (Mini-) Organ: Nrf2 Activation Protects against Oxidative Stress-Induced Hair Growth Inhibition. Journal of Investigative Dermatology. 2017;137(2):295–304. doi: 10.1016/j.jid.2016.08.035 [DOI] [PubMed] [Google Scholar]
- 133.Jiang W, Li S, Chen X, et al. Berberine protects immortalized line of human melanocytes from H2O2-induced oxidative stress via activation of Nrf2 and Mitf signaling pathway. Journal of Dermatological Science. 2019;94(1):236–243. doi: 10.1016/j.jdermsci.2019.03.007 [DOI] [PubMed] [Google Scholar]
- 134.Schallreuter KU, Rokos H, Chavan B, et al. Quinones are reduced by 6-tetrahydrobiopterin in human keratinocytes, melanocytes, and melanoma cells. Free Radical Biology and Medicine. 2008;44(4):538–546. doi: 10.1016/j.freeradbiomed.2007.10.043 [DOI] [PubMed] [Google Scholar]
- 135.Tan DX, Manchester LC, Terron MP, Flores LJ, Tamura H, Reiter RJ. Melatonin as a naturally occurring co-substrate of quinone reductase-2, the putative MT3 melatonin membrane receptor: hypothesis and significance. J Pineal Res. 2007;43(4):317–320. doi: 10.1111/j.1600-079X.2007.00513.x [DOI] [PubMed] [Google Scholar]
- 136.Kurek D, Garinis GA, van Doorninck JH, van der Wees J, Grosveld FG. Transcriptome and phenotypic analysis reveals Gata3-dependent signalling pathways in murine hair follicles. Development. 2007;134(2):261–272. doi: 10.1242/dev.02721 [DOI] [PubMed] [Google Scholar]
- 137.León J, Escames G, Rodríguez MI, et al. Inhibition of neuronal nitric oxide synthase activity by N1-acetyl-5-methoxykynuramine, a brain metabolite of melatonin. J Neurochem. 2006;98(6):2023–2033. doi: 10.1111/j.1471-4159.2006.04029.x [DOI] [PubMed] [Google Scholar]
- 138.Sarti P, Magnifico MC, Altieri F, Mastronicola D, Arese M. New Evidence for Cross Talk between Melatonin and Mitochondria Mediated by a Circadian-Compatible Interaction with Nitric Oxide. Int J Mol Sci. 2013;14(6):11259–11276. doi: 10.3390/ijms140611259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Soto-Vega E, Meza I, Ramírez-Rodríguez G, Benitez-King G. Melatonin stimulates calmodulin phosphorylation by protein kinase C. J Pineal Res. 2004;37(2):98–106. doi: 10.1111/j.1600-079X.2004.00141.x [DOI] [PubMed] [Google Scholar]
- 140.Emerit I, Filipe P, Freitas J, Vassy J. Protective effect of superoxide dismutase against hair graying in a mouse model. Photochem Photobiol. 2004;80(3):579–582. doi: [DOI] [PubMed] [Google Scholar]
- 141.Hollfelder B, Blankenburg G, Wolfram LJ, Höcker H. Chemical and physical properties of pigmented and non-pigmented hair ('grey hair’). Int J Cosmet Sci. 1995;17(2):87–89. doi: 10.1111/j.1467-2494.1995.tb00112.x [DOI] [PubMed] [Google Scholar]
- 142.Seiberg M. Age-Induced Hair Graying and Oxidative Stress. In: Farage MA, Miller KW, Maibach HI, eds. Textbook of Aging Skin. Springer Berlin Heidelberg; 2015:1–14. doi: 10.1007/978-3-642-27814-3_117-1 [DOI] [Google Scholar]
- 143.Bertazzo A, Biasiolo M, Costa CVL, Cardin de Stefani E, Allegri G. Tryptophan in human hair: correlation with pigmentation. Il Farmaco. 2000;55(8):521–525. doi: 10.1016/S0014-827X(00)00038-0 [DOI] [PubMed] [Google Scholar]
- 144.Qiao Z, Xu Z, Xiao Q, et al. Dysfunction of ATG7-dependent autophagy dysregulates the antioxidant response and contributes to oxidative stress-induced biological impairments in human epidermal melanocytes. Cell Death Discov. 2020;6(1):1–12. doi: 10.1038/s41420-020-0266-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Fischer TW, Sweatman TW, Semak I, Sayre RM, Wortsman J, Slominski A. Constitutive and UV-induced metabolism of melatonin in keratinocytes and cell-free systems. FASEB J. 2006;20(9):1564–1566. doi: 10.1096/fj.05-5227fje [DOI] [PubMed] [Google Scholar]
- 146.Nahhas AF, Abdel-Malek ZA, Kohli I, Braunberger TL, Lim HW, Hamzavi IH. The potential role of antioxidants in mitigating skin hyperpigmentation resulting from ultraviolet and visible light-induced oxidative stress. Photodermatol Photoimmunol Photomed. 2019;35(6):420–428. doi: 10.1111/phpp.12423 [DOI] [PubMed] [Google Scholar]
- 147.Poljšak B, Dahmane R. Free radicals and extrinsic skin aging. Dermatol Res Pract. 2012;2012:135206. doi: 10.1155/2012/135206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Chaiprasongsuk A, Janjetovic Z, Kim TK, et al. Protective effects of novel derivatives of vitamin D3 and lumisterol against UVB-induced damage in human keratinocytes involve activation of Nrf2 and p53 defense mechanisms. Redox Biol. 2019;24:101206. doi: 10.1016/j.redox.2019.101206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Chaiprasongsuk A, Janjetovic Z, Kim TK, et al. Hydroxylumisterols, Photoproducts of Pre-Vitamin D3, Protect Human Keratinocytes against UVB-Induced Damage. IJMS. 2020;21(24):9374. doi: 10.3390/ijms21249374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Slominski AT, Chaiprasongsuk A, Janjetovic Z, et al. Photoprotective Properties of Vitamin D and Lumisterol Hydroxyderivatives. Cell Biochem Biophys. 2020;78(2):165–180. doi: 10.1007/s12013-020-00913-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Slominski AT, Janjetovic Z, Kim TK, et al. Novel non-calcemic secosteroids that are produced by human epidermal keratinocytes protect against solar radiation. The Journal of Steroid Biochemistry and Molecular Biology. 2015;148:52–63. doi: 10.1016/j.jsbmb.2015.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Rosenberg AM, Rausser S, Ren J, et al. Quantitative mapping of human hair greying and reversal in relation to life stress. Elife. 2021;10:e67437. doi: 10.7554/eLife.67437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Stout R, Birch-Machin M. Mitochondria’s Role in Skin Ageing. Biology. 2019;8(2):29. doi: 10.3390/biology8020029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.van der Bliek AM, Sedensky MM, Morgan PG. Cell Biology of the Mitochondrion. Genetics. 2017;207(3):843–871. doi: 10.1534/genetics.117.300262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Venegas C, García JA, Escames G, et al. Extrapineal melatonin: analysis of its subcellular distribution and daily fluctuations. Journal of Pineal Research. 2012;52(2):217–227. doi: 10.1111/j.1600-079X.2011.00931.x [DOI] [PubMed] [Google Scholar]
- 156.Huo X, Wang C, Yu Z, et al. Human transporters, PEPT1/2, facilitate melatonin transportation into mitochondria of cancer cells: An implication of the therapeutic potential. Journal of Pineal Research. 2017;62(4):e12390. doi: 10.1111/jpi.12390 [DOI] [PubMed] [Google Scholar]
- 157.Suofu Y, Li W, Jean-Alphonse FG, et al. Dual role of mitochondria in producing melatonin and driving GPCR signaling to block cytochrome c release. Proc Natl Acad Sci U S A. 2017;114(38):E7997–E8006. doi: 10.1073/pnas.1705768114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Semak I, Naumova M, Korik E, Terekhovich V, Wortsman J, Slominski A. A novel metabolic pathway of melatonin: oxidation by cytochrome C. Biochemistry. 2005;44(26):9300–9307. doi: 10.1021/bi050202d [DOI] [PubMed] [Google Scholar]
- 159.Reiter RJ, Tan DX, Rosales-Corral S, Galano A, Jou MJ, Acuna-Castroviejo D. Melatonin Mitigates Mitochondrial Meltdown: Interactions with SIRT3. Int J Mol Sci. 2018;19(8). doi: 10.3390/ijms19082439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Tan DX, Manchester LC, Qin L, Reiter RJ. Melatonin: A Mitochondrial Targeting Molecule Involving Mitochondrial Protection and Dynamics. Int J Mol Sci. 2016;17(12). doi: 10.3390/ijms17122124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Paradies G, Paradies V, Ruggiero FM, Petrosillo G. Changes in the mitochondrial permeability transition pore in aging and age-associated diseases. Mechanisms of Ageing and Development. 2013;134(1):1–9. doi: 10.1016/j.mad.2012.12.006 [DOI] [PubMed] [Google Scholar]
- 162.Annapoorna S, Aguilera-Aguirre L, Singh KK. Mitochondria in skin health, aging, and disease. Cell Death and Disease. 2020;11(6). doi: 10.1038/s41419-020-2649-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Fischer TW, Zmijewski MA, Wortsman J, Slominski A. Melatonin maintains mitochondrial membrane potential and attenuates activation of initiator (casp-9) and effector caspases (casp-3/casp-7) and PARP in UVR-exposed HaCaT keratinocytes. J Pineal Res. 2008;44(4):397–407. doi: 10.1111/j.1600-079X.2007.00542.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Glick D, Barth S, Macleod KF. Autophagy: cellular and molecular mechanisms. J Pathol. 2010;221(1):3–12. doi: 10.1002/path.2697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Scherz-Shouval R, Elazar Z. Regulation of autophagy by ROS: physiology and pathology. Trends in Biochemical Sciences. 2011;36(1):30–38. doi: 10.1016/j.tibs.2010.07.007 [DOI] [PubMed] [Google Scholar]
- 166.Coto-Montes A, Boga JA, Rosales-Corral S, Fuentes-Broto L, Tan DX, Reiter RJ. Role of melatonin in the regulation of autophagy and mitophagy: A review. Molecular and Cellular Endocrinology. 2012;361(1):12–23. doi: 10.1016/j.mce.2012.04.009 [DOI] [PubMed] [Google Scholar]
- 167.He Y, Li S, Zhang W, et al. Dysregulated autophagy increased melanocyte sensitivity to H2O2-induced oxidative stress in vitiligo. Sci Rep. 2017;7(1):42394. doi: 10.1038/srep42394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Jeong D, Qomaladewi NP, Lee J, Park SH, Cho JY. The Role of Autophagy in Skin Fibroblasts, Keratinocytes, Melanocytes, and Epidermal Stem Cells. Journal of Investigative Dermatology. 2020;140(9):1691–1697. doi: 10.1016/j.jid.2019.11.023 [DOI] [PubMed] [Google Scholar]
- 169.Bomirski A, Wrzołkowa T, Arendarczyk M, et al. Pathology and ultrastructural characteristics of a hypomelanotic variant of transplantable hamster melanoma with elevated tyrosinase activity. J Invest Dermatol. 1987;89(5):469–473. doi: 10.1111/1523-1747.ep12460928 [DOI] [PubMed] [Google Scholar]
- 170.Bomirski A, Słominski A, Bigda J. The natural history of a family of transplantable melanomas in hamsters. Cancer Metastasis Rev. 1988;7(2):95–118. doi: 10.1007/BF00046481 [DOI] [PubMed] [Google Scholar]
- 171.Murase D, Hachiya A, Takano K, et al. Autophagy Has a Significant Role in Determining Skin Color by Regulating Melanosome Degradation in Keratinocytes. J Invest Dermatol. 2013;133(10):2416–2424. doi: 10.1038/jid.2013.165 [DOI] [PubMed] [Google Scholar]
- 172.Zhu W, Zhao Z, Cheng B. The role of autophagy in skin pigmentation. Eur J Dermatol. 2020;30(6):655–662. doi: 10.1684/ejd.2020.3930 [DOI] [PubMed] [Google Scholar]
- 173.Boga JA, Caballero B, Potes Y, et al. Therapeutic potential of melatonin related to its role as an autophagy regulator: A review. Journal of Pineal Research. 2019;66(1):e12534. doi: 10.1111/jpi.12534 [DOI] [PubMed] [Google Scholar]
- 174.Jenwitheesuk A, Nopparat C, Mukda S, Wongchitrat P, Govitrapong P. Melatonin Regulates Aging and Neurodegeneration through Energy Metabolism, Epigenetics, Autophagy and Circadian Rhythm Pathways. International Journal of Molecular Sciences. 2014;15(9):16848–16884. doi: 10.3390/ijms150916848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Parodi C, Hardman JA, Allavena G, et al. Autophagy is essential for maintaining the growth of a human (mini-)organ: Evidence from scalp hair follicle organ culture. PLoS Biol. 2018;16(3):e2002864. doi: 10.1371/journal.pbio.2002864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Green DR, Galluzzi L, Kroemer G. Mitochondria and the Autophagy–Inflammation–Cell Death Axis in Organismal Aging. Science. 2011;333(6046):1109–1112. doi: 10.1126/science.1201940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Yessenkyzy A, Saliev T, Zhanaliyeva M, et al. Polyphenols as Caloric-Restriction Mimetics and Autophagy Inducers in Aging Research. Nutrients. 2020;12(5):E1344. doi: 10.3390/nu12051344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Lee IH, Cao L, Mostoslavsky R, et al. A role for the NAD-dependent deacetylase Sirt1 in the regulation of autophagy. Proc Natl Acad Sci U S A. 2008;105(9):3374–3379. doi: 10.1073/pnas.0712145105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Xu C, Wang L, Fozouni P, et al. SIRT1 is downregulated by autophagy in senescence and ageing. Nat Cell Biol. 2020;22(10):1170–1179. doi: 10.1038/s41556-020-00579-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Stefan J, Kim TK, Schedel F, et al. Differential and Overlapping Effects of Melatonin and Its Metabolites on Keratinocyte Function: Bioinformatics and Metabolic Analyses. Antioxidants (Basel). 2021;10(4). doi: 10.3390/antiox10040618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Beker MC, Caglayan B, Caglayan AB, et al. Interaction of melatonin and Bmal1 in the regulation of PI3K/AKT pathway components and cellular survival. Sci Rep. 2019;9(1):19082. doi: 10.1038/s41598-019-55663-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Boutin JA, Witt-Enderby PA, Sotriffer C, Zlotos DP. Melatonin receptor ligands: A pharmaco-chemical perspective. J Pineal Res. 2020;69(3):e12672. doi: 10.1111/jpi.12672 [DOI] [PubMed] [Google Scholar]
- 183.Bora NS, Mazumder B, Mandal S, et al. Amelioration of UV radiation-induced photoaging by a combinational sunscreen formulation via aversion of oxidative collagen degradation and promotion of TGF-β-Smad-mediated collagen production. European Journal of Pharmaceutical Sciences. 2019;127:261–275. doi: 10.1016/j.ejps.2018.11.004 [DOI] [PubMed] [Google Scholar]
- 184.Guan LL, Lim HW, Mohammad TF. Sunscreens and Photoaging: A Review of Current Literature. Am J Clin Dermatol. Published online August 13, 2021. doi: 10.1007/s40257-021-00632-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Skobowiat C, Brożyna AA, Janjetovic Z, et al. Melatonin and its derivatives counteract the ultraviolet B radiation-induced damage in human and porcine skin ex vivo. Journal of Pineal Research. 2018;65(2):e12501. doi: 10.1111/jpi.12501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Bocheva G, Slominski RM, Slominski AT. Neuroendocrine Aspects of Skin Aging. Int J Mol Sci. 2019;20(11):E2798. doi: 10.3390/ijms20112798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Rusanova I, Martínez-Ruiz L, Florido J, et al. Protective Effects of Melatonin on the Skin: Future Perspectives. International Journal of Molecular Sciences. 2019;20(19):4948. doi: 10.3390/ijms20194948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Scheuer C, Pommergaard HC, Rosenberg J, Gögenur I. Melatonin’s protective effect against UV radiation: a systematic review of clinical and experimental studies. Photodermatology, Photoimmunology & Photomedicine. 2014;30(4):180–188. doi: 10.1111/phpp.12080 [DOI] [PubMed] [Google Scholar]
- 189.Fisher GJ, Kang S, Varani J, et al. Mechanisms of Photoaging and Chronological Skin Aging. Archives of Dermatology. 2002;138(11):1462–1470. doi: 10.1001/archderm.138.11.1462 [DOI] [PubMed] [Google Scholar]
- 190.Janjetovic Z, Nahmias ZP, Hanna S, et al. Melatonin and its metabolites ameliorate ultraviolet B-induced damage in human epidermal keratinocytes. J Pineal Res. 2014;57(1):90–102. doi: 10.1111/jpi.12146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Kleszczynski K, Fischer TW. Melatonin and human skin aging. Dermato-Endocrinology. 2012;4(3):245–252. doi: 10.4161/derm.22344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Fernández A, Ordóñez R, Reiter RJ, González-Gallego J, Mauriz JL. Melatonin and endoplasmic reticulum stress: relation to autophagy and apoptosis. J Pineal Res. 2015;59(3):292–307. doi: 10.1111/jpi.12264 [DOI] [PubMed] [Google Scholar]
- 193.Yang F, Yang L, Wataya-Kaneda M, et al. Dysregulation of autophagy in melanocytes contributes to hypopigmented macules in tuberous sclerosis complex. J Dermatol Sci. 2018;89(2):155–164. doi: 10.1016/j.jdermsci.2017.11.002 [DOI] [PubMed] [Google Scholar]
- 194.Scheibye-Knudsen M, Ramamoorthy M, Sykora P, et al. Cockayne syndrome group B protein prevents the accumulation of damaged mitochondria by promoting mitochondrial autophagy. Journal of Experimental Medicine. 2012;209(4):855–869. doi: 10.1084/jem.20111721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Fischer TW, Greif C, Fluhr JW, Wigger-Alberti W, Elsner P. Percutaneous penetration of topically applied melatonin in a cream and an alcoholic solution. Skin Pharmacol Physiol. 2004;17(4):190–194. doi: 10.1159/000078822 [DOI] [PubMed] [Google Scholar]
- 196.Bora NS, Mazumder B, Mandal S, et al. Protective effect of a topical sunscreen formulation fortified with melatonin against UV-induced photodermatitis: an immunomodulatory effect via NF-κB suppression. Immunopharmacology and Immunotoxicology. 2019;41(1):130–139. doi: 10.1080/08923973.2019.1566358 [DOI] [PubMed] [Google Scholar]
- 197.Scheuer C, Pommergaard HC, Rosenberg J, Gögenur I. Dose dependent sun protective effect of topical melatonin: A randomized, placebo-controlled, double-blind study. J Dermatol Sci. 2016;84(2):178–185. doi: 10.1016/j.jdermsci.2016.08.007 [DOI] [PubMed] [Google Scholar]
- 198.Hatem S, Nasr M, Moftah NH, Ragai MH, Geneidi AS, Elkheshen SA. Clinical cosmeceutical repurposing of melatonin in androgenic alopecia using nanostructured lipid carriers prepared with antioxidant oils. Expert Opinion on Drug Delivery. 2018;15(10):927–935. doi: 10.1080/17425247.2018.1517740 [DOI] [PubMed] [Google Scholar]
- 199.Chuffa LG de A, Seiva FRF, Novais AA, et al. Melatonin-Loaded Nanocarriers: New Horizons for Therapeutic Applications. Molecules. 2021;26(12):3562. doi: 10.3390/molecules26123562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Shukla R, Tiwari G, Tiwari R, Rai AK. Formulation and evaluation of the topical ethosomal gel of melatonin to prevent UV radiation. Journal of Cosmetic Dermatology. 2020;19(8):2093–2104. doi: 10.1111/jocd.13251 [DOI] [PubMed] [Google Scholar]
- 201.Bertolini M, Ramot Y, Gherardini J, et al. Theophylline exerts complex anti-ageing and anti-cytotoxicity effects in human skin ex vivo. Int J Cosmet Sci. 2020;42(1):79–88. doi: 10.1111/ics.12589 [DOI] [PubMed] [Google Scholar]
- 202.Reiter RJ, Sharma R, Rosales-Corral S. Anti-Warburg Effect of Melatonin: A Proposed Mechanism to Explain its Inhibition of Multiple Diseases. Int J Mol Sci. 2021;22(2):E764. doi: 10.3390/ijms22020764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Majidinia M, Reiter RJ, Shakouri SK, Yousefi B. The role of melatonin, a multitasking molecule, in retarding the processes of ageing. Ageing Research Reviews. 2018;47:198–213. doi: 10.1016/j.arr.2018.07.010 [DOI] [PubMed] [Google Scholar]
- 204.Ashrafizadeh M, Najafi M, Kavyiani N, Mohammadinejad R, Farkhondeh T, Samarghandian S. Anti-Inflammatory Activity of Melatonin: a Focus on the Role of NLRP3 Inflammasome. Inflammation. 2021;44(4):1207–1222. doi: 10.1007/s10753-021-01428-9 [DOI] [PubMed] [Google Scholar]
- 205.Sheikholeslami S, Aryafar T, Abedi-Firouzjah R, et al. The role of melatonin on radiation-induced pneumonitis and lung fibrosis: A systematic review. Life Sciences. 2021;281:119721. doi: 10.1016/j.lfs.2021.119721 [DOI] [PubMed] [Google Scholar]
- 206.Pham L, Baiocchi L, Kennedy L, et al. The interplay between mast cells, pineal gland, and circadian rhythm: Links between histamine, melatonin, and inflammatory mediators. J Pineal Res. 2021;70(2):e12699. doi: 10.1111/jpi.12699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Moreno ACR, Saito R de F, Tiago M, et al. Melatonin inhibits human melanoma cells proliferation and invasion via cell cycle arrest and cytoskeleton remodeling. Melatonin Res. 2020;3(2):194–209. doi: 10.32794/mr11250057 [DOI] [Google Scholar]
- 208.Alvarez-Artime A, Cernuda-Cernuda R, Francisco-Artime-Naveda, et al. Melatonin-Induced Cytoskeleton Reorganization Leads to Inhibition of Melanoma Cancer Cell Proliferation. International Journal of Molecular Sciences. 2020;21(2):548. doi: 10.3390/ijms21020548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Samanta S. Melatonin: an endogenous miraculous indolamine, fights against cancer progression. J Cancer Res Clin Oncol. 2020;146(8):1893–1922. doi: 10.1007/s00432-020-03292-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Kleszczyński K, Bilska B, Stegemann A, et al. Melatonin and Its Metabolites Ameliorate UVR-Induced Mitochondrial Oxidative Stress in Human MNT-1 Melanoma Cells. Int J Mol Sci. 2018;19(12):3786. doi: 10.3390/ijms19123786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Baltatu OC, Senar S, Campos LA, Cipolla-Neto J. Cardioprotective Melatonin: Translating from Proof-of-Concept Studies to Therapeutic Use. IJMS. 2019;20(18):4342. doi: 10.3390/ijms20184342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Xu Z, You W, Liu J, Wang Y, Shan T. Elucidating the Regulatory Role of Melatonin in Brown, White, and Beige Adipocytes. Advances in Nutrition. Published online July 29, 2019:nmz070. doi: 10.1093/advances/nmz070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Munmun F, Witt-Enderby PA. Melatonin effects on bone: Implications for use as a therapy for managing bone loss. Journal of Pineal Research. 2021;71(1):e12749. doi: 10.1111/jpi.12749 [DOI] [PubMed] [Google Scholar]
- 214.Pal PK, Sarkar S, Chattopadhyay A, Tan DX, Bandyopadhyay D. Enterochromaffin cells as the source of melatonin: key findings and functional relevance in mammals. Melatonin Res. 2019;2(4):61–82. doi: 10.32794/mr11250041 [DOI] [Google Scholar]
- 215.Segovia-Roldan M, Diez ER, Pueyo E. Melatonin to Rescue the Aged Heart: Antiarrhythmic and Antioxidant Benefits. Oxidative Medicine and Cellular Longevity. 2021;2021:e8876792. doi: 10.1155/2021/8876792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Mehraein F, Talebi R, Jameie B, Joghataie MT, Madjd Z. Neuroprotective Effect of Exogenous Melatonin on Dopaminergic Neurons of the Substantia Nigra in Ovariectomized Rats. Iran Biomed J. 2011;15(1-2):44–50. [PMC free article] [PubMed] [Google Scholar]
- 217.Chen T, Luo Y, Li Q, et al. Melatonin reduces radiation damage in inner ear. Journal of Radiation Research. 2021;62(2):217–225. doi: 10.1093/jrr/rraa137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Li Y, Wu H, Liu N, et al. Melatonin exerts an inhibitory effect on insulin gene transcription via MTNR1B and the downstream Raf-1/ERK signaling pathway. Int J Mol Med. Published online December 1, 2017. doi: 10.3892/ijmm.2017.3305 [DOI] [PubMed] [Google Scholar]
- 219.Sato K, Meng F, Francis H, et al. Melatonin and circadian rhythms in liver diseases: Functional roles and potential therapies. J Pineal Res. 2020;68(3). doi: 10.1111/jpi.12639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Niu YJ, Zhou W, Nie ZW, Shin KT, Cui XS. Melatonin enhances mitochondrial biogenesis and protects against rotenone-induced mitochondrial deficiency in early porcine embryos. Journal of Pineal Research. 2020;68(2):e12627. doi: 10.1111/jpi.12627 [DOI] [PubMed] [Google Scholar]
- 221.Dudka Y, Zamorskii I, Shchudrova T, Petriuk A, Kopchuk T, Drachuk V. Nephroprotective mechanisms, therapeutic potential and perspective on melatonin use for drug-induced nephropathy. Published online 2021:7. [Google Scholar]
- 222.Alghamdi BS. The neuroprotective role of melatonin in neurological disorders. J Neurosci Res. 2018;96(7):1136–1149. doi: 10.1002/jnr.24220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Huang R, Xu Y, Lu X, et al. Melatonin protects inner retinal neurons of newborn mice after hypoxia-ischemia. Journal of Pineal Research. 2021;71. doi: 10.1111/jpi.12716 [DOI] [PubMed] [Google Scholar]
- 224.Diéguez HH, Fleitas MFG, Aranda ML, et al. Melatonin protects the retina from experimental nonexudative age-related macular degeneration in mice. Journal of Pineal Research. 2020;68(4):e12643. doi: 10.1111/jpi.12643 [DOI] [PubMed] [Google Scholar]
- 225.Cipolla-Neto J, Amaral FG, Soares JM Jr, et al. The crosstalk between melatonin and sex steroid hormones. Neuroendocrinology. Published online March 26, 2021. doi: 10.1159/000516148 [DOI] [PubMed] [Google Scholar]
- 226.Roy D, Belsham DD. Melatonin Receptor Activation Regulates GnRH Gene Expression and Secretion in GT1–7 GnRH Neurons. Journal of Biological Chemistry. 2002;277(1):251–258. doi: 10.1074/jbc.M108890200 [DOI] [PubMed] [Google Scholar]
- 227.González A, Alonso-González C, González-González A, Menéndez-Menéndez J, Cos S, Martínez-Campa C. Melatonin as an Adjuvant to Antiangiogenic Cancer Treatments. Cancers. 2021;13(13):3263. doi: 10.3390/cancers13133263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Kleszczyński K, Böhm M. Can melatonin and its metabolites boost the efficacy of targeted therapy in patients with advanced melanoma? Experimental Dermatology. 2020;29(9):860–863. doi: 10.1111/exd.14144 [DOI] [PubMed] [Google Scholar]
- 229.Pariente R, Bejarano I, Rodríguez AB, Pariente JA, Espino J. Melatonin increases the effect of 5-fluorouracil-based chemotherapy in human colorectal adenocarcinoma cells in vitro. Mol Cell Biochem. 2018;440(1-2):43–51. doi: 10.1007/s11010-017-3154-2 [DOI] [PubMed] [Google Scholar]
- 230.Reiter RJ, Rosales-Corral SA, Tan DX, et al. Melatonin, a Full Service Anti-Cancer Agent: Inhibition of Initiation, Progression and Metastasis. International Journal of Molecular Sciences. 2017;18(4):843. doi: 10.3390/ijms18040843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Zhang J, Xie T, Zhong X, et al. Melatonin reverses nasopharyngeal carcinoma cisplatin chemoresistance by inhibiting the Wnt/β-catenin signaling pathway. Aging (Albany NY). 2020;12(6):5423–5438. doi: 10.18632/aging.102968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Dong K, Goyarts E, Rella A, Pelle E, Wong YH, Pernodet N. Age Associated Decrease of MT-1 Melatonin Receptor in Human Dermal Skin Fibroblasts Impairs Protection Against UV-Induced DNA Damage. Int J Mol Sci. 2020;21(1):E326. doi: 10.3390/ijms21010326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Koçtürk S, Yüksel Egrilmez M, Aktan Ş, et al. Melatonin attenuates the detrimental effects of UVA irradiation in human dermal fibroblasts by suppressing oxidative damage and MAPK/AP-1 signal pathway in vitro. Photodermatol Photoimmunol Photomed. 2019;35(4):221–231. doi: 10.1111/phpp.12456 [DOI] [PubMed] [Google Scholar]
- 234.Kaczmarek-Szczepańska B, Ostrowska J, Kozłowska J, et al. Evaluation of Polymeric Matrix Loaded with Melatonin for Wound Dressing. Int J Mol Sci. 2021;22(11):5658. doi: 10.3390/ijms22115658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Houssay AB, Pazo JH, Epper CE. Effects of the pineal gland upon the hair cycles in mice. J Invest Dermatol. 1966;47(3):230–234. doi: 10.1038/jid.1966.135 [DOI] [PubMed] [Google Scholar]
- 236.Rust CC, Meyer RK. Hair color, molt, and testis size in male, short-tailed weasels treated with melatonin. Science. 1969;165(3896):921–922. doi: 10.1126/science.165.3896.921 [DOI] [PubMed] [Google Scholar]
- 237.Allain D, Rougeot J. Induction of autumn moult in mink (Mustela vison Peale and Beauvois) with melatonin. Reprod Nutr Dev. 1980;20(1A):197–201. doi: 10.1051/rnd:19800114 [DOI] [PubMed] [Google Scholar]
- 238.Lincoln GA, Ebling FJP. Effect of constant-release implants of melatonin on seasonal cycles in reproduction, prolactin secretion and moulting in rams. Reproduction. 1985;73(1):241–253. doi: 10.1530/jrf.0.0730241 [DOI] [PubMed] [Google Scholar]
- 239.Allain D, Ravault JP, Panaretto BA, Rougeot J. Effects of Pinealectomy on Photoperiodic Control of Hair Follicle Activity in the Limousine Ram: Possible Relationships With Plasma Prolactin Levels. Journal of Pineal Research. 1986;3(1):25–32. doi: 10.1111/j.1600-079X.1986.tb00723.x [DOI] [PubMed] [Google Scholar]
- 240.Rose J, Oldfield J, Stormshak F. Apparent role of melatonin and prolactin in initiating winter fur growth in mink. General and Comparative Endocrinology. 1987;65(2):212–215. doi: 10.1016/0016-6480(87)90168-7 [DOI] [PubMed] [Google Scholar]
- 241.Welch RAS, Gurnsey MP, Betteridge K, Mitchell RJ Goat fibre response to melatonin given in spring in two consecutive years. Proc N Z Soc Anim. 1990;50:335–338. [Google Scholar]
- 242.Webster JR, Suttie JM, Corson ID. Effects of melatonin implants on reproductive seasonality of male red deer (Cervus elaphus). Reproduction. 1991;92(1):1–11. doi: 10.1530/jrf.0.0920001 [DOI] [PubMed] [Google Scholar]
- 243.McCloghry E, Foldes A, Hollis D, et al. Effects of pinealectomy on wool growth and wool follicle density in Merino sheep. Journal of Pineal Research. 1992;13(3):139–144. doi: 10.1111/j.1600-079X.1992.tb00068.x [DOI] [PubMed] [Google Scholar]
- 244.Nixon AJ, Choy VJ, Parry AL, Pearson AJ. Fiber growth initiation in hair follicles of goats treated with melatonin. Journal of Experimental Zoology. 1993;267(1):47–56. doi: 10.1002/jez.1402670108 [DOI] [PubMed] [Google Scholar]
- 245.Ibraheem M, Galbraith H, Scaife J, Ewen S. Growth of secondary hair follicles of the Cashmere goat in vitro and their response to prolactin and melatonin. J Anat. 1994;185 (Pt 1):135–142. [PMC free article] [PubMed] [Google Scholar]
- 246.Paterson AM, Foldes A. Melatonin and farm animals: Endogenous rhythms and exogenous applications. Journal of Pineal Research. 1994;16(4):167–177. doi: 10.1111/j.1600-079X.1994.tb00097.x [DOI] [PubMed] [Google Scholar]
- 247.Nixon AJ, Ashby MG, Saywell DP, Pearson AJ. Seasonal fiber growth cycles of ferrets (Mustela putorius furo) and long-term effects of melatonin treatment. Journal of Experimental Zoology. 1995;272(6):435–445. doi: 10.1002/jez.1402720605 [DOI] [PubMed] [Google Scholar]
- 248.Xiao Y, Forsberg M, Laitinen JT, Valtonen M. Effects of melatonin implants in spring on testicular regression and moulting in adult male raccoon dogs (Nyctereutes procynoides). Reproduction. 1995;105(1):9–15. doi: 10.1530/jrf.0.1050009 [DOI] [PubMed] [Google Scholar]
- 249.Diaz SF, Torres SMF, Nogueira SAF, Gilbert S, Jessen CR. The impact of body site, topical melatonin and brushing on hair regrowth after clipping normal Siberian Husky dogs. Vet Dermatol. 2006;17(1):45–50. doi: 10.1111/j.1365-3164.2005.00497.x [DOI] [PubMed] [Google Scholar]
- 250.Feng Y, Gun S. Melatonin supplement induced the hair follicle development in offspring rex rabbits. J Anim Physiol Anim Nutr (Berl). 2021;105(1):167–174. doi: 10.1111/jpn.13417 [DOI] [PubMed] [Google Scholar]
- 251.Liu J, Mu Q, Liu Z, et al. Melatonin Regulates the Periodic Growth of Cashmere by Upregulating the Expression of Wnt10b and β-catenin in Inner Mongolia Cashmere Goats. Front Genet. 2021;0. doi: 10.3389/fgene.2021.665834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Fischer TW, Fischer A, Knöll B, Hipler UC, Elsner P. Melatonin in low doses enhances in vitro human hair follicle proliferation and inhibits hair growth in high doses. Arch Derm Res. 2000;292:147. [Google Scholar]
- 253.Fischer TW, Burmeister G, Schmidt HW, Elsner P. Melatonin increases anagen hair rate in women with androgenetic alopecia or diffuse alopecia: results of a pilot randomized controlled trial. British Journal of Dermatology. 2004;150(2):341–345. doi: 10.1111/j.1365-2133.2004.05685.x [DOI] [PubMed] [Google Scholar]
- 254.Clive D, Snell RS. Effect of Melatonin on Mammalian Hair Color**From the Department of Anatomy, The New Jersey College of Medicine and Dentistry, Jersey City, N.J. 07304. Journal of Investigative Dermatology. 1969;53(2):159–162. doi: 10.1038/jid.1969.123 [DOI] [PubMed] [Google Scholar]
- 255.Hoffmann K. Photoperiodic effects in the Djungarian hamster: one minute of light during darktime mimics influence of long photoperiods on testicular recrudescence, body weight and pelage colour. Experientia. 1979;35(11):1529–1530. doi: 10.1007/BF01962828 [DOI] [PubMed] [Google Scholar]
- 256.Logan A, Weatherhead B. Post-tyrosinase Inhibition of Melanogenesis by Melatonin in Hair Follicles in Vitro. Journal of Investigative Dermatology. 1980;74(1):47–50. doi: 10.1111/1523-1747.ep12514608 [DOI] [PubMed] [Google Scholar]
- 257.Thody AJ, Ridley K, Carter RJ, Lucas AM, Shuster S. α-MSH and coat color changes in the mouse. Peptides. 1984;5(6):1031–1036. doi: 10.1016/0196-9781(84)90166-9 [DOI] [PubMed] [Google Scholar]
- 258.Küderling I, Cedrini MC, Fraschini F, Spagnesi M. Season-dependent effects of melatonin on testes and fur color in mountain hares (Lepus timidus L.). Experientia. 1984;40(5):501–502. doi: 10.1007/BF01952407 [DOI] [PubMed] [Google Scholar]
- 259.Duncan MJ, Goldman BD, Di Pinto MN, Stetson MH. Testicular function and pelage color have different critical daylengths in the Djungarian hamster, Phodopus sungorus sungorus. Endocrinology. 1985;116(1):424–430. doi: 10.1210/endo-116-1-424 [DOI] [PubMed] [Google Scholar]
- 260.Lerchl A, Schlatt S. Influence of Photoperiod on Pineal Melatonin Synthesis, Fur Color, Body Weight, and Reproductive Function in the Female Djungarian Hamster, Phodopus sungorus. NEN. 1993;57(2):359–364. doi: 10.1159/000126380 [DOI] [PubMed] [Google Scholar]
- 261.Boutin JA. Quinone reductase 2 as a promising target of melatonin therapeutic actions. Expert Opin Ther Targets. 2016;20(3):303–317. doi: 10.1517/14728222.2016.1091882 [DOI] [PubMed] [Google Scholar]
- 262.Vella F, Ferry G, Delagrange P, Boutin JA. NRH:quinone reductase 2: an enzyme of surprises and mysteries. Biochem Pharmacol. 2005;71(1-2):1–12. doi: 10.1016/j.bcp.2005.09.019 [DOI] [PubMed] [Google Scholar]
- 263.Benedusi M, Frigato E, Beltramello M, Bertolucci C, Valacchi G. Circadian clock as possible protective mechanism to pollution induced keratinocytes damage. Mech Ageing Dev. 2018;172:13–20. doi: 10.1016/j.mad.2017.08.017 [DOI] [PubMed] [Google Scholar]
- 264.Reppert SM, Weaver DR. Molecular Analysis of Mammalian Circadian Rhythms. Annual Review of Physiology. 2001;63(1):647–676. doi: 10.1146/annurev.physiol.63.1.647 [DOI] [PubMed] [Google Scholar]
- 265.Vitaterna MH, Selby CP, Todo T, et al. Differential regulation of mammalian Period genes and circadian rhythmicity by cryptochromes 1 and 2. Proceedings of the National Academy of Sciences. 1999;96(21):12114–12119. doi: 10.1073/pnas.96.21.12114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Sarkar S, Porter KI, Dakup PP, et al. Circadian clock protein BMAL1 regulates melanogenesis through MITF in melanoma cells. Pigment Cell & Melanoma Research. 2021;34(5):955–965. doi: 10.1111/pcmr.12998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Coelho LA, Andrade-Silva J, Motta-Teixeira LC, Amaral FG, Reiter RJ, Cipolla-Neto J. The Absence of Pineal Melatonin Abolishes the Daily Rhythm of Tph1 (Tryptophan Hydroxylase 1), Asmt (Acetylserotonin O-Methyltransferase), and Aanat (Aralkylamine N-Acetyltransferase) mRNA Expressions in Rat Testes. Mol Neurobiol. 2019;56(11):7800–7809. doi: 10.1007/s12035-019-1626-y [DOI] [PubMed] [Google Scholar]
- 268.Coelho LA, Peres R, Amaral FG, Reiter RJ, Cipolla-Neto J. Daily differential expression of melatonin-related genes and clock genes in rat cumulus-oocyte complex: changes after pinealectomy. J Pineal Res. 2015;58(4):490–499. doi: 10.1111/jpi.12234 [DOI] [PubMed] [Google Scholar]
- 269.Al-Nuaimi Y, Hardman JA, Bíró T, et al. A Meeting of Two Chronobiological Systems: Circadian Proteins Period1 and BMAL1 Modulate the Human Hair Cycle Clock. Journal of Investigative Dermatology. 2014;134(3):610–619. doi: 10.1038/jid.2013.366 [DOI] [PubMed] [Google Scholar]
- 270.Slominski A, Paus R, Costantino R. Differential expression and activity of melanogenesis-related proteins during induced hair growth in mice. J Invest Dermatol. 1991;96(2):172–179. doi: 10.1111/1523-1747.ep12460956 [DOI] [PubMed] [Google Scholar]
- 271.Lee J, Moulik M, Fang Z, et al. Bmal1 and -Cell Clock Are Required for Adaptation to Circadian Disruption, and Their Loss of Function Leads to Oxidative Stress-Induced -Cell Failure in Mice. Molecular and Cellular Biology. 2013;33(11):2327–2338. doi: 10.1128/MCB.01421-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Geyfman M, Kumar V, Liu Q, et al. Brain and muscle Arnt-like protein-1 (BMAL1) controls circadian cell proliferation and susceptibility to UVB-induced DNA damage in the epidermis. Proceedings of the National Academy of Sciences. 2012;109(29):11758–11763. doi: 10.1073/pnas.1209592109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Jeayeng S, Wongkajornsilp A, Slominski AT, Jirawatnotai S, Sampattavanich S, Panich U. Nrf2 in keratinocytes modulates UVB-induced DNA damage and apoptosis in melanocytes through MAPK signaling. Free Radic Biol Med. 2017;108:918–928. doi: 10.1016/j.freeradbiomed.2017.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Bocheva G; Slominski RM; Janjetovic Z; Kim T-K; Böhm M; Steinbrink K; Reiter RJ; Kleszczyński K; Slominski AT Protective Role of Melatonin and Its Metabolites in Skin Aging. Int. J. Mol. Sci 2022, 23, 1238. 10.3390/ijms23031238 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.